Abstract
Adenosine compartmentalization has a profound impact on immune cell function by regulating adenosine localization and, therefore, extracellular signaling capabilities, which suppresses immune cell function in the tumor microenvironment. Nucleoside transporters, responsible for the translocation and cellular compartmentalization of hydrophilic adenosine, represent an understudied yet crucial component of adenosine disposition in the tumor microenvironment. In this review article, we will summarize what is known regarding nucleoside transporter’s function within the purinome in relation to currently devised points of intervention (i.e., ectonucleotidases, adenosine receptors) for cancer immunotherapy, alterations in nucleoside transporter expression reported in cancer, and potential avenues for targeting of nucleoside transporters for the desired modulation of adenosine compartmentalization and action. Further, we put forward that nucleoside transporters are an unexplored therapeutic opportunity, and modulation of nucleoside transport processes could attenuate the pathogenic buildup of immunosuppressive adenosine in solid tumors, particularly those enriched with nucleoside transport proteins.
Keywords: Nucleoside, Adenosine, Transporter, Receptor, Drug, Immunosuppression, Signaling
1. Introduction
The purinergic signaling complex of a cell frequently referred to as “purinome”, comprises numerous proteins and cofactors that define purine-based cellular responses. Although the term purinome was first coined by Timothy Haystead to describe the >2000 proteins that bind purines from the perspective of screening potential drug targets (Haystead, 2006), for the scope of this review, the purinome will be described in the manner put forth by Volonté and D’Ambrosi, who elegantly reviewed the concept previously. The term was defined as “the molecular complex responsible for the biological effects of extracellular purine and pyrimidine ligands, thus consists of ectonucleotidemetabolizing enzymes hydrolyzing nucleoside phosphates, purinergic receptors classified as P1 for adenosine/AMP and P2 for nucleosides tri-/diphosphates, nucleotide channels and finally, nucleoside transporters” (Volonte & D’Ambrosi, 2009). Nucleoside transporters play an integral role within biological membranes by transporting nucleosides, including the signaling nucleoside adenosine and various nucleoside analog drugs, across both intracellular and extracellular membranes. Although integral to the membrane purinome that could directly modulate purinergic receptor signaling, nucleoside transporters’ impact on purinergic signaling is poorly understood. This review will investigate the potential interplay between adenosine transporters and receptors in the context of immunosuppressive adenosinergic signaling within the tumor microenvironment. Understanding the therapeutic potential of the adenosine transporters in relation to other established targets within the purinome may facilitate rational development of new drug candidates not only for cancer immunotherapy but also for amelioration of other diseases such as cardiac diseases, Parkinson’s disease, psoriasis, thrombosis, dry eye, cystic fibrosis, and glaucoma where purinergic signaling plays crucial roles (Boison, 2013; Burnstock, 2006; Gendron et al., 2002; Jacobson, Balasubramanian, Deflorian, & Gao, 2012).
2. Concentrative nucleoside transporters (CNTs) and equilibrative nucleoside transporters (ENTs) are understudied components of the purinome
The two groups of nucleoside transporters are concentrative nucleoside transporters (CNTs), which are categorized within the solute carrier family 28 (SLC28), and equilibrative nucleoside transporters (ENTs), which fall within solute carrier family 29 (SLC29) (Pastor-Anglada & Perez-Torras, 2018a). Three distinct CNTs concentrate nucleosides within the cell through unidirectional transport. Each of the four ENTs instead work bidirectionally to equilibrate nucleosides between the cell and its surroundings and potentially across intracellular compartments. For CNTs to function in a single direction, CNTs use a sodium or proton gradient to help move nucleosides across membranes. CNT1 and CNT2 both have a 1:1 cation to nucleoside gradient, whereas CNT3 has a 2:1 cation to nucleoside gradient and a stronger affinity for protons over sodium ions. Alternatively, ENTs operate by facilitated diffusion (JYoung, Yao, Baldwin, Cass, & Baldwin, 2013). The multiplicity of nucleoside transporters in mammals alludes to the existing differences in their subcellular location, permeant selectivity, kinetics, and regulation.
Each of the CNT transporters has a unique role. CNT1 prefers pyrimidine substrates, CNT2 prefers purine substrates, and CNT3 transports both purines and pyrimidines (Mirabet et al., 1999). Additionally, all CNTs can transport uridine (Pastor-Anglada & Perez-Torras, 2018a). The CNTs are primarily localized to the plasma membrane of the cell and on the apical surface of polarized epithelial cells, although CNT3 is occasionally found within intracellular membranes (Young et al., 2013). CNTs also tend to have much higher affinities for their substrates as opposed to the ENT family of transporters (Pastor-Anglada & Perez-Torras, 2018a).
ENT transporters also have individualized roles. Both ENT1 and ENT2 can transport purine nucleosides and pyrimidine nucleosides with varied affinities (Pastor-Anglada & Perez-Torras, 2018a). These transporters are primarily found within the basolateral plasma membrane of the polarized cell but can be found in some intracellular membranes (e.g., nuclear membranes). Unlike the other ENTs, ENT3 is primarily found within the intracellular membranes of the cell both in lysosomal as well as mitochondrial membranes (Young et al., 2013). ENT4 is set apart in that it preferentially transports monoamines such as dopamine or serotonin and is evolutionarily distinct from the other three ENT transporters (Pastor-Anglada & Perez-Torras, 2018b).
2.1. Nucleoside transporters facilitate adenosine transport
All nucleoside transporters play at least some role in the transport of adenosine, including the pyrimidine preferring CNT1 which transports adenosine at a relatively low affinity than its counterparts (CNT2 and CNT3) (Pastor-Anglada & Perez-Torras, 2018b). While CNTs are responsible for concentrating adenosine within the cell against the concentration gradient, ENTs, in general, equilibrate adenosine concentrations across the membrane. However, the metabolism of adenosine within the cell to AMP via phosphorylation may help allow CNTs to build up intracellular adenosine concentrations (Pastor-Anglada & Perez-Torras, 2018b). Within the ENT family, ENT1 and ENT2 are the primary transporter proteins believed to be responsible for determining intracellular adenosine concentrations as ENT3 is more directly involved in intracellular rather than extracellular transport and ENT4’s role is still yet to be fully understood (Pastor-Anglada & Perez-Torras, 2018a, 2018b). In addition, ENT3 is an obligatory, acidic pH-dependent nucleoside transporter that facilitates the lysosomal exit of adenosine derived from the hydrolytic degradation of nucleic acids arising from the phagocytic and endocytic pathways. It was also reported that ENT4 transport of adenosine is optimal at acidic pH in cardiomyocytes and therefore likely to play a role in ischemic conditions (Yang & Leung, 2015). ENT transport of adenosine across the plasma membrane is reliant upon the concentration gradient as well as its intracellular metabolism, as mentioned above. It is worth mentioning that a Ph-dependent adenosine transport activity has been described for ENT4 which is optimal at acidic pH (pH 5.5) and absent in neutral conditions (pH 7.4). This pH-selective transport is adenosineselective, Na+-independent, mildly affected by ENT inhibitors, and does not impact the ENT4-mediated transport of other metabolites such as serotonin (Barnes et al., 2006; Tandio, Vilas, & Hammond, 2019). The equilibrative transport serves as a counter to CNT unidirectional transport and allows for excess adenosine and nucleosides to leave the cell. These roles in adenosine transport across cellular membranes influence adenosine’s ability to accumulate at the extracellular side and interact with receptors and exert regulatory effects upon biological processes (Young et al., 2013).
Studies have demonstrated that both CNT and ENT mediate the translocation of adenosine in different human tissues. There is a notable expression of different CNT isoforms on the apical surface of epithelial cells lining the pulmonary epithelium in the airway. CNT2 is mostly localized in nasal epithelia and CNT3 is expressed in both nasal and bronchial epithelial cells. CNT2 and CNT3 are responsible for the permeability of airway epithelia to airway surface liquid adenosine as well as mediating translocation of inosine generated by adenosine deaminase (ADA) (Hirsh et al., 2007; Ritzel et al., 2001a; Ritzel et al., 2001b). The role of ENTs, especially ENT1 and ENT2, in regulating apical adenosine uptake in airway epithelia is also well-defined (Lu, Gong, Monks, Zaharevitz, & Moscow, 2002; Szkotak et al., 2001). Similarly, intracellular, or extracellular adenosine formed in the brain and spinal cord is available for membrane translocation via nucleoside transporters localized to many CNS regions (Bynoe, Viret, Yan, & Kim, 2015; Jennings et al., 2001; Liu et al., 2019; Pinto-Duarte, Coelho, Cunha, Ribeiro, & Sebastiao, 2005). Such spatiotemporal and regional differences in ENT and CNT expression are prevalent in different body tissues. Quantitative studies on tissue localization of CNTs and ENTs have demonstrated a similar pattern, with a few interspecies and gender-linked variations (Gray et al., 2004; Lu, Chen, & Klaassen, 2004; Pastor-Anglada & Perez-Torras, 2018a; Young et al., 2013). A high expression of both CNT1 and CNT2 is reported in the small intestine, followed by the kidney, liver, testes, thymus, and spleen. CNT3 has elevated expression in brain tissues, salivary glands, skin, breast, placenta, urinary bladder, and lung with moderate expression in the small intestine. ENT1 and ENT2 are abundant in skeletal muscles along with some expression in the heart, small intestine, testes, lungs, and liver. ENT3 is widely expressed throughout the body tissues including the testis, uterus, ovaries, breast, pancreas, small intestine, kidney, lung, liver, and so on. Table 1 describes the tissue distribution and cellular location of CNTs and ENTs along with their affinity for adenosine.
Table 1.
Adenosine Transport Characteristics of ENTs and CNTs
| Nucleoside Transporter | Solute Carrier Family | Adenosine Affinity [Km (mM)] | Tissue Distribution | Subcellular Localization | Reference |
|---|---|---|---|---|---|
| CNT1 | SLC28A1 | -- | Small intestine, Kidney, Liver, | Plasma membrane | (Gray, et al., 2004; J. D. Young, et al., 2013) |
| CNT2 | SLC28A2 | 0.008 | Small intestine, Testis, skeletal muscle, liver, kidney, intestine, pancreas, placenta, brain | Plasma membrane | (Duflot, et al., 2004; Gray, et al., 2004; Pastor-Anglada & Perez-Torras, 2018a, 2018b; J. D. Young, et al., 2013) |
| CNT3 | SLC28A3 | 0.0024–0.015 | Mammary gland, pancreas, bone marrow, trachea, intestine | Plasma membrane, sometimes intracellular | (Pastor-Anglada & Perez-Torras, 2018a, 2018b; J. D. Young, et al., 2013) |
| ENT1 | SLC29A1 | 0.04–0.78 | Widely expressed with high levels in skeletal and smooth muscle, lung, thymus | Plasma, nuclear, and mitochondrial membranes | (Parkinson, et al., 2011; Ward, Sherali, Mo, & Tse, 2000) |
| ENT2 | SLC29A2 | 0.14–0.78 | Widely expressed, especially skeletal muscle | Plasma and nuclear membranes | (Parkinson, et al., 2011; Ward, et al., 2000) |
| ENT3 | SLC29A3 | 0.78–1.86 | Widely expressed in most tissues | Endosomal, lysosomal, and mitochondrial membranes | (Baldwin, et al., 2005; Kang, et al., 2010; Parkinson, et al., 2011; Pastor-Anglada & Perez-Torras, 2018a, 2018b; Rahman, Askwith, & Govindarajan, 2017) |
| ENT4 | SLC29A4 | 0.78 | Heart, brain, skeletal muscle | Plasma membrane | (Barnes, et al., 2006; Parkinson, et al., 2011; Pastor-Anglada & Perez-Torras, 2018a) |
Despite the increasing understanding of the role of nucleoside transporters in adenosine translocation in different tissues, the extent to which the differential expression and regulation levels of different transporter families and subfamilies can affect purinergic signaling is still unresolved. More recent findings suggest a more active role for nucleoside transporters in central nervous system (CNS) beyond the default transportation abilities (Bynoe et al., 2015; Choi et al., 2004; Choudhury, Chellappan, Sengupta, Pandey, & Gorain, 2019; Liu et al., 2019; Pasquini et al., 2022). A bidirectional interaction of adenosine with nucleoside transporters as well as excitatory and inhibitory adenosine receptors suggests a dual role for nucleoside transporters.
3. Nucleoside transporters and cross talk with other members of the purinome
Nucleoside transporters are likely to impact purinergic signaling, mainly through modulating existing adenosine levels inside and outside the cell. These can also terminate downstream adenosine receptor-dependent signaling by clearing the extracellular milieu of adenosine. These effector functions highlight the possibility of regulatory feedback loops, which connect receptor signaling with transporter function. Despite the perceived critical role of nucleoside transporters as modulators of purinergic signaling in the human body, their role within the context of the purinome is not yet explored to its full potential.
The localization of ENTs and CNTs on plasma membranes of synaptic vesicles confirms their role in mediating efflux and influx of adenosine in the intracellular sites. The release of trapped adenosine from these sites via Ca+-dependent excitation-secretion mechanism (Melani et al., 2012; Wall & Dale, 2013), suggests the causal involvement of these transporters in both purine nucleoside uptake and release under physiological conditions. ENTs are principal transporters that facilitate intracellular to extracellular flux of the adenosine in the CNS (Latini & Pedata, 2001; Paes-de-Carvalho et al., 2005; Zamzow, Bose, & Parkinson, 2009), whereas, in the cardiovascular system (CVS), ENTs mediate the uptake of extracellular adenosine, thus terminating adenosine receptor signaling by clearing the extracellular milieu of the purine nucleoside (Loffler, Morote-Garcia, Eltzschig, Coe, & Eltzschig, 2007; Rose et al., 2010). Recent evidence also suggests a significant role of CNT2 in the modulation of adenosine levels in the CNS. CNT2 mRNA levels vary with changes in extracellular adenosine concentration (Guillen-Gomez et al., 2004; Medina-Pulido et al., 2013), while ENT1 mRNA levels remain unchanged. Additional studies have demonstrated CNT2 expression on the plasma and vesicle membranes isolated from rat striatum, supporting the relevance of this transporter as a member of the purinome (Melani et al., 2012).
3.1. Nucleoside transporters act as gatekeepers within the purinome for adenosine compartmentalization in tumor tissues
CNTs and ENTs facilitate the transmembrane flux of adenosine acting as conduits mediating the adenosine flow in a highly regulated manner. When inside, intracellular metabolism of adenosine is most readily performed by adenosine kinase (ADK), and the addition of a phosphate group to adenosine prevents efflux via nucleoside transporters. Alternatively, ADA can metabolize adenosine to inosine. Cellular stress, mechanical injury, or metabolic changes can all contribute to the release of nucleotides from intracellular storage by exocytosis or conductive release by pannexin and connexin channels or adenosine triphosphate (ATP)-conduction anion channels. Adenosine is produced from extracellular ATP by dephosphorylation reactions carried out sequentially by ectonucleotidases CD39 and CD73.
Although a key player in multiple metabolic pathways, adenosine has been recognized as an important modulator of immune cell function in the tumor microenvironment (TME) (Ohta, 2016). It serves as a constituent of ATP, the primary cellular energy source, and as a critical component of nucleic acid synthesis. In the extracellular space, adenosine is available to activate G-protein coupled adenosine receptors (ARs) on the cell surface, affecting acute inflammation, immune response, vascular tension, and neurotransmission (Eltzschig, 2013; Karmouty-Quintana et al., 2013; Karmouty-Quintana, Xia, & Blackburn, 2013). Elevated levels of extracellular adenosine have been noted in solid tumors, reaching immunosuppressive concentrations (Blay, White, & Hoskin, 1997). A multitude of processes in summation determines the concentration of extracellular adenosine. Adenosine is produced in the extracellular space by the breakdown of nucleotides that are released from intracellular stores. Intracellular compartmentalization of adenosine by nucleoside transporters facilitates the localization of adenosine, although efflux via ENTs is also possible based on the concentration gradient of extracellular and intracellular and is especially likely in scenarios where adenosine transport and metabolism are not tightly coupled.
The cumulative contribution of ATP released from intracellular stores, adenosine generated extracellularly by ectonucleotidases, combined with nucleosides available in the blood or interstitial fluid, is counter-balanced by metabolic pathways that breakdown adenosine in the extracellular space, integrated with transporter-mediated intracellular translocation and the intracellular metabolism of adenosine that traps it inside of cells preventing efflux. Therefore, it is important to keep in mind the interdependence of the various aspects of the purinome, particularly in the context of adenosine transport. Pharmacologic blockade or activation of a single aspect will impact the function of all others and the overall balance. Therefore, careful experimentation and evaluation of the nucleoside transporters within the purinome is needed to fully validate new therapies.
Due to the complexity of the purinome, it is likely that targeting only one aspect would not be sufficient to intervene in the pathogenic accumulation of extracellular adenosine. Already, the inhibition of ectonucleotide-metabolizing enzymes, CD39 and CD73 has shown efficacy in cutting down adenosine, although clinical trials failed to show any improvement over current standard-of-care treatment options. Most research efforts up to this point have been focused on understanding and targeting the ectonucleotidase enzymes CD39 and CD73, which contribute to adenosine production by the removal of phosphate groups from extracellular ATP. Yet as Boison and Yegutkin point out in their recent article, this is only one element of the complex system that regulates adenosine levels (Boison & Yegutkin, 2019; Yegutkin, 2008). Fig. 1 shows a schematic of the various elements of the purinome that work together to determine the levels of extracellular adenosine.
Fig. 1.
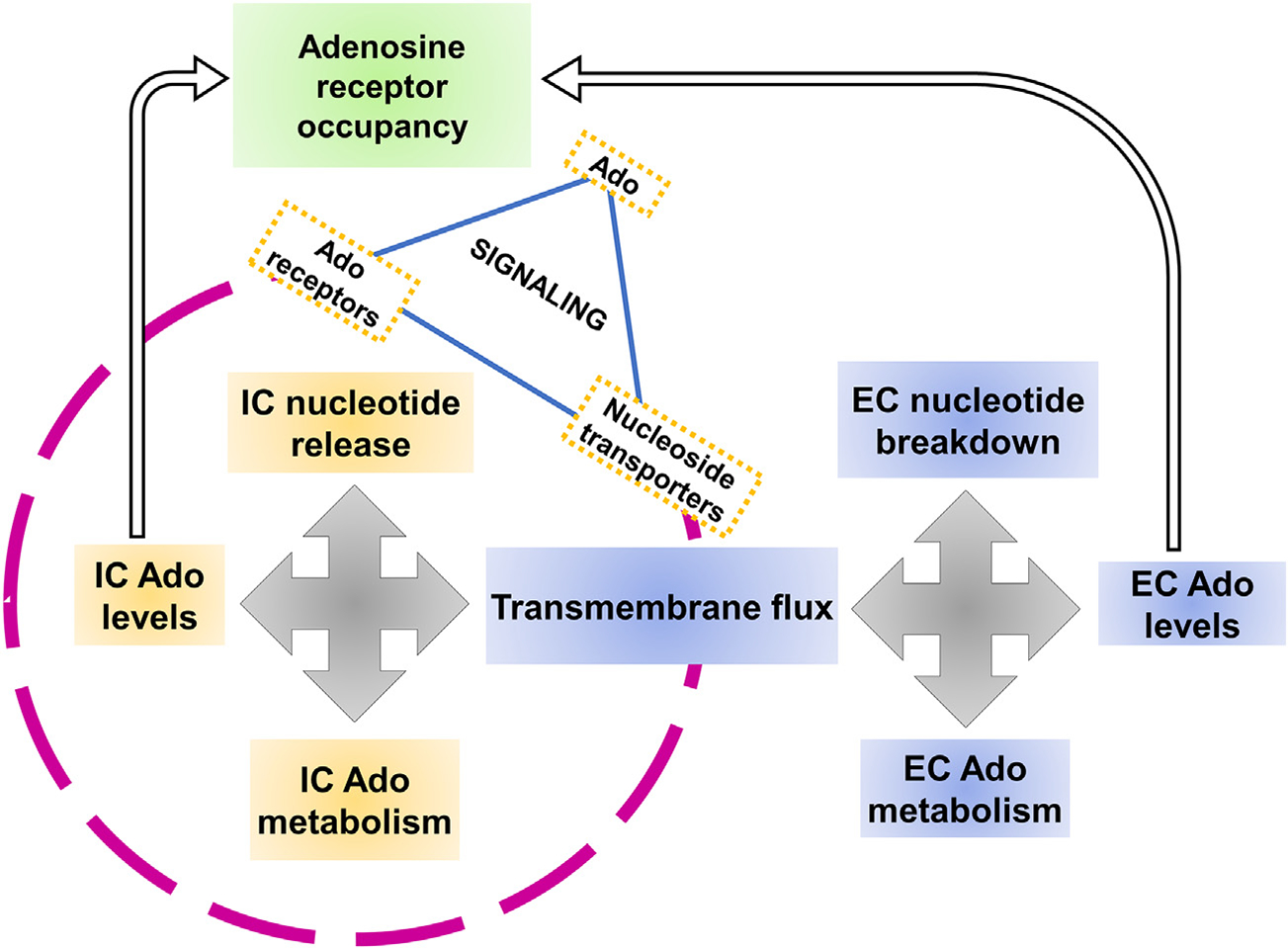
Adenosine transporter-receptor Interplay in Purinergic Signaling. Both intracellular and extracellular components determine the adenosine levels in the cellular milieu.
The source of extracellular adenosine which accumulates in solid tumors has been attributed to multiple sources, including ATP release from necrotic or mechanically damaged cells (Wang et al., 2013; Yin, Xu, Zhang, Kumar, & Yu, 2007), and cells undergoing inflammatory stress (Bodin & Burnstock, 1998). Additionally, the vesicular release of ATP occurs and can take part, such as that demonstrated in neurotransmission, when released from synaptic vesicles upon stimulus (Zimmermann, 2008). Further, treatment with cytotoxic chemotherapy drugs which induce apoptosis also induces tumor cell release of ATP (Martins et al., 2009). ENTs may also perform efflux of adenosine depending upon the concentration gradient of intracellular and extracellular adenosine. Besides exocytotic pathways, other conductive ATP release mechanisms include connexin and pannexin channels, and ATP-conducting anion channels, which are shown to modulate adenosine/ATP levels (Lazarowski, 2012).
Once released into the extracellular space, ATP can undergo a variety of fates. Perhaps the best-explored pathway is metabolism by ectonucleotidases CD39 and CD73 which decorate the cell surface of tumor cells and, often, infiltration of immune cells and resident stromal cells. CD39 and CD73 are upregulated in many cancers and significantly contribute to the pathogenic production of extracellular adenosine. Yet it is also possible that the reverse reaction could occur and regenerate ATP from adenosine. Adenosine itself can undergo intracellular compartmentalization by nucleoside transport processes or be further metabolized to inosine.
After intracellular translocation by ENTs or CNTs, ADK rapidly adds a phosphate group to adenosine. The addition of a phosphate group bearing a negative charge excludes AMP from efflux via ENTs whose selectivity for nucleosides does not permit the passage of AMP. Therefore, it is predicted that under normal conditions, a steady state equilibrium exists between the levels of extracellular adenosine (influenced by blood levels of adenosine, expression of ectonucleotidases, conditions that modulate ATP efflux) and the rate of intracellular adenosine metabolism, primarily performed by ADK which sequesters adenosine inside of cells which ENTs facilitating transmembrane flux according to the concentration gradient of adenosine (Antonioli et al., 2008a, 2008b; Fornai et al., 2009; Livingston, Heaney, & Ennis, 2004; Polosa & Holgate, 2006; Poulsen & Quinn, 1998). ADA inhibition is known for its neuroprotective function in pain and inflammation (Mark & Don, 2007). During metabolic stress, the release and degradation of precursor adenine nucleotides (i.e., ATP, ADP, and AMP) also contribute to adenosine accumulation (Sachdeva & Gupta, 2013). Fig. 2 is a transportercentric representation of the interplay between different members of purinome and the regulation of adenosine signaling.
Fig. 2.
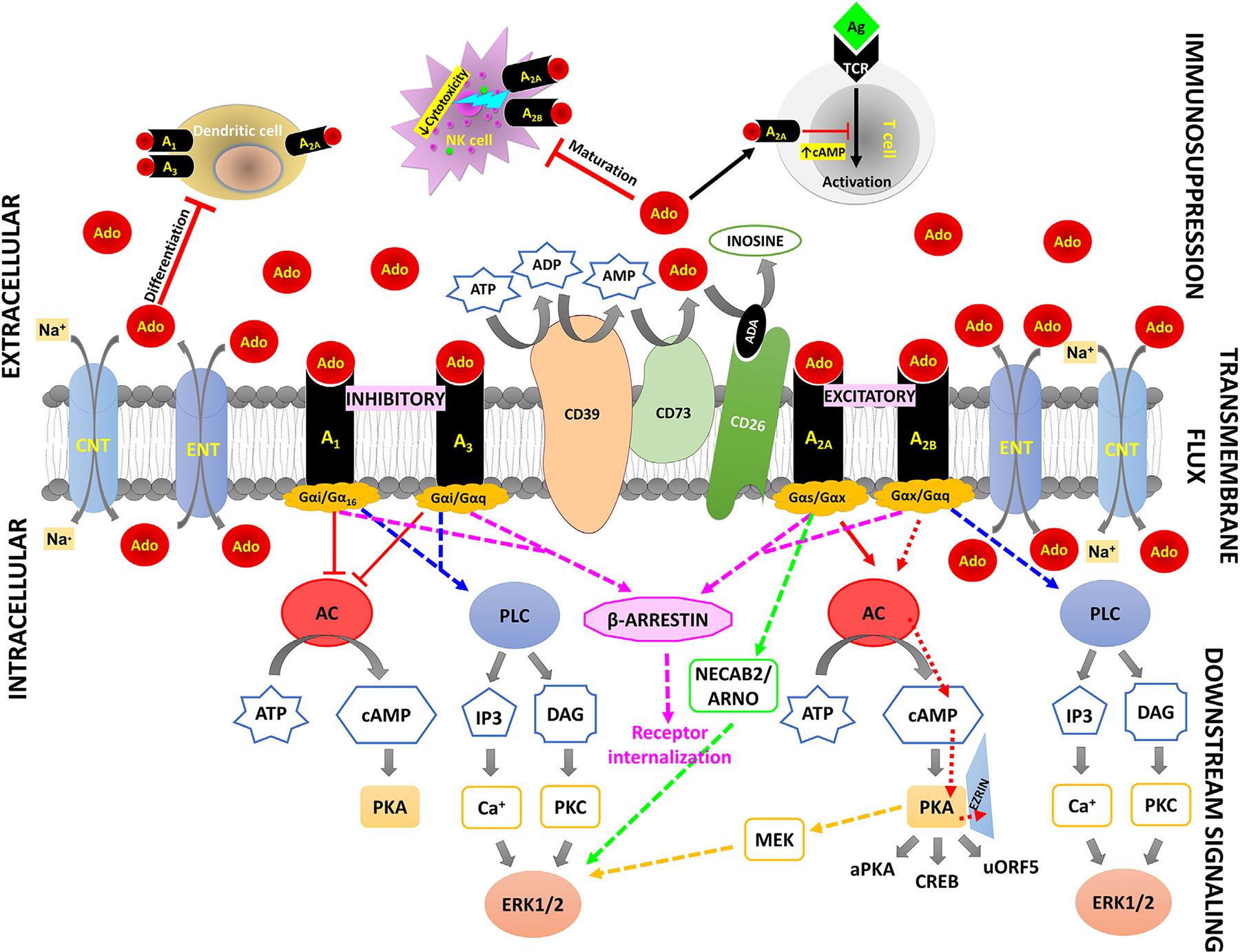
Nucleoside transporters within the purinome. A transporter-centric view of the purinome describing adenosine fate within and outside a cell.
This model has optimal utility in tissue types where the expression of ENTs or CNTs predominates; however, in many epithelial cell types CNTs are primarily located on the apical surface exposed to the extracellular environment and drive inward-directed movement of nucleosides. Further, many solid tumor types are of epithelial origin and would display the asymmetric distribution of ENTs and CNTs in which apically localized CNTs would have a larger role in adenosine uptake from the extracellular environment. CNTs do not depend on the concentration gradient for adenosine flux but instead concentrate nucleosides by cotransport of sodium or hydrogen ions. However, due to their high affinities, they are likely to get saturated much earlier than ENTs. How CNTs contribute to the steady state equilibrium described when ENTs are coupled tightly to the intracellular metabolism of adenosine is yet unclear. We predict that in many early-stage epithelial tumor types, the role of CNTs is prominent and underappreciated for the sequestration of extracellular adenosine. In addition, in late-stage poorly differentiated or undifferentiated tumors, the spatial organization of ENTs and CNTs would be severely impaired to affect the adenosine disposition through transporters.
3.2. Bidirectional dialogue of adenosine with nucleoside transporters and G protein-coupled receptors
Nucleoside transporters’ transmembrane flux of adenosine is the most defining event, which is coupled to signaling responses triggered by the downstream effectors within the cells producing real-time reactions in response to the ever-changing dynamics of the extracellular environment. This downstream signaling is initiated via the binding of adenosine to G protein-coupled adenosine receptors (GPCRs). There are four main types of adenosine receptors: A1, A2A, A2B, and A3. A1 and A3 receptors are typically classified as inhibitory, and A2A and A2B receptors are typically classified as excitatory. The receptor signaling centers around the excitation and inhibition of secondary messengers to incite or prohibit different signaling cascades, based on the type of adenosine receptor activated. These receptors also show considerable variation in expression among different tissues of the body (Table 1). A1, A2A, and A2B receptors are mostly conserved among mammals, however, A3 receptors are structurally diverse. The binding of ligands leads to the dissociation of, the Gα subunit of the heterotrimeric G protein which then regulates downstream effectors. The identity of the Gα subunit determines the activity of adenylate cyclase enzymes which modulate the formation of cyclic adenosine monophosphate (cAMP) (Rosenbaum, Rasmussen, & Kobilka, 2009). The facilitatory A2A and A2B receptors are coupled to Gαs protein complexes, and the binding of extracellular adenosine increases intracellular cAMP levels (Fredholm, Johansson, & Wang, 2011; Gao & Jacobson, 2019; Jacobson & Gao, 2006). In contrast, the inhibitory A1 and A3 receptors are paired with Gαi proteins which block the generation of cAMP (Jacobson & Gao, 2006). βγ subunits of G proteins also act through the mitogen-activated protein kinase (MAPK) and phospholipase C (PLC) pathways, respectively. The general cytoprotective functions modulated by extracellular adenosine are regulated by adenosine receptors. A1 receptor regulates sleep, vasoconstriction, and inhibition of the release of neurotransmitters; A2A receptor also modulates sleep along with angiogenesis, and immunosuppression; A2B receptor is associated with cardiovascular function including vascular integrity and myocardial preconditioning; and A3 receptor controls mast cell regulation and myocardial preconditioning (Fredholm et al., 2011; Jacobson & Gao, 2006). Fig. 3 lists the major pathways affected by activated adenosine receptors along with associated pro- and anti-tumoral effects.
Fig. 3.
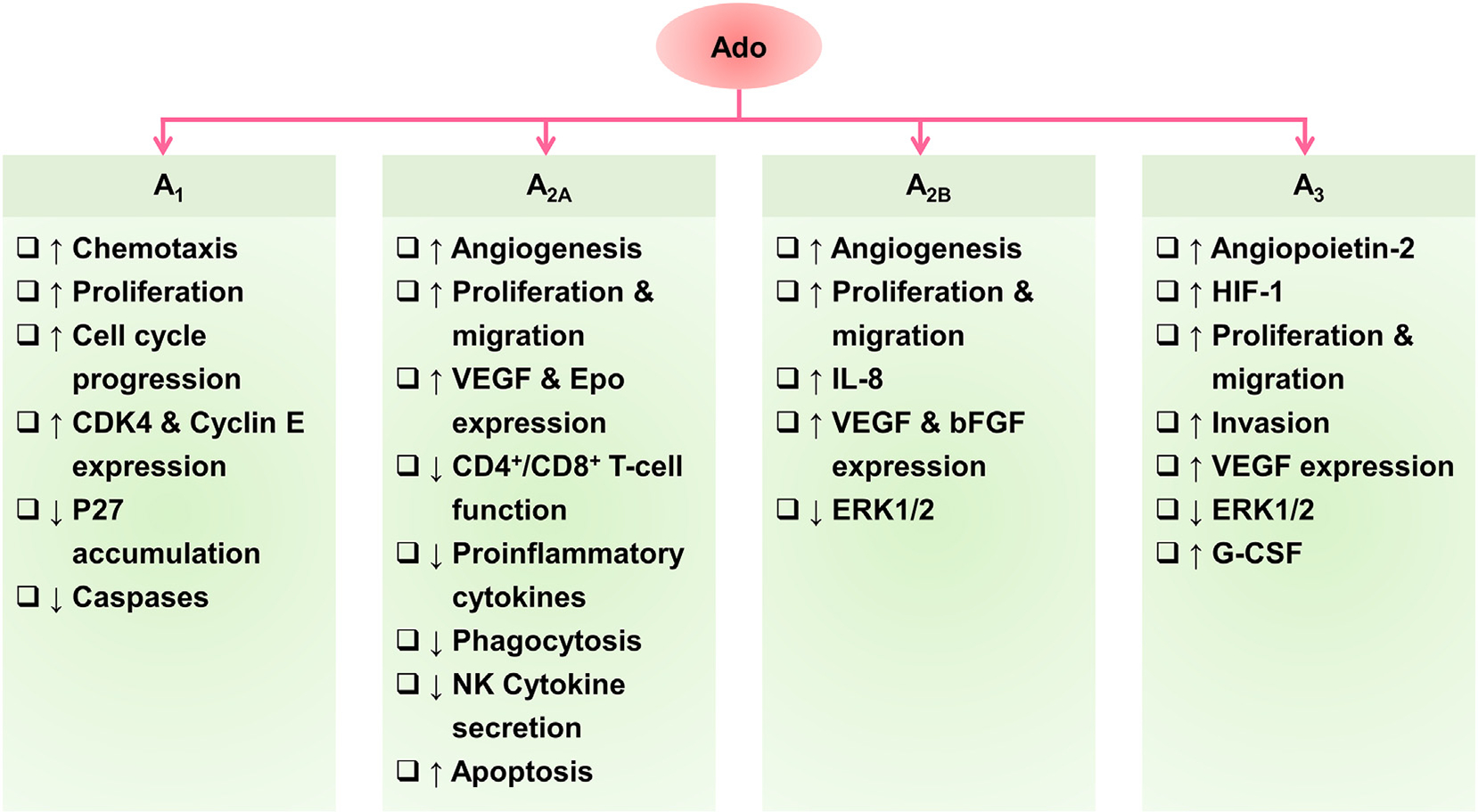
Tumoral effects of activated adenosine receptors.
All four adenosine receptors are widely expressed in the central nervous system, along with peripheral tissues of the cardiovascular, respiratory, renal, and immune systems (Table 2), where they act with specific pharmacological features and signaling abilities. Due to the colocalization of all four receptors within a cell and the overlapping of receptor function, monitoring the overall function of adenosine is a challenging task. Although extracellular adenosine serves as the endogenous agonist for all four receptors under physiological conditions, variability in adenosine binding affinities, tissue expression, and associated G protein types give each subtype a distinct signaling profile (Cieslak, Komoszynski, & Wojtczak, 2008; Fredholm, 2014). Another notoriously challenging aspect of GPCRs is their structure determination, largely owing to their conformationally dynamic nature and poor thermostability after extraction from the plasma membrane. Despite all the challenges, researchers have been able to decipher the structure of the A2A receptor which is currently the best structurally characterized GPCR among all four receptors (Carpenter & Tate, 2016; Carpenter & Tate, 2016; Jaakola et al., 2008; Lebon, Bennett, Jazayeri, & Tate, 2011; Lebon & Tate, 2011; Lebon et al., 2011; Xu et al., 2011), followed by some success with A1 receptor. The other AR subtypes have proven more difficult to crystallize for structural analysis, compared to these two receptors (Cheng et al., 2017; Glukhova et al., 2017). Several selective agonists and antagonists with variable ligand affinities have been synthesized and characterized for each of the receptors (summarized in Table 3). As A2B receptors’ threshold adenosine occupancy is achieved at higher ligand concentrations as compared to the rest of the three receptors, it is often considered a “bad copy” of the A2A receptor owing to its low adenosine affinity.
Table 2:
Key Characteristics of Adenosine Receptors
| Receptor | Structural information (Accession No.) | Chromosomal location (h) | G-protein coupling | Tissue expression | Physiological function(s) | Reference |
|---|---|---|---|---|---|---|
| A1 | h 326 aa (P30542) m 326 aa (Q60612) |
1q32.1 | Gi, Go | Brain tissues, Pancreas, Heart muscle, Testis, Spleen, Kidney, Lipocytes | Bradycardia; inhibition of lipolysis; reduced glomerular filtration; tubero-glomerular feedback; antinociception; reduction of sympathetic and parasympathetic activity; presynaptic inhibition; neuronal hyperpolarization; ischemic preconditioning; sleep cycle regulation | (Babich, Vadnagara, & Di Sole, 2015; J. F. Chen, et al., 2014; Fozard, 2003; Fredholm, 2007; Fredholm, et al., 2000; Hoffman, Chang, Dall’Aglio, & Reaven, 1986; Koeppen, Eckle, & Eltzschig, 2009; Satoh, et al., 2000; Vallon, Muhlbauer, & Osswald, 2006; Yoon, Bae, & Choi, 2005; Yun, et al., 2019) |
| A2A | h 410 aa (P29274) m 409 aa (UO5672) |
22q11.2 | Gs, Golf | Basal ganglia, Thymus, Lymph node, Heart muscle, Kidney, Liver, Lung, Striatum, Nucleus accumbens, Olfactory tubercle, Immune cells, Heart, Lung, Blood vessels, Retina, Aorta | Sensorimotor integration regulation in basal ganglia; sensory nerve stimulation; inhibition of polymorphonuclear leukocytes; inhibition of platelet aggregation; vasodilatation, protection against ischemic damage | (Conti, et al., 1993; Dixon, et al., 1996; Fredholm, 2007; Martin, Ueeda, & Olsson, 1993; Salmon & Cronstein, 1990) |
| A2B | h 328 aa (P29275) m 332 aa (UO5673) |
17p11.2–12 | Gs, Gq | Urinary bladder, Esophagus, Colon, Rectum, Brain tissues, Vagina, Placenta, Skin, Caecum, Large intestine, Bladder | Smooth muscle relaxation in vasculature and intestine; stimulation of mast cell mediator release; inhibition of monocyte and macrophage function | (Dixon, et al., 1996; Fredholm, 2007) |
| A3 | h 328 aa (P29275) m 332 aa (UO5673) |
17p11.2–12 | Gi | Brain, Stomach, Duodenum, Small intestine, Parathyroid gland, Liver, Gall bladder, Urinary bladder, breast, Lymph node, Thymus, eyes | Enhancement of mediator release from mast cells; preconditioning | (Borea, Gessi, Bar-Yehuda, & Fishman, 2009; J. F. Chen, et al., 2014; Fozard, 2003; Fredholm, et al., 2000; Gaytan, et al., 2006; Jacobson & Gao, 2006; Jacobson, et al., 2018; Mohamadi, Aghaei, & Panjehpour, 2018; Zhou, et al., 1992) |
Table 3:
Substrate Binding Affinity of Adenosine Receptors
| Receptor | Selective agonists | Selective antagonists | Radioligands | Reference | |||
|---|---|---|---|---|---|---|---|
| compound | affinity | compound | affinity | compound | affinity | ||
| A1 | Adenosine | 70nM-0.31μM (EC50) | DPCPX | 0.3–3.9nM | [3H]-CCPA | 0.6 (Kd) | (J. F. Chen, et al., 2014; Fredholm, et al., 2007; Geiger, LaBella, & Nagy, 1984; Klotz, 2000; Klotz, Keil, Zimmer, & Schwabe, 1990; Kreft, Bier, Holschbach, Schulze, & Coenen, 2017; Martin, Wysocki, Barrett, May, & Linden, 1996; Stockwell, Jakova, & Cayabyab, 2017; Yun, et al., 2019) |
| CPA | 8.9 (pKi) | 8-cyclopentyltheophylline | 7.5–8.0pM (Ki) | [3H]-R-PIA | 2.0 (Kd) | ||
| CCPA | 800 (pKi) | WRC0571 | 8.8pM (Ki) | [3H]-CHA | - | ||
| CHA | 3.6 | - | - | [3H] NECA | 14 (Kd) | ||
| S-ENBA | 0.3nM | - | - | 125I-AB-MECA | 3.4–8.5 | ||
| LUF6944 | 7.6 (Ki) | - | - | [3H]-DPCPX | 0.3–3.9 (Kd) | ||
| LUF6941 | 7.9 (Ki) | - | - | CPMMCB | 3.73nM (Ki) | ||
| - | - | - | - | CPFPX | 3.49nM (Ki) | ||
| - | - | - | - | CBCPM | 8.68nM (Ki) | ||
| A2A | Adenosine | 0.7μM (EC50) | Caffeine | 43,000nM (Ki) | [3H]-CGS 21680 | 32nM (Kd) | (de Lera Ruiz, et al., 2014; Dionisotti, et al., 1997; Fernandez-Duenas, et al., 2014; Fredholm, 2007; Fredholm, et al., 2001; Grahner, Winiwarter, Lanzner, & Muller, 1994; Klotz, et al., 1998; C. E. Muller, et al., 1998; Murphree, Marshall, Rieger, MacDonald, & Linden, 2002; Nonaka, Ichimura, et al., 1994; Nonaka, Mori, et al., 1994; O’Malley, et al., 2009; Okusa, Linden, Macdonald, & Huang, 1999; Olah, Jacobson, & Stiles, 1994; Palmer, Poucher, Jacobson, & Stiles, 1995; Preti, Baraldi, Moorman, Borea, & Varani, 2015; van der Walt & Terre’Blanche, 2018) |
| C2-/C8-substituted adenosine | 7.19nM (Ki) | SCH 58261 | 0.6 nM (Ki) | [3H]-NECA, 125 | 20nM (Kd) | ||
| 2-((4-aryl(alkyl)piperazin-1-yl)alkylamino)-5′-N-ethylcarboxamidoadenosine (2-F, 4-Cl) | 4.78nM (Ki) | ZM241385 | 1.4 nM (Ki) | I-ZM 241385 | 0.7nM (Kd) | ||
| CGS 21680 | 72±10nM (Kd) | KF 17387, CSC | 1± 0.057nM (Ki) | [3H]-MSX-2 | 8nM (Kd) | ||
| HE-NECA | 36±2nM (Kd) | 5-hydroxy-2-(3-hydroxyphenyl)-4H-1-benzopyran-4-one | 1.44uM (Ki) | 125I-AB-MECA | 25nM (Kd) | ||
| CV-1808 | 76 nM (Ki) | Istradefylline | 36nM (Ki) | [3H]-SCH 58261 | 2nM (Kd) | ||
| CV-1674 | - | - | - | - | - | ||
| ATL146e | 0.2nM (Ki) | - | - | [3H]-ZM-241385 | - | ||
| A2B | Adenosine | 24μM (EC50) | MRS1754 (enprofylline) | 2nM (Ki) | [3H]-DPCPX) | 56 nM (Ki) | (Fredholm, 2007; Fredholm, et al., 2001; Jacobson & Gao, 2006; Linden, Thai, Figler, Jin, & Robeva, 1999; Preti, et al., 2015) |
| LUF5835 | 10nM (Ki) | MRE 2029-F20 | 3nM (Ki) | 125 I-ABOPX | 36 nM (Kd) | ||
| 2-((4-aryl(alkyl)piperazin-1-yl)alkylamino-5’-Nethylcarboxamidoadenosines scaffold | 1487nM (Ki) | OSIP-339391 | 0.5nM (Ki) | [3H]-ZM-241385 | 33 nM (Kd) | ||
| A3 | Adenosine | 0.29μM (EC50) 6500nM | MRS 1220 | 0.7 0.65 |
[3H]-PIA | 16 (Kd) | (J. F. Chen, et al., 2014; Jacobson, et al., 2012; Jacobson, et al., 2018; Y. C. Kim, Ji, & Jacobson, 1996; Klotz, et al., 1998; A. H. Li, Moro, Melman, Ji, & Jacobson, 1998; Olah, et al., 1994; Stockwell, et al., 2017; van Muijlwijk-Koezen, Timmerman, Link, van der Goot, & AP, 1998; van Muijlwijk-Koezen, Timmerman, Link, van der Goot, & Ijzerman, 1998; Varani, et al., 2000) |
| 2-Cl-IB-MECA | 11 | MRE 3008-F20 | 9.54 (pKi) | [3H]-NECA | 6.2 (Kd) | ||
| CCPA | 18.8μM (Ki) 42 | MRS 1191 | 31.4 | 125I-AB-MECA | 0.6–1.5 | ||
| - | - | MRS 1523 | 18.93nM (Ki) | [3H]-MRE 3008-F20 | 0.8 (Kd) | ||
| - | - | VUF 8504 | 17nM | [3H]-MRS7799 | 0.55nM (Kd) | ||
3.3. Receptor classification and G-protein signaling pathways in cancer relevant functions
3.3.1. A1 receptor
A1 receptors inhibit cAMP production by binding and activating intracellular heterotrimeric G proteins Gi and Go. Activation of these proteins in turn inhibits adenylate cyclase (AC), which when inhibited, cannot activate cyclic AMP. Overall, this leads to an inhibition of cAMP production within the cell, thus making A1 receptors classified as inhibitory (Chen, 2014; Chen, 2014). Additionally, A1 receptor binding can target the inhibition of calcium and initiation of potassium signaling within the brain specifically (Kirsch, Codina, Birnbaumer, & Brown, 1990). However, the binding of the A1 receptor to the membrane does have excitatory effects on other molecules. For example, the A1 receptor binding to the membrane activates G protein Gα16, which in turn causes the β and γ subunits to release. This release causes another signaling cascade by activating PLC to release inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), which in turn cause the release of calcium and protein kinase C (PKC), respectively (Fenton, Shea, Doddi, & Dobson Jr., 2010). The binding of the A1 receptor is also thought to activate β-arrestin1 in a self-downregulating process. As adenosine binds to A1 receptors on the membrane, this stimulates beta-arrestin1, which in turn activates extracellular signal-regulated kinases 1 and 2 (ERK1/2). ERK1/2 promotes the uncoupling of adenosine from the A1 receptor, thus downregulating expression. However, this mechanism of uncoupling and downregulating is seen to simultaneously prepare the cell for increased activity by mediating long-term agonist exposure (Jajoo, Mukherjea, Watabe, & Ramkumar, 2009). Overall, A1 receptors are classified as inhibitory due to their inhibitory effects on AC and in turn cAMP. The expression levels and function of the A1 receptor in different cancer types are highly variable. Studies have reported variable A1 receptor expression levels in tumor cell lines and cancer tissues in comparison to healthy controls (Gessi et al., 2011; Gessi, Merighi, Sacchetto, Simioni, & Borea, 2011; Panjehpour, Hemati, & Forghani, 2012). Despite its role in tumorigenesis, few studies have reported anti-tumor effects exerted by the A1 receptor including increased apoptosis by activating caspases (Fishman et al., 2001; Gessi, Merighi, Fazzi, et al., 2011; Gessi, Merighi, Sacchetto, et al., 2011; Panjehpour et al., 2012; Regan et al., 2003).
3.3.2. A2A receptor
A2A receptor is a G-coupled protein receptor with the highest affinity for adenosine out of the four adenosine receptors. The binding affinity of this receptor for adenosine is ~0.7 μM (EC50) (Fredholm, Irenius, Kull, & Schulte, 2001). A2A receptor is present in several tissues of the human body including, but not limited to, the striatum, nucleus accumbens, olfactory tubercle, immune cells, heart, lungs, blood vessels, retina, aorta, and a variety of blood cells (Conti, Monopoli, Gamba, Borea, & Ongini, 1993; Dixon, Gubitz, Sirinathsinghji, Richardson, & Freeman, 1996; Fredholm, 2007; Lee et al., 2003; McIntosh & Blazynski, 1994). The A2A and A2B receptors are classified separately from A1 and A3 receptors because of their property of activating AC (Chen, Lee, & Chern, 2014; de Lera Ruiz, Lim, & Zheng, 2014). This activation leads to an increase in cAMP levels. Subsequently, Protein kinase A (PKA) is activated and phosphorylated to stimulate cAMP-responsive binding protein (CREB) (de Lera Ruiz et al., 2014). In monocytic THP 1 cells, the activated A2AR uses G-coupled protein receptor kinase 2 (GRK2) to send β-arrestin to the plasma membrane of the cell to desensitize A2A (Khoa, Postow, Danielsson, & Cronstein, 2006). Evidence shows that the activation of other MAPKs and ERKs results from the activation of A2AR (Schulte & Fredholm, 2000). Several cancer cell lines overexpress A2A receptor which is associated with changes in cell proliferation, apoptosis, angiogenesis, anti-tumor protective abilities, and endothelial cell tube formation (Cekic, Day, Sag, & Linden, 2014; Fishman et al., 2003; Gessi, Merighi, Sacchetto, et al., 2011; Ohta, 2016; Sun, Wang, & Hao, 2022; Yu, Zhu, Xie, & Wang, 2020).
3.3.3. A2B receptor
A2B receptor has the lowest binding affinity for adenosine out of the four classified adenosine receptors, with an EC50 value of 24 μM (Fredholm et al., 2001). A2B receptor primarily acts under physiopathological conditions due to its requirement for high adenosine. As an example, A2B receptors are notably expressed during conditions of hypoxia and ischemic pre-conditioning (Aherne, Kewley, & Eltzschig, 2011; Eltzschig, 2013; Kong, Westerman, Faigle, Eltzschig, & Colgan, 2006). A2B receptors are mainly located in the gastrointestinal tract, urinary bladder, lung tissues, immune cells, and cecum (Dixon et al., 1996; Jacobson & Gao, 2006). Like A2A, the A2B receptor stimulates AC thus increasing cAMP levels followed by PKA activation and CREB stimulation (Feoktistov, Murray, & Biaggioni, 1994). β-arrestin plays a role in the desensitization of A2B like other adenosine receptors (Mundell, Loudon, & Benovic, 1999). A2B is considered of less significance than A2A, however recent data advocate a specific role of this receptor in pathophysiological conditions including cancer (Gessi, Merighi, Fazzi, et al., 2011; Gessi, Merighi, Sacchetto, et al., 2011). A2B receptor is considered to play a pro-angiogenic role in cancer with demonstrated reduced tumor growth and increased survival rate (Gao & Jacobson, 2019; Sorrentino, Miele, Porta, Pinto, & Morello, 2015). The promoter region of this receptor contains a functional binding site for hypoxiainducible factor (HIF), which leads to the induction of A2B under hypoxic conditions (Kong et al., 2006). A2B receptor-dependent signaling in immune cells is exploited by tumor cells for their survival, and several agonists and antagonist compounds have been discovered for targeting this receptor for therapies (Allard, Turcotte, & Stagg, 2017; Gessi, Merighi, Fazzi, et al., 2011; Gessi, Merighi, Sacchetto, et al., 2011; Sitkovsky, 2009).
3.3.4. A3 receptor
A3 receptors follow a similar pathway to A1 receptors and inhibit cAMP production via binding activation of Gαi. Once activated, this protein inhibits AC, resulting in a lack of production of cAMP. Therefore, the binding of adenosine to an A3 receptor ultimately results in the inhibition of cAMP production, making it an inhibitory adenosine receptor (Chen, 2014). However, the secondary messenger cascades following activation of the A3 receptor are arguably more complicated and less studied than those of the A1 receptor. A3 receptor activation also initiates the PLC pathway, which sets off several secondary messenger pathways (Chen, 2014). Activation of the PLC pathway by the A3 receptor cascades into activating IP3 and DAG, which in turn increase intracellular calcium and activates PKC (Hammarberg, Schulte, & Fredholm, 2003). Like A1R receptors, this process activates ERK1/2. However, an additional pathway activated by A3 receptors also results in ERK1/2 activation. When A3 receptors bind and release the trimeric G-protein, beta and gamma G-proteins are thought to activate the phosphatidylinositol-3-kinase (PI3K) pathway (Schulte & Fredholm, 2000). Increased activity in this pathway is widely accepted as a sign of cancer, which is pertinent to investigating A3 receptors and immunosuppression interplay (Fruman et al., 2017). PI3K activates Ras, a guanine nucleoside protein, and Akt (protein kinase B), which then together activate the rapidly accelerated fibrosarcoma 1 (RAF1) gene. This gene encodes the protein that makes mitogen-activated protein kinase kinase (MEK), which is the final step in contributing to the activation of the ERK1/2 pathway. Interactions between these two separate pathways that end in ERK1/2 activation are less studied. However, like other adenosine receptors, A3 receptors are known to recruit β-arrestin, eventually causing receptor desensitization and internalization (Chen, 2014). Various sources have reported ligands that prefer A3 receptor-mediated β-arrestin translocation, indicating A3 receptor is a potential avenue for drug and therapy development (Gao & Jacobson, 2008; Gao et al., 2011). A3 receptor is also over-expressed in different types of cancer cells (Fishman, Bar-Yehuda, Madi, & Cohn, 2002; Gessi, Merighi, Fazzi, et al., 2011; Gessi, Merighi, Sacchetto, et al., 2011; Mazziotta et al., 2022) compared to their normal counterparts. The role of the A3 receptor in mediating anti-tumor actions has been demonstrated in in vitro and in vivo models. A3 receptor agonists are known to increase natural killer (NK) cell activity, thus promoting killing of tumor cells (Harish, Hohana, Fishman, Arnon, & Bar-Yehuda, 2003; Jacobson et al., 2018). Another study demonstrated that A3 activation suppresses high ROS levels in prostate cancer cells via inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Jajoo et al., 2009). Further, a reduced activity of ERK1/2 has also been associated with A3 receptor activation.
4. Adenosine receptor and transporter interplay in cancers
Although adenosine receptors and transporters share the common factor of adenosine itself, the connection is relatively under-studied. While the exact mechanisms are unknown, research suggests a connection between A1 receptors and CNT2 transporters that is modulated by varying glucose concentrations in the cell, suggesting that extracellular conditions may bring the two together (Duflot et al., 2004). Additionally, researchers found that A1 receptors activate ATP-sensitive potassium channels which in turn regulate CNT2 transporters, suggesting that different ion channels may potentially be the link between the receptors and transporters (Duflot et al., 2004). Although more research is needed to determine whether these observations apply to the broader spectrum of adenosine receptors and transporters, these findings show that investigating ion channels and outside cellular conditions might further support the link between the transporter and receptor. Potentially, adenosine receptors activate ATP-dependent ion channels through the adenosine exchange, which in turn encourages the flow of additional adenosine through CNTs, most likely CNT2 and CNT3 due to CNT1’s lack of high affinity for adenosine. Therefore, therapies that target the receptors themselves could in turn influence the functionality and expression of transporters, potentially resulting in less toxic and more easily developed therapies. This also begs the question of whether targeting the interplay mechanism, for instance, the potassium-dependent ATP channels, could be the key to regulating transporter levels in cancer patients.
5. Adenosine compartmentalization in immune cells within the tumor microenvironment
Nucleoside transporter proteins have been considered a static component of the purinome up to this point, facilitating the intracellular compartmentalization of adenosine at a constant rate that depends upon the flux between intracellular and extracellular adenosine levels. Notwithstanding the potential for efflux of adenosine under conditions where nucleoside transport and intracellular metabolism are uncoupled, there is evidence that the expression of nucleoside transporter proteins and therefore adenosine compartmentalization is altered in several cancer subtypes (Farre et al., 2004; Pennycooke, Chaudary, Shuralyova, Zhang, & Coe, 2001). Table 4 summarizes previously published studies that have documented alterations in nucleoside transporter expression or function in various human malignancies.
Table 4:
Nucleoside Transporter Alterations in Cancer
In addition to differences in nucleoside transporter expression between normal and tumor tissues, it is also noteworthy to mention that extensive interpatient variability exists in transporter expression. In hepatocellular carcinoma, a study investigating the CNT1 expression in patient samples, found an overall downward trend between normal liver and cancerous samples, although a wide range of variability existed between individual patients (Hesler et al., 2016). Likewise, a downward trend in CNT1 expression was noted in pancreatic cancer samples with distinct interpatient variability (Fotoohi, Lindqvist, Peterson, & Albertioni, 2006). In acute myeloid leukemia (AML), genetic polymorphisms in ENT1 lead to differences in sensitivity to cytarabine (Kim et al., 2016).
Although the altered purinergic signaling during immunosuppression in different cancers is well documented, knowledge of alterations in adenosine receptors on the acting immune cells is also accumulating. It is apparent that cancer cells are sometimes able to evade immunosurveillance and elimination and manage to proliferate and form malignant tumors. Immunosuppression is achieved by multiple mechanisms not limited to the expression of immune checkpoints, downregulation of antigen presentation, and accumulation of immunosuppressive factors. A continually expanding body of research aims to fully appreciate how the immune system is reprogrammed to ignore cancer cells and the mechanisms by which cancer cells evade immune detection to permit tumor growth. Studies have reported the up- and downregulation of ARs, ENTs, and CNTs specific to immune cells during inflammatory stress, as discussed in subsequent sections.
5.1. T cells
T cells express all four adenosine receptors (Gessi et al., 2004; Koshiba, Rosin, Hayashi, Linden, & Sitkovsky, 1999; Mastelic-Gavillet et al., 2019; Mirabet et al., 1999), yet A2A receptor-mediated downstream adenosine signaling dominates functional regulation of T cells since A2A receptor is predominantly expressed (Gessi et al., 2004). Additionally, the expression of A2A and A2B receptors is induced by hypoxia via HIF-α1 (Sitkovsky, 2009) and Egr-2/Egr-3 anergic stimulation (Beavis et al., 2017; Safford et al., 2005), both conditions characteristic of the TME which often has a limited blood supply and infiltration of CD8+ T cells rendered anergic due to the lack of proper priming by co-stimulation. The upregulation of A2A and A2B receptors on T cells further magnifies the impact of adenosine ligation. Increased levels of cAMP in T cells activate PKA which in turn phosphorylates and induces the activity of transcription factors CREB, cAMP responsive element modulator (CREM), and activating transcription factor-1 (ATF-1) (Linden & Cekic, 2012). CREB, CREM, and ATF-1 activation modulate the expression of several genes related to inflammation including IL-3, IL-4, IL-17, IFN-γ, and IL-2 (Vigano et al., 2019). Another downstream effector of cAMP, guanine nucleotide exchange factor EPAC1 which is also known as exchange protein activated by cAMP or EPAC, prevents the release of Rap1 from the plasma membrane which is required for TCR-mediated signal transduction via the MEK-ERK pathway (Boussiotis, Freeman, Berezovskaya, Barber, & Nadler, 1997). CD8+ T cells perform targeted killing of cells displaying neoantigens on MHC class I molecules. Stimulation of A2A receptor on CD8+ T cells leads to suppression of proliferation and cytotoxic effector function (Mastelic-Gavillet et al., 2019; Ohta et al., 2009). Furthermore, the differentiation of T cells towards an immunosuppressive phenotype called T regulatory cell (Treg), is induced by A2A receptor signaling (Ohta & Sitkovsky, 2014). Additionally, the secretion of cytokines is suppressed in CD4+ T helper (Th) cells with these cells exhibiting a decreased secretion of IFN-γ and decreased secretion of IL-4, IL-5, and IL-10 by Th2 cells (Csoka et al., 2008).
It is known that T cells predominantly express ENTs which promote T cell effector function and expansion (McCaw et al., 2019; Naes, Ab-Rahim, Mazlan, & Abdul Rahman, 2020; Pastor-Anglada & PerezTorras, 2018b). ENT promotes T cell effector function and expansion. ENT3 is abundantly expressed in peripheral T cells, while ENT1 is also expressed in peripheral T cells at lower levels. ENT3 is also suspected of playing a role in peripheral T cell homeostasis, regulation of cell sizes and organelles, and cell survival and proliferation (Hsu et al., 2012; Wei et al., 2018). In the absence of ENT3, peripheral T cells would have dysregulations such as failure to maintain cellular homeostasis, irregular quantities of organelles, failure to proliferate, and undergoing apoptosis. In addition to these factors, the absence of ENT3 in cells showed more disorganization of mitochondria and an increased number of vacuoles within the cells. The absence of ENT3 in cells also showed an increase in size compared to ENT3-proficient cells. The absence of ENT3 in cells also displayed negative effects on autophagy (Nair et al., 2019; Wei et al., 2018). Autophagy allows for the removal of unwanted mitochondria in T cells. Finally, the absence of ENT3 in cells also showed an increase in DNA damage within the affected cells (McCaw et al., 2019; Wei et al., 2018).
5.2. B cells
B cells are a component of humoral immunity and function to secrete antibodies, flagging invading cells for engulfment by circulating phagocytes, and have minor roles in antigen presentation and cytokine secretion. A subset of B cells (B reg) is identified with high expression of extracellular adenosinergic enzymes CD39 and CD73 and is capable of suppressing T cell proliferation and cytokine production (Figueiro et al., 2016; Jeske et al., 2020; Saze et al., 2013). A follow-up study demonstrated that IL-10 may be linked to CD73-mediated immune suppression, although the mechanism is yet unclear (Kaku, Cheng, Al-Abed, & Rothstein, 2014). A new role of B cells in HIV-1 pathology is reported where a skewed CD39/CD73/adenosine pathway leads to activation of the innate immune response, thus, opening new avenues for the treatment of HIV-1 patients (Song et al., 2019).
In the context of nucleoside transport, previous studies have reported significant expression of both ENTs and CNTs (mostly CNT2) transporters in human B cell lines (Raji) (Flanagan & Meckling-Gill, 1997; Soler, Felipe, Casado, Celada, & Pastor-Anglada, 2000). Further, B-cell activators such as phorbol esters (PMA) and bacterial lipopolysaccharide (LPS), were found to upregulate the level of CNTs and downregulate ENTs. A significant up-regulation of CNT3 after PMA treatment in HL-60 cells suggests its potential involvement in the immune regulation of these cells (Ritzel et al., 2001b). Significant changes in nucleoside transporter (ENT1, ENT2, and CNT2) mRNA levels have been observed in B lymphocytes isolated from diabetic rats, where transport alterations are independently and differentially regulated by glucose and insulin (Sakowicz, Szutowicz, & Pawelczyk, 2005).
5.3. Natural killer cells
NK cells are an integral component of the innate immune response and are involved in immunosurveillance by recognizing and eliminating tumorigenic cells. Adenosine has profound immunosuppressive implications for NK cell function in solid tumors. In A2A receptor-deficient mice, the proportion of terminally mature NK cells is significantly increased compared to mice with intact A2A receptor signaling, suggesting that adenosine suppresses the development of NK cells (Young et al., 2018). Regarding NK effector function, IL-2 stimulated NK cell cytotoxicity was impaired in both the Fas-ligand and perforinmediated pathways by Gαs signaling via A2A and A2B activation (Raskovalova et al., 2005). Interestingly, Chambers and colleagues found that cytokine priming of NK cells is an important determinant of the cellular pathways activated in response to adenosine signaling, and that pre-treatment with unique cytokine signatures elicited varied NK cell responses (Chambers et al., 2018). IL-2 in presence of adenosine inhibited NK cell cytotoxic function, cytokine production (especially IFN-γ and TNF-α), and associated signaling pathways (Chambers & Matosevic, 2019; Lokshin et al., 2006; Raskovalova et al., 2005; Raskovalova, Lokshin, Huang, Jackson, & Gorelik, 2006). These cells modulate their metabolism to meet the high energy demands during cell proliferation and functionality during inflammatory stress.
An up-regulation in the expression of several solute carrier transporters in NK cells is reported during inflammation (Khan & Khan, 2021). NK cells express key transporters like ENT1, ENT2, and CNT3 which are involved in the uptake and activation of fludarabine (a nucleoside analog) to its active metabolite, fludarabine triphosphate (F-ara-ATP) (Woodahl, Wang, Heimfeld, Sandmaier, & McCune, 2009).
5.4. Dendritic cells
Responsible for antigen processing and presentation, dendritic cells (DCs) are critical for proper training of the adaptive immune system to respond to foreign antigens. There are several subsets of DCs classified mainly based on peculiar cell-surface markers (Collin, McGovern, & Haniffa, 2013; McDonald et al., 2003; Patente, Pelgrom, & Everts, 2019). The classical or conventional myeloid DCs typically express myeloid antigens CD11c, CD13, CD33, and CD11b, whereas plasmacytoid DCs lack the characteristic myeloid antigens and express CD123, CD303, and CD304 respectively. Langerhans cells are another selfrenewing DC population expressed predominantly in the brain. Monocyte-related DCs have numerous diverse subsets including inflammatory non-classical DCs with a range of CD surface markers. Expression of all four AR subtypes has been found on the surface of human DCs, although A1 and A3 receptors are expressed during development while the A2A receptor subtype is most highly expressed after maturation (Panther et al., 2001; Panther et al., 2003; Schnurr et al., 2004). A1 and A3 receptors mediate PLC/DAG/IP3/Ca+ signaling in immature DCs, while A2A acts through elevating cAMP levels implicated in the down-regulation of cytokine-producing capacity in mature DCs (Panther et al., 2001; Panther et al., 2003; Schnurr et al., 2004). Notably, the differentiation of dendritic cells in the presence of adenosine is skewed towards a tolerogenic phenotype with decreased cytokine (IL-2) secretion in response to LPS exposure and defective T-cell priming (Challier, Bruniquel, Sewell, & Laugel, 2013). A suppressive tumor-promoting DC phenotype with increased IL-10 secretion was also observed by AR signaling which could be mimicked by treatment with cAMP and PKA analogs (Kayhan, Koyas, Akdemir, Savas, & Cekic, 2019).
While there is an abundance of human nucleoside transporters in dendritic cells in general (Minuesa et al., 2008), notably, ENT1, ENT2, and CNT3 are highly expressed in monocyte-derived dendritic cells (Arimany-Nardi et al., 2014; Minuesa et al., 2008). ENT1 is also, be located on dendritic plasma membranes (Anderson et al., 1999).
5.5. Macrophages
Macrophages originate from undifferentiated stem cells in the bone marrow and proliferate, become activated, or differentiate in the presence of specific growth factors or cytokines. Within the tumor microenvironment, macrophages undergo phenotypic polarization into M1 (classically activated) and M2 (alternatively activated) phenotypes in response to tumor-derived signals (Italiani & Boraschi, 2014; Murray et al., 2014). Both tumor associated macrophage (TAM)1 and TAM2 phenotypes are involved in tumor-related inflammatory reactions, however, M2 is primarily associated with cancer progression via angiogenesis, neovascularization, stromal activation, and remolding (Afik et al., 2016; Ries et al., 2014; Ruffell & Coussens, 2015; Tiainen et al., 2015). TAMs may generate additional extracellular adenosine which can, in turn, activate immunosuppressive adenosine signaling in neighboring immune cells in a paracrine manner. A recent study demonstrated that the generation of adenosine by ovarian cancer cells favored the differentiation of myeloid cells to TAMs which also created extracellular adenosine by overexpression of CD39 and CD73 (Montalban Del Barrio et al., 2016). A separate study showed that treatment of CD39+ TAMs with POM-1 (a CD39 inhibitor) or ADA was both able to diminish some of the immunosuppressive functions of TAMs including the secretion of IL-10 (d’Almeida et al., 2016).
Studies have reported co-expression of CNT1, CNT2, ENT1, and ENT2 in murine bone marrow samples (Kong, Engel, & Wang, 2004; PastorAnglada et al., 2001). The findings reveal that nucleoside transporters selectively regulate macrophage proliferation and activation depending on their specific requirements for DNA and RNA synthesis (MecklingGill, Guilbert, & Cass, 1993; Pastor-Anglada et al., 2001; Soler et al., 2001; Soler et al., 2001). Further, IFN-γ differentially regulates transporter expression (CNT1, CNT2, and ENT1) in macrophages via STAT1-dependent and/or independent manner (Soler et al., 2003). ENT3 is an essential nucleoside transporter crucial for lysosomal nucleoside trafficking. A high ENT3 expression in macrophages is associated with the development of lysosomal storage disorder in ENT3 mutant mice (Hsu et al., 2012). ENT3 deficiency triggered myelopoiesis which significantly enhanced the number of splenic macrophages, with evident organ infiltration leading to histiocytic sarcoma in mice. Since ENT3 is known to coordinate lysosomal function with nucleoside availability in immune cells (Wei et al., 2018), transporter loss renders these cells with dysfunctional lysosomal abilities incapable of executing normal clearance and defense functions (Hsu et al., 2012). Abundant immunosuppressive adenosine in TME affects the phagocytic activity of TAMs, via nucleoside transporters (ENTs, CNTs) and/or adenosine receptors (A1, A2A, A2B, and A3) (Li et al., 2016). A2A receptor activation reduces M1 polarization, whereas A2B activation induces M2 macrophage polarization. Hypoxic conditions in TME further facilitate these processes by transcriptional induction of CD39/CD73 and transcriptional downregulation of ENTs (Eltzschig et al., 2005). The precise changes in the nucleoside transport profiles of M1 and M2 macrophages are understudied.
5.6. Myeloid-derived suppressor cells
Myeloid-derived suppressor cells (MDSCs) are formed in the TME when infiltrating myeloid cells are exposed to tumor-associated factors and accumulate as an immunosuppressive cell population in tumors, lymphoid organs, and peripheral blood. They encompass immature myeloid cells from various lineages. In the context of the TME, T-cell responses are suppressed by MDSCs by their production of arginase, reactive oxidative species, and cytokines. Interestingly, MDSC expansion is promoted by A2B receptor signaling, and the generation of adenosine by CD73 may further augment this expansion in an autocrine fashion (Ryzhov et al., 2011). Additionally, A2B receptor signaling promotes the production of vascular endothelial growth factor (VEGF) and angiogenesis in MDSCs, whereas pharmacological blockade of the A2B receptor resulted in decreased immunosuppression and tumor growth in a mouse model of melanoma (Iannone, Miele, Maiolino, Pinto, & Morello, 2013; Sorrentino et al., 2015). The expression of nucleoside transporters in MDSCs is not fully characterized.
6. Regulation of nucleoside transporters in cancer
The function of nucleoside transporters, both ENTs, and the less studied CNTs, as a point of regulation for adenosine compartmentalization in cancer, is less exploited. Summarized below are potential therapeutic avenues for attenuation of adenosine buildup by modulating nucleoside transport processes including mechanisms that alter nucleoside transporters in various physio-pathological states, which could be potentially exploited for the intended purpose.
6.1. Drug treatment
Many nucleoside analogs are used as anti-cancer drugs and rely on nucleoside transporters for intracellular accumulation. This includes nucleoside analog drugs that resemble both purine and pyrimidine nucleosides, such as cytarabine (pyrimidine type) and fludarabine (purine type), both used in the treatment of leukemia (Pastor-Anglada & Perez-Torras, 2015). Numerous reports have studied the relevance of nucleoside transporter expression and function for nucleoside analog chemosensitivity. Interestingly, treatment with chemotherapy can lead to the downregulation of nucleoside transporter expression, compromising the intracellular accumulation of nucleoside analog drugs. This is the case for CNT2 and ENT2 in T-lymphoblastic cell lines resistant to thiopurine nucleoside analogs (Fotoohi et al., 2006). Notably, drug treatment can modulate nucleoside transporter function as well. For instance, HeLa cells were treated with over 60 different cytotoxic drugs, and etoposide was found to significantly enhance the uptake of [18F] fluor thymidine, a positron emission tomography (PET) tracer used to monitor cancer treatment (Lee & Lee, 2013). On the other hand, treatment of K562 leukemia cells with tyrosine-kinase inhibitors can inhibit nucleoside transporter function (Huang, Wang, Mitchell, & Graves, 2004). Hence, there is potential for repurposing these drugs for targeting the nucleoside transporters to ultimately either improve drug uptake or reduce the extracellular adenosine, especially in a chemotherapy setting. Wang et al. published an extensive compilation of the currently marketed drugs as well as those under clinical trials targeting the SLC family of proteins (Wang, Gallo, Jadhav, Hawkins, & Parker, 2020). So far, there are two approved vasodilator drugs namely, dipyridamole and dilazep, that are known to directly target ENT1. Table 5 lists all the clinically approved drugs and non-drug use commercial inhibitors, that can potentially impact the nucleoside transporter function.
Table 5:
Clinical Drugs/Inhibitors Inhibiting ENT And CNT-mediated Nucleoside Transport
| Drug Type | Name | Cell lines/animals | Dose | Transporter | [3H]nucleosidee uptake (%Inhibition/IC50) | Reference |
|---|---|---|---|---|---|---|
| Nucleoside Reverse Transcriptase inhibitor (NRTIs) | Entecavir (ETV) | HeLa | 1mM | ENT1 ENT2 |
3.05±1.56mM ([3H] uridine) 2.07±0.29mM ([3H] uridine) |
(Miller, et al., 2020) |
| Abacavir (ABC) | 1mM | ENT1 ENT2 |
0.11±0.02mM ([3H] uridine) 0.22±0.01mM ([3H] uridine) |
|||
| Zidovudine (AZT) | 1mM | ENT1 ENT2 |
2.54±0.26mM ([3H] uridine) 1.64±0.36mM ([3H] uridine) |
|||
| Pyrimidopyrimidines and Pteridines | Dipyridamole | PK15NTD | 10μM | ENT1 ENT2 |
5.0±0.9nM ([3H]NBMPR) 356±13nM ([3H]NBMPR) |
(Boswell-Casteel & Hays, 2017; Boyer, Karjian, Wahl, Pegram, & Neuteboom, 2002; Ward, et al., 2000; J. D. Young Yao, Sun, Cass, & Baldwin, 2008); |
| Alkyl, Cycloalkyldiamine and Piperazine compounds | Dilazep | PK15NTD (hENT1 and hENT2) H9c2 (rENT2) |
10μM | ENT1 ENT2 |
17.5nM ([3H]-5-uridine) 8800nM ([3H]-5-uridine) |
(Playa, et al., 2014) |
| Draflazine | U-2 OS | 10-11 to 10-6 | ENT1 ENT2 |
5.3nM ([3H]adenosine) 15.85nM ([3H]adenosine) 16.0± 5.9nM ([3H] uridine) 2400± 400nM ([3H] uridine) |
(Bohm, et al., 1994; Hammond, 2000; Noji, Karasawa, & Kusaka, 2004; Vlachodimou, Konstantinopoulou, AP, & Heitman, 2020) | |
| Cannabinoids | Tetrahydrocannabinol (THC) | EOC-20 RAW264.7 | 100–1000nM | ENT1 | 0.17μM ([3H]thymidine) 0.27μM ([3H]adenosine) |
(Carrier, et al., 2006; Stollenwerk, Pollock, & Hillard, 2021) |
| Cannabidiol (CBD) | 100–1000nM | ENT1 | 0.19μM ([3H]thymidine) 0.12μM ([3H]adenosine) |
|||
| Quinolinone derivative | Cilostazol (Pletal) | Cardiac ventricular myocytes, Coronary artery smooth muscle, Endothelial cells | 5–10μM | ENT1 | ~10μM (EC50 ([3H]adenosine) | (Y. Liu, et al., 2000) |
| Receptor inhibitors | Propentofylline | L1210/B23.1 cells; L1210/C2; Walker 256; L1210/MA27.1 |
es
ei cif |
9μM ([3H]adenosine) 170μM ([3H]adenosine) 6mM ([3H]adenosine) |
(Parkinson, Paterson, Young, & Cass, 1993; Parkinson, Rudolphi, & Fredholm, 1994) | |
| Ticagrelor | MDCK | 0.1–100 nM/L; 1–100 μM/L | ENT1 ENT2 |
6.59+0.09μM ([3H]adenosine) 4.76+0.03μM ([3H]adenosine) |
(Armstrong, et al., 2014) | |
| Thiazolidinediones | Troglitazone | HASMC | 30μM | ENT1 | 2.35±0.35μM ([3H]adenosine) 4.38±0.34μM ([3H]uridine) 3.99±0.57μM ([3H]NBMPR) |
(Leung, Man, & Tse, 2005) |
| Pioglitazone | 30μM | ENT1 | 13% ([3H]adenosine) | |||
| Ciglitazone | 30μM | ENT1 | 8% ([3H]adenosine) | |||
| Antiviral drugs | Remdesivir | HeLa | - | ENT1 ENT2 |
38±2μM ([3H]uridine) 73±14μM ([3H]uridine) |
(Miller, et al., 2021) |
| EIDD-1931 | - | ENT1 ENT2 |
259±118μM ([3H]uridine) 467±101μM ([3H]uridine) |
|||
| Molnupiravir | - | ENT1 ENT2 |
701±294μM ([3H]uridine) 851±152μM ([3H]uridine) |
|||
| Dihydrochalcones | Phloridzin | - | - | CNT3 | 16±0.01μM (Ki) | (Gupte & Buolamwini, 2009) |
| Tyrosine kinase inhibitors (TKIs) | Erlotinib | AsPc-1 A549, H292, H1975 cells, Yeast | 0–100μM | CNT1 CNT3 ENT1 |
160±20μM ([3H]-uridine) 11±1μM ([3H]-uridine) 1.6±0.4 to 34±6μM ([3H]-uridine) |
(Damaraju, et al., 2014) |
| Gefitinib | CNT1 ENT1 |
37±11μM ([3H]-uridine) 2.0±0.6 to 14±6μM ([3H]-uridine) |
||||
| Vandetanib | CNT1 CNT2 CNT3 ENT1 ENT2 |
64±17μM ([3H]-uridine) 82±4μM ([3H]-uridine) 28±9μM ([3H]-uridine) 11±1 to 33±8μM ([3H]-uridine) 89±17μM ([3H]-uridine) |
||||
| Imatinib | Saccharomyces cerevisiae | 0–300μM | CNT2 ENT1 |
2.3μM ([3H]uridine) 110μM ([3H]uridine) |
(Damaraju, Weber, Kuzma, Cass, & Sawyer, 2016) | |
| Dasatinib | ENT1 | 60μM ([3H]uridine) | ||||
| Bosutinib | ENT1 | 13μM ([3H]uridine) | ||||
| Nilotinib | ENT1 | 0.7μM ([3H]uridine) | ||||
| Ponatinib | ENT1 | 9μM ([3H]uridine) | ||||
| Abemaciclib | HAP1-ENT2 KO | 10μM | ENT1 | 25.0±7.9% ([3H]-uridine) | (Jouan, et al., 2021) | |
| Acalabrutinib | 10μM | ENT1 | 42.6±10.4% ([3H]-uridine) | |||
| Afatinib | 10μM | ENT1 | 54.5±2.4% ([3H]-uridine) | |||
| Alectinib | 10μM | ENT1 | 54.0±4.9% ([3H]-uridine) | |||
| Brigatinib | 10μM | ENT1 | 52.5±11.1% ([3H]-uridine) | |||
| Cabozantinib | 10μM | ENT1 | 48.8±2.5% ([3H]-uridine) | |||
| Ceritinib | 10μM | ENT1 | 26.3±1.7% ([3H]-uridine) | |||
| Crizotinib | 10μM | ENT1 | 34.7±2.7% ([3H]-uridine) | |||
| Dacomitinib | 10μM | ENT1 | 43.9±2.3% ([3H]-uridine) | |||
| Entrectinib | 10μM | ENT1 | 22.9±5.2% ([3H]-uridine) | |||
| Ibrutinib | 10μM | ENT1 | 72.7±1.1% ([3H]-uridine) | |||
| Itacitinib | 10μM | ENT1 | 21.9±13.3% ([3H]-uridine) | |||
| Lapatinib | 10μM | ENT1 | 25.7±15.2% ([3H]-uridine) | |||
| Lenvatinib | 10μM | ENT1 | 63.1±5.9% ([3H]-uridine) | |||
| Lorlatinib | 10μM | ENT1 | 86.6±6.4% ([3H]-uridine) | |||
| Neratinib | 10μM | ENT1 | 61.0±8.5% ([3H]-uridine) | |||
| Nintedanib | 10μM | ENT1 | 46.9±9.6% ([3H]-uridine) | |||
| Osimertinib | 10μM | ENT1 | 32.3±11.3% ([3H]-uridine) | |||
| Pacritinib | 10μM | ENT1 | 61.6±8.1% ([3H]-uridine) | |||
| Regorafenib | 10μM | ENT1 | 21.4±2.7% ([3H]-uridine) | |||
| Ribociclib | 10μM | ENT1 | 17.8±10.2% ([3H]-uridine) | |||
| Ruxolitinib | 10μM | ENT1 | 34.1±13.2% ([3H]-uridine) | |||
| Tofacitinib | 10μM | ENT1 | 4.8±8.3% ([3H]-uridine) | |||
| Vemurafenib | 10μM | ENT1 | 27.3±10.9% ([3H]-uridine) | |||
| Inhibitors not approved for drug use | S-(4-nitrobenzyl)-6-thioinosine (NBMPR) | PK15NTD | 1μM | ENT1, 2 | 0.4± 0.1nM ([3H]NBMPR) 2.8±0.3mM ([3H]NBMPR) |
(Ward, et al., 2000) |
| FPMINT | 10nM-100μM | ENT1 ENT2 |
8.04±1.04μM ([3H]-uridine) 1.34±0.154μM ([3H]-uridine) |
(R. Li, et al., 2022) | ||
| Rapadocin | 10μM | ENT1 | 3.3nM ([3H]-thymidine) | (Y. Wang, et al., 2021) |
6.2. Epithelial-mesenchymal transition
The epithelial-mesenchymal transition (EMT) is a set of phenotypic changes that confer cancer cells with migratory and invasive properties. During EMT, cells undergo morphologic changes driven by EMT master-regulator transcription factors such as SNAI1 and TWIST that result in the rearrangement of cytoskeletal elements, changes in cell polarity, and loss of cell-cell adhesion (Nieto, Huang, Jackson, & Thiery, 2016). Zheng and colleagues demonstrated that silencing the EMT program in a spontaneous mouse model of pancreatic cancer resulted in increased expression of ENT2 and CNT3 (Zheng et al., 2015). A recent study on the loss of ENT1 in pancreatic cancer further established the role of EMT in regulating nucleoside transporter expression, identifying that altered expression of adhesion molecules, especially the downregulation of E-cadherin and EpCAM and upregulation of N-cadherin, correlated with decreased ENT1 at the plasma membrane (Weadick et al., 2021). Interestingly, mesenchymal-type pancreatic cancer mouse xenografts had a loss of ENT1 and decreased sensitivity to gemcitabine (a mainline agent for treating pancreatic cancer) treatment, resulting in larger tumors and more extensive metastatic spread (Weadick et al., 2021). In addition, the loss of cellular polarity in EMT may shift the directionality of vectorial transport of nucleosides mediated by ENTs and thereby outcomes. Elaskalani and colleagues studied the role of platelet-derived ATP and ADP and found that ADP-mediated P2Y12 receptor purinergic signaling regulated the expression of EMT transcription factor SLUG, which in turn suppressed ENT1 expression in pancreatic cancer cells (Elaskalani, Falasca, Moran, Berndt, & Metharom, 2017).
6.3. Secreted factors in the TME
The TME fosters an environment that favors tumor progression, drug resistance, and metastasis. Besides tumor cells, the TME can include cancer-associated fibroblasts, stromal cells, stellate cells, and immune cells. Many of these cell types secrete soluble factors, i.e., cytokines, that modulate tumor cells, including the expression and function of nucleoside transporters. In leukemia, the TME consists of bone marrow that harbors a cancer-promoting niche that includes stromal cells which influence leukemic cells. In particular, the sensitivity of AML cells to nucleoside analog cytarabine was decreased by co-culture with stromal cells, and mechanistic studies found that ENT1 transport capacity was reduced by over 50% due to soluble factors secreted by stromal cells (Macanas-Pirard et al., 2012). In pancreatic cancer, a negative association between the mRNA expression of matricellular protein CYR61 in pancreatic stellate cells and ENT1 and CNT3 led the researchers to further investigate. They found that transforming growth factor beta (TGF-β) signaling induced the expression of CYR61 in stellate cells, which decreased gemcitabine chemosensitivity by downregulation of ENT1 and CNT3 (Hesler et al., 2016). Both studies point to the role of the TME in regulating the expression of nucleoside transporters.
It is a distinct possibility that the dysfunction of nucleoside transport processes contributes to the buildup of extracellular adenosine by failure to facilitate the uptake of adenosine. One cannot safely assume that adenosine intracellular compartmentalization, performed exclusively by nucleoside transporters, is static in the tumor microenvironment. Depending on the discrepancy between normal tissue expression of nucleoside transporters and expression level in cancer, loss of a nucleoside transporter could be a highly significant factor. This may provide further explanation to what is currently known about extracellular adenosine accumulation; at least partially influenced by ATP release from cancer cells driven by metabolic changes and stress, and the overexpression of CD39/CD73 that metabolizes ATP to adenosine in the extracellular space.
6.4. Soluble factors in the TME
Notably, some soluble factors that are characteristic of the tumor-friendly TME have demonstrated the capability to influence nucleoside transporter expression or function. Cytokines are chemical messengers secreted by immune cells and stromal cells that modulate cell behavior in both autocrine and paracrine manners. The cytokine profile in tumor tissues can vary greatly from normal tissues, and cytokine signaling is involved in the selective process that drives the evolution of cancer cells. IFN-γ is a cytokine that modulates innate and adaptive immune responses. Interestingly, IFN-γ inhibited ENT1 activity via signal transduction and activator of transcription 1 (STAT-1) while CNT1 and CNT2 were upregulated by STAT-1 independent mechanisms (Soler et al., 2003). TGF-β is commonly found in the TME at elevated concentration levels and can lead to the downregulation of CNT1 and CNT3 in pancreatic cancer by induced expression of CYR61 in stromal cells (Hesler et al., 2016). In chronic lymphocytic leukemia, however, CNT3 plasma membrane trafficking and function are enhanced by treatment with all-trans retinoic acid by activation of TFG-β whose downstream effectors promote CNT3 trafficking (Fernandez-Calotti & Pastor-Anglada, 2010).
The TME of many solid tumors is lacking in oxygenation as angiogenesis struggles to keep up with the growing demand for blood flow to cancer cells. Under hypoxic conditions, expression of the transcription factor HIF-1 is increased, reprogramming cellular metabolism and homeostasis to adapt to anaerobic conditions. HIF-1 binds to the promoter of ENT1, repressing the expression of this transporter protein, and decreasing the uptake of adenosine, ultimately leading to enhanced extracellular adenosine (Eltzschig et al., 2005). Based on these findings, the authors speculate that the hypoxia phenomenon may be a putative mechanism that drives the accumulation of extracellular adenosine.
7. Targeting nucleoside transport to curtail immunosuppressive adenosine signaling
Situated as the shuttles between intracellular and extracellular adenosine levels, nucleoside transporter proteins can play a central role in regulating adenosine signaling capabilities. Therefore, manipulation of transporter expression or function can modulate levels of extracellular adenosine and the impact of adenosine receptor signaling. Considering the buildup of extracellular adenosine in the TME and its overall immunosuppressive qualities, an ideal intervention would limit this accumulation of adenosine outside of cells. One can conceive that this could be accomplished primarily by enhancing the intracellular translocation of adenosine via CNTs and ENTs. Yet, it is possible that in scenarios where nucleoside transport and intracellular metabolism are uncoupled, significant adenosine efflux could occur via ENTs, in which case inhibiting transporter function would be beneficial.
7.1. Pharmacologic targeting of nucleoside transporters
Indeed, inhibition of nucleoside transporters is already in use in the cardiovascular system. Adenosine reuptake inhibitor drugs such as dilazep and dipyridamole block individual or multiple ENTs to raise levels of extracellular adenosine and augment the cardioprotective function of adenosine receptor signaling. While blocking ENTs could raise adenosine levels and potentiate immunosuppressive signaling, analysis of the purinome may reveal that efflux via ENTs contributes to the buildup of adenosine, and inhibition of this efflux would help contain adenosine inside cells. In this case, currently approved adenosine reuptake inhibitors may provide therapeutic benefits (Table 5). Considering the multiple genetic and epigenetic alterations in cancer cells and the abnormal conditions of the TME, it is reasonable to hypothesize that significant disruption of purine homeostasis may involve adenosine efflux.
Some of the clinical trials and lab-scale pilot studies have targeted the anti-tumor aspect of inhibitor drugs, dipyridamole, and dilazep in combination treatment with other compounds. The studies as summarized in Table 6, advocate a significant downfall in cancer risk among patients. However, there is limited clinical data available to support the in vivo applications. Moreover, the studies largely involved insufficient patient cohorts to reach any evident conclusion. Therefore, it calls for further extensive and high-throughput studies focused on implementing these treatments in patients.
Table 6:
Clinical Trials and Studies Involving Inhibitor Drugs Targeting Nucleoside Transporters
| Human trials | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor | Cancer | Study Phase | Study type | Identifier/Approval | Purpose | Outcomes | Reference | |||||
| Dipyridamole | Ovarian | Phase 2 | Interventional | NCT00002487 | Studied the effectiveness of combining methotrexate and dipyridamole | Not reported | - | |||||
| Lymphoid neoplasms | - | Interventional | Dnr 2012/795 | Exploring the chemo-preventive potential of dipyridamole | Significant reduction in risk of lymphoid neoplasms (95% CI) | (W. Huang, Sundquist, Sundquist, & Ji, 2022) | ||||||
| Several cancers | - | Observational | - | Antiplatelet agents aspirin and dipyridamole and the risk of different carcinoma in patients with type 2 diabetes mellitus | dipyridamole and aspirin decreased the risk of liver cancer | (H. Y. Huang, et al., 2022) | ||||||
| In vitro studies | ||||||||||||
| Inhibitor | Cancer | Cell line | Concentration | Outcomes | Reference | |||||||
| Dipyridamole | Colorectal cancer | HCT-8, CD133+/CD44+ HCT-8, U937 cells | 0–20μM | Decreased proliferation of cancer cells | (Abdelghany El-Mahdy, Kawabata, Goto, & Li, 2021) | |||||||
| Leukemia | CFU-GEMM, BFU-E, CFU-GM, HL-60/C1, CCRF-CEM | 0.1μM-10μM | reduced toxicity of tubercidin | (Cass, King, Montano, & Janowska-Wieczorek, 1992) | ||||||||
| Dilazep | Prostate cancer | LNCaP, LNCaP-Abl, 22Rv1, LAPC4 | 0–50μM | inhibit proliferation and AR signaling | (Kaochar, et al., 2021) | |||||||
| Leukemia | CFU-GEMM, BFU-E, CFU-GM, HL-60/C1, CCRF-CEM | 0.1μM-10μM | reduced toxicity of tubercidin | (Cass, et al., 1992) | ||||||||
7.2. Genetic regulation of nucleoside transporters
Evidence in the literature points to epigenetic regulation of nucleoside transporter expression, which is a potential point of therapeutic intervention. CNT1 is heavily expressed in the liver, kidneys, and intestines, and its expression can be induced by the transcription factor hepatocyte nuclear factor 4 alpha (HNF4α), which acts by binding to the CNT1 promoter (Klein, Kullak-Ublick, Wagner, Trauner, & Eloranta, 2009). Further, the expression of ENT1 was reduced in a chronic myelogenous leukemia mouse model in response to chemical stress mediated by JNK activation and c-Jun binding to the mENT1 promoter (Leisewitz et al., 2011). Similarly, the expression of zinc transporter ZIP4 activated ZEB1 and induced integrin signaling causing reduced expression of ENT1 via JNK/c-Jun signaling (Liu et al., 2020). Further, loss of histone 3 lysine 27 demethylase KDM6A expression led to nucleoside analog drug resistance in acute myeloid leukemia cells by reducing the acetylation at the ENT1 locus (Stief et al., 2020). Together, these studies show that epigenetic changes modulate the expression of nucleoside transporters. Activation of the signaling pathways known to affect transporter expression, such as JNK/c-Jun and ENT1, could be an alternative to the use of pharmacologic inhibitors. In the case of HNF4α, inducing this transcription factor in tumors to increase the expression of CNT1 could increase the intracellular compartmentalization of adenosine, especially considering that CNTs work against the concentration gradient to concentrate nucleosides inside of cells. In that manner, induction of CNTs could convert tumor cells into immunosuppressive adenosine “sinks.”
Different gene editing methods have been explored to mitigate the effects caused due to transporter loss, by upregulating transporter genes via gene promotors, and at the transcriptional level, targeting epigenetic regulation. Restoring the expression of hCNT1 in NP-9 and NP29 cells via adenoviral vectors affected cell-cycle progression, cell migration, and basal phosphorylation status of selected signaling kinases and significantly reduced tumor growth in an ectopic mouse model of human adenocarcinoma (Perez-Torras et al., 2013). Garcia-Manteiga et al. reported increased gemcitabine sensitivity in pancreatic neoplasia cells (NP9, NP18, NP29, and NP31) heterologously expressing hCNT1, despite high constitutive hENT1 activity (Garcia-Manteiga, MolinaArcas, Casado, Mazo, & Pastor-Anglada, 2003). Similarly, Bhutia et al. showed that hCNT1 overexpression improved the anti-tumorigenic and chemosensitivity potential of pancreatic cancer cells (Bhutia, Hung, Patel, Lovin, & Govindarajan, 2011). Overexpression of hENT1 enhanced gemcitabine response in human pancreatic cancer, and cells lacking hENT1 expression are highly resistant to gemcitabine (Mori et al., 2007; Perez-Torras et al., 2008; Perez-Torras et al., 2013).
Micro RNAs (miRs) represent another form of epigenetic regulation that could potentially be harnessed to modulate nucleoside transporters. Although there is still much to be learned about how miRs may downregulate the expression of nucleoside transporters, a recent study evaluating gene-miR interactions identified 175 miRs that may target ENT1. Interestingly, 4 ENT1-miR hits are upregulated in pancreatic cancer (Randazzo et al., 2020). CRISPR-Cas9 gene editing represents the gold standard for precise programming of the genetic code. In addition, there is a significant difference in CNT1 mRNA and protein in human pancreatic cancers, likely due to miR dysregulation, as demonstrated by independent studies (Bhutia et al., 2011; Boces-Pascual et al., 2021). The CRISPR technology could be used to deliver nucleoside transporter genes to tumor tissues which may display loss of expression. Conversely, CRISPR-based deletion of ENTs may be strategic in situations where efflux of adenosine is observed. Table 7 is a compilation of the nucleic acid therapeutics, particularly miRs and siRNA-based silencing of nucleoside transporters with potential application in cancer therapy.
Table 7:
Nucleic Acid-Based Approaches Targeting Nucleoside Transporters
| Type | Name | Disease state | Animal/Cell lines | Nucleoside transporter targeted | Outcome | Reference |
|---|---|---|---|---|---|---|
| Micro-RNA | MiR-26b | Cirrhotic portal hypertension | Rats | ENT1 | Regulate the invasion and migration ability by targeting hENT1 depending on the RhoA/ROCK-1 pathway | (Y. Chen & Tian, 2019; Y. Gao & Yang, 2017) |
| Lung cancer | A549 cells | |||||
| miR-106a | Colorectal carcinoma and Pancreatic cancer | NP9; CP15T; CaCo2; HT29 | CNT1 | hCNT1 inhibition could contribute towards chemoresistance to fluoropyrimidine-based treatments | (Boces-Pascual, et al., 2021) | |
| miR-17 | ||||||
| hsa-miR-221-5p | Pancreatic cancer | - | ENT1 | Downregulation of hENT1 in pancreatic cancer contributes to chemoresistance | (SenGupta, et al., 2002) | |
| hsa-miR-23b-3p | - | ENT1 | (Jaramillo, et al., 2020) | |||
| hsa-miR-155-5p | - | ENT1 | (Sundaram, et al., 1998) | |||
| hsa-miR-196a-3p | - | ENT1 | (Mantini, et al., 2020; Randazzo, et al., 2020) | |||
| Small-interfering RNA (siRNA) | - | Astrocytes | Primary astrocytes | ENT3 | ENT3 downregulation abolished the release of gliotransmitter ATP via inhibition of adenosine uptake in vesicles | (D. Song, et al., 2014) |
| - | Leukemia | MOLT4 | CNT3, ENT1, ENT2 | Inhibition of nucleoside transport resulted in increased 6-MP tolerability | (Fotoohi, et al., 2006) | |
| - | Prostate cancer | PC3 cells | ENT1 | Impaired ENT activity imparts anti-proliferative potential to Cimicifuga racemosa extract BNO-1055 | (Dueregger, et al., 2013) | |
| Overexpression | Recombinant adenovirus (Ad-hENT1) | Pancreatic cancer | NP-9, NP-18, NP-2 | ENT1 | Improved the therapeutic response to gemcitabine | (Perez-Torras, et al., 2008) |
| Nucleic acid conjugates | Lipid conjugates | Pancreatic cancer | HEK293 | CNT3 | Delivery of hCNT3 cDNA using ultrasound and microbubbles reversed gemcitabine resistance | (Paproski, et al., 2013) |
7.3. Nanocarriers: drug delivery via transporter-independent mode
Drug delivery via transporter-independent mode has shown promising potential in overcoming chemoresistance due to transporter loss. Nano-formulations of nucleoside analog drugs such as gemcitabine overcame chemoresistance in ENT1-deficient mouse lung cancer cells (Wonganan et al., 2013) and ovarian cancer cells with low hCNT1 expression (Hung et al., 2015). Table 8 lists the various types of nanomedicines employed for increasing gemcitabine delivery into different cancer cell lines. This strategy could be adopted when nucleoside transporters are required to be inhibited in which case the hindered chemotherapeutic uptake can be circumvented using nanocarriers.
Table 8:
Nanocarriers for Gemcitabine Delivery into Cancer Cells
| Nanomedicine | Ingredient | Cell system | Reference |
|---|---|---|---|
| Nanoparticles | PLGA, Chitosan, BSA, Soy lecithin, GMS, Tween 20, PLGA-b-PEG-OH, Stearic acid, Squalene, Lipid bilayer-MSNP | Panc-1, MIA PaCa-2, AsPc-1, CEM-AraC-8C, CEM-AraC-8D, L1210R | (Arya, Vandana, Acharya, & Sahoo, 2011; Chung, Sandoval, Sloat, Lansakara, & Cui, 2012; Cosco, et al., 2012; Daman, Ostad, Amini, & Gilani, 2014; Hung, et al., 2015; Lansakara, Rodriguez, & Cui, 2012; J. Li, et al., 2013; Meng, et al., 2015; Papa, et al., 2012; Reddy, et al., 2007; Sloat, et al., 2011; Wonganan, et al., 2013) |
| Nanoassemblies | Ethanol, aqueous dextrose solution | Panc-1, L1210 10K cells | (Maksimenko, Caron, Mougin, Desmaele, & Couvreur, 2015) |
| Liposomes | DPPC, Chol, DSPE-PEG2000, DOPE, CHEMS, DSPE-mPEG2000 | MDA-MB-231 breast cancer cells, MIA PaCa-2 | (Papa, et al., 2013; Y. Xu & Meng, 2016) |
| Micelles | PEG-PLA, Stearic acid, PEG-PCC, Stearoyl-PEG | AsPc-1, MIA PaCa-2 | (Chitkara, Mittal, Behrman, Kumar, & Mahato, 2013; Daman, et al., 2014; Papa, et al., 2012; Zhu, Budin, & Szostak, 2013) |
7.4. Improving the efficacy of nucleoside transporters
The efficacy of nucleoside transporters in TME is jeopardized due to the overexpression of mucin proteins in certain cancer types like pancreatic cancer. This results in the formation of a mucoid barrier between cancer cells and the extracellular matrix. The disruption of mucolytic layers produced by mucins on cancerous cells enables the effective uptake of cytotoxic drugs into cells resulting in better patient survival. Therefore, several agents with mucolytic properties have been investigated for targeting mucins, especially in pancreatic cancer (Gautam et al., 2017; Spratlin et al., 2004). A variety of miRNAs, pharmacological and natural agents have also been investigated for their ability to limit pancreatic cancer progression by specifically targeting and downregulating well-known mucins such as MUC1 and MUC4 (Srivastava et al., 2011; Torres, Chakraborty, Souchek, & Batra, 2012). Four key miRNAs (miR-Let-7b, miR-150, miR-219-1-3p, and miR-200c) have been investigated and found to downregulate MUC4 expression (Arora et al., 2014; Lahdaoui et al., 2015; Seshacharyulu et al., 2015; Wang et al., 2013). Mucins can also be controlled through anti-inflammatory drugs, corticosteroids such as dexamethasone, as well as pan-EGFR family inhibitors, afatinib and canertinib (Gollub, Waksman, Goswami, & Marom, 1995; Macha et al., 2013; Torres et al., 2012). MUC4 mucin is reported to negatively regulate the CNT1 transporter expression via the NF-κB pathway and alter the drug-susceptibility of pancreatic tumor cells. MUC4-deficient pancreatic cancer cell lines (CAPAN-1 and CAPAN-1) were found to be sensitive to gemcitabine and reduced activation of NF-κB-mediated signaling pathways in MUC4-deficient cells is associated with gemcitabine sensitivity and regulation of CNT1 (Carter, Mekkawy, & Morris, 2021; Skrypek et al., 2013). Such studies emphasize the therapeutic value of strategies targeting the down-regulation of mucin proteins to enhance transporter expression and/or function as well as highlight the potential of exogenous transporter overexpression for controlling mucin production in cancer cells.
Bromelain (a cysteine proteolytic enzyme extracted from the stem of Ananas comosus) has been used for its anti-inflammatory and mucolytic properties for the treatment of ovarian and breast cancer with an evident decrease in metastasis and tumor growth. Patients treated with bromelain in combination with chemotherapeutic agents such as 5-FU resulted in greater patient outcomes with more tumor regression (Amini, Masoumi-Moghaddam, Ehteda, Liauw, & Morris, 2016; Carter et al., 2021; Gautam et al., 2017; Pillai et al., 2020; Suh, Pillai, & Morris, 2017; Zheng, 2017; Pillai et al., 2020).
7.5. Other routes to modulate nucleoside transporters
The dietary intervention of naturally occurring compounds such as Indole-3-carbinol (I3C) has shown promising chemopreventive effects for breast cancer prevention (Wong et al., 1997). I3C, in combination with clinically relevant concentrations of gemcitabine, has been reported to enhance cancer cell death through up-regulation of hENT1 in pancreatic cancer (Wang, Word, & Lyn-Cook, 2011).
8. Future directions
Nucleoside transporters are central players in dictating the levels of extracellular adenosine, which has a profound effect on tumor immunity. Adenosine recycling between various metabolic and signaling pathways would be severely limited without the transmembrane flux of adenosine conducted by nucleoside transporters. Although traditionally thought of as only facilitating the inward-directed transfer of adenosine, the possibility of efflux via ENTs highlights the versatility and dynamic nature of nucleoside transport processes within the purinome. Up to this point, most research on nucleoside transporter pharmacologic intervention has been in the cardiovascular and nervous systems. However, minimal studies have been conducted on the function of nucleoside transporters in cancer as it pertains to the regulation of adenosine concentration levels. Below we will describe some of the most important topics for further study that will hasten the development of therapies targeting nucleoside transporters in cancer.
8.1. Evaluating potentially important, yet understudied, component of the purinome: CNTs
Concentrative nucleoside transporters remain underexplored compared to ENTs, and much is still to be determined about their function in the purinome. Bidirectional ENTs play a major role in maintaining nucleoside homeostasis, whereas CNTs contribute to nucleoside sensing and signal transduction. The putative role of CNTs as transceptors is abundantly evident from recent research. The interacting proteins identified for hCNT2 suggest a link to energy metabolism. Being purine transporters, CNT2 and CNT3 also play a role in purinergic signaling by mediating adenosine transport.
As mentioned earlier, previous studies on nucleoside transporter proteins and their contribution to adenosine levels have been conducted in the central nervous system or cardiovascular system. For example, ENTs regulate vascular tension by modulation of adenosine receptor signaling in the cardiovascular system. However, these tissues specifically rely on ENTs with little or no contribution of CNTs. Both CNTs and ENTs are likely involved in regulating adenosine levels in tumors. CNTs may be especially important in early-stage tumors of epithelial origin, as epithelial-type cells classically display asymmetric distribution of ENTs and CNTs, with CNTs decorating the apical surface of cells that directly interfaces with the extracellular environment. As dedifferentiation is frequently observed in cancer progression, it will be important to study changes in CNTs that may occur during cancer cell transformations as more malignant cells gain survival advantages in response to drug treatment and other selective pressures in the TME. Additionally, CNTs may have a more pronounced role in cancers arising from tissue types with high endogenous levels of CNTs. Nevertheless, further research is warranted for cases such as the involvement of CNT3 in pancreatic cancer, as CNT3 is highly expressed in pancreas acinar cells. The development of CNT knockout mice models is also lacking, and experimentation using model animal systems will be critical for understanding the physiological relevance of these transporters. Additionally, CNT knockout mouse models could be used to differentiate between adenosine-dependent tumor cell-intrinsic and extrinsic influences on tumor growth. Such studies are likely to uncover the role of adenosine transporters in tumor cell-stromal cell and tumor cell-immune cell interplays.
All in vitro and in vivo studies have provided significant insights into the tissue-specific distribution and functionality of different nucleoside transporters. The gene knockdown/knockout investigations have been beneficial in figuring out the transporter-specific functions and crosstalk with other signaling players. The exogenous transporter expression studies demonstrated their potential in overcoming drug chemoresistance in tumor cells. The in silico computational and molecular modeling has deciphered the structural aspects of drug-transporter interactions. In nutshell, prior research has improved the understanding of nucleoside transporter-adenosine receptor interplay. However, there are critical challenges to the therapeutic success of these strategies. The application of CNT-based approaches requires a parallel inhibition of predominant ENT transporters to prevent leaching out of adenosine and counterbalance the nucleoside flux. However, specific, and complete inhibition of a transporter is not always achievable and presents a major challenge in studying the role of individual transporter in signaling pathways. Another bottleneck is the shared substrate selectivity and often tissue localization of both ENTs and CNTs, which could lead to undesirable off-target effects of inhibitor compounds and drugs targeting nucleoside transport.
Non-specific targeting by drugs could result in systemic inhibition of nucleoside transporters, disrupting the normal flow of nucleosides across cellular compartments. Increased extracellular levels of nucleosides such as adenosine might trigger the inflammatory pathways, especially affecting CNS activity, causing sleep disturbances and drowsiness (Bjorness & Greene, 2009; Sims, Wu, & Dale, 2013). However, not all nucleoside transporters cause adverse effects upon inhibition or downregulation. Studies on ENT-null mice have demonstrated that adenosine uptake is almost completely abolished in ENT1−/− mice along with a slightly decreased body weight, increased alcohol consumption, and reduced hypnotic and ataxic responses to ethanol due to low adenosine levels (Choi et al., 2004; http://www.informatics.jax. org/marker/MGI:1927073). ENT2-null mice exhibited normal adenosine uptake in erythrocytes with protective effects in case of acute lung injury is observed (http://www.informatics.jax.org/marker/MGI:1345278). In contrast, a variety of severe health effects including lymphadenopathy, splenomegaly, histiocytic sarcoma, anemia, and premature death associated with extramedullary hematopoiesis, increased macrophage proliferation, apoptosis, and abnormal lysosome function have been reported in ENT3-null mice (http://www.informatics.jax.org/marker/MGI:1918529). Studies in mice models with knockout genotype for the key effectors involved in purinergic signaling (adenosine receptors, protein kinases) demonstrated a neutral unaltered phenotype compared to wildtype (Braz et al., 2004; Chen et al., 1999; Gimenez-Llort et al., 2002; Hua et al., 2007; Johansson et al., 2001; Ledent et al., 1997; Salvatore et al., 2000; Xiao, Liu, Jacobson, Gavrilova, & Reitman, 2019), with no significant effect on growth, metabolism, breeding, and body temperature regulation. To summarize, systemic effects are mostly observed with inhibition or knockout of predominant ENT transporters, especially ENT3 and a mild phenotype is evident with the absence of ENT1 and ENT2. CNTs are facilitatory transporters working alongside ENTs, there will likely be minimal adverse effects under conditions of their complete systemic inhibition/down-regulation.
The reversible competitive uptake of nucleoside analogs by CNTs/ENTs is another common phenomenon reported in numerous uptake studies (Carrier, Auchampach, & Hillard, 2006; Huang, Zeng, Shi, & Liu, 2017; Scholtissek, 1968). It is certainly useful for short-term assessment studies but as soon as the optimal concentration of a specific substrate is restored, the transport may come back to the default setting. CNTs and ENTs have a relatively short half-life and soon be replaced with nascent proteins via cellular protein synthesis machinery. Therefore, it is highly likely that the effects due to irreversible inhibition would not last long enough to cause any significant effect on cellular function. Lastly, the lack of in vivo data limits the therapeutic potential of most in vitro approaches. The generation of knockout mice and xenograft models, which closely mimic the human tumor microenvironment, is a very expensive and time-consuming process. Apart from ethical concerns associated with animal use in research, the huge failure rate in the accurate translation of animal data in phase I clinical trials and approval of new drugs have raised concerns about the importance of these models (Plenge, Scolnick, & Altshuler, 2013; Swaminathan, Kumar, & Kaul, 2019). Technological advances in tissue engineering, ‘omics’ approaches, and in silico modeling are enabling. With advances in high throughput cutting-edge instrumentation, ‘omics’ approaches, in silico modeling, 3D organoid development, etc., scientists can conduct their research with limited use of animal models.
8.2. Nucleoside transporters and intracellular metabolism coupling
Researchers have established a solid understanding of the metabolic pathways instrumental for the fate of adenosine in the extracellular and intracellular milieu (Boison & Yegutkin, 2019; Borbath et al., 2012; Burnstock, 2017; Fox & Kelley, 1978; Fredholm et al., 2011; Fredholm, Chern, Franco, & Sitkovsky, 2007; Goldthwait, 1957; Hasko, Antonioli, & Cronstein, 2018; Katori & Berne, 1966; Newton & Perry, 1960; Pastor-Anglada & Perez-Torras, 2018b). Extracellular adenosine levels are determined by the balance between its synthesis and degradation. In most cases adenosine is generated because of the sequential metabolic action of various ectonucleotidases (CD73, CD39) on nucleotide precursors namely, ATP, ADP, and AMP (Dos Santos-Rodrigues, GraneBoladeras, Bicket, & Coe, 2014; Nguyen, Ross, Ryals, Lee, & Venton, 2015; Pastor-Anglada & Perez-Torras, 2018b) and sometimes released from the cells (Almeida, Rodrigues, de Mendonca, Ribeiro, & Cunha, 2003). The disposal of extracellular adenosine is mediated by either metabolism, i.e., conversion to inosine via adenosine deaminase, or by its uptake into cells followed by metabolic trapping as AMP after being phosphorylated by adenosine kinase. The transport across the cell membranes occurs via selected adenosine transporter proteins, which makes transport processes key modulators of extracellular adenosine disposal.
The direct coupling of influx performed by nucleoside transporters and intracellular metabolism facilitated primarily by adenosine kinase and to a lesser extent, adenosine deaminase, is assumed to be the driving force that pulls adenosine inward. By the addition of a phosphate group by adenosine kinase or removal of the amino group by adenosine deaminase, adenosine is altered to prevent efflux via nucleoside transporters, thus trapping it inside cells. Further, once intracellular metabolism has taken place, these adenosine metabolites no longer factor into the concentration gradient. Thus, extracellular adenosine levels rapidly overtake the intracellular levels driving inward movement by ENTs. While this system maintains a homeostatic mechanism for the salvage of nucleosides, it cannot be counted on to function as normal under pathological conditions such as cancer. In the above section, we have outlined changes in the expression and function of each nucleoside transporter across multiple cancer subtypes. These alterations alone may disrupt the balance in transport and metabolism coupling.
Additionally, the expression of intracellular or extracellular adenosine metabolizing enzymes may be changed in cancer. One could reasonably project that the downregulation of adenosine kinase may have a significant influence on the sequestration and concentration of adenosine inside cells. Centelles presented a model for adenosine transport and metabolism in different steady states based on their kinetic behavior (Centelles, Cascante, Canela, & Franco, 1992). It was observed that CD73 regulates the fluxes towards intracellular adenine nucleosides in some circumstances and nucleoside transporters in others. The model predicts that a change in one independent variable such as extracellular AMP, re-routes the system towards a completely new steady state with a different control pattern. The pharmacological inhibition (50%) of CNT2, CNT3, and ENT1 only slightly alters the adenosine fluxes as the concentrations of adenosine and inosine change to counterbalance the effect. The results suggest a key role for the nucleoside carriers that deal with adenosine and inosine, which maintain the adenosine influx following the chemical gradient for the Na+-dependent transporter, however, an efflux of inosine against the gradient with an adenosine influx down its concentration gradient is observed in Na+-independent transporter. ENT inhibition alone affects the extracellular adenosine levels with minimal change in the intracellular milieu. Rose et al. reported higher levels of extracellular adenosine in ENT1-null cardiomyocytes (4360 ± 1840 pmol/mg protein) compared to wild-type cardiomyocytes (3035 ± 730 pmol/mg protein, p < 0.05) after hypoxic challenge. Furthermore, the pharmacological inhibition of ENT2 led to a further increase in extracellular adenosine in KO (6805 ± 1830 pmol/mg protein) versus WT cells (4260 ± 1225 pmol/mg protein, p < 0.05) under the same treatments revealing a significant contribution of ENT2 to adenosine uptake. No difference in intracellular adenosine levels was observed between ENT1-null and wild-type cardiomyocytes under normoxia or hypoxia, supporting an outside-to-inside gradient for adenosine. The study also ruled out the effect of metabolic enzymes such as CD39, and CD73 on extracellular levels of adenosine. However, adenosine kinase which can affect intracellular levels (Eltzschig, 2009), showed significantly lower expression compared to wild-type cardiomyocytes. Further research is required to ascertain how the alteration of both metabolic enzymes and nucleoside transporters affects the capability of cells to concentrate adenosine.
Computational modeling theories and software have been quite useful for in silico prediction and correlation analysis so far and keep on improving with advancements in technology. Petri net theory was successfully applied to study the Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulation network (Hardy & Robillard, 2008). BioWays software has been used for the quantitative modeling and analysis of the glycogen breakdown pathway and the HIV life cycle (Chiarugi, Falaschi, Hermith, Guzman, & Olarte, 2013). All these tools could be applied to study the adenosine transporter-receptor interplay in various cancer settings. The resulting data will aid in narrowing down the focus on significant effectors by filtering out the neutral players. However, only detailed experimental testing would confirm the reliability of such approaches for therapeutic targeting and precisely study how perturbations in a single transporter component in the purinome are likely to impact the adenosine sequestration and consequent effects.
To conclude, elaborated and more affirmative outcomes are needed for applying these models to oncology settings. The current studies have been beneficial in breakdown a lot of complexities in the nucleoside transporter-mediated adenosine signaling network. However, the quantitative information is still not enough to distinguish each component’s role separately in the context of the tumor microenvironment.
8.3. Simultaneous targeting of multiple components of the purinome
Up to this point, multiple elements of the purinome have been targeted to limit the accumulation of adenosine inside cells. Yet to date, none of these therapies that have shown efficacy in in vivo models have been successfully transferred to cancer patients. Here, we suggest the consideration of modulating multiple components of the purinome. Due to the redundancy and complexity of pathways that all contribute to adenosine concentration levels, targeting one aspect may likely allow for compensatory mechanisms to overcome the therapeutic targeting applied. We suggest that further research focus on strategies that involve multiple elements of the purinome. For instance, perhaps the inhibition of ectonucleotidase enzymes combined with the induction of CNT transporters would both curtail the production of extracellular adenosine while enhancing the uptake of existing pools of adenosine.
In summary, we see nucleoside transporter research in the context of the purinome as a whole to be an important missing link in understanding how adenosine builds up in the extracellular space of cancer cells. Surprisingly, nucleoside transporter processes have not yet been targeted to counteract high levels of extracellular adenosine, although modulation of nucleoside transport has resulted in approved therapies in other disease types. Therefore, we call for a more thoughtful study of the dynamic processes that control adenosine levels and tumors to underpin the rational design of new drugs to boost anti-tumor immunity.
Funding
The project was supported by the National Institutes of Health grant R01GM143217 and R03CA262490 awarded to R.G.
Abbreviations:
- A1
adenosine 1 receptor
- A2A
adenosine 2A receptor
- A2B
adenosine 2B receptor
- A3
adenosine 3 receptor
- AC
adenylate cyclase
- ADA
adenosine deaminase
- ADK
adenosine kinase
- AKT
protein kinase B
- AML
acute myeloid leukemia
- AMP
adenosine monophosphate
- ARs
adenosine receptors
- ATF-1
activating transcription factor-1
- ATP
adenosine triphosphate
- BSA
bovine serum albumin
- cAMP
cyclic adenosine monophosphate
- CaMKII
calcium/calmodulin-dependent protein kinase II
- CHEMS
cholesterol hemisuccinate
- CNS
central nervous system
- CNT
concentrative nucleoside transporter
- CREB
cAMP responsive binding protein
- CREM
cAMP responsive element modulator
- DAG
diacylglycerol
- DC
dendritic cells
- DOPE
dioleoylphosphatidylethanolamine
- DPPC
dipalmitoylphosphatidylcholine
- DSPE
1.2-distearoyl-sn-glycro-3-phosphorylethanolamine
- EC50
half maximal effective concentration
- EPAC1
exchange protein activated by cAMP 1
- ENT
equilibrative nucleoside transporter
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- GMS
glyceryl monostearate
- GPCR
G-protein coupled receptor
- GRK2
G-coupled protein receptor kinase 2
- HASMCs
human aortic smooth muscle cell
- HIF
hypoxia-inducible factor
- HNF4-α
hepatocyte nuclear factor 4 alpha
- IFN-γ
interferon-γ
- IL
Interleukin
- IP3
inositol 1,4,5-trisphosphate
- MAPK
mitogen-activated protein kinase
- MDSCs
myeloid-derived suppressor cells
- MEK
MAPK/ERK kinase
- miRs
micro RNAs
- mRNA
messenger RNA
- NADPH
nicotinamide adenine dinucleotide phosphate
- NK
natural killer
- PCC
precipitated calcium carbonate
- PEG
polyethylene glycol
- PET
positron emission tomography
- PI3K
phosphatidylinositol-3-kinase
- PLA
polylactic acid
- PLC
phospholipase C
- PLGA
poly (lactic-co-glycolic acid)
- PKA
protein kinase A
- SLC28
solute carrier family 28
- SLC29
solute carrier family 29
- STAT1
signal transducer and activator of transcription 1
- TAMs
tumor-associated macrophages
- TGF-β
transforming growth factor beta
- Th
t-helper
- TME
tumor microenvironment
- TNF-α
tumor necrosis factor alpha
- Treg
immunosuppressive regulatory T cell
- VEGF
Vascular endothelial growth factor
Footnotes
Declaration of Competing Interest
The authors declare no potential conflict of interests.
Data availability
No data was used for the research described in the article.
References
- Abdelghany L, El-Mahdy N, Kawabata T, Goto S, & Li TS (2021). Dipyridamole induces the phosphorylation of CREB to promote cancer cell proliferation. Oncology Letters 21, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Varatharajan S, Karathedath S, Philip C, Lakshmi KM, Jayavelu AK, … Balasubramanian P (2015). RNA expression of genes involved in cytarabine metabolism and transport predicts cytarabine response in acute myeloid leukemia. Pharmacogenomics 16, 877–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, Shenoy A, Bassat E, Halpern Z, Geiger T, Sagi I, & Varol C (2016). Tumor macrophages are pivotal constructors of tumor collagenous matrix. The Journal of Experimental Medicine 213, 2315–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aherne CM, Kewley EM, & Eltzschig HK (2011). The resurgence of A2B adenosine receptor signaling. Biochimica et Biophysica Acta 1808, 1329–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon S, Toro MLA, Villarreal C, Melo R, Fernandez R, Ayuso Sacido A, … Quezada C (2020). Decreased equilibrative nucleoside transporter 1 (ent1) activity contributes to the high extracellular adenosine levels in mesenchymal glioblastoma stemlike cells. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard D, Turcotte M, & Stagg J (2017). Targeting A2 adenosine receptors in cancer. Immunology and Cell Biology 95, 333–339. [DOI] [PubMed] [Google Scholar]
- Almeida T, Rodrigues RJ, de Mendonca A, Ribeiro JA, & Cunha RA (2003). Purinergic P2 receptors trigger adenosine release leading to adenosine A2A receptor activation and facilitation of long-term potentiation in rat hippocampal slices. Neuroscience 122, 111–121. [DOI] [PubMed] [Google Scholar]
- Amini A, Masoumi-Moghaddam S, Ehteda A, Liauw W, & Morris DL (2016). Potentiation of chemotherapeutics by bromelain and N-acetylcysteine: Sequential and combination therapy of gastrointestinal cancer cells. American Journal of Cancer Research 6, 350–369. [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Xiong W, Geiger JD, Young JD, Cass CE, Baldwin SA, & Parkinson FE (1999). Distribution of equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporters (ENT1) in brain. Journal of Neurochemistry 73, 867–873. [DOI] [PubMed] [Google Scholar]
- Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, & Blandizzi C (2008a). Pharmacological modulation of adenosine system: Novel options for treatment of inflammatory bowel diseases. Inflammatory Bowel Diseases 14, 566–574. [DOI] [PubMed] [Google Scholar]
- Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Del Tacca M, & Blandizzi C (2008b). Regulation of enteric functions by adenosine: Pathophysiological and pharmacological implications. Pharmacology & Therapeutics 120, 233–253. [DOI] [PubMed] [Google Scholar]
- Arimany-Nardi C, Errasti-Murugarren E, Minuesa G, Martinez-Picado J, Gorboulev V, Koepsell H, & Pastor-Anglada M (2014). Nucleoside transporters and human organic cation transporter 1 determine the cellular handling of DNA-methyltransferase inhibitors. British Journal of Pharmacology 171, 3868–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, & van Giezen JJ (2014). Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. Journal of Cardiovascular Pharmacology and Therapeutics 19, 209–219. [DOI] [PubMed] [Google Scholar]
- Arora S, Swaminathan SK, Kirtane A, Srivastava SK, Bhardwaj A, Singh S, … Singh AP (2014). Synthesis, characterization, and evaluation of poly (D,L-lactide-co-glycolide)-based nanoformulation of miRNA-150: Potential implications for pancreatic cancer therapy. International Journal of Nanomedicine 9, 2933–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya G, Vandana M, Acharya S, & Sahoo SK (2011). Enhanced antiproliferative activity of Herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomedicine 7, 859–870. [DOI] [PubMed] [Google Scholar]
- Babich V, Vadnagara K, & Di Sole F (2015). Dual Effect of Adenosine A1 Receptor Activation on Renal O2 Consumption. Journal of Cellular Physiology 230, 3093–3104. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, … Young JD (2005). Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. The Journal of Biological Chemistry 280, 15880–15887. [DOI] [PubMed] [Google Scholar]
- Barnes K, Dobrzynski H, Foppolo S, Beal PR, Ismat F, Scullion ER, … Baldwin SA (2006). Distribution and functional characterization of equilibrative nucleoside transporter-4, a novel cardiac adenosine transporter activated at acidic pH. Circulation Research 99, 510–519. [DOI] [PubMed] [Google Scholar]
- Beavis PA, Henderson MA, Giuffrida L, Mills JK, Sek K, Cross RS, … Darcy PK (2017). Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. The Journal of Clinical Investigation 127, 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia YD, Hung SW, Patel B, Lovin D, & Govindarajan R (2011). CNT1 expression influences proliferation and chemosensitivity in drug-resistant pancreatic cancer cells. Cancer Research 71, 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorness TE, & Greene RW (2009). Adenosine and sleep. Current Neuropharmacology 7, 238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blay J, White TD, & Hoskin DW (1997). The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Research 57, 2602–2605. [PubMed] [Google Scholar]
- Boces-Pascual C, Mata-Ventosa A, Martin-Satue M, Boix L, Gironella M, Pastor-Anglada M, & Perez-Torras S (2021). OncomiRs miR-106a and miR-17 negatively regulate the nucleoside-derived drug transporter hCNT1. Cellular and Molecular Life Sciences 78, 7505–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock AJ, Dong HP, Trope CG, Staff AC, Risberg B, & Davidson B (2012). Nucleoside transporters are widely expressed in ovarian carcinoma effusions. Cancer Chemotherapy and Pharmacology 69, 467–475. [DOI] [PubMed] [Google Scholar]
- Bodin P, & Burnstock G (1998). Increased release of ATP from endothelial cells during acute inflammation. Inflammation Research 47, 351–354. [DOI] [PubMed] [Google Scholar]
- Bohm M, Weinhold C, Schwinger RH, Muller-Ehmsen J, Bohm D, Reichenspurner H, … Erdmann E (1994). Studies of the nucleoside transporter inhibitor, draflazine, in the human myocardium. British Journal of Pharmacology 112, 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D (2013). Role of adenosine in status epilepticus: A potential new target? Epilepsia 54(Suppl. 6), 20–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, & Yegutkin GG (2019). Adenosine metabolism: Emerging concepts for cancer therapy. Cancer Cell 36, 582–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbath I, Verbrugghe L, Lai R, Gigot JF, Humblet Y, Piessevaux H, & Sempoux C (2012). Human equilibrative nucleoside transporter 1 (hENT1) expression is a potential predictive tool for response to gemcitabine in patients with advanced cholangiocarcinoma. European Journal of Cancer 48, 990–996. [DOI] [PubMed] [Google Scholar]
- Borea PA, Gessi S, Bar-Yehuda S, & Fishman P (2009). A3 adenosine receptor: Pharmacology and role in disease. Handbook of Experimental Pharmacology, 297–327. [DOI] [PubMed] [Google Scholar]
- Boswell-Casteel RC, & Hays FA (2017). Equilibrative nucleoside transporters-A review. Nucleosides, Nucleotides & Nucleic Acids 36, 7–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, & Nadler LM (1997). Maintenance of human T cell anergy: Blocking of IL-2 gene transcription by activated Rap1. Science 278, 124–128. [DOI] [PubMed] [Google Scholar]
- Boyer CR, Karjian PL, Wahl GM, Pegram M, & Neuteboom ST (2002). Nucleoside transport inhibitors, dipyridamole and p-nitrobenzylthioinosine, selectively potentiate the antitumor activity of NB1011. Anti-Cancer Drugs 13, 29–36. [DOI] [PubMed] [Google Scholar]
- Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, … Molkentin JD (2004). PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nature Medicine 10, 248–254. [DOI] [PubMed] [Google Scholar]
- Burnstock G (2006). Purinergic signalling–an overview. Novartis Foundation Symposium 276, 26–48 discussion 48–57, 275–281. [PubMed] [Google Scholar]
- Burnstock G (2017). Purinergic signalling: Therapeutic developments. Frontiers in Pharmacology 8, 661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bynoe MS, Viret C, Yan A, & Kim DG (2015). Adenosine receptor signaling: A key to opening the blood-brain door. Fluids and Barriers of the CNS 12, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B, & Tate CG (2016). Engineering a minimal G protein to facilitate crystallisation of G protein-coupled receptors in their active conformation. Protein Engineering, Design & Selection 29, 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA, & Hillard CJ (2006). Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proceedings of the National Academy of Sciences of the United States of America 103, 7895–7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CJ, Mekkawy AH, & Morris DL (2021). Role of human nucleoside transporters in pancreatic cancer and chemoresistance. World Journal of Gastroenterology 27, 6844–6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass CE, King KM, Montano JT, & Janowska-Wieczorek A (1992). A comparison of the abilities of nitrobenzylthioinosine, dilazep, and dipyridamole to protect human hematopoietic cells from 7-deazaadenosine (tubercidin). Cancer Research 52, 5879–5886. [PubMed] [Google Scholar]
- Cekic C, Day YJ, Sag D, & Linden J (2014). Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Research 74, 7250–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centelles JJ, Cascante M, Canela EI, & Franco R (1992). A model for adenosine transport and metabolism. The Biochemical Journal 287(Pt 2), 461–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challier J, Bruniquel D, Sewell AK, & Laugel B (2013). Adenosine and cAMP signalling skew human dendritic cell differentiation towards a tolerogenic phenotype with defective CD8(+) T-cell priming capacity. Immunology 138, 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AM, & Matosevic S (2019). Immunometabolic dysfunction of natural killer cells mediated by the hypoxia-CD73 axis in solid tumors. Frontiers in Molecular Biosciences 6, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AM, Wang J, Lupo KB, Yu H, Atallah Lanman NM, & Matosevic S (2018). Adenosinergic signaling alters natural killer cell functional responses. Frontiers in Immunology 9, 2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF (2014). Adenosine receptor control of cognition in normal and disease. International Review of Neurobiology 119, 257–307. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, … Schwarzschild MA (1999). A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. The Journal of Neuroscience 19, 9192–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Lee CF, & Chern Y (2014). Adenosine receptor neurobiology: Overview. International Review of Neurobiology 119, 1–49. [DOI] [PubMed] [Google Scholar]
- Chen Y, & Tian Y (2019). Influence of miR-26b on hepatic cirrhosis and portal pressure in rats with cirrhotic portal hypertension by targeting hENT1 depending on RhoA/ROCK-1 pathway. European Review for Medical and Pharmacological Sciences 23, 1668–1673. [DOI] [PubMed] [Google Scholar]
- Cheng RKY, Segala E, Robertson N, Deflorian F, Dore AS, Errey JC, … Cooke RM (2017). Structures of Human A1 and A2A Adenosine Receptors with Xanthines Reveal Determinants of Selectivity. Structure 25(1275–1285), e1274. [DOI] [PubMed] [Google Scholar]
- Chiarugi D, Falaschi M, Hermith D, Guzman M, & Olarte C (2013). Simulating signalling pathways with BioWayS. Electronic Notes in Theoretical Computer Science 293, 17–34. [Google Scholar]
- Chitkara D, Mittal A, Behrman SW, Kumar N, & Mahato RI (2013). Self-assembling, amphiphilic polymer-gemcitabine conjugate shows enhanced antitumor efficacy against human pancreatic adenocarcinoma. Bioconjugate Chemistry 24, 1161–1173. [DOI] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, … Messing RO (2004). The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nature Neuroscience 7, 855–861. [DOI] [PubMed] [Google Scholar]
- Choudhury H, Chellappan DK, Sengupta P, Pandey M, & Gorain B (2019). Adenosine Receptors in Modulation of Central Nervous System Disorders. Current Pharmaceutical Design 25, 2808–2827. [DOI] [PubMed] [Google Scholar]
- Chung WG, Sandoval MA, Sloat BR, Lansakara PD, & Cui Z (2012). Stearoyl gemcitabine nanoparticles overcome resistance related to the over-expression of ribonucleotide reductase subunit M1. Journal of Controlled Release 157, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslak M, Komoszynski M, & Wojtczak A (2008). Adenosine A(2A) receptors in Parkinson’s disease treatment. Purinergic Signal 4, 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, McGovern N, & Haniffa M (2013). Human dendritic cell subsets. Immunology 140, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti A, Monopoli A, Gamba M, Borea PA, & Ongini E (1993). Effects of selective A1 and A2 adenosine receptor agonists on cardiovascular tissues. Naunyn-Schmiedeberg’s Archives of Pharmacology 348, 108–112. [DOI] [PubMed] [Google Scholar]
- Cosco D, Rocco F, Ceruti M, Vono M, Fresta M, & Paolino D (2012). Self-assembled squalenoyl-cytarabine nanostructures as a potent nanomedicine for treatment of leukemic diseases. International Journal of Nanomedicine 7, 2535–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, … Hasko G (2008). Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. The FASEB Journal 22, 3491–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Almeida SM, Kauffenstein G, Roy C, Basset L, Papargyris L, Henrion D, … Tabiasco J (2016). The ecto-ATPDase CD39 is involved in the acquisition of the immunoregulatory phenotype by M-CSF-macrophages and ovarian cancer tumor-associated macrophages: Regulatory role of IL-27. Oncoimmunology 5, e1178025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daman Z, Ostad S, Amini M, & Gilani K (2014). Preparation, optimization and in vitro characterization of stearoyl-gemcitabine polymeric micelles: A comparison with its self-assembled nanoparticles. International Journal of Pharmaceutics 468, 142–151. [DOI] [PubMed] [Google Scholar]
- Damaraju VL, Scriver T, Mowles D, Kuzma M, Ryan AJ, Cass CE, & Sawyer MB (2014). Erlotinib, gefitinib, and vandetanib inhibit human nucleoside transporters and protect cancer cells from gemcitabine cytotoxicity. Clinical Cancer Research 20, 176–186. [DOI] [PubMed] [Google Scholar]
- Damaraju VL, Weber D, Kuzma M, Cass CE, & Sawyer MB (2016). Selective inhibition of human equilibrative and concentrative nucleoside transporters by BCRABL kinase inhibitors: Identification of key hENT1 amino acid residues for interaction with BCR-ABL kinase inhibitors. The Journal of Biological Chemistry 291, 18809–18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisotti S, Ongini E, Zocchi C, Kull B, Arslan G, & Fredholm BB (1997). Characterization of human A2A adenosine receptors with the antagonist radioligand [3H]-SCH 58261. British Journal of Pharmacology 121, 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, & Freeman TC (1996). Tissue distribution of adenosine receptor mRNAs in the rat. British Journal of Pharmacology 118, 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos-Rodrigues A, Grane-Boladeras N, Bicket A, & Coe IR (2014). Nucleoside transporters in the purinome. Neurochemistry International 73, 229–237. [DOI] [PubMed] [Google Scholar]
- Dueregger A, Guggenberger F, Barthelmes J, Stecher G, Schuh M, Intelmann D, Abel G, Haunschild J, Klocker H, Ramoner R, & Sampson N (2013). Attenuation of nucleoside and anti-cancer nucleoside analog drug uptake in prostate cancer cells by Cimicifuga racemosa extract BNO-1055. Phytomedicine 20, 1306–1314. [DOI] [PubMed] [Google Scholar]
- Duflot S, Riera B, Fernandez-Veledo S, Casado V, Norman RI, Casado FJ, … Pastor-Anglada M (2004). ATP-sensitive K(+) channels regulate the concentrative adenosine transporter CNT2 following activation by A(1) adenosine receptors. Molecular and Cellular Biology 24, 2710–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaskalani O, Falasca M, Moran N, Berndt MC, & Metharom P (2017). The role of platelet-derived ADP and ATP in promoting pancreatic cancer cell survival and gemcitabine resistance. Cancers (Basel), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK (2009). Adenosine: An old drug newly discovered. Anesthesiology 111, 904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK (2013). Extracellular adenosine signaling in molecular medicine. Journal of Molecular Medicine (Berlin, Germany) 91, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Abdulla P, Hoffman E, Hamilton KE, Daniels D, Schonfeld C, … Colgan SP (2005). HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. The Journal of Experimental Medicine 202, 1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza JA, Garcia P, Bizama C, Leal JL, Riquelme I, Weber H, … Nervi B (2016). Low expression of equilibrative nucleoside transporter 1 is associated with poor prognosis in chemotherapy-naive pT2 gallbladder adenocarcinoma patients. Histopathology 68, 722–728. [DOI] [PubMed] [Google Scholar]
- Farre X, Guillen-Gomez E, Sanchez L, Hardisson D, Plaza Y, Lloberas J, … Pastor-Anglada M (2004). Expression of the nucleoside-derived drug transporters hCNT1, hENT1 and hENT2 in gynecologic tumors. International Journal of Cancer 112, 959–966. [DOI] [PubMed] [Google Scholar]
- Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, … Mackey JR (2009). Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology 136, 187–195. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Shea LG, Doddi C, & Dobson JG Jr. (2010). Myocardial adenosine A(1)-receptor-mediated adenoprotection involves phospholipase C, PKC-epsilon, and p38 MAPK, but not HSP27. American Journal of Physiology. Heart and Circulatory Physiology 298, H1671–H1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistov I, Murray JJ, & Biaggioni I (1994). Positive modulation of intracellular Ca2+ levels by adenosine A2b receptors, prostacyclin, and prostaglandin E1 via a cholera toxin-sensitive mechanism in human erythroleukemia cells. Molecular Pharmacology 45, 1160–1167. [PubMed] [Google Scholar]
- Fernandez-Calotti P, & Pastor-Anglada M (2010). All-trans-retinoic acid promotes trafficking of human concentrative nucleoside transporter-3 (hCNT3) to the plasma membrane by a TGF-beta1-mediated mechanism. The Journal of Biological Chemistry 285, 13589–13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duenas V, Gomez-Soler M, Lopez-Cano M, Taura JJ, Ledent C, Watanabe M, … Ciruela F (2014). Uncovering caffeine’s adenosine A2A receptor inverse agonism in experimental parkinsonism. ACS Chemical Biology 9, 2496–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiro F, Muller L, Funk S, Jackson EK, Battastini AM, & Whiteside TL (2016). Phenotypic and functional characteristics of CD39(high) human regulatory B cells (Breg). Oncoimmunology 5, e1082703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S, Ardon E, Rath-Wolfson L, Barrer F, Ochaion A, & Madi L (2003). Targeting the A3 adenosine receptor for cancer therapy: Inhibition of prostate carcinoma cell growth by A3AR agonist. Anticancer Research 23, 2077–2083. [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S, Barer F, Madi L, Multani AS, & Pathak S (2001). The A3 adenosine receptor as a new target for cancer therapy and chemoprotection. Experimental Cell Research 269, 230–236. [DOI] [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S, Madi L, & Cohn I (2002). A3 adenosine receptor as a target for cancer therapy. Anti-Cancer Drugs 13, 437–443. [DOI] [PubMed] [Google Scholar]
- Flanagan SA, & Meckling-Gill KA (1997). Characterization of a novel Na+-dependent, guanosine-specific, nitrobenzylthioinosine-sensitive transporter in acute promyelocytic leukemia cells. The Journal of Biological Chemistry 272, 18026–18032. [DOI] [PubMed] [Google Scholar]
- Fornai M, Antonioli L, Colucci R, Ghisu N, Buccianti P, Marioni A, Chiarugi M, Tuccori M, Blandizzi C, & Del Tacca M (2009). A1 and A2a receptors mediate inhibitory effects of adenosine on the motor activity of human colon. Neurogastroenterology and Motility 21, 451–466. [DOI] [PubMed] [Google Scholar]
- Fotoohi AK, Lindqvist M, Peterson C, & Albertioni F (2006). Involvement of the concentrative nucleoside transporter 3 and equilibrative nucleoside transporter 2 in the resistance of T-lymphoblastic cell lines to thiopurines. Biochemical and Biophysical Research Communications 343, 208–215. [DOI] [PubMed] [Google Scholar]
- Fox IH, & Kelley WN (1978). The role of adenosine and 2′-deoxyadenosine in mammalian cells. Annual Review of Biochemistry 47, 655–686. [DOI] [PubMed] [Google Scholar]
- Fozard JR (2003). The case for a role for adenosine in asthma: almost convincing? Current Opinion in Pharmacology 3, 264–269. [DOI] [PubMed] [Google Scholar]
- Fredholm BB (2007). Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death and Differentiation 14, 1315–1323. [DOI] [PubMed] [Google Scholar]
- Fredholm BB (2014). Adenosine–a physiological or pathophysiological agent? Journal of Molecular Medicine (Berlin, Germany) 92, 201–206. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, & Wasserman W (2000). Structure and function of adenosine receptors and their genes. Naunyn-Schmiedeberg’s Archives of Pharmacology 362, 364–374. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chern Y, Franco R, & Sitkovsky M (2007). Aspects of the general biology of adenosine A2A signaling. Progress in Neurobiology 83, 263–276. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Irenius E, Kull B, & Schulte G (2001). Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochemical Pharmacology 61, 443–448. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Johansson S, & Wang YQ (2011). Adenosine and the regulation of metabolism and body temperature. Advances in Pharmacology 61, 77–94. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, & Abraham RT (2017). The PI3K pathway in human disease. Cell 170, 605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, & Yang F (2017). MiR-26b regulates invasion and migration of lung cancer cells through targeting hENT1 depending on RhoA/ROCK-1 pathway. Zhong Nan Da Xue Xue Bao. Yi Xue Ban 42, 755–761. [DOI] [PubMed] [Google Scholar]
- Gao ZG, & Jacobson KA (2008). Translocation of arrestin induced by human A(3) adenosine receptor ligands in an engineered cell line: comparison with G protein-dependent pathways. Pharmacological Research 57, 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, & Jacobson KA (2019). A2B adenosine receptor and cancer. International Journal of Molecular Sciences 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Verzijl D, Zweemer A, Ye K, Goblyos A, Ijzerman AP, & Jacobson KA (2011). Functionally biased modulation of A(3) adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochemical Pharmacology 82, 658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Manteiga J, Molina-Arcas M, Casado FJ, Mazo A, & Pastor-Anglada M (2003). Nucleoside transporter profiles in human pancreatic cancer cells: Role of hCNT1 in 2′,2′-difluorodeoxycytidine- induced cytotoxicity. Clinical Cancer Research 9, 5000–5008. [PubMed] [Google Scholar]
- Gautam SK, Kumar S, Cannon A, Hall B, Bhatia R, Nasser MW, … Jain M (2017). MUC4 mucin- a therapeutic target for pancreatic ductal adenocarcinoma. Expert Opinion on Therapeutic Targets 21, 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan SP, Saadani-Makki F, Bodineau L, Frugiere A, Larnicol N, & Pasaro R (2006). Effect of postnatal exposure to caffeine on the pattern of adenosine A1 receptor distribution in respiration-related nuclei of the rat brainstem. Autonomic Neuroscience 126–127, 339–346. [DOI] [PubMed] [Google Scholar]
- Geiger JD, LaBella FS, & Nagy JI (1984). Ontogenesis of adenosine receptors in the central nervous system of the rat. Brain Research 315, 97–104. [DOI] [PubMed] [Google Scholar]
- Gendron FP, Benrezzak O, Krugh BW, Kong Q, Weisman GA, & Beaudoin AR (2002). Purine signaling and potential new therapeutic approach: Possible outcomes of NTPDase inhibition. Current Drug Targets 3, 229–245. [DOI] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Fazzi D, Stefanelli A, Varani K, & Borea PA (2011). Adenosine receptor targeting in health and disease. Expert Opinion on Investigational Drugs 20, 1591–1609. [DOI] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Sacchetto V, Simioni C, & Borea PA (2011). Adenosine receptors and cancer. Biochimica et Biophysica Acta 1808, 1400–1412. [DOI] [PubMed] [Google Scholar]
- Gessi S, Varani K, Merighi S, Cattabriga E, Avitabile A, Gavioli R, … Borea PA (2004). Expression of A3 adenosine receptors in human lymphocytes: up-regulation in T cell activation. Molecular Pharmacology 65, 711–719. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Fernandez-Teruel A, Escorihuela RM, Fredholm BB, Tobena A, Pekny M, & Johansson B (2002). Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. The European Journal of Neuroscience 16, 547–550. [DOI] [PubMed] [Google Scholar]
- Gloeckner-Hofmann K, Guillen-Gomez E, Schmidtgen C, Porstmann R, Ziegler R, Stoss O, … Pastor-Anglada M (2006). Expression of the high-affinity fluoropyrimidine-preferring nucleoside transporter hCNT1 correlates with decreased disease-free survival in breast cancer. Oncology 70, 238–244. [DOI] [PubMed] [Google Scholar]
- Glukhova A, Thal DM, Nguyen AT, Vecchio EA, Jorg M, Scammells PJ, … Christopoulos A (2017). Structure of the adenosine A1 receptor reveals the basis for subtype selectivity. Cell 168(867–877), e813. [DOI] [PubMed] [Google Scholar]
- Goldthwait DA (1957). Mechanisms of synthesis of purine nucleotides in heart muscle extracts. The Journal of Clinical Investigation 36, 1572–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollub EG, Waksman H, Goswami S, & Marom Z (1995). Mucin genes are regulated by estrogen and dexamethasone. Biochemical and Biophysical Research Communications 217, 1006–1014. [DOI] [PubMed] [Google Scholar]
- Grahner B, Winiwarter S, Lanzner W, & Muller CE (1994). Synthesis and structure-activity relationships of deazaxanthines: analogs of potent A1- and A2-adenosine receptor antagonists. Journal of Medicinal Chemistry 37, 1526–1534. [DOI] [PubMed] [Google Scholar]
- Gray JH, Mangravite LM, Owen RP, Urban TJ, Chan W, Carlson EJ, … Giacomini KM (2004). Functional and genetic diversity in the concentrative nucleoside transporter, CNT1, in human populations. Molecular Pharmacology 65, 512–519. [DOI] [PubMed] [Google Scholar]
- Guillen-Gomez E, Calbet M, Casado J, de Lecea L, Soriano E, Pastor-Anglada M, & Burgaya F (2004). Distribution of CNT2 and ENT1 transcripts in rat brain: Selective decrease of CNT2 mRNA in the cerebral cortex of sleep-deprived rats. Journal of Neurochemistry 90, 883–893. [DOI] [PubMed] [Google Scholar]
- Gupte A, & Buolamwini JK (2009). Synthesis and biological evaluation of phloridzin analogs as human concentrative nucleoside transporter 3 (hCNT3) inhibitors. Bioorganic & Medicinal Chemistry Letters 19, 917–921. [DOI] [PubMed] [Google Scholar]
- Hagmann W, Jesnowski R, & Lohr JM (2010). Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia 12, 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg C, Schulte G, & Fredholm BB (2003). Evidence for functional adenosine A3 receptors in microglia cells. Journal of Neurochemistry 86, 1051–1054. [DOI] [PubMed] [Google Scholar]
- Hammond JR (2000). Interaction of a series of draflazine analogues with equilibrative nucleoside transporters: Species differences and transporter subtype selectivity. Naunyn-Schmiedeberg’s Archives of Pharmacology 361, 373–382. [DOI] [PubMed] [Google Scholar]
- Hardy S, & Robillard PN (2008). Petri net-based method for the analysis of the dynamics of signal propagation in signaling pathways. Bioinformatics 24, 209–217. [DOI] [PubMed] [Google Scholar]
- Harish A, Hohana G, Fishman P, Arnon O, & Bar-Yehuda S (2003). A3 adenosine receptor agonist potentiates natural killer cell activity. International Journal of Oncology 23, 1245–1249. [PubMed] [Google Scholar]
- Hasko G, Antonioli L, & Cronstein BN (2018). Adenosine metabolism, immunity and joint health. Biochemical Pharmacology 151, 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haystead TA (2006). The purinome, a complex mix of drug and toxicity targets. Current Topics in Medicinal Chemistry 6, 1117–1127. [DOI] [PubMed] [Google Scholar]
- Hesler RA, Huang JJ, Starr MD, Treboschi VM, Bernanke AG, Nixon AB, … Blobe GC (2016). TGF-beta-induced stromal CYR61 promotes resistance to gemcitabine in pancreatic ductal adenocarcinoma through downregulation of the nucleoside transporters hENT1 and hCNT3. Carcinogenesis 37, 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh AJ, Stonebraker JR, van Heusden CA, Lazarowski ER, Boucher RC, & Picher M (2007). Adenosine deaminase 1 and concentrative nucleoside transporters 2 and 3 regulate adenosine on the apical surface of human airway epithelia: implications for inflammatory lung diseases. Biochemistry 46, 10373–10383. [DOI] [PubMed] [Google Scholar]
- Hoffman BB, Chang H, Dall’Aglio E, & Reaven GM (1986). Desensitization of adenosine receptor-mediated inhibition of lipolysis. The mechanism involves the development of enhanced cyclic adenosine monophosphate accumulation in tolerant adipocytes. The Journal of Clinical Investigation 78, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homminga I, Zwaan CM, Manz CY, Parker C, Bantia S, Smits WK, … Meijerink JP (2011). In vitro efficacy of forodesine and nelarabine (ara-G) in pediatric leukemia. Blood 118, 2184–2190. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Lin W, Seshasayee D, Chen YH, Ding X, Lin Z, … Martin F (2012). Equilibrative nucleoside transporter 3 deficiency perturbs lysosome function and macrophage homeostasis. Science 335, 89–92. [DOI] [PubMed] [Google Scholar]
- Hu Q, Qin Y, Zhang B, Liang C, Ji S, Shi S, Xu W, Xiang J, Liang D, Ni Q, Yu X, & Xu J (2017). FBW7 increases the chemosensitivity of pancreatic cancer cells to gemcitabine through upregulation of ENT1. Oncology Reports 38, 2069–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Kovarova M, Chason KD, Nguyen M, Koller BH, & Tilley SL (2007). Enhanced mast cell activation in mice deficient in the A2b adenosine receptor. The Journal of Experimental Medicine 204, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Lu TW, Liang HL, Hsu WH, Sung YW, & Lee MY (2022). Antiplatelet agents aspirin and dipyridamole, and the risk of different carcinoma in patients with type 2 diabetes mellitus: A Taiwan retrospective cohort study. Medicine (Baltimore) 101, e30468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Wang Y, Mitchell BS, & Graves LM (2004). Regulation of equilibrative nucleoside uptake by protein kinase inhibitors. Nucleosides, Nucleotides & Nucleic Acids 23, 1445–1450. [DOI] [PubMed] [Google Scholar]
- Huang W, Sundquist K, Sundquist J, & Ji J (2022). Use of dipyridamole is associated with lower risk of lymphoid neoplasms: A propensity score-matched cohort study. British Journal of Haematology 196, 690–699. [DOI] [PubMed] [Google Scholar]
- Huang W, Zeng X, Shi Y, & Liu M (2017). Functional characterization of human equilibrative nucleoside transporter 1. Protein & Cell 8, 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SW, Marrache S, Cummins S, Bhutia YD, Mody H, Hooks SB, … Govindarajan R (2015). Defective hCNT1 transport contributes to gemcitabine chemoresistance in ovarian cancer subtypes: Overcoming transport defects using a nanoparticle approach. Cancer Letters 359, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone R, Miele L, Maiolino P, Pinto A, & Morello S (2013). Blockade of A2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloidderived suppressor cells in a mouse model of melanoma. Neoplasia 15, 1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiani P, & Boraschi D (2014). From Monocytes to M1/M2 Macrophages: Phenotypical vs. functional differentiation. Frontiers in Immunology 5, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, … Stevens RC (2008). The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322, 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Balasubramanian R, Deflorian F, & Gao ZG (2012). G protein-coupled adenosine (P1) and P2Y receptors: Ligand design and receptor interactions. Purinergic Signal 8, 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, & Gao ZG (2006). Adenosine receptors as therapeutic targets. Nature Reviews. Drug Discovery 5, 247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Merighi S, Varani K, Borea PA, Baraldi S, Aghazadeh Tabrizi M, … Gessi S (2018). A3 adenosine receptors as modulators of inflammation: From medicinal chemistry to therapy. Medicinal Research Reviews 38, 1031–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajoo S, Mukherjea D, Watabe K, & Ramkumar V (2009). Adenosine A(3) receptor suppresses prostate cancer metastasis by inhibiting NADPH oxidase activity. Neoplasia 11, 1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo AC, Hubeek I, Broekhuizen R, Pastor-Anglada M, Kaspers GJL, Jansen G, … Peters GJ (2020). Expression of the nucleoside transporters hENT1 (SLC29) and hCNT1 (SLC28) in pediatric acute myeloid leukemia. Nucleosides, Nucleotides & Nucleic Acids 39, 1379–1388. [DOI] [PubMed] [Google Scholar]
- Jennings LL, Hao C, Cabrita MA, Vickers MF, Baldwin SA, Young JD, & Cass CE (2001). Distinct regional distribution of human equilibrative nucleoside transporter proteins 1 and 2 (hENT1 and hENT2) in the central nervous system. Neuropharmacology 40, 722–731. [DOI] [PubMed] [Google Scholar]
- Jeske SS, Brand M, Ziebart A, Laban S, Doescher J, Greve J, … Schuler PJ (2020). Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunology, Immunotherapy 69, 1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson K, Ramaswamy S, Ljungcrantz C, Knecht W, Piskur J, Munch-Petersen B, Eriksson S, & Eklund H (2001). Structural basis for substrate specificities of cellular deoxyribonucleoside kinases. Nature Structural Biology 8, 616–620. [DOI] [PubMed] [Google Scholar]
- Jouan E, Moreau A, Bruyere A, Alim K, Denizot C, Parmentier Y, & Fardel O (2021). Differential inhibition of equilibrative nucleoside transporter 1 (ENT1) activity by tyrosine kinase inhibitors. European Journal of Drug Metabolism and Pharmacokinetics 46, 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku H, Cheng KF, Al-Abed Y, & Rothstein TL (2014). A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. Journal of Immunology 193, 5904–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Jun AH, Bhutia YD, Kannan N, Unadkat JD, & Govindarajan R (2010). Human equilibrative nucleoside transporter-3 (hENT3) spectrum disorder mutations impair nucleoside transport, protein localization, and stability. The Journal of Biological Chemistry 285, 28343–28352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaochar S, Rusin A, Foley C, Rajapakshe K, Robertson M, Skapura D, … Mitsiades N (2021). Inhibition of GATA2 in prostate cancer by a clinically available small molecule. Endocrine-Related Cancer 29, 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty-Quintana H, Weng T, Garcia-Morales LJ, Chen NY, Pedroza M, Zhong H, … Blackburn MR (2013). Adenosine A2B receptor and hyaluronan modulate pulmonary hypertension associated with chronic obstructive pulmonary disease. American Journal of Respiratory Cell and Molecular Biology 49, 1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty-Quintana H, Xia Y, & Blackburn MR (2013). Adenosine signaling during acute and chronic disease states. Journal of Molecular Medicine (Berlin, Germany) 91, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katori M, & Berne RM (1966). Release of adenosine from anoxic hearts: Relationship to coronary flow. Circulation Research 19, 420–425. [DOI] [PubMed] [Google Scholar]
- Kawamoto M, Umebayashi M, Tanaka H, Koya N, Nakagawa S, Kawabe K, Onishi H, Nakamura M, & Morisaki T (2018). Combined gemcitabine and metronidazole is a promising therapeutic strategy for cancer stem-like cholangiocarcinoma. Anticancer Research 38, 2739–2748. [DOI] [PubMed] [Google Scholar]
- Kayhan M, Koyas A, Akdemir I, Savas AC, & Cekic C (2019). Adenosine receptor signaling targets both PKA and Epac pathways to polarize dendritic cells to a suppressive phenotype. Journal of Immunology 203, 3247–3255. [DOI] [PubMed] [Google Scholar]
- Kenny LM, Contractor KB, Stebbing J, Al-Nahhas A, Palmieri C, Shousha S, … Aboagye EO (2009). Altered tissue 3′-deoxy-3′-[18F]fluorothymidine pharmacokinetics in human breast cancer following capecitabine treatment detected by positron emission tomography. Clinical Cancer Research 15, 6649–6657. [DOI] [PubMed] [Google Scholar]
- Khan MA, & Khan A (2021). Role of NKT cells during viral infection and the development of NKT cell-based nanovaccines. Vaccines (Basel), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoa ND, Postow M, Danielsson J, & Cronstein BN (2006). Tumor necrosis factor-alpha prevents desensitization of Galphas-coupled receptors by regulating GRK2 association with the plasma membrane. Molecular Pharmacology 69, 1311–1319. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim H, Lee JC, Kim JW, Paik WH, Lee SH, … Kim YT (2018). Human equilibrative nucleoside transporter 1 (hENT1) expression as a predictive biomarker for gemcitabine chemotherapy in biliary tract cancer. PLoS One 13, e0209104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee C, Cheong HS, Koh Y, Ahn KS, Kim HL, … Yoon SS (2016). SLC29A1 (ENT1) polymorphisms and outcome of complete remission in acute myeloid leukemia. Cancer Chemotherapy and Pharmacology 78, 533–540. [DOI] [PubMed] [Google Scholar]
- Kim YC, Ji XD, & Jacobson KA (1996). Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) are selective for the human A3 receptor subtype. Journal of Medicinal Chemistry 39, 4142–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch GE, Codina J, Birnbaumer L, & Brown AM (1990). Coupling of ATP-sensitive K + channels to A1 receptors by G proteins in rat ventricular myocytes. The American Journal of Physiology 259, H820–H826. [DOI] [PubMed] [Google Scholar]
- Klein K, Kullak-Ublick GA, Wagner M, Trauner M, & Eloranta JJ (2009). Hepatocyte nuclear factor-4alpha and bile acids regulate human concentrative nucleoside transporter-1 gene expression. American Journal of Physiology. Gastrointestinal and Liver Physiology 296, G936–G947. [DOI] [PubMed] [Google Scholar]
- Klotz KN (2000). Adenosine receptors and their ligands. Naunyn-Schmiedeberg’s Archives of Pharmacology 362, 382–391. [DOI] [PubMed] [Google Scholar]
- Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, & Lohse MJ (1998). Comparative pharmacology of human adenosine receptor subtypes - characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedeberg’s Archives of Pharmacology 357, 1–9. [DOI] [PubMed] [Google Scholar]
- Klotz KN, Keil R, Zimmer FJ, & Schwabe U (1990). Guanine nucleotide effects on 8-cyclopentyl-1,3-[3H]dipropylxanthine binding to membrane-bound and solubilized A1 adenosine receptors of rat brain. Journal of Neurochemistry 54, 1988–1994. [DOI] [PubMed] [Google Scholar]
- Koeppen M, Eckle T, & Eltzschig HK (2009). Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One 4, Article e6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong T, Westerman KA, Faigle M, Eltzschig HK, & Colgan SP (2006). HIF-dependent induction of adenosine A2B receptor in hypoxia. The FASEB Journal 20, 2242–2250. [DOI] [PubMed] [Google Scholar]
- Kong W, Engel K, & Wang J (2004). Mammalian nucleoside transporters. Current Drug Metabolism 5, 63–84. [DOI] [PubMed] [Google Scholar]
- Koshiba M, Rosin DL, Hayashi N, Linden J, & Sitkovsky MV (1999). Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Molecular Pharmacology 55, 614–624. [PubMed] [Google Scholar]
- Kreft S, Bier D, Holschbach MH, Schulze A, & Coenen HH (2017). New potent A1 adenosine receptor radioligands for positron emission tomography. Nuclear Medicine and Biology 44, 69–77. [DOI] [PubMed] [Google Scholar]
- Lahdaoui F, Delpu Y, Vincent A, Renaud F, Messager M, Duchene B, Leteurtre E, Mariette C, Torrisani J, Jonckheere N, & Van Seuningen I (2015). miR-219–1-3p is a negative regulator of the mucin MUC4 expression and is a tumor suppressor in pancreatic cancer. Oncogene 34, 780–788. [DOI] [PubMed] [Google Scholar]
- Lansakara PD, Rodriguez BL, & Cui Z (2012). Synthesis and in vitro evaluation of novel lipophilic monophosphorylated gemcitabine derivatives and their nanoparticles. International Journal of Pharmaceutics 429, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini S, & Pedata F (2001). Adenosine in the central nervous system: release mechanisms and extracellular concentrations. Journal of Neurochemistry 79, 463–484. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER (2012). Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal 8, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon G, Bennett K, Jazayeri A, & Tate CG (2011). Thermostabilisation of an agonistbound conformation of the human adenosine A(2A) receptor. Journal of Molecular Biology 409, 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon G, & Tate CG (2011). Structure of the adenosine-bound conformation of the human adenosine A(2A) receptor. Medical Science (Paris) 27, 926–928. [DOI] [PubMed] [Google Scholar]
- Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, & Tate CG (2011). Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 474, 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, … Parmentier M (1997). Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388, 674–678. [DOI] [PubMed] [Google Scholar]
- Lee EJ, & Lee SJ (2013). Etoposide increases equilibrative nucleoside transporter 1 activity and fluorothymidine uptake: screening of 60 cytotoxic agents. Oncology Reports 29, 763–770. [DOI] [PubMed] [Google Scholar]
- Lee YC, Chien CL, Sun CN, Huang CL, Huang NK, Chiang MC, … Chern Y (2003). Characterization of the rat A2A adenosine receptor gene: a 4.8-kb promoter-proximal DNA fragment confers selective expression in the central nervous system. The European Journal of Neuroscience 18, 1786–1796. [DOI] [PubMed] [Google Scholar]
- Leisewitz AV, Zimmerman EI, Huang M, Jones SZ, Yang J, & Graves LM (2011). Regulation of ENT1 expression and ENT1-dependent nucleoside transport by c-Jun N-terminal kinase. Biochemical and Biophysical Research Communications 404, 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lera Ruiz M, Lim YH, & Zheng J (2014). Adenosine A2A receptor as a drug discovery target. Journal of Medicinal Chemistry 57, 3623–3650. [DOI] [PubMed] [Google Scholar]
- Leung GP, Man RY, & Tse CM (2005). Effect of thiazolidinediones on equilibrative nucleoside transporter-1 in human aortic smooth muscle cells. Biochemical Pharmacology 70, 355–362. [DOI] [PubMed] [Google Scholar]
- Li AH, Moro S, Melman N, Ji XD, & Jacobson KA (1998). Structure-activity relationships and molecular modeling of 3, 5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. Journal of Medicinal Chemistry 41, 3186–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GG, Guo ZZ, Ma XF, Cao N, Geng SN, Zheng YQ, … Du GJ (2016). The M2 macrophages induce autophagic vascular disorder and promote mouse sensitivity to urethane-related lung carcinogenesis. Developmental and Comparative Immunology 59, 89–98. [DOI] [PubMed] [Google Scholar]
- Li J, Di Y, Jin C, Fu D, Yang F, Jiang Y, Yao L, Hao S, Wang X, Subedi S, & Ni Q (2013). Gemcitabine-loaded albumin nanospheres (GEM-ANPs) inhibit PANC-1 cells in vitro and in vivo. Nanoscale Research Letters 8, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Mak WW, Li J, Zheng C, Shiu PH, Seto SW, … Leung GP (2022). Structure-activity relationship studies of 4-((4-(2-fluorophenyl)piperazin-1-yl)methyl)-6-imino-N-(naphthalen-2-yl)-1,3,5-tr iazin-2-amine (FPMINT) analogues as inhibitors of human equilibrative nucleoside transporters. Frontiers in Pharmacology 13, 837555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J, & Cekic C (2012). Regulation of lymphocyte function by adenosine. Arteriosclerosis, Thrombosis, and Vascular Biology 32, 2097–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J, Thai T, Figler H, Jin X, & Robeva AS (1999). Characterization of human A (2B) adenosine receptors: radioligand binding, western blotting, and coupling to G (q) in human embryonic kidney 293 cells and HMC-1 mast cells. Molecular Pharmacology 56, 705–713. [PubMed] [Google Scholar]
- Liu M, Zhang Y, Yang J, Cui X, Zhou Z, Zhan H, … Li M (2020). ZIP4 increases expression of transcription factor ZEB1 to promote integrin alpha3beta1 signaling and inhibit expression of the gemcitabine transporter ENT1 in pancreatic cancer cells. Gastroenterology 158(679–692), e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fong M, Cone J, Wang S, Yoshitake M, & Kambayashi J (2000). Inhibition of adenosine uptake and augmentation of ischemia-induced increase of interstitial adenosine by cilostazol, an agent to treat intermittent claudication. Journal of Cardiovascular Pharmacology 36, 351–360. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Chen J, Li X, Zhou X, Hu YM, Chu SF, … Chen NH (2019). Research progress on adenosine in central nervous system diseases. CNS Neuroscience & Therapeutics 25, 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston M, Heaney LG, & Ennis M (2004). Adenosine, inflammation and asthma–a review. Inflammation Research 53, 171–178. [DOI] [PubMed] [Google Scholar]
- Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, & Eltzschig HK (2007). Physiological roles of vascular nucleoside transporters. Arteriosclerosis, Thrombosis, and Vascular Biology 27, 1004–1013. [DOI] [PubMed] [Google Scholar]
- Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, & Gorelik E (2006). Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Research 66, 7758–7765. [DOI] [PubMed] [Google Scholar]
- Lu H, Chen C, & Klaassen C (2004). Tissue distribution of concentrative and equilibrative nucleoside transporters in male and female rats and mice. Drug Metabolism and Disposition 32, 1455–1461. [DOI] [PubMed] [Google Scholar]
- Lu X, Gong S, Monks A, Zaharevitz D, & Moscow JA (2002). Correlation of nucleoside and nucleobase transporter gene expression with antimetabolite drug cytotoxicity. Journal of Experimental Therapeutics & Oncology 2, 200–212. [DOI] [PubMed] [Google Scholar]
- Macanas-Pirard P, Leisewitz A, Broekhuizen R, Cautivo K, Barriga FM, Leisewitz F, … Nervi B (2012). Bone marrow stromal cells modulate mouse ENT1 activity and protect leukemia cells from cytarabine induced apoptosis. PLoS One 7, e37203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macha MA, Rachagani S, Gupta S, Pai P, Ponnusamy MP, Batra SK, & Jain M (2013). Guggulsterone decreases proliferation and metastatic behavior of pancreatic cancer cells by modulating JAK/STAT and Src/FAK signaling. Cancer Letters 341, 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimenko A, Caron J, Mougin J, Desmaele D, & Couvreur P (2015). Gemcitabine-based therapy for pancreatic cancer using the squalenoyl nucleoside monophosphate nanoassemblies. International Journal of Pharmaceutics 482, 38–46. [DOI] [PubMed] [Google Scholar]
- Mantini G, Valles AM, Le Large TYS, Capula M, Funel N, Pham TV, … Jimenez CR (2020). Co-expression analysis of pancreatic cancer proteome reveals biology and prognostic biomarkers. Cellular Oncology (Dordrecht) 43, 1147–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RB, & Don JM (2007). Drug-induced disorders of the nervous system. Clinical Medicine (London, England) 7, 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PL, Ueeda M, & Olsson RA (1993). 2-Phenylethoxy-9-methyladenine: An adenosine receptor antagonist that discriminates between A2 adenosine receptors in the aorta and the coronary vessels from the guinea pig. The Journal of Pharmacology and Experimental Therapeutics 265, 248–253. [PubMed] [Google Scholar]
- Martin PL, Wysocki RJ Jr., Barrett RJ, May JM, & Linden J (1996). Characterization of 8-(N-methylisopropyl)amino-N6-(5′-endohydroxy- endonorbornyl)-9-methyladenine (WRC-0571), a highly potent and selective, non-xanthine antagonist of A1 adenosine receptors. The Journal of Pharmacology and Experimental Therapeutics 276, 490–499. [PubMed] [Google Scholar]
- Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, … Kroemer G (2009). Chemotherapy induces ATP release from tumor cells. Cell Cycle 8, 3723–3728. [DOI] [PubMed] [Google Scholar]
- Mastelic-Gavillet B, Navarro Rodrigo B, Decombaz L, Wang H, Ercolano G, Ahmed R, … Vigano S (2019). Adenosine mediates functional and metabolic suppression of peripheral and tumor-infiltrating CD8(+) T cells. Journal for Immunotherapy of Cancer 7, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta C, Rotondo JC, Lanzillotti C, Campione G, Martini F, & Tognon M (2022). Cancer biology and molecular genetics of A3 adenosine receptor. Oncogene 41, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaw TR, Li M, Starenki D, Liu M, Cooper SJ, Arend RC, … Randall TD (2019). Histone deacetylase inhibition promotes intratumoral CD8(+) T-cell responses, sensitizing murine breast tumors to anti-PD1. Cancer Immunology, Immunotherapy 68, 2081–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, & Hope TJ (2003). Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300, 1295–1297. [DOI] [PubMed] [Google Scholar]
- McIntosh HH, & Blazynski C (1994). Characterization and localization of adenosine A2 receptors in bovine rod outer segments. Journal of Neurochemistry 62, 992–997. [DOI] [PubMed] [Google Scholar]
- Meckling-Gill KA, Guilbert L, & Cass CE (1993). CSF-1 stimulates nucleoside transport in S1 macrophages. Journal of Cellular Physiology 155, 530–538. [DOI] [PubMed] [Google Scholar]
- Medina-Pulido L, Molina-Arcas M, Justicia C, Soriano E, Burgaya F, Planas AM, & Pastor-Anglada M (2013). Hypoxia and P1 receptor activation regulate the high-affinity concentrative adenosine transporter CNT2 in differentiated neuronal PC12 cells. The Biochemical Journal 454, 437–445. [DOI] [PubMed] [Google Scholar]
- Melani A, Corti F, Stephan H, Muller CE, Donati C, Bruni P, … Pedata F (2012). Ecto-ATPase inhibition: ATP and adenosine release under physiological and ischemic in vivo conditions in the rat striatum. Experimental Neurology 233, 193–204. [DOI] [PubMed] [Google Scholar]
- Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, … Nel AE (2015). Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 9, 3540–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SR, Hau RK, Jilek JL, Morales MN, Wright SH, & Cherrington NJ (2020). Nucleoside reverse transcriptase inhibitor interaction with human equilibrative nucleoside transporters 1 and 2. Drug Metabolism and Disposition 48, 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SR, Lane TR, Zorn KM, Ekins S, Wright SH, & Cherrington NJ (2021). Multiple computational approaches for predicting drug interactions with human equilibrative nucleoside transporter 1. Drug Metabolism and Disposition 49, 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuesa G, Purcet S, Erkizia I, Molina-Arcas M, Bofill M, Izquierdo-Useros N, … Martinez-Picado (2008). Expression and functionality of anti-human immunodeficiency virus and anticancer drug uptake transporters in immune cells. The Journal of Pharmacology and Experimental Therapeutics 324, 558–567. [DOI] [PubMed] [Google Scholar]
- Mirabet M, Herrera C, Cordero OJ, Mallol J, Lluis C, & Franco R (1999). Expression of A2B adenosine receptors in human lymphocytes: Their role in T cell activation. Journal of Cell Science 112(Pt 4), 491–502. [DOI] [PubMed] [Google Scholar]
- Mohamadi A, Aghaei M, & Panjehpour M (2018). Estrogen stimulates adenosine receptor expression subtypes in human breast cancer MCF-7 cell line. Research in Pharmaceutical Sciences 13, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Arcas M, Bellosillo B, Casado FJ, Montserrat E, Gil J, Colomer D, & Pastor-Anglada M (2003). Fludarabine uptake mechanisms in B-cell chronic lymphocytic leukemia. Blood 101, 2328–2334. [DOI] [PubMed] [Google Scholar]
- Montalban Del Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wockel A, … Hausler SFM (2016). Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages - a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. Journal for Immunotherapy of Cancer 4, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R, Ishikawa T, Ichikawa Y, Taniguchi K, Matsuyama R, Ueda M, … Shimada H (2007). Human equilibrative nucleoside transporter 1 is associated with the chemosensitivity of gemcitabine in human pancreatic adenocarcinoma and biliary tract carcinoma cells. Oncology Reports 17, 1201–1205. [PubMed] [Google Scholar]
- van Muijlwijk-Koezen JE, Timmerman H, Link R, van der Goot H, & AP IJ (1998). A novel class of adenosine A3 receptor ligands. 1. 3-(2-Pyridinyl)isoquinoline derivatives. Journal of Medicinal Chemistry 41, 3987–3993. [DOI] [PubMed] [Google Scholar]
- van Muijlwijk-Koezen JE, Timmerman H, Link R, van der Goot H, & Ijzerman AP (1998). A novel class of adenosine A3 receptor ligands. 2. Structure affinity profile of a series of isoquinoline and quinazoline compounds. Journal of Medicinal Chemistry 41, 3994–4000. [DOI] [PubMed] [Google Scholar]
- Muller CE, Sandoval-Ramirez J, Schobert U, Geis U, Frobenius W, & Klotz KN (1998). 8-(Sulfostyryl)xanthines: Water-soluble A2A-selective adenosine receptor antagonists. Bioorganic & Medicinal Chemistry 6, 707–719. [DOI] [PubMed] [Google Scholar]
- Muller PJ, Dally H, Klappenecker CN, Edler L, Jager B, Gerst M, … Risch A (2009). Polymorphisms in ABCG2, ABCC3 and CNT1 genes and their possible impact on chemotherapy outcome of lung cancer patients. International Journal of Cancer 124, 1669–1674. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Loudon RP, & Benovic JL (1999). Characterization of G protein-coupled receptor regulation in antisense mRNA-expressing cells with reduced arrestin levels. Biochemistry 38, 8723–8732. [DOI] [PubMed] [Google Scholar]
- Murphree LJ, Marshall MA, Rieger JM, MacDonald TL, & Linden J (2002). Human A(2A) adenosine receptors: High-affinity agonist binding to receptor-G protein complexes containing Gbeta(4). Molecular Pharmacology 61, 455–462. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, … Wynn TA (2014). Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 41, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naes SM, Ab-Rahim S, Mazlan M, & Abdul Rahman A (2020). Equilibrative nucleoside transporter 2: Properties and physiological roles. BioMed Research International 2020, 5197626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Strohecker AM, Persaud AK, Bissa B, Muruganandan S, McElroy C, … Govindarajan R (2019). Adult stem cell deficits drive Slc29a3 disorders in mice. Nature Communications 10, 2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AA, & Perry SV (1960). The incorportion of 15Ninto adenine nucleotides and their formation from inosine monophosphate by skeletal-muscle preparations. The Biochemical Journal 74, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Ross AE, Ryals M, Lee ST, & Venton BJ (2015). Clearance of rapid adenosine release is regulated by nucleoside transporters and metabolism. Pharmacology Research & Perspectives 3, Article e00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Huang RY, Jackson RA, & Thiery JP (2016). Emt: 2016. Cell 166, 21–45. [DOI] [PubMed] [Google Scholar]
- Nishio R, Tsuchiya H, Yasui T, Matsuura S, Kanki K, Kurimasa A, Hisatome I, & Shiota G (2011). Disrupted plasma membrane localization of equilibrative nucleoside transporter 2 in the chemoresistance of human pancreatic cells to gemcitabine (dFdCyd). Cancer Science 102, 622–629. [DOI] [PubMed] [Google Scholar]
- Noji T, Karasawa A, & Kusaka H (2004). Adenosine uptake inhibitors. European Journal of Pharmacology 495, 1–16. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Ichimura M, Takeda M, Nonaka Y, Shimada J, Suzuki F, Yamaguchi K, & Kase H (1994). KF17837 ((E)-8-(3,4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine), a potent and selective adenosine A2 receptor antagonist. European Journal of Pharmacology 267, 335–341. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Mori A, Ichimura M, Shindou T, Yanagawa K, Shimada J, & Kase H (1994). Binding of [3H]KF17837S, a selective adenosine A2 receptor antagonist, to rat brain membranes. Molecular Pharmacology 46, 817–822. [PubMed] [Google Scholar]
- Ohta A (2016). A Metabolic immune checkpoint: Adenosine in tumor microenvironment. Frontiers in Immunology 7, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Ohta A, Madasu M, Kini R, Subramanian M, Goel N, & Sitkovsky M (2009). A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. Journal of Immunology 183, 5487–5493. [DOI] [PubMed] [Google Scholar]
- Ohta A, & Sitkovsky M (2014). Extracellular adenosine-mediated modulation of regulatory T cells. Frontiers in Immunology 5, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Macdonald T, & Huang L (1999). Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. The American Journal of Physiology 277, F404–F412. [DOI] [PubMed] [Google Scholar]
- Olah ME, Jacobson KA, & Stiles GL (1994). Identification of an adenosine receptor domain specifically involved in binding of 5′-substituted adenosine agonists. The Journal of Biological Chemistry 269, 18016–18020. [PMC free article] [PubMed] [Google Scholar]
- O’Malley MA, Mancini JD, Young CL, McCusker EC, Raden D, & Robinson AS (2009). Progress toward heterologous expression of active G-protein-coupled receptors in Saccharomyces cerevisiae: Linking cellular stress response with translocation and trafficking. Protein Science 18, 2356–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paes-de-Carvalho R, Dias BV, Martins RA, Pereira MR, Portugal CC, & Lanfredi C (2005). Activation of glutamate receptors promotes a calcium-dependent and transporter-mediated release of purines in cultured avian retinal cells: possible involvement of calcium/calmodulin-dependent protein kinase II. Neurochemistry International 46, 441–451. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Poucher SM, Jacobson KA, & Stiles GL (1995). 125I-4-(2-[7-amino-2[2-furyl][1,2,4]triazolo[2,3-a][1,3,5] triazin-5-yl-amino]ethyl)phenol, a high affinity antagonist radioligand selective for the A2a adenosine receptor. Molecular Pharmacology 48, 970–974. [PMC free article] [PubMed] [Google Scholar]
- Panjehpour M, Hemati S, & Forghani MA (2012). Expression of A1 and A3 adenosine receptors in human breast tumors. Tumori 98, 137–141. [DOI] [PubMed] [Google Scholar]
- Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, & Norgauer J (2003). Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood 101, 3985–3990. [DOI] [PubMed] [Google Scholar]
- Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, … Norgauer J (2001). Expression and function of adenosine receptors in human dendritic cells. The FASEB Journal 15, 1963–1970. [DOI] [PubMed] [Google Scholar]
- Papa AL, Basu S, Sengupta P, Banerjee D, Sengupta S, & Harfouche R (2012). Mechanistic studies of Gemcitabine-loaded nanoplatforms in resistant pancreatic cancer cells. BMC Cancer 12, 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa AL, Sidiqui A, Balasubramanian SU, Sarangi S, Luchette M, Sengupta S, & Harfouche R (2013). PEGylated liposomal gemcitabine: Insights into a potential breast cancer therapeutic. Cellular Oncology (Dordrecht) 36, 449–457. [DOI] [PubMed] [Google Scholar]
- Paproski RJ, Yao SY, Favis N, Evans D, Young JD, Cass CE, & Zemp RJ (2013). Human concentrative nucleoside transporter 3 transfection with ultrasound and microbubbles in nucleoside transport deficient HEK293 cells greatly increases gemcitabine uptake. PLoS One 8, Article e56423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson FE, Damaraju VL, Graham K, Yao SY, Baldwin SA, Cass CE, & Young JD (2011). Molecular biology of nucleoside transporters and their distributions and functions in the brain. Current Topics in Medicinal Chemistry 11, 948–972. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Paterson AR, Young JD, & Cass CE (1993). Inhibitory effects of propentofylline on [3H]adenosine influx. A study of three nucleoside transport systems. Biochemical Pharmacology 46, 891–896. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Rudolphi KA, & Fredholm BB (1994). Propentofylline: A nucleoside transport inhibitor with neuroprotective effects in cerebral ischemia. General Pharmacology 25, 1053–1058. [DOI] [PubMed] [Google Scholar]
- Pasquini S, Contri C, Merighi S, Gessi S, Borea PA, Varani K, & Vincenzi F (2022). Adenosine receptors in neuropsychiatric disorders: Fine regulators of neurotransmission and potential therapeutic targets. International Journal of Molecular Sciences 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Anglada M, Casado FJ, Valdes R, Mata J, Garcia-Manteiga J, & Molina M (2001). Complex regulation of nucleoside transporter expression in epithelial and immune system cells. Molecular Membrane Biology 18, 81–85. [DOI] [PubMed] [Google Scholar]
- Pastor-Anglada M, & Perez-Torras S (2015). Nucleoside transporter proteins as biomarkers of drug responsiveness and drug targets. Frontiers in Pharmacology 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Anglada M, & Perez-Torras S (2018a). Emerging roles of nucleoside transporters. Frontiers in Pharmacology 9, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Anglada M, & Perez-Torras S (2018b). Who is who in adenosine transport. Frontiers in Pharmacology 9, 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patente TA, Pelgrom LR, & Everts B (2019). Dendritic cells are what they eat: How their metabolism shapes T helper cell polarization. Current Opinion in Immunology 58, 16–23. [DOI] [PubMed] [Google Scholar]
- Pennycooke M, Chaudary N, Shuralyova I, Zhang Y, & Coe IR (2001). Differential expression of human nucleoside transporters in normal and tumor tissue. Biochemical and Biophysical Research Communications 280, 951–959. [DOI] [PubMed] [Google Scholar]
- Perez-Torras S, Garcia-Manteiga J, Mercade E, Casado FJ, Carbo N, Pastor-Anglada M, & Mazo A (2008). Adenoviral-mediated overexpression of human equilibrative nucleoside transporter 1 (hENT1) enhances gemcitabine response in human pancreatic cancer. Biochemical Pharmacology 76, 322–329. [DOI] [PubMed] [Google Scholar]
- Perez-Torras S, Vidal-Pla A, Cano-Soldado P, Huber-Ruano I, Mazo A, & Pastor-Anglada M (2013). Concentrative nucleoside transporter 1 (hCNT1) promotes phenotypic changes relevant to tumor biology in a translocation-independent manner. Cell Death & Disease 4, e648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai K, Mekkawy AH, Akhter J, Badar S, Dong L, Liu AI, & Morris DL (2020). Enhancing the potency of chemotherapeutic agents by combination with bromelain and N-acetylcysteine - an in vitro study with pancreatic and hepatic cancer cells. American Journal of Translational Research 12, 7404–7419. [PMC free article] [PubMed] [Google Scholar]
- Pinto-Duarte A, Coelho JE, Cunha RA, Ribeiro JA, & Sebastiao AM (2005). Adenosine A2A receptors control the extracellular levels of adenosine through modulation of nucleoside transporters activity in the rat hippocampus. Journal of Neurochemistry 93, 595–604. [DOI] [PubMed] [Google Scholar]
- Playa H, Lewis TA, Ting A, Suh BC, Munoz B, Matuza R, … Buolamwini JK (2014). Dilazep analogues for the study of equilibrative nucleoside transporters 1 and 2 (ENT1 and ENT2). Bioorganic & Medicinal Chemistry Letters 24, 5801–5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenge RM, Scolnick EM, & Altshuler D (2013). Validating therapeutic targets through human genetics. Nature Reviews. Drug Discovery 12, 581–594. [DOI] [PubMed] [Google Scholar]
- Polosa R, & Holgate ST (2006). Adenosine receptors as promising therapeutic targets for drug development in chronic airway inflammation. Current Drug Targets 7, 699–706. [DOI] [PubMed] [Google Scholar]
- Poulsen SA, & Quinn RJ (1998). Adenosine receptors: New opportunities for future drugs. Bioorganic & Medicinal Chemistry 6, 619–641. [DOI] [PubMed] [Google Scholar]
- Preti D, Baraldi PG, Moorman AR, Borea PA, & Varani K (2015). History and perspectives of A2A adenosine receptor antagonists as potential therapeutic agents. Medicinal Research Reviews 35, 790–848. [DOI] [PubMed] [Google Scholar]
- Rahman MF, Askwith C, & Govindarajan R (2017). Molecular determinants of acidic pH-dependent transport of human equilibrative nucleoside transporter 3. The Journal of Biological Chemistry 292, 14775–14785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo O, Papini F, Mantini G, Gregori A, Parrino B, Liu DSK, … Giovannetti E (2020). “Open Sesame?”: Biomarker status of the human equilibrative nucleoside transporter-1 and molecular mechanisms influencing its expression and activity in the uptake and cytotoxicity of gemcitabine in pancreatic cancer. Cancers (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskovalova T, Huang X, Sitkovsky M, Zacharia LC, Jackson EK, & Gorelik E (2005). Gs protein-coupled adenosine receptor signaling and lytic function of activated NK cells. Journal of Immunology 175, 4383–4391. [DOI] [PubMed] [Google Scholar]
- Raskovalova T, Lokshin A, Huang X, Jackson EK, & Gorelik E (2006). Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: Involvement of protein kinase A isozyme I (PKA I). Immunologic Research 36, 91–99. [DOI] [PubMed] [Google Scholar]
- Reddy LH, Dubernet C, Mouelhi SL, Marque PE, Desmaele D, & Couvreur P (2007). A new nanomedicine of gemcitabine displays enhanced anticancer activity in sensitive and resistant leukemia types. Journal of Controlled Release 124, 20–27. [DOI] [PubMed] [Google Scholar]
- Regan SE, Broad M, Byford AM, Lankford AR, Cerniway RJ, Mayo MW, & Matherne GP (2003). A1 adenosine receptor overexpression attenuates ischemiareperfusion-induced apoptosis and caspase 3 activity. American Journal of Physiology. Heart and Circulatory Physiology 284, H859–H866. [DOI] [PubMed] [Google Scholar]
- Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, … Ruttinger D (2014). Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25, 846–859. [DOI] [PubMed] [Google Scholar]
- Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, … Young JD (2001a). Recent molecular advances in studies of the concentrative Na+-dependent nucleoside transporter (CNT) family: Identification and characterization of novel human and mouse proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). Molecular Membrane Biology 18, 65–72. [DOI] [PubMed] [Google Scholar]
- Ritzel MW, Ng AM, Yao SY, Graham K, Loewen SK, Smith KM, … Young JD (2001b). Molecular identification and characterization of novel human and mouse concentrative Na+-nucleoside cotransporter proteins (hCNT3 and mCNT3) broadly selective for purine and pyrimidine nucleosides (system cib). The Journal of Biological Chemistry 276, 2914–2927. [DOI] [PubMed] [Google Scholar]
- Rose JB, Naydenova Z, Bang A, Eguchi M, Sweeney G, Choi DS, … Coe IR (2010). Equilibrative nucleoside transporter 1 plays an essential role in cardioprotection. American Journal of Physiology. Heart and Circulatory Physiology 298, H771–H777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, & Kobilka BK (2009). The structure and function of G-protein-coupled receptors. Nature 459, 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffell B, & Coussens LM (2015). Macrophages and therapeutic resistance in cancer. Cancer Cell 27, 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, … Feoktistov I (2011). Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. Journal of Immunology 187, 6120–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva S, & Gupta M (2013). Adenosine and its receptors as therapeutic targets: An overview. Saudi Pharmaceutical Journal 21, 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safford M, Collins S, Lutz MA, Allen A, Huang CT, Kowalski J, … Powell JD (2005). Egr-2 and Egr-3 are negative regulators of T cell activation. Nature Immunology 6, 472–480. [DOI] [PubMed] [Google Scholar]
- Sakowicz M, Szutowicz A, & Pawelczyk T (2005). Differential effect of insulin and elevated glucose level on adenosine transport in rat B lymphocytes. International Immunology 17, 145–154. [DOI] [PubMed] [Google Scholar]
- Salmon JE, & Cronstein BN (1990). Fc gamma receptor-mediated functions in neutrophils are modulated by adenosine receptor occupancy. A1 receptors are stimulatory and A2 receptors are inhibitory. Journal of Immunology 145, 2235–2240. [PubMed] [Google Scholar]
- Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, & Jacobson MA (2000). Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. The Journal of Biological Chemistry 275, 4429–4434. [DOI] [PubMed] [Google Scholar]
- Satoh A, Shimosegawa T, Satoh K, Ito H, Kohno Y, Masamune A, Fujita M, & Toyota T (2000). Activation of adenosine A1-receptor pathway induces edema formation in the pancreas of rats. Gastroenterology 119, 829–836. [DOI] [PubMed] [Google Scholar]
- Saze Z, Schuler PJ, Hong CS, Cheng D, Jackson EK, & Whiteside TL (2013). Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood 122, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr M, Toy T, Shin A, Hartmann G, Rothenfusser S, Soellner J, … Maraskovsky E (2004). Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood 103, 1391–1397. [DOI] [PubMed] [Google Scholar]
- Scholtissek C (1968). Studies on the uptake of nucleic acid precursors into cells in tissue culture. Biochimica et Biophysica Acta 158, 435–447. [DOI] [PubMed] [Google Scholar]
- Schulte G, & Fredholm BB (2000). Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Molecular Pharmacology 58, 477–482. [PubMed] [Google Scholar]
- SenGupta DJ, Lum PY, Lai Y, Shubochkina E, Bakken AH, Schneider G, & Unadkat JD (2002). A single glycine mutation in the equilibrative nucleoside transporter gene, hENT1, alters nucleoside transport activity and sensitivity to nitrobenzylthioinosine. Biochemistry 41, 1512–1519. [DOI] [PubMed] [Google Scholar]
- Seshacharyulu P, Ponnusamy MP, Rachagani S, Lakshmanan I, Haridas D, Yan Y, … Batra SK (2015). Targeting EGF-receptor(s) - STAT1 axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer. Oncotarget 6, 5164–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RE, Wu HH, & Dale N (2013). Sleep-wake sensitive mechanisms of adenosine release in the basal forebrain of rodents: an in vitro study. PLoS One 8, Article e53814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitkovsky MV (2009). T regulatory cells: Hypoxia-adenosinergic suppression and redirection of the immune response. Trends in Immunology 30, 102–108. [DOI] [PubMed] [Google Scholar]
- Skrypek N, Duchene B, Hebbar M, Leteurtre E, van Seuningen I, & Jonckheere N (2013). The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the concentrative nucleoside transporter family. Oncogene 32, 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloat BR, Sandoval MA, Li D, Chung WG, Lansakara PD, Proteau PJ, … Cui Z (2011). In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles. International Journal of Pharmaceutics 409, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler C, Felipe A, Casado FJ, Celada A, & Pastor-Anglada M (2000). Nitric oxide regulates nucleoside transport in activated B lymphocytes. Journal of Leukocyte Biology 67, 345–349. [DOI] [PubMed] [Google Scholar]
- Soler C, Felipe A, Garcia-Manteiga J, Serra M, Guillen-Gomez E, Casado FJ, … Celada A (2003). Interferon-gamma regulates nucleoside transport systems in macrophages through signal transduction and activator of transduction factor 1 (STAT1)-dependent and -independent signalling pathways. The Biochemical Journal 375, 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler C, Garcia-Manteiga J, Valdes R, Xaus J, Comalada M, Casado FJ, … Felipe A (2001). Macrophages require different nucleoside transport systems for proliferation and activation. The FASEB Journal 15, 1979–1988. [DOI] [PubMed] [Google Scholar]
- Soler C, Valdes R, Garcia-Manteiga J, Xaus J, Comalada M, Casado FJ, … Pastor-Anglada M (2001). Lipopolysaccharide-induced apoptosis of macrophages determines the up-regulation of concentrative nucleoside transporters Cnt1 and Cnt2 through tumor necrosis factor-alpha-dependent and -independent mechanisms. The Journal of Biological Chemistry 276, 30043–30049. [DOI] [PubMed] [Google Scholar]
- Song D, Xu J, Bai Q, Cai L, Hertz L, & Peng L (2014). Role of the intracellular nucleoside transporter ENT3 in transmitter and high K+ stimulation of astrocytic ATP release investigated using siRNA against ENT3. ASN Neuro 6 1759091414543439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JW, Huang HH, Zhang C, Yang HG, Zhang JY, Xu RN, … Jiao YM (2019). Expression of CD39 is correlated with HIV DNA levels in naive tregs in Chronically infected ART naive patients. Frontiers in Immunology 10, 2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino C, Miele L, Porta A, Pinto A, & Morello S (2015). Myeloid-derived suppressor cells contribute to A2B adenosine receptor-induced VEGF production and angiogenesis in a mouse melanoma model. Oncotarget 6, 27478–27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratlin J, Sangha R, Glubrecht D, Dabbagh L, Young JD, Dumontet C, … Mackey JR (2004). The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine-treated pancreas adenocarcinoma. Clinical Cancer Research 10, 6956–6961. [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Bhardwaj A, Singh S, Arora S, Wang B, Grizzle WE, & Singh AP (2011). MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis 32, 1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stief SM, Hanneforth AL, Weser S, Mattes R, Carlet M, Liu WH, … Spiekermann K (2020). Loss of KDM6A confers drug resistance in acute myeloid leukemia. Leukemia 34, 50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell J, Jakova E, & Cayabyab FS (2017). Adenosine A1 and A2A receptors in the brain: Current research and their role in neurodegeneration. Molecules 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollenwerk TM, Pollock S, & Hillard CJ (2021). Contribution of the adenosine 2A receptor to behavioral effects of tetrahydrocannabinol, cannabidiol and PECS-101. Molecules 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenaga M, Schirripa M, Cao S, Zhang W, Yang D, Dadduzio V, … Lenz HJ (2017). Potential role of polymorphisms in the transporter genes ENT1 and MATE1/OCT2 in predicting TAS-102 efficacy and toxicity in patients with refractory metastatic colorectal cancer. European Journal of Cancer 86, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Pillai K, & Morris DL (2017). Mucins in pancreatic cancer: biological role, implications in carcinogenesis and applications in diagnosis and therapy. American Journal of Cancer Research 7, 1372–1383. [PMC free article] [PubMed] [Google Scholar]
- Sun C, Wang B, & Hao S (2022). Adenosine-A2A Receptor Pathway in Cancer Immunotherapy. Frontiers in Immunology 13, Article 837230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram M, Yao SY, Ng AM, Griffiths M, Cass CE, Baldwin SA, & Young JD (1998). Chimeric constructs between human and rat equilibrative nucleoside transporters (hENT1 and rENT1) reveal hENT1 structural domains interacting with coronary vasoactive drugs. The Journal of Biological Chemistry 273, 21519–21525. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Kumar V, & Kaul R (2019). Need for alternatives to animals in experimentation: An Indian perspective. The Indian Journal of Medical Research 149, 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkotak AJ, Ng AM, Sawicka J, Baldwin SA, Man SF, Cass CE, … Duszyk M (2001). Regulation of K(+) current in human airway epithelial cells by exogenous and autocrine adenosine. American Journal of Physiology. Cell Physiology 281, C1991–C2002. [DOI] [PubMed] [Google Scholar]
- Takagaki K, Katsuma S, Kaminishi Y, Horio T, Nakagawa S, Tanaka T, Ohgi T, & Yano J (2004). Gene-expression profiling reveals down-regulation of equilibrative nucleoside transporter 1 (ENT1) in Ara-C-resistant CCRF-CEM-derived cells. Journal of Biochemistry 136, 733–740. [DOI] [PubMed] [Google Scholar]
- Tandio D, Vilas G, & Hammond JR (2019). Bidirectional transport of 2-chloroadenosine by equilibrative nucleoside transporter 4 (hENT4): Evidence for allosteric kinetics at acidic pH. Scientific Reports 9, 13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiainen S, Tumelius R, Rilla K, Hamalainen K, Tammi M, Tammi R, … Auvinen P (2015). High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology 66, 873–883. [DOI] [PubMed] [Google Scholar]
- Torres MP, Chakraborty S, Souchek J, & Batra SK (2012). Mucin-based targeted pancreatic cancer therapy. Current Pharmaceutical Design 18, 2472–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V, Muhlbauer B, & Osswald H (2006). Adenosine and kidney function. Physiological Reviews 86, 901–940. [DOI] [PubMed] [Google Scholar]
- Varani K, Merighi S, Gessi S, Klotz KN, Leung E, Baraldi PG, … Borea PA (2000). [(3)H]MRE 3008F20: A novel antagonist radioligand for the pharmacological and biochemical characterization of human A(3) adenosine receptors. Molecular Pharmacology 57, 968–975. [PubMed] [Google Scholar]
- Vigano S, Alatzoglou D, Irving M, Menetrier-Caux C, Caux C, Romero P, & Coukos G (2019). Targeting adenosine in cancer immunotherapy to enhance T-cell function. Frontiers in Immunology 10, 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachodimou A, Konstantinopoulou K, AP IJ, & Heitman LH (2020). Affinity, binding kinetics and functional characterization of draflazine analogues for human equilibrative nucleoside transporter 1 (SLC29A1). Biochemical Pharmacology 172, 113747. [DOI] [PubMed] [Google Scholar]
- Volonte C, & D’Ambrosi N (2009). Membrane compartments and purinergic signalling: The purinome, a complex interplay among ligands, degrading enzymes, receptors and transporters. The FEBS Journal 276, 318–329. [DOI] [PubMed] [Google Scholar]
- Wall MJ, & Dale N (2013). Neuronal transporter and astrocytic ATP exocytosis underlie activity-dependent adenosine release in the hippocampus. The Journal of Physiology 591, 3853–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Walt MM, & Terre’Blanche G (2018). Benzopyrone represents a privilege scaffold to identify novel adenosine A1/A2A receptor antagonists. Bioorganic Chemistry 77, 136–143. [DOI] [PubMed] [Google Scholar]
- Wang C, Lin W, Playa H, Sun S, Cameron K, & Buolamwini JK (2013). Dipyridamole analogs as pharmacological inhibitors of equilibrative nucleoside transporters. Identification of novel potent and selective inhibitors of the adenosine transporter function of human equilibrative nucleoside transporter 4 (hENT4). Biochemical Pharmacology 86, 1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Word BR, & Lyn-Cook BD (2011). Enhanced efficacy of gemcitabine by indole-3-carbinol in pancreatic cell lines: The role of human equilibrative nucleoside transporter 1. Anticancer Research 31, 3171–3180. [PubMed] [Google Scholar]
- Wang WW, Gallo L, Jadhav A, Hawkins R, & Parker CG (2020). The druggability of solute carriers. Journal of Medicinal Chemistry 63, 3834–3867. [DOI] [PubMed] [Google Scholar]
- Wang Y, Martins I, Ma Y, Kepp O, Galluzzi L, & Kroemer G (2013). Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 9, 1624–1625. [DOI] [PubMed] [Google Scholar]
- Wang Y, Peng H, Guo Z, Ullman BR, Yamamoto K, Hong SY, & Liu JO (2021). Influence of stereochemistry on the activity of rapadocin, an isoform-specific inhibitor of the nucleoside transporter ENT1. Chemical Science 12, 11484–11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JL, Sherali A, Mo ZP, & Tse CM (2000). Kinetic and pharmacological properties of cloned human equilibrative nucleoside transporters, ENT1 and ENT2, stably expressed in nucleoside transporter-deficient PK15 cells. Ent2 exhibits a low affinity for guanosine and cytidine but a high affinity for inosine. The Journal of Biological Chemistry 275, 8375–8381. [DOI] [PubMed] [Google Scholar]
- Weadick B, Nayak D, Persaud AK, Hung SW, Raj R, Campbell MJ, … Govindarajan R (2021). EMT-induced gemcitabine resistance in pancreatic cancer involves the functional loss of equilibrative nucleoside transporter 1. Molecular Cancer Therapeutics 20, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CW, Lee CY, Lee DJ, Chu CF, Wang JC, Wang TC, … Hsu CL (2018). Equilibrative nucleoside transporter 3 regulates T cell homeostasis by coordinating lysosomal function with nucleoside availability. Cell Reports 23, 2330–2341. [DOI] [PubMed] [Google Scholar]
- Wong GY, Bradlow L, Sepkovic D, Mehl S, Mailman J, & Osborne MP (1997). Doseranging study of indole-3-carbinol for breast cancer prevention. Journal of Cellular Biochemistry. Supplement 28–29, 111–116. [DOI] [PubMed] [Google Scholar]
- Wonganan P, Lansakara PD, Zhu S, Holzer M, Sandoval MA, Warthaka M, & Cui Z (2013). Just getting into cells is not enough: mechanisms underlying 4-(N)-stearoyl gemcitabine solid lipid nanoparticle’s ability to overcome gemcitabine resistance caused by RRM1 overexpression. Journal of Controlled Release 169, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodahl EL, Wang J, Heimfeld S, Sandmaier BM, & McCune JS (2009). Intracellular disposition of fludarabine triphosphate in human natural killer cells. Cancer Chemotherapy and Pharmacology 63, 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Liu N, Jacobson KA, Gavrilova O, & Reitman ML (2019). Physiology and effects of nucleosides in mice lacking all four adenosine receptors. PLoS Biology 17, e3000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, … Stevens RC (2011). Structure of an agonist-bound human A2A adenosine receptor. Science 332, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, & Meng H (2016). Paclitaxel-loaded stealth liposomes: Development, characterization, pharmacokinetics, and biodistribution. Artificial Cells, Nanomedicine, and Biotechnology 44, 350–355. [DOI] [PubMed] [Google Scholar]
- Yang C, & Leung GP (2015). Equilibrative nucleoside transporters 1 and 4: which one is a better target for cardioprotection against ischemia-reperfusion injury? Journal of Cardiovascular Pharmacology 65, 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG (2008). Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochimica et Biophysica Acta 1783, 673–694. [DOI] [PubMed] [Google Scholar]
- Yin J, Xu K, Zhang J, Kumar A, & Yu FS (2007). Wound-induced ATP release and EGF receptor activation in epithelial cells. Journal of Cell Science 120, 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MH, Bae HB, & Choi JI (2005). Antinociception of intrathecal adenosine receptor subtype agonists in rat formalin test. Anesthesia and Analgesia 101, 1417–1421. [DOI] [PubMed] [Google Scholar]
- Young A, Ngiow SF, Gao Y, Patch AM, Barkauskas DS, Messaoudene M, … Smyth MJ (2018). A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Research 78, 1003–1016. [DOI] [PubMed] [Google Scholar]
- Young JD, Yao SY, Baldwin JM, Cass CE, & Baldwin SA (2013). The human concentrative and equilibrative nucleoside transporter families, SLC28 and SLC29. Molecular Aspects of Medicine 34, 529–547. [DOI] [PubMed] [Google Scholar]
- Young JD, Yao SY, Sun L, Cass CE, & Baldwin SA (2008). Human equilibrative nucleoside transporter (ENT) family of nucleoside and nucleobase transporter proteins. Xenobiotica 38, 995–1021. [DOI] [PubMed] [Google Scholar]
- Yu F, Zhu C, Xie Q, & Wang Y (2020). Adenosine A2A receptor antagonists for cancer immunotherapy. Journal of Medicinal Chemistry 63, 12196–12212. [DOI] [PubMed] [Google Scholar]
- Yun Y, Chen J, Liu R, Chen W, Liu C, Wang R, Hou Z, Yu Z, Sun Y, & AP IJ, Heitman LH, Yin X, & Guo D (2019). Long residence time adenosine A1 receptor agonists produce sustained wash-resistant antilipolytic effect in rat adipocytes. Biochemical Pharmacology 164, 45–52. [DOI] [PubMed] [Google Scholar]
- Zamzow CR, Bose R, & Parkinson FE (2009). N-methyl-D-aspartate-evoked adenosine and inosine release from neurons requires extracellular calcium. Canadian Journal of Physiology and Pharmacology 87, 850–858. [DOI] [PubMed] [Google Scholar]
- Zheng HC (2017). The molecular mechanisms of chemoresistance in cancers. Oncotarget 8, 59950–59964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, … Kalluri R (2015). Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Li C, Olah ME, Johnson RA, Stiles GL, & Civelli O (1992). Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proceedings of the National Academy of Sciences of the United States of America 89, 7432–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu TF, Budin I, & Szostak JW (2013). Preparation of fatty acid micelles. Methods in Enzymology 533, 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman EI, Huang M, Leisewitz AV, Wang Y, Yang J, & Graves LM (2009). Identification of a novel point mutation in ENT1 that confers resistance to Ara-C in human T cell leukemia CCRF-CEM cells. FEBS Letters 583, 425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H (2008). ATP and acetylcholine, equal brethren. Neurochemistry International 52, 634–648. [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Fickert P, Silbert D, Fuchsbichler A, Zatloukal K, Denk H, & Trauner M (2005). Hepatobiliary transporter expression in human hepatocellular carcinoma. Liver International 25, 367–379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


