Abstract
Purpose:
Disseminated tumor cells (DTCs) expressing epithelial markers in the bone marrow are associated with recurrence and death, but little is known about risk factors predicting their occurrence. We detected EPCAM+/CD45- cells in bone marrow from early-stage breast cancer patients after neoadjuvant chemotherapy (NAC) in the I-SPY 2 Trial and examined clinicopathologic factors and outcomes.
Methods:
Patients who signed consent for SURMOUNT, a sub-study of the I-SPY 2 Trial (NCT01042379), had bone marrow collected after NAC at the time of surgery. EPCAM+CD45- cells in 4 mLs of bone marrow aspirate were enumerated using immunomagnetic enrichment/flow cytometry (IE/FC). Patients with >4.16 EPCAM+CD45- cells per mL of bone marrow were classified as DTC-positive. Tumor response was assessed using the residual cancer burden (RCB), a standardized approach to quantitate the extent of residual invasive cancer present in the breast and the axillary lymph nodes after NAC. Association of DTC-positivity with clinicopathologic variables and survival was examined.
Results:
A total of 73 patients were enrolled, 51 of whom had successful EPCAM+CD45- cell enumeration. Twenty-four of 51 (47.1%) were DTC-positive. The DTC-positivity rate was similar across receptor subtypes, but DTC-positive patients were significantly younger (p=0.0239) and had larger pretreatment tumors compared to DTC-negative patients (p=0.0319). Twenty of 51 (39.2%) achieved a pathologic complete response (pCR). While DTC-positivity was not associated with achieving pCR, it was significantly associated with higher RCB class (RCB-II/III, 62.5% vs. RCB-0/I; 33.3%; Chi-squared p=0.0373). No significant correlation was observed between DTC-positivity and distant recurrence-free survival (p=0.38, median follow-up=3.2 years).
Conclusion:
DTC-positivity at surgery after NAC was higher in younger patients, those with larger tumors, and those with residual disease at surgery.
Keywords: disseminated tumor cells, neoadjuvant chemotherapy, pathologic complete response, residual cancer burden
INTRODUCTION
Disseminated tumor cells (DTCs) detected in the bone marrow (bone marrow) after neoadjuvant chemotherapy (NAC) are thought to represent dormant residual disease that could ultimately give rise to distant metastases. These cells are believed to have a non-proliferative phenotype, utilizing pathways for survival that are distinct from proliferating cells in the primary tumor or growing distant metastases [2]. Several previous clinical studies in the neoadjuvant setting for treatment of early-stage breast cancer have shown that DTCs detected at surgery after completion of NAC or within the post-surgical follow-up period are independently predictive of breast cancer recurrence and survival [9, 10, 18]. However, none of these studies showed a significant correlation between DTCs and response to NAC.
Achieving a pathologic complete response (pCR) or RCB-0 provides a significant survival advantage over those who have residual disease after NAC [13]. There are no well-established molecular and cell-based biomarkers that can accurately predict pCR or identify those at risk of recurrence after surgery. Additional molecular and cellular information available at the time of surgery may help fine-tune the prognostic value of pCR as an early surrogate endpoint of survival. Detection of DTCs in bone marrow may complement pathologic evaluation of the primary tumor and could potentially supplement the prognostic value of pCR.
In this study, we assessed the clinical significance of DTCs in SURMOUNT, a sub-study of the neoadjuvant I-SPY 2 Trial—an ongoing, multicenter, and adaptive Phase 2 trial—that investigates new agents combined with standard NAC for the treatment of locally advanced breast cancer [20, 21, 25]. We sought to characterize patients who are DTC-positive after NAC and assess whether DTC-positivity was associated with baseline clinicopathologic characteristics, response to NAC, or longer-term outcomes in the I-SPY 2 Trial.
PATIENTS AND METHODS
Patient population.
Patients enrolled in the I-SPY 2 Trial (NCT01042379) were recruited to participate in a sub-study called SURMOUNT (Surveillance Markers of Utility for Recurrence after Neoadjuvant Therapy for Breast Cancer) (Supplementary Figure 1, Supplementary Table 1). Eligibility criteria for the I-SPY 2 Trial have been described in detail in previous reports [20, 21]. The study included female patients, 18 years of age or older, diagnosed with high-risk, stage II/III breast cancer and a tumor at least 2.5 cm in diameter. To be eligible for the SURMOUNT study, patients required consent for bone marrow aspiration obtained at surgery and collection of blood samples after NAC.
Ethics declaration.
Six I-SPY 2 Trial sites participated in the SURMOUNT sub-study, obtaining Institutional Review Board approval for this additional consent form at each site, and all patients provided written informed consent to the sub-study.
Data acquisition.
5 mLs of bone marrow aspirate were collected from the posterior superior iliac crest while the patient was under anesthesia immediately before surgery (Supplementary Figure 2). Samples were drawn into ethylenediaminetetraacetic acid (EDTA) tubes and shipped overnight in a cold pack to the John Park Laboratory at the University of California San Francisco using an overnight courier. Samples were processed immediately after receipt.
Cells positive for expression of an epithelial marker (EPCAM) and negative for a leukocyte-specific marker (CD45), were enumerated in 4 mLs of bone marrow using an immunomagnetic enrichment/flow cytometry (IE/FC) assay as previously described [7, 16]. Briefly, magnetic beads coated with anti-EPCAM (epithelial cell adhesion molecule) monoclonal antibodies were used to enrich for EPCAM-expressing cells in the bone marrow. After adding a nuclear stain and fluorochrome-conjugated antibodies to EPCAM and CD45, the enriched sample was analyzed by flow cytometry.
To determine the presence of background EPCAM+CD45- cells, bone marrow samples from 8 individuals with no history of cancer were subjected to IE/FC (Supplementary Table 2). The cutoff for DTC-positivity was then set at two standard deviations above the mean of EPCAM+CD45- cells per mL present in the samples (Supplementary Table 2). Bone marrow samples with >4.16 EPCAM+CD45- cells per mL of bone marrow from patients in this cohort were classified as DTC-positive.
Evaluation and reporting of the biomarker in this study were compliant with the REMARK guidelines [19].
Study design.
The response endpoints were pCR and residual cancer burden (RCB) as determined routinely on the I-SPY 2 Trial and previously described [13]. pCR was defined as the complete eradication of invasive cancer in both the breast and regional lymph nodes determined at the time of surgery. Residual cancer in the breast and the nodes was also evaluated using the RCB method [23]. This method classified patients into 4 groups with increasing amounts of residual disease: RCB-0 (equivalent to pCR), RCB-I (minimal), RCB-II (moderate), and RCB-III (extensive). Distant recurrence-free survival (DRFS) was calculated from the date of patient consent for treatment to the date of clinical diagnosis of metastatic recurrence or death by any cause. Patients lost to follow-up were censored at the time of their last visit. Survival analysis was performed on follow-up data available as of June 2020.
Statistical Methods.
To determine associations between DTC-positivity and categorical variables, including menopausal status, clinical T and N stage, receptor subtypes, MammaPrint status, race, pCR, and RCB, we used the Chi-square test for proportions. T-test was used to compare means of continuous variables—age at screening, longest tumor diameter by magnetic resonance imaging (MRI), and RCB index—between groups stratified according to DTC status (DTC-positive vs. DTC-negative). Logistic regression was used to estimate the odds ratio (OR) and 95% confidence interval (CI). Survival in DTC-positive and DTC-negative groups was visualized using Kaplan-Meier analysis and compared using the log-rank test. The hazard ratio and 95% confidence interval were estimated using Cox proportional hazard regression.
RESULTS
A total of 73 patients from 6 participating I-SPY 2 sites were enrolled between July 2014 and June 2017 (Supplementary Figure 1, Supplementary Table 1). Of the 73 enrolled patients, 53 (72%) had bone marrow samples successfully collected, of which 51 (96.2%) were successfully analyzed for the presence of EPCAM+CD45- cells (Supplementary Figure 2). The distribution of study patients by study site and treatment arm is shown in Supplementary Figure 1. EPCAM+CD45- cells were detected in 46 (90.2%) patients, ranging from 0 to 104.5 EPCAM+CD45- cells per mL (Supplementary Figure 2). The median EPCAM+CD45- cells per mL of bone marrow was 4.03. Using the cutoff >4.16 EPCAM+CD45- cells per mL (Supplementary Table 2), 24 (47.1%) were classified as DTC-positive.
Association between DTCs and clinicopathologic variables.
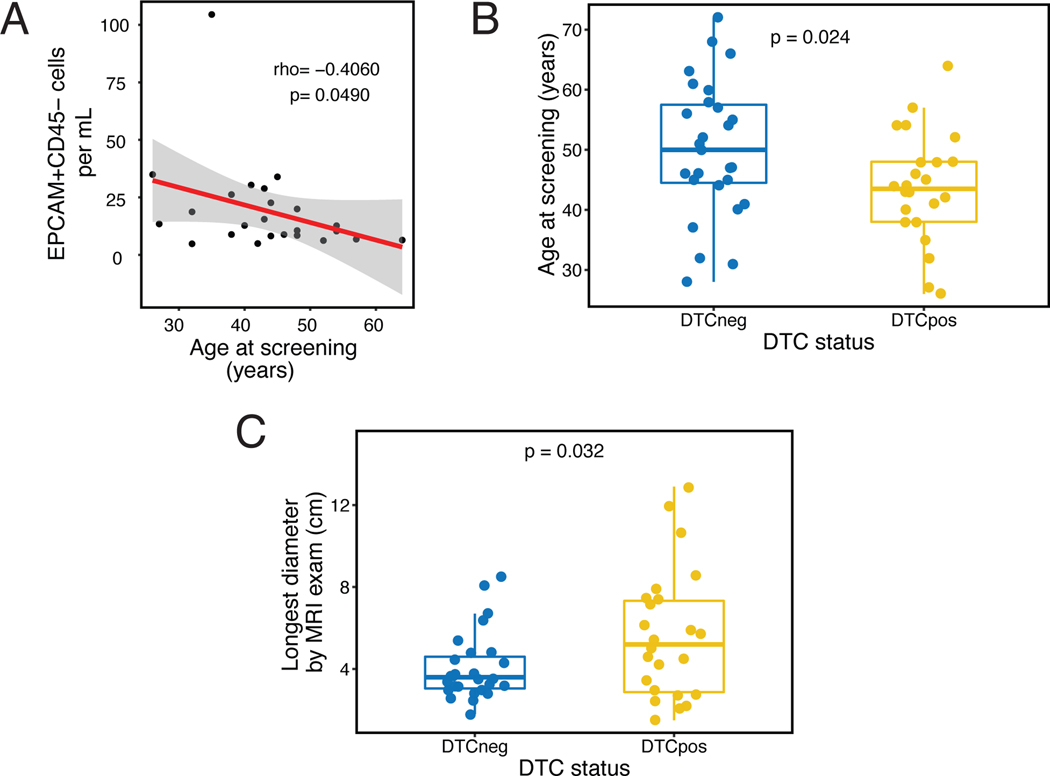
Younger age was significantly associated with higher levels of EPCAM+CD45- cells in DTC-positive patients (correlation coefficient=−0.41, Spearman rank p=0.0490) (Figure 1A). The mean age was significantly lower among DTC-positive patients compared to DTC-negative patients (43.4. vs. 50.1, t-test p=0.0239) (Table 1, Figure 1B). However, there was no association with menopausal status (Table 1, Supplementary Figure 3). DTC-positivity was also significantly associated with non-white race, with 8 of 9 (88.9%) non-white patients DTC-positive compared to only 16 of 42 (38.1%) white patients (Chi-squared p=0.0056). Regarding tumor factors, the length of the longest tumor diameter by MRI at pretreatment was significantly greater in DTC-positive compared to DTC-negative patients (t-test p=0.0319, Figure 1C). Notably, no significant associations were observed between DTC-positivity and nodal status or receptor subtype. Regarding the latter, it is noteworthy that 50% of patients were classified as DTC-positive in each receptor subtype when classified as HR+/HER2-, any HER2+, and triple-negative breast cancer.
Figure 1. DTCs after neoadjuvant therapy vs. age and tumor burden at pretreatment.
A. Correlation between EPCAM+CD45- cells per mL and age at screening in DTC-positive patients. Correlation coefficient (rho) and p-value were calculated using Spearman’s rank correlation test; B. Association between DTC status and age at screening; C. Association between DTC status and longest tumor diameter by magnetic resonance imaging (MRI) at pretreatment. Means between groups (in B and C) were compared using Wilcoxon signed rank test.
Table 1.
Clinicopathologic characteristics of 51 patients according to DTC status after NAC.
| DTC- | % | DTC+ | % | Total | % | p value* | |
|---|---|---|---|---|---|---|---|
| Menopausal status (n=47) | 0.1884 | ||||||
| Post | 11 | 61.1 | 7 | 38.9 | 18 | 38.3 | |
| Pre | 12 | 41.4 | 17 | 58.6 | 29 | 61.7 | |
| Tumor size (n=49) | 0.5604 | ||||||
| T1 and T2 | 20 | 55.6 | 16 | 44.4 | 36 | 73.5 | |
| T3 and T4 | 6 | 46.2 | 7 | 53.8 | 13 | 26.5 | |
| Nodal status (n=48) | 0.7904 | ||||||
| Node-negative | 14 | 53.8 | 12 | 46.2 | 26 | 54.2 | |
| Node-positive | 11 | 50.0 | 11 | 50.0 | 22 | 45.8 | |
| Subtype | 1.0000 | ||||||
| HER2+ | 9 | 52.9 | 8 | 47.1 | 17 | 33.3 | |
| HR+HER2- | 9 | 52.9 | 8 | 47.1 | 17 | 33.3 | |
| Triple negative | 9 | 52.9 | 8 | 47.1 | 17 | 33.3 | |
| MammaPrint | 0.6678 | ||||||
| High 1 | 13 | 50.0 | 13 | 50.0 | 26 | 51.0 | |
| High 2 | 14 | 56.0 | 11 | 44.0 | 25 | 49.0 | |
| Race | 0.0056 | ||||||
| Non-white | 1 | 11.1 | 8 | 88.9 | 9 | 17.6 | |
| White | 26 | 61.9 | 16 | 38.1 | 42 | 82.4 | |
| Pathological complete response | 0.1658 | ||||||
| Yes | 13 | 65.0 | 7 | 35.0 | 20 | 39.2 | |
| No | 14 | 45.2 | 17 | 54.8 | 31 | 60.8 | |
| RCB class | 0.1138 | ||||||
| 0 | 13 | 65.0 | 7 | 35.0 | 20 | 74.1 | |
| I | 5 | 71.4 | 2 | 28.6 | 7 | ||
| II | 5 | 29.4 | 12 | 70.6 | 17 | ||
| III | 4 | 57.1 | 3 | 42.9 | 7 | 25.9 | |
| RCB class (binary) | 0.0373 | ||||||
| 0 and I | 18 | 66.7 | 9 | 33.3 | 27 | 52.9 | |
| II and III | 9 | 37.5 | 15 | 62.5 | 24 | 47.1 | |
| RCB index | 0.1516 | ||||||
| mean (range) | 0.5 | 0–3.8 | 1.6 | 0–3.4 | |||
| Age | 0.0239 | ||||||
| mean (range) [Years] | 50.1 | 28–72 | 43.4 | 26–64 | |||
| Longest tumor diameter by MRI (pretreatment) | 0.0319 | ||||||
| mean and range [cm] | 4.1 | 1.8–8.5 | 5.6 | 1.5–12.9 |
For continuous clinicopathologic variables (age and tumor size), association with DTC-positivity was assessed using a t-test. For categorical variables, association with DTC-positivity was assessed using a Chi-squared test. RCB-residual cancer burden.
To determine which factors were independently associated with DTC-positivity, we built logistic regression models using the factors that were significant in univariate analysis. The univariate analysis showed that age (p=0.0329), longest tumor diameter by MRI at pretreatment (p=0.0366), and race (p=0.0205) were significant predictors of DTC-positivity (Table 2). In a multivariate model containing these three predictors, longer tumor diameter by MRI at pretreatment (p=0.0444) and non-white race (p=0.0250) remained significantly independently associated with being positive for DTCs.
Table 2.
Logistic regression models for prediction of DTC-positivity after neoadjuvant chemotherapy. OR-odds ratio, CI-confidence interval
| Predictors | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| p value | Odds ratio | lower 95% CI | higher 95% CI | p value | |
| Age at screening | 0.0329 | 0.97 | 0.90 | 1.03 | 0.3379 |
| Longest tumor diameter by MRI (pretreatment) | 0.0366 | 1.37 | 1.03 | 1.94 | 0.0444 |
| Non-white vs. White | 0.0205 | 13.86 | 1.90 | 290.21 | 0.0250 |
A total of 20/51 patients (39.2%) achieved pCR and there was no significant association between pCR and DTC-positivity (Chi-squared p=0.1658) (Table 1, Supplementary Figure 4). Association between DTC-positivity and RCB classes was not significant (Chi-squared p=0.1138). When dichotomized as RCB-0/I vs. RCB-II/III, the association between DTC-positivity and RCB was significant (Chi-squared p=0.0373), i.e., the proportion of DTC-positive patients was significantly higher in patients with moderate or extensive residual cancer (RCB-II/III, 62.5%) compared to those with no or minimal residual cancer (RCB-0/I, 33.3%). The mean RCB index was also numerically higher, but not statistically significant, in DTC-positive patients compared to DTC-negative patients (1.6 vs. 1.0, t-test p=0.1516).
At the time of this analysis, the median follow-up of the I-SPY 2 Trial DTC cohort was 3.2 years (range of 0.9–6.1 years). 8/51 (15.7%) patients experienced distant recurrence, 6 (11.8%) of whom died. In this small sample, no significant difference was seen in DRFS between DTC-positive and DTC-negative patients (log-rank p=0.38) (Supplementary Figure 5) even after adjusting for subtype (Supplementary Table 3).
DISCUSSION
We examined the relationship of DTC-positivity detected after NAC with standard clinicopathologic variables, response to NAC, and risk of metastatic recurrence and death in locally advanced high-risk breast cancer patients in the SURMOUNT study, a subset of the I-SPY 2 Trial.
In univariate analysis, we found that DTC-positive patients were significantly younger and had larger pretreatment tumors by MRI compared to those who were DTC-negative. Preliminary findings based on our small dataset showed a significant association between DTC-positivity and race: non-white patients had a higher DTC-positivity rate compared to white patients. In multivariate analysis, pretreatment tumor size by MRI and race but not age remained significant predictors of DTC-positivity.
Our results showed no significant association between DTC-positivity after NAC and pCR. However, when the response variable RCB was dichotomized (RCB-0/I vs. RCB-II/III), we found that the proportion of DTC-positives was significantly higher in patients with moderate and extensive residual disease (RCB-II/III, 62.5%) compared those with no or minimal residual disease (RCB-0/I, 33.3%).
Our findings that DTC-positivity after NAC reflect aggressive disease (e.g., larger tumors at pretreatment) provide additional clinical evidence that DTCs are an intermediate step in the metastatic process. The finding of a higher DTC-positivity rate in non-whites vs. white patients was unanticipated and not clearly due to the confounding effects of more advanced stage, warranting further investigation.
To our knowledge, our study is the first to report on a significant association between DTC-positivity and response to NAC. Here, DTCs were detected using the IE/FC assay, a method that involves enrichment and detection of cells based on EPCAM expression. Tumor cells with low expression of EPCAM, e.g., those undergoing an epithelial-to-mesenchymal transition [12], will be missed by IE/FC and thus represents a limitation of the assay. Another limitation involves the detection of false positives in bone marrow samples from individuals with no history of cancer. Testing of the IE/FC assay in non-cancer controls during initial development revealed the presence of EPCAM+CD45- events by flow cytometry in non-cancer controls (mean: 1.31 DTC/mL, standard deviation: 1.43). Upon further investigation, we found cells with a weak autofluorescence in the EPCAM channel that resulted in what appears to be false-positive events. In this study, we used a cutoff based on two standard deviations above the mean EPCAM+CD45- cells per mL in the bone marrow of non-cancer controls for DTC-positivity. Samples in this present cohort with >4.16 EPCAM+CD45- cells per mL were classified as DTC-positive. Since false positives were detected in non-cancer controls, we acknowledge that not all EPCAM+CD45- cells in bone marrow detected by IE/FC may represent bona fide DTCs with malignant characteristics. We, therefore, consider the results of our study as tentative findings that warrant future confirmatory studies.
In addition to EPCAM, other studies have used cytokeratin expression for detecting DTCs in bone marrow using standard immunocytochemistry (ICC) [6, 14]. In this study, we observed that 47.1% of bone marrow was DTC-positive. In a large, pooled DTC study using ICC, the positivity rate was 30.6% [5]. The IE/FC approach used in this study detected higher numbers of DTCs compared to ICC. The difference in detection rates may be due to the number of cells used as input for each assay. The IE/FC method routinely uses 4 mLs of bone marrow, which is equivalent to ~176 million mononuclear cells per sample, while the standard ICC evaluates 4–8 million cells per sample [8]. Therefore, IE/FC interrogates >20-fold more cells than ICC and thus may reflect the higher sensitivity of the IE/FC, but its specificity may be lower compared to that of the cytokeratin-based ICC assay. It is also possible that the IE/FC and ICC methods detect overlapping, but distinct populations of cells, since the former is based on flow cytometry detection of EPCAM expression, whereas the latter is based on the immunohistochemical detection of cytokeratin expression.
In contrast to previous reports by Hall [9] and Hartkopf [10], our survival analysis did not reveal any significant correlation between DTC-positivity after NAC and increased risk of disease relapse or death. This may be due to the limited sample size, heterogeneity of clinical characteristics across breast cancer subtypes and the treatment received, and the relatively short follow-up of 3.2 years. Also, previous studies that have demonstrated the prognostic impact of DTCs have used cytokeratin-based approaches to detect these cells in the bone marrow [9, 10]. Our previous studies using IE/FC to detect DTCs in treatment-naïve early-stage breast cancer did show that DTC-positive patients who were also CTC-positive (DTC+CTC+) at surgery had the worst breast cancer-specific survival compared to other groups: DTC+CTC-, DTC-CTC+, and DTC-CTC- [17]. Also, higher pretreatment levels of DTCs, detected by IE/FC, were a significant predictor of recurrence and death in stage I-III breast cancer patients receiving adjuvant zoledronic acid [24].
The promise of DTCs lies in their potential utility for guiding patient selection [4] and improving risk stratification by adding prognostic information to the response endpoints (e.g., pCR and RCB) to accurately estimate the risk of recurrence. For example, the patient who had the highest levels of EPCAM+CD45- cells (104.5 cells per mL) achieved a pCR but experienced an ipsilateral breast cancer recurrence. Survival analysis using data from the whole population, however, did not show that DTCs were significantly associated with survival. Importantly, we found that DTCs after NAC were significantly associated with the presence of moderate and extensive residual disease (RCB II/III), a finding that warrants further study in larger populations. Identifying patients with systemic residual cancer, using DTCs as a surrogate marker, could inform therapeutic decisions in the adjuvant setting. In addition, the presence of DTCs after NAC may identify those who may benefit from more aggressive and/or targeted therapy (escalation) vs. those who can forgo additional treatment (de-escalation).
DTCs also provide an opportunity to target tumor dormancy, to eliminate the reservoir of cells that can ultimately reactivate and travel to distant sites. Previous work on genomic analysis of EPCAM+/CD45- cells isolated by IE/FC from the bone marrow of early-stage breast cancer patients have revealed malignant characteristics of these cells [15]. DTCs detected after NAC represent genetic subclones that have escaped therapy [11]. Molecular characterization of these cells has revealed novel therapeutic targets, which could facilitate the development of more effective treatments in the neoadjuvant setting [1, 3, 22]. Such trials are ongoing, including the CLEVER (NCT03032406), PALAVY (NCT04841148), and ABBY (NCT04523857) trials.
CONCLUSIONS
Higher rates of DTC-positivity after NAC were observed in younger patients, those with larger tumors, and those with residual disease at surgery. The analyses were, however, limited by the small sample size. Further studies in larger cohorts are needed to confirm the predictive and prognostic impact of DTCs as well as their utility for guiding neoadjuvant therapy to improve patient outcomes.
Supplementary Material
Acknowledgments.
The authors thank the patients and their families and the I-SPY 2 TRIAL Investigators for their participation in this study.
STATEMENTS & DECLARATIONS
Funding.
This project was funded by the “2-PREVENT” Breast Cancer Translational Center of Excellence at the University of Pennsylvania through funds provided by the University of Pennsylvania Health System. MJMM received support from the Breast Cancer Research Foundation and a fellowship from the Cancer Cell Mapping Initiative (U54 CA209891).
Competing interests.
LV is a parttime employee of Agendia; and holds stock options from this employment. ASC reports grants and institutional research funding from Novartis and Lilly; and reports an uncompensated position on the Scientific Steering Committee for Novartis. AJC reports grants and institutional research funding from Merck, Amgen, Puma and SeaGen. ML reports grants and institutional research funding from Eisai, Exact Sciences, Genentech, Genomic Health, GRAIL, Menarini Silicon Biosystems, Merck, Novartis, Seattle Genetics, and Tesaro; reports travel support from AstraZeneca, Genomic Health, and Ionis; reports institutional support for Advisory Boards from AstraZeneca, Celgene, Roche/Genentech, Genomic Health, GRAIL, Ionis, Merck, Pfizer, Seattle Genetics, Syndax; became an employee of Natera in 2022; and holds stock options from this employment. HH reports institutional research funding from Arvinas, Abbvie, Celcuity, GSK, G1 therapeutics, Quantum Leap Healthcare Collaborative, Pfizer, and Zymeworks; reports participation on Advisory Boards for Novartis, AstraZeneca and Gilead; and received a grant from the Department of Defense. CY reports grants and institutional research funding from the National Cancer Institute, and Quantum Leap Healthcare Collaborative; and received travel reimbursement from Quantum Leap Healthcare Collaborative. JP reports participation on a Data Safety Monitoring Board for QuantumLEAD; reports participation on an Advisory Board for VIVLI, University of Wisconsin SPORE, and a reviewer for PCORI; received travel reimbursement for ASCO and SABCS; served as an ISPY Trial Advocate Lead; and serves as a faculty member for Methods in Clinical Research. EC reports institutional research funding from Oncocyte, C2i Genomics, the Parker Institute, UHG, ChipDX, Tempus, Merck, Becton Dickinson, Menarini/Janssen, and AstraZeneca; reports honoraria from AstraZeneca, GuardantHealth, and BMS; and received research support from Personalis. LE is an unpaid member of the Board of Directors for Quantum Leap Healthcare Collaborative; reports institutional research funding from Quantum Leap Healthcare Collaborative, Merck, and the National Cancer Institute (P-01); serves on the Blue Cross Medical Advisory Panel, and receives reimbursement for travel, and honorarium for her participation. JP reports honoraria from Roche, Gilead, AstraZeneca, and Daiichi Sankyo. LAC has served as an expert consultant for Teva Pharmaceuticals, Eisai, Sanofi, Lilly, Imerys, Colgate, Whittaker, Clark and Daniels, and Sterigenics in litigation. AD reports institutional research funding from Pfizer, Genentech, Novartis, Inivata, and Calithera; reports unpaid leadership roles for American Society of Clinical Oncology, AACR San Antonio Breast Cancer Symposium, and ECOG/ACRIN Cooperative Group; received honorarium from the NCI Breast Cancer Steering Committee; and reports participation on a Data Safety Monitoring Board for QuantumLEAD; reports participation on an Advisory Board for VIVLI, University of Wisconsin SPORE, and a reviewer for PCORI.
Footnotes
All other participating authors have no disclosures to report. The rest of the authors declare no competing interests.
Ethics approval and. The I-SPY 2 Trial sites that participated in the SURMOUNT sub-study obtained Institutional Review Board approval at each site.
Consent to participate. All patients provided written informed consent to the sub-study.
Consent for publication. Not Applicable.
Data availability.
The datasets used for the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Abravanel DL, Belka GK, Pan TC, Pant DK, Collins MA, Sterner CJ, Chodosh LA (2015) Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. J Clin Invest 125:2484–2496. doi: 10.1172/JCI74883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre-Ghiso JA, Sosa MS (2018) Emerging Topics on Disseminated Cancer Cell Dormancy and the Paradigm of Metastasis. Annual Review of Cancer Biology 2:377–393. doi: 10.1146/annurev-cancerbio-030617-050446 [DOI] [Google Scholar]
- 3.Alvarez JV, Pan TC, Ruth J, Feng Y, Zhou A, Pant D, Grimley JS, Wandless TJ, Demichele A, ISPY Trial Investigators, Chodosh LA (2013) Par-4 downregulation promotes breast cancer recurrence by preventing multinucleation following targeted therapy. Cancer Cell 24:30–44. doi: 10.1016/j.ccr.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayne L, Nivar I, Goodspeed B, Deluca S, Wileyto EP, Shih N, Nayak A, Feldman MD, Edwards J, Fox K, Matro JM, Domchek S, Knollman H, Jankowitz R, Bradbury A, Shah PD, Graves J, Woodfield G, Chislock E, Wang J, Belka G, Chodosh LA, Clark AS, DeMichele A (2021) Abstract PD9–11: Identifying breast cancer survivors with dormant disseminated tumor cells: The PENN-SURMOUNT screening study. Cancer Research 81:PD9–11-PD19–11. doi: 10.1158/1538-7445.Sabcs20-pd9-11 [DOI] [Google Scholar]
- 5.Braun S, Pantel K, Muller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, Riethmuller G, Schlimok G (2000) Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 342:525–533. doi: 10.1056/NEJM200002243420801 [DOI] [PubMed] [Google Scholar]
- 6.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GY, Bliss J, Vincent-Salomon A, Pantel K (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353:793–802. doi: 10.1056/NEJMoa050434 [DOI] [PubMed] [Google Scholar]
- 7.Campbell MJ, Scott J, Maecker HT, Park JW, Esserman LJ (2005) Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res Treat 91:163–171. doi: 10.1007/s10549-004-7048-0 [DOI] [PubMed] [Google Scholar]
- 8.Fehm T, Braun S, Muller V, Janni W, Gebauer G, Marth C, Schindlbeck C, Wallwiener D, Borgen E, Naume B, Pantel K, Solomayer E (2006) A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer 107:885–892. doi: 10.1002/cncr.22076 [DOI] [PubMed] [Google Scholar]
- 9.Hall C, Krishnamurthy S, Lodhi A, Bhattacharyya A, Anderson A, Kuerer H, Bedrosian I, Singh B, Lucci A (2012) Disseminated tumor cells predict survival after neoadjuvant therapy in primary breast cancer. Cancer 118:342–348. doi: 10.1002/cncr.26202 [DOI] [PubMed] [Google Scholar]
- 10.Hartkopf AD, Taran FA, Wallwiener M, Hagenbeck C, Melcher C, Krawczyk N, Hahn M, Wallwiener D, Fehm T (2013) The presence and prognostic impact of apoptotic and nonapoptotic disseminated tumor cells in the bone marrow of primary breast cancer patients after neoadjuvant chemotherapy. Breast Cancer Res 15:R94. doi: 10.1186/bcr3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseini H, Obradovic MMS, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C, Maneck M, Patwary N, Haunschild G, Guzvic M, Reimelt C, Grauvogl M, Eichner N, Weber F, Hartkopf AD, Taran FA, Brucker SY, Fehm T, Rack B, Buchholz S, Spang R, Meister G, Aguirre-Ghiso JA, Klein CA (2016) Early dissemination seeds metastasis in breast cancer. Nature 540:552–558. doi: 10.1038/nature20785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyun KA, Koo GB, Han H, Sohn J, Choi W, Kim SI, Jung HI, Kim YS (2016) Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 7:24677–24687. doi: 10.18632/oncotarget.8250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ISPY Trial Consortium, Yee D, DeMichele AM, Yau C, Isaacs C, Symmans WF, Albain KS, Chen YY, Krings G, Wei S, Harada S, Datnow B, Fadare O, Klein M, Pambuccian S, Chen B, Adamson K, Sams S, Mhawech-Fauceglia P, Magliocco A, Feldman M, Rendi M, Sattar H, Zeck J, Ocal IT, Tawfik O, LeBeau LG, Sahoo S, Vinh T, Chien AJ, Forero-Torres A, Stringer-Reasor E, Wallace AM, Pusztai L, Boughey JC, Ellis ED, Elias AD, Lu J, Lang JE, Han HS, Clark AS, Nanda R, Northfelt DW, Khan QJ, Viscusi RK, Euhus DM, Edmiston KK, Chui SY, Kemmer K, Park JW, Liu MC, Olopade O, Leyland-Jones B, Tripathy D, Moulder SL, Rugo HS, Schwab R, Lo S, Helsten T, Beckwith H, Haugen P, Hylton NM, Van’t Veer LJ, Perlmutter J, Melisko ME, Wilson A, Peterson G, Asare AL, Buxton MB, Paoloni M, Clennell JL, Hirst GL, Singhrao R, Steeg K, Matthews JB, Asare SM, Sanil A, Berry SM, Esserman LJ, Berry DA (2020) Association of Event-Free and Distant Recurrence-Free Survival With Individual-Level Pathologic Complete Response in Neoadjuvant Treatment of Stages 2 and 3 Breast Cancer: Three-Year Follow-up Analysis for the I-SPY2 Adaptively Randomized Clinical Trial. JAMA Oncol. doi: 10.1001/jamaoncol.2020.2535 [DOI] [PMC free article] [PubMed]
- 14.Magbanua MJ, Das R, Polavarapu P, Park JW (2015) Approaches to isolation and molecular characterization of disseminated tumor cells. Oncotarget 6:30715–30729. doi: 10.18632/oncotarget.5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magbanua MJM, Rugo HS, Hauranieh L, Roy R, Scott JH, Lee JC, Hsiao F, Sosa EV, Van’t Veer L, Esserman LJ, Park JW (2018) Genomic and expression profiling reveal molecular heterogeneity of disseminated tumor cells in bone marrow of early breast cancer. NPJ Breast Cancer 4:31. doi: 10.1038/s41523-018-0083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magbanua MJM, Solanki TI, Ordonez AD, Hsiao F, Park JW (2017) Enumeration of Circulating Tumor Cells and Disseminated Tumor Cells in Blood and Bone Marrow by Immunomagnetic Enrichment and Flow Cytometry (IE/FC). Methods Mol Biol 1634:203–210. doi: 10.1007/978-1-4939-7144-2_17 [DOI] [PubMed] [Google Scholar]
- 17.Magbanua MJM, Yau C, Wolf DM, Lee JS, Chattopadhyay A, Scott JH, Bowlby-Yoder E, Hwang ES, Alvarado M, Ewing CA, Delson AL, Van’t Veer LJ, Esserman L, Park JW (2019) Synchronous Detection of Circulating Tumor Cells in Blood and Disseminated Tumor Cells in Bone Marrow Predicts Adverse Outcome in Early Breast Cancer. Clin Cancer Res 25:5388–5397. doi: 10.1158/1078-0432.CCR-18-3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathiesen RR, Borgen E, Renolen A, Lokkevik E, Nesland JM, Anker G, Ostenstad B, Lundgren S, Risberg T, Mjaaland I, Kvalheim G, Lonning PE, Naume B (2012) Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res 14:R117. doi: 10.1186/bcr3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of NCIEWGoCD (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235. doi: 10.1007/s10549-006-9242-8 [DOI] [PubMed] [Google Scholar]
- 20.Park JW, Liu MC, Yee D, Yau C, van ‘t Veer LJ, Symmans WF, Paoloni M, Perlmutter J, Hylton NM, Hogarth M, DeMichele A, Buxton MB, Chien AJ, Wallace AM, Boughey JC, Haddad TC, Chui SY, Kemmer KA, Kaplan HG, Isaacs C, Nanda R, Tripathy D, Albain KS, Edmiston KK, Elias AD, Northfelt DW, Pusztai L, Moulder SL, Lang JE, Viscusi RK, Euhus DM, Haley BB, Khan QJ, Wood WC, Melisko M, Schwab R, Helsten T, Lyandres J, Davis SE, Hirst GL, Sanil A, Esserman LJ, Berry DA, Investigators IS (2016) Adaptive Randomization of Neratinib in Early Breast Cancer. N Engl J Med 375:11–22. doi: 10.1056/NEJMoa1513750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rugo HS, Olopade OI, DeMichele A, Yau C, van ‘t Veer LJ, Buxton MB, Hogarth M, Hylton NM, Paoloni M, Perlmutter J, Symmans WF, Yee D, Chien AJ, Wallace AM, Kaplan HG, Boughey JC, Haddad TC, Albain KS, Liu MC, Isaacs C, Khan QJ, Lang JE, Viscusi RK, Pusztai L, Moulder SL, Chui SY, Kemmer KA, Elias AD, Edmiston KK, Euhus DM, Haley BB, Nanda R, Northfelt DW, Tripathy D, Wood WC, Ewing C, Schwab R, Lyandres J, Davis SE, Hirst GL, Sanil A, Berry DA, Esserman LJ, Investigators IS (2016) Adaptive Randomization of Veliparib-Carboplatin Treatment in Breast Cancer. N Engl J Med 375:23–34. doi: 10.1056/NEJMoa1513749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sosa MS (2016) Dormancy programs as emerging antimetastasis therapeutic alternatives. Mol Cell Oncol 3:e1029062. doi: 10.1080/23723556.2015.1029062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, Walls A, Bousamra A, Ramineni M, Sinn B, Hunt K, Buchholz TA, Valero V, Buzdar AU, Yang W, Brewster AM, Moulder S, Pusztai L, Hatzis C, Hortobagyi GN (2017) Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol 35:1049–1060. doi: 10.1200/JCO.2015.63.1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidula N, Greenberg S, Petrillo L, Hwang J, Melisko M, Goga A, Moasser M, Magbanua M, Park JW, Rugo HS (2021) Evaluation of disseminated tumor cells and circulating tumor cells in patients with breast cancer receiving adjuvant zoledronic acid. NPJ Breast Cancer 7:113. doi: 10.1038/s41523-021-00323-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf DM, Yau C, Sanil A, Glas A, Petricoin E, Wulfkuhle J, Severson TM, Linn S, Brown-Swigart L, Hirst G, Buxton M, DeMichele A, Hylton N, Symmans F, Yee D, Paoloni M, Esserman L, Berry D, Rugo H, Olopade O, van ‘t Veer L (2017) DNA repair deficiency biomarkers and the 70-gene ultra-high risk signature as predictors of veliparib/carboplatin response in the I-SPY 2 breast cancer trial. NPJ Breast Cancer 3:31. doi: 10.1038/s41523-017-0025-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used for the current study are available from the corresponding author upon reasonable request.