Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine-induced systemic antibody profiles are well characterized; however, little is known about whether intranasal mucosal antibodies are induced or can neutralize virus in response to mRNA vaccination.
Objective
We sought to evaluate intranasal mucosal antibody production with SARS-CoV-2 mRNA vaccination.
Methods
SARS-CoV-2–specific IgG and IgA concentrations and neutralization activity from sera and nasal mucosa via nasal epithelial lining fluid (NELF) collection were measured in SARS-CoV-2 mRNA–vaccinated healthy volunteers (N = 29) by using multiplex immunoassays. Data were compared before and after vaccination, between mRNA vaccine brands, and by sex.
Results
SARS-CoV-2 mRNA vaccination induced an intranasal immune response characterized by neutralizing mucosal antibodies. IgG antibodies displayed greater Spike 1 (S1) binding specificity than did IgA in serum and nasal mucosa. Nasal antibodies displayed greater neutralization activity against the receptor-binding domain than serum. Spikevax (Moderna)-vaccinated individuals displayed greater SARS-CoV-2–specific IgG and IgA antibody concentrations than did Comirnaty (BioNTech/Pfizer)-vaccinated individuals in their serum and nasal epithelial lining fluid. Sex-dependent differences in antibody response were not observed.
Conclusion
SARS-CoV-2 mRNA vaccination induces a robust systemic and intranasal antibody production with neutralizing capacity. Spikevax vaccinations elicit a greater antibody response than does Comirnaty vaccination systemically and intranasally.
Key words: SARS-CoV-2, mRNA vaccine, antigens, antibodies, systemic immune response, mucosal immune response, serum, NELF, neutralization, epitope
The nasal cavity acts as a primary site of infection and the first line of defense against many respiratory pathogens, including the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. The nose provides a physical barrier against inhaled particles, functions to trap and clear invading pathogens through the mucosal membrane and mucocilliary clearance, and is a site of immune cell surveillance and signaling.1 In addition, the nasal mucosa is a source of antiviral antibodies (immunoglobulin). Prior studies have found that virus-specific antibodies, including IgG and secretory IgA, are produced within the nasal cavity.2 IgG is the most abundant antibody found in human serum and is involved in opsonization for phagocytosis, neutralization, and activation of the complement system.3 IgGs can also access the nasal mucosal surface by passive diffusion across epithelium.4 IgA antibodies are found systemically and at mucosal surfaces, and they can exist in both the monomeric and dimeric form. Whereas monomeric IgA is found systemically and performs opsonizing functions, dimeric secretory IgA is the primary immunoglobulin subtype that mediates mucosal immunity through neutralization and is secreted by plasma cells adjacent to the mucosal epithelial layer in mucosal tissue, such as the nasal cavity.5 Despite this, only the live attenuated influenza virus vaccine uses the nasal cavity as a target for antibody development. Furthermore, the nasal mucosa is not frequently utilized as a sample site for biomarkers indicating immunologic change against vaccination or respiratory virus infection.
With the sudden global emergence of the SARS-CoV-2 virus, quick development of an effective vaccine was necessary, and the novel mRNA-based vaccine was produced within 1 year.6 Whereas traditional attenuated vaccines rely on directly introducing the target epitope, mRNA vaccines utilize modified viral mRNA encapsulated in lipid nanoparticles encoding for specific antigen targets of interest.7 On the basis of effectiveness studies, we know that intramuscularly administered mRNA vaccination exponentially increased the systemic production of SARS-CoV-2–specific IgG and IgA antibodies.8,9 However, whether and how mRNA-based vaccination translates to antibody production within the nasal mucosa is unknown and presents a critical knowledge gap. Although initially boasted greater than 90% efficacy in preventing coronavirus disease 2019 (COVID-19) illness, continued viral evolution has resulted in multiple SARS-CoV-2 variants with increasing prior infection–induced immune evasiveness and transmissibility.10, 11, 12 Thus, ongoing research continues to pursue vaccination methods that prevent SARS-CoV-2 infection and boost immune response to infection. This research includes exploring the benefit of heightened mucosal IgA, as it has been shown to be effective in viral neutralization and priming of the immune response to infection in response to other viruses, including influenza.13 This strategy may also prove beneficial in the fight against SARS-CoV-2, as evidenced by a preclinical study examining the effectiveness of targeting the nasal cavity as a primary site of infection. That study found that unadjuvanted intranasal vaccination following intramuscular mRNA vaccination elicits a robust mucosal immune response within the respiratory tract in mice, thereby providing complete protection and partial immunity against lethal SARS-CoV-2 infection.14 The importance of mucosal immunity has also been addressed in other studies exploring potential protective roles of cell types, such as CD8+ T cells. Specifically, in a study of SARS-CoV-2–challenged mice CD8+ T-cell protection was found to be redundant in the presence of respiratory mucosal neutralizing antibodies. Furthermore, protection afforded by CD8+ T cells alone was insufficient in preventing infection within the lungs of CD4+ T-cell–depleted mice, emphasizing the critical role of mRNA vaccine-induced neutralizing antibodies.15 Taken together, these findings indicate that localized production of virus-specific antibodies within primary sites of infection, such as the nasal passages, may be crucial in preventing infection and transmission of respiratory pathogens, such as SARS-CoV-2.
In the study presented here, we examined virus-specific antibody profiles and neutralization activity of SARS-CoV-2 mRNA–vaccinated individuals at 2 different sites, in the nasal mucosa and in circulating serum. We hypothesize that mRNA vaccination against the SARS-CoV-2 virus induces virus-specific antibody production within the nasal cavity. Through use of our well-established nasal epithelial lining fluid (NELF) sampling method, the study presented here compares virus-specific antibody concentrations derived from the nasal cavity and serum across vaccine status, sex, and vaccine brands.16
Methods
Study protocol
Blood and NELF samples were collected from healthy human volunteers from March 2021 to December 2021 to examine changes in virus-specific antibody levels in response to the novel mRNA-based SARS-CoV-2 vaccine. Subjects were recruited as a convenience sample from the local area, including the university campus and surrounding communities, through informational campus-wide emails and listserv announcements. The exclusion criteria called for excluding immunocompromised individuals or those currently taking immunosuppressive medication, cigarette smokers or those with significant smoking history, individuals who refused vaccination, and vaccinated individuals who did not receive either the Spikevax (Moderna, Cambrideg, Mass; mRNA-1273) or Comirnaty (BioNTech/Pfizer (Mainz, Germany; BNT162b2) vaccine. Subject visits were categorized as either preimmunity or postimmunity visits. The participants in the preimmunity group included those whose visit occurred before 2 weeks after the second SARS-CoV-2 vaccination, whereas the postimmunity group consisted of participants whose visit took place at least 2 weeks after the second SARS-CoV-2 vaccination. We chose 2 weeks after the second vaccination as a time point on the basis of prior research observing peak virus-specific antibody concentrations at 2 weeks following the second mRNA-based SARS-CoV-2 vaccination.17 When possible, subjects were sampled before vaccination, between the first and second vaccinations, and again at least 2 weeks after administration of the second vaccination. Participants with a history of SARS-CoV-2 were sampled if their most recent positive test result occurred at least 6 months before sampling and they no longer displayed associated symptoms. Additionally, data from individuals with a history of SARS-CoV-2 infection were included only if their antibody concentrations before complete vaccination were comparable to those of uninfected individuals, indicating similar baseline antibody levels.
During visit 1, participants completed the consent process; were examined by a physician; and self-reported their age, sex, race, body mass index (BMI), and health history, including SARS-CoV-2 vaccination status, vaccine brand administered, dates of vaccination(s), and any history of SARS-CoV-2 diagnosis. Blood was collected via venipuncture by a trained phlebotomist, and serum was isolated through centrifugation. NELF was collected through methods previously reported.16 Serum and NELF samples were stored at –80°C and –20°C, respectively, until analysis. The protocol was submitted to and approved by the University of North Carolina Biomedical institutional review board under institutional review board no. 21-0371, and all methods were performed in accordance with the relevant guidelines and regulations.
Sample analysis
Total IgA and IgG concentrations.
Serum and NELF samples were analyzed via singleplex electrochemiluminescence indirect-sandwich ELISAs targeting human IgA (Human IgA ELISA Kit [reference no. BMS2096], Invitrogen, Waltham, Mass) and IgG (Human IgG ELISA Kit [reference no. BMS2091], Invitrogen) per the manufacturer's instructions. The dilutions used for measuring total human IgG were 1:500,000 and 1:10,000 for serum and NELF, respectively. The dilutions used for measuring total human IgA were 1:10,000 and 1:1,000 for serum and NELF, respectively. Summary data available in Table E1 (in the Online Repository at www.jaci-global.org).
SARS-CoV-2–specific IgA and IgG concentrations
Serum and NELF samples were analyzed via multiplex electrochemiluminescence indirect-sandwich ELISAs targeting IgA (V-PLEX SARS-CoV-2 Panel 1 Kit [catalog no. K15361U], Meso Scale Discovery, Rockville, Md) and IgG (V-PLEX SARS-CoV-2 Panel 1 Kit [catalog no. K15359U], Meso Scale Discovery) per the manufacturer's instructions. As the SARS-CoV-2 mRNA vaccines encode for the systemic production of antibodies targeting epitopes of the SARS-CoV-2 Spike protein (most notably, the receptor-binding domain [RBD], but also the N-terminal domain [NTD] and Spike 2 [S2]), these epitopes were primary targets of analysis, with S2 binding measured indirectly.10 The dilutions used for measuring SARS-CoV-2–specific IgG and IgA were 1:40,000 and 1:2,500 for serum and NELF, respectively. Calculated virus-specific IgA and IgG concentrations were normalized to total IgA and IgG as seen in Equation 1.
| (Equation 1) |
S1 binding specificity
Serum and NELF IgA and IgG percentage of binding specificity toward the Spike 1 (S1) subunit against overall Spike protein binding in the postimmunity group was calculated as seen in Equation 2.
| (Equation 2) |
Serum and NELF SARS-CoV-2 S1 RBD neutralization
Antibody neutralization activity in serum and NELF samples toward the SARS-CoV-2 Spike S1 RBD antigen was measured via multiplex neutralization ELISAs (SARS-CoV-2 Panel 11 Kit [catalog no. K15458U], Meso Scale Discovery). The dilutions used for measuring SARS-CoV-2 S1 RBD-specific IgG and IgA neutralization activity were 1:400 and 1:5, respectively. Percent inhibition was calculated as seen in Equation 3. Percent inhibition when controlling for virus-specific antibody concentrations was calculated as in accordance with Equation 4, in arbitrary units (AU).
| (Equation 3) |
| (Equation 4) |
Statistical analysis
Comparisons of serum and NELF IgA and IgG concentrations against target antibodies across vaccine status, vaccine brands, and sex, as well as postimmunity neutralization activity analysis, was completed after Shapiro-Wilk normality testing. Unpaired t tests and Mann-Whitney tests were used to compare IgG and IgA concentrations and neutralization activity across groups. Analyses were completed and graphs were plotted in GraphPad Prism 9.2.0 (GraphPad Software, San Diego, Calif). Differences with a P value less than .05 were considered statistically significant.
z Score values of age, BMI, and antibody concentrations were measured for contribution toward variance in serum and NELF IgA and IgG concentrations against SARS-CoV-2 antigens through principal component analysis (see Figs E1-E5 in the Online Repository at www.jaci-global.org). Analyses were completed in R-4.2.0 using the FactoMineR18 and Factoextra19 packages.
Results
Demographics
Subject demographics are summarized in (Table I). There were no differences among groups in terms of age or BMI. As suggested in prior studies, data on potential sex differences in response to vaccination were analyzed by sex.20,21 However, sex-dependent differences in serum and NELF antibody concentrations and neutralization activity were not observed for this study. This finding may be limited by a small sample size, and future studies with a larger population are needed to confirm a lack of sex differences.
Table I.
Study demographics
| Characteristic | N = 29 | n1 = 19 (preimmunity visits) | n2 = 24 (postimmunity visits) | n3 = 14 (Comirnaty postimmunity visits) | n4 = 10 (Spikevax postimmunity visits) |
|---|---|---|---|---|---|
| Age (y), mean ± SD | 25.80 ± 7.23 | 24.29 ± 5.71 | 26.64 ± 7.60 | 27.5 ± 8.54 | 25.43 ± 6.28 |
| Male-to-female ratio | 13:16 | 11:8 | 14:10 | 5:9 | 5:5 |
| BMI (kg/m2), mean ± SD | 25.20 ± 6.09 | 25.89 ± 6.67 | 25.05 ± 6.32 | 24.1 ± 4.49 | 26.37 ± 8.34 |
| Race | |||||
| Black | 3 | 2 | 2 | 2 | 0 |
| Asian | 2 | 2 | 2 | 2 | 0 |
| White | 22 | 13 | 19 | 10 | 9 |
| Black/Asian | 1 | 1 | 1 | 0 | 1 |
| Asian/White | 1 | 1 | 0 | 0 | 0 |
Preimmunity versus postimmunity
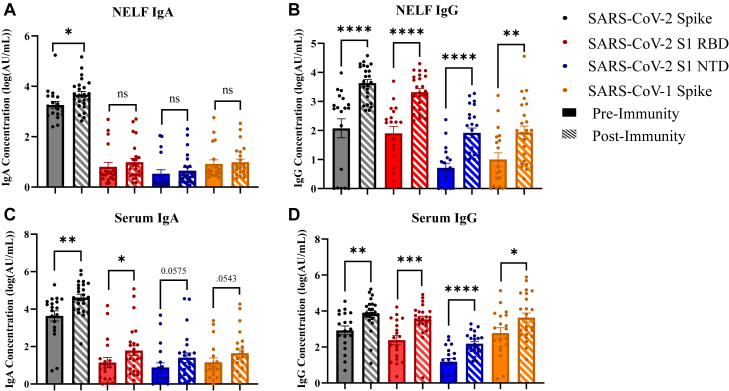
SARS-CoV-2 protein–specific IgA and IgG concentrations were compared between the preimmunity and postimmunity groups in both serum and NELF samples (see Tables E2-E8 in the Online Repository at www.jaci-global.org). Spike protein–specific IgA and IgG concentrations were greater in the postimmunity group than the preimmunity group for both NELF (Fig 1, A and B) and serum (Fig 1, C and D) samples. Nasal mucosal IgA and IgG antibody concentrations (14,116 ± 30,586 AU and 54,434 ± 8,195 AU, respectively) were an order of magnitude lower than those the serum concentrations (154,245 ± 265,080 AU and 15,546 ± 31,600 AU, respectively). S1 RBD protein–specific IgG concentrations were greater in the postimmunity group than in the preimmunity group for both serum and NELF samples (Fig 1, B and D). Only the serum-derived S1 RBD protein–specific IgA concentration was greater in the postimmunity group than in the preimmunity group, indicating a weaker IgA immune response to this antigen within the nasal cavity (Fig 1, C). S1 NTD protein–specific IgG concentrations were greater in the postmmunity group than in the preimmunity group for both serum and NELF samples (Fig 1, B and D). However, a difference in S1 NTD protein–specific IgA concentrations was not observed in either the serum or NELF samples, indicating a weaker IgA immune response systemically and within the nasal cavity to this antigen (Fig 1, A and C). No difference in SARS-CoV-2 nucleocapsid protein–specific IgA and IgG concentrations was found when the preimmunity and postimmunity groups were compared across serum and NELF samples (see Table E7). Severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) Spike protein–specific IgG concentrations were greater in the postimmunity group than in the preimmunity group for both serum and NELF samples (Fig 1, B and D). However, a difference in SARS-CoV-1 Spike protein–specific IgA concentration was not found in either the serum and NELF samples (Fig 1, A and C). As both SARS-CoV-2 and SARS-CoV-1 share similar binding mechanisms utilizing the Spike protein, these results may indicate conserved epitopes for antibody recognition.22
Fig 1.
Preimmunity and postimmunity SARS-CoV-2 virus–specific IgG and IgA antibody concentrations in NELF and serum. Antibody concentrations were reported as log10 values. A, NELF IgA. B, NELF IgG. C, Serum IgA. D, Serum IgG. Unpaired t test and Mann-Whitney test with Shapiro Wilk normality testing. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Figures generated in GraphPad Prism 9.2.0.
S1 subunit binding specificity
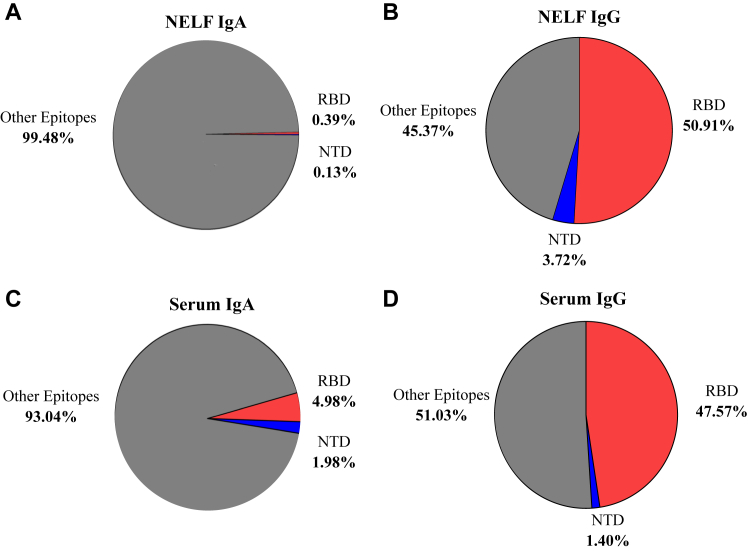
The ratio of RBD and NTD to SARS-CoV-2 Spike protein–specific antibody concentration in the postimmunity group was calculated to measure S1 subunit binding specificity in NELF and serum samples. S1 subunit IgG binding accounted for 54.63% (50.91% derived from RBD and 3.72% derived from NTD) and 48.97% (47.57% derived from RBD and 1.40% derived from NTD) of the total binding to the SARS-CoV-2 Spike protein in the NELF and serum samples, respectively (Fig 2, A and B). In contrast, S1 subunit IgA binding accounted for 0.52% (0.39% derived from RBD and 0.13% derived from NTD) and 6.96% (4.98% derived from RBD and 1.98% derived from NTD) of the total binding to the SARS-CoV-2 Spike protein in the NELF and serum samples, respectively (Fig 2, B and C). Thus, it is plausible that IgA antibodies may have more affinity to S2 epitopes; however this was not measured directly in this study.
Fig 2.
Ratio of postimmunity SARS-CoV-2 S1 RBD and NTD to Spike-specific antibody concentrations in NELF and serum. A, NELF IgA. B, NELF IgG. C, Serum IgA. D, Serum IgG. Figures generated in GraphPad Prism 9.2.0.
Spikevax versus Comirnaty
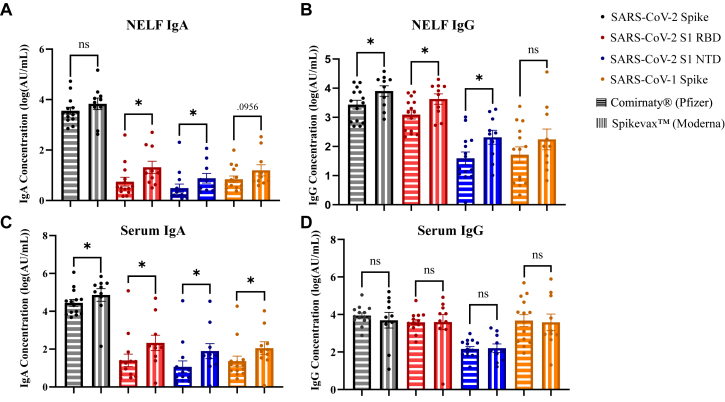
SARS-CoV-2 protein–specific IgA and IgG concentrations were compared between Spikevax- and Comirnaty-vaccinated individuals in both serum and NELF samples (see Table E9 in the Online Repository at www.jaci-global.org). Spike protein–specific serum IgA and NELF IgG concentrations were greater in Spikevax-vaccinated individuals than in Comirnaty-vaccinated individuals across postimmunity visits (Fig 1, B and C). S1 RBD protein–specific serum and NELF IgA and NELF IgG concentrations were greater in Spikevax-vaccinated individuals than in Comirnaty-vaccinated individuals across postimmunity visits (Fig 3, A-C). S1 NTD protein–specific serum and NELF IgA and NELF IgG concentrations were greater in Spikevax-vaccinated individuals than in Comirnaty-vaccinated individuals across postimmunity visits (Fig 3, A-C). Nucleocapsid protein–specific serum IgG concentrations were greater in Spikevax-vaccinated individuals than in Comirnaty-vaccinated individuals across postimmunity visits. However, SARS-CoV-2 nucleocapsid protein–specific NELF IgA concentrations were greater in Comirnaty-vaccinated individuals than in Spikevax-vaccinated individuals. As neither vaccine brand encodes for the nucleocapsid protein, this difference in concentration could be due to unreported SARS-CoV-2 infection or potential antibody cross-reactivity. SARS-CoV-1 Spike protein–specific serum IgA concentrations were greater in Spikevax vaccinated individuals compared to Comirnaty across postimmunity visits (Fig 3, C).
Fig 3.
Comirnaty and Spikevax postimmunity SARS-CoV-2 virus–specific IgG and IgA antibody concentrations in NELF and serum. Antibody concentrations were reported as log10 values. A, NELF IgA. B, NELF IgG. C, Serum IgA. D, Serum IgG. Unpaired t test and Mann-Whitney test with Shapiro Wilk normality testing. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Figures generated in GraphPad Prism 9.2.0.
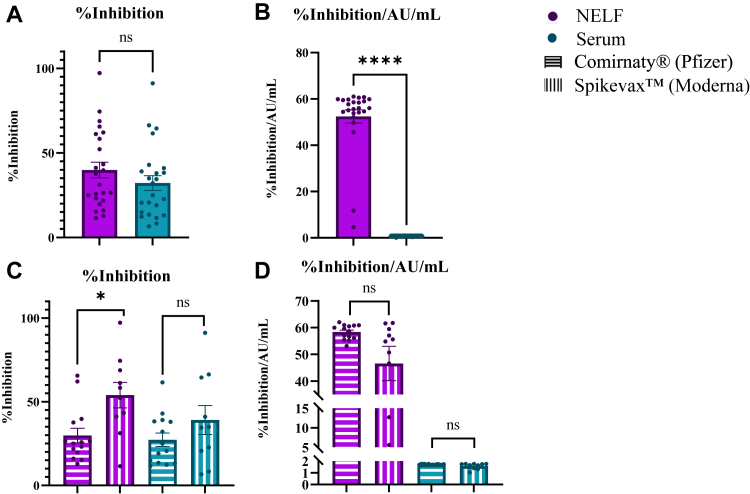
SARS-CoV-2 S1 RBD neutralization activity
A difference in percent inhibition was not observed when comparing serum and NELF, with signal levels falling within the standard curve for both sample types, thus indicating appropriate dilution concentrations for both sample types (Fig 4, A). When the concentration of virus-specific antibodies was controlled for, nasal antibodies displayed greater neutralization activity than did serum antibodies in the postimmunity group (Fig 4, B). In NELF samples, percent inhibition was elevated in Spikevax-vaccinated individuals versus in Comirnaty-vaccinated individuals (Fig 4, C). However, when concentration of virus-specific antibodies was controlled for, a difference in percent inhibition in serum and NELF was not found when Comirnaty- and Spikevax-vaccinated individuals were compared (Fig 4, D).
Fig 4.
Postimmunity SARS-CoV-2 S1 RBD–specific neutralization activity in NELF and serum. A, Percent inhibition in NELF and serum. B, Percent inhibition (reported as AU/mL) in NELF and serum. C, Percent inhibition comparing Comirnaty and Spikevax in NELF and serum. D, Percent inhibition (reported as AU/mL) comparing the Comirnaty and Spikevax vaccines in NELF and serum. Unpaired t test and Mann-Whitney test with Shapiro-Wilk normality testing. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. Figures generated in GraphPad Prism 9.2.0.
Discussion
The purpose of this study was to evaluate the hypothesis that intramuscular mRNA-based vaccination against the SARS-CoV-2 virus induces virus-specific antibody production within the nasal cavity, which is a primary site of infection. To assess this hypothesis, we sampled nasal epithelial lining fluid to collect soluble biomarkers within the nasal cavity and systemically through serum. Virus-specific antibody concentration and neutralization activity were measured across vaccine status, mRNA-based vaccine brands, and sex. Our results demonstrate that mRNA-based intramuscular vaccination against the SARS-CoV-2 virus induces virus-specific antibody production within the nasal cavity and systemically (Fig 1). Additionally, our results suggest IgG specificity toward the S1 subunit and potentially IgA specificity to the S2 subunit of the SARS-CoV-2 Spike protein (Fig 2). Spikevax vaccination appears to induce a greater concentration of systemic and nasal mucosal antibodies than Comirnaty vaccination does (Fig 3). Lastly, our results demonstrate that nasal-derived antibodies display significantly greater neutralizing characteristics than serum-derived antibodies do (Fig 4, B).
We have demonstrated that intramuscular mRNA vaccination against the SARS-CoV-2 virus induces a robust systemic and intranasal IgG and IgA antibody response against the SARS-CoV-2 Spike protein following intramuscular vaccination, thereby demonstrating the capability of mRNA vaccination to induce a localized immune response within the nasal cavity, which is a primary site of infection (Fig 1). Systemic and nasal IgG targeting the RBD and NTD epitopes of the S1 subunit was found to be elevated after vaccination (Fig 1, B and D). Despite observing an elevated systemic and intranasal IgA response to the SARS-CoV-2 Spike protein, we did not observe a similar degree of increase in systemic and nasal IgA concentrations targeting the RBD and NTD epitopes after vaccination (Fig 1, A and C). This indicates that IgA may not target the RBD and NTD of the S1 subunit but instead bind to other domains found on either the S1 or S2 subunit (Fig 2). We believe that the elevated systemic and intranasal IgA response to the SARS-CoV-2 Spike protein may be attributed to IgA antibodies targeting epitopes of the S2 subunit of the SARS-CoV-2 Spike protein; however, other studies that include S2-specific assessments are needed to verify this hypothesis.
Longitudinal observation of SARS-CoV-2 vaccine response in naive subjects found a significant increase in systemic IgG compared with systemic IgA targeting the RBD-domain 6 months after vaccination.23 This coincides with our results, indicating greater IgG binding specificity toward the RBD-domain than IgA binding specificity toward the RBD-domain. Furthermore, although most ongoing SARS-CoV-2 vaccine research has focused on the S1 subunit, specifically, the RBD, several studies have characterized vaccine-induced antibody response against the S2 subunit as well as its potential as a target in vaccine development. The S2 subunit of the Spike protein is a highly conserved region across coronaviruses that mediates viral fusion to the host membrane following angiotensin-converting enzyme 2 (ACE2) binding.24 A study profiling SARS-CoV-2 Spike mRNA vaccine–induced antibodies identified 42 mAbs targeting the epitopes of the full-length Spike protein in 3 naive vaccinated subjects. Of the mAbs identified, 12 bound specifically to the RBD whereas 10 antibodies bound specifically to the NTD. Surprisingly, 17 antibodies bound specifically to the S2 subunit, providing evidence that SARS-CoV-2 Spike mRNA vaccination induces antibody production targeting the S2 subunit.24 Additionally, vaccination induced a robust population of S2-specific memory B cells 6 months after vaccination, accounting for 40% to 80% of the Spike-specific memory B-cell population.23 This suggests that in addition to the S2 subunit being a target for antibody binding, S2-specific antibodies may play an important role in providing long-term immunity against SARS-CoV-2 infection. However, this is speculation, as no studies quantifying vaccine-induced IgA-specific binding to epitopes found on the S2 subunit have been conducted.
This study has also demonstrated that compared with Comirnaty vaccination, Spikevax vaccination induces a greater level of virus-specific antibody production systemically and intranasally (Fig 3, A-C). Nasal-derived IgG and IgA targeting the RBD and NTD were elevated in Spikevax-vaccinated individuals versus in Comirnaty-vaccinated individuals (Fig 3, A and B). This may indicate a slightly more protective immune response within the nasal cavity, a primary site of infection, in Spikevax-vaccinated individuals. Systemic IgG concentrations targeting the full-length Spike, RBD, and NTD did not significantly differ between Spikevax- and Comirnaty-vaccinated individuals (Fig 3, D). However, systemic IgA concentrations targeting these domains were consistently elevated in Spikevax-vaccinated individuals versus in Comirnaty-vaccinated individuals (Fig 3, C). This coincides with current literature observing elevated anti-Spike and anti-RBD antibody concentrations in Spikevax-vaccinated individuals versus in Comirnaty-vaccinated individuals, resulting in slightly greater vaccine effectiveness and lower associated hospitalizations in Spikevax recipients than in Comirnaty recipients.8,25 Nonetheless, little is known regarding the optimal intranasal antibody concentrations needed to confer protection, and therefore, we cannot confirm whether the observed differences in concentration result in altered functional outcomes.
In addition to determining levels of antibodies in serum and nasal mucosa, we also evaluated neutralization as a measure of immune competence. When we controlled for concentration, nasal antibodies displayed significantly greater levels of neutralization activity toward SARS-CoV-2 RBD than serum antibodies did (Fig 4, B). Another study observed significantly greater proportions of systemic nonneutralizing anti-RBD antibodies than neutralizing antibodies in mRNA-based SARS-CoV-2–vaccinated adults, indicating that systemic antibodies derived from the mRNA-based vaccine may be less involved in neutralization activity.26 Furthermore, mucosal-derived oral fluids have previously demonstrated neutralizing capabilities in response to the Wuhan strain, along with Delta, Alpha, Beta, and Gamma variants of concern in naive vaccinated individuals.27 This indicates that SARS-CoV-2 vaccination may direct an immune response guided by production of neutralizing antibodies within the nasal cavity and other mucosal surfaces. However, as the assay utilized measures overall antibody neutralization against the RBD antigen, we were unable to measure Ig-specific neutralization activity. The production of neutralizing antibodies within the nasal cavity, a primary site of infection for the SARS-CoV-2 virus, may confer further protection by preventing initial dissemination of the virus, and it is encouraging as a starting point for developing intranasal vaccines or boosters for even greater neutralization activity.
Because of declining efficacy of vaccine-induced antibody production against new strains of SARS-CoV-2, different types of boosters, including several targeting viral neutralization in the nasal mucosa, are currently in development.28,29 Studies have shown that priming systemically followed by intranasal boosting may result in systemic immunity similar to that resulting from systemic priming and boosting, but with the additional benefit of eliciting more robust mucosal immunity.30 In a mouse model of waning immunity, intranasal boosting resulted in protection against a lethal dose of SARS-CoV-2. This protection was characterized by elevated numbers of antigen-specific CD8+ tissue-resident memory T cells within the lungs as well as IgA and IgG in bronchial alveolar lavage fluid.14 However, intranasal vaccination alone may not be sufficient to induce a robust immune response. For example, in a recent phase I trial funded by AstraZeneca, 2-dose primary intranasal vaccination utilizing their adeno-associated virus–based ChAdOXI nCoV-19 in vaccine-naive adults induced a weaker anti-Spike mucosal response than in convalescent subjects. Systemic immunity following both intranasal doses was also much weaker than 2 intramuscular vaccine doses.31 It should be noted that this study does have limitations; they include lack of optimization of the intramuscular ChAdOXI nCoV-19 vector for intranasal use. Overall, intranasal booster vaccination following intramuscular priming may contribute significantly to preventing respiratory infection by conferring mucosal immunity and neutralizing pathogens directly at the site of infection.
This study, although novel and informative, does include limitations. A cross-sectional convenience sample with a limited sample size of 29 was utilized for this study, and it may not fully represent the outcomes of all vaccinated individuals. We were also unable to sample all subjects before vaccination to conduct matched analyses. Participants were asked to self-report COVID-19 diagnosis, and we cannot confirm whether or when they had received PCR confirmation. To be conservative, all subjects were included in this study. Additionally, nonmatched comparisons were used during the statistical analysis, thereby reducing the power of comparison. Finally, our sample consisted mainly of young White adults with a healthy BMI, and it may not accurately represent other groups. Although no studies have compared nasal mucosal antibody levels across the life span, what is known about antibody production differences across the life span is that systemic vaccine-induced antibody levels decrease in older populations, and depending on dose, the antibody responses in children may surpass those in adults.32 In studies evaluating antibody levels across age, with Comirnaty vaccine, vaccine-induced Spike-specific antibody levels decreased with increasing age in blood.33 In a head-to-head comparison of Comirnaty and Spikevax, vaccination with the Comirnaty vaccine was observed to result in lower antibody levels in postbooster older adults than in younger individuals, but similar levels were observed across ages for Spikevax.34 In children, the adult dose of Spikevax induced an IgG-dominant response surpassing the levels induced in adults; however, responses were more variable at the pediatric dose.35 Further, children receiving 2 doses of Comirnaty had enhanced IgM and similar IgG cross-reactivity to variants of concern versus that in adults.36 Similarly, there are no studies comparing nasal mucosal antibody levels in individuals with preexisting disease, but in systemic studies, there is some evidence for waning antibody levels over time after vaccination. For example, in individuals with asthma being treated with biologics, IgG levels were lower than in healthy adults 90 days after second vaccination.37 If the change in nasal mucosal antibody levels with age and preexisting disease mirrors systemic trends, we might expect children to have the same or more mucosal antibodies, older populations to have decreased levels, and individuals with preexisting respiratory disease to have varying antibody responses compared with those of young adults. This is plausible on the basis of prior studies in SARS-CoV-2–infected individuals, in which correlation between systemic and mucosal antibody production was observed.38 However, future research is needed to fully evaluate this hypothesis.
Overall, this study demonstrates that intramuscular mRNA vaccination against the SARS-CoV-2 virus elicits a robust immune response within the nasal cavity, as well as systemically (Fig 1). Our results suggest systemic and nasal IgG specificity toward epitopes of the S1 subunit, including the RBD and NTD. Additionally, systemic and nasal IgA may target epitopes of the S2 subunit, such as the heptad repeat (HR) domain (Fig 2); however, further studies would be needed to confirm S2 specificity. We also found that compared with Comirnaty vaccination, Spikevax vaccination elicits a greater antibody response against SARS-CoV-2 systemically and intranasally (Fig 3, A-C). Whereas differences in systemic antibody concentration between Comirnaty and Spikevax do not appear to greatly affect immune response, the optimal intranasal antibody concentrations against SARS-CoV-2 are unknown, and we are unsure whether this concentration difference results in altered functional outcome. Additionally, our results demonstrate that nasal antibodies display significantly greater levels of neutralizing characteristics against the SARS-CoV-2 RBD antigen than systemic antibodies do (Fig 4, B). This suggests an immune response consisting of neutralizing antibodies localized at mucosal primary sites of infection (such as the nasal cavity) and nonneutralizing antibodies located systemically. As SARS-CoV-2 variants continue to emerge and vaccine effectiveness wanes, we believe that intranasal boosting following intramuscular priming while targeting the S2 subunit of the SARS-CoV-2 Spike protein may not only greatly reduce the risk of viral dissemination and infection but also provide immunity against multiple variants.
Data availability
The data that support the findings of this article are publicly available through the UNC Dataverse at https://doi.org/10.15139/S3/YTXJB9.39
Disclosure statement
Supported by a National Institute of Environmental Health Sciences T32 Training Grant in Toxicology and Environmental Health (grant T32 ES007126) and cooperative agreements with the US Environmental Protection Agency (EPA) (agreements CR83578501 and CR84033801). Although this research was supported in part by EPA cooperative agreements, it has not been subjected to review and does not necessarily reflect EPA policy.
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
Key messages.
-
•
Intramuscular mRNA vaccination results in the production of neutralizing antibodies in the nasal mucosa.
-
•
Binding specificity and overall antibody concentrations differ by immunoglobulin class and vaccine brand.
Acknowledgments
We thank the subjects who provided their time, effort, and samples to conduct this research.
Supplementary data
References
- 1.Gallo O., Locatello L.G., Mazzoni A., Novelli L., Annunziato F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol. 2021;14:305–316. doi: 10.1038/s41385-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gianchecchi E., Manenti A., Kistner O., Trombetta C., Manini I., Montomoli E. How to assess the effectiveness of nasal influenza vaccines? Role and measurement of sIgA in mucosal secretions. Influenza Other Respir Viruses. 2019;13:429–437. doi: 10.1111/irv.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janeway C., editor. Immunobiology: the immune system in health and disease [animated CD-ROM inside] 5th ed. Garland Publishing; New York, NY: 2001. p. 732. [Google Scholar]
- 4.Ainai A., Suzuki T., Tamura S.I., Hasegawa H. intranasal administration of whole inactivated influenza virus vaccine as a promising influenza vaccine candidate. Viral Immunol. 2017;30:451–462. doi: 10.1089/vim.2017.0022. [DOI] [PubMed] [Google Scholar]
- 5.Steffen U., Koeleman C.A., Sokolova M.V., Bang H., Kleyer A., Rech J., et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun. 2020;11:120. doi: 10.1038/s41467-019-13992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball P. The lightning-fast quest for COVID vaccines — and what it means for other diseases. Nature. 2021;589:16–18. doi: 10.1038/d41586-020-03626-1. [DOI] [PubMed] [Google Scholar]
- 7.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajema K.L., Dahl R.M., Evener S.L., Prill M.M., Rodriguez-Barradas M.C., Marconi V.C., et al. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans — five veterans affairs medical centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1700–1705. doi: 10.15585/mmwr.mm7049a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisnewski A.V., Campillo Luna J., Redlich C.A. Human IgG and IgA responses to COVID-19 mRNA vaccines. PloS One. 2021;16 doi: 10.1371/journal.pone.0249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chagla Z. In high-risk adults, the Moderna vaccine had 94% efficacy against COVID-19 ≥14 d after the 2nd dose. Ann Intern Med. 2021;174:JC28. doi: 10.7326/ACPJ202103160-028. [DOI] [PubMed] [Google Scholar]
- 12.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Riet E., Ainai A., Suzuki T., Hasegawa H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine. 2012;30:5893–5900. doi: 10.1016/j.vaccine.2012.04.109. [DOI] [PubMed] [Google Scholar]
- 14.Mao T., Israelow B., Peña-Hernández M.A., Suberi A., Zhou L., Luyten S., et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science. 2022;378:eabo2523. doi: 10.1126/science.abo2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingstad-Bakke B., Lee W., Chandrasekar S.S., Gasper D.J., Salas-Quinchucua C., Cleven T., et al. Vaccine-induced systemic and mucosal T cell immunity to SARS-CoV-2 viral variants. Proc Natl Acad Sci. 2022;119 doi: 10.1073/pnas.2118312119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebuli M.E., Speen A.M., Clapp P.W., Jaspers I. Novel applications for a noninvasive sampling method of the nasal mucosa. Am J Physiol Lung Cell Mol Physiol. 2017;312:L288–L296. doi: 10.1152/ajplung.00476.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Röltgen K., Boyd S.D. Antibody and B cell responses to SARS-CoV-2 infection and vaccination. Cell Host Microbe. 2021;29:1063–1075. doi: 10.1016/j.chom.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lê S., Josse J., Husson F. FactoMineR: an R package for multivariate analysis. J Stat Softw. 2008;25:1–18. [Google Scholar]
- 19.Galili T. dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics. 2015;31:3718–3720. doi: 10.1093/bioinformatics/btv428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischinger S., Boudreau C.M., Butler A.L., Streeck H., Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen A., Stromme M., Moyassari S., Chadha A.S., Tartaglia M.C., Szoeke C., et al. COVID-19 vaccines: considering sex differences in efficacy and safety. Contemp Clin Trials. 2022;115 doi: 10.1016/j.cct.2022.106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi G.A., Sacco O., Mancino E., Cristiani L., Midulla F. Differences and similarities between SARS-CoV and SARS-CoV-2: spike receptor-binding domain recognition and host cell infection with support of cellular serine proteases. Infection. 2020;48:665–669. doi: 10.1007/s15010-020-01486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel R.R., Painter M.M., Apostolidis S.A., Mathew D., Meng W., Rosenfeld A.M., et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amanat F., Thapa M., Lei T., Ahmed S.M.S., Adelsberg D.C., Carreño J.M., et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell. 2021;184:3936–3948.e10. doi: 10.1016/j.cell.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Self W.H., Tenforde M.W., Rhoads J.P., Gaglani M., Ginde A.A., Douin D.J., et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions — United States, March-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amanat F., Thapa M., Lei T., Sayed Ahmed S.M., Adelsberg D.C., Carreno J.M., et al. The plasmablast response to SARS-CoV-2 mRNA vaccination is dominated by non-neutralizing antibodies and targets both the NTD and the RBD. Infectious Diseases (except HIV/AIDS) 2021. http://medrxiv.org/lookup/doi/10.1101/2021.03.07.21253098 Available at:
- 27.Longet S., Hargreaves A., Healy S., Brown R., Hornsby H.R., Meardon N., et al. mRNA vaccination drives differential mucosal neutralizing antibody profiles in naive and SARS-CoV-2 previously-infected individuals. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.953949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Link-Gelles R., Ciesla A.A., Fleming-Dutra K.E., Smith Z.R., Britton A., Wiegand R.E., et al. Effectiveness of bivalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection — increasing community access to testing program, United States, September-November 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1526–1530. doi: 10.15585/mmwr.mm7148e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas C., Vogels C.B.F., Yildirim I., Rothman J.E., Lu P., Monteiro V., et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature. 2021;600:523–529. doi: 10.1038/s41586-021-04085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiorino F., Pettini E., Pozzi G., Medaglini D., Ciabattini A. Prime-boost strategies in mucosal immunization affect local IgA production and the type of th response. Front Immunol. 2013;4:128. doi: 10.3389/fimmu.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madhavan M., Ritchie A.J., Aboagye J., Jenkin D., Provstgaad-Morys S., Tarbet I., et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: an open-label partially-randomised ascending dose phase I trial. eBioMedicine. 2022;85 doi: 10.1016/j.ebiom.2022.104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palacios-Pedrero M.Á., Jansen J.M., Blume C., Stanislawski N., Jonczyk R., Molle A., et al. Signs of immunosenescence correlate with poor outcome of mRNA COVID-19 vaccination in older adults. Nat Aging. 2022;2:896–905. doi: 10.1038/s43587-022-00292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collier D.A., Ferreira I.A.T.M., Kotagiri P., Datir R.P., Lim E.Y., Touizer E., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards N.E., Keshavarz B., Workman L.J., Nelson M.R., Platts-Mills T.A.E., Wilson J.M. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.24331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartsch Y.C., St. Denis K.J., Kaplonek P., Kang J., Lam E.C., Burns M.D., et al. SARS-CoV-2 mRNA vaccination elicits robust antibody responses in children. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abn9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartsch Y.C., Chen J.W., Kang J., Burns M.D., St Denis K.J., Sheehan M.L., et al. BNT162b2 induces robust cross-variant SARS-CoV-2 immunity in children. Npj Vaccines. 2022;7:158. doi: 10.1038/s41541-022-00575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Runnstrom M.C., Morrison-Porter A., Ravindran M., Quehl H., Ramonell R.P., Woodruff M., et al. Reduced COVID-19 vaccine response in patients treated with biologic therapies for asthma. Am J Respir Crit Care Med. 2022;205:1243–1245. doi: 10.1164/rccm.202111-2496LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright P.F., Prevost-Reilly A.C., Natarajan H., Brickley E.B., Connor R.I., Wieland-Alter W.F., et al. Longitudinal systemic and mucosal immune responses to SARS-CoV-2 infection. J Infect Dis. 2022;226:1204–1214. doi: 10.1093/infdis/jiac065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao K., Cobos-Uribe C., Knight N., Jonnalgadda R., Robinette C., J I, et al. Replication data for: SARS-CoV-2 mRNA vaccination induces an intranasal mucosal response characterized by neutralizing antibodies. Available at: [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this article are publicly available through the UNC Dataverse at https://doi.org/10.15139/S3/YTXJB9.39