Abstract
Objectives
This study aimed to establish a standard for selecting bone graft type for thoracolumbar spinal tuberculosis surgery based on the spinal instability neoplastic score (SINS).
Methods
Patients with thoracolumbar tuberculosis who underwent one-stage debridement posteriorly and instrumentation were divided into a structural bone graft group (SBG) (51 cases) and a non-structural bone graft group (NSBG) (54 cases) according to their SINS. SBG was performed when the SINS was ≥ 13 and NSBG was performed when it was 7 ≤ SINS ≤ 12. Baseline data, clinical outcomes, and imaging outcomes were collected and statistically analyzed between the two groups.
Results
Significant improvements in clinical and imaging outcomes were achieved in both groups. Compared to the SBG group, the operation time of the NSBG group was shorter, the intraoperative blood loss of the NSBG group was less, the bone fusion time of the NSBG group was faster.
Conclusion
Non-structural and structural bone grafting can achieve comparable therapeutic effects in patients with spinal tuberculosis, and a suitable selection of bone grafts based on quantitative SINS will make full use of the advantages of different bone grafts.
Keywords: Thoracic vertebrae, Lumbar vertebrae, Tuberculosis, Spinal, Bone transplantation, Surgical procedures, Operative
Introduction
Spinal tuberculosis is the most common form of extrapulmonary tuberculosis, accounting for approximately half of all cases of skeletal tuberculosis [1]. Spinal tuberculosis typically destroys the intervertebral discs and adjacent vertebrae, leading to spinal instability and kyphosis [2–4]. When surgery is indicated, the affected vertebral bone and disc should be removed, which always requires bone grafting. Many types of bone grafts have been successfully reported, and are mainly divided into non-structural and structural types, including the ilium, titanium mesh, and rib bone [5–7]. However, precise criteria for selecting the appropriate bone graft type are lacking [8]. Spinal instability is a surgical indication for many spinal disorders, such as spinal tuberculosis, and many criteria have been proposed and reported. However, there is no consensus regarding the criteria for evaluating the stability of tuberculosis-infected spinal columns. The spinal instability neoplastic score (SINS) has been widely used to evaluate the stability of the tumor-involved spine by assessing six factors: location, mechanical pain, bone lesion quality, spinal alignment, vertebral body collapse, and posterolateral involvement of spinal elements [9]. Inspired by the SINS, we aimed to use it to evaluate the spinal stability of tuberculosis-infected spines.
The objective of the present retrospective cohort study was to assess the results of patients with thoracolumbar tuberculosis who underwent reconstruction using non-structural or structural bone grafts after one-stage posterior debridement and internal fixation determined by SINS, to establish a rule for selecting bone grafts for spinal reconstruction and fusion of thoracolumbar tuberculosis.
Materials and methods
The Ethics Committee of The First Affiliated Hospital of Chongqing Medical University approved this study (2019 − 123), and informed consent was obtained from all patients in this research. All methods were carried out in accordance with relevant guidelines and regulations.
Selection of patients
Between 2016 and 2020, 105 patients who were diagnosed with thoracolumbar tuberculosis and underwent posterior surgery were retrospectively reviewed. The selection criteria were as follows: (1) pathologically confirmed thoracolumbar spinal tuberculosis; (2) single-segment tuberculosis; (3) surgical treatment with one-stage posterior debridement, bone graft fusion, and instrumentation; (4) a structural bone graft (SBG) (titanium mesh bone graft) or non-structural bone graft (NSBG) (autologous granular bone graft) was used in the procedure; (5) the follow-up time was no less than two years; and (6) all required data had been collected completely. The exclusion criteria were as follows: (1)spinal tuberculosis involving the cervical spine; (2) patients who have previously undergone spinal procedures; and (3) patients with primary or metastatic tumors and active pulmonary tuberculosis.
Preoperative management
Four anti-tuberculosis drugs (isoniazid 0.3 g qd; rifampicin 0.45 g qd; pyrazinamide 1.5 g qd; and ethambutol 0.75 g qd) were used for 2–4 weeks before the operation. Toxic manifestations of tuberculosis were alleviated, and the patients’ comorbidities were controlled preoperatively.
Operative procedure
All patients received general anesthesia. A longitudinal midline incision was made in the prone position to expose the posterior elements of the spine. One or two normal vertebral bodies above and below the lesion segments were instrumented using suitable posterior pedicle screws. Temporary stability was maintained using a rod that was fixed on the side of the debridement. Unilateral laminectomy or pedicle resection was performed to remove the necrotic tissue, bone fragments, and necrotic discs.
SBG was performed when SINS was ≥ 13 and NSBG was performed when 7 ≤ SINS ≤ 12. In the SBG group (Fig. 1), a sizable titanium mesh filled with crushed granular bone particles and 1.0 g of streptomycin was implanted into the vertebra. In the NSBG group (Fig. 2), the vertebral body was implanted with granular bones from the spinous process and vertebral plate that was harvested intraoperatively. The posterior part of the vertebral body was covered by a gelatin sponge containing isoniazid when granular bones entered the spinal canal. After washing with enough normal saline, isoniazid 0.3 g and streptomycin powder 1.0 g were placed in the surgical site for local treatment. Finally, the rods were locked and the surgical incision was sutured.
Fig. 1.
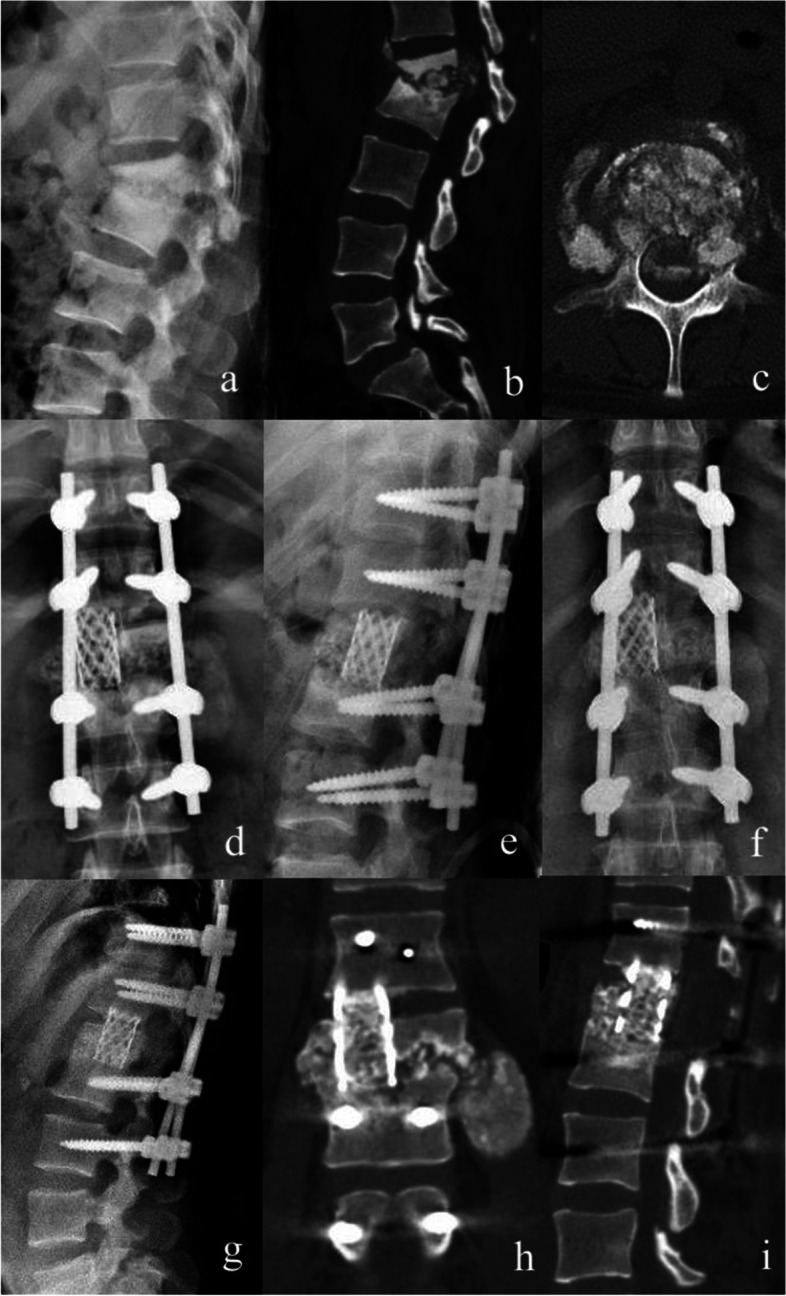
A 31 years old female diagnosed as L1-2 tuberculosis and treated with structural bone graft. a-c Preoperative X-ray and CT scan. d-e Postoperative X-ray. f-i X-ray and CT scan at last follow-up
Fig. 2.
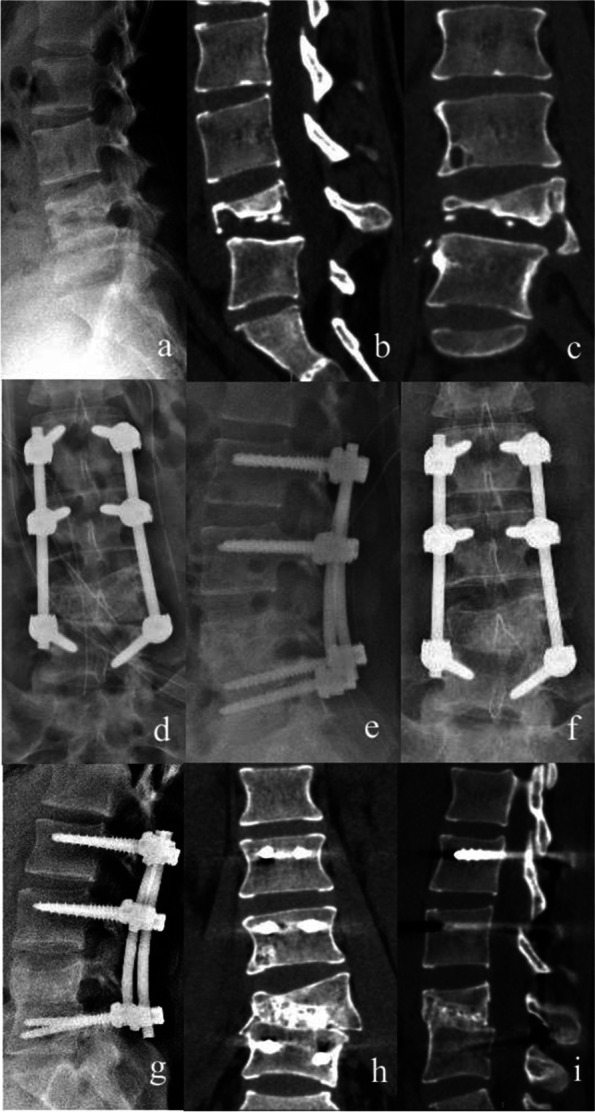
A 22 years old female diagnosed as L4-5 tuberculosis and treated with non-structural bone graft. a-c Preoperative X-ray and CT scan. d-e Postoperative X-ray. f-i X-ray and CT scan at last follow-up
Postoperative management
Antibiotics were administered within 3 days to prevent infection. The drainage tube removal criterion was a drainage volume of less than 40 mL/day. The patients were treated with the same anti-tuberculosis drugs for 18–24 months. Braces were used for 3–6 months after surgery. A high-protein nutritional diet was provided throughout the treatment period. Routine blood examinations, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), liver function, kidney function, X-ray, computed tomography, and magnetic resonance imaging, when necessary, were performed during follow-up at 1, 3, 6, and 12 months postoperatively.
Follow-up index
The operation time, bleeding volume, and length of hospital stay were recorded. Visual analog scale (VAS), Oswestry disability index (ODI), ESR, CRP, and American Spinal Injury Association (ASIA) grade of neurological function were assessed before surgery and at the last follow-up. The criterion of Bridwell et al. was used to evaluate the bone graft fusion time according to the results of computed tomography scans during each follow-up [10]. The four grades were described by Bridwell et al. [10]. In the present study, bone graft fusion was defined as grade I or II. The correction of the Cobb angle was defined as the difference between post- and pre-operative Cobb angles. Loss of Cobb angle was defined as the difference between the last follow-up Cobb angle and the postoperative Cobb angle.
Statistical analysis
SPSS 21.0 was used for analyzing data. The Kolmogorov–Smirnov test was used to assess if the values were normally distributed. An independent sample t-test was used to compare normally distributed continuous variables (presented as the mean ± standard deviation) between the two groups. The Mann-Whitney U test was used to compare non-normally distributed continuous variables (presented as median and range) between the two groups. The chi-squared test was used to compare categorical variables between the two groups. A matched t-test was used for intragroup comparisons of normally distributed continuous variables. The significance level was set as 0.05.
Results
Patients’ baseline information
During the surgical procedure, 51 and 54 patients received structural and non-structural bone grafts, respectively. The patients were followed for 24–66 months. No significant differences were found in sex, age, body mass index, paravertebral abscess percentage, or follow-up time between the SBG and NSBG groups (Table 1).
Table 1.
Comparison of baseline data between the two groups
| Structural bone graft (N = 51) | Non-structural bone graft (N = 54) | P Value | |
|---|---|---|---|
| Gender (male / female)a | 24/27 | 31/23 | 0.289 |
| Age (year)b | 34(22–76) | 35(21–75) | 0.783 |
| BMI (kg/m2) c | 21.1 ± 2.3 | 21.5 ± 2.5 | 0.353 |
| Paravertebral abscess (yes/no) a | 35/16 | 35/19 | 0.679 |
| Follow up time (month) b | 31 (24–66) | 32 (24–63) | 0.835 |
a The categorical variables were compared by Chi-square test
b The non-normally distributed continuous variables were presented as median (range) and compared by Mann-Whitney U test
c The normally distributed continuous variables were presented as mean ± standard deviation and compared by independent sample t-test
Clinical outcomes
The operation time in the SBG group was longer than that in the NSBG group (P = 0.000). Intraoperative blood loss in the SBG group was higher than that in the NSBG group (P = 0.000). The length of hospital stay did not differ significantly between the two groups. No significant differences were found in preoperative VAS and last follow-up VAS scores between the SBG and NSBG groups. The preoperative ODI of the SBG group was significantly higher than that of the NSBG group (P = 0.041), while no difference in the final follow-up ODI was found between the groups. Compared with the SBG group, the preoperative ESR of the NSBG group was significantly lower (P = 0.005), while no difference was found in the last follow-up ESR between the groups. No significant differences were found in preoperative CRP and last follow-up CRP levels between the SBG and NSBG groups (Table 2). Compared to the corresponding preoperative parameters, the VAS, ODI, ESR, and CRP were all remarkably improved in the last follow-up of each group according to results of matched t-tests (P = 0.000).
Table 2.
Comparison of clinical outcomes between the two groups
| Structural bone graft (N = 51) | Non-structural bone graft (N = 54) | P Value | |
|---|---|---|---|
| Operation time (min) a | 209.7 ± 31.9 | 175.8 ± 33.8 | 0.000 |
| Intraoperative blood loss (ml) a | 552.9 ± 198.3 | 340.7 ± 122.1 | 0.000 |
| Hospital stay (d) a | 15.0 ± 2.8 | 15.7 ± 2.9 | 0.213 |
| Preoperative VAS a | 6.0 ± 1.6 | 5.8 ± 1.7 | 0.421 |
| Last follow up VAS b | 1 (0–5) | 1 (0–4) | 0.568 |
| Preoperative ODI a | 45.1 ± 14.9 | 38.8 ± 16.1 | 0.041 |
| Last follow up ODI a | 16.0 ± 5.8 | 15.0 ± 5.8 | 0.381 |
| Preoperative ESR (mm/h) a | 48.9 ± 14.2 | 41.4 ± 12.7 | 0.005 |
| Last follow up ESR (mm/h) a | 16.4 ± 5.2 | 14.8 ± 5.4 | 0.123 |
| Preoperative CRP (mg/L) a | 26.1 ± 12.6 | 23.9 ± 10.8 | 0.347 |
| Last follow up CRP (mg/L) a | 6.5 ± 3.1 | 7.5 ± 4.3 | 0.163 |
a The normally distributed continuous variables were presented as mean ± standard deviation and compared by independent sample t-test
b The non-normally distributed continuous variables were presented as median (range) and compared by Mann-Whitney U test
Imaging outcomes
No implant failure was observed at the last follow-up. Compared to the SBG group, the preoperative Cobb angle, postoperative Cobb angle, last follow-up Cobb angle, and correction of the Cobb angle were all lower in the NSBG group (P = 0.000, 0.000, 0.002, and 0.019, respectively). The reason may be that the SBG group patients’ kyphosis is generally more serious than that in USBG group patients. There was no significant difference between the loss of Cobb angles in both groups. The bone graft fusion time in the NSBG group was significantly shorter than that in the SBG group (P = 0.007) (Table 3).
Table 3.
Comparison of imaging outcomes between the two groups
| Structural bone graft (N = 51) | Non-structural bone graft (N = 54) | P Value | |
|---|---|---|---|
| Preoperative Cobb angle (°) a | 21.5 ± 6.3 | 14.8 ± 10.5 | 0.000 |
| Postoperative Cobb angle (°) a | 9.2 ± 5.6 | 5.0 ± 6.0 | 0.000 |
| Last follow-up Cobb angle (°) a | 11.6 ± 5.8 | 7.7 ± 7.1 | 0.002 |
| Correction of Cobb angle (°) a | 12.3 ± 4.4 | 9.8 ± 6.3 | 0.019 |
| Loss of Cobb angle (°) a | 2.3 ± 1.5 | 2.7 ± 2.4 | 0.419 |
| Bone graft fusion time (month) b | 7 (4–18) | 6 (4–12) | 0.007 |
a The normally distributed continuous variables were presented as mean ± standard deviation and compared by independent sample t-test
b The non-normally distributed continuous variables were presented as median (range) and compared by Mann-Whitney U test
Neurological function and complications
At the last follow-up in the SBG group, the ASIA scale of two patients improved from C to D, that of two patients improved from C to E, and that of nine patients improved from D to E. At the last follow-up in the NSBG group, the ASIA scale of two patients improved from C to D, that of one patient improved from C to E, and that of 14 patients improved from D to E.
In the SBG group, 13 patients (25.5%) had complications: one patient had leakage of cerebrospinal fluid, two had kidney function lesions, three had liver function lesions, one had a urinary tract infection, two had pulmonary infections, two had deep venous thrombosis, and two had sinus formation. In the NSBG group, 15 patients (27.8%) had complications, including two cases of liver function lesions, two of kidney function lesions, three of deep venous thrombosis, two of sinus formation, three of urinary tract infections, and three of pulmonary infections. According to the chi-square test, the number of postoperative complications was comparable between the SBG and NSBG groups (P = 0.791). After conservative treatment, all complications resolved without serious consequences.
Discussion
Typically, spinal tuberculosis always destroys the intervertebral disc and deconstructs the spinal bone, leading to spinal instability. Surgical management of spinal tuberculosis includes complete debridement, decompression of neurological deficits, correction of deformities, bone grafting, and stable internal fixation to achieve solid fusion [11]. Several types of bone grafts, which are mainly categorized as non-structural or structural grafts, have been successfully used for intervertebral reconstruction and fusion, including the iliac bone, titanium mesh, and rib bone [5–7]. However, the type of bone graft that should be properly selected during surgery remains controversial.
Many studies have reported the merits of using a titanium mesh as a dependable reconstruction method for satisfactory sagittal profile maintenance and bone fusion rate, with fewer problems in implants [12]. Titanium mesh eliminates the need of harvesting iliac bone as the adequate resource of local bone either from lamina or spinal process, decreasing donor site complications. However, it is technically demanding to insert a titanium mesh into the intervertebral space, and in this process, the operation time and the incidence of nerve injury will increase [13]. Autogenous bone harvested from the iliac crest is the gold standard for bone defect repair owing to its good biocompatibility, bone conductibility, and osteogenesis [14]. Several studies [15–18] have reported that NSBGs could reduce surgical difficulty, surgical injury, and procedure time, and the outcomes of NSBGs are similar to those of titanium mesh with autogenous bone in reconstruction of the spine. In addition, NSBGs with granular bone can be placed into the intervertebral space more easily than titanium mesh cage, and is associated with a lower incidence of nerve injury. A meta-analysis by He et al. also supported this view and declared that titanium meshes did not show an advantage, as reported by previous studies, when considering imaging outcomes, effectiveness, or surgical complications [19].
The Denis three-column theory and loading-sharing principles may be violated when using the NSBG method [20, 21]. However, the use of NSBG in spinal tuberculosis has been proven to be successful in a few studies [16–18]. Our previous research also revealed that NSBG had satisfactory clinical and imaging outcomes when used to treat tuberculosis involving a single-segment of the thoracic spine compared to the outcomes of cases treated with the SBG method [15]. Owing to the rigidity of pedicle screw placement, the use of NSBG in our study did not result in instrumentation failure or recurrence of tuberculosis. The results of this study revealed significant improvements in clinical and imaging outcomes in both the NSBG and SBG groups, and the NSBG had less procedure time, bone fusion time, and intraoperative blood loss. Therefore, we believe that using NSBGs in selected cases is safe and effective, and spinal stability plays a key role in determining the style of the bone graft. In previous clinical practice, NSBG was selected under the following conditions: newly formed bone bridging the affected vertebra, at least one side of the lateral wall of the vertebral body was intact; at least one side of the pedicle was intact for placing a pedicle screw; the local Cobb angle was less than 20°; and the bone defect was less than half the height of the vertebral body. However, these criteria cannot be accurately quantified, and generally accepted standards for choosing NSBG or SBG should be developed. Moreover, developing a simple, easy-to-identify, reliable, and effective standard can help select appropriate treatment methods and facilitate peer communication and scientific research [22]. Spinal stability is a crucial factor when choosing the procedure for many spinal disorders, including spinal tuberculosis. In addition to debridement and anti-tuberculosis medication, restoring and maintaining spinal stability is important for controlling tuberculosis infection. Instability of the spine is defined as the loss of spinal integrity due to a pathological change with motion-associated pain and progressive deformity, with or without nerve defects [23]. So far, there are few reports on scenarios of judging the spinal stability of a tuberculosis-infected spine and directing the choice of bone graft material [24, 25]. Spinal tuberculosis has clinical manifestations that are similar to those of spinal metastasis, including back pain, weight loss, weakness, and even neurological dysfunction, as well as imaging features, such as vertebral destruction, pathological fracture, and kyphosis deformity [26]. In this study, we applied the SINS to evaluate the stability of the spine and expected to develop a quantitative standard for guiding the selection of bone grafts in patients with tuberculosis of the thoracolumbar spine.
First published in 2010, the SINS was originally developed as the standard for assessing the level of spinal stability in patients with spinal tumors. It including six items (posterolateral involvement of spinal elements, vertebral body collapse, spinal alignment, bone lesion quality, mechanical pain, and lesion location) and had a maximum score of 18 [9].
This research has some limitations. First, the number of patients included in this retrospective study was small. Prospective research at multiple centers with more cases should be performed in the future. Second, spinal tuberculosis is intrinsically different from spinal metastasis in the style of bone destruction, as hyperostosis and bone bridges frequently occur in spinal tuberculosis. To develop an accurate evaluation system for spinal tuberculosis, the bone destruction score should be modified according to studies with more spinal tuberculosis cases. However, the present study provides a reference standard for choosing bone graft materials for cases of spinal tuberculosis.
Conclusion
NSBGs and SBGs can achieve comparable therapeutic effects in patients with spinal tuberculosis, and a suitable selection of bone grafts based on quantitative SINS will make full use of the advantages of different bone grafts.
Acknowledgements
Not applicable.
Abbreviations
- ASIA
American Spinal Injury Association
- CRP
C-reactive protein
- ESR
Erythrocyte sedimentation rate
- NSBG
Non-structural bone graft group
- ODI
Oswestry disability index
- SBG
Structural bone graft group
- SINS
Spinal instability neoplastic score
- VAS
Visual analog scale
Authors’ contributions
Study conception and design: Tianji Huang, Zenghui Zhao.Acquisition, analysis of data: Tianji Huang, Zhenghan Han, Wei Luo, Bin He, Yong Zhu Interpretation of data: Tianji Huang, Zenghui Zhao.Draft the manuscript: Tianji Huang, Zhenghan Han.
Funding
This study was funded by the Higher education scientific research planning project (22SZH0422), the Teaching Innovation Project of the First Affiliated Hospital of Chongqing Medical University (CMER202204) and the Program for Youth Innovation in Future Medicine of Chongqing Medical University (Nos. W0086).
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Ethics Committee of The First Affiliated Hospital of Chongqing Medical University approved this study (2019-123), and informed consent was obtained from all patients in this research. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tianji Huang, Email: huangtianji@cqmu.edu.cn.
Zhenghan Han, Email: 2021120205@stu.cqmu.edu.cn.
Wei Luo, Email: 723162925@qq.com.
Bin He, Email: binheing@163.com.
Yong Zhu, Email: 568731668@qq.com.
Zenghui Zhao, Email: 203210@hospital.cqmu.edu.cn.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Jain AK, Kumar J. Tuberculosis of spine: neurological deficit. Eur Spine J. 2013;22:624–33. doi: 10.1007/s00586-012-2335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng H, Zhang P, Shen X, et al. One-stage posterior-only approach in surgical treatment of single-segment thoracic spinal tuberculosis with neurological deficits in adults: a retrospective study of 34 cases. BMC Musculoskelet Disord. 2015;16:186. doi: 10.1186/s12891-015-0640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Güven O, Kumano K, Yalçin S, Karahan M, Tsuji S. A single stage posterior approach and rigid fixation for preventing kyphosis in the treatment of spinal tuberculosis. Spine. 1994;199:1039–43. doi: 10.1097/00007632-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Qian J, Rijiepu A, Zhu B, Tian D, Chen L, Jing J. Outcomes of radical debridement versus no debridement for the treatment of thoracic and lumbar spinal tuberculosis. Int Orthop. 2016;40(10):2081–8. doi: 10.1007/s00264-016-3234-z. [DOI] [PubMed] [Google Scholar]
- 6.Ozdemir HM, Us AK, Oğu¨n T. The role of anterior spinal instrumentation and allograft fibula for the treatment of pott disease. Spine. 2003;28:474–9. doi: 10.1097/01.BRS.0000048666.17934.17. [DOI] [PubMed] [Google Scholar]
- 7.Christodoulou AG, Givissis P, Karataglis D, Symeonidis PD, Pournaras J. Treatment of tuberculos pondylitis with anterior stabilization and titanium cage. Clin Orthop Relat Res. 2006;444:60–5. doi: 10.1097/01.blo.0000201175.87635.28. [DOI] [PubMed] [Google Scholar]
- 8.Rajasekaran S, Soundararajan DCR, Reddy GJ, Shetty AP, Kanna RM. A Validated Score for Evaluating Spinal Instability to Assess Surgical Candidacy in Active Spinal Tuberculosis-An Evidence Based Approach and Multinational Expert Consensus Study. Global Spine J. 2022 Feb 26:21925682221080102. doi: 10.1177/21925682221080102 . Epub ahead of print. PMID: 35220789. [DOI] [PMC free article] [PubMed]
- 9.Fisher CG, DiPaola CP, Ryken TC, Bilsky MH, Shaffrey CI, Berven SH, Harrop JS, Fehlings MG, Boriani S, Chou D, Schmidt MH, Polly DW, Biagini R, Burch S, Dekutoski MB, Ganju A, Gerszten PC, Gokaslan ZL, Groff MW, Liebsch NJ, Mendel E, Okuno SH, Patel S, Rhines LD, Rose PS, Sciubba DM, Sundaresan N, Tomita K, Varga PP, Vialle LR, Vrionis FD, Yamada Y, Fourney DR. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the spine oncology Study Group. Spine. 2010;35(22):E1221–1229. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]
- 10.Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine. 1995;20(12):1410–8. doi: 10.1097/00007632-199506020-00014. [DOI] [PubMed] [Google Scholar]
- 11.Moon Tuberculosis of the spine. Controversies and a new challenge. Spine. 1997;22:1791–7. doi: 10.1097/00007632-199708010-00022. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Zeng K, Yin X, Huang J, Tang M, Guo C. Debridement, internal fixation, and reconstruction using titanium mesh for the surgical treatment of thoracic and lumbar spinal tuberculosis via a posterior only approach: a 4-year follow-up of 28 patients. J Orthop Surg Res. 2015;10:150. doi: 10.1186/s13018-015-0292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin HX, Liu ZK, He BR, Hao DJ. Single posterior surgical management for lumbosacral tuberculosis: titanium mesh versus iliac bone graft. Med (Baltim) 2017;96:e9449. doi: 10.1097/MD.0000000000009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundararaj GD, Amritanand R, Venkatesh K, Arockiaraj J. The use of titanium mesh cages in the reconstruction of anterior column defects in active spinal infections: can we rest the crest? Asian Spine 2011; J 5: 155–61. [DOI] [PMC free article] [PubMed]
- 15.Du X, Ou Y, Zhu Y, et al. One stage posterior debridement, non-structural bone graft in the surgical treatment of single segment thoracic tuberculosis: a retrospective single-center cohort study. Int J Surg. 2019;65:134–9. doi: 10.1016/j.ijsu.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Wang X, Wu P, et al. Surgical treatment for mono-segmental lumbar tuberculosis by single-stage posterior debridement, compact bone grafting and posterior single-segment? Injury. 2015;46:1311–6. doi: 10.1016/j.injury.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Liu JM, Zhou Y, Peng AF, et al. One-stage posterior surgical management of lumbosacral spinal tuberculosis with non-structural autograft. Clin Neurol Neurosurg. 2017;153:67–72. doi: 10.1016/j.clineuro.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Liu JM, Chen XY, Zhou Y, et al. Is non-structural bone graft useful in surgical treatment of lumbar spinal tuberculosis? A retrospective case-control study. Medicine(Baltim) 2016;95:e4677. doi: 10.1097/MD.0000000000004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Z, Ou Y, Hou B, Wei J, Mu X. A meta–analysis of the safety and effectiveness of titanium mesh versus bone graft alone for the treatment of thoracolumbar tuberculosis. Eur Spine J. 2020;29(7):1505–17. doi: 10.1007/s00586-019-06260-2. [DOI] [PubMed] [Google Scholar]
- 20.Denis F. The three-column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817–31. doi: 10.1097/00007632-198311000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Mccormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine. 1994;19:1741–4. doi: 10.1097/00007632-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29(22):3072–7. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 23.Andreas K, Filis KV, Aghayev JJ, Doulgeris SA, Gonzalez-Blohm Frank D Vrionis. Spinal neoplastic instability: biomechanics and current management options. Cancer Control. 2014;21(2):144–50. doi: 10.1177/107327481402100207. [DOI] [PubMed] [Google Scholar]
- 24.Arana E, Kovacs FM, Royuela A, Asenjo B, Pérez-Ramírez Ú, Zamora J. Spine instability neoplastic score: agreement across different medical and surgical specialties. Spine J. 2016;16(5):591–9. doi: 10.1016/j.spinee.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Ulrike H, Mitchell J, Hurrell Clinical spinal instability: 10 years since the derivation of a clinical prediction rule. A narrative literature review. J Back Musculoskelet Rehabil. 2019;32(2):293–8. doi: 10.3233/BMR-181239. [DOI] [PubMed] [Google Scholar]
- 26.Paus B. Tumour, tuberculosis and osteomyelitis of the spine. Differential diagnostic aspects. Acta Orthop Scand. 1973;44:372–82. doi: 10.3109/17453677308989073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.


