Abstract
Bone marrow mesenchymal stem cell (BMSC)-derived exosomes are a promising therapeutic agent for human disease, but their effects on neural stem cells (NSCs) subject to spinal cord ischaemia-reperfusion injury (SCIRI) remain unknown. Here, we examine the impact of miR-199a-5p-enriched exosomes derived from BMSCs on NSC proliferation. We establish a rat model of aortic cross-clamping to induce SCIRI in vivo and a primary NSC model of oxygen-glucose deprivation/reoxygenation (OGD/R) to simulate SCIRI in vitro. CCK8, EdU, and BrdU assays are performed to evaluate the proliferation of NSCs. Hematoxylin and eosin (H&E) staining is used to determine the number of surviving neurons. The Basso, Beattie, and Bresnahan (BBB) scale and inclined plane test (IPT) are used to evaluate hind limb motor function. DiO-labelled exosomes are efficiently internalized by NSCs and increase ectopic amounts of miR-199a-5p, which promotes the proliferation of NSCs. In contrast, exosomes derived from miR-199a-5p-depleted BMSCs exert fewer beneficial effects. MiR-199a-5p targets and negatively regulates glycogen synthase kinase 3β (GSK-3β) and increases nuclear β-catenin and cyclin D1 levels. miR-199a-5p inhibition reduces the total number of EdU-positive NSCs after OGD/R, but the GSK-3β inhibitor CHIR-99021 reverses this effect. In vivo, intrathecal injection of BMSC-derived exosomes increases the proliferation of endogenous spinal cord NSCs after SCIRI. In addition, more proliferating NSCs are found in rats intrathecally injected with exosomes overexpressing miR-199a-5p. In summary, miR-199a-5p in BMSC-derived exosomes promotes NSC proliferation via GSK-3β/β-catenin signaling.
Keywords: bone marrow mesenchymal stem cells, exosome, neural stem cells, GSK-3β, miR-199a-5p
Introduction
As a catastrophic complication of aortic aneurysm and aortic dissection surgery, spinal cord ischaemia-reperfusion injury (SCIRI) may cause irreversible paraplegia [1]. The nonregenerative property of neurons is the main cause of permanent lower extremity dysfunction after SCIRI. Endogenous neural stem cells (NSCs) are multipotent stem cells with high self-renewal capacity and the ability to differentiate into neurons, astrocytes, or oligodendrocytes. Mammalian NSCs reside in the central nervous system (CNS) and have an extensive range of functions [2]. During development, NSCs in the brain are distributed in multiple areas, such as the hippocampus and striatum [3]. Adult NSCs are mainly confined to the lateral ventricle (subventricular zone), the hippocampal dentate gyrus (subgranular zone), and the central canal of the spinal cord (ependyma and submembrane zone) [ 4, 5] . NSCs usually remain inactive. However, under pathological conditions of inflammation, ischemia, and hypoxia, NSCs become activated, proliferate and differentiate into new neurons, astrocytes, and oligodendrocytes [ 6, 7] . Endogenous neurogenesis is the generation of new neural cell types from NSCs and is important for the recovery of neural function following injury. Unfortunately, low levels of proliferation and differentiation limit its regenerative potential.
Exosomes are a subset of extracellular vesicles of 70–160 nm in diameter that are secreted by many cell types [8]. Similar to the cytosol of their origin cells, exosomes contain diverse signaling molecules, including proteins, lipids, mRNAs, and noncoding RNAs. As a cell-free treatment, exosomes from bone marrow mesenchymal stem cells (BMSCs) possess many of the same therapeutic biological molecules but are safer and more efficient than BMSC transplantation [9]. Current mechanistic studies on exosomes focus on the transport of microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) [ 10– 13] . A recent study reported that miR-124-3p in BMSC-derived exosomes reduces neuronal injury after SCIRI by regulating Ern1 and promoting M2 macrophage polarization [14]. In addition, miR-29b-3p from BMSC-derived exosomes induces PTEN-mediated Akt signaling and protects the rat brain from hypoxic-ischemic injury [11]. miR-25-enriched BMSC-derived exosomes upregulate miR-25 and enhance the neuroprotective effects against SCIRI [12]. However, the function of exosomes derived from BMSCs on NSCs and the underlying mechanisms have not yet been clarified.
Wnt signaling is one of the main pathways involved in NSC proliferation [15]. Glycogen synthase kinase-3β (GSK-3β) is an important effector of Wnt/β-catenin signaling. Inhibition of GSK-3β can induce Wnt signaling, upregulate β-catenin, and promote neurogenesis in the adult hippocampus [16], while increased expression of GSK-3β in human NSCs inhibits neurogenesis [17]. A previous study reported that miR-199a overexpression in PC12 cells reduces the expression of GSK-3β, inhibits autophagy, enhances cell viability, and improves cell survival [18]. We recently found that miR-199a-5p protects the rat spinal cord against ischaemia-reperfusion injury and promotes neuron survival [19]. However, the regulatory role of miR-199a-5p on the proliferation of rat NSCs remains unknown.
In this study, an oxygen-glucose deprivation/reoxygenation (OGD/R) model using NSCs was established to examine the proliferation of NSCs treated with exosomes derived from BMSCs and explore the underlying mechanisms. In addition, rats subject to experimental SCIRI were assessed to examine the effects of BMSC-derived and miR-199a-5p-enriched exosomes on endogenous NSC proliferation.
Materials and Methods
Animals
Adult male and pregnant female Sprague-Dawley (SD) rats of SPF grade were provided by the Animal Center of China Medical University (Shenyang, China). All animals were housed in individual cages at 22°C±2°C and 50%±10% relative humidity under a 12 h/ 12 h light-dark cycle. All animal experiments were approved by the Ethics Committee of China Medical University (CMU2021368) and conformed to the National Institutes of Health Guide for the Use and Care of Laboratory Animals (NIH Publications No. 80-23, revised 1996).
NSC isolation, identification, and culture
NSCs were isolated from E14–E16 embryonic rat brains, as previously described [ 7, 20] . Briefly, the heads of fetal rats were amputated, followed by bilateral hippocampal isolation and incubation for 20 min at 37°C with 0.125% ethylenediaminetetraacetic acid (EDTA) solution. The strained NSCs were washed with Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco, Grand Island, USA) and complete NSC culture medium (Gibco). The harvested cells were resuspended in DMEM/F12 with 2% B27, 20 ng/mL recombinant rat basic fibroblast growth factor (bFGF; Gibco), epidermal growth factor (EGF; Gibco), 100 μg/mL penicillin, and 100 μg/mL streptomycin (Gibco) [21]. The medium was changed every 3 days, and NSCs were passaged when the neurospheres had diameters of 50–100 μm. Third-passage cells were seeded on poly-L-lysine-coated plates (Sigma, Shanghai, China) for further experiments.
NSCs in suspension and adherent cultures were identified by immunofluorescence staining for nestin. Cells were fixed with 4% formalin (30 min) at ambient temperature, rinsed three times with phosphate-buffered saline (PBS), and permeabilized with 0.3% Triton Χ-100 in PBS (30 min). Blocking was carried out for 30 min at 37°C. Next, anti-nestin antibody (1:200; Affinity, Changzhou, China) was incubated overnight at 4°C, followed by a 90-min incubation at 37°C with CY3-linked secondary antibodies (1:200; Servicebio, Wuhan, China). Finally, DAPI (Servicebio) counterstaining was performed for 5 min in the dark before the Eclipse Ti2 fluorescence microscopy (Nikon, Tokyo, Japan).
BMSC-derived exosome isolation and identification
BMSCs from SD rats were provided by Cyagen Biosciences (RASMX-01001; Guangzhou, China). After passage 4–6 BMSCs grew to 80%–90% confluency, the culture medium was aspirated, followed by the addition of exosome-depleted FBS medium. To obtain exosome-depleted serum, FBS centrifugation was carried out at 120,000 g for 2 h. Exosomes were isolated at 4°C, as previously described [12]. First, the collected cell medium was centrifuged at 2000 g for 10 min to remove dead cells and debris. Second, the samples were centrifuged at 10,000 g for 30 min before the final centrifugation of the resulting supernatant at 100,000 g for 3 h. The pellet obtained was resuspended in PBS. The particle size of the microspheres was assessed by nanoparticle tracking analysis, and the morphology and size were visualized by transmission electron microscopy (TEM; Hitachi, Tokyo, Japan). The expression of the exosome surface markers CD9, CD63, and TSG101 was evaluated by western blot analysis.
Internalization of BMSC-derived exosomes into NSCs
In vitro, BMSC-derived exosomes were labelled with DiO fluorochrome (Thermo Fisher, Waltham, USA) according to the manufacturer’s instructions and incubated with NSCs for 12 h [22]. The following day, the DiO-labelled exosomes were observed by the Eclipse Ti2 fluorescence microscopy.
OGD/R and cell treatment
To induce ischemia in cultured cells, the OGD/R model was established with complete medium replaced by glucose-free DMEM. Incubation was carried out in humid anaerobic chambers containing 94% N 2, 5% CO 2 and 1% O 2 at 37°C for 4 h [ 23, 24] . After OGD, the cells were cultured under normoxia with BMSC-derived exosomes (1×10 9 particles/mL) or exosomes from miR-199a-depleted BMSCs. To determine whether miR-199a-5p promotes NSC proliferation through GSK-3β/β-catenin signaling, the GSK-3β inhibitor CHIR-99021 (10 nM; MCE, Shanghai, China) and a lentivirus vector expressing the miR-199a-5p inhibitor were added to the cell medium for 48 h.
Virus construction and transfection
To perform transfection, an adenoviral vector overexpressing GSK-3β (oe-GSK-3β) purchased from HanBio (Shanghai, China) was injected intrathecally 48 h prior to modelling. NSCs were infected using lentiviral vectors (HanBio) expressing the miR-199a-5p mimic (5′-CCCAGTGTTCAGACTACCTGTTC-3′), miR-199a-5p mimic NC (5′-UCACAACCUCCUAGAAAGAGUAGA-3′), miR-199a-5p inhibitor (5′-GAACAGGTAGTCTGAACACTGGG-3′), and miR-199a-5p inhibitor NC (5′-UCACAACCUCCUAGAAAGAGUAGA-3′). In addition, the miR-199a-5p mimic and miR-199a-5p inhibitor were used to transfect BMSCs. Three days after transfection, green fluorescent protein levels were examined by the Eclipse Ti2 fluorescence microscopy. The optimal multiplicities of infection values were 30 and 5 for BMSCs and NSCs, respectively, with a transfection efficiency of 95%.
CCK-8 assay
NSCs were seeded in 96-well plates at 5×10 3 cells/well, and the CCK-8 assay was performed to quantify cell viability. CCK-8 (10 μL; Yobibio, Shanghai, China) was added to every well after treatment, followed by a 2-h incubation at 37°C. The optical density (OD) values were determined at a wavelength of 450 nm using a microplate reader (Thermo Fisher). Assays were performed in triplicate for each sample, and three independent experiments were used for statistical analysis.
EdU labelling
An EdU assay kit (Beyotime Biotechnology, Shanghai, China) was employed to evaluate the proliferation of NSCs. Briefly, after OGD/R for 24 h, incubation was carried out with EdU (10 μM) for another 24 h at 37°C and 5% CO 2. This was followed by fixation with 4% PEA and permeabilization with 0.3% Triton X-100. Further fixation was performed using a click reaction solution for 30 min incubated in the dark. DAPI counterstaining was performed 10 min before analysis.
Quantitative reverse-transcription PCR (qRT-PCR)
Total RNA from NSCs, BMSCs, and exosomes with different transfections or treatments was isolated using TRIzol reagent (Takara, Dalian, China). The cDNA Reverse Transcription Kit (Takara) or MicroRNA Reverse Transcription Kit (Takara) was used to reverse transcribe 450 ng of RNA. Quantitative real-time PCR analysis was performed with Applied Biosystems 7500 real time PCR system (Thermal Fisher, Waltham, USA) using TB Green Premix Ex Taq II (Takara, Dalian, China). The reaction conditions were: 40 cycles, 95°C for 5s, and 60°C for 34 s. The specific primers used were as follows: miR-199a-5p (F: 5′-CCCAGUGUUCAGACUACCUGUUC-3′), miR-26a-5p (F: 5′-GCCGGTTCAAGTAATCCAG-3′), miR-185-3p (F: 5′-CACTCCAGCTGGGTTTCCTCTGGTCC-3′), miR-6314 (F: 5′-CAGGCACTGACAGATCTGATGGT-3′), U6 (F: 5′-CTCGCTTCGGCAGCACA-3′; R: 5′-AACGCTTCACGAATTTGCGT-3′), GSK-3β (F: 5′-CACAGAACCTCTTGCTGGAT-3′; R: 5′-GGTGCCCTGTAGTACCGAGA-3′), and GAPDH (F: 5′-GTCGGTGTGAACGGATTTG-3′; R: 5′-TCCCATTCTCAGCCTTGAC-3′). The expression of miRNAs were normalized to the expression of U6 and calculated by using the 2 −ΔΔCT method.
Western blot analysis
Total protein from the tissue or cell samples was isolated using RIPA lysis buffer (Beyotime Biotechnology). Protein concentrations were determined using the BCA protein assay kit (Beyotime Biotechnology). The remaining steps were performed according to previously described methods [ 25, 26] . The primary antibodies were as follows: GSK-3β (1:1000; CST, Boston, USA), β-catenin (1:1000; CST), nestin (1:1000; Abcam, Cambridge, UK), cyclin D1 (1:1000; CST), β-actin (1:10,000; Proteintech, Wuhan, China), and histone 3 (1:1000; Abmart, Shanghai, China). ImageJ (NIH, Bethesda, USA) was used for quantification.
Dual-luciferase reporter assay
A luciferase reporter assay was performed as previously reported [27]. Briefly, 4×10 4 cells were seeded into each well and transfected with miR-199a-5p mimic or miR-199a-5p mimic NC together with GSK-3β-WT/GSK-3β-MUT. After 48 h, relative luciferase activity was determined using a double luciferase reporter gene detection kit (Promega, Madison, USA).
SCIRI model, intrathecal administration and BrdU injection
The SCIRI model was induced in rats as described previously [28]. Briefly, the aortic arch was crossed between the left carotid and subclavian arteries to block arterial flow towards the spinal cord for 14 min before reperfusion. The sham animals underwent aortic arch exposure without arterial occlusion.
Intrathecal administration of exosomes was performed as previously described [ 12, 29] . Exosomes or miR-199a-5p-overexpressing exosomes (10 μL at 2 μg/μL in PBS) were injected into the lumbar cistern at the L4–L5 level using a Hamilton syringe within 30 s daily for 3 consecutive days after SCIRI. The IR group was administered with an equivalent volume of phosphate-buffered saline (PBS). The correct position of the needle tip was confirmed by observation of a sudden tail flick.
To label proliferative cells in vivo, three consecutive intraperitoneal injections of 5-bromo-2′-deoxyuridine (BrdU; 50 mg/kg; Sigma) at 10 mg/mL in saline were administered every 8 h after 48 h of recovery from surgery.
Spinal cord tissue preparation and double immunofluorescence
After the rats were euthanized, the spinal cord at L4–L6 was quickly removed and fixed overnight with 4% paraformaldehyde at 4°C. The tissue was dehydrated with 20% and 30% sucrose for 24–48 h. Six-micron slices were obtained from fresh spinal specimens. Double immunofluorescence staining was performed to examine endogenous neurogenesis using antibodies directed against BrdU (1:1000; Bosterbio, Pleasanton, USA) and nestin (1:1000; Affinity).
Hematoxylin and eosin (H&E) staining
Three days after reperfusion, rats were euthanized, and spinal cord tissue from L4 to L6 was collected for analysis, fixed in 4% paraformaldehyde, and then embedded in paraffin wax. Paraffin-embedded sections were dewaxed in xylene, dehydrated using a graded ethanol series, and washed with distilled water. The sections were stained with hematoxylin and eosin, examined and photographed by a Win20 slice scanner (Winmedic, Jinan, China).
Long-term neurological function analysis
The Basso, Beattie, and Bresnahan (BBB) scale was used to evaluate hindlimb motor function in rats with SCIRI. The behavior of the trunk, tail, and hind limbs of SCIRI rats were assessed by open field test. The inclined plane test (IPT) involves using a board secured at one end, with the free edge of the board gradually raised to increase the angle of inclination. The maximum angle at which the rats maintained their stability for at least 5 s was recorded as the inclined plane test angle. Both tests were conducted 1, 2, 3, 5, 7, 10, and 14 days after surgery.
Statistical analysis
Data were analysed using IBM SPSS Statistics 25.0 (SPSS, Chicago, USA) and presented as the mean±standard deviation (SD). Student’s t-test or ANOVA with Tukey’s post hoc test was performed for group comparisons. Spearman’s correlation analysis was performed to study the association between miR-199a-5p and GSK-3β expression. P<0.05 indicated statistical significance.
Results
Characterization of NSCs and BMSC-derived exosomes
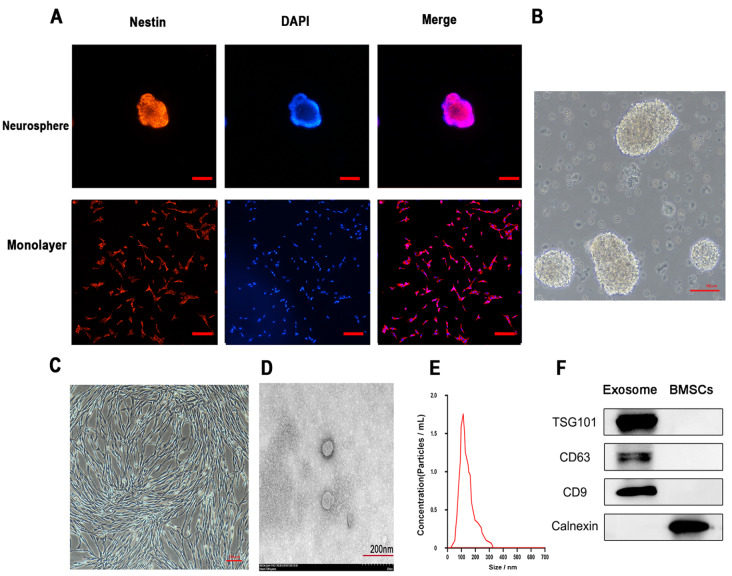
Nestin protein (red fluorescence), a specific marker of NSCs, was expressed in both neurospheres and NSCs in adherent cultures after three passages of purified cells ( Figure 1A). Primary NSCs were isolated from the rat hippocampus and cultured for 3 days before observation. The cell spheres were enlarged with few protrusions, good light transmittance, a strong stereoscopic effect, and high cell density ( Figure 1B). These results indicated that the cultured cells were NSCs.
Figure 1 .
Characterization of NSCs and BMSC-derived exosomes
(A) Identification of suspension and adherent cultured NSCs by immunofluorescence microscopy. Scale bar: 50 μm. Primary hippocampal NSCs from fetal rats underwent immunolabelling with antibodies targeting nestin (red). DAPI (blue) counterstaining was performed. (B) Neurosphere formation by NSCs. Scale bar: 100 μm. (C) Morphology of BMSCs observed under a microscope. Scale bar: 100 μm. (D) Morphological assessment of BMSC-derived exosomes by transmission electron microscopy. Scale bar: 200 nm. (E) Size distribution of exosomes examined by NTA. (F) Exosomal markers (CD63, TSG101 and CD9) and negative control (Calnexin) examined by western blot analysis in BMSC-derived exosomes and cell culture supernatants.
When BMSCs were cultured to the fourth passage, they gradually displayed a fibroblast-like, spindle-shaped morphology and were clustered ( Figure 1C). Exosomes were obtained from the BMSC culture supernatant and examined by TEM, NTA and western blot analysis. TEM demonstrated that BMSC-derived exosomes were spherical ( Figure 1D). The average particle diameter was approximately 130 nm, and the concentration was 3×10 10 particles/mL, according to NTA ( Figure 1E). Western blot analysis showed that BMSC-derived exosomes contained exosomal markers CD63, TSG101, and CD9 ( Figure 1F). These data suggested that BMSC-derived exosomes were successfully isolated.
BMSC-derived exosomes promote the proliferation of NSCs after OGD/R induction
To investigate whether BMSC-derived exosomes are taken up by NSCs, exosomes were labelled with DiO and incubated with NSCs for 12 h. NSCs effectively took up DiO-labelled exosomes ( Figure 2A,B). To mimic in vivo ischemia/reperfusion injury, cultured NSCs underwent OGD/R [30]. Then, CCK-8 was used to evaluate the effects of BMSC-derived exosomes on NSC proliferation 24, 48, 72, and 96 h after reoxygenation. BMSC-derived exosomes significantly enhanced the proliferation of NSCs ( Figure 2C). Double-immunofluorescent staining indicated that exosomes elevated the number of EdU and Nestin dual-positive cells ( Figure 2D).
Figure 2 .
BMSC-derived exosomes promote proliferation in NSCs subject to OGD/R
(A) DiO-labelled exosomes were observed by upright microscopy after coincubation with NSCs. Scale bar: 100 μm. (B) DiO-labelled exosomes were observed by fluorescence microscopy after coincubation with NSCs. Scale bar: 100 μm. (C) The CCK-8 assay was used to examine proliferation in NSCs at 0 h, 24 h, 48 h, 72 h and 96 h after reoxygenation. (D) Representative immunofluorescence staining images and quantitative analysis of the rate of EdU and nestin double-positive cells. Scale bar: 100 μm. * P<0.05, ** P<0.01 vs OGD/R.
GSK-3β is a direct target of miR-199a-5p
miRNAs are involved in SCIRI pathogenesis and can exert protective effects as important constituents of exosomes [ 12, 28, 31] . Wnt signaling plays beneficial roles in self-renewal and tissue repair by NSCs [32]. GSK-3β plays a critical role in NSC proliferation as an essential effector of Wnt signaling [ 33, 34] . In this study, we investigated whether certain miRNAs in BMSC-derived exosomes could regulate GSK-3β expression, thereby affecting NSC proliferation. Based on the predictions from three databases (TargetScan, miRDB, and mirWalk), four potential miRNAs (miR-199a-5p, miR-26a-5p, miR-6314, and miR-185-3p) were identified ( Figure 3A). Furthermore, qRT-PCR demonstrated that miR-199a-5p expression was the highest among the four miRNAs in the BMSC-derived exosomes ( Figure 3B). miR-199a-5p is associated with nerve regeneration after SCIRI [ 10, 19] . Hence, miR-199a-5p was selected as the candidate for further studies. The luciferase activity of GSK-3β-WT was markedly reduced by the miR-199a-5p mimic, whereas GSK-3β-MUT showed no obvious changes, suggesting that GSK-3β is a target of miR-199a-5p ( Figure 3C). In addition, miR-199a-5p expression was negatively correlated with GSK-3β mRNA levels in NSCs ( Figure 3D).
Figure 3 .
Identification of candidate miRNAs related to GSK-3β
(A) Venn diagram showing unique and shared miRNAs in the TargetScan, miRDB and miRWalk databases. (B) Relative expression levels of miRNAs in BMSC-derived exosomes, as determined by qRT‒PCR. (C) Luciferase reporter assay was performed in 293T cells cotransfected with the WT or MUT 3′UTR of GSK-3β and miR-199a-5p mimic or miR-199a-5p mimic NC. (D) Correlation analysis of miR-199a-5p and GSK-3β mRNA.
miR-199a-5p in BMSC-derived exosomes promotes the proliferation of NSCs subject to OGD/R
To further examine the function of miR-199a-5p in BMSC-derived exosomes, BMSCs were transfected with the miR-199a-5p inhibitor or miR-199a-5p inhibitor NC, and exosomes were isolated from BMSC culture supernatants. The results of the qRT-PCR analysis confirmed the silencing efficiency of the miR-199a-5p inhibitor. The expression of miR-199a-5p in BMSCs and BMSC-derived exosomes was significantly reduced by the miR-199a-5p inhibitor ( Figure 4A). Next, mature miR-199a-5p and pri-miR-199a-5p levels were measured in NSCs after reoxygenation for 48 h following OGD to investigate whether BMSC-derived exosome-induced miR-199a-5p upregulation is mediated by exosomal transport or exosome-induced synthesis. High levels of mature miR-199a-5p were found in NSCs incubated with BMSC-derived exosomes but not exosomes from miR-199a-5p-depleted BMSCs. Meanwhile, BMSC-derived exosomes increased the expression of mature miR-199a-5p but not pri-miR-199a-5p in NSCs ( Figure 4B). Collectively, these findings indicated that BMSC-derived exosomes increased miR-199a-5p expression in NSCs through direct transport.
Figure 4 .
NSC proliferation is controlled by exosomal miR-199a-5p derived from BMSCs
(A) qRT-PCR assessment of the relative expression of miR-199a-5p in BMSCs (** P<0.01 vs BMSCs) and BMSC-Exos (** P<0.01 vs BMSCs). (B) Relative expressions of mature miR-199a-5p and pri-miR-199a-5p determined in NSCs by qRT-PCR. ** P<0.01 vs OGD/R. (C) Representative immunofluorescent images and percentages of EdU and Nestin double-positive cells. Scale bar: 100 μm. ** P<0.01 vs OGD/R; ## P<0.01 vs Exo. (D) Relative GSK-3β, total β-catenin, Cyclin D1 and nuclear β-catenin protein levels in NSCs, as measured by western blot analysis. * P<0.05, ** P<0.01 vs OGD/R; # P<0.05, ## P<0.01 vs Exo.
In the EdU staining assay, incubation with BMSC-derived exosomes increased the percentage of EdU and nestin double-positive NSCs. In contrast, the percentage of double-positive NSCs decreased after the administration with exosomes from miR-199a-5p-depleted BMSCs ( Figure 4C). To further determine the effects of exosomal miR-199a-5p on the GSK-3β/β-catenin pathway, western blot analysis was performed to assess GSK-3β, total β-catenin, nuclear β-catenin, and cyclin D1 protein expressions. BMSC-derived exosomes markedly reduced GSK-3β expression and increased total β-catenin, nuclear β-catenin, and cyclin D1 protein expressions. In contrast, exosomes from miR-199a-5p-depleted BMSCs showed no significant change ( Figure 4D).
miR-199a-5p promotes the proliferation of NSCs subject to OGD/R
Subsequently, we examined the effects of miR-199a-5p on NSC proliferation. As demonstrated by EdU staining, transfection with the miR-199a-5p mimic significantly elevated the number of EdU-positive NSCs. However, the number of EdU-positive NSCs was reduced after transfection with the miR-199a-5p inhibitor. The NC mimic and NC inhibitor had no effect ( Figure 5A). As shown in Figure 5B, GSK-3β protein expression was decreased, while the expressions of total β-catenin, nuclear β-catenin, and cyclin D1 were increased in NSCs after transfection with the miR-199a-5p mimic. Opposite results were obtained in NSCs transfected with the miR-199a-5p inhibitor. The qRT-PCR assessment of GSK-3β mRNA showed consistent changes ( Figure 5C).
Figure 5 .
miR-199a-5p promotes the proliferation of OGD/R-stimulated NSCs
(A) Representative immunofluorescence images and percentages of EdU-positive cells. Scale bar: 100 μm. ** P<0.01 vs OGD/R. (B) Relative GSK-3β, total β-catenin, Cyclin D1 and nuclear β-catenin protein levels in NSCs, as assessed by western blot analysis. * P<0.05, ** P<0.01 vs OGD/R. (C ) Relative expression of GSK-3β mRNA in NSCs determined by qRT-PCR. * P<0.05, ** P<0.01 vs OGD/R.
miR-199a-5p increases NSC proliferation subject to OGD/R by targeting GSK-3β
CHIR-99021 is a highly selective small molecule inhibitor of GSK-3β. EdU labelling demonstrated that the miR-199a-5p inhibitor decreased the percentage of EdU-positive NSCs after OGD/R, and CHIR-99021 elevated the rate of EdU-positive cells. Meanwhile, the reduction in miR-199a-5p inhibitor-induced EdU-positive cells was reversed by CHIR-99021 ( Figure 6A). Similarly, incubation with CHIR-99021 rescued the miR-199a-5p inhibitor-induced increase in GSK-3β protein expression and the reduction in total β-catenin, nuclear β-catenin, and cyclin D1 protein expression in NSCs ( Figure 6B).
Figure 6 .
miR-199a-5p increases proliferation in OGD/R-stimulated NSCs by targeting GSK-3β
(A) Representative immunofluorescence images and percentages of EdU-positive cells. Scale bar: 100 μm. * P<0.05, ** P<0.01 vs OGD/R; ## P<0.01 vs miR-199a-5p inhibitor. (B) Western blot analysis was performed to quantitate the relative protein levels of GSK-3β, total β-catenin, Cyclin D1 and nuclear β-catenin in NSCs. * P<0.05, ** P<0.01 vs OGD/R; ## P<0.01 vs miR-199a-5p inhibitor.
miR-199a-5p in BMSC-derived exosomes promotes endogenous NSC proliferation in the rat spinal cord following SCIRI
To determine the effects and underlying mechanism of BMSC-derived exosomes on the proliferation of endogenous NSCs in the spinal cord after SCIRI, exosomes derived from miR-199a-5p mimic- and mimic NC-transfected BMSCs and adenovirus overexpressing GSK-3β were intrathecally injected ( Figure 7A). qRT-PCR confirmed the high overexpression efficiency of the miR-199a-5p mimic ( Figure 7B). The proliferation of endogenous NSCs in the spinal cord was investigated by BrdU assay. The percentage of BrdU+Nestin double-positive cells in the spinal cord displayed a notable increase after the intrathecal injection of BMSC-derived exosomes ( Figure 7C). In addition, a higher number of BrdU+Nestin double-positive cells appeared in rats that were intrathecally injected with exosomes overexpressing miR-199a-5p. Notably, negligible amounts of BrdU+Nestin double-positive cells appeared along the central canal, and most activated NSCs exhibiting BrdU+Nestin double positivity were located outside the central canal. In contrast, rats overexpressing GSK-3β had a significantly lower number of BrdU+Nestin double-positive cells after SCIRI compared to the IR group. Moreover, the proliferation of spinal cord NSCs was significantly inhibited by simultaneous overexpression of the Exo-miR-199a-5p mimic and GSK-3β compared to rats in the Exo-miR-199a-5p mimic group. Therefore, miR-199a-5p in BMSC-derived exosomes could promote the proliferation of endogenous NSCs after SCIRI through the targeting regulatory effect of miR-199a-5p on GSK-3β. H&E staining results showed that the Exo-miR-199a-5p mimic group had the highest number of surviving neurons, with reduced degeneration and vacuolization, which was reversed by GSK-3β overexpression ( Figure 7D). Finally, the BBB score and IPT results were used to evaluate the motor function of the hind limbs. As expected, higher BBB scores ( Figure 7E) and larger angles of incline ( Figure 7F) were observed in the Exo-miR-199a-5p mimic group than in the IR group. However, the improved effect of Exo-miR-199a-5p on motor function was similarly suppressed by the overexpression of GSK-3β.
Figure 7 .
miR-199a-5p from BMSC-derived exosomes promotes proliferation of endogenous NSCs in rat spinal cords following SCIRI
(A) Diagram of the experimental protocols. (B) Relative miR-199a-5p levels in BMSCs and BMSC-Exos measured by qRT-PCR. ** P<0.01 vs BMSCs+ miR-199a-5p mimic NC. (C) Representative double immunofluorescence staining for nestin and BrdU in cross-sections of spinal cord tissue specimens; enlargement of the central canal area and outside central canal area. Scale bar: 200 μm. ** P<0.01 vs IR; ## P<0.01 vs Exo-miR-199a-5p mimic NC. ※※ P<0.01 vs Exo-miR-199a-5p. (D) Representative sections of L4–L6 spinal cord segments stained with hematoxylin and eosin 3 days after I/R; scale bar: 100 μm. * P<0.05, ** P<0.01 vs IR; # P<0.05 vs Exo-miR-199a-5p mimic NC: ※※ P<0.01 vs Exo-miR-199a-5p. (E) The BBB scores were higher in the Exo-miR-199a-5p mimic group. * P<0.05 vs IR, *** P<0.0005 vs IR; **** P<0.0001 vs IR; # P<0.05 vs Exo-miR-199a-5p mimic NC; ※ P<0.05 vs Exo-miR-199a-5p; ※※ P<0.01 vs Exo-miR-199a-5p. (F) The angles of incline were larger in the Exo-miR-199a-5p mimic group. ** P<0.01 vs IR; *** P<0.0005 vs IR; ## P<0.01 vs Exo-miR-199a-5p mimic NC; ※※ P<0.01 vs Exo-miR-199a-5p.
Figure 8 .
miR-199a-5p from BMSC-derived exosomes promotes the proliferation of NSCs via the GSK-3β/β-catenin signaling pathway
After internalization by NSCs, exosomal miR-199a-5p interacts with GSK-3β’s 3′UTR, and RNA-induced silencing complex (RISC) further induces the degradation of GSK-3β mRNA. GSK-3β downregulation depolymerizes the β-catenin destruction complex-axin/adenomatous polyposis coli (APC)/casein kinase 1 (CK1)/GSK-3β complex and inhibits β-catenin degradation. Thus, β-catenin undergoes nuclear translocation and binds to the transcription factor T-cell factor/lymphoid enhancer factor (TCF/LEF) for the formation of a transcription complex, which regulates downstream target genes to promote NSC proliferation.
Discussion
This study is the first to demonstrate that miR-199a-5p in BMSC-derived exosomes promotes NSC proliferation via GSK-3β/β-catenin signaling. Notably, exosomes overexpressing miR-199a-5p showed a more pronounced effect.
Endogenous NSCs are activated in the damaged adult mammalian spinal cord and produce new neurocytes to repair lost tissues [35]. Normally, adult endogenous NSCs maintain a quiescent state, but hypoxia or ischemia results in limited proliferation capacity. Recent studies on therapies for neurodegeneration have focused on strategies for enhancing the proliferation of NSCs [ 36, 37] . Exosomes possess many advantages over their donor cells as therapeutic agents, such as biocompatibility, penetrability across blood-tissue barriers, bioengineering potential, and abundance [38]. Recent evidence suggests that the application of exosomes from human placental mesenchymal stem cells is a promising method for improving NSC proliferation [39]. Consistent with this, our data showed that BMSC-derived exosomes enhanced the proliferation of NSCs after SCIRI. The ependyma in the central canal region of the spinal cord was once considered to be the source of NSCs. However, NSCs located outside of the central canal are also important. According to a recent study, NSCs in the central canal are not activated after spinal cord injury, while those found outside the central canal are activated [40]. Another study on spinal cord samples from patients with spinal cord injury demonstrated that ependymal cells located in the central canal lack proliferative ability after injury [41]. In the present study, almost all EdU and Nestin dual-positive cells were located outside the central canal following SCIRI.
The unique composition of a particular exosome depends mainly on its cellular origin and type [42]. Exosomes contain various cargos, such as DNAs, RNAs, lipids, metabolites, and proteins. Exosomal RNAs may escape degradation by blood-derived ribonucleases with better systemic retention than liposomes. Exosomes can also be enriched with miRNAs. Notably, unique exosomal miRNAs target neuronal signaling pathways, especially the Wnt pathway [43]. A single miRNA can perform various cellular functions by regulating several genes [44]. A specific miRNA may play different roles at different stages of development in the same cell type or different roles at the same stage of development in different cell types. For example, miR-188 is a key factor regulating the switch between osteogenesis and adipogenesis in BMSCs, and this regulatory role is exerted mainly in BMSCs from adult mice (18 months) but not in those from young mice (3 months) [45]. miR-34a promotes adipogenesis in HepG2 cells by binding to the 3′UTR site of AdipoR2 mRNA and inhibiting the PPARα signaling pathway [46]; however, miR-34a inhibits lipid droplet formation during adipogenesis in porcine adipocytes by targeting the ACSL4 gene [47]. miR-199a-5p protected against SCIRI by targeting ECE1 [19]. Meanwhile, miR-199a-5p aggravated OGD/R-related oxidative stress and apoptosis by targeting Brg1 in HT22 neurons [48]. In this study, miR-199a-5p exerted beneficial effects on NSCs. In vitro, NSCs transfected with miR-199a-5p mimic showed significantly enhanced proliferation. However, proliferation was suppressed in NSCs transfected with the miR-199a-5p inhibitor. Additionally, BMSC-derived exosomes transported miR-199a-5p into NSCs and promoted their proliferation. However, this beneficial effect was reduced when exosomes were derived from miR-199a-5p-knockdown BMSCs. Furthermore, we confirmed that this beneficial effect was produced by direct transport of mature miR-199a-5p by BMSC-derived exosomes. pri-miR-199a-5p is the major transcriptional product produced in the nucleus and represents the endogenous occurrence of miR-199a-5p. Here, qRT-PCR assays showed that exosomes did not affect the expression of pri-miR-199a-5p while altering the expression of mature miR-199a-5p in NSCs. Thus, the effect of exosomes on NSCs is mediated by direct transport of miR-199a-5p rather than indirect stimulation of endogenous miR-199a-5p in NSCs. In vivo, endogenous NSCs were activated by intrathecal injection of BMSC-derived exosomes, and the proliferation of NSCs was increased in animals treated with exosomes overexpressing miR-199a-5p.
In this study, the dual-luciferase reporter assay highlighted that GSK-3β is a direct miR-199a-5p target. Consistently, the miR-199a-5p mimic downregulated GSK-3β expression, whereas miR-199a-5p inhibition exerted the opposite effect. The miR-199a-5p mimic and GSK-3β inhibitor enhanced NSC proliferation by increasing β-catenin nuclear translocation to upregulate cyclin D1. As expected, GSK-3β inhibition reversed the effect of the miR-199a-5p inhibitor. Thus, miR-199a-5p promotes NSC proliferation by downregulating GSK-3β expression. GSK-3β attracted our attention because it is a core molecule that regulates NSC fate. Administration of a GSK-3β inhibitor causes nuclear and perinuclear accumulation of β-catenin in ependymal stem/progenitor cells (ESPCs) and promotes adult neurogenesis after spinal cord injury [33]. Inhibition of Wnt signaling leads to degradation of cytoplasmic β-catenin by the polyprotein complex [38]. Conversely, activation of the Wnt pathway stabilizes β-catenin. At this time, GSK-3β expression is downregulated, and β-catenin accumulates in the cytoplasm and then undergoes nuclear translocation. In the nuclear compartment, β-catenin interacts with the transcription factors TCF and LEF to affect the proliferation and differentiation of neuronal precursor cells [39].
We are the first to demonstrate that miR-199a-5p in BMSC-derived exosomes promotes NSC proliferation through GSK-3β/β-catenin signaling. At the end of the study, intrathecal injection of the Exo-miR-199a-5p mimic significantly improved neuronal survival and recovery of motor scores in rats after SCIRI. Therefore, the use of BMSC-derived exosomes to activate endogenous NSCs is promising for SCIRI treatment.
In conclusion, miR-199a-5p in BMSC-derived exosomes promotes the proliferation of NSCs via GSK-3β/β-catenin signaling. This suggests that BMSC-derived exosomal transport may be a potential treatment approach for SCIRI.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (No. 81971152) and the Natural Science Foundation of Liaoning Province (No. 2019-ZD-0742).
References
- 1.Werlin EC, Kaushik S, Gasper WJ, Hoffman M, Reilly LM, Chuter TA, Hiramoto JS. Multibranched endovascular aortic aneurysm repair in patients with and without chronic aortic dissections. J Vascular Surg. . 2019;70:1419–1426. doi: 10.1016/j.jvs.2019.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierdominici-Sottile G, Moffatt L, Palma J. The dynamic behavior of the P2X4 ion channel in the closed conformation. Biophys J. . 2016;111:2642–2650. doi: 10.1016/j.bpj.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zahr SK, Kaplan DR, Miller FD. Translating neural stem cells to neurons in the mammalian brain. Cell Death Differ. . 2019;26:2495–2512. doi: 10.1038/s41418-019-0411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Chen Z. Employing endogenous NSCs to promote recovery of spinal cord injury. Stem Cells Int. . 2019;2019:1–10. doi: 10.1155/2019/1958631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Li G, Ye J, Lu D, Chen Z, Xiang AP, Jiang MH. Substance P enhances endogenous neurogenesis to improve functional recovery after spinal cord injury. Int J Biochem Cell Biol. . 2017;89:110–119. doi: 10.1016/j.biocel.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Cusimano M, Brambilla E, Capotondo A, De Feo D, Tomasso A, Comi G, D′Adamo P, et al. Selective killing of spinal cord neural stem cells impairs locomotor recovery in a mouse model of spinal cord injury. J Neuroinflamm. . 2018;15:58. doi: 10.1186/s12974-018-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S, Jin T, Wang L, Liu W, Zhang Y, Zheng Y, Lin Y, et al. Electro-acupuncture promotes the differentiation of endogenous neural stem cells via exosomal microRNA 146b after ischemic stroke. Front Cell Neurosci. . 2020;14:223. doi: 10.3389/fncel.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. . 2015;4:1131–1143. doi: 10.5966/sctm.2015-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. . 2018;20:291–301. doi: 10.1016/j.jcyt.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Luan L, Liu Q, Liu Y, Lan X, Li Z, Liu W. MiRNA-199a-5p protects against cerebral ischemic injury by down-regulating DDR1 in rats. World Neurosurg. . 2019;131:e486–e494. doi: 10.1016/j.wneu.2019.07.203. [DOI] [PubMed] [Google Scholar]
- 11.Hou K, Li G, Zhao J, Xu B, Zhang Y, Yu J, Xu K. RETRACTED ARTICLE:Bone mesenchymal stem cell-derived exosomal microRNA-29b-3p prevents hypoxic-ischemic injury in rat brain by activating the PTEN-mediated Akt signaling pathway. J Neuroinflamm. . 2020;17:46. doi: 10.1186/s12974-020-1725-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Zhao L, Jiang X, Shi J, Gao S, Zhu Y, Gu T, Shi E. Exosomes derived from bone marrow mesenchymal stem cells overexpressing microRNA-25 protect spinal cords against transient ischemia. J Thoracic Cardiovasc Surg. . 2019;157:508–517. doi: 10.1016/j.jtcvs.2018.07.095. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Chen W, Zhao L, Li Y, Liu Z, Gao H, Bai X, et al. Obesity regulates miR‐467/HoxA10 axis on osteogenic differentiation and fracture healing by BMSC‐derived exosome LncRNA H19. J Cell Mol Med. . 2021;25:1712–1724. doi: 10.1111/jcmm.16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li R, Zhao K, Ruan Q, Meng C, Yin F. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther. . 2020;22:75. doi: 10.1186/s13075-020-2146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Song Y, Deng R, Li X, Cheng Y, Zhang Z, Sun F, et al. Mallotus oblongifolius extracts ameliorate ischemic nerve damage by increasing endogenous neural stem cell proliferation through the Wnt/β-catenin signaling pathway . Food Funct. . 2020;11:1027–1036. doi: 10.1039/c9fo01790a. [DOI] [PubMed] [Google Scholar]
- 16.Varela-Nallar L, Arredondo SB, Tapia-Rojas C, Hancke J, Inestrosa NC. Andrographolide stimulates neurogenesis in the adult hippocampus. Neural Plast. . 2015;2015:1–13. doi: 10.1155/2015/935403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coronel R, Lachgar M, Bernabeu-Zornoza A, Palmer C, Domínguez-Alvaro M, Revilla A, Ocaña I, et al. Neuronal and glial differentiation of human neural stem cells is regulated by amyloid precursor protein (APP) levels. Mol Neurobiol. . 2019;56:1248–1261. doi: 10.1007/s12035-018-1167-9. [DOI] [PubMed] [Google Scholar]
- 18.Ba RQ, Liu J, Fan XJ, Jin GL, Huang BG, Liu MW, Yang JS. Effects of miR-199a on autophagy by targeting glycogen synthase kinase 3β to activate PTEN/AKT/mTOR signaling in an MPP + in vitro model of Parkinson’s disease . Neurol Res. . 2020;42:308–318. doi: 10.1080/01616412.2020.1726584. [DOI] [PubMed] [Google Scholar]
- 19.Bao N, Fang B, Lv H, Jiang Y, Chen F, Wang Z, Ma H. Upregulation of mir-199a-5p protects spinal cord against ischemia/reperfusion-induced injury via downregulation of ECE1 in rat. Cell Mol Neurobiol. . 2018;38:1293–1303. doi: 10.1007/s10571-018-0597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Bai H, Li Q, Li J, Wan F, Tian M, Li Y, et al. In vitro investigation of the mechanism underlying the effect of ginsenoside on the proliferation and differentiation of neural stem cells subjected to oxygen-glucose deprivation/reperfusion. Int J Mol Med. . 2018;41:353–363. doi: 10.3892/ijmm.2017.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huan X, Oumei C, Hongmei Q, Junxia Y, Xiaojiao M, Qingsong J. PDE9 inhibition promotes proliferation of neural stem cells via cGMP-PKG pathway following oxygen-glucose deprivation/reoxygenation injury in vitro. Neurochem Int. . 2020;133:104630. doi: 10.1016/j.neuint.2019.104630. [DOI] [PubMed] [Google Scholar]
- 22.Zhang G, Zhu Z, Wang H, Yu Y, Chen W, Waqas A, Wang Y, et al. Exosomes derived from human neural stem cells stimulated by interferon gamma improve therapeutic ability in ischemic stroke model. J Adv Res. . 2020;24:435–445. doi: 10.1016/j.jare.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K, Yang Y, Ge H, Wang J, Chen X, Lei X, Zhong J, et al. Artesunate promotes the proliferation of neural stem/progenitor cells and alleviates Ischemia-reperfusion Injury through PI3K/Akt/FOXO-3a/p27kip1 signaling pathway. Aging. . 2020;12:8029–8048. doi: 10.18632/aging.103121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu P, Shi X, Zhang X, Liu Q, Xie Y, Hong Y, Li J, et al. Overexpression of BRCA1 in neural stem cells enhances cell survival and functional recovery after transplantation into experimental ischemic stroke. Oxid Med Cell Longev. . 2019;2019:1–13. doi: 10.1155/2019/8739730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jing N, Fang B, Li Z, Tian A. Exogenous activation of cannabinoid-2 receptor modulates TLR4/MMP9 expression in a spinal cord ischemia reperfusion rat model. J Neuroinflamm. . 2020;17:101. doi: 10.1186/s12974-020-01784-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q, Tan Y, Qu T, Zhang J, Duan X, Xu H, Mu Y, et al. Therapeutic mechanism of human neural stem cell-derived extracellular vesicles against hypoxia-reperfusion injury in vitro. Life Sci. . 2020;254:117772. doi: 10.1016/j.lfs.2020.117772. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Chen FS, Zhang ZL, Zhou HX, Ma H, Li XQ. MiR-126-3p-enriched extracellular vesicles from hypoxia-preconditioned VSC 4.1 neurons attenuate ischaemia-reperfusion-induced pain hypersensitivity by regulating the PIK3R2-mediated pathway. Mol Neurobiol. . 2021;58:821–834. doi: 10.1007/s12035-020-02159-y. [DOI] [PubMed] [Google Scholar]
- 28.Jia H, Li Z, Chang Y, Fang B, Zhou Y, Ma H. Downregulation of long noncoding RNA TUG1 attenuates MTDH-mediated inflammatory damage via targeting miR-29b-1-5p after spinal cord ischemia reperfusion . J Neuropathol Exp Neurol. . 2021;80:254–264. doi: 10.1093/jnen/nlaa138. [DOI] [PubMed] [Google Scholar]
- 29.Noori L, Arabzadeh S, Mohamadi Y, Mojaverrostami S, Mokhtari T, Akbari M, Hassanzadeh G. Intrathecal administration of the extracellular vesicles derived from human Wharton’s jelly stem cells inhibit inflammation and attenuate the activity of inflammasome complexes after spinal cord injury in rats. Neurosci Res. 2021, 170: 87–98 . [DOI] [PubMed]
- 30.Xu B, Xu J, Cai N, Li M, Liu L, Qin Y, Li X, et al. Roflumilast prevents ischemic stroke-induced neuronal damage by restricting GSK3β-mediated oxidative stress and IRE1α/TRAF2/JNK pathway. Free Radical Biol Med. . 2021;163:281–296. doi: 10.1016/j.freeradbiomed.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 31.Fang H, Yang M, Pan Q, Jin H, Li H, Wang R, Wang Q, et al. MicroRNA‐22‐3p alleviates spinal cord ischemia/reperfusion injury by modulating M2 macrophage polarization via IRF5. J Neurochem. . 2021;156:106–120. doi: 10.1111/jnc.15042. [DOI] [PubMed] [Google Scholar]
- 32.Gao J, Liao Y, Qiu M, Shen W. Wnt/β-Catenin signaling in neural stem cell homeostasis and neurological diseases. Neuroscientist. . 2021;27:58–72. doi: 10.1177/1073858420914509. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Jimenez FJ, Vilches A, Perez-Arago MA, Clemente E, Roman R, Leal J, Castro AA, et al. Activation of neurogenesis in multipotent stem cells cultured in vitro and in the spinal cord tissue after severe injury by inhibition of glycogen synthase kinase-3. Neurotherapeutics. . 2021;18:515–533. doi: 10.1007/s13311-020-00928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbloom AB, Tarczyński M, Lam N, Kane RS, Bugaj LJ, Schaffer DV. β-Catenin signaling dynamics regulate cell fate in differentiating neural stem cells. Proc Natl Acad Sci USA. . 2020;117:28828–28837. doi: 10.1073/pnas.2008509117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furube E, Ishii H, Nambu Y, Kurganov E, Nagaoka S, Morita M, Miyata S. Neural stem cell phenotype of tanycyte-like ependymal cells in the circumventricular organs and central canal of adult mouse brain. Sci Rep. . 2020;10:2826. doi: 10.1038/s41598-020-59629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang B, Zang L, Cui J, Wei L. Circular RNA TTC3 regulates cerebral ischemia-reperfusion injury and neural stem cells by miR-372-3p/TLR4 axis in cerebral infarction. Stem Cell Res Ther. . 2021;12:125. doi: 10.1186/s13287-021-02187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan Y, Kong L, Xia Y, Liang W, Wang L, Song J, Yao Y, et al. Osthole promotes endogenous neural stem cell proliferation and improved neurological function through Notch signaling pathway in mice acute mechanical brain injury. Brain Behav Immun. . 2018;67:118–129. doi: 10.1016/j.bbi.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Kalluri R, LeBleu VS. The biology , function , and biomedical applications of exosomes . Science. . 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W, Silva M, Feng C, Zhao S, Liu L, Li S, Zhong J, et al. Exosomes derived from human placental mesenchymal stem cells enhanced the recovery of spinal cord injury by activating endogenous neurogenesis. Stem Cell Res Ther. . 2021;12:174. doi: 10.1186/s13287-021-02248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shu M, Xue X, Nie H, Wu X, Sun M, Qiao L, Li X, et al. Single-cell RNA sequencing reveals Nestin(+) active neural stem cells outside the central canal after spinal cord injury. Sci China Life Sci. 2022, 65: 295–308 . [DOI] [PubMed]
- 41.Paniagua-Torija B, Norenberg M, Arevalo-Martin A, Carballosa-Gautam MM, Campos-Martin Y, Molina-Holgado E, Garcia-Ovejero D. Cells in the adult human spinal cord ependymal region do not proliferate after injury. J Pathol. . 2018;246:415–421. doi: 10.1002/path.5151. [DOI] [PubMed] [Google Scholar]
- 42.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. . 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. . 2014;3:23743. doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S, Lin Y, Zhong Y, Zhao M, Yao W, Ren X, Wang Q, et al. The long noncoding RNA HCG18 participates in PM2.5-mediated vascular endothelial barrier dysfunction. Aging. . 2020;12:23960–23973. doi: 10.18632/aging.104073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY, Xia ZY, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. . 2015;125:1509–1522. doi: 10.1172/JCI77716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen F, An C, Wu X, Yang Y, Xu J, Liu Y, Wang C, et al. MiR-34a regulates mitochondrial content and fat ectopic deposition induced by resistin through the AMPK/PPARα pathway in HepG2 cells. Int J Biochem Cell Biol. . 2018;94:133–145. doi: 10.1016/j.biocel.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Wang W, Li X, Ding N, Teng J, Zhang S, Zhang Q, Tang H. miR-34a regulates adipogenesis in porcine intramuscular adipocytes by targeting ACSL4. BMC Genet. . 2020;21:33. doi: 10.1186/s12863-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li F, Liang J, Tong H, Zhu S, Tang D. Inhibition of microRNA‐199a‐5p ameliorates oxygen‐glucose deprivation/reoxygenation‐induced apoptosis and oxidative stress in HT22 neurons by targeting Brg1 to activate Nrf2/HO‐1 signalling. Clin Exp Pharmaco l Physiol. . 2020;47:1020–1029. doi: 10.1111/1440-1681.13265. [DOI] [PubMed] [Google Scholar]