Abstract
Introduction: Dental caries is one of the most common childhood diseases. This study purposed to investigate the prediction capability of potential renal acid load (PRAL), salivary buffer capacity (SBC), and Healthy Eating Index (HEI) on children's dental caries.
Methods: The decay, missing, filing, and teeth for primary teeth (dmft)/Decay, Missing, Filling, and Teeth for permanent teeth (DMFT) indexes of the children aged 7-12 years who applied to our faculty were recorded. Approximately 1 mL of unstimulated saliva samples were collected, and SBC was evaluated. PRAL and HEI scores were calculated by entering the data in the form of a daily nutrition record of the children into the BeBiS software (Ebispro for Windows, Stuttgart, Germany). The association of dental caries indices with PRAL, SBC, and HEI was analyzed using an independent sample t-test. A binomial logistic regression analysis was performed to predict the dental caries burden. The statistical significance level was adjusted to a=0.05.
Results: A total of 150 children, 88 (58.6%) females and 62 (41.4%) males, were included in the study. Significant differences were found between the low and high dental caries groups for dmft regarding PRAL and SBC (p<0.001). A significant difference was found between the low and high dental caries groups for DMFT in terms of SBC (p<0.05).
Conclusion: In our study, established regression models significantly predicted dental caries in primary teeth. SBC was the most influential factor in predicting dental caries compared to PRAL and HEI. There was a significant relationship between SBC, PRAL, and caries in primary teeth. In the model we created, the strongest predictor was SBC.
Keywords: healthy eating index, salivary buffer capacity, potential renal acid load, dental caries, child
Introduction
Dental caries is a chronic, infectious, and multifactorial disease in which tooth structure is destroyed by acidic by-products produced by the fermentation of dietary carbohydrates [1,2]. Caries risk indicators are the variables that are assumed to directly cause or contribute to caries formation. The most commonly used caries risk indicators are low salivary flow rate, cariogenic microflora, presence of caries lesions, presence of visible plaque on the teeth, sugar consumption, presence of appliances in the mouth, difficulty in accessing oral and dental health care and sociodemographic and socioeconomic factors [1-4].
Saliva, which contains many different organic and inorganic substances, is an essential element that affects the oral cavity and tooth surfaces [5]. Changes in the amount and structure of saliva may cause a decrease in moisturizing, lubricating, remineralization, buffering, and antimicrobial effects. Thus, as the salivary pH (SpH) decreases, the risk of infection increases, and dental caries occur [6]. Saliva is considered the most critical host-related factor, which can shift the direction of dental caries activity to remineralization [7,8].
There is a strong connection between dental and overall health, and diet is an essential step in this regard [9,10]. Nutrition is crucial in developing and protecting oral and dental tissues, preventing diseases, and treating them when necessary [11,12]. Potential renal acid load (PRAL) determines the acid loads of foods or diets [13]. The decrease in the PRAL in the negative (-) direction indicates that the diet shifts to alkaline, while its increase in the positive (+) direction indicates that the diet shifts to acidity [14].
The Healthy Eating Index (HEI) is a diet quality index that measures compliance with the Diet Guidelines for Americans (DGA) and includes 13 dietary components [15]. HEI is essential in evaluating the relationship between diet quality and caries [16]. There has been limited research attention on the collective predictive role of salivary buffer capacity (SBC), PRAL, HEI, and dietary factors on dental caries. This study aimed to examine the prediction capability of SBC, PRAL, and HEI on children’s dental caries. The null hypothesis of the study was that dental caries cannot be predicted using SBC, PRAL, and HEI.
Materials and methods
This is a cross-sectional study involving children who applied to Recep Tayyip Erdogan University, Faculty of Dentistry, Department of Pediatric Dentistry. The study was conducted between July and December 2021. Ethics committee approval was retrieved from Recep Tayyip Erdogan University, Faculty of Medicine, Ethics Committee (155/2021). The parents were informed in writing and verbally about the content, duration, and possible benefits of the study and were asked to sign the declaration of consent.
A simple random sampling method was preferred to represent the universe in the sample selection. In the literature review [16], the total sample size was found to be 138 using the G-Power software (Sydney, Australia) with a 0.3 (Cohen) effect size, 95% power, and 0.05 margin of error, based on the percentage measurement values of the methods. Thus, 150 patients were included in the study in order to prevent possible data loss.
Inclusion criteria
Patients aged 7-12 years, without any systemic disease or physical-mental disability, who agreed to answer the saliva collection procedure and the questionnaire and who consumed their meal and brushed their teeth at least two hours before the saliva collection process were included in the study.
Questionnaire (Hanna Instruments-HI-83141)
The language of the questionnaires was Turkish, and these were translated into English. The questionnaires had adequate reliability with Cronbach’s alpha coefficient (>0.70). Questions regarding demographic features were asked and recorded.
Dental examination
Before the examinations, the principal investigator (E.K) was initially trained and calibrated according to the WHO Basic Surveys Calibration Protocol for caries detection, coding findings, and recording. Firstly, the consistency of the decay, missing, filing, and teeth for primary teeth (dmft)/Decay, Missing, Filling, and Teeth for permanent teeth (DMFT) measurements of the investigator (E.K) via obtained data from the first 20 patients who was examined twice with an interval of two weeks. Concordance correlation analysis was used to estimate the intra-rater reliability and sufficient reliability was obtained (r=0.84). A dental examination was performed using a sterile dental Shepherd’s Hook explorer and mirror. Caries evaluation was performed by visual and digital panoramic radiographs. In cases in which caries detection was challenging, the bitewing radiography technique was used to support the panoramic radiograph. After the tooth was dried, each tooth was recorded as decayed (d), missing (m), or filled (f), and the burden of caries was determined according to the dmft/DMFT index. The patients were divided into two parts: subjects with low (dmft≤5, DMFT≤1) and high (dmft>5, DMFT>1) dental caries.
Collection of saliva samples and measurement of salivary buffer capacity
Approximately 1 mL of unstimulated saliva was collected using the Ericsson Method [17] in the next control session between 9-12 hours in the morning, in a calm environment, without any stress. As soon as saliva samples were taken, their buffering capacity was measured with a pH meter (Hanna Instruments-HI-83141). Since the measurement was made immediately, there was no need to store the saliva samples. The children spit into the phantom tubes, and after mixing the saliva with the calibrated tip of the pH meter device (Hanna Instruments-HI-83141), the pH was measured and recorded.
Dietary acidity
Macro- and micronutrient contents of foods (especially protein, phosphorus (P3-), potassium (K2+), magnesium (Mg2+), and calcium (Ca2+)) with a retrospective one-day nutritional record were obtained by using the BeBiS software (BeBiS 9, Turkey). The PRAL was calculated with these data. The formula arranged by Remer [14] according to the kidney acid load formed by the nutrients is as follows:
PRAL (mEq/day)=0.49 × protein (g/day) + 0.037 × phosphorus (mg/day) - 0.021 × potassium (mg/day) - 0.026 × magnesium (mg/day) - 0.013 × calcium (mg/day).
Healthy Eating Index
The foods' macro- and micronutrients were obtained using the BeBiS software (Ebispro for Windows, Stuttgart, Germany) with a retrospective one-day nutritional record. The HEI score was determined with the data obtained with this software. This index has some limitations as it analyzes only one day's nutrition record.
Statistical analysis
Statistical analysis was performed using Jamovi Software (Version: 2.3.21; Sydney, Australia). The relationship between demographic characteristics and caries indices was analyzed using the chi-square test. The distribution of data was tested with the Kolmogorov-Smirnov test. Because the data were normally distributed, the association of dental caries indices with PRAL, SBC, and HEI was analyzed using an independent sample t-test. A binomial logistic regression analysis was performed to predict the dental caries burden. The statistical significance level was adjusted to a=0.05.
Results
A total of 150 children, 88 (58.6%) females and 62 (41.4%) males, were included in the study. No significant relationship was found between dmft/DMFT indices and demographic characteristics (Table 1).
Table 1. Association between dental caries and some demographic attributes.
dmft: decay, missing, filing, and teeth for primary teeth; DMFT: Decay, Missing, Filling, and Teeth for permanent teeth.
| dmft | DMFT | ||||||
| >5 (N=73) | ≤5 (N=77) | p value | >1 (N=70) | ≤1 (N=80) | p value | Total (N=150) | |
| Gender | 0.348 | 0.33 | |||||
| Female | 40.0 (54.8%) | 48.0 (62.3%) | 44.0 (62.9%) | 44.0 (55.0%) | 88.0 (58.7%) | ||
| Male | 33.0 (45.2%) | 29.0 (37.7%) | 26.0 (37.1%) | 36.0 (45.0%) | 62.0 (41.3%) | ||
| Age range | 0.463 | 0.181 | |||||
| 7-10 | 46.0 (63.0%) | 44.0 (57.1%) | 38.0 (54.3%) | 52.0 (65.0%) | 90.0 (60.0%) | ||
| 11-12 | 27.0 (37.0%) | 33.0 (42.9%) | 32.0 (45.7%) | 28.0 (35.0%) | 60.0 (40.0%) | ||
| Socioeconomic status | 0.337 | 0.109 | |||||
| Low | 23.0 (31.5%) | 27.0 (35.1%) | 25.0 (35.7%) | 25.0 (31.2%) | 50.0 (33.3%) | ||
| Mediocre | 29.0 (39.7%) | 22.0 (28.6%) | 28.0 (40.0%) | 23.0 (28.8%) | 51.0 (34.0%) | ||
| High | 21.0 (28.8%) | 28.0 (36.4%) | 17.0 (24.3%) | 32.0 (40.0%) | 49.0 (32.7%) | ||
| Toothbrushing habit | 0.881 | 0.31 | |||||
| Always | 14.0 (19.2%) | 14.0 (18.2%) | 11.0 (15.7%) | 17.0 (21.2%) | 28.0 (18.7%) | ||
| Sometimes | 55.0 (75.3%) | 60.0 (77.9%) | 54.0 (77.1%) | 61.0 (76.2%) | 115.0 (76.7%) | ||
| Never | 4.0 (5.5%) | 3.0 (3.9%) | 5.0 (7.1%) | 2.0 (2.5%) | 7.0 (4.7%) | ||
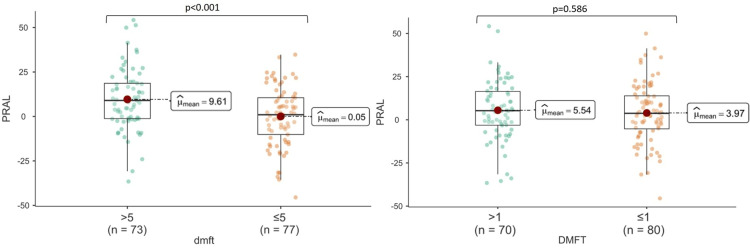
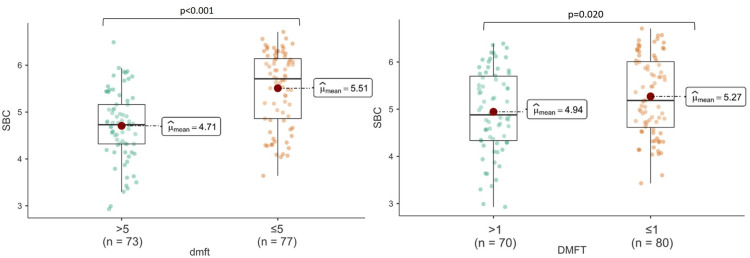
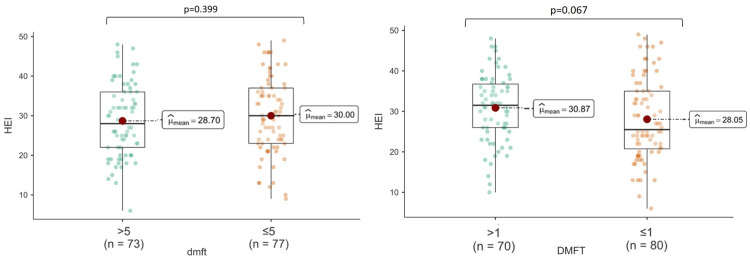
Significant differences were found between the low and high dental caries groups for dmft regarding PRAL and SBC (p<0.001); however, there was no significant difference regarding HEI (p>0.05). A significant difference was found between the low and high dental caries groups for DMFT in terms of SBC (p<0.05); however, there was no significant difference in terms of PRAL and HEI (Figures 1-3).
Figure 1. The relationship between PRAL and dental caries indices.
PRAL: potential renal acid load; dmft: decay, missing, filing, and teeth for primary teeth; DMFT: Decay, Missing, Filling, and Teeth for permanent teeth.
Figure 2. The relationship between SBC and dental caries indices.
SBC: salivary buffer capacity; dmft: decay, missing, filing, and teeth for primary teeth; DMFT: Decay, Missing, Filling, and Teeth for permanent teeth.
Figure 3. The relationship between HEI and dental caries indices.
HEI: Healthy Eating Index; dmft: decay, missing, filing, and teeth for primary teeth; DMFT: Decay, Missing, Filling, and Teeth for permanent teeth.
PRAL (OR=0.99, p=0.466), SBC (OR=3.5, p<0.001), and HEI (OR=1.02, p=0.331) were added to model 1, and the model was adjusted with gender, age range, socioeconomic features, and tooth brushing habit. The model explained 20% of the variance (McFadden’s R2=0.20) (Table 2).
Table 2. Binominal logistic regression analysis for dmft.
Adjusted with gender, age range, socioeconomic features, and tooth brushing habit. PRAL: potential renal acid load, SBC: salivary buffer capacity, HEI: Healthy Eating Index.
| Predictor | Estimate | SE | Z | p | Odds ratio | 95% CI Lower | 95% CI Upper | McFadden’s R2 |
| Model 1 | 0.20 | |||||||
| Intercept | -6.76 | 1.6 | -4.22 | 0 | 0 | 0.03 | ||
| SBC | 1.25 | 0.27 | 4.59 | 3.5 | 2.05 | 5.97 | ||
| PRAL | -0.01 | 0.01 | -0.73 | 0.466 | 0.99 | 0.97 | 1.02 | |
| HEI | 0.02 | 0.02 | 0.97 | 0.331 | 1.02 | 0.98 | 1.06 |
PRAL (OR=1.01, p=0.373), SBC (OR=1.68, p=029), and HEI (OR=0.96, p=0.058) were added to model 2, and the model was adjusted with gender, age range, socioeconomic features, and tooth brushing habit. The model explained 8% of the variance (McFadden’s R2=0.08) (Table 3).
Table 3. Binominal logistic regression analysis for DMFT.
Adjusted with gender, age range, socioeconomic features, and tooth brushing habit. PRAL: potential renal acid load, SBC: salivary buffer capacity, HEI: Healthy Eating Index.
| Predictor | Estimate | SE | Z | p | Odds ratio | 95% CI Lower | 95% CI Upper | McFadden’s R2 |
| Model 2 | 0.08 | |||||||
| Intercept | -1.5 | 1.38 | -1.09 | 0.276 | 0.22 | 0.02 | 3.31 | |
| SBC | 0.52 | 0.24 | 2.19 | 0.029 | 1.68 | 1.06 | 2.68 | |
| PRAL | 0.01 | 0.01 | 0.89 | 0.373 | 1.01 | 0.99 | 1.03 | |
| HEI | -0.04 | 0.02 | -1.89 | 0.058 | 0.96 | 0.93 | 1 |
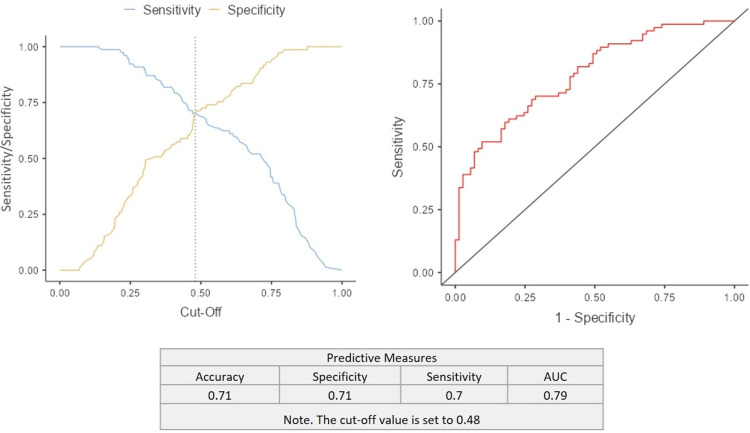
In the receiving operating characteristic (ROC) analysis, the optimal cut-off value of 0.48 for dmft was seen to have 70% sensitivity and 71% specificity. The cut-off plot and ROC curve are presented in Figure 4.
Figure 4. Cut-off plot and ROC curve presentation.
AUC: area under curve; ROC: receiving operating characteristic.
Discussion
In our study, there was a significant relationship between SBC, PRAL, and caries in primary teeth. In the model we created using SBC, PRAL, and HEI, the strongest predictor was SBC. We can say that if we know SBC, we can make a significant guess whether the child has dental caries or not; at the same time, we can say that SBC affects dental caries much more than other factors.
The effectiveness of SBC depends on various factors such as systematic, genetic diseases, diet, hygiene habits, and medicines used [18-20]. Several studies have shown that salivary pH is a significant predictive factor for caries development [21-23], in agreement with the present study. In this study, SBC was the most effective predictor for dmft. The buffering capacity of saliva and fermentable carbohydrates affects the plaque's pH. When the saliva is unstimulated, the pH in the plaque is approximately between 6 and 7.3. The pH rises during the first five minutes after ingestion of most foods. Then, the pH drops to its lowest level, 6.1 or even less, approximately 15 minutes after food consumption. Plaque pH gradually returns to its resting pH unless there is an additional intake of fermentable carbohydrates. Demineralization occurs when acids diffuse through the plaque and pellicle into the liquid phase of the enamel between the enamel crystals. The resulting dissolution occurs at a pH of 5-5.5, the critical pH range for caries development. The dissolved minerals diffuse out of the tooth structure and into the saliva surrounding the tooth. The buffering capacity of saliva significantly affects the plaque's pH surrounding the enamel, thus preventing the progression of caries [7,8,24-26].
Acid-base balance affected by dietary habits is becoming increasingly noteworthy in medical fields. PRAL enables the estimation of endogenous acid production exceeding the alkaline level produced for specific amounts of food ingested daily [7]. Akyüz et al. [26] found that the PRAL value is positively correlated with grain and meat consumption and negatively correlated with fruit and vegetable consumption. Also, a negative correlation was exhibited between the number of caries and the consumption of milk and dairy products and between the number of fillings and fruit consumption. In our current work, a positive correlation was found with the number of primary tooth fillings. Also, it has been reported that the PRAL does not significantly correlate with the number of primary tooth caries, dmft, and dmft index scores.
Nowadays, foods with cariostatic and cariogenic properties are included in most meals. Therefore, the relationship between diet content and caries should be assessed. The negative correlation between HEI and dental caries has been shown in several studies [16,27]. However, the present study uncovered no association between HEI and dental caries, probably due to the presence of weak HEI (<50) in all children. The fact that the participants were from the same region and had similar socioeconomic conditions may have made it complicated to determine the relationship between HEI and dental caries. For a more accurate assessment, including children from different regions with different socioeconomic backgrounds may provide a more precise inference.
This study had some limitations; the generalizability was low because the involved population was in a limited region. Furthermore, dental caries has multifactorial etiology, and adding all the potential factors in the model is unfeasible. Also, each age has different deciduous and permanent teeth combinations; generalizing models for all ages may decrease predictive robustness.
Conclusions
Within the limitations of the study, the established regression models have significantly predicted dental caries in deciduous teeth. SBC was the most influential factor in predicting dental caries compared to PRAL and HEI. There was a significant relationship between SBC, PRAL, and caries in deciduous teeth. In the model we created, the strongest predictor was SBC. However, the variance explanation capacity of dental caries in permanent teeth was not strong as in deciduous teeth.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Recep Tayyip Erdogan University, Faculty of Medicine, Ethics Committee issued approval 2021/155. This is a cross-sectional study involving children who applied to Recep Tayyip Erdogan University, Faculty of Dentistry, Department of Pediatric Dentistry. The study was conducted between July and December 2021. Ethics committee approval was retrieved from Recep Tayyip Erdogan University, Faculty of Medicine, Ethics Committee (2021/155). The parents were informed in writing and verbally about the content, duration and possible benefits of the study and were asked to sign the declaration of consent.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Evaluation of the effect of etiological risk factors on caries risk. (Article in Turkish) Çayır I, Karabekiroğlu S. Selcuk Dent J. 2021;8:313–321. [Google Scholar]
- 2.Maternal risk indicators for childhood caries in an inner city population. Smith RE, Badner VM, Morse DE, Freeman K. Community Dent Oral Epidemiol. 2002;30:176–181. doi: 10.1034/j.1600-0528.2002.300303.x. [DOI] [PubMed] [Google Scholar]
- 3.The effect of parents' socio-economic status and home oral hygiene habits on early childhood caries. (Article in Turkish) Yiğit T, Küçükeşmen Ç. Curr Res Dent Sci. 2020;30:366–372. [Google Scholar]
- 4.Risk factors and patterns related to dental caries evaluated with caries assessment spectrum and treatment (cast) among schoolchildren of Bhubaneswar, India. Nagarajappa R, Naik D, Satyarup D, Dalai RP. https://pubmed.ncbi.nlm.nih.gov/32227790/ Rocz Panstw Zakl Hig. 2020;71:113–122. doi: 10.32394/rpzh.2020.0103. [DOI] [PubMed] [Google Scholar]
- 5.Saliva as a diagnostic tool in oral diseases. Kart Ö, Yarat A. Experimed. 2020;10:135–139. [Google Scholar]
- 6.The effect of milk and casein proteins on the adherence of Streptococcus mutans to saliva-coated hydroxyapatite. Vacca-Smith AM, Van Wuyckhuyse BC, Tabak LA, Bowen WH. Arch Oral Biol. 1994;39:1063–1069. doi: 10.1016/0003-9969(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 7.Diet, nutrition and the prevention of dental diseases. Moynihan P, Petersen PE. Public Health Nutr. 2004;7:201–226. doi: 10.1079/phn2003589. [DOI] [PubMed] [Google Scholar]
- 8.Association of dermatoglyphic patterns and salivary pH with DMFT index of patients in Riyadh. Lingam AS. Niger J Clin Pract. 2022;25:294–298. doi: 10.4103/njcp.njcp_1490_21. [DOI] [PubMed] [Google Scholar]
- 9.The impact of diet, nutrition and nutraceuticals on oral and periodontal health. Isola G. Nutrients. 2020;12:2724. doi: 10.3390/nu12092724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eating habits, smoking and toothbrushing in relation to dental caries: a 3-year study in Swedish female teenagers. Bruno-Ambrosius K, Swanholm G, Twetman S. Int J Paediatr Dent. 2005;15:190–196. doi: 10.1111/j.1365-263X.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 11.Oral health-related risk factors among students in Southeast Serbia. Bojović MD, Kesić LG, Mitić AN, et al. Med Sci Monit. 2021;27:0. doi: 10.12659/MSM.929375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Is obesity associated to dental caries in Brazilian schoolchildren? Fernández MR, Goettems ML, Demarco FF, Corrêa MB. Braz Oral Res. 2017;31:0. doi: 10.1590/1807-3107BOR-2017.vol31.0083. [DOI] [PubMed] [Google Scholar]
- 13.Effect of potential renal acid load of foods on urinary citrate excretion in calcium renal stone formers. Trinchieri A, Lizzano R, Marchesotti F, Zanetti G. Urol Res. 2006;34:1–7. doi: 10.1007/s00240-005-0001-9. [DOI] [PubMed] [Google Scholar]
- 14.Acid-base in renal failure: influence of diet on acid-base balance. Remer T. Semin Dial. 2001;13:221–226. doi: 10.1046/j.1525-139x.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- 15.Update of the Healthy Eating Index: HEI-2015. Krebs-Smith SM, Pannucci TE, Subar AF, et al. J Acad Nutr Diet. 2018;118:1591–1602. doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Is diet quality associated with early childhood caries in preschool children? A descriptive study. İnan-Eroğlu E, Özşin-Özler C, Erçim RE, Büyüktuncer Z, Uzamış-Tekçiçek M, Güçiz-Doğan B. Turk J Pediatr. 2017;59:537–547. doi: 10.24953/turkjped.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Simplified method to estimate salivary buffer capacity. Ericson D, Bratthall D. Scand J Dent Res. 1989;97:405–407. doi: 10.1111/j.1600-0722.1989.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 18.Association between asthma and caries-related salivary factors: a meta-analysis. Hatipoğlu Ö, Pertek Hatipoğlu F. J Asthma. 2022;59:38–53. doi: 10.1080/02770903.2020.1826045. [DOI] [PubMed] [Google Scholar]
- 19.Salivary flow rate, pH, and buffer capacity in the individuals with obesity and overweight: a meta-analysis. Hatipoglu O, Maras E, Hatipoglu FP, Saygin AG. Niger J Clin Pract. 2022;25:1126–1142. doi: 10.4103/njcp.njcp_1760_21. [DOI] [PubMed] [Google Scholar]
- 20.Caries-related salivary parameters and oral microbial flora in patients with type 1 diabetes: a meta-analysis. Hatipoğlu Ö, Önsüren AS, Hatipoğlu FP, Kurt A. Diabetes Metab Res Rev. 2022;38:0. doi: 10.1002/dmrr.3527. [DOI] [PubMed] [Google Scholar]
- 21.Oral pH value predicts the incidence of radiotherapy related caries in nasopharyngeal carcinoma patients. Li Z, Wu Q, Meng X, et al. Sci Rep. 2021;11:12283. doi: 10.1038/s41598-021-91600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correlation between caries activity and salivary microbiota in preschool children. Lin X, Wang Y, Ma Z, et al. Front Cell Infect Microbiol. 2023;13:1141474. doi: 10.3389/fcimb.2023.1141474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evaluation of the Healthy Eating Index-2015. Reedy J, Lerman JL, Krebs-Smith SM, et al. J Acad Nutr Diet. 2018;118:1622–1633. doi: 10.1016/j.jand.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The relationship between salivary flow rate and pH and dental caries in children. Kılınç G, Çetin M, Ellidokuz H. J Pediatr Res. 2015;2:87–91. [Google Scholar]
- 25.Saliva and dental caries. Lenander-Lumikari M, Loimaranta V. Adv Dent Res. 2000;14:40–47. doi: 10.1177/08959374000140010601. [DOI] [PubMed] [Google Scholar]
- 26.Dietary habits and oral health of children in deciduous, early and late mixed dentition. Akyüz S, Doğan BN, Kuru L. https://eds.p.ebscohost.com/eds/pdfviewer/pdfviewer?vid=0&sid=9ffcb59e-d9fa-45c4-a56e-09614fae4b4b%40redis MUSBED. 2012;2:113–118. [Google Scholar]
- 27.The Healthy Eating Index and coronal dental caries in US adults: National Health and Nutrition Examination Survey 2011-2014. Kaye EA, Sohn W, Garcia RI. J Am Dent Assoc. 2020;151:78–86. doi: 10.1016/j.adaj.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]