Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) envelope (E) and RNA-dependent RNA polymerase (RdRP) genes were detected via electrochemical measurements using a screen-printed carbon electrode (SPCE) (3-electrode system) coupled with a battery-operated thin-film heater based on the loop-mediated isothermal amplification (LAMP) technique. The working electrodes of the SPCE sensor were decorated with synthesized gold nanostars (AuNSs) to obtain a large surface area and improve sensitivity. The LAMP assay was enhanced using a real-time amplification reaction system to detect the optimal target genes (E and RdRP) of SARS-CoV-2. The optimized LAMP assay was performed with diluted concentrations (from 0 to 109 copies) of the target DNA using 30 μM of methylene blue as a redox indicator. Target DNA amplification was conducted for 30 min at a constant temperature using a thin-film heater, and the final amplicon electrical signals were detected based on cyclic voltammetry curves. Our electrochemical LAMP analysis of SARS-CoV-2 clinical samples showed an excellent correlation with the Ct value of real-time reverse transcriptase-polymerase chain reaction, indicating successful validation of results. A linear relationship between the peak current response and the amplified DNA was observed for both genes. The AuNS-decorated SPCE sensor with the optimized LAMP primer enabled accurate analysis of both SARS-CoV-2-positive and -negative clinical samples. Therefore, the developed device is suitable for use as a point-of-care test DNA-based sensor for the diagnosis of SARS-CoV-2.
Keywords: coronavirus, Loop-mediated isothermal amplification, SARS-CoV-2, COVID-19, Electrochemical sensing
Graphical abstract
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the viral respiratory illness that caused the Coronavirus disease 2019 (COVID-19) pandemic [[1], [2], [3], [4], [5]]. Early diagnosis of COVID-19 infection is crucial because the global spread of the virus subsequently impacts the healthcare systems, causing widespread social and economic disruption [[5], [6], [7], [8], [9], [10], [11]]. Hence, a point-of-care test for accurate and rapid detection of SARS-CoV-2 is warranted to reduce the transmissibility of this disease [12]. Serological tests, such as an enzyme-linked immunosorbent assay (ELISA) or a lateral flow serological assay, performed in clinical laboratories are available for the diagnosis of COVID-19. However, these tests cannot be used for early diagnosis, as antibodies cannot be accurately detected during the first days of the infection [13,14]. Currently, the molecular reverse transcription-quantitative polymerase chain reaction (RT-qPCR) is the only accurate method for the detection and diagnosis of COVID-19 [4,8,[15], [16], [17], [18]]. Numerous potential molecular targets of coronaviruses, such as the E and RNA-dependent RNA polymerase (RdRP) genes, can be used for RT-qPCR [4,19]. It is common to either verify two nucleocapsid protein targets, N1 and N2 [4], or to perform first-line screening using the envelope E gene standard assay and a sensitive assay using RdRP genes [5]. Loop-mediated isothermal amplification (LAMP) and recombinase polymerase amplification are isothermal techniques that can replace RT-qPCR, the gold standard, for providing results with sensitivity and specificity within a short time window [1,20,21]. Isothermal amplification techniques, such as recombinase polymerase amplification (RPA), rolling circle amplification (RCA) and loop-mediated isothermal amplification (LAMP) can be performed without a thermocycler and coupled with biosensors to achieve a more straightforward, low-cost, and rapid diagnosis [22,23].
The LAMP assay is a single-step nucleic acid amplification procedure based on six target sequences simultaneously identified in the same reaction using specific primers [12,24]. Amplified specific viral sequences can be detected using either fluorescence, colorimetric, mechanical (turbidimeter), or electrical techniques [[24], [25], [26], [27]]. The LAMP method has been successfully used to detect different RNA viruses such as Zika and MERS [24,27,28]. LAMP assays have previously been used for detecting SARS-Cov-2 in different types of samples, such as gargle water and oropharyngeal swab samples [15,16,21,29,30]. Most reported methods are based on either fluorescence or colorimetric detection, reducing the accuracy and selectivity compared to conventional reverse transcriptase-polymerase chain reactions (RT-PCR) [8,12,16,31]. However, sensitivity of the LAMP method can be enhanced by analyzing the electrical properties after the amplification process, thereby increasing the accuracy, minimizing the use of samples, and reducing the total procedure time [32]. In a typical configuration, electrochemical DNA sensors use a single-stranded capture probe sequence immobilized on a working electrode, where the complementary target DNA is hybridized. Electrochemical methods often facilitate label-free DNA detection, which does not require sandwich assays or additional signaling molecules that are not the probe and target nucleotides. Another approach uses nonintercalating ligands that electrostatically bind to the anionic DNA backbone [33]. In this case, the presence of the amplicon suppresses the catalyzed oxidation of the substrate, resulting in a significant decrease in the electrochemical signal. Decorating the working electrodes with synthesized gold nanostars (AuNSs) increases the surface area and improves the current density of the electrochemical signals. Although a few studies have been conducted on the label-free electrochemical detection of COVID-19, they rely on the redox response of sensors and use immobilized antibodies and nanocomposites to enhance detection [[34], [35], [36], [37], [38]]. Most techniques rely on the decrease in current response of a free-to-diffuse intercalating redox reporter during the reaction processes [39,40]. Some studies have also reported the use of field-effect transistor devices for the detection of SARS-CoV-2; however, this approach has a drawback of high noise signals [32,41,42].
In this study, we electrochemically detected SARS-CoV-2 E and RdRP genes using disposable AuNS-SPCE sensors based on an optimized LAMP assay. Screen-printed sensors were treated with gold nanostars to amplify the signal and to increase the sensitivity of the working electrode. Methylene blue (MB) was used as a redox probe. The sensor was connected to a battery-operated thin-film heater to maintain a constant temperature. During the on-chip LAMP amplification, the free-state MB intercalated with an increasing number of DNA molecules, allowing for the detection of the target analyte based on the electrical properties of the sample in less than 30 min [Fig. 1 (a) and (b)].
Fig. 1.
Schematic diagram of the LAMP (a) amplification and (b) electrochemical DNA sensor.
3. Material and methods
3.1. Reagents and apparatus
Methylene blue, hydrochloric acid (HCl), silver nitrate (AgNO3), ascorbic acid, gold (III) chloride hydrate (HAuCl4), potassium ferricyanide (K3 [Fe(CN)6]), and polyvinylpyrrolidone (PVP) were purchased from Sigma-Aldrich (USA). LAMP 2 × Master Mix was supplied by New England Biolabs, Inc. (USA). Primer sequences were synthesized by Bionics, Inc. (Daejeon, Korea). The template for the LAMP assay, plasmids containing the E and RdRP gene sequences from SARS-CoV-2, were ordered from GenScript (USA). The screen-printed carbon electrodes (Model 220) were obtained from QSTAG (Seoul, Korea). The, electrochemical instrument CHI 830B was used to measure the screen-printed sensors. LAMP assay reactions were monitored using RT-PCR.
3.2. Synthesis of AuNSs and electrochemical deposition on SPCE
AuNSs were prepared using a previously reported method with minor modifications [43]. First, 10 μL of a 1 M HCl solution was diluted with 10 mL of de-ionized (DI) water. Subsequently, 50 μL of a 50 mM HAuCl4 solution was added. Then, 50 μL of a 100 mM ascorbic acid solution and 50 μL of a 10 mM AgNO3 solution were added simultaneously, and the solution was stirred slowly (300 rpm). Next, 500 μL of a 2.5 mM PVP solution was included, and the mixed solution was centrifuged at 13,000 rpm for 30 min. To deposit the synthesized gold nanostars on the SPCE, the nanomaterial was electrodeposited onto the working electrodes through cyclic voltammetry (CV) using a 0.1 V/s scan rate with a potential range from −0.2 to 0.4 V over five cycles. Subsequently, the AuNSs/SPCEs were rinsed with DI water and incubated at 75 °C. The characterization of the device was measured by the CV method from −0.2 V to 0.4 V potential range at 0.1 V/s in a buffer containing 5 mM K3 [Fe(CN)6] and 0.1 M KCl.
3.3. Loop-mediated isothermal amplification assay
The target templates of SARS-CoV-2, namely the E and RdRP genes, were serially diluted with DEPC-treated water from 1.48×102 copies/μL to 1.48×109 copies/μL. The LAMP reaction was prepared in 9.5 μL of DEPC-treated water with target DNA (1 μL), gene primer mix (1.6 μM of forward inner primer (FIP), 1.6 μM of backward inner primer (BIP), 0.2 μM of forward outer primer (F3), and 0.2 μM of backward outer primer (B3)), and 12.5 μL of LAMP 2 × Master Mix. The prepared solutions were injected inside the O-ring chamber, and the surface was covered by mineral oil to prevent evaporation during LAMP amplification at 65 °C. The LAMP reactions were monitored using RT-PCR and electrochemical measurements with the AuNS/SPCE sensor coupled with the O-ring chamber. For the LAMP reaction on the SPCE sensor, a thin-film heater connected to a battery was used to maintain the temperature. After 30 min, the final amplified LAMP assays were analyzed in real time using an RT-PCR system and cyclic voltammetry curves.
3.4. Measurement of the of the modified screen-printed electrodes
The CHI 830B instrument was used to measure CV and differential pulse voltammetry (DPV) electrochemical techniques. CVs provided the characteristics of the SPCE sensors at 0.1 V/s from −0.2 V to 0.4 V. Additionally, the final amplified on-chip LAMP assay was analyzed with CV at a scan rate of 0.1 V/s with a potential range from −0.6 V to −0.2 V. The DPV technique was performed with a 0.05 V pulse amplitude, with a potential range from −0.2 V to 0.4 V, and a 0.05 s pulse width, to measure the peak current based on the MB concentration. All the electrochemical analyses were performed under the same environmental conditions.
4. Results and discussion
4.1. AuNSs and methylene blue optimization on screen-printed electrodes
The proposed method comprised of LAMP assays to amplify the SARS-CoV-2 E and RdRP genes and to detect targets using electrochemical approaches with disposable SPCE sensors coupled with a battery-operated thin-film heater. Fig. S1(a) shows an image of the SPCE coupled to an 8 mm diameter O-ring chamber. The modified SPCE was coupled to a thin-film heater powered by four double-A batteries [Fig. S1 (b)]. The temperatures at three random spots on the battery-operated thin-film heater were measured by inserting a thin thermocouple [Fig. S1(c)]. In each real-time measurement spot, the temperature was adjusted within 5 min, and the standard deviation temperature of the last 30 min was 65.4 °C ± 0.83, which indicates a stable value for LAMP assay amplification.
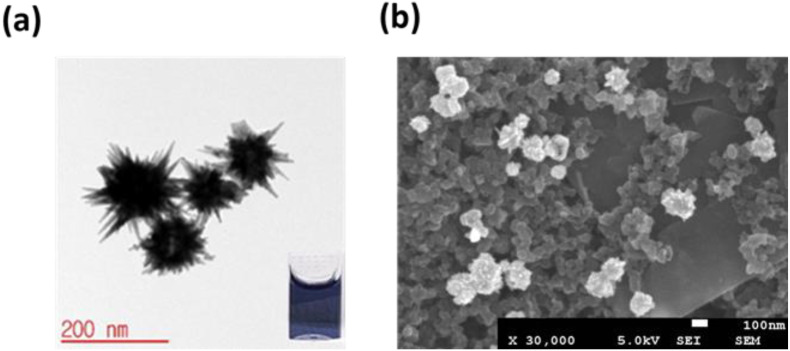
The carbon working electrode of the SPCE sensor was modified with the synthesized AuNSs to increase its surface area. AuNSs were synthesized using a seedless method [43]. Fig. 2 (a) shows the size of the AuNSs measured by transmission electron microscopy (TEM); the sizes of the AuNPs with star-like spikes, as determined using the ImageJ software, were between 161 nm and 188 nm. Spikes were synthesized using silver nitrate, turning the solution blue within a few seconds, as shown in the inset of Fig. 2 (a) [44]. The working electrodes of the SPCE sensor were decorated with synthesized gold nanostars (AuNSs) to obtain a large surface area and improve sensitivity. Decorating the working electrodes with synthesized gold nanostars (AuNSs) increases the surface area and improves the current density of the electrochemical signals. The gold nanostars (10 μL) were electrodeposited on the carbon working electrode surface for five cycles at 0.1 V/s with a potential range from −0.2 to 0.4 V. SPCE decorated with AuNSs prepared by electrochemical deposition were analyzed with scanning electron microscopy (SEM), shown in Fig. 2 (b).
Fig. 2.
(a) TEM images of synthesis AuNPs. Inset: solution color of AuNPs. (b) SEM images of AuNSs -SPCE after electrodeposition.
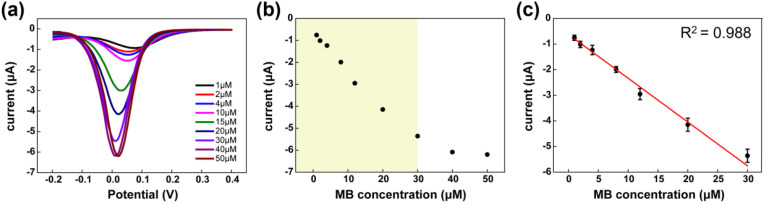
The working electrodes were decorated with AuNSs, which increased the surface area, amplifying and improving the sensitivity of the electrochemical signals. After the sensor treatment, the peak currents of the SPCE sensors were measured with DPV techniques using different concentrations (ranging from 1 μM to 50 μM) of MB as a redox indicator. Compared to other dyes, methylene blue is an excellent dye for electrochemical measurements [45]. During the reaction process for the PCR and LAMP assays, MB did not affect amplification [46]. DPV was analyzed from a potential range of −0.2 V to 0.4 V, and the peak current increased until the concentration of MB was 30 μM [Fig. 3 (a)]. However, the current peak was saturated at higher concentrations (between 40 μM and 50 μM). Fig. 3 (b) and (c) show the recorded peak current to MB concentrations of 1 μM–30 μM, plotted with a linear relationship (R2 = 0.988). Therefore, an MB concentration of 30 μM was selected to maximize assay sensitivity.
Fig. 3.
(a) The DPV measurement of MB with concentrations between 1 and 50 μM and (b) the peak current of the full concentration range. (c) Linear correlation between 1 and 30 μM MB concentration and peak currents.
4.2. Electrochemical characterization of the modified screen-printed electrodes
The modified electrode was characterized using CV measurements, as shown in Fig. 4 (a). The performances of the electron transfer of the bare SPCE, SPCE/AuNSs, and SPCE/AuNSs/MB were measured by the CV method at a potential range of −0.2 V–0.4 V at 0.1 V/s in a solution of 5 mM K3 [Fe(CN)6] with 0.1 M KCl. After decorating the working electrodes with AuNSs (SPCE/AuNSs), the surface area andthe current density of the electrochemical signals increased. Under the same conditions, the optimized concentration of MB (30 μM) was added to the analyte as a redox probe, which enhanced electron transfer on the carbon working electrode surface with AuNSs.
Fig. 4.
(a) CV measurements and (b)EIS measurements for bare SPCE, SPCE/AuNSs and SPCE/AuNSs/MB in the buffer of K3 [Fe(CN)6] with 0.1 M KCl as buffer. (c) E gene and (d)RdRP gene was measured CV with and without AuNSs in LAMP assay with MB.
As shown in Fig. 4 (b), the CV measurements were confirmed by electrochemical impedance spectroscopy (EIS). The Nyquist plots of EIS can be analyzed in two parts: the semi-circular and linear regions. The semicircular region represents Rct and the linear region represents the surface diffusion process. SPCE/AuNSs/MB had the smallest Rct value, and MB, as a redox probe, effectively improved the electron transfer on the carbon working electrode surface with AuNSs.
Bare SPCE and SPCE/AuNSs were measured with CV to define the sensitivity improvement from a potential of −0.6 V to −0.2 V at 0.1 V/s in 25 μl of amplified LAMP assay containing 30 mM MB as a redox probe. Approximately 1.48 × 105 copies of template DNA of the E and RdRP genes were measured on bare SPCE and SPCE/AuNSs, as shown in Fig. 4 (c) and (d), respectively. This result indicated that the sensitivity of the SPCE/AuNSs device was higher than that of the bare SPCE. The current peak is reduced more in AuNS-modified devices because of the strong surface adsorption between AuNS and the amplified DNA, which interrupts the electron transfer on the surface [18]. In addition, MB is a double-stranded DNA-binding redox probe. During LAMP amplification, free-state MB is intercalated into an increasing number of DNA molecules. Owing to the decrease in free-state MB, the redox peak current decreased.
4.3. Sensitivity ccomparisons between RT-LAMP and EC-LAMP measurements
To obtain higher-sensitivity amplification, the LAMP assay was optimized using a real-time amplification reaction system. To detect the optimal target genes, SARS-CoV-2 RdRP genes [Fig. S2 (a)], E genes [Fig. S2 (b)], and LAMP primers were designed in 16 sets. Colorimetry and gel electrophoresis were used to screen for primers with the most efficient color visualization and agarose gel intensity (Fig. S2). A selected LAMP primer (Fig. S3) mixture was added to the LAMP assay, including the E and RdRP gene targets (serially diluted from 0 to 1.48 × 109 copies per reaction) and the no-template control (NTC).
The limit of detection of each E and RdRP gene in the LAMP reaction was analyzed in 30 min using 65 °C by the real-time LAMP reaction system [Fig. 5 (a) and (c)]. The real-time amplification fluorescence curve of a 10-fold serially diluted target was recorded every 30 s, and the relative intensity threshold value was used. On this graph, the x axis displays time in minutes and the y axis displays the fluorescence intensity. The fluorescence curves were indicated by the threshold time (Tt) and log dilution factor (Fig. S4). The linear relationship of Tt (n = 3) was determined as R2 > 0.95 for the E gene and R2 > 0.99 for the RdRP gene. To compare the results of the limit of detection for each E and RdRP gene, the electrochemical LAMP (EC-LAMP) reaction was carried out under the same temperature conditions as those of the real-time LAMP reaction using a battery-operated thin-film heater [Fig. 5 (b) and (d)]. The current curve of the 10-fold serially diluted target was recorded every 5 min. The y-axis represents the normalized cyclic voltammetry current peak. These comparison results indicated that the optimized LAMP reaction's detection limits were approximately 1.48 × 102 and 1.48 × 103 for the E gene and the RdRP gene, respectively.
Fig. 5.
Detection of each E and RdRP gene in the LAMP reaction was analyzed at within 30 min at 65 °C by RT-LAMP (a) and (c) and by EC-LAMP (b) and (d), respectively.
4.4. End-point detection of EC-LAMP reaction
A AuNS-decorated SPCE sensor with a battery-operated thin-film heater was constructed for electrochemical measurements. LAMP assays separately optimized for E and RdRP genes with diluted target DNA from 0 to 109 copies were prepared with 30 μM of MB. The assay was amplified for 30 min at 65 °C using the thin-film heater, and the final amplicon signals were collected by cyclic voltammetry [Fig. 6 (a) and (c)]. CV curves were recorded from −0.6 V to −0.2 V, with a scan rate of 0.1 V/s, in a 25 μL LAMP assay containing MB. Methylene blue is a double-stranded DNA-binding redox probe, and during LAMP amplification, free-state MB is intercalated into an increasing number of DNA molecules. Owing to the decrease in free-state MB, the redox peak current decreased. Linear plots of the current peak, with error bars (n = 3), were determined to be R2 > 0.982 for the E gene and R2 > 0.953 for the RdRP gene [Fig. 6 (b) and (d)].
Fig. 6.
The end-point measurements of E and RdRP genes with diluted target DNA from 109 to 0 copies were prepared with 30 μM of MB on the AuNS-decorated SPCE sensor(a) and (c), respectively. The linearity plot of the CV measurement by diluted target DNA concentrations for (b) E gene and (d) for RdRP gene.
4.5. Detection of clinical sample using EC-LAMP measurement
Electrochemical LAMP measurements on the AuNS-decorated SPCE sensors were performed using patient samples for validation. RNA was extracted from a total of 20 clinical samples obtained from Samsung Medical Center, Seoul, Korea; the cycle threshold (Ct) value from the RT-PCR test targeting the E gene sequence was calculated. The Ct values of the 20 clinical samples were defined as the range of 21.99–36.24 (13 known positives, high positive to low positive) and undetermined (seven known negatives, N/A). The peak current response of electrochemical LAMP measurements of positive and negative clinical samples was defined as the Delta (Δ) currents for the Ct values of RT-PCR (Fig. 7 ). All 13 positive clinical samples with different ranges of Ct values (21.99 cycles to 36.24 cycles) were positively identified by Δ currents (2.11 μA–0.649 μA), which indicated an elevated correlation of high positive to low positive with the Ct value. In comparison, less than 0.124 μA Δ currents, which is an almost negligible value, identified the 7 negative clinical samples with undetermined (N/A) Ct values of RT-PCR. The measurement results of the AuNS-decorated SPCE sensor using the optimized LAMP primer showed high agreement between the Δ currents and the Ct values of RT-PCR.
Fig. 7.
EC-LAMP measurement on AuNSs-decorated SPCE sensor with a total of 20 clinical samples for validation. 13 samples were known as positive, and 7 samples were known negatives (N/A).
5. Conclusions
In this study, SARS-CoV-2 E and RdRP genes were detected by an optimized LAMP assay using electrochemical techniques by measuring the current response in an AuNS-decorated SPCE sensor with a battery-operated thin-film heater. AuNSs were electrodeposited on the working electrode to increase the surface area, and methylene blue was used as a redox probe. Using a real-time LAMP reaction system, the LAMP primers were optimized to target 1.48 × 102 bases for the E gene and 1.48 × 103 bases for the RdRP gene. During the on-chip LAMP amplification, the free-state MB was intercalated into an increasing number of DNA molecules. Owing to the decrease in free-state MB and the strong surface adsorption between AuNS and the amplified DNA, which interrupted electron transfer on the surface, and the redox current peak decreased. The comparison results between using an RT-LAMP assay and our developed EC-LAMP system indicated that the optimized LAMP reaction's detection limit was both 1.48 × 102 for the E gene and 1.48 × 103 for the RdRP gene. The positive and negative clinical sample measurement results on the AuNS-decorated SPCE sensor using the optimized LAMP primers showed high agreement between the Δ currents and Ct values of RT-PCR. The features of the developed immunosensor indicate its potential for use as a point-of-care test DNA-based sensor for the diagnosis of SARS-CoV-2, providing highly sensitive detection while using a reliable technique.
Credit author statement
Hyo Eun Kim: Conceptualization, methodology, investigation, software, formal analysis, writing-original draft, writing-review, and editing. Ariadna Schuck: Software, formal analysis, and writing-original draft. Hyeonseek Park: Validation, writing-review, and editing. Hee Jae Huh: Resources, writing-review, and editing. Minhee Kang: Formal analysis, resources, visualization, validation, writing-review & editing, supervision. Yong-Sang Kim: Writing–review and editing, supervision, funding acquisition.
Funding
This research was funded by the National R&D Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2021M3H4A4079521) and supported by the Basic Science Research Program through the NRF funded by the Ministry of Education (grant number) (NRF-2022R1I1A1A01073043). This work was supported by the BK21 FOUR Project.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Future Medicine 2030 Project of the Samsung Medical Center (SMX1230761).
Handling Editor: J.-M. Kauffmann
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.talanta.2023.124841.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Zhou P., Lou Yang X., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Di Jiang R., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huajun B., Cai X., Zhang X. OSF; 2020. Landscape Coronavirus Disease 2019 Test (COVID-19 Test) in Vitro -- A Comparison of PCR vs Immunoassay vs Crispr-Based Test. [DOI] [Google Scholar]
- 5.Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C.-Y., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee D., Lee J. Testing on the move: South Korea's rapid response to the COVID-19 pandemic. Transp. Res. Interdiscip. Perspect. 2020;5 doi: 10.1016/j.trip.2020.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silveira C., Monteiro T., Almeida M. Biosensing with paper-based miniaturized printed electrodes–A modern trend. Biosensors. 2016;6:51. doi: 10.3390/bios6040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan X., Yang C., He Q., Chen J., Yu D., Li J., Zhai S., Qin Z., Du K., Chu Z., Qin P. Current and perspective diagnostic techniques for COVID-19. ACS Infect. Dis. 2020;6:1998–2016. doi: 10.1021/acsinfecdis.0c00365. [DOI] [PubMed] [Google Scholar]
- 9.Won J., Lee S., Park M., Kim T.Y., Park M.G., Choi B.Y., Kim D., Chang H., Kim V.N., Lee C.J. Development of a laboratory-safe and low-cost detection protocol for SARS-CoV-2 of the Coronavirus Disease 2019 (COVID-19) Exp. Neurobiol. 2020;29:107–119. doi: 10.5607/en20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Z., Tian D., Liu Y., Lin Z., Lyon C.J., Lai W., Fusco D., Drouin A., Yin X., Hu T., Ning B. Ultra-sensitive and high-throughput CRISPR-p owered COVID-19 diagnosis, Biosens. Bioelectron. 2020;164 doi: 10.1016/j.bios.2020.112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter L.J., Garner L.V., Smoot J.W., Li Y., Zhou Q., Saveson C.J., Sasso J.M., Gregg A.C., Soares D.J., Beskid T.R., Jervey S.R., Liu C. Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 2020;6:591–605. doi: 10.1021/acscentsci.0c00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashir J., Yaqinuddin A. Loop mediated isothermal amplification (LAMP) assays as a rapid diagnostic for COVID-19. Med. Hypotheses. 2020;141 doi: 10.1016/j.mehy.2020.109786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GeurtsvanKessel C.H., Okba N.M.A., Igloi Z., Bogers S., Embregts C.W.E., Laksono B.M., Leijten L., Rokx C., Rijnders B., Rahamat-Langendoen J., van den Akker J.P.C., van Kampen J.J.A., van der Eijk A.A., van Binnendijk R.S., Haagmans B., Koopmans M. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat. Commun. 2020;11:1–5. doi: 10.1038/s41467-020-17317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng W., Newbigging A., Le C., Pang B., Peng H., Cao Y., Wu J., Abbas G., Song J., Wang D.-B., Cui M., Tao J., Tyrrell D.L., Zhang X.-E., Zhang H., Le X.C. Molecular diagnosis of COVID-19: challenges and research needs. Anal. Chem. 2020;92:10196–10209. doi: 10.1021/acs.analchem.0c02060. [DOI] [PubMed] [Google Scholar]
- 15.Kilic T., Weissleder R., Lee H. Molecular and immunological diagnostic tests of COVID-19 – current status and challenges. iScience. 2020;23 doi: 10.1016/j.isci.2020.101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Odiwuor N., Xiong J., Sun L., Nyaruaba R.O., Wei H., Tanner N. Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimetric. LAMP. 2020;2 doi: 10.1101/2020.02.26.20028373. [DOI] [Google Scholar]
- 17.Chan J.F.-W., Yip C.C.-Y., To K.K.-W., Tang T.H.-C., Wong S.C.-Y., Leung K.-H., Fung A.Y.-F., Ng A.C.-K., Zou Z., Tsoi H.-W., Choi G.K.-Y., Tam A.R., Cheng V.C.-C., Chan K.-H., Tsang O.T.-Y., Yuen K.-Y. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 2020;58:1–10. doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H.E., Schuck A., Lee S.H., Lee Y., Kang M., Kim Y.-S. Sensitive electrochemical biosensor combined with isothermal amplification for point-of-care COVID-19 tests. Biosens. Bioelectron. 2021;182 doi: 10.1016/j.bios.2021.113168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joung J., Ladha A., Saito M., Segel M., Bruneau R., Huang M.-L.W., Kim N.-G., Yu X., Li J., Walker B.D., Greninger A.L., Jerome K.R., Gootenberg J.S., Abudayyeh O.O., Zhang F. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. MedRxiv Prepr. Serv. Heal. Sci. 2020 doi: 10.1101/2020.05.04.20091231. [DOI] [Google Scholar]
- 21.Kim J.H., Kang M., Park E., Chung D.R., Kim J., Hwang E.S. A simple and multiplex loop-mediated isothermal amplification (LAMP) assay for rapid detection of SARS-CoV. BioChip J. 2019;13:341–351. doi: 10.1007/s13206-019-3404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng Y., Zhou T., Peng Y., Wang M., Xiang L., Zhang Y., Li J., Yang J., Li G. Dual-gene-controlled rolling circle amplification strategy for SARS-CoV-2 analysis. Anal. Chem. 2023;95(6):3358–3362. doi: 10.1021/acs.analchem.2c04572. [DOI] [PubMed] [Google Scholar]
- 23.Arbaciauskaite S. Direct self-sampled gargle water LAMP as a screening method for the detection of SARS-CoV-2 infections. Eesti Arst. 2022;101(Supplement 4):25. doi: 10.3390/diagnostics12040775. https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-2111899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esbin M.N., Whitney O.N., Chong S., Maurer A., Darzacq X., Tjian R. Overcoming the bottleneck to widespread testing: a rapid review of nucleic acid testing approaches for COVID-19 detection. RNA. 2020;26:771–783. doi: 10.1261/rna.076232.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan M., Wang R., Li B., Liu P., Weng Q., Chen Q. Comparative evaluation of the LAMP assay and PCR-based assays for the rapid detection of Alternaria solani. Front. Microbiol. 2018;9:1–11. doi: 10.3389/fmicb.2018.02089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svendsen W.E. Springer International Publishing; Cham: 2015. Lab-on-a-Chip Devices and Micro-total Analysis Systems. [DOI] [Google Scholar]
- 27.Song J., Liu C., Mauk M.G., Rankin S.C., Lok J.B., Greenberg R.M., Bau H.H. Two-stage isothermal enzymatic amplification for concurrent multiplex molecular detection. Clin. Chem. 2017;63:714–722. doi: 10.1373/clinchem.2016.263665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong Y.-P., Othman S., Lau Y.-L., Radu S., Chee H.-Y. Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018;124:626–643. doi: 10.1111/jam.13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L., Wu S., Hao X., Li X., Liu X., Ye S., Han H., Dong X., Li X., Li J., Liu N., Liu J., Zhang W., Pelechano V., Chen W.H., Yin X. Rapid colorimetric detection of COVID-19 coronavirus using a reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform: iLACO. Clin. Chem. 2020;66:975–977. doi: 10.1101/2020.02.20.20025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbaciauskaite S., Babakhani P., Sandetskaya N., Vitkus D., Jancoriene L., Karosiene D., Karciauskaite D., Zablockiene B., Kuhlmeier D. Self-Sampled gargle water direct RT-LAMP as a screening method for the detection of SARS-CoV-2 infections. Diagnostics. 2022;12:775. doi: 10.3390/diagnostics12040775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang T., Wang Y.C., Shen C.F., Cheng C.M. Point-of-care RNA-based diagnostic device for Covid-19. Diagnostics. 2020;10:9–11. doi: 10.3390/diagnostics10030165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selvarajan R.S., Gopinath S.C.B., Zin N.M., Hamzah A.A. Infection-mediated clinical biomarkers for a covid-19 electrical biosensing platform. Sensors. 2021;21:3829. doi: 10.3390/s21113829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balkourani G., Brouzgou A., Archonti M., Papandrianos N., Song S., Tsiakaras P. Emerging materials for the electrochemical detection of COVID-19. J. Electroanal. Chem. 2021;893 doi: 10.1016/j.jelechem.2021.115289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji T., Liu Z., Wang G., Guo X., Akbar khan S., Lai C., Chen H., Huang S., Xia S., Chen B., Jia H., Chen Y., Zhou Q. Detection of COVID-19: a review of the current literature and future perspectives. Biosens. Bioelectron. 2020;166 doi: 10.1016/j.bios.2020.112455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lomae A., Preechakasedkit P., Hanpanich O., Ozer T., Henry C.S., Maruyama A., Pasomsub E., Phuphuakrat A., Rengpipat S., Vilaivan T., Chailapakul O., Ruecha N., Ngamrojanavanich N. Label free electrochemical DNA biosensor for COVID-19 diagnosis. Talanta. 2023;253 doi: 10.1016/j.talanta.2022.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chandra P. Miniaturized label-free smartphone assisted electrochemical sensing approach for personalized COVID-19 diagnosis. Sensors Int. 2020;1 doi: 10.1016/j.sintl.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho H., Shim S., Cho W.W., Cho S., Baek H., Lee S.-M., Shin D.-S. Electrochemical impedance-based biosensors for the label-free detection of the nucleocapsid protein from SARS-CoV-2. ACS Sens. 2022;7:1676–1684. doi: 10.1021/acssensors.2c00317. [DOI] [PubMed] [Google Scholar]
- 38.Shoute L.C.T., Abdelrasoul G.N., Ma Y., Duarte P.A., Edwards C., Zhuo R., Zeng J., Feng Y., Charlton C.L., Kanji J.N., Babiuk S., Chen J. Label-free impedimetric immunosensor for point-of-care detection of COVID-19 antibodies. Microsystems Nanoeng. 2023;9:3. doi: 10.1038/s41378-022-00460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Won B.Y., Shin S., Baek S., Jung Y.L., Li T., Shin S.C., Cho D.-Y., Lee S.B., Park H.G. Investigation of the signaling mechanism and verification of the performance of an electrochemical real-time PCR system based on the interaction of methylene blue with DNA. Analyst. 2011;136:1573. doi: 10.1039/c0an00695e. [DOI] [PubMed] [Google Scholar]
- 40.Fang T.H., Ramalingam N., Xian-Dui D., Ngin T.S., Xianting Z., Lai Kuan A.T., Peng Huat E.Y., Hai-Qing G. Real-time PCR microfluidic devices with concurrent electrochemical detection. Biosens. Bioelectron. 2009;24:2131–2136. doi: 10.1016/j.bios.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Seo G., Lee G., Kim M.J., Baek S.-H., Choi M., Ku K.B., Lee C.-S., Jun S., Park D., Kim H.G., Kim S.-J., Lee J.-O., Kim B.T., Park E.C., Il Kim S. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 42.Alnaji N., Wasfi A., Awwad F. The design of a point of care FET biosensor to detect and screen COVID-19. Sci. Rep. 2023;13:4485. doi: 10.1038/s41598-023-31679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phiri M.M., Mulder D.W., Vorster B.C. Seedless gold nanostars with seed-like advantages for biosensing applications. R. Soc. Open Sci. 2019;6 doi: 10.1098/rsos.181971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulder D.W., Phiri M.M., Jordaan A., Vorster B.C. Modified HEPES one-pot synthetic strategy for gold nanostars. R. Soc. Open Sci. 2019;6 doi: 10.1098/rsos.190160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao F., Zhang H., Zheng J. Novel electrochemical biosensing platform for microRNA detection based on G-quadruplex formation in nanochannels. Sensor. Actuator. B Chem. 2021;327 doi: 10.1016/j.snb.2020.128898. [DOI] [Google Scholar]
- 46.Martin A., Grant K.B., Stressmann F., Ghigo J.-M., Marchal D., Limoges B. Ultimate single-copy DNA detection using real-time electrochemical LAMP. ACS Sens. 2016;1:904–912. doi: 10.1021/acssensors.6b00125. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.