Abstract
Background and Aims
Quetiapine is an atypical antipsychotic predominantly metabolized by the cytochrome P450 3A4 (CYP3A4) enzyme. We studied the risk of adverse events following coprescription of clarithromycin (a strong CYP3A4 inhibitor) versus azithromycin (not a CYP3A4 inhibitor) in quetiapine users.
Materials and Methods
This was a population‐based retrospective cohort study from 2004 to 2020 in Ontario, Canada in adult quetiapine users newly co‐prescribed clarithromycin (n = 16,909) or azithromycin (n = 25,267). The primary outcome was the composite of hospital encounters with encephalopathy (defined as a diagnosis of delirium, disorientation, transient alteration of awareness, transient ischemic attack, or unspecified dementia), a fall, or a fracture within 30 days of new coprescription. Secondary outcomes were individual components of the composite outcome, hospital encounter with computed tomography (CT) head scan, and all‐cause mortality.
Results
Coprescription of clarithromycin versus azithromycin with quetiapine was associated with a higher risk of the primary composite outcome (365 of 16,909 clarithromycin users [2.2%] vs. 309 of 16,929 azithromycin users [1.8%]; absolute risk increase, 0.34% [95% confidence interval, CI, 0.04–0.63]; relative risk [RR], 1.19 [95% CI, 1.02–1.38]). This was primarily driven by an increase in fragility fractures (78 of 16,909 clarithromycin users [0.5%] vs. 45 of 16,923 azithromycin users [0.3%]; absolute risk increase, 0.20% [95% CI, 0.07–0.32]; RR, 1.74 [95% CI, 1.21–2.52]). Hospital encounters with a CT head scan were higher in clarithromycin users (220 of 16,909 [1.3%] vs. 175 of 16,923 azithromycin users [1.0%]; absolute risk increase, 0.27% [95% CI, 0.04–0.50]; RR, 1.26 [95% CI, 1.04–1.54]), but there was no difference in hospital encounters with encephalopathy, falls, or all‐cause mortality between macrolide groups.
Conclusion
Among adults taking quetiapine, concurrent use of clarithromycin compared with azithromycin was associated with a small but statistically greater 30‐day risk of a hospital encounter for encephalopathy, falls, or fracture, which was predominantly related to a higher rate of fragility fractures.
Keywords: drug interactions, epidemiology, pharmacovigilance, polypharmacy
1. INTRODUCTION
Quetiapine is a widely prescribed second‐generation antipsychotic agent approved for the treatment of schizophrenia, bipolar disorder, and major depressive disorder with the extended‐release formulation. There can be side effects with use of quetiapine, particularly when used off‐label. Despite this, off‐label low‐dose quetiapine usage now accounts for 92% of prescriptions for nonapproved indications including behavioral and psychological symptoms of dementia, anxiety disorders, insomnia, and posttraumatic stress disorder. 1 , 2 , 3 , 4 By 2010, quetiapine was the fifth highest‐selling pharmaceutical medicine with estimated annual sales of $6.8 billion. 5 Second‐generation antipsychotics like quetiapine have increased in popularity due to lower incidence of extrapyramidal symptoms and tardive dyskinesia. 6 Quetiapine exerts its biological effect in schizophrenia and bipolar disorder through antagonism of dopamine 2 receptors, with recommended daily dosages ranging from 150 to 800 mg to achieve full dopamine 2 receptor antagonism. 7 , 8 , 9
At low unapproved dosages (frequently ≤25 mg daily), quetiapine predominantly antagonizes histamine‐1 receptor and alpha‐1 adrenergic receptors resulting in sedative and anxiolytic effect but may also be associated with orthostatic hypotension. 10 Moreover, in 2005 the United States Food and Drug Administration issued a black box warning for antipsychotics due to the association with increased mortality including sudden cardiac death. 11 , 12 Atypical antipsychotic usage is also associated with increased risk of nonvertebral osteoporotic fractures, falls, and pneumonia in the elderly. 13 , 14 , 15 , 16 , 17 Further, clinicians often prescribe quetiapine as an alternative to benzodiazepines, 5 but the rates of quetiapine‐related poison control center calls in France increased from 2011 to 2017 with 21% of cases being unintentional overdoses. 18 , 19 In the overdose setting, patients most commonly present with drowsiness, coma, tachycardia, postural hypotension, and sinus tachycardia with dosages of >1500 mg associated with severe poisoning. In comparison to olanzapine and risperidone, quetiapine appears to have a lower risk of mortality, but is associated with higher risk for falls. 4
Among second‐generation antipsychotics, quetiapine is also unique in that it is metabolized predominantly by the cytochrome P450 3A4 (CYP3A4) 4 to the active metabolite norquetiapine. 2 Both quetiapine and norquetiapine are pharmacologically active. When used concomitantly with a strong inhibitor of CYP3A4, it is recommended that the quetiapine dosage be reduced by sixfold and titrated to clinical effect, although no specific guidance is provided for moderate CYP3A4 inhibitors. Clarithromycin and azithromycin are macrolide antibiotics commonly prescribed for the treatment of community‐acquired pneumonia. We have previously demonstrated that macrolides are helpful for assessing population‐based drug interactions as clarithromycin is a moderate inhibitor of CYP3A4 while azithromycin does not affect CYP3A4 metabolism. 20 , 21 , 22 , 23
A clinically relevant drug interaction between macrolides and quetiapine has been observed. A previous study in 19 patients coadministered erythromycin (which also inhibits CYP3A4) and quetiapine demonstrated that the elimination half‐life of quetiapine increased by 92% and recommended dosage modification of quetiapine. 24 Furthermore, elderly individuals have been found to have 30–50% slower metabolism of quetiapine than younger individuals likely related to aging‐related changes in drug metabolizing enzymes. 25 These studies and case reports have suggested that coadministration of quetiapine with CYP3A4 inhibitors may represent a clinically significant drug–drug interaction risk, with the elderly particularly prone to adverse events including delirium. 26 Therefore, we conducted this population‐based study to estimate the 30‐day risk for hospitalization with encephalopathy, falls, or fragility fractures following coprescription of clarithromycin compared to azithromycin in adults taking quetiapine.
2. MATERIALS AND METHODS
2.1. Design and setting
We conducted a population‐based, retrospective cohort study using linked healthcare databases in Ontario, Canada between June 1, 2002, and March 1, 2020. Ontario has a population of 14.6 million residents who have universal access to hospital care and physician services, with individuals 65 years of age or older receiving universal drug coverage (14% of the population or 2 million persons). Emigration from the province, which occurs at a rate of 0.5% per year, was the only reason for loss to follow‐up. Individuals under the age of 65 are eligible for drug coverage if they receive disability support, receive social assistance, or have high drug costs relative to their income. Data sets were linked using unique encoded identifiers and analyzed at ICES. The use of administrative data in this project was authorized under section 45 of Ontario's Personal Health Information Protection Act which does not require review by a research ethics board. The full data set creation plan and underlying analytic code are available from the authors upon request. The reporting of this study follows the REporting of studies Conducted using Observational Routinely Collected Health Data for Pharmacoepidemiology Research (RECORD‐PE) which is an extension of the STROBE guideline (Supporting Information: Table S1).
2.2. Data sources
We ascertained patient characteristics, drug use, covariate information, and outcome data using linked administrative databases (Supporting Information: Table S2). We obtained vital statistics from the Ontario Registered Persons Database, which contains demographic information on all Ontario residents ever issued a health card. We used the Ontario Drug Benefit program database to determine prescription drug use. This database contains accurate records of all dispensed outpatient prescriptions, with an error rate of 0.7%. 27 We identified diagnostic and procedural information on all hospitalizations and emergency department visits from the Canadian Institute for Health Information Discharge Abstract Database and the National Ambulatory Care Reporting System database, respectively. We also obtained diagnostic and procedural information from the Ontario Health Insurance Plan database, which includes health claims for physician services. We used the ICES Physician Database to ascertain drug prescriber information. Mental health diagnoses were obtained from the Ontario Mental Health Reporting System.
International Classification of Diseases, 9th revision (ICD‐9; pre‐2002) and 10th revision (ICD‐10; post‐2002) codes were used to assess baseline comorbidities in the 5 years before receipt of a prescription (Supporting Information: Table S3). Only ICD‐10 codes were used to ascertain outcomes, as all outcomes would have occurred after implementation of this coding system in April 2002. Hospital admission and diagnoses are coded by trained personnel using physician‐recorded diagnoses.
2.3. Cohort build and exposure categorization
We established two cohorts of patients in Ontario, Canada with evidence of a new outpatient prescription for either azithromycin or clarithromycin of 5–14 days duration, dispensed between June 1, 2002, and March 1, 2020, with concurrent quetiapine usage. The date of antibiotic dispensing served as the start date for follow‐up, also referred to as the index date. Azithromycin users were selected as the referent group. Both clarithromycin and azithromycin in Ontario are prescribed for near‐identical infections (e.g., respiratory tract, sinus, and oropharyngeal infections), and typically prescribed by primary care physicians to patients with similar comorbidities (e.g., not significantly different in their risk of hospitalization with neuroimaging as a proxy for delirium in the absence of another interacting drug (clarithromycin vs. azithromycin: adjusted relative risk, RR, 1.04 [95% confidence interval, CI, 0.93–1.18]). 20 We a priori decided not to include the CYP3A4 inhibitor erythromycin, because in Ontario this drug was prescribed less than once for every 20 clarithromycin prescriptions. 20 , 21 From both groups we excluded the following patients: (1) age less than 18 years on index date, (2) less than two prescription for quetiapine in the 210 days before and including the index date to ensure ongoing quetiapine usage (3) evidence of more than one antibiotic prescription on the index date or any other antibiotic prescription in the 30 days before the index date (4) those who had a hospital discharge or emergency department visit in the 2 days before index date (to ensure these were new outpatient antibiotic prescriptions) (5) evidence of usage of a non‐study antipsychotic agent in the 180 days before and including the index date (Supporting Information: Table S3) (6) evidence of a strong CYP3A4 inhibitor on or in the 180 days before and including the index date (Supporting Information: Table S4). Patients with multiple eligible antibiotic study prescriptions were restricted to the first eligible study prescription. Dosage of antibiotics were restricted to valid dosages of azithromycin (250–500 mg daily) and clarithromycin (500–1000 mg daily).
2.4. Outcomes
2.4.1. Primary outcome
All outcomes were prespecified. We assessed the primary composite outcome of hospital encounter (hospitalization or emergency department visit) with encephalopathy, fall, or fragility fracture, within 30 days following the index date with a maximum follow‐up of March 31, 2020. Fragility fractures included vertebral, humeral, radial, ulnar, or pelvic fractures. This composite outcome was selected given that the main toxicity of quetiapine overdose is oversedation or coma which may lead to hospital visits for encephalopathy and given known associations of atypical antipsychotic usage with falls and fractures in the elderly.
We assessed outcomes using ICD‐10 diagnosis codes (Supporting Information: Table S2) that are well‐validated in Ontario, we have previously used a composite outcome for encephalopathy which includes delirium, unspecified dementia (unclear diagnosis of dementia), transient cerebral ischemic attack, disorientation, or a transient alteration of awareness. 28 , 29 , 30 ICD‐10 codes for encephalopathy have previously been compared to a reference standard in a cohort of patient undergoing cardiac surgery with a diagnosis of delirium obtained from the hospital clinical data with high specificity (98% [interquartile range, IQR, 93%–100%] but low sensitivity 18% [IQR, 10%–30%]. 31 In a sensitivity analysis, we excluded ICD‐10 codes for transient ischemic attacks given that aphasia has only rarely been reported in patients with delirium related to quetiapine. 32 We used a validated algorithm to identify fractures which has been used in previous research studies which includes hip, forearm, vertebral, humeral, and pelvic fracture ICD‐10 codes, in addition to physician billing codes. 33 , 34 , 35 The follow‐up period of 30 days was chosen to focus on acute adverse events, the short duration of antibiotic prescription, and to avoid potential crossovers between the study groups that might occur with longer follow‐up.
2.4.2. Secondary outcomes
We assessed the individual components of our composite primary outcome separately: hospital encounter with encephalopathy, fall, fragility fracture. We also assessed a computed tomography (CT) scan of the brain as a proxy for the work‐up of delirium. Overall all‐cause mortality was also evaluated.
2.5. Statistical analyses
Baseline characteristics were reported as frequencies and proportions for categorical measures and mean (standard deviation, SD) or median (25th percentile, 75th percentile) for continuous variables. Standardized differences were used to compare baseline characteristics between clarithromycin and azithromycin users, with a value greater than 10% indicating imbalance. 36
Propensity scores estimating the probability of clarithromycin prescription were derived using multivariable logistic regression with 71 measured baseline characteristics. Inverse probability of treatment weights based on the propensity scores was used to form a weighted cohort in which the baseline characteristics were independent of the antibiotic treatment. Patients in the azithromycin group were weighted (propensity score/[1−propensity score]). This method produced a weighted pseudo‐sample of patients in the azithromycin group with the same distribution of measured covariates as the clarithromycin group. Variables included in the propensity score model are shown in Supporting Information: Table S5 and were selected to account for potential confounders.
Logistic regression modeling to estimate odds ratios (ORs) and 95% CIs with and without inverse probability weighting was performed for the primary and secondary outcomes. Each OR was approximated to be the RR which was appropriate given the incidences observed. In all outcome analyses, we interpreted two‐tailed p values < 0.05 as statistically significant.
A prespecified sensitivity analysis using receipt of a CT scan of the head in emergency department as an alternative definition of encephalopathy. Post hoc sensitivity analyses included restricting only to quetiapine immediate release formulations which may have a more immediate profound sedating effect, and an E‐value analysis for unmeasured confounding. We performed all analyses using SAS version 9.4 (SAS Institute).
3. RESULTS
3.1. Baseline characteristics
From June 1, 2002, to March 1, 2020, we identified 42,176 quetiapine users with a new macrolide prescription (16,909 were prescribed clarithromycin and 25,267 azithromycin) who met the study criteria. Over the accrual period, the number of prescriptions for clarithromycin began to decline in 2012 but was still widely co‐prescribed with quetiapine throughout the period of observation (Supporting Information: Table S6). The study flow diagram for creation of the cohorts is shown in Figure 1.
Figure 1.
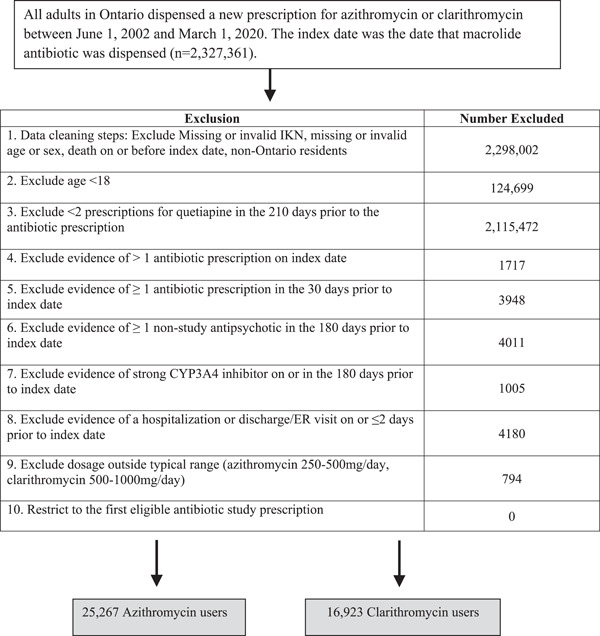
Study flow diagram describing the cohort creation of azithromycin and clarithromycin users after applying inclusion and exclusion criteria.
Both clarithromycin and azithromycin groups were well balanced on demographics, comorbidities, medication usage, and healthcare utilization before and following weighting with a mean age of 57. In the overall cohort, 37% of individuals were older adults (age ≥ 65), and 64% were female. The prevalence of psychiatric disorders was high in the cohorts: 34% had bipolar disorder, 28% had schizophrenia or another psychotic disorder, and 49% had unipolar depression or anxiety. In the prior year, 12% of individuals had an episode of encephalopathy, 10% had a fall, and 4% had experienced a fragility fracture. Following propensity score‐based weighting, standardized differences were <10% for all baseline characteristics (Table 1 and Supporting Information: Table S7).
Table 1.
Baseline characteristics of quetiapine users with new prescription for azithromycin and clarithromycin before and following weighting.
| Characteristic, n (%) | Unweighted | Weighteda | ||||
|---|---|---|---|---|---|---|
| Azithromycin (n = 25,267) | Clarithromycin (n = 16,909) | Standardized difference (%) | Azithromycin (n = 16,923) | Clarithromycin (n = 16,909) | Standardized difference (%) | |
| Demographics | ||||||
| Age (years), mean (SD) | 57.7 (20.2) | 57.2 (19.9) | 3 | 56.9 (16.7) | 57.2 (19.9) | 1 |
| Age ≥65 | 10,194 (40.0) | 6306 (37.3) | 6 | 6207 (36.7) | 6306 (37.3) | 1 |
| Female | 16,130 (63.8) | 10,813 (63.9) | 0 | 10,756 (63.6) | 10,813 (63.9) | 1 |
| Income quintile | ||||||
| 1 (lowest income) | 9257 (36.6) | 6438 (38.1) | 3 | 6457 (38.2) | 6438 (38.1) | 0 |
| 2 | 5679 (22.6) | 3621 (21.4) | 3 | 3662 (21.6) | 3621 (21.4) | 0 |
| 3 | 4260 (16.9) | 2760 (16.3) | 2 | 2760 (16.3) | 2760 (16.3) | 0 |
| 4 | 3322 (13.2) | 2286 (13.6) | 1 | 2265 (13.4) | 2286 (13.5) | 0 |
| 5 (highest income) | 2749 (10.9) | 1804 (10.7) | 1 | 1779 (10.5) | 1804 (10.7) | 1 |
| Long‐term care | 3011 (11.9) | 2910 (17.2) | 15 | 2868 (16.9) | 2910 (17.2) | 1 |
| Rural residence | 2423 (9.6) | 1456 (8.6) | 3 | 1488 (8.8) | 1456 (8.6) | 1 |
| Comorbidities in the prior 5 years | ||||||
| Charlson comorbidity index, mean (SD)b | 0.49 (1.2) | 0.5 (1.2) | 0 | 0.5 (0.95) | 0.49 (1.2) | 2 |
| Acute kidney injury | 1213 (4.8) | 569 (3.4) | 7 | 548 (3.2) | 569 (3.4) | 1 |
| Alcoholism | 1097 (4.3) | 756 (4.5) | 1 | 757 (4.5) | 756 (4.5) | 0 |
| Angina | 2734 (10.8) | 1972 (11.7) | 3 | 1989 (11.8) | 1972 (11.7) | 0 |
| Atrial fibrillation/flutter | 3710 (14.7) | 2198 (13) | 5 | 2150 (12.7) | 2198 (13) | 1 |
| Bipolar disorder | 8622 (34.1) | 5732 (33.9) | 0 | 5713 (33.8) | 5732 (33.9) | 0 |
| Cancer | 2041 (8.1) | 1267 (7.5) | 2 | 1256 (7.4) | 1267 (7.5) | 0 |
| Chronic kidney disease | 2639 (10.4) | 1439 (8.5) | 6 | 1410 (8.3) | 1439 (8.5) | 1 |
| Chronic liver disease | 2226 (8.8) | 1385 (8.2) | 2 | 1404 (8.3) | 1385 (8.2) | 0 |
| Chronic lung disease | 10,109 (0.4) | 7289 (43.1) | 6 | 7297 (43.1) | 7289 (43.1) | 0 |
| Congestive heart failure | 2558 (10.1) | 1628 (9.6) | 2 | 1601 (9.5) | 1628 (9.6) | 0 |
| Coronary artery disease | 5405 (21.4) | 3676 (21.7) | 1 | 3669 (21.7) | 3676 (21.7) | 0 |
| Delirium | 1476 (5.8) | 817 (4.8) | 4 | 792 (4.7) | 817 (4.8) | 0 |
| Dementia | 6312 (25) | 4393 (26) | 2 | 4270 (25.2) | 4393 (26) | 2 |
| Diabetes | 4009 (15.9) | 2339 (13.8) | 6 | 2318 (13.7) | 2339 (13.8) | 0 |
| Encephalopathy | 2889 (11.4) | 1970 (11.7) | 1 | 1940 (11.5) | 1970 (11.7) | 1 |
| Falls (1 year prior) | 2533 (10) | 1630 (9.6) | 1 | 1609 (9.5) | 1630 (9.6) | 0 |
| Fractures (1 year prior) | 1043 (4.1) | 683 (4) | 1 | 673 (4) | 683 (4) | 0 |
| Hypotension | 595 (2.4) | 367 (2.2) | 1 | 357 (2.1) | 367 (2.2) | 1 |
| Hypertension | 11425 (45.2) | 7487 (44.3) | 2 | 7446 (44) | 7487 (44.3) | 1 |
| Myocardial infarction | 658 (2.6) | 380 (2.2) | 3 | 376 (2.2) | 380 (2.2) | 0 |
| Osteoporosis | 2198 (8.7) | 1454 (8.6) | 0 | 1489 (8.8) | 1454 (8.6) | 1 |
| Parkinson's disease | 1110 (4.4) | 802 (4.7) | 1 | 779 (4.6) | 802 (4.7) | 0 |
| Peripheral vascular disease | 256 (1) | 152 (0.9) | 1 | 153 (0.9) | 152 (0.9) | 0 |
| Respiratory infection | 12,295 (48.7) | 8173 (48.3) | 1 | 8163 (48.2) | 8173 (48.3) | 0 |
| Rheumatoid arthritis | 1269 (5) | 873 (5.2) | 1 | 871 (5.1) | 873 (5.2) | 0 |
| Schizophrenia or other psychotic disorders | 6922 (27.4) | 4746 (28.1) | 2 | 4743 (28) | 4746 (28.1) | 0 |
| Seizure | 327 (1.3) | 182 (1.1) | 2 | 176 (1) | 182 (1.1) | 1 |
| Stroke/TIA | 770 (3) | 605 (3.6) | 3 | 589 (3.5) | 605 (3.6) | 1 |
| Unipolar depression or anxiety disorder | 12,341 (48.8) | 8229 (48.7) | 0 | 8279 (48.9) | 8229 (48.7) | 0 |
| Ventricular tachycardia | 76 (0.3) | 36 (0.2) | 2 | 38 (0.2) | 36 (0.2) | 0 |
| Prescribing physician characteristics | ||||||
| Family physician | 21,543 (85) | 14,322 (85) | 0 | 14,303 (84.5) | 14,322 (84.7) | 1 |
| Healthcare contacts in prior year | ||||||
| Hospitalizations, mean (SD) | 0.3 (0.9) | 0.3 (0.9) | 2 | 0.3 (0.7) | 0.3 (0.9) | 1 |
| ER visits, mean (SD) | 1.7 (3.6) | 1.8 (3.5) | 0 | 1.8 (2.9) | 1.8 (3.5) | 0 |
| Primary care physician visits, mean (SD) | 15.8 (16.7) | 16.9 (17.2) | 7 | 16.9 (14) | 16.9 (17.2) | 0 |
| CT head (prior 365 days) | 3756 (14.9) | 2444 (14.5) | 1 | 2416 (14.3) | 2444 (14.5) | 1 |
| MRI head (prior 365 days) | 688 (2.7) | 499 (3) | 2 | 485 (2.9) | 499 (3) | 1 |
| Electroencephalography (prior 365 days) | 427 (1.7) | 274 (1.6) | 1 | 274 (1.6) | 274 (1.6) | 0 |
| Medication use (120 days) | ||||||
| Number of unique drugs prescribed, mean (SD) | 4.21 (6) | 3.8 (5.7) | 7 | 3.7 (4.6) | 3.8 (5.7) | 2 |
| Acetylsalicylic acid (ASA) | 306 (1.2) | 229 (1.4) | 2 | 221 (1.3) | 229 (1.4) | 1 |
| Anticonvulsants | 1868 (7.4) | 923 (5.5) | 8 | 903 (5.3) | 923 (5.5) | 1 |
| Antidepressants | 5766 (22.8) | 3671 (21.7) | 3 | 3611 (21.3) | 3671 (21.7) | 1 |
| Antiemetics | 175 (0.7) | 137 (0.8) | 1 | 138 (0.8) | 137 (0.8) | 0 |
| Antihistamines | 14 (0.1) | 18 (0.1) | 0 | 3184 (18.8) | 3189 (18.9) | 0 |
| Antihypertensives | 5446 (21.6) | 3189 (18.9) | 7 | 10 (0.1) | 18 (0.1) | 0 |
| Antiparkinson drugs | 60 (0.2) | 34 (0.2) | 0 | 39 (0.2) | 34 (0.2) | 0 |
| Antispasmodic drugs | 27 (0.1) | 18 (0.1) | 0 | 355 (2.1) | 350 (2.1) | 0 |
| Antipsychotics | 489 (1.9) | 350 (2.1) | 1 | 14 (0.1) | 18 (0.1) | 0 |
| Barbiturates | 29 (0.1) | 23 (0.1) | 0 | 17 (0.1) | 23 (0.1) | 0 |
| Benzodiazepines | 3382 (13.4) | 2235 (13.2) | 1 | 2209 (13.1) | 2235 (13.2) | 0 |
| Beta‐blockers | 2991 (11.8) | 1724 (10.2) | 5 | 1699 (10) | 1724 (10.2) | 1 |
| Bisphosphonates | 1605 (6.4) | 1092 (6.5) | 0 | 1062 (6.3) | 1092 (6.5) | 1 |
| Cholinesterase inhibitor | 2154 (8.5) | 1622 (9.6) | 4 | 1574 (9.3) | 1622 (9.6) | 1 |
| Digoxin | 333 (1.3) | 254 (1.5) | 2 | 243 (1.4) | 254 (1.5) | 1 |
| Diuretics | 3076 (12.2) | 2058 (12.2) | 0 | 2012 (11.9) | 2058 (12.2) | 1 |
| Oral hypoglycemics | 1769 (7) | 970 (5.7) | 5 | 928 (5.5) | 970 (5.7) | 1 |
| Insulin | 586 (2.3) | 366 (2.2) | 1 | 350 (2.1) | 366 (2.2) | 1 |
| Lithium | 254 (1) | 142 (0.8) | 2 | 134 (0.8) | 142 (0.8) | 0 |
| NSAIDs (excluding ASA) | 1401 (5.5) | 887 (5.2) | 1 | 891 (5.3) | 887 (5.2) | 0 |
| Opioids | 2461 (9.7) | 1546 (9.1) | 2 | 1516 (9) | 1546 (9.1) | 0 |
| Overactive bladder medications | 435 (1.7) | 284 (1.7) | 0 | 271 (1.6) | 284 (1.7) | 1 |
| Statins | 4464 (17.7) | 2255 (13.3) | 12 | 2198 (13) | 2255 (13.3) | 1 |
| Steroids | 696 (2.8) | 410 (2.4) | 3 | 399 (2.4) | 410 (2.4) | 0 |
| QT‐interval prolonging drugs | 3700 (14.6) | 2454 (14.5) | 0 | 2444 (14.4) | 2454 (14.5) | 0 |
| Quetiapine dosage | ||||||
| ≤25 mg/day | 6188 (24.5) | 3870 (22.9) | 4 | 3826 (22.6) | 3870 (22.9) | 1 |
| >25 mg/day | 19,079 (75.5) | 13,039 (77.1) | 4 | 13,097 (77.4) | 13,039 (77.1) | 1 |
Abbreviations: CT, computed tomography; ER, emergency department; MRI, magnetic resonance imaging; NSAID, nonsteroidal anti‐inflammatory drug; SD, standard deviation; TIA, transient ischemic attack.
Weighted using inverse probability of treatment weighting, based on propensity scores. The propensity score was estimated using multivariable logistic regression with covariates (defined in Supporting Information: Table 7). Patients in the reference group (azithromycin) were weighted as (propensity score/[1−propensity score]).
The Charlson Comorbidity Index was calculated based on hospital admission data in the 3‐year period before the index date. The Charlson score considers each person with a hospital admission with the disease of interest. For each person, it assigns a point score based on disease mortality risk and sums them to generate an overall score of disease burden. The final risk scores range between 0 and 13, with higher values associated with higher mortality. Each disease in the index has an assigned weight of 1,2,3, or 6, with HIV/AIDS and metastatic cancer having the highest weight of 6.
3.2. Prescription information
Family physicians provided 85% of quetiapine prescriptions, and 77% of participants were taking high‐dose quetiapine defined as >25 mg per day. Median (25th percentile, 75th percentile) daily quetiapine dosages were 53.6 mg (37.5–150) in azithromycin users and 75 mg (50–150) in clarithromycin users (Supporting Information: Table S8). Immediate‐release formulations comprised 97% of overall quetiapine prescriptions. Median (25th percentile, 75th percentile) duration of prescription of macrolide antibiotic was 5 (5–5) days in azithromycin users and 10 (7–10) days in clarithromycin users (Supporting Information: Table S9).
3.3. Primary composite outcome
There were 844 hospitalizations or emergency department visits for encephalopathy, fall, or fragility fracture within 30 days of initiating a macrolide antibiotic over the study period. After propensity score weighting, the risk of the primary composite outcome was 19% higher in new clarithromycin users in comparison to new azithromycin users (365 of 16,909 patients taking clarithromycin [2.2%] vs. 309 of 16,923 patients taking azithromycin [1.8%]; absolute risk increase, 0.34% [95% CI, 0.04–0.63]; RR, 1.19 [95% CI, 1.02–1.38]; Table 2).
Table 2.
Composite primary outcome and secondary outcomes.
| Unweighted | Weighted | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of events (%) | No. of events (%) | |||||||
| Azithromycin (ref) (n = 25,267) | Clarithromycin (n = 16,909) | Risk difference (95% CI) | Odds ratioa (95% CI) | Azithromycin (ref) (n = 16,923) | Clarithromycin (n = 16,909) | Risk difference (95% CI) | Odds ratiob (95% CI) | |
| Hospital encounter with encephalopathy, fragility fracture, or fall | 479 (1.9%) | 365 (2.2%) | 0.26% (−0.01 to 0.54) | 1.14 (1.00–1.31) | 309 (1.8%) | 365 (2.2%) | 0.34% (0.04–0.63) | 1.19 (1.02–1.38) |
| Hospital encounter with encephalopathyb | 226 (0.9%) | 181 (1.1%) | 0.18% (−0.02 to 0.37) | 1.20 (0.99–1.46) | 148 (0.9%) | 181 (1.1%) | 0.20% (−0.01 to 0.41) | 1.23 (0.99–1.53) |
| Hospital encounter with fragility fracture | 69 (0.3%) | 78 (0.5%) | 0.19% (0.07–0.31) | 1.69 (1.22–2.34) | 45 (0.3%) | 78 (0.5%) | 0.20% (0.07–0.32) | 1.74 (1.21–2.52) |
| Hospital encounter with fall | 245 (1.0%) | 164 (1.0%) | 0.00% (−0.19 to 0.19) | 1.00 (0.82–1.22) | 154 (0.9%) | 164 (1.0%) | 0.06% (−0.15 to 0.27) | 1.07 (0.85–1.35) |
| Hospital encounter with CT scan of the headc | 306 (1.2%) | 220 (1.3%) | 0.09% (−0.13 to 0.31) | 1.08 (0.90–1.28) | 175 (1.0%) | 220 (1.3%) | 0.27% (0.04–0.50) | 1.26 (1.04–1.54) |
| All‐cause mortality | 257 (1.0%) | 232 (1.4%) | 0.35% (0.14–0.57) | 1.35 (1.13–1.62) | 204 (1.2%) | 232 (1.4%) | 0.17% (−0.07 to 0.41) | 1.14 (0.94–1.38) |
Abbreviations: CI, confidence interval; CT, computed tomography.
All odds ratios were approximated to be the relative risk based on the rare incidence of outcome events.
Category indicates the 30‐day risk of hospital admission with encephalopathy, defined as a diagnosis of delirium, disorientation, transient alteration of awareness, transient cerebral ischemic attack, or unspecified dementia.
Category indicates computed tomography scans of the head performed within 30 days of the index date.
3.4. Secondary outcomes
Clarithromycin usage was associated with a higher risk of hospital encounter with a fragility fracture (78 of 16,909 patients taking clarithromycin [0.5%] vs. 45 of 16,923 patients taking azithromycin [0.3%]; absolute risk increase, 0.20% [95% CI, 0.07–0.32]; RR, 1.74 [95% CI, 1.21–2.52]). Hospital encounter with a CT head scan was more common among clarithromycin users (220 of 16,909 patients taking clarithromycin [1.3%] vs. 175 of 16,923 patients taking azithromycin [1.0%]; absolute risk increase, 0.27% [95% CI, 0.04–0.50]; RR, 1.26 [95% CI, 1.04–1.54]). There was no significant difference in hospital encounters for encephalopathy (181 of 16,909 patients taking clarithromycin [1.1%] vs. 148 of 16,923 patients taking azithromycin [1.8%]; absolute risk increase, 0.20% [95% CI, −0.01 to 0.41]; RR, 1.23 [95% CI, 0.99–1.53]), falls (164 of 16,909 patients taking clarithromycin [1.0%] vs. 154 of 16,923 patients taking azithromycin [0.9%]; absolute risk increase, 0.06% [95% CI, −0.15 to 0.27]; RR, 1.07 [95% CI, 0.85–1.35]), nor all‐cause mortality (232 of 16,909 patients taking clarithromycin [1.4%] vs. 204 of 16,923 patients taking azithromycin [1.2%]; absolute risk increase, 0.17% [95% CI, −0.07 to 0.41]; RR, 1.14 [95% CI, 0.94–1.38]).
3.5. Sensitivity analyses
In a sensitivity analysis restricted to immediate‐release quetiapine formulations, use of clarithromycin remained associated with a higher risk for the primary composite outcome (363 of 16,633 patients taking clarithromycin [2.2%] vs. 303 of 16,645 patients taking azithromycin [1.8%]; absolute risk increase, 0.36% [95% CI, 0.06–0.66]; RR,1.20 [95% CI, 1.03–1.41]). We conducted an additional sensitivity analysis, for the primary composite outcome excluding transient ischemic attack (TIA) from the definition for encephalopathy given that it would be rare for quetiapine toxicity to be coded as a TIA. Using this alternative definition of encephalopathy our results remained similar with a 19% increased risk of the primary composite outcome in new clarithromycin users: (358 of 16,909 patients taking clarithromycin [2.1%] vs. 304 of 16,923 patients taking azithromycin [1.8%]; absolute risk increase, 0.32% [95% CI, 0.02–0.61]; RR, 1.18 [95% CI, 1.01–1.38]).
In an additional analysis, we excluded individuals (<6) with evidence of a trauma code on the same day as the CT head to exclude differences in trauma evaluations. After this exclusion, the finding of higher rates of hospital encounters with a CT head scan among clarithromycin users remained unchanged with an absolute risk increase of 0.27% [95% CI, 0.04–0.50]; RR, 1.26 [95% CI, 1.04–1.55]).
E‐value analysis was used to determine the extent to which unmeasured confounding would be required to negate the observed results. 37 For the primary composite outcome the E‐value was 1.67 and 1.16 for the lower bound of the 95% CI, which indicates that the observed risk ratio for the primary outcome of 1.19 could be explained by an unmeasured confounder with an association between the exposure and outcome with a risk ratio of 1.67 above and beyond the measured confounders, and the confidence interval for the primary composite outcome could be moved to the null by an unmeasured confounder associated with both the exposure and outcome by a risk ratio of 1.16 each, indicating a relatively modest association. The E‐value was 2.87 for the association with fragility fracture and 1.71 for the lower bound of the 95% CI.
4. DISCUSSION
In this study, the coprescription of clarithromycin, as compared to azithromycin, with quetiapine was associated with a 19% increase in the relative risk of the primary composite outcome of a hospital encounter with encephalopathy, fall, or fragility fracture with an absolute risk increase of 0.34%. This outcome was primarily driven by a hospital encounter for a fragility fracture which was likely caused by falls given the short duration of medication usage.
These results support that a clinically significant adverse drug interaction exists between clarithromycin and quetiapine in a predominantly elderly population. This is in keeping with previous work which has clearly shown that CYP3A4 inhibitor drug interactions have clinically significant adverse effects with a wide range of common medications including calcium channel blockers, 21 , 23 statins, 22 and calcineurin inhibitors. 38
Off‐label prescription for quetiapine is an increasing phenomenon with a 300% increase in prescriptions for quetiapine by family physicians between 2005 and 2012 in Canada compared to 37.1% for olanzapine, and 37.4% for risperidone. 2 Efforts have been made to attempt to curtail excessive off‐label use of quetiapine, and peer comparison letters on prescribing habits have been successful in primary care providers. 39
Notably, prescriptions for clarithromycin in our study declined over time, potentially as recognition of adverse events related to this medication have increased. Beyond the adverse events investigated in this study, the CLARICOR trial which included 4733 patients with stable coronary artery disease, found that clarithromycin was associated with increased cardiovascular mortality over 10‐year follow‐up although the precise mechanism is unknown. 40 However, despite decreasing clarithromycin prescriptions, given the widespread nature of quetiapine prescriptions, CYP3A4 drug interactions remain relevant in daily clinical practice. For example, nirmatrelvir/ritonavir (Paxlovid) has emerged as a common medication used to reduce COVID‐19 disease severity. Ritonavir is a well‐known strong CYP3A4 inhibitor, specifically used to boost the plasma concentration of nirmatrelvir. Therefore, coadministration of Paxlovid with quetiapine could lead to similar CYP3A4 inhibition‐mediated adverse effects observed in this study, and guidance has been issued regarding coprescriptions with medications metabolized by CYP3A4. 41
In our study, the point estimates for all‐cause mortality were higher among clarithromycin users but this was not statistically significant after weighting. Both clarithromycin and azithromycin are known to be associated with a prolonged QT interval which may rarely be associated with Torsades de Pointes. 42 However, previous studies have demonstrated that macrolide antibiotics compared to nonmacrolide antibiotics were not associated with a higher 30‐day risk of arrhythmia or all‐cause mortality suggesting that arrhythmia is unlikely to be the principal mechanism underlying the adverse events observed in this study. 43
Prior population‐based studies have found that the new receipt of an atypical antipsychotic including quetiapine is associated with a 54% increased risk of a serious fall and a 51% increased risk of nonvertebral osteoporotic fracture. 15 A prior meta‐analysis of 19 observational studies which included 544,811 participants with 80,835 fractures found that antipsychotic usage was associated with higher risk of fractures including a 47% increased risk among quetiapine users. 44 In our study, we found a 74% higher risk of hospital encounter with fragility fracture among new clarithromycin users in comparison to new azithromycin users but did not find a statistically significant difference in the risk of fall between groups, suggesting that we were able to detect a difference in falls more likely to be injurious leading to fragility fractures.
Strengths of this study include the large number of participants, and the rich population‐based databases used to assess outcomes from an interaction between quetiapine and macrolide antibiotics which allow for external generalizability of our findings. We used propensity score‐based weighting which resulted in well‐balanced study groups across a wide number of demographics and comorbidities. The outcomes in this study have previously been used in other pharmacoepidemiology studies to evaluate adverse events of baclofen on encephalopathy and we confirmed the robustness of our results in sensitivity analyses including restricting to only immediate release formulations. 28 , 29 , 30
This study has several limitations. Although study groups were well balanced on a wide variety of demographics, comorbidities, medication usage, and healthcare utilization, residual confounding remained a possibility. E‐value analysis indicated that the association for the primary composite outcome was modest, and that residual confounding with a risk ratio of 1.67 could markedly attenuate the association found in this study although a residual confounder with a risk ratio of 2.87 would be required to negate the association with fragility fractures. Indeed, since CYP3A4 is involved in the metabolism of nearly half of all prescription medications, 45 inhibition of CYP3A4 could have contributed to other drug–drug interactions in this cohort, although baseline usage of other common CYP3A4 substrates was well balanced and we excluded those with a prescription for another strong CYP3A4 inhibitor. In addition, the definition of encephalopathy in this study was broad although we performed a sensitivity analysis using an alternative definition of encephalopathy which excluded TIA, which did not change the underlying conclusion. While a trend towards a higher rate of a hospital encounter with encephalopathy was noted, this did not reach statistical significance despite rates of CT scans of the head, a common component of the workup for delirium, being more frequent in clarithromycin users.
In conclusion, in this study, we found that new prescription for clarithromycin was associated with increased fragility fracture risk when co‐prescribed with quetiapine, and clinicians should consider this drug interaction when considering macrolide antibiotic prescription in the elderly.
AUTHOR CONTRIBUTIONS
Kevin Yau: Conceptualization; data curation; formal analysis; investigation; methodology; visualization; writing—original draft; writing—review & editing. Eric McArthur: data curation; formal analysis; methodology; validation; writing—review & editing. Nivethika Jeyakumar: Data curation; formal analysis; investigation; methodology; project administration; validation; writing—review and editing. Flory Tsobo Muanda: Formal analysis; investigation; methodology; writing—review and editing. Richard B. Kim: Investigation; methodology; validation; writing—review and editing. Kristin K. Clemens: Investigation; methodology; validation; writing—review and editing. Ron Wald: Investigation; methodology; validation; writing—review and editing. Amit X. Garg: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; writing—original draft; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
Kevin Yau has received speaking honoraria from AstraZeneca unrelated to the current manuscript. Kristin Clemens has received honoraria for delivering certified medical education from the Canadian Medical and Surgical Knowledge Translation Research Group and the CPD Network unrelated to the current manuscript.
ETHICS STATEMENT
The use of administrative data in this project was authorized under section 45 of Ontario's Personal Health Information Protection Act which does not require review by a research ethics board.
TRANSPARENCY STATEMENT
The lead author Kevin Yau affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
Parts of this material are based on data and information compiled and provided by the Ontario Ministry of Health (MOH) and the Canadian Institute for Health Information (CIHI). Income quintile analysis was adapted from Statistics Canada Postal CodeOM Conversion File and/or Postal CodesOM by Federal Ridings File and/or Postal CodeOM Conversion File Plus (November 2018), which is based on data licensed from Canada Post Corporation. This study was supported by the ICES Western site. ICES is funded by an annual grant from the Ontario Ministry of Health (MOH) and Long‐Term Care (MLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). The research was conducted by members of the ICES Kidney, Dialysis, and Transplantation team at the ICES Western facility, and they are supported by a grant from the Canadian Institutes of Health Research. Kevin Yau is supported by the Eliot Phillipson Clinician‐Scientist Training Program, Department of Medicine, University of Toronto, Banting and Best Diabetes Centre Postdoctoral Fellowship, University of Toronto, and KRESCENT Postdoctoral Fellowship from the Kidney Foundation of Canada. Richard B. Kim was supported by the Wolfe Medical Research Chair in Pharmacogenomics. Amit X. Garg was supported by Dr. Adam Linton Chair in Kidney Health Analytics and a Clinician Investigator Award from the Canadian Institutes of Health Research.
Yau K, McArthur E, Jeyakumar N, et al. Adverse events with quetiapine and clarithromycin coprescription: a population‐based retrospective cohort study. Health Sci Rep. 2023;6:e1375. 10.1002/hsr2.1375
DATA AVAILABILITY STATEMENT
The analysis was conducted by members of the ICES Kidney Dialysis & Transplantation (KDT) team at the ICES Western facility (London, Ontario). The protocol can be obtained by emailing KY (kevin.yau@mail.utoronto.ca).
REFERENCES
- 1. Duncan D, Cooke L, Symonds C, Gardner D, Pringsheim T. Quetiapine use in adults in the community: a population‐based study in Alberta, Canada. BMJ Open. 2016;6(3):e010861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pringsheim T, Gardner DM. Dispensed prescriptions for quetiapine and other second‐generation antipsychotics in Canada from 2005 to 2012: a descriptive study. CMAJ Open. 2014;2(4):E225‐E232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee TC, Desforges P, Murray J, Saleh RR, McDonald EG. Off‐label use of quetiapine in medical inpatients and postdischarge. JAMA Internal Med. 2016;176(9):1390‐1391. [DOI] [PubMed] [Google Scholar]
- 4. El‐Saifi N, Moyle W, Jones C, Tuffaha H. Quetiapine safety in older adults: a systematic literature review. J Clin Pharm Ther. 2016;41(1):7‐18. [DOI] [PubMed] [Google Scholar]
- 5. Kelly M, Dornan T, Pringsheim T. The lesser of two evils: a qualitative study of quetiapine prescribing by family physicians. CMAJ Open. 2018;6(2):E191‐E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first‐ and second‐generation antipsychotics: a state‐of‐the‐art clinical review. Ther Clin Risk Manag. 2017;13:757‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson SL, Vande Griend JP. Quetiapine for insomnia: a review of the literature. Am J Health Syst Pharm. 2014;71(5):394‐402. [DOI] [PubMed] [Google Scholar]
- 8. Coe HV, Hong IS. Safety of low doses of quetiapine when used for insomnia. Ann Pharmacother. 2012;46(5):718‐722. [DOI] [PubMed] [Google Scholar]
- 9. Sparshatt A, Jones S, Taylor D. Quetiapine: dose‐response relationship in schizophrenia. CNS Drugs. 2008;22(1):49‐68. [DOI] [PubMed] [Google Scholar]
- 10. Huthwaite M, Tucker M, McBain L, Romans S. Off label or on trend: a review of the use of quetiapine in New Zealand. N Z Med J. 2018;131(1474):45‐50. [PubMed] [Google Scholar]
- 11. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hwang YJ, Dixon SN, Reiss JP, et al. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population‐based cohort study. Ann Intern Med. 2014;161(4):242‐248. [DOI] [PubMed] [Google Scholar]
- 13. Trifirò G. Association of community‐acquired pneumonia with antipsychotic drug use in elderly patients: a nested case‐control study. Ann Intern Med. 2010;152(7):418‐425. [DOI] [PubMed] [Google Scholar]
- 14. Brännström J, Lövheim H, Gustafson Y, Nordström P. Antipsychotic drugs and hip fracture: associations before and after the initiation of treatment. J Am Med Dir Assoc. 2020;21(11):1636‐1642. [DOI] [PubMed] [Google Scholar]
- 15. Fraser LA, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population‐based cohort study. JAMA Intern Med. 2015;175(3):450‐452. [DOI] [PubMed] [Google Scholar]
- 16. Trifirò G. Antipsychotic drug use and community‐acquired pneumonia. Curr Infect Dis Rep. 2011;13(3):262‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pratt N, Roughead EE, Ramsay E, Salter A, Ryan P. Risk of hospitalization for hip fracture and pneumonia associated with antipsychotic prescribing in the elderly: a self‐controlled case‐series analysis in an Australian health care claims database. Drug Saf. 2011;34(7):567‐575. [DOI] [PubMed] [Google Scholar]
- 18. Balit CR, Isbister GK, Hackett LP, Whyte IM. Quetiapine poisoning. Ann Emerg Med. 2003;42(6):751‐758. [DOI] [PubMed] [Google Scholar]
- 19. Peridy E, Hamel JF, Rolland AL, Gohier B, Boels D. Quetiapine poisoning and factors influencing severity. J Clin Psychopharmacol. 2019;39(4):312‐317. [DOI] [PubMed] [Google Scholar]
- 20. Fleet JL, Shariff SZ, Bailey DG, et al. Comparing two types of macrolide antibiotics for the purpose of assessing population‐based drug interactions. BMJ Open. 2013;3(7):e002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gandhi S, Fleet JL, Bailey DG, et al. Calcium‐channel blocker‐clarithromycin drug interactions and acute kidney injury. JAMA. 2013;310(23):2544‐2553. [DOI] [PubMed] [Google Scholar]
- 22. Patel AM, Shariff S, Bailey DG, et al. Statin toxicity from macrolide antibiotic coprescription: a population‐based cohort study. Ann Intern Med. 2013;158(12):869‐876. [DOI] [PubMed] [Google Scholar]
- 23. Wright AJ, Gomes T, Mamdani MM, Horn JR, Juurlink DN. The risk of hypotension following co‐prescription of macrolide antibiotics and calcium‐channel blockers. Can Med Assoc J. 2011;183(3):303‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li KY, Li X, Cheng ZN, Zhang BK, Peng WX, Li HD. Effect of erythromycin on metabolism of quetiapine in Chinese suffering from schizophrenia. Eur J Clin Pharmacol. 2005;60(11):791‐795. [DOI] [PubMed] [Google Scholar]
- 25. Jaskiw GE, Thyrum PT, Fuller MA, Arvanitis LA, Yeh C. Pharmacokinetics of quetiapine in elderly patients with selected psychotic disorders. Clin Pharmacokinet. 2004;43(14):1025‐1035. [DOI] [PubMed] [Google Scholar]
- 26. Kennedy WK, Jann MW, Kutscher EC. Clinically significant drug interactions with atypical antipsychotics. CNS Drugs. 2013;27(12):1021‐1048. [DOI] [PubMed] [Google Scholar]
- 27. Levy AR, O'brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Canadian J Clin Pharmacol. 2003;10(2):67‐71. [PubMed] [Google Scholar]
- 28. Chauvin KJ, Blake PG, Garg AX, et al. Baclofen has a risk of encephalopathy in older adults receiving dialysis. Kidney Int. 2020;98(4):979‐988. [DOI] [PubMed] [Google Scholar]
- 29. Muanda FT, Weir MA, Bathini L, et al. Association of baclofen with encephalopathy in patients with chronic kidney disease. JAMA. 2019;322(20):1987‐1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muanda FT, Blake PG, Weir MA, et al. Association of baclofen with falls and fractures in patients with CKD. Am J Kidney Dis. 2021;78(3):470‐473. [DOI] [PubMed] [Google Scholar]
- 31. Kim DH, Lee J, Kim CA, et al. Evaluation of algorithms to identify delirium in administrative claims and drug utilization database. Pharmacoepidemiol Drug Saf. 2017;26(8):945‐953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chien CF, Huang P, Hsieh SW. Reversible global aphasia as a side effect of quetiapine: a case report and literature review. Neuropsychiatr Dis Treat. 2017;13:2257‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cowan A, Jeyakumar N, Kang Y, et al. Fracture risk of sodium‐glucose cotransporter‐2 inhibitors in chronic kidney disease. Clin J Am Soc Nephrol. 2022;17(6):835‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Donnell S. Use of administrative data for national surveillance of osteoporosis and related fractures in Canada: results from a feasibility study. Arch Osteoporosis. 2013;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Papaioannou A, Kennedy CC, Ioannidis G, et al. Comparative trends in incident fracture rates for all long‐term care and community‐dwelling seniors in Ontario, Canada, 2002‐2012. Osteoporos Int. 2016;27(3):887‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Comm Stat Simulation Comput. 2009;38(6):1228‐1234. [Google Scholar]
- 37. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167(4):268‐274. [DOI] [PubMed] [Google Scholar]
- 38. Jeong R, Quinn RR, Lentine KL, et al. Outcomes following macrolide use in kidney transplant recipients. Can J Kidney Health Dis. 2019;6:205435811983070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sacarny A, Barnett ML, Le J, Tetkoski F, Yokum D, Agrawal S. Effect of peer comparison letters for high‐volume primary care prescribers of quetiapine in older and disabled adults: a randomized clinical trial. JAMA Psychiatry. 2018;75(10):1003‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Winkel P, Hilden J, Hansen JF, et al. Clarithromycin for stable coronary heart disease increases all‐cause and cardiovascular mortality and cerebrovascular morbidity over 10 years in the CLARICOR randomised, blinded clinical trial. Int J Cardiol. 2015;182:459‐465. [DOI] [PubMed] [Google Scholar]
- 41. Marzolini C, Kuritzkes DR, Marra F, et al. Recommendations for the management of drug‐drug interactions between the COVID‐19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications. Clin Pharmacol Ther. 2022;112:1191‐1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shaffer D, Singer S, Korvick J, Honig P. Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis. 2002;35(2):197‐200. [DOI] [PubMed] [Google Scholar]
- 43. Trac MH, McArthur E, Jandoc R, et al. Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. Can Med Assoc J. 2016;188(7):E120‐E129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee SH, Hsu WT, Lai CC, et al. Use of antipsychotics increases the risk of fracture: a systematic review and meta‐analysis. Osteoporos Int. 2017;28(4):1167‐1178. [DOI] [PubMed] [Google Scholar]
- 45. Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352(21):2211‐2221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The analysis was conducted by members of the ICES Kidney Dialysis & Transplantation (KDT) team at the ICES Western facility (London, Ontario). The protocol can be obtained by emailing KY (kevin.yau@mail.utoronto.ca).


