Abstract
Aims
The effectiveness of sodium–glucose cotransporter 2 inhibitors (SGLT2i) in patients with heart failure (HF) in routine clinical practice is not extensively studied. This study aimed to evaluate the comparative effectiveness of SGLT2i vs. sitagliptin in older adults with HF and type 2 diabetes and to investigate whether there were any differences between agents within the SGLT2i class or for reduced and preserved ejection fraction.
Methods and results
Using Medicare claims data (April 2013 to December 2019), 16 253 SGLT2i initiators vs. 43 352 initiators of sitagliptin aged ≥65 years with type 2 diabetes and HF were included. The primary outcome was a composite of all-cause mortality, hospitalization for HF or urgent visit requiring intravenous diuretics; secondary outcomes included its individual components. Propensity score fine stratification weighted Cox regression was used to adjust for 100 pre-exposure characteristics. Mean age was 74 years; 49.8% were women. Initiation of SGLT2i vs. sitagliptin was associated with a lower risk of the primary composite outcome [adjusted hazard ratio (HR) 0.72; 95% confidence interval 0.67–0.77]. The adjusted HRs were 0.70 (0.63–0.78) for all-cause mortality, 0.64 (0.58–0.70) for hospitalization for HF, and 0.77 (0.69–0.86) for urgent visit requiring intravenous diuretics. Similar associations with the primary composite outcome were observed for all three agents within the SGLT2i class, for reduced and preserved ejection fraction, and subgroups based on demographics, comorbidities, and other HF treatments. Bias-calibrated HRs for the primary endpoint using negative and positive control outcomes ranged between 0.81 and 0.89, suggesting that the observed benefit could not be fully explained by residual confounding.
Conclusion
In routine US clinical practice, SGLT2i demonstrated robust clinical effectiveness in older adults with HF and type 2 diabetes compared with sitagliptin, with no evidence of heterogeneity across the SGLT2i class or across ejection fraction.
Keywords: Heart failure, Sodium–glucose cotransporter 2 inhibitors, Type 2 diabetes mellitus, Cohort studies, Cardiovascular diseases
Structured Graphical Abstract
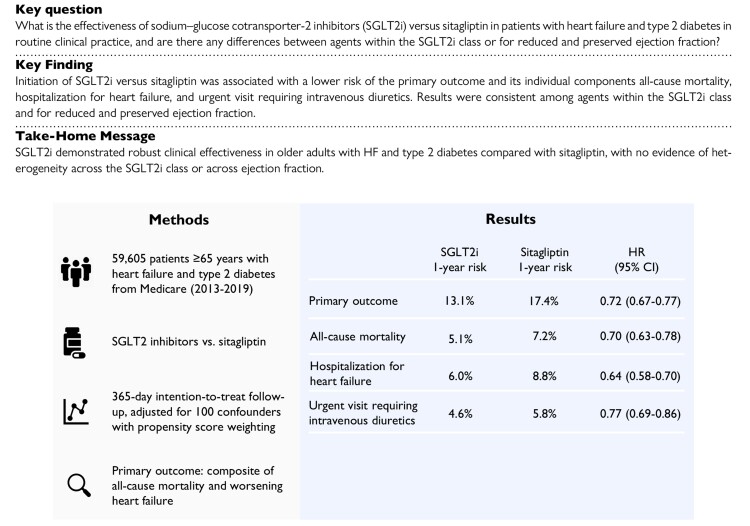
Structured Graphical Abstract.
This study investigated the effectiveness of SGLT2i vs. sitagliptin in patients with heart failure and type 2 diabetes in routine clinical practice. Initiation of SGLT2i vs. sitagliptin was associated with a lower risk of the primary outcome and its individual components all-cause mortality, hospitalization for heart failure, and urgent visit requiring intravenous diuretics, with no evidence of heterogeneity across the SGLT2i class or across ejection fraction. CI, confidence interval; HR, hazard ratio; SGLT2i, sodium–glucose cotransporter 2 inhibitors.
See the editorial comment for this article ‘SGLT2 inhibitors in heart failure and type 2 diabetes: from efficacy in trials towards effectiveness in the real world’, by N. Marx and D. Müller-Wieland, https://doi.org/10.1093/eurheartj/ehad282.
Introduction
Type 2 diabetes mellitus and heart failure (HF) are conditions that frequently coexist, with up to 22% of older adults with type 2 diabetes also having HF.1,2 Importantly, the presence of diabetes in HF patients is associated with reduced survival and higher hospitalization rates.3 Over half of patients with HF have a mildly reduced or preserved ejection fraction (HFmrEF/HFpEF),4,5 and their prevalence is increasing due to population aging and a rise in risk factors such as obesity and diabetes.6–8 Although morbidity and mortality in HFpEF may be as high as in HFrEF,9 disease-modifying treatment options in HFpEF have been limited.
Randomized controlled trials have shown that sodium–glucose cotransporter 2 inhibitors (SGLT2i) improve cardiovascular outcomes in patients with HFrEF, regardless of diabetes status,10,11 and SGLT2i are now strongly recommended as a cornerstone of medical therapy in these patients.12,13 Recently, the EMPEROR-Preserved and DELIVER trials showed that SGLT2i also improved cardiovascular outcomes in patients with HFpEF.14–16 A meta-analysis of five HF trials showed that SGLT2i reduced the composite of cardiovascular death or hospitalization for HF [hazard ratio (HR) 0.77; 95% confidence interval (CI) 0.72–0.82] compared with placebo, with consistent benefits across ejection fraction.17 Although these data provide strong evidence for the cardiovascular efficacy of SGLT2i in patients with HF, complementary data are needed to understand their cardiovascular effectiveness in a broad group of HF patients in routine clinical practice. Furthermore, it remains uncertain whether all agents within the SGLT2i class confer similar benefits in patients with HF.
We therefore used nationwide data from US Medicare beneficiaries to evaluate the effectiveness of SGLT2i in older adults with HF and type 2 diabetes in routine clinical practice. Our secondary aims were to investigate whether there were any differences between agents within the SGLT2i class or for reduced and preserved ejection fraction.
Methods
Data source
We used data from Medicare fee-for-service claims from April 2013 through December 2019. Medicare is a federal health insurance program and provides healthcare coverage for residents of the USA aged at least 65 years and older and patients aged <65 with disabilities. The database covers ∼50 million people and contains longitudinal information including patient demographics, inpatient and outpatient medical diagnoses and procedures, and prescription-dispensing records. Medicare Part A (inpatient coverage), Part B (outpatient coverage), and Part D (prescription medications) claims are available for research purposes through the Centers for Medicare & Medicaid Services (CMS). A signed use agreement with the CMS was available. This study was approved with waiver of informed consent by the Brigham and Women’s Hospital institutional review board (#2019P001953).
Study design and study population
An overview of the study design is given in Supplementary material online, Figure S1.18 We conducted an active comparator new user cohort study of patients who newly initiated an SGLT2i, i.e. canagliflozin, dapagliflozin, or empagliflozin, or the dipeptidyl peptidase-4 inhibitor (DPP4i) sitagliptin between 1 April 2013 (consistent with the release of the first SGLT2i in the USA) and 31 December 2019. New initiation was defined as a dispensation for SGLT2i or sitagliptin, with no previous dispensation of either drug in the previous 365 days. We chose sitagliptin as an active comparator to reduce confounding by indication,19 since clinical guidelines during the study period recommended both SGLT2i and DPP4i as second- or third-line glucose-lowering drugs.20,21 We explicitly chose sitagliptin since a large randomized controlled cardiovascular outcome trial has shown that sitagliptin does not influence the cardiovascular outcomes under study,22 whereas there have been some concerns for increased HF risks for saxagliptin.23–26 Specifically, the randomized TECOS trial including 14 671 patients found no differences between sitagliptin or placebo for hospitalization for HF (HR 1.00; 95% CI 0.83–1.20) or cardiovascular outcomes (HR 0.98; 95% CI 0.88–1.09).21 Furthermore, linagliptin is preferentially prescribed to patients with renal impairment since it does not require dose adjustments according to kidney function, which may lead to increased confounding compared with using sitagliptin.27,28 We did not use glucagon-like peptide-1 receptor agonists as an active comparator because they have been shown to lower the risk of all-cause mortality, cardiovascular mortality, and potentially HF.29 Glucagon-like peptide-1 receptor agonists would therefore not be a neutral comparator.
Eligible patients were required to have at least 12 months of continuous enrollment in Medicare Parts A, B, and D preceding the cohort entry date. Eligible individuals were required to be aged 65 years or older and have a diagnosis of type 2 diabetes in the year prior to cohort entry and a recorded diagnosis of HF within 6 months prior to the cohort entry date. We excluded individuals with a history of type 1 diabetes, secondary or gestational diabetes, HIV, organ transplant, end-stage kidney disease or dialysis, left ventricular assist device, missing age or sex, or a nursing home admission in the 12 months prior to cohort entry.
Drug exposure and follow-up
The study exposure was initiation of SGLT2i or sitagliptin. Follow-up began on the day after cohort entry and continued in a modified 365-day intention-to-treat approach until the earliest of outcome occurrence, death, Medicare disenrollment, 31 December 2019, or 365 days of follow-up, regardless of treatment discontinuation or switch. We chose a 365-day intention-to-treat follow-up rather than indefinite follow-up to account for the high discontinuation rate in routine clinical practice, which biases results toward the null.
Study outcomes
The primary outcome was a composite of all-cause mortality, hospitalization for HF (with HF code in primary position), or an emergency visit with HF where treatment with intravenous diuretics (furosemide, bumetanide, and torsemide) was administered. Secondary outcomes included the individual components of the primary composite endpoint. All-cause mortality was ascertained from claims data. A validation study showed that all-place all-cause mortality ascertained from Medicare claims data has excellent sensitivity (>99%) compared with the National Death Index (NDI).30
Covariates
Patient baseline characteristics were measured during the 365 days before cohort entry date. Based on subject matter knowledge and previous studies evaluating outcomes of medication use in older adults with HF and diabetes,31–33 we chose covariates that were associated with the outcome or represented proxy measurements for possible underlying confounders. Covariates of interest included (i) demographics including age, sex, race, and proxies of socioeconomic status (low-income subsidy receipt and a composite index34); (ii) comorbid conditions; (iii) diabetes-specific complications; (iv) use of HF and diabetes drugs; (v) use of other comedications; (vi) healthcare utilization markers (including number of hospitalizations, number of emergency department visits, number of cardiologist visits, and number of laboratory tests) as markers of overall health, healthcare access, surveillance, and intensity of care; (vii) healthy behavior markers, including use of screening services and vaccinations; and (viii) calendar year. To address potential confounding by frailty, we also adjusted for a claims-based frailty index.35 Patient characteristics were defined using ICD-9 or ICD-10 diagnosis or procedure codes, Current Procedural Terminology, 4th Edition procedure codes, and National Drug Code (pharmacy). A full list of all patient baseline characteristics is provided in Supplementary material online, Table S1.
Statistical analysis
We used propensity score (PS)-based fine stratification and weighting to adjust for confounding.36,37 We estimated the probability of receiving SGLT2i vs. sitagliptin as a function of 100 pre-exposure covariates (all covariates listed in Supplementary material online, Table S1) using a multivariable logistic regression model. After trimming the non-overlapping regions of the PS distribution to focus on individuals with probability to receive both treatments, 50 strata were created based on the PS distribution of the SGLT2i group. We weighted sitagliptin initiators proportional to the distribution of SGLT2i initiators in the PS stratum within which they fell. This type of weighting estimates an average treatment effect of the treated (ATT).38 Covariate balance before and after weighting was assessed using standardized mean differences.39,40 Post-weighting C statistics were reported as a measure of overall balance.41 Weighted cause-specific Cox proportional hazards regression models were used to estimate HRs with 95% CIs calculated using robust variance estimation to account for weighting. Furthermore, we estimated absolute risks and absolute risks differences between treatment groups using the Kaplan–Meier estimator for the primary endpoint and all-cause death and cumulative incidence functions for the other endpoints, which does not overestimate absolute risks in the presence of the competing risk of death.42 All analyses were performed using R version 4.1.3.
Secondary analysis: effectiveness of individual agents and stratification by ejection fraction
To investigate potential differences between agents within the SGLT2i class, we assessed the associations between canagliflozin, dapagliflozin, or empagliflozin vs. sitagliptin on the primary outcome. For this analysis, we constructed three separate cohorts, where each cohort was restricted to the dates when both drugs under comparison were on the market (i.e. April 2013 for canagliflozin vs. sitagliptin, January 2014 for dapagliflozin vs. sitagliptin, and August 2014 for empagliflozin vs. sitagliptin). In each of the three cohorts, we re-estimated the PS model and calculated HRs using PS fine stratification weighted Cox regression. We assessed heterogeneity between effect estimates using the Q statistic.
As Medicare claims do not contain ejection fraction measurements and ICD codes are non-specific for HF subtypes,43 we applied a validated claims-based prediction model to stratify our HF cohort into patients with predicted HFrEF and predicted HFpEF.44 This model predicts the probability of having HFrEF (defined as ejection fraction <45%) or HFpEF (defined as ejection fraction ≥45%) based on 35 variables, including demographics, comorbidities, and medications. The model was developed in a cohort of 11 073 HF patients for which Medicare claims were linked to electronic medical records containing ejection fraction measurements. The positive predictive value in the original cohort was 73% for HFrEF and 84% HFpEF, meaning that 73% of the patients classified as HFrEF by the algorithm truly had an ejection fraction <45% and 84% of patients classified as HFpEF truly had an ejection fraction ≥45%. An external validation study found virtually identical positive predictive values for HFrEF and HFpEF of 72% and 81%, respectively.45
Subgroup and additional analyses
We performed subgroup analyses in the following prespecified strata: age (65–74 vs. ≥75 years), sex, race, baseline chronic kidney disease, baseline atrial fibrillation, baseline angiotensin-converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor–neprilysin inhibitor (ACEi/ARB/ARNI) use, baseline mineralocorticoid receptor antagonist (MRA) use, and prior HF failure hospitalization within the previous 12 months. Within each subgroup, we re-estimated the PS, and fine stratification weighted Cox model was reperformed. We tested effect modification on a multiplicative scale by including an interaction term between treatment status and subgroup to the Cox model. In addition, we performed the following additional analyses: first, we applied an as-treated follow-up, which censored patients if and when they discontinued or switched treatment. This analysis was performed to avoid the exposure misclassification that often occurs in observational intention-to-treat analyses, which typically bias findings toward the null. However, this analysis assumes that discontinuation is non-informative. Discontinuation was defined as no prescription refill for the index exposure within the 30 days after the most recent prescription had ended. Second, we performed a ‘time of first statistical significance’ analysis to investigate how early the beneficial associations for SGLT2i were observed. To determine the time point when statistical significance was reached and maintained for the first time, Cox regression models were fitted and sequentially censored at increasing number of days since treatment initiation, yielding a continuous display of HRs with confidence bands. Third, we assessed a composite endpoint of cardiovascular death or hospitalization for HF, consistent with the primary endpoint of the EMPEROR-Reduced and EMPEROR-Preserved trials.11,14 In addition, we assessed a composite outcome of cardiovascular death and worsening HF (the primary endpoint of the DELIVER and DAPA-HF trials) and cardiovascular death separately (a key secondary endpoint of all these trials). We ascertained the exact cause of death through linkage with the NDI file. Since data on cause of death were available until December 2016, we restricted this analysis to the period April 2013 to December 2016. Fourth, we implemented a high-dimensional PS adjustment algorithm to adjust for 200 empirically identified confounding variables prioritized based on the Bross bias formula in addition to all prespecified covariates.46
Sensitivity analyses for unmeasured confounding: positive and negative control calibration
We conducted three bias calibration analyses in which we leveraged two negative control outcomes and one positive control outcome to adjust our HRs for residual bias due to unmeasured confounding and measurement error (detailed explanations are provided in Supplementary material online, Methods).47–49
Results
Study population
A total of 59 605 individuals were included in the analysis, of which 16 253 initiated SGLT2i and 43 352 initiated sitagliptin (see Supplementary material online, Figure S2). Before PS weighting, the SGLT2i group was younger and had less comorbidities and less healthcare use compared with the sitagliptin group (Table 1 and Supplementary material online, Table S1). After PS weighting, balance was achieved across treatment groups for all patient characteristics, with all standardized mean differences <0.10 and a post-weighting C statistic of 0.53. In the weighted cohort, median age was 73 years, 58% were men, and 83% were White. The most commonly used medications were statins (83%), ACEi (43%), ARB (39%), beta blockers (82%), and loop diuretics (66%). Metformin (49%), sulfonylureas (32%), and insulin (28%) were also commonly used. Supplementary material online, Figures S3 and S4 show the PS distributions before and after weighting, as well as the distribution of PS weights.
Table 1.
Selected patient characteristics in patients with HF and type 2 diabetes stratified by SGLT2i vs. sitagliptin initiation, before and after propensity score weighting
| Unweighted | Propensity score weighted | |||||
|---|---|---|---|---|---|---|
| SGLT2i | Sitagliptin | SMD | SGLT2i | Sitagliptin | SMD | |
| Total | 16 253 | 43 352 | 16 185 | 42 960 | ||
| Demographics | ||||||
| Age; median (IQR), mean (SD) | 73 (69–77) 74 (6) |
76 (71–82) 77 (7) |
−0.51 | 73 (69–77) 74 (6) |
73 (69–78) 74 (6) |
−0.01 |
| Men; n (%) | 9482 (58.3) | 20 992 (48.4) | 0.20 | 9426 (58.2) | 24 549 (57.1) | 0.02 |
| Race; n (%) | ||||||
| White | 13 433 (82.6) | 33 871 (78.1) | 0.11 | 13 371 (82.6) | 35 469 (82.6) | 0.00 |
| Black | 1273 (7.8) | 5098 (11.8) | −0.13 | 1272 (7.9) | 3310 (7.7) | 0.01 |
| Low-income subsidy recipients; n (%) | 5025 (30.9) | 16 212 (37.4) | −0.14 | 5014 (31) | 13 469 (31.4) | −0.01 |
| Socioeconomic status index; median (IQR), mean (SD) | 56 (51–63) 57 (7) |
56 (51–62) 56 (7) |
0.06 | 56 (51–63) 57 (7) |
56 (51–62) 57 (7) |
0.02 |
| Predicted HFpEF | 14 708 (90.5) | 39 529 (91.2) | −0.02 | 14 648 (90.5) | 39 096.82 (91) | −0.02 |
| Predicted HFrEF | 1545 (9.5) | 3823 (8.8) | 0.02 | 1537 (9.5) | 3863.18 (9) | 0.02 |
| Comorbidities; n (%) | ||||||
| Hypertension | 15 948 (98.1) | 42 477 (98) | 0.01 | 15 880 (98.1) | 42 126 (98.1) | 0.00 |
| Smoking | 6228 (38.3) | 15 775 (36.4) | 0.04 | 6196 (38.3) | 16 334 (38) | 0.01 |
| Obesity | 8707 (53.6) | 16 657 (38.4) | 0.31 | 8649 (53.4) | 22 857 (53.2) | 0.00 |
| Stable angina | 3358 (20.7) | 6854 (15.8) | 0.13 | 3335 (20.6) | 8905 (20.7) | 0.00 |
| Coronary revascularization | 1401 (8.6) | 3129 (7.2) | 0.05 | 1390 (8.6) | 3600 (8.4) | 0.01 |
| Ischemic stroke | 1585 (9.8) | 7081 (16.3) | −0.20 | 1582 (9.8) | 4251 (9.9) | 0.00 |
| Peripheral vascular disease | 5550 (34.1) | 16 452 (37.9) | −0.08 | 5525 (34.1) | 14 769 (34.4) | −0.01 |
| Atrial fibrillation | 6629 (40.8) | 19 257 (44.4) | −0.07 | 6600 (40.8) | 17 507 (40.8) | 0.00 |
| Hypertensive nephropathy | 3864 (23.8) | 15 828 (36.5) | −0.28 | 3859 (23.8) | 10 165 (23.7) | 0.00 |
| Acute kidney injury | 2485 (15.3) | 12 468 (28.8) | −0.33 | 2484 (15.3) | 6572 (15.3) | 0.00 |
| Chronic kidney disease stages 3 and 4 | 3972 (24.4) | 16 371 (37.8) | −0.29 | 3968 (24.5) | 10 372 (24.1) | 0.01 |
| Anemia | 5961 (36.7) | 20 996 (48.4) | −0.24 | 5944 (36.7) | 15 649 (36.4) | 0.01 |
| Chronic obstructive pulmonary disease | 5351 (32.9) | 16 266 (37.5) | −0.10 | 5333 (33) | 14 229 (33.1) | 0.00 |
| Burden of comorbidities | ||||||
| Frailty score; median (IQR), mean (SD) | 0.20 (0.17–0.23) 0.20 (0.04) |
0.21 (0.18–0.25) 0.22 (0.05) |
−0.30 | 0.20 (0.17–0.23) 0.20 (0.04) |
0.20 (0.18–0.23) 0.20 (0.04) |
0.00 |
| Combined comorbidity score; median (IQR), mean (SD) | 5 (4–7) 5.6 (2.6) |
6 (4–8) 6.2 (2.8) |
−0.23 | 5 (4–7) 5.6 (2.6) |
5 (4–7) 5.6 (2.5) |
0.00 |
| Diabetes-related conditions; n (%) | ||||||
| Diabetic nephropathy | 4682 (28.8) | 12 915 (29.8) | −0.02 | 4661 (28.8) | 12 149 (28.3) | 0.01 |
| Diabetes with peripheral circulatory disorders | 3105 (19.1) | 7155 (16.5) | 0.07 | 3089 (19.1) | 8121 (18.9) | 0.00 |
| Diabetic neuropathy | 6320 (38.9) | 13 946 (32.2) | 0.14 | 6272 (38.8) | 16 831 (39.2) | −0.01 |
| Diabetic retinopathy | 515 (3.2) | 2442 (5.6) | −0.12 | 515 (3.2) | 1352 (3.1) | 0.00 |
| Hyperglycemia | 8211 (50.5) | 15 602 (36) | 0.30 | 8157 (50.4) | 21 366 (49.7) | 0.01 |
| Heart failure medications; n (%) | ||||||
| ACEi | 7051 (43.4) | 19 611 (45.2) | −0.04 | 7024 (43.4) | 18 569 (43.2) | 0.00 |
| ARB | 6266 (38.6) | 15 335 (35.4) | 0.07 | 6235 (38.5) | 16 540 (38.5) | 0.00 |
| ARNI | 899 (5.5) | 654 (1.5) | 0.22 | 881 (5.4) | 2346 (5.5) | 0.00 |
| Mineralocorticoid receptor antagonist | 3562 (21.9) | 7895 (18.2) | 0.09 | 3536 (21.8) | 9339 (21.7) | 0.00 |
| Beta blockers | 13 273 (81.7) | 34 898 (80.5) | 0.03 | 13 215 (81.6) | 35 059 (81.6) | 0.00 |
| Digoxin | 1630 (10) | 5350 (12.3) | −0.07 | 1628 (10.1) | 4386 (10.2) | 0.00 |
| Loop diuretics | 10 692 (65.8) | 30 477 (70.3) | −0.10 | 10 652 (65.8) | 28 106 (65.4) | 0.01 |
| Nitrates | 4119 (25.3) | 11 111 (25.6) | −0.01 | 4095 (25.3) | 10 900 (25.4) | 0.00 |
| Diabetes medications; n (%) | ||||||
| Number of antidiabetic drugs at cohort entry; median (IQR), mean (SD) | 1 (1–2) 1.3 (0.9) |
1 (0–1) 1.0 (0.8) |
0.34 | 1 (1–2) 1.3 (0.9) |
1 (1–2) 1.3 (0.9) |
0.00 |
| Concomitant initiation or current use of metformin | 8024 (49.4) | 17 304 (39.9) | 0.19 | 7977 (49.3) | 21 011 (48.9) | 0.01 |
| Concomitant initiation or current use of sulfonylureas | 5011 (30.8) | 15 773 (36.4) | −0.12 | 5005 (30.9) | 13 758 (32) | −0.02 |
| Concomitant initiation or current use of GLP-1 receptor agonists | 1845 (11.4) | 497 (1.1) | 0.43 | 1777 (11) | 4454 (10.4) | 0.02 |
| Concomitant initiation or current use of insulin | 4734 (29.1) | 6273 (14.5) | 0.36 | 4676 (28.9) | 12 135 (28.2) | 0.01 |
| Baseline hospitalizations in previous year; n (%) | ||||||
| For heart failure | ||||||
| Zero | 14 710 (90.5) | 37 025 (85.4) | 0.16 | 14 651 (90.5) | 38 908 (90.6) | 0.00 |
| One | 1250 (7.7) | 4988 (11.5) | −0.13 | 1243 (7.7) | 3269 (7.6) | 0.00 |
| Two | 227 (1.4) | 1000 (2.3) | −0.07 | 227 (1.4) | 615 (1.4) | 0.00 |
| Three or more | 66 (0.4) | 339 (0.8) | −0.05 | 64 (0.4) | 169 (0.4) | 0.00 |
| For other reasons | ||||||
| Zero | 10 842 (66.7) | 23 245 (53.6) | 0.16 | 10 789 (66.7) | 28 840 (67.1) | −0.01 |
| One | 3505 (21.6) | 11 793 (27.2) | −0.13 | 3493 (21.6) | 9213 (21.4) | 0.00 |
| Two | 1236 (7.6) | 5031 (11.6) | −0.07 | 1234 (7.6) | 3118 (7.3) | 0.01 |
| Three or more | 670 (4.1) | 3283 (7.6) | −0.05 | 669 (4.1) | 1789 (4.2) | 0.00 |
| Healthcare utilization markers | ||||||
| Emergency room visits; median (IQR), mean (SD) | 1 (0–2) 1.3 (2.0) |
1 (0–2) 1.7 (2.4) |
−0.21 | 1 (0–2) 1.3 (2.0) |
1 (0–2) 1.3 (1.9) |
0.00 |
| Cardiologist visits; median (IQR), mean (SD) | 4 (1–8) 6.2 (7.8) |
4 (2–9) 6.9 (8.3) |
−0.08 | 4 (1–8) 6.2 (7.8) |
4 (1–8) 6.1 (7.5) |
0.02 |
| Endocrinologist visits; median (IQR), mean (SD) | 0 (0–0) 0.9 (2.1) |
0 (0–0) 0.5 (1.9) |
0.17 | 0 (0–0) 0.9 (2.1) |
0 (0–0) 0.8 (2.1) |
0.03 |
| Internal medicine visits; median (IQR), mean (SD) | 9 (5–15) 11.3 (9.8) |
11 (6–18) 14.1 (14.4) |
−0.23 | 9 (5–15) 11.3 (9.8) |
9 (5–15) 11.5 (10.6) |
−0.01 |
| Nephrologist visits; median (IQR), mean (SD) | 0 (0–0) 0.4 (2.0) |
0 (0–0) 1.3 (6.1) |
−0.19 | 0 (0–0) 0.4 (2.0) |
0 (0–0) 0.4 (2.3) |
0.00 |
| Healthy behavior markers; n (%) | ||||||
| Colonoscopy | 1527 (9.4) | 3840 (8.9) | 0.02 | 1519 (9.4) | 3939 (9.2) | 0.01 |
| Fecal occult blood test | 825 (5.1) | 2316 (5.3) | −0.01 | 822 (5.1) | 2147 (5) | 0.00 |
| Flu vaccination | 9634 (59.3) | 17 947 (41.4) | 0.36 | 9583 (59.2) | 25 616 (59.6) | −0.01 |
| Mammography | 2000 (12.3) | 3236 (7.5) | 0.16 | 1996 (12.3) | 5365 (12.5) | 0.00 |
| Pneumococcal vaccine | 3127 (19.2) | 7339 (16.9) | 0.06 | 3113 (19.2) | 8058 (18.8) | 0.01 |
| Prostate specific antigen test | 2974 (18.3) | 6411 (14.8) | 0.09 | 2950 (18.2) | 7831 (18.2) | 0.00 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; COPD, chronic obstructive pulmonary disease; HbA1c, hemoglobin A1c; IQR, interquartile range; n, number of patients; No., number of; NSAID, non-steroidal anti-inflammatory drug; SGLT2i, sodium–glucose cotransporter 2 inhibitor; SD, standard deviation; SMD, standardized mean difference.
Effectiveness of sodium–glucose cotransporter 2 inhibitors vs. sitagliptin
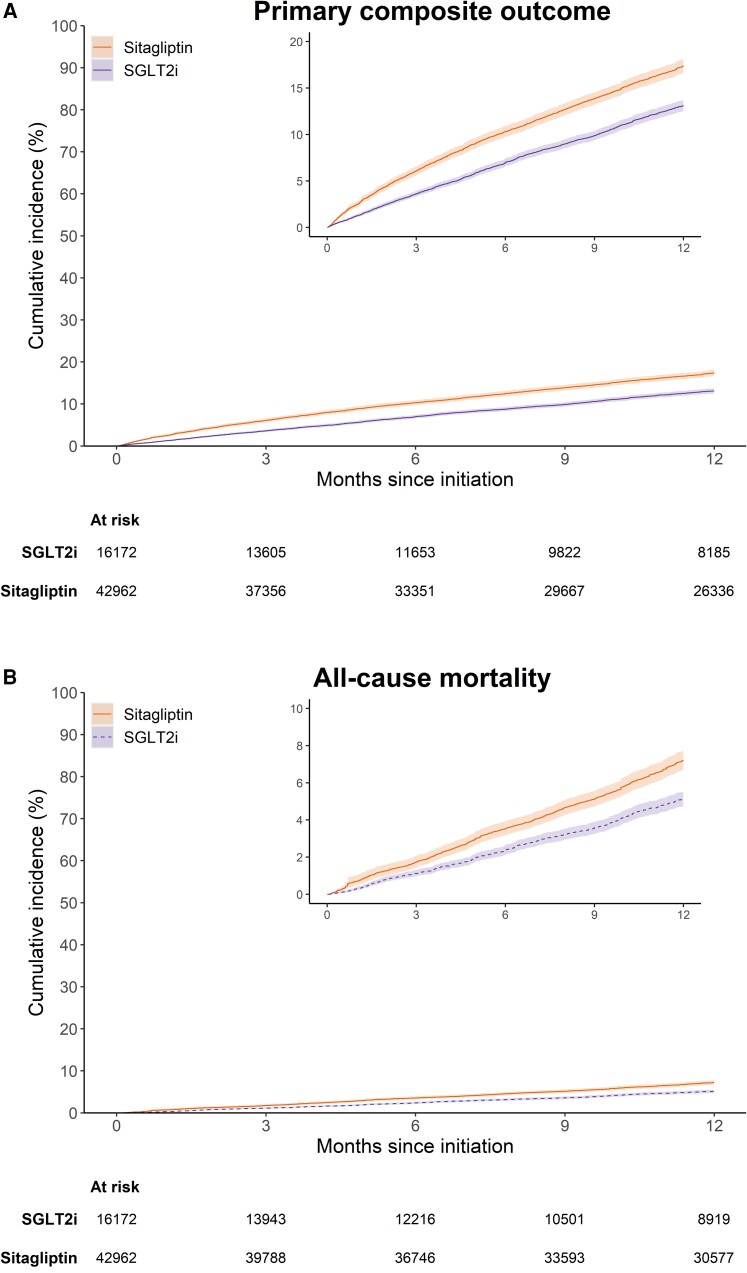
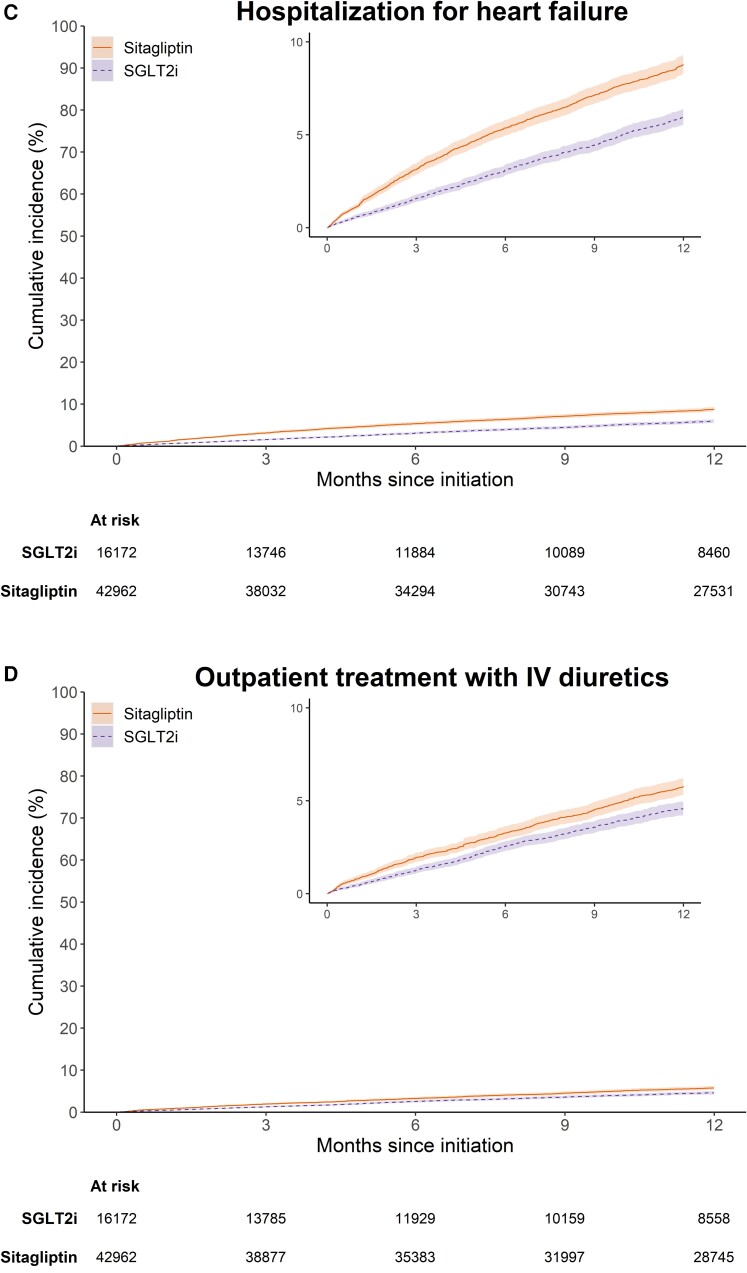
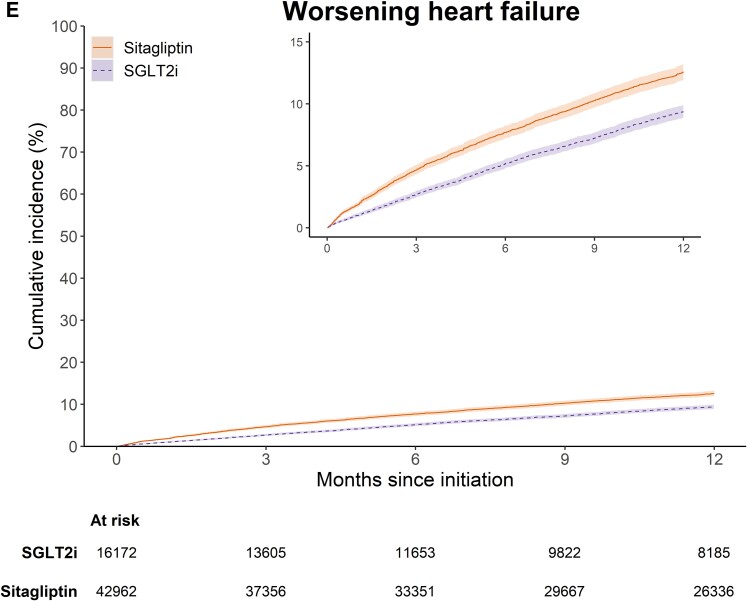
After PS weighting, the mean and median follow-ups for the primary composite outcome were 9.3 and 12 months (interquartile range 6.4–12 months). Weighted cumulative incidence curves for the primary composite outcome and its components are shown in Figure 1. The curves for the primary composite outcome and hospitalization for HF separated early and remained parallel from 6 months of follow-up onward (Figure 1A and C). The 1-year weighted cumulative incidence for the primary composite endpoint was 13.1% (95% CI 12.6%–13.8%) in the SGLT2i group and 17.4% (95% CI 16.7%–18.2%) in the sitagliptin group, with an absolute risk difference of −4.3% (95% CI −5.2%, −3.3%) (see Supplementary material online, Table S2). Among individual components, compared with sitagliptin, the 1-year cumulative incidence for SGLT2i was 2.1% (95% CI 1.4%–2.7%) lower for all-cause mortality, 2.8% (2.2%–3.5%) lower for HF hospitalization, and 1.2% (0.6%–1.8%) lower for urgent treatment with intravenous diuretics. Table 2 shows the number of events, incidence rates, and HRs before and after PS weighting. After PS weighting, the HRs (95% CI) were 0.72 (0.67–0.77) for the primary composite endpoint, 0.70 (0.63–0.78) for all-cause mortality, 0.64 (0.58–0.70) for hospitalization for HF, and 0.77 (0.69–0.86) for urgent visit requiring intravenous diuretics.
Figure 1.
Weighted cumulative incidence for (A) the primary composite outcome of all-cause mortality or worsening heart failure, (B) all-cause mortality, (C) heart failure hospitalization, (D) treatment with intravenous diuretics in outpatient setting, and (E) worsening heart failure under 365-day intention-to-treat follow-up, stratified by sodium–glucose cotransporter 2 inhibitors or sitagliptin initiation.
Table 2.
Comparative outcomes in patients with HF and type 2 diabetes initiating SGLT2i vs. sitagliptin under 365-day intention-to-treat follow-up
| Exposure group | Unweighted | PS-weighted | ||
|---|---|---|---|---|
| SGLT2i | Sitagliptin | SGLT2i | Sitagliptin | |
| Sample size | 16 253 | 43 352 | 16 172 | 42 962 |
| Primary composite endpoint of all-cause mortality or worsening HF | ||||
| Total events | 1672 | 9787 | 1667 | 6204 |
| Follow-up, person-years | 11 815 | 33 903 | 11 780 | 31 314 |
| Incidence rate (95% CI)/100 person-years | 14.2 (13.5–14.8) | 28.9 (28.3–29.4) | 14.2 (13.5–14.8) | 19.8 (19.3–20.3) |
| HR (95% CI) | 0.49 (0.46–0.51) | Ref | 0.72 (0.67–0.77) | Ref |
| Single components of the primary composite endpoint | ||||
| All-cause mortality | ||||
| Total events | 637 | 4769 | 636 | 2468 |
| Follow-up, person-years | 12 316 | 37 018 | 12 280 | 33 384 |
| Incidence rate (95% CI)/100 person-years | 5.2 (4.8–5.6) | 12.9 (12.5–13.3) | 5.2 (4.8–5.6) | 7.4 (7.1–7.7) |
| HR (95% CI) | 0.40 (0.37–0.44) | Ref | 0.70 (0.63–0.78) | Ref |
| Hospitalization for heart failure | ||||
| Total events | 755 | 4989 | 752 | 3147 |
| Follow-up, person-years | 12 015 | 34 769 | 11 980 | 32 014 |
| Incidence rate (95% CI)/100 person-years | 6.3 (5.8–6.7) | 14.3 (14.0–14.8) | 6.3 (5.8–6.7) | 9.8 (9.5–10.2) |
| HR (95% CI) | 0.43 (0.40–0.47) | Ref | 0.64 (0.58–0.70) | Ref |
| Urgent visit requiring intravenous diuretics | ||||
| Total events | 584 | 2645 | 583 | 2041 |
| Follow-up, person-years | 12 068 | 35 774 | 12 033 | 32 431 |
| Incidence rate (95% CI)/100 person-years | 4.8 (4.5–5.2) | 7.4 (7.1–7.7) | 4.8 (4.5–5.3) | 6.3 (6.0–6.6) |
| HR (95% CI) | 0.65 (0.59–0.71) | Ref | 0.77 (0.69–0.86) | Ref |
CI, confidence interval; HF, heart failure; HR, hazard ratio; PS, propensity score; SGLT2i, sodium–glucose cotransporter 2 inhibitors. Hazard ratios are shown in bold.
Effectiveness of canagliflozin, empagliflozin, and dapagliflozin vs. sitagliptin
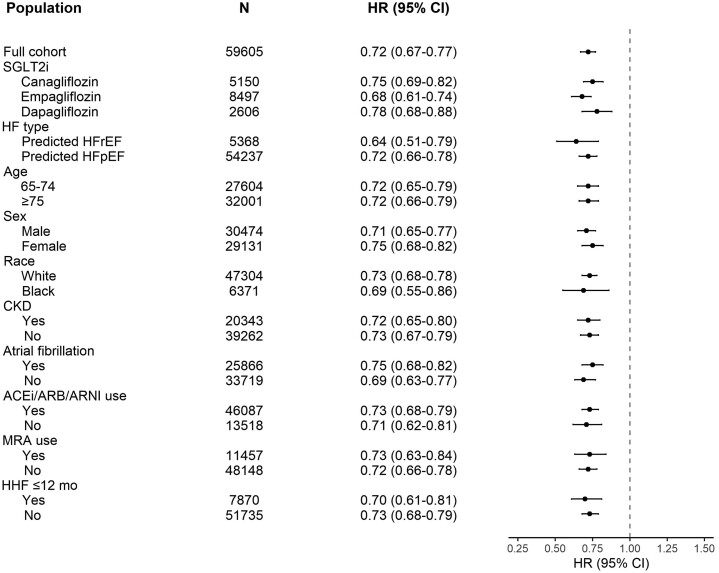
Among 16 253 individuals initiating SGLT2i, 5150 (31.7%) initiated canagliflozin, 8497 (52.3%) empagliflozin, and 2606 (16.0%) dapagliflozin. The weighted HRs (95% CI) for the primary composite endpoint were 0.75 (0.69–0.82) for canagliflozin, 0.68 (0.61–0.74) for empagliflozin, and 0.78 (0.68–0.88) for dapagliflozin, with a P-value for heterogeneity of 0.36 (Table 3 and Figure 2).
Table 3.
Comparative outcomes for the primary composite endpoint in patients with HF and type 2 diabetes initiating canagliflozin, empagliflozin, or dapagliflozin vs. sitagliptin under 365-day intention-to-treat follow-up
| Exposure group | Unweighted | Propensity score weighted | ||
|---|---|---|---|---|
| Canagliflozin | Sitagliptin | Canagliflozin | Sitagliptin | |
| Sample size | 5150 | 43 352 | 5144 | 43 098 |
| Total events | 633 | 9787 | 633 | 6936 |
| Follow-up, person-years | 4432 | 33 903 | 4426 | 36 361 |
| Incidence rate (95% CI)/100 person-years | 14.3 (13.2–15.4) | 28.9 (28.3–29.4) | 14.3 (13.2–15.5) | 19.1 (18.6–19.5) |
| HR (95% CI) | 0.50 (0.46–0.54) | Ref | 0.75 (0.69–0.82) | Ref |
| Exposure group | Empagliflozin | Sitagliptin | Empagliflozin | Sitagliptin |
| Sample size | 8497 | 43 352 | 8452 | 42 025 |
| Total events | 765 | 9787 | 763 | 5676 |
| Follow-up, person-years | 5505 | 33 903 | 5485 | 27 564 |
| Incidence rate (95% CI)/100 person-years | 13.9 (12.9–14.9) | 28.9 (28.3–29.4) | 13.9 (12.9–14.9) | 20.6 (20.1–21.1) |
| HR (95% CI) | 0.47 (0.43–0.50) | Ref | 0.68 (0.61–0.74) | Ref |
| Exposure group | Dapagliflozin | Sitagliptin | Dapagliflozin | Sitagliptin |
| Sample size | 2606 | 43 352 | 2596 | 41 747 |
| Total events | 274 | 9787 | 273 | 5814 |
| Follow-up, person-years | 1877 | 33 903 | 1873 | 30 981 |
| Incidence rate (95% CI)/100 person-years | 14.6 (12.9–16.4) | 28.9 (28.3–29.4) | 14.6 (12.9–16.4) | 18.8 (18.3–19.3) |
| HR (95% CI) | 0.50 (0.44–0.56) | Ref | 0.78 (0.68–0.88) | Ref |
CI, confidence interval; HR, hazard ratio.
Figure 2.
Adjusted hazard ratios for the subgroup analyses for the primary composite outcome of all-cause mortality or worsening heart failure under 365-day intention-to-treat follow-up.
Effectiveness of sodium–glucose cotransporter 2 inhibitors in predicted heart failure with reduced ejection fraction and heart failure with preserved ejection fraction
The prediction model classified 5368 patients as HFrEF and 54 237 patients as HFpEF (Tables 4 and 5). For predicted HFrEF, the weighted HRs (95% CI) for SGLT2i vs. sitagliptin were 0.64 (0.51–0.79) for the primary composite outcome, 0.76 (0.56–1.02) for all-cause mortality, 0.65 (0.50–0.83) for hospitalization for HF, and 0.60 (0.42–0.84) for urgent visit requiring intravenous diuretics. For predicted HFpEF, the weighted HRs were 0.72 (0.66–0.78), 0.70 (0.63–0.79), 0.64 (0.58–0.71), and 0.80 (0.71–0.90), respectively (Tables 4 and 5 and Figure 2).
Table 4.
Comparative outcomes in patients with predicted HFrEF and type 2 diabetes initiating SGLT2i vs. sitagliptin under 365-day intention-to-treat follow-up
| Exposure group | Unweighted | Propensity score weighted | ||
|---|---|---|---|---|
| SGLT2i | Sitagliptin | SGLT2i | Sitagliptin | |
| Sample size | 1545 | 3823 | 1511 | 3755 |
| Primary composite endpoint of all-cause mortality or worsening HF | ||||
| Total events | 156 | 941 | 154 | 600 |
| Follow-up, person-years | 1042 | 2728 | 1027 | 2539 |
| Incidence rate (95% CI)/100 person-years | 15.0 (12.7–17.5) | 34.5 (32.3–36.8) | 15.0 (12.7–17.6) | 23.6 (21.8–25.6) |
| HR (95% CI) | 0.43 (0.36–0.50) | Ref | 0.64 (0.51–0.79) | Ref |
| Single components of the primary composite endpoint | ||||
| All-cause mortality | ||||
| Total events | 71 | 578 | 71 | 244 |
| Follow-up, person-years | 1098 | 3158 | 1083 | 2812 |
| Incidence rate (95% CI)/100 person-years | 6.5 (5.1–8.2) | 18.3 (16.8–19.9) | 6.6 (5.1–8.3) | 8.7 (7.6–9.8) |
| HR (95% CI) | 0.35 (0.27–0.45) | Ref | 0.76 (0.56–1.02) | Ref |
| Hospitalization for heart failure | ||||
| Total events | 112 | 767 | 110 | 431 |
| Follow-up, person-years | 1059 | 2827 | 1044 | 2638 |
| Incidence rate (95% CI)/100 person-years | 10.6 (8.7–12.7) | 27.1 (25.2–29.1) | 10.5 (8.7–12.7) | 16.3 (14.8–18.0) |
| HR (95% CI) | 0.38 (0.31–0.47) | Ref | 0.65 (0.50–0.83) | Ref |
| Urgent visit requiring intravenous diuretics | ||||
| Total events | 64 | 334 | 64 | 273 |
| Follow-up, person-years | 1074 | 3001 | 1059 | 2683 |
| Incidence rate (95% CI)/100 person-years | 6.0 (4.6–7.6) | 11.1 (10.0–12.4) | 6.0 (4.7–7.7) | 10.2 (9.0–11.5) |
| HR (95% CI) | 0.52 (0.40–0.68) | Ref | 0.60 (0.42–0.84) | Ref |
CI, confidence interval; HF, heart failure; HR, hazard ratio; SGLT2i, sodium–glucose cotransporter 2 inhibitors.
Table 5.
Comparative outcomes in patients with predicted HFpEF and type 2 diabetes initiating SGLT2i vs. sitagliptin under 365-day intention-to-treat follow-up
| Exposure group | Unweighted | Propensity score weighted | ||
|---|---|---|---|---|
| SGLT2i | Sitagliptin | SGLT2i | Sitagliptin | |
| Sample size | 14 708 | 39 529 | 14 641 | 39 198 |
| Primary composite endpoint of all-cause mortality or worsening HF | ||||
| Total events | 1045 | 5712 | 1042 | 3887 |
| Follow-up, person-years | 10 772 | 31 175 | 10 743 | 28 776 |
| Incidence rate (95% CI)/100 person-years | 9.7 (9.1–10.3) | 18.3 (17.9–18.8) | 9.7 (9.1–10.3) | 13.5 (13.1–13.9) |
| HR (95% CI) | 0.52 (0.49–0.56) | Ref | 0.72 (0.66–0.78) | Ref |
| Single components of the primary composite endpoint | ||||
| All-cause mortality | ||||
| Total events | 566 | 4191 | 566 | 2199 |
| Follow-up, person-years | 11 218 | 33 860 | 11 188 | 30 561 |
| Incidence rate (95% CI)/100 person-years | 5.0 (4.6–5.5) | 12.4 (12.0–12.8) | 5.1 (4.7–5.5) | 7.2 (7.0–7.5) |
| HR (95% CI) | 0.41 (0.37–0.45) | Ref | 0.70 (0.63–0.79) | Ref |
| Hospitalization for heart failure | ||||
| Total events | 643 | 4222 | 641 | 2689 |
| Follow-up, person-years | 10 956 | 31 942 | 10 926 | 29 374 |
| Incidence rate (95% CI)/100 person-years | 5.9 (5.4–6.3) | 13.2 (12.8–13.6) | 5.9 (5.4–6.3) | 9.2 (8.8–9.5) |
| HR (95% CI) | 0.44 (0.40–0.48) | Ref | 0.64 (0.58–0.71) | Ref |
| Urgent visit requiring intravenous diuretics | ||||
| Total events | 520 | 2311 | 519 | 1761 |
| Follow-up, person-years | 10 994 | 32 773 | 10 964 | 29 743 |
| Incidence rate (95% CI)/100 person-years | 4.7 (4.3–5.2) | 7.1 (6.8–7.3) | 4.7 (4.3–5.2) | 5.9 (5.6–6.2) |
| HR (95% CI) | 0.66 (0.60–0.73) | Ref | 0.80 (0.71–0.90) | Ref |
CI, confidence interval; HF, heart failure; HR, hazard ratio; SGLT2i, sodium–glucose cotransporter 2 inhibitors.
Subgroup and additional analyses
Sodium–glucose cotransporter 2 inhibitors were associated with lower HRs for the primary composite endpoint within all subgroups of age (65–74 vs. ≥75 years), sex, race, chronic kidney disease, atrial fibrillation, ACEi/ARB/ARNI use, MRA use, or hospitalization for HF in the previous year (Figure 2).
Results were consistent in as-treated analyses, although HRs were stronger. The weighted HR was 0.59 (0.55–0.64) for the primary composite endpoint, 0.59 (0.52–0.68) for all-cause mortality, and 0.51 (0.46–0.57) for HF hospitalization (see Supplementary material online, Table S3). The weighted cumulative incidence curves for the primary composite endpoint and HF hospitalization diverged early during follow-up and stayed parallel after 12 months (see Supplementary material online, Figure S5). The early benefit was confirmed in the analysis of time to first statistical significance, with statistical significance for the primary endpoint achieved for the first time and maintained on day 5 of follow-up (see Supplementary material online, Figure S6). Consistent with the primary endpoint, we observed a lower HR for the composite endpoint of cardiovascular death or HF hospitalization (HR 0.71; 95% CI 0.61–0.81), cardiovascular death or worsening HF (0.77; 0.69–0.87), and cardiovascular death (0.79; 0.62–1.00) in the NDI-linked Medicare data (see Supplementary material online, Table S4 and Figure S7). Hazard ratios were consistent when using a high-dimensional PS (HR 0.72; 95% CI 0.67–0.77). The HR (95% CI) for non-cardiovascular death was 0.81 (0.65–1.01) (see Supplementary material online, Table S5). Bias-calibrated HRs (i.e. adjusted for residual bias) when using the negative control outcome non-cardiovascular death were 0.89 (0.72–1.11) for the primary endpoint, 0.87 (0.71–1.10) for all-cause mortality, and 0.78 (0.62–0.99) for HF hospitalization. When using ischemic stroke as negative control, bias-calibrated HRs were 0.86 (0.67–1.10) for the primary endpoint, 0.84 (0.65–1.09) for all-cause death, and 0.77 (0.60–0.99) for HF hospitalization. Similar results were obtained when using the positive control outcome HF hospitalization (see Supplementary material online, Table S5).
Discussion
Main findings
In this large nationwide study of older US Medicare beneficiaries with HF and type 2 diabetes, we found that SGLT2i use was associated with a reduced risk of the primary composite endpoint of all-cause mortality and worsening HF compared with sitagliptin, as well as a reduced risk of its individual components (Structured Graphical Abstract). Benefits appeared to be consistent across individual agents within the SGLT2i class (canagliflozin, dapagliflozin, and empagliflozin) and across predicted ejection fraction status. Furthermore, associations were similar across subgroups of demographics, comorbidities, and baseline HF medications.
Implications
Our study has important clinical implications. The number of HF patients is expected to increase due to population aging and an increase in associated cardio–renal–metabolic risk factors. Our results suggest that the cardiovascular efficacy of SGLT2i observed in controlled trial settings is consistent in the broad group of patients with HF and type 2 diabetes from routine clinical practice.
Interpretation and comparison with other studies
A number of randomized trials have assessed the effects of SGLT2i on cardiovascular outcomes in patients with HF, with or without diabetes. A recent meta-analysis pooled data from five randomized controlled trials of SGLT2i (EMPEROR-Reduced, EMPEROR-Preserved, DAPA-HF, DELIVER, and SOLOIST-WHF) and found that SGLT2i reduced the hazard of cardiovascular death or hospitalization for HF (pooled HR 0.77; 95% CI 0.72–0.82);17 this effect was identical in patients with type 2 diabetes (0.77; 0.70–0.84). Furthermore, SGLT2i also reduced the risk of hospitalization for HF (HR 0.72; 95% CI 0.67–0.78), cardiovascular death (0.87; 0.79–0.95), and all-cause mortality (0.92; 0.86–0.99). In accordance with these findings, we observed that SGLT2i use was associated with a reduction of the composite endpoint of all-cause mortality, hospitalization for HF, or urgent visit requiring intravenous diuretics in our study of patients with HF and type 2 diabetes (HR 0.72; 95% CI 0.67–0.77). We also found reductions in hospitalization for HF, cardiovascular death, and all-cause death. The early benefit of SGLT2i driven by an early reduction in hospitalization for HF is noteworthy, with statistically significant results at Day 5 of follow-up. This rapid benefit is consistent with evidence from several other SGLT2i trials.10,14,15,50–53
In line with the meta-analysis (which only included dapagliflozin and empagliflozin),17 we found consistent benefit across agents within the SGLT2i class, including canagliflozin. Moreover, results were consistent for predicted HFrEF and HFpEF. However, the mortality benefit observed in our study was stronger than the pooled estimate from the meta-analysis, especially for HFpEF. Whereas EMPEROR-Preserved and DELIVER showed no benefit for all-cause death (pooled HR 0.97; 95% CI 0.88–1.06), our study found a HR of 0.70 (95% CI 0.63–0.79). The strong mortality signal in this subgroup of our Medicare-based study is suggestive of residual confounding, although our estimates showed a benefit even after adjusting for the remaining unmeasured confounding. It is noteworthy that mortality benefits have been observed in the EMPA-REG OUTCOME trial of patients with type 2 diabetes (HR 0.68; 95% CI 0.57–0.82), the DAPA-CKD trial of patients with chronic kidney disease (HR 0.69; 95% CI 0.53–0.88),51,54 and the DAPA-HF trial of patients with HFrEF (HR 0.83; 95% CI 0.71–0.97).10 Furthermore, another meta-analysis that also included cardiovascular outcome and kidney trials showed that SGLT2i reduced all-cause mortality (HR 0.85; 95% CI 0.78–0.92; 95% prediction interval 0.68–1.05) and cardiovascular mortality (HR 0.84; 95% CI 0.77–0.93; 95% prediction interval 0.66–1.09), although effects were heterogeneous between individual agents and across different populations.55
Lastly, two prior small observational studies have investigated the associations between SGLT2i and cardiovascular outcomes. Becher et al.56 analyzed 6805 patients from the Swedish Heart Failure Registry, of which 376 (5.5%) received SGLT2i. In this analysis, SGLT2i users had a lower risk of cardiovascular death/hospitalization for HF (HR 0.70; 95% CI 0.52–0.95), which was consistent regardless of ejection fraction. Furthermore, Lam et al.57 compared 5307 SGLT2i inhibitor users with 5307 users of other glucose-lowering drugs from Israel who had type 2 diabetes and available ejection fraction measurements. They observed that SGLT2i were associated with lower risk of HF hospitalization or death (HR 0.57; 95% CI 0.46–0.70), regardless of ejection fraction.
Our findings need to be interpreted in light of the chosen comparator, the DPP4i sitagliptin. There is experimental evidence that DPP4i potentiate the effects of stromal cell-derived factor 1 (SDF-1), enhancing cardiac fibrosis.58,59 The SAVOR-TIMI 53 trial found that the DPP4i saxagliptin was associated with an increased risk of HF compared with placebo (HR 1.27; 95% CI 1.07–1.51).23 However, we specifically chose sitagliptin as comparator in our study because the randomized TECOS trial has not found any differences between sitagliptin or placebo for hospitalization for HF (HR 1.00; 95% CI 0.83–1.20) or cardiovascular outcomes (HR 0.98; 95% CI 0.88 to 1.09),22 which support the notion that sitagliptin’s effects on outcomes are neutral.
Strengths and limitations of the study
Strengths of our study include its active comparator new user design, nationwide nature, large sample size, and adjustment for a large number of potential confounders. Furthermore, we tested the robustness of our findings in several supplemental analyses. The Medicare data set was also suited to address our research question as the majority of HF patients in the USA receive health insurance through the Medicare program.60 We also used a validated predictive algorithm to differentiate between patients with HFrEF or HFpEF.44,45 This prediction model has superior performance compared with diagnostic codes alone, has high positive predictive value (72% and 81% for HFrEF and HFpEF, respectively), and has been shown to accurately replicate patient characteristics and mortality incidence of HFrEF and HFpEF.44,45,61
Our study also has limitations. First, we cannot rule out potential residual confounding. Indeed, our bias calibration sensitivity analysis using two negative control outcomes (non-cardiovascular death and ischemic stroke) and one positive control outcome (hospitalization for HF) indicated that some bias remained after adjustment for measured confounders. Nevertheless, our estimates showed a benefit for SGLT2i even after adjusting for the remaining net bias (point estimates for the primary outcome after bias calibration ranged between 0.81 and 0.89), indicating that residual confounding cannot fully explain away the observed beneficial effects of SGLT2i. All three control outcomes may have had different unmeasured confounders but led to similar corrected point estimates, and this triangulation therefore adds further robustness to our findings.62 Second, we lacked ejection fraction measurements and used a prediction model to identify the HF subtype. Our model may have misclassified a proportion of patients. Third, our study had a relatively short follow-up. Future studies should investigate the long-term effects of SGLT2i in patients with HF. Nevertheless the beneficial effects of SGLT2i on HF hospitalization are immediately observed and strongest within the first 6 months of follow-up.15,63 Fourth, our study included only patients with type 2 diabetes due to the limited number of patients with HF without concomitant diabetes. Lastly, causes of death were not adjudicated in our study, and there may be misclassification between cardiovascular and non-cardiovascular death.64
Conclusion
In routine US clinical practice, SGLT2i demonstrated robust clinical effectiveness with respect to the composite outcome indicative of worsening HF in older adults with HF and comorbid type 2 diabetes compared with sitagliptin, without apparent heterogeneity across the class. These observational data suggest that SGLT2i prevent adverse HF events in a broad range of patients with HF and type 2 diabetes.
Supplementary data
Supplementary data are available at European Heart Journal online.
Supplementary Material
Contributor Information
Edouard L Fu, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 1620 Tremont St., BC-3030, Boston, MA 02120, USA.
Elisabetta Patorno, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 1620 Tremont St., BC-3030, Boston, MA 02120, USA.
Brendan M Everett, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, USA; Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, USA.
Muthiah Vaduganathan, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, USA.
Scott D Solomon, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, USA.
Raisa Levin, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 1620 Tremont St., BC-3030, Boston, MA 02120, USA.
Sebastian Schneeweiss, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 1620 Tremont St., BC-3030, Boston, MA 02120, USA.
Rishi J Desai, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 1620 Tremont St., BC-3030, Boston, MA 02120, USA.
Data availability
Data may be obtained from a third party and are not publicly available. Patient-level data are not available for sharing due to restrictions imposed under data use agreement by the Centers for Medicare and Medicaid Services. All aggregate-level data are presented in the manuscript and supplemental content.
Funding
E.L.F. is supported by a Rubicon Grant from the Netherlands Organization for Scientific Research. E.P. is supported by PCORI grant #DB-2020C2–20326 and a career development grant (K08AG055670) from the National Institute on Aging. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
E.L.F., E.P., and R.J.D. initiated the study. E.L.F. and R.J.D. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. E.L.F. drafted the manuscript. R.L. performed the statistical analysis. All authors contributed to the design of the study; the acquisition, analysis, and interpretation of data; and the critical revision of the manuscript for important intellectual content. E.L.F. and R.J.D. are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. E.L.F. and R.J.D. affirm that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
References
- 1. Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004;27:699–703. 10.2337/diacare.27.3.699 [DOI] [PubMed] [Google Scholar]
- 2. Boonman-de Winter LJ, Rutten FH, Cramer MJ, Landman MJ, Liem AH, Rutten GE, et al. High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 2012;55:2154–2162. 10.1007/s00125-012-2579-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cas AD, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail 2015;3:136–145. [DOI] [PubMed] [Google Scholar]
- 4. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo-Leiro MG, Harjola VP, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: an analysis of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail 2017;19:1574–1585. 10.1002/ejhf.813 [DOI] [PubMed] [Google Scholar]
- 5. Savarese G, Lund LH. Global Public Health Burden of Heart Failure. Card Fail Rev 2017;3:7–11. 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail 2018;6:678–685. 10.1016/j.jchf.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251–259. 10.1056/NEJMoa052256 [DOI] [PubMed] [Google Scholar]
- 8. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017;14:591–602. 10.1038/nrcardio.2017.65 [DOI] [PubMed] [Google Scholar]
- 9. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012;126:65–75. 10.1161/CIRCULATIONAHA.111.080770 [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 11. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 12. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 13. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022;145:e895–e1032. [DOI] [PubMed] [Google Scholar]
- 14. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 15. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-Preserved trial. Circulation 2021;144:1284–1294. 10.1161/CIRCULATIONAHA.121.056824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–1098. 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 17. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet 2022;400:757–767. 10.1016/S0140-6736(22)01429-5 [DOI] [PubMed] [Google Scholar]
- 18. Schneeweiss S, Rassen JA, Brown JS, Rothman KJ, Happe L, Arlett P, et al. Graphical depiction of longitudinal study designs in health care databases. Ann Intern Med 2019;170:398–406. 10.7326/M18-3079 [DOI] [PubMed] [Google Scholar]
- 19. Lund JL, Richardson DB, Sturmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2015;2:221–228. 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Diabetes Association . 8. Pharmacologic approaches to glycemic treatment. Diabetes Care 2017;40:S64–S74. 10.2337/dc17-S011 [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association . 8. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 2018;41:S73–S85. 10.2337/dc18-S008 [DOI] [PubMed] [Google Scholar]
- 22. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–242. 10.1056/NEJMoa1501352 [DOI] [PubMed] [Google Scholar]
- 23. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–1326. 10.1056/NEJMoa1307684 [DOI] [PubMed] [Google Scholar]
- 24. Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014;130:1579–1588. 10.1161/CIRCULATIONAHA.114.010389 [DOI] [PubMed] [Google Scholar]
- 25. Toh S, Hampp C, Reichman ME, Graham DJ, Balakrishnan S, Pucino F, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med 2016;164:705–714. 10.7326/M15-2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Filion KB, Azoulay L, Platt RW, Dahl M, Dormuth CR, Clemens KK, et al. A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med 2016;374:1145–1154. 10.1056/NEJMoa1506115 [DOI] [PubMed] [Google Scholar]
- 27. Gallwitz B. Safety and efficacy of linagliptin in type 2 diabetes patients with common renal and cardiovascular risk factors. Ther Adv Endocrinol Metab 2013;4:95–105. 10.1177/2042018813486165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Patorno E, Gopalakrishnan C, Bartels DB, Brodovicz KG, Liu J, Schneeweiss S. Preferential prescribing and utilization trends of diabetes medications among patients with renal impairment: emerging role of linagliptin and other dipeptidyl peptidase 4 inhibitors. Endocrinol Diabetes Metab 2018;1:e00005. 10.1002/edm2.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:653–662. 10.1016/S2213-8587(21)00203-5 [DOI] [PubMed] [Google Scholar]
- 30. Desai RJ, Levin R, Lin KJ, Patorno E. Bias implications of outcome misclassification in observational studies evaluating association between treatments and all-cause or cardiovascular mortality using administrative claims. J Am Heart Assoc 2020;9:e016906. 10.1161/JAHA.120.016906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Desai RJ, Patorno E, Vaduganathan M, Mahesri M, Chin K, Levin R, et al. Effectiveness of angiotensin-neprilysin inhibitor treatment versus renin-angiotensin system blockade in older adults with heart failure in clinical care. Heart 2021;107:1407–1416. 10.1136/heartjnl-2021-319405 [DOI] [PubMed] [Google Scholar]
- 32. Patorno E, Pawar A, Franklin JM, Najafzadeh M, Deruaz-Luyet A, Brodovicz KG, et al. Empagliflozin and the risk of heart failure hospitalization in routine clinical care. Circulation 2019;139:2822–2830. 10.1161/CIRCULATIONAHA.118.039177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Desai RJ, Glynn RJ, Everett BM, Schneeweiss S, Wexler DJ, Bessette LG, et al. Comparative effectiveness of empagliflozin in reducing the burden of recurrent cardiovascular hospitalizations among older adults with diabetes in routine clinical care. Am Heart J 2022;254:203–215. 10.1016/j.ahj.2022.09.008 [DOI] [PubMed] [Google Scholar]
- 34. Agency for Healthcare Research and Quality . Creation of New Race-ethnicity Codes and Socioeconomic Status (Ses) Indicators for Medicare Beneficiaries. 2008. [DOI] [PubMed]
- 35. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci 2018;73:980–987. 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology 2017;28:249–257. 10.1097/EDE.0000000000000595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fu EL, Groenwold RHH, Zoccali C, Jager KJ, van Diepen M, Dekker FW. Merits and caveats of propensity scores to adjust for confounding. Nephrol Dial Transplant 2019;34:1629–1635. 10.1093/ndt/gfy283 [DOI] [PubMed] [Google Scholar]
- 38. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 2019;367:l5657. 10.1136/bmj.l5657 [DOI] [PubMed] [Google Scholar]
- 39. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 2009;38:1228–1234. 10.1080/03610910902859574 [DOI] [Google Scholar]
- 40. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Stat Med 2014;33:1685–1699. 10.1002/sim.6058 [DOI] [PubMed] [Google Scholar]
- 42. Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389–2430. 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 43. Masri A, Althouse AD, McKibben J, Lee JS, Mulukutla SR. Limitations of administrative data for studying patients hospitalized with heart failure. Ann Intern Med 2017;166:916–917. 10.7326/L17-0077 [DOI] [PubMed] [Google Scholar]
- 44. Desai RJ, Lin KJ, Patorno E, Barberio J, Lee M, Levin R, et al. Development and preliminary validation of a Medicare claims-based model to predict left ventricular ejection fraction class in patients with heart failure. Circ Cardiovasc Qual Outcomes 2018;11:e004700. 10.1161/CIRCOUTCOMES.118.004700 [DOI] [PubMed] [Google Scholar]
- 45. Mahesri M, Chin K, Kumar A, Barve A, Studer R, Lahoz R, et al. External validation of a claims-based model to predict left ventricular ejection fraction class in patients with heart failure. PLoS One 2021;16:e0252903. 10.1371/journal.pone.0252903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology 2009;20:512–522. 10.1097/EDE.0b013e3181a663cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shi X, Miao W, Tchetgen ET. A selective review of negative control methods in epidemiology. Curr Epidemiol Rep 2020;7:190–202. 10.1007/s40471-020-00243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fu EL, van Diepen M, Xu Y, Trevisan M, Dekker FW, Zoccali C, et al. Pharmacoepidemiology for nephrologists (part 2): potential biases and how to overcome them. Clin Kidney J 2021;14:1317–1326. 10.1093/ckj/sfaa242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arnold BF, Ercumen A, Benjamin-Chung J, Colford JM Jr. Brief report: negative controls to detect selection bias and measurement bias in epidemiologic studies. Epidemiology 2016; 27:637–641. 10.1097/EDE.0000000000000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 51. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 52. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128. 10.1056/NEJMoa2030183 [DOI] [PubMed] [Google Scholar]
- 53. Butler J, Siddiqi TJ, Filippatos G, Ferreira JP, Pocock SJ, Zannad F, et al. Early benefit with empagliflozin in heart failure with preserved ejection fraction: insights from the EMPEROR-Preserved trial. Eur J Heart Fail 2022;24:245–248. 10.1002/ejhf.2420 [DOI] [PubMed] [Google Scholar]
- 54. Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 55. Johansen ME, Argyropoulos C. The cardiovascular outcomes, heart failure and kidney disease trials tell that the time to use sodium–glucose cotransporter 2 inhibitors is now. Clin Cardiol 2020;43:1376–1387. 10.1002/clc.23508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Becher PM, Schrage B, Ferrannini G, Benson L, Butler J, Carrero JJ, et al. Use of sodium–glucose co-transporter 2 inhibitors in patients with heart failure and type 2 diabetes mellitus: data from the Swedish Heart Failure Registry. Eur J Heart Fail 2021;23:1012–1022. 10.1002/ejhf.2131 [DOI] [PubMed] [Google Scholar]
- 57. Lam CSP, Karasik A, Melzer-Cohen C, Cavender MA, Kohsaka S, Norhammar A, et al. Association of sodium–glucose cotransporter-2 inhibitors with outcomes in type 2 diabetes with reduced and preserved left ventricular ejection fraction: analysis from the CVD-REAL 2 study. Diabetes Obes Metab 2021;23:1431–1435. 10.1111/dom.14356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Packer M. Do DPP-4 inhibitors cause heart failure events by promoting adrenergically mediated cardiotoxicity? Clues from laboratory models and clinical trials. Circ Res 2018;122:928–932. 10.1161/CIRCRESAHA.118.312673 [DOI] [PubMed] [Google Scholar]
- 59. Chu PY, Zatta A, Kiriazis H, Chin-Dusting J, Du XJ, Marshall T, et al. CXCR4 Antagonism attenuates the cardiorenal consequences of mineralocorticoid excess. Circ Heart Fail 2011;4:651–658. 10.1161/CIRCHEARTFAILURE.110.960831 [DOI] [PubMed] [Google Scholar]
- 60. Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail 2018;11:e004873. 10.1161/CIRCHEARTFAILURE.117.004873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Desai RJ, Mahesri M, Chin K, Levin R, Lahoz R, Studer R, et al. Epidemiologic characterization of heart failure with reduced or preserved ejection fraction populations identified using Medicare claims. Am J Med 2021;134:e241–ee51. 10.1016/j.amjmed.2020.09.038 [DOI] [PubMed] [Google Scholar]
- 62. Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol 2016;45:1866–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zou X, Shi Q, Vandvik PO, Guyatt G, Lang CC, Parpia S, et al. Sodium–glucose cotransporter-2 inhibitors in patients with heart failure: a systematic review and meta-analysis. Ann Intern Med 2022;175:851–861. 10.7326/M21-4284 [DOI] [PubMed] [Google Scholar]
- 64. Quin JA, Hattler B, Shroyer ALW, Kemp D, Almassi GH, Bakaeen FG, et al. Concordance between administrative data and clinical review for mortality in the randomized on/off bypass follow-up study (ROOBY-FS). J Card Surg 2017;32:751–756. 10.1111/jocs.13379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Patient-level data are not available for sharing due to restrictions imposed under data use agreement by the Centers for Medicare and Medicaid Services. All aggregate-level data are presented in the manuscript and supplemental content.