Abstract
Patients with post-acute or long COVID-19 syndrome (PA-COVID) develop persistent symptoms and complications that last beyond 4 weeks of the initial infection. There is limited information regarding the pulmonary pathology in PA-COVID patients that require bilateral orthotopic lung transplantation (BOLT). Our experience with 40 lung explants from 20 PA-COVID patients that underwent BOLT is described. Clinico-pathologic findings are correlated with best evidence from literature. The lung parenchyma showed bronchiectasis (n=20) and severe interstitial fibrosis with areas resembling the nonspecific interstitial pneumonia (NSIP) pattern of fibrosis (n=20), interstitial fibrosis NOS (n=20) and fibrotic cysts (n=9). None of the explants exhibited a usual interstitial pneumonia (UIP) pattern of fibrosis. Other parenchymal changes included multinucleated giant cells (n=17), hemosiderosis (n=16), peribronchiolar metaplasia (n=19), obliterative bronchiolitis (n=6) and microscopic honeycombing (n=5). Vascular abnormalities included thrombosis of a lobar artery (n=1) and microscopic thrombi in small vessels (n=7). Systematic literature review identified 7 articles reporting the presence in 12 patients of interstitial fibrosis showing NSIP pattern (n=3), organizing pneumonia/diffuse alveolar damage (DAD) (n=4) and NOS (n=3) patterns. All but one of these studies also reported the presence of multinucleated giant cells and none of the studies reported the presence of severe vascular abnormalities.
PA-COVID patients undergoing BOLT show a pattern of fibrosis that resembles mixed cellular-fibrotic NSIP pattern and generally lack severe vascular complications. As the NSIP pattern of fibrosis is often associated with autoimmune diseases, additional studies are needed to understand the mechanism of disease and learn whether this information can be used for therapeutic purposes.
Keywords: Post-acute COVID-19, Long COVID-19, Lung, Pathology, Lung transplantation, Nonspecific Interstitial Pneumonia Pattern
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has posed the greatest medical and public health challenge in decades, with more than 450 million total cases and 6 million deaths worldwide [1]. The SARS-CoV-2 virus infects the respiratory tract and other organs, resulting in a multi organ disease with respiratory, cardiac, neurologic, and other manifestations [[2], [3], [4], [5], [6], [7]]. Approximately 33% of hospitalized COVID-19 patients develop acute respiratory distress syndrome and pneumonia that can progress to respiratory failure requiring Extracorporeal Membrane Oxygenation (ECMO)/mechanical ventilation [[1], [2], [3], [4], [5], [6], [7], [8]]. A recent systematic review and meta-analysis by Bertini et al of over 4,000 COVID-19 patients treated with ECMO reported a 39% in-hospital mortality and a 1.34 higher risk mortality ratio in these patients than in those with influenza treated in a similar manner [8]. Several autopsy studies have shown that the lungs of patients dying from COVID-19 often show diffuse alveolar damage (DAD) changes that are often associated with intraalveolar hemorrhage, superimposed bacterial or fungal pneumonias, multiple thromboemboli, and/or vasculitis in pulmonary arteries [[9], [10], [11], [12], [13], [14], [15], [16], [17]]. A systematic review by Caramaschi et al of the histopathological findings in 28 autopsy studies of COVID-19 patients described that 53% of patients died during the acute phase of DAD. Seventy-seven (77%) and 18% of autopsy cases showed DAD in the acute-proliferative and proliferative phases of the syndrome respectively. Other pulmonary findings included interstitial/alveolar edema, interstitial lymphocytic infiltrates, pneumocyte reactive hyperplasia, multinucleated giant cells, arteriolar microthrombi, organizing pneumonia and others [18]. Extrapulmonary findings included myocarditis, acute tubular injury, gastric hemorrhage, shock liver, encephalitis, and many others.
Post-acute or long COVID-19 syndrome (PA-COVID) is defined by the Center for Disease Control (CDC) as the presence of persistent symptoms and delayed or long-term complications beyond 4 weeks of the initial infection [19]. Various clinical problems have been described, such as progressive interstitial fibrosis, superimposed bacterial and other infectious pneumonias, pulmonary thromboemboli, pulmonary hypertension and others. The etiology of PA-COVID syndrome is under investigation; it has been suggested that may result from the development of autoimmune response with cytokine storm and cellular damage [19].
There is only limited information in case reports and small case series about the histopathologic findings in the lungs of patients with post-acute COVID-19 syndrome that suffer from chronic pulmonary disease [[20], [21], [22]]. Aesif et al reported the presence of diffuse interstitial fibrosis and lung infarction in a small retrospective series of 3 patients [23]. Konopka et al reported a spectrum of pathologic changes including organizing diffuse alveolar damage, usual interstitial fibrosis and other patterns of interstitial fibrosis in surgical lung biopsies from 18 patients [20]. Rohr el al described the presence of pulmonary hypertensive changes in a single patient with post-acute COVID-19 syndrome that underwent bilateral lung transplantation [21]. Flaifel at al reported a spectrum of fibrotic changes in the explants of 3 patients that underwent lung transplantation.
We performed a systematic literature review and reviewed our experience with the clinical, radiologic, and histopathologic findings in 40 lung explants from post-acute COVID 19 patients that underwent bilateral orthotopic lung transplantation (BOLT) at Cedars-Sinai Medical Center (CSMC) to gather best available evidence about the pulmonary pathologic manifestations in patients with post-acute COVID-19 syndrome.
2. Methods
The files of the Department of Pathology and Laboratory Medicine of Cedars-Sinai Medical Center (CSMC) were queried for patients without a prior history of chronic lung disease who underwent BOLT for post-acute COVID-19 syndrome from April 2021 until October 2022. One patient was excluded because of a known clinical history of Common Variable Immunodeficiency with recurrent bronchiectasis prior to COVID-19 syndrome. One other patient underwent a single lung transplant and was not included in this series. The following demographic and clinical data were obtained from Electronic Medical Records: age, sex, race, BMI, comorbidities, vaccination status at the time of admission to CSMC, medical treatment prior to transplant, time interval between initial acute COVID-19 syndrome and BOLT, duration of ECMO exposure, COVID-19-related complications prior to transplantation, post-operative complications and the latest clinical follow up. Reports of chest computed tomography (CT) scans performed prior to BOLT were reviewed.
The gross pathology and microscopic findings in all lung explants were reviewed by two lung pathologists (AM, MV) and a pathology resident (SM) as a group and differences in scoring were reconciled by interactive discussion. Six sections of alveolated lung parenchyma from the right lung and 4 from the left lung (2 sections per each lobe, total 10 slides per each case) were reviewed. The histopathologic abnormalities were classified into three major categories: parenchymal, airways and vascular. The extent of each finding was arbitrarily semi-quantitated as 1+ to 4+ based on the number of histologic glass slides showing an abnormality and as follows: 1+: single slide, 2+: 2-4 slides, 3+: 5-6 slides, 4+: more than 6 slides. Patterns of interstitial fibrosis were classified, using the American Thoracic Society/European Respiratory Society (ATS/ERS) classification of interstitial fibrosis [24, 25]. Histochemical stains such as GMS, PAS, trichrome and others and immunohistochemical stains were performed in selected cases.
A systematic review of the English literature was performed using the PubMed database of the National Library of Medicine and the Web of Science databases and the search terms "post-acute COVID”, “long COVID”, “pathology”, “lung pathology” “lung biopsy” and lung transplantation". Reports in literature were categorized into studies evaluating lung pathology in post-acute COVID19 patients treated with lung transplantation and those reporting changes on patients with the syndrome that underwent only lung biopsies to diagnose a pulmonary syndrome in patients that didn’t suffer from severe pulmonary insufficiency.
3. Results
3.1. Clinical findings
Twenty patients from different ethnicities, including 13 Hispanic, 6 White and 1 African American, underwent BOLT for post-acute COVID-19 syndrome during the 19 months selected for study. Their relevant clinical and imaging findings are summarized in table 1 . Median age at transplant was 39-year-old (24–66-year-old). Twelve patients were male, and 8 patients were female. Median BMI was 24.7 (22.1-35.6). Comorbidities included obesity (4/20), hypertension (4/20), diabetes/hyperlipidemia (5/20), chronic deep vein thrombosis (1/20), drug/alcohol abuse (1/20), and congenital heart disease (1/20). One other patient had Ramsay-Hunt Syndrome, and two other patients were pregnant at 30- and 36-weeks gestational age when they developed acute COVID-19 syndrome. All patients had been diagnosed with COVID-19 at the referring institutions using polymerase-chain reaction (PCR) testing and were transferred to Cedars from an outside hospital for lung transplant consideration and received various medications prior to transplant including corticosteroids (18/20), Remdesivir (14/20), Tocilizumab (7/20), Baricitinib (3/20), convalescent plasma (1/20) and others. At the time of admission to CSMC five patients were vaccinated with a COVID vaccine (5/20) and 14 patients were unvaccinated; the vaccination history of one other patient is unknown. Six of 20 patients tested positive for COVID using a PCR test on admission to CSMC. Chest computed tomography (CT) performed prior to BOLT demonstrated consolidation with ground glass opacification and traction bronchiectasis (15/20), air-containing cysts (3/20), enlarged main pulmonary artery (3/20) and apical cystic formation (1/20). None of the chest CT showed honeycomb changes. The median interval time from initial COVID-19 syndrome and lung transplantation was 129 days (63-711 days). Sixteen patients required veno-venous extracorporeal membrane oxygenation (ECMO) prior to lung transplantation for a median duration of 86 days (25-157 days).
Table 1.
Clinical findings in patients with post-acute COVID-19 syndrome undergoing bilateral orthotopic lung transplantation.
| Age/Sex | Race | BMI | Comorbidities | Vaccination status on admission to CSMC | Medical treatment received before transplant | Duration of ECMO exposure prior to transplant | Time interval between initial diagnosis and transplant | Covid related complications prior to transplant | Clinical follow-up (days after transplant) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 35/F | Caucasian | 22.96 | Pregnancy | Unvaccinated | Convalescent plasma, Remdesivir | 119 | 129 | Stroke, thrombosis, compartment syndrome | 459 |
| Case 2 | 40/M | Hispanic | 26.59 | Ramsay-Hunt syndrome, hypertension | Unvaccinated | Steroid, Remdesivir | 82 | 100 | Bacteremia | 495 |
| Case 3 | 34/F | Hispanic | 28.11 | None | Unvaccinated | Steroid | 87 | 111 | Infection | 382 |
| Case 4 | 51/M | Hispanic | 28.5 | Chronic deep vein thrombosis | Unvaccinated | Steroid Remdesivir, Tociliuzumab | 145 | 199 | Brain hemorrhage | Dead (305) |
| Case 5 | 52/M | African American | 24.12 | Drug/alcohol use | Unvaccinated | Steroid, Remdesivir | 104 | 63 | Pneumothorax, pneumomediastinum, pleural effusion | 381 |
| Case 6 | 55/M | Hispanic | 22.36 | Hypertension | Unknown | Steroid, Remdesivir, Tociliuzumab | 122 | 130 | Stroke, candida sepsis, RV mass, endocarditis | Dead (13) |
| Case 7 | 37/M | Hispanic | 26.73 | Obesity, fatty liver | Unvaccinated | Steroid, Remdesivir, Tociliuzumab | 0 | 71 | None | 360 |
| Case 8 | 38/F | White | 32.7 | Pregnancy | Unvaccinated | Steroid, Remdesivir, Tocilizumab | 78 | 92 | Aspergillus empyema, cardiac tamponade, cardiogenic shock, acute kidney injury | Dead (19) |
| Case 9 | 33/M | Caucasian | 25.24 | None | Unvaccinated | Steroid, Remdesivir | 94 | 106 | None | 244 |
| Case 10 | 46/M | Non-Hispanic | 24.42 | Diabetes | Unvaccinated | Steroid, Remdesivir, Baricitinib | 57 | 121 | Ventilator-associated pneumonia | 246 |
| Case 11 | 61/M | Non-Hispanic | 23.97 | Hyperlipidemia | Unvaccinated | Steroid | 0 | 197 | Steroid-induced hyperglycemia and myopathy, DVT | 217 |
| Case 12 | 33/F | White | 29.68 | Hypothyroidism, depression, obesity | Vaccinated | Steroid, Remdesivir, Baricitinib | 85 | 93 | Pneumothorax, pneumomediastinum, ischemic stroke, hyponatremia, bacteremia with Stenotrophomonas | 205 |
| Case 13 | 66/F | Hispanic | 19.79 | Hypertension, diabetes, ovarian cancer | Unvaccinated | Steroid, Remdesivir | 0 | 409 | Thrombocytopenia, bleeding | 211 |
| Case 14 | 66/M | Hispanic | 21.35 | Hypertension, hyperlipidemia, COPD, former smoker | Unvaccinated | Steroid | 0 | 711 | Pneumothorax, bronchopleural fistula | 244 |
| Case 15 | 38/M | Hispanic | 22.1 | None | Unvaccinated | Not provided | 139 | 150 | Pneumothorax | 198 |
| Case 16 | 24/F | Hispanic | 27.02 | Obesity | Unvaccinated | Steroid | 157 | 169 | Hemothorax, pneumonia | 189 |
| Case 17 | 35/M | Hispanic | 22.77 | None | Vaccinated | Steroid, Remdesivir, Baricitinib | 76 | 79 | None | 153 |
| Case 18 | 57/F | Hispanic | 22.44 | Diabetes, hyperlipidemia, asthma | Vaccinated | Steroid, Remdesivir, Tocilizumab | 57 | 144 | DVT, pulmonary emboli, thrombocytopenia, pneumonia | 125 |
| Case 19 | 52/M | Hispanic | 24.91 | Atrial septal defect | Vaccinated | Steroid, Remdesivir, Tocilizumab | 52 | 401 | Cerebrovascular accident | 90 |
| Case 20 | 25/F | Hispanic | 35.6 | Obesity | Vaccinated | Steroid, Tocilizumab | 25 | 232 | Tension pneumothorax, PEA arrest | 12 |
Abbreviations: BMI: Body mass index, CSMC: Cedars-Sinai Medical Center, ECMO: Extracorporeal membrane oxygenation, RV: Right ventricle, DVT: Deep vein thrombosis, COPD: Chronic obstructive pulmonary disease, PEA: Pulseless electrical activity.
The median post-operative follow-up period after transplantation was 217 days (12-495 days). Hospital course was complicated with vascular events in 7 patients, including stroke, thrombosis, and pulmonary embolism. Eight patients developed post-operative infections and sepsis, including bacterial infections (6/8) and fungal infections by Candida sp. (1/8) and Aspergillus sp. (1/8). Six patients developed post-operative pneumothorax and pneumomediastinum. One patient was diagnosed with acute myelogenous leukemia on bone marrow biopsy 98 days after BOLT. Three patients died at 13, 19 and 305 post-operative days, respectively.
3.2. Pathology
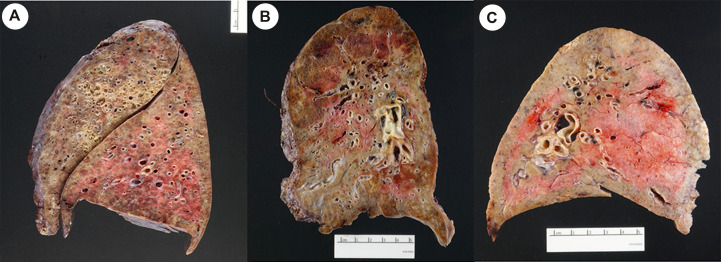
All 40 lung explants showed at least focal, bilateral fibrous adhesions and showed somewhat small lungs with diffuse consolidation and a granular pleural surface. On section, all explants showed a variable number of bronchiectasis (Fig. 1 A), small subpleural fibrotic cysts and extensive areas with firm, gray fibrosis involving all lobes, without a particular lobar distribution (Fig. 1B). Patchy areas of softer, gray consolidation were also present (Fig. 1C). No honeycomb changes were seen in any of the gross specimens.
Fig. 1.
A. Explant from patient # 15 showing bronchiectasis with recent hemorrhage B. Explant from patient # 6 showing small subpleural fibrotic cysts and extensive fibrosis C. Explant from patient # 11 showing patchy consolidation.
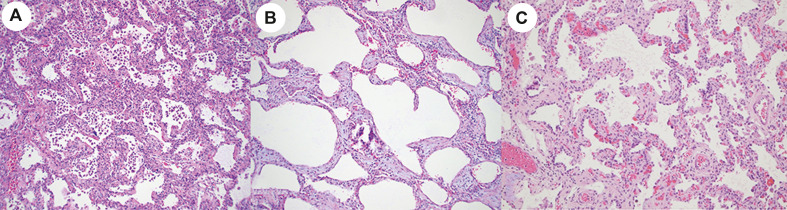
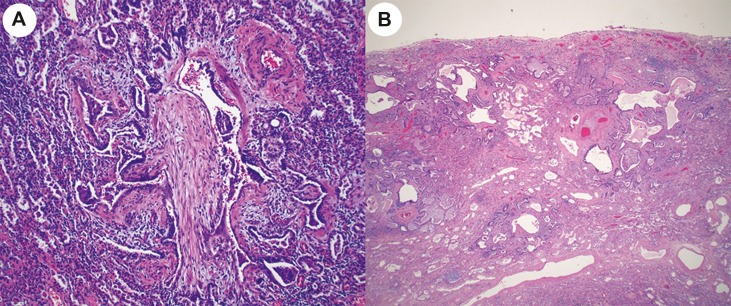
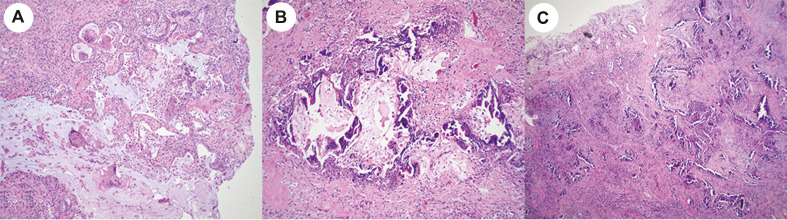
The microscopic findings are summarized in Table 2 . All 40 explants showed a variable amount of bronchiectasis with moderate to severe bronchial chronic inflammation, focal squamous metaplasia of respiratory epithelium and variable peribronchial fibrosis. Peribronchial lymph nodes were generally hyperplastic. The lung parenchyma of all explants showed diffuse, severe interstitial fibrosis but the pattern of fibrosis was difficult to classify in all lung areas according to current ATS/ERS guidelines. In multifocal areas the lungs showed diffuse alveolar infiltrates by lymphoid cells resembling the histopathologic findings seen in patients with community acquired pneumonias or cellular nonspecific interstitial pneumonia (NSIP) pattern (Fig. 2 A). Other areas showed extensive and diffuse interstitial fibrosis with mild to moderate alveolar thickening, as seen in patients with the fibrotic phase of NSIP (Fig. 2B), interspersed with foci of dense fibrosis (Fig. 2C) that could not be classified according to ATS/ERS guidelines and are listed in table 2 as interstitial fibrosis not otherwise specified (NOS). Fibrotic areas of the lung also showed macroscopic and microscopic cysts with fibrotic walls in 9/20 cases and obliterative bronchiolitis in 6/20 cases (Fig. 3 A). Five explants showed focal prominent peribronchiolar metaplasia that resembled microscopic honeycombing (Fig. 3B), but no fibroblastic foci or other features of usual interstitial pneumonia (UIP) pattern of fibrosis were seen. None of the cases showed findings characteristic of proliferative and fibrotic diffuse alveolar damage or cryptogenic organizing pneumonia. As shown in Table 1, Table 3, there were no apparent pathologic changes in the lung explants from the 4 patients that had been treated with prolonged mechanical ventilation and the 16 patients that were treated with ECMO. A somewhat unusual finding seen in 17 cases was the presence of multinucleated giant cells (Fig. 4 A) adjacent to dilated bronchioles, cysts with fibrotic and delicately calcified walls (Fig. 4B) or small arterioles with linear calcifications (Fig. 4C). No viral inclusions, as described in cases of acute COVID-19 patients, were seen. 9/20 cases showed parenchymal macro and micro cystic formation. Other lung parenchyma changes included multiple areas of subpleural microscopic peribronchiolar metaplasia, (Fig. 5 ), focal squamous metaplasia, focal microscopic honeycombing, hemosiderosis with hemosiderin-laden macrophages filling the alveolar spaces in 16/20 patients, and well-formed epithelioid granulomas (1/20).
Table 2.
Microscopic findings in 40 lung explants from 20 post-acute COVID-19 syndrome undergoing bilateral orthotopic lung transplantation.
| Parenchymal |
Airways |
Vascular |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Interstitial fibrosis NOS | NSIP fibrotic pattern | NSIP cellular pattern | Focal microscopic honeycombing | Cysts with fibrosis | Acute alveolitis | Multinucleated giant cells | Peribronchiolar metaplasia | Obliterative bronchiolitis | Alveolar hemosiderosis | Thrombosis | |
| Case1 | 4+ | 4+ | 4+ | 0 | 0 | 0 | 1+ | 4+ | 1+ | 4+ | 1+ |
| Case2 | 3+ | 1+ | 4+ | 0 | 1+ | 2+ | 2+ | 3+ | 0 | 4+ | 0 |
| Case3 | 4+ | 1+ | 4+ | 0 | 0 | 0 | 2+ | 4+ | 1+ | 4+ | 1+ |
| Case4 | 4+ | 1+ | 4+ | 1+ | 1+ | 0 | 1+ | 4+ | 0 | 4+ | 0 |
| Case5 | 4+ | 4+ | 4+ | 0 | 0 | 1+ | 2+ | 4+ | 1+ | 3+ | 2+ |
| Case6 | 4+ | 4+ | 4+ | 1+ | 0 | 3+ | 1+ | 4+ | 0 | 4+ | 3+ |
| Case7 | 4+ | 4+ | 3+ | 0 | 3+ | 4+ | 3+ | 0 | 0 | 3+ | 1+ |
| Case8 | 4+ | 4+ | 1+ | 0 | 0 | 4+ | 2+ | 2+ | 0 | 4+ | 0 |
| Case9 | 4+ | 4+ | 4+ | 1+ | 1+ | 0 | 2+ | 3+ | 1+ | 4+ | 0 |
| Case10 | 4+ | 1+ | 4+ | 0 | 0 | 0 | 0 | 2+ | 0 | 4+ | 0 |
| Case11 | 4+ | 4+ | 1+ | 0 | 4+ | 1+ | 1+ | 3+ | 0 | 0 | 0 |
| Case12 | 4+ | 1+ | 4+ | 0 | 0 | 1+ | 2+ | 4+ | 3+ | 0 | 0 |
| Case13 | 4+ | 4+ | 4+ | 0 | 0 | 1+ | 1+ | 4+ | 0 | 1+ | 0 |
| Case14 | 4+ | 4+ | 4+ | 0 | 0 | 2+ | 0 | 4+ | 0 | 0 | 1+ |
| Case15 | 4+ | 1+ | 4+ | 0 | 2+ | 0 | 2+ | 1+ | 0 | 0 | 0 |
| Case16 | 4+ | 1+ | 4+ | 0 | 0 | 0 | 1+ | 4+ | 1+ | 4+ | 1+ |
| Case17 | 4+ | 1+ | 4+ | 0 | 1+ | 4+ | 1+ | 4+ | 0 | 4+ | 0 |
| Case18 | 4+ | 4+ | 4+ | 1+ | 2+ | 0 | 2+ | 4+ | 0 | 1+ | 0 |
| Case19 | 4+ | 4+ | 4+ | 0 | 1+ | 0 | 4+ | 4+ | 0 | 4+ | 0 |
| Case20 | 4+ | 1+ | 4+ | 1+ | 0 | 0 | 0 | 4+ | 0 | 4+ | 0 |
1 Abbreviations: NOS: Not otherwise specified, NSIP: Nonspecific interstitial pneumonia.
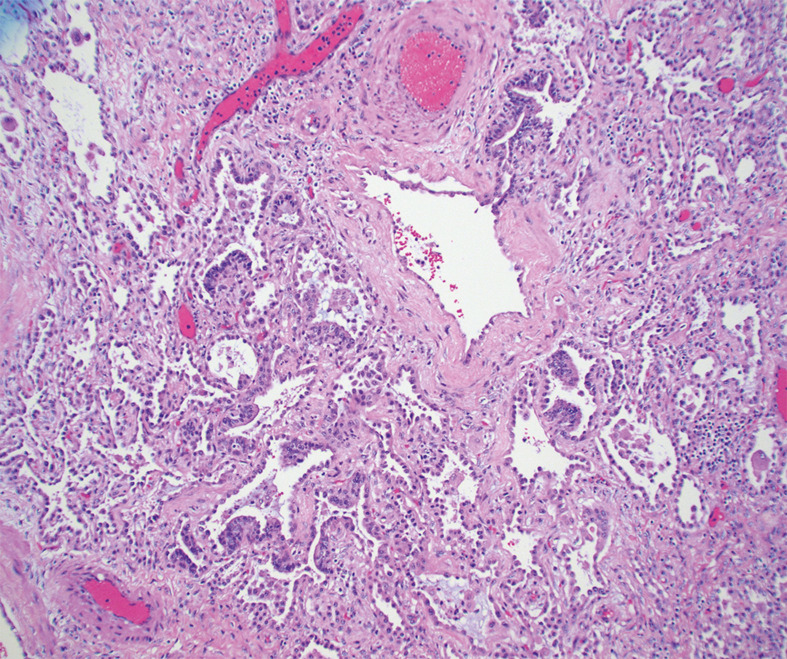
Fig. 2.
A. Explant from patient # 12 showing diffuse alveolar infiltration by lymphoid cells, resembling a cellular NSIP pattern of interstitial lung disease (hematoxylin-eosin, 20x magnification) B. The same explant shows areas of diffuse fibrosis resembling a fibrotic NSIP pattern of interstitial disease (hematoxylin-eosin, 20x magnification) C. Explant from patient # 20 showing interstitial fibrosis, NOS (hematoxylin-eosin, 10x magnification).
Fig. 3.
A. Explant from patient # 12 showing obliteration of the bronchiolar wall and lumen by fibrous tissue (hematoxylin-eosin, 20x magnification) B. Explant from patient # 4 showing microscopic honeycombing. However, areas of predominantly subpleural patchy fibrosis, residual normal alveoli, and fibroblastic foci, as seen in usual interstitial pneumonia (UIP) were not seen in this explant or any other case (hematoxylin-eosin, 20x magnification).
Table 3.
Literature review: Histopathologic findings in explants and surgical biopsy specimens from post-acute COVID-19.
| Pathologic findings in explants |
||||||
|---|---|---|---|---|---|---|
| Fibrosis | Peribronchiolar metaplasia/bronchiolization of alveoli | Delicate alveolar septal calcification | Multinucleated giant cells | Microscopic honeycombing | Cyst formation with giant cell reaction | |
| Luo 2020 (N#1) | Interstitial (#1) | Present (#1) | None | Present (viral inclusion bodies) (#1) | None | None |
| Chen 2020 (N#1) | Interstitial (#1) | None | None | Present (#1) | None | None |
| Bharat 2020 (N#3) | Interstitial (#3) | Present (#3) | None | Present (#1) | Present (#1) | Present (#1) |
| Aesif 2021 (N#1) | Vaguely resemble NSIP (#1) | Present (#1) | None | None | Present (#1) | None |
| Flaifel 2022 (N#3) | Proliferative & fibrotic DAD (#3) | Present (#3) | Present (#1) | Present (#1) | None | None |
| Roden 2022 (N#2) | NSIP (#NS) | Present (#NS) | None | Present (#NS) | Present (#NS) | Present (#NS) |
| Rohr 2022 (N#1) | Organizing DAD with prominent fibrosis (#1) | Present (#1) | None | Present (histoplasma granuloma) (#1) |
None | None |
| Pathologic findings in surgical lung biopsies | ||||||
| Konopka 2021 (N#18) | UIP (#9) | Present (#1) | None | None | Present (#2) | None |
| Aesif 2021 (N#2) | NSIP (#1) | None | None | None | Present (#1) | None |
| Baldi 2022 (N#6) | Peri bronchial (#5) organizing pneumonia (#1) | None | Present (#1) | None | None | None |
| Gagiannis 2021 (N#3) | Organizing pneumonia (#1) | None | None | Present (#1) | None | None |
| Roden 2022 (N#30) | NSIP (#NS) | Present (#NS) | None | Present (#NS) | Present (#NS) | None |
1 Abbreviations: NSIP: Nonspecific interstitial pneumonia, DAD: Diffuse alveolar damage, NS: Not specified, UIP: Usual interstitial pneumonia.
Fig. 4.
A. Explant from patient # 1 showing multinucleated giant cells (hematoxylin-eosin, 20x magnification) B. The multinucleated giant cells were often seen by bronchioloectatic small airways and adjacent to fibrotic cysts that often showed delicately calcified walls as seen in this explant from patient # 7 (hematoxylin-eosin, 20x magnification) C. Explant from patient # 17 showing small arterioles with linear calcifications (hematoxylin-eosin, 10x magnification).
Fig. 5.
Explant from patient # 11 showing peribronchiolar metaplasia in the right upper lobe (hematoxylin-eosin, 20x magnification).
Chronic pleuritis with dense adhesions was seen in 15 patients (15/20), one of them also showed pleural calcification (patient # 14). Three explants showed fibrinous pleuritis (3/20).
Vascular changes were not prominent in most of the 40 explants. Only one explant showed an organizing thrombus in a lobar pulmonary artery and few recent thrombi were present in small arterioles of 7 cases.
Ten patients had bacterial infections at the time of transplantation, with acute bronchopneumonia (10/20) and lung abscess formation (2/20). Bacterial infections were documented in all 10 patients prior to transplant by either sputum culture (4/10), bronchial washing culture (2/10), tracheal aspirate (2/10) or lung tissue culture (2/10). Cultures showed Escherichia coli (3/10), Klebsiella Oxytoca (2/10), Klebsiella Aerogenes (2/10), Coagulase negative Staph (1/10), Staph Aureus (3/10), Yeast (3/10), and Aspergillus (2/10). GMS and PAS stains were also performed on these ten cases which revealed invasive aspergillosis in two patients with cavitary abscess (2/10) and was suggestive of coccidiomycosis in one patient (1/10).
4. Systematic literature review
To our knowledge, only 7 studies have described the lung pathology findings in explants of 12 patients that underwent orthotopic lung transplantation to treat several pulmonary complications of PA-COVID syndrome (Table 3 ) [20, 21, 23, [26], [27], [28], [29], [30]]. The NSIP pattern of fibrosis was described by Aesif et al and Roden et al. in 3 patients [23, 30]. Other studies did not specify a particular fibrosis pattern. Multinucleated giant cells and other features were seen in a small number of explants. The studies by Aesif et al. and Roden et al. and 3 others reported the results of lung biopsies from 59 patients that suffered from presumably less severe forms of PA-COVID syndrome (Table 3) [22, 23, [30], [31], [32]]. The pattern of fibrosis is not clearly specified in most PA-COVID patients that underwent lung biopsies. Aesif et al described the presence of NSIP pattern in their patient and Roden et al described NSIP pattern without specifying the number of cases. Konopka reported the presence of UIP pattern of fibrosis in 9 patients and concluded that “Usual Interstitial Pneumonia is the Most Common Finding in Surgical Lung Biopsies from Patients with Persistent Interstitial Lung Disease Following Infection with SARS-CoV-2” [22]. Baldi et al. and Gagiannis et al. described the presence of organizing pneumonia in 2 cases [31, 32]. Table 3 shows that peribronchiolar metaplasia, bronchiolization of airspaces, alveolar septal calcifications, multinucleated giant cells, microscopic honeycombing, and fibrotic cysts were present in a small number of biopsies.
5. Discussion
The mechanism of pulmonary fibrosis in patients with coronavirus infections such as SARS-CoV-2 and others is under investigation. These viruses can induce a significant degree of intracellular endoplasmic reticulum stress, deposition of excessive extracellular matrix (ECM), persistent infiltration of the interstitium by macrophages and long-lived circulating inflammatory monocytes and autoimmune phenomena that promote a profibrotic milieu in the lung despite complete viral clearance [[33], [34], [35]]. Luo et al. first described the histopathologic findings in the explants from a patient that developed respiratory failure secondary to PA-COVID in Wuhan, China and was treated with bilateral orthotopic lung transplantation at approximately 5-6 weeks following the onset of severe disease [26]. The most prominent histopathologic findings included hemorrhagic necrosis, viral inclusions, interstitial fibrosis, vascular microthrombi, necrotizing bronchiolitis, multinucleated giant cells and diffuse alveolar damage in exudative and early proliferative phase. The findings in this patient are similar to those described in multiple autopsy reports of patients dying from COVID-19 syndrome during the acute phase of the disease [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18]]. The reports by Flaifel et al, Roden et al, Rohr et al and Gagiannis et al describe the presence of organizing and fibrotic diffuse alveolar damage in a small number of patients with PA-COVID, but this pattern of fibrosis is not the most important feature of disease evolution in most patients developing progressive diffuse lung disease.
Indeed, best current evidence from literature and our own experience suggests that PA-COVID patients that develop interstitial fibrosis as a result of SARS-CoV-2 infection and do not die within the first two months of the disease probably develop a fibrotic process that resembles the NSIP pattern of fibrosis admixed with patchy areas of organizing pneumonia and irregular areas of fibrosis that cannot be characterized according to ATS/ERS diagnostic guidelines [24]. The NSIP pattern of fibrosis is not unique to SARS-CoV-2 infections and has been described as a complication of human immunodeficiency virus (HIV) and other lung infections [36, 37]. It is also frequently associated with connective tissue disorders, autoimmune disorders, and other etiologies [24, 38]. In contrast, usual interstitial pneumonia (UIP) pattern of fibrosis is not frequently seen in association with viral pneumonias, so its presence in the PA-COVID cases described by Konopka et al is unusual [22]. The conclusion in this study that UIP is the most common pattern of fibrosis seen in patients with PA-COVID, is not supported by our experience or other reports shown in Table 3.
The presence of a NSIP pattern of fibrosis in PA-COVID patients may have therapeutic implications, as NSIP patients with idiopathic disease or syndrome associated with connective tissue disorders often respond to steroid and/or immunosuppressive therapy and are generally not treated with anti-fibrotic agents [39, 40]. However, this is speculative, as our cases were not studied with immunostains to characterize the nature of the inflammatory cells in areas with cellular NSIP pattern. Future studies comparing the immune microenvironment in fibrotic lungs from patients with post-acute COVID-19 syndrome and those with idiopathic NSIP and NSIP associated with connective tissue disorders are needed to provide insight into the pathogenesis of this syndrome.
The majority (16/20) of our patients were treated with ECMO for up to 409 days prior to BOLT, and the other 4 patients were treated with prolonged mechanical ventilation. We have no detailed information about the treatment of these patients in outside institutions to exclude the possibility that patients on ECMO had been previously treated with prolonged mechanical ventilation. No significant pathologic differences were seen between these two groups. To our knowledge, the development of progressive diffuse lung disease has not been described in patients treated with ECMO that survive the procedure [8, 41, 42]. For example, in a review of the histopathologic findings in lungs of 76 patients treated with ECMO, pathologic changes included pulmonary hemorrhage (63.3%), acute lung injury (60.5%), thromboembolic disease (47.4%), calcifications (28.9%), vascular changes (21.1%) and hemorrhagic infarct (21.1%) [43]. Prolonged mechanical ventilation can result in ventilator-induced lung injury (VILI) with the development of pneumonia, sepsis, acute respiratory distress syndrome, atelectasis, and pulmonary edema [44]. To our knowledge, severe interstitial fibrosis with NSIP or other patterns of interstitial fibrosis has not been described as a complication of either ECMO or prolonged mechanical ventilation, but the possibility that these therapeutic methods played a role in the development of fibrosis or the other pathologic changes observed in the lung explants from our patients cannot be entirely ruled out.
BOLT provides a valuable last resort therapeutic option to PA-COVID patients that suffer from severe respiratory insufficiency secondary to diffuse lung disease and cannot be removed from ECMO therapy, although there are still concerns regarding possible recurrence of viral pneumonia in the allograft, severe deconditioning associated with lengthy illness prior to a long surgery that might complicate the recovery, and the uncertainty of the outcome of the spontaneous repair after severe COVID [[45], [46], [47], [48]]. To our knowledge, there are still evidence-based guidelines to support the selection of patients that should be considered for lung transplant. Proposed criteria are evidence of irreversible lung damage at least 4 weeks since the onset of ARDS, confirmed by at least two physicians from two different specialties, The experience in our medical center with the largest to date cohort treated with BOLT offers hope. Seventeen of 20 patients of both genders ranging in age from 24-year-old to 66-year-old are alive up to 495 days following the procedure in spite of the development of a variety of complications shown in Table 1.
6. Conclusion
In conclusion, current best evidence suggests that mixed cellular-fibrotic pattern of NSIP is probably the most frequent pattern of interstitial fibrosis in PA-COVID patients suffering from severe diffuse lung disease. Future studies are needed to investigate the mechanism of disease, the role of steroid and immunosuppressive therapy in treatment and the development of evidence-based guidelines to determine eligibility for BOLT.
Declarations of interest
None.
References
- 1.John Hopkin’s COVID-19 database. 2022. https://coronavirus.jhu.edu/map.html
- 2.Canatan D., Vives Corrons J.L., De Sanctis V. The Multifacets of COVID-19 in Adult Patients: A Concise Clinical Review on Pulmonary and Extrapulmonary Manifestations for Healthcare Physicians. Acta Biomed. 2020;91 doi: 10.23750/abm.v91i4.10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson K.D., Harris C., Cain J.K., Hummer C., Goyal H., Perisetti A. Pulmonary and Extra-Pulmonary Clinical Manifestations of COVID-19. Front Med (Lausanne) 2020;7:526. doi: 10.3389/fmed.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elrobaa I.H., New K.J. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.711616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakur V., Ratho R.K., Kumar P., et al. Multi-Organ Involvement in COVID-19: Beyond Pulmonary Manifestations. J Clin Med. 2021;10 doi: 10.3390/jcm10030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw B., Daskareh M., Gholamrezanezhad A. The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19) Radiol Med. 2021;126:40–46. doi: 10.1007/s11547-020-01295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai C.C., Hsu C.K., Yen M.Y., Lee P.I., Ko W.C., Hsueh P.R. Long COVID: An inevitable sequela of SARS-CoV-2 infection. J Microbiol Immunol Infect. 2022 doi: 10.1016/j.jmii.2022.10.003. https://doi.org/10.1016%2Fj.jmii.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertini P., Guarracino F., Falcone M., et al. ECMO in COVID-19 Patients: A Systematic Review and Meta-analysis. J Cardiothorac Vasc Anesth. 2022;36:2700–2706. doi: 10.1053/j.jvca.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichmann D., Sperhake J.P., Lutgehetmann M., et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/m20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duarte-Neto A.N., Monteiro R.A.A., da Silva L.F.F., et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosmuller H., Traxler S., Bitzer M., et al. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Arch. 2020;477:349–357. doi: 10.1007/s00428-020-02881-x. https://doi.org/10.1007%2Fs00428-020-02881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calabrese F., Pezzuto F., Fortarezza F., et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020;477:359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Nemer A. Histopathologic and Autopsy Findings in Patients Diagnosed With Coronavirus Disease 2019 (COVID-19): What We Know So Far Based on Correlation With Clinical, Morphologic and Pathobiological Aspects. Adv Anat Pathol. 2020;27:363–370. doi: 10.1097/pap.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 14.Maiese A., Manetti A.C., La Russa R., et al. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol. 2021;17:279–296. doi: 10.1007/s12024-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borczuk A.C. Pulmonary pathology of COVID-19: a review of autopsy studies. Curr Opin Pulm Med. 2021;27:184–192. doi: 10.1097/mcp.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 16.Bryce C., Grimes Z., Pujadas E., et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Von Stillfried S., Bulow R.D., Rohrig R., Boor P., German Registry of Covid-19 Autopsies DC First report from the German COVID-19 autopsy registry. Lancet Reg Health Eur. 2022;15 doi: 10.1016/j.lanepe.2022.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caramaschi S., Kapp M.E., Miller S.E., et al. Histopathological findings and clinicopathologic correlation in COVID-19: a systematic review. Mod Pathol. 2021;34:1614–1633. doi: 10.1038/s41379-021-00814-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long Covid or Post Covid Conditions. 2022. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
- 20.Flaifel A., Kwok B., Ko J., et al. Pulmonary Pathology of End-Stage COVID-19 Disease in Explanted Lungs and Outcomes After Lung Transplantation. Am J Clin Pathol. 2022;157:908–926. doi: 10.1093/ajcp/aqab208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohr J.M., Strah H., Berkheim D., Siddique A., Radio S.J., Swanson B.J. Pulmonary Hypertensive Changes Secondary to COVID-19 Pneumonia in a Chronically SARS-CoV-2-Infected Bilateral Lung Explant. Int J Surg Pathol. 2022;30:443–447. doi: 10.1177/10668969211064208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konopka K.E., Perry W., Huang T., Farver C.F., Myers J.L. Usual Interstitial Pneumonia is the Most Common Finding in Surgical Lung Biopsies from Patients with Persistent Interstitial Lung Disease Following Infection with SARS-CoV-2. EClinicalMedicine. 2021;42 doi: 10.1016/j.eclinm.2021.101209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aesif S.W., Bribriesco A.C., Yadav R., et al. Pulmonary Pathology of COVID-19 Following 8 Weeks to 4 Months of Severe Disease: A Report of Three Cases, Including One With Bilateral Lung Transplantation. Am J Clin Pathol. 2021;155:506–514. doi: 10.1093/ajcp/aqaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Travis W.D., Costabel U., Hansell D.M., et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483st. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariri L.P., Smith M.L., Mino-Kenudson M., et al. Pulmonary Pathology Society Perspective on the 2018 American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society Idiopathic Pulmonary Fibrosis Clinical Practice Guidelines. Ann Am Thorac Soc. 2020;17:550–554. doi: 10.1513/annalsats.201910-801ps. [DOI] [PubMed] [Google Scholar]
- 26.Luo W.R., Yu H., Gou J.Z., et al. Histopathologic Findings in the Explant Lungs of a Patient With COVID-19 Treated With Bilateral Orthotopic Lung Transplant. Transplantation. 2020;104:e329–e331. doi: 10.1097/tp.0000000000003412. [DOI] [PubMed] [Google Scholar]
- 27.Chen X.J., Li K., Xu L., et al. Novel insight from the first lung transplant of a COVID-19 patient. Eur J Clin Invest. 2021;51 doi: 10.1111/eci.13443. [DOI] [PubMed] [Google Scholar]
- 28.Bharat A., Querrey M., Markov N.S., et al. Lung transplantation for pulmonary fibrosis secondary to severe COVID-19. medRxiv. 2020 https://doi.org/10.1101%2F2020.10.26.20218636. [Google Scholar]
- 29.Bharat A., Querrey M., Markov N.S., et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roden A.C., Boland J.M., Johnson T.F., et al. Late Complications of COVID-19. Arch Pathol Lab Med. 2022;146:791–804. doi: 10.5858/arpa.2021-0519-sa. [DOI] [PubMed] [Google Scholar]
- 31.Baldi B.G., Fabro A.T., Franco A.C., et al. Clinical, radiological, and transbronchial biopsy findings in patients with long COVID-19: a case series. J Bras Pneumol. 2022;48 doi: 10.36416/1806-3756/e20210438. https://doi.org/10.36416%2F1806-3756%2Fe20210438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagiannis D., Umathum V.G., Bloch W., et al. Antemortem vs Postmortem Histopathologic and Ultrastructural Findings in Paired Transbronchial Biopsy Specimens and Lung Autopsy Samples From Three Patients With Confirmed SARS-CoV-2. Am J Clin Pathol. 2022;157:54–63. doi: 10.1093/ajcp/aqab087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giacomelli C., Piccarducci R., Marchetti L., Romei C., Martini C. Pulmonary fibrosis from molecular mechanisms to therapeutic interventions: lessons from post-COVID-19 patients. Biochem Pharmacol. 2021;193 doi: 10.1016/j.bcp.2021.114812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saifi M.A., Bansod S., Godugu C. COVID-19 and fibrosis: Mechanisms, clinical relevance, and future perspectives. Drug Discov Today. 2022;27 doi: 10.1016/j.drudis.2022.103345. https://doi.org/10.1016%2Fj.drudis.2022.103345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parimon T., Espindola M., Marchevsky A., Rampolla R., Chen P., Hogaboam C.M. Potential Mechanisms for Lung Fibrosis Associated with COVID-19 Infection. QJM. 2022 doi: 10.1093/qjmed/hcac206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sattler F., Nichols L., Hirano L., et al. Nonspecific interstitial pneumonitis mimicking Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 1997;156:912–917. doi: 10.1164/ajrccm.156.3.9612050. [DOI] [PubMed] [Google Scholar]
- 37.Azadeh N., Limper A.H., Carmona E.M., Ryu J.H. The Role of Infection in Interstitial Lung Diseases: A Review. Chest. 2017;152:842–852. doi: 10.1016/j.chest.2017.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enomoto N., Sumikawa H., Sugiura H., et al. Clinical, radiological, and pathological evaluation of "NSIP with OP overlap" pattern compared with NSIP in patients with idiopathic interstitial pneumonias. Respir Med. 2020;174 doi: 10.1016/j.rmed.2020.106201. [DOI] [PubMed] [Google Scholar]
- 39.Li C., Wang Y., Liu Q., et al. Clinical, radiologic, and physiologic features of idiopathic pulmonary fibrosis (IPF) with and without emphysema. Expert Rev Respir Med. 2022;16:813–821. doi: 10.1080/17476348.2022.2093717. [DOI] [PubMed] [Google Scholar]
- 40.Domiciano D.S., Bonfa E., Borges C.T., et al. A long-term prospective randomized controlled study of non-specific interstitial pneumonia (NSIP) treatment in scleroderma. Clin Rheumatol. 2011;30:223–229. doi: 10.1007/s10067-010-1493-4. [DOI] [PubMed] [Google Scholar]
- 41.Tavares E.P., Rebolo J.R., Pimentel R., Roncon-Albuquerque R.L., Jr. Bleeding and Thrombotic Complications in COVID-19-Associated ARDS Requiring ECMO. Respir Care. 2022 doi: 10.4187/respcare.10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee Y.J., Kim D.J., Kim J.S., et al. Experience and results with VV-ECMO for severe acute respiratory failure: weaning versus nonweaning. ASAIO J. 2015;61:184–189. doi: 10.1097/mat.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 43.Lee H.F., Yi E.S., Rabatin J.T., Bohman J.R., et al. Histopathologic findings in lungs of patients treated with extracorporeal membrane oxygenation. Chest. 2018;153:825–833. doi: 10.1016/j.chest.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Haribhai S, Mahboobi SK. Ventilator complications https://www.ncbi.nim.nih.gov/books/NBK560535/ [PubMed]
- 45.Messika J., Roux A., Dauriat G., Pavec J.L. Groupe de Transplantation Pulmonaire de la Societe de Pneumologie de Langue F. Lung transplantation in the COVID-19 Era: A multi-faceted challenge. Respir Med Res. 2022;81 doi: 10.1016/j.resmer.2021.100866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang C., Ritschl V., Augustin F., et al. Clinical relevance of lung transplantation for COVID-19 ARDS: a nationwide study. Eur Respir J. 2022 doi: 10.1183/13993003.02404-2021. https://doi.org/10.1183%2F13993003.02404-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin A.K., Shah S.Z., Guru P.K., et al. Multidisciplinary Approach for Lung Transplantation due to COVID-19. Mayo Clin Proc Innov Qual Outcomes. 2022;6:200–208. doi: 10.1016/j.mayocpiqo.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ko R.E., Oh D.K., Choi S.M., et al. Lung transplantation for severe COVID-19-related ARDS. Ther Adv Respir Dis. 2022;16 doi: 10.1177/17534666221081035. [DOI] [PMC free article] [PubMed] [Google Scholar]