Abstract
In chronic infections and cancers, T lymphocytes (T cells) are exposed to persistent antigen or inflammatory signals. The condition is often associated with a decline in T-cell function: a state called “exhaustion”. T cell exhaustion is a state of T cell dysfunction characterized by increased expression of a series of inhibitory receptors (IRs), decreased effector function, and decreased cytokine secretion, accompanied by transcriptional and epigenetic changes and metabolic defects. The rise of immunotherapy, particularly the use of immune checkpoint inhibitors (ICIs), has dramatically changed the clinical treatment paradigm for patients. However, its low response rate, single target and high immunotoxicity limit its clinical application. The multiple immunomodulatory potential of traditional Chinese medicine (TCM) provides a new direction for improving the treatment of T cell exhaustion. Here, we review recent advances that have provided a clearer molecular understanding of T cell exhaustion, revealing the characteristics and causes of T cell exhaustion in persistent infections and cancers. In addition, this paper summarizes recent advances in improving T cell exhaustion in infectious diseases and cancer with the aim of providing a comprehensive and valuable source of information on TCM as an experimental study and their role in collaboration with ICIs therapy.
Keywords: T cell exhaustion, Traditional Chinese medicine, Infection, Cancer, Immune checkpoint inhibitors
Introduction
T cell exhaustion is a state of T cell dysfunction, which is mainly characterized by increased expression of a series of inhibitory receptors (IRs), low effector function and reduced cytokine secretion [1, 2]. Moreover, it also accompanied by changes in gene expression related to T cell chemotaxis, adhesion and migration, changes in transcription factor expression profile and metabolic function defects [3]. T cell exhaustion was first observed in mice with chronic viral infection and had subsequently been found in many animal models or patients with chronic viral and parasitic infections and tumors [4].
In the process of acute infection, when antigen is cleared or inflammation subside, effector CD8+ T lymphocytes (T cells) further differentiate into functional memory CD8+T cells, which produce interferon⁃γ (IFN⁃γ), tumor necrosis factor (TNF), interleukin-2 (IL-2), etc. When secondary infection occurs, effector CD8+ T cells can produce strong memory response quickly and efficiently, and carry out immune defense. These memory T cells can also be renewed by IL⁃7 and IL⁃15 driven homeostast to maintain their active state for a long time [5].
In chronic infection, such as hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), Corona Virus Disease 2019 (COVID-19) [6] and mycobacterium tuberculosis (MTB) [7, 8], and cancer [9–14], due to chronic antigenic stimulation T cells enter a “exhausted” state. Exhausted T cells lost complete effector function and were highly expressed in a variety of IRs, including programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), T cell immunoglobulin domain and mucin domain protein 3 (TIM-3) and lymphocyte activation gene 3 protein (LAG-3) [15, 16]. Exhausted T cells had unique differentiation, phenotype, and function, and stable epigenetic inheritance [17]. In the early stages of infection, virus-specific T cells initially acquire effector function, but gradually deplete and gradually lose their effector function due to persistent viral antigen and inflammatory or infective stimulation [2] (Generally, T cells first lose their ability to proliferate and produce IL-2. In the intermediate stages of cell failure, other T cells properties are lost, including the ability to produce tumor necrosis factor. When the cell is severely exhausted, T cells no longer produce large amounts of IFN-γ or β-chemokines or degranulation. When T cells are completely exhausted, they become exhausted T cells and their effector function is completely lost [18]). However, exhausted T cells are not completely unresponsive but retain some effector functions, allowing the host to control the pathogen without deleterious immunopathology. It turns out that exhausted T cells lead to lethal infections [19].
Reversing T cell exhaustion is critical for inhibiting tumor growth. Exhausted T cells expressed immunosuppressive receptors. Immune checkpoint inhibitors (ICIs) or combination of inhibitor receptors could block these signals, enhancing the effect of immunotherapy and reversing T cell exhaustion to a certain extent [20]. However, ICIs were only effective in a small number of patients, and even when ICIs work in patients, further T cell exhaustion occurred later in immunotherapy, or severe immune-related adverse events, leading to treatment failure [21–24]. In addition, in some cases, clinical efficacy might fall short of expectations due to the complex regulatory mechanisms of PD-1/PD-L1 or CTLA-4 in cancer immunity. These studies suggested that understanding the characteristics and causes of T cell failure and developing more effective, safer and cost-effective drugs are of great significance to ameliorate T cell exhaustion [25].
Due to its characteristics of multiple targets and multiple components, the application of traditional Chinese medicine (TCM) to improve human immunity has been widely used in clinical practice, especially in improving the immune function of tumor patients, with remarkable efficacy. Modern studies proved that TCM compounds and active ingredients improved T cell exhaustion to some extent by affecting T cells subsets in virus or tumor patients [26]. In this paper, we review the characteristics and causes of T cell exhaustion, and TCM targeting T cell exhaustion, providing a systematically reviewed the experimental progress of TCM regulating T cell exhaustion in infectious diseases and cancer in recent years. Although the current studies are mostly limited to animal and cell experiments, this review provides a comprehensive and valuable source of information for the development of subsequent experimental studies and clinical applications needed to further determine the role of TCM in improving the treatment of T cell exhaustion in infectious diseases and cancer.
Characteristics of T cell exhaustion
T cell exhaustion is manifested by progressive loss of effector function. Exhausted T cells in infections and cancers often express high levels of IRs, including PD-1, CTLA-4, LAG-3, TIM-3, etc. These IRs are also known as immune checkpoints. Exhausted T cells can co-express multiple IRs. Thus, IRs can be used as a characteristic of T cell exhaustion. Recent studies have revealed the epigenetic and transcriptomic landscapes of T cell exhaustion, identifying several key depletion related molecules such as DNA methyltransferase 3 alpha (DNMT3A), thymocyte selection-associated high mobility group box (TOX), NR4a and Eomesodermin (Eomes). Disorders of depleted T cell metabolism have also been reported. These studies reveal patterns and mechanisms of T cell exhaustion, which may provide promising strategies for restoring T cell function. Strategies to reverse T cell exhaustion are as follows (Table 1).
Table 1.
Strategies to reverse T cell exhaustion
| Disease types | Intervention methods | Target | References |
|---|---|---|---|
| HIV | Anti-PD-L1 | PD-1 | [30] |
| HBV | Anti-PD-L1 | PD-1 | [31] |
| LCMV | Anti-PD-L1, anti-LAG-3 | PD-1, LAG-3 | [32, 33] |
| HIV | Knock-down of PD-L1 and PD-L2 | PD-L1, PD-L2 | [34] |
| HIV | Anti-CTLA-4 | CTLA-4 | [36] |
| HBV | Anti-CTLA-4 | CTLA-4 | [37] |
| Bladder cancer | Anti-CTLA-4 | CTLA-4 | [38] |
| LCMV | Anti-TIM-3 | TIM-3 | [39] |
| HCV | Anti-TIM-3 | TIM-3 | [42] |
| HIV | Anti-TIM-3 | TIM-3 | [43] |
| HCC | Anti-TIM-3 | TIM-3 | [45] |
| LCMV | Anti-LAG-3 | LAG-3 | [46] |
| RCC | Knock out of VISTA | VISTA | [49, 50] |
| HCV | Anti-PD-L1, anti-CTLA-4 | PD-1, CTLA-4 | [51] |
| HBV | Anti-PD-L1, anti-CTLA-4 | PD-1, CTLA-4 | [52] |
| LCMV | Anti-PD-L1, anti-TIM-3 | PD-1, TIM-3 | [53] |
| GC | Anti-PD-1, anti-PD-L1, anti-LAG-3, anti-TIM-3 | PD-1, TIM-3, LAG-3 | [54] |
| Osteosarcoma | Knockout of DNMT3A | DNMT3A | [56, 57] |
| LCMV | Knockout of TOX | TOX | [58] |
| Melanoma | Knockout of TOX | TOX | [59] |
| LCMV | Knockout of NR4A1 | NR4A1 | [61, 62] |
| LCMV | Knockout of Eomes | Eomes | [66] |
| LCMV | Knockout of T-bet | T-bet | [67] |
| CLL | BTK inhibitor | Glycolysis | [72] |
| LCMV | Anti-PD-L1 | Glycolysis | [73] |
| Sarcoma | Anti-PD-1, anti-PD-L1, anti-CTLA-4 | Glycolysis | [75] |
| NSCLC | Anti-PD-1 | Glucose, lipid and mitochondrial metabolism | [76] |
| Melanoma | Knock down and overexpress of XBP1s | Cholesterol | [77, 78] |
| Lymphoma | Glutamine metabolism inhibitors | Glutamine metabolism | [79] |
| Breast cancer, cervical cancer | D-1MT | Tryptophan catabolism | [81] |
| HBV | Anti-CD3, and anti-CD28 | Arginine | [82] |
| HIV | IL-15 | Glycolysis | [84] |
| HCV | Histone methyltransferase inhibitors | Glycolytic and mitochondrial functions | [85] |
| Colon cancer, lymphoma, melanoma | DON, JHU083 | Glutamine | [87] |
| HBV | Mitochondrion-targeted antioxidants | Mitochondria | [86] |
| ccRCC | Metformin | Mitochondrial ROS | [89] |
| Melanoma | Axitinib, anti-PD-1, anti-CTLA-4 | Mitochondrial ROS | [91] |
| Melanoma, lung cancer, colorectal cancer | Overexpress of PGC1α | Mitochondrial metabolism | [92] |
| LCMV, melanoma | Knock out of VHL | HIF-1α, HIF-2α | [94] |
| Breast cancer | Knock out of HIF-1α | HIF-1α, VEGF-A | [95] |
RCC renal cell carcinoma, GC gastric cancer, CLL chronic lymphocytic leukemia, BTK Bruton’s tyrosine kinase, d-1MT 1-methyl-d-tryptophan, NSCLC non-small-cell lung cancer, IL-15 interleukin-15, DON 6-diazo-5-oxo-l-norleucine, ccRCC clear cell renal cell carcinoma
Dysfunction and loss of function
In terms of effector function, the exhausted T cells show progressive dysfunction and even loss of function. T cells usually lose their ability to proliferate and kill first. During the intermediate stages of dysfunction, T cells lose other functions, such as the ability to produce TNF. Severe exhaustion of T cells eventually results in a partial or complete lack of ability to produce IFN⁃γ, β-chemokines or degranulation [27]. The function of the exhausted T cells is completely lost, ultimately resulting in the inability to eliminate the virus or tumor cells.
The expression of inhibitory receptors
PD-1
Persistent high expression of inhibitory receptors and co-expression of multiple inhibitory receptors are important features of the immune response of exhausted T cells in chronic infection or cancer. PD-1 is one of the most widely studied inhibitory receptors in chronic viral infections and cancer. Its two known ligands are PD-L1 (B7-H1) and PD-L2 (B7-DC). PD-1 is mainly expressed on T cells, B cells, natural killer cell (NK), dendritic cell (DC) and macrophages, while PD-L1 is expressed on many cells, including tumor cells, B cells, T cells and macrophages, etc. The combination of PD-1 and PD-L1 can lead to T cell dysfunction. PD-1/PD-L1 immune checkpoints function in peripheral tissues and act as negative regulators of T cells to help control local inflammatory responses and maintain auto tolerance [28]. PD-1 on the surface of T cells is coupled with PD-L1 on the surface of tumor cells, resulting in Tyr phosphorylation in the ITSM domain in the cytoplasmic region of T cells. The phosphorylated Tyr can recruit SHP-1 and SHP-2 (Src homology region 1, 2, Src homology domains 1 and 2), SHP-2 dephosphorylates TCR-associated CD3ζ and zeta chain associated protein kinase 70 (ZAP70), inhibits downstream signaling and interferes with IL-2 secretion. The activation of immune cells by TCR/CD28 signals is weakened, inhibiting T cell proliferation and cytokine secretion [29]. Inhibition of PD-1 induced HIV or HBV-specific CD8 T cells to produce cytokines, such as IFN-γ, enhancing the immune response [30, 31]. A comparison of the gene expression profiles of virus-specific CD8 T cells from acute or chronic lymphocytic choriomeningitis virus (LCMV)-infected mice showed that the inhibitory receptor PD-1 was highly expressed in chronic infection [32], and was associated with an exhausted CD8 T cells phenotype [33]. In HIV infection, by blocking PD-L1, T cells regained their ability to secrete cytokines IFN-γ, IL-2, and IL-12 after restimulation [34]. In addition, blocking PD-1/PD-L1 partially improved the effector function of exhausted T cells in vivo [5].
CTLA-4
In addition to PD-1, exhausted T cells expressed a lot of inhibitory receptors, including CTLA-4, TIM-3, LAG-3, and VISTA. Although individual expression of PD-1 or its inhibitory receptors was not sufficient to indicate T cell exhaustion, co-expression of multiple inhibitory receptors was thought to be a major feature of T cell exhaustion [35]. CTLA-4 was found to be associated with T cell exhaustion. Studies indicated that high expression of CTLA-4 affected the quality of T cell response function after infection with HIV [36] and HBV [37]. The high expression of CTLA-4 also weakened T cell function in cancer [38].
TIM-3
In addition, high expression of TIM-3 on effector T cells also indicated severe T cells failure or dysfunction [39–41]. TIM-3 frequency and expression levels were positively correlated with T cell exhaustion status and infection severity in HIV, HCV, and LCMV infections [39, 42, 43]. High expression of TIM-3 also led to T-cell exhaustion [44]. Moreover, TIM-3 was significantly up-regulated in NK in HCC, and inhibited its cytokine production and cytotoxic activity by inhibiting phosphatidylinositol-3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR)1 signaling pathway, leading to dysfunction of NK cells in hepatocellular carcinoma (HCC) [45].
LAG-3
In addition to these IRs, a growing number of studies have linked the expression of inhibitory receptors such as LAG-3 and VISTA to T cell exhaustion in chronic infections and some cancers. The expression of LAG-3 was positively correlated with T cell exhaustion status and infection severity in LCMV infections [46]. A study showed that LAG-3 activation enhanced intra-tumoral Tregs activity, blocking improved T cells function and reactivating tumor-infiltrating CD8+ T cells (CD8+TIL) to eliminate tumor cells [47].
VISTA
As a ligand of VISTA, V-Set and immunoglobulin domain containing 3 (VSIG-3) blocked the production of cytokines and chemokines [48]. The expression of VISTA or VSIG-3 was elevated in many cancers. Studies found that VISTA was a regulatory immune checkpoint. The blockade of VISTA/VSIG-3 could be used as a new target for immune checkpoint therapy. Spontaneous T cell activation occurred in VISTA-deficient mice [49]. Moreover, VISTA was associated with T cell exhaustion marker TOX, and VISTA positive was associated with a poorer prognosis in clear cell renal cell carcinoma (ccRCC) [50].
In addition, exhausted CD8+T cells expressed multiple IRs. The co-expression pattern of IRs affected the function of T cells during chronic infection, and the severity of chronic infection was related to the number and intensity of IRs expressed. In general, the more IRs exhausted T cells co-expresses, the more severe its exhaustion state [33]. Studies showed that the effector function of patient-specific CD8 T cells was restored by co-inhibition of PD-1 and CTLA-4 expression in chronic HCV [51] or HBV infection [52]. Studies showed that CD8+T cells that co-expression of TIM-3 and PD-1(TIM-3+PD-1+) had more severe exhaustion than those that only express PD-1(TIM-3−PD-1+). Compared with blocking either pathway alone, the blocking of TIM-3 and PD-1 pathways at the same time had a synergistic effect in restoring the antiviral immunity of T cells [53] and tumor inhibition [54]. Similarly, studies found that blocking LAG-3 alone had a minimal effect on the reversal of CD8+T cell exhaustion, but the combined blocking of LAG-3 and PD-1 collaboratively improved CD8+T cell response and significantly reduced viral load in vivo [33]. These studies suggested that co-expression of multiple IRs might play a synergistic role in T cell exhaustion.
Changes in the epigenetic and transcriptional landscape
T cell exhaustion results in extensive changes in transcription profile and chromatin. After chronic LCMV infection, the accessibility of T cell chromatin in exhausted T cells showed that exhausted T cells exhibited different chromatin phenotypes from effector T cells and memory T cells. These findings suggested that exhaustion was a specific T cell fate associated with distinct changes in chromatin phenotype [55]. DNA methylation induced by DNMT3A has been shown to play a key role in chromatin accessibility and T-cell exhaustion, and the tumor killing effect of chimeric antigen receptor T-cell immunotherapy (CAR-T) after DNMT3A knockout is significantly enhanced [56, 57]. Furthermore, studies showed that DNA-binding protein TOX affected the survival and development of T cell exhaustion. It was involved in epigenetic remodeling during T cell exhaustion in chronic viral infections and cancer [58, 59]. Moreover, TOX deletion of virus-specific CD8+ T cells did not prevent the formation of effector T cells and memory T cells during acute LCMV infection. However, in the case of chronic LCMV infections and cancer, TOX deficiencies had improved antiviral and anti-tumor activity, and were associated with decreased expression of CD8+ T cells suppressor receptors. However, CD8+ T cells with TOX deficiency did not persist during chronic viral infection and cancer development. This suggested that TOX mediated signaling and epigenetic programming sustained the survival of exhausted T cells in the presence of prolonged exposure to antigens [60].
In addition to DNMT3A and TOX, nuclear receptor transcription factor NR4a drove T cell exhaustion by coordinating epigenetic and transcriptomic changes associated with exhaustion in CD8+ TILs and CAR-T cells. In adoptive cell transfer of tumor-specific CD8+ T cells and CAR-T cells, the absence of NR4a increased the antitumor activity of T cells [61, 62]. Moreover, in CD8+ TILs, interactions between TOX and NR4a might further enhance phenotypic and epigenetic expression of exhausted T cells.
Furthermore, studies showed that Eomes and T-bet were transcription factors that regulated T cells differentiation [63, 64]. And up-regulation of Eomes was necessary for the formation of long-term memory-like cytotoxic T cells [65]. Loss of Eomes led to defects in memory T-cell populations [66]. Conversely, T-bet induced the production of inhibitory receptor molecules, such as PD-1 and TIGIT [67]. Exhausted CD8+ T (Tex) cells contain two subpopulations: Tex progenitor cells and terminal Tex cells. Tex progenitor cells highly expressed T-bet (T-betHi PD-1Int), regenerated by division and maintain virus specificity. However, they differentiated to express Eomes (EomesHiPD-1Hi). This subgroup was more effective against the virus, but cannot self-replicate [27, 68, 69].
An additional consideration was that the exhausted epigenetic state was largely irreversible. Analysis of chromatin accessibility in HCV and HIV-specific responses identified a core epigenetic program for CD8+ T cell exhaustion that undergone only limited remodeling before and after resolution of infection [70]. In cancer therapy, ICIs could often transiently reprogram or activate T cells to restore effector cell-like transcriptional and functional phenotypes. However, under the influence of DNA methylation and histone modification, the chromatin state of T cells was still largely stable, which could quickly disappear the effect of ICIs and T cells return to exhaustion. This regulatory mechanism was also referred to as “epigenetic traces”. Collectively, these findings demonstrated that epigenetic alterations played an important role in orchestrating T-cell exhaustion.
Metabolic reprogramming
Carbohydrate metabolism
Studies indicated that histone acetylation required acetyl-CoA, which was produced by the catabolism of acetate, citrate or pyruvate. Limited glucose availability or glycolytic metabolism might affect chromatin accessibility and further hinder T cells immunity. Lactate dehydrogenase A (LDHA) depletion decreased the acetylation of histone H3 lysine 9 residue (H3K9) at the Ifng site in mouse CD4+ T cells by inhibiting aerobic glycolysis, resulting in decreased acetyl-CoA levels. Acetate supplementation promoted histone acetylation and chromatin accessibility, restoring IFN-γ expression in CD8+ T cells under glucose limitation [71]. Studies showed that immune checkpoints inhibited T cells function by inhibiting glycolysis in immune cells, and ICIs restored glucose uptake by immune cells [72]. For example, the interaction of PD-1 with its ligand PD-L1 regulated early glycolysis and mitochondrial changes and inhibited the transcriptional coactivator PGC-1α. Overexpression of PGC-1α reversed the function of exhausted CD8 T cells by improving biological energy, and treated with anti-PD-L1 could reprogram the metabolism of a portion of exhausted CD8 T cells [73]. In addition, impaired glycolysis and reduced metabolic responses was also found in HBV, HCV, or HIV infected patients, as well as in LCMV infected mice. Although the microenvironment of viral infection was quite different from that of TME, PD-1 signaling prevented aerobic glycolysis, which led to metabolic impairment during chronic LCMV infection. Thus, chronic viral infection led to similar impairment of T cells metabolism by promoting glucose deprivation and pseudohypoxia [74]. A study also found that increasing the glucose uptake and utilization ability of T cells was beneficial to the activation of T cells in cancer [75].
Lipid metabolism
Exhausted T cells from both chronic viral infection and cancer exhibited metabolic abnormalities, and lipid metabolism contributed to CD8+T cell exhaustion. PD-1hi CD8+ TILs had higher lipid content than PD-1lo CD8+ TILs in non-small cell lung cancer patients. Abnormal lipid metabolism promoted T cell exhaustion, and the promotion of lipid catabolism effectively enhanced the effector function and tumor killing activity of CD8+ TILs [76, 77]. Moreover, due to the accumulation of adipocytes in tumors and the up-regulation of fatty acid synthesis in cancer cells, cholesterol and other lipids in tumor microenvironment (TME) caused T cell exhaustion through the perturbation of ER stress response and ferroapoptosis in CD8+ TILs [74]. Although little is known about the role of cholesterol metabolism in chronic infection, it is widely accepted that cholesterol metabolism is critical in T-cell function and cancer failure. Studies showed that cholesterol accumulation promoted the expression of inhibitory receptors such as PD-1 by increasing ER stress, leading to CD8+ T cell exhaustion. Inhibition of ER stress sensor XBP1 or cholesterol reduction could effectively restore effector T cells function [77, 78].
Amino acid metabolism
Glutamine played an important role in the rapid proliferation of CD8+T cells, especially in effector CD8+T cells with highly active glutamine metabolism. Effector T cells consumed glutamine as a non-glucose fuel for mitochondrial oxidation and retained glucose for the production of macromolecules required for rapid proliferation. Glutamine deficiency increased the expression of inhibitory receptors such as PD-1 and LAG-3, leading to CD8+ T cell exhaustion [79]. Since arginase and indoleamine-pyrrole 2,3-dioxygenase (IDO) were highly expressed in DCs, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs), the absence of amino acids such as arginine and tryptophan could further impair metabolic fitness and alter the activation and differentiation of TILs [80]. Arginine depletion promoted a decrease in T-cell proliferation, cytokine production, and TCR expression. The high activity of IDO consumed tryptophan in the TME and impaired the activity of mTOR through the activation of the kinase GCN2, leading to T cells dysfunction [81]. In addition, kynurenine produced by tryptophan degradation inhibited T cells immunity by activating aromatic hydroxyl receptors [82]. T cells activation was also required large amounts of methionine, which acted as a donor of methyl groups during cellular methylation, affecting epigenetic reprogramming of T cells differentiation [83]. Studies indicated that viral antigen-specific exhausted CD8+ T cells exhibited decreased metabolic fitness in patients infected with HBV, HCV, or HIV [84–86]. Blocking the tumor metabolism of glutamine not only reduced hypoxia, acidosis and nutrient consumption in the tumor microenvironment, but also promoted T cell activation and prolong the life of T cells [87]. These studies showed that metabolic reprogramming influenced T cell exhaustion.
Mitochondrial fitness
As a key regulator of energy, current studies confirmed that the importance of mitochondria in coordinating the function of CD8+T cells and their importance in the generation of CD8+T cell exhaustion. Mitochondria were key organelles that regulated cell metabolism and activity [88]. In chronic renal LCMV-infected mice, early activated LCMV-specific CD8+ T cells contained depolarized mitochondria that impede T cell metabolic adaptation [73]. In HBV infection, after depletion of CD8+ T cells, the expression of genes encoding mitochondrial proteins and regulators of mitochondrial biogenesis was also greatly decreased and contained more depolarized mitochondria [86]. The accumulation of depolarized mitochondria led to the production of mitochondrial reactive oxygen species (mtROS). Exhaustion of mtROS effectively restored the effector and antiviral response of HBV-specific exhausted T cells [89]. Thus, targeted clearance of mtROS and improvement of depolarized mitochondria may prevent T cells from differentiating into a state of terminal depletion and maintain their reactivity to ICIs therapy [90]. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) acted as a transcriptional coactivator to promote the function of CD8+TILs by coordinating mitochondrial biogenesis and antioxidant activity [91]. In early activated LCMV viral infection, over-expression of PGC1α in specific CD8+ T cells reduced the exhaustion phenotype and alleviated mitochondrial dysfunction, improving metabolic adaptation [92]. Blymphocyte-induced maturation protein 1 (Blimp-1), a transcriptional repressor, was highly expressed in terminally exhausted CD8+T cells in the B16 melanoma model. Blimp-1 also accelerated the exhaustion of CD8+T cells by inhibiting the metabolic changes of PGC-1α [92].
Hypoxia
Hypoxia is another key factor that dampens T-cell function in response to oxygen deprivation and pseudohypoxia in the context of chronic infection and tumors [93]. Hypoxia has a unique two-sided effect on immune cell function. Hypoxia-inducible factor (HIF), a key factor in the hypoxic response, was activated in response to hypoxia and inactivation of proline hydroxylase. HIF signaling pathway played an important role in the induction of glycolysis in chronic LCMV infection and melanoma. Moreover, HIF activation during chronic infection led to over-activation of T cells [94], while the absence of HIF in T cells impaired the activity of tumor-induced T cells and led to a reduced T cell activation phenotype in lung cancer and melanoma [95]. In mouse breast cancer, HIF-1α deficiency reduced tumor infiltration and cytotoxic function of CD8+T cells [95].
Factors related to the occurrence and development of T cell exhaustion
T cell exhaustion is influenced by a variety of factors, the level and duration of antigen persistence, inhibitory and promotive cytokines, and immunomodulatory cells.
The level and duration of antigenic stimulation
A key feature of chronic infection or cancer was constant exposure of T cells to antigens, with both high antigen load and prolonged antigen exposure, leading to more severe T cell exhaustion. During more protracted infections, effector T-cell numbers and the functional quality of the response generally decrease, with a further exhaustion phenotype developing as the viral load remains high. Mice infected with chronic strains of LCMV differentiated into fully CD8+ memory T cells if they were isolated from sustained antigenic stimulation early (about 1 week after infection). However, these T cells were exposed to persistent antigens for 2–4 weeks, T cells became exhausted and failed to resume normal memory differentiation [96]. In HIV, persistent antigen exposure (or co-stimulation) in the absence of IL-1β results in systemic CD4+ T-cell exhaustion. In this context, the deleterious effects of HIV infection on CD4+ bone marrow cells, including APCs, capable of producing IL-1β, might lead to altered T cell lineage homeostasis and reduced cell division [97]. These studies suggested that chronic antigenic stimulation and its duration might be a key factor in T cell exhaustion.
Cytokines
Similar to the inhibitory receptors described above, cytokines affect the function and status of T cells in chronic infections and tumors. Cytokines that promoted T cell exhaustion included IL-10, transforming growth factor-beta (TGF-β), IL-6 and tumor necrosis factor-alpha (TNF-α), as well as chemokines. IL-10 and TGF-β promoted PD-1 expression on the surface of T cells and were involved in the induction of T cell exhaustion and viral persistence [98–101]. Studies showed that the expression of IL-10 was upregulated during persistent infection of several viruses, including HBV, HCV, HIV, and LCMV. Blocking the expression of IL-10 enhanced viral control and improved responses to exhausted T cells [102–105]. Blocking T cell acceptance of TGF-β signaling during chronic LCMV infection improved CD8+T cell function and prevented severe exhaustion of CD8+T cells [106]. The presence of IL-10 and TGF-β was also found to inhibit T cells function in cancer [107, 108]. Studies also found that other immunomodulatory cytokines such as IL-6 and TNF-α also played a role in CD8 T cell exhaustion in chronic infection and cancer. Inhibition of these molecules or the receptors that bind to them serves as a potential strategy for reversing T cell exhaustion [2]. Studies showed that IL-6 promoted T cell exhaustion through IL-6/STAT3/PD-1 transcriptional regulation [98], TNF-α induced PD-L1 expression and attenuated immune cell activation, and blockade of TNF-α also reversed T cell exhaustion [109]. Studies found that in COVID-19, it did not directly attack T cells, but triggered the release of cytokines, which in turn drove the exhaustion of T cells. CD4+ and CD8+ T cells were significantly decreased in COVID-19 patients, the number of T cells was negatively correlated with serum IL-6, IL-10 and TNF-α concentrations. The level of PD-1, a marker of T cell exhaustion, was significantly increased [110]. In addition, cytokine storm promoted T cell exhaustion through the cytokine-receptor axis. Blocking the cytokine receptor axis, such as CCL2-CCR2, CCL3-CCR1, CCL3-CCR5, CCL4-CCR5, CCL3L1-VSIR, and CCL3L1-CCR1, might be a new strategy to reverse T cell exhaustion and further treat the immune failure associated with severe coronavirus pneumonia with cytokine storm [111].
Cytokines that antagonized T cell exhaustion included IL-2 and IL-12. IL-2, known as T cells growth factor, was essential for T cell proliferation and survival and for the formation of effector and memory cells [112]. By IL-2 supply, defective virus-specific CD8 T cells restored the proliferation and differentiation capacity of IFN-γ-producing cells [113]. However, IL-2 played a bidirectional role in the regulation of T cells in tumors. In the early stage of tumor, IL-2 induced the differentiation of effector CD8+T cells in an autocrine manner, and promoted the activation and proliferation of CD8+T cells [114]. However, in the late stage of tumor, IL-2 induced CD8+ T cell exhaustion in the tumor microenvironment by activating STAT5-5-HTP-AhR pathway [115]. IL-12 was produced by activated antigen-presenting cells (APC). It promoted the development of Th1 responses and was a potent inducer of IFN-γ production by T cells and NK cells. IL-12 induced NK cell and T cells proliferation, enhancing cytotoxic mediator expression and cytokine production, especially IFN-γ [114]. Moreover, IL-12 alone could effectively restore the secretion of multiple cytokines and the cytotoxic activity of HBV-specific CD8 T cells. In addition, IL-12 restored IFN-γ production in HBV-specific CD8 T cells [116], but the effects of different cytokines on exhausted T cells needed to be further confirmed.
Other immune cells
In chronic infection, CD4+T cells played an important role in regulating the response of CD8+T cells. CD4+T cells activated antigen-presenting cells through CD40 and CD40 ligand interaction to secrete chemokines and cytokines. At the same time, CD4+T cells produced IL-2 and IL-21 cytokines that directly acted on CD8+T cells [117, 118]. Short-term depletion of CD4+T cells in mice chronically infected with HIV led to high viral load and severe exhaustion of CD8+T cells [119]. A study showed that in LCMV mice, effective adjuvant action of CD4+T cells with initial and antigen-specific effects could reverse exhaustion of CD8+T cells in vivo [120]. In addition, CD8+T cell exhaustion status was also influenced by NK cells, which produced IL-10 and TGF-β to inhibit T cell activation and led to T cell exhaustion by impacting APC function during persistent viral infection. NK cell exhaustion significantly reduced the expression of inhibitory receptors such as PD-1 and LAG-3 in T cell [121, 122].
Other immune regulatory cells such as regulatory T cells (Tregs), MDSCs, M2 macrophages are frequently increased in infections and tumors [27]. Treg cells often abnormally accumulated in TME and expressed immunosuppressive molecules, including the cytokines IL-10, IL-35, and TGF-β, which suppressed the anti-tumor effects of T cells [123–125]. In addition, studies showed that intra-tumoral Treg cells promoted T-cell exhaustion in CD8+TILs through interactions with IL-10 and IL-35 in melanoma [125]. Large accumulation of TAMs impaired T cell-mediated anti-tumor responses and promoted T cell exhaustion due to IL-10 production and PD-L1 expression [60]. MDSCs suppressed the innate immune system mainly by secreting IL-10 and TGF-β, which induced macrophages to develop an immunosuppressive M2 phenotype and negatively affected NK cells maturation and DC function [126]. MDSCs promoted the expression of PD-1 [127, 128], PD-L1, LAG3, CTLA-4 and TIM-3 on CD4 cells, and partially induced the exhaustion of activated T cells through IFN-γ [129].
The occurrence and development of T cell exhaustion was a complex process, which was influenced by many factors. Changes in any aspect of antigenic stimulation, cytokines or immune cells might lead to poor response to chronic infections or cancer, further promoting the development of a state of exhaustion.
Traditional Chinese medicine and T cell exhaustion
Traditional Chinese medicine compound
Bushen Jianpi recipe
TCM compounds played an important role in the treatment of infectious diseases and cancer (Table 2). Studies also found that Bushen Jianpi Recipe intervention in a mouse model of T cell exhaustion induced by continuous stimulation of purified protein derivative (PPD) significantly restored the number of CD4+ and CD8+T cells in the spleen, and significantly reduced the expression of PD-1 on CD4+T cells. Moreover, the levels of IL-2 and IFN-γ secreted by splenic lymphocytes were significantly increased, and the ability of anti-infection of mice was significantly enhanced [130].
Table 2.
The effect of TCM compounds and active components on the regulation mechanism of T cell exhaustion
| Drugs | Disease type | Mechanism of effect | Cell type/model | References |
|---|---|---|---|---|
| Bushen Jianpi Recipe | Mycobacterium bovis BCG purified protein derivative | Enhances IFN-γ and IL-2 expression, up-regulates CD4+ and CD8+ T cells and inhibits PD-1 expression level on CD4+ T cells | Mice in vivo model | [130] |
| Dahuang Fuzi Baijiang Decoction | Colorectal cancer | Inhibits the PD-1hiTIM3+ subset and CCL2 expression and enhances the PD-1intTCF+ population | MC38 cell line and mice in vivo model | [131] |
| Yangyin Fuzheng Jiedu Prescription | Hepatocellular carcinoma | Enhances the ratio of CD3+ and CD8+ T cells in the peripheral blood, spleen, and tumor tissue and the expression of TNF-α, and IFN-γ and down-regulates the expression of PD-1, TIGIT, TIM-3 in CD8+ T cells and IL-1β, IL-6, and IL-10 in the serum and tumor tissues | Mouse HCC cell line H22 and mice in vivo model | [132] |
| ShenQi FuZheng Injection | Lung cancer-related fatigue | Inhibits PD-L1, TIM-3 and FOXP3 and IL-2, IFN-γ and TNF-α expression in serum | Lewis Lung carcinoma and mice in vivo model | [133] |
| Yangyin Fuzheng Decoction | Lung cancer | Inhibits the expression of PD-1+ CD8+ T cells and PD-1, PD-L1, TIM-3 in tumor tissues and increases PD-1−CD8+ T cells in peripheral blood and CD4+CD25+ FoxP+ T cell in tumor tissues | PD-L1+Lewis and Lewis lung cancer cells and mice in vivo model | [134, 135] |
| Qiyusanlong Decoction | Lung cancer | Increases TNF-α, IL-1, IL-12 and inhibits CXCL-9, CCL-17, STAT6 and MTOR expression | Lewis lung cancer cells and mice in vivo model | [138] |
| Compound kushen injection | Hepatocellular carcinoma | Reduces the distribution and polarization of M2-TAM, promotes M1-TAM | Hepa1-6 or LPC-H12 cell lines and mice in vivo model | [139] |
| Astragalus membranaceus polysaccharide | Breast cancer | Enhances IFN-γ and IL-2 expression | 4T1 breast tumor cell line and mice in vivo model | [144] |
| Dihydroartemisinin | Hepatocellular carcinoma | Promotes CD4+ T cell infiltration in spleen and CD8+ T cell infiltration in tumor tissue | HepG2215 cell line and mice in vivo model | [145] |
| Extract derived from the sporoderm-breaking spores of G. lucidum | Breast cancer | Increases cytotoxic T cell (Tc) population and the ratio of Tc to helper T cell (Th) and decreases PD-1 and CTLA-4 expression | Breast cancer 4T1-cell line and mice in vivo model | [146] |
| Hirsutella sinensis fungus | Breast cancer | Increases CD44LowCD62LHi and CD44HiCD62LLow populations in the tumor-infiltrating CD8+ T cells and enhances IFN-γ and granzyme B and reduces PD-1, TIGIT, CTLA-4 expression | 4T1-Luc cells and mice in vivo model | [147] |
| Pinellia pedatisecta Schott extract | Cervical cancer | Up-regulates the expression of MHCII, CD80, CD86, IL-12 and promotes CD4+ and CD8+ T cells and induces the differentiation of IFN-γ+CD4+ and GZMB+CD8+ T cells | TC-1 tumor cell line and mice in vivo model | [151, 152] |
| Astragalus membranaceus and extract | Renal cell carcinoma | Increases IL-21 expression | Renal cell carcinoma patients | [153] |
| Lycium barbarum polysaccharide | Liver cancer | Increases CD8+ T cells and decreases TGF-β and IL-10 expression | Mouse HCC cell line H22 and mice in vivo model | [154] |
| Luteolin and its derivative apigenin | Lung cancer | Inhibition of STAT3 phosphorylation, down-regulates IFN-γ-induced PD-L1 expression and increases CD8+ T cells, IFN-γ, TNF-α and Granzyme B | KRAS-mutation NSCLC (H460, H358and A549) cells and mice in vivo model | [155] |
| Bisdemethoxycurcumin | Bladder cancer | Enhances IFN-γ, granzyme B, and perforin expression | MB49 cells and mice in vivo model | [156] |
Dahuang Fuzi Baijiang decoction
Moreover, studies found that obesity increased the occurrence of colorectal cancer, and it led to T cell exhaustion by increasing PD-1 expression and reducing IFN-γ and TNF-α production in TILs. PD- 1hiTIM3+showed a defect in IFN-γ production and a residual capacity in TNF-ɑ production. The PD-1hiTIM3+ subset was typically defined as terminally differentiated, exhausted T cells, whereas the co-existence of intermediate expressed PD-1 (PD-1int) and TCF-1 gave Tex stem cell properties. Dahuang Fuzi Baijiang Decoction (DFB) enhanced the efficacy of PD-1 checkpoint blockade by restricting the PD-1hiTIM3+ subset and amplifying the PD-1int TCF+ to suppress tumor growth in high-fat diet-induced obese mice. The recombinant chemokine C–C-motif receptor 2 (CCR2) +CD8+ subset was defined as Tex terminals and differentiation from progenitor cells to Tex terminals was driven, at least in part, by the chemokine (C–C motif) ligand 2 (CCL2)/CCR2 axis, CCR2 inhibitors enhanced the response to anti-PD-1 by promoting Tex progenitor cell counts. In conclusion, DFB inhibited CCL2 and retained progenitor Tex to suppress CRC progression in the obese microenvironment [131].
Yangyin Fuzheng Jiedu prescription
TCM compounds also play an important role in improving T cell exhaustion in a variety of cancers. Studies indicated that Yangyin Fuzheng Jiedu Prescription (YFJP) effectively inhibited solid tumor growth and spleen index in mice with liver cancer. In addition, YFJP maintained high percentages of CD3+ and CD8+ T cells in peripheral blood, spleen and tumor tissues, while reducing the expression of multiple inhibitory receptors on CD8+ T cells. These included PD-1, TIGIT and TIM-3. In addition, YFJP significantly reduced the expression of inflammatory and immunosuppressive cytokines, including IL-1β, IL-6 and IL-10, while increased the expression of cellular effectors TNF-α and IFN-γ in serum and tumor tissues. It also restored the cytotoxicity of tumor-infiltrating T cells. It has also been found that PD-1+TIM-3+ double positive T cells have a more sensitive and effective prognostic value than PD-1+ or TIM-3+CD8+ T cells. Surprisingly, PD-1/TIM-3 double-positive T cells were significantly reduced in peripheral blood and tumor tissue in the YFJP group [132].
ShenQi FuZheng injection
In addition, Chinese Medicinal Preparations also played an important role in the treatment of T cell exhaustion. Chinese Medicinal Preparations increased the number of CD4+ T cells, CD8+ T cells and the ratio of CD4+/CD8+, regulating the body's immune function and enhancing the immune response. Studies also found that ShenQi FuZheng Injection (SFI) inhibited the growth of lung cancer in mice and reduced the depression-like behavior of tumor-bearing mice. The level of CD19 protein and CD4/CD8 were increased in SFI group, indicating that SFI could enhance the immune function of tumor-bearing mice. SFI also significantly down-regulated the expression levels of PD-L1, TIM-3 and FOXP3 in tumors and decreased the expression of FOXP3 in spleen. In addition, SFI reduced the levels of proinflammatory cytokines IL-2, IFN-γ, and TNF-α in spleen, However, the mRNA levels of IL-2, IFN-γ and TNF-α in tumor tissues were significantly increased, while IL-6 mRNA levels were significantly decreased. These results suggested that SFI improved symptoms of fatigue, alleviated dysfunction of T cell exhaustion by inhibiting proinflammatory cytokines produced by peripheral immune cells, and enhanced antitumor immunity through PD-L1, TIM-3, and FOXP3 [133].
Yangyin Fuzheng decoction
In lung cancer, Yangyin Fuzheng Decoction promoted the secretion of IL-2 and IFN-γ by splenic lymphocytes, inhibited the secretion of IL-10 and TGF-β by splenic lymphocytes. Moreover, it also reduced the number of PD-L1+CD8+T cells in peripheral blood and increased the number of PD-L1−CD8+T cells. It also reduced the expression of CD4+CD25+FoxP+T, PD-L1, PD-1, TIM-3 protein in tumor tissues and the expression of PD-L1 and PD-1 mRNA in tumor tissues, increasing the effect of atezolizumab on the proliferation of lung cancer cells [134, 135].
Qiyusanlong decoction
Moreover, studies also indicated that Qiyusanlong Decoction (QYSL) significantly promoted CD8 T cell activation in lung cancer. QYSL promoted the polarization of M1 macrophages and inhibited tumor growth by promoting the expression of TNF-α, IL-1, and IL-12. Studies showed that M2 macrophages inhibited the activation of effector T cells by promoting the high expression of CCL17. Furthermore, M2 macrophages also inhibited CD8+ T cell infiltration by decreasing the expression of CXCL9 [136, 137]. A study found that M2 macrophages were significantly increased in tumors. QYSL could inhibit the tumor growth by down-regulating the expression of M2 macrophage-related genes CXCL-9 and CCL-17 and inhibiting the activation of M2 macrophages. STAT6 and MTOR played important roles in the polarization of M2 macrophages. QYSL inhibited the expression of STA6 and MTOR and effectively inhibited the polarization of M2 macrophages. Collectively, QYSL suppressed tumor growth by inhibiting M2 macrophage polarization and modulating the tumor immune microenvironment [138].
Compound kushen injection
A study showed that compound kushen injection (CKI) activated the pro-inflammatory response of tumor-associated macrophages in the HCC microenvironment and reduced immunosuppression through triggering tumor necrosis factor receptor superfamily member 1 (TNFR1)-mediated cascade of NF-κB and p38 MAPK signaling. CKI also reduced the distribution and polarization of M2-TAM, promoted M1-TAM, and significantly promoted the proliferation and cytotoxic ability of CD8+ T cells, thus leading to the apoptosis of HCC cells [139].
Active ingredients of traditional Chinese medicine
The active ingredients of traditional Chinese medicine also play an important role in restoring T cell exhaustion (Table 2).
Polyphenolic compounds
Polyphenolic compounds significantly regulated PD-L1 expression through a variety of pathways and increased gene transcription by targeting epigenetic processes, affecting the stability of transcription factors or mRNA. In addition, polyphenolic compounds affected the production and structure of PD-L1 through post-translational modification processes, such as ubiquitination and glycosylation. It also blocked the PD-1/PD-L1 pathway by directly blocking or increasing PD-L1 degradation, activating exhausted T cells and anti-tumor immunity [140, 141]. Studies indicated that polyphenolic compounds modulated TIL cell subsets by cytokine secretion, increasing the proportion of CD4+CD8+ TILs, and enhancing the activity of CD8+ CTLs in TME [142, 143]. These studies suggested that TCM had immunomodulatory effects and improved the state of T cell exhaustion in infectious diseases and cancer to a certain extent, which was beneficial for anti-infection and anti-tumor treatment.
Astragalus membranaceus polysaccharide
Furthermore, Astragalus membranaceus polysaccharide (APS), one of the active components of Astragalus membranaceus, promoted the expression of IL-2 and IFN-γ in the serum of mice, and induced low dose anti-PD-1 response. And it could delay the occurrence and development of 4T1 breast cancer by increasing the activity of T cells. Anti-PD-1 produced by APS stimulation. The isolated single strand fragment variable (scFv) S12 had the highest binding affinity with PD-1 (20 nM), which completed the interaction between PD-1 and PD-L1, and blocked the effect of PD-L1 inducing T cell failure in peripheral blood monocytes in vitro [144].
Dihydroartemisinin
The efficacy of anti-PD-1 therapy in HCC is not as expected. YAP1 was highly expressed and activated in liver cancer tissues. The mechanism of YAP1 in immune escape of liver cancer remains unclear. Anti-PD-1 treatment increased YAP1 expression in liver cancer cells and increased the expression of exhausted CD4+ and CD8+ T cells in blood and spleen of liver tumor mice. Knockdown of YAP1 inhibited the expression of PD-L1 and promoted the proportion of CD4+ and CD8+ T cells in liver tumor microenvironment. Dihydroartemisinin (DHA) inhibited YAP1 expression and broke immune escape in liver tumor microenvironment, which was manifested by decreased PD-L1 and increased infiltration of CD8+ T cells in liver tumor cells. DHA combined with anti-PD-1 treatment promoted CD4+ T cell infiltration in spleen and CD8+ T cell infiltration in tumor tissue. This suggests that DHA improved the effect of anti-PD-1 [145].
Extract derived from the sporoderm-breaking spores of G. lucidum
In breast cancer, studies showed that extract derived from the sporoderm-breaking spores of G. lucidum (ESG) inhibited the growth of 4T1 in vivo, and significantly increased the number of cytotoxic T cells (Tc) and the ratio of Tc to helper T cells (Th) in peripheral blood. A similar Tc promotion was found in tumor-infiltrating lymphocytes. In addition, ESG significantly reduced the expression levels of PD-1 and CTLA-4 in spleen and tumors, indicating that ESG effectively restored T cells function by inhibiting the co-inhibitory checkpoint to restore the exhaustion state [146].
Hirsutella sinensis fungus
In breast cancer, hirsutella sinensis fungus (HSF) inhibited tumor growth and lung metastasis of mice, decreased the expression of inhibitory receptors such as PD-1, TIGIT and CTLA-4, and suppressed the percentages of PD-1Int and PD-1HiCD8+T populations. Furthermore, the PD-1IntCD8+T cell population highly expressed T-bet both in vivo and in vitro, HSF inhibited T-bet expression in CD8 cells as well as PD-1Lo and PD-1Hicell populations. In addition, Eomes was highly expressed in PD-1Int and PD-1Hi cell populations, HSF promoted Eomes expression in CD8 T cells, PD-1Lo PD-1Int and PD-1Hi cell populations. These findings suggested that HSF improved T cell function mainly by promoting the expression of Eomes [147].
Pinellia pedatisecta Schott extract
Immune cells played a key role in initiating, stimulating and maintaining immune responses against tumor progression [148]. DCs have dual effects on immune stimulation or suppression [149]. Immature DCs expressed low MHC and costimulatory molecules such as CD40, CD80, CD83 and CD86, maintaining immune tolerance by inducing T cells loss or anergy and inhibiting T cell differentiation [148, 150]. Upon maturation, DCs had high expression of major histocompatibility complex class II (MHCII) and costimulatory molecules and stable production of IL-12, which further promoted CTL and Th1 responses. Pinellia pedatisecta Schott extract (PE) promoted the expression of MHCII, CD80, CD86 and IL-12, the proliferation of CD4+ and CD8+ T cells, and induced the differentiation of IFN-y+CD4+ and GZMB+CD8+ T cells in tumor-associated dendritic cells (TADC). PE-treated TADCs also elicited stronger antigen-specific CTL responses. Furthermore, PE enhanced the proliferative capacity of anti-tumor T cells (increased expression of Ki67, CD137, GZMB or IFN-γ, TNF-a) and reversed T cell exhaustion (impaired CD95 or PD-1 expression). SOCS1 and SOCS3 were important negative regulators of tumor-associated immune cells during tumor development [151], PE inhibited the activation of JAK2 in SOCS1 [152].
Astragalus membranaceus
T cells from patients with renal cell carcinoma (RCC) exhibited multiple features of injury and depletion, as shown by the down-regulation of IL-21 expression associated with the dysfunction of CXCR5+ Tfh-like cells. Astragalus membranaceus could significantly increase the expression of IL-21 in a dose-dependent manner depending on the presence of APCs. APCs induced by astragalus extract also promoted the expression of IL-21 in Tfh-like cells. Interestingly, astragalus stimulated Tfh-like cells exhibited enhanced helper function, higher humoral responses and better CD8 T cells survival, which depended on the presence of IL-21 [153].
Lycium barbarum polysaccharide
Studies showed that Lycium barbarum polysaccharide (LBP), one of the active ingredients of Lycium barbarum, inhibited the tumor growth of H22 tumor-bearing mice. In addition, LBP could maintain high levels of T cells in peripheral blood, tumor-draining lymph nodes (TDLN), and tumor tissues. It also inhibited the increase of Tregs and TGF-β and IL-10, while promoted CD8+ T cells infiltration in tumor tissues, reducing the exhaustion phenotype of T cells and maintaining lymphocyte cytotoxicity [154].
Luteolin and apigenin
In KRAS-mutant NSCLC cancer cells, luteolin and apigenin inhibited IFN-γ-induced PD-L1 expression upregulation and STAT3 phosphorylation. PD-1 antibody combined with luteolin or apigenin treatment significantly increased the frequency and number of CD8+ T lymphocytes, and CD8+ T cells produced more IFN-γ, TNF-α and Granzyme B. Moreover, the ratio of CD8/ CD4 T cells in serum was increased, and the proportion of Treg cells (CD4+FoxP3+T cells) in spleen and blood was decreased in apigenin treatment group [155].
Bisdemethoxycurcumin
Bisdemethoxycurcumin (BDMC) combined with anti-PD-L1 antibody significantly prolonged the survival of bladder cancer mice, increased CD8+ T cells number, and promoted the secretion of IFN-γ, granzyme B, and perforin, improving the function of CD8+T cells. In addition, the combination group reduced the proportion of exhausted T cells and increased the number of intra-tumoral cytotoxic T lymphocyte (CTL), protecting effector T cells from exhaustion. MDSCs had a highly immunosuppressive effect in TME. The combination treatment also significantly reduced the number of MDSCs in the tumor [156].
In conclusion, according to current studies, in infection and cancer, TCM mainly reduces the expression of inhibitory receptors, inhibitory cells and cytokines, improving T cell exhaustion. However, the researches on transcription, epigenetic and metabolism related to T cell exhaustion have not been in-depth enough, which will be the focus of TCM research on improving T cell exhaustion in the future.
Conclusions and perspective
Although T-cell exhaustion differs between infection and cancer, both share similar features, including impaired cytokine production, cytotoxicity, and elevated levels of multiple inhibitory receptors [13]. Moreover, different diseases do not exist in isolation, they are interrelated, and the occurrence of viruses will lead to the occurrence of cancer. Oncogenic viruses (oncoviruses) are implicated in approximately 12% of all human cancers [157]. For example, HBV and HCV remain the most important risk factors for HCC, and effective treatment of chronic HBV and HCV viral infections helps to reduce the incidence of viral-associated HCC [158]. T cell exhaustion is not the result of a single factor, but is affected by multiple factors. It is the result of the superposition of multiple states. Moreover, a variety of inhibitory receptors such as PD-1, PD-L1, LAG-3, TIM-3 in T cell exhaustion also affect T cell metabolism [159]. Therapeutic blockade of PD-1 reactivates the exhausted T-cell immune response by reprogramming metabolic processes, promoting proliferation, and upregulating the expression of effector molecules [5]. Studies showed that inhibition of PD-1 and PD-L1 increased glucose utilization in TME and enhanced the glycolytic activity of T cells [160]. Studies also found that after T cell exhaustion, the application of anti-PD-L1 or anti-PD-1 improved the proliferation of CD8+T cells, increased the production of cytokines and enhanced the cytolytic activity, reducing the viral load [32]. In addition, anti-PD-1 antibody enhanced the glycolytic capacity and effector function of exhausted CD8+T cells. Therapeutic blockade of anti-PD-L1 reactivated and improved the metabolic function of partially exhausted CD8+T cells in tumors [161]. Furthermore, TIM-3 changed T cells metabolism by disrupting the PI3K/AKT/mTOR signaling pathway [162]. The basal apoptosis rate and aerobic glycolysis of CD4+ T cells lacking LAG-3 were significantly increased, indicating that LAG-3 reduced the metabolic fitness of T cells [163]. All these studies suggested that the various factors of T cell exhaustion influenced each other. In addition, although exhausted T cells exhibited immune dysfunction and lose effective control of chronic infections and tumor cells, T cell exhaustion was not an irreversible terminal state [20]. Studies showed that blocking inhibitory receptors improved the functional status of T cells to a certain extent, and improved the clearance rate of viruses, tumor cells and bacteria. Blockade of PD-1, LAG-3, TIM-3, BTLA, and CTLA-4 alone or in combination with other therapies reversed T cell exhaustion during chronic infection or tumor [53, 164]. Compared with single blockade, combined blockade showed greater advantages, but the adverse reactions of combined blockade were more obvious than single blockade. In addition, it was only effective in some people, and most patients had to discontinue treatment because of serious immune-related adverse events, such as immune-related toxicity and excessive disease progression [23, 24].
TCM has a history of thousands of years in China, which is not only well respected but also has a good track record [165]. Although in recent years, TCM has been criticized for being “unscientific” or “imprecise” in oncology, an increasing number of scientific reports have confirmed the scientific basis of TCM [26]. In addition, in the study of T cell exhaustion, it was found that both TCM compounds and TCM ingredients played a role in improving T cell exhaustion through multiple channels, such as Dahuang Fuzi Baijiang Decoction [131], Yangyin Fuzheng Jiedu Prescription [132], Yangyin Fuzheng Decoction [134, 135] and HSF [147] all played a therapeutic role by inhibiting two or more IRs. Qiyusanlong Decoction [138], CKI [139], PE [151, 152], LBP [154], luteolin and apigenin [155] not only inhibited the expression of IRs, but also regulated immunosuppressive cells and immunosuppressive cytokines, so as to improve the function T cell exhaustion. In addition, there were abundant clinical research data of TCM. Shenqi Fuzheng injection had good curative effect on Chinese patients with relapsed metastatic or advanced (stage IIIB/IV) NSCLC, and significantly improved Qi deficiency constitution [166]. A meta-analysis of CKI confirmed that CKI plus transcatheter arterial chemoembolization (TACE) was superior to TACE alone in patients with unresectable hepatocellular carcinoma (UHCC) [167]. Yangyin Fuzheng Jiedu Prescription has been used in the treatment of liver cancer in the Center of Integrated Chinese and Western Medicine of Beijing Ditan Hospital (affiliated to Capital Medical University) for many years. Based on the research of the prescription has been registered in the clinical trial system (https://www.clinicaltrials.gov/), the registration number for NCT02927626. A recent meta-analysis of epidemiological studies found that apigenin intake reduced the risk of colorectal cancer [0.82(0.70–0.97)] but had no effect on colon cancer [0.88(0.69–1.13)] [168]. However, there are few TCM clinical studies on T cell exhaustion, most of which are still in the preclinical stage. However, preclinical studies of TCM show the advantages of studying T-cell exhaustion.
Acknowledgements
The authors acknowledge using Biorender (https://app.biorender.com/user/signin) to create the schemata (Figs. 1, 2, 3).
Fig. 1.
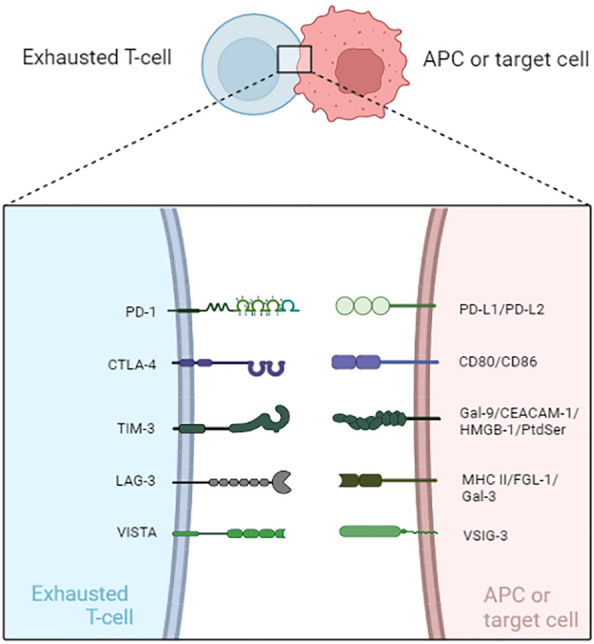
Inhibitory receptors associated with T cell exhaustion and related ligands. APC antigen-presenting cell, PD-1 programmed cell death protein 1, PD-L1 programmed cell death 1 ligand 1, PD-L2 programmed cell death 1 ligand 2, CTLA-4 cytotoxic T-lymphocyte-associated protein 4, TIM-3 T cell immunoglobulin domain and mucin domain protein 3, Gal-9 Galectin-9, CEACAM1 carcinoembryonic antigen-related cell adhesion molecule 1, HMGB-1 high-mobility
Fig. 2.
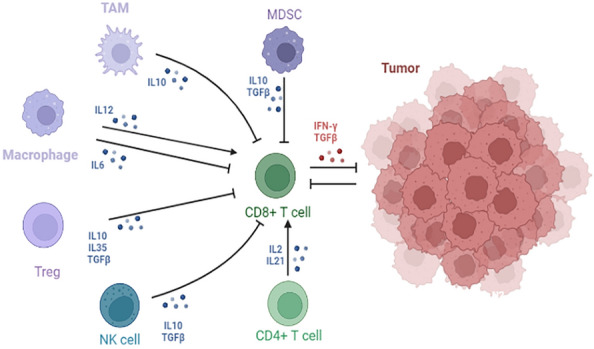
Interaction and functions of immune cells in tumor microenvironment. Cancer cells, Tregs, NK, TAM, MDSC and CD4 T cells are important immune cells that regulate CD8 T-cell exhaustion. IL-2, IL-6, IL-10, IL12, TGF-β, TNF-α and IFN-γ are all important exogenous cytokines involved in exhausted process of T cells. TAM tumor-associated macrophage, Treg regulatory cells, NK natural killer cell, MDSC myeloid-derived suppressor cells
Fig. 3.
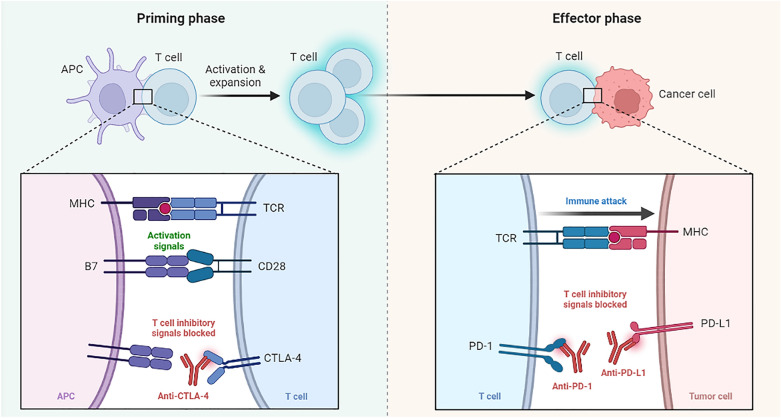
Blockade of CTLA-4, or PD-1, or PD-L1 signaling in tumor immunotherapy. The activation of T cells requires double signaling. The first signal of T cell activation comes from the specific binding of TCR to MHC. The second signal of T cell activation comes from costimulatory molecules, namely signals mediated by the interaction of APC-expressed costimulatory molecules with corresponding receptors or ligands on the T cell surface. Costimulatory molecules can be divided into positive co-stimulatory molecules and negative co-stimulatory molecules. CD28/B7 is an important positive co-stimulatory molecule, and CTLA-4, which is highly homologous to CD28 molecule, has a ligand of B7, but the binding of CTLA-4 and B7 mediates the conduction of negative signals. Anti-CTLA-4 maintains T cell activation by blocking the transmission of inhibitory signals by inhibiting the binding of CTLA-4 to CD80 or CD86 molecules. When T cells kill tumor cells, the combination of PD-L1 expressed on tumor cells and PD-1 expressed on T cells will transmit negative regulatory signals to T cells, causing T cells to be unable to recognize cancer cells and tumor cells to achieve “immune escape”. Anti-PD-1 or anti-PD-L1 prevents PD-L1 from binding to PD-1, thereby reactivating T cells to kill tumors
Abbreviations
- T cells
T lymphocytes
- IFN⁃γ
Interferon⁃γ
- TNF
Tumor necrosis factor
- IL-2
Interleukin-2
- COVID-19
Corona Virus Disease 2019
- PD-1
Programmed cell death protein 1
- LAG-3
Lymphocyte activation gene 3 protein
- VISTA
V-domain Ig suppressor of T-cell activation
- TIM-3
T cell immunoglobulin domain and mucin domain protein 3
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- COTL
Coactosin-like protein
- HMGB1
High mobility group protein B1
- HSPD1
Heat shock protein family member D1
- HSP90AA1
Heat shock protein 90 alpha family class A member 1
- BIRC5
Baculoviral IAP repeat-containing protein 5
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- MTB
Mycobacterium tuberculosis
- PD-L1
Programmed cell death 1 ligand 1
- TIL
Tumor infiltrating lymphocyte
- Tex
Exhausted CD8+ T cells
- ICIs
Immune checkpoint inhibitors
- TCM
Traditional Chinese medicine
- NK
Natural killer cell
- DC
Dendritic cell
- ZAP70
Zeta chain associated protein kinase 70
- LCMV
Lymphocytic choriomeningitis virus
- PI3K
Phosphatidylinositol-3-kinase
- mTOR
Mammalian target of rapamycin
- VSIG-3
V-Set and immunoglobulin domain containing 3
- APC
Antigen-presenting cell
- Gal-9
Galectin-9
- CEACAM1
Carcinoembryonic antigen-related cell adhesion molecule 1
- HMGB-1
High-mobility group box
- PtdSer
Phosphatidylserine
- MHC II
Major histocompatibility complex class II
- FGL-1
Fibrinogen-like protein 1
- Gal-3
Galectin-3
- DNMT3A
DNA methyltransferase 3 alpha
- TOX
Thymocyte selection-associated high mobility group box
- Eomes
Eomesodermin
- CAR-T
Chimeric antigen receptor T-cell immunotherapy
- LDHA
Lactate dehydrogenase A
- H3K9
H3 lysine 9 residue
- IDO
Indoleamine-pyrrole 2,3-dioxygenase
- MDSCs
Myeloid-derived suppressor cells
- TAMs
Tumor-associated macrophages
- mtROS
Mitochondrial reactive oxygen species
- PGC-1α
Peroxisome proliferator-activated receptor-γ coactivator 1α
- Blimp-1
Blymphocyte-induced maturation protein 1
- HIF
Hypoxia-inducible factor
- Tregs
Regulatory T cells
- PPD
Purified protein derivative
- YFJP
Yangyin Fuzheng Jiedu Prescription
- DFB
Dahuang Fuzi Baijiang Decoction
- CCR2
Chemokine C–C-motif receptor 2
- QYSL
Qiyusanlong Decoction
- SFI
ShenQi FuZheng Injection
- A. indica
Azadirachta indica
- SEB
Staphylococcal Enterotoxin B
- GK
Guttiferone K
- Tc
Cytotoxic T cells
- Th
Helper T cells
- HSF
Hirsutella sinensis fungus
- APS
Astragalus membranaceus polysaccharide
- RCC
Renal cell carcinoma
- PE
Pinellia pedatisecta Schott extract
- LBP
Lycium barbarum polysaccharide
- BDMC
Bisdemethoxycurcumin
- CTL
Cytotoxic T lymphocyte
- TCRs
T cell receptors
Author contributions
XH and SL designed research; SL, LH, JZ and JD wrote the manuscript with contributions from all authors. All authors read and approved the initial manuscript.
Funding
The present study was financially supported by the Sichuan Provincial Administration of Traditional Chinese Medicine Major science and technology projects (2021XYCZ004); National Natural Science Foundation of China (No. 81973840 and No. 81273748); National science and Technology major projects of the 13th Five-Year Plan (2018ZX10303502); Science and Technology Program of Hebei (223777156D).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shenghao Li and Liyuan Hao contributed equally.
References
- 1.Gallimore A, Glithero A, Godkin A, Tissot AC, Plückthun A, Elliott T, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187(9):1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 3.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27(4):670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138(1):30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 5.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Pan Y, Hu Z, Wu M, Wang C, Feng Z, et al. Thymosin Alpha 1 reduces the mortality of severe coronavirus disease 2019 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin Infect Dis. 2020;71(16):2150–2157. doi: 10.1093/cid/ciaa630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayaraman P, Jacques MK, Zhu C, Steblenko KM, Stowell BL, Madi A, et al. TIM3 mediates T cell exhaustion during Mycobacterium tuberculosis infection. PLoS Pathog. 2016;12(3):e1005490. doi: 10.1371/journal.ppat.1005490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen L, Gao Y, Liu Y, Zhang B, Liu Q, Wu J, et al. PD-1/PD-L pathway inhibits M.tb-specific CD4(+) T-cell functions and phagocytosis of macrophages in active tuberculosis. Sci Rep. 2016;6:38362. doi: 10.1038/srep38362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipp JJ, Wang L, Yang H, Yao F, Harrer N, Müller S, et al. Functional and molecular characterization of PD1(+) tumor-infiltrating lymphocytes from lung cancer patients. Oncoimmunology. 2022;11(1). [DOI] [PMC free article] [PubMed]
- 11.Ma J, Zheng B, Goswami S, Meng L, Zhang D, Cao C, et al. PD1(Hi) CD8(+) T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J Immunother Cancer. 2019;7(1):019–0814. doi: 10.1186/s40425-019-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baitsch L, Baumgaertner P, Devêvre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 15.Martinez GJ, Pereira RM, Äijö T, Kim EY, Marangoni F, Pipkin ME, et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity. 2015;42(2):265–278. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JC, Xu Y, Huang ZM, Lu XJ. T cell exhaustion in cancer: mechanisms and clinical implications. J Cell Biochem. 2018;119(6):4279–4286. doi: 10.1002/jcb.26645. [DOI] [PubMed] [Google Scholar]
- 17.Mann TH, Kaech SM. Tick-TOX, it's time for T cell exhaustion. Nat Immunol. 2019;20(9):1092–1094. doi: 10.1038/s41590-019-0478-y. [DOI] [PubMed] [Google Scholar]
- 18.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philip M, Schietinger A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr Opin Immunol. 2019;58:98–103. doi: 10.1016/j.coi.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu N, Xiu Y, Duan D. Research progress in mechanisms of T cell exhaustion. Chinese J Immunol. 2021;37(01):103–108. [Google Scholar]
- 21.Ren D, Hua Y, Yu B, Ye X, He Z, Li C, et al. Predictive biomarkers and mechanisms underlying resistance to PD1/PD-L1 blockade cancer immunotherapy. Mol Cancer. 2020;19(1):19. doi: 10.1186/s12943-020-1144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Chen M, Xu D, Li TE, Zhang Z, Li JH, et al. The combination of PD-1 blockade with interferon-α has a synergistic effect on hepatocellular carcinoma. Cell Mol Immunol. 2022;19(6):726–737. doi: 10.1038/s41423-022-00848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. 2022;23(5):660–670. doi: 10.1038/s41590-022-01141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016;44(6):1255–1269. doi: 10.1016/j.immuni.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Wu M, Huang Q, Xie Y, Wu X, Ma H, Zhang Y, et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. 2022;15(1):022–01242. doi: 10.1186/s13045-022-01242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsao SY. Perspectives of traditional Chinese medicine to patch up immune checkpoint blockers. Explor Target Antitumor Ther. 2022;3(5):676–693. doi: 10.37349/etat.2022.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 29.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21(1):24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer BE, Neff CP, Lecureux J, Ehler A, Dsouza M, Remling-Mulder L, et al. In vivo blockade of the PD-1 receptor suppresses HIV-1 viral loads and improves CD4+ T cell levels in humanized mice. J Immunol. 2013;190(1):211–219. doi: 10.4049/jimmunol.1201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang ZS, Hao YH, Zhang EJ, Xu CL, Zhou Y, Zheng X, et al. CD28 family of receptors on T cells in chronic HBV infection: expression characteristics, clinical significance and correlations with PD-1 blockade. Mol Med Rep. 2016;14(2):1107–1116. doi: 10.3892/mmr.2016.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 33.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthumani K, Shedlock DJ, Choo DK, Fagone P, Kawalekar OU, Goodman J, et al. HIV-mediated phosphatidylinositol 3-kinase/serine-threonine kinase activation in APCs leads to programmed death-1 ligand upregulation and suppression of HIV-specific CD8 T cells. J Immunol. 2011;187(6):2932–2943. doi: 10.4049/jimmunol.1100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rha MS, Shin EC. Activation or exhaustion of CD8(+) T cells in patients with COVID-19. Cell Mol Immunol. 2021;18(10):2325–2333. doi: 10.1038/s41423-021-00750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elahi S, Shahbaz S, Houston S. Selective upregulation of CTLA-4 on CD8+ T cells restricted by HLA-B*35Px renders them to an exhausted phenotype in HIV-1 infection. PLoS Pathog. 2020;16(8):e1008696. doi: 10.1371/journal.ppat.1008696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53(5):1494–1503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Shi L, Zhao Z, Du P, Ye X, Li D, et al. Disruption of CTLA-4 expression on peripheral blood CD8 + T cell enhances anti-tumor efficacy in bladder cancer. Cancer Chemother Pharmacol. 2019;83(5):911–920. doi: 10.1007/s00280-019-03800-x. [DOI] [PubMed] [Google Scholar]
- 39.Avery L, Filderman J, Szymczak-Workman AL, Kane LP. Tim-3 co-stimulation promotes short-lived effector T cells, restricts memory precursors, and is dispensable for T cell exhaustion. Proc Natl Acad Sci USA. 2018;115(10):2455–2460. doi: 10.1073/pnas.1712107115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrando-Martinez S, Snell Bennett A, Lino E, Gehring AJ, Feld J, Janssen HLA, et al. Functional exhaustion of HBV-specific CD8 T cells impedes PD-L1 blockade efficacy in chronic HBV infection. Front Immunol. 2021;12:648420. doi: 10.3389/fimmu.2021.648420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao H, Li B, Pang N, Li Z, Aibibula M, Tian F, et al. High expression of Tim-3 in alveolar echinococcosis mediates depletion of CD8(+) T cell function. Ann Clin Lab Sci. 2021;51(6):827–836. [PubMed] [Google Scholar]
- 42.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83(18):9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205(12):2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang R, Zhu X, Lan T, Ding D, Zheng Z, Chen T, et al. TIGIT promotes CD8(+)T cells exhaustion and predicts poor prognosis of colorectal cancer. Cancer Immunol Immunother. 2021;70(10):2781–2793. doi: 10.1007/s00262-021-02886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan S, Xu Y, Wang Z, Wang T, Du X, Song X, et al. Tim-3 hampers tumor surveillance of liver-resident and conventional NK cells by disrupting PI3K signaling. Can Res. 2020;80(5):1130–1142. doi: 10.1158/0008-5472.CAN-19-2332. [DOI] [PubMed] [Google Scholar]
- 46.Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int Immunol. 2010;22(1):13–23. doi: 10.1093/intimm/dxp107. [DOI] [PubMed] [Google Scholar]
- 47.Lecocq Q, Keyaerts M, Devoogdt N, Breckpot K. The next-generation immune checkpoint LAG-3 and its therapeutic potential in oncology: third time's a charm. Int J Mol Sci. 2020;22(1):75. doi: 10.3390/ijms22010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Wu G, Manick B, Hernandez V, Renelt M, Erickson C, et al. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology. 2019;156(1):74–85. doi: 10.1111/imm.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci USA. 2015;112(21):6682–6687. doi: 10.1073/pnas.1420370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zapała Ł, Kunc M, Sharma S, Pęksa R, Popęda M, Biernat W, et al. Immune checkpoint receptor VISTA on immune cells is associated with expression of T-cell exhaustion marker TOX and worse prognosis in renal cell carcinoma with venous tumor thrombus. J Cancer Res Clin Oncol. 2022;30(10):022–04329. doi: 10.1007/s00432-022-04329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho H, Kang H, Lee HH, Kim CW. Programmed cell death 1 (PD-1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) in viral hepatitis. Int J Mol Sci. 2017;18(7):1517. doi: 10.3390/ijms18071517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng G, Luo B, Li J, Zhao D, Wu W, Chen F, et al. Hepatitis B e-antigen persistency is associated with the properties of HBV-specific CD8 T cells in CHB patients. J Clin Immunol. 2011;31(2):195–204. doi: 10.1007/s10875-010-9483-5. [DOI] [PubMed] [Google Scholar]
- 53.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107(33):14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mimura K, Kua LF, Xiao JF, Asuncion BR, Nakayama Y, Syn N, et al. Combined inhibition of PD-1/PD-L1, Lag-3, and Tim-3 axes augments antitumor immunity in gastric cancer-T cell coculture models. Gastric Cancer. 2021;24(3):611–623. doi: 10.1007/s10120-020-01151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354(6316):1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, et al. De Novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell. 2017;170(1):142–157. doi: 10.1016/j.cell.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prinzing B, Zebley CC, Petersen CT, Fan Y, Anido AA, Yi Z, et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med. 2021;13(620):17. doi: 10.1126/scitranslmed.abh0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571(7764):211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571(7764):270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Franco F, Jaccard A, Romero P, Yu YR, Ho PC. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020;2(10):1001–1012. doi: 10.1038/s42255-020-00280-9. [DOI] [PubMed] [Google Scholar]
- 61.Seo H, Chen J, González-Avalos E, Samaniego-Castruita D, Das A, Wang YH, et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8(+) T cell exhaustion. Proc Natl Acad Sci U S A. 2019;116(25):12410–12415. doi: 10.1073/pnas.1905675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, Wang Y, Lu H, Li J, Yan X, Xiao M, et al. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature. 2019;567(7749):525–529. doi: 10.1038/s41586-019-0979-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pauken KE, Wherry EJ. SnapShot: T cell exhaustion. Cell. 2015;163(4):10381-e1. doi: 10.1016/j.cell.2015.10.054. [DOI] [PubMed] [Google Scholar]
- 64.Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, et al. The epigenetic landscape of T cell exhaustion. Science. 2016;354(6316):1165–1169. doi: 10.1126/science.aae0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302(5647):1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 66.Chen Z, Ji Z, Ngiow SF, Manne S, Cai Z, Huang AC, et al. TCF-1-centered transcriptional network drives an effector versus exhausted CD8 T cell-fate decision. Immunity. 2019;51(5):840–855. doi: 10.1016/j.immuni.2019.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37(6):1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338(6111):1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beltra JC, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, et al. Developmental relationships of four exhausted CD8(+) T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. 2020;52(5):825–41.e8. doi: 10.1016/j.immuni.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yates KB, Tonnerre P, Martin GE, Gerdemann U, Al Abosy R, Comstock DE, et al. Epigenetic scars of CD8(+) T cell exhaustion persist after cure of chronic infection in humans. Nat Immunol. 2021;22(8):1020–1029. doi: 10.1038/s41590-021-00979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peng M, Yin N, Chhangawala S, Xu K, Leslie CS, Li MO. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science. 2016;354(6311):481–484. doi: 10.1126/science.aaf6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qorraj M, Bruns H, Böttcher M, Weigand L, Saul D, Mackensen A, et al. The PD-1/PD-L1 axis contributes to immune metabolic dysfunctions of monocytes in chronic lymphocytic leukemia. Leukemia. 2017;31(2):470–478. doi: 10.1038/leu.2016.214. [DOI] [PubMed] [Google Scholar]
- 73.Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity. 2016;45(2):358–373. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Møller SH, Hsueh PC, Yu YR, Zhang L, Ho PC. Metabolic programs tailor T cell immunity in viral infection, cancer, and aging. Cell Metab. 2022;34(3):378–395. doi: 10.1016/j.cmet.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med. 2018;24(7):994–1004. doi: 10.1038/s41591-018-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30(1):143–156. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma X, Yi Q. Cholesterol induces T cell exhaustion. Aging (Albany NY). 2019;11(18):7334–7335. doi: 10.18632/aging.102305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nabe S, Yamada T, Suzuki J, Toriyama K, Yasuoka T, Kuwahara M, et al. Reinforce the antitumor activity of CD8(+) T cells via glutamine restriction. Cancer Sci. 2018;109(12):3737–3750. doi: 10.1111/cas.13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016;16(10):599–611. doi: 10.1038/nri.2016.80. [DOI] [PubMed] [Google Scholar]
- 81.Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by d-1-methyl-tryptophan. Oncoimmunology. 2012;1(9):1460–1468. doi: 10.4161/onci.21716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Das A, Hoare M, Davies N, Lopes AR, Dunn C, Kennedy PT, et al. Functional skewing of the global CD8 T cell population in chronic hepatitis B virus infection. J Exp Med. 2008;205(9):2111–2124. doi: 10.1084/jem.20072076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinclair LV, Howden AJ, Brenes A, Spinelli L, Hukelmann JL, Macintyre AN, et al. Antigen receptor control of methionine metabolism in T cells. Elife. 2019;27(8):44210. doi: 10.7554/eLife.44210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Angin M, Volant S, Passaes C, Lecuroux C, Monceaux V, Dillies MA, et al. Metabolic plasticity of HIV-specific CD8(+) T cells is associated with enhanced antiviral potential and natural control of HIV-1 infection. Nat Metab. 2019;1(7):704–716. doi: 10.1038/s42255-019-0081-4. [DOI] [PubMed] [Google Scholar]
- 85.Barili V, Fisicaro P, Montanini B, Acerbi G, Filippi A, Forleo G, et al. Targeting p53 and histone methyltransferases restores exhausted CD8+ T cells in HCV infection. Nat Commun. 2020;11(1):019–14137. doi: 10.1038/s41467-019-14137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fisicaro P, Barili V, Montanini B, Acerbi G, Ferracin M, Guerrieri F, et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat Med. 2017;23(3):327–336. doi: 10.1038/nm.4275. [DOI] [PubMed] [Google Scholar]
- 87.Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366(6468):1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Buck MD, O'Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166(1):63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siska PJ, Beckermann KE, Mason FM, Andrejeva G, Greenplate AR, Sendor AB, et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight. 2017;2(12):15. doi: 10.1172/jci.insight.93411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li W, Cheng H, Li G, Zhang L. Mitochondrial damage and the road to exhaustion. Cell Metab. 2020;32(6):905–907. doi: 10.1016/j.cmet.2020.11.004. [DOI] [PubMed] [Google Scholar]
- 91.Scharping NE, Rivadeneira DB, Menk AV, Vignali PDA, Ford BR, Rittenhouse NL, et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol. 2021;22(2):205–215. doi: 10.1038/s41590-020-00834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45(2):374–388. doi: 10.1016/j.immuni.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27(9):3282–3289. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013;14(11):1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palazon A, Tyrakis PA, Macias D, Veliça P, Rundqvist H, Fitzpatrick S, et al. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell. 2017;32(5):669–683. doi: 10.1016/j.ccell.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol. 2012;86(15):8161–8170. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ladell K, Hazenberg MD, Fitch M, Emson C, McEvoy-Hein Asgarian BK, Mold JE, et al. Continuous antigenic stimulation of DO11.10 TCR transgenic mice in the presence or absence of IL-1β: possible implications for mechanisms of T cell depletion in HIV disease. J Immunol. 2015;195(9):4096–4105. doi: 10.4049/jimmunol.1500799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuo IY, Yang YE, Yang PS, Tsai YJ, Tzeng HT, Cheng HC, et al. Converged Rab37/IL-6 trafficking and STAT3/PD-1 transcription axes elicit an immunosuppressive lung tumor microenvironment. Theranostics. 2021;11(14):7029–7044. doi: 10.7150/thno.60040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li C, Zuo W. IL-10 in vitro could enhance IFNγ expression and suppress PD-1 expression in CD8 T cells from esophageal cancer patients. Exp Cell Res. 2019;379(2):159–165. doi: 10.1016/j.yexcr.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 100.Jimbu L, Mesaros O, Neaga A, Nanut AM, Tomuleasa C, Dima D, et al. The potential advantage of targeting both PD-L1/PD-L2/PD-1 and IL-10-IL-10R pathways in acute myeloid leukemia. Pharmaceuticals (Basel, Switzerland). 2021;14(11):1105. doi: 10.3390/ph14111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554(7693):538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 102.Li M, Sun R, Xu L, Yin W, Chen Y, Zheng X, et al. Kupffer cells support hepatitis B virus-mediated CD8+ T cell exhaustion via hepatitis B Core antigen-TLR2 interactions in mice. J Immunol. 2015;195(7):3100–3109. doi: 10.4049/jimmunol.1500839. [DOI] [PubMed] [Google Scholar]
- 103.Barathan M, Mohamed R, Vadivelu J, Chang LY, Vignesh R, Krishnan J, et al. CD8+ T cells of chronic HCV-infected patients express multiple negative immune checkpoints following stimulation with HCV peptides. Cell Immunol. 2017;313:1–9. doi: 10.1016/j.cellimm.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Li M, Guo W, Dong Y, Wang X, Dai D, Liu X, et al. Elevated exhaustion levels of NK and CD8(+) T cells as indicators for progression and prognosis of COVID-19 disease. Front Immunol. 2020;11:580237. doi: 10.3389/fimmu.2020.580237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oldstone MB. A Jekyll and Hyde Profile: type 1 interferon signaling plays a prominent role in the initiation and maintenance of a persistent virus infection. J Infect Dis. 2015;1(Suppl 1):S31–S36. doi: 10.1093/infdis/jiu501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boettler T, Cheng Y, Ehrhardt K, von Herrath M. TGF-β blockade does not improve control of an established persistent viral infection. Viral Immunol. 2012;25(3):232–238. doi: 10.1089/vim.2011.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xie F, Zhou X, Su P, Li H, Tu Y, Du J, et al. Breast cancer cell-derived extracellular vesicles promote CD8(+) T cell exhaustion via TGF-β type II receptor signaling. Nat Commun. 2022;13(1):022–31250. doi: 10.1038/s41467-022-31250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin HT, Jeong YH, Park HJ, Ha SJ. Mechanism of T cell exhaustion in a chronic environment. BMB Rep. 2011;44(4):217–231. doi: 10.5483/BMBRep.2011.44.4.217. [DOI] [PubMed] [Google Scholar]
- 109.Totzke J, Gurbani D, Raphemot R, Hughes PF, Bodoor K, Carlson DA, et al. Takinib, a selective TAK1 inhibitor, broadens the therapeutic efficacy of TNF-α inhibition for cancer and autoimmune disease. Cell Chem Biol. 2017;24(8):1029–1039. doi: 10.1016/j.chembiol.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang M, Lin C, Wang Y, Chen K, Han Y, Zhang H, et al. Cytokine storm promoting T cell exhaustion in severe COVID-19 revealed by single cell sequencing data analysis. Precis Clin Med. 2022;5(2). [DOI] [PMC free article] [PubMed]
- 112.Kalia V, Sarkar S. Regulation of effector and memory CD8 T cell differentiation by IL-2—a balancing act. Front Immunol. 2018;9:2987. doi: 10.3389/fimmu.2018.02987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bénéchet AP, De Simone G, Di Lucia P, Cilenti F, Barbiera G, Le Bert N, et al. Dynamics and genomic landscape of CD8(+) T cells undergoing hepatic priming. Nature. 2019;574(7777):200–205. doi: 10.1038/s41586-019-1620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abbas AK, Trotta E, D RS, Marson A, Bluestone JA. Revisiting IL-2: biology and therapeutic prospects. Sci Immunol. 2018;3(25). [DOI] [PubMed]
- 115.Liu Y, Zhou N, Zhou L, Wang J, Zhou Y, Zhang T, et al. IL-2 regulates tumor-reactive CD8(+) T cell exhaustion by activating the aryl hydrocarbon receptor. Nat Immunol. 2021;22(3):358–369. doi: 10.1038/s41590-020-00850-9. [DOI] [PubMed] [Google Scholar]
- 116.Schurich A, Pallett LJ, Jajbhay D, Wijngaarden J, Otano I, Gill US, et al. Distinct metabolic requirements of exhausted and functional virus-specific CD8 T cells in the same host. Cell Rep. 2016;16(5):1243–1252. doi: 10.1016/j.celrep.2016.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462(7272):510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324(5934):1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, et al. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol. 2011;85(5):2316–2324. doi: 10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, et al. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci USA. 2011;108(52):21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cook KD, Whitmire JK. The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J Immunol. 2013;190(2):641–649. doi: 10.4049/jimmunol.1202448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med. 2009;206(10):2235–2251. doi: 10.1084/jem.20082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang H, Franco F, Ho PC. Metabolic regulation of Tregs in cancer: opportunities for immunotherapy. Trends Cancer. 2017;3(8):583–592. doi: 10.1016/j.trecan.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 124.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sawant DV, Yano H, Chikina M, Zhang Q, Liao M, Liu C, et al. Adaptive plasticity of IL-10(+) and IL-35(+) T(reg) cells cooperatively promotes tumor T cell exhaustion. Nat Immunol. 2019;20(6):724–735. doi: 10.1038/s41590-019-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Markowitz J, Wesolowski R, Papenfuss T, Brooks TR, Carson WE., 3rd Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res Treat. 2013;140(1):13–21. doi: 10.1007/s10549-013-2618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy C, et al. Targeting tumor-associated macrophages and granulocytic myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J Clin Investig. 2020;130(10):5380–5396. doi: 10.1172/JCI137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Holtzhausen A, Harris W, Ubil E, Hunter DM, Zhao J, Zhang Y, et al. TAM family receptor kinase inhibition reverses MDSC-mediated suppression and augments Anti-PD-1 therapy in melanoma. Cancer Immunol Res. 2019;7(10):1672–1686. doi: 10.1158/2326-6066.CIR-19-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu H, Gu Y, Xue Y, Yuan M, Cao X, Liu Q. CXCR2(+) MDSCs promote breast cancer progression by inducing EMT and activated T cell exhaustion. Oncotarget. 2017;8(70):114554–114567. doi: 10.18632/oncotarget.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gan C, Li X, Liu X, Li F, Niu H, Zhu B. T cell exhaustion induced by persistent mycobacterium bovis BCG purified protein derivative stimulation could be restored by a Chinese Herbal Bushen Jianpi Recipe in mice model. Chin J Immunol. 2017;33(12):1804–1809. [Google Scholar]
- 131.Xu Y, Wang H, Wang T, Chen C, Sun R, Yao W, et al. Dahuang Fuzi Baijiang decoction restricts progenitor to terminally exhausted T cell differentiation in colorectal cancer. Cancer Sci. 2022;113(5):1739–1751. doi: 10.1111/cas.15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan F, Wang X, Xie Y, Liu X, Yu L, Wang P, et al. Yangyin Fuzheng Jiedu Prescription exerts anti-tumor immunity in hepatocellular carcinoma by alleviating exhausted T cells. Phytomedicine. 2021;91(153722):19. doi: 10.1016/j.phymed.2021.153722. [DOI] [PubMed] [Google Scholar]
- 133.Zhu G, Zhang B, Jiang F, Zhao L, Liu F. ShenQi FuZheng injection ameliorates fatigue-like behavior in mouse models of cancer-related fatigue. Biomed Pharmacother Biomed Pharmacother. 2019;111:1376–1382. doi: 10.1016/j.biopha.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 134.Chen H, Sun X, Wei D, Jiang M, Chen M, Liu L, et al. Research on mechanism of regulating PD-1/PD-L1 pathway by Yangyin Fuzheng decoction to reverse T cell depletion. Chin J Tradit Med Sci Technol. 2022;29(02):190–194. [Google Scholar]
- 135.Chen H. Molecular mechanism of Yangyin Fuzheng Prescription regulating PD-1/PD-L1 signaling pathway in the treatment of non-small cell lung cancer. 2020. (in Chinese)
- 136.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;816460(10):18. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pu J, Xu Z, Nian J, Fang Q, Yang M, Huang Y, et al. M2 macrophage-derived extracellular vesicles facilitate CD8+T cell exhaustion in hepatocellular carcinoma via the miR-21-5p/YOD1/YAP/β-catenin pathway. Cell Death Discov. 2021;7(1):182. doi: 10.1038/s41420-021-00556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen Y, Wu H, Jiao AN, Tong JB, Zhu J, Zhang M, et al. Chinese herbal prescription QYSL prevents progression of lung cancer by targeting tumor microenvironment. Oncologie. 2022;24(2):295–307. doi: 10.32604/oncologie.2022.022116. [DOI] [Google Scholar]
- 139.Yang Y, Sun M, Yao W, Wang F, Li X, Wang W, et al. Compound kushen injection relieves tumor-associated macrophage-mediated immunosuppression through TNFR1 and sensitizes hepatocellular carcinoma to sorafenib. J Immunother Cancer. 2020;8(1):2019–000317. doi: 10.1136/jitc-2019-000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Verdura S, Cuyàs E, Cortada E, Brunet J, Lopez-Bonet E, Martin-Castillo B, et al. Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging. 2020;12(1):8–34. doi: 10.18632/aging.102646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chun KS, Kim DH, Raut PK, Surh YJ. Anticancer natural products targeting immune checkpoint protein network. Semin Cancer Biol. 2022;86(Pt 3):1008–1032. doi: 10.1016/j.semcancer.2021.11.006. [DOI] [PubMed] [Google Scholar]
- 142.Liu L, Lim MA, Jung SN, Oh C, Won HR, Jin YL, et al. The effect of Curcumin on multi-level immune checkpoint blockade and T cell dysfunction in head and neck cancer. Phytomedicine. 2021;92(153758):16. doi: 10.1016/j.phymed.2021.153758. [DOI] [PubMed] [Google Scholar]
- 143.Espinoza JL, Trung LQ, Inaoka PT, Yamada K, An DT, Mizuno S, et al. The repeated administration of resveratrol has measurable effects on circulating T-Cell subsets in humans. Oxid Med Cell Longev. 2017;6781872(10):4. doi: 10.1155/2017/6781872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chang FL, Tsai KC, Lin TY, Yang TW, Lo YN, Chen WC, et al. Astragalus membranaceus-derived anti-programmed death-1 monoclonal antibodies with immunomodulatory therapeutic effects against tumors. Biomed Res Int. 2020;2020:1–11. doi: 10.1155/2020/3415471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peng Q, Li S, Shi X, Guo Y, Hao L, Zhang Z, et al. Dihydroartemisinin broke the tumor immunosuppressive microenvironment by inhibiting YAP1 expression to enhance anti-PD-1 efficacy. Phytotherapy research : PTR. 2023;37(5):1740–1753. doi: 10.1002/ptr.7695. [DOI] [PubMed] [Google Scholar]
- 146.Su J, Su L, Li D, Shuai O, Zhang Y, Liang H, et al. Antitumor activity of extract from the sporoderm-breaking spore of ganoderma lucidum: restoration on exhausted cytotoxic T cell with gut microbiota remodeling. Front Immunol. 2018;9:1765. doi: 10.3389/fimmu.2018.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jin L, Wu R, Liu X, Zhu X, Shou Q, Fu H. Hirsutella Sinensis fungus regulates CD8(+) T cell exhaustion through involvement of T-Bet/Eomes in the tumor microenvironment. Front Pharmacol. 2021;11(612620). [DOI] [PMC free article] [PubMed]
- 148.Gonzalez-Gugel E, Saxena M, Bhardwaj N. Modulation of innate immunity in the tumor microenvironment. Cancer Immunol Immunother CII. 2016;65(10):1261–1268. doi: 10.1007/s00262-016-1859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma Y, Shurin GV, Peiyuan Z, Shurin MR. Dendritic cells in the cancer microenvironment. J Cancer. 2013;4(1):36–44. doi: 10.7150/jca.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 151.Jiang M, Zhang WW, Liu P, Yu W, Liu T, Yu J. Dysregulation of SOCS-mediated negative feedback of cytokine signaling in carcinogenesis and its significance in cancer treatment. Front Immunol. 2017;8:70. doi: 10.3389/fimmu.2017.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y, Huang H, Yao S, Li G, Xu C, Ye Y, et al. A lipid-soluble extract of Pinellia pedatisecta Schott enhances antitumor T cell responses by restoring tumor-associated dendritic cell activation and maturation. J Ethnopharmacol. 2019;241(111980):28. doi: 10.1016/j.jep.2019.111980. [DOI] [PubMed] [Google Scholar]
- 153.Dong Q, Pu J, Du T, Xu S, Liu W, Liu L, et al. Astragalus-mediated stimulation on antigen-presenting cells could result in higher IL-21 production from CXCR5(+) Tfh-like cells and better IL-21-mediated effector functions. Hum Immunol. 2021;82(6):429–437. doi: 10.1016/j.humimm.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 154.Deng X, Luo S, Luo X, Hu M, Ma F, Wang Y, et al. Polysaccharides from Chinese Herbal Lycium barbarum induced systemic and local immune responses in H22 tumor-bearing mice. J Immunol Res. 2018;2018:3431782. doi: 10.1155/2018/3431782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jiang ZB, Wang WJ, Xu C, Xie YJ, Wang XR, Zhang YZ, et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021;515:36–48. doi: 10.1016/j.canlet.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 156.Shao Y, Zhu W, Da J, Xu M, Wang Y, Zhou J, et al. Bisdemethoxycurcumin in combination with α-PD-L1 antibody boosts immune response against bladder cancer. Onco Targets Ther. 2017;10:2675–2683. doi: 10.2147/OTT.S130653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Warowicka A, Nawrot R, Broniarczyk J, Węglewska M, Goździcka-Józefiak A. Oncogenic viruses and cancer. Postepy Biochem. 2021;66(4):336–355. doi: 10.18388/pb.2020_360. [DOI] [PubMed] [Google Scholar]
- 158.McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;1(Suppl 1):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li X, Wenes M, Romero P, Huang SC, Fendt SM, Ho PC. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat Rev Clin Oncol. 2019;16(7):425–441. doi: 10.1038/s41571-019-0203-7. [DOI] [PubMed] [Google Scholar]
- 160.Cascone T, McKenzie JA, Mbofung RM, Punt S, Wang Z, Xu C, et al. Increased tumor glycolysis characterizes immune resistance to adoptive T cell therapy. Cell Metab. 2018;27(5):977–987. doi: 10.1016/j.cmet.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.He QF, Xu Y, Li J, Huang ZM, Li XH, Wang X. CD8+ T-cell exhaustion in cancer: mechanisms and new area for cancer immunotherapy. Brief Funct Genomics. 2019;18(2):99–106. doi: 10.1093/bfgp/ely006. [DOI] [PubMed] [Google Scholar]
- 162.Ferris RL, Lu B, Kane LP. Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J Immunol. 2014;193(4):1525–1530. doi: 10.4049/jimmunol.1400557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Previte DM, Martins CP, O'Connor EC, Marre ML, Coudriet GM, Beck NW, et al. Lymphocyte activation Gene-3 maintains mitochondrial and metabolic quiescence in Naive CD4(+) T cells. Cell Rep. 2019;27(1):129–141. doi: 10.1016/j.celrep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 164.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aboushanab TS, AlSanad S. Cupping therapy: an overview from a modern medicine perspective. J Acupunct Meridian Stud. 2018;11(3):83–87. doi: 10.1016/j.jams.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 166.Liu Q, Lou Y, Li L, Yang G, Cui H, Cheng Z, et al. A single-arm phase II study to evaluate efficacy and safety of first-line treatment with DCVAC/LuCa, standard of care chemotherapy and Shenqi Fuzheng injection in advanced (Stage IIIB/IV) non-small cell lung cancer patients. Integr Cancer Ther. 2022;21:15347354221083968. doi: 10.1177/15347354221083968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sun Q, Ma W, Gao Y, Zheng W, Zhang B, Peng Y. Meta-analysis: therapeutic effect of transcatheter arterial chemoembolization combined with compound Kushen injection in hepatocellular carcinoma. Afr J Tradit Complement Altern Med AJTCAM. 2012;9(2):178–188. doi: 10.4314/ajtcam.v9i2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chang H, Lei L, Zhou Y, Ye F, Zhao G. Dietary flavonoids and the risk of colorectal cancer: an updated meta-analysis of epidemiological studies. Nutrients. 2018;10(7):950. doi: 10.3390/nu10070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.