Abstract
Background
Among adults with cancer, malnutrition is associated with decreased treatment completion, more treatment harms and use of health care, and worse short-term survival. To inform the National Institutes of Health Pathways to Prevention workshop, “Nutrition as Prevention for Improved Cancer Health Outcomes,” this systematic review examined the evidence for the effectiveness of providing nutrition interventions before or during cancer therapy to improve outcomes of cancer treatment.
Methods
We identified randomized controlled trials enrolling at least 50 participants published from 2000 through July 2022. We provide a detailed evidence map for included studies and grouped studies by broad intervention and cancer types. We conducted risk of bias (RoB) and qualitative descriptions of outcomes for intervention and cancer types with a larger volume of literature.
Results
From 9798 unique references, 206 randomized controlled trials from 219 publications met the inclusion criteria. Studies primarily focused on nonvitamin or mineral dietary supplements, nutrition support, and route or timing of inpatient nutrition interventions for gastrointestinal or head and neck cancers. Most studies evaluated changes in body weight or composition, adverse events from cancer treatment, length of hospital stay, or quality of life. Few studies were conducted within the United States. Among intervention and cancer types with a high volume of literature (n = 114), 49% (n = 56) were assessed as high RoB. Higher-quality studies (low or medium RoB) reported mixed results on the effect of nutrition interventions across cancer and treatment-related outcomes.
Conclusions
Methodological limitations of nutrition intervention studies surrounding cancer treatment impair translation of findings into clinical practice or guidelines.
Among adults with cancer, malnutrition is associated with decreased treatment completion, greater treatment-related adverse events and health-care use, and worse survival (1-4). Cancer-related malnutrition results from inadequate nutrition intake due to the systemic effects of the disease, adverse or negative effects of cancer and treatment, and other factors (5,6). Adults with cancer commonly experience malnutrition, with estimates ranging between 25% and 80% across patient populations (7-9). However, malnutrition substantially varies by patient characteristics, such as age at diagnosis, cancer type, stage of disease, type of cancer treatment, and preexisting conditions (eg, diabetes), among other factors (8,10). Further, many factors may increase risk or severity of malnutrition, including cancer location (head and neck or gastrointestinal), symptoms (eg, anorexia, early satiety, and fatigue), treatment modalities (surgery, chemo or radiotherapy), complications (eg, mucositis, nausea, taste changes), and psychological distress. In individuals with cancer, malnutrition often goes unrecognized by clinicians and patients and family or caregivers (11). Even when recognized, malnutrition may not be adequately addressed. Only 30% to 50% of cancer patients at risk for malnutrition receive nutrition intervention (12,13). Considering that an estimated 1.9 million individuals were diagnosed with cancer in 2021, between 570 000 and 950 000 individuals may have or be at risk for malnutrition.
Both the American Society for Parenteral and Enteral Nutrition and the American College of Surgeons Commission on Cancer recommend initial malnutrition screening and subsequent periodic reassessment during the course of cancer treatment and survivorship (6,14,15). However, no guidelines based on comprehensive, high-quality evidence exist for prevention, screening, or treatment of malnutrition in adults with cancer, creating challenges for individuals with cancer and their clinicians. Furthermore, we do not know how treatment benefits and harms may be affected by patient characteristics (eg, age, race and ethnicity, family or other support, socioeconomic status including food security, prediagnosis obesity, or sarcopenia), cancer-related factors (eg, cancer type, stage), treatment type (chemotherapy, radiation, surgery), treatment timing (before or after treatment), and provider, hospital, and geographic characteristics (eg, presence of integrated nutrition programs; specialist type, availability, and insurance coverage; and rural or urban location). Understanding the most effective interventions for nutrition in this population is critical, given the poor access to outpatient nutrition care for cancer patients across the United States (16).
To summarize the evidence, we conducted a systematic literature review that examined the effectiveness of nutrition interventions before or during cancer therapy to improve outcomes of cancer treatment. Additionally, we identified research gaps and challenges to inform expert and stakeholder discussions at the National Institutes of Health Pathways to Prevention workshop, “Nutrition as Prevention for Improved Cancer Health Outcomes,” which took place July 26-28, 2022. We also aimed to inform development of a research agenda for evaluating nutrition interventions in inpatient and outpatient cancer care in the United States. Our results can inform clinical guidelines on the prevention and treatment of malnutrition in cancer care.
Methods
The review was guided by a set of key questions (KQ) and contextual questions (CQ), including:
In adults diagnosed with cancer who have or are at risk for cancer-associated malnutrition, what is the effect of nutrition interventions before (KQ 1) or during (KQ 2) cancer treatment in preventing negative treatment outcomes such as effects on dose tolerance, hospital use, adverse events, and survival?
What is the effect of nutrition interventions before or during cancer treatment on associated symptoms such as fatigue, nausea and vomiting, appetite, physical and functional status (eg, frailty), and quality of life (KQ 3)?
In adults with cancer who are overweight or obese, what is the effect of nutrition interventions intended for weight loss before or during cancer treatment in preventing negative treatment outcomes such as effects on dose, hospital use, adverse events, and survival (KQ 4)?
What evidence is available on the cost-effectiveness of nutrition interventions for preventing negative outcomes associated with cancer treatment (CQ)?
For KQs 1-3, we further examined the evidence on variation in effects of nutrition interventions 1) by cancer type, treatment type, and stage of disease; 2) across the lifespan (eg, adults aged ≥65 years vs <65 years); 3) in adults with or without muscle wasting; and 4) across special populations (eg, individuals with multiple comorbid conditions).
We conducted a comprehensive literature search in July 2022, searching MEDLINE (Ovid), Embase (Ovid), and Cochrane Central Register of Controlled Trials (Wiley) for studies that evaluated a broad range of nutrition interventions (eg, dietary supplements, nutrition support, nutrition counseling) for preventing and treating negative outcomes of cancer and its treatment (see Supplementary Materials, available online for our complete search strategy). The search included literature published from 2000 through July 2022 to encompass contemporary cancer treatments. We included studies that met our prespecified criteria outlined in Table 1. We further limited our search to randomized controlled trials (RCTs) published in English in a peer-reviewed journal. To identify the literature with the highest likelihood of having statistical power to detect an effect from a nutrition intervention, we limited included studies to those randomly assigning at least 50 participants (ie, approximately 25 individuals per arm). Studies that described the cost or value (eg, cost-effectiveness, cost-benefit) of nutrition interventions were eligible for inclusion in the CQ.
Table 1.
Population, intervention, comparator, outcome, timing, and setting (PICOTS) for included studies
| KQ | KQ1 | KQ2 | KQ3 | KQ4 |
|---|---|---|---|---|
| Population | Adults diagnosed with cancer at or after age 18 y who have or are at risk for cancer-associated malnutrition Subgroups:
|
Adults diagnosed with cancer at or after age 18 y who have or are at risk for cancer-associated malnutrition Subgroups:
|
Adults diagnosed with cancer at or after age 18 y who have or are at risk for cancer-associated malnutrition Subgroups:
|
Overweight (BMI 25 to <30)/obese (BMI ≥30) adults aged ≥18 y diagnosed with cancer |
| Intervention | Nutrition interventions under supervision of nutrition professional (eg, dietitian, nutritionist, or other licensed clinicians)
|
Nutrition interventions under supervision of nutrition professional (eg, dietitian, nutritionist, or other licensed clinicians)
|
Nutrition interventions under supervision of nutrition professional (eg, dietitian, nutritionist, or other licensed clinicians)
|
Nutrition interventions intended for weight loss (includes both PNIs and NIDTs) |
| Comparators |
|
|
|
|
| Outcomes |
|
|
|
|
| Timing | Nutrition interventions delivered precancer treatment (KQ1, KQ3, KQ4) and during cancer treatment (KQ2, KQ3, KQ4) | Nutrition interventions delivered precancer treatment (KQ1, KQ3, KQ4) and during cancer treatment (KQ2, KQ3, KQ4) | Nutrition interventions delivered precancer treatment (KQ1, KQ3, KQ4) and during cancer treatment (KQ2, KQ3, KQ4) | Nutrition interventions delivered precancer treatment (KQ1, KQ3, KQ4) and during cancer treatment (KQ2, KQ3, KQ4) |
| Setting | Outpatient oncology care, ambulatory care, cancer treatment centers, inpatient, home based, hospice, telemedicine | Outpatient oncology care, ambulatory care, cancer treatment centers, inpatient, home based, hospice, telemedicine | Outpatient oncology care, ambulatory care, cancer treatment centers, inpatient, home based, hospice, telemedicine | Outpatient oncology care, ambulatory care, cancer treatment centers, inpatient, home based, hospice, telemedicine |
AE = adverse event; BMI = body mass index; ER = emergency room; KQ = key question; NI = nutrition intervention; NIDT = nutrition intervention during treatment; PNI = pretreatment nutrition intervention; RCT = randomized controlled trial.
To identify eligible studies, search results were downloaded to PICO Portal (17), an online systematic review platform, for screening. Two trained, independent investigators reviewed titles and abstracts for identified studies meeting population, intervention, comparator, outcome, timing, and setting framework and study selection criteria. Two reviewers independently performed full-text screening to determine whether studies met inclusion criteria. Differences in screening decisions were resolved by consultation between reviewers, and, if necessary, consultation with a third investigator. All citations deemed appropriate for inclusion through title and abstract review by both reviewers were then examined in the full text. We documented inclusion and exclusion status of citations, noting reasons for exclusion. Throughout the screening process, members of the review team regularly met to discuss training material and issues as they arose to ensure that inclusion criteria were consistently applied.
Among studies deemed eligible for inclusion in one of the KQs, 2 independent reviewers assessed risk of bias (RoB) on a subset of eligible studies based on Agency for Healthcare Research and Quality guidance. This subset of studies included those that had a relatively large number of studies within an intervention and cancer type. We used a threshold of 10 studies within a specific intervention or cancer type; dietary supplements and nutrition support for gastrointestinal cancers were frequent categories for RoB assessment, which included all studies of dietary supplements and nutrition support for gastrointestinal cancers across KQs. Any discrepancies in overall RoB assessments were resolved through discussion. We classified overall RoB for each study as low, moderate, or high based on the collective RoB inherent in each domain and confidence that the results are believable given the study’s limitations.
We then extracted basic study information from all eligible studies that met the inclusion criteria. Among studies for which RoB was assessed, we further abstracted information on intervention duration, comparisons, and outcomes for those studies deemed to have low or medium RoB. Meta-analysis was generally not feasible or appropriate because of the considerable heterogeneity of intervention types, comparators, outcomes, and timing evaluated within KQs. We organized the results by KQ then broadly by type of nutrition intervention and type of cancer. Given the lack of recognized classification systems for grouping or describing nonpharmacologic nutrition interventions, we grouped studies into single intervention types based on the content and intent of the intervention and the intended audience, using study author-supplied taxonomies and definitions where available (Table 2).
Table 2.
Systematic review intervention categories and descriptions
| Intervention category | Description |
|---|---|
| Nutrition counseling | Nutrition counseling involves an individualized nutrition assessment followed by personalized care of nutrition and diet-related needs with the goal of achieving and maintaining optimal nutrition status across the continuum of care. |
| Dietary supplements | Dietary supplements include products (eg, added arginine, glutamine, fish oil) containing 1 or more ingredients meant to supplement the diet for improved nutrition status but not meant to replace calories. Vitamins, minerals, and antioxidants were not included. |
| Special diets | Special diets include the use of defined nutrition plans or approaches such as fasting (intermittent or short term), calorie restriction, ketogenic, Mediterranean, high-calorie, high-protein diets to support cancer care, and others. |
| Route or timing of nutrition interventions | Route or timing interventions involve testing only the route (eg, percutaneous endoscopic gastrostomy [PEG] tube, enteral feeding) or timing (eg, initiation or duration) of nutrition interventions with similar nutrition contents. |
| Nutrition support including oral nutrition supplements | Nutrition support interventions involve the use of parenteral nutrition, enteral nutrition (tube feeding), and oral nutrition supplements (eg, Ensure, Boost, immunonutrition [oral nutrition supplements that include a set of nutrients meant to have an effect of the immune system]) (240) to maintain or improve nutrition status. |
| Multi-component interventions | Multi-component interventions involve multiple strategies for nutrition interventions, such as counseling plus use of dietary supplements. |
The final protocol was posted online on October 8, 2021 (https://effectivehealthcare.ahrq.gov/products/improved-cancer-outcomes/protocol). We registered the protocol on PROSPERO (CRD42021282881). Additional review details, including our RoB assessment tool, can be found as part of the full systematic review posted on the Agency for Healthcare Research and Quality and Pathways to Prevention websites.
Results
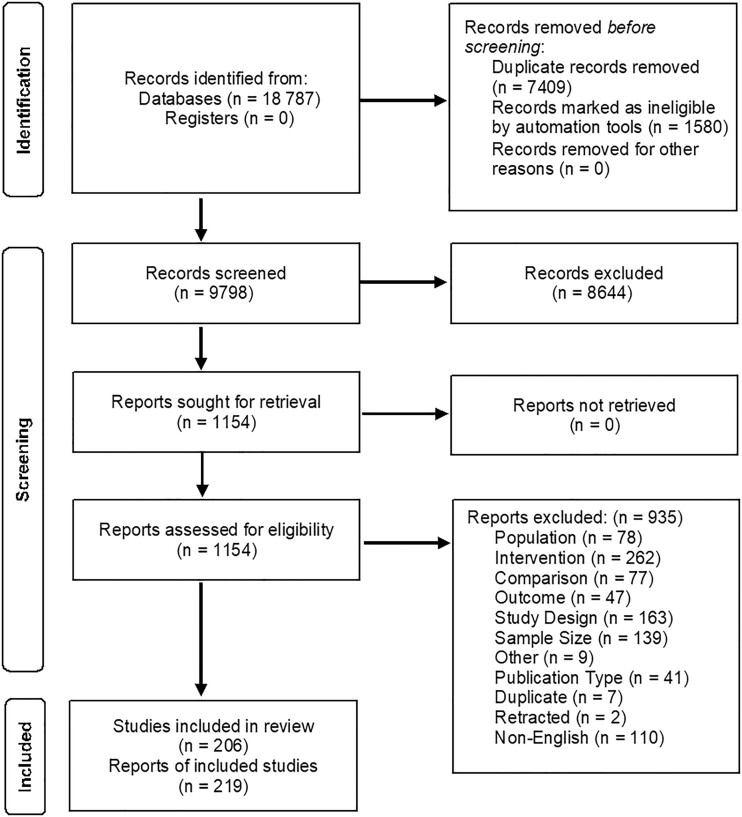
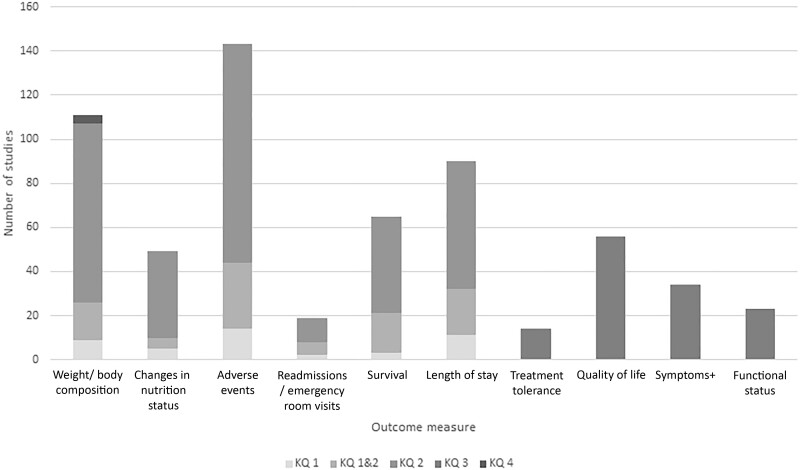
From 9798 unique references, we identified 206 RCTs described in 219 publications on nutrition interventions to improve cancer treatment outcomes that met inclusion criteria (Figure 1). The randomized trial evidence on nutrition interventions for adults before and/or during cancer treatment was extremely broad and focused on dietary supplements, nutrition support (enteral and parenteral nutrition, including oral nutrition supplements), and the route or timing of nutrition interventions (Table 3). Studies were predominantly conducted in populations with gastrointestinal and head and neck cancers. Less than one-half of enrollees had American Joint Commission on Cancer Stage IV disease among studies reporting tumor stage. Studies included both inpatient surgical and outpatient settings (Table 3) and focused on evaluating changes in body weight or composition, treatment-related adverse events, length of hospital stay, and quality of life (Figure 2). Most studies included patients considered “at risk” for (eg, gastrointestinal cancers, receiving inpatient surgical treatment, advanced disease) malnutrition (Table 4). A variety of malnourishment screening tools were used, with many studies not reporting malnourishment assessment method. Few studies were conducted within the US setting. Among studies with a high volume of literature, which predominately included studies of dietary supplements and nutrition support in gastrointestinal and head and neck cancers, 11% (n = 12) were rated as having low RoB (higher quality), 40% (n = 46) medium RoB, and 49% (n = 56) high RoB (low quality) (Table 3). Overall, studies widely differed in type of nutrition interventions administered, route and/or dose of administration, and control populations, even within KQ and intervention categories.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) diagram.
Table 3.
Included studies by nutrition intervention and cancer type across key questions (KQ) (N = 206)a
| KQ | Intervention | Head and neck cancer | Gastrointestinal cancer | Multiple cancers | Other cancer typesb | Totalc |
|---|---|---|---|---|---|---|
| Nutrition interventions before cancer treatment (KQ1) | Nutrition counseling | 0 | 0 | 0 | 0 | 0 |
| Dietary supplements | 1 |
|
0 | 2 | 5 | |
| Special diets | 0 | 0 | 0 | 0 | 0 | |
| Route or timing of nutrition interventions | 0 | 0 | 0 | 0 | 0 | |
| Nutrition support including oral nutrition supplements | 0 |
|
0 | 0 | 15 | |
| Multi-component interventions | 0 | 0 | 0 | 0 | 0 | |
| Total | 20 | |||||
| Nutrition interventions before and including initiation of cancer treatment (spans KQ 1 and 2) | Nutrition counseling | 0 | 0 | 0 | 0 | 0 |
| Dietary supplements | 2 |
|
2 | 1 | 15 | |
| Special diets | 0 | 0 | 0 | 0 | 0 | |
| Route or timing of nutrition interventions | 2 | 6 | 1 | 0 | 9 | |
| Nutrition support including oral nutrition supplements | 1 |
|
0 | 2 | 13 | |
| Multi-component interventions | 0 | 0 | 1 | 0 | 1 | |
| Total | 38 | |||||
| Nutrition interventions after treatment began (KQ2) | Nutrition counseling | 3 | 4 | 6 | 2 | 15 |
| Dietary supplements |
|
|
3 | 5 | 34 | |
| Special diets | 0 | 1 | 2 | 5 | 8 | |
| Route or timing of nutrition interventions | 4 |
|
1 | 2 | 31 | |
| Nutrition support including oral nutrition supplements | 4 |
|
4 | 8 | 43 | |
| Multi-component interventions | 2 | 2 | 2 | 4 | 10 | |
| Total | 141 | |||||
| Effect of nutrition interventions on symptoms (KQ3) | Nutrition counseling | 1 | 4 | 4 | 3 | 12 |
| Dietary supplements | 1 |
|
3 | 1 | 10 | |
| Special diets | 0 | 1 | 2 | 7 | 10 | |
| Route or timing of nutrition interventions | 2 | 4 | 1 | 1 | 8 | |
| Nutrition support including oral nutrition supplements | 5 |
|
3 | 4 | 32 | |
| Multi-component interventions | 2 | 0 | 3 | 2 | 7 | |
| Total | 79 | |||||
|
Nutrition counseling | 0 | 0 | 0 | 0 | 0 |
| Dietary supplements | 0 | 0 | 0 | 0 | 0 | |
| Special diets | 0 | 0 | 0 | 4 | 4 | |
| Route or timing of nutrition interventions | 0 | 0 | 0 | 0 | 0 | |
| Nutrition support including oral nutrition supplements | 0 | 0 | 0 | 0 | 0 | |
| Multi-component interventions | 0 | 0 | 0 | 0 | 0 | |
| Total | 4 |
Totals are not mutually exclusive to number of included studies. Studies addressing KQ3 could also address KQ1, KQ2, or span KQ1 and 2. RoB = risk of bias.
Other cancer types include studies evaluating all remaining cancer types not included in previous categories (eg, breast, prostate, lung cancer). Includes a total of 206 unique studies across 219 publications. KQs: In adults diagnosed with cancer who have or are at risk for cancer-associated malnutrition: What is the effect of nutrition interventions before (KQ1) or during (KQ2) cancer treatment in preventing negative treatment outcomes such as effects on dose tolerance, hospital use, adverse events, and survival? What is the effect of nutrition interventions before or during cancer treatment on associated symptoms such as fatigue, nausea and vomiting, appetite, physical and functional status (eg, frailty), and quality of life (KQ3)? In adults with cancer who are overweight or obese, what is the effect of nutrition interventions before or during cancer treatment in preventing negative treatment outcomes such as effects on dose, hospital use, adverse events, and survival (KQ4)?
Indicates studies in which RoB was assessed.
Figure 2.
Number of studies evaluating outcomes by key question (KQ), N = 206.
Studies may evaluate more than 1 outcome; + Symptoms may include cancer or cancer treatment related symptoms such as fatigue, nausea and vomiting, appetite. KQs: In adults diagnosed with cancer who have or are at risk for cancer-associated malnutrition: What is the effect of nutrition interventions before (KQ1) or during (KQ2) cancer treatment in preventing negative treatment outcomes such as effects on dose tolerance, hospital use, adverse events, and survival? What is the effect of nutrition interventions before or during cancer treatment on associated symptoms such as fatigue, nausea and vomiting, appetite, physical and functional status (eg, frailty), and quality of life (KQ3)? In adults with cancer who are overweight or obese, what is the effect of nutrition interventions before or during cancer treatment in preventing negative treatment outcomes such as effects on dose, hospital use, adverse events and survival (KQ4)?
Table 4.
Characteristics of included studies examining the impact of nutrition interventions before or during cancer treatment (N = 206)
| Characteristics | KQ1 | Spans KQ1 and 2 | KQ2 | KQ3 | KQ4 |
|---|---|---|---|---|---|
| Total included studiesa | 20 Studies | 38 Studies | 141 studies | 79 studies | 4 studies |
| Intervention type |
|
|
|
|
4 Special diets |
| Study sample size |
|
|
|
|
|
| Cancer type |
|
|
|
|
4 Other |
| Intervention delivery setting |
|
|
|
|
|
| Dominant cancer treatment type of participants |
|
|
|
|
|
| Limited to malnourished patients |
|
|
|
|
2 No |
| Malnourishment screening tool used |
|
|
|
|
4 Not reported |
| Provider prescribing or delivering the intervention |
|
|
|
|
|
| Route of administration |
|
|
|
|
4 Oral |
| Geographic region of intervention |
|
|
|
|
|
| No, % of participants with stage IV disease |
|
|
|
|
4, 0-25 |
| No, % female participants |
|
|
|
|
4, 76-100 |
| Mean age of participants |
|
|
|
|
4, 50-64 |
| Outcomes evaluatedb |
|
|
|
|
4 Weight/body composition changes |
Totals are not mutually exclusive to number of included studies. Studies addressing key question (KQ)3 could also address KQ1, KQ2, or span KQ1 and 2.
Studies may evaluate multiple outcomes: KQs: In adults diagnosed with cancer who have or are at risk for cancer-associated malnutrition: What is the effect of nutrition interventions before (KQ1) or during (KQ2) cancer treatment in preventing negative treatment outcomes such as effects on dose tolerance, hospital use, adverse events, and survival? What is the effect of nutrition interventions before or during cancer treatment on associated symptoms such as fatigue, nausea and vomiting, appetite, physical and functional status (eg, frailty), and quality of life (KQ3)? In adults with cancer who are overweight or obese, what is the effect of nutrition interventions before or during cancer treatment in preventing negative treatment outcomes such as effects on dose, hospital use, adverse events, and survival (KQ4)?
Nutrition interventions before cancer treatment
For KQ1, we identified 20 unique studies that examined nutrition interventions before the initiation of cancer treatment. Studies examined the use of dietary supplements (n = 5) and nutrition support (n = 15). Table 3 includes basic characteristics of all included studies.
Dietary supplements
We identified 5 unique studies examining dietary supplements used before cancer treatment (Table 4) (18-22). Studies most commonly evaluated outcomes including body weight or composition changes, adverse events, and survival. One study compared parenteral fish oil lipid emulsion (19), and another evaluated omega-3 fatty acids (20). Two studies evaluated the use of arginine, omega-3 fatty acids, and nucleotides in lung (21) and head and neck cancer patients (18). A final study evaluated preoperative oral carbohydrate loading before breast cancer surgery (22). Two studies were conducted in Brazil (19,20), 1 in Norway (22), 1 in Thailand (18), and 1 in Turkey (21).
Both studies evaluated for RoB were assessed as high RoB (low quality, Table 3), and outcomes were not evaluated (19,20).
Nutrition support
We identified 15 unique studies examining nutrition support interventions before cancer treatment (Table 4) (23-37). Studies most commonly evaluated adverse events, length of stay, and body weight or composition changes.
Most interventions were preoperatively delivered in a surgical setting. Two studies evaluated daily oral supplementation with Fortisip oral nutrition support (23,24). Three studies examined preoperative use of immunonutrition, including use of oral immunonutrition in advanced pancreatic adenocarcinoma (31), enteral immunonutrition among patients with colorectal or gastric carcinoma (36), and immunonutrient-enriched supplementation in patients with colon cancer. Seven studies examined the use of more diverse oral nutrition support across gastrointestinal cancers, including use of preoperative Nutricia (35), hypercaloric supplementation (Nutridrink Protein) (28), peripheral intravenous nutrition for patients undergoing workup for biliopancreatic masses (29), oral nutrition combined with microbial preparations (33), preoperative oral supplementation for colorectal (34) and gastrointestinal cancers (27), and the use of Nutrison Fiber in patients with adenocarcinomas (37). The final 3 studies examined carbohydrate-rich beverages (26,32) as well as the use of 10% glucose solution before radical gastrectomy (25).
Among the 15 studies assessed for RoB (Table 3), 8 were assessed as low or medium RoB and reported mixed results on development of complications, improvements in weight loss, and length of hospital stay (Table 5) (23-25,27,30,32,34,36).
Table 5.
Outcomes reported for low- and medium- risk-of-bias studies by key question (KQ) and intervention typea
| KQ | Intervention type | RoB | No. range | Weight/body comp. | Changes in nutrition status | Adverse events | Readmissions/emergency room visits | Survival | LOS | Treatment tolerance | QoL | Symptoms | Functional status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrition interventions before cancer treatment (KQ1) | Nutrition support including oral nutrition supplements | 50-161 | 1 ↔ (23) | 2 ↔ (25,27) | 1 ↔ (23) | — | — | — | — | ||||
| Nutrition interventions before and including initiation of cancer treatment (spans KQ 1 and 2) | Dietary supplements | 4 Medium (44,47-50) | 60-195 | — | 4 ↔ (44,47-50) | 2 ↔ (47-49) | 4 ↔ (44,47-50) | 4 ↔ (44,47-50) | — | — | — | — | |
| Nutrition support including oral nutrition supplements | 120-317 | — | — | — | — | — | — | ||||||
| Nutrition interventions after treatment began (KQ2) | Dietary supplements | 50-229 | 1 ↔ (117) | — | — | — | — | ||||||
| Route or timing of nutrition interventions | 60-317 | 1 ↓ (139) | 1 ↔ (139) | — | — | — | — | ||||||
| Nutrition support including oral nutrition supplements | 77-1003 | 1 ↑ (177) | 2 ↔ (172,212) | — | — | — | — | ||||||
| Effect of nutrition interventions on symptoms (KQ3) | Dietary supplements | 3 Medium (47,107,124) | 71-229 | — | — | — | — | — | — | — | — | ||
| Nutrition support including oral nutrition supplements | 50-353 | — | — | — | — | — | — | 1 ↑ (197,198) | 2 ↔ (212,217) |
Studies that reported statistically significant results for 1 aspect of an outcome and not statistically significant in another aspect of an outcome were recorded as separate instances. ↑ = Intervention group had a statistically significantly better outcome than comparison group (eg, fewer AEs, shorter LOS than comparison group); ↓ = intervention group had a statistically significantly worse outcome than comparison group (eg, more AEs, longer LOS); ↔ = no statistically significant difference between groups; AEs = adverse events; LOS = length of stay; QoL = quality of life; RoB = risk of bias.
Nutrition interventions before and including initiation of cancer treatment
We identified 38 unique studies across 42 publications that examined nutrition interventions conducted both before and after initiation of cancer treatment. Studies examined the use of dietary supplements (n = 15), route or timing of nutrition interventions (n = 9), use of nutrition support (n = 13), and multicomponent interventions (n = 1). Table 3 includes basic characteristics of all included studies.
Dietary supplements
We identified 15 unique studies across 16 publications examining the use of dietary supplements before and continued after the initiation of cancer treatment (Table 4) (38-53). Studies most commonly evaluated outcomes including adverse events, body weight or composition changes, survival, and length of stay. One group of studies examined the use of a single supplement for immunonutrition (eicosapentaenoic—[EPA], omega-3 fatty acid, or glutamine-enriched nutrition), but interventions varied by population and had a wide range of intervention doses and durations around the time of cancer surgery (42-46,48-52). The second group of studies examined use of supplements for immunonutrition, including a combination of L-arginine, omega-3 fatty acids, and nucleotides, a combination of arginine, nucleotides, and fatty acids, and immunomodulating nutrition (Oral Impact) relative to standard nutrition (38,40,53). The remaining studies evaluated a variety of other supplement combinations, including nutrition counseling with oral whey protein supplementation (39), omega-3 fatty acid and vitamin D (41), and perioperative oral protein supplementation rich in arginine and omega-6 (47). Studies were primarily conducted in Europe and Asia.
Among the 10 studies assessed for RoB (Table 3), 4 medium-RoB studies across 5 publications showed mixed results for the effect of dietary supplements on weight changes, readmissions, length of hospital stay, development of complications, and survival (Table 5) (44,47-50).
Route or timing of nutrition interventions
We identified 9 unique studies that examined the route or timing of nutrition interventions initiated before and continued after cancer treatment (Table 4) (54-62). Studies most commonly evaluated adverse events, body weight or composition changes, and length of stay. One study evaluated enteral nutrition immediately initiated after placement of a prophylactic gastrostomy tube (55). A 3-armed trial evaluated changes in the contents and duration of perioperative whole oral immunonutrition (57). Six studies examined the timing of nutrition support in individuals with gastrointestinal cancer, including variation in the timing and/or duration of perioperative nutrition (54,56,58-60,62). Finally, 1 study evaluated consumption of a milk-based oral nutrition supplement (61). These non-US studies took place primarily in Europe and Asia.
Nutrition support
We identified 13 unique studies across 16 publications examining nutrition support interventions initiated before and continued after the start of cancer treatment (Table 4) (63-78). Studies most commonly evaluated adverse events, length of stay, and survival. Ten studies examined nutrition support in gastrointestinal cancer, using a broad range of nutrition support, including perioperative use of an oral nutrition supplement (69), oral immunonutrition (71), an EPA-enriched supplement (66-68), multi-oil fat emulsion, total parenteral nutrition (63), and whole enteral nutrition in liver cancer (78). The remainder of these 10 studies evaluated use of broader types of nutrition support, including perioperative use of parenteral or enteral nutrition in gastric and colorectal cancer patients (77), peripheral parenteral nutrition in colorectal cancer (74,75), early oral feeding (65), and the use of a preoperative carbohydrate drink (70). The final 3 studies examined peri-treatment nutrition support, including the use of an omega-3 fatty acid-enriched oral nutrition supplement in bladder cancer (73), the use of an amino acid-enriched oral nutrition support in hepatocellular carcinoma (72), and an immune-enhancing diet in head and neck cancer (76). One study was United States based (73), with the majority based in Asia.
Of the 10 studies assessed for RoB (Table 3), 2 low-RoB and 4 medium-RoB studies across 7 publications examined outcomes in surgical inpatients with gastrointestinal cancer (63-65,70,71,74,75). Reported results were mixed, with some studies reporting a benefit and some reporting no difference in improving development of adverse events, length of hospital stay, readmission and emergency room visits, and survival (Table 5).
Multi-component interventions
One 4-arm study tested a multi-component intervention to improve nutrition, examining the use of dietary advice with nutrition support supplements in England (79).
Nutrition interventions after treatment began
For KQ2, we identified 141 studies across 150 publications that examined nutrition interventions after cancer treatment began. Studies examined nutrition counseling (n = 15), dietary supplements (n = 34), special diets (n = 8), route or timing of nutrition interventions (n = 31), nutrition support including oral nutrition supplements (n = 43), and multi-component interventions (n = 10) (Table 3).
Nutrition counseling
We identified 15 unique studies that examined nutrition counseling during cancer treatment (Table 3) (80-94). Studies most commonly included the following outcomes: body weight or composition changes, changes in nutrition status, adverse events, and survival.
Four studies evaluated standardized intensive nutrition counseling across diverse populations, including patients with head and neck cancer undergoing chemoradiotherapy (86), multiple cancer types during and after radiotherapy (92), patients with gastrointestinal and head and neck cancer undergoing radiotherapy (83), and radiation therapy for prostate cancer (82). A second group of studies evaluated individualized nutrition counseling in patients with head and neck cancer undergoing chemoradiotherapy (84), patients with colorectal cancer undergoing radiotherapy (89) and chemotherapy (93), and patients undergoing radiation therapy across multiple cancer types (94). A third group of studies examined face-to-face interviewing among individuals with multiple cancer types undergoing chemotherapy (80,90). The remaining studies were more diverse, evaluating a standardized dietary advice approach for foods to avoid and consume (87); nutrition counseling and meal planning posthospital discharge for multiple cancers; a whole-course nutrition management model (88); the use of individualized dietary planning, nutrition education, and pharmacotherapy; and the use of a motivational interviewing and cognitive behavioral therapy program (81). Most of these studies were conducted in Europe and Asia.
Dietary supplements
We identified 34 unique studies across 35 publications that examined dietary supplements administered after the start of cancer treatment (Table 4) (95-129). Studies most commonly evaluated body weight or composition changes, adverse events, length of stay, and survival.
Several studies examined the effects of a single supplement, but interventions varied by cancers studied, cancer treatments, length of follow-up, and comparators. Single supplements included the use of arginine-enhanced oral or enteral nutrition support (98,100,102-104,112); omega-3 fatty acids, fish oil, or amino acids (95,99,101,107-109,115,118,119,123,125,126,128,129); glutamine (97,110,113,116,122); EPA (120); and Echium oil (117). A second set of studies examined diverse multi-component supplements across a wide range of cancer populations and treatments, including the use of EPA and gamma-linolenic acid (114); triglycerides and protein (124); arginine, omega-3 fatty acids, and RNA (105); arginine, glutamine, and cysteine (111); protein and arginine (121); arginine, glutamine, and fish oil (96); EPA and docosahexaenoic acid (106); and omega-3 fatty acid, glutamine, and probiotics (127). Studies in dietary supplements were predominately conducted within Europe and Asia.
Among the 26 studies assessed for RoB (Table 3; 4 low-RoB and 14 medium-RoB studies), results were mixed, with most studies reporting no benefit of added dietary supplements on body weight, adverse events, length of hospital stay, or survival (Table 5) (100-103,105,107,109,111,113,115-119,123,124,126,129).
Special diets
We identified 8 unique studies across 9 publications that examined special diets after the initiation of cancer treatment (Table 4) (130-138). Studies evaluated changes in body weight or composition and adverse events. Two studies evaluated the use of special drinks, including wine (132) or grape juice (131) before meals. Three studies evaluated fasting or calorie restriction, including calorie-restricted ketogenic diets (133,134,137) or a fasting-mimicking diet (135). The remaining studies examined modified diets by consistency or contents, including early initiation of a solid vs liquid diet after surgery (136), a diet containing no raw fruits or vegetables (130), and a low-fat or modified-fat diet (138). Studies were predominantly conducted in North America and Europe.
Route or timing of nutrition interventions
We identified 31 unique studies that examined the route or timing of nutrition interventions delivered, at least in part, during cancer treatment (Table 4) (139-169). Studies most commonly evaluated adverse events and length of stay. Seventeen studies compared enteral nutrition vs parenteral nutrition, predominately in postoperative individuals (140-142,146-153,156,158,164,166-168). Six studies compared oral feeding vs enteral or parenteral methods (139,144,145,157,160,162). Three studies evaluated the effects of different enteral or parenteral methods, including use of percutaneous endoscopic gastrostomy tube vs a nasogastric tube (159), jejunostomy feeding vs nasogastric feeding (163), and use of a peripherally inserted central catheter for administration of parenteral nutrition vs a central venous catheter (169). Finally, 5 studies examined variation in the timing of oral feeding after cancer surgery (143,154,155,161,165). Studies were predominately conducted in Asia and Europe.
Among 24 studies assessed for RoB (Table 3), 1 low-RoB and 10 medium-RoB studies that examined route and timing of nutrition interventions conducted during cancer treatment demonstrated mixed results (139,141,142,144,149,153,162,163,165-167). The majority reported no difference for body weight, adverse events, readmissions, or death, but one-half reported reduced length of hospital stay (Table 5).
Nutrition support
We identified 43 unique studies across 49 publications that examined nutrition support interventions during cancer treatment (Table 4) (170-218). Studies most commonly evaluated adverse events and body weight or composition changes.
Twenty-one studies from 26 publications examined oral or enteral nutrition support after cancer surgery, and they varied in the route and contents of nutrition support delivered as well as the timing of the comparisons. Specifically, several studies examined immunonutrition after surgery, and another compared early enteral nutrition vs parenteral nutrition (200). Among the remaining studies, the nutrition support varied in contents and quantity, including nutrition support supplements such as Elental (182,183), Jevity (193), Racol (199), Nutren (197,198), and Nutrison Fibre (214), and other enteral nutrition formulations across diverse cancer types after surgery (171,172,175,176,178,180,212,215,217). Finally, 1 distinct study evaluated the use of iEAT, food disintegrated on the tongue, vs standard care after surgery for gastrointestinal cancer (207).
Seventeen studies from 18 publications examined oral or enteral nutrition support during chemotherapy or radiation, and they varied in the route and contents of nutrition support delivered as well as the timing of comparisons. Three studies examined EPA-enriched oral nutrition support (170,186,205). Another 3 studies examined hyper-protein and omega-3 fatty acid-enhanced nutrition support (174,179,218). The remaining studies evaluated standard nutrition support across diverse populations and intervention dose.
Finally, 4 studies examined total parenteral nutrition (TPN) across cancer and treatment types (173,185,209,211), with 1 additional study examining combined enteral and parenteral nutrition after esophageal cancer surgery (177). Among 27 studies assessed for RoB (Table 3), 3 low-RoB and 8 medium-RoB studies evaluated the use of nutrition support during cancer treatment (172,177,188-192,194,197-199,211,212,214,217). Two low- (197,217), and 3 medium-RoB studies (199,211,212) reported improvements in body weight or composition with postoperative nutrition support. Four out of 10 studies (172,177,194,214) reported improvements in adverse events and 3 reported reductions in length of hospital stay (172,177,214) across diverse enteral and oral nutrition support interventions.
Multi-component interventions
Ten studies across 11 publications examined multi-component interventions initiated after cancer treatment began (Table 4) (219-229). Studies most commonly evaluated body weight or composition changes as well as changes in nutrition status.
Multi-component interventions varied in the interventions administered, cancer type, comparators, and cancer treatments. Four studies examined individualized diet or nutrition plans combined with additional interventions such as education or counseling (219,223,228,229). The remaining studies widely varied in interventions administered, including the use of individual recipes developed by patients, caregivers, and clinical specialists (221); a calcium-rich, low-fat and high-fruit and vegetable diet plus exercise (220); prophylactic percutaneous endoscopic gastrostomy tube along with nutrition advice (224,225); oral, enteral, or TPN nutrition followed by nutrition education; and in-hospital nutrition education and early enteral nutrition support (227).
Effect of nutrition interventions on symptoms
For KQ3, we identified 79 studies across 83 publications that examined nutrition interventions before or during cancer treatment on symptoms. Studies examined nutrition counseling (n = 12), dietary supplements (n = 10), special diets (n = 10), route or timing of nutrition interventions (n = 8), nutrition support (including oral nutrition supplements) (n = 32), and multi-component interventions (n = 7) (Table 3).
Nutrition counseling
We identified 12 unique studies examining nutrition counseling on improving cancer symptoms before or during cancer treatment (Table 4) (81-83,85,87-90,92-94,230). Studies most commonly included the following outcomes: quality of life, symptoms, and functional status. Three studies evaluated standardized intensive nutrition counseling (82,83,92). Another group of studies evaluated individualized nutrition counseling or face-to-face interviewing (89,90,93,94,230). The remaining studies were more diverse, including standardized dietary advice for foods to avoid or consume (87), a whole-course nutrition management model (88), individualized dietary planning plus nutrition education and pharmacotherapy, and use of motivational interviewing and cognitive behavioral therapy (81). Most studies were conducted in Europe and Asia.
Dietary supplements
We identified 10 unique studies that examined dietary supplements initiated before or during cancer treatment and evaluated the impact of the intervention on symptoms (18,39,47,51,106-108,120,122,124). Studies most commonly evaluated quality of life and symptoms.
Seven studies examined a single supplement in a variety of populations with a wide range of durations and treatment contexts, including use of omega-3 fatty acids (107), EPA-enriched supplements (106), orally administered amino acid jelly (108), glutamine injection, oral whey protein supplementation (39), and amino acid supplements (51). One 5-arm study evaluated the combinations of EPA, l-carnitine, thalidomide, and medroxyprogesterone acetate or megestrol acetate. Three studies examined multiple supplements to improve cancer treatment–related symptoms, including oral protein supplementation rich in arginine and omega-6 (47); fatty acids, arginine, fiber, and nucleotides (18); and medium-chain triglycerides and protein (124).
Of the 5 studies assessed for RoB (Table 3), 3 medium-RoB studies in dietary supplements reported mixed results for patient-reported symptoms (47,107,124). One study of probiotics and omega-3 fatty acids reported improved quality of life (107), whereas another reported no benefit (47). Two studies reported mixed outcomes for patient-reported symptoms, with 1 reporting benefit (107) and the other reporting no difference (Table 5) (124).
Special diets
We identified 10 unique studies across 11 publications that examined the effects of special diets before or during cancer treatment on treatment-related symptoms (130-138,231,232). Studies most commonly evaluated quality of life and symptoms.
Two studies evaluated the use of specific drinks, with 1 evaluating consumption of a glass of wine (132) or grape juice (131) before meals. Another 4 studies examined fasting or calorie restriction, including a calorie-restricted ketogenic diet (133,134,137), a fasting-mimicking diet (135), and calorie restriction with synbiotics (232). The remaining studies examined modified diets by consistency or contents, including evaluation of early initiation of a solid diet (136), a diet containing no raw fruits or vegetables (130), ginger (231), and a low or modified-fat diet (138). Most of these studies were conducted in North America, Europe, and Asia.
Route or timing of nutrition intervention
We identified 8 studies that examined whether the route or timing of nutrition interventions delivered, at least in part, during cancer treatment affected cancer or cancer treatment-related symptoms, with most studies evaluating quality of life (55,60,61,144,157,159,162,163).
Of the 8 studies, 4 compared early oral feeding vs enteral nutrition. Another evaluated timing of delivery of a milk-based oral nutrition supplement (61). The remaining studies were more varied but focused on evaluating the mode of nutrition interventions on symptoms of cancer treatment, including the use of a percutaneous endoscopic gastrostomy tube vs a nasogastric tube (159), the use of jejunostomy feeding or nasogastric feeding (163), and total parenteral nutrition vs an oral diet (157).
Nutrition support
We identified 32 studies across 34 publications that examined the effect of nutrition support interventions during cancer treatment on cancer treatment-related symptoms (25,27,29,32,34,65,69,72,76,171,173,174,176,179-181,184-186,195,197,198,202-205,208-213,217,218).
Fifteen studies examined oral or enteral nutrition support before or after cancer surgery; the studies varied in the route and contents of nutrition support delivered and the timing of the intervention and comparison groups. Thirteen studies examined oral or enteral nutrition support before or after chemotherapy and/or radiation; however, they also considerably varied in the route and contents of nutrition support delivered, populations, and timing of the intervention and comparison groups (72,89,174,179,181,184,186,195,205,208,210,213,218). Finally, 4 studies examined TPN across cancer and treatment types (173,185,209,211).
Of the 20 studies assessed for RoB (Table 3), 5 low-RoB and 4 medium-RoB studies of nutrition support reported mixed results (13,15,16,18,28,66,68,69,71,72). Two studies showed mixed results on functional status, with 1 showing a benefit and the other reporting no difference (28,66). Two low-RoB studies reported improvement in nausea for individuals receiving preoperative oral carbohydrate drinks (28,66). A third low-RoB study reported improvement in treatment tolerance and symptoms after use of oral nutrition supplements and dietary advice (Table 5) (66,68).
Multi-component interventions
Seven studies across 8 publications examined multi-component nutrition interventions administered before or during cancer treatment. Two studies examined individualized diet plans combined with interventions such as education or counseling (222,228). The remaining studies varied in the types of interventions delivered, including evaluation of a calcium-rich, low-fat, and high-fruit-and-vegetable diet (220); dietary advice plus a supplement (79); in-hospital nutrition education and early enteral nutrition support; avoidance of food high in dietary fiber and lactose (233); and a prophylactic percutaneous endoscopic gastrostomy tube along with nutrition advice (224,225).
Variation in the effect of nutrition interventions (KQs 1-3)
Although studies addressing KQ1-3 enrolled varied samples (eg, cancer type and stage, treatment, age, comorbid conditions, and degree of muscle wasting), no eligible studies specifically evaluated whether the effects of nutrition interventions on preventing negative outcomes varied across these characteristics.
Effect of nutrition interventions on weight loss
Four studies reported the effects of nutrition interventions intended for body weight loss for overweight or obesity using special diets among individuals with breast cancer (Table 4). Studies varied in the enrolled populations, interventions. and comparison groups. Studies included evaluation of the effect of calorie restriction with synbiotics (232), a diet designed to prevent weight gain, the use of a Mediterranean diet and dietary advice, and intermittent 25% energy restriction. Studies were conducted predominantly in Europe and Asia.
Cost or value of nutrition interventions
Among studies evaluating the effectiveness of nutrition interventions included in our KQs, few (8/206) published any cost or value (eg, cost-effectiveness, cost-benefit) information related to the intervention. Most often, the cost-related information focused on the total cost of care for 1 group vs a comparison group, making it challenging to identify the exact cost of the intervention or its components. In a grey literature search (Supplementary Materials), we identified a few additional studies that examined the cost or value of nutrition interventions, including examination of the cost-effectiveness of preoperative immunonutrition (234,235), perioperative enteral nutrition in colorectal cancer, supplemental parenteral nutrition for inoperable pancreatic cancer (236), and a value analysis of nutrition interventions for gastrointestinal cancer. These studies were predominantly conducted in inpatient settings in non-US health systems.
Discussion
Two decades of randomized trial evidence on nutrition interventions for adults prior and/or during cancer treatment provide only limited high-quality evidence to inform supplemental nutrition recommendations that could improve cancer treatment-related outcomes.
The questions of whether nutrition interventions (or components) can prevent negative health outcomes, for whom, and under what circumstances are of vital importance to patients and clinicians. The number of individuals at risk for cancer-related malnutrition remains substantial, with estimates ranging from 25% to 80% across patient populations (7-9). Because cancer risk (and malnutrition) increases with age, the rapidly growing older population in the United States will increase the demand for cancer care and, by extension, nutrition therapy over the coming decades (237).
There are sizeable limitations in the evidence base on nutrition interventions in adult cancer care. The topic of nutrition interventions to prevent adverse effects of treatments for cancer patients at risk for malnutrition is extremely broad. This breadth creates challenges for adequately categorizing and summarizing clinical evidence. Although we identified many studies, they widely differed by population, intervention type, intent, timing, mode of delivery, comparators, cancer types, treatments, and outcomes. Even within intervention categories, we found substantial heterogeneity, making aggregation infeasible. Furthermore, definitions of malnutrition (or at risk for) widely varied, and many interventions (eg, dietary supplements) are unlikely to improve malnutrition outcomes such as weight loss in the time frame of the studies. Additionally, most studies were small and of a short duration.
Based on our systematic review, we note several potential areas to target to improve research strategies in cancer-associated malnutrition to better inform clinical practice and policy. First, the literature broadly lacked a clear conceptual framework describing how each intervention would be expected to improve outcomes. As a result, the field could benefit from development of a comprehensive and clear conceptual framework addressing how specific nutrition interventions could improve the key outcomes most important to stakeholders. Second, we were struck by the lack of adherence to basic reporting standards within these publications, including missing information on the random assignment process, blinding of participants and assessors, and populations analyzed. Future RCT research on nutrition interventions should emphasize consistent use of criteria laid out in the CONSORT statement (238).
Third, although clinical stakeholders were eager to understand the available literature within specific populations of individuals receiving nutrition interventions (eg, adults ≥65 years old, those with muscle wasting, individuals with comorbid conditions), studies rarely reported results according to these characteristics. Rather, studies tended to focus on very specific populations (eg, early-stage gastric cancer), and almost all were conducted outside of the United States, mainly in Asia and Europe, where generalizability of nutrition norms, costs of care, and available resources differ greatly from the United States.
Fourth, definitions of malnutrition widely varied. Although weight loss is one common definition, many studies enrolled individuals considered malnourished based on biochemical or micronutrient abnormalities rather than weight. Furthermore, many individuals with localized cancer may be malnourished for reasons not due to cancer.
Fifth, we noted considerable diversity in the nutrition interventions tested, where outcomes were focused on the reduction of the negative effects of cancer treatment and associated symptoms. Nutrition interventions were extremely heterogeneous, even within intervention types. For example, studies using dietary supplements included omega-3 fatty acids, glutamine, or arginine, among others. Even when the studies used the same interventions, they varied considerably in the populations studied, duration (eg, 2 days vs 3 months), dosage, route, and type of cancer treatment. Heterogeneity in intervention type and reporting, as well as the absence of key information, limited our ability to conduct meta-analyses.
Sixth, outcomes collected to assess the impact of nutrition interventions were diverse and ranged from intermediate outcomes, such as changes in body weight, to longer-term outcomes, such as changes in overall survival. Outcomes were often poorly defined, with few details provided on the timing, definitions, or evaluation of outcomes (how, by whom, over what time frame, and using what criteria). One crucial way to improve outcome assessment in future nutrition intervention studies is to use standardized assessments with common, validated tools coupled with clearly defined assessment time periods that are consistent between the intervention and comparison group.
Finally, we focused our report on individuals judged to have physiologic or biochemical measures of, or at risk for, malnutrition. We did not evaluate interventions specifically targeted to individuals at risk for malnutrition due to sociodemographic factors, including food insecurity and healthy dietary choices. To better inform future implementation, additional research should examine the effects of interventions across populations and ensure they are applicable to future real-world implementation.
We acknowledge the following limitations of the review. We used broad definitions of nutrition interventions, thereby increasing the scope, breadth, and heterogeneity of the included literature to better assess the range and depth of available evidence. This decision allowed for a demonstration of the diffuse literature set on the topic and highlighted the predominantly low quality of the literature where there were concentrations of similar intervention types. However, this required focusing on high-level directionality of intervention effects across a broader range of nutrition interventions rather than detailed, precise estimates of intervention effects. Nonetheless, we failed to find strong signals indicating clear, consistent benefits of nutrition interventions in any populations or cancer therapies. Our review did not assess the long-term effects of nutrition interventions on cancer outcomes including cancer control and cancer-specific mortality. We presumed that nutrition interventions that improved effective cancer treatment initiation, adherence, and tolerance and reduced treatment-related adverse effects would lead to improved long-term cancer-related outcomes.
Overall, these findings should serve to encourage and suggest ways to bolster the rigor and reporting of future research to inform clinical practice and guideline development on nutrition interventions for cancer. Future investigation of the role of malnutrition screening would fill an important gap but was not addressed by this review. Another important future direction would be investigations to optimize and standardize methods for widespread nutrition assessment and intervention implementation in clinical settings. Future funding efforts, which should align with the priorities of the National Institutes of Health (NIH) Precision Nutrition Initiative (239), include 1) standardizing definitions and taxonomies for populations, interventions, and outcomes, including identifying those with and at risk for malnutrition; 2) improving rigor in the primary intent, design, and reporting of nutrition interventions; and 3) coordinating efforts to develop detailed conceptual frameworks for mechanisms of nutrition interventions effects across patient nutrition risk categories, cancers, and cancer treatments. These efforts will help prioritize research agendas as well as inform study designs and, ultimately, clinical practice and health policy.
Supplementary Material
Acknowledgements
We thank many individuals for their contributions to this project: Suchitra Iyer (Task Order Officer) and Nora Mueller from the Agency for Healthcare Research and Quality (AHRQ); Carrie Klabunde, Keisha Shropshire, and Elizabeth Vogt, from the National Institutes of Health (NIH) Office of Disease Prevention (ODP); Christopher Lynch and Roberto Flores from the Office of Nutrition Research (ONR); Karen Regan from the Office of Dietary Supplements (ODS); Elaine Trujillo, Sharon Ross, Joanne Elena, and Linda Nebeling from the National Cancer Institute (NCI); Ashley Vargas and Kimberlea Gibbs from the National Institute of Child Health and Human Development (NICHD); and Marcel Salive and Yih-Woei Fridell from the National Institute on Aging (NIA). We also thank Jeannine Ouellette for her exceptional editing, Bessie Peterson for her professional report preparation, and Eric Linskens and Lauren McKenzie for their assistance with abstract reviews.
The National Institutes of Health (NIH) planning committee developed the KQs that guided the systematic review. However, the funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
The findings and conclusions in this document are those of the authors, who are responsible for its contents; the content does not necessarily represent the official views of or imply endorsement by AHRQ or the US Department of Health and Human Services. AHRQ retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency’s contract with the author.
This work was presented in part at the National Institutes of Health Pathways to Prevention Program workshop titled “Nutrition as Prevention for Improved Cancer Outcomes” on July 26-28, 2022.
Contributor Information
Helen M Parsons, Minnesota Evidence-Based Practice Center, Division of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Mary L Forte, Minnesota Evidence-Based Practice Center, Division of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Hamdi I Abdi, Minnesota Evidence-Based Practice Center, Division of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Sallee Brandt, Minnesota Evidence-Based Practice Center, Division of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Amy M Claussen, Minnesota Evidence-Based Practice Center, Division of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Timothy Wilt, Minnesota Evidence-Based Practice Center, Division of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, MN, USA; Minneapolis VA Center for Care Delivery and Outcomes Research, Minneapolis, MN, USA; School of Medicine, University of Minnesota, Minneapolis, MN, USA; Minneapolis VA Healthcare System, Minneapolis, MN, USA.
Mark Klein, School of Medicine, University of Minnesota, Minneapolis, MN, USA; Minneapolis VA Healthcare System, Minneapolis, MN, USA.
Elizabeth Ester, University of Minnesota Physicians, Minneapolis, MN, USA.
Adrienne Landsteiner, Minneapolis VA Center for Care Delivery and Outcomes Research, Minneapolis, MN, USA.
Aasma Shaukut, New York University Langone, New York, NY, USA.
Shalamar S Sibley, School of Medicine, University of Minnesota, Minneapolis, MN, USA; Minneapolis VA Healthcare System, Minneapolis, MN, USA.
Joanne Slavin, Department of Food Science and Nutrition, College of Food, Agricultural and Natural Resource Sciences, St. Paul, MN, USA.
Catherine Sowerby, Minneapolis VA Center for Care Delivery and Outcomes Research, Minneapolis, MN, USA.
Weiwen Ng, Minnesota Evidence-Based Practice Center, Division of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Mary Butler, Minnesota Evidence-Based Practice Center, Division of Health Policy and Management, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Author contributions
Helen M. Parsons, PhD, MPH (Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing), Mary Forte, PhD (Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft), Hamdi Abdi, MPH (Data curation; Formal analysis; Methodology; Writing – original draft), Sallee Brandt, MPH (Data curation; Formal analysis; Writing – original draft), Amy Claussen, MLIS (Data curation; Formal analysis; Writing – original draft), Timothy Wilt, MD (Conceptualization; Methodology; Writing – original draft), Mark Klein, md (Conceptualization; Writing – review & editing), Elizabeth Ester, MD (Conceptualization; Writing – review & editing), Adrienne Landsteiner, PhD (Data curation; Formal analysis; Writing – original draft), Aasma Shaukut, MD (Conceptualization; Writing – review & editing), Shalamar Sibley, MD (Writing—review and editing), Joanne Slavin, PhD (Conceptualization; Writing – review & editing), Catherine Sowerby, BA (Data curation; Formal analysis; Writing – review & editing), Weiwen Ng, MPH (Data curation; Formal analysis; Writing – review & editing), and Mary Butler, PhD (Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Writing – original draft).
Funding
This work was supported under Contract No 75Q80120D00008 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (HHS). The National Institutes of Health (NIH) funded the report.
Conflicts of interest
HMP, MF, HIA, SB, AC, TW, MK, EE, AL, AS, SS, JS, CS, WN, and MB have no disclosures to report.
References
- 1. Aaldriks AA, Maartense E, Nortier HJ, et al. Prognostic factors for the feasibility of chemotherapy and the Geriatric Prognostic Index (GPI) as risk profile for mortality before chemotherapy in the elderly. Acta Oncol. 2016;55(1):15-23. [DOI] [PubMed] [Google Scholar]
- 2. van Deudekom FJ, van der Velden LA, Zijl WH, et al. Geriatric assessment and 1-year mortality in older patients with cancer in the head and neck region: a cohort study. Head Neck. 2019;41(8):2477-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aparicio T, Bouché O, Francois E, et al. ; for PRODIGE 20 investigators. Geriatric analysis from PRODIGE 20 randomized phase II trial evaluating bevacizumab+chemotherapy versus chemotherapy alone in older patients with untreated metastatic colorectal cancer. Eur J Cancer. 2018;97:16-24. [DOI] [PubMed] [Google Scholar]
- 4. Guner A, Kim SY, Yu JE, et al. Parameters for predicting surgical outcomes for gastric cancer patients: simple is better than complex. Ann Surg Oncol. 2018;25(11):3239-3247. [DOI] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Arends J.. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl 2):S51-S63. [DOI] [PubMed] [Google Scholar]
- 6. Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187-1196. [DOI] [PubMed] [Google Scholar]
- 7. Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado CM.. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75(2):199-211. [DOI] [PubMed] [Google Scholar]
- 8. Caillet P, Liuu E, Raynaud Simon A, et al. Association between cachexia, chemotherapy and outcomes in older cancer patients: a systematic review. Clin Nutr. 2017;36(6):1473-1482. [DOI] [PubMed] [Google Scholar]
- 9. Muscaritoli M, Lucia S, Farcomeni A, et al. ; PreMiO Study Group. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884-79896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marshall KM, Loeliger J, Nolte L, Kelaart A, Kiss NK.. Prevalence of malnutrition and impact on clinical outcomes in cancer services: a comparison of two time points. Clin Nutr. 2019;38(2):644-651. [DOI] [PubMed] [Google Scholar]
- 11. Gyan E, Raynard B, Durand JP, et al. Malnutrition in patients with cancer: comparison of perceptions by patients, relatives, and physicians-results of the nutricancer 2012 study. JPEN J Parenter Enteral Nutr. 2018;42(1):255-260. [DOI] [PubMed] [Google Scholar]
- 12. Planas M, Álvarez-Hernández J, León-Sanz M, Celaya-Pérez S, Araujo K, García de Lorenzo A; PREDyCES® researchers. Prevalence of hospital malnutrition in cancer patients: a sub-analysis of the PREDyCES® study. Support Care Cancer 2016;24(1):429-435. [DOI] [PubMed] [Google Scholar]
- 13. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F.. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. 2014;38(2):196-204. [DOI] [PubMed] [Google Scholar]
- 14. Walsh D, Szafranski M, Aktas A, Kadakia KC.. Malnutrition in cancer care: time to address the elephant in the room. J Oncol Pract. 2019;15(7):357-359. [DOI] [PubMed] [Google Scholar]
- 15. American College of Surgeons. Optimal Resources for Cancer Care (2020 Standards). Published 2022. https://www.facs.org/quality-programs/cancer-programs/commission-on-cancer/standards-and-resources/2020/. Accessed November 12, 2022.
- 16. Trujillo EB, Claghorn K, Dixon SW, et al. Inadequate nutrition coverage in outpatient cancer centers: results of a national survey. J Oncol. 2019;2019:7462940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PICO Portal. PICO Portal Transforming research findings into decision-ready evidence with ease. Published 2022. https://picoportal.org. Accessed 2022.
- 18. Dechaphunkul T, Arundon T, Raungkhajon P, Jiratrachu R, Geater SL, Dechaphunkul A.. Benefits of immunonutrition in patients with head and neck cancer receiving chemoradiation: a phase II randomized, double-blind study. Clin Nutr. 2022;41(2):433-440. [DOI] [PubMed] [Google Scholar]
- 19. de Miranda Torrinhas RS, Santana R, Garcia T, et al. Parenteral fish oil as a pharmacological agent to modulate post-operative immune response: a randomized, double-blind, and controlled clinical trial in patients with gastrointestinal cancer. Clin Nutr. 2013;32(4):503-510. [DOI] [PubMed] [Google Scholar]
- 20. Feijo PM, Rodrigues VD, Viana MS, et al. Effects of omega-3 supplementation on the nutritional status, immune, and inflammatory profiles of gastric cancer patients: a randomized controlled trial. Nutrition. 2019;61:125-131. [DOI] [PubMed] [Google Scholar]
- 21. Kaya SO, Akcam TI, Ceylan KC, Samancılar O, Ozturk O, Usluer O.. Is preoperative protein-rich nutrition effective on postoperative outcome in non-small cell lung cancer surgery? A prospective randomized study. J Cardiothorac Surg. 2016;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lende TH, Austdal M, Varhaugvik AE, et al. Influence of pre-operative oral carbohydrate loading vs. standard fasting on tumor proliferation and clinical outcome in breast cancer patients horizontal line a randomized trial. BMC Cancer 2019;19(1):1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burden ST, Gibson DJ, Lal S, et al. Pre-operative oral nutritional supplementation with dietary advice versus dietary advice alone in weight-losing patients with colorectal cancer: single-blind randomized controlled trial. J Cachexia Sarcopenia Muscle. 2017;8(3):437-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burden ST, Hill J, Shaffer JL, Campbell M, Todd C.. An unblinded randomised controlled trial of preoperative oral supplements in colorectal cancer patients. J Hum Nutr Diet. 2011;24(5):441-448. [DOI] [PubMed] [Google Scholar]
- 25. Chen X, Li K, Yang K, et al. Effects of preoperative oral single-dose and double-dose carbohydrates on insulin resistance in patients undergoing gastrectomy: a prospective randomized controlled trial. Clin Nutr. 2021;40(4):1596-1603. [DOI] [PubMed] [Google Scholar]
- 26. Hamamoto H, Yamamoto M, Masubuchi S, et al. The impact of preoperative carbohydrate loading on intraoperative body temperature: a randomized controlled clinical trial. Surg Endosc. 2018;32(11):4393-4401. [DOI] [PubMed] [Google Scholar]
- 27. He FJ, Wang MJ, Yang K, et al. Effects of preoperative oral nutritional supplements on improving postoperative early enteral feeding intolerance and short-term prognosis for gastric cancer: a prospective, single-center, single-blind, randomized controlled trial. Nutrients. 2022;14(7):1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kabata P, Jastrzębski T, Kąkol M, et al. Preoperative nutritional support in cancer patients with no clinical signs of malnutrition--prospective randomized controlled trial. Support Care Cancer. 2015;23(2):365-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kruger J, Meffert PJ, Vogt LJ, et al. Early parenteral nutrition in patients with biliopancreatic mass lesions, a prospective, randomized intervention trial. PLoS One. 2016;11(11):e0166513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee SY, Lee J, Park HM, Kim CH, Kim HR.. Impact of preoperative immunonutrition on the outcomes of colon cancer surgery: results from a randomized controlled trial. Ann Surg. 2023;277(3):381-386. [DOI] [PubMed] [Google Scholar]
- 31. Martin RC 2nd, Agle S, Schlegel M, et al. Efficacy of preoperative immunonutrition in locally advanced pancreatic cancer undergoing irreversible electroporation (IRE). Eur J Surg Oncol 2017;43(4):772-779. [DOI] [PubMed] [Google Scholar]
- 32. Rizvanovic N, Nesek Adam V, Causevic S, Dervisevic S, Delibegovic S.. A randomised controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing colorectal surgery. Int J Colorectal Dis. 2019;34(9):1551-1561. [DOI] [PubMed] [Google Scholar]
- 33. Shen Y, Zhao X, Zhao H, et al. Clinical application of enteral nutrition combined with microbial preparation for intestinal preparation in elderly patients with colorectal cancer. Med Sci Monit. 2022;28:e935366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tesar M, Kozusnikova V, Martinek L, Durdik S, Ihnat P.. Preoperative nutritional support for patients undergoing elective colorectal cancer surgery - does it really work? Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2022. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 35. Wang F, Hou MX, Wu XL, Bao LD, Dong PD.. Impact of enteral nutrition on postoperative immune function and nutritional status. Genet Mol Res. 2015;14(2):6065-6072. [DOI] [PubMed] [Google Scholar]
- 36. Xu J, Zhong Y, Jing D, Wu Z.. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. 2006;30(7):1284-1289. [DOI] [PubMed] [Google Scholar]
- 37. Zhao Q, Li Y, Yu B, et al. Effects of preoperative enteral nutrition on postoperative recent nutritional status in patients with Siewert II and III adenocarcinoma of esophagogastric junction after neoadjuvant chemoradiotherapy. Nutr Cancer. 2018;70(6):895-903. [DOI] [PubMed] [Google Scholar]
- 38. Celik JB, Gezginc K, Ozcelik K, Celik C.. The role of immunonutrition in gynecologic oncologic surgery. Eur J Gynaecol Oncol. 2009;30(4):418-421. [PubMed] [Google Scholar]
- 39. Cereda E, Turri A, Klersy C, et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019;8(16):6923-6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghosh S, Dempsey G, Skelly R, et al. A double blind, randomised, placebo controlled, feasibility phase III clinical trial of peri-operative immune-enhancing enteral nutrition in patients undergoing surgery for advanced head and neck cancer. e-SPEN J. 2012;7(3):e107-e114. [Google Scholar]
- 41. Haidari F, Abiri B, Iravani M, Ahmadi-Angali K, Vafa M.. Randomized study design to test effects of vitamin D and omega-3 fatty acid supplementation as adjuvant therapy in colorectal cancer patients. Methods Mol Biol. 2020;2138:337-350. [DOI] [PubMed] [Google Scholar]
- 42. Healy LA, Ryan A, Doyle SL, et al. Does prolonged enteral feeding with supplemental omega-3 fatty acids impact on recovery post-esophagectomy: results of a randomized double-blind trial. Ann Surg. 2017;266(5):720-728. [DOI] [PubMed] [Google Scholar]
- 43. Jantharapattana K, Orapipatpong O.. Efficacy of EPA-enriched supplement compared with standard formula on body weight changes in malnourished patients with head and neck cancer undergone surgery: a randomized study. Head Neck. 2020;42(2):188-197. [DOI] [PubMed] [Google Scholar]
- 44. Jo S, Choi SH, Heo JS, et al. Missing effect of glutamine supplementation on the surgical outcome after pancreaticoduodenectomy for periampullary tumors: a prospective, randomized, double-blind, controlled clinical trial. World J Surg. 2006;30(11):1974-1982. Discussion 1983–1974. [DOI] [PubMed] [Google Scholar]
- 45. Oguz M, Kerem M, Bedirli A, et al. L-alanin-L-glutamine supplementation improves the outcome after colorectal surgery for cancer. Colorectal Dis. 2007;9(6):515-520. [DOI] [PubMed] [Google Scholar]
- 46. Ryan AM, Reynolds JV, Healy L, et al. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: results of a double-blinded randomized controlled trial. Ann Surg. 2009;249(3):355-363. [DOI] [PubMed] [Google Scholar]
- 47. Serrano PE, Parpia S, Simunovic M, et al. Perioperative optimization with nutritional supplements in patients undergoing gastrointestinal surgery for cancer: a randomized, placebo-controlled feasibility clinical trial. Surgery. 2022;172(2):670-676. [DOI] [PubMed] [Google Scholar]
- 48. Sorensen LS, Thorlacius-Ussing O, Schmidt EB, et al. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. Br J Surg. 2014;101(2):33-42. [DOI] [PubMed] [Google Scholar]
- 49. Sorensen LS, Rasmussen SL, Calder PC, Yilmaz MN, Schmidt EB, Thorlacius-Ussing O.. Long-term outcomes after perioperative treatment with omega-3 fatty acid supplements in colorectal cancer. BJS Open. 2020;4(4):678-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sultan J, Griffin SM, Di Franco F, et al. Randomized clinical trial of omega-3 fatty acid-supplemented enteral nutrition versus standard enteral nutrition in patients undergoing oesophagogastric cancer surgery. Br J Surg. 2012;99(3):346-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tsuchiya T, Honda H, Oikawa M, et al. Oral administration of the amino acids cystine and theanine attenuates the adverse events of S-1 adjuvant chemotherapy in gastrointestinal cancer patients. Int J Clin Oncol. 2016;21(6):1085-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vidal-Casariego A, Calleja-Fernández A, de Urbina-González JJO, Cano-Rodríguez I, Cordido F, Ballesteros-Pomar MD.. Efficacy of glutamine in the prevention of acute radiation enteritis: a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2014;38(2):205-213. [DOI] [PubMed] [Google Scholar]
- 53. Yeğen SF, Kafadar MT, Gök MA.. Comparison of perioperative standard and immunomodulating enteral nutrition in patients received major abdominal cancer surgery: a prospective, randomized, controlled clinical trial. Indian J Surg 2020;82(5):828-834. [Google Scholar]
- 54. Braga M, Gianotti L, Nespoli L, Radaelli G, Di Carlo V.. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg. 2002;137(2):174-180. [DOI] [PubMed] [Google Scholar]
- 55. Brown T, Banks M, Hughes BGM, Lin C, Kenny LM, Bauer JD.. Impact of early prophylactic feeding on long term tube dependency outcomes in patients with head and neck cancer. Oral Oncol. 2017;72:17-25. [DOI] [PubMed] [Google Scholar]
- 56. Ding D, Feng Y, Song B, Gao S, Zhao J.. Effects of preoperative and postoperative enteral nutrition on postoperative nutritional status and immune function of gastric cancer patients. Turk J Gastroenterol. 2015;26(2):181-185. [DOI] [PubMed] [Google Scholar]
- 57. Falewee MN, Schilf A, Boufflers E, et al. Reduced infections with perioperative immunonutrition in head and neck cancer: exploratory results of a multicenter, prospective, randomized, double-blind study. Clin Nutr 2014;33(5):776-784. [DOI] [PubMed] [Google Scholar]
- 58. Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V.. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122(7):1763-1770. [DOI] [PubMed] [Google Scholar]
- 59. Miyata H, Yano M, Yasuda T, et al. Randomized study of clinical effect of enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Clin Nutr. 2012;31(3):330-336. [DOI] [PubMed] [Google Scholar]
- 60. Mudge LA, Watson DI, Smithers BM, et al. ; Australian Immunonutrition Study Group. Multicentre factorial randomized clinical trial of perioperative immunonutrition versus standard nutrition for patients undergoing surgical resection of oesophageal cancer. Br J Surg. 2018;105(10):1262-1272. [DOI] [PubMed] [Google Scholar]
- 61. Wong TX, Wong WX, Chen ST, et al. Effects of perioperative oral nutrition supplementation in Malaysian patients undergoing elective surgery for breast and colorectal cancers-a randomised controlled trial. Nutrients. 2022;14(3):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wu S, You D, Lu L, et al. Effect of enteral nutrition support on the curative effect and immune system in patients with rectal cancer during fast track surgery. Int J Clin Exp Med. 2020;13(8):6065-6073. [Google Scholar]
- 63. Bozzetti F, Gavazzi C, Miceli R, et al. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr. 2000;24(1):7-14. [DOI] [PubMed] [Google Scholar]
- 64. Chen H, Pan D, Li L.. The effects of multi-oil fat emulsion on older patients with gastric cancer. Biomed Res (India). 2017;28(1):4270-4276. [Google Scholar]
- 65. Feng J, Xu R, Li K, et al. Effects of preoperative oral carbohydrate administration combined with postoperative early oral intake in elderly patients undergoing hepatectomy with acute-phase inflammation and subjective symptom burden: a prospective randomized controlled study. Asian J Surg. 2022;45(1):386-395. [DOI] [PubMed] [Google Scholar]
- 66. Ida S, Hiki N, Cho H, et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br J Surg. 2017;104(4):377-383. [DOI] [PubMed] [Google Scholar]
- 67. Aoyama T, Yoshikawa T, Ida S, et al. Effects of perioperative eicosapentaenoic acid-enriched oral nutritional supplement on lean body mass after total gastrectomy for gastric cancer. J Cancer. 2019;10(5):1070-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aoyama T, Yoshikawa T, Ida S, et al. Effects of perioperative eicosapentaenoic acid-enriched oral nutritional supplement on the long-term oncological outcomes after total gastrectomy for gastric cancer. Oncol Lett 2022;23(5):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kong SH, Lee HJ, Na JR, et al. Effect of perioperative oral nutritional supplementation in malnourished patients who undergo gastrectomy: a prospective randomized trial. Surgery. 2018;164(6):1263-1270. [DOI] [PubMed] [Google Scholar]
- 70. Lidder P, Thomas S, Fleming S, Hosie K, Shaw S, Lewis S.. A randomized placebo controlled trial of preoperative carbohydrate drinks and early postoperative nutritional supplement drinks in colorectal surgery. Colorectal Dis. 2013;15(6):737-745. [DOI] [PubMed] [Google Scholar]
- 71. Moya P, Miranda E, Soriano-Irigaray L, et al. Perioperative immunonutrition in normo-nourished patients undergoing laparoscopic colorectal resection. Surg Endosc. 2016;30(11):4946-4953. [DOI] [PubMed] [Google Scholar]
- 72. Poon RT, Yu WC, Fan ST, Wong J.. Long-term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: a randomized trial. Aliment Pharmacol Ther. 2004;19(7):779-788. [DOI] [PubMed] [Google Scholar]
- 73. Ritch CR, Cookson MS, Clark PE, et al. Perioperative oral nutrition supplementation reduces prevalence of sarcopenia following radical cystectomy: results of a prospective randomized controlled trial. J Urol. 2019;201(3):470-477. [DOI] [PubMed] [Google Scholar]
- 74. Sanchez-Guillen L, Soriano-Irigaray L, Lopez-Rodriguez-Arias F, et al. Effect of early peripheral parenteral nutrition support in an enhanced recovery program for colorectal cancer surgery: a randomized open trial. J Clin Med. 2021;10(16):3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lopez-Rodriguez-Arias F, Sanchez-Guillen L, Lillo-Garcia C, et al. Assessment of body composition as an indicator of early peripheral parenteral nutrition therapy in patients undergoing colorectal cancer surgery in an enhanced recovery program. Nutrients. 2021;13(9):3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sittitrai P, Ruenmarkkaew D, Booyaprapa S, Kasempitakpong B.. Effect of a perioperative immune-enhancing diet in clean-contaminated head and neck cancer surgery: a randomized controlled trial. Int J Surg. 2021;93:106051. [DOI] [PubMed] [Google Scholar]
- 77. Wu GH, Liu ZH, Wu ZH, Wu ZG.. Perioperative artificial nutrition in malnourished gastrointestinal cancer patients. World J Gastroenterol. 2006;12(15):2441-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yan X, Liu L, Zhang Y, et al. Perioperative enteral nutrition improves postoperative recovery for patients with primary liver cancer: a randomized controlled clinical trial. Nutr Cancer. 2021;73(10):1924-1932. [DOI] [PubMed] [Google Scholar]
- 79. Baldwin C, Spiro A, McGough C, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet. 2011;24(5):431-440. [DOI] [PubMed] [Google Scholar]
- 80. Bourdel-Marchasson I, Blanc-Bisson C, Doussau A, et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two-year randomized controlled trial. PLoS One. 2014;9(9):e108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Britton B, Baker AL, Wolfenden L, et al. Eating As Treatment (EAT): a stepped-wedge, randomized controlled trial of a health behavior change intervention provided by dietitians to improve nutrition in patients with head and neck cancer undergoing radiation therapy (TROG 12.03). Int J Radiat Oncol Biol Phys. 2019;103(2):353-362. [DOI] [PubMed] [Google Scholar]
- 82. Forslund M, Ottenblad A, Ginman C, Johansson S, Nygren P, Johansson B.. Effects of a nutrition intervention on acute and late bowel symptoms and health-related quality of life up to 24 months post radiotherapy in patients with prostate cancer: a multicentre randomised controlled trial. Support Care Cancer. 2020;28(7):3331-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Isenring EA, Capra S, Bauer JD.. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer. 2004;91(3):447-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Loser A, Abel J, Kutz LM, et al. Head and neck cancer patients under (chemo-)radiotherapy undergoing nutritional intervention: results from the prospective randomized HEADNUT-trial. Radiother Oncol. 2021;159:82-90. [DOI] [PubMed] [Google Scholar]
- 85. Movahed S, Seilanian Toussi M, Pahlavani N, et al. Effects of medical nutrition therapy compared with general nutritional advice on nutritional status and nutrition-related complications in esophageal cancer patients receiving concurrent chemoradiation: a randomized controlled trial. MNM. 2020;13(3):265-276. [Google Scholar]
- 86. Orell H, Schwab U, Saarilahti K, Österlund P, Ravasco P, Mäkitie A.. Nutritional counseling for head and neck cancer patients undergoing (chemo) radiotherapy-a prospective randomized trial. Front Nutr. 2019;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pettersson A, Johansson B, Persson C, Berglund A, Turesson I.. Effects of a dietary intervention on acute gastrointestinal side effects and other aspects of health-related quality of life: a randomized controlled trial in prostate cancer patients undergoing radiotherapy. Radiother Oncol. 2012;103(3):333-340. [DOI] [PubMed] [Google Scholar]
- 88. Qiu Y, You J, Wang K, et al. Effect of whole-course nutrition management on patients with esophageal cancer undergoing concurrent chemoradiotherapy: a randomized control trial. Nutrition 2020;69:110558. [DOI] [PubMed] [Google Scholar]
- 89. Ravasco P, Monteiro-Grillo I, Camilo M.. Individualized nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of nutritional therapy. Am J Clin Nutr. 2012;96(6):1346-1353. [DOI] [PubMed] [Google Scholar]
- 90. Regueme SC, Echeverria I, Moneger N, et al. Protein intake, weight loss, dietary intervention, and worsening of quality of life in older patients during chemotherapy for cancer. Support Care Cancer. 2021;29(2):687-696. [DOI] [PubMed] [Google Scholar]
- 91. Tu MY, Chien TW, Lin HP, Liu MY.. Effects of an intervention on nutrition consultation for cancer patients. Eur J Cancer Care (Engl). 2013;22(3):370-376. [DOI] [PubMed] [Google Scholar]
- 92. Um MH, Choi MY, Lee SM, Lee IJ, Lee CG, Park YK.. Intensive nutritional counseling improves PG-SGA scores and nutritional symptoms during and after radiotherapy in Korean cancer patients. Support Care Cancer. 2014;22(11):2997-3005. [DOI] [PubMed] [Google Scholar]
- 93. van der Werf A, Langius JAE, Beeker A, et al. The effect of nutritional counseling on muscle mass and treatment outcome in patients with metastatic colorectal cancer undergoing chemotherapy: a randomized controlled trial. Clin Nutr. 2020;39(10):3005-3013. [DOI] [PubMed] [Google Scholar]
- 94. Zhang Z, Zhu Y, Zhang L, et al. Nutritional education and counseling program for adult cancer patients during radiotherapy: a cluster-randomized clinical trial. Support Care Cancer. 2022;30(4):3279-3289. [DOI] [PubMed] [Google Scholar]
- 95. Cheng M, Zhang S, Ning C, Huo Q.. Omega-3 fatty acids supplementation improve nutritional status and inflammatory response in patients with lung cancer: a randomized clinical trial. Front Nutr. 2021;8:686752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chitapanarux I, Traisathit P, Chitapanarux T, et al. Arginine, glutamine, and fish oil supplementation in cancer patients treated with concurrent chemoradiotherapy: a randomized control study. Curr Probl Cancer. 2020;44(1):100482. [DOI] [PubMed] [Google Scholar]
- 97. da Gama Torres HO, Vilela EG, da Cunha AS, et al. Efficacy of glutamine-supplemented parenteral nutrition on short-term survival following allo-SCT: a randomized study. Bone Marrow Transplant. 2008;41(12):1021-1027. [DOI] [PubMed] [Google Scholar]
- 98. de Luis DA, Izaola O, Cuellar L, Terroba MC, Aller R.. Randomized clinical trial with an enteral arginine-enhanced formula in early postsurgical head and neck cancer patients. Eur J Clin Nutr. 2004;58(11):1505-1508. [DOI] [PubMed] [Google Scholar]
- 99. de Luis DA, Izaola O, Aller R, Cuellar L, Terroba MC.. A randomized clinical trial with oral immunonutrition (omega3-enhanced formula vs. arginine-enhanced formula) in ambulatory head and neck cancer patients. Ann Nutr Metab. 2005;49(2):95-99. [DOI] [PubMed] [Google Scholar]
- 100. de Luis DA, Izaola O, Cuellar L, Terroba MC, Martin T, Aller R.. Clinical and biochemical outcomes after a randomized trial with a high dose of enteral arginine formula in postsurgical head and neck cancer patients. Eur J Clin Nutr. 2007;61(2):200-204. [DOI] [PubMed] [Google Scholar]
- 101. de Luis DA, Izaola O, Aller R, Cuellar L, Terroba MC, Martin T.. A randomized clinical trial with two omega 3 fatty acid enhanced oral supplements in head and neck cancer ambulatory patients. Eur Rev Med Pharmacol Sci. 2008;12(3):177-181. [PubMed] [Google Scholar]
- 102. De Luis DA, Izaola O, Cuellar L, Terroba MC, Martin T, Aller R.. High dose of arginine enhanced enteral nutrition in postsurgical head and neck cancer patients. A randomized clinical trial. Eur Rev Med Pharmacol Sci. 2009;13(4):279-283. [PubMed] [Google Scholar]
- 103. De Luis DA, Izaola O, Cuellar L, Terroba MC, Martin T, Ventosa M.. A randomized double-blind clinical trial with two different doses of arginine enhanced enteral nutrition in postsurgical cancer patients. Eur Rev Med Pharmacol Sci. 2010;14(11):941-945. [PubMed] [Google Scholar]
- 104. De Luis DA, Izaola O, Terroba MC, Cuellar L, Ventosa M, Martin T.. Effect of three different doses of arginine enhanced enteral nutrition on nutritional status and outcomes in well nourished postsurgical cancer patients: a randomized single blinded prospective trial. Eur Rev Med Pharmacol Sci. 2015;19(6):950-955. [PubMed] [Google Scholar]
- 105. Farreras N, Artigas V, Cardona D, Rius X, Trias M, González JA.. Effect of early postoperative enteral immunonutrition on wound healing in patients undergoing surgery for gastric cancer. Clin Nutr. 2005;24(1):55-65. [DOI] [PubMed] [Google Scholar]
- 106. Fietkau R, Lewitzki V, Kuhnt T, et al. A disease-specific enteral nutrition formula improves nutritional status and functional performance in patients with head and neck and esophageal cancer undergoing chemoradiotherapy: results of a randomized, controlled, multicenter trial. Cancer. 2013;119(18):3343-3353. [DOI] [PubMed] [Google Scholar]
- 107. Golkhalkhali B, Rajandram R, Paliany AS, et al. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia Pac J Clin Oncol. 2018;14(3):179-191. [DOI] [PubMed] [Google Scholar]
- 108. Iwase S, Kawaguchi T, Yotsumoto D, et al. Efficacy and safety of an amino acid jelly containing coenzyme Q10 and L-carnitine in controlling fatigue in breast cancer patients receiving chemotherapy: a multi-institutional, randomized, exploratory trial (JORTC-CAM01). Support Care Cancer. 2016;24(2):637-646. [DOI] [PubMed] [Google Scholar]
- 109. Jiang ZM, Wilmore DW, Wang XR, et al. Randomized clinical trial of intravenous soybean oil alone versus soybean oil plus fish oil emulsion after gastrointestinal cancer surgery. Br J Surg. 2010;97(6):804-809. [DOI] [PubMed] [Google Scholar]
- 110. Kiek S, Kulig J, Szczepanik AM, Jedrys J, Kotodziejczyk P.. The clinical value of parenteral immunonutrition in surgical patients. Acta Chir Belg. 2005;105(2):175-179. [PubMed] [Google Scholar]
- 111. Lobo DN, Williams RN, Welch NT, et al. Early postoperative jejunostomy feeding with an immune modulating diet in patients undergoing resectional surgery for upper gastrointestinal cancer: a prospective, randomized, controlled, double-blind study. Clin Nutr 2006;25(5):716-726. [DOI] [PubMed] [Google Scholar]
- 112. Adiamah A, Rollins KE, Kapeleris A, et al. Postoperative arginine-enriched immune modulating nutrition: long-term survival results from a randomised clinical trial in patients with oesophagogastric and pancreaticobiliary cancer. Clin Nutr. 2021;40(11):5482-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lu CY, Shih YL, Sun LC, et al. The inflammatory modulation effect of glutamine-enriched total parenteral nutrition in postoperative gastrointestinal cancer patients. Am Surg. 2011;77(1):59-64. [PubMed] [Google Scholar]
- 114. Matsuda Y, Habu D, Lee S, Kishida S, Osugi H.. Enteral diet enriched with omega-3 fatty acid improves oxygenation after thoracic esophagectomy for cancer: a randomized controlled trial. World J Surg. 2017;41(6):1584-1594. [DOI] [PubMed] [Google Scholar]
- 115. Miyata H, Yano M, Yasuda T, et al. Randomized study of the clinical effects of omega-3 fatty acid-containing enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Nutrition. 2017;33:204-210. [DOI] [PubMed] [Google Scholar]
- 116. Pathak S, Soni TP, Sharma LM, Patni N, Gupta AK.. A randomized controlled trial to evaluate the role and efficacy of oral glutamine in the treatment of chemo-radiotherapy-induced oral mucositis and dysphagia in patients with oropharynx and larynx carcinoma. Cureus. 2019;11(6):e4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pottel L, Lycke M, Boterberg T, et al. Echium oil is not protective against weight loss in head and neck cancer patients undergoing curative radio(chemo)therapy: a randomised-controlled trial. BMC Complement Altern Med. 2014;14:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sun LC, Shih YL, Lu CY, et al. Randomized, controlled study of branched chain amino acid-enriched total parenteral nutrition in malnourished patients with gastrointestinal cancer undergoing surgery. Am Surg. 2008;74(3):237-242. [PubMed] [Google Scholar]
- 119. Takeshita S, Ichikawa T, Nakao K, et al. A snack enriched with oral branched-chain amino acids prevents a fall in albumin in patients with liver cirrhosis undergoing chemoembolization for hepatocellular carcinoma. Nutr Res. 2009;29(2):89-93. [DOI] [PubMed] [Google Scholar]
- 120. Tanca FM, Madeddu C, Macciò A, et al. New perspective on the nutritional approach to cancer-related anorexia/cachexia: preliminary results of a randomised phase III clinical trial with five different arms of treatment. Med J Nutrition Metab. 2009;2(1):29-36. [Google Scholar]
- 121. Tumas J, Tumiene B, Jurkeviciene J, Jasiunas E, Sileikis A.. Nutritional and immune impairments and their effects on outcomes in early pancreatic cancer patients undergoing pancreatoduodenectomy. Clin Nutr. 2020;39(11):3385-3394. [DOI] [PubMed] [Google Scholar]
- 122. Wang J, Li Y, Qi Y.. Effect of glutamine-enriched nutritional support on intestinal mucosal barrier function, MMP-2, MMP-9 and immune function in patients with advanced gastric cancer during perioperative chemotherapy. Oncol Lett. 2017;14(3):3606-3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang WP, Yan XL, Ni YF, et al. Effects of lipid emulsions in parenteral nutrition of esophageal cancer surgical patients receiving enteral nutrition: a comparative analysis. Nutrients. 2013;6(1):111-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang X, Pan L, Zhang P, et al. Enteral nutrition improves clinical outcome and shortens hospital stay after cancer surgery. J Invest Surg. 2010;23(6):309-313. [DOI] [PubMed] [Google Scholar]
- 125. Wu Z, Qin J, Pu L.. Omega-3 fatty acid improves the clinical outcome of hepatectomized patients with hepatitis B virus (HBV)-associated hepatocellular carcinoma. J Biomed Res. 2012;26(6):395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yang J, Zhang X, Li K, et al. Effects of EN combined with PN enriched with n-3 polyunsaturated fatty acids on immune related indicators and early rehabilitation of patients with gastric cancer: a randomized controlled trial. Clin Nutr. 2022;41(6):1163-1170. [DOI] [PubMed] [Google Scholar]
- 127. Yeh KY, Wang HM, Chang JW, et al. Omega-3 fatty acid-, micronutrient-, and probiotic-enriched nutrition helps body weight stabilization in head and neck cancer cachexia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(1):41-48. [DOI] [PubMed] [Google Scholar]
- 128. Zhang B, Wei G, Li R, et al. n-3 fatty acid-based parenteral nutrition improves postoperative recovery for cirrhotic patients with liver cancer: a randomized controlled clinical trial. Clin Nutr. 2017;36(5):1239-1244. [DOI] [PubMed] [Google Scholar]
- 129. Zhu MW, Tang DN, Hou J, et al. Impact of fish oil enriched total parenteral nutrition on elderly patients after colorectal cancer surgery. Chin Med J (Engl). 2012;125(2):178-181. [PubMed] [Google Scholar]
- 130. Gardner A, Mattiuzzi G, Faderl S, et al. Randomized comparison of cooked and noncooked diets in patients undergoing remission induction therapy for acute myeloid leukemia. J Clin Oncol. 2008;26(35):5684-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ingersoll GL, Wasilewski A, Haller M, et al. Effect of concord grape juice on chemotherapy-induced nausea and vomiting: results of a pilot study. Oncol Nurs Forum. 2010;37(2):213-221. [DOI] [PubMed] [Google Scholar]
- 132. Jatoi A, Qin R, Satele D, et al. “Enjoy glass of wine before eating:” a randomized trial to test the orexigenic effects of this advice in advanced cancer patients. Support Care Cancer. 2016;24(9):3739-3746. [DOI] [PubMed] [Google Scholar]
- 133. Khodabakhshi A, Akbari ME, Mirzaei HR, Mehrad-Majd H, Kalamian M, Davoodi SH.. Feasibility, safety, and beneficial effects of MCT-based ketogenic diet for breast cancer treatment: a randomized controlled trial study. Nutr Cancer. 2020;72(4):627-634. [DOI] [PubMed] [Google Scholar]
- 134. Khodabakhshi A, Seyfried TN, Kalamian M, Beheshti M, Davoodi SH.. Does a ketogenic diet have beneficial effects on quality of life, physical activity or biomarkers in patients with breast cancer: a randomized controlled clinical trial. Nutr J. 2020;19(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lugtenberg RT, de Groot S, Kaptein AA, et al. Dutch Breast Cancer Research Group (BOOG). Quality of life and illness perceptions in patients with breast cancer using a fasting mimicking diet as an adjunct to neoadjuvant chemotherapy in the phase 2 DIRECT (BOOG 2013-14) trial. Breast Cancer Res Treat. 2021;185(3):741-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Miyakawa A, Kodera S, Sakuma Y, et al. Effects of early initiation of solid versus liquid diet after endoscopic submucosal dissection on quality of life and postoperative outcomes: a prospective pilot randomized controlled trial. Digestion. 2019;100(3):160-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Voss M, Wagner M, von Mettenheim N, et al. ERGO2: a prospective, randomized trial of calorie-restricted ketogenic diet and fasting in addition to reirradiation for malignant glioma. Int J Radiat Oncol Biol Phys. 2020;108(4):987-995. [DOI] [PubMed] [Google Scholar]
- 138. Wedlake LJ, McGough C, Shaw C, et al. Clinical trial: efficacy of a low or modified fat diet for the prevention of gastrointestinal toxicity in patients receiving radiotherapy treatment for pelvic malignancies. J Hum Nutr Diet. 2012;25(3):247-259. [DOI] [PubMed] [Google Scholar]
- 139. Berkelmans GHK, Fransen LFC, Dolmans-Zwartjes ACP, et al. Direct oral feeding following minimally invasive esophagectomy (NUTRIENT II trial): an international, multicenter, open-label randomized controlled trial. Ann Surg. 2020;271(1):41-47. [DOI] [PubMed] [Google Scholar]
- 140. Boelens PG, Heesakkers FF, Luyer MD, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann Surg. 2014;259(4):649-655. [DOI] [PubMed] [Google Scholar]
- 141. Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L.. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001;358(9292):1487-1492. [DOI] [PubMed] [Google Scholar]
- 142. Braga M, Gianotti L, Gentilini O, Parisi V, Salis C, Di Carlo V.. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med. 2001;29(2):242-248. [DOI] [PubMed] [Google Scholar]
- 143. Dag A, Colak T, Turkmenoglu O, Gundogdu R, Aydin S.. A randomized controlled trial evaluating early versus traditional oral feeding after colorectal surgery. Clinics (Sao Paulo). 2011;66(12):2001-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Feo CV, Romanini B, Sortini D, et al. Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg. 2004;74(5):298-301. [DOI] [PubMed] [Google Scholar]
- 145. Gao L, Zhao Z, Zhang L, Shao G.. Effect of early oral feeding on gastrointestinal function recovery in postoperative gastric cancer patients: a prospective study. J Buon. 2019;24(1):194-200. [PubMed] [Google Scholar]
- 146. Huang D, Sun Z, Huang J, Shen Z.. Early enteral nutrition in combination with parenteral nutrition in elderly patients after surgery due to gastrointestinal cancer. Int J Clin Exp Med. 2015;8(8):13937-13945. [PMC free article] [PubMed] [Google Scholar]
- 147. Hyltander A, Bosaeus I, Svedlund J, et al. Supportive nutrition on recovery of metabolism, nutritional state, health-related quality of life, and exercise capacity after major surgery: a randomized study. Clin Gastroenterol Hepatol. 2005;3(5):466-474. [DOI] [PubMed] [Google Scholar]
- 148. Kita R, Miyata H, Sugimura K, et al. Clinical effect of enteral nutrition support during neoadjuvant chemotherapy on the preservation of skeletal muscle mass in patients with esophageal cancer. Clin Nutr. 2021;40(6):4380-4385. [DOI] [PubMed] [Google Scholar]
- 149. Kurbanalievich SD, Vladimirovich DV, Kabildina NA.. Nutritional support for patients with diseases of hepatopancreotoduodenal zone in the early after the operational period. Open Access Maced J Med Sci. 2020;8(B):769-774. [Google Scholar]
- 150. Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ.. The postoperative clinical outcomes and safety of early enteral nutrition in operated gastric cancer patients. J Buon. 2015;20(2):468-472. [Mismatch [PubMed] [Google Scholar]
- 151. Liu C, Du Z, Lou C, et al. Enteral nutrition is superior to total parenteral nutrition for pancreatic cancer patients who underwent pancreaticoduodenectomy. Asia Pac J Clin Nutr. 2011;20(2):154-160. No doi number available. [PubMed] [Google Scholar]
- 152. Luo Z, Wang J, Zhang Z, et al. Efficacy of early enteral immunonutrition on immune function and clinical outcome for postoperative patients with gastrointestinal cancer. JPEN J Parenter Enteral Nutr. 2018;42(4):758-765. doi: 10.1177/0148607117715439. Epub 2017 Dec 20. [DOI] [PubMed] [Google Scholar]
- 153. Ma BQ, Chen SY, Jiang ZB, et al. Effect of postoperative early enteral nutrition on clinical outcomes and immune function of cholangiocarcinoma patients with malignant obstructive jaundice. World J Gastroenterol. 2020;26(46):7405-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mahmoodzadeh H, Shoar S, Sirati F, Khorgami Z.. Early initiation of oral feeding following upper gastrointestinal tumor surgery: a randomized controlled trial. Surg Today. 2015;45(2):203-208. [DOI] [PubMed] [Google Scholar]
- 155. Minig L, Biffi R, Zanagnolo V, et al. Reduction of postoperative complication rate with the use of early oral feeding in gynecologic oncologic patients undergoing a major surgery: a randomized controlled trial. Ann Surg Oncol. 2009;16(11):3101-3110. [DOI] [PubMed] [Google Scholar]
- 156. Perinel J, Mariette C, Dousset B, et al. Early enteral versus total parenteral nutrition in patients undergoing pancreaticoduodenectomy: a randomized multicenter controlled trial (Nutri-DPC). Ann Surg. 2016;264(5):731-737. [DOI] [PubMed] [Google Scholar]
- 157. Roberts S, Miller J, Pineiro L, Jennings L.. Total parenteral nutrition vs oral diet in autologous hematopoietic cell transplant recipients. Bone Marrow Transplant. 2003;32(7):715-721. [DOI] [PubMed] [Google Scholar]
- 158. Ryu J, Nam BH, Jung YS.. Clinical outcomes comparing parenteral and nasogastric tube nutrition after laryngeal and pharyngeal cancer surgery. Dysphagia. 2009;24(4):378-386. [DOI] [PubMed] [Google Scholar]
- 159. Sadasivan A, Faizal B, Kumar M.. Nasogastric and percutaneous endoscopic gastrostomy tube use in advanced head and neck cancer patients: a comparative study. J Pain Palliat Care Pharmacother. 2012;26(3):226-232. [DOI] [PubMed] [Google Scholar]
- 160. Seven H, Calis AB, Turgut S.. A randomized controlled trial of early oral feeding in laryngectomized patients. Laryngoscope. 2003;113(6):1076-1079. [DOI] [PubMed] [Google Scholar]
- 161. Sousa AA, Porcaro-Salles JM, Soares JM, et al. Does early oral feeding increase the likelihood of salivary fistula after total laryngectomy? J Laryngol Otol. 2014;128(4):372-378. [DOI] [PubMed] [Google Scholar]
- 162. Sun HB, Li Y, Liu XB, et al. ; behalf of the AME Thoracic Surgery Collaborative Group. Early oral feeding following McKeown minimally invasive esophagectomy: an open-label, randomized, controlled, noninferiority trial. Ann Surg. 2018;267(3):435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Tao Z, Zhang Y, Zhu S, et al. A prospective randomized trial comparing jejunostomy and nasogastric feeding in minimally invasive McKeown esophagectomy. J Gastrointest Surg. 2020;24(10):2187-2196. [DOI] [PubMed] [Google Scholar]
- 164. van Barneveld KW, Smeets BJ, Heesakkers FF, et al. Beneficial effects of early enteral nutrition after major rectal surgery: a possible role for conditionally essential amino acids? Results of a randomized clinical trial. Crit Care Med. 2016;44(6):e353-361-e361. [DOI] [PubMed] [Google Scholar]
- 165. Wang Q, Yang KL, Guo BY, et al. Safety of early oral feeding after total laparoscopic radical gastrectomy for gastric cancer (SOFTLY-1): a single-center randomized controlled trial. Cancer Manag Res. 2019;11:4839-4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang J, Zhao J, Zhang Y, Liu C.. Early enteral nutrition and total parenteral nutrition on the nutritional status and blood glucose in patients with gastric cancer complicated with diabetes mellitus after radical gastrectomy. Exp Ther Med. 2018;16(1):321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Xiao-Bo Y, Qiang L, Xiong Q, et al. Efficacy of early postoperative enteral nutrition in supporting patients after esophagectomy. Minerva Chir. 2014;69(1):37-46. [PubMed] [Google Scholar]
- 168. Xu B, Ma J, Chen X, Zhang S.. The effect of early enteral nutrition on the postoperative immune function and inflammatory indexes in patients with digestive tract cancers. Int J Clin Exp Med. 2020;13(4):2541-2547. [Google Scholar]
- 169. Zhang J, Si X, Li W, Yang J, Cao Y.. Effect of peripherally inserted central catheter (PICC) parenteral nutrition on immune function and nutritional support after radical gastrectomy for gastric cancer. Pak J Pharm Sci. 2019;32(3 Special):1441-1445. [PubMed] [Google Scholar]
- 170. Akita H, Takahashi H, Asukai K, et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: prospective randomized control study. Clin Nutr ESPEN. 2019;33:148-153. [DOI] [PubMed] [Google Scholar]
- 171. Baker J, Janda M, Graves N, et al. Quality of life after early enteral feeding versus standard care for proven or suspected advanced epithelial ovarian cancer: results from a randomised trial. Gynecol Oncol. 2015;137(3):516-522. [DOI] [PubMed] [Google Scholar]
- 172. Barlow R, Price P, Reid TD, et al. Prospective multicentre randomised controlled trial of early enteral nutrition for patients undergoing major upper gastrointestinal surgical resection. Clin Nutr. 2011;30(5):560-566. [DOI] [PubMed] [Google Scholar]
- 173. Bouleuc C, Anota A, Cornet C, et al. Impact on health-related quality of life of parenteral nutrition for patients with advanced cancer cachexia: results from a randomized controlled trial. Oncologist. 2020;25(5):e843-e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Cereda E, Cappello S, Colombo S, et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother Oncol. 2018;126(1):81-88. [DOI] [PubMed] [Google Scholar]
- 175. Chen X, Zhao G, Zhu L.. Home enteral nutrition for postoperative elderly patients with esophageal cancer. Ann Palliat Med. 2021;10(1):278-284. [DOI] [PubMed] [Google Scholar]
- 176. Chen T, Jiang W, He G.. Effect of family enteral nutrition on nutritional status in elderly patients with esophageal carcinoma after minimally invasive radical surgery: a randomized trial. Ann Palliat Med. 2021;10(6):6760-6767. [DOI] [PubMed] [Google Scholar]
- 177. Chu L, Ren Y, Zhang L, Yu X.. Evaluation of effects of nutritional risk assessment and enteral and parenteral nutritional interventions after esophageal cancer surgery. Int J Clin Exp Med. 2018;11(5):5110-5116. [Google Scholar]
- 178. Deibert CM, Silva MV, RoyChoudhury A, et al. A prospective randomized trial of the effects of early enteral feeding after radical cystectomy. Urology. 2016;96:69-73. [DOI] [PubMed] [Google Scholar]
- 179. Faccio AA, Mattos C, Santos E, et al. Oral nutritional supplementation in cancer patients who were receiving chemo/chemoradiation therapy: a multicenter, randomized phase II study. Nutr Cancer. 2021;73(3):442-449. [DOI] [PubMed] [Google Scholar]
- 180. Gavazzi C, Colatruglio S, Valoriani F, et al. Impact of home enteral nutrition in malnourished patients with upper gastrointestinal cancer: a multicentre randomised clinical trial. Eur J Cancer. 2016;64:107-112. [DOI] [PubMed] [Google Scholar]
- 181. Huang S, Piao Y, Cao C, et al. A prospective randomized controlled trial on the value of prophylactic oral nutritional supplementation in locally advanced nasopharyngeal carcinoma patients receiving chemo-radiotherapy. Oral Oncol. 2020;111:105025. [DOI] [PubMed] [Google Scholar]
- 182. Imamura H, Nishikawa K, Kishi K, et al. Effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients: a randomized controlled clinical trial. Ann Surg Oncol. 2016;23(9):2928-2935. [DOI] [PubMed] [Google Scholar]
- 183. Kimura Y, Nishikawa K, Kishi K, et al. Long-term effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients (KSES002). Ann Gastroenterol Surg. 2019;3(6):648-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Jiang W, Ding H, Li W, Ling Y, Hu C, Shen C.. Benefits of oral nutritional supplements in patients with locally advanced nasopharyngeal cancer during concurrent chemoradiotherapy: an exploratory prospective randomized trial. Nutr Cancer. 2018;70(8):1299-1307. [DOI] [PubMed] [Google Scholar]
- 185. Jin Y, Yong C, Ren K, Li D, Yuan H.. Effects of post-surgical parenteral nutrition on patients with gastric cancer. Cell Physiol Biochem. 2018;49(4):1320-1328. [DOI] [PubMed] [Google Scholar]
- 186. Kanat O, Cubukcu E, Avci N, et al. Comparison of three different treatment modalities in the management of cancer cachexia. Tumori. 2013;99(2):229-233. [DOI] [PubMed] [Google Scholar]
- 187. Katada C, Fukazawa S, Sugawara M, et al. Randomized study of prevention of gastrointestinal toxicities by nutritional support using an amino acid-rich elemental diet during chemotherapy in patients with esophageal cancer (KDOG 1101). Esophagus. 2021;18(2):296-305. [DOI] [PubMed] [Google Scholar]
- 188. Klek S, Kulig J, Sierzega M, et al. Standard and immunomodulating enteral nutrition in patients after extended gastrointestinal surgery--a prospective, randomized, controlled clinical trial. Clin Nutr. 2008;27(4):504-512. [DOI] [PubMed] [Google Scholar]
- 189. Klek S, Sierzega M, Szybinski P, et al. The immunomodulating enteral nutrition in malnourished surgical patients - a prospective, randomized, double-blind clinical trial. Clin Nutr. 2011;30(3):282-288. [DOI] [PubMed] [Google Scholar]
- 190. Klek S, Scislo L, Walewska E, Choruz R, Galas A.. Enriched enteral nutrition may improve short-term survival in stage IV gastric cancer patients: a randomized, controlled trial. Nutrition. 2017;36:46-53. [DOI] [PubMed] [Google Scholar]
- 191. Klek S, Sierzega M, Szybinski P, et al. Perioperative nutrition in malnourished surgical cancer patients - a prospective, randomized, controlled clinical trial. Clin Nutr. 2011;30(6):708-713. [DOI] [PubMed] [Google Scholar]
- 192. Klek S, Szybinski P, Szczepanek K.. Perioperative immunonutrition in surgical cancer patients: a summary of a decade of research. World J Surg. 2014;38(4):803-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ.. Impact of early postoperative enteral nutrition on clinical outcomes in patients with gastric cancer. Genet Mol Res. 2015;14(2):7136-7141. [DOI] [PubMed] [Google Scholar]
- 194. Li C, Ni L, Liu C.. Early enteral immunonutrition support protects the cellular and humoral immune functions of patients with pancreatic cancer after chemotherapy. Int J Clin Exp Med. 2020;13(2):700-708. [Google Scholar]
- 195. Lyu J, Shi A, Li T, et al. Effects of enteral nutrition on patients with oesophageal carcinoma treated with concurrent chemoradiotherapy: a prospective, multicentre, randomised, controlled Study . Front Oncol. 2022;12:839516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. McGough C, Wedlake L, Baldwin C, et al. Clinical trial: Normal diet vs. partial replacement with oral E028 formula for the prevention of gastrointestinal toxicity in cancer patients undergoing pelvic radiotherapy. Aliment Pharmacol Ther. 2008;27(11):1132-1139. [DOI] [PubMed] [Google Scholar]
- 197. Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin Nutr. 2021;40(1):40-46. [DOI] [PubMed] [Google Scholar]
- 198. Tan S, Meng Q, Jiang Y, et al. Impact of oral nutritional supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: a randomised clinical trial. Clin Nutr. 2021;40(1):47-53. [DOI] [PubMed] [Google Scholar]
- 199. Miyazaki Y, Omori T, Fujitani K, et al. ; Osaka University Clinical Research Group for Gastroenterological Study. Oral nutritional supplements versus a regular diet alone for body weight loss after gastrectomy: a phase 3, multicenter, open-label randomized controlled trial. Gastric Cancer. 2021;24(5):1150-1159. [DOI] [PubMed] [Google Scholar]
- 200. Nie J, Su X, Wei L, Li H.. Early enteral nutrition support for colon carcinoma patients can improve immune function and promote physical recovery. Am J Transl Res. 2021;13(12):14102-14108. eCollection 2021. No doi number available. [PMC free article] [PubMed] [Google Scholar]
- 201. Ohkura Y, Ueno M, Shindoh J, Iizuka T, Udagawa H.. Randomized controlled trial on efficacy of oligomeric formula (HINE E-GEL(R)) versus polymeric formula (MEIN(R)) enteral nutrition after esophagectomy for esophageal cancer with gastric tube reconstruction. Dis Esophagus. 2019;32(5):doy084. doi: 10.1093/dote/doy084. [DOI] [PubMed] [Google Scholar]
- 202. Okabayashi T, Iyoki M, Sugimoto T, Kobayashi M, Hanazaki K.. Oral supplementation with carbohydrate- and branched-chain amino acid-enriched nutrients improves postoperative quality of life in patients undergoing hepatic resection. Amino Acids. 2011;40(4):1213-1220. [DOI] [PubMed] [Google Scholar]
- 203. Ravasco P, Monteiro-Grillo I, Marques Vidal P, Camilo ME.. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck. 2005;27(8):659-668. [DOI] [PubMed] [Google Scholar]
- 204. Ravasco P, Monteiro-Grillo I, Vidal PM, Camilo ME.. Dietary counseling improves patient outcomes: a prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J Clin Oncol. 2005;23(7):1431-1438. [DOI] [PubMed] [Google Scholar]
- 205. Sanchez-Lara K, Turcott JG, Juarez-Hernandez E, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr. 2014;33(6):1017-1023. [DOI] [PubMed] [Google Scholar]
- 206. Scislo L, Pach R, Nowak A, et al. The impact of postoperative enteral immunonutrition on postoperative complications and survival in gastric cancer patients - randomized clinical trial. Nutr Cancer. 2018;70(3):453-459. [DOI] [PubMed] [Google Scholar]
- 207. Shimizu N, Oki E, Tanizawa Y, et al. Effect of early oral feeding on length of hospital stay following gastrectomy for gastric cancer: a Japanese multicenter, randomized controlled trial. Surg Today. 2018;48(9):865-874. [DOI] [PubMed] [Google Scholar]
- 208. Sim E, Kim JM, Lee SM, et al. The effect of omega-3 enriched oral nutrition supplement on nutritional indices and quality of life in gastrointestinal cancer patients: a randomized clinical trial. Asian Pac J Cancer Prev. 2022;23(2):485-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Vidal A, Arnold N, Vartolomei MD, et al. Oncological and functional outcomes of postoperative total parenteral nutrition after radical cystectomy in bladder cancer patients: a single-center randomized trial. Int J Urol. 2016;23(12):992-999. [DOI] [PubMed] [Google Scholar]
- 210. Wang J, Wang L, Zhao M, et al. Effect of early enteral nutrition support combined with chemotherapy on related complications and immune function of patients after radical gastrectomy. J Healthc Eng. 2022;2022:1531738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Wu W, Zhong M, Zhu DM, et al. Effect of early full-calorie nutrition support following esophagectomy: a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2017;41(7):1146-1154. [DOI] [PubMed] [Google Scholar]
- 212. Xie H, Chen X, Xu L, et al. A randomized controlled trial of oral nutritional supplementation versus standard diet following McKeown minimally invasive esophagectomy in patients with esophageal malignancy: a pilot study. Ann Transl Med. 2021;9(22):1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Yang L, Gao J, Zhou Y, et al. Effect of oral nutritional supplements on patients with esophageal cancer during radiotherapy. Cancer Biother Radiopharm. 2023;38(2):89-94. [DOI] [PubMed] [Google Scholar]
- 214. Yao R, Zhang T, Zhang J, et al. Effects of postoperative enteral nutrition combined with adjuvant radiotherapy on inflammatory response, nutrition, healing and prognosis in patients receiving radical surgery for esophageal carcinoma. J Buon. 2019;24(4):1673-1678. [PubMed] [Google Scholar]
- 215. Zhang Y, Liu L, Li D, Zhou D.. Effectiveness of noninvasive positive pressure ventilation combined with enteral nutrition in the treatment of patients with combined respiratory failure after lung cancer surgery and its effect on blood gas indexes. Emerg Med Int. 2022;2022:1508082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Zhao M, Li XG, Ma YY, et al. Application of enteral nutrition during perichemotherapy of acute non-lymphocytic leukemia. J Chem Pharm Res. 2014;6(6):768-771. ISSN: 0975-7384. CODEN(USA): JCPRC5. [Google Scholar]
- 217. Zhu MW, Yang X, Xiu DR, et al. Effect of oral nutritional supplementation on the post-discharge nutritional status and quality of life of gastrointestinal cancer patients after surgery: a multi-center study. Asia Pac J Clin Nutr. 2019;28(3):450-456. doi: 10.6133/apjcn.201909_28(3).0004. [DOI] [PubMed] [Google Scholar]
- 218. Ziętarska M, Krawczyk-Lipiec J, Kraj L, Zaucha R, Małgorzewicz S.. Chemotherapy-related toxicity, nutritional status and quality of life in precachectic oncologic patients with, or without, high protein nutritional support. A prospective, randomized study. Nutrients. 2017;9(10):1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Abdollahi R, Najafi S, Razmpoosh E, et al. The effect of dietary intervention along with nutritional education on reducing the gastrointestinal side effects caused by chemotherapy among women with breast cancer. Nutr Cancer 2019;71(6):922-930. [DOI] [PubMed] [Google Scholar]
- 220. Demark-Wahnefried W, Case LD, Blackwell K, et al. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin Breast Cancer. 2008;8(1):70-79. [DOI] [PubMed] [Google Scholar]
- 221. Lin JX, Chen XW, Chen ZH, et al. A multidisciplinary team approach for nutritional interventions conducted by specialist nurses in patients with advanced colorectal cancer undergoing chemotherapy: a clinical trial. Medicine (Baltimore). 2017;96(26):e7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Poulsen GM, Pedersen LL, Osterlind K, Baeksgaard L, Andersen JR.. Randomized trial of the effects of individual nutritional counseling in cancer patients. Clin Nutr. 2014;33(5):749-753. [DOI] [PubMed] [Google Scholar]
- 223. Qin N, Jiang G, Zhang X, Sun D, Liu M.. The effect of nutrition intervention with oral nutritional supplements on ovarian cancer patients undergoing chemotherapy. Front Nutr. 2021;8:685967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Silander E, Nyman J, Bove M, Johansson L, Larsson S, Hammerlid E.. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: a randomized study. Head Neck. 2012;34(1):1-9. [DOI] [PubMed] [Google Scholar]
- 225. Silander E, Jacobsson I, Berteus-Forslund H, Hammerlid E.. Energy intake and sources of nutritional support in patients with head and neck cancer--a randomised longitudinal study. Eur J Clin Nutr. 2013;67(1):47-52. [DOI] [PubMed] [Google Scholar]
- 226. Skaarud KJ, Veierød MB, Lergenmuller S, Bye A, Iversen PO, Tjønnfjord GE.. Body weight, body composition and survival after 1 year: follow-up of a nutritional intervention trial in allo-HSCT recipients. Bone Marrow Transplant. 2019;54(12):2102-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Song G, Liu H.. Effect of hospital to home nutrition management model on postoperative clinical outcomes of patients with laryngeal carcinoma. Oncol Lett. 2017;14(4):4059-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Uster A, Ruefenacht U, Ruehlin M, et al. Influence of a nutritional intervention on dietary intake and quality of life in cancer patients: a randomized controlled trial. Nutrition 2013;29(11-12):1342-1349. [DOI] [PubMed] [Google Scholar]
- 229. Xie FL, Wang YQ, Peng LF, Lin FY, He YL, Jiang ZQ.. Beneficial effect of educational and nutritional intervention on the nutritional status and compliance of gastric cancer patients undergoing chemotherapy: a randomized trial. Nutr Cancer. 2017;69(5):762-771. [DOI] [PubMed] [Google Scholar]
- 230. Najafi S, Haghighat S, Raji Lahiji M, et al. Randomized study of the effect of dietary counseling during adjuvant chemotherapy on chemotherapy induced nausea and vomiting, and quality of life in patients with breast cancer. Nutr Cancer. 2019;71(4):575-584. [DOI] [PubMed] [Google Scholar]
- 231. Ansari M, Porouhan P, Mohammadianpanah M, et al. Efficacy of ginger in control of chemotherapy induced nausea and vomiting in breast cancer patients receiving doxorubicin-based chemotherapy. Asian Pac J Cancer Prev. 2016;17(8):3877-3880. [PubMed] [Google Scholar]
- 232. Vafa S, Zarrati M, Malakootinejad M, et al. Calorie restriction and synbiotics effect on quality of life and edema reduction in breast cancer-related lymphedema, a clinical trial. Breast. 2020;54:37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Pettersson A, Nygren P, Persson C, Berglund A, Turesson I, Johansson B.. Effects of a dietary intervention on gastrointestinal symptoms after prostate cancer radiotherapy: long-term results from a randomized controlled trial. Radiother Oncol. 2014;113(2):240-247. [DOI] [PubMed] [Google Scholar]
- 234. Braga M, Gianotti L.. Preoperative immunonutrition: cost-benefit analysis. JPEN J Parenter Enteral Nutr. 2005;29(suppl 1):S57-S61. [DOI] [PubMed] [Google Scholar]
- 235. Martin B, Cereda E, Caccialanza R, Pedrazzoli P, Tarricone R, Ciani O.. Cost-effectiveness analysis of oral nutritional supplements with nutritional counselling in head and neck cancer patients undergoing radiotherapy. Cost Eff Resour Alloc. 2021;19(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Webb N, Fricke J, Hancock E, et al. The clinical and cost-effectiveness of supplemental parenteral nutrition in oncology. ESMO Open. 2020;5(3):e000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. DeSantis CE, Miller KD, Dale W, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin. 2019;69(6):452-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726-732. [DOI] [PubMed] [Google Scholar]
- 239. National Institutes of Health Office of Nutrition Research. 2020-2030 Strategic plan for NIH nutrition research. National Institutes of Health Office of Nutrition Research. Published 2022. https://dpcpsi.nih.gov/onr/strategic-plan. Accessed April 25, 2022.
- 240. Grimble RF. Basics in clinical nutrition: immunonutrition–nutrients which influence immunity: effect and mechanism of action. e-SPEN Eur e-J Clin Nutr Metabol. 2009;4(1):e10-e13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.