Abstract
Aims
To perform evaluation of widely embraced bone scintigraphy-based non-biopsy diagnostic criteria (NBDC) for ATTR amyloid cardiomyopathy (ATTR-CM) in clinical practice, and to refine serum free light chain (sFLC) ratio cut-offs that reliably exclude monoclonal gammopathy (MG) in chronic kidney disease.
Methods and results
A multi-national retrospective study of 3354 patients with suspected or histologically proven cardiac amyloidosis (CA) referred to specialist centres from 2015 to 2021; evaluations included radionuclide bone scintigraphy, serum and urine immunofixation, sFLC assay, eGFR measurement and echocardiography. Seventy-nine percent (1636/2080) of patients with Perugini grade 2 or 3 radionuclide scans fulfilled NBDC for ATTR-CM through absence of a serum or urine monoclonal protein on immunofixation together with a sFLC ratio falling within revised cut-offs incorporating eGFR; 403 of these patients had amyloid on biopsy, all of which were ATTR type, and their survival was comparable to non-biopsied ATTR-CM patients (p = 0.10). Grade 0 radionuclide scans were present in 1091 patients, of whom 284 (26%) had CA, confirmed as AL type (AL-CA) in 276 (97%) and as ATTR-CM in only one case with an extremely rare TTR variant. Among 183 patients with grade 1 radionuclide scans, 122 had MG of whom 106 (87%) had AL-CA; 60/61 (98%) without MG had ATTR-CM.
Conclusion
The NBDC for ATTR-CM are highly specific [97% (95% CI 0.91-0.99)] in clinical setting, and diagnostic performance was further refined here using new cut-offs for sFLC ratio in patients with CKD. A grade 0 radionuclide scan all but excludes ATTR-CM but occurs in most patients with AL-CA. Grade 1 scans in patients with CA and no MG are strongly suggestive of early ATTR-type, but require urgent histologic corroboration.
Keywords: Amyloid, Amyloidosis, ATTR cardiomyopathy, eGFR, Radionuclide scintigraphy
Structured Graphical Abstract
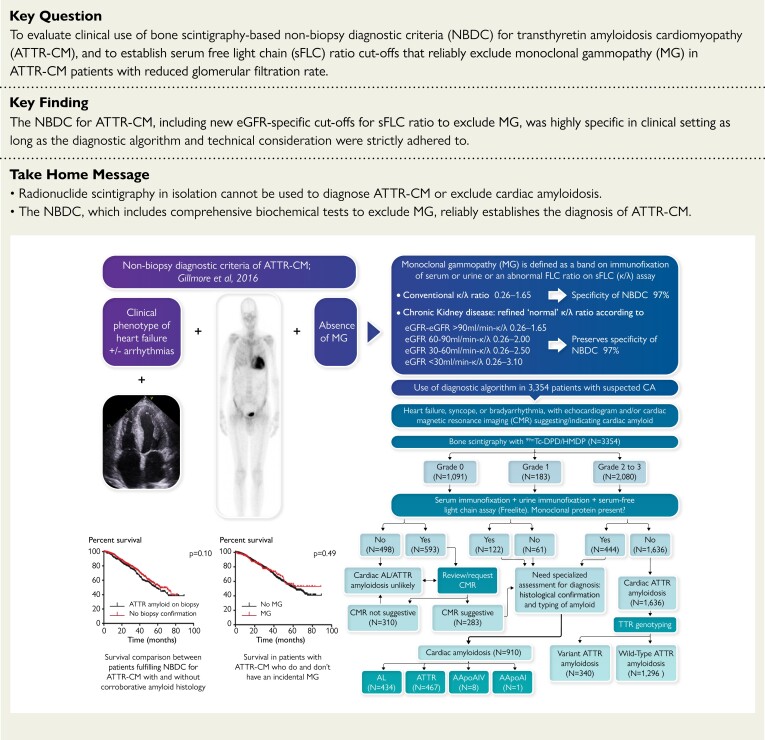
Structured Graphical Abstract.
A real-world analysis of 3354 patients referred to specialist centres with suspected or proven cardiac amyloidosis showed that the non-biopsy diagnostic criteria for ATTR-CM remain highly specific with inclusion of refined cut-offs for sFLC ratio in CKD, as long as the diagnostic algorithm is carefully adhered to and technical considerations are met. Legend: AL, light chain amyloidosis; ATTR, transthyretin amyloidosis; ATTR-CM, transthyretin amyloid cardiomyopathy; CA, cardiac amyloidosis; IFE, immunofixation electrophoresis; CA, cardiac amyloidosis; CMR, cardiac magnetic resonance; NBDC, non-biopsy diagnostic criteria; eGFR, estimated glomerular filtration rate; MG, monoclonal gammopathy; sFLC, serum free light chains; TTR, transthyretin.
See the editorial comment for this article ‘Expanding indications for non-biopsy diagnosis of transthyretin amyloid cardiomyopathy’, by S. Dorbala, https://doi.org/10.1093/eurheartj/ehad281.
Introduction
Cardiac amyloidosis (CA) is caused by extra-cellular deposition of insoluble amyloid fibrils in the myocardial space. Transthyretin (TTR), produced by the liver, and monoclonal immunoglobulin light chains, produced by clonal plasma cells, comprise the amyloid fibril in transthyretin amyloid cardiomyopathy (ATTR-CM) and light chain cardiac amyloidosis (AL-CA), respectively, and account for the vast majority of cases of CA. However, other proteins such as apolipoprotein A-I and apolipoprotein A-IV can also form amyloid fibrils which infiltrate the myocardium.1,2 Over the past 15 years, CA has been increasingly recognized as a major cause of heart failure with preserved ejection fraction (HFpEF) which previously went undiagnosed.3 The failure to diagnose CA was due to a range of factors including the poor diagnostic performance of echocardiography and the requirement for a tissue diagnosis in the form of an endomyocardial biopsy (EMB).4 Histological identification and typing of amyloid has long been considered the gold standard in the diagnosis of amyloidosis. More recently, however, advances in imaging have enabled non-biopsy diagnosis of CA, such that ∼70% patients with ATTR-CM are now diagnosed without recourse to EMB.5 In particular, the development and wide application of technetium-labelled radionuclide bone tracers over the last 10 years has proven to be critical in establishing the diagnosis of ATTR-CM, with 99mTc-DPD and 99mTc-HMDP used in Europe and 99mTc-PYP used in the USA.1 A diagnostic algorithm, first proposed in 2016,6 and since validated,7 has been widely adopted in clinical practice. This algorithm requires a clinical phenotype of cardiomyopathy, evidence of CA on echocardiography or cardiac magnetic resonance (CMR), absence of biochemical evidence of a monoclonal gammopathy (MG) on serum and urine immunofixation and by serum free light chain (sFLC) assay, and grade 2 or 3 cardiac uptake on a radionuclide bone scan using one of the three tracers listed above to establish a diagnosis of ATTR-CM. The original multicentre study which gave rise to this non-biopsy diagnostic algorithm suggested a very high specificity for ATTR-CM using this approach.6 The diagnosis of AL-CA does not specifically rely on clinical and functional features and is currently defined by the echocardiographic finding of left ventricular wall thickness ≥ 12 mm and/or an N-terminal fragment of the pro–B-type natriuretic peptide (NT-proBNP) > 332 pg/mL in the absence of any other underlying cause and in the presence of a MG and any biopsy showing AL amyloid.3
A significant existing limitation of the non-biopsy diagnostic criteria (NBDC) for ATTR-CM includes a lack of clarity regarding interpretation of sFLC results in patients with reduced glomerular filtration rate (GFR). The development of the sFLC assay established a reference polyclonal range of kappa-to-lambda ratio (κ/λ) of 0.26–1.65 with values outside this range indicating monoclonality.8 However, the preferential clearance of lambda free light chains by the reticuloendothelial system in individuals with impaired renal clearance coupled with the increased physiological production of kappa free light chains progressively increases the polyclonal reference range as GFR falls such that Hutchison et al. proposed a polyclonal κ/λ FLC ratio reference range of 0.37–3.1 in patients with an estimated GFR (eGFR) below 60 mL/min/1.73 m2.9,10
In November 2021, we established a collaboration with several international amyloidosis centres to examine the specificity of the NBDC for ATTR-CM in clinical practice whilst simultaneously attempting to refine the polyclonal κ/λ free light chain ratio reference range according to eGFR.
Methods
Patients and study design
This was a multicentre multinational retrospective study of 3354 patients with suspected or proven cardiac amyloidosis who were referred for evaluation to the following specialist amyloidosis centres in Europe between 2015 and 2021: National Amyloidosis Centre, London, UK; Tuscan Amyloid Referral Centre, Careggi University Hospital, Florence, Italy; Cardiologic Centre, University of Ferrara, Italy; Maria Cecilia Hospital, GVM Care & Research, Cotignola (Ravenna), Italy; Cardiologic Centre, Azienda Ospedaliero Universitaria of Ferrara, Italy; Cardiology Unit, St. Orsola Hospital, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; and Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Italy.
All patients underwent 99mtechnetium-labelled bone scintigraphy (99mTc-DPD or 99mTc-HMDP) together with immunofixation electrophoresis (IFE) of both serum and urine, serum free light chain (sFLC) assay, and echocardiography. Prior CMRs were reviewed centrally, or new CMRs were obtained at the specialist centres in accordance with local clinical practice. Final diagnosis was established on the basis of the results of all investigations alongside results of histological examination of available biopsy specimens and results of gene sequencing.
Follow-up protocols were consistent between centres comprising routine annual follow-up in all patients. Selected patients with cardiac AL amyloidosis underwent additional follow-up visits (followed once every 6 months) during active treatment with chemotherapy. Median follow-up in the whole cohort was 25.5 months (95% confidence interval [CI] 0.24–0.26), and a total of 833 (25%) deaths were recorded. No patients were lost to follow-up.
Patients were managed in accordance with the Declaration of Helsinki and provided written informed consent for retrospective analysis and publication of their data with approval from the Royal Free Hospital ethics committee (ref: 06/Q0501/42).
Echocardiography and cardiac magnetic resonance
Detailed echocardiography was performed at each specialist amyloid centre and included measurement of left and right ventricular wall thickness, an extensive analysis of left ventricular function including global and regional longitudinal strain, and atrial parameters. Diastolic function was analysed by tissue Doppler imaging. Features that were characteristic of amyloid were defined as the combined findings of thickened left ventricular walls, impaired global strain with relative sparing of the apical region compared with the base of the heart, and abnormal diastolic physiology.
Cardiac magnetic resonance was performed at the specialist amyloid centres as previously described.11,12 Features that were characteristic of amyloid were defined as the presence of diffuse subendocardial or transmural late gadolinium enhancement coupled with abnormal myocardial and blood pool gadolinium kinetics. The diagnostic criteria utilized to identify cardiac amyloidosis by echocardiography and CMR imaging were in accordance with the recently published position statement on myocardial and pericardial diseases.7
Bone (99mTc-DPD/99mTc-HMDP) scintigraphy
Patients were intravenously administered ∼700 MBq of 99mTc-DPD (n = 3085) or 99mTc-HMDP (n = 269), providing an expected radiation dose of ∼4 mSv per patient. Scintigraphy was mostly performed at the specialist centres although a small proportion were acquired in referring centres. Whole body planar images were acquired 3 hours post-injection using low-energy high-resolution collimators. This was followed immediately by a single-photon emission computed tomography (SPECT) with a low-dose, non-contrast computed tomography (CT) scan of the heart. Cardiac retention of all radionuclide scans was defined by a single experienced reader at each specialist centre (where necessary, after image transfer from referral centres) according to the grading devised by Perugini et al.13
Histology, immunohistochemistry, and proteomic analysis
All formalin-fixed paraffin-embedded biopsies were stained with Congo red dye and viewed under crossed polarized light. The definitive amyloid fibril type was established by immunohistochemical staining of amyloid deposits using a panel of monospecific antibodies reactive with serum amyloid A protein (SAA); kappa and lambda immunoglobulin light chains; transthyretin; apolipoprotein A-I and apolipoprotein A-IV, as previously described;14 and/or by microdissection of amyloid deposits and proteomic analysis, as previously described.15
Monoclonal protein studies and estimated glomerular filtration rate measurement
The presence of a MG was sought by IFE of serum, by IFE of urine, and by sFLC assay (Freelite, The Binding Site)16,17 in all patients. The presence of a MG was defined as the presence of a band on IFE of serum or urine or an abnormal FLC ratio on sFLC assay. ‘Refined’ normal sFLC ratio was defined according to standardized four-variable Modification of Diet in Renal Disease (MDRD) eGFR equation18 as follows: eGFR > 90 mL/min, ratio 0.26–1.65; eGFR 60–90 mL/min, ratio 0.26–2.00; eGFR 30–60 mL/min, ratio 0.26–2.50; and eGFR < 30 mL/min, ratio 0.26–3.10. Chronic kidney disease was classified in five stages (I–V) according to eGFR categories in line with KDIGO clinical practice guidelines.19,20
Additionally, three different approaches to define ‘normal’ and ‘abnormal’ sFLC ratio in the chronic kidney disease population were compared; ‘conventional’ (normal ratio 0.26–1.26), ‘Hutchison’ (0.37–3.10),9 and ‘refined’ (defined above, according to eGFR ranges). Lastly, a comparison of the effect on the NBDC for ATTR-CM of employing the 2021 standardized race-free Chronic Kidney Disease Epidemiology Collaboration (CKD–EPI) equation, used worldwide to define eGFR,21 rather than the MDRD formula was conducted.
Gene sequencing
All patients with cardiac ATTR amyloidosis underwent sequencing of the transthyretin (TTR) gene. DNA was extracted from whole blood and amplified by polymerase chain reaction assay, and the whole coding region of the TTR gene was sequenced, as previously described.22 Selected patients underwent sequencing of the APOA1 gene.
Statistical analyses
Statistical analyses including sensitivity, specificity, and positive predictive value analyses were conducted using Stata statistical software (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.). Kaplan Meier survival curves were created using GraphPad Prism Version 5. Cox proportional hazards regression analysis was used to determine the hazard ratio, with its 95% confidence interval, and test its significance. The proportional hazards assumption was satisfied in each analysis. A significance level of 5% was used for all hypothesis tests.
Results
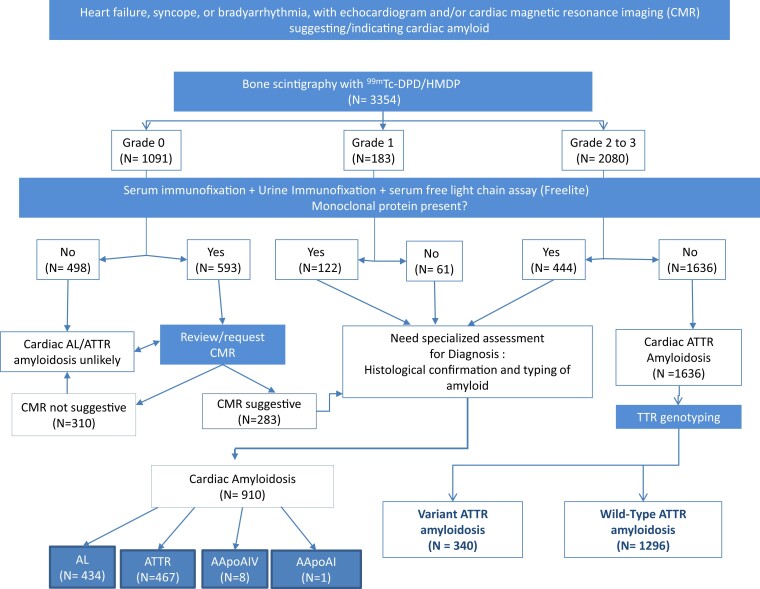
The relevant patient diagnostic characteristics are summarized in Table 1, and final diagnoses among all 3354 patients from the cohort are shown in Table 2. The final diagnosis, according to the previously published diagnostic algorithm for suspected CA, is shown in Figure 1.
Table 1.
Patient characteristics stratified by grade of cardiac uptake on bone scintigraphy
| Parameter | All (n = 3354) |
Perugini 0 (n = 1091) |
Perugini 1 (n = 183) |
Perugini ≥ 2 (n = 2080) |
|---|---|---|---|---|
| Age at diagnosis; median (IQR) | 78 (70–83) | 70 (60–78) | 74 (65–83) | 80 (75–85) |
| Male gender, n (%) | 2724 (81%) | 767 (70%) | 140 (77%) | 1817 (87%) |
| Creatinine (μmol/L), median (IQR) | 102 (82–126) | 95 (75–126) | 102 (83–137) | 105 (86–126) |
| MDRD eGFR (mL/min/1.73m²) median (IQR) | 62 (47–79) | 67 (48–87) | 63 (42–78) | 60 (47–74) |
| CKD stages | ||||
| CKD stage I, n (%) | 463 (13.8%) | 241 (22%) | 25 (13.7%) | 197 (9.4%) |
| CKD stage II, n (%) | 1295 (38.7%) | 419 (39%) | 69 (37.7%) | 807 (38.8%) |
| CKD stage III, n (%) | 1351 (40.3%) | 304 (28%) | 68 (37.2%) | 979 (47.1%) |
| CKD stage IV, n (%) | 183 (5.5%) | 80 (7%) | 14 (7.7%) | 89 (4.3%) |
| CKD stage V, n (%) | 58 (1.7%) | 43 (4%) | 7 (3.8%) | 8 (0.4%) |
| MG; conventional sFLC ratio, n (%) | 1618 (48%) | 674 (62%) | 131 (71.5%) | 813 (39%) |
| MG; Hutchison sFLC ratio, n (%) | 1053 (31%) | 560 (51%) | 121 (66%) | 372 (18%) |
| MG; sFLC ratio refined for eGFRa, n (%) | 1159 (35%) | 593 (54%) | 122 (67%) | 444 (21%) |
| Serum paraprotein by IF | ||||
| Absent, n (%) | 2455 (73.6%) | 605 (56%) | 88 (49%) | 1762 (85%) |
| Present, n (%) | 880 (26.4%) | 469 (44%) | 93 (51%) | 318 (15%) |
| Urine BJP by IF | ||||
| Absent, n (%) | 2814 (86%) | 727 (71%) | 98 (57%) | 1989 (95.6%) |
| Present, n (%) | 465 (14%) | 300 (29%) | 74 (43%) | 91 (4.4%) |
| sFLC ratio (conventional) | ||||
| Normal (0.26–1.65), n (%) | 2041 (61%) | 539 (50.3%) | 76 (43%) | 1426 (68.5%) |
| Abnormal (<0.26−>1.65), n (%) | 1287 (39%) | 532 (49.7%) | 101 (57%) | 654 (31.5%) |
| sFLC ratio (Hutchison range) | ||||
| Normal (0.37–3.10), n (%) | 2713 (82%) | 678 (63%) | 81 (46%) | 1954 (94%) |
| Abnormal (<0.37, > 3.10), n (%) | 615 (18%) | 393 (37%) | 96 (54%) | 126 (6%) |
| sFLC ratio (refined for eGFR a ) | ||||
| Normal, n (%) | 2578 (77%) | 644 (60%) | 87 (49%) | 1847 (89%) |
| Abnormal (defined below), n (%) | 750 (23%) | 427 (40%) | 90 (51%) | 233 (11%) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease; MG, monoclonal gammopathy; sFLC ratio, serum free light chain ratio; urine BJP, urine Bence–Jones protein; IF, immunofixation; % in each case depicts percentage within a Perugini grade.
Normal sFLC ratio refined according to eGFR; eGFR > 90 mL/min, ratio 0.26–1.65; eGFR 60–90 mL/min, ratio 0.26–2.00; eGFR 30–60 mL/min, ratio 0.26–2.50; eGFR < 30 mL/min, ratio 0.26–3.10.
Table 2.
Final diagnosis in 3354 patients referred with suspected or proven cardiac amyloidosis
| Number of patients (n) | |
|---|---|
| Cardiac amyloidosis | 2546 |
| Cardiac ATTR amyloidosis | 2103 |
| ATTRwt-CM | 1650 |
| ATTRv-CM | 453 |
| Cardiac AL amyloidosis | 434 |
| Cardiac AApoAI amyloidosis | 1 |
| Cardiac AApoAIV amyloidosis | 8 |
| No cardiac amyloidosis | 808 |
| Non-amyloid cardiomyopathy | 204 |
| No systemic amyloidosis | 359 |
| AL amyloidosis with no cardiac involvement | 157 |
| AApoAI amyloidosis with no cardiac involvement | 5 |
| AA amyloidosis with no cardiac involvement | 1 |
| TTR mutation carrier with no amyloidosis | 47 |
| ATTRv-PN with no cardiac involvement | 35 |
Bold values are indicative of major groups which are further broken down in subgroups.
ATTRwt-CM, wild-type ATTR amyloid cardiomyopathy; ATTRv-CM, variant ATTR amyloid cardiomyopathy; PN, polyneuropathy.
Figure 1.
Real-world use of the previously published diagnostic algorithm in 3354 patients with suspected cardiac amyloidosis to establish a definitive diagnosis.
Perugini grade 2 or 3 radionuclide bone scans
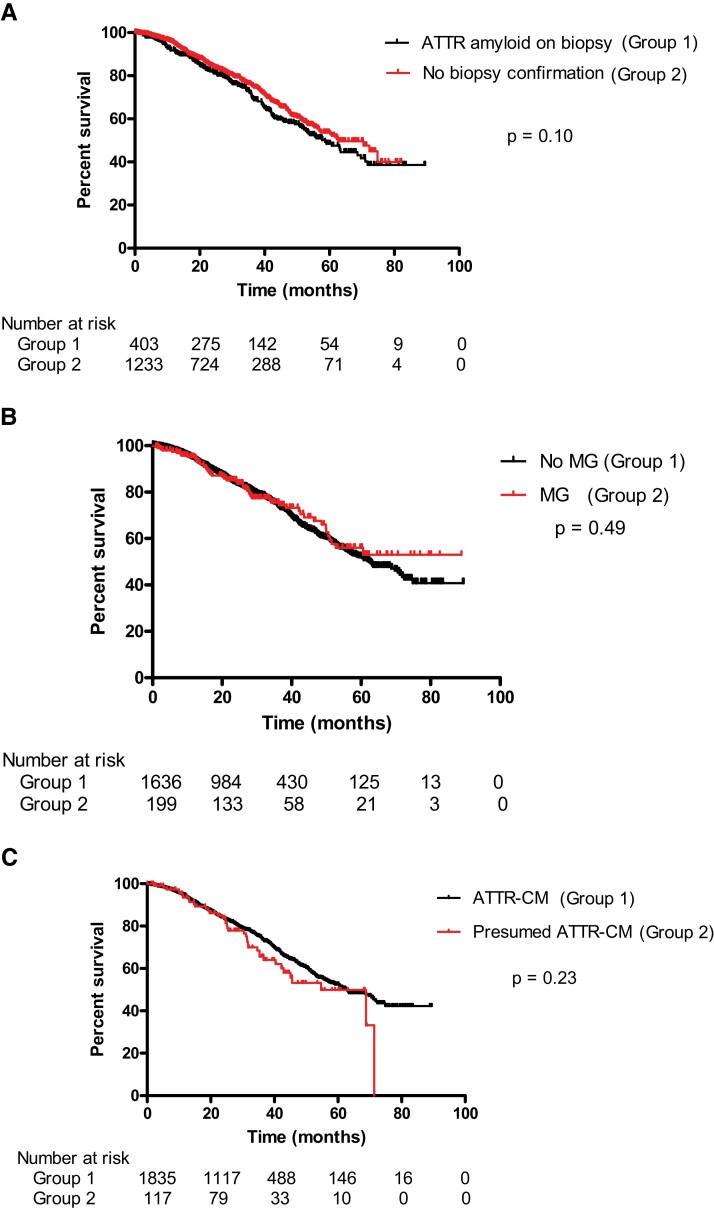
There were 2080 patients with a grade ≥2 Tc-DPD/HMDP scan, 1636 (79%) of whom did not have evidence of a MG, thereby fulfilling the NBDC for ATTR-CM, and 444 (21%) of whom did have evidence of a MG. Among the 1636 patients with ATTR-CM by NBDC, 134 (8%) underwent EMB and 697 (43%) underwent extra-cardiac biopsies, including 540 fat aspirates. ATTR amyloid was present in 132/134 EMBs; the remaining 2 EMBs did not show any evidence of amyloid; one was from a patient receiving hydroxychloroquine in whom the Tc-DPD scan was a likely false positive and the other was a scanty biopsy sample thought to be a likely false negative EMB. ATTR amyloid was detected in 271/697 (39%) extra-cardiac biopsies; the remaining 426 extra-cardiac biopsies, of which 391 (92%) were fat aspirates or bone marrow trephine biopsies, did not show evidence of amyloid. Importantly, every 1 of 403 biopsies in which amyloid was detected from the 1636 patients who fulfilled NBDC for ATTR-CM showed that the amyloid type was ATTR, corroborating the non-biopsy diagnosis (Table 3). Furthermore, there was no significant difference in survival between the 403 patients with histological evidence of ATTR amyloid corroborating a non-biopsy diagnosis of ATTR-CM and the remaining 1233 patients who fulfilled NBDC for ATTR-CM without histological ‘proof’ of amyloid [Figure 2A, HR 1.19 (95% CI 0.97–1.46), P = 0.10], providing further evidence of ATTR-CM being the correct diagnosis in those without histological confirmation of the presence or type of amyloid.
Table 3.
Patients with Perugini grade 2/3 radionuclide bone scan
| N | Amyloid type by histology +/− proteomics | |||||
|---|---|---|---|---|---|---|
| ATTR amyloid | AL amyloid | Other amyloid | No amyloid | No biopsy taken | ||
| Patients with no monoclonal gammopathy | 1636a | 403 | 0 | 0 | 428 | 805 |
| Endomyocardial biopsy | 134 | 132 | 0 | 0 | 2b | |
| Extra-cardiac biopsy | 697 | 271 | 0 | 0 | 426c | |
| Patients with monoclonal gammopathy | 444 | 199 | 40 | 0 | 126 | 79 |
| Endomyocardial biopsy | 101 | 85 | 15 | 0 | 1b | |
| Extra-cardiac biopsy | 264 | 114 | 25 | 0 | 125 | |
| Total | 2080 | 602 | 40 | 0 | 554 | 884 |
Bold values are indicative of major groups which are further broken down in subgroups.
Fulfilling non-biopsy diagnostic criteria for ATTR-CM.
Potential false positive radionuclide scans or false negative EMBs.
Three hundred ninety-one/426 biopsies in which there was no amyloid were fat aspirates or bone marrow trephine biopsies.
Figure 2.
(A) Survival among patients fulfilling non-biopsy diagnostic criteria for ATTR-CM stratified by whether there was (n = 403) or was not (n = 1233) corroborating evidence of ATTR amyloid in a biopsy specimen (log rank test, P = 0.10). (B) Survival among patients with ATTR-CM stratified by the presence (n = 199) or absence (n = 1636) of a MG (log rank test, P = 0.49). (C) Comparison of survival between patients with a confirmed diagnosis of ATTR-CM (on the basis of non-biopsy diagnostic criteria or histology, n = 1835) and patients with presumed ATTR-CM (characteristic clinical phenotype and imaging for ATTR-CM but presence of a MG and no histology confirming the amyloid type, n = 117) (log rank test, P = 0.23).
Among 444 patients with a grade ≥2 radionuclide scan and evidence of a MG, 365 (82%) underwent biopsies (101 EMB and 264 extra-cardiac) to determine the amyloid type; the 79 (18%) remaining patients were deemed to be too unwell for either enrolment into a clinical trial (for ATTR-CM) or chemotherapy (for AL-CA) such that a definitive diagnosis via histology was not pursued (Table 3). Two hundred thirty-nine out of 365 (65%) biopsies showed the presence of amyloid and 126 did not show amyloid. Of the 239 biopsies containing amyloid, 199 (83%) were ATTR type and 40 (17%) were AL type. Among the 126 patients without amyloid on histological examination of a biopsy, an indication for chemotherapy due to the presence of visceral amyloid deposits on 123I-labelled serum amyloid P component (SAP) scintigraphy (confirming AL-type amyloid) or symptomatic multiple myeloma in 9 patients precluded the pursuit of an EMB to exclude the possibility of ATTR-CM. The remaining 117 patients were given a presumptive diagnosis of ATTR-CM, 29 of whom probably did not have a MG (i.e. negative serum and urine IF and FLC ratio < 3.1 but slightly outside the GFR criteria proposed in this manuscript) and 88 of whom did not wish to pursue a definitive diagnosis following their analysis of the risk/benefit ratio of EMB. There was no difference in survival between 1636 patients with ATTR-CM in the absence of a MG and the 199 patients with biopsy-proven ATTR-CM who did have a MG [Figure 2B, HR 0.90 (95% CI 0.67–1.20), P = 0.49]. There was no significant difference in survival between the 117 patients with presumed ATTR-CM and the 1835 patients with a firm diagnosis of ATTR-CM [Figure 2C, HR 0.82 (95% CI 0.59–1.13), P = 0.23].
Perugini grade 0 radionuclide bone scans
There were 1091 patients with a grade 0 radionuclide bone scan, 807 (74%) of whom did not have CA and 284 of whom did have CA (Table 4). Among those with CA, 276/284 (97%) were AL type, 7/284 (3%) were AApoAIV type, and one had EMB-proven ATTR-CM in association with the rare pathogenic p.(Y134C) TTR variant. The 807 patients without CA comprised 609 (75%) patients without systemic amyloidosis including 47 TTR mutation carriers without disease and 198 patients with systemic amyloidosis who did not have cardiac involvement [157 AL, 35 variant ATTR (ATTRv), 5 AApoAI, and 1 AA amyloidosis]. The absence of cardiac involvement in patients with AL, ATTRv, AApoAI, and AA amyloidosis was determined by echocardiography and corroborated in cases of doubt by CMR. The absence of cardiac involvement in ATTRv amyloidosis was entirely consistent with that expected according to age and TTR mutation findings (i.e. 17/35 early-onset p.(V50M)-associated ATTRv amyloidosis and 18/35 with other TTR mutations). The presence of cardiac amyloidosis was supported by EMB in 46/284 cases including 6 of 7 with AApoAIV amyloid cardiomyopathy, and the absence of cardiac amyloid was corroborated by EMB in 29/807 patients (Table 4).
Table 4.
Patients with Perugini grade 0 radionuclide bone scan
| N | Histology | |||
|---|---|---|---|---|
| EMB | Extra-cardiac biopsy | No biopsy | ||
| No cardiac amyloidosis | 807 | 29 | 464 | 314 |
| No amyloidosis | 609 | 27 | 293 | 289 |
| AL amyloidosis without cardiac involvementa | 157 | 2 | 152 | 3 |
| Hereditary ATTR amyloidosis without cardiac involvementb | 35 | 0 | 15 | 20 |
| Hereditary AApoAI amyloidosis without cardiac involvementa | 5 | 0 | 3 | 2 |
| AA amyloidosis without cardiac involvementa | 1 | 0 | 1 | 0 |
| Cardiac amyloidosis | 284 | 46 | 219 | 19 |
| Cardiac AL amyloidosis | 276 | 39 | 218 | 19 |
| Cardiac AApoAIV amyloidosis | 7 | 6 | 1 | 0 |
| Cardiac ATTR amyloidosis | 1 | 1 | 0 | 0 |
| Total | 1091 | 75 | 683 | 333 |
Bold values indicate major groups and italics represent breakdown of these into subgroups.
Absence of cardiac involvement in AL, AA, and AApoAI amyloidosis was on the basis of echocardiography and corroborated in cases of doubt by CMR findings.
Absence of cardiac involvement in hereditary ATTR amyloidosis was established in each case by both echocardiography and CMR.
Perugini grade 1 radionuclide bone scans
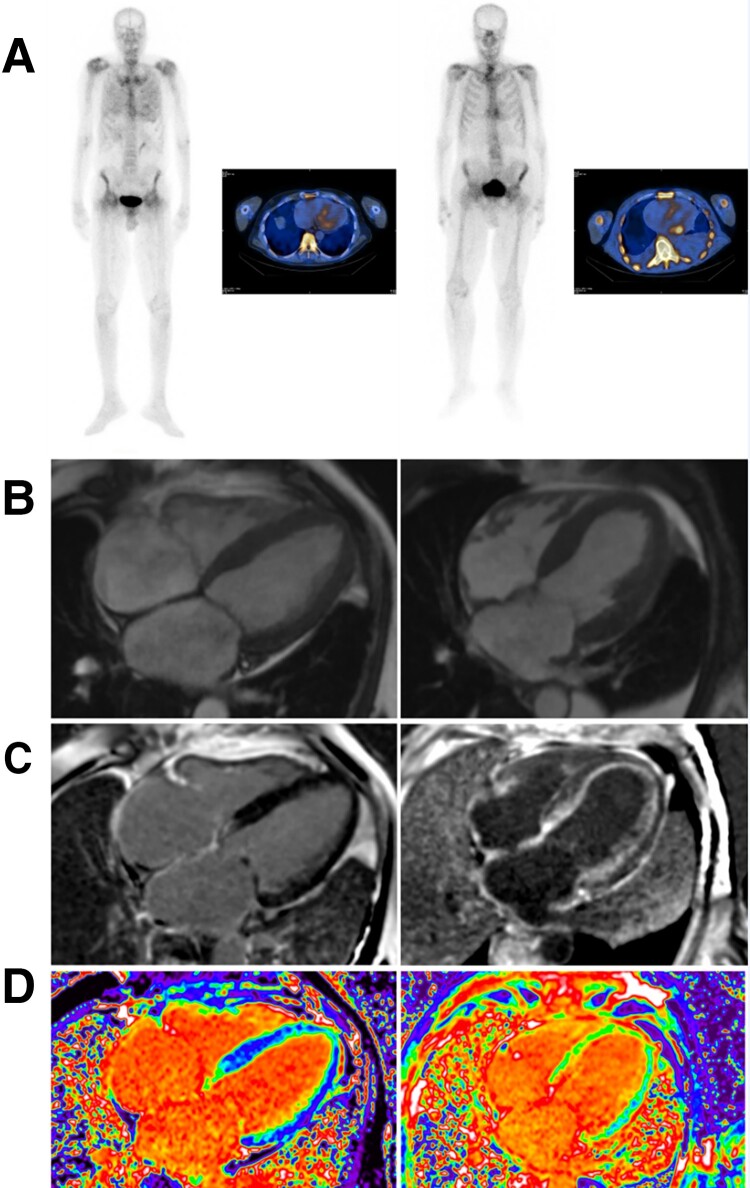
There were 183 patients with a grade 1 radionuclide bone scan, 122 (67%) of whom did have biochemical evidence of a MG and 61 of whom did not have a MG (Table 5). Among those with a MG, 106 (87%) had AL-CA, 14 had ATTR-CM, and 1 had cardiac AApoAIV amyloidosis. The remaining patient, who was known to have cardiac sarcoidosis and end-stage renal disease, was considered to have a false positive Tc-DPD scan following an EMB in which no amyloid was detected. Among 61 patients with a grade 1 Tc-DPD/HMDP scan and no MG, 60 (98%) had ATTR amyloidosis and 1 had cardiac AApoAI amyloidosis. A comparison between patients with a grade 1 radionuclide scan and AL-CA and patients with a grade 1 radionuclide scan and ATTR-CM showed that NT-proBNP was < 1000 ng/L in only 2/106 patients with AL-CA but was < 1000 ng/L in a majority (43/74, 58%) of those with ATTR-CM. Similarly, echocardiography and CMR findings in those with ATTR-CM and a grade 1 radionuclide scan indicated earlier stage disease in the vast majority [with the exception of patients carrying the p.(S97Y) TTR variant] on the basis of maximal septal wall thickness (mean ± SD; 12.9 ± 2.63 mm), global longitudinal strain (mean ± SD; −16.6 ± 4.46), LVEF (mean ± SD; 62% ± 10.8), mid-wall subendocardial late gadolinium enhancement (LGE) (87%), and extracellular volume (ECV) (mean ± SD; 32 ± 6.7%), whereas a grade 1 radionuclide scan in patients with AL-CA amyloidosis was typically associated with more advanced stage disease according to CMR and/or echocardiographic criteria (Figure 3) as evidenced by greater maximal septal wall thickness (mean ± SD; 15.7 ± 2.32 mm), worse global longitudinal strain (mean ± SD; −9.84 ± 4.15), lower LVEF (mean ± SD; 52 ± 13%), transmural LGE (74%), and higher ECV (mean ± SD; 55% ± 8.4).
Table 5.
Patients with Perugini grade 1 radionuclide bone scan
| N | Amyloid type by histology +/− proteomics | ||||||
|---|---|---|---|---|---|---|---|
| ATTR amyloid | AL amyloid | AApoAIV amyloid | AApoAI amyloid | No amyloid | No biopsy taken | ||
| Patients with no monoclonal gammopathy | 61 | 25 | 0 | 0 | 0 | 19 | 17 |
| Endomyocardial biopsy | 1 | 1 | 0 | 0 | 0 | 0 | - |
| Extra-cardiac biopsy | 43 | 24 | 0 | 0 | 0 | 19 | - |
| Patients with monoclonal gammopathy | 122 | 9 | 83 | 1 | 0 | 14 | 15 |
| Endomyocardial biopsy | 20 | 3 | 15 | 0 | 0 | 2* | - |
| Extra-cardiac biopsy | 87 | 6 | 68 | 1 | 0 | 12 | - |
| Total | 183 | 34 | 83 | 1 | 0 | 33 | 32 |
Bold values are indicative of major groups which are further broken down in subgroups.
Possible false positive radionuclide scan or false negative EMB; one patient was felt to have a false positive radionuclide scan due to cardiac sarcoidosis and/or ESRD; the other was felt to have a false negative EMB on the basis of a scanty tissue sample and characteristic cardiac imaging for amyloid and was treated with chemotherapy for cardiac AL amyloidosis.
Figure 3.
Comparison of CMR findings between a patient with grade 1 DPD scintigraphy with ATTR-CM (left) and AL-CA (right). (A) Anterior view of grade 1 Tc-DPD scan including SPECT-CT in a patient with ATTR-CM and AL-CA. (B) Cine CMR in ATTR-CM showing normal cardiac structure and in AL-CA showing increased left ventricular wall thickness and mass. (C) Subtle subendocardial late gadolinium enhancement (LGE) on CMR in ATTR-CM and diffuse subendocardial LGE in AL-CA. (D) Mildly elevated extra-cellular volume (ECV) of 0.33 in ATTR-CM and markedly increased ECV of 0.54 in AL-CA. The CMR findings indicate earlier stage disease in ATTR-CM and more advanced disease in AL-CA despite similar cardiac uptake of Tc-DPD.
Serum free light chain ratio
Importantly, the sFLC ratio was considered normal in 2041 patients by the conventional method and 2713 patients by the Hutchison method, including in 2 patients diagnosed with AL amyloidosis. The refined criteria, incorporating different sFLC ratio cut-offs for different GFR bands, defined the sFLC ratio as normal in 2578 patients with the 2 patients with AL amyloidosis who were defined as having no MG by the Hutchison method now being defined as having a MG (thus necessitating histological confirmation of the amyloid type). The specificity of the NBDC for ATTR-CM using conventional sFLC ratio (0.26–1.65) is shown in supplementary data online, Tables A and B. The specificity of the NBDC for ATTR-CM was similar upon substitution of the CKD–EPI formula for the MDRD formula for calculation of eGFR (see supplementary data online, Tables C and E).
Discussion
This multicentre, multinational retrospective study of radionuclide bone scintigraphy in more than 3300 patients referred to specialist amyloidosis centres for evaluation of suspected CA indicates that the NBDC established in 2016 for ATTR-CM, if followed to the letter, remain highly specific [specificity 97% (95% CI 91%–99.7%)] for the diagnosis assuming EMB as gold standard. A recently published meta-analysis showed a prevalence of ATTR-CM of 11% in HFpEF,23 and the prevalence of ATTR-CM was 63% (2013/3354) in the current cohort. A high positive predictive value (PPV) for the NBDC to diagnose ATTR-CM is desirable in order to minimize any risk of false positive diagnoses, including patients with AL-CA who require urgent chemotherapy being incorrectly diagnosed as having ATTR-CM. This study indicates a PPV of 98.5% (95% CI 94.7%–99.8%) for the NBDC with a specificity and PPV of up to 99.5% (95% CI 98.5–100) and 99.5% (95% CI 98.2%–99.9%), respectively, if one includes all extra-cardiac diagnostic biopsies in the cohort. The authors acknowledge the modest sensitivity of the NBDC for ATTR-CM (∼60%), mainly due to presence of an ‘incidental’ MG. Up to 40% patients with ATTR-CM therefore continue to require biopsy proof of amyloid type in order for a firm diagnosis to be established.
One important challenge with respect to these NBDC for ATTR-CM has been the interpretation of the sFLC ratio in the context of a reduced GFR. This is due to the fact that the normal ‘polyclonal’ kappa/lambda sFLC ratio rises progressively with advancing renal impairment.8 Here, using eGFR-specific cut-offs to indicate a normal sFLC ratio, we were able to preserve the high specificity of the NBDC for ATTR-CM regardless of eGFR at the time of diagnosis whilst simultaneously avoiding the need for a potential EMB in 369/1636 (23%) patients who, by conventional sFLC ratio criteria (0.26–1.65), would have been labelled as having a MG and thereby failed to fulfil the NBDC for ATTR-CM. On the other hand, there is no doubt that using an upper sFLC ratio cut-off of 3.1 in any patient with an eGFR of < 60 mL/min, as proposed by Hutchison et al.,9 introduces a risk of diagnosing ATTR-CM by NBDC in a patient who in fact has AL-CA associated with a low-level kappa light chain secreting MG, as evidenced by two patients in this cohort.8 We would therefore suggest that the eGFR-specific cut-offs proposed here should be adopted in clinical practice to indicate a ‘normal’ FLC ratio within the context of excluding a MG, an essential component of the diagnostic algorithm for ATTR-CM. Of note, the use of these eGFR-specific FLC cut-offs resulted in 29 (9%) patients (from the cohort of 316 patients with confirmed or presumed ATTR-CM) who almost certainly did not have a MG being incorrectly labelled as MG + ve thereby potentially subjecting them to an EMB to establish the diagnosis of ATTR-CM; however, the authors firmly believe that the priority with respect to the NBDC for ATTR-CM should be to preserve the high diagnostic specificity, potentially at the expense of sensitivity, in order to avoid patients with possible AL-CA from being incorrectly diagnosed with ATTR-CM and thus denied life-prolonging chemotherapy.
We also conducted an analysis of our findings using the CKD–EPI formula to estimate GFR rather than MDRD, given the widespread use of this formula in clinical practice. Reassuringly, there were only a few patients in whom there was a discrepancy between the two formulae in defining the presence or absence of a MG, notably due to higher values of eGFR by the CKD–EPI equation. Most importantly, there was no significant loss of specificity of the NBDC for ATTR-CM using the CKD–EPI formula.
It is important to consider the extremely rare cases in which the NBDC for the diagnosis of ATTR-CM are met but no amyloid is detected on EMB of which there were two in our cohort. One such patient was receiving hydroxychloroquine which has previously been reported to be associated with radionuclide imaging characteristics mimicking ATTR-CM,24 and the other had a tiny EMB sample which, given the patchy distribution of amyloid deposits within tissues,25 is likely to represent a false negative EMB rather than a false positive Tc-DPD scan. It is crucial to stress here that all images were acquired 3 hours after administration of the bone tracer, including SPECT-CT scans to confirm specific myocardial uptake since residual blood pool signal can give rise to false positive diagnosis of CA on limited planar 2D views particularly if acquired sooner after radiotracer administration.
The absence of cardiac uptake on radionuclide scintigraphy reliably excluded ATTR-CM in all but 1 of 1091 (> 99.9%) patients. This particular patient had an extremely rare pathogenic TTR variant p.(Y134C), which has previously been reported to be associated with less than expected cardiac uptake by radionuclide scintigraphy.26 Conversely, 276 of 434 (64%) patients with AL-CA from the whole cohort had a Perugini grade 0 Tc-DPD/HMDP scan, such that radionuclide scintigraphy cannot be used to exclude CA. However, if an echocardiogram or preferably a CMR is characteristic of CA and the radionuclide bone scan is Perugini grade 0, the presence of a MG indicates likely AL-CA, and the absence of a MG should prompt sequencing of the TTR and APOA1 genes and pursuit of an EMB to exclude rare amyloid cardiomyopathies such as cardiac AApoAIV amyloidosis and cardiac AApoAI amyloidosis.
Among 183 patients with grade 1 cardiac uptake on radionuclide bone scintigraphy, 106 (58%) had AL-CA all of whom had a MG, and 75 (41%) had ATTR-CM. With the exception of six patients who carried the p.(S97Y) TTR variant, all of those with ATTR-CM had earlier stage disease on the basis of echocardiographic and CMR criteria compared with those with AL-CA, as previously reported.27 Like the p.(Y134C) TTR variant highlighted earlier in this manuscript, the p.(S97Y) variant has also previously been reported to be associated with less than expected cardiac uptake on radionuclide scintigraphy.28 Importantly, among the 61 patients with a grade 1 radionuclide scan and no MG, 60 had ATTR-CM. These results further highlight the essential requirement for genetic sequencing and histological identification and typing of CA in patients who do not fulfil the NBDC for ATTR-CM.
It is particularly important to note that 52/2080 (2.5%) patients with Perugini grade 2 or 3 radionuclide scintigraphy had a final diagnosis of AL-CA, which highlights the fact that radionuclide scintigraphy should not be used in isolation to diagnose CA since it is not specific for ATTR-CM regardless of the intensity of cardiac uptake. In fact, among 434 patients in the study with AL-CA, 276 (64%), 106 (24%), and 52 (12%) had grade 0, 1, and ≥ 2 radionuclide scans, respectively. These findings highlight, once again, the essential requirement of undertaking biochemical tests to search for a MG in any patient with suspected CA who undergoes radionuclide bone scintigraphy whilst also underlining the fact that both serum and urine immunofixation and the sFLC assay are required to rule out MG since patients from the cohort with isolated abnormalities of each of the three tests with both grade 1 and grade ≥2 radionuclide scans were diagnosed with cardiac AL amyloidosis.
Conclusions
Radionuclide scintigraphy with Tc-DPD or Tc-HMDP is a very sensitive imaging modality for identifying possible ATTR-CM, but it cannot be used alone to establish this diagnosis. Nor can radionuclide scintigraphy be used to exclude CA. However, the use of radionuclide scintigraphy with comprehensive biochemical tests for a MG can reliably establish ATTR-CM without a biopsy, as long as the previously published non-biopsy diagnostic algorithm for ATTR-CM is followed to the letter. The inclusion of novel sFLC ratio cut-offs according to eGFR for exclusion of a MG further refines the NBDC for ATTR-CM.
Supplementary Material
Acknowledgements
We thank our many physician colleagues for referring the patients. M.U.R., A.P., M.F., P.N.H., and J.D.G. were responsible for conceiving the study, interpreting the results, and drafting the manuscript. F.C., F.P., M.Z., A.A., N.S., S.L., Y.R., J.B., S.R., I.A., R.P., A.P., D.F.H., S.M., B.W., A.M.N., L.V., C.W., D.R., J.A.G., H.J.L., A.D.W., C.R., M.S., P.M., A.G.C., A.P., A.A., A.G., G.S., M.S., C.G., E.B., and S.L. were responsible for data collection, data interpretation, and editing of the manuscript.
We authors would like particularly to acknowledge the late Professor Claudio Rapezzi who was a giant of the amyloid field and made a significant contribution to this study.
Contributor Information
Muhammad Umaid Rauf, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Philip N Hawkins, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Francesco Cappelli, Tuscan Amyloid Referral Centre, Careggi University Hospital, Florence, Italy.
Federico Perfetto, Tuscan Amyloid Referral Centre, Careggi University Hospital, Florence, Italy.
Mattia Zampieri, Tuscan Amyloid Referral Centre, Careggi University Hospital, Florence, Italy.
Alessia Argiro, Tuscan Amyloid Referral Centre, Careggi University Hospital, Florence, Italy.
Aviva Petrie, Eastman Dental Institute, University College London (UCL), London, UK.
Steven Law, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Aldostefano Porcari, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK; Centre for Diagnosis and Treatment of Cardiomyopathies, Department of Cardiovascular, Azienda Sanitaria Universitaria Giuliano Isontina (ASUGI), University of Trieste, Italy.
Yousuf Razvi, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Joshua Bomsztyk, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Sriram Ravichandran, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Adam Ioannou, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Rishi Patel, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Neasa Starr, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
David F Hutt, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Shameem Mahmood, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Brendan Wisniowski, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Ana Martinez–Naharro, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Lucia Venneri, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Carol Whelan, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Dorota Roczenio, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Janet Gilbertson, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Helen J Lachmann, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Ashutosh D Wechalekar, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Claudio Rapezzi, Cardiologic Centre, University of Ferrara, Italy; Maria Cecilia Hospital, GVM Care & Research, Cotignola (Ravenna), Italy.
Matteo Serenelli, Cardiologic Centre, Azienda Ospedaliero Universitaria di Ferrara, Italy.
Paolo Massa, Cardiology Unit, Department of Cardiac Thoracic and Vascular, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Italy.
Angelo Giuseppe Caponetti, Cardiology Unit, Department of Cardiac Thoracic and Vascular, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Italy.
Alberto Ponziani, Cardiology Unit, Department of Cardiac Thoracic and Vascular, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Italy.
Antonella Accietto, Cardiology Unit, Department of Cardiac Thoracic and Vascular, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Italy.
Alessandro Giovannetti, Cardiology Unit, Department of Cardiac Thoracic and Vascular, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Italy.
Giulia Saturi, Cardiology Unit, Department of Cardiac Thoracic and Vascular, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Italy.
Maurizio Sguazzotti, Cardiology Unit, Department of Cardiac Thoracic and Vascular, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Italy.
Christian Gagliardi, Cardiology Unit, Department of Cardiac Thoracic and Vascular, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; European Reference Network for rare, low-prevalence, or complex diseases of the heart (ERN GUARD-Heart).
Elena Biagini, Cardiology Unit, Department of Cardiac Thoracic and Vascular, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; European Reference Network for rare, low-prevalence, or complex diseases of the heart (ERN GUARD-Heart).
Simone Longhi, Cardiology Unit, Department of Cardiac Thoracic and Vascular, IRCCS Azienda Ospedaliero—Universitaria di Bologna, Italy; European Reference Network for rare, low-prevalence, or complex diseases of the heart (ERN GUARD-Heart).
Marianna Fontana, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Julian D Gillmore, National Amyloidosis Centre, University College London, Royal Free Campus, Rowland Hill Street, NW3 2PF London, UK.
Author contributions
Muhammad Umaid Rauf (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Writing—original draft: Lead; Writing—review & editing: Lead), Helen J Lachmann (Data curation: Supporting; Writing—review & editing: Supporting), Ashutosh D Wechalekar (Data curation: Supporting; Writing—review & editing: Supporting), Claudio Rapezzi (Data curation: Supporting; Methodology: Supporting; Writing—review & editing: Supporting), Matteo Serenelli (Data curation: Supporting; Writing—review & editing: Supporting), Paolo Massa (Data curation: Supporting; Writing—review & editing: Supporting), Angelo Giuseppe Caponetti (Data curation: Supporting; Writing—review & editing: Supporting), Alberto Ponziani (Data curation: Supporting; Writing—review & editing: Supporting), Janet Gilbertson (Data curation: Supporting; Writing—review & editing: Supporting), Antonella Accietto (Data curation: Supporting; Writing—review & editing: Supporting), Giulia Saturi (Data curation: Supporting; Writing—review & editing: Supporting), Maurizio Sguazzotti (Data curation: Supporting; Writing—review & editing: Supporting), Christian Gagliardi (Data curation: Supporting; Writing—review & editing: Supporting), Elena Biagini (Data curation: Supporting; Writing—review & editing: Supporting), Simone Longhi (Data curation: Supporting; Writing—review & editing: Supporting), Marianna Fontana (Conceptualization: Equal; Data curation: Equal; Investigation: Equal; Methodology: Equal; Project administration: Equal; Supervision: Lead; Writing—original draft: Lead; Writing—review & editing: Lead), Julian D Gillmore (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Supervision: Lead; Writing—original draft: Lead; Writing—review & editing: Lead), Alessandro Giovannetti (Data curation: Supporting; Writing—review & editing: Supporting), Neasa Starr (Data curation: Supporting; Writing—review & editing: Equal), Dorota Roczenio (Data curation: Supporting; Writing—review & editing: Supporting), Lucia Venneri (Data curation: Supporting; Writing—review & editing: Supporting), Philip N Hawkins (Conceptualization: Equal; Data curation: Equal; Supervision: Equal; Writing—original draft: Equal; Writing—review & editing: Equal), Francesco Cappelli (Data curation: Supporting; Writing—review & editing: Supporting), Federico Perfetto (Data curation: Supporting; Writing—review & editing: Supporting), Mattia Zampieri (Data curation: Supporting; Writing—review & editing: Supporting), Alessia Argiro (Data curation: Supporting; Writing—review & editing: Supporting), Steven Law (Data curation: Supporting; Writing—review & editing: Supporting), Yousuf Razvi (Data curation: Supporting; Writing—review & editing: Supporting), Carol Whelan (Data curation: Supporting; Writing—review & editing: Supporting), Joshua Bomsztyk (Data curation: Supporting; Writing—review & editing: Supporting), Adam Ioannou (Data curation: Supporting; Writing—review & editing: Supporting), Rishi Patel (Data curation: Supporting; Writing—review & editing: Supporting), Aldostefano Porcari (Data curation: Supporting; Formal analysis: Supporting; Writing—review & editing: Supporting), David F Hutt (Data curation: Supporting; Methodology: Supporting; Writing—review & editing: Supporting), Shameem Mahmood (Data curation: Supporting; Writing—review & editing: Supporting), Brendan Wisniowski (Data curation: Supporting; Writing—review & editing: Supporting), Ana Martinez–Naharro (Data curation: Supporting; Writing—review & editing: Supporting), Sriram Ravichandran (Data curation: Supporting; Writing—review & editing: Supporting), and Aviva Petrie (Formal analysis: Lead; Investigation: Equal; Methodology: Equal; Software: Lead; Writing—review & editing: Equal)
Supplementary data
Supplementary data is available at European Heart Journal online.
Funding
This work was funded via core support for the UK National Amyloidosis Centre by the National Health Service England and by the Italian Ministry of Health, RC-2022–2773270 project.
References
- 1. Singh V, Falk R, Di Carli MF, Kijewski M, Rapezzi C, Dorbala S. State-of-the-art radionuclide imaging in cardiac transthyretin amyloidosis. J Nucl Cardiol 2019;26:158–173. 10.1007/s12350-018-01552-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanna M, Ruberg FL, Maurer MS, Dispenzieri A, Dorbala S, Falk RH, et al. Cardiac scintigraphy with technetium-99m-labeled bone-seeking tracers for suspected amyloidosis: JACC review topic of the week. J Am Coll Cardiol 2020;75:2851–2862. 10.1016/j.jacc.2020.04.022 [DOI] [PubMed] [Google Scholar]
- 3. AbouEzzeddine OF, Davies DR, Scott CG, Fayyaz AU, Askew JW, McKie PM, et al. Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. JAMA Cardiol 2021;6:1267–1274. 10.1001/jamacardio.2021.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation 2005;112:2047–2060. 10.1161/CIRCULATIONAHA.104.489187 [DOI] [PubMed] [Google Scholar]
- 5. Lane T, Fontana M, Martinez-Naharro A, Quarta CC, Whelan CJ, Petrie A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation 2019;140:16–26. 10.1161/CIRCULATIONAHA.118.038169 [DOI] [PubMed] [Google Scholar]
- 6. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–2412. 10.1161/CIRCULATIONAHA.116.021612 [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail 2021;23:512–526. 10.1002/ejhf.2140 [DOI] [PubMed] [Google Scholar]
- 8. Molina-Andujar A, Robles P, Cibeira MT, Montagud-Marrahi E, Guillen E, Xipell M, et al. The renal range of the kappa/lambda sFLC ratio: best strategy to evaluate multiple myeloma in patients with chronic kidney disease. BMC Nephrol 2020;21:111. 10.1186/s12882-020-01771-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hutchison CA, Plant T, Drayson M, Cockwell P, Kountouri M, Basnayake K, et al. Serum free light chain measurement aids the diagnosis of myeloma in patients with severe renal failure. BMC Nephrol 2008;9:11. 10.1186/1471-2369-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diamantidis MD, Ioannidou-Papagiannaki E, Ntaios G. Novel extended reference range for serum kappa/lambda free light chain ratio in diagnosing monoclonal gammopathies in renal insufficient patients. Clin Biochem 2009;42:1202–1203. 10.1016/j.clinbiochem.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 11. Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005;111:186–193. 10.1161/01.CIR.0000152819.97857.9D [DOI] [PubMed] [Google Scholar]
- 12. Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2015;132:1570–1579. 10.1161/CIRCULATIONAHA.115.016567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perugini E, Guidalotti PiL, Salvi F, Cooke RMT, Pettinato C, Riva L, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005;46:1076–1084. 10.1016/j.jacc.2005.05.073 [DOI] [PubMed] [Google Scholar]
- 14. Tennent GA, Cafferty KD, Pepys MB, Hawkins PN. Congo red overlay immunohistochemistry aids classification of amyloid deposits. In: Kyle RA, Gertz MA (eds.), Amyloid and Amyloidosis, 1998. Parthenon Publishing, 1999, 160–162. [Google Scholar]
- 15. Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 2009;114:4957–4959. 10.1182/blood-2009-07-230722 [DOI] [PubMed] [Google Scholar]
- 16. Drayson M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free light-chain measurements for identifying and monitoring patients with nonsecretory multiple myeloma. Blood 2001;97:2900–2902. 10.1182/blood.V97.9.2900 [DOI] [PubMed] [Google Scholar]
- 17. Bradwell AR, Carr-Smith D, Mead GP, Tang LX, Showell PJ, Drayson MT, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 2001;47:673–680. 10.1093/clinchem/47.4.673 [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–254. 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 19. Levey AS, Eckardt K-U, Dorman NM, Christiansen SL, Hoorn EJ, Ingelfinger JR, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) consensus conference. Kidney Int 2020;97:1117–1129. 10.1016/j.kint.2020.02.010 [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Eckardt K-U, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005;67:2089–2100. 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]
- 21. Miller WG, Kaufman HW, Levey AS, Straseski JA, Wilhelms KW, Yu HYE, et al. National Kidney Foundation Laboratory Engagement Working Group recommendations for implementing the CKD-EPI 2021 race-free equations for estimated glomerular filtration rate: practical guidance for clinical laboratories. Clin Chem 2022;68:511–520. 10.1093/clinchem/hvab278 [DOI] [PubMed] [Google Scholar]
- 22. Rowczenio DM, Noor I, Gillmore JD, Lachmann HJ, Whelan C, Hawkins PN, et al. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum Mutat 2014;35:E2403-12. 10.1002/humu.22619 [DOI] [PubMed] [Google Scholar]
- 23. Magdi M, Mostafa MR, Abusnina W, Al-Abdouh A, Doss R, Mohamed S, et al. A systematic review and meta-analysis of the prevalence of transthyretin amyloidosis in heart failure with preserved ejection fraction. Am J Cardiovasc Dis 2022;12:102–111. [PMC free article] [PubMed] [Google Scholar]
- 24. Chang ICY, Bois JP, Bois MC, Maleszewski JJ, Johnson GB, Grogan M. Hydroxychloroquine-mediated cardiotoxicity with a false-positive (99 m)technetium-labeled pyrophosphate scan for transthyretin-related cardiac amyloidosis. Circ Cardiovasc Imaging 2018;11:e007059. 10.1161/CIRCIMAGING.117.007059 [DOI] [PubMed] [Google Scholar]
- 25. Sawabe M, Hamamatsu A, Ito, Arai T, Ishikawa K, Chida K, et al. Early pathogenesis of cardiac amyloid deposition in senile systemic amyloidosis: close relationship between amyloid deposits and the basement membranes of myocardial cells. Virchows Arch 2003;442:252–257. 10.1007/s00428-003-0759-5 [DOI] [PubMed] [Google Scholar]
- 26. Razvi Y, Patel RK, Fontana M, Gillmore JD. Cardiac amyloidosis: a review of current imaging techniques. Front Cardiovasc Med 2021;8:751293. 10.3389/fcvm.2021.751293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ioannou A, Patel RK, Razvi Y, Porcari A, Knight D, Martinez-Naharro A, et al. Multi-imaging characterization of cardiac phenotype in different types of amyloidosis. JACC Cardiovasc Imaging. 2022:7–13. 10.1016/j.jcmg.2022.07.008 [DOI] [PubMed] [Google Scholar]
- 28. Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, et al. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol 2017;70:466–477. 10.1016/j.jacc.2017.05.053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.