Abstract
Our goal in this manuscript is to advance the assessment and treatment of monkey species in neuroscience research. We hope to begin a discussion and establish baseline data on how complications are identified and treated. We surveyed the neuroscience research community working with monkeys and compiled responses to questions about investigator demographics, assessment of animal wellbeing, treatment choices, and approaches to mitigate risks associated with CNS procedures and promote monkey health and wellbeing. The majority of the respondents had worked with nonhuman primates (NHP) for over 15 y. Identification of procedure-related complications and efficacy of treatment generally rely on common behavioral indices. Treatments for localized inflammatory responses are generally successful, whereas the treatment success for meningitis or meningoencephalitis, abscesses, and hemorrhagic stroke are less successful. Behavioral signs of pain are treated successfully with NSAIDs and opioids. Our future plans are to collate treatment protocols and develop best practices that can be shared across the neuroscience community to improve treatment success rates and animal welfare and therefore science. Human protocols can be used to develop best practices, assess outcomes, and promote further refinements in treatment practices for monkeys to enhance research outcomes.
Abbreviation: LIR, localized inflammatory response
Introduction
Advances in neuroscience depend on computer, human, and animal models to understand, explain, and predict biologic and behavioral processes. The use of animals allows neuroscientists to test hypotheses and conduct research that has applications in clinical human and veterinary medicine. Monkey genera and species comprise a critical minority of all the biomedical research performed with animals. While nonhuman primates (NHP) represent only 0.09% of the animals used in research in the United States and 0.08% of those used in the European Union, the resulting work constitutes approximately 19% of published neuroscience studies.6,12,58 NHP are critical to biomedical and behavioral research, given that they are close relatives to humans with regard to biology, visuomotor, social and cognitive capabilities, and brain organization.4,17,31
The aging human population and the position of age as the highest risk factor for many neurodegenerative diseases30,47,52 mandate the continued need for neuroscience research with NHP. Many neuroscience experiments involve surgery, such as installation of cranial hardware and recording electrodes allowing access to and monitoring of brain activity. These invasive procedures are similar to those performed in humans and are performed under general anesthesia, using aseptic technique with appropriate perioperative medication, including analgesia and antibiotics as required by IACUC in the United States or equivalent national or local authorities elsewhere. Trained neuroscientists and veterinarians execute these procedures, and skilled researchers, veterinarians, and technicians provide pre-, peri-, and postoperative care. Nevertheless, neurosurgical procedures with all animals including humans, carry risks, including infection, seizures, or stroke. NHP used in such studies must be monitored carefully for adverse effects, with interventions implemented rapidly to ensure their wellbeing and the success of these vital experiments.
Developing a set of best practices will enhance the ability of the neuroscience community to care for NHP involved in research. By analyzing standard procedures, we aim to identify effective diagnostic guidelines and treatment strategies and to highlight areas that need additional research. However, several barriers impede accurate neurologic and pain assessment in research settings and the prevention of pain, including 1) a lack of knowledge regarding pain management techniques for NHP compared with other animals, such as dogs, cats, and rodents;10,27,53 2) the belief that prey animals mask pain, despite sparse evidence for this belief;5 and 3) the subjective nature of pain identification, given that different species have different responses to pain and personnel require training to recognize symptoms that indicate pain in animal.5, Our goal here is to summarize current protocols used for the identification, treatment, and management of NHP in neuroscience research and to stimulate further research on pain mitigation strategies.
We surveyed researchers and veterinarians who evaluate and maintain NHP welfare. Surveys are imperfect but useful tools for identifying trends in practices. Previous surveys on animal welfare assess pain documentation methods in the United Kingdom and gauge the availability of NHP for research in North America.21,28 Our survey builds on these efforts by examining pain management and welfare of NHP used in research. We hope to generate a discussion of effective treatments for neurologic illness to improve care for these NHP. This survey is focused on current practices. We hope that our data will help investigators and veterinarians work toward standardizing the most effective treatments and developing evidence-based practices for the assessment and optimal care of NHP in neuroscience research.
Materials and Methods
We assembled responses detailing how neuroscientists and veterinarians around the world evaluate and treat clinical cases of neurologic illnesses and identify pain in NHP. The demographic survey provided an overview of the staff working with NHP, the NHP themselves, and how NHP are used in research. The data regarding the prevalence of neurologic clinical cases included overviews of the protocols used to identify and treat clinical cases. The pain assessment survey addressed the methods used to identify pain and determine efficacy of pain management approaches. Because our study is the first to consider the demographics of neuroscience research specifically with NHP, we do not have any comparative data to determine whether we have obtained a representative sample.
Our 3 surveys were developed by using Google Forms and were analyzed by using Google Sheets via Google Apps. All surveys were anonymous, and email addresses or other personal or institutionally identifying information were not collected from respondents. All surveys included a mixture of yes–no, multiple-choice single-answer, multiple-choice multiple-answer, and open-ended questions. Respondents were not required to fill out the surveys in order. Answers were not required for any of the questions. After development, survey questions were refined by all coauthors, and then distributed to potential respondents.
In the first survey, Nonhuman Primates (NHP) Questionnaire Part I (Supplemental Figure 1), we asked about careers in research and work with NHP, the number of NHP used in a single study, brain regions studied, the ratio of males to females, and the types of housing. The demographic survey contained 22 questions: 2 yes–no questions, 8 multiple-choice single-answer questions, 6 multiple-choice multiple-answer questions, and 6 open-ended questions. Two of the multiple choice single-answer questions and 4 of the multiple-choice multiple-answer questions allowed respondents to write in their answers.
The second survey, Nonhuman Primates (NHP) Questionnaire Part II (Supplemental Figure 2), assessed neurologic clinical cases, including identification, treatment, and outcomes. This survey had 22 questions in total: 2 yes–no questions, 8 multiple-choice single-answer questions, 6 multiple-choice multiple-answer questions, and 6 open-ended questions. Six of the multiple-choice multiple-answer questions allowed respondents to write in their answer.
The third survey, Nonhuman Primates (NHP) Questionnaire Part III (Supplemental Figure 3), assessed clinical signs of pain, medications used for treatment, and assessment of treatment efficacy. This survey also included questions on anesthesia induction protocols for juvenile and geriatric NHP. Survey III had 24 questions: 3 yes–no questions, 5 multiple-choice multiple-answer questions, and 16 open-ended questions. One of the multiple-choice multiple-answer questions allowed respondents to write in their answers.
A link to the surveys was distributed anonymously to 550 people in at least 17 countries in North America, Europe, and Asia who were known to participate in neuroscience research with NHP. Recipients of the survey were asked to forward the survey to anyone they thought might be interested, such that the overall return rate may underestimate the actual return rate. The response rates for the 3 separate surveys were calculated by using the initial distribution list of 550 people. The study was reviewed by the University of California–Los Angeles Chancellor’s Animal Research Committee (IACUC) and the Institutional Review Board for human subjects; these committees determined that because neither personal information nor animal use was involved, formal review was unnecessary. The first survey was sent 2 wk before the second and third surveys, which were sent together. Follow-up reminders were sent 2 wk after the second two surveys were sent out. All surveys were sent in August 2021, and data collection was closed on October 3, 2021.
Results
Demographic survey.
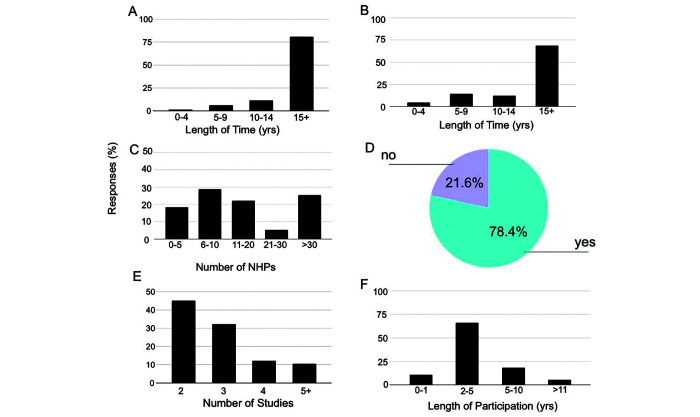
The purpose of this survey was to gain a baseline understanding of the demographics of those working with NHP in neuroscience research and how NHP are used. In total, 155 respondents replied to the demographic survey (28%). The data appear in Figure 1, which indicates the length of time that researchers have been involved with NHP, the number of studies in which NHP are involved, and the average length of time that individual NHPs are assigned to studies .
Figure 1.
Average use of non-human primates (NHP) in neuroscientific research. (a) Length of time (in years) that the survey respondents have been involved in research. (b) Frequency histogram indicating the length of time respondents have been conducting research on or working with NHP. (c) Frequency histogram showing the number of NHPsNHP that respondents have worked with in the last five years. (d) Percentage of respondents that use NHP in multiple studies. (e) Average number (x-axis) of studies for each NHP. (f) Frequency histogram showing the length of time (in years) that NHP remain in study.
Eighty-one percent (81%) of the respondents had been working in research for over 15 y, 11% for 10 to 14 y, and 7% for less than 10 y (Figure 1A). Sixty-nine percent of respondents had worked with NHP for over 15 y (69%), 12% for 10 to 14 y, and 19% for less than 10 y (Figure 1 B).
During the past 5 y, 25% of respondents worked with over 30 NHP, 29% with 6 to 10 NHP and 5% with 21 to 30 NHP. 78% of respondents used NHP in multiple studies. For NHP that were used in multiple studies, 45% in 2 studies, and 11% in over 5 studies we used. In addition, 66% of respondents indicated that NHP spent approximately 2 to 5 y participating in any one study, whereas only 5% indicated that NHP spent more than 11 y participating in any one study (Figure 1 C through F).
Figure 2 shows the species, age range, placement, and type of housing of NHP. Fifty-eight percent of respondents worked with rhesus macaques (Macaca mulatta), and 18% with cynomolgus macaques (Macaca fascicularis). Other species included common marmosets (Callithrix jacchus, 11%), pigtailed macaques (Macaca nemestrina, 4%), common squirrel monkeys (Saimiri sciureus, 3%); primates of the Chlorocebus species (e.g., African green monkeys, 3%), baboons (Papio spp., 2%), capuchins (Cebus spp., 1%), bonnet macaques (Macaca radiata, 1%); and white-eared titis (Plecturocebus donacophilus, <1%). Animal ages ranged from younger than 5 y to older than 20 y, with the greatest number (36%) between the ages of 6 and 10 y (Figure 2 A and B).
Figure 2.
Demographics of non-human primates (NHP) used in neuroscientific research. (a) Frequency of monkey species. (b) Frequency of age in years (yrs). (c) Frequency of housing type (indoor, outdoor, or both). (d) Frequency of pair and social housing for all housing types.
Three major types of housing configurations were used: fully indoors, indoors with outdoor access, and fully outdoors. Of these types, 84% of respondents indicated that the NHP were housed fully indoors, whereas 11% and 6% of respondents indicated that NHP were housed indoors with outdoor access and outdoors, respectively. Regardless of housing, 46% of responses indicated that NHP were pair housed, 28% were singly housed units, 18% were group-housed, and 8% were housed in family units (relevant for marmosets; Figure 2 C and D).
We next addressed the methods used during neuroscience experiments of respondents. Surface cerebral cortical regions were most studied (38%), with deep cerebral cortical regions next (33%), followed by subcortical regions (e.g., amygdala, basal ganglia, thalamus; 26%). The most studied brain region was the frontal lobe (24%), and the least studied was the cerebellum (6%). Four percent of respondents answered ‘not applicable,’ indicating that they did not study cerebral cortical regions or brain regions specified in our survey (Figure 3 A and B). Most of the respondents (73%) used both stereotaxic-guided and MRI-guided techniques for neurosurgical procedures. Twelve percent of respondents used only stereotaxic-guided techniques whereas 6% only used MRI-guided techniques. The 10% of respondents who used neither technique conducted primarily behavioral or cognitive research (Figure 3 C).
Figure 3.
Brain regions studied in non-human primates (NHP) and prominent use of guidance techniques for targeting and recording (a) Proportion of experiments performed in cortical and subcortical brain areas. N/A = did not answer or specify brain area. (b) Frontal cortex is a target of a majority of respondents’ NHP neuroscientific experiments. (c) Most experiments rely on MRI and stereotaxic guidance. (d) The majority of experiments use single and array electrodes. The frequency histogram of responses for each method is shown. V, S Probes refers to electrodes with recording ability. DREADDs stands for Designer Receptors Exclusively Activated by Designer Drugs.
One of the fundamental goals of neuroscience is to understand and map neural connections and brain activity that allow for cognition and action.38 To do this requires the use of various recording and mapping instruments. The most used methods reported include single electrodes (29%), electrode bundles and arrays (24%), multicontact recording probes – V,S probes (20%), injectrodes (10%), optrodes (8%), and neuropixels (6%).Responses indicated that cannulas for injection, Hamilton syringes, functional fMRI and MRI, EEG electrodes, ultrasonography, designer receptors exclusively activated by designer drugs (DREADDs), and 2-photon calcium imaging were used less frequently (Figure 3 D).
Neurologic clinical cases survey.
Our second survey assessed neurologic clinical cases. This survey had a return rate of 6%, calculated from the total number of surveys sent and thus had a lower number of responses that did the first survey (28%). Assuming that the responses came only from those individuals who had responded to the first survey, then the response rate was 17%. Our goal was to determine not only which areas of the brain are studied but also what common conditions arise in particular models so that we can then develop an evidenced-based set of best practices based on the identification of complications and assessment of the most effective treatments. For our purposes, treatment success was defined as the NHP being healthy enough (as determined by a veterinarian) to return to study.
First, we surveyed the number of respondents who reported the identification of neurologic illnesses or traumas within the last 10 y. Bacterial colonization around or within an implant (i.e., local inflammatory response, LIR) occurred most frequently (reported by 33% of respondents). Other reported illnesses included cerebral edema (16%), stroke (13%), parenchymal infection (12%), meningitis or meningoencephalitis (4%), and seizure (4%); 7% of respondents indicated a zero incidence of neurologic health conditions during the last 10 y. The number of clinical cases in the cerebral cortical regions (2.2 ± 3.2) did not differ significantly from that reported in deep, subcortical regions (1.3 ± 1.6; mean difference = 0.09, P > 0.05; Figure 4).
Figure 4.

Neurological trauma and illness is rare in neuroscientific experiments. (a) The 10 year average frequency of occurrence of each event identified on the x axis is plotted on the y axis. (b) The frequency of neurological traumas or illness for cerebral cortical experiments. (c) Same as in (b) for subcortical and deep cortical experiments. The difference in average number of clinical cases of cerebral cortical and deep, subcortical regions was not significant (mean difference = 0.9, p > 0.05).
Consistent with a commitment to 3Rs ethical principles—to replace animal models whenever possible, to reduce numbers whenever possible, and to refine procedures—the use of custom-designed cranial implants is increasing in frequency in the neuroscience community.44 Supporting this trend, 69% of respondents used custom-designed cranial implants, 28% did not, and 3% responded ‘not applicable,’ implying no use of implants. In addition, 39% of respondents had the impression that custom implants reduced the incidence of LIR compared with implants that were not customized (Figure 5).
Figure 5.

The majority of scientists use custom implants. (a) Percentage of respondents using custom-designed cranial implants. (b) Impression that custom implants are beneficial over ready-made implants.
The 3 most used methods to assess LIR were the appearance of purulent exudate (31%), identification of an odor (28%), and culture results (26%). Other methods of identification reported include changes in behavior (for example, decreased appetite, 3%), routine assessments (1%), and redness of tissue (1%); 7% replied ‘not applicable,’ suggesting that either respondents saw no clinical cases of LIR or that they did not report methods of identification.
The most common treatments of LIR reported include an increase of routine cleaning frequency (28%), use of a topical antibiotic (27%), and use of a systemic antibiotic (24%), the latter depending on culture results. Another treatment was removal of the implant (21%). Over 75% of respondents reported a treatment success rate of over 75%. However, 33% and 3% of respondents respectively reported having to remove 1 to 5 or 6 to 10 subjects from studies (Figure 6).
Figure 6.
Localized Inflammatory Responses (LIR) are common and successfully treatable. (a) Frequency histogram of different methods of identification of implant issues (CR = culture result; RA = routine assessment). N/A = did not answer or specify method of identification. (b) Frequency histogram of treatment type used for LIRs. Antibiotic treatments are all based on culture results. (c) Success rate of LIR treatment. (d) Implant issues infrequently require removal from study.
Respondents used at least 1 of 8 methods to identify meningitis, brain abscesses, and hemorrhagic stroke (Figure 7 A). These methods reported include MRI (39%), blood analysis (16%), clinical signs (6%), and CSF analysis (4%). T1, T2, and diffusion MRI were used to determine both presence and type of parenchymal lesion.1,29 Clinical signs ranged from changes in the normal appearance of the dura, suggesting a localized issue, to lateralizing signs, suggesting a larger, perhaps more serious issue. Other identification methods reported include X-rays, behavioral indicators, opisthotonos, experimental history, and necropsy findings. Thirty percent of respondents indicated no known incidents or did not report their methods of identification (Figure 7 A).
Figure 7.
Identifying and treating infections of the cerebrospinal fluid (CSF) space or meninges and parenchymal lesions. (a) Methods used to identify clinical cases of infections of the CSF space or meninges and type of parenchymal lesion, such as abscess, cyst, ischemic or hemorrhagic stroke. N/A = did not answer or specify method of identification. N/A = did not answer or specify method of identification. (b) Frequency of treatments used for clinical cases of infections of CSF space or meninges. (c) Success rate of respondents who treated infections of the CSF space or meninges, wherein, the animal continued in experimental study. (d) Frequency of the number of NHP removed from a study due to infection of the CSF space or meninges.
Treatment methods for infection of CSF space or meninges included medical (50%) and surgical (43%) options and euthanasia when animal welfare could no longer be guaranteed (7%). 86% of respondents reported success rates of less than 75% for medical and surgical interventions, whereas 14% of respondents reported success rates exceeding 75%. 40% of respondents indicated that 1 to 5 subjects had to be removed from study, whereas 60% indicated that no subjects were removed from study (Figure 7 B through D). Therefore, medical and surgical interventions used for infection of CSF space or meningitis appear to have limited success. Developing evidence-based best practices that can be shared across the neuroscience community could improve success rates and animal welfare and is an important area for further effort.
Thirty-eight percent of respondents indicated that they treated cerebral cortical or deep brain abscesses medically and surgically, whereas 12.5% used only surgical intervention. Medical interventions included antibiotic therapy and steroid treatment. Surgical interventions reported include subdural aspiration and increased frequency of cleaning the area. In addition, 38% of respondents indicated that brain abscesses were typically fatal or that the animals were euthanized; 13% of respondents did not report their treatment, if any. Regarding localized brain abscess, 36% of respondents reported a success rate greater than 75%, 18% had a success rate of 21% to 50%, 9% had a success rate of 11% to 20%, and 36% had a success rate of less than 10%. Only 29% of respondents indicated that subjects had to be removed from study (Figure 8). Thus, treatment of deep brain abscess appears to have a higher success rate than treatment of infection of the CSF space or meninges. A next step would be to collate the specific treatment protocols and develop best practices that can be shared across the neuroscience community to improve treatment success and animal welfare. Human treatment practices can be used to develop and assess best practices for monkeys.
Figure 8.

Treatment of cerebral cortical or deep brain abscesses for those that indicated encounters of such clinical cases. (a) Frequency histogram of treatments used for clinical cases of cerebral cortical or deep brain abscesses. (b) Success rate of respondents who treated cerebral cortical or deep brain abscesses, as in the animal continued in experimental study. (c) Frequency histogram of the number of NHP removed from a study due to cerebral cortical or deep brain abscesses.
Similar to reports for humans,61,63 40% of respondents observed and monitored resolution of hemorrhagic strokes without intervention, and 20% intervened surgically after presentation of injury. Fifty-seven percent of respondents had a success rate that exceeded 75%, whereas 43% had a success rate of less than 10%. The majority (83%) did not have to remove subjects from study (Figure 9). Although the success rate for treatment of hemorrhagic stroke is already relatively high, developing best practices that can be shared across the neuroscience community would nonetheless benefit both the NHP and neuroscience research.
Figure 9.

Treatment of hemorrhagic stroke for those that indicated encounters of such clinical cases. (a) Frequency histogram of treatments used for clinical cases of hemorrhagic stroke. (b) Success rate of respondents who treated hemorrhagic stroke, as in the animal continued in experimental study. (c) Frequency of the number of NHP removed from a study due to hemorrhagic stroke.
Pain and welfare assessment survey.
Because the third survey had a return rate of 5% and with the assumption that responses came only from people who responded to the first survey, responses to the third survey could range between 5% (out of the number of individuals that received the first survey) and 16% (out of the total number of respondents to the first survey). Pain indicators could be classified into three major categories: behavioral observations (70%), clinical assessments (20%), and stereotypies (10% Table 1, Figure 10 A). Behavioral observations, such as personality changes and vocalizations, and stereotypies are sometimes difficult to measure and require understanding of the animal’s normal behavior because changes can be subtle. Clinical assessments such as blood chemistry are measurable but may not be sufficiently sensitive.51 Standardization of assessments and treatment options would help to improve animal welfare.
Table 1.
The specific indicators or signs that fall under the 3 major classifications—behavioral observation, clinical assessment, and stereotypy—used to identify generalized or localized pain
| Classification | Specific indicators or signs |
|---|---|
| Behavioral observation | Personality changes, aggression, changes to interest in enrichment, changes in lab responses or task completion, interactions with handler, vocalization, facial expression, appetite, grooming behavior, favoring limb, head holding, posture, huddling, sitting, changes in alertness or activity |
| Clinical assessment | Wound sites, quality of coat or skin, weight changes, changes to urine or stool, physiologic changes, vomiting or diarrhea, seizures, eyes, nose |
| Stereotypy | Stereotyped behavior, locomotor behavior, repetitive behaviors |
Figure 10.
Identification and treatment of pain and assessment of efficacy. (a) Indicators or signs used to infer general issues of well-being, e.g. pain, illness, discomfort, etc. (b) Indicators or signs used to infer central nervous system (CNS)-related pain (BP = blood pressure; Oph. = ophthalmic). N/A = did not specify clinical signs for CNS-related pain. (c) Frequency histogram of medications used to treat generalized or localized pain. (d) Frequency histogram of medications used to treat CNS-related pain. N/A = did not specify clinical signs for CNS-related pain. (e) Indicators or signs used to assess efficacy of CNS-related pain medication. N/A = did not specify clinical signs for CNS-related pain.
Lethargy and vomiting were the 2 clinical signs of CNS-related illness with the most responses (16% for both). Head holding (15%), changes in appetite (9%), and signs of discomfort (e.g., wincing, squinting, grimacing; 6%) were the next most common. The results of the pain survey appear in Figure 10 B.
The majority of respondents reported using NSAIDs and opioids to treat generalized or localized pain (77%) and CNS-related pain (62%). Twelve percent of respondents specified that NSAIDs were the first line of management for pain, and opioids were used if there was no response to NSAID treatment. Combining NSAIDs and opioids can effectively achieve analgesic effects with minimal unwanted effects.10,42 Respondents indicated that NSAIDs were the most prescribed analgesia for generalized or localized pain (42%) and CNS-related pain (33%). Meloxicam was the most commonly used NSAID analgesic, with dosages ranging from 0.1 to 0.2 mg/kg PO. Two other commonly used NSAIDs were carprofen and ketoprofen. Opioids were the second most prescribed analgesic for generalized or localized pain (35%) and CNS-related pain (28%). The most prescribed opioid was buprenorphine, with dosages ranging from 0.005 to 0.1 mg/kg IM. Buprenorphine is used for analgesia after craniotomies, intracerebral injections, and placement of electrodes.10
Because buprenorphine has a longer duration of effect than other opioids and less potential for unwanted effects, it is used more frequently in research animal medicine than are other similar opioids.10,39 After neurosurgery in humans, the use of opioids is limited due to side effects, such as nausea, vomiting, and increased intracranial pressure.57 Antidiarrheal and antiemetic medications are also used to manage similar side effects in NHP (Figure 10 C and D), including gastrointestinal complications due to the use of NSAID. Twenty-three percent of respondents stated that veterinarians determined appropriate treatments and dosages (Table 2).
Table 2.
Medications used to treat pain and their associated dosages
| Medication | Dosage |
|---|---|
| NSAID | |
| Meloxicam | 0.1–0.2 mg/kg (IM or PO) |
| Carprofen | 2–5 mg/kg (PO, BID) |
| Ketoprofen | 2–5 mg/kg [IM, SID or BID) |
| Ibuprofen | 7–10 mg/kg (PO, BID) |
| Tolfenamic Acid | 4 mg/kg |
| Steroids | |
| Dexamethasone | 0.05–4 mg/kg (IM) |
| Prednisone | None specified |
| Opioids | |
| Buprenorphine | 0.005–0.03 mg/kg (IM, BID) |
| Buprenorphine, sustained release | 0.12 mg/kg |
| Tilidine (with naloxone) | 0.8–0.13 mg/kg |
| Hydromorphone | None specified |
| Butorphanol | None specified |
| Analgesics | |
| Lidocaine | 10–40 mg/kg |
| Ketamine | None specified |
| Gabapentin | None specified |
| Cerenia | None specified |
| Amantadine | None specified |
| Selective serotonin reuptake inhibitors (SSRI) | None specified |
| Serotonin and norepinephrine reuptake inhibitors (SNRI) | None specified |
| Bupivacaine | None specified |
| Ropivacaine | None specified |
| Dexmedetomidine | None specified |
BID, twice daily
IM, intramuscular
PO, by mouth
SID, once daily
Respondents did not specify dosage for some medications
Many respondents reported that any shift back to the animal’s typical behavioral state was considered to indicate pain reduction. Improved behavior and mood and recovery of appetite were considered to be the best predictors that the treatment was efficacious and the animal’s pain had been mitigated (Figure 10 E).
Discussion
Research using NHP allows scientists to study brain function and develop clinical therapies for brain disorders, and these animals are an essential resource in neuroscience research.35 To improve their welfare and quality of life, current treatments for their neurologic illnesses and pain must be evaluated for effectiveness. By surveying the neuroscience research community, we received an aggregate of responses regarding assessment of and treatment protocols for NHP. Many researchers working with NHP have lengthy experience with these animals. This experience must at least in part underlie high success rates for treatment of neurologic illnesses and substantial retention of animals on study protocols. Evaluations of pain include objective and subjective measures that make accurate diagnosis and quick intervention possible. Although response rates were low, the surveys captured assessments of current treatment methods for pain and neurologic illnesses used by the research community. Overall, this report provides a baseline understanding of assessment and treatment of NPH used for neuroscience research with NHP. These observations and interventions can later be evaluated for effectiveness toward the long-term goal of improving animal wellbeing.
The demographic data showed that a large proportion of researchers had been working with NHP for more than 15 y. A small percentage of researchers had been working with NHP for less than 4 y—a possible signal of fewer researchers starting work with NHP or a reluctance to share data. Although we cannot confirm that our sample is representative of neuroscientists working with NHP, we can at least assess what many established researchers are doing.
NHP are among the most expensive and complex of research animals.31 Rhesus and cynomolgus macaques and common marmosets are most cost-effective with regard to breeding and housing.28 The type of research study and expected lifespan influence the repeated use of NHP in multiple studies.31 Macaques can be used for brain aging studies, and advancements in transgenic techniques in common marmosets are providing new models for the study of neurodegenerative diseases.25 The median lifespan of rhesus macaques is approximately 29 y, whereas common marmosets are elderly at 8 y. If their health is maintained, and with appropriate regulatory approval, these species can be used in multiple experiments over time.31,33 The current shortage of rhesus macaques makes the development of alternative NHP models imperative.28 The issue of reuse is an important topic due to the ethical principle of reduction in the 3Rs.43
Prioritizing the social needs of NHP is essential to support and maintain their health and wellbeing. Accommodation of the social needs of NHP through their housing environments can also have a positive impact on the quality of research data.49 Pair-housing NHP requires training and expertise, with knowledge of the species’ social environment in their native habitats and in captivity, nuances of their behavior toward animals of the same and opposite sex, and consideration of the particular requirements of the experimental research design. Marmosets, for example, require a social environment and typically live in pairs or small family groups.31 Macaques also need a social environment. Creating a macaque pair requires familiarization of the 2 animals and evaluation of compatibility.49 For male cynomolgus and rhesus macaques with cranial implants, pair housing provides social enrichment but does not result in more injuries.46 Larger group housing of mature cynomolgus males together can lead to increased inter-animal aggression.20 Ultimately, NHP researchers must prioritize the social nature of their animals when determining the optimal housing configurations.56
A range of guiding and recording techniques is necessary to conduct research in different brain regions. Use of both stereotaxic and MRI guiding has many positive benefits for accurate 3D targeting of brain regions. Recording instruments, such as single electrodes and electrode bundles and arrays, must be high quality and have low potential for tissue damage after insertion.36,55 With optrodes and designer receptors that are activated only by designer drugs, researchers can record activity after direct manipulation. However, these methods are currently not widely used due to a lack of reliable instruments and challenges with implementation and specificity.9,45
Using any NHP species in neuroscience research, like performing neurosurgical procedures on humans,18,63 creates a possibility for neurologic trauma and illness. We focused on incidents resulting from implants, surgeries, or experimental procedures performed for the purpose of neuroscience research. We considered the identification and treatment of 4 main types of neurologic damage: LIR, infection of the CSF space or meninges, deep brain abscess, and hemorrhagic stroke. Clinical cases are more frequent in surface or cerebral cortical areas, given that more research is conducted in these regions (Figure 4 B). Identifying patterns in the presentation and management of neurologic illnesses will improve diagnosis and treatment in NHP.
Individuals who are certified and permitted to perform surgical procedures, implantation procedures, or even routine veterinary procedures must know the basic principles of asepsis, which prevent or reduce the likelihood of infection. Current research shows that the microbial commensal life on and in our bodies is much more extensive than previously understood.2,22,54 Although some organs, like the ocular surface, were previously thought to be sterile,2,22,50,54 we now know that these areas have normal microbial colonization. Balance of the normal microbiome with routine health care is critical for maintaining the wellbeing of any research animal species. Therefore, the historical philosophy of keeping implants and chambers ‘sterile’ is likely a misunderstanding of the homeostasis between normal microbiome and unwanted microbial invaders. Our approach to maintaining the health of implanted animals, using standardized, routine practices of cleaning, asepsis, and treatment, have thus far been successful. Moving forward, successful approaches should be independently evaluated and the best practices reported to the community.
The natural responses of the body to implants and microbial colonization understandably lead to inflammation. Because infection is technically not diagnosed without a positive confirmation by culture or by microscopy, we broadened our terminology surrounding the clinical signs that present in an implanted animal. These signs—for example, exudate, granulation tissue development, fibrosis—are clear indicators of inflammation and are highly localized to specific sites, such as inside chambers or along implant edges. LIR indicate the presence of an inflammatory response ranging from mild, localized inflammation to outright infection. Within that range, standardized approaches of care coupled with routine asepsis and specific treatments can maintain the health of an implanted NHP; evaluating and reporting best practices to the community are critical next steps.
Intracranial implants allow scientists to record brain activity from underlying regions while a subject is either conscious or anesthetized. Colonization around an implant refers to contamination of the tissue around the implant with commensal or pathogenic bacteria and can lead to LIR.23 Prompt treatment can prevent spread to areas such as the meninges or brain parenchyma. Typically, more frequent cleaning and the use of antibiotics can eliminate LIR and preventing severe tissue damage. Implant removal may be necessary if LIR cannot be cleared or has detrimental effects. A step-wise approach can be effective in maintaining craniotomies without using antibiotics for routine care. Although custom implants may improve fit and reduce infection, our data indicate that researchers believe they are no more effective than ready-made implants (Figure 5 B).8 These 2 options would benefit from rigorous assessment and comparison.1
In a study of polyetheretherketone cranial implants in humans, 8% of 66 cases were infected; the primary infectious agent was methicillin-resistant Staphylococcus aureus, MRSA.48 All infected implants were removed due to the cranioplasty site and the infectious agent.48 In contrast, in NHP, custom polyetheretherketone hydroxyapatite-coated implants improved the health of the skin margin and quality of functional MRI images.40 Prophylactic measures lower rates of infection for surgical sites in human patients by reducing the bacterial count and propensity for infection, and can reasonably do the same for NHP.3,7 Data on the incidence of infection in implanted NHP are sparse, leaving us reliant upon human studies, and indicating that studies of NHP are warranted.
Brain abscesses and infections of the CSF space or meninges can occur if LIR progresses over time or presents immediately after neurosurgery.29 MRI can pinpoint infection-related tissue damage for accurate diagnosis, although treatment may precede the scan if MRI is not available.1,29 Typically, such infections are rare in both humans and NHP, likely because of the availability of vaccines and immune system resistance to adverse clinical outcomes.11,19,29,31 In humans, incidences of bacterial meningitis and brain abscesses after neurosurgery were 0.3% and 0.2%, respectively, among 2111 procedures.34 An infection incidence of 3.6% was reported in 528 human subjects within a year after deep brain stimulation surgery on human patients; all were treated successfully with antibiotic therapy.14 Further study in NHP may indicate whether rates in humans are similar to those seen in NHP. Medical management consists of antibiotic therapy,16 whereas surgical methods completely remove the infected area. Because many clinical signs of brain abscess in NHP are nonspecific and varied, in contrast to the situation in humans,29 diagnosing the infections is difficult and is sometimes not accomplished until necropsy.15,29,60 The varied success rates and numbers of NHP removed from study for both illnesses reflect that treatment does not guarantee success.
In humans, hemorrhagic strokes are fatal more often than ischemic strokes, but comparable data on spontaneous strokes in NHP is scarce.41 Observation of symptom progression is necessary to assess severity. Administration of various treatments may be beneficial both and after recovery. Studies on human patients show that minimally invasive surgery is more beneficial than invasive surgery in reducing the hemorrhagic mass and overall mortality.13 Treatment success depends on the location and the amount of damage that may occur due to intervention or natural progression. Therefore, preventative measures and monitoring must be emphasized to decrease prevalence of these clinical cases.
Overall, the best treatments for LIR are more frequent cleaning and use of topical and systemic antibiotics, as indicated by our data on success rates and NHP removal numbers. However, treatments for meningitis and brain abscess are not as widely effective. Rapid progression and delayed detection due to nonspecific clinical signs may affect treatment success.29 Observation is used to monitor the course of a hemorrhagic stroke, and surgical intervention can be successful, as indicated in our data by the success rates and numbers of NHP removed from study. Meningitis, brain abscess, and hemorrhagic stroke are all relatively rare, so few NHP are negatively affected.19,29
Finally, assessment of pain is a widespread concern in veterinary medicine, in part due to the lack of standardization. In human medicine, most patients are verbal and can respond to questions; verbal responses are not available to veterinarians. Medical specialties, such as neonatal medicine, have developed standardized assessments of their nonverbal or preverbal patients. Using this approach in NHP requires the input of researchers.
All individuals who assess general pain or distress must have a detailed knowledge of the animal’s normal behavior in order to accurately evaluate potential clinical signs.37,56 Behavioral changes and physiologic indicators (i.e., blood pressure, heart rate) are both common clinical signs. NSAID and opioids are the most prescribed treatments for pain; however, for humans, acetaminophen is used more commonly than opioids, partly due to the risk of addiction in humans.24 For NHP, combinations of different classes of analgesics are effective and are commonly prescribed after neurosurgery.10,42 Other medications, such as antibiotics and gastric protectants, are used during perioperative care to prevent infection and protect against the unwanted adverse effects of analgesics, respectively.42 Rigorous assessments of efficacy will provide important data toward ensuring mitigation of pain in NHP.
CNS-related pain is expressed both behaviorally and physiologically. Knowing the cause of pain is vital to treating it in a timely manner. After neurosurgery in human patients, pain was greatest during the first 24 h and in posterior regions due to greater muscle reflection.57,59 Attention to the surgical site and routine administration of analgesics may improve pain management of NHP after surgery. In humans, opioids can cause multiple adverse effects and the trend is toward using nonopioid or multimodal analgesia in humans.26 Evaluation of such trends could help veterinarians to determine effective analgesic protocols after neurosurgery. Identifying clinical signs and anticipating potential for pain will improve welfare. However, treatments directed at reducing pain and minimizing distress are not always successful. Euthanasia is essential when the animal is not responding to the prescribed treatment strategy.62 In addition, institutional policy should require that any treatment protocol, whether for a neurologic illness or pain, must be directed by a veterinarian. For both generalized pain and CNS-related pain, recovery from the initial presenting clinical signs is a good indication of pain relief.
We obtained a 28% response rate for our demographic survey. For the other 2 surveys, the response rates were 6% and 5%, respectively, out of the intial distribution list to 550 individuals. However, the response rates for the second and third surveys, when calculated based on the 155 individuals who responded to the first survey, were 17% and 16%, respectively. Thus, our findings are constrained by this low response rate and consequently probably do not fully represent the entire neuroscience research community working with NHP. Although this issue is common in survey studies, several factors may underlie our low return. Potential respondents may hesitate to answer questions about situations with negative outcomes or may lack of experience, given that many neurosurgical procedures must be performed by certified individuals. Another factor that may have contributed to the low return rate may have been the timing of the survey release, such that potential respondents may have missed the second 2 surveys that were released after the first one. In addition, privacy and security concerns may have deterred people from responding.32 Lastly, response bias could be present in that respondents may not have been completely accurate due to concerns about the implications and the effect of the data on NHP research.28 Strategies to improve the response rate moving forward may be to release all surveys at the same time, minimizing the possibility of respondents missing the follow-up surveys. Moreover, respondents would be required to answer the surveys in sequential order to ensure that the responding populations are not distinct. In addition, more reminders may be helpful. Another way to increase response rates may be to send out a single survey including all 3 parts. Although this survey would be longer, it would not require respondents to reply to multiple surveys.
Although the response rates for the second 2 surveys were low, responses to the demographic survey confirm that a large community would benefit from continued research on NHP experimental and veterinary protocols. We want to begin the dialogue to ensure that all animals used in neuroscience research receive the best possible care. Our study has identified current practices used by researchers and veterinarians working with NHP. This information allows us to identify areas requiring further study and begin to evaluate animal care based on effectiveness, with the long-term goal of enhancing animal welfare.
Acknowledgments
AKJ and MAB are supported by NIHEY013692, AG063090, and UF1NS107668, and MAB is also supported by P51OD010425 and U42OD011123 to WaNPRC. ASM is supported by the Wellcome Trust 110157/Z/15/Z.
Supplemental Materials
Nonhuman primates (NHP) questionnaire, part 1.
Nonhuman primates (NHP) questionnaire, part 2.
Nonhuman primates (NHP) questionnaire, part 3.
References
- 1.Basso MA, Frey S, Guerriero KA, Jarraya B, Kastner S, Koyano KW, Leopold DA, Murphy K, Poirier C, Pope W, Silva AC, Tansey G, Uhrig L. 2021. Using noninvasive neuroimaging to enhance the care, well-being, and experimental outcomes of laboratory nonhuman primates (monkeys). Neuroimage 228:117667. 10.1016/j.neuroimage.2020.117667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benakis C, Martin-Gallausiaux C, Trezzi J-P, Melton P, Liesz A, Wilmes P. 2020. The microbiome–gut–brain axis in acute and chronic brain diseases. Curr Opin Neurobiol 61:1–9. 10.1016/j.conb.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia R, Dalton A, Richards M, Hopkins C, Aziz T, Nandi D. 2011. The incidence of deep brain stimulator hardware infection: The effect of change in antibiotic prophylaxis regimen and review of the literature. Br J Neurosurg 25:625–631. 10.3109/02688697.2011.566384. [DOI] [PubMed] [Google Scholar]
- 4.Buffalo EA, Movshon JA, Wurtz RH. 2019. From basic brain research to treating human brain disorders. Proc Natl Acad Sci USA 116:26167–26172. 10.1073/pnas.1919895116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone L. 2020. Do “prey species” hide their pain? J Appl Anim Ethics Res 2:216–236. [Google Scholar]
- 6.Carlsson H-E, Schapiro SJ, Farah I, Hau J. 2004. Use of primates in research: A global overview. Am J Primatol 63:225–237. 10.1002/ajp.20054. [DOI] [PubMed] [Google Scholar]
- 7.Cavanaugh DL, Berry J, Yarboro SR, Dahners LE. 2009. Better prophylaxis against surgical site infection with local as well as systemic antibiotics. An in vivo study. J Bone Joint Surg Am 91:1907–1912. 10.2106/JBJS.G.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Possel JK, Wacongne C, van Ham AF, Klink PC, Roelfsema PR. 2017. 3D printing and modelling of customized implants and surgical guides for nonhuman primates. J Neurosci Methods 286:38–55. 10.1016/j.jneumeth.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark AM, Ingold A, Reiche CF, Cundy DI, Balsor JL, Federer F, McAlinden N, Cheng Y, Rolston JD, Rieth L, et al. 2022. An Optrode Array for Spatiotemporally Precise Large-Scale Optogenetic Stimulation of Deep Cortical Layers in Nonhuman Primates. BioRxiv. Cold Spring Harbor: Cold Spring Harbor Laboratory Press. 10.1101/2022.02.09.479779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiVincenti L. 2013. Analgesic use in nonhuman primates undergoing neurosurgical procedures. J Am Assoc Lab Anim Sci 52:10–16. [PMC free article] [PubMed] [Google Scholar]
- 11.Dorsett M, Liang SY. 2016. Diagnosis and treatment of central nervous system infections in the emergency department. Emerg Med Clin North Am 34:917–942. 10.1016/j.emc.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Commission. 2018. ALURES – ANIMAL USE REPORTING - EU SYSTEM. In: Commission E, editor. EU STATISTICS DATABASE ON THE USE OF ANIMALS FOR SCIENTIFIC PURPOSES UNDER DIRECTIVE 2010/63/EU. European Commission: European Union. [Google Scholar]
- 13.Fayad PB, Awad IA. 1998. Surgery for intracerebral hemorrhage. Neurology 51 Suppl 3:S69–S73. 10.1212/WNL.51.3_Suppl_3.S69. [DOI] [PubMed] [Google Scholar]
- 14.Feldmann LK, Neumann W-J, Faust K, Schneider G-H, Kühn AA. 2021. Risk of infection after deep brain stimulation surgery with externalization and local-field potential recordings: Twelve-year experience from a single institution. Stereotact Funct Neurosurg 99:512–520. 10.1159/000516150. [DOI] [PubMed] [Google Scholar]
- 15.Ferrecchia CE, Ducore RM, Colgin LMA, Lewis AD. 2015. Spontaneous nocardial brain abscess in a juvenile rhesus macaque (Macaca mulatta). J Med Primatol 44:45–48. 10.1111/jmp.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorello CV, Heard DJ, Heller HLB, Russell K. 2006. Medical management of Toxoplasma meningitis in a white-throated capuchin (Cebus capucinus). J Zoo Wildl Med 37:409–412. 10.1638/05-058.1. [DOI] [PubMed] [Google Scholar]
- 17.Friedman H, Ator N, Haigwood N, Newsome W, Allan JS, Golos TG, Kordower JH, Shade RE, Goldberg ME, Bailey MR, Bianchi P. 2017. The critical role of nonhuman primates in medical research. Pathog Immun 2:352–365. 10.20411/pai.v2i3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fugate JE. 2015. Complications of neurosurgery. Continuum (Minneap Minn) 21:1425–1444. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert SG, Reuhl KR, Wong JH, Rice DC. 1987. Fatal pneumococcal meningitis in a colony-born monkey (Macaca fascicularis). J Med Primatol 16:333–338. 10.1111/j.1600-0684.1987.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 20.Goosen C, Van der Gulden W, Rozemond H, Balner H, Bertens A, Boot R, Brinkert J, Dienske H, Janssen G, Lammers A. 1984. Recommendations for the housing of macaque monkeys. Lab Anim 18:99–102. 10.1258/002367784780891316. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins P. 2002. Recognizing and assessing pain, suffering, and distress in laboratory animals: A survey of current practice in the UK with recommendations. Lab Anim 36:378–395. 10.1258/002367702320389044. [DOI] [PubMed] [Google Scholar]
- 22.Horai R, Caspi RR. 2019. Microbiome and autoimmune uveitis. Front Immunol 10:232. 10.3389/fimmu.2019.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston JM, Cohen YE, Shirley H, Tsunada J, Bennur S, Christison-Lagay K, Veeder CL. 2016. Recent refinements to cranial implants for rhesus macaques (Macaca mulatta). Lab Anim (NY) 45:180–186. 10.1038/laban.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. 2002. Recent patterns of medication use in the ambulatory adult population of the United States. JAMA 287:337–344. 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 25.Kishi N, Sato K, Sasaki E, Okano H. 2014. Common marmoset as a new model animal for neuroscience research and genome editing technology. Dev Growth Differ 56:53–62. 10.1111/dgd.12109. [DOI] [PubMed] [Google Scholar]
- 26.Kvolik S, Koruga N, Skiljic S. 2022. Analgesia in the neurosurgical intensive care unit. Front Neurol 12:819613. 10.3389/fneur.2021.819613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamont LA. 2008. Multimodal pain management in veterinary medicine: The physiologic basis of pharmacologic therapies. Vet Clin North Am Small Anim Pract 38:1173–1186. 10.1016/j.cvsm.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Lankau EW, Turner PV, Mullan RJ, Galland GG. 2014. Use of nonhuman primates in research in North America. J Am Assoc Lab Anim Sci 53:278–282. [PMC free article] [PubMed] [Google Scholar]
- 29.Leblanc M, Berry K, McCort H, Reuter JD. 2013. Brain abscess in a rhesus macaque (Macaca mulatta) with a cephalic implant. Comp Med 63:367–372. [PMC free article] [PubMed] [Google Scholar]
- 30.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. 2012. Age-related macular degeneration. Lancet 379:1728–1738. 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 31.Magden ER, Mansfield KG, Simmons JH, Abee CR, Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT. 2015. Chapter 17. Nonhuman primates, p 771–930. Laboratory animal medicine (3rd edition). Boston (MA): Academic Press. [Google Scholar]
- 32.Manfreda KL, Berzelak J, Vehovar V, Bosnjak M, Haas I. 2008. Web surveys versus other survey modes: A meta-analysis comparing response rates. Int J Mark Res 50:79–104. 10.1177/147078530805000107. [DOI] [Google Scholar]
- 33.Mattison JA, Vaughan KL. 2017. An overview of nonhuman primates in aging research. Exp Gerontol 94:41–45. 10.1016/j.exger.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClelland S, Hall WA. 2007. Postoperative central nervous system infection: Incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis 45:55–59. 10.1086/518580. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell AS, Thiele A, Petkov CI, Roberts A, Robbins TW, Schultz W, Lemon R. 2018. Continued need for nonhuman primate neuroscience research. Curr Biol 28:R1186–R1187. 10.1016/j.cub.2018.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitz AR, Bartolo R, Saunders RC, Browning PG, Talbot T, Averbeck BB. 2017. High channel count single-unit recordings from nonhuman primate frontal cortex. J Neurosci Methods 289:39–47. 10.1016/j.jneumeth.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals. 2009. Recognition and alleviation of pain in laboratory animals. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 38.O’Shea DH, Troutman E, Shandrasekaran C, Stavisky S, Kao JC, Sahani M, Ryu S, Deisseroth K, Shenoy KV. 2017. The need for calcium imaging in nonhuman primates: New motor neuroscience and brain-machine interfaces. Exp Neurol 287:437–451. 10.1016/j.jneumeth.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohtani M. 2007. Basic pharmacology of buprenorphine. Eur J Pain Suppl 1 S1:69–73. 10.1016/S1754-3207(08)60017-6. [DOI] [Google Scholar]
- 40.Ortiz-Rios M, Haag M, Balezeau F, Frey S, Thiele A, Murphy K, Schmid MC. 2018. Improved methods for MRI-compatible implants in nonhuman primates. J Neurosci Methods 308:377–389. 10.1016/j.jneumeth.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perna R, Temple J. 2015. Rehabilitation outcomes: Ischemic versus hemorrhagic strokes. Behav Neurol 2015:891651. 10.1155/2015/891651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perry BAL, Mason S, Nacef J, Waddle A, Hynes B, Bergmann C, Schmid MC, Petkov CI, Thiele A, Mitchell AS. 2021. Protective cranial implant caps for macaques. J Neurosci Methods 348:108992. 10.1016/j.jneumeth.2020.108992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prescott MJ, Langermans JA, Ragan I. 2017. Applying the 3Rs to nonhuman primate research: Barriers and solutions. Drug Discov Today Dis Models 23:51–56. 10.1016/j.ddmod.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prescott MJ, Poirier C. 2021. The role of MRI in applying the 3Rs to non-human primate neuroscience. NeuroImage 225:117521. [DOI] [PubMed] [Google Scholar]
- 45.Raper J, Galvan A. 2022. Applications of chemogenetics in nonhuman primates. Curr Opin Pharmacol 64:102204. 10.1016/j.coph.2022.102204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts SJ, Platt ML. 2005. Effects of isosexual pair-housing on biomedical implants and study participation in male macaques. Contemp Top Lab Anim Sci 44:13–18. [PubMed] [Google Scholar]
- 47.Rodriguez M, Rodriguez-Sabate C, Morales I, Sanchez A, Sabate M. 2015. Parkinson’s disease as a result of aging. Aging Cell 14:293–308. 10.1111/acel.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenthal G, Ng I, Moscovici S, Lee KK, Lay T, Martin C, Manley GT. 2014. Polyetheretherketone implants for the repair of large cranial defects: A 3-center experience. Neurosurgery 75:523–529, discussion 528–529. 10.1227/NEU.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 49.Seelig D. 2007. A tail of 2 monkeys: Social housing for nonhuman primates in the research laboratory setting. J Appl Anim Welf Sci 10:21–30. 10.1080/10888700701277279. [DOI] [PubMed] [Google Scholar]
- 50.Shivaji S. 2021. A systematic review of gut microbiome and ocular inflammatory diseases: Are they associated? Indian J Ophthalmol 69:535–542. 10.4103/ijo.IJO_1362_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith JJ, Hadzic V, Li X, Liu P, Day T, Utter A, Kim B, Washington IM, Basso MA. 2006. Objective measures of health and well-being in laboratory rhesus monkeys (Macaca mulatta). J Med Primatol 35:388-396. [DOI] [PubMed] [Google Scholar]
- 52.Sosa-Ortiz AL, Acosta-Castillo I, Prince MJ. 2012. Epidemiology of dementias and Alzheimer’s disease. Arch Med Res 43:600–608. 10.1016/j.arcmed.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JCS, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. 2011. The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain 7:55. 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.St Leger AJ, Caspi RR. 2018. Visions of eye commensals: The known and the unknown about how the microbiome affects eye disease. BioEssays 40:1800046. 10.1002/bies.201800046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. 2005. Reliability of signals from a chronically implanted, silicon-based electrode array in nonhuman primate primary motor cortex. IEEE Trans Neural Syst Rehabil Eng 13:524–541. 10.1109/TNSRE.2005.857687 [DOI] [PubMed] [Google Scholar]
- 56.Tardif SD, Coleman K, Hobbs TR, Lutz C. 2013. IACUC review of nonhuman primate research. ILAR J 54:234–245. 10.1093/ilar/ilt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thibault M, Girard F, Moumdjian R, Chouinard P, Boudreault D, Ruel M. 2007. Craniotomy site influences postoperative pain following neurosurgical procedures: A retrospective study. Can J Anaesth 54:544–548. 10.1007/BF03022318. [DOI] [PubMed] [Google Scholar]
- 58.United States Department of Agriculture - Animal and Plant Health Inspection Service. 2019. [Internet]. Total number of animals research facilities used in regulated activities. [Cited 22 November 2022]. Available at: https://www.aphis.usda.gov/animal_welfare/downloads/reports/fy19-summary-report-column-F.pdf.
- 59.Vadivelu N, Kai AM, Tran D, Kodumudi G, Legler A, Ayrian E. 2016. Options for perioperative pain management in neurosurgery. J Pain Res 9:37. 10.2147/JPR.S85782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villano JS, Ogden B, Goh A, Hui LS, Chow PKH. 2008. Cerebellar abscess in a cynomolgus macaque (Macaca fascicularis). J Med Primatol 37:82–87. 10.1111/j.1600-0684.2007.00254.x. [DOI] [PubMed] [Google Scholar]
- 61.Waziry R, Heshmatollah A, Bos D, Chibnik LB, Ikram MA, Hofman A, Ikram MK. 2020. Time trends in survival following first hemorrhagic or ischemic stroke between 1991 and 2015 in the Rotterdam study. Stroke 51:824–829. 10.1161/STROKEAHA.119.027198. [DOI] [PubMed] [Google Scholar]
- 62.Weichbrod RH, Thompson GA, Norton JN. 2018. Management of animal care and use programs in research, education, and testing. Weichbrod RH, Thompson GA, Norton JN, eds. Boca Raton (FL): CRC Press. [PubMed] [Google Scholar]
- 63.Zrinzo L, Foltynie T, Limousin P, Hariz MI. 2012. Reducing hemorrhagic complications in functional neurosurgery: A large case series and systematic literature review. J Neurosurg 116:84–94. 10.3171/2011.8.JNS101407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nonhuman primates (NHP) questionnaire, part 1.
Nonhuman primates (NHP) questionnaire, part 2.
Nonhuman primates (NHP) questionnaire, part 3.