Abstract
Purpose
This study aimed to compare the success and complication rates among patients implanted with Ahmed Glaucoma Valve (AGV) using the short and long tunnel technique through retrospective chart review.
Patients and Methods
We reviewed 54 charts of adult patients who underwent AGV implantation using a Short-Needle Track (SNT) or Long-Needle Track (LNT) technique. Intraocular pressures (IOP), Best Corrected Visual Acuity (BCVA) and number of medications were recorded pre-operatively, and at Day 1, 3, 7, Month 1, 3, 6 post-operatively. Treatment success, occurrence of Hypertensive Phase (HP), complication and procedures done after AGV implantation were compared between the two groups using one-tailed Z-test of proportions.
Results
A total of 20 (LNT) and 21 (SNT) charts were included in the study. There was no significant difference between the median postoperative IOP, BCVA, and number of anti-glaucoma medications between the two groups at each time interval. The comparison between the occurrence of HP (P = 0.435) and success rates (P = 0.476) between the two groups yielded no significant difference. Flat/shallow anterior chamber (AC) was seen exclusively in three eyes (14%) in the SNT group (P = 0.039). There was one occurrence of plate exposure in the LNT group (P = 0.149).
Conclusion
The LNT technique of AGV Implantation may be used as an alternative to the traditional SNT (with autologous graft). The long needle track offers the advantage reducing the risk of complications arising from shallow anterior chamber post-operatively.
Keywords: glaucoma drainage device, Ahmed glaucoma valve, long needle track technique, short needle track technique
Introduction
Incisional surgical treatment of glaucoma involves trabeculectomy, and in cases where the risk for scarring is high, implantation of a Glaucoma Drainage Device (GDD) is indicated. In GDD surgery, a Gauge-23 (G-23) needle is traditionally used to create a short scleral tunnel 2–3 mm away from the corneal limbus (short needle track, SNT). This allows for an easy insertion of the GDD tube into the AC. However, this technique has two disadvantages. First, the remaining exposed tube needs to be patched. Second, the short tunnel is prone to peritubular leakage.1–3
To address the first disadvantage, the remaining exposed tube can be patched with various non-autologous grafts such as pericardium, fascia lata, tectonic cornea, amniotic membrane, and donor sclera to prevent Conjunctival Tube Erosion (CTE).4,5 Despite these augmentations, sight-threatening complications related to post-operative CTE including endophthalmitis are still reported.6 Other problems with the use patch grafts include: induction of an immunologic reaction that can erode the overlying conjunctiva, unacceptable cosmetic appearance, and additional cost.7 Due to these possible complications, autologous scleral patch grafts or flaps offer a readily available alternative.8,9
Peritubular filtration is the seepage of aqueous humor between the Ahmed Glaucoma Valve (AGV) tube and the sclera even though a G-23 needle is used to create the track. This was thought to be a result of peritubular leakage from excessive manipulation of the needle track.2 Tightly suturing the scleral flap may be done to avoid peritubular leakage of aqueous humor in a short scleral tunnel but there might be an increased chance of tube erosion as a long-term post-operative complication. Irritation of the conjunctiva and an inflammatory reaction may also occur due to the presence of many suture ends.10
A long tunnel variation (Long Needle Track, LNT) has been developed to forego the use of patch grafts and lessen incidence of peritubular leakage.2 This technique has been reported to be safe and may decrease operating time compared to the conventional SNT technique.11 It allows for maintenance of the normal contour between the conjunctiva and the cornea, which reduces the risk of dellen, foreign body sensation, and bleb leaks.12
At present, the creation of a long scleral tunnel via the needle track method is done in our institution on the hypothesis that greater AGV tube coverage and lesser tube movements are protective against conjunctival erosion. In addition, the LNT is assumed to prevent peritubular filtration which reduces the risk of post-operative hypotony or a shallow AC. This study compared the success and complication rates between the two surgical techniques of a graft-free AGV implantation (LNT vs SNT) through a retrospective chart review. After an extensive literature search (PubMed, Google Scholar, Philippine Academy of Ophthalmology Archives, etc.) no published articles of similar nature were identified.
Materials and Methods
Study Design
This study involved a retrospective review of consecutive cases of AGV implantation using the SNT technique using an autologous scleral flap done from year 2015–2017 and was compared to cases done from year 2018–2021 using the LNT technique by review of chart records/electronic database.
Ethical Considerations
This study was designed and conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. Informed consent was waived due to the retrospective nature of the study. All data collection materials did not include any of the participants’ names. Paper data collection forms were stored so that only the primary investigator will have access to them. Programs or electronic material containing participant data were password-protected. The master list matching participant names and the number codes was kept by the principal investigator. No conflict of interest exists among the investigators which may affect the outcome of the study. The research protocol was submitted to the St. Cabrini Medical Center Asian Eye Institute (SCMC-AEI) Review Committee for ethical review and was conducted upon approval (ERC#2021-017, November 2, 2021).
Setting
This study included all surgeries done by a single surgeon (EUL) in a single center.
Study Population
We reviewed the charts of adult patients who underwent AGV implantation using SNT or LNT and who have at least 6 months of follow up. Patients with missing preoperative or postoperative data were excluded in this study. Out of 54 charts reviewed, a total of 20 eyes in the LNT group and 21 eyes in the SNT which satisfied the set criteria were included in this study.
Study Outcome
Primary outcome measures included post-operative IOP, presence of complications (such as AC shallowing, hyphema, corneal decompensation, choroidal effusion, and conjunctival erosion/tube exposure) and presence of hypertensive phase. Secondary outcome measures included BCVA (expressed in logMAR units) and number of glaucoma medications on Day 1, 3, 7 and Month 1, 3, 6 post-operatively.
Treatment success was defined as an IOP between 6 mm Hg and 21 mm Hg with or without medications at the end of the 6 months follow-up. Treatment failure was defined as IOP more than 21 mm Hg or hypotony less than 6 mm Hg at the end of the 6 months follow-up, a requirement for cyclodestructive procedure, or further glaucoma surgery such as bleb revision. Occurrence of HP (when the IOP increases above 21 mm Hg after an initial postoperative IOP reduction to 21 mm Hg or less during the first 3 post-operative months) was recorded. The results were presented in tabular form.
Surgical Procedure
After induction of intravenous anesthesia and instillation of topical proparacaine eye drops, asepsis/ antisepsis technique was performed. Traction with Vicryl 8.0 (Ethicon, Inc., Bridgewater, New Jersey) suture was then applied to superotemporal corneal limbus and the eye rotated downwards inferonasally. A conjunctival snip was performed at the superotemporal limbus, followed by sub-Tenon’s infiltration with Lidocaine 2%. A two-clock hour curvilinear incision of conjunctiva and Tenon’s at the limbus with Tenon’s dissection carried posteriorly to create a pocket for the valve plate in between the recti muscles. Hemostasis was done with underwater cautery. Ahmed valve FP-7 (New World Medical, Rancho Cucamonga, USA) was primed using a gauge 27 cannula and 3 mL of Balanced Saline Solution (BSS). The plate was tucked into the pocket with its anterior border 8 mm from the limbus. Vicryl 8–0 was used to secure the plate through the eyelets followed by Nylon 9–0 (Ethicon, Inc., Bridgewater, New Jersey) sutures which were applied over the anterior edge of the valve plate.
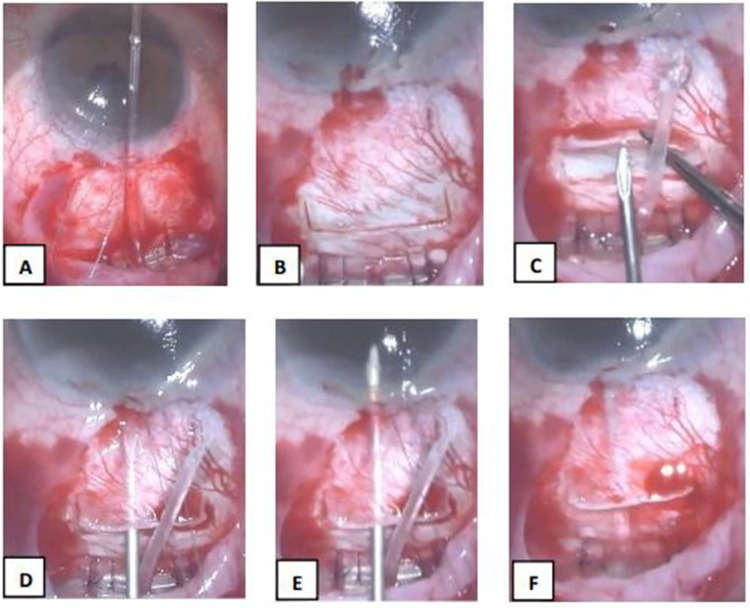
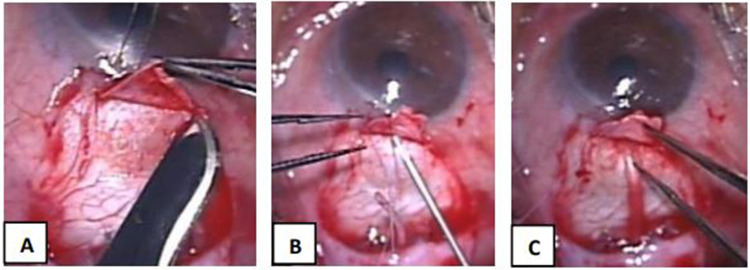
In the LNT method, a 2 mm (length, L) × 4 mm (width, W) rectangular scleral flap was created 2 mm from the anterior border of the plate. A G-23 needle was used to create a 4 mm long tube track commencing at an area under the scleral flap hinge and passed through the scleral stroma until the bevel was visualized to enter the anterior chamber at an angle parallel to the iris. The tube was trimmed to length bevel up then inserted into the tube track leaving 1–1.5 mm of visible tube into the AC (Figure 1). In the SNT method, a 4 mm (L) × 5 mm (W) scleral flap was created commencing 3 mm from the anterior edge of the Ahmed GDD plate. A G-23 needle was used to create a 1–2 mm short tube track commencing at an area under the scleral flap hinge which passed through the scleral stroma into the AC. The tube was then cut and inserted into the short scleral track until 1–1.5 mm of the bevel up end is visible in AC (Figure 2).
Figure 1.
Ahmed Glaucoma Valve Implantation using the LNT technique (A–G). (A) AGV was sutured in place using Vicryl 8–0 and Nylon 9–0 sutures; (B) A 2 (L) × 4 (W) mm scleral flap was created 2 mm anterior to the edge of the plate; (C) G-23 needle was used to create a long needle track; (D) Bevel tip of the G-23 needle near the limbus; (E) Entry of G-23 needle to the anterior chamber parallel to iris plane; (F) Ahmed tube was cut leaving 1–1.5 mm of visible tube inserted in the AC.
Figure 2.
Ahmed Glaucoma Valve Implantation using the SNT technique (A–C). (A) Creation of a 4 mm (L) x 5 mm (W) scleral flap; (B) 2 mm short needle track created with a G-23 needle; (C) Insertion of Ahmed tube with 1–1.5mm intracameral portion.
Inferotemporal paracentesis was done with a blade 11 or a 15-degree stab knife. The tube was sutured onto sclera followed by securing the scleral flap in place both using Nylon 10–0 suture. Conjunctival closure was done using Vicryl 8–0. Leak test was performed. Vancomycin wash was administered. Subtenon’s triamcinolone injection was administered over the valve plate. The corneal bridle and speculum were removed.
Statistical Analysis
Sample Size
The power of analysis was computed using the G*Power. Power of analysis is commonly used to examine the reliability of the sample size as a representative of the target population. The effect size was set at 0.80 based on the study titled “Outcomes and Complications of Ahmed Tube Implantation in Asian Eyes” by Choo et al. According to this, there was significant reduction in IOP (mean reduction, 25.9%; P < 0.001) and number of IOP-lowering medications (mean reduction, 77.8%; P < 0.001) at 3 years. The effect size of 0.80 was set according to effect size convention of G*Power (0.10 low, 0.5 medium, 0.8 high). Given the significant results of the reference study, 0.80 was the chosen effect size. The computed power of analysis was 89.7% which means that the sample size of 41 (20 and 21) are good enough as a representative of the target population of this study.
Data Analysis
Mann–Whitney U-test was used to compare median IOP, BCVA, and number of antiglaucoma medications of the LNT and SNT groups at each time point from preoperative to 6th month. A one-tailed Z-test of proportion was used to compare the occurrence of complications and additional eye procedures, occurrence of hypertensive phase, success rates and failure rates between the two groups. SAS On Demand™ (SAS Institute, USA) was used for data analysis. The null hypothesis was rejected at 0.05-alpha level of significance.
Results
A total of 20 charts for the LNT group and 21 charts for the SNT group were included in this study. There was no significant difference in the median IOP, logMAR BCVA, and median anti-glaucoma medications of LNT and SNT groups at each time interval (Table 1). Based on the data gathered, antiglaucoma medications were started in six eyes in the LNT group without IOP elevation >21 mm Hg compared to two eyes in the SNT. In addition, two eyes in the SNT group were not started with anti-glaucoma medications after needling was done.
Table 1.
Comparison of the Pre-Operative and Post-Operative Median Intraocular Pressure, Best Corrected Visual Acuity and Number of Medications Between Long Needle Track and Short Needle Track Groups
| Pre-op | Day 1 | Day 3 | Day 7 | Month 1 | Month 3 | Month 6 | |
|---|---|---|---|---|---|---|---|
| AGV technique | Median Intraocular Pressure (mmHg) and interquartile range | ||||||
| LNT | 30 (21.5–36.5) | 12 (10–15) | 10 (8–14.3) | 10.5 (8–12.3) | 16 (12–21.3) | 14.5 (12–21.3) | 15 (12–17.3) |
| SNT | 28 (20–33) | 10 (9–13) | 10 (8–12) | 11 (8–12) | 16 (11–20) | 17 (14–19) | 16 (15–19) |
| P-value | 0.695 | 0.070 | 0.331 | 0.926 | 0.714 | 0.695 | 0.417 |
| AGV technique | Median Visual Acuity (LogMAR) and interquartile range | ||||||
| LNT | 1.2 (0.4–2.7) | 1.3 (0.5–1.4) | 1.4 (0.4–1.4) | 1.2 (0.3–1.4) | 0.7 (0.3–1.4) | 0.6 (0.3–1.4) | 0.4 (0.3–1.3) |
| SNT | 1.3 (0.6–2.7) | 1.4 (0.7–2.7) | 1.4 (0.7–2.7) | 1.3 (0.7–1.4) | 1.3 (0.5–2.7) | 1.3 (0.7–1.4) | 1.3 (0.5–1.4) |
| P-value | 0.792 | 0.428 | 0.671 | 0.225 | 0.115 | 0.176 | 0.135 |
| AGV technique | Median number of anti-glaucoma medications and interquartile range | ||||||
| LNT | 2.5 (2–3.3) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 1 (0.8–2) | 1 (0.8–1.3) | 1 (1–2) |
| SNT | 3 (1–4) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0 (0–1) |
| P-value | 0.792 | 0.428 | 0.671 | 0.225 | 0.115 | 0.176 | 0.135 |
Table 2 shows the list of post-operative complications in both groups. Immediate postoperative flat/shallow AC occurred exclusively in the SNT group (P = 0.039). In patients with shallow AC, two phakic eyes were diagnosed with neovascular glaucoma while one pseudophakic eye was diagnosed with secondary glaucoma following penetrating keratoplasty. Due to the AC shallowing, one eye developed tube iris plug (5%). This was resolved after AC reformation with viscoelastic. Postoperative hypotony occurred in two eyes in the SNT group (9.5%) compared to one eye in the LNT group (5%). Decompression retinopathy occurred in 1 eye in the SNT group (4.8%). Plate exposure occurred in one eye (5%) in the LNT group which was noted at three and a half months postoperatively. The patient was female, non-diabetic, phakic, and was diagnosed with neovascular glaucoma. The patient underwent repair of plate and tube exposure with improvement of vision at 6 months post-operatively.
Table 2.
Occurrence of Complications in Long Needle Track and Short Needle Track Ahmed Glaucoma Valve Implantation
| Group | Total N = 41 | P-value | ||
|---|---|---|---|---|
| Long N = 20 | Short N = 21 | |||
| Complications | ||||
| Corneal Bullae | 1 (5%) | 0 (0%) | 1 (2.4%) | 0.149 |
| Filamentary Keratitis | 0 (0%) | 1 (4.8%) | 1 (2.4%) | 0.161 |
| Early flat chamber / Shallow AC | 0 (0%) | 3 (14.3%) | 3 (7.3%) | 0.039* |
| Iris plug | 0 (0%) | 1 (4.8%) | 1 (2.4%) | 0.161 |
| Malignant Glaucoma | 1 (5%) | 0 (0%) | 1 (2.4%) | 0.149 |
| Plate exposure | 1 (5%) | 0 (0%) | 1 (2.4%) | 0.149 |
| Post operative hyphema | 1 (5%) | 1 (4.8%) | 2 (4.9%) | 0.484 |
| Post-operative hypotony | 1 (5%) | 2 (9.5%) | 3 (7.3%) | 0.287 |
| Decompression retinopathy | 0 (0%) | 1 (4.8%) | 1 (2.4%) | 0.161 |
| Vitreous hemorrhage | 1 (5%) | 0 (0%) | 1 (2.4%) | 0.149 |
Note: *Statistically significant.
Complications such as post-operative hyphema, vitreous hemorrhage, post-operative hypotony, decompression retinopathy, filamentary keratitis were transient and did not cause any change in BCVA at 6 months post operatively. Complications such as malignant glaucoma and tube and plate exposure were associated with treatment failure. Corneal bullae formation occurring 4 months post-surgery had resolved with treatment of sodium chloride eye drops and did not cause a decline in BCVA at 6 months post-operatively. The occurrence of decompression retinopathy did not cause any decline in BCVA and remained stable at 2.70 logMAR unit (hand movement). There was no significant difference in the occurrence of postoperative HP between the two groups (P = 0.435) (Table 3).
Table 3.
The Occurrence of Hypertensive Phase Between the Two Groups
| Group | Total N = 41 | P-value | ||
|---|---|---|---|---|
| LNT N = 20 | SNT N = 21 | |||
| Hypertensive phase* | 9 (45%) | 12 (57.1%) | 21 (51.2%) | 0.435 |
Notes: *Hypertensive phase - when the IOP increases above 21 mm Hg after an initial postoperative IOP reduction to 21 mm Hg or less during the first 3 post-operative months.
Table 4 shows the percentage rate of treatment success and treatment failure between the two groups. Since in some of the patients, anti-glaucoma medications were started before a rise in IOP >21 mm Hg was detected, pooled treatment success rates (with or without medications) were considered in this study. Ocular massage (observed in LNT and SNT) and bleb needling (observed in SNT only) were not considered as treatment failure. Success and treatment failure rates were statistically similar for both groups.
Table 4.
Comparison of Treatment Success and Treatment Failure Between the Long Needle Track and Short Needle Track Groups
| Group | Total N = 41 | P-value | ||
|---|---|---|---|---|
| LNT N = 20 | SNT N = 21 | |||
| Treatment successa | 17 (85.0%) | 18 (85.7%) | 35 (85.3%) | 0.476 |
| Treatment failureb | 3 (15%) | 3 (14.3%) | 6 (14.7%) | 0.476 |
Notes: aTreatment success - an IOP between 6 mm Hg and 21 mm Hg with or without medications at the end of the end of the 6 months follow-up. bTreatment failure – an IOP more than 21 mm Hg or hypotony less than 6 mm Hg at the end of the 6 months of follow-up, or a requirement for cyclodestructive procedure, or further glaucoma surgery such as bleb revision.
Table 5 shows the comparison of LNT and SNT based on additional procedures done after AGV Implantation. All patients with shallow/flat AC post-operatively underwent AC reformation with viscoelastic in the operating room. Needling was done exclusively in 6 eyes of the SNT group (28.6%, P = 0.0048). Based on review of records, IOP control in patients belonging to the LNT group (2018–2021) is managed using ocular massage and anti-glaucoma medications. On the other hand, IOP control in patients from the SNT (2015–2017) group consisted of needling, ocular massage and anti-glaucoma medications. Cases wherein needling was done included, juvenile open angle glaucoma (2 eyes), aphakic glaucoma (1 eye), aniridia with secondary glaucoma (2 eyes), and glaucoma status post trabeculectomy, vitrectomy for bleb-related endophthalmitis (1 eye). In three out of six eyes, needling was done as a primary treatment for IOP elevation, while the remaining three combined both needling and initiation of anti-glaucoma medications. In eyes that underwent needling, 3 were associated with treatment failure.
Table 5.
Comparison of Additional Procedures Done in Long Needle Track and Short Needle Track Groups After Ahmed Glaucoma Valve Drainage Device Implantation
| Group | Total N = 41 | P-value | ||
|---|---|---|---|---|
| LNT N = 20 | SNT N = 21 | |||
| Additional Procedures | ||||
| AC reformation | 0 (0%) | 3 (14.3%) | 3 (7.3%) | 0.039* |
| Repair of plate and tube exposure | 1 (5%) | 0 (0%) | 1 (2.4%) | 0.149 |
| Anterior chamber washout | 1 (5%) | 0 (0%) | 1 (2.4%) | 0.149 |
| Revision of encapsulation | 1 (5%) | 0 (0%) | 1 (2.4%) | 0.149 |
| Needling | 0 (0%) | 6 (28.6%) | 6 (14.6%) | 0.0048* |
Note: *Statistically significant.
Discussion
Early post-operative hypotony (9–31%) or flat/shallow AC (8%) is a serious complication following AGV device implantation.13–16 Untreated, these complications can lead to corneal decompensation and peripheral anterior synechia from corneolenticular touch, choroidal detachment, hypotony maculopathy, hyphema, suprachoroidal hemorrhage, peripheral iris synechiae, or cataract.17 Reasons for development of such complications include hyposecretion of aqueous humor in cases of uveitic glaucoma, valve device defect or from over priming, and employing a SNT technique leading to peritubular leakage.2,10,18,19 Several authors have suggested ways to manage post-operative hypotony such as injection of ocular viscoelastic device, tube ligation and intraluminal tube stenting.20–22 We hypothesize that using the LNT technique in AGV implantation reduces the risk for developing hypotony or flat/shallow AC by increasing the surface area of the scleral track in contact with the tube, hence increasing extraluminal resistance to aqueous flow.
In our study 14% (n = 3) developed post-operative shallow/flat AC exclusively in the SNT group (P = 0.039). Our results are similar to the study of Chang Kyu Lee et al in which 14% of patients who underwent the SNT method were observed to develop post-operative shallow AC. Garcia-Feijoo et al identified peritubular filtration as the cause of immediate post-operative hypotony following AGV implantation.2 It is our impression that a SNT can predispose to peritubular filtration due to a reduced amount of surface area available for tube-sclera contact. One patient in the SNT group developed a shallow anterior chamber associated with a tube-iris plug, which was resolved after intracameral injection of an Ocular Viscoelastic Device (OVD). In this patient, the anterior chamber shallowing was thought to be a result of peritubular leakage.
Another factor that can influence peritubular leakage is the shape of the tube track. In the LNT method using a G-22 or G-23 needle, Albis-Donado reported the rate of shallow AC at 3%.12 On the other hand, LNT method by which scleral tunnel constructed using a 2.0 mm crescent knife resulted to shallow AC in 16%.4 The presence of peritubular leakage may be more in the LNT method in which the crescent knife was used, but currently there is no study that can support this hypothesis. In both the SNT and LNT techniques, a G-23 needle was used in the creation of the needle track.
The occurrence of HP is a risk factor for AGV failure.23 The reported incidence of HP in LNT ranges between 27% and 82%.12,15,24 In our study, there was no significant difference in the occurrence of HP in both groups. HP was observed in 45% (n = 9) of patients in LNT and 57.1% (n = 12) in the SNT group (P = 0.435). However, the occurrence of HP in both groups could not be analyzed adequately due to the use of antiglaucoma medications to mitigate the rise in IOP >21 mm Hg and was continued until the 6 months of follow-up. Early administration of anti-glaucoma medications even before IOP elevation >21 mm Hg was done in 30% (n = 6) of patients in the LNT group and 9.5% (n = 2) in the SNT group. Early use of aqueous suppressants is associated with higher long-term success rates and lower occurrence of a hypertensive phase.25,26 In our study, treatment success based on IOP control was 85% and 85.7% in the LNT and SNT groups, respectively. Failure rates based on IOP control (LNT 15%, SNT 14.3%) were similar at the end of the 6th month of follow-up.
There was one occurrence (5%, P = 0.149) of plate exposure in the LNT group at 3 months post-operatively. The patient was a 72-year-old female diagnosed with neovascular glaucoma with no previous eye surgeries. She was previously maintained on two anti-glaucoma eye drops. In previous studies, even with the placement of a patch graft, erosion rates of about 8% have been reported.27 Other studies have cited no incidence of tube erosion with LNT technique in the long-term follow-up.24 Local ischemia and excessive implant mobility were identified to be the causes of late plate exposure (>3 months post-operatively).28 Previous ocular surgery was identified as a significant risk factor for GDD implant exposure.29
There are several limitations in our study. First, the sample size is small. Since the LNT technique of AGV implantation was adapted fairly recently in our institution, only a limited number of cases were compared to the SNT technique. Further study with a larger sample size and longer follow up period is recommended. Second, there were differences in IOP control between the two groups. In the LNT group, IOP control was managed using ocular massage and anti-glaucoma medications while in the SNT group, IOP control consisted of needling, ocular massage and anti-glaucoma medications.
Conclusion
The LNT technique of AGV implantation may be used as an alternative to the traditional SNT (with autologous graft) with similar outcomes in BCVA, IOP, and number of anti-glaucoma medications, success rates and HP. Our findings suggest that the long needle track offers the advantage reducing the risk of complications arising from shallow anterior chamber post-operatively.
Acknowledgments
The authors would like to thank Ms. Angelica Q. Fabillar for lending her expertise in the statistical analysis of the data.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Riva I, Roberti G, Oddone F, Konstas AGP, Quaranta L. Ahmed glaucoma valve implant: surgical technique and complications. Clin Ophthalmol. 2017;11:357–367. doi: 10.2147/OPTH.S104220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Feijoo J, Cuiña-Sardiña R, Méndez-Fernández C, Castillo-Gómez A, García-Sánchez J. Peritubular filtration as cause of severe hypotony after Ahmed valve implantation for glaucoma. Am J Ophthalmol. 2001;132:571–572. doi: 10.1016/S0002-9394(01)01057-1 [DOI] [PubMed] [Google Scholar]
- 3.Choo J, Chen ZD, Koh V, et al. Outcomes and complications of Ahmed tube implantation in Asian eyes. J Glaucoma. 2018;27:733–738. doi: 10.1097/IJG.0000000000001004 [DOI] [PubMed] [Google Scholar]
- 4.Eslami Y, Azaripour E, Mohammadi M, et al. Single long scleral tunnel technique for prevention of Ahmed valve tube exposure. Eur J Ophthalmol. 2018;9(1):52–56. [DOI] [PubMed] [Google Scholar]
- 5.Lama P, Fechtner R. Tube erosion following insertion of a glaucoma drainage device with a pericardial patch graft. Arch Ophthalmol. 1999;117(9):1243–1244. doi: 10.1001/archopht.117.9.1243 [DOI] [PubMed] [Google Scholar]
- 6.Kalamkar C, Radke N, Mukherjee A, Radke S. A new surgical technique of intra-scleral tube fixation in Ahmed glaucoma valve implantation: ‘scleral sleeve method’. Saudi J Ophthalmol. 2017;31:234–237. doi: 10.1016/j.sjopt.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gdih G, Jiang K. Graft-free Ahmed valve implantation through a 6 mm scleral tunnel. Canad J Ophalmol. 2017;2(1):85–91. doi: 10.1016/j.jcjo.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 8.Wolfe A, Hod Y, Buckman G, Stein N, Geyer O. Use of autologous scleral graft in Ahmed glaucoma valve surgery. J Glaucoma. 2016;25:365–370. doi: 10.1097/IJG.0000000000000232 [DOI] [PubMed] [Google Scholar]
- 9.Rivera J, Leuenberger E, Veloso MI. A retrospective review of autologous scleral flap versus donor scleral graft. Philipp J Ophthalmol. 2008;33(1):17–21. [Google Scholar]
- 10.Valimaki J. Fibrin glue for preventing immediate postoperative hypotony following glaucoma drainage implant surgery. Acta Ophthalmol Scand. 2006;84:372–374. doi: 10.1111/j.1600-0420.2006.00653.x [DOI] [PubMed] [Google Scholar]
- 11.Leong JK, McCluskey P, Lightman S, Towler HM. Outcome of graft free Molteno tube insertion. Br J Ophthalmol. 2006;90:501–505. doi: 10.1136/bjo.2005.079087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albis-Donado O, Gil-Carrasco F, Romero-Quijada R, Thomas R. Evaluation of Ahmed glaucoma valve implantation through a needle- generated scleral tunnel in Mexican children with glaucoma. Indian J Ophthalmol. 2010;58:365–373. doi: 10.4103/0301-4738.67039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D-H, Park C-K, Ahn M-D. Clinical results of Ahmed valve implantation in the aspects of complications. J Korean Ophthalmol Soc. 2003;44:888–895. [Google Scholar]
- 14.Huang MC, Netland PA, Coleman AL, Siegner SW, Moster MR, Hill RA. Intermediate term clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1999;127:27–33. doi: 10.1016/S0002-9394(98)00394-8 [DOI] [PubMed] [Google Scholar]
- 15.Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology. 1998;105:1968–1976. doi: 10.1016/S0161-6420(98)91049-1 [DOI] [PubMed] [Google Scholar]
- 16.Taglia DP, Perkins TW, Gangnon R, Heatley GA, Kaufman PL. Comparison of the Ahmed glaucoma valve, the Krupin eye valve with disk, and the double-plate Molteno implant. J Glaucoma. 2002;11:347–353. doi: 10.1097/00061198-200208000-00012 [DOI] [PubMed] [Google Scholar]
- 17.Park HY, Lee NY, Park CK. Risk factors of shallow anterior chamber other than hypotony after Ahmed glaucoma valve implant. J Glaucoma. 2009;18:44–48. doi: 10.1097/IJG.0b013e31816b2fe7 [DOI] [PubMed] [Google Scholar]
- 18.Chow A, Burkemper B, Varma R, Rodger DC, Rao N, Richter GM. Comparison of surgical outcomes of trabeculectomy, Ahmed shunt, and Baerveldt shunt in uveitic glaucoma. J Ophthalmic Inflamm Infect. 2018;8:9. doi: 10.1186/s12348-018-0150-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones E, Alaghband P, Cheng J, Beltran-Agullo L, Lim KS. Preimplantation flow testing of Ahmed glaucoma valve and the early postoperative clinical outcome. J Cur Glauco Pract. 2013;7(1):1–5. doi: 10.5005/jp-journals-10008-1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiam PJ, Chen X, Haque MS, Sung V. Outcome of fixed volume intracameral sodium hyaluronate 1.4% injection for early post-operative hypotony after Baerveldt glaucoma implant. Clin Exp Ophthalmol. 2018;46(9):1035–1040. doi: 10.1111/ceo.13347 [DOI] [PubMed] [Google Scholar]
- 21.Stein JD, McCoy AN, Asrani S, et al. Surgical management of hypotony owing to overfiltration in eyes receiving glaucoma drainage devices. J Glaucoma. 2009;18(8):638–641. doi: 10.1097/IJG.0b013e31819aa4e0 [DOI] [PubMed] [Google Scholar]
- 22.Feinstein M, Moussa K, Han Y. Ab interno tube occlusion for postoperative hypotony in a patient with an Ahmed glaucoma drainage device. J Glaucoma. 2018;27(3):e61–e63. doi: 10.1097/IJG.0000000000000869 [DOI] [PubMed] [Google Scholar]
- 23.Perez C, Verdaguer S, Khaliliyeh D, Maul M, Ou Y, Han Y. Subconjunctival injections of Mitomycin C are associated with a lower incidence of hypertensive phase in eyes with Ahmed Glaucoma Valve. Ophthalmol Glaucoma. 2021;4(3):322–329. doi: 10.1016/j.ogla.2020.10.004 [DOI] [PubMed] [Google Scholar]
- 24.Arpa P, Arpa C. Ahmed glaucoma valve in vitrectomized eyes: an implantation technique to minimize early postoperative fluid-loss hypotony. Eur J Ophthalmol. 2021;31(5):2759–2764. doi: 10.1177/11206721211012200 [DOI] [PubMed] [Google Scholar]
- 25.Jeong HJ, Young H, Park L, Park CK. Effects of early postoperative intraocular pressure after Ahmed glaucoma valve implantation on long-term surgical outcomes. Korean J Ophthalmol. 2018;32(5):391–399. doi: 10.3341/kjo.2017.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pakravan M, Rad SS, Yazdani S, Ghahari E, Yaseri M. Effect of early treatment with aqueous suppressants on Ahmed glaucoma valve implantation outcome. Ophthalmol Amn Acad Ophthalmol. 2014;121(9):1693–1698. [DOI] [PubMed] [Google Scholar]
- 27.Wishart PK, Choudhary A, Wong D. Ahmed glaucoma valves in refractory glaucoma: a 7-year audit. Clinical Science Br J Ophthalmol. 2015;94:1174e1179. [DOI] [PubMed] [Google Scholar]
- 28.Roy AK, Senthil S. Management of implant plate exposure of silicone Ahmed glaucoma valve: a review of six cases. GMS Ophthalmol Cas. 2016;6. doi: 10.3205/oc000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byun YS, Lee NY, Park CK. Risk factors of implant exposure outside the conjunctiva after Ahmed glaucoma valve implantation. Jpn J Ophthalmol. 2009;53(2):114–119. doi: 10.1007/s10384-008-0630-y [DOI] [PubMed] [Google Scholar]