Abstract
Objectives:
To describe the methods for the in-person assessment of the RISE FOR HEALTH (RISE) study, a population-based multicenter prospective cohort study designed to identify factors that promote bladder health and/or prevent lower urinary tract symptoms in adult women, conducted by the Prevention of Lower Urinary Tract Symptoms Research Consortium (PLUS).
Methods and Results:
A subset of RISE participants who express interest in the in-person assessment will be screened to ensure eligibility (planned n=525). Eligible consenting participants are asked to complete 15 physical assessments in addition to height and weight, to assess pelvic floor muscle function, musculoskeletal status, and pain, and to provide urogenital microbiome samples. Pelvic floor muscle assessments include presence of prolapse, strength, levator attachment integrity (tear) and myofascial pain. Musculoskeletal tests evaluate core stability, lumbar spine, pelvic girdle and hip pain and function. Participants are asked to complete the Short Physical Performance Battery to measure balance, lower extremity strength, and functional capacity. All participants are asked to provide a voided urine sample and a vaginal swab for microbiome analyses; a subset of 100 are asked to contribute additional samples for feasibility and validation of a home collection of urinary, vaginal and fecal biospecimens.
Results:
Online and in-person training sessions were used to certify research staff at each clinical center prior to the start of RISE in-person assessments. Standardized protocols and data collection methods are employed uniformly across sites.
Conclusions:
The RISE in-person assessmentis an integral portion of the overall population-based RISE study and represents an innovative approach to assessing factors hypothesized to promote bladder health and/or prevent lower urinary tract symptoms. Data collected from this assessment will be used to prioritize future research questions and prevention strategies and interventions. This description of the assessment methods is intended to provide methodologic transparency and inform other researchers who join efforts to understand and improve bladder health.
Keywords: women, bladder, musculoskeletal assessment, pelvic floor, microbiome
Introduction
The Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium was formed with support of the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) in 2015 to develop an evidence base for promotion of bladder health (BH) and prevention of lower urinary tract symptoms (LUTS) in adult and adolescent women. Consistent with the World Health Organization’s definition of health,1 the PLUS Consortium conceptualizes BH as “a complete state of physical, mental and social well-being related to bladder function, and not merely the absence of LUTS”2 with function that “permits daily activities, adapts to short term physical or environmental stressors, and allows optimal well-being (e.g., travel, exercise, social, occupational, or other activities).” Healthy bladder function, as conceived by PLUS, encompasses storage, emptying and bio-regulatory functions of the bladder.3
PLUS investigators designed the RISE FOR HEALTH (RISE) study to investigate BH within a population-based prospective longitudinal cohort of community-dwelling adult U.S. women. The RISE study has two major scientific goals: 1) to investigate individual and multi-level factors that promote bladder health and/or prevent LUTS, focusing primarily on modifiable factors and 2) estimate the distribution of BH (from very healthy to very unhealthy) and BH knowledge, attitudes, and beliefs in women across the life course. The methods of the overall study are described in a companion manuscript in this issue. After two initial surveys, a nested sample of individuals within the cohort will be eligible to participate in an in-person visit. This sample is included to augment the RISE survey data with physical and biologic factors associated with BH that may be identified as potential areas of study in future intervention trials.
The RISE in-person assessment will collect data to address five specific research questions: 1) Is optimal musculoskeletal (MSK) health (i.e., functional mobility, perceived MSK pain and psychological factors) associated with BH?; 2) Are women able to perform a pelvic floor muscle contraction properly (including contraction duration, frequency, and correct technique) and is this associated with BH?; 3) Is levator ani muscle tear (i.e., integrity of the pubovisceral/pubococcygeus muscle) associated with BH?; 4) Is pelvic floor myofascial pain (PFMP), assessed by a clinical evaluator, associated with BH?; and, 5) Is the urogenital microbiome related to BH?
Based on formative studies to inform our study design4 we elected not to include specific bladder measures (e.g., bladder diaries) or physiological testing, such as non-invasive uroflowmetry and post-void residual volumes. In our validation study of the Bladder Health Scales and Bladder Function Indices (BHS/BFI), we found poor associations between BH and bladder physiological measures or bladder diary data. Given our emphasis on BH (not LUTS), the RISE study focuses on pelvic floor and musculoskeletal components that may inform future intervention studies related to BH and the prevention of LUTS. This manuscript describes the methods used to develop the RISE in-person assessment protocol.
Materials & Methods
The RISE n-person assessment is planned as a multicenter observational study of a subset of women in the RISE population-based cohort study (n=525). Eligible participants who have expressed interest in participating in the in-person assessment are contacted following completion of the initial baseline bladder health scale (BHS) and bladder function indices (BFI)4 to measure BH along with other baseline demographics and medical history. The PLUS Scientific and Data Coordinating Center (SDCC) notifies clinical research sites (CRC) if a RISE participant indicates interest in being screened for the in-person assessment, which, consistent with BHS/BFI validation parameters, occurs within 8 weeks of BHS/BFI completion.
Screening: A PLUS research coordinator (RC) screens each RISE participant who volunteers for the in-person assessment by telephone (Table 1) prior to the in-person assessment. During the screening call, the RC will establish eligibility, review specifics of the evaluation, and obtain verbal consent for continuation with research contact. Screening information is used to implement a distress protocol for RISE participants with a history of sexual violence/trauma, based on responses to preliminary screening queries, as the study protocol includes four pelvic examination components. Written informed research consent is obtained prior to conducting any research tasks within the in-person assessment. Figure 1 displays the flow of potential participants through this phase of the RISE study.
Table 1:
List of eligibility criteria for RISE in person assessment.
| Inclusion criteria: |
| • Community-dwelling |
| • Age ≥18 years Identify or born as female |
| • Stand independently without human assistance (e.g., can use cane/walker) for 3 minutes |
| • Able to access bathroom and use toilet independently, without assistance from another person |
| • Able to read and understand English or Spanish |
| • Able to provide informed consent |
| • Able to attend an in-person assessment at one of the clinical research centers within 8 weeks of BHI completion |
| • Able and willing to provide the following during in-person assessment: |
| ○ Biospecimen Pilot (initial n=100): Able and willing to provide urine (2), vaginal (2) and stool (1) sample collection at home and ship specimens on day of collection (see Appendix B for instructions). If unable or unwilling to do home specimen, may be included in the in-person only cohort (n=425). This will constitute a protocol deviation and participants will be continued to be asked to participate in the home specimen group until the quota for the site is met. |
| • Willing and able to undergo clinical measures including MSK and pelvic examination (no contraindications to examination – e.g., post operative pelvic surgery, etc). |
| Exclusion criteria |
| • Men |
| • Unable to schedule in-person assessment within 8 weeks of BHI completion |
| • Recent (6 weeks) pelvic, bladder, abdominal, lower extremity, low back surgery, hip replacement or vaginal birth preventing ability to perform MSK or pelvic examination. |
| • Self-catheterizing or has an indwelling catheter by self-report |
| • Unable to stand for 3 minutes |
| • Not able to toilet independently. |
| • Physical or mental condition that would prohibit self-collection of vaginal, urine, or stool specimens or prevent the participant from participating in the study. |
| • Former or current participation in a bladder-related research study or LUTS or has participated in a PLUS study (e.g. VIEW, CLEAR, KAB, LatinX, Where-I-Go, SHARE) |
| • Currently pregnant |
Participants who have recently used, or are currently using, systemic antibiotics will not be excluded but their use will be recorded, recognizing the known effects on the human microbiome.
Figure 1.
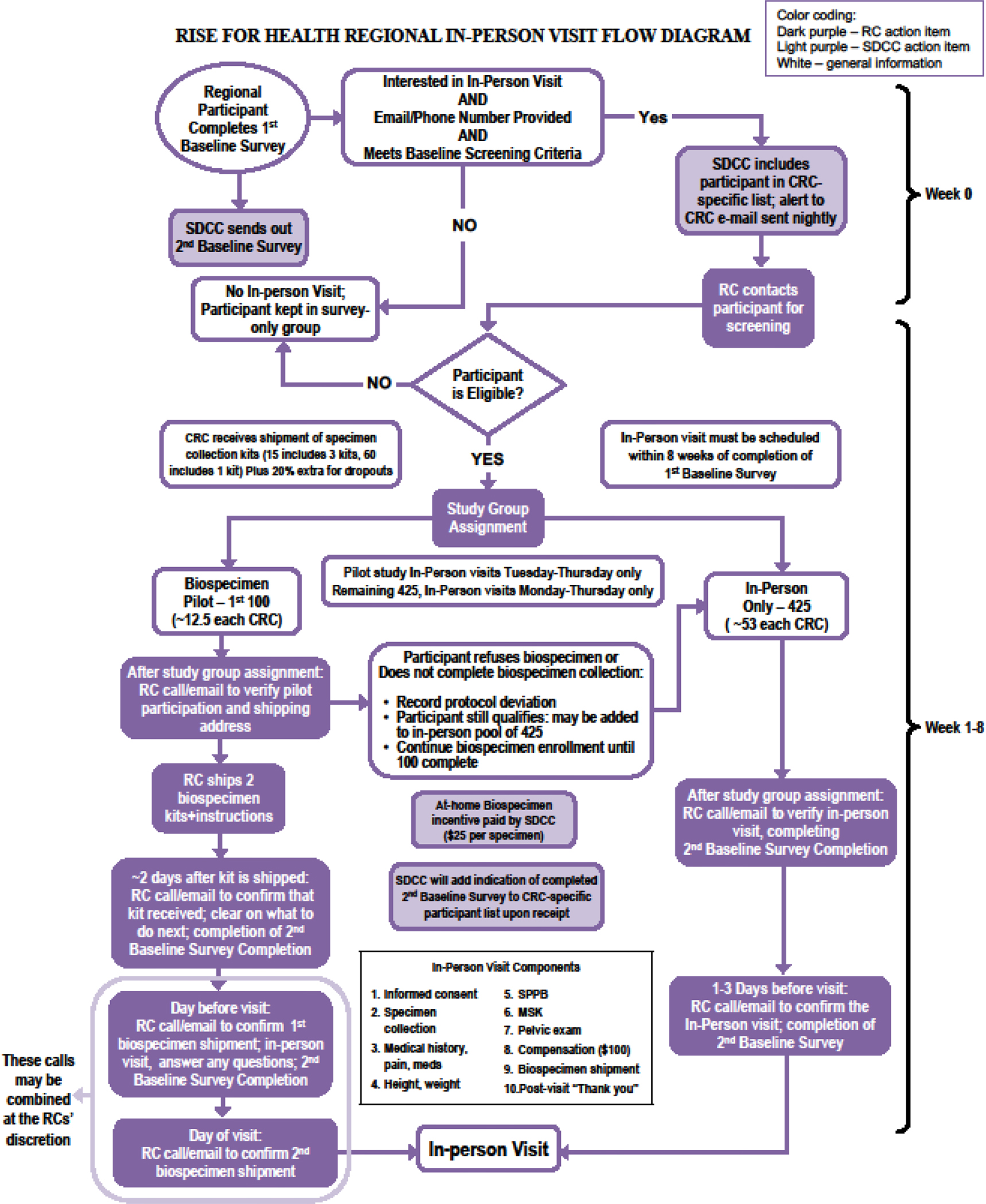
Flow of potential participants in the RISE in-person assessment.
The estimated time to for the participant to complete the in-person assessment is approximately 60 minutes. At the time of the assessment, participants will be asked to complete a short medical history, including current pain medication use, to determine any changes since completion of the baseline surveys. Physical assessments will provide data on factors related to MSK status and pelvic floor function. The planned clinical measures include 15 components. Table 2 highlights the measures and types of data collected along with the specific mapping to key research questions related to BH. Height and weight are recorded. The Short Physical Performance Battery5 (SPPB), an objective assessment tool developed by the National Institute on Aging, is administered to evaluate balance, gait speed, lower extremity strength, and mobility.
Table 2:
Measures and the types of data that will be collected along with the specific mapping to key research questions related to bladder health.
| Measures | RISE Research Question |
|---|---|
| Urine and vaginal specimens (self-collected) | What is the relationship of the urogenital microbiome to bladder health and lower urinary tract symptoms? |
| Medical History & medications | Do women with multiple chronic conditions (specifically higher risk conditions) have lower bladder health status at study baseline and a decreased ability to maintain bladder health over time? Do women who take multiple medications (polypharmacy), especially medications with higher anticholinergic drug burden, have poorer bladder health status at baseline and a decreased ability to maintain bladder health over time? |
| Musculoskeletal (MSK) Measures | Is optimal MSK associated with bladder health and lower urinary tract symptoms? a) Is functional mobility related to MSK conditions associated with bladder health and lower urinary tract symptoms? b) Is perceived MSK pain (location, duration, intensity) associated with bladder health and lower urinary tract symptoms? |
| MSK -Core stability | |
| MSK -Lumbar spine | |
| MSK - External Pelvic Girdle | |
| MSK -Hip Provocation | |
| MSK -Pelvic Girdle Provocation | |
| MSK -Pelvic Girdle Functional Stability | |
| Short Physical Performance Battery | |
| Pelvic Examination | |
| Pelvic Floor Muscle Evaluation | Are women performing pelvic floor muscle exercises (including duration, frequency, and correct performance) and is this associated with bladder health and lower urinary tract symptoms? |
| Palpatory Assessment Pubovisceral Muscle Body Integrity | Is levator ani tear (and the integrity of the levator ani muscles supporting the bladder) associated with bladder health and lower urinary tract symptoms? |
| Myofascial Pain Screening | Is pelvic floor myofascial pain with palpation associated with bladder health, lower urinary tract symptoms, and symptom severity? |
The RISE MSK examination includes six separate tests which are performed on all participants regardless of age: 1) four core stability assessments (single leg squat, supine bridge, prone bridge and side-lying bridge); 2) lumbar spine seated slump test; 3) external pelvic girdle palpation tests involving pain assessments of the sacroiliac joint, anterior superior iliac spine and pubic symphysis); 4) hip pain provocation tests (hip flexion, adduction and internal rotation and hip flexion, abduction and external rotation); 5) pelvic posterior girdle pain provocation test; and 6) pelvic girdle functional stability test: active straight leg raise. The data collection record will note whether a participant was unable to complete any component of the SPPB or MSK tests and document the reason; further analysis of those unable to complete tasks will be considered as data become available.
Following the MSK assessment, women will undergo a focused pelvic floor examination including four specific components: 1) pelvic organ prolapse (POP) assessment; 2) pubovisceral (PV) muscle integrity; 3) PFM strength and endurance; and 4) pelvic floor myofascial pain (PFMP). POP are assessed using a visual assessment for the presence of prolapse protruding beyond the introitus at rest and with Valsalva. PV muscle (also known as pubococcygeus) assessment are performed using a transvaginal palpation technique to assess muscle body integrity where it passes along the vaginal sidewall; both right and left sides are separately assessed and pain documented as: PV present (the muscle body is definitely felt), PV equivocal (the examiner cannot clearly and confidently feel the PV is present or absent ) or PV absent (the muscle body is not felt).6 PFM strength and endurance are assessed using a modified Oxford scale described by Newman, et al.7–10 Pelvic floor myofascial pain (PFMP) are assessed using the method described by Meister et al.11 The PFMP assessment evaluates the obturator internus (OI) and levator ani (LA) muscles for tenderness to palpation. Additional details are provided in companion manuscripts in this issue.
During the in-person assessment at the clinical center, all participants are asked to provide a voided urine sample using the Peezy™ clean catch collection device and a vaginal swab for microbiome analyses. A subset of 100 RISE participants is invited to contribute additional pre-assessment samples in order to assess the feasibility and validation of a home collection of urinary, vaginal, and stool biospecimens. These 100 participants are mailed specimen collection kits to perform clean catch collection voided urine and vaginal swab collections with instructions for shipping to a central biorepository on the day prior to and the day of the In-Person Assessment. An additional stool sample collection is included to be returned with one of the home kits, in order to allow subsequent testing of relationships between pelvic niche microbiomes. Urine samples are shipped in prefilled vials containing Assay Assure DNA protectant and vaginal and stool samples are shipped in ethanol.
Statistical Analysis
Analyses of factors hypothesized to promote bladder health and/or prevent LUTS measured during the in-person assessment will be analyzed as outlined for other self-reported factors with the BHS/BFI serving as the primary outcome. With a sample size of 525 participants, in-person assessment measurements, and a factor prevalence of 10% (e.g., levator tear in vaginally parous women), we will have 90% power to detect differences of 0.47 SD of a BHI score. We will have higher power for factors with higher prevalence. Exploratory analyses of the urogenital microbiome will be conducted and will include alpha and beta diversity of samples collected at the in-person assessment.
For the pilot participants, feasibility of home specimen collection will be assessed using rates of successful sample return and integrity. In addition, we will evaluate the comparability of the measured biome across the three different time frames and methods of collection: first, at home on the day prior to in-person assessment, second, at home on the day of in-person assessment, and third, in research setting at the CRC. The focus will be on the reproducibility of taxa counts and taxa representation across samples that ideally should be identical. Paired differences in proportions across all identified operational taxonomic units (OTUs) will be evaluated. In addition to the paired analysis, we will also use non-metric multidimensional scaling (NMDS) and principal component analysis (PC-A) to provide visual representation, calculate Bray-Curtis distances between the specimen types, and compare with permutational multivariate analysis of variance (PERMANOVA).
Results
Description of the In-Person Assessment Training
Assessment of clinical measures and tests are conducted by trained and certified PLUS research staff (e.g., RCs, clinical evaluators [MD, NP, or CNM] who have experience in performing pelvic examinations) at each clinical center (Table 3). Prior to the start of RISE in-person assessment, participating PLUS research staff completed training on all clinical assessments. Table 4 summarizes the PLUS RISE certification electronic learning (e-Learning) modules and hands-on training. Training included on-line modules, videos and a 2-day in-person, hands-on training; the detailed training and certification approaches are provided in a companion manuscript in this issue. Briefly, in-person training was conducted at a university-based training facility using a “train-the-trainer” model. Compensated patient educators (aka, simulated patients, standardized patients) and other trained professionals were utilized to optimize the training (see agenda/supplement). A certification checklist for each in-person assessment task was used to verify competency. Each clinical center identified the certified trainer(s) who could train and certify additional team members at their own institutions. Consistent with compliance for local licensure, most clinical centers plan to have RCs complete the musculoskeletal assessments, including the SPPB while trained clinical providers will complete the modified pelvic examination components and assessments.
Table 3:
PLUS RISE In Person Training Trainee Credentials
| Research Task | MD | Nurse Practitioner |
Certified Nurse Midwife |
RN | Research Coordinator (not listed in other category) | Total |
|---|---|---|---|---|---|---|
| SPPB | 11 | 3 | 1 | 0 | 13 | 27 |
| MSK | 11 | 3 | 1 | 0 | 10 | 25 |
| PFM | 11 | 4 | 1 | 0 | 0 | 16 |
| Study Coordination | 0 | 0 | 0 | 0 | 9 | 9 |
Table 4:
PLUS RISE certification electronic learning (e-Learning) modules, hands-on training and completion rates.
| e-Learning Modules | Content | Completion Rate n=40 |
| Overview of RISE Clinical Assessments PowerPoint recording | • Pelvic floor anatomy • Description of clinical test/measure |
N = 39 (97.5%) |
| SPPB PowerPoint recording and videos of test | • Complete SPPB Assessment: Part 1- Balance Test and Part 2 - Gait Speed Test, Chair Stand Test • Demonstration of Balance Test • Demonstration of Gait Speed Test • Demonstration of Chair Stand Test |
N = 38 (95%) |
| MSK PowerPoint recording and videos of test | • Complete MSK Assessmenta ○ Demonstration of 4 core stability tests: Single Leg Squat, Supine Bridge, Side Bridge and Prone Bridge ○ Lumbar Spine Seated Slump Test ○ External Pelvic Girdle Palpation Test - Sacroiliac joint (SIJ) ○ External Pelvic Girdle Palpation Test - Anterior Superior Iliac Spine ○ Pubic Symphysis Palpation Test ○ FADIR Supine Test ○ FABER Supine Test ○ Pelvic Girdle Pain Provocation Test ○ Pelvic Girdle Functional Stability Test - Active Straight Leg Raise |
N = 37 (92.5%) |
| Pelvic examination PowerPoint recording | • POP assessment with pictures of prolapse beyond the introitus • Pubovisceral muscle integrity (tear) • Pelvic Floor Myofascial Pain (PFMP) Screening • PFMP Examination Videob,c |
N = 36 (90%) |
| Average completion rate | 93.8% | |
| Hands-on Training | Content | Average Competency Score |
| SPPB | • Assembles equipment • Demonstration with instructions for each test • Recording of score on CRF • SPPB Competency Checklist |
N = 29 Average score = 79.8/80 (99.8%) |
| MSK Exam | • Room and table set-up • Orients “patient educator” to MSK exam • Instructs “patient educator” on components of each test • Recording of score for each individual test on CRF • MSK Competency Checklist |
N = 26 Average score= 292.6/300 (97.5%) |
| Pelvic examination | • Orients “patient educator” to external observation for POP and internal pelvic exam • Instructs “patient educator” on components of each test • Recording of score for each individual test on CRF • Pelvic Exam Competency Checklist |
N = 17 Average score = 115.5/124 (93.1%) |
| Research Coordinator Training | ||
| Study Coordination Overview - PowerPoint |
• Screening • Informed Consent • Site preparation for visit • Review of checklists • Handling a Participant’s Emotional Distress • Handling unusual situations |
N =11 Average score = 131.7/136 (96.8%) |
| Biospecimens Collection - PowerPoint |
• Biospecimens Pilot study • In-Person study biospecimens collection |
NA |
| Redcap overview - PowerPoint |
• Screening • CRFs |
NA |
| Questions and Discussion | • All Research Coordinator Training Content | NA |
Friedrich J, Brakke R, Akuthota V, Sullivan W. Reliability and Practicality of the Core Score: Four Dynamic Core Stability Tests Performed in a Physician Office Setting. Clin J Sport Med. 2017 Jul;27(4):409–414. doi: 10.1097/JSM.0000000000000366
Meister MR, Sutcliffe S, Ghetti C, Chu CM, Spitznagle T, Warren DK, Lowder JL. Development of a standardized, reproducible screening examination for assessment of pelvic floor myofascial pain. Am J Obstet Gynecol. 2019 Mar;220(3):255.e1–255.e9. doi: 10.1016/j.ajog.2018.11.1106.
Research Appreciation for Participation in In-Person Assessment
Research participant appreciation is shown through payment of $100, based on an estimated 1.5 hours to complete the full assessment on each participant, regardless of any participants inability or unwillingness to complete a specific assessment. Participants who provide additional home-collected biospecimens are eligible to receive an additional $50 ($25 for collection of each home biospecimen kit).
Discussion
The development of the RISE in-person assessment protocol challenged the investigators to utilize validated assessments where they existed, and, where they did not, develop novel techniques for the evaluation of the pelvic floor and overall musculoskeletal and BH of participants. The wholistic approach to assessing the biologic and physical contributors to overall BH is novel and will provide a wealth of data from which to establish preliminary hypotheses to test in subsequent prevention trials. While there have been efforts to identify specific factors associated with LUTS, the concept of BH and individual and multi-level factors that promote bladder health are not well established. Thus, the RISE in-person assessment is designed to better understand the factors associated with a healthy bladder as opposed to conventional reports identifying such factors for specific LUTS. We present here the methods for identifying potential physical and biologic factors hypothesized to promote bladder health and/or prevent LUTS. This innovative approach aims to identify novel associations of BH with musculoskeletal function and pain, PFM integrity and function, and urogenital microbial characteristics. Using a transdisciplinary approach, the protocol development group capitalized on expertise in physical medicine and rehabilitation, urogynecology, urology, geriatrics, and microbiome research to establish a protocol that would best capture these elements. Together with other aspects of the study, RISE will evaluate factors associated with BH and/or prevent of LUTS across the biopsychosocial model.12
The RISE in-person assessment is designed to address several knowledge gaps: it systematically assess pelvic floor muscle integrity, strength, and pain with palpation using a standardized examination approach in a multicenter study; it incorporates focused, validated musculoskeletal assessments of overall lower extremity and musculoskeletal function and pain in a diverse population of women recruited from nine US centers; it collects urine, vaginal and stool samples for assessments of the microbiome of these pelvic niches; and it informs the feasibility of home biologic specimen collections in a diverse longitudinal cohort of women for long term follow up of modifiable factors hypothesized to be associated with BH.
Unique to the RISE study, investigators will assess MSK health status, as both low back pain and urinary symptoms have high prevalence throughout the female lifecourse.13,14 Globally, female individuals, ages 40–80, have the highest prevalence of low back pain.15 Seventy-eight percent of childbearing women with low back pain report urinary incontinence (UI) and demonstrate signs of dysfunctional pelvic floor muscles (PFM).16 Patients with chronic MSK conditions (including spine, peripheral joint, muscle, and tendon) are more likely to have decreased physical activity and quality of life, and number of body regions with pain (<3 vs >3) determines this risk; these relationships are more pronounced in women.16 The SPPB is an objective assessment of physical function which decreases with age. The relationship of LUTS, specifically UI, with declining physical function is thought to be bidirectional and may be amenable to physical activity interventions although there are few prospective data on these relationships.17
PV muscle tear has been identified as a risk factor for two important pelvic floor disorders: POP and possibly stress UI.18–21 Pelvic floor myofascial pain (PFMP) may occur in conjunction with, or as sequelae of, diseases of the urinary, genital, colorectal, or musculoskeletal systems, or it may arise independently and has been observed in patients with LUTS and pelvic floor dysfunction.9,22,23
Establishment of this cohort and decisions for inclusion of important in-person assessment tasks followed an iterative process; challenges related to what elements to include were apparent. Where possible, we selected validated measures. However, some musculoskeletal and pelvic floor constructs lacked validated, standardized assessments. We arrived at the included assessments through rigorous review of existing literature and consensus of expert opinion. Selection of assessments also considered standardization across centers, necessitating development of a rigorous training and certification program. Microbiome collection required establishing collection, preservation, shipping and storage procedures to ensure stability of specimens for sufficient duration to allow for home collection.24 Testing in our pilot study will ensure feasibility of home shipping procedures prior to establishment of a repository for use in future correlation of the urogenital microbiome with BH.
Conclusions
The PLUS investigators contribute this methods description for the RISE in-person assessment to facilitate understanding of the study protocol. The methodological rigor of this protocol is enhanced by standardized training, assessment of training efficacy leading to certification for RISE research tasks, multi-site implementation, and a detailed protocol, which incorporates key assessments relevant to the RISE research questions. The methodology is designed to achieve high standards for complete data collection. The novel use of an embedded biospecimen collection pilot will inform the feasibility of home collection for urobiome specimens in future population-based studies, as well as the short-term variability of the bladder, vaginal and colonic microbiome. Given the growing awareness of the relationship between BH and musculoskeletal health, the detailed assessment of musculoskeletal findings with both questionnaire and physical examination is novel. This work will help inform future investigators as to the need for a MSK questionnaire alone, a physical examination MSK assessment alone, or whether both questionnaire and examination MSK assessments are needed in studying BH.
This in-person assessment is an integral portion of the overall RISE study, an innovative approach to assessing BH utilizing prioritized research questions. This description of the in-person assessment methods is intended to provide methodologic transparency and inform other research teams who join our efforts to improve BH.
Acknowledgements:
We gratefully acknowledge the collegial research work of the Prevention of Lower Urinary Tract Symptoms (PLUS) Research Consortium members
Loyola University Chicago - Maywood, IL (U01DK106898)
Multi-Principal Investigators: Linda Brubaker, MD; Elizabeth R. Mueller, MD, MSME
Investigators: Marian Acevedo-Alvarez, MD; Colleen M. Fitzgerald, MD, MS; Cecilia T. Hardacker, MSN, RN, CNL; Jeni Hebert-Beirne, PhD, MPH; Missy Lavender, MBA.
Northwestern University - Chicago IL (U01DK126045)
Multi-Principal Investigators: James W. Griffith, PhD; Kimberly Sue Kenton, MD; Melissa Simon, MD, MPH; Investigator: Julia Geynisman-Tan, MD;
University of Alabama at Birmingham - Birmingham, AL (U01DK106858)
Principal Investigator: Alayne D. Markland, DO, MSc
Investigators: Tamera Coyne-Beasley, MD, MPH, FAAP, FSAHM; Kathryn L. Burgio, PhD; Cora E. Lewis, MD, MSPH; Gerald McGwin, Jr., MS, PhD; Camille P. Vaughan, MD, MS; Beverly Rosa Williams, PhD.
University of California San Diego - La Jolla, CA (U01DK106827)
Principal Investigator: Emily S. Lukacz, MD
Investigators: Sheila Gahagan, MD, MPH; D. Yvette LaCoursiere, MD, MPH; Jesse Nodora, DrPH.
University of Michigan - Ann Arbor, MI (U01DK106893)
Principal Investigator: Janis M. Miller, PhD, APRN, FAAN
Investigators: Lisa Kane Low, PhD, CNM, FACNM, FAAN.
University of Minnesota (Scientific and Data Coordinating Center) - Minneapolis MN (U24DK106786)
Multi-Principal Investigators: Bernard L. Harlow, PhD; Kyle D. Rudser, PhD
Investigators: Sonya S. Brady, PhD; Haitao Chu, MD, PhD; Cynthia S. Fok, MD, MPH; Peter Scal, PhD; Todd Rockwood, PhD.
University of Pennsylvania – Philadelphia, PA (U01DK106892)
Principal Investigator: Multi-Principal Investigators: Diane K. Newman, DNP FAAN; Ariana L. Smith, MD
Investigators: Amanda Berry, MSN, CRNP; Heather Klusaritz, PhD, MSW; Ann E. Stapleton, MD; Jean F. Wyman, PhD.
Washington University in St. Louis - Saint Louis, MO (U01DK106853)
Principal Investigator: Siobhan Sutcliffe, PhD, ScM, MHS
Investigators: Aimee S. James, PhD, MPH;Jerry L. Lowder, MD, MSc; Melanie R. Meister, MD, MSCI.
Yale University - New Haven, CT (U01DK106908)
Principal Investigator: Leslie M. Rickey, MD, MPH
Investigators: Marie A. Brault, PhD; Deepa R. Camenga, MD, MHS; Shayna D. Cunningham, PhD.
Steering Committee Chair: Linda Brubaker, MD. UCSD, San Diego. (January 2021-)
NIH Program Office: National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urologic, and Hematologic Diseases, Bethesda, MD.
NIH Project Scientist: Julia Barthold, M.D.
Funding:
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (NIH) by cooperative agreements [U24 DK106786, U01 DK106853, U01 DK126045, U01 DK106858, U01 DK106898, U01 DK106893, U01 DK106827, U01 DK106908, U01 DK106892]. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional funding from: National Institute on Aging, NIH Office of Research on Women’s Health.
Footnotes
| Brubaker: | Editorial stipends from JAMA, Urogynecology (formerly Female Pelvic Medicine and Reconstructive Surgery and UptoDate |
| Barthold: | None |
| Fitzgerald | Royalties: UptoDate, Expert Witness: Salvi, Schostok & Pritchard |
| Kenton: | Expert Witness: Butler Snow/Ethicon, Grant Funding: Axonics |
| Lewis | None |
| Lowder: | Expert witness |
| Lukacz: | Consultant: Axonics, Pathnostics, Urovant, BioElectronics; Research Funding: Boston Scientific, Cogentix/Uroplasty; Royalties: UpToDate |
| Markland | None |
| Meister | None |
| Miller | None |
| Mueller: | Royalties: UpToDate |
| Rudser | None |
| Smith | None |
| Newman | Consultant: EBT Medical, COSM, Urovant; Research Funding: Society for Urologic Nurses and Associates; Editorial: Digital Science Press; Royalties: Springer |
Ethics approval Statement: This manuscript is the original work of the authors and has not been submitted for publication elsewhere.
Patient (Participant) Consent Statement: None needed for methods paper
The RISE FOR HEALTH study is registered at clinicaltrials.gov: NCT05365971
Data Availability Statement:
no data
References
- 1.World Health Organization (WHO). International Health Regulations. 2005.
- 2.Lukacz ES, Bavendam TG, Berry A, et al. A Novel Research Definition of Bladder Health in Women and Girls: Implications for Research and Public Health Promotion. J Womens Health (Larchmt). 2018;27(8):974–981. doi: 10.1089/jwh.2017.6786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady SS, Brubaker L, Fok CS, et al. Development of Conceptual Models to Guide Public Health Research, Practice, and Policy: Synthesizing Traditional and Contemporary Paradigms. Health Promot Pract. January 2020:1524839919890869. doi: 10.1177/1524839919890869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantine M, Rockwood T, Rickey L, et al. The Validation of Novel Bladder Health Scales and Function Indices for Research with Women. Am J Obstet Gynecol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welch SA, Ward RE, Beauchamp MK, Leveille SG, Travison T, Bean JF. The Short Physical Performance Battery (SPPB): A Quick and Useful Tool for Fall Risk Stratification Among Older Primary Care Patients. J Am Med Dir Assoc. 2021;22(8):1646–1651. doi: 10.1016/j.jamda.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheng Y, Low LK, Liu X, Ashton-Miller JA, Miller JM. Association of index finger palpatory assessment of pubovisceral muscle body integrity with MRI-documented tear. Neurourol Urodyn. 2019;38(4):1120–1128. doi: 10.1002/nau.23967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chevalier F, Fernandez-Lao C, Cuesta-Vargas AI. Normal reference values of strength in pelvic floor muscle of women: a descriptive and inferential study. BMC Womens Health. 2014;14:143. doi: 10.1186/s12905-014-0143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman DK, Borello-France D, Sung VW. Structured behavioral treatment research protocol for women with mixed urinary incontinence and overactive bladder symptoms. Neurourol Urodyn. 2018;37(1):14–26. doi: 10.1002/nau.23244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bo K, Frawley HC, Haylen BT, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Neurourol Urodyn. 2017;36(2):221–244. doi: 10.1002/nau.23107 [DOI] [PubMed] [Google Scholar]
- 10.Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24(4):374–380. doi: 10.1002/NAU.20144 [DOI] [PubMed] [Google Scholar]
- 11.Meister M, Shivakumar N, Sutcliffe S, Spitznagle TM, Lowder J. Physical examination techniques for the assessment of pelvic floor myofascial pain: a systematic review. Am J Obstet Gynecol. 2018;(In Review). doi: 10.1016/j.ajog.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brady SS, Bavendam TG, Berry A, et al. The Prevention of Lower Urinary Tract Symptoms (PLUS) in girls and women: Developing a conceptual framework for a prevention research agenda. Neurourol Urodyn. 2018;37(8):2951–2964. doi: 10.1002/nau.23787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–2037. doi: 10.1002/art.34347 [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Yoshida H, Hu X, et al. Association between self-reported urinary incontinence and musculoskeletal conditions in community-dwelling elderly women: a cross-sectional study. Neurourol Urodyn. 2015;34(4):322–326. doi: 10.1002/nau.22567 [DOI] [PubMed] [Google Scholar]
- 15.Eliasson K, Elfving B, Nordgren B, Mattsson E. Urinary incontinence in women with low back pain. Man Ther. 2008;13(3):206–212. doi: 10.1016/j.math.2006.12.006 [DOI] [PubMed] [Google Scholar]
- 16.Majlesi J Patients with Chronic Musculoskeletal Pain of 3–6-Month Duration Already Have Low Levels of Health-Related Quality of Life and Physical Activity. Curr Pain Headache Rep. 2019;23(11):81. doi: 10.1007/s11916-019-0817-6 [DOI] [PubMed] [Google Scholar]
- 17.Hassani D, Arya L, Andy U. Continence: Bowel and Bladder and Physical Function Decline in Women. Curr Geriatr reports. 2020;9(2):64–71. doi: 10.1007/s13670-020-00313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JM, Low LK, Zielinski R, Smith AR, Delancey JOL, Brandon C. Evaluating maternal recovery from labor and delivery: Bone and levator ani injuries. Am J Obstet Gynecol. 2015;213(2):188.e1–188.e11. doi: 10.1016/j.ajog.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Low LK, Zielinski R, Tao Y, Galecki A, Brandon CJ, Miller JM. Predicting Birth-Related Levator Ani Tear Severity in Primiparous Women: Evaluating Maternal Recovery from Labor and Delivery (EMRLD Study). Open J Obstet Gynecol. 2014;4(6):266–278. doi: 10.4236/ojog.2014.46043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandon C, Jacobson JA, Low LK, Park L, Delancey J, Miller J. Pubic bone injuries in primiparous women: Magnetic resonance imaging in detection and differential diagnosis of structural injury. Ultrasound Obstet Gynecol. 2012;39(4):444–451. doi: 10.1002/uog.9082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kearney R, Miller JM, Ashton-Miller JA, DeLancey JOL. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstet Gynecol. 2006;107(1):144–149. doi: 10.1097/01.AOG.0000194063.63206.1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams K, Gregory WT, Osmundsen B, Clark A. Levator myalgia: why bother? Int Urogynecol J. 2013;24(10):1687–1693. doi: 10.1007/s00192-013-2089-8 [DOI] [PubMed] [Google Scholar]
- 23.Pastore EA, Katzman WB. Recognizing myofascial pelvic pain in the female patient with chronic pelvic pain. J Obstet Gynecol neonatal Nurs JOGNN. 2012;41(5):680–691. doi: 10.1111/j.1552-6909.2012.01404.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung CE, Chopyk J, Shin JH, et al. Benchmarking urine storage and collection conditions for evaluating the female urinary microbiome. Sci Rep. 2019;9(1):13409. doi: 10.1038/s41598-019-49823-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
no data


