Abstract
Globally, salinity and drought are severe abiotic stresses that presently threaten vegetable production. This study investigates the potential exogenously-applied glutathione (GSH) to relieve water deficits on Phaseolus vulgaris plants cultivated in saline soil conditions (6.22 dS m−1) by evaluating agronomic, stability index of membrane, water satatus, osmolytes, and antioxidant capacity responses. During two open field growing seasons (2017 and 2018), foliar spraying of glutathione (GSH) at 0.5 (GSH1) or 1.0 (GSH1) mM and three irrigation rates (I100 = 100%, I80 = 80% and I60 = 60% of the crop evapotranspiration) were applied to common bean plants. Water deficits significantly decreased common bean growth, green pods yield, integrity of the membranes, plant water status, SPAD chlorophyll index, and photosynthetic capacity (Fv/Fm, PI), while not improving the irrigation use efficiency (IUE) compared to full irrigation. Foliar-applied GSH markedly lessened drought-induced damages to bean plants, by enhancing the above variables. The integrative I80 + GSH1 or GSH2 and I60 + GSH1 or GSH2 elevated the IUE and exceeded the full irrigation without GSH application (I100) treatment by 38% and 37%, and 33% and 28%, respectively. Drought stress increased proline and total soluble sugars content while decreased the total free amino acids content. However, GSH-supplemented drought-stressed plants mediated further increases in all analyzed osmolytes contents. Exogenous GSH enhanced the common bean antioxidative machinery, being promoted the glutathione and ascorbic acid content as well as up-regulated the activity of superoxide dismutase, catalase, ascorbate peroxidase, and glutathione peroxidase. These findings demonstrate the efficacy of exogenous GSH in alleviating water deficit in bean plants cultivated in salty soil.
Keywords: Antioxidant, Drought stress, Growth, Osmotic stress, Water status, Yield
Introduction
Plants are subjected to recurring abiotic stresses during their growth, threatening the production of vegetable crops. Water stress and salinity alone or in combination are the main yield-limiting factors (Awad et al., 2012; Semida et al., 2020). Forecasts based on the integration of crop growth models and climate change estimated further yield losses (Waqas et al., 2019), associated with population growth that would require an increase in food production, consequently increasing the demands for irrigated agriculture, which requires heightening the irrigation use efficiency (IUE) (Abdelkhalik et al., 2019c; Abd El-mageed et al., 2021).
Soil water deficit alters many vital processes and causes severe damage to the plant. A plant’s primary response to soil water reduction is a decrease in cell turgor pressure, which causes osmotic stress, resulting in several cellular signaling pathways that disrupt different physio-biochemical activities in plant cells (Xiong & Zhu, 2002; Abdelkhalik et al., 2019c). Later, drought/osmotic stress leads to excessive accumulation of reactive oxygen species (ROS; e.g., OH −, H2O2, and O2•−) produced from different plant organelles; mitochondria, chloroplasts, and peroxisomes (Rady et al., 2021; Abd El-mageed et al., 2022). Hyperproduction of ROS destructs the normal balance between ROS formation and scavenging that not only inhibits several enzymes activity but also triggers cellular oxidative damage like protein, DNA, and lipids even can lead to cell damage (Zhu et al., 2020; Abd El-mageed et al., 2022). Simultaneously, ROS provokes chlorophyll degradation and membrane lipid peroxidation, decreasing membrane stability, selectivity, and fluidity, and disturbing cell redox homeostasis (Rady, Taha & Kusvuran, 2018; Semida et al., 2021a). Under water stress, the lower tissue water content becomes limiting for physiological and biochemical processes in the plant (Tombesi et al., 2015; Abdelkhalik et al., 2019a). Besides stomatal closure is a primary factor that decreases photosynthesis, ROS generated in the chloroplasts can damage the photosynthetic pigments, thylakoid membrane, and enzymes, as well as decrease or inhibits the photosynthetic capacity of photosystem II (PSII) (Ma, Dias & Freitas, 2020; Semida et al., 2021b).
The plant has adapted several regulatory signaling mechanisms to withstand water stress, including activating the plant’s defense system, which helps the plant modulate metabolism, maintain protective regimes, and redox homeostasis (Rady et al., 2019). The abundance of osmoprotectants in plant tissues involves osmotic adjustment, the maintenance of cell turgor, and the control of water influx/efflux (Blum, 2017; Turner, 2018). Furthermore, increasing the activity of antioxidants both enzymatic and non-enzymatic helps to reduce ROS and protect membrane lipids from peroxidation under drought stress (Farooq et al., 2009; Semida, Hemida & Rady, 2018; Zhu et al., 2020). However, a continuous water deficit can trigger an imbalance among scavenge and the production of ROS, which inhibits the antioxidative machinery’s scavenging action. In that case, the endogenous plant defense mechanisms are unable to completely protect plants from the harmful effects of drought stress, thus impairing plant growth and reducing yield (Pal et al., 2016; Rady et al., 2021). Therefore, exogenous use of auxiliary compounds such as antioxidative compounds or another effective approach to support plant defense system, allows plants to perform well under water deficit.
Glutathione (GSH) is a major free thiol tripeptide with a low molecular weight that acts as an antioxidant to relieve environmental stresses in several ways (Ding et al., 2016; Ashraf et al., 2019). GSH participates in the antioxidant defense, hormone or redox molecule signaling, transmembrane of amino acids transport, and detoxification of ROS, methylglyoxal, and xenobiotics (Hasanuzzaman, Nahar & Anee, 2017; Zhou et al., 2019). GSH is a small molecular weight of a thiol group that presents the GSH as a powerful ROS scavenger (Gill et al., 2017), it can also scavenge ROS indirectly through the AsA-GSH cycle, which removes harmful peroxides (Rady & Hemida, 2016; Hossain et al., 2017). GSH is an active redox compound that is typically found in reduced glutathione (GSH) form which may be oxidized by ROS to disulfide glutathione (GSSG) form that is recirculated to GSH by NADPH-dependent glutathione reductase (Noctor et al., 2012). The balance of GSH and GSSG in the cell is related to its redox state, in the sense that a higher GSH or GSH/GSSG ratio is important for many physiological mechanisms and regulates various adaptations to abiotic stress resilience (Ding et al., 2016; Hossain et al., 2017). Furthermore, GSH contributes to plasma membrane stability by reducing lipid peroxidation, as well as osmotic adjustment for abiotic stress tolerance (Hasanuzzaman, Nahar & Anee, 2017; Rehman et al., 2021). Exogenous GSH has been shown in recent studies to improve plant growth, leaf water status, and photosynthesis, and reduce oxidative damage indicators, along with up-regulated some osmolytes, the antioxidant capacity, and induced cellular redox hemostasis under abiotic stress such as drought stress (Nahar et al., 2015), high temperature (Ding et al., 2016) and salt stress (Rady & Hemida, 2016).
The Phaseolus vulgaris, a common bean, is among the world’s largest legume crops, which are produced and consumed on a large scale (Biddle, 2017). The world’s green pod production is approximately 27 million Mg, grown on an area of approximately 1.65 million ha, and Egypt is one of the world’s major producers and exporters of green beans (FAOSTAT, 2020). Irrigation is critical at all growth stages of bean plants, which requires adequate water to achieve an important yield (Sezen et al., 2008). Furthermore, common beans are considered a highly salt-sensitive crop, being exhibit growth reduction and injury symptoms upon exposure to salinity (Maas & Grattan, 1999).
However, little information exists regarding the influence of the foliar application of antioxidative compounds like GSH on drought-stressed common bean plants cultivated in salt-affected soil in open field conditions. Therefore, the current research was intended to look into the potential use of GSH to reduce the adverse consequences of water shortage on common beans. In this study, the potential changes in physio-biochemical attributes, osmolytes, and antioxidative molecules of Phaseolus vulgaris were examined under combined exogenously-applied GSH and water deficit. Furthermore, the expected enhancements in water status, membrane stability, photosynthetic efficiency, growth parameters, pod yield, and IUE of common bean induced by GSH under water deficits were assessed.
Material and Methods
Experimental field site
The field experiments were performed on a private farm in Fayoum governorate, Egypt, 29.5004 N, 30.8767 E, during the two seasons of 2017 and 2018. Before the start of each experiment, soil samples were collected to a depth of 25 cm from the experimental site and analyzed for physical and chemical properties (Table 1) according to Klute (1986) and Page, Miller & Keeney (1982). The average monthly climatic data of El-Fayoum during the study period (September −November) are presented in Table 2. The study area has a hyperarid climate as identified by the aridity index (Ponce, Pandey & Ercan, 2000).
Table 1. Some initial physico-chemical characteristics of the studied soil.
| EC (dS/m) | pH | OM % | CaCO3 | Particle size distribution | Texture class | ρd g.cm−3 | Ksat cm h−1 | Soil moisture content at | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand % | Silt % | Clay % | FC % | WP % | AW % | |||||||
| 6.22 | 7.66 | 1.13 | 4.51 | 13.0 | 12.8 | 74.2 | LS | 1.58 | 2.10 | 21.03 | 10.55 | 10.48 |
Notes.
- EC
- electrical conductivity
- OM
- Organic matter content %
- LS
- loamy sand
- ρd
- Bulk density
- Ksat
- Hydraulic conductivity
- FC
- Field capacity
- WP
- wilting point
- AW
- Available water
Table 2. Monthly weather data at Fayoum, Egypt as an average for 2017–2018.
| Month | Tmax (°C) | aTmin (°C) | Tavg (°C) | RHavg (%) | U2 ms−1 | ETo (mmd−1) |
|---|---|---|---|---|---|---|
| September | 38.3 | 23.6 | 30.95 | 37.0 | 2.1 | 5.85 |
| October | 34.0 | 22.4 | 28.2 | 40.0 | 1.95 | 4.7 |
| November | 27.8 | 15.4 | 21.6 | 41.5 | 2.2 | 2.15 |
Notes.
Tmax , Tavg, and Tmin are average, maximum, and minimum temperatures, respectively.
- RHavg
- average relative humidity
- U2
- average wind speed
- EP
- average of measured pan evaporation class A
Irrigation applied (IA) and treatments
Every two days, the growing Phaseolus vulgaris plants were irrigated with varying amounts of irrigation water. The amount of irrigation applied (m3) to each experimental unit was calculated by using the following equation:
where; A is the experimental unit area (m2), ETc is the crop evapotranspiration (mm day−1), Ii is the intervals between irrigation events (day), Kr is the covering factor, Ea is the application efficiency (%), and LR is the leaching requirements.
The daily reference evapotranspiration; ETo (mm day−1) was computed using the evaporation registered from class A pan (Epan) and appropriate pan coefficient (Kpan) for the experimental area as follows:
The crop water evapotranspiration (ETC) was estimated using the crop coefficient according to the following equation:
where ETo = the reference evapotranspiration (mm day−1) and Kc = the crop coefficient. The duration of the initial, crop development, mid-season, and late-season stages were 15, 25, 25, and 10 days, respectively. The Phaseolus vulgaris Kc according to Allen et al. (1998) was 0.50, 1.05, and 0.90, corresponding to the initial, mid, and end stages, respectively.
In a preliminary pot experiment the GSH was foliar-applied with 0.5 and 1.0 mM at 25 days after sowing and applied again after 10 days. The spray solution was prepared by dissolving GSH in distilled water with adding Tween-20 (0.1%, v/v) as a surfactant to increase its retention on the leaves, thus fast penetration through the leaves. For each experimental plot (9 m2), a volume of 9 L of spraying solution was specified per each application time. Foliar spraying GSH at the concentrations and times obtained from the initial pot experiment achieved the highest growth of green bean plants grown under three irrigation rates 100, 80, and 60% of ETc. For the current study, there were two factors including GSH (0, 0.5 mM = GSH1 and 1.0 mM = GSH2), and irrigation regimes as a percentage of the ETc (100% = I100, 80% = I80, and 60% =160). Therefore, nine treatments were established as follows; I100 (full irrigation without GSH application), I80 (irrigation with 80% of the ETc without GSH application), I60 (irrigation with 60% of the ETc without GSH application), I100 + GSH1, I100 + GSH2, I80 + GSH1, I80 + GSH2, I60 + GSH1, and I60 + GSH2.
Plant materials and experimental layout
The experimental layout was a randomized complete block design with three replications. The total experimental included 27 plots; each one was approximately 9 m2 (15 m in length × 0.6 m row width) each plot included 2 planting rows placed 30 cm apart with a distance of 10 cm between plants within rows. Two drip lines were placed 0.3 m apart in each elementary test plot. Phaseolus vulgaris (cv. Bronco) seeds were planted on 6 September and harvested on 28 November in both growing seasons. All treatments were separated and surrounded by a 1m non-irrigated area. Plants were adequately watered during the first irrigation. One week after complete germination, irrigation treatments were started. All experimental units received identical doses of N, P2O5, and K150 kg N ha−1, 60 kg P ha−1, and 70 K kg ha−1 orderly. The other cultural practices for commercial bean production were carried out according to the instructions of the Ministry of Agriculture and Land Reclamation.
Growth and yield-related attributes
After 50 days of sowing in each season, three random plants from each experimental unit were taken to measure morphological characteristics, and another group of three plants was taken to determine physio-biochemical traits. The lengths of the shoots were measured on a meter scale, and the number of leaves per plant was counted. A digital planimeter (Planix 7, Tamaya Technics Inc., Tokyo, Japan) was used to measure the leaf area per plant. Plant shoots were weighed to determine their fresh weight before being oven dried at 70 °C until their weight stabilized. Green pods were collected at the harvest stage from all plants in each plot to determine the average number of pods per plant, green pod weight per plant, and green pod yield per hectare in a ton.
Leaf relative water content, membrane stability, and irrigation use efficiency
The relative water content (%) in bean leaves were assessed (Osman & Rady, 2014). After excluding the midrib, 2-cm discs were taken and the fresh mass (FM) was weighed. Immediately, the discs were submerged in distilled water for 24 h, after which they were extracted and weighed to determine the saturated mass (SM). The dry mass of discs after dehydrating at 70 °C for 48 h was recorded. The RWC was calculated using the formula:
Duplicate samples of fully-expanded fresh leaves tissue, each weighing 0.2 g were used to determine the stability index of the cellular membrane (MSI) (Abdelkhalik et al., 2019b). The first leaf sample was placed in a test tube with 10 ml of double-distilled water and heated in a water bath at 40 °C for 30 min. The electrical conductivity of the solution was measured and denoted a C1. The same previous steps were performed with the second leaf sample but the samples were heated at 100 °C for 10 min, and electric conductivity was measured and denoted a C2. The MSI was calculated as follows:
Irrigation use efficiency (IUE) values were calculated for different treatments as kg green pods per cubic meter (m3) of applied water using the following equation (Jensen, 1983):
Photosynthetic efficiency
Using a chlorophyll meter; SPAD-502 (Minolta, Tokyo, Japan) fully developed leaves were collected from the top of each plant to determine the relative chlorophyll content (SPAD value). The chlorophyll fluorescence parameters were measured with one leaf per plant on two different sunny days using a portable fluorometer (Handy PEA, Hansatech Instruments Ltd., Kings Lynn, UK). The maximum quantum yield (Fv/Fm) was calculated using the formula: Fv/ Fm = ( Fm −F0)/ Fm (Maxwell & Johnson, 2000). According to Clark et al. (2000), the photosynthetic performance index (PI) was determined.
Quantification of osmoprotectants and non-enzymatic antioxidant contents
Free proline content was quantified using the rapid colorimetric method described by Bates, Waldren & Teare (1973). Rosen’s (1957) method was used to determine the total free amino acid content of dry leaves. Leaf-soluble sugar content was determined after extraction with 96% (v/v) ethanol, as outlined by Irigoyen, Einerich & Sánchez-Díaz (1992). The extract was reacted with anthrone reagent, the obtained mix was boiled for 10 min. After cooling, the samples were read at 625 nm using a spectrophotometer (a Bausch and Lomb-2000).
Leaf ascorbic acid (AsA) content was extracted and quantified according to the methods of Kampfenkel, Van Montagu & Inzé (1995). To assay AsA content, a leaf sample of 1.0 g was homogenized and extracted with 5% (w/v) trichloroacetic acid (TCA) with liquid N2 then the mixture was centrifuged (15,600 × g, 4 °C, 5 min). 1.0 ml of the supernatant was carefully taken and placed in the vessel tube with 0.5% (v/v) nethylmaleimide, 10 mM DTT, 10% (w/v) TCA, 4% (v/v) 2,2′-dipyridyl, 42% (v/v) H3PO4, 3% (w/v) FeCl3, in addition to 0.2 M phosphate buffer with pH 7.4. For GSH contents determination by Griffith (1980), homogenization of fresh leaf tissue (50 mg) was exercised in two mL of 2% (v/v) metaphosphoric acid, and centrifugation was then applied at 17,000 × g for 10 min. The supernatant was neutralized with sodium citrate of 10% (w/v). Assessments of 3 replicates were made for each sample. A composition of 700 µL of 0.3 mM NADPH, 100 µL of 6 mM 5,50 -dithiol-bis-2- nitro benzoic acid, 100 µL distilled water, and 100 µL of the extract was of each assay (1.0 mL) that was stabilized at 25 °C for 3–4 min, and GSH reductase (10 µL of 50 units mL−1) was then added and the absorbances were read at 412 nm to calculate GSH contents from a standard curve.
Enzymatic antioxidant assay
For obtaining the enzyme extracts, 200 mg freeze-fresh leaf was homogenized in a cold mortar with 2 ml of extraction buffer prepared from potassium phosphate buffer (100 mM, pH 7.0) containing 0.1 mM ethylenediaminetetraacetic acid (EDTA) (Bradford, 1976). For assaying the APX activity (µmol H2O2 min−1 g−1 protein) (Nakano & Asada, 1981), 2 mM AsA was added to the extraction buffer. The homogenate was filtered and then centrifuged at 12,000 × g for 15 min. All steps were completed under 4 °C. The mixture (2 ml) was spotted for 2 min at 290 nm with a spectrophotometer measuring the AsA oxidation, and an extinction coefficient of 2.8 mM−1 cm−1 was used. The activity (µmol H2O2 min−1 g−1 protein) of CAT (EC 1.11.1.6) was quantified as described by Havir & McHale (1987), by measuring the decrease in absorbance read at 240 nm caused by H2O2 breakdown (ɛ = 36 M−1 cm−1). The activity (U mg−1 protein) of SOD (EC 1.15.1.1) was measured by determining its ability to inhibit nitro blue tetrazolium (NBT) photochemical decrement (Beauchamp & Fridovich, 1971). The enzyme amount required to inhibit half of the NBT photoreduction rate (%) was assigned as one unit of SOD activity. As outlined by Martinez et al. (2018) the GPX activity was quantified with a glutathione peroxidase assay kit (Ref. ab102530; Abcam, Cambridge, UK) measuring the reduction of NADPH at 340 nm, and extinction coefficient of 6.22 mM−1 cm−1 was used.
Statistical analysis
Data from both field experimental seasons were analyzed using GenStat 19th Edition (VSN International Ltd, Hemel Hempstead, UK). Differences between the treatments were compared using Tukey’s Honest Significant Difference test at P ≤ 0.05.
Results
Growth characteristics and green pods yield
Results in Table 3 exhibited that deficit irrigation (I80 or I60) unfavorable affected all growth parameters; i.e., shoot length, plant leaf area, the number of leaves per plant, and shoot dry weight plant−1. However, foliar-applied GSH (0.5 or 1.0 mM) corrected this growth inhibition and increased all growth parameters compared to deficit irrigation treatment (I80, or I60) in both seasons. Generally, the maximum values of all analyzed growth traits were recorded under I100 + GSH1 treatment. However, foliar-applied GSH (0.5 mM) to 20% water-stressed common bean plants increased the aforementioned growth parameters and registered similar or higher values than fully irrigation plants untreated with GSH (I100 treatment).
Table 3. Effect of exogenous spray applications of glutathione (GSH; 0.5 or 1.0 mM) on vegetative growth characteristics of common beans plants grown under different irrigation levels in 2017 (SI) and 2018 (SII) seasons.
| Treatment | Shoot length (cm) | Number of leaves plant−1 | Leaf area plant−1 (dm2) | Shoot dry weight plant−1 (g) | ||||
|---|---|---|---|---|---|---|---|---|
| SI | SII | SI | SII | SI | SII | SI | SII | |
| I100 | 79.3b | 78.3c | 27.0a | 29.3a | 20.7a | 22.5a | 22.8a | 20.4a |
| I80 | 78.3b | 76.3cd | 23.7b | 27.0b | 18.3bc | 20.0b | 19.3b | 18.6b |
| I60 | 68.3d | 59.7e | 18.3c | 20.0d | 16.3d | 12.9d | 13.2c | 16.6c |
| I100+ GSH1 | 86.0a | 87.0a | 27.0a | 28.3a | 21.0a | 23.8a | 23.4a | 21.4a |
| I100+ GSH2 | 74.7bc | 80.3bc | 27.3a | 28.0a | 20.6a | 22.9a | 22.2a | 20.0a |
| I80+ GSH1 | 78.0b | 78.0c | 27.0a | 29.0a | 20.3a | 22.4a | 22.8a | 18.6b |
| I80+ GSH2 | 75.0b | 80.3bc | 26.3a | 29.0a | 20.0ab | 22.6a | 22.0a | 20.4a |
| I60+ GSH1 | 70.0cd | 71.3d | 22.3b | 24.0c | 19.5ab | 20.4b | 23.2a | 19.9a |
| I60+ GSH2 | 72.0cd | 63.0e | 22.7b | 23.7d | 17.1cd | 15.5c | 19.8b | 16.7c |
Notes.
# Mean values in each column followed by a different lower-case-letter are significantly different by Tukey’s Honest Significant Difference test at P ≤ 0.05.
- I100
- irrigation with 100% of ETc
- I80
- irrigation with 80% of ETc
- I60
- irrigation with 60% of ETc
In both seasons, the gradual reduction of irrigation from 100% (I100) to 60% (I60) of ETc significantly decreased gradually the number of pods (up to 61%), green pods weight (up to 48%), and green pods yield ha−1 (up to 47%) (Table 4). However, exogenously-applied GSH to common bean plants grown under water stress recovered the yield losses by inducing considerable increases in the number of pods, green pods weight, and green pods yield compared to the respective control. Foliar spraying bean plants grown under 20% water deficit with GSH (0.5 mM) increased the abovementioned traits by 38% and 37%, 24% and 23%, and 24% and 18% (seasons average) respectively, when compared with the corresponding control, with similar values as those observed under full irrigation (I100). However, integrative I60 + GSH1 or GSH2 elevated the green pod’s yield and its component compared to the corresponding control but did not reach those observed under optimum irrigation without GSH application (I100).
Table 4. Effect of exogenous spray applications of glutathione (GSH; 0.5 or 1.0 mM) on the productivity of common beans plants grown under different irrigation levels in 2017 (SI) and 2018 (SII) seasons.
| Treatment | Number of pods plant−1 | Green pods weight plant−1 | Green pods yield (ton ha−1) | |||
|---|---|---|---|---|---|---|
| SI | SII | SI | SII | SI | SII | |
| I100 | 26.7*a | 29.3a | 49.0a | 51.7a | 9.61a | 10.15a |
| I80 | 19.0b | 22.3b | 40.7b | 41.0b | 8.63b | 9.04b |
| I60 | 10.7c | 11.0c | 25.8e | 26.7d | 4.87c | 5.65d |
| I100+ GSH1 | 27.0a | 29.7a | 50.7a | 51.1a | 9.73a | 11.02a |
| I100+ GSH2 | 28.3a | 29.3a | 49.7a | 50.7a | 9.73a | 10.70a |
| I80+ GSH1 | 27.7a | 28.9a | 50.3a | 51.3a | 10.87a | 10.97a |
| I80+ GSH2 | 27.3a | 29.0a | 49.7a | 51.0a | 9.93a | 11.00a |
| I60+ GSH1 | 19.0b | 22.3b | 36.0c | 36.3c | 8.20b | 8.99b |
| I60+ GSH2 | 19.0b | 21.7b | 31.7d | 37.0c | 7.97b | 8.02c |
Notes.
Mean values in each column followed by a different lower-case-letter are significantly different by Tukey’s Honest Significant Difference test at P ≤ 0.05.
- I100
- irrigation with 100% of ETc
- I80
- irrigation with 80% of ETc
- I60
- irrigation with 60% of ETc
Membrane integrity, water status, and irrigation use efficiency
As presented in Table 5, deficit irrigation (I80 and I60) induced stress in bean plants, being reduced the membrane stability index (MSI) by 14% and 40% and leaf relative water contents (RWC) by 6% and 17% (seasons average) respectively, compared to well-watered plants without application of GSH (I100). Nevertheless, GSH supplementation attenuated the water deficit-induced damages as the same values of MSI and RWC were observed under fully irrigated plants untreated with GSH (I100). Reducing irrigation by up to 60% of ETc markedly decreased the irrigation use efficiency (IUE) by 13% in comparison with full irrigation (I100). However, combined externally applied GSH and water deficit substantially elevated the IUE. The integrative I80 + GSH1 or GSH2 and I60 + GSH1 or GSH2 exceeded the full irrigation without GSH application (I100) treatment by 38% and 37%, and 33% and 28% (seasons average) respectively.
Table 5. Effect of exogenous spray applications of glutathione (GSH; 0.5 or 1.0 mM) on membrane stability index (MSI), relative water content (RWC) and irrigation use efficiency (IUE) of common beans plants grown under different irrigation levels in 2017 (SI) and 2018 (SII) seasons.
| Treatment | MSI (%) | RWC (%) | IUE (kg pods m−3 of water) | |||
|---|---|---|---|---|---|---|
| SI | SII | SI | SII | SI | SII | |
| I100 | 52.3*a | 57.9a | 87.9a | 89.8a | 2.57d | 3.35de |
| I80 | 46.4b | 47.8b | 83.6b | 84.1b | 2.90cd | 3.76c |
| I60 | 30.6d | 36.0d | 72.7c | 75.2c | 2.08e | 3.14e |
| I100+ GSH1 | 53.7a | 58.6a | 88.3a | 89.7a | 2.78cd | 3.74c |
| I100+ GSH2 | 52.8a | 57.4a | 88.5a | 88.9a | 2.78cd | 3.57cd |
| I80+ GSH1 | 52.2a | 57.2a | 87.2a | 88.3a | 3.88a | 4.16b |
| I80+ GSH2 | 51.9a | 56.7a | 86.1a | 88.0a | 3.55ab | 4.58a |
| I60+ GSH1 | 46.3b | 47.5b | 86.9a | 84.1b | 3.43b | 4.44ab |
| I60+ GSH2 | 40.7c | 42.7c | 83.1b | 83.9b | 3.13bc | 4.52a |
Notes.
Mean values in each column followed by a different lower-case-letter are significantly different by Tukey bc Honest Significant deffghgi]g test at P ≤ 0.05.
- I100
- irrigation with 100% of ETc
- I80
- irrigation with 80% of ETc
- I60
- irrigation with 60% of ETc
Chlorophyll a fluorescence and SPAD value
Data of chlorophyll fluorescence (i.e., Fv/Fm and PI) and SPAD chlorophyll content of common bean plants in response to the application of GSH and water deficits are shown in Table 6. Compared to fully irrigated plants (I100), common bean plants subjected to water deficits (I80 and I60) exhibited lower values of Fv/Fm (by 3% and 7%), PI (24% and 42%), and SPAD value (by 29% and 47%) (seasons average), respectively. However, foliage spraying GSH was observed to adjust the drought-impacted Fv/Fm, PI, and SPAD values of bean plants. In this respect, spraying with 0.5 or 1 mM GSH to water-stressed bean plants at 20% showed similar values of the SPAD value, Fv/Fm, and PI to bean plants subjected to full irrigation without GSH application (I100).
Table 6. Effect of exogenous spray applications of glutathione (GSH; 0.5 or 1.0 mM) on chlorophyll a fluorescence and relative chlorophyll content (SPAD value) of common beans plants grown under different irrigation levels in 2017 (SI) and 2018 (SII) seasons.
| Treatment | Fv/Fm | PI | SPAD chlorophyll | |||
|---|---|---|---|---|---|---|
| SI | SII | SI | SII | SI | SII | |
| I100 | 0.81*a | 0.82a | 2.41b | 2.54a | 35.3a | 35.4a |
| I80 | 0.79b | 0.79c | 1.60c | 2.17b | 25.1c | 25.2c |
| I60 | 0.76c | 0.76d | 1.15d | 1.72c | 16.8d | 20.5d |
| I100+ GSH1 | 0.82a | 0.82a | 2.41b | 2.58a | 37.3a | 36.7a |
| I100+ GSH2 | 0.82a | 0.83a | 2.76a | 2.64a | 35.9a | 36.2a |
| I80+ GSH1 | 0.81a | 0.82a | 2.42b | 2.51a | 34.1a | 34.7a |
| I80+ GSH2 | 0.82a | 0.82a | 2.41b | 2.55a | 34.2a | 34.5a |
| I60+ GSH1 | 0.81a | 0.80bc | 1.62c | 2.18b | 31.0b | 29.4b |
| I60+ GSH2 | 0.80ab | 0.79c | 1.20d | 2.17b | 31.1b | 28.7b |
Notes.
Mean values in each column followed by a different lower-case-letter are significantly different by Tukey’s Honest Significant Difference test at P ≤ 0.05.
- I100
- irrigation with 100% of ETc
- I80
- irrigation with 80% of ETc
- I60
- irrigation with 60% of ETc
Plant defense system: osmolytes and antioxidants
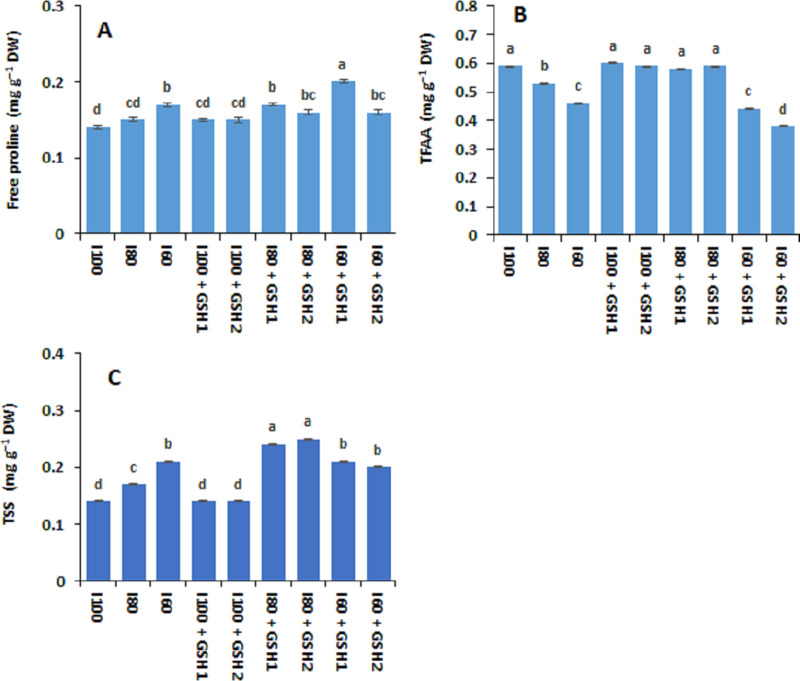
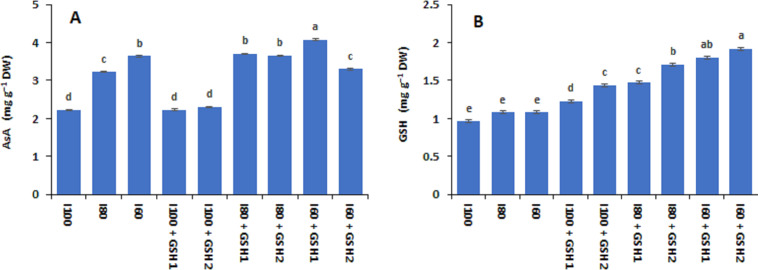
Figures 1 and 2 show that the contents of total soluble sugars, free proline, AsA, and GSH (not significant) increased in Phaseolus vulgaris leaves in response to the water deficit exposure (I80 and I60), whereas the contents of total free amino acids decreased (I100). Under water deficits, exogenous GSH-mediated further increases in total free amino acids, free proline, and total soluble sugars, as well as AsA and GSH compared to stressed plants untreated with GSH (I80 and I60). In this regard, the maximum values of free proline, AsA, and GSH corresponded with the integrative I60 + GSH1 treatment while the integrative I80 + GSH1 or GSH2 produced the highest total soluble sugars and total free amino acids levels.
Figure 1. Effect of exogenous spray applications of glutathione (GSH; 0.5 or 1.0 mM) on the contents of free proline (A), total free amino acids (TFAA) (B), and total soluble sugars (TSS) (C) of common beans plants grown under different irrigation levels (seasons average).
The vertical bar represents the standard error. Different letters on the bar indicate a significant difference by Tukey’s Honest Significant Difference test at P ≤ 0.05.
Figure 2. Effect of exogenous spray applications of glutathione (GSH; 0.5 or 1.0 mM) on the contents of ascorbic acid (AsA) (A) and glutathione (GSH) (B) of common beans plants grown under different irrigation levels (seasons average).
The vertical bar represents the standard error. Different letters on the bar indicate a significant difference by Tukey’s Honest Significant Difference test at P ≤ 0.05.
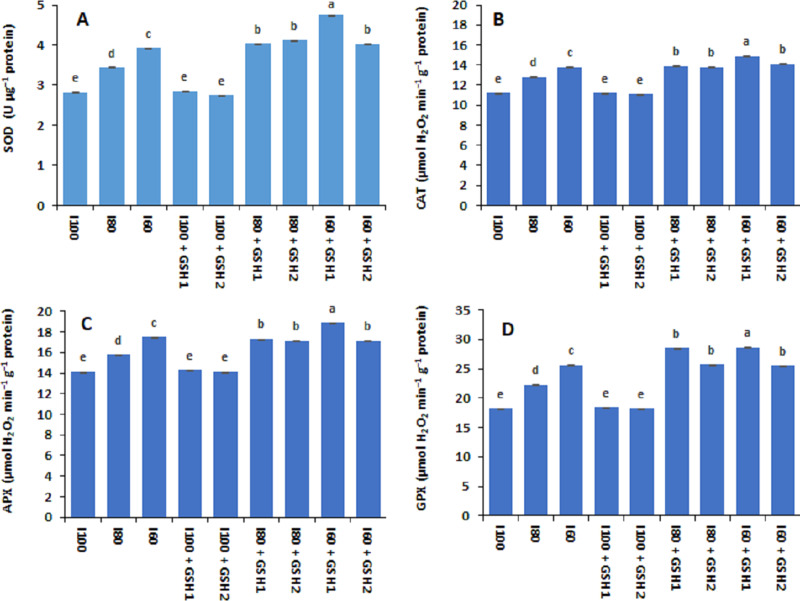
Drought stress (I80 and I60) increased significantly the activities of antioxidant enzymes in terms of SOD, CAT, APX, and GPX in common bean plants compared to normal conditions (I100) as shown in Fig. 3. The aforementioned enzymes activities were at lower level corresponding to the I100, I100 + GSH1, or I100 + GSH2 treatments. Nonetheless, exogenous GSH to water-stressed Phaseolus vulgaris induced additional increases in the antioxidative compounds. The magnitude of antioxidant enzyme activity response was more pronounced with integrative I60 + GSH1 treatment, followed by integrative I60 + GSH1, and I80 + GSH1 or GH2 treatments.
Figure 3. Effect of exogenous spray applications of glutathione (GSH; 0.5 or 1.0 mM) on the activities antioxidant enzymes; superoxide dismutase (SOD) (A), catalase (CAT) (B), ascorbate peroxidase (APX) (C), and glutathione peroxidase (GPX) (D) of common beans plan.
The vertical bar represents the standard error. Different letters on the bar indicate a significant difference by Tukey’s Honest Significant Difference test at P ≤ 0.05.
Discussion
As expected, decreasing soil water content under high soil salinity conditions (6.22 dS m−1; Table 1) significantly reduced bean plant growth (i.e., shoot height, leaf area, number of leaves, and dry biomass), pods yield, and the IUE (Tables 3–5). Environmental stresses; drought and salinity mediate the loss of cell turgor and impede cell division and elongation, consequently diminishing bean growth and development (Taiz & Zeiger, 2010; Dawood, Abdelhamid & Schmidhalter, 2014; Fahad et al., 2017). However, externally-applied GSH ameliorated drought-induced damage to bean plants, showing that it increased their growth, dry matter, and productivity (yield and IUE) (Tables 3–5) due to increased membrane integrity, tissue water content, and photosynthetic efficiency (Tables 4–6). The application of GSH to water-stressed plants at 20%, yielded better results, whereas when applied to severe water deficit (60% of ETc) improved growth characteristics but not to the same extent as observed under fully irrigated plants. Growth and productivity restoration of water-stressed bean plants by GSH application demonstrated that GSH may involve in mechanisms for drought stress tolerance. GSH is thought to have growth-regulating properties, as increased endogenous GSH promotes cell division in the apical meristem of the root (Vernoux et al., 2000; Hossain et al., 2017), this root elongation is a key morphological adaptation to water deficit (Hasanuzzaman, Nahar & Anee, 2017).
Exogenous GSH increased yield-linked parameters, which could be consequent to the increase of plant leaf area, as well as higher photosynthetic pigments content. Both of these characteristics contribute to increased photosynthetic efficiency and sink capability, which is met by a steady supply of metabolites necessary for the development of bean pods (Thomas & Howarth, 2000; Rehman et al., 2021). Our results are consistent with a previous study (Al-Elwany et al., 2020), which found that foliarly applied GSH increased leaf number, leaf area, shoot dry weight, fruit yield and water use efficiency of chili pepper under water deficits.
Soil water deficit and salinity stress provoke lower cell turgor pressure and soil water potential, increasingly less available water and nutrients acquisition by the plant, thus decreasing the leaf’s RWC (Table 5) (Mahajan & Tuteja, 2005; Giménez, Gallardo & Thompson, 2013; Sarker & Oba, 2018). This water stress within the membrane lipid bilayer may result in the degradation of the membrane protein by ROS activity which triggers lipid peroxidation and loss of membrane integrity (Table 5) or even induces complete membrane denaturation (Mahajan & Tuteja, 2005; Abdelkhalik et al., 2020). However, the negative impacts of decreased RWC and increased cell membranes injured by drought stress were ameliorated in GSH-treated bean plants under water deficit. Therefore, fully irrigated or water-stressed plants at 20% treated with GSH achieved the highest RWC and MSI followed by integrative I60 + GSH1 or GH2. High endogenous GSH levels were linked to the regulation of leaf RWC, suggesting that GSH can play a role in leaf rolling control induced by drought stress (Hasanuzzaman, Nahar & Anee, 2017). Exogenous GSH has been shown to improve RWC, MSI, and electrolyte leakage in similar studies (Sohag et al., 2020; Rehman et al., 2021). These improvements in water status and stable cell membrane could be attributed to foliar-applied GSH increased osmolytes accumulation (Fig. 1), which improves osmotic adjustment for maintaining turgor pressure and increases water diffusion into the cell while also increasing antioxidant activities (Figs. 2 and 3) for ROS detoxification and protecting cell membranes (Nahar et al., 2015; Pei et al., 2019; Rehman et al., 2021). These findings highlight the role of GSH in stabilizing membrane integrity for normal functions and increasing tissue water content as metabolically available water, allowing plants to maintain physiological and metabolism activities (Abid et al., 2018; Huang et al., 2020).
Water deficits reduced the relative chlorophyll content and photosynthetic capacity of the PSII including Fv/Fm and PI. This could be attributed to reduced essential nutrient uptake by roots that are necessary for chlorophyll biosynthesis (Etienne et al., 2018; Semida et al., 2021b), concomitant with inhibition of D1 protein synthesis and degradation of the thylakoid membrane owing to oxidative stress (Tian et al., 2013; Wang et al., 2018), indicating inhibition of electron transport chain and the light-harvesting complex of PSII in drought-stressed common bean plants. Nevertheless, GSH-mediated recovery of the relative chlorophyll content and photosynthetic capacity of common bean plants, showed that GSH increased the SPAD chlorophyll, maximal quantity yield (Fv/Fm), and the performance index (PI) of the PSII (Table 6). These findings may be linked to improving plant water status (RWC) and cell membrane stability index (MSI, Table 5) by exogenous GSH for restoring the damaged chloroplast and increasing chlorophyll contents (Nahar et al., 2015; Al-Elwany et al., 2020). Besides the role of GSH in scavenging ROS via the AsA-GSH cycle, GSH-mediated increases in the antioxidative compounds; enzymatic and non-enzymatic (Figs. 2 and 3) that participated in ROS scavenging and inhibition of chlorophyll degradation (Hasanuzzaman, Nahar & Anee, 2017; Hossain et al., 2017) thus increasing the photosynthetic efficiency. The increment in root growth and biomass has been linked to the up-regulation of cysteine and GSH concentrations to contract water stress in maize plants (Ahmad et al., 2016), which may increase water and element uptake and enhance photosynthetic capacity.
In osmotically-stressed common bean plants, clear increases in osmolytes accumulation; total free amino acids, proline, and total soluble sugars were observed in GSH-treated plants. These osmoprotectants might help in water stress tolerance in bean plants by enhancing osmotic adjustment for maintaining cell turgor, protecting cell membranes and proteins against ROS-induced oxidative damage, thus recovering the cellular functions and metabolism as adaptive mechanisms under stressors (Turner, 2018; Sharma et al., 2019). In previous researches, exogenous GSH was found to up-regulate the accumulation of osmolytes such as proline in mung beans grown under high-temperature stress (Nahar et al., 2015), proline and soluble sugars in chili pepper grown under water stress (Al-Elwany et al., 2020). Also, higher osmolytes concentration after foliar spraying GSH may have a valuable role in ameliorating oxidative damage and acting as an osmoprotectant to prevent water loss and increase the RWC (Nahar et al., 2015; Turner, 2018).
Antioxidant defense system components help to lessen oxidative damage and confer drought stress tolerance in plants. Among them, GSH is one of the most remarkable antioxidants, which forms a crucial portion of the AsA-GSH cycle (Bartoli et al., 2017; Hasanuzzaman, Nahar & Anee, 2017). Common bean plants exposed to water deficits exhibited higher GSH and AsA contents than those well-watered. However, exogenous GSH application mediated further elevation in AsA and GSH content in drought-stressed plants (especially at severe levels), indicating an enhancement in the AsA-GSH cycle, and up-regulation associated enzymes activity as an effective pathway to ameliorate the oxidative damage in cellular organelles under abiotic stress (Hasanuzzaman et al., 2019; Semida et al., 2021a). GSH and AsA predominately quench O2 directly or by enzyme catalysis (Sarker & Oba, 2018). GSH and AsA have high redox potentials, and interactions with a variety of components and pathways to maintain a normally reduced state. As a result, AsA and GSH correlate with the activity of various enzymes (DHAR, GR and MDHAR, and APX) during the ROS detoxification process by donating electrons or reducing equivalents (Foyer & Noctor, 2011; Hasanuzzaman et al., 2019). Supplementation of GSH to water-stressed mung bean markedly decreased the indicators of oxidative stress; H2O2, OH −, O2•−−, and lipid peroxidation, showing the effective role of GSH in reducing oxidative damage (Nahar et al., 2015).
Furthermore, our results exhibited that foliar-applied GSH elevated the enzymatic antioxidant; SOD, CAT, APX, and GPX capacity in leaves of Phaseolus vulgaris plants grown under water deficit (Fig. 3), which is paramount in the removal of ROS in plant tissues. The balance among SOD and APX or CAT activities in the plant cell is critical to determine the stable state of the level of H2O2 and O2•−− to inhibit the forming of the highly toxic OH− (Mittler, 2002). The SOD is found in nearly all tissues and serves as the primary line of defense in the ROS detoxification approach by disputing the O2•−− into O2 and H2O2. After that, the CAT dismutase the H2O2 to H2O during stress, or the H2O2 enters the AsA-GSH cycle, where the APX utilizes the AsA as an electron donor to convert H2O2 to H2O (Das & Roychoudhury, 2014; Hasanuzzaman, Nahar & Anee, 2017). Also, GPX used the GSH as a substrate during scavenging H2O2 and lipid hydroperoxides (Noctor et al., 2012; Zhang et al., 2019), thus increasing the endogenous GSH associated with up-regulation of the activity of GPX may help to scavenge ROS in bean plants under water stress.
In the present study, it was observed that the higher concentration of glutathione led to similar or lower values than the lower concentration. The response of plants to different GSH concentrations varies depending on the crop (Hasanuzzaman, Nahar & Anee, 2017). In stress responses to GSH, an initial response phase (change in GSH redox state) will be followed by an acclimatization phase in which a new steady state is established (increase GSH level and related enzymes activity or/and more reduced GSH redox state). Alternately, system degradation will occur if successful acclimatization is not accomplished (Tausz, Šircelj & Grill, 2004). According to various studies, GSH levels may increase, not change, or decrease under stress. GSH redox potential, which depends on both the GSH/GSSG ratio and GSH concentration, and the redox state of GSH/GSSG may change to become more oxidized, more reduced, or not change at all (Dorion, Ouellet & Rivoal, 2021; Ito & Ohkama-Ohtsu, 2023). Indeed, further investigations with more related measurements are needed to determine the exact reasons for the difference of some parameters under different GSH concentrations. Externally-applied GSH alleviated the water stress (especially at a moderate level) on bean plants grown under salt-affected soil conditions, while under acute water stress improved to some extent the growth and productivity of bean plants.
Conclusions
In summary, reducing irrigation to common bean plants under moderate soil salinity conditions decreased the leaf water content, membrane integrity, SPAD chlorophyll, and photosynthetic efficiency of the PSII, resulting in reduced growth and pods yield while not improving the irrigation use efficiency. On the other hand, exogenously applied GSH mitigated the adverse effects of deficit irrigation on common beans, GSH-directed improvements in the growth, yield, and physio-biochemical properties of bean plants. The relative water content, membrane stability, and photosynthetic efficiency of the PSII were increased in water-stressed bean plants by GSH application. GSH-induced drought stresses tolerance by up-regulating total free amino acids, free proline, and total soluble sugars, as well as AsA and GSH and enzymatic antioxidants (APX, CAT, SOD, and GPX) for osmotic adjustment and stabilizing membrane integrity of bean plants. The integrative I80 + GSH1 or GH2 were more pronounced, recording similar or higher values than well-watered plants untreated with GSH(I100). Therefore, integrating GSH and water deficit is recommended for future application in order to improve Phaseolus vulgaris performance under soil salinity conditions.
Supplemental Information
Funding Statement
This study was a portion of Research Project No. 11030129 corroborative by the National Research Centre, Cairo, Egypt. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Magdi Abdelhamid & Othmane Merah are Academic Editors for PeerJ.
Author Contributions
Taia A. Abd El Mageed conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Wael Semida conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Khoulood A. Hemida conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Mohammed A.H. Gyushi conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Mostafa M. Rady conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Abdelsattar Abdelkhalik conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Othmane Merah analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Marian Brestic analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Heba I. Mohamed analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Ayman El Sabagh analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Magdi T. Abdelhamid conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in the Supplemental File.
References
- Abd El-mageed et al. (2021).Abd El-mageed TA, Abdelkhalik A, Abd El-Mageed SA, Semida WM. Co-composted poultry litter biochar enhanced soil quality and eggplant productivity under different irrigation regimes. Journal of Soil Science and Plant Nutrition. 2021;2021(3):1917–1933. doi: 10.1007/s42729-021-00490-4. [DOI] [Google Scholar]
- Abd El-mageed et al. (2022).Abd El-mageed TA, Gyushi MAH, Hemida KA, El-Saadony MT, Abd El-Mageed SA, Abdalla H, AbuQamar SF, El-Tarabily KA, Abdelkhalik A. Coapplication of effective microorganisms and nanomagnesium boosts the defenses against salt stress in Ipomoea batatas. Frontiers in Plant Science. 2022;13:883274. doi: 10.3389/fpls.2022.883274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelkhalik et al. (2019c).Abdelkhalik A, Pascual B, Nájera I, Baixauli C, Pascual-Seva N. Deficit irrigation as a sustainable practice in improving irrigation water use efficiency in cauliflower under Mediterranean conditions. Agronomy. 2019c;9:732. doi: 10.3390/agronomy9110732. [DOI] [Google Scholar]
- Abdelkhalik et al. (2020).Abdelkhalik A, Pascual B, Nájera I, Domene MA, Baixauli C, Pascual-Seva N. Effects of deficit irrigation on the yield and irrigation water use efficiency of drip-irrigated sweet pepper (Capsicum annuum L.) under Mediterranean conditions. Irrigation Science. 2020;38:89–104. doi: 10.1007/s00271-019-00655-1. [DOI] [Google Scholar]
- Abdelkhalik et al. (2019b).Abdelkhalik A, Pascual-Seva N, Nájera I, Giner A, Baixauli C, Pascual B. Yield response of seedless watermelon to different drip irrigation strategies under Mediterranean conditions. Agricultural Water Management. 2019b;212:99–110. doi: 10.1016/j.agwat.2018.08.044. [DOI] [Google Scholar]
- Abdelkhalik et al. (2019a).Abdelkhalik A, Pascual-Seva N, Nájera I, MÁ Domene, Baixauli C, Pascual B. Effect of deficit irrigation on the productive response of drip-irrigated onion (Allium cepa L.) in mediterranean conditions. The Horticulture Journal. 2019a;88:488–498. doi: 10.2503/hortj.UTD-081. [DOI] [Google Scholar]
- Abid et al. (2018).Abid M, Ali S, Qi LK, Zahoor R, Tian Z, Jiang D, Snider JL, Dai T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.) Scientific Reports. 2018;8:1–15. doi: 10.1038/s41598-018-21441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad et al. (2016).Ahmad N, Malagoli M, Wirtz M, Hell R. Drought stress in maize causes differential acclimation responses of glutathione and sulfur metabolism in leaves and roots. BMC Plant Biology. 2016;16:1–15. doi: 10.1186/s12870-016-0940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Elwany et al. (2020).Al-Elwany OAAI, Mohamed GF, Abdurrahman HA, Rady MM, Latef AAA. Exogenous glutathione-mediated tolerance to deficit irrigation in salt-affected Capsicum frutescence (L.) plants is connected with higher antioxidant content and ionic homeostasis. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2020;48:1957–1979. [Google Scholar]
- Allen et al. (1998).Allen RG, Pereira LS, Raes D, Smith M. FAO Irrigation and Drainage Paper No. 56 - Crop Evapotranspiration 1998
- Ashraf et al. (2019).Ashraf MA, Riaz M, Arif MS, Rasheed R, Iqbal M, Hussain I, Mubarik MS. Plant tolerance to environmental stress. CRC Press; 2019. The role of non-enzymatic antioxidants in improving abiotic stress tolerance in plants; pp. 129–144. [Google Scholar]
- Awad et al. (2012).Awad NM, Turky AS, Abdelhamid MT, Attia M. Ameliorate of environmental salt stress on the growth of the growth of Zea mays L. plants by exopolysaccharides producing bacteria. Journal of Applied Sciences Research. 2012;8:2033–2044. [Google Scholar]
- Bartoli et al. (2017).Bartoli CG, Buet A, Grozeff GGergoff, Galatro A, Simontacchi M. Ascorbate-glutathione cycle and biotic stress tolerance in plants. In: Hossain MA, Munné-Bosch S, Burritt DJ, Diaz-Vivancos P, Fujita M, Lorence A, editors. Ascorbic acid in plant growth, development and stress tolerance. Springer International Publishing; Cham, Switzerland: 2017. pp. 177–200. [DOI] [Google Scholar]
- Bates, Waldren & Teare (1973).Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;207:205–207. [Google Scholar]
- Beauchamp & Fridovich (1971).Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Biddle (2017).Biddle AJ. Crop production science in horticulture. CABI International; 2017. Harvesting, nutritional value and uses. [DOI] [Google Scholar]
- Blum (2017).Blum A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell and Environment. 2017;40:4–10. doi: 10.1111/pce.12800. [DOI] [PubMed] [Google Scholar]
- Bradford (1976).Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark et al. (2000).Clark AJ, Landolt W, Bucher JB, Strasser RJ. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environmental Pollution. 2000;109:501–507. doi: 10.1016/S0269-7491(00)00053-1. [DOI] [PubMed] [Google Scholar]
- Das & Roychoudhury (2014).Das K, Roychoudhury A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Frontiers in Environmental Science. 2014;2:1–13. doi: 10.3389/fenvs.2014.00053. [DOI] [Google Scholar]
- Dawood, Abdelhamid & Schmidhalter (2014).Dawood MG, Abdelhamid MT, Schmidhalter U. Potassium fertiliser enhances the salt-tolerance of common bean (Phaseolus vulgaris L.) Journal of Horticultural Science and Biotechnology. 2014;89:185–192. doi: 10.1080/14620316.2014.11513067. [DOI] [Google Scholar]
- Ding et al. (2016).Ding X, Jiang Y, He L, Zhou Q, Yu J, Hui D, Huang D. Exogenous glutathione improves high root-zone temperature tolerance by modulating photosynthesis, antioxidant and osmolytes systems in cucumber seedlings. Scientific Reports. 2016;6:1–12. doi: 10.1038/srep35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorion, Ouellet & Rivoal (2021).Dorion S, Ouellet JC, Rivoal J. Glutathione metabolism in plants under stress: beyond reactive oxygen species detoxification. Metabolites. 2021;11(9):641. doi: 10.3390/metabo11090641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne et al. (2018).Etienne P, Diquelou S, Prudent M, Salon C, Maillard A, Ourry A. Macro and micronutrient storage in plants and their remobilization when facing scarcity: the case of drought. Agriculture. 2018;8(1):14. doi: 10.3390/agriculture8010014. [DOI] [Google Scholar]
- Fahad et al. (2017).Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J. Crop production under drought and heat stress: plant responses and management options. Frontiers in Plant Science. 2017;8:1–16. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAOSTAT (2020).FAOSTAT Food and agriculture organization of the united nations. 2020. http://www.fao.org/faostat/en/#data/QC. [02 September 2022]. http://www.fao.org/faostat/en/#data/QC
- Farooq et al. (2009).Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development. 2009;29:185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- Foyer & Noctor (2011).Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiology. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill et al. (2017).Gill RA, Ali B, Yang S, Tong C, Islam F, Gill MB, Mwamba TM, Ali S, Mao B, Liu S, Zhou W. Reduced glutathione mediates pheno-ultrastructure, kinome and transportome in chromium-induced Brassica napus L. Frontiers in Plant Science. 2017;8:1–24. doi: 10.3389/fpls.2017.02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez, Gallardo & Thompson (2013).Giménez C, Gallardo M, Thompson RB. Plant–water relations. Reference Module in Earth Systems and Environmental Sciences. Elsevier; 2013. Reference module in earth systems and environmental sciences; pp. 1–8. [DOI] [Google Scholar]
- Griffith (1980).Griffith W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analytical Biochemistry. 1980;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman et al. (2019).Hasanuzzaman M, Borhannuddin Bhuyan MHM, Anee TI, Parvin K, Nahar K, Mahmud JAl, Fujita M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;8(9):384. doi: 10.3390/antiox8090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanuzzaman, Nahar & Anee (2017).Hasanuzzaman M, Nahar K, Anee TI. Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiology and Molecular Biology of Plants. 2017;23:249–268. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havir & McHale (1987).Havir EA, McHale NA. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiology. 1987;84:450–455. doi: 10.1104/pp.84.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain et al. (2017).Hossain MA, Mostofa MG, Diaz-Vivancos P, Burritt DJ, Fujita M, Tran L-SP. Glutathione in plant growth, development, and stress tolerance. Springer International Publishing AG; Gewerbestrasse: 2017. [DOI] [Google Scholar]
- Huang et al. (2020).Huang H, Ran J, Ji M, Wang Z, Dong L, Hu W, Deng Y, Hou C, Niklas KJ, Deng J. Water content quantitatively affects metabolic rates over the course of plant ontogeny. New Phytologist. 2020;228:1524–1534. doi: 10.1111/nph.16808. [DOI] [PubMed] [Google Scholar]
- Irigoyen, Einerich & Sánchez-Díaz (1992).Irigoyen JJ, Einerich DW, Sánchez-Díaz M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiologia Plantarum. 1992;84:55–60. doi: 10.1111/j.1399-3054.1992.tb08764.x. [DOI] [Google Scholar]
- Ito & Ohkama-Ohtsu (2023).Ito T, Ohkama-Ohtsu N. Degradation of glutathione and glutathione conjugates in plants. Journal of Experimental Botany. 2023:erad018. doi: 10.1093/JXB/ERAD018. [DOI] [PubMed] [Google Scholar]
- Jensen (1983).Jensen ME. Design and operation of farm irrigation systems. American Society of Agricultural Engineers; Michigan: 1983. p. 827. [Google Scholar]
- Kampfenkel, Van Montagu & Inzé (1995).Kampfenkel K, Van Montagu M, Inzé D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Analytical Biochemistry. 1995;225:165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- Klute (1986).Klute A. Methods of soil analysis: part 1 physical and mineralogical methods. the American Society of Agronomy, Inc. Soil Science Society of America, Inc.; Madison: 1986. [DOI] [Google Scholar]
- Ma, Dias & Freitas (2020).Ma Y, Dias MC, Freitas H. Drought and salinity stress responses and microbe-induced tolerance in plants. Frontiers in Plant Science. 2020;11:591911. doi: 10.3389/fpls.2020.591911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas & Grattan (1999).Maas EV, Grattan SR. Crop yields as affected by salinity. In: Skaggs RW Van Schilfgaarde J eds., editor. Agricultural drainage, Agronomy. American Society of Agronomy, Crop Science Society of America, Soil Science Society of America; Madison: 1999. pp. 55–108. [DOI] [Google Scholar]
- Mahajan & Tuteja (2005).Mahajan S, Tuteja N. Cold, salinity and drought stresses: an overview. Archives of Biochemistry and Biophysics. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Martinez et al. (2018).Martinez V, Nieves-Cordones M, Lopez-Delacalle M, Rodenas R, Mestre TC, Garcia-Sanchez F, Rubio F, Nortes PA, Mittler R, Rivero RM. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules. 2018;23:1–20. doi: 10.3390/molecules23030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell & Johnson (2000).Maxwell K, Johnson GN. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Mittler (2002).Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Nahar et al. (2015).Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants. 2015;7:1–18. doi: 10.1093/aobpla/plv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano & Asada (1981).Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- Noctor et al. (2012).Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH. Glutathione in plants: an integrated overview. Plant, Cell and Environment. 2012;35:454–484. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- Osman & Rady (2014).Osman AS, Rady MM. Effect of humic acid as an additive to growing media to enhance the production of eggplant and tomato transplants. Journal of Horticultural Science and Biotechnology. 2014;89:237–244. doi: 10.1080/14620316.2014.11513074. [DOI] [Google Scholar]
- Page, Miller & Keeney (1982).Page AL, Miller RH, Keeney DR. Methods of soil analysis Part 2. Chemical and microbiological properties. American Society of Agronomy, Inc; Madison: 1982. [Google Scholar]
- Pal et al. (2016).Pal S, Zhao J, Khan A, Yadav NS, Batushansky A, Barak S, Rewald B, Fait A, Lazarovitch N, Rachmilevitch S. Paclobutrazol induces tolerance in tomato to deficit irrigation through diversified effects on plant morphology, physiology and metabolism. Scientific Reports. 2016;6:1–13. doi: 10.1038/srep39321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei et al. (2019).Pei L, Che R, He L, Gao X, Li W, Li H. Role of exogenous glutathione in alleviating abiotic stress in maize (Zea mays L.) Journal of Plant Growth Regulation. 2019;38:199–215. doi: 10.1007/s00344-018-9832-9. [DOI] [Google Scholar]
- Ponce, Pandey & Ercan (2000).Ponce VM, Pandey RP, Ercan S. Characterization of drought across climatic spectrum. Journal of Hydrologic Engineering. 2000;5:222–224. doi: 10.1061/(ASCE)1084-0699(2000)5:2(222). [DOI] [Google Scholar]
- Rady et al. (2021).Rady MM, Boriek SHK, El-mageed TAA, El-yazal MAS, Ali EF, Hassan FAS, Abdelkhalik A. Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants,antioxidants, nutrients, and phytohormones. Plants. 2021;10:748. doi: 10.3390/plants10040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rady & Hemida (2016).Rady MM, Hemida KA. Sequenced application of ascorbate-proline-glutathione improves salt tolerance in maize seedlings. Ecotoxicology and Environmental Safety. 2016;133:252–259. doi: 10.1016/j.ecoenv.2016.07.028. [DOI] [PubMed] [Google Scholar]
- Rady, Taha & Kusvuran (2018).Rady MM, Taha SS, Kusvuran S. Integrative application of cyanobacteria and antioxidants improves common bean performance under saline conditions. Scientia Horticulturae. 2018;233:61–69. doi: 10.1016/j.scienta.2018.01.047. [DOI] [Google Scholar]
- Rady et al. (2019).Rady MM, Talaat NB, Abdelhamid MT, Shawky BT, Desoky ESM. Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. Journal of Horticultural Science and Biotechnology. 2019;94:777–789. doi: 10.1080/14620316.2019.1626773. [DOI] [Google Scholar]
- Rehman et al. (2021).Rehman HU, Alharby HF, Bamagoos AA, Abdelhamid MT, Rady MM. Sequenced application of glutathione as an antioxidant with an organic biostimulant improves physiological and metabolic adaptation to salinity in wheat. Plant Physiology and Biochemistry. 2021;158:43–52. doi: 10.1016/j.plaphy.2020.11.041. [DOI] [PubMed] [Google Scholar]
- Rosen (1957).Rosen H. A modified ninhydrin colorimetric analysis for amino acids. Archives of Biochemistry and Biophysics. 1957;67:10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Sarker & Oba (2018).Sarker U, Oba S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Scientific Reports. 2018;8:1–12. doi: 10.1038/s41598-018-34944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semida et al. (2021b).Semida WM, Abdelkhalik A, Mohamed GF, Abd El-mageed TA, El-Mageed SAA, Rady MM, Ali EF. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.) Plants. 2021b;10:421. doi: 10.3390/plants10020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semida et al. (2020).Semida WM, Abdelkhalik A, Rady MOA, Marey RA, Abd El-Mageed TA. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Scientia Horticulturae. 2020;272:109580. doi: 10.1016/j.scienta.2020.109580. [DOI] [Google Scholar]
- Semida et al. (2021a).Semida WM, El-mageed TAAbd, Abdelkhalik A, Hemida KA, Abdurrahman HA, Howladar SM, Leilah AAA, Rady MOA. Selenium modulates antioxidant activity, osmoprotectants, and photosynthetic efficiency of onion under saline soil conditions. Agronomy. 2021a;11:855. doi: 10.3390/agronomy11050855. [DOI] [Google Scholar]
- Semida, Hemida & Rady (2018).Semida WM, Hemida KA, Rady MM. Sequenced ascorbate-proline-glutathione seed treatment elevates cadmium tolerance in cucumber transplants. Ecotoxicology and Environmental Safety. 2018;154:171–179. doi: 10.1016/j.ecoenv.2018.02.036. [DOI] [PubMed] [Google Scholar]
- Sezen et al. (2008).Sezen SM, Yazar A, Akyildiz A, Dasgan HY, Gencel B. Yield and quality response of drip irrigated green beans under full and deficit irrigation. Scientia Horticulturae. 2008;117:95–102. doi: 10.1016/j.scienta.2008.03.032. [DOI] [Google Scholar]
- Sharma et al. (2019).Sharma A, Shahzad B, Kumar V, Kohli SK, Sidhu GPS, Bali AS, Handa N, Kapoor D, Bhardwaj R, Zheng B. Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules. 2019;9(7):285. doi: 10.3390/biom9070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohag et al. (2020).Sohag AAM, Tahjib-Ul-Arif M, Polash MAS, Belal Chowdhury M, Afrin S, Burritt DJ, Murata Y, Hossain MA, Afzal Hossain M. Exogenous glutathione-mediated drought stress tolerance in rice (Oryza sativa L.) is associated with lower oxidative damage and favorable ionic homeostasis. Iranian Journal of Science and Technology, Transaction A: Science. 2020;44:955–971. doi: 10.1007/s40995-020-00917-0. [DOI] [Google Scholar]
- Taiz & Zeiger (2010).Taiz L, Zeiger E. Plant physiology. Sinauer Associates Inc; Sunderland: 2010. [Google Scholar]
- Tausz, Šircelj & Grill (2004).Tausz M, Šircelj H, Grill D. The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? Journal of Experimental Botany. 2004;55:1955–1962. doi: 10.1093/jxb/erh194. [DOI] [PubMed] [Google Scholar]
- Thomas & Howarth (2000).Thomas H, Howarth CJ. Five ways to stay green. Journal of Experimental Botany. 2000;51:329–337. doi: 10.1093/jexbot/51.suppl_1.329. [DOI] [PubMed] [Google Scholar]
- Tian et al. (2013).Tian F, Gong J, Zhang J, Zhang M, Wang G, Li A, Wang W. Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. Journal of Experimental Botany. 2013;64:1509–1520. doi: 10.1093/jxb/ert004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombesi et al. (2015).Tombesi S, Nardini A, Frioni T, Soccolini M, Zadra C, Farinelli D, Poni S, Palliotti A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Scientific Reports. 2015;5:12449. doi: 10.1038/srep12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner (2018).Turner NC. Turgor maintenance by osmotic adjustment: 40 years of progress. Journal of Experimental Botany. 2018;69:3223–3233. doi: 10.1093/jxb/ery181. [DOI] [PubMed] [Google Scholar]
- Vernoux et al. (2000).Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, May MJ, Sung ZR. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell. 2000;12:97–109. doi: 10.2307/3871032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang Z, Li G, Sun H, Ma L, Guo Y, Zhao Z, Gao H, Mei L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biology Open. 2018;7:1–9. doi: 10.1242/bio.035279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waqas et al. (2019).Waqas MA, Kaya C, Riaz A, Farooq M, Nawaz I, Wilkes A, Li Y. Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Frontiers in Plant Science. 2019;10:1–14. doi: 10.3389/fpls.2019.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong & Zhu (2002).Xiong L, Zhu JK. Molecular and genetic aspects of plant responses to osmotic stress. Plant, Cell and Environment. 2002;25:131–139. doi: 10.1046/j.1365-3040.2002.00782.x. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2019).Zhang L, Wu M, Teng Y, Jia S, Yu D, Wei T, Chen C, Song W. Overexpression of the glutathione peroxidase 5 (RcGPX5) gene from Rhodiola crenulata increases drought tolerance in Salvia miltiorrhiza. Frontiers in Plant Science. 2019;9:1–16. doi: 10.3389/fpls.2018.01950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2019).Zhou Y, Diao M, Chen X, Cui J, Pang S, Li Y. Application of exogenous glutathione confers salinity stress tolerance in tomato seedlings by modulating ions homeostasis and polyamine metabolism. Scientia Horticulturae. 2019;250:45–58. doi: 10.1016/j.scienta.2019.02.026. [DOI] [Google Scholar]
- Zhu et al. (2020).Zhu Y, Luo X, Nawaz G, Yin J, Yang J. Physiological and biochemical Responses of four cassava cultivars to drought stress. Scientific Reports. 2020;10(1):6968. doi: 10.1038/s41598-020-63809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in the Supplemental File.