Abstract
Key Clinical Message
This case report highlights the potential of belinostat for the treatment of relapsed/refractory peripheral T‐cell lymphomas, for which effective therapies are still scarce.
Abstract
Peripheral T‐cell lymphomas have an aggressive disease course associated with poor outcomes. We report a young patient with highly pretreated relapsed/refractory nodal follicular helper T‐cell lymphoma (angioimmunoblastic‐type [nTFHL‐AI]), who successfully received an allogeneic stem cell transplantation following belinostat therapy. The complete hematologic response achieved has lasted more than 2 years.
Keywords: allogenic stem cell transplantation, belinostat, complete response, HDAC inhibitor, nTFHL‐AI, PTCL
1. INTRODUCTION
Peripheral T‐cell lymphomas (PTCL) are a rare and heterogeneous group of highly aggressive non‐Hodgkin lymphomas (NHL). With a 5‐year survival rate of approximately 30%, they are associated with poor treatment outcome. 1 The most common subtypes of PTCL include nodal follicular helper T‐cell (nTFH) lymphoma whose prototype is nTFHL angioimmunoblastic‐type (nTFHL‐AI), PTCL not otherwise specified (PTCL‐NOS), and anaplastic large cell lymphoma (ALCL). 2 , 3
For decades, cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) has been the standard first‐line therapy of PTCL. Although efforts have been made to improve treatment outcomes by replacing CHOP with another chemotherapy regimen or adding new therapeutic agents, limited progress has been made. 4 In recent years, new innovative agents specifically designed for the treatment of relapsed/refractory (R/R) PTCL have received accelerated approval in the United States; they include pralatrexate (a folate antagonist), brentuximab vedotin (an antibody‐drug conjugate targeting CD30 protein), and the histone deacetylase inhibitors (HDACis) romidepsin and belinostat. 5 , 6 , 7 , 8 However, most patients with PTCL still experience relapse, and durable, well‐tolerated disease control is achieved in only a minority of them. 9 Promising phase II clinical trials with the HDAC inhibitor belinostat as monotherapy showed durable response rates with a manageable safety profile in heavily pretreated PTCL patients. 8 , 10 nTFHL‐AI, one of the most common types of PTCL, typically presents with an advanced disease, systemic symptoms, and an immune deregulation. Its prognosis is mainly guided by age, C‐reactive protein level, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and β2‐microglobulin level, all these factors being combined in the angioimmunoblastic T‐cell lymphoma (AITL) score. 11
In this report, we describe the case of a young patient with multiple pretreated R/R nTFHL‐AI, who successfully received an allogeneic stem cell transplantation after belinostat therapy and achieved a durable complete hematologic response.
2. CASE PRESENTATION
2.1. Medical history
In May 2018, a 33‐year‐old patient consulted the Department of Infectious Diseases at Jacques Monod hospital (Le Havre, France) with complaints of persistent fever ranging from 38 to 39°C since May 2018, night sweats within the last 2 months, pruritus since April 2018, and recent weight loss of 7 kg (actual weight of 80 kg). Despite undergoing antibiotic therapy, he did not experience any improvement. At the time of consultation, his general health status was classified as PS grade 1. The patient's medical history revealed that he had a Crohn's disease, which had been well controlled with adalimumab for approximately 30 months. Although an initial suspicion of an Epstein–Barr virus (EBV) infection was raised, serology showed a negative virus load. The patient did not present any allergies or any intoxication with alcohol or tobacco. In his family history, his father had a sarcoidosis, and an aunt on the maternal family side had lymphoma (unknown subtype).
2.2. Diagnostic procedures
In June 2018, a positron emission tomography combined with a computed tomography (PET‐CT) scan revealed an intense hypermetabolism of the supra‐ and subdiaphragmatic lymph nodes, splenomegaly, at least two pulmonary nodules, and an involvement of the entire skeleton to a lesser extent. Consequently, the patient was referred to the Hematology Department of Centre Henri Becquerel (a hospital in Rouen, France) for further evaluation of the suspected lymphatic disease. In July 2018, after an initial biopsy in the left groin and molecular‐pathological analyses of the sample, the patient was diagnosed with an nTFHL‐AI (Figure 1), with the Ann‐Arbor stage being classified as IV. A clonality test identified a low‐intensity T‐cell receptor gamma (TCR‐γ) rearrangement. According to a RACE (rapid amplification of cDNA ends) analysis, a dominance of a TCR‐beta clone (43% of detected TCRs) were detected (Figure 2A). Next‐Generation Sequencing (NGS) revealed a mutation in RHOA (c.50G > T, p.Gly17Val, Tier 2) as well as a detrimental TET2 mutation (c.4138C > A, p.His1380Asn, Tier 2) with allele frequencies of 13% and 17%, respectively. No informative karyotyping could be performed. Gene expression profiling (GEP) assay confirmed nTFHL‐AI signature with RHOA G17V hotspot mutation 12 (Figure 3). Moreover, a reassessment PET‐CT in August 2018 (Figure 4) revealed an increase in supra‐ and subdiaphragmatic lymphadenopathies and multiple jugulocarotid‐, sub‐maxillary‐, and spinal adenomegalies (max. 11 mm). In addition, multiple mediastinal (17 mm) and axillar pulmonal lesions (16 mm) on the right side, as well as a pathological hypermetabolism in liver and bone were detected. A bone marrow biopsy (BMB) showed a lymphoid infiltration of the bone marrow, but no malignant bone lesions were observed. A blood analysis at that time showed a moderate microcytic anemia (11 g/dL) and light alteration of the renal function (creatinine, 110 μmol/L), as well as elevated lactate dehydrogenase (LDH) (530 U/L) and C‐reactive protein (CRP) (32 mg/L) levels. The patient was in good general condition (PS1).
FIGURE 1.
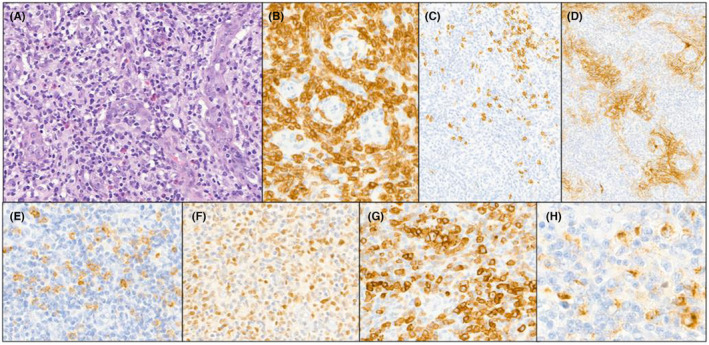
Biopsy at diagnosis. (A) The lymph node architecture is completely effaced by a polymorphous infiltrate, composed of small reactive lymphocytes, histiocytes, eosinophils, plasma cells and small to medium size atypical lymphocytes adjacent to high endothelial venules (HE × 400) (B) Atypical T‐cell lymphocytes expressed CD3 (C) CD20 immunostaining showed scarce B‐cell lymphocytes D) CD21 highlighted an extensive follicular dendritic cells meshwork (E–H) Expression of TFH markers by the atypical T‐cells (E:CD10, F: BCL6, G: PD1, H: CXCL13).
FIGURE 2.
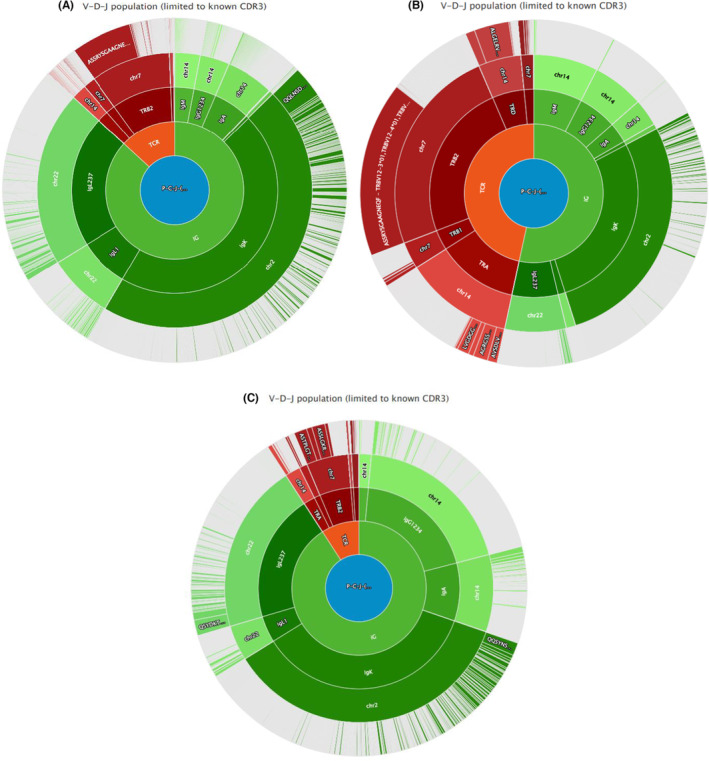
(A) Rapid amplification of cDNA ends (RACE) analysis at diagnosis (July 2018) showing a dominance of a TCR‐beta clone (43% of detected TCRs). (B) RACE analysis at first relapse (January 2019) showing the persistence of the dominant TCR‐beta clone (30%–35%) and the detection of two very low‐abundant TCR‐beta clones (0.5%) and one TCR‐delta clone (6%). (C) RACE analysis at 2nd relapse showing the dominance of the two original minor TCR‐beta clones (14% each), while the TCR‐beta clone that was dominant in 2018 had declined to 5%–6%. Additionally, a TCR‐delta clone was again detected at 2.4%, and a new TCR‐alpha clone also appeared at 4.5%. This suggests a sub clonal evolution of the disease.
FIGURE 3.
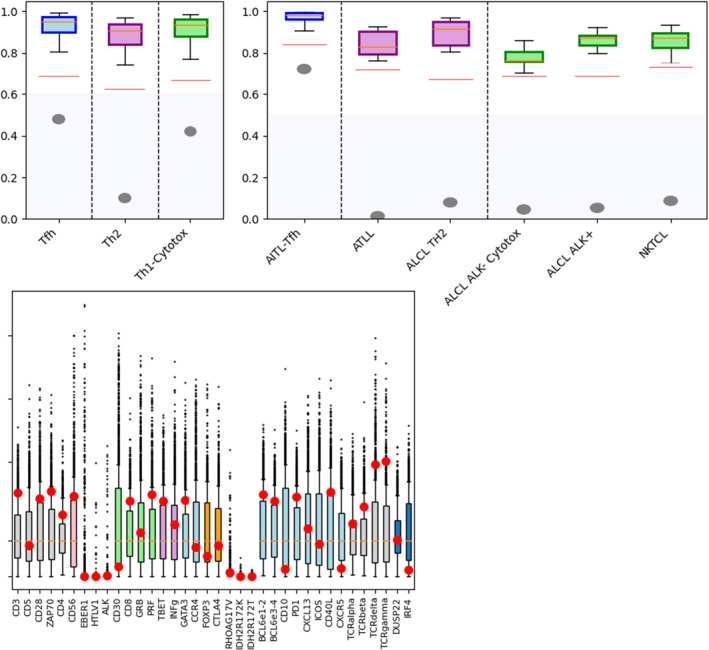
Gene expression profiling (GEP) assay confirmed nTFHL‐AI (AITL‐TFH) signature with RHOA G17V hotspot mutation.
FIGURE 4.
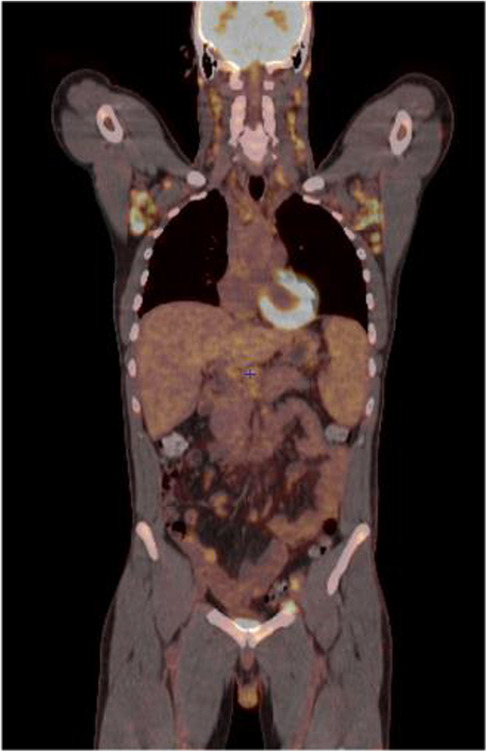
Initial PET‐CT performed in August 2018 showing intense hypermetabolism of the supra‐ and subdiaphragmatic lymph nodes, splenomegaly, at least two pulmonary nodules, and to a lesser extent of the entire skeleton. PET‐CT, positron emission tomography‐computed tomography.
2.3. Treatments and outcome
At the end of August 2018, an improvement of the patient's B‐symptoms (weight loss, fever, night sweats, and pruritus) was observed following a corticoid therapy that had been started 2 weeks earlier. Six cycles of first‐line chemotherapy were administered between September and December 2018 using the CHOEP protocol (cyclophosphamide [750 mg/m2, Day 1], doxorubicin [50 mg/m2, Day 1], etoposide [100 mg/m2, Day 1–Day 3], vincristine [1.4 mg/m2, Day 1], and prednisone [40 mg/m2, Day 1–Day 5]), as well as four prophylactic methotrexate (15 mg) intrathecal injections. After three cycles of CHOEP in 3‐week cycles, a partial response (PR) occurred with the disappearance of liver and bone hypermetabolism, with a Deauville score (DS) of 5 (DS5). After three additional cycles of CHOEP, a progressive disease (PD) was observed in form of a hypermetabolic state of the spleen hilum with a DS5. However, lymphadenopathies above and below the diaphragm persisted, in addition to the onset of a new hypermetabolism in the splenic hilum lymph nodes. A second biopsy of the right axilla was performed in January 2019 which confirmed the persistence of an nTFHL‐AI. A new RACE analysis showed the persistence of the dominant TCR‐beta clone (30%–35%) and detected two very low‐abundant TCR‐beta clones (0.5%) and one TCR‐delta clone (6%) (Figure 2B). The following karyotype was established on the lymph node biopsy 52,XY,+5,+10,+15,+17+18+21[4]/46,XY, 13 trisomy 5 and trisomy 21 being consistent with an nTFHL‐AI diagnosis. 14
From mid‐February to end of April 2019, the patient received three cycles of gemcitabine (1200 mg/m2, Day 1, Day 8, and Day 15) as second‐line therapy. At the start of the therapy, the patient was in good general health (PS0), but his condition slightly deteriorated in mid‐May 2019 (PS1) with the apparition of a facial edema without vena cava syndrome. A follow‐up PET‐CT in May 2019 showed progressive disease (PD) with an increase in size (23 × 15 mm cervical, 22 × 21 mm axillar, 20 × 40 mm sub‐carinar), number, and intensity of the supra‐ and subdiaphragmatic lymph node hypermetabolism (DS5).
Subsequently, a third‐line chemotherapy with ICE regimen (ifosamide [5 g/m2, Day 2], carboplatin [AUC 5 mg/mL min, Day 2], and etoposide [100 mg/m2, Day 1–Day3]) was administered from mid‐May to late June 2019; this new therapy was well tolerated and resulted in a PR with a DS4 after three cycles, as confirmed by a new PET‐CT.
The PR allowed for a BEAM (carmustine, etoposide, cytarabine, and melphalan)‐conditioned autologous stem cell transplantation (ASCT) to be performed at the end of July 2019. However, this transplantation failed, and, in August 2019, a follow‐up PET‐CT showed a major PD with a DS5. A biopsy of a right inguinal lymph node revealed the persistence of the nTFHL‐AI. The RACE analysis showed the dominance of the two original minor TCR‐beta clones (14% each), while the TCR‐beta clone that was dominant in 2018 had declined to 5%–6%. Additionally, a TCR‐delta clone was again detected at 2.4%, and a new TCR‐alpha clone also appeared at 4.5% (Figure 2C). Finally, the RHOA gene mutation was still detected by NGS.
Because of the presence of the TET2 mutation, 15 a treatment with azacytidine (75 mg/m2, Day 1–Day 7)—a hypomethylating agent–was then initiated end of September 2019 and administered until mid‐January 2020 with the goal of subsequently performing an allogeneic stem cell transplantation (allo‐SCT). After the first cycle of azacytidine, the PET‐CT performed by the end of October 2019 initially showed a complete metabolic response (CMR) with a DS3. However, further therapy with azacytidine was suboptimal. Bacteremia (Staphylococcus haemolyticus) of the implanted central venous access port (CVAP) was diagnosed in December 2019, which completely resolved following the administration of daptomycin (10 mg/kg) for 14 days. However, patients remained transfusion‐dependent with severe pancytopenia. Due to a new episode of antibiotic/antifungal therapy‐resistant and prolonged febrile aplasia, a BMB was performed in February 2020 and showed a bone marrow infiltration by the nTFHL‐AI. PET‐CT only revealed an intense and heterogeneous uptake of the bone marrow without any lymph node involvement.
Due to PD proven on BMB, the allo‐SCT was postponed, and the decision was made to treat the patient with belinostat through a managed access program. Between the end of February 2020 and September 2020, a total of eight cycles of belinostat (1000 mg/m2, Day 1–Day 5 of a 21‐day cycle) were administered. The treatment with belinostat was well tolerated and the patient's general condition improved significantly over the course of therapy. Follow‐up PET‐CT analyses showed minimal progression after Cycle 2 (DS5) (Figure 5A) resembling “tumor flare” (pseudo‐progression) with complete disappearance of B‐symptoms and clinical improvement, PR after Cycle 4 and Cycle 6 (both DS4) (Figure 5B, Figure 5C) with BMB complete response (CR) at the end of July 2020, and minimal progression after Cycle 8 (DS5) (Figure 5D). Another BMB performed at the end of September 2020 confirmed persistent complete response at the bone marrow level. The patient was clinically considered to present a very good partial response, with no nTFHL‐AI‐related symptoms; therefore, a decision was made to perform a consolidation allo‐SCT at the end of October 2020.
FIGURE 5.
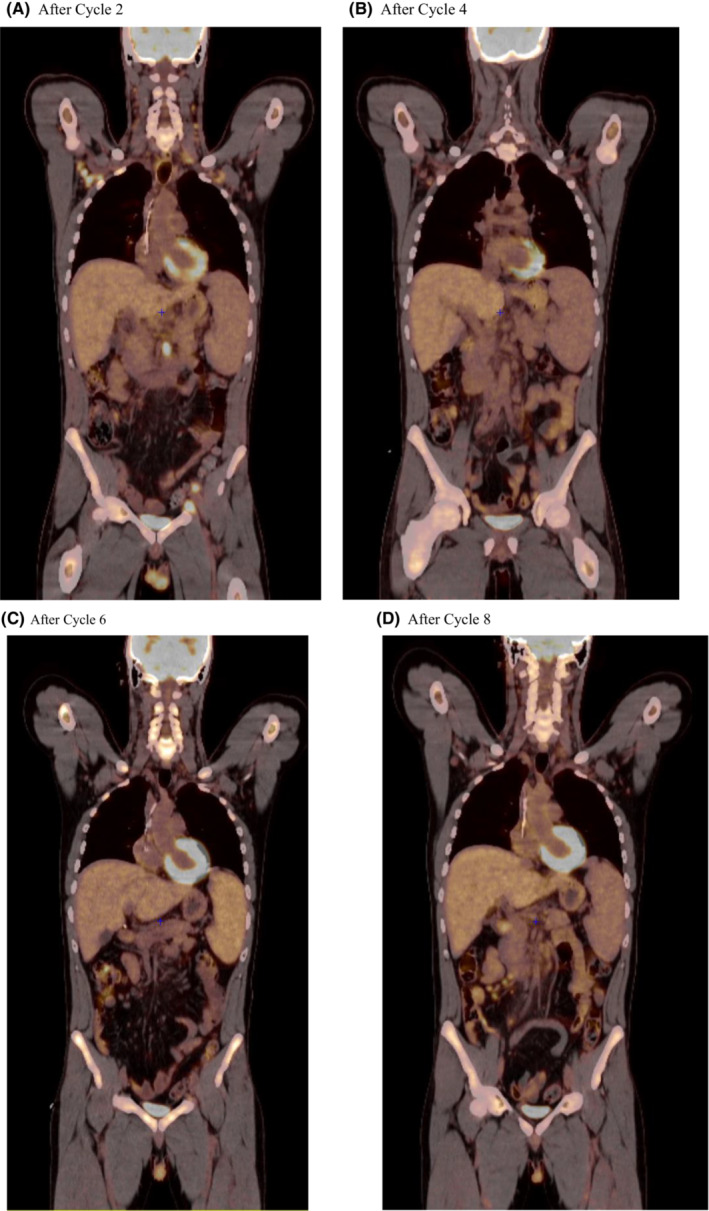
PET‐CT performed during belinostat therapy (1000 mg/m2): (A) after two cycles of belinostat therapy (DS 5, April 2020); (B) after four cycles of belinostat therapy (DS 4, June 2020); (C) after six cycles of belinostat therapy (DS 4, August 2020); (D) after eight cycles of belinostat therapy (DS 5, September 2020). PET‐CT, positron emission tomography‐computed tomography; DS, Deauville score.
We utilized an adapted TEC‐RIC sequential conditioning regimen consisting of a sequential chemotherapy with thiotepa (5 mg/kg, Day 14), etoposide (100 mg/m2, Day 13–Day 10), and cyclophosphamide (400 mg/m2, Day 13–Day 10) (TEC) followed by a reduced intensity conditioning (RIC) regimen with fludarabine (150 mg/m2, i.v.), busulfan (6.4 mg/kg), and thymoglobulin (5 mg/kg, Day 6–Day 2). The donor was a 10/10 matched unrelated donor and we used peripheral blood stem cell (PBSC) donation. Both donor and recipient presented with a blood group O+, a negative cytomegalovirus (CMV) serology, as well as a positive serology for the Epstein–Barr virus (EBV) in favor of a prior EBV immunity. As prophylaxis of the graft‐versus‐host (GvH) disease, the patient received anti‐thymocyte globulin (ATG) pre‐allo‐SCT, as well as cyclosporine 3 mg/kg/day and mycophenolate mofetil 1000 mg twice per day post‐allo‐SCT. However, during the transplant, a hepatic veno‐occlusive disease with a severity of 6 out of 12 criteria on the hepatic Doppler‐ultrasonography 16 and a high level of total bilirubin of 51 μmol/L (normal value <21 μmol/L on November 7, 2020) was diagnosed and showed a favorable course under defibrotide (25 mg/kg/day for 1 month). Additionally, the patient experienced a severe and acute GvH disease of the skin (grade 3) and in the digestive tract including a rectorrhagia (grade 4, with a hemorrhagic shock which required a transfer to the intensive care unit). Histological analyses confirmed the cutaneous and digestive (from colonic biopsies through digestive endoscopy and colonoscopy) GvH disease which responded well to corticosteroids (the patient received 2 mg/kg/day of prednisone for 2 weeks). Progressive discontinuation of corticosteroids led to a recurrence of minimal cutaneous GvH reactions. A post‐transplant PET‐CT performed at the end of November 2020 (Day+30) demonstrated a partial regression of the hypermetabolic lymph node metabolism on the right side, as well as a complete regression of the other metabolisms (DS4, partial metabolic response). Complete donor chimerism was achieved at Day+30 with donor/host chimerism results of 99.7% of the total blood population/100.0% of the myeloid population/96.0% of the lymphoid population, and 100.0/100.0/99.3%, respectively, at Day+100. Due to an EBV reactivation, the patient had to receive four weekly injections of rituximab (375 mg/m2, Day 1, Day 8, Day 15, and Day 21), the last one in mid‐December 2020. No EBV recurrence happened after. Despite a minimal persistent mucosal GvH disease, a withdrawal of the corticosteroids (4 months post‐allo‐SCT on February 26, 2021) and of the immunosuppressors (on June 4, 2021, for the mycophenolate mofetil, and on August 16, 2021, for the cyclosporine) was performed. Although a high risk of relapse post‐allo‐SCT existed, neither a reinjection of donor lymphocytes post‐allo‐SCT—because of the prior severe digestive GvH disease–, nor a belinostat maintenance therapy—because maintenance is not a standard of care in T‐cell lymphoma–, were considered.
At his last follow‐up appointment in October 2022—2 years after the allo‐SCT–the complete hematologic response was confirmed. No further GvH reactions occurred, and a non‐severe post‐transfusional hemochromatosis (ferritin, 3420 ng/mL) was treated with monthly phlebotomies of 400 mL. The patient stated that he was looking forward for work, exercising regularly on his exercise bike, and seeing a urologist for a libido disorder. His next control visit at the hospital is planned in May 2023.
3. DISCUSSION
We reported here a very rare case of a heavily refractory nTFHL‐AI in a young patient that was successfully treated with belinostat and consolidation allo‐SCT. The diagnosis of nTFHL‐AI in this patient was unusual due to his young age (33 years), as the median age at diagnosis of nTFHL‐AI in the literature is 65 years. 17
Because of the rarity and heterogeneity of PTCL, few randomized clinical trials are available. Thus, strategies for first‐line treatment have often been adopted from treatment paradigms for aggressive B‐cell lymphomas. 18 Anthracycline‐based therapeutic regimens such as CHOP or CHOEP are still commonly used as first‐line therapy, but their efficacy in PTCL has not been prospectively proven, and generally does not lead to durable remissions. 13 The role of ASCT as a first‐line consolidation treatment in patients achieving complete or partial response is still highly debated, all reports suffering from both positive and negative biases. However, in a multicenter study, ASCT in first CR significantly improved OS and PFS for patients with nTFHL‐AI compared to other PTCL subtypes. A superior survival was noted in patients with advanced stage and high IPI scores. 19 On the other hand, patients undergoing an allo‐SCT are usually fitter, younger, and have responded well to induction regimens, but they may also present with more aggressive disease features than patients who do not undergo an allo‐SCT. On the other hand, an allo‐SCT usually offers the chance of long‐term remission for eligible patients in the R/R setting. Promising results have been achieved with a 2‐ and 5‐year PFS of 45% and 53%, respectively, in nodal PTCL, and up to 81% in nTFHL‐AI; however, only few patients are eligible for this therapy. 9 The patient described in this case report did not achieve a disease control even after undergoing three different commonly used lines of therapy (CHOEP, gemcitabine, ICE) and an autologous transplantation. Additional therapy with the hypomethylating agent azacytidine, which was expected to induce a good response due to the presence of a TET2 mutation, was also not successful. 15 After several prior treatments, it was the belinostat therapy which induced a sufficient response to allow the patient to get an allo‐SCT; this clinical case is, however, a good illustration of a graft‐versus‐leukemia (GvL) effect of the allo‐SCT in nTFHL‐AI. Before the allo‐SCT, we had concerns about a higher risk for a digestive GvH disease because of the patient's medical history of Crohn's disease; however, since the allo‐SCT, the patient did not present any further Crohn's disease onset, although he did not receive any specific therapy (no mesalamine treatment) since the allo‐SCT.
The HDAC inhibitor belinostat was first approved by the U.S. FDA in 2014 for the treatment of R/R PTCL; it inhibits the tumor cell growth by inducing cell cycle arrest and apoptosis. In addition, the inhibition of HDAC causes a histone acetylation, leading to an increased expression of tumor suppressor genes. 20 Compared to other HDAC inhibitors that selectively inhibit specific HDAC enzymes (such as the class‐I selective HDACi romidepsin), belinostat is a pan‐HDAC inhibitor that blocks all zinc‐dependent HDAC enzymes with high affinity. It is believed that a broader spectrum of inhibition results in better efficacy. 21 The administration of belinostat for the treatment of R/R PTCL has already been well documented in the literature. The phase II BELIEF clinical study evaluated belinostat as monotherapy in 120 patients with R/R PTCL and demonstrated an overall response rate (ORR) of 26% (complete response, CR: 10.8%) with a median duration of response (DoR) of 13.6 months (95% CI, 4.5–29.4). 8 In this study, the most common (>25%) treatment‐emergent adverse events (TEAEs) were nausea, fatigue, pyrexia, anemia, and vomiting. Grade 3/4 toxicities (≥5.0%) included anemia, thrombocytopenia, dyspnea, neutropenia, fatigue, and pneumonia. 8 An impressive case of an elderly patient with a chemorefractory PTCL showed a particularly long‐lasting efficacy of over 39 months after treatment with belinostat. 22 Among the PTCL patients enrolled in the BELIEF trial, 22 patients had nTFHL‐AI; an analysis of this subgroup showed an even higher ORR of 45% (CR: 18%) compared with the overall study population. 23
Belinostat is also currently being evaluated as consolidation therapy in combination with zidovudine for adult T‐cell leukemia‐lymphoma (ATLL); belinostat is able to reactivate HTLV‐1 provirus in ATLL cells and infected T‐cell reservoirs, thus eliciting an immune response against virus infected cells. 24
nTFHL‐AI has been associated with frequent mutations in epigenome‐regulating genes, such as TET2 (80%), DNMT3A (20% to 30%), IDH2 (20% to 30%) and RHOA (50% to 70%). 25 It is suggested that HDAC inhibitors may help maintain an acetylated and active TET2 and therefore prevent the abnormal DNA methylation that occurs in cancer. 23 Using a gene set cancer analysis (GSCALite), both TET1 and TET2 genes were shown to be sensitive to belinostat. 26 , 27 Mutations in TET2 and RHOA were also present in our patient case, and the use of belinostat may have eventually led to a very good partial response of the disease, allowing an allo‐SCT to be performed. A sub‐analysis of the BELIEF study presented data from patients who underwent a hematopoietic SCT (HSCT) after belinostat treatment: a total of 12 patients, including two patients with nTFHL‐AI, received either an ASCT (four patients) or an allo‐SCT (eight patients). In total, one patient achieved a PR with an overall survival (OS) of 19.9 months, and one patient was not evaluable with an OS of 9.4 months. 28
This is, to our knowledge, the first report of an allo‐SCT following belinostat therapy in a young patient presenting with a multiple R/R nTFHL‐AI that resulted in a durable CR. Our patient has been able to maintain a complete hematologic response for at least 2 years, up to the writing of this case report. Multiple R/R PTCLs are difficult to treat, and the chances of a successful outcome are quite low. Currently, there is no clear consensus on the optimal treatment strategy, and physicians must rely on existing clinical trials and treatment options. The main clinical significance of this clinical case is that (i) belinostat is able to catch up with very refractory disease situations, (ii) it is necessary to wait for several cycles of treatment before judging the effectiveness due to a possible flare‐up effect at the start of treatment, and (iii) the clinical improvement of the patient's symptoms must be taken into account and not based solely on the DS to assess the patient's response. Indeed, our patient never had a strictly negative PET before the allo‐SCT, but his clinical symptoms had disappeared under belinostat and the bone marrow biopsy was also negative under the action of this treatment. This case report does not aim to draw definitive conclusions on the efficacy of belinostat in R/R nTFHL‐AI but rather to illustrate its practical use in very refractory situations, as a bridge to transplant, and also to testify of the GvL effect provided by the graft in nTFHL‐AI patients.
4. CONCLUSION
R/R nTFHL‐AI is a difficult‐to‐treat hematological malignancy that can occur in very young patients. We presented here the case of a young patient with R/R nTFHL‐AI after multiple unsuccessful prior treatment regimens who achieved a durable and stable CR after an allo‐SCT bridged by belinostat. The presence of epigenetic alterations such as TET2 or RHOA in this patient may have contributed to his sensitivity to belinostat.
AUTHOR CONTRIBUTIONS
Vincent Camus: Conceptualization; data curation; investigation; methodology; project administration; supervision; validation; writing – original draft; writing – review and editing. Pascaline Etancelin: Formal analysis; methodology; validation; writing – review and editing. Fanny Drieux: Methodology; validation; writing – review and editing. Elena‐Liana Veresezan: Formal analysis; methodology; validation; writing – review and editing. Jean‐Michel Picquenot: Formal analysis; resources; validation; writing – review and editing. Dominique Penther: Resources; writing – review and editing. Mathieu Viennot: Formal analysis; resources; writing – review and editing. Philippe Ruminy: Formal analysis; methodology; resources; writing – review and editing. Nathalie Contentin: Resources; writing – review and editing. Emilie Lemasle: Resources; writing – review and editing. Stéphane Leprêtre: Resources; writing – review and editing. Sydney Dubois: Resources; writing – review and editing. Juliette Penichoux: Formal analysis; validation; writing – review and editing. Aspasia Stamatoullas: Resources; writing – review and editing. Alexandra Zduniak: Formal analysis; validation; writing – review and editing. Helene Lanic: Resources; validation; writing – review and editing. Fabrice Jardin: Formal analysis; resources; supervision; validation; writing – review and editing.
FUNDING INFORMATION
The publication of this work was sponsored by IDEOGEN AG (Switzerland).
CONFLICT OF INTEREST STATEMENT
Vincent Camus received honoraria from IDEOGEN AG (Switzerland). The other authors declare no conflict of interest regarding this manuscript.
CONSENT
This case report was published with written consent of the patient.
ACKNOWLEDGMENTS
The authors would like to thank Florence Boulmé, PhD, for writing assistance.
Camus V, Etancelin P, Drieux F, et al. Complete hematologic response after belinostat treatment and allogeneic stem cell transplantation for multiple relapsed/refractory angioimmunoblastic T‐cell lymphoma: A case report. Clin Case Rep. 2023;11:e7623. doi: 10.1002/ccr3.7623
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Savage KJ. Peripheral T‐cell lymphomas. Blood Rev. 2007;21(4):201‐216. [DOI] [PubMed] [Google Scholar]
- 2. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of Haematolymphoid Tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720‐1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foss F. Evolving therapy of peripheral T‐cell lymphoma: 2010 and beyond. Ther Adv Hematol. 2011;2(3):161‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sibon D. Peripheral T‐cell lymphomas: therapeutic approaches. Cancers (Basel). 2022;14(9):2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Connor OA, Pro B, Pinter‐Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T‐cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN‐35) in patients with relapsed or refractory systemic anaplastic large‐cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190‐2196. [DOI] [PubMed] [Google Scholar]
- 7. Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T‐cell lymphoma. Blood. 2011;117(22):5827‐5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T‐cell lymphoma: results of the pivotal phase II BELIEF (CLN‐19) study. J Clin Oncol. 2015;33(23):2492‐2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moskowitz AJ. Practical treatment approach for Angioimmunoblastic T‐cell lymphoma. J Oncol Pract. 2019;15(3):137‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foss F, Advani R, Duvic M, et al. A phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T‐cell lymphoma. Br J Haematol. 2015;168(6):811‐819. [DOI] [PubMed] [Google Scholar]
- 11. Advani RH, Skrypets T, Civallero M, et al. Outcomes and prognostic factors in angioimmunoblastic T‐cell lymphoma: final report from the international T‐cell project. Blood. 2021;138(3):213‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drieux F, Ruminy P, Abdel‐Sater A, et al. Defining signatures of peripheral T‐cell lymphoma with a targeted 20‐marker gene expression profiling assay. Haematologica. 2020;105(6):1582‐1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Leary HM, Savage KJ. Novel therapies in peripheral T‐cell lymphomas. Curr Oncol Rep. 2008;10(5):404‐411. [DOI] [PubMed] [Google Scholar]
- 14. Schlegelberger B, Zhang Y, Weber‐Matthiesen K, Grote W. Detection of aberrant clones in nearly all cases of angioimmunoblastic lymphadenopathy with dysproteinemia‐type T‐cell lymphoma by combined interphase and metaphase cytogenetics. Blood. 1994;84(8):2640‐2648. [PubMed] [Google Scholar]
- 15. Itzykson R, Kosmider O, Cluzeau T, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25(7):1147‐1152. [DOI] [PubMed] [Google Scholar]
- 16. Lassau N, Auperin A, Leclere J, Bennaceur A, Valteau‐Couanet D, Hartmann O. Prognostic value of doppler‐ultrasonography in hepatic veno‐occlusive disease. Transplantation. 2002;74(1):60‐66. [DOI] [PubMed] [Google Scholar]
- 17. Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T‐cell lymphoma: analysis of the international peripheral T‐cell lymphoma project. J Clin Oncol. 2013;31(2):240‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T‐cell lymphomas. Blood. 2014;123(17):2636‐2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park SI, Horwitz SM, Foss FM, et al. The role of autologous stem cell transplantation in patients with nodal peripheral T‐cell lymphomas in first complete remission: report from COMPLETE, a prospective, multicenter cohort study. Cancer. 2019;125(9):1507‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee HZ, Kwitkowski VE, Del Valle PL, et al. FDA approval: Belinostat for the treatment of patients with relapsed or refractory peripheral T‐cell lymphoma. Clin Cancer Res. 2015;21(12):2666‐2670. [DOI] [PubMed] [Google Scholar]
- 21. Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769‐784. [DOI] [PubMed] [Google Scholar]
- 22. Reimer P, Chawla S. Long‐term complete remission with belinostat in a patient with chemotherapy refractory peripheral T‐cell lymphoma. J Hematol Oncol. 2013;6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sawas A, Ma H, Shustov A, et al. Characterizing the belinostat response in patients with relapsed or refractory angioimmunoblastic T‐cell lymphoma. Leuk Lymphoma. 2020;61(8):2003‐2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramos JC, Chapman JR, Komanduri KV, Barber GV. Trial in Progress: a phase II trial of Belinostat As consolidation therapy with zidovudine for adult T‐cell leukemia‐lymphoma (ATLL). Blood. 2021;138(Suppl. 1):2477. [Google Scholar]
- 25. Lemonnier F, Gaulard P, de Leval L. New insights in the pathogenesis of T‐cell lymphomas. Curr Opin Oncol. 2018;30(5):277‐284. [DOI] [PubMed] [Google Scholar]
- 26. Liu CJ, Hu FF, Xia MX, Han L, Zhang Q, Guo AY. GSCALite: a web server for gene set cancer analysis. Bioinformatics. 2018;34(21):3771‐3772. [DOI] [PubMed] [Google Scholar]
- 27. Huang Y, Wei J, Huang X, et al. Comprehensively analyze the expression and prognostic role for ten‐eleven translocations (TETs) in acute myeloid leukemia. Transl Cancer Res. 2020;9(11):7259‐7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shustov A, Al‐Ali H, Wulf G, et al. Subsequent hematopoietic stem cell transplantation in Belinostat‐treated patients with relapsed/refractory peripheral T‐cell lymphoma (R/R PTCL). Blood. 2014;124:5438. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


