Abstract
Aims
A comprehensive nationwide study on the incidence and outcomes of COVID-19 vaccination-related myocarditis (VRM) is in need.
Methods and results
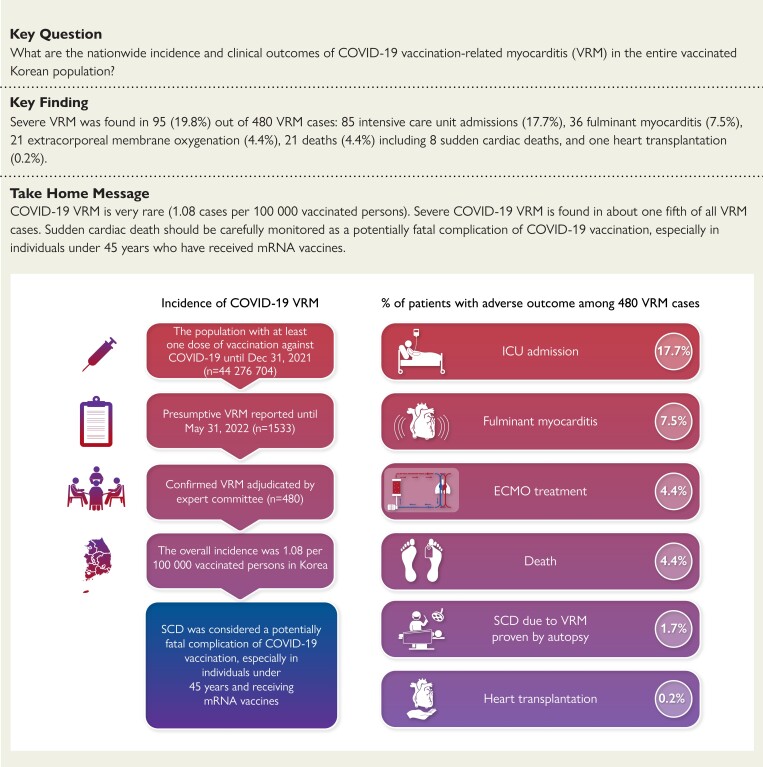
Among 44 276 704 individuals with at least 1 dose of COVID-19 vaccination, the incidence and clinical courses of VRM cases confirmed by the Expert Adjudication Committee of the Korea Disease Control and Prevention Agency were analyzed. COVID-19 VRM was confirmed in 480 cases (1.08 cases per 100 000 persons). Vaccination-related myocarditis incidence was significantly higher in men than in women (1.35 vs. 0.82 per 100 000 persons, P < 0.001) and in mRNA vaccines than in other vaccines (1.46 vs. 0.14 per 100 000 persons, P < 0.001). Vaccination-related myocarditis incidence was highest in males between the ages of 12 and 17 years (5.29 cases per 100 000 persons) and lowest in females over 70 years (0.16 cases per 100 000 persons). Severe VRM was identified in 95 cases (19.8% of total VRM, 0.22 per 100 000 vaccinated persons), 85 intensive care unit admission (17.7%), 36 fulminant myocarditis (7.5%), 21 extracorporeal membrane oxygenation therapy (4.4%), 21 deaths (4.4%), and 1 heart transplantation (0.2%). Eight out of 21 deaths were sudden cardiac death (SCD) attributable to VRM proved by an autopsy, and all cases of SCD attributable to VRM were aged under 45 years and received mRNA vaccines.
Conclusion
Although COVID-19 VRM was rare and showed relatively favorable clinical courses, severe VRM was found in 19.8% of all VRM cases. Moreover, SCD should be closely monitored as a potentially fatal complication of COVID-19 vaccination.
Keywords: COVID-19, Myocarditis, Vaccination, Outcomes
Structured Graphical Abstract
Structured Graphical Abstract.
Study flowchart and summary of the percentage of patients with adverse outcomes among 480 cases of COVID-19 vaccination-related myocarditis. VRM, vaccine-related myocarditis; SCD, sudden cardiac death; ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation
Introduction
Vaccine-related myocarditis (VRM) is a rare complication of COVID-19 vaccines.1 Large cohort and epidemiologic studies have suggested that COVID-19 VRM is associated with the use of mRNA vaccines, especially in young males and after the second dose of vaccination, and usually develops within 7 days after vaccination.2–6
The reported incidence of COVID-19 VRM varies among studies with 1.4–5.0 per 100 000 vaccinated persons because of the differences in the study population. COVID-19 VRM is generally known to be mild in severity with favorable short-term clinical outcomes.2–4 However, there are several reported COVID-19 VRM cases showing unfavorable clinical outcomes such as fulminant myocarditis (FM) or even death.7–9
Therefore, further studies involving entire nationwide vaccinated people with a consistent reporting system will be needed to minimize selection bias. Because the Korea Disease Control and Prevention Agency (KDCA) organized a national reporting system for adverse events associated with COVID-19 vaccination, we analyzed the nationwide incidence and clinical outcomes of COVID-19 VRM in the entire vaccinated Korean population.
Methods
Data collection
The Korean government organized the COVID-19 Vaccination Task Force Adverse Event Investigation Team within the KDCA to screen for all adverse events associated with COVID-19 vaccination. Medical institutions, vaccinated individuals, or guardians can report any adverse events after COVID-19 vaccination to the official website (nip.kdca.go.kr) of the KDCA or regional public health centers. The Korea Disease Control and Prevention Agency also established a reporting system with a legal obligation for special adverse events including myocarditis and pericarditis after COVID-19 vaccination. Physicians or healthcare providers who treat or detect special adverse events should make a legal report to the KDCA.
To evaluate all reported cases of suspected myocarditis or pericarditis after COVID-19 vaccination, the KDCA organized the Expert Adjudication Committee on COVID-19 Vaccination Pericarditis/Myocarditis. The committee comprised 7 experts in cardiology, 1 in infectious disease, 2 in epidemiology, epidemiologic investigators in 16 regional centers, and officials from the KDCA. Epidemiologic investigators collected and investigated all medical records and provided those data to the committee.
Study design and population
This is a retrospective nationwide report including all vaccinated Korean people, and the institutional review board of the KDCA approved the study protocol (IRB number: 2022-03-07-PE-A).
From 26 February to 31 December 2021, 44 276 704 individuals older than 12 years (94.4% of Korean older than 12 years) were vaccinated with at least one dose of COVID-19 vaccines: ChAdOx1 (n = 11 156 646), BNT162b2 (n = 24 828 152), mRNA-1273 (n = 6 781 796), or Ad26.COV2.S (n = 1 510 110). The second dose of COVID-19 vaccines was given in 41 084 830 persons: ChAdOx1 (n = 11 093 528), BNT162b2 (n = 23 369 725), or mRNA-1273 (n = 6 621 577). A third dose of COVID-19 vaccines was administered in 18 411 821 persons: BNT162b2 (n = 11 458 290), mRNA-1273 (n = 6 930 450), or Ad26.COV2.S (n = 23 081).
Diagnosis of COVID-19 VRM
Among 44 276 704 subjects vaccinated from 26 February to 31 December 2021, 1533 cases of suspected myocarditis were reported to the KDCA. The Expert Adjudication Committee on COVID-19 Vaccination Pericarditis/Myocarditis has been held every week, and the committee reviewed all records for the verification of VRM diagnosis.
The committee adopted the myocarditis case definition and classification of the Brighton Collaboration (BC) for the diagnosis and degree of certainty of VRM diagnosis [BC. Myocarditis/pericarditis case definition. The Task Force for Global Health, 16 July 2021 (https://brightoncollaboration.us/myocarditis-case-definition-update/)]. Acute myocarditis developed within 42 days after COVID-19 vaccination was considered as COVID-19 VRM. To minimize the risk of an inaccurate diagnosis, the committee rejected the level 3 BC case definition of myocarditis and the level 2 BC case without elevation of cardiac troponin and cases with a positive result for COVID-19 infection. Furthermore, the adjudication committee has examined other potential causes of myocarditis, such as the presence of antibodies against various viruses and autoimmune markers in their review of the medical records.
Initial cardiac troponin was measured when patients visited the hospital with symptoms after vaccination and followed up on a daily basis until the values normalize.
Vaccination-related myocarditis requiring intensive care unit (ICU) admission, fulminant myocarditis (FM), the use of extracorporeal membrane oxygenation (ECMO), death, or heart transplantation was considered severe VRM in this study.
Statistical analysis
Continuous variables were described as mean (standard deviation) or medians with interquartile ranges (IQR) compared using Student’s t-test or Mann–Whitney U test. Categorical variables were compared using the χ2 test or Fisher’s exact test. Kaplan–Meier analysis was performed to estimate the cumulative incidence of myocarditis at 42 days after the last vaccine dose. Receiver operating characteristic curve analysis was performed to identify the cut-off values of age and systolic blood pressure for prediction of severe VRM. Binary logistic regression was used to determine the independent predictors of severe VRM. Variables with P < 0.1 on univariate regression analysis and clinically relevant variables were tested in the model. All statistical tests were two-tailed, and P values <0.05 were considered significant. All analyses were performed using the Statistical Package for Social Sciences, version 21.0 (SPSS-PC, Chicago, IL) and R Statistical Software version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Among 1533 cases of suspected acute myocarditis reported to the KDCA, the Expert Adjudication Committee on COVID-19 Vaccination Pericarditis/Myocarditis confirmed 480 cases of COVID-19 VRM. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline characteristics
| Characteristics | Korean population (n = 51 349 116) | Vaccinated population (n = 44 276 704) | VRM (n = 480) |
|---|---|---|---|
| Median age (years), median (IQR) | 45 (27–60) | 47 (32–61) | 30 (20–45) |
| Age group (years), n (%) | |||
| < 12 | 4 441 020 (9) | ||
| 12–17 | 2 768 836 (5) | 2 076 259 (5) | 78 (16) |
| 18–29 | 7 619 756 (15) | 7 415 068 (17) | 161 (34) |
| 30–39 | 6 686 639 (13) | 6 313 754 (14) | 86 (18) |
| 40–49 | 8 109 221 (16) | 7 706 804 (17) | 63 (13) |
| 50–59 | 8 570 076 (17) | 8 361 166 (19) | 55 (12) |
| 60–69 | 7 140 703 (14) | 6 913 088 (16) | 23 (5) |
| ≥ 70 | 6 012 865 (11) | 5 490 565 (12) | 14 (3) |
| Sex, n (%) | |||
| Female | 25 746 790 (50) | 22 118 827 (50) | 181 (38) |
| Male | 25 602 326 (50) | 22 157 877 (50) | 299 (62) |
| Symptoms or signs, n (%) | |||
| Chest pain or discomfort | 287 (60) | ||
| Dyspnea | 128 (27) | ||
| Fever | 76 (16) | ||
| Palpitation | 60 (13) | ||
| Vital signs, mean (SD) | |||
| Systolic BP (mmHg) | 121. 2 (25.3) | ||
| Diastolic BP (mmHg) | 74. 4 (17.1) | ||
| Heart rate (b.p.m.) | 86.4 (22.1) | ||
| Body temperature (°C) | 36. 9 (0.8) | ||
| Coexisting illness, n (%) | |||
| Diabetes mellitus | 6 (2) | ||
| Hypertension | 26 (8) | ||
| Dyslipidemia | 10 (3) | ||
| CAD | 2 (1) | ||
| CVA | 1 (0) | ||
| Type of vaccine, n (%) | |||
| BNT162b2 | 24 828 152 (56) | 306 (64) | |
| mRNA-1273 | 6 781 796 (15) | 156 (33) | |
| ChAdOx1 | 11 156 646 (25) | 15 (3) | |
| Ad26 | 1 510 110 (3) | 3 (1) |
The population of Korea is from Korean Statistical Information Service (http://kosis.kr/eng/).
VRM, vaccine-related myocarditis; BP, blood pressure; CAD, coronary artery disease; CVA, cerebrovascular accident; SD, standard deviation; IQR, interquartile range.
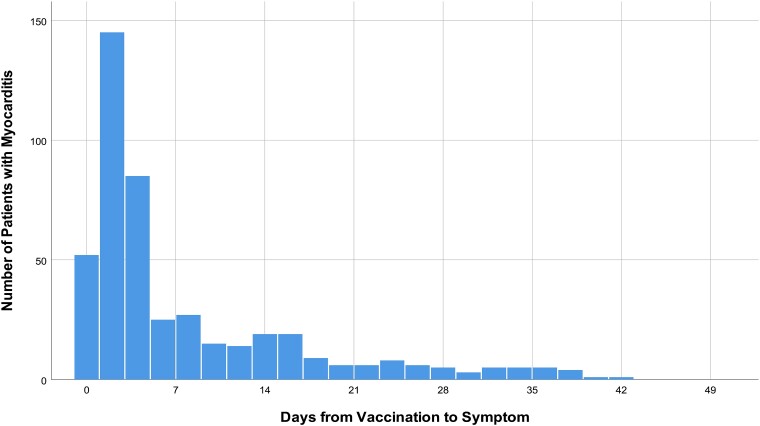
COVID-19 VRM was common in males (62.3%), ages under 40 years (67.9%), and after the use of mRNA vaccines (96.3%). Chest pain or discomfort was the most common presenting symptom, and the median time from the vaccination to symptom onset was 3 days (IQR, 1–10 days) (Figure 1).
Figure 1.
Time from COVID-19 vaccination to symptom onset.
Incidence of COVID-19 VRM
The incidence of COVID-19 VRM is summarized in Table 2.
Table 2.
Incidence of COVID-19 VRM
| Vaccinated population (no.) |
VRM (no.) |
Incidence of VRM no./100 000 persons (95% CI) |
|
|---|---|---|---|
| Total | 44 276 704 | 480 | 1.08 (0.99–1.19) |
| 12–17 years | 2 076 259 | 78 | 3.76 (2.97–4.69) |
| 18–29 years | 7 415 068 | 161 | 2.17 (1.85–2.53) |
| 30–39 years | 6 313 754 | 86 | 1.36 (1.09–1.68) |
| 40–49 years | 7 706 804 | 63 | 0.82 (0.63–1.05) |
| 50–59 years | 8 361 166 | 55 | 0.66 (0.50–0.86) |
| 60–69 years | 6 913 088 | 23 | 0.33 (0.21–0.50) |
| ≥ 70 years | 5 490 565 | 14 | 0.26 (0.14–0.43) |
| Males | 22 157 877 | 299 | 1.35 (1.20–1.51) |
| 12–17 years | 1 171 550 | 62 | 5.29 (4.06–6.78) |
| 18–29 years | 3 920 277 | 115 | 2.93 (2.42–3.52) |
| 30–39 years | 3 381 709 | 52 | 1.54 (1.15–2.02) |
| 40–49 years | 3 954 215 | 27 | 0.68 (0.45–0.99) |
| 50–59 years | 4 245 958 | 19 | 0.45 (0.27–0.70) |
| 60–69 years | 3 297 193 | 15 | 0.46 (0.26–0.75) |
| ≥ 70 years | 2 186 975 | 9 | 0.41 (0.19–0.78) |
| Females | 22 118 827 | 181 | 0.82 (0.70–0.95) |
| 12–17 years | 1 105 176 | 16 | 1.45 (0.83–2.35) |
| 18–29 years | 3 544 543 | 46 | 1.30 (0.95–1.73) |
| 30–39 years | 2 977 337 | 34 | 1.14 (0.79–1.60) |
| 40–49 years | 3 797 674 | 36 | 0.95 (0.66–1.31) |
| 50–59 years | 4 173 776 | 36 | 0.86 (0.60–1.19) |
| 60–69 years | 3 431 287 | 8 | 0.23 (0.10–0.46) |
| ≥ 70 years | 3 089 034 | 5 | 0.16 (0.05–0.38) |
VRM, vaccine-related myocarditis; CI, confidence interval.
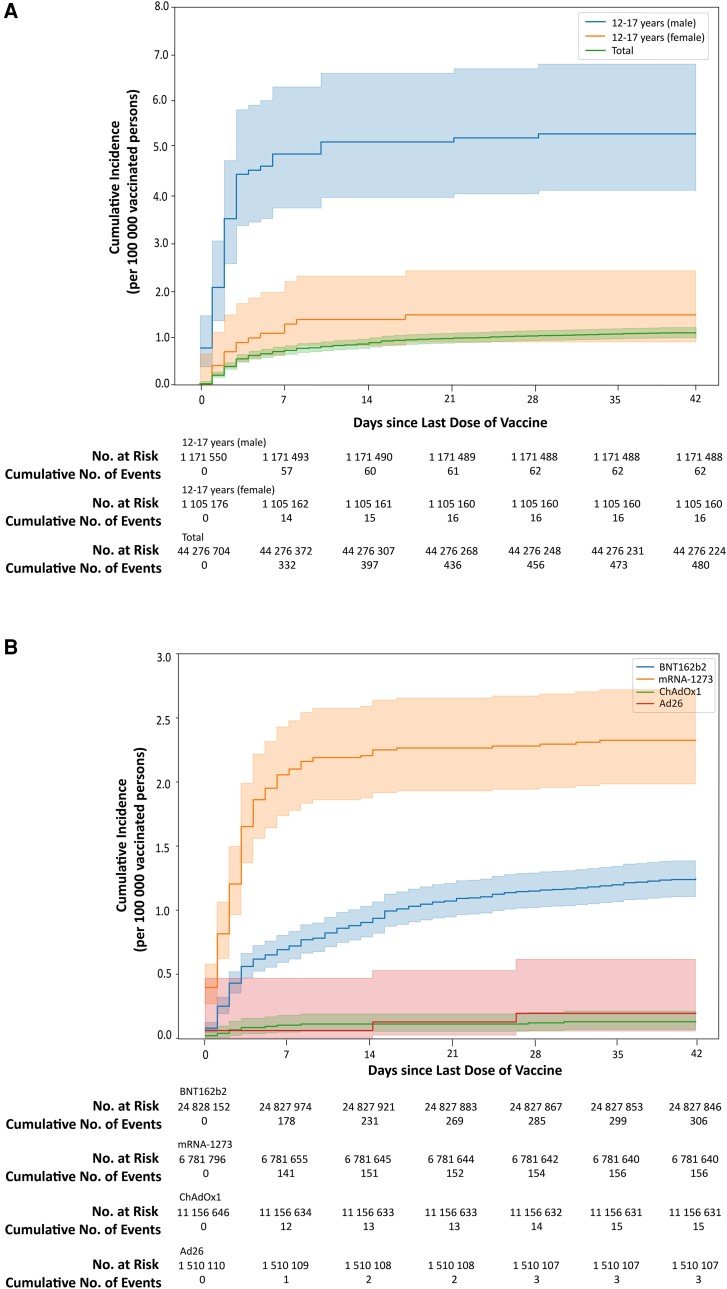
The overall incidence was 1.08 per 100 000 vaccinated persons (Figure 2A). COVID-19 VRM incidence was significantly higher in men than in women (1.35 vs. 0.82 per 100 000 persons, P < 0.001).
Figure 2.
Kaplan–Meier estimates of the incidence of COVID-19 vaccine-related myocarditis. (A) Cumulative incidence per 100 000 persons in the total vaccinated population and males and females between age 12 and 17 and (B) cumulative incidence per 100 000 persons in the total vaccinated population according to vaccine type.
COVID-19 VRM incidence was highest in mRNA-1273 vaccine [2.30 per 100 000 persons (95% CI, 1.95 to 2.69)] and followed by BNT162b2 [1.23 per 100 000 persons (95% CI, 1.10 to 1.38)], Ad26 (0.20 [95% CI, 0.04 to 0.58] per 100 000 persons), and ChAdOx1 [0.14 (95% CI, 0.08 to 0.22) per 100 000 persons] (Figure 2B). VRM incidence was significantly higher in mRNA vaccines than in other vaccines (1.46 [95% CI, 1.33 to 1.60] vs. 0.14 [95% CI, 0.08 to 0.23] per 100 000 persons, P < 0.001).
The incidence of VRM was highest in males between the ages of 12 and 17 years [5.29 (95% CI, 4.06 to 6.78) per 100 000 persons] followed by males between the ages of 18 and 29 years 2.93 (95% CI, 2.42 to 3.52) and lowest in females aged more than 70 years [0.16 (95% CI, 0.05 to 0.38) per 100 000 persons].
COVID-19 VRM incidence was 0.47 per 100 000 persons after the first vaccination dose, 0.55 per 100 000 persons after the second vaccination dose, and 0.24 per 100 000 persons after the third vaccination dose. COVID-19 VRM incidence was not different between the first and second vaccination doses, but it was significantly lower in the third vaccination dose than in the first or second vaccination dose.
COVID-19 VRM incidence after the first vaccination dose was not different between men and women [0.47 (95% CI, 0.39 to 0.57) vs. 0.47 (95% CI, 0.38 to 0.57) per 100 000 persons, P = 1.000]. However, the incidence of VRM after the second vaccination dose was significantly higher in men than in women [0.76 (95% CI, 0.65 to 0.89) vs. 0.31 (95% CI, 0.24 to 0.39) per 100 000 persons, P < 0.001].
There were only two heterologous vaccination schemes in Korea during the study period: ChAdOx1 followed by BNT162b2 (n = 1 789 915) and mRNA-1273 followed by BNT162b2 (n = 98 761). There were no VRM reported with mRNA-1273 followed by BNT162b2. However, 8 individuals were diagnosed with VRM out of 1 789 915 ChAdOx1 followed by BNT162b2 heterologous vaccination scheme [0.45 cases per 100 000 persons (95% CI, 0.19 to 0.88)].
Laboratory and imaging findings
Cardiac troponin levels were elevated in 464 COVID-19 VRM cases (96.7%). Vaccine-related myocarditis was confirmed by the endo-myocardial biopsy (see Supplementary data online, Figure S1) or cardiac magnetic resonance (CMR) in the remaining 16 cases, even though cardiac troponin levels were not measured or elevated.
Electrocardiography (ECG) was available in 322 cases (67.1%), and ECG findings are summarized in Supplementary data online, Table S1. Electrocardiography findings were normal only in 104 out of 322 VRM cases (32.3%). The most common ECG abnormality was ST-segment elevation (134 cases: 41.6%). During hospitalization, sustained ventricular tachycardia or fibrillation was observed in 16 cases (5.0%).
Echocardiography was available in 285 cases (59.4%). Left ventricular ejection fraction (LVEF) < 50% was found in 66 out of 285 VRM cases (23.2%).
Cardiac magnetic resonance was available in 78 cases (16.3%), and 61 cases (72.7%) showed compatible findings of acute myocarditis. Left ventricular ejection fraction <50% was found in 26 cases (33.3%), and late gadolinium enhancement was shown in 54 cases (69.2%).
Clinical courses of COVID-19 vaccine-related myocarditis
Severe COVID-19 VRM was identified in 95 cases (19.8%), 85 ICU admissions (17.7%), 36 FM (7.5%), 21 ECMO therapies (4.4%), 21 deaths (4.4%), and 1 heart transplantation (0.2%).
Fulminant myocarditis cases are summarized in Supplementary data online, Table S2. Extracorporeal membrane oxygenation was applied in 21 out of 36 FM cases (58.3%). During hospitalization, 13 out of 36 FM cases (36.1%) died (9 deaths in ECMO cases). Heart transplantation was done successfully in one case, and the histopathologic examination of the explanted heart demonstrated giant cell myocarditis.
Autopsy studies were done for sudden death cases after COVID-19 vaccination in Korea because of an issue regarding the National Compensation Liability and identified eight sudden cardiac death (SCD) cases attributable to COVID-19 VRM. Eight autopsy-proven VRM cases are summarized in Table 3. Sudden cardiac death was developed within a week after the vaccination and in individuals aged under 45 years in all cases. All SCD cases received mRNA vaccines.
Table 3.
Sudden cardiac death cases attributable to COVID-19 VRM
| Case | Age | Sex | Vaccination to death (days) | Types of vaccine | Order of vaccination | Histopathologic findings |
|---|---|---|---|---|---|---|
| 1 | 22 | M | 6 | BNT162b2 | 1 | Diffuse inflammatory infiltration, with neutrophil and histiocyte predominance in both atria and near AV node and SA node. Free of inflammatory infiltrates in ventricular myocardium. |
| 2 | 30 | F | 3 | BNT162b2 | 1 | Diffuse inflammatory cell infiltration, myocardial fiber disarray, interstitial fibrosis, and localized necrosis of myocyte. |
| 3 | 45 | M | 3 | BNT162b2 | 2 | Localized infiltration of neutrophils, lymphocytes, histocyte, and a few eosinophils was noted. A small number of cardiomyocyte necrosis were also seen. |
| 4 | 25 | M | 3 | BNT162b2 | 2 | Myocarditis |
| 5 | 45 | M | 3 | BNT162b2 | 2 | Interstitial infiltration of various inflammatory cells including lymphocyte, neutrophil, eosinophil, and focal necrosis suggesting the diagnosis of myocarditis. |
| 6 | 36 | F | 2 | mRNA-1273 | 1 | Neutrophil, eosinophil, and histiocyte infiltration in the myocardium suggesting acute myocarditis. |
| 7 | 33 | M | 1 | mRNA-1273 | 2 | Multiple focal infiltrations of acute inflammatory cells and chronic inflammatory cells in the myocardium. |
| 8 | 33 | M | 3 | mRNA-1273 | 2 | Various inflammatory cells such as neutrophils, eosinophils, lymphocytes, macrophages, and cardiomyocyte necrosis in the myocardial interstitium and epicardium suggested myocarditis. |
VRM, vaccine-related myocarditis; AV, atrioventricular; SA, sinoatrial.
Comparisons between severe and non-severe COVID-19 VRM are summarized in Table 4. Female sex, older age, symptoms of dyspnea or fever, and low blood pressure were more significantly associated with severe COVID-19 VRM. Multivariate analysis showed that low systolic blood pressure (<100 mmHg) and older age (>40 years) were independent predictors of developing severe VRM (see Supplementary data online, Table S3).
Table 4.
Comparisons between severe and non-severe COVID-19 VRM
| Characteristics | Severe VRM (n = 95) | Non-severe VRM (n = 385) | P value |
|---|---|---|---|
| Median age (years), median (IQR) | 36 (22–51) | 28 (20–43) | 0.017 |
| Age group (years), n (%) | 0.006 | ||
| 12–17 | 15 (16) | 63 (16) | |
| 18–29 | 23 (24) | 138 (36) | |
| 30–39 | 14 (15) | 72 (19) | |
| 40–49 | 18 (19) | 45 (12) | |
| 50–59 | 11 (12) | 44 (11) | |
| 60–69 | 10 (11) | 13 (3) | |
| ≥ 70 | 4 (4) | 10 (3) | |
| Sex, n (%) | 0.016 | ||
| Female | 46 (48) | 135 (35) | |
| Male | 49 (52) | 250 (65) | |
| Presenting symptoms and signs, n (%) | |||
| Chest pain or discomfort | 56 (59) | 231 (60) | 0.851 |
| Dyspnea | 38 (40) | 90 (23) | 0.001 |
| Fever | 26 (27) | 51 (13) | <0.001 |
| Palpitations | 5 (5) | 55 (14) | 0.017 |
| Vital signs on admission, mean (SD) | |||
| Systolic BP (mmHg) | 104.6 (29.2) | 126.6 (21.3) | <0.001 |
| Diastolic BP (mmHg) | 65.0 (19.2) | 77.5 (15.2) | <0.001 |
| Heart rate (b.p.m.) | 88.2 (31.2) | 85.9 (18.2) | 0.554 |
| Body temperature (°C) | 37.0 (1.0) | 36.8 (0.7) | 0.151 |
| Comorbidity, n (%) | |||
| Diabetes mellitus | 2 (2) | 4 (1) | 0.339 |
| Hypertension | 10 (11) | 17 (4) | 0.021 |
| Dyslipidemia | 4 (4) | 7 (2) | 0.240 |
| Coronary artery disease | 0 (0.0) | 2 (1) | 1.000 |
VRM, vaccine-related myocarditis; BP, blood pressure; IQR, interquartile range; SD, standard deviation.
Third vaccination dose and COVID-19 vaccine-related myocarditis
COVID-19 VRM after the third vaccination dose comprised 44 out of 480 cases (9.2%). COVID-19 VRM incidence after the third COVID-19 vaccination was 0.24 per 100 000 persons and significantly lower than that of the first or second vaccination dose (Table 5). Among 44 cases, severe VRM was identified in 4 cases (9.1%), 4 ICU admissions (9.1%), 2 FM (4.6%), and 2 deaths (4.6%).
Table 5.
Incidence of COVID-19 VRM according to the type of vaccines and order of vaccination
| Type of vaccine | Overall | First vaccination | Second vaccination | Third vaccination | ||||
|---|---|---|---|---|---|---|---|---|
| n | Incidence (95% CI) no./100 000 persons | n | Incidence (95% CI) no./100 000 persons | n | Incidence (95% CI) no./100 000 persons | n | Incidence (95% CI) no./100 000 persons | |
| BNT162b2 | 306 | 1.23 (1.10–1.38) |
133 | 0.54 (0.45–0.64) |
140 | 0.60 (0.50–0.71) | 33 | 0.30 (0.21–0.42) |
| mRNA-1273 | 156 | 2.30 (1.95–2.69) |
68 | 1.00 (0.78–1.27) |
77 | 1.16 (0.92–1.45) | 11 | 0.19 (0.10–0.32) |
| ChAdOx1 | 15 | 0.14 (0.08–0.22) |
5 | 0.05 (0.02–0.11) |
10 | 0.09 (0.04–0.17) |
- | - |
| Ad26 | 3 | 0.20 (0.04–0.58) |
3 | 0.20 (0.04–0.58) |
- | - | 0 | 0.00 |
| Total | 480 | 1.08 (0.99–1.19) |
209 | 0.47 (0.41–0.54) |
227 | 0.55 (0.48–0.63) |
44 | 0.24 (0.17–0.32) |
VRM, vaccine-related myocarditis; CI, confidence interval.
Discussion
The present nationwide study involving more than 44 million vaccinated individuals in Korea demonstrated several clinically important findings on acute myocarditis after COVID-19 vaccination. First, VRM was a very rare complication of COVID-19 vaccination (1.08 cases per 100 000 vaccinated persons) and mainly developed in association with mRNA vaccines, especially in young males. Second, the demographic characteristics of COVID-19 VRM differed from those of the previous studies. Third, notably, we demonstrated severe COVID-19 VRM including FM or death was not uncommon (19.8% of total VRM). Sudden cardiac death attributable to COVID-19 VRM demonstrated in this study warrants the careful monitoring or warning of SCD as a potentially fatal complication of COVID-19 vaccination, especially in individuals who are ages under 45 years with mRNA vaccination. Fourth, the incidence of severe cases of COVID-19 VRM significantly decreased in the third vaccination than in the first or second COVID-19 vaccination (Structured Graphical Abstract).
The incidence of COVID-19 VRM was 1.08 cases per 100 000 vaccinated persons for all vaccines (2.30 for mRNA-1273, 1.23 for BNT162b2) in this nationwide Korean report. The reported incidences of myocarditis after COVID-19 vaccination vary among studies with 1.4–5.0 per 100 000 vaccinated persons.2–4 In a large cohort study in Israel,2 VRM was identified in 54 cases out of 2.5 million vaccinated individuals, and the estimated incidence of myocarditis after mRNA vaccination was 2.13 per 100 000 vaccinated persons within the 42 days after the first vaccine dose. An analysis of the Vaccine Adverse Event Reporting System (VAERS) identified 1626 VRM cases out of 192 405 448 persons after COVID-19 mRNA vaccination in the USA.3 In the study of Denmark,4 the incidence of myocarditis was 1.4 per 100 000 vaccinated individuals with BNT162b2 vaccine and 4.2 per 100 000 with mRNA-1273 within 28 days of vaccination. These varying incidences of myocarditis among studies may be associated with the differences in the COVID-19 vaccines used and the risk period for myocarditis after vaccination. The ethnic difference may also be a possible explanation.
Previous studies have suggested that COVID-19 VRM is associated with the use of mRNA vaccines, especially in young males and after the second dose of vaccination.2–5 The present study also demonstrated similar findings that COVID-19 VRM develops predominantly in young adult and adolescent males and in association with mRNA vaccines. However, epidemiologic characteristics of COVID-19 VRM incidence in Korea were different from those of the previous studies: (i) male predominance seems to be weak in Koreans, (ii) no remarkable difference between the first and second vaccination dose, and (iii) COVID-19 VRM was not uncommon in individuals aged between 40 and 60 years.
The clinical course or severity of VRM in our study was considerably different from the previous reports. COVID-19 VRM has been known to be mild in severity with favorable short-term clinical outcomes. In a large cohort study in Israel,2 among 54 cases of COVID-19 VRM, cardiogenic shock leading to ECMO developed in only one patient. In a study including 40 hospitals in the USA,10 all COVID-19 VRM patients were discharged after a median of 2 days (IQR 2–3 days) and there were no readmissions or deaths. In the present study, however, we found 95 severe COVID-19 VRM cases (19.8% of total VRM) including 36 FM and 21 death cases. Furthermore, we identified eight SCD cases only confirmed by an autopsy. Because our data incorporated more than 44 million people, there might be a possibility that more deaths could have occurred than in other studies with smaller populations. However, there was no death-related COVID-19 VRM in the largest cohort including 192 405 448 persons in the USA.3 In that report, they analyzed clinical events in 826 cases of myocarditis among younger than 30 years of age with detailed clinical information. Although we did not know the exact reason for this discrepancy between the two countries, the difference in the used case reporting system may be a possible explanation. Most studies from the USA used the VAERS,11 but this system is a passive reporting system that allows for underreporting or overreporting. On the contrary, the Korean government made a reporting system for all adverse events before starting COVID-19 vaccination and established a national compensation system for all medical expenses related to adverse events of COVID-19 vaccination. Besides these systems, because VRM was the special adverse reaction of COVID-19 vaccination with a legal obligation that should be reported to the KDCA, the risk of underreporting for VRM might be minimized. The Korean government organized the causality assessment committee to review and confirm cases associated with the vaccination; the possibility of the overreporting of VRM could also be minimized.
The main problems regarding the BC criteria in the diagnosis of myocarditis would be overreporting and underreporting. In some cases with limited imaging study and biomarkers, possible diagnosis can be made with only symptoms and ECG (BC level 3 criteria). However, to increase the accuracy and robustness of the diagnosis, modifications to the criteria are necessary. The use of endomyocardial biopsy, echocardiography, and biomarkers is critical in diagnosing myocarditis in clinical practice, and positive CMR imaging findings can further improve diagnostic accuracy.12 To reduce the risk of underreporting, suspected myocarditis patients may require more extensive cardiac imaging or biomarker assessments, while the use of level 3 criteria should be avoided to prevent overreporting. Although there was substantial missing data for echocardiography or CMR imaging in the present study, the presence of positive cardiac troponin results in patients without cardiac imaging, along with the presence of robust symptoms of myocarditis, increased the reliability of the diagnosis.
Sudden cardiac death was the most serious and worrisome adverse reaction of COVID-19 vaccination in our study. In eight SCD cases, VRM was not suspected as a clinical diagnosis or a cause of death before performing an autopsy. All SCD cases attributable to COVID-19 VRM were aged under 45 years and received mRNA vaccines. Vaccine-related myocarditis was the only possible cause of death in all SCD cases. Therefore, SCD attributable to COVID-19 VRM demonstrated in this study warrants the careful monitoring or warning of SCD as a potentially fatal complication of COVID-19 vaccination, especially in individuals who are ages under 45 years and receiving mRNA vaccination.
This study has several limitations. First, the present study has a possibility of underestimation of the incidence of COVID-19 VRM due to the nature of the current reporting system, even though the KDCA has tried many efforts to reduce the underreporting. In addition, cases of suspected myocarditis that meet BC level 2 criteria and do not have elevated cardiac troponin have been rejected for overreporting by the adjudication committee, except in cases where there is clear evidence from both histopathology and CMR. Second, there can be a possibility of myocarditis caused by other causes rather than COVID-19 vaccination because etiologic evaluations including viral marker testing were not performed in all cases of COVID-19 VRM. However, the Expert Adjudication Committee of the KDCA did thorough investigations to detect other causes of myocarditis rather than vaccination. Third, the echocardiography and CMR findings should be interpreted with caution and cannot be generalized because not all patients had echocardiography or CMR data. Although this could be the cause of the underreporting usually, thorough measurement of cardiac troponin and endomyocardial biopsy could minimize those underreporting in the present study. Fourth, due to the fact that covariates such as baseline characteristics of the vaccinated individuals without VRM are not included in the data reported to the KDCA, it was not possible to identify independent predictors for VRM in the entire vaccinated population. However, predictors for severe VRM were identified from the total cases of VRM. Finally, it was difficult to explain the reason for some of the patients developing myocarditis very early such as several hours to a day. Hypersensitivity reaction was proposed as a potential mechanism.13
Conclusion
COVID-19 VRM was rare (1.08 cases per 100 000 vaccinated persons) and mainly developed in association with mRNA vaccines, especially in young males, in Korea. COVID-19 VRM showed relatively favorable clinical courses, but severe VRM cases including death or FM were found in 95 cases (19.8%). Furthermore, all eight SCDs from myocarditis were seen in relatively young people within a week after mRNA COVID-19 vaccination. Severe COVID-19 VRM including SCD should be carefully monitored as a potentially fatal complication of COVID-19 vaccination, especially in individuals who are aged under 45 years and receiving mRNA vaccines.
Supplementary Material
Acknowledgements
The authors express deep appreciation to all regional epidemiologic investigators for their sincere efforts to investigate or collect medical records related to COVID-19 VRM cases. Also, the authors would like to express special thanks to Prof Bonggyun Ko, Department of Statistics, Chonnam National University, Korea, for statistical consultation.
Contributor Information
Jae Yeong Cho, Department of Cardiovascular Medicine, Chonnam National University Medical School, 160 Baekseo-ro, Dong-gu, Gwangju 61469, Korea; Department of Cardiovascular Medicine, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea.
Kye Hun Kim, Department of Cardiovascular Medicine, Chonnam National University Medical School, 160 Baekseo-ro, Dong-gu, Gwangju 61469, Korea; Department of Cardiovascular Medicine, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea.
Nuri Lee, Department of Cardiovascular Medicine, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea; Department of Cardiovascular Medicine, Chonnam National University Hwasun Hospital, 322 Seoyang-ro, Hwasun, Jeollanam-do 58128, Korea.
Soo Hyeon Cho, COVID-19 Vaccination Task Force Adverse Event Investigation Team, Korea Disease Control and Prevention Agency, Osong Health Technology Administration Complex, 187, Osongsaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju, Chungcheongbuk-do 28159, Korea.
Seung Yun Kim, COVID-19 Vaccination Task Force Adverse Event Investigation Team, Korea Disease Control and Prevention Agency, Osong Health Technology Administration Complex, 187, Osongsaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju, Chungcheongbuk-do 28159, Korea.
Eun Kyoung Kim, COVID-19 Vaccination Task Force Adverse Event Investigation Team, Korea Disease Control and Prevention Agency, Osong Health Technology Administration Complex, 187, Osongsaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju, Chungcheongbuk-do 28159, Korea.
Jae-Hyeong Park, Department of Cardiology in Internal Medicine, Chungnam National University Hospital, Chungnam National University, 282 Munhwa-ro, Jung-gu, Daejeon 35015, Korea.
Eui-Young Choi, Division of Cardiology, Heart Center, Gangnam Severance Hospital, Yonsei University College of Medicine, 211, Eonju-ro, Gangnam-gu, Seoul 06273, Korea.
Jin-Oh Choi, Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-Ro Gangnam-gu, Seoul 06351, Korea.
Hyukjin Park, Department of Cardiovascular Medicine, Chonnam National University Hwasun Hospital, 322 Seoyang-ro, Hwasun, Jeollanam-do 58128, Korea.
Hyung Yoon Kim, Department of Cardiovascular Medicine, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea.
Hyun Ju Yoon, Department of Cardiovascular Medicine, Chonnam National University Medical School, 160 Baekseo-ro, Dong-gu, Gwangju 61469, Korea; Department of Cardiovascular Medicine, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea.
Youngkeun Ahn, Department of Cardiovascular Medicine, Chonnam National University Medical School, 160 Baekseo-ro, Dong-gu, Gwangju 61469, Korea; Department of Cardiovascular Medicine, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea.
Myung Ho Jeong, Department of Cardiovascular Medicine, Chonnam National University Medical School, 160 Baekseo-ro, Dong-gu, Gwangju 61469, Korea; Department of Cardiovascular Medicine, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea.
Jeong Gwan Cho, Department of Cardiovascular Medicine, Chonnam National University Medical School, 160 Baekseo-ro, Dong-gu, Gwangju 61469, Korea; Department of Cardiovascular Medicine, Chonnam National University Hospital, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea.
Author contributions
J.Y.C., K.H.K., and E.K.K. were responsible for the conception of the work. J.-H.P., E.-Y.C., J.-O.C., H.P., H.Y.K., and H.J.Y. reviewed the design of the work. J.Y.C., N.L., S.H.C., and S.Y.K. contributed to the acquisition, analysis, and curation of data. J.Y.C., K.H.K., J.-H.P., E.-Y.C., J.-O.C., H.P., H.Y.K., H.J.Y., Y.A., M.H.J., and J.G.C. contributed to the validation and interpretation of the data. All authors have contributed to the writing and editing of the manuscript. K.H.K., J.-H.P., E.-Y.C., and J.-O.C. provided oversight for the work, and S.H.C., S.Y.K., and E.K.K. contributed to project administration. J.Y.C., N.L., and K.H.K. had full access to all data in the study and were responsible for the decision to submit the manuscript. All authors critically revised the manuscript for intellectual content, approved the final version, and met the ICMJE criteria for authorship. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The study-related documents can be made available where needed, by contacting the corresponding author. De-identified participant data will not be shared without approval from the KDCA.
Funding
This study was supported by a fund (2021-05-007) of the KDCA.
References
- 1. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–484. 10.1161/CIRCULATIONAHA.121.056135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after COVID-19 vaccination in a large health care organization. N Engl J Med 2021;385:2132–2139. 10.1056/NEJMoa2110737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA 2022;327:331–340. 10.1001/jama.2021.24110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Husby A, Hansen JV, Fosbøl E, Thiesson EM, Madsen M, Thomsen RW, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ 2021;375:e068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patone M, Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 2022;28:410–422. 10.1038/s41591-021-01630-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong HL, Hu M, Zhou CK, Lloyd PC, Amend KL, Beachler DC, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet 2022;399:2191–2199. 10.1016/S0140-6736(22)00791-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi S, Lee S, Seo JW, Kim MJ, Jeon YH, Park JH, et al. Myocarditis-induced sudden death after BNT162b2 mRNA COVID-19 vaccination in Korea: case report focusing on histopathological findings. J Korean Med Sci 2021;36:e286. 10.3346/jkms.2021.36.e286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim Y, Kim MC, Kim KH, Jeong IS, Cho YS, Choi YD, et al. Case report: acute fulminant myocarditis and cardiogenic shock after messenger RNA coronavirus disease 2019 vaccination requiring extracorporeal cardiopulmonary resuscitation. Front Cardiovasc Med 2021;8:758996. 10.3389/fcvm.2021.758996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verma AK, Lavine KJ, Lin CY. Myocarditis after COVID-19 mRNA vaccination. N Engl J Med 2021;385:1332–1334. 10.1056/NEJMc2109975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA 2021;326:1210–1212. 10.1001/jama.2021.13443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015;33:4398–4405. 10.1016/j.vaccine.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648. 10.1093/eurheartj/eht210 [DOI] [PubMed] [Google Scholar]
- 13. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol 2022;19:75–77. 10.1038/s41569-021-00662-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study-related documents can be made available where needed, by contacting the corresponding author. De-identified participant data will not be shared without approval from the KDCA.