Abstract
COVID-19 is an immune-mediated disease whose pathophysiology uses SAMHD1 tetramerization and cGAS–STING signaling, toll-like receptor 4 (TLR4) cascade, spike protein– inflammasome activation, and neuropilin 1 (NRP1) signaling. Variants of concern, such as SARS-CoV-2 Omicron Subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, BA.2.75.2, and other mutants, have emerged. The longitudinal memory T-cell response to SARS-CoV-2 persists for eight months after symptom onset. Therefore, we must achieve viral clearance to coordinate immune cell reactions. Aspirin, dapsone, and dexamethasone as anticatalysis medicines have been used to treat COVID-19. They are shown to work harmoniously with modulating ILCs. Therefore, it needs to prescribe this immune triad to alleviate the clinical pathologic course and block exacerbation mechanisms due to diverse SARS-CoV-2 variants.
Keywords: ACE2, Aspirin, cGAS–STING, Dapsone, Dexamethasone, Immunologic engram, Inflammasome, SAMHD1, SARS-CoV-2 spike protein, TLR4
1. Introduction
The pathophysiologic mechanisms of acute COVID-19 include direct toxicity through virus-dependent mechanisms, including alveolar cells and microvascular endothelial injury, and virus-independent mechanisms, such as immunological damage and an immune-dysregulated hyperinflammatory state, including perivascular inflammation and hypercoagulability with resultant in situ thrombosis, as well as the maladaptation of ACE2 (the angiotensin-converting enzyme 2) pathway [1].
In the early Human Immunodeficiency Virus (HIV)- Acquired Immune Deficiency Syndrome (AIDS) experience, as with COVID-19 today, controlling the virus in severely ill vulnerable patients was problematic. However, funneling resources into identifying and blocking the pathological process might be more effective than continuously developing a vaccine for each variant. There has been no cure for HIV, but as of 2020, 95% of people with HIV are on treatment with highly active antiretroviral therapy (HAART), which is available per patient [2]. We are confronting other new Omicron variants with past COVID-19 vaccines amidst the current pandemic. Variants of concern, such as SARS-CoV-2 Omicron Subvariants, have emerged: BQ.1, BQ.1.1, XBB.1.5, BF.7, XBB, and other mutants. The longitudinal memory T-cell response to SARS-CoV-2 persists for eight months after symptom onset [3].
Convalescent plasma transfusion for COVID-19 was clinically safe, but was not significant in critically ill patients [4]. Different immune response to COVID-19 shows the different up- or non-responses of macrophages cultured from human pluripotent stem cells [5]. Exogenous interleukin-1β (IL-1β) activates IRF3 (interferon regulatory factor 3) in myeloid, fibroblast, and epithelial cells. This cell-intrinsic response depends on cGAS (Cyclic GMP-AMP synthase) and STING (stimulator of interferon genes, also known as TMEM173); however, exogenous IL-1β activates IRF3 in epithelial and myeloid cells. IL-1R (IL-1 receptor type 1) signaling stimulates mitochondria to release and detect cytosolic mtDNA (mitochondrial DNA) [6]. IL-1β treatment resulted in interferon (IFN) production, activated signaling, and directed an innate immune reaction, which restricts dengue virus infection [7]. Acquired somatic mutations are a universal feature of aging in hematopoietic stem and progenitor cells. In addition, they are related to an increased hazard of cardiovascular and malignant diseases. A proinflammatory immunologic profile of clonal hematopoiesis (CH) is associated with worse results in COVID-19. Acquired somatic mutations in CH consisting of hematopoietic stem and progenitor cells, particularly noncancer gene mutations, are associated with severe COVID-19 outcomes. They are also significantly associated with the danger of Clostridium difficile, Streptococcus, or Enterococcus infections [8]. After receiving select cytotoxic therapies for solid tumors, persons with gene mutations have the highest possibility for the subsequent diagnosis of myeloid neoplasms. The heavy mutations confer a cumulative risk of hematologic malignancy, and CH-related inflammation is a typical driver of several chronic diseases. Precise anti-cytokine therapies are needed for crashing the feedforward loop between CH-related inflammation and the subsequent chronic end-organ disorders [9]. cGAS–STING–NLRP3 (NLR family pyrin domain-containing 3) pathway provides critical insight into the complex relationship between the COVID-19 severity, hematological malignancy, and subsequent age-related diseases. While immunomodulation is a promising way to improve treatment outcomes for COVID-19, we have yet to find the most effective strategy.
2. Experiences of the with-coronavirus policy in South Korea
2.1. mRNA vaccine encoding the full-length S protein
A lipid nanoparticle-encapsulated mRNA encoding the S protein (or an adenovirus vector) can enter host cells at the injection site. The host then expresses the S protein and can present it on the cell surface or secret outside. Activated B and T lymphocytes produce a robust adaptive immune response when antigen-presenting cells (APCs) transfer signals to immune cells at the draining lymph nodes. Although the S protein may be limited primarily to APCs at the injection site or nearby lymph nodes, S protein can be expressed in distant organs. Some mRNA vaccines showed a systemic spread of some degree of expression [10,11]. Lipid nanoparticles encode the formulated nucleoside-modified mRNA of the SARS-CoV-2 spike glycoprotein. Lipid nanoparticles are stabilized in their prefusion conformation and induce an immune reaction of IL-2+ CD8+ and CD4+ T helper type 1 cell or IFNγ+ [12].
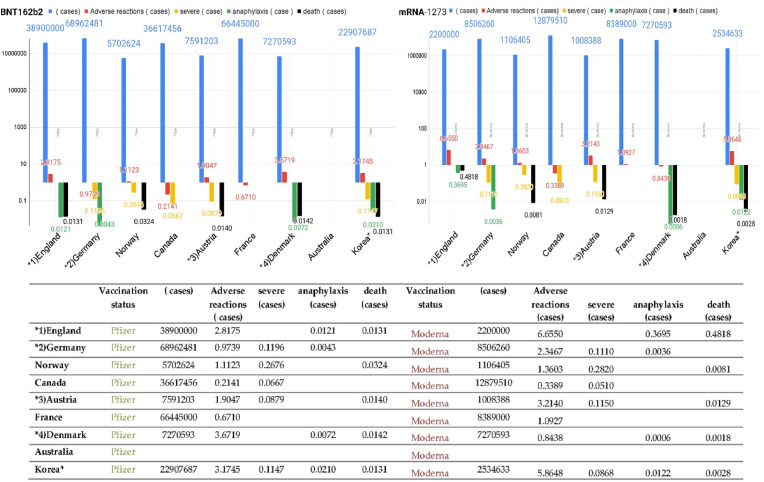
Memory B cells appear five months after immunization of naive individuals. Memory B cells express antibodies similar to those at the initial response. The overall neutralization effect of plasma is more significant after vaccination. However, when selected over time by natural infections, individual memory antibodies have greater efficacy and breadth than antibodies induced by immunization [13] (Table 1 ) (Fig. 1 ).
Table 1.
Adverse reactions after vaccination against COVID-19 in nine countries.
| Vaccination status | (cases) | Adverse reactions (cases) | severe (case) | anaphylaxis (case) | death (cases) | Vaccination status | (cases) | Adverse reactions (cases) | severe (case) | anaphylaxis (case) | death (cases) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∗1)England | Pfizer | 38,900,000 | 109,600 | - | 472 | 509 | Moderna | 2,200,000 | 14,641 | - | 813 | 1060 | Summary of yellow card reporting, Medicines & Healthcare products Regulatory Agency (MHRA) |
| ∗2)Germany | Pfizer | 68,962,481 | 67,165 | 8248 | 294 | Moderna | 8,506,260 | 19,962 | 944 | 31 | Paul-Ehrlilch-Institut (PEI) | ||
| Norway | Pfizer | 5,702,624 | 6343 | 1526 | 185 | Moderna | 1,106,405 | 1505 | 312 | 9 | Norway Medicine Agency | ||
| Canada | Pfizer | 36,617,456 | 7840 | 2442 | Moderna | 12,879,510 | 4365 | 657 | Government of Canada | ||||
| ∗3)Austria | Pfizer | 7,591,203 | 14,459 | 667 | 106 | Moderna | 1,008,388 | 3241 | 116 | 13 | Bericht BASG | ||
| Nebenwirkungsmeldungen | |||||||||||||
| France | Pfizer | 66,445,000 | 44,587 | Moderna | 8,389,000 | 9167 | ANSM-Agence nationale | ||||||
| de sécurité du | |||||||||||||
| médicament et des | |||||||||||||
| produits de santé | |||||||||||||
| ∗4)Denmark | Pfizer | 7,270,593 | 26,697 | 52 | 103 | Moderna | 7,270,593 | 6135 | 4 | 13 | Indberettede | ||
| bivirkninger ved | |||||||||||||
| Covid-19vaccine | |||||||||||||
| Australia | Pfizer | 20,940 | Moderna | Therapeutic Goods | |||||||||
| Administration | |||||||||||||
| Korea∗ | Pfizer | 22,907,687 | 72,720 | 2628 | 480 | 299 | Moderna | 2,534,633 | 14,865 | 220 | 31 | 7 | Korea Disease Control and Prevention Agency |
∗ 1) In the case of the UK, both anaphylaxis and anaphylaxis-like reactions are included.
∗ 2) Death status not updated in Germany (week 15∼)
∗ 3) Severe patients in Austria are hospitalized
∗ 4) Danish anaphylaxis is based on completed evaluation
∗∗ Data collection methods vary by country, making simple comparison difficult∗∗
Korea∗
a. It was reported as a suspected adverse reaction after vaccination against COVID-19, and it was calculated based on information reported by medical institutions. It is not intended to suggest a causal relationship between an adverse reaction and an adverse event. Report status classification may be changed when new information is added.
b. General adverse reactions include symptoms that commonly occur after vaccination, such as redness, pain, swelling, muscle pain, fever, headache, and chills at the vaccination site.
c. Serious adverse events include.
① death, ② Suspected anaphylaxis (including anaphylactic reaction)
③ Major adverse reactions: adverse event special interest (AESI), ICU admission, life-threatening, permanent disability/sequelae, etc. (from Status of reports of adverse reactions after overseas COVID-19 vaccination < Adverse Response Team, Vaccination Response Promotion Team of Korea CDC, ‘21.9.6.)
Fig. 1.
The report of adverse events of special interest (AESI) after vaccination.
2.2. cGAS–STING signaling provokes the pathogenesis of diverse diseases
ACE2 and TL4 on cell surfaces increase caspase-1. Caspase-1 is the downstream mediator of NLRP3 inflammasome. Exposure to the S protein provokes the expression of proteins involved in the positive stimulation of TLR4 signaling and NLRP3 activation pathway [14]. TLR4 activation in microglia induces neuroinflammation. In addition, TLR4 mediates the activation of nuclear factor-κB (NF-κB) and p38 MAPK (mitogen-activated protein kinase) as a route [15].
TLR4 has been an essential intermediate of neurotoxicity induced by α-synuclein (α-Syn) oligomers. Incorrectly folded α-Syn induces a neuro-inflammatory response. Extracellular α-Syn in astrocytes can activate the proinflammatory TLR4 pathway. Specific gene transcription activation of NF-kB may cause the release of proinflammatory cytokines, leading to nerve injury and pathological alteration of α-Syn. The neurofilament light chain (NFL) was higher in COVID-19 subjects than in the control group. Serum NFL is a specific biomarker of nerve damage. High NFL levels were associated with nerve damage in severe COVID-19 patients. They may have caused central nervous system neuroinflammation by activating astrocytes and microglial cells that can promote pathology like prions. S proteins can provoke intracellular TLR4 signaling cascades. S proteins contain several prion-producing domains and are similar to other prion proteins. Therefore, their direct toxic effects can cause neurodegenerative conditions, which mimic a pathology identical to prion disease [16].
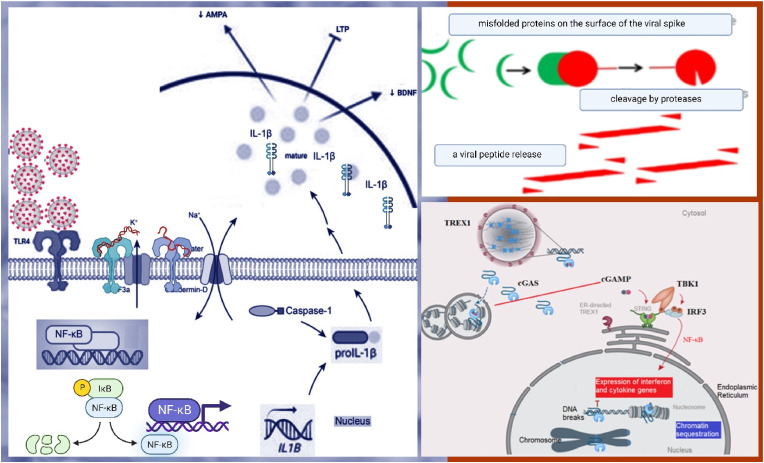
The apoptosis is associated with antiviral activity, including IFN production, NF-κB expression, and autophagy. The biological implications of nuclear cGAS and chromatin interactions induce autoimmune by cGAS. The IFN-independent antiviral function of the cGAS-STING pathway has been a significant nucleic acid recognition pathway. cGAS is activated upon binding to aberrant DNA [17] (Fig. 2 ).
Fig. 2.
cGAS–STING signaling is linked to the pathogenesis of the central nervous system.
S protein activated inflammasome reactions in macrophages from isolated convalescent in COVID-19 patients. The responses were correlated with distinct genetic and epigenetic expression signatures [18]. After lipid nanoparticle-encapsulated mRNA encoding S protein vaccination, most multiple myeloma patients (55%) among 103 have impaired responses to detect IgG antibodies to SARS-CoV-2 S protein [19]. The interaction of ACE2 with SARS-CoV-2 S protein in human VSELs (very small embryonic-like stem cells) and HSCs (hematopoietic stem cells) activates the NLRP3. The exposure of human VSELs to recombinant S protein led to the upregulation of NLRP3 mRNA expression. In addition, IL-1β levels in conditioned media were elevated for cells exposed to S protein. Thus, SARS-CoV-2 could damage human VSELs in adult tissues [20], and mRNA S proteins hurt them (Table 2 ).
Table 2.
The manifestations of SARS-CoV-2, AESI, and Inflammasome disease in humans.
| Clinical Manifestation | COVID-19 symptoms [28] | mRNA Vaccine AESI [114,115] | Inflammasome disease [28] |
|---|---|---|---|
| skin vascular symptoms, lymphadenopathy, fever | violaceous macules with porcelain appearance, livedo of the trunk with chest pain and cough, violaceous macule and Raynaud's phenomenon 10 days after fever and cough, eruptive cherry angioma 21 days after COVID-19 healing of clinical symptoms, chilblain appearance and Raynaud's phenomenon in a patient with anosmia, fever, and cough | lymphadenopathy | autoinflammatory diseases associated with aberrant cell death and inflammasome activation. |
| hypersensitivity reactions | the clinical triad: fever, rash, and systemic involvement, which can cause severe organ dysfunction (heart, kidney, lung, brain, etc.) | mRNA vaccine induced the clinical triad of fever, rash, and systemic involvement. Anaphylaxis | Inflammasome produces immune modulators and cytokines. |
| hematology laboratory | decreased lymphocytes, decreased eosinophils, increased neutrophils, lymphopenia, leukocytosis, neutrophilia, thrombocytopenia, focal fibrin clusters mixed with mononuclear inflammatory cells, | Thrombocytopenia [114] | Thrombocytopenia |
| anemia | thrombocytopenia, consumptive coagulopathy | the interplay of inflammasome mediators with immune modulators and transcription factors to have a significant role in the development of myeloid diseases | |
| liver disease, pancreatic disease | significant liver injury - clinically uncommon, pancreatic cells - highly expressed ACE2 | Appendicitis | steatohepatitis, colitis, diabetes, Inflammatory bowel disease |
| renal disease | acute tubular necrosis, severe collapsing focal segmental glomerulosclerosis | NLRP3 inflammasome is activated in acute kidney injury, chronic kidney diseases, diabetic nephropathy, and hyperuricemic nephropathy. | |
| cardiac disease | acute myocardial injury, chronic damage to the cardiovascular system | myocarditis, and pericarditis [114] | arrhythmias, atherosclerosis, myocardial infarction. |
| pulmonary disease | coronavirus disease (COVID-19)-related pneumonia | Overall increased risk of Bell's palsy in Hong Kong [116] | chronic obstructive pulmonary disease (COPD) development via activation of NLRP3-mediated pyroptosis [124]. |
| neurologic disease | ischemic stroke, Neurological complications range from potentially fatal encephalopathy and stroke to the onset of headaches and dizziness | cerebral venous sinus thrombosis [114] | cognitive impairment in Alzheimer's disease [52,88,95], autoimmune encephalomyelitis, stroke [89], traumatic brain injury. |
| others | uveitis, Herpes zoster infection [115] | diverse elderly disease care [29,125] |
We summarized prophylactic treatment candidates for the treatment of inflammasome diseases against NLRP3.
Canakinumab binds to IL-1β and inhibits their interaction with the IL-1 cell surface receptor. However, canakinumab did not substantially increase the probability of survival without IMV (invasive mechanical ventilation) [21,22].
Colchicine disrupts inflammasome activation, suppressing the activation of caspase-1 and the subsequent release of IL-1β and IL-18. Colchicine can block NLRP3 and thus IL-1β production [23]. Therefore, it is impossible to make sure that colchicine reduces the mortality of COVID-19 [24].
CY-09, (Z)-4-((4-Oxo-2-thioxo-3-(3-(trifluoromethyl)benzyl)thiazolidin-5-ylidene)methyl)benzoic acid), binds the NACHT domain. CY-09 limits NLRP3 oligomerization and assembly, and CY-09 inhibits NLRP3 activation of ATPase. Thus, CY-09 decreases platelet aggregation by modifying the threshold concentration of collagen and weakening clot contraction in platelets [25].
Dapansutrile (OLT1177) is an orally active β-sulfonyl nitrile molecule. Dapansutrile inhibits the activation of NLRP3, and nanomolar concentrations of dapansutrile reduced IL-1β and IL-18 release following canonical and noncanonical NLRP3 activation in vivo. Dapansutrile showed no effect on the NLRC4 (NLR Family CARD Domain Containing 4) and AIM2 (absent in melanoma 2) inflammasomes, suggesting specificity for NLRP3. Healthy subjects receiving 1000 mg of dapansutrile daily for eight days exhibited neither adverse effects nor biochemical or hematological changes [26]. Thus, dapansutrile is a therapeutic option for AD [27].
Dapsone is a small-molecule competitor of the NLRPS inflammasome [28]. Dapsone acts as a treatment for MCI, AD, and COVID-19 ARDS [29].
MCC950, a small-molecule inhibitor of NLRP3, reduces infarct size and preserves cardiac function in a blinded translational randomized controlled trial (RCT) of large animal myocardial infarct model. NLRP3 inflammasome inhibition of MCC950 may have therapeutic potential in patients with AMI (acute myocardial infarct) [27]. MCC950 is a small molecular inhibitor of the NLRP3 inflammasome [14]. Nucleocapsid (N)-induced lung injury and cytokine production by SARS-CoV-2 are blocked by MCC950 [30].
PEDF (pigment epithelium-derived factor) reduced NLRP3 inflammasome activation in a rabbit model. This result is suppoded by inhibiting mitochondrial division through PEDFR/iPLA2 (pigment epithelial-derived factor receptor/calcium-independent phospholipase A2). It indicates that PEDF can be used as a treatment for AMI and ischemia/reperfusion (I/R) injury [31,32]. PEDF-induced signaling involves the crucial cytokine signaling in the immune system [33].
Statins inhibit endothelial dysfunction. They can reduce the expression of the NLRP3 and the downstream factors IL-1β and IL-18 and may treat ischemia-reperfusion injury by inhibiting the NLRP3 inflammasome [34,35]. Preceding statin use in patients hospitalized with COVID-19 is associated with lower COVID-19 mortality [36,37].
Tocilizumab administration in all seven patients in one trial led to prompt recovery as the anti-IL-6 receptor antibody from fever and malaise in all seven patients. It displayed lower oxygen intake after receiving tocilizumab treatment. IL-6 signaling plays a key role in endothelial cell dysfunction during cytokine release syndrome (CRS) [38].). It indicates that IL-6 signaling blockade has the potential as a therapy for CRS. Specifically, the pathogenesis of CRS in patients with sepsis, ARDS, and burns involves the IL-6 and plasminogen activator inhibitor-1 (PAI-1). Furthermore, tocilizumab decreased PAI-1 production and alleviated clinical manifestations in severe COVID-19 patients [38]. However, tocilizumab did not reduce the risk of clinically worsening COVID-19 pneumonia [39].
Total flavones (TFs) inhibit cell death and reduce oxidative stress and inflammation. TFs have a definite role in treating cerebrovascular diseases. Studies in ischemia-reperfusion mice have revealed that total flavones inhibit the activation of the NLRP3 inflammasome and have a positive therapeutic effect on ischemia-reperfusion [31,40]. Furthermore, flavonoids are potential modulators of COVID-19-related inflammation, immune deregulations, and inflammatory signaling associated with SARS-CoV-2 [41,42]. Quercetin in combination with remdesivir and favipiravir reduced q-CRP (C-Reactive Protein Blood Test), LDH (lactate dehydrogenase), and ALP (alkaline phosphatase) in severe COVID-19 [43,44].
Tranilast showed significant therapeutic and preventive outcomes in mouse models of gout, cryopyrin-associated periodic syndrome, and type 2 diabetes. In pigs, tranilast reduced ARDS and acute lung injury, preventing pulmonary and airway vascular permeability and hypoxemia. In addition, it reduces cardiomyopathy in patients with muscular dystrophy after administration of 300 mg/day for three months [45]. Tranilast suppressed NLRP3 activation in low density lipoprotein receptor and apolipoprotein E deficient macrophages [46].
Triptolide relieves cardiac hypertrophy induced by isoproterenol in mice. Low-dose triptolide improves mouse cardiac fibrosis induced by isoproterenol or angiotensin II (in vitro) by blocking NLRP3 assembly. It inhibits the activation of the NLRP3-TGF-β1 (transforming growth factor-beta) -Smad pathway [31,47].
Thiolutin blocks NLRP3 activation by alternative, canonical, noncanonical, and transcription-independent pathways at nanomolar concentrations. Thiolutin effectively suppressed the activation of multiple NLRP3 mutants linked with CAPS (cryopyrin-associated periodic syndromes) [48] (Table 3 ).
Table 3.
The mechanism of pharmaceutical action on the NLRP3 inflammasome with deterioration factors.
| Mechanism of action | Specificity | Applicable | ||
|---|---|---|---|---|
| Ageing | A deterioration of mitochondrial performance, diminished metabolism, increased oxidative stress, higher levels of mtROS production, the release of mtDNA | mtDNA | NLRP3 | Deterioration factor |
| Smoking | increases the expression of ACE2 receptor | ACE2 | ACE2 | Deterioration factor |
| Canakinumab | Inhibited the expression of IL-1 associated genes; targeted for IL-1β anti-inflammatory therapy | Heart | an IL-1β neutralizing antibod | X |
| Colchicine | Reduced acute inflammation in infarct areas, improved survival, inhibited heart failure, reduced ventricular remodelling and maintaining stability in cardiac function; colchicine reduces cytokine levels as well as the activation of macrophages, neutrophils, and the inflammasome. | Heart | caspase-1, NLRP3 | X |
| CY-09 | Binds to the NACHT domain, which limits NLRPS oligomerization and assembly of the inflammasome; inhibited NLRP3-mediated activation of ATPase selectively | ATP | NLRP3 | ?a |
| Dapansutrile (OLT1177) | A selective inhibitor of the NLRP3 inflammasome, with unique properties to reverse the metabolic costs of inflammation and to treat IL-1β– and IL-18–mediated diseases | Joint, Heart | NLRP3 | ? |
| Dapsone (Dapsone) | Reducing the inflammatory response of cells by binding myeloperoxidase. Dapsone compete with Ub and NLRP3. | Brain Heart | Inflammasome | Oa |
| MCC950 | Inhibited the NLRP3 inflammasome selectively. The diarylsulfonylurea compound MCC950 (originally reported as CRID3/CP-456773) | Heart | NLRP3 | ? |
| PEDF | Inhibited mitochondrial division through PEDFR/IPLA2 & mitochondrial fission-induced NLRP3inflammasome activation. | Heart | NLRP3 | ? |
| Statin | Reduced the expression if IL-1 associated genes; targeted for IL-1β and IL-18 | Heart | NLRP3 | O |
| TA (total flavones) | Inhibited cell death and reduced oxidative stress and inflammation | Heart | NLRP3 | ? |
| Tocilizumab | Decreased PAI-1 production and alleviated clinical manifestations in severe Covid-19patients | Heart | NLRP3 | X |
| TP (triptolide) | Inhibited the expression of NLRP3 and ASC as well as inflammasome assembly and blocked the NLRP3-TGFβ1-Smad pathway | Heart | NLRP3 | ? |
| Tranilast | Reduced cardiomyopathy in patients with muscular dystrophy. Acting as direct NLRP3 inhibitors | Heart | NLRP3 | ? |
| Thiolutin | Thiolutin nhibited the activation of multiple NLRP3 mutants linked with cryopyrin-associated periodic syndromes | Heart | NLRP3 | ? |
X: Clinically vague or negated, ?: No clinical study reports, O: Reported to be clinically applicable
SARS-CoV-2 S protein damages hematopoietic stem and progenitor cells via pyroptosis in an NLRP3 inflammasome-dependent manner. MCC950, an inhibitor of NLRP3, inhibited NLPR3 activation [14], but MCC950 has no clinical study reports. Statin and dapsone are clinically applicable for SARS-CoV-2 inflammasomes. Therefore, we selected a small molecule compound, dapsone, to pass the blood-brain barrier in cerebral diseases. We suggest that dapsone could control appendicitis, hypersensitivity reactions, acute myocardial infarction, and cerebrovascular accidents caused by mRNA vaccination. In addition, it might treat AESI and unknown inflammasome diseases reported in nine countries as a supplement. The specific targeting of the NLRP3 inflammasome by dapsone might reduce AESI or other inflammasome diseases among mRNA vaccine recipients.
2.3. Viral respiratory diseases can provoke a storm of bronchitis, COPD, and dementia
The virus uses endogenous cellular machinery inside the cell to replicate itself [49]. Lee et al. analyzed 9649 HD patients in the Sorokdo National Hospital. Among those, 4685 were randomized to the dapsone use (DDS(+)) group and 4964 to the dapsone nonuse (DDS(-)) group: 6983 to the viral respiratory disease (VRD)-diagnosed (+) group, and 2666 to the VRD-undiagnosed (-) group from 2005 to 2020 on Sorok Island of South Korea. The prevalence of Immune-related viral inflammatory diseases showed a sharp increase in prevalence from 2008 to 2015, followed by a decrease. COPD slowly increased from 2008 to 2011 and rapidly increased in 2012 and 2013, followed by a reduction. Bronchitis levels quickly rose from 2012 to 2014 and then decreased, and pneumonia increased sharply in 2013. 2008-2010 was endemic on Sorok Island. Since 2012, COPD has increased, as have bronchitis and pneumonia frequencies [50]. Dementia patients have increased: Dementia (+) subjects are 37 (2005), 57 (2006), 73 (2007), 80 (20 [50]08), 85 (2009), 107 (2010), 133 (2011), 174 (2012), 206 (2013), 215 (2014), 268 (2015), 288 (2016), 305 (2017), 337 (2018), 380 (2019), and 384 (2020) [51] [52] (Fig. 3 ).
Fig. 3.
Viral correlation factor with bronchitis, pneumonia, and COPD.
In observation, centralized national health insurance systems such as China, Singapore, and South Korea controlled the more effectively to the pandemic in terms of lower infection and fatality [[53], [54], [55]]. New treatments were developed. For example, nirmatrelvir plus ritonavir treatment reduced the risk of progression to severe COVID-19 by 89% lower than the placebo group [56]. Dexamethasone use for patients hospitalized with COVID-19 decreased 28-day mortality among those receiving intensive mechanical ventilation or oxygen alone but did not lower the mortality of those not receiving respiratory support [57]. Nirmatrelvir-ritonavir is used for mild patients and dexamethasone for severe patients with vaccinations. In a real-life study, we observed a low hospitalization rate, death, and adverse drug reactions in mostly vaccinated patients treated with oral antivirals [58].
China eased Covid measures and trimmed quarantine time for international travelers by two days. China tracked no longer beyond close contacts of COVID infections. The new standards emphasized home quarantine, but the reform window will not be wide enough to overcome the entrenched path dependency [55]. China rolled Beijing into a kind of Covid island for the party congress despite repeated assurances that the country's rollback of restrictions is under control. It had become challenging to enter the city: China still tracks residents' PCR tests and other health indicators with an app on residents' mobile phones. Their health code should be green to get into any building or travel.
Nevertheless, their health codes turn red or receive a nationwide pop-up warning [59]. Recurrent SARS-CoV-2 symptoms have been reported after treatment with nirmatrelvir–ritonavir in a Centers for Disease Control and Prevention Health Advisory [60]. Viral load rebound may be a feature of our previous study and some SARS-CoV-2 infections. The natural history of VRDs requires continued studies and more basic pathologic blockers.
3. Treatment mechanism of triad
3.1. Dexamethasone treatment metabolism
Dexamethasone is one of the essential medicines on the WHO list. Dexamethasone treats the emetic effects of cancer therapy and epicondylitis and has anti-inflammatory effects [61]. Dexamethasone has been used to treat rheumatic problems, several skin diseases, a variety of allergies, asthma, other lung diseases, and brain swelling to improve neonatal outcomes after preterm labor. As a direct chemotherapeutic agent, dexamethasone counteracts the development of edema in metastatic brain tumors. It treats in combination with other chemotherapeutic drugs in hematological malignancies, especially in treating multiple myeloma. COVID-19 requires supplemental oxygen (without ventilation) or mechanical ventilation [62].
Dexamethasone attenuated the neutrophil expression of IFN pathways more broadly [6]. Neutrophil IFN responses during infections are generally considered homogenous with a uniform proinflammatory capacity. In COVID-19 neutrophils, neutrophil state-specific markers such as interferon response genes: 1) IFITM1 (interferon-induced transmembrane protein, 2) RSAD2 (radical S-adenosyl methionine domain containing, 3) IFI6 (interferon-inducible protein 6), and 4) ISG10 (IFN-stimulated genes 10) are more highly expressed [63]. The most prominent discriminating features of dexamethasone treatment were the downregulation of IFITM1 and upregulation of IL1R2 (interleukin-1 receptor type II), which encodes a decoy receptor that sequesters IL-1. Dexamethasone expanded immunosuppressive immature neutrophils by changing neutrophils from information receivers into information providers. Dexamethasone remodeled cellular interactions: affected circulating neutrophils, changed IFNactive neutrophils, downregulated IFN-stimulated genes (IFITM1, RSAD2, IFI6, and ISG10), and activated IL1R2+ neutrophils during severe COVID-19 [63]. The activation of IFN-1 signaling pathways with notable increases in PTGER4 (prostaglandin E receptor 4) and PTGS2 (prostaglandin-endoperoxide synthase 2 or COX2) encodes a proposed target in COVID-19. However, the lineage relationship was unclear for COVID-19-enriched PGactive clusters in COVID-19 [63]. COVID-19 has been associated with the preferential expansion of interferon (IFNactive) and prostaglandin (PGactive) neutrophil states. The SARS-CoV-2-induced gene PTGS2, encoding COX-2, is expressed in human cells and mice [64].
Glucocorticosteroid hormones of the adrenal cortex have striking pharmacologic effects on lymphoid tissues and cells [65]. Innate lymphoid cells (ILCs) responded directly to steroid treatment despite the persistence of steroid-resistant IL-17+ cells and eosinophils [66]. Liu et al. reported that dexamethasone inhibited type 2 cytokine production by blood group 2 innate lymphoid cells (ILC2s) [67].
The exact incidence of dexamethasone's adverse effects is unknown, but of the 464 patients without a prior diagnosis of diabetes mellitus, 52 (11%) developed hyperglycemia [68]. Dexamethasone is a glucocorticoid anti-inflammatory drug used to regulate systemic and pulmonary inflammation progression in sepsis and ARDS via downregulation of NF-κB activity [69]. It significantly decreased NF-κB and IL-8 in the cytoplasm and mononuclear cell nuclei after dexamethasone therapy [70,71].
Dexamethasone use was associated with reduced mortality only in patients requiring respiratory support (i.e., oxygen or mechanical ventilation) at a cumulative dose between 60 and 150 mg either orally or by intravenous injection in a multicenter observational study of patients hospitalized for COVID-19. In addition, dexamethasone administered at 6 mg once per day for ten days is associated with reduced mortality only in patients with COVID-19 requiring respiratory support [72].
3.2. Dapsone treatment metabolism
Dapsone, as an antibiotic, acts by limiting microbial dihydrofolic acid synthesis and was used in clinical practice as the first modern antibiotic. It is active in leprosy, Mycobacteria pneumonia, toxoplasmosis, plasmodia, and pneumocystis [73,74]. Dapsone represses disease activity in bullous pemphigoid, dermatitis herpetiformis, and cutaneous lupus [28,29].
Dapsone regulates hypochlorite production from neutrophil extracellular traps (NETs). It increases cell survival from MPO-DNA NETs [28]. The nucleophilic and electrophilic zone in dapsone interacts with amino acids at noncovalent and covalent bonding sites. The methionine residue initiates neurotoxicity, aggregation, and free-radical formation at position 35 in the Aβ C-terminal domain [75,76]. The two-electron oxidation of bicarbonate is facilitated by hydrogen peroxide in the production of peroxymonocarbonate (HCO4−). The bicarbonate/carbon dioxide pair also accelerates mono-electron oxidation. Carbonate-radical anions (CO3•ㅡ) facilitate one-electron oxidation that can efficiently oxidize thioester sulfur to radical sulfur cations in Methionine residue (MetS•+) [77].
The human genome encodes approximately 100 deubiquitinating enzymes (DUBs). The ability for ubiquitination with DUBs acts as an essential regulatory layer in the ubiquitin (Ub) system. DUBs are involved in pathologies of neurodegeneration and cancer [78]. DUBs can reverse the conjugation of Ub. This reflects additional regulation of Ubiquitin [79]. The covalent attachment of Ub to substrates has supposed that dapsone could compete with pathogenic targets in Ub-conjugating targeting chimeras [80].
The interaction/binding relative binding energy between dapsone and DNA is −6.22 kcal mol-1, as estimated in silico studies. According to spectroscopic, viscometric, and molecular docking studies, dapsone binds to AT-rich DNA regions and noncovalently binds in the minor groove of DNA [81]. Dapsone decreased the paraquat-induced generation of superoxide anions in lung fibroblasts in mice. Dapsone can reduce the local expression of transcripts of mRNA encoding inflammation-related molecules: endothelin-1, MIP-1α (macrophage inflammatory protein-1-alpha), and TGF-β [82].
The administration of dapsone reversed the alterations made by doxorubicin in CK-MB (creatine kinase-MB fraction) serum levels, ECG (electrocardiogram) parameters, papillary muscle contractility, and excitation. Dapsone significantly reduced oxidative stress in tissue by measuring malondialdehyde, SOD (superoxide dismutase), and TNF-α levels. They are consistent with histopathological analysis [28]. Dapsone prevents ischemic injury and results in functional improvement after postischemia. Dapsone inhibits the proapoptotic proteins: Calpain, Caspase-3, c-Jun N-Terminal Kinases (JNK), Phosphatase, and Tensin Homolog (PTEN) in cerebral ischemia. Dapsone activates the pro-survival protein, Brain-Derived Neurotrophic Factor (BDNF) [83]. Dapsone protects the oxidation of high-fat diet-induced low-density lipoprotein (LDL) and keeps microvascular integrity from the oxidation attack [84]. Dapsone reduced the acetic acid-induced inflammatory response by inhibiting the NF-kB signaling pathway in rat experiments [85]. Dapsone decreased tissue edema, the tissue concentration of TNF-α, and interferon γ (INFγ). As a result, it hindered inflammatory cell infiltration [86]. Idiopathic pulmonary fibrosis (IPF) is a chronic interstitial disease of the lung tissue. Inflammatory factors and oxidative stress cause IPF. Bleomycin also induces IPF in an in vivo model, but dapsone decreases the total number of inflammatory cells in bleomycin-induced IPF, such as neutrophils and eosinophils [87]. Dapsone treats neuroinflammasome diseases such as mild cognitive impairment, exacerbated Alzheimer's conditions, and Stroke [52,88,89]. These data represent an essential avenue for further therapeutic development (e.g., NET-targeted or inflammasome or cGAS-STING therapies) in the battle against COVID-19 ARDS [6] (Fig. 4 ).
Fig. 4.
Treatment mechanisms of dapsone.
Dapsone was used to be part of standard care in COVID-19 and related acute inflammatory lung dysfunctions [28,29,90]. Neutrophils have destructed a significant tissue element of COVID-19 lung [[91], [92], [93], [94]]. On this basis, Badar et al. prescribed dapsone alongside standard intensive care unit (ICU) care to patients in Hunt Regional Medical Center. They administered standard treatment with dexamethasone plus dapsone for COVID-19 patients with dyspnea from December 21 to December 29, 2020, as the case-control study of 22 control and 22 trial. As a result, The mortality rates were 0 (5.9)% and 40% at the ARDS-onset stage. None with dapsone died except for one subject without dapsone after the relapse of COVID-19. The 17 participants with dapsone were compared to 20 without dapsone at the ARDS-onset stage. Eight of the twenty who received standard treatment with dexamethasone and without dapsone died. They examined the mortality relationship between those with dapsone and without dapsone [95]. The ARDS onset group with dapsone survived more than the ARDS onset without dapsone. Dapsone uses reduced COVID-19 mortality in ICU patients (relative ratio (RR) = 0.52, 95% confidence interval (CI): 0.32 to 0.84) and had more chance of being discharged home (RR = 2.7, 95% CI: 1.2 to 5.9) [96,97]. Patients infrequently received 400 mg cimetidine three times daily to weaken dapsone-related methemoglobinemia [98,99].
3.3. Aspirin treatment metabolism
Aspirin was used for potent analgesic, antipyretic and anti-inflammatory properties and was successfully used as an antithrombotic agent. The cox enzyme has been a target of drug interventions against the inflammatory process. However, aspirin remains topical after two centuries of evaluation, and new therapeutic indications are increasingly being studied [100]. Dai et al. discovered that aspirin acetylates cGAS directly only and suppresses cGAS-mediated interferon production in 2019 [101]. Aspirin inhibits cGAS-mediated immune responses and efficiently prevents death by COVID-19 [6].
COVID-19 is associated with the favored expansion of interferon (IFNactive) and prostaglandin (PGactive) neutrophil states. The SARS-CoV-2-induced gene PTGS2 is expressed in human cells [64]. This indicates that NSAIDs (which inhibit COX-2) could modulate COVID-19 severity by reducing the production of neutralizing antibodies and inflammatory cytokines [64]. Aspirin directly acetylates cGAS and suppresses cGAS-mediated type I interferonopathies. As a result, aspirin efficiently inhibits cGAS-mediated immune responses [6,101]. AERD (Aspirin-Exacerbated Respiratory Disease) is distinguished by tissue eosinophilia and mast cell activation, which ILC2s promote, including large PGD2 (prostaglandin D2) production. ILC2s go through chemotaxis and cytokine production in response to PGD2. ILC2s are significantly increased in the nasal mucosa and decreased in blood during COX-1 (cyclooxygenase 1) inhibitor reactions in AERD patients. This correlates with enhanced production of prostaglandins and leukotrienes [102]. However, aspirin therapy adjusts clinical and inflammatory markers through several unique mechanisms [103]. The activation of cGAS-STING developed higher expression levels of cGAS, STING, and p-IRF3 in aged human aortic intima tissue than in young aortic intima tissue [104]. A vital role of the cGAS-STING pathway in aging-related endothelial dysfunction explains the indications of aspirin use in elderly individuals [104] (Fig. 5 ).
Fig. 5.
Aspirin inhibited cGAS-mediated cytokine production.
In a study of veteran patients, 35,370 patients were involved in the 14-day mortality cohort from March 2 to September 13, 2020, and 32,836 patients in the 30-day mortality cohort from March 2 to August 28, 2020. Among the COVID-19-positive veterans, a prior aspirin prescription was statistically and clinically significantly associated with a decrease in overall mortality at 14 days and 30 days [105]. Ten observational studies and one RCT were analyzed from the results of RECOVERY and the retrospective cohort study on COVID-19. The total subjects were 136,695, of which 27,168 were in the aspirin-prescribed (+) group, and 109,527 were in the aspirin-nonprescribed (-) group. All-cause mortality was decreased in aspirin use was associated with a 14% decrease in the aspirin (+) group compared with the aspirin (-) group in patients hospitalized with COVID-19 [106]. Another meta-analysis also found that aspirin use was associated with a reduction in mortality and not an increased risk of bleeding in patients with COVID-19 [107]. However, low-dose aspirin is not preventive for exacerbated COVID-19 because the acetylation action needs a sufficient dose of over 100 mg [6,108].
Early in the COVID-19 pandemic, there was worry that NSAIDs could harm cardiovascular and pulmonary outcomes with NSAID prescriptions for COVID-19 patients [109]. As a result, many aspirin contraindications for DIC and other bleeding disorders exist. In addition, aspirin use may cause Reye syndrome in children. However, there was no evidence of a dangerous influence of NSAIDs on COVID-19-related deaths. Therefore, the threats do not need to influence the therapeutic use of COVID-19 [110]. Furthermore, according to the collective data meta-analysis of fourteen studies, 164,539 COVID-19 patients preceding aspirin prescription showed a reduced risk of in-hospital mortality in eight studies [111].
3.4. Aspirin, dapsone, and dexamethasone lower the mortality of COVID-19 patients
cGAS–STING–NLRP3 pathway constitutes the primary inflammasome reaction during microbial infections in human myeloid cells. cGAS–STING–NLRP3 pathway ameliorates the pathology in inflammatory conditions linked with cytosolic DNA sensing [112]. However, the pathway exacerbated COVID-19 by sensing cytosolic DNA from senescent cells or aberrant DNA by SARS-CoV-2 [113]. We have experiences based on clinical practice prescribing aspirin, dapsone, and dexamethasone to inhibit cGAS-STING activation by SARS-CoV-2 [6]. The immune triad has been implemented to prevent and treat COVID-19 patients with severe inflammation [[95], [96], [97]]. Side effects are well known, and dapsone uses cimetidine or famotidine to avoid methemoglobinemia (Table 4 ).
Table 4.
Treatment protocol recommendation for Immune Triad.
| Home | Admission | O2 supply 5 | O2 supply 10 | O2 supply 15 | Remark | |
|---|---|---|---|---|---|---|
| aSaO2 | Above 90 | Above 90 | Above 90 | Above 90 | Above 90 | |
| O2 supply | 2 L/min | 5 L/min | 10 L/min | 15 L/min | ||
| CRP | Check | Check | Check | Check | ||
| Platelet | Check | Check | Check | Check | ||
| Treatment | Anti-platelet treatment | Anti-platelet treatment | Anti-platelet treatment | Anti-platelet treatment | ||
| Aspirin | 300-500 mg bid∗ | 300-500 mg tid∗∗ or iv∗∗∗ | 300-500 mg tid or iv | 300-500 mg tid or iv | 300-500 mg tid or iv | |
| Dapsone | 100 mg bid | 100 mg biad | 100 mg bid | 200 mg tid | 200 mg tid | Check methemoglobinemia with/without Cimetidine∗! |
| Dexamethasone | 3 mg bid | 6 mg bid | iv | iv | iv |
Saturation of O2, ∗Two times a day, ∗∗three times a day, ∗∗∗within a vein, ∗! Cimetidine is the antidote for methemoglobinemia. This protocol is for adults.
3.5. When meets the adverse event of special interest
SARS-CoV-2 mRNA vaccines showed anaphylaxis, appendicitis, cerebral venous sinus thrombosis, herpes zoster infection, hypersensitivity reactions, increased risk of Bell's palsy in Hong Kong, lymphadenopathy, myocarditis, pericarditis, thrombocytopenia, and uveitis [[114], [115], [116]]. Inflammasomes cause arrhythmias, atherosclerosis, autoimmune encephalomyelitis, cardiac arrhythmias, cognitive impairment in AD, colitis, diabetes, inflammatory bowel disease, myocardial infarction, steatohepatitis, Stroke, traumatic brain injury, and thrombocytopenia [117]. mRNA vaccine S protein can damage human cells via cGAS–STING–NLRP3 pathway [28]. Barhoumi et al. reported S protein-induced apoptosis and inflammatory and oxidative stress responses [118].
mRNA vaccine S protein could induce adverse reactions by inflammasomes. Therefore, we recommend the immune triad for the treatment of AESI.
4. Conclusion
It is necessary to confirm their use as a first-line therapeutic triad through more systematic clinical studies of the immune triad as COVID-19 treatment agents.
Adverse reaction cases, severe cases, anaphylaxis cases, and death cases were obtained as crude rates. Moreover, in the graph, the Y-axis was drawn on a logarithmic scale, and the X-axis was compared for each country. The graphs of the UK and Korea, which have similar National Health Insurance Systems, have similar patterns. The overall seroprevalence of Korean anti-SARS-CoV-2 was very low, but adverse events of special interest (AESI) prevalence was very high among nine countries. This suggests that AESI might originate from inflammasomes induced by mRNA vaccines.
Peripheral immune insults activate the pattern recognition receptor TLR4, and TLR4 activates nuclear factor-κB (NF-κB) and, ultimately, produces immature IL-1β (pro-IL-1β). Caspase-1 cleaves pro-IL-1β, and inflammasome components turn it into mature IL-1β, which is out into the extracellular fluid. As a result, IL-1β provokes multiple effects on synaptic-plasticity-related processes, and IL-1β suppress brain-derived neurotrophic factor (BDNF) production, reduces α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor membrane expression, and inhibits long-term potentiation (LTP).
The respiratory virus binds the spike protein to cell surface receptors for cellular entry. This makes available the fusion of the virus to the cellular membrane with proteases such as TMPRSS2 (transmembrane serine protease 2) and furin to be involved in priming the spike protein. Next, virions are taken up into endosomes, where the virus may be cleaved and possibly activated by the cysteine protease. Pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) in the cell may present respiratory symptoms. Viral respiratory disease (VRD) showed a sharp increase from 2008, followed by bronchitis and COPD, increasing in 2012 and declining from 2014. Pneumonia also rose sharply in 2013 compared to previous years.
The purpose of dapsone administration is to reduce the activity of the inflammasome. Dapsone was used for the treatment of immune thrombocytopenia [119]. Dapsone also reduced doxorubicin's cardiac toxicity. Dapsone suppressed and decreased the mRNA of TNF-α.
Dapsone binds noncovalent to the minor groove of DNA. To date, scientists have regarded dapsone as an adjuvant, alternative, augmentation or active ingredient for patient improvements. It is a newly developed drug that treats significant diseases, and dapsone manages side effects and improves treatment efficiency. Dapsone was the treatment and prevention drug for familial Mediterranean fever by mutations in a pyrin coding gene [120,121], immune thrombocytopenia [119], mild cognitive impairment, Alzheimer's disease [88], seizure [122], infective endocarditis [89], stroke [89,123], and COVID-19 ARDS [95].
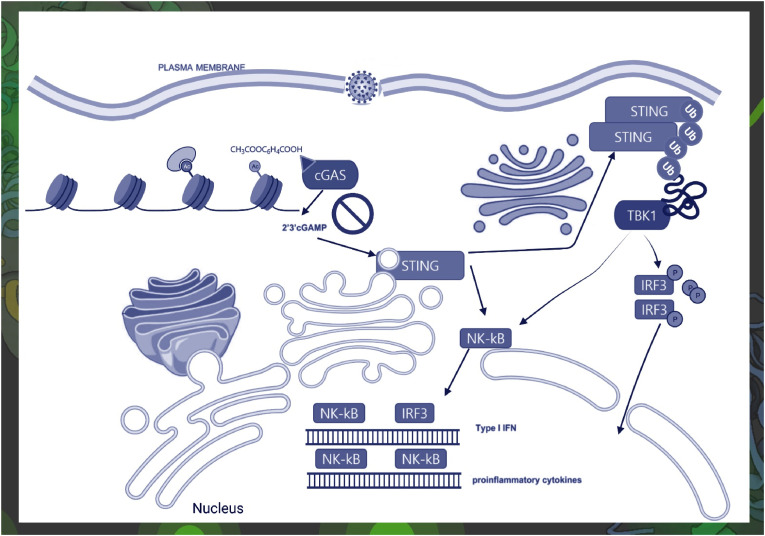
Acetylation contributes to cyclic-GMP-AMP synthase (cGAS) control and delivers a potential therapy by restricting the activation of both interferon regulatory factor 3 (IRF3) signaling and NK-κB. cGAS activates downstream IRF3 signaling and NK-κB signaling via the stimulator of interferon genes (STING) signaling. cGAS and STING are involved in interferon (IFN) production, and NK-κB can adjust the expression of some interferon-stimulated genes (ISGs). Aspirin strongly inhibited cGAS-mediated TBK1 (tank-binding kinase 1)–IRF3 signaling, but aspirin did not inhibit direct STING-associated activation. cGAS–STING pathway can activate IRF3 and NK-κB by cytosolic nucleic acids and promote the transcription of IFN-1 and other proinflammatory cytokines. But acetylation modulates antigen presentation and immune responses [6].
Funding sources
None.
List of abbreviations
- ACE
Angiotensin-converting enzyme
- AD
Alzheimer's disease
- AESI
Adverse events of special interest
- AIDS
Acquired Immune Deficiency Syndrome
- AGS
Aicardi–Goutières syndrome
- AIM2
Absent in melanoma 2
- ALP
alkaline phosphatase
- α-Syn
α-synuclein
- AMI
Acute myocardial infarction
- AMI I/R injury
AMI ischemia–reperfusion injury
- APC
Antigen-presenting cells
- ARDS
Acute respiratory distress syndrome
- ASC
Apoptosis-associated speck-like protein containing a CARD
- ATF4
Activated parkin via protein kinase RNA-like endoplasmic reticulum kinase-activating transcription factor 4
- BDNF
Brain-derived neurotrophic factor
- BiPAP
Bilevel positive airway pressure
- BV-2
A type of microglial cell derived from C57/BL6 mice
- CAPS
Cryopyrin-associated periodic syndromes
- CARD
Caspase activation and recruitment domain
- CCNE2
Essential for the control of the cell cycle at the late G1 and early S phases; belongs to the cyclin family
- CCR5
C–C motif chemokine receptor 5
- CH
Clonal hematopoiesis, hematopoietic stem and progenitor cells
- CI
Confidence interval
- CK-MB
Creatine kinase-MB fraction
- COPD
Chronic obstructive pulmonary disease
- COX-1
Cyclooxygenase 1
- CRP
C-reactive protein
- CRS
Cytokine release syndrome
- CtIP
C-terminal binding protein 1 (CtBP1) interacting protein
- Cyclin E2
Cyclin E2 is a protein that in humans is encoded by the CCNE2 gene
- CXCR-4
C-X-C chemokine receptor type 4
- DDS
4,4′-Diaminodiphenyl sulfone (dapsone)
- DIC
Disseminated intravascular coagulation
- ECG
Electrocardiogram
- G6PDH
Glucose-6-phosphate dehydrogenase
- HAART
Highly active antiretroviral therapy
- HIV
Human Immunodeficiency Virus
- HLA
Human leukocyte antigen
- HLA-DRB1
Major histocompatibility complex, class II, DR beta 1
- HSPC
hematopoietic stem/progenitor cell
- ICU
Intensive care unit
- IFN
Interferon
- IFNAR2
Interferon-alpha and beta receptor subunit 2
- IL
Interleukin
- IL-1β
Interleukin-1 beta
- IMV
intensive mechanical ventilation
- IRF3
Interferon regulatory factor 3
- JNK
Jun N-terminal kinases
- LDH
Lactate dehydrogenase
- LDL
Low-density lipoprotein
- LL
Lepromatous leprosy
- MADDS
Monoacetyldapsone
- MAPK
Mitogen-activated protein kinase
- MCI
Mild cognitive impairment
- MHC
Major histocompatibility complex
- MIS-C/A
Multisystem inflammation syndrome in children and adults
- MPO
Myeloperoxidase
- NOD2
Nucleotide-binding oligomerization domain containing 2
- mRNA
Messenger RNA
- mtDNA
Mitochondrial DNA
- NACHT
Domain conserved in NAIP, CIITA, HET-E, and TP1
- NFL
Neurofilament light chain
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRC4
NLR Family CARD Domain Containing 4
- NLRP3
NOD-, LRR-, and pyrin domain-containing protein 3
- NLR
family pyrin domain-containing 3
- PAI-1
Plasminogen activator inhibitor-1
- PAMPs
Pathogen-associated molecular patterns
- PBMCs
Human peripheral blood mononuclear cells
- PD
Parkinson's disease
- PEDF
Pigment epithelium-derived factor
- PEDFR/iPLA2
PEDF/calcium-independent phospholipase A2
- Phospho-p65
Anti-phospho-NFkB p65 (Ser536) monoclonal antibody (T.849.2)
- Phospho-IκBα
Phospho-IκBα (Ser32/36) (5A5) mouse mAb #9246
- PRMT5
Protein arginine methyltransferase 5
- PTGS2
Prostaglandin synthase 2
- PTM
Multiple posttranslational modification
- ROS
Reactive oxygen species
- S
Full-length prefusion spike glycoprotein of SARS-CoV-2
- S1
SARS-CoV-2 spike protein subunit 1
- SCLS
Systemic capillary leak syndrome
- RCT
Randomized controlled trial
- SOD
Superoxide dismutase
- TGF-β
Transforming growth factor-beta
- THP-1
A spontaneously immortalized monocyte-like cell line
- TNF
Tumor necrosis factor
- TLR
Toll-like receptor
- TMPRSS2
Transmembrane protease serine subtype 2
- TTS
Thrombosis with thrombocytopenia syndrome
- TYK2
Tyrosine kinase 2
- VRD
Viral respiratory disease
- VSEL
Very small embryonic-like stem cell
References
- 1.Gupta A., et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barda N., et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang C.K., et al. Longitudinal analysis of human memory T-cell response according to the severity of illness up to 8 Months after severe acute respiratory syndrome coronavirus 2 infection. J. Infect. Dis. 2021;224(1):39–48. doi: 10.1093/infdis/jiab159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L., et al. Convalescent plasma is of limited clinical benefit in critically ill patients with coronavirus disease-2019: a cohort study. J. Transl. Med. 2021;19(1):365. doi: 10.1186/s12967-021-03028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lian Q., et al. Differential effects of macrophage subtypes on SARS-CoV-2 infection in a human pluripotent stem cell-derived model. Nat. Commun. 2022;13(1):2028. doi: 10.1038/s41467-022-29731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J.H., et al. COVID-19 molecular pathophysiology: acetylation of repurposing drugs. Int. J. Mol. Sci. 2022;23(21) doi: 10.3390/ijms232113260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aarreberg L.D., et al. Interleukin-1β induces mtDNA release to activate innate immune signaling via cGAS-STING. Mol. Cell. 2019;74(4):801–815.e6. doi: 10.1016/j.molcel.2019.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolton K.L., et al. Clonal hematopoiesis is associated with risk of severe Covid-19. Nat. Commun. 2021;12(1):5975. doi: 10.1038/s41467-021-26138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond D., Loghavi S. Clonal haematopoiesis of emerging significance. Pathology. 2021;53(3):300–311. doi: 10.1016/j.pathol.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Pardi N., et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Contr. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahl K., et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 2017;25(6):1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahin U., et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021:1–6. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 13.Cho A., et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature. 2021;600:517–522. doi: 10.1038/s41586-021-04060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucia M., et al. 2021. An Evidence that SARS-Cov-2/covid-19 Spike Protein (SP) Damages Hematopoietic Stem/progenitor Cells in the Mechanism of Pyroptosis in Nlrp3 Inflammasome-dependent Manner. Leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olajide O.A., et al. SARS-CoV-2 spike glycoprotein S1 induces neuroinflammation in BV-2 microglia. Mol. Neurobiol. 2022;59:445–458. doi: 10.1007/s12035-021-02593-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti S.S., et al. Rapidly progressive dementia with asymmetric rigidity following ChAdOx1 nCoV-19 vaccination. Aging and disease. 2021:0. doi: 10.14336/AD.2021.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domizio J.D., et al. The cGAS–STING pathway drives type I IFN immunopathology in COVID-19. Nature. 2022;603(7899):145–151. doi: 10.1038/s41586-022-04421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theobald S.J., et al. Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19. EMBO Mol. Med. 2021;13(8) doi: 10.15252/emmm.202114150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stampfer S.D., et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia. 2021;35(12):3534–3541. doi: 10.1038/s41375-021-01354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratajczak M.Z., et al. SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45− precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome. Stem Cell Reviews and Reports. 2021;17(1):266–277. doi: 10.1007/s12015-020-10010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caricchio R., et al. Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA. 2021;326(3):230–239. doi: 10.1001/jama.2021.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cremer P.C., et al. Double-blind randomized proof-of-concept trial of canakinumab in patients with COVID-19 associated cardiac injury and heightened inflammation. European Heart Journal Open. 2021;1(1) doi: 10.1093/ehjopen/oeab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martínez G.J., Celermajer D.S., Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269:262–271. doi: 10.1016/j.atherosclerosis.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 24.Lopes M.I., et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7(1) doi: 10.1136/rmdopen-2020-001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang H., et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017;214(11):3219–3238. doi: 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchetti C., et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. U. S. A. 2018;115(7):E1530–E1539. doi: 10.1073/pnas.1716095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonnemann N., et al. The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2020;117(50):32145–32154. doi: 10.1073/pnas.2009680117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J.-h., et al. 4,4′-Diaminodiphenyl sulfone (DDS) as an inflammasome competitor. Int. J. Mol. Sci. 2020;21(17):5953. doi: 10.3390/ijms21175953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khattak A., et al. Commentary for the elderly in the pandemic era. Dement. Geriatr. Cogn. Dis. Extra. 2021;11(2):168–171. doi: 10.1159/000515926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan P., et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021;12(1):4664. doi: 10.1038/s41467-021-25015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An N., et al. Regulatory mechanisms of the NLRP3 inflammasome, a novel immune-inflammatory marker in cardiovascular diseases. Front. Immunol. 2019;10:1592. doi: 10.3389/fimmu.2019.01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Z., et al. PEDF inhibits the activation of NLRP3 inflammasome in hypoxia cardiomyocytes through PEDF receptor/phospholipase A2. Int. J. Mol. Sci. 2016;17(12):2064. doi: 10.3390/ijms17122064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morenikeji O.B., et al. Evolutionarily conserved long non-coding RNA regulates gene expression in cytokine storm during COVID-19. Front. Bioeng. Biotechnol. 2021;8(1330) doi: 10.3389/fbioe.2020.582953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu S.Y., et al. Statin protects the heart against ischemia-reperfusion injury via inhibition of the NLRP3 inflammasome. Int. J. Cardiol. 2017;229:23–24. doi: 10.1016/j.ijcard.2016.11.219. [DOI] [PubMed] [Google Scholar]
- 35.Satoh M., et al. NLRP3 inflammasome activation in coronary artery disease: results from prospective and randomized study of treatment with atorvastatin or rosuvastatin. Clin. Sci. (Lond.) 2014;126(3):233–241. doi: 10.1042/CS20130043. [DOI] [PubMed] [Google Scholar]
- 36.Gupta A., et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat. Commun. 2021;12(1):1325. doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daniels L.B., et al. Relation of prior statin and anti-hypertensive use to severity of disease among patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 Cardiovascular Disease Registry. PLoS One. 2021;16(7) doi: 10.1371/journal.pone.0254635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang S., et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. U. S. A. 2020;117(36):22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvarani C., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern. Med. 2021;181(1):24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lv D., et al. The cardioprotective effect of total flavonoids on myocardial ischemia/reperfusion in rats. Biomed. Pharmacother. 2017;88:277–284. doi: 10.1016/j.biopha.2017.01.060. [DOI] [PubMed] [Google Scholar]
- 41.Liskova A., et al. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 2021;138 doi: 10.1016/j.biopha.2021.111430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alzaabi M.M., et al. Phytochemistry Reviews; 2021. Flavonoids Are Promising Safe Therapy against COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shohan M., et al. The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: a randomized controlled trial. Eur. J. Pharmacol. 2022;914 doi: 10.1016/j.ejphar.2021.174615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soleiman-Meigooni S., et al. Efficacy of a standardized herbal formulation from Glycyrrhiza glabra L. as an adjuvant treatment in hospitalized patients with COVID-19: a Randomized Controlled trial. J. Ayurveda Integr. Med. 2022;13(4) doi: 10.1016/j.jaim.2022.100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quagliariello V., et al. SARS-CoV-2 infection: NLRP3 inflammasome as plausible target to prevent cardiopulmonary complications? Eur. Rev. Med. Pharmacol. Sci. 2020;24(17):9169–9171. doi: 10.26355/eurrev_202009_22867. [DOI] [PubMed] [Google Scholar]
- 46.Chen S., et al. Novel role for tranilast in regulating NLRP3 ubiquitination, vascular inflammation, and atherosclerosis. J. Am. Heart Assoc. 2020;9(12) doi: 10.1161/JAHA.119.015513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding Y.Y., et al. Triptolide upregulates myocardial forkhead helix transcription factor p3 expression and attenuates cardiac hypertrophy. Front. Pharmacol. 2016;7:471. doi: 10.3389/fphar.2016.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren G.-M., et al. Pharmacological targeting of NLRP3 deubiquitination for treatment of NLRP3-associated inflammatory diseases. Science Immunology. 2021;6(58) doi: 10.1126/sciimmunol.abe2933. [DOI] [PubMed] [Google Scholar]
- 49.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metabol. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J.H., et al. Naunyn-Schmiedeberg's Archives of Pharmacology; 2023. Bronchitis, COPD, and Pneumonia after Viral Endemic of Patients with Leprosy on Sorok Island in South Korea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J.H. Naunyn-Schmiedeberg's Archives of Pharmacology; 2022. The Listed, Delisted, and Sustainability of Therapeutic Medicines for Dementia Patients: the Study Is Specific to South Korea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J.H., et al. iScience; 2022. Dapsone Is an Anticatalysis for Alzheimer's Disease Exacerbation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He A.J., Shi Y., Liu H. Crisis governance, Chinese style: distinctive features of China's response to the Covid-19 pandemic. Policy Design and Practice. 2020;3(3):242–258. [Google Scholar]
- 54.Woo J.J. Policy capacity and Singapore's response to the COVID-19 pandemic. Policy and Society. 2020;39(3):345–362. doi: 10.1080/14494035.2020.1783789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bali A.S., He A.J., Ramesh M. Health policy and COVID-19: path dependency and trajectory. Policy and Society. 2022;41(1):83–95. [Google Scholar]
- 56.Hammond J., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19. N. Engl. J. Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Group T.R.C. Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 2020;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gentile I., et al. Nirmatrelvir/ritonavir and molnupiravir in the treatment of mild/moderate COVID-19: results of a real-life study. Vaccines. 2022;10(10):1731. doi: 10.3390/vaccines10101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolfe J. The New York Times. 2022. China's Covid politics. (USA) [Google Scholar]
- 60.Anderson A.S., Caubel P., Rusnak J.M. Nirmatrelvir–ritonavir and viral load rebound in covid-19. N. Engl. J. Med. 2022;387(11):1047–1049. doi: 10.1056/NEJMc2205944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grunwald H.W., Rosner F. Dexamethasone as an antiemetic during cancer chemotherapy. Ann. Intern. Med. 1984;101(3) doi: 10.7326/0003-4819-101-3-398_1. 398-398. [DOI] [PubMed] [Google Scholar]
- 62.Cohen S.P., et al. Epidural steroids: a comprehensive, evidence-based review. Reg. Anesth. Pain Med. 2013;38(3):175–200. doi: 10.1097/AAP.0b013e31828ea086. [DOI] [PubMed] [Google Scholar]
- 63.Sinha S., et al. Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19. Nat. Med. 2022;28:201–211. doi: 10.1038/s41591-021-01576-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J.S., et al. Nonsteroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection. J. Virol. 2021;95(7) doi: 10.1128/JVI.00014-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Claman H.N. Corticosteroids and lymphoid cells. N. Engl. J. Med. 1972;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- 66.Nagakumar P., et al. Pulmonary type-2 innate lymphoid cells in paediatric severe asthma: phenotype and response to steroids. Eur. Respir. J. 2019;54(2) doi: 10.1183/13993003.01809-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu S., et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: the role of thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 2018;141(1):257–268.e6. doi: 10.1016/j.jaci.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Younes Y.R., et al. COVID-19 and dexamethasone-induced hyperglycaemia: workload implications for diabetes inpatient teams. Diabet. Med. 2022;39(2) doi: 10.1111/dme.14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meduri G.U., et al. Nuclear factor-ĸB-and glucocorticoid receptor α-mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Neuroimmunomodulation. 2005;12(6):321–338. doi: 10.1159/000091126. [DOI] [PubMed] [Google Scholar]
- 70.Aghai Z.H., et al. Dexamethasone suppresses expression of Nuclear Factor-kappaB in the cells of tracheobronchial lavage fluid in premature neonates with respiratory distress. Pediatr. Res. 2006;59(6):811–815. doi: 10.1203/01.pdr.0000219120.92049.b3. [DOI] [PubMed] [Google Scholar]
- 71.Yamamoto Y., Gaynor R.B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001;107(2):135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoertel N., et al. Dexamethasone use and mortality in hospitalized patients with coronavirus disease 2019: a multicentre retrospective observational study. Br. J. Clin. Pharmacol. 2021;87(10):3766–3775. doi: 10.1111/bcp.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolf R., Matz H., Orion E. Cimetidine and dapsone-mediated methaemoglobinaemia. Dermatol. Online J. 2002;8(1):2. [PubMed] [Google Scholar]
- 74.Ghaoui N., et al. Update on the use of dapsone in dermatology. Int. J. Dermatol. 2020;59(7):787–795. doi: 10.1111/ijd.14761. [DOI] [PubMed] [Google Scholar]
- 75.Varadarajan S., et al. Methionine residue 35 is important in amyloid β-peptide-associated free radical oxidative stress. Brain Res. Bull. 1999;50(2):133–141. doi: 10.1016/s0361-9230(99)00093-3. [DOI] [PubMed] [Google Scholar]
- 76.Enache T.A., Oliveira-Brett A.M. Alzheimer's disease amyloid beta peptides in vitro electrochemical oxidation. Bioelectrochemistry. 2017;114:13–23. doi: 10.1016/j.bioelechem.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Francioso A., et al. One- and two-electron oxidations of β-Amyloid25-35by carbonate radical anion (CO3•−) and peroxymonocarbonate (HCO4−): role of sulfur in radical reactions and peptide aggregation. Molecules. 2020;25(4):961. doi: 10.3390/molecules25040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Cesare V., et al. Deubiquitinating enzyme amino acid profiling reveals a class of ubiquitin esterases. Proc. Natl. Acad. Sci. U.S.A. 2021;118(4) doi: 10.1073/pnas.2006947118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clague M.J., Urbe S., Komander D. Breaking the chains: deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 2019;20(6):338–352. doi: 10.1038/s41580-019-0099-1. [DOI] [PubMed] [Google Scholar]
- 80.Burslem G.M., Crews C.M. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell. 2020;181(1):102–114. doi: 10.1016/j.cell.2019.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakraborty A., et al. DNA minor groove binding of a well known anti-mycobacterial drug dapsone: a spectroscopic, viscometric and molecular docking study. Arch. Biochem. Biophys. 2019;665:107–113. doi: 10.1016/j.abb.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 82.Cho S.C., et al. Protective effect of 4,4'-diaminodiphenylsulfone against paraquat-induced mouse lung injury. Exp. Mol. Med. 2011;43(9):525–537. doi: 10.3858/emm.2011.43.9.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahale A., et al. Dapsone prolong delayed excitotoxic neuronal cell death by interacting with proapoptotic/survival signaling proteins. J. Stroke Cerebrovasc. Dis. 2020;29(8) doi: 10.1016/j.jstrokecerebrovasdis.2020.104848. [DOI] [PubMed] [Google Scholar]
- 84.Zhan R., et al. Dapsone protects brain microvascular integrity from high-fat diet induced LDL oxidation. Cell Death Dis. 2018;9(6):683. doi: 10.1038/s41419-018-0739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rashidian A., et al. Dapsone reduced acetic acid-induced inflammatory response in rat colon tissue through inhibition of NF-kB signaling pathway. Immunopharmacol. Immunotoxicol. 2019;41(6):607–613. doi: 10.1080/08923973.2019.1678635. [DOI] [PubMed] [Google Scholar]
- 86.Mohammad Jafari R., et al. Dapsone ameliorates colitis through TLR4/NF-kB pathway in TNBS induced colitis model in rat. Arch. Med. Res. 2021;52(6):595–602. doi: 10.1016/j.arcmed.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Yousefi-Manesh H., et al. Protective effect of dapsone against bleomycin-induced lung fibrosis in rat. Exp. Mol. Pathol. 2022;124 doi: 10.1016/j.yexmp.2021.104737. [DOI] [PubMed] [Google Scholar]
- 88.Lee J.H., et al. Recovery of dementia syndrome following treatment of brain inflammation. Dement. Geriatr. Cogn. Dis. Extra. 2020;10(1):1–12. doi: 10.1159/000504880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee J.-h., et al. The neuroinflammasome in alzheimer's disease and cerebral stroke. Dement. Geriatr. Cogn. Dis. Extra. 2021;11(2):159–167. doi: 10.1159/000516074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schön M.P., et al. COVID-19 and immunological regulations–from basic and translational aspects to clinical implications. JDDG J. der Deutschen Dermatol. Gesellschaft. 2020;18(8):795–807. doi: 10.1111/ddg.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McKenna E., et al. Neutrophils in COVID-19: not innocent bystanders. Front. Immunol. 2022:2548. doi: 10.3389/fimmu.2022.864387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silberberg E., Filep J.G., Ariel A. Weathering the storm: harnessing the resolution of inflammation to limit COVID-19 pathogenesis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.863449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Codd A.S., et al. Neutrophilia, lymphopenia and myeloid dysfunction: a living review of the quantitative changes to innate and adaptive immune cells which define COVID-19 pathology. Oxford open immunology. 2021;2(1) doi: 10.1093/oxfimm/iqab016. iqab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Conrad C., Looney M.R. Is neutrophilic inflammation treatable in COVID-19? Lancet Respir. Med. 2022;10(12):1100–1101. doi: 10.1016/S2213-2600(22)00293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanwar B., Lee C.J., Lee J.-H. Specific treatment exists for SARS-CoV-2 ARDS. Vaccines. 2021;9(6):635. doi: 10.3390/vaccines9060635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kanwar B.A., et al. Benefits of using dapsone in patients hospitalized with COVID-19. Vaccines. 2022;10(2):195. doi: 10.3390/vaccines10020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanwar B., Khattak A., Kast R.E. Dapsone lowers neutrophil to lymphocyte ratio and mortality in COVID-19 patients admitted to the ICU. Int. J. Mol. Sci. 2022;23(24) doi: 10.3390/ijms232415563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coleman M.D. Improvement of patient tolerance to dapsone: current and future developments. Dermatol. Online J. 2007;13(4) [PubMed] [Google Scholar]
- 99.Rhodes L., et al. Cimetidine improves the therapeutic/toxic ratio of dapsone in patients on chronic dapsone therapy. Br. J. Dermatol. 1995;132(2):257–262. doi: 10.1111/j.1365-2133.1995.tb05022.x. [DOI] [PubMed] [Google Scholar]
- 100.Lévesque H., Lafont O. [Aspirin throughout the ages: a historical review] Rev. Med. Interne. 2000;21(Suppl 1):8s–17s. doi: 10.1016/s0248-8663(00)88720-2. [DOI] [PubMed] [Google Scholar]
- 101.Dai J., et al. Acetylation blocks cGAS activity and inhibits self-DNA-induced autoimmunity. Cell. 2019;176(6):1447–1460.e14. doi: 10.1016/j.cell.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eastman J.J., et al. Group 2 innate lymphoid cells are recruited to the nasal mucosa in patients with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2017;140(1):101–108.e3. doi: 10.1016/j.jaci.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wangberg H., White A.A. Aspirin-exacerbated respiratory disease. Curr. Opin. Immunol. 2020;66:9–13. doi: 10.1016/j.coi.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 104.Yu H., et al. Role of the cGAS-STING pathway in aging-related endothelial dysfunction. Aging Dis. 2022;13(6):1901–1918. doi: 10.14336/AD.2022.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Osborne T.F., et al. Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baral N., et al. All-cause and in-hospital mortality after aspirin use in patients hospitalized with COVID-19: a systematic review and meta-analysis. Biology Methods and Protocols. 2022;7(1) doi: 10.1093/biomethods/bpac027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma S., et al. Does aspirin have an effect on risk of death in patients with COVID-19? A meta-analysis. Eur. J. Clin. Pharmacol. 2022;78(9):1403–1420. doi: 10.1007/s00228-022-03356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eikelboom J.W., et al. Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial. Lancet Respir. Med. 2022;10(12):1169–1177. doi: 10.1016/S2213-2600(22)00298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Little P. British Medical Journal Publishing Group; 2020. Non-steroidal Anti-inflammatory Drugs and Covid-19. [DOI] [PubMed] [Google Scholar]
- 110.Collaborative T.O., et al. 2020. OpenSAFELY: Do Adults Prescribed Non-steroidal Anti-inflammatory Drugs Have an Increased Risk of Death from COVID-19? medRxiv; p. 2020. 08.12.20171405. [Google Scholar]
- 111.Srinivasan A., et al. Aspirin use is associated with decreased inpatient mortality in patients with COVID-19: a meta-analysis. Am. Heart J.: Cardiol. Res. Pract. 2022;20 doi: 10.1016/j.ahjo.2022.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gaidt M.M., et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171(5):1110–1124.e18. doi: 10.1016/j.cell.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu G., Gack M.U. Insights into pandemic respiratory viruses: manipulation of the antiviral interferon response by SARS-CoV-2 and influenza A virus. Curr. Opin. Immunol. 2022 doi: 10.1016/j.coi.2022.102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu Q., et al. Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC Med. 2021;19(1):173. doi: 10.1186/s12916-021-02059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barda N., et al. Safety of the BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wan E.Y.F., et al. The Lancet Infectious Diseases; 2021. Bell's Palsy Following Vaccination with mRNA (BNT162b2) and Inactivated (CoronaVac) SARS-CoV-2 Vaccines: a Case Series and Nested Case-Control Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Swanson K.V., Deng M., Ting J.P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barhoumi T., et al. SARS-CoV-2 coronavirus spike protein-induced apoptosis, inflammatory, and oxidative stress responses in THP-1-like-macrophages: potential role of angiotensin-converting enzyme inhibitor (perindopril) Front. Immunol. 2021;12(3771) doi: 10.3389/fimmu.2021.728896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Colella M.P., et al. A retrospective analysis of 122 immune thrombocytopenia patients treated with dapsone: efficacy, safety and factors associated with treatment response. J. Thromb. Haemostasis. 2021;19(9):2275–2286. doi: 10.1111/jth.15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park Y.H., et al. Ancient familial Mediterranean fever mutations in human pyrin and resistance to Yersinia pestis. Nat. Immunol. 2020;21(8):857–867. doi: 10.1038/s41590-020-0705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salehzadeh F., Jahangiri S., Mohammadi E. Dapsone as an alternative therapy in children with familial Mediterranean fever. Iran. J. Pediatr. (Engl. Ed.) 2012;22(1):23. [PMC free article] [PubMed] [Google Scholar]
- 122.López-Gómez M., et al. Safety and tolerability of dapsone for the treatment of patients with drug-resistant, partial-onset seizures: an open-label trial. Neurol. Sci. 2011;32(6):1063–1067. doi: 10.1007/s10072-011-0612-6. [DOI] [PubMed] [Google Scholar]
- 123.Nader-Kawachi J., et al. Neuroprotective effect of dapsone in patients with acute ischemic stroke: a pilot study. Neurol. Res. 2007;29(3):331–334. doi: 10.1179/016164107X159234. [DOI] [PubMed] [Google Scholar]
- 124.Jong hoon Lee B.K., Khattak Asif, Altschuler Eric, Sergi Consolato, Lee So Jeong, Choi Su-Hee, Park Jungwuk. Naunyn-Schmiedeberg's Archives of Pharmacology; 2022. Michael Coleman, and Jean Bourbeau, Bronchitis, COPD, and Pneumonia After Viral Endemic Of Patients with Leprosy on Sorok Island In South Korea. (Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee J.H. The listed, delisted, and sustainability of therapeutic medicines for dementia patients: the study is specific to South Korea. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022;395(5):535–546. doi: 10.1007/s00210-022-02209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]