Graphical Abstract
Graphical abstract.
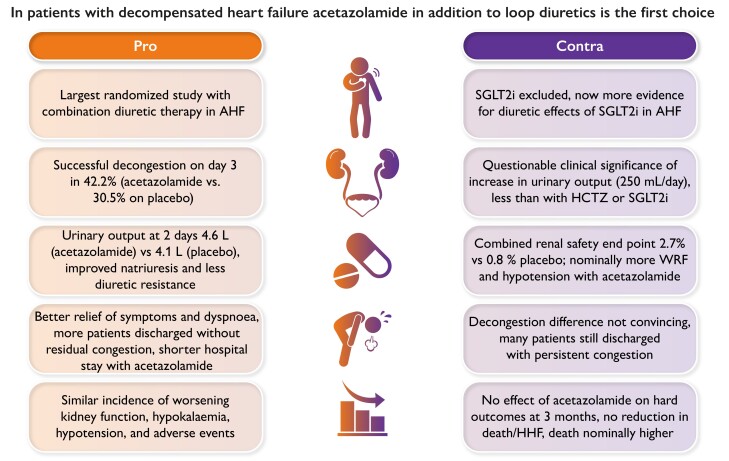
Key points in favour or against the addition of acetazolamide to loop diuretics as the first choice in patients with decompensated heart failure. Abbreviations: AHF, acute heart failure; HCTZ, hydrochlorothiazide; HHF, hospitalization for heart failure; SGLT2i, sodium–glucose cotransporter 2 inhibitor; WRF, worsening renal function.
Keywords: Acute heart failure, Diuretic therapy, Acetazolamide, SGLT2 inhibitors
Introduction
Medical therapy for heart failure (HF) has made substantial progress during recent years, and the ‘fantastic four’ drugs angiotensin receptor–neprilysin inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and sodium–glucose cotransporter 2 inhibitors (SGLT2i) markedly improve prognosis in patients with HF with reduced ejection fraction (HFrEF).1,2 Early initiation of guideline-directed medical therapy (GDMT) is key in patients diagnosed with HF as most drugs have been shown to reduce hard clinical endpoints such as cardiovascular death and HF hospitalization as early as some weeks after randomization.3 STRONG-HF has proved that an intensified treatment strategy of rapid up-titration of GDMT and close follow-up compared with usual care after an acute heart failure (AHF) admission reduced symptoms, improved quality of life, and decreased the risk of all-cause death or HF readmission after 6 months.4
In sharp contrast to the myriad of randomized trials constituting the evidence base for GDMT in HFrEF, until recently, there has been virtually no randomized study investigating the most important medical therapy for all patients with acute decompensated or chronic HF with congestion, i.e. diuretics. While placebo-controlled larger studies with diuretics in HF are absent, the Diuretic Optimization Strategies Evaluation (DOSE) study in 2011 found no difference between bolus dosages of furosemide vs. continuous infusion but a non-significant trend towards greater symptomatic improvement in the high-dose group.5 In clinical practice, adequate treatment of congestion and volume overload is key for symptom improvement in all patients with decompensated HF; frequent challenges include the assessment of diuretic response/resistance especially in the early phase and the best approach towards stepped pharmacologic diuretic strategies by combining additional diuretics with furosemide.6 Two-month treatment with tolvaptan initiated for acute treatment of patients hospitalized with HF had no effect on its primary endpoint of long-term mortality or HF-related morbidity in 4133 patients included in the EVEREST trial.7 It did show a significantly greater decrease in weight on Day 1. However, while statistically significant, the percentage of patients with at least a two-grade improvement in oedema at 7 days was 73.8% vs. 70.5%, leading to a number needed to treat (NNT) of 30.3. Furthermore, tolvaptan is an aquaretic agent that induces more water loss in contrast to other diuretic agents, which also induce natriuresis.
Given the lack of scientific evidence, the 2021 European Society of Cardiology (ESC) guidelines on HF recommend intravenous (i.v.) loop diuretics for all patients with AHF (Class I, level of evidence C, i.e. expert consensus) and consider the combination of a loop diuretic with thiazide-type diuretics in patients with resistant oedema (IIa, B).1
Luckily, in 2022, three seminal trials on medical treatments to relieve congestion additive to loop diuretics have been published.8
In patients with AHF (reduced and preserved ejection fractions) and volume overload in the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial, i.v. application of the carbonic anhydrase inhibitor acetazolamide for 3 days in addition to i.v. loop diuretics led to more efficient decongestion and higher cumulative urine output and natriuresis.9 The CLOROTIC (Safety and Efficacy of the Combination of Loop with Thiazide-type Diuretics in Patients with Decompensated Heart Failure) trial demonstrated that the addition of hydrochlorothiazide (HCTZ) to i.v. loop diuretic therapy improved the diuretic response in patients with AHF, although there was no significant difference in patient-reported dyspnoea.10 In a novel design using a ‘win ratio’ as the primary endpoint, the EMPULSE (EMPagliflozin 10 mg compared with placebo, initiated in patients hospitalized for acUte heart faiLure who have been StabilisEd) study randomized patients with acute de novo or decompensated HF after stabilization (3 days of median time from hospital admission to randomization) to the SGLT2i empagliflozin or placebo.11 After 90 days, a clinical benefit occurred significantly more often in patients treated with empagliflozin vs. placebo; also, total mortality and HF readmissions were reduced.11 Furthermore, in a subanalysis, all decongestion-related endpoints such as weight loss, haemoconcentration, and clinical congestion score (dyspnoea, orthopnoea, and fatigue) were favourably affected by the addition of empagliflozin to furosemide.12
These three randomized studies with combined diuretic treatment regimens (SGLT2i are no classic ‘diuretics’ but display some diuretic features) are very useful to define the best diuretic strategy in patients with decompensated HF and congestion/diuretic resistance. Clearly, these data remind us that we need to do more than just apply some i.v. furosemide: we need to monitor the diuretic response shortly after the start of therapy (either by determining urinary sodium content after 2 or 6 h and/or by measuring the hourly urine output), although there is no scientific evidence that such an approach improves clinical outcomes of patients. A satisfactory diuretic response can be defined as a urine sodium content >50–70 mEq/L at 2 h and/or by a urine output >100–150 mL/h during the first 6 h.1,6 In case of an insufficient diuretic response, doubling of the i.v. loop diuretic dose is recommended, with a further assessment of diuretic response. The most important question arising from ADVOR, CLOROTIC, and EMPULSE is now as follows: which diuretic strategy additive to i.v. loop diuretics is the preferred one in contemporary clinical practice? This is the core of the current debate. Two groups of authors share their views and arguments.
While the very early addition of acetazolamide or thiazide diuretics has proved better decongestion, both ADVOR and CLOROTIC did not show improved outcomes after 90 days. EMPULSE showed a reduction in hard clinical endpoints after 90 days; however, patients were randomized mainly after the first days of admission, during which better decongestion is required. A potential approach was suggested combining the administration of loop diuretics with acetazolamide initially followed by the introduction of SGLT2i thereafter.13
All regimens for acute and chronic decongestion need to take into account effects on neurohormonal and sympathetic nervous system activation associated with the administration of loop diuretics. Acetazolamide may acutely counteract neurohormonal stimulation with more proximal sodium reabsorption, especially in patients with advanced kidney disease not receiving a renin–angiotensin system blocker and in those patients with increased bicarbonate levels at baseline and/or during monotherapy with loop diuretics. A diuretic agent working more distally in the renal tubular loop may not affect the physiological consequences of increased proximal sodium retention. However, also SGLT2i may act as neurohormonal antagonists and display a similar pattern on cellular signalling and cardiac remodelling such as classical neurohormonal antagonists like angiotensin-converting enzyme inhibitors, MRA, and beta-blockers.14
Clearly, all combination diuretic regimens require careful monitoring of serum electrolytes and renal function, as profound hypokalaemia and worsening of renal function may occur. While diuretic treatment is inevitable to relieve acute decongestion in patients with HF, it is important to note that there remains considerable controversy and uncertainty regarding the long-term use of diuretics after restoration of euvolaemia and hospital discharge. In any case, especially in HFrEF and HF with mildly reduced ejection fraction, progressive implementation of oral GDMT with renin–angiotensin–aldosterone blockade, beta-blockers, and SGLT2i is mandatory to further relieve congestion and prevent recurrent decompensation as demonstrated in STRONG-HF.5,13
Pre-registered clinical trial number
Not applicable.
Ethical approval
Ethical approval was not required.
Data availability
No data were generated or analysed for or in support of this paper.
Funding
Jo.B. was supported by the Deutsche Forschungsgemeinschaft (KFO 311).
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J 2021;42:681–683. 10.1093/eurheartj/ehaa1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abdin A, Bauersachs J, Soltani S, Eden M, Frey N, Böhm M. A practical approach to the guideline-directed pharmacological treatment of heart failure with reduced ejection fraction. ESC Heart Fail 2023;10:24–31. 10.1002/ehf2.14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mebazaa A, Davison B, Chioncel O, Cohen-Solal A, Diaz R, Filippatos G, et al. . Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet 2022;400:1938–1952. 10.1016/S0140-6736(22)02076-1 [DOI] [PubMed] [Google Scholar]
- 5. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. . Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805. 10.1056/NEJMoa1005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. . The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:137–155. 10.1002/ejhf.1369 [DOI] [PubMed] [Google Scholar]
- 7. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. . Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 2007;297:1319–1331. 10.1001/jama.297.12.1319 [DOI] [PubMed] [Google Scholar]
- 8. de Boer RA, Bauersachs J. The year in cardiovascular medicine 2022: the top 10 papers in heart failure and cardiomyopathies. Eur Heart J 2023;44:342–344. 10.1093/eurheartj/ehac781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. . Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med 2022;387:1185–1195. 10.1056/NEJMoa2203094 [DOI] [PubMed] [Google Scholar]
- 10. Trullàs JC, Morales-Rull JL, Casado J, Carrera-Izquierdo M, Sánchez-Marteles M, Conde-Martel A, et al. . Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J 2023;44:411–421. 10.1093/eurheartj/ehac689 [DOI] [PubMed] [Google Scholar]
- 11. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. . The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022;28:568–574. 10.1038/s41591-021-01659-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Biegus J, Voors AA, Collins SP, Kosiborod MN, Teerlink JR, Angermann CE, et al. . Impact of empagliflozin on decongestion in acute heart failure: the EMPULSE trial. Eur Heart J 2023;44:41–50. 10.1093/eurheartj/ehac530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mebazaa A, Solal AC, Colombo PC. Assessing and treating congestion in acute decompensated heart failure: are we seeing the light at the end of the tunnel? Eur Heart J 2023;44:51–53. 10.1093/eurheartj/ehac680 [DOI] [PubMed] [Google Scholar]
- 14. Packer M. Molecular, cellular, and clinical evidence that sodium-glucose cotransporter inhibitors act as neurohormonal antagonists when used for the treatment of chronic heart failure. J Am Heart Assoc 2020;9:e016270. 10.1161/JAHA.120.016270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Pro
Objectives of diuretic therapy
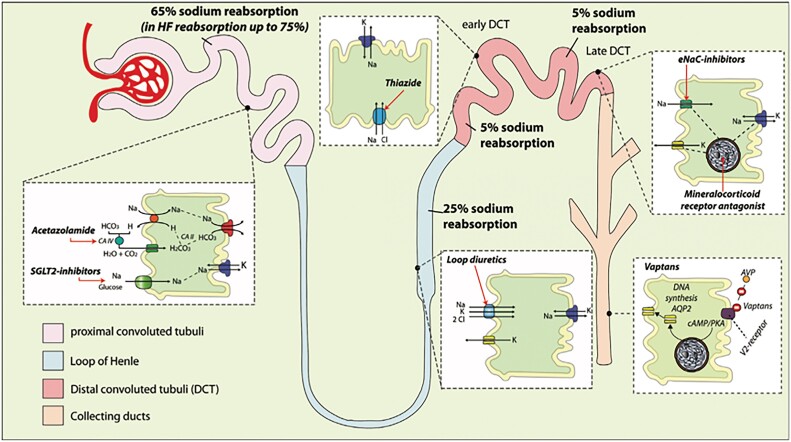
Loop diuretics have a Class I recommendation in all ejection fraction classes to treat signs and symptoms of congestion, but in contrast to other medical therapies, robust clinical trial evidence to guide the use of loop diuretics is sparse, which is reflected in a Class C recommendation.1 Moreover, the appropriate use of diuretics remains challenging, especially in the setting of worsening renal function (WRF), diuretic resistance, and electrolyte disturbances. Thorough knowledge of the pharmacokinetics and pharmacodynamics of diuretics is therefore mandatory to use them effectively (Figure 1).2
Figure 1.
Side and mode of action of different diuretics (adapted from Mullens et al.2).
Despite routine use of high-dose loop diuretics, many patients are discharged with residual clinical signs of volume overload, which is a strong predictor of poor outcomes.3,4 Although the ESC HF guidelines have a Class I recommendation (level of evidence C), only 15% of patients were free from clinical congestion after 72 h of treatment in the DOSE study.5 Sequential diuretic therapy has been suggested as a way to achieve better decongestion, but reliable evidence on the optimal diuretic agents, dosing schedule, and route of administration has been lacking.1,2 Importantly, the ESC HF guidelines have a Class I recommendation (level of evidence C) that patients hospitalized for HF should not be discharged with persistent signs of congestion.1
Why should acetazolamide be used as a first-line agent in addition to loop diuretics in acute heart failure with volume overload?
To treat congestion, a Class I recommendation
ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) was a prospective, multicentre, double-blind, placebo-controlled trial (n = 519, mean age 78 years, 63% male), which clearly demonstrated that the addition of 3 days of 500 mg of i.v. acetazolamide added to standardized i.v. loop diuretic therapy (twice home dose intravenously) was associated with a higher incidence of successful decongestion within 3 days after randomization (30.5% vs. 42.2%, P = .0009) but also at discharge (62.5% vs. 78.8%, P = .0001).6 The administration of acetazolamide did not lead to an increase in pre-defined adverse events (i.e. metabolic acidosis, renal safety endpoints, hypokalaemia, or hypotension). Importantly, ADVOR chose successful decongestion, defined as the absence of any clinical sign of fluid overload (i.e. oedema, pleural effusion, or ascites), which is the best clinical reflection of extracellular volume overload, as its primary endpoint.7 While assessment of weight might appear to be a simpler measurement, it is actually technically challenging, and fluctuations in weight, especially during a hospital admission, might not represent changes in fluid status.8 Furthermore, simply measuring urine output is insufficient since there is a poor correlation between this and weight loss due to inaccuracies of fluid intake.8 The CLOROTIC trial examined the effect of the addition of HCTZ to i.v. loop diuretic therapy on a coprimary endpoint of changes in body weight and patient-reported dyspnoea 72 h after randomization. The addition of HCTZ led to a moderate increase in weight loss (−2.3 vs. −1.5 kg) without significant differences in patient-reported dyspnoea.9 Unfortunately, CLOROTIC did not provide data on natriuresis and volume scoring, and the benefit on urine output was rather limited at 24 h (1775 vs. 1400 mL, P = .05).
While congestion is a universal phenomenon occurring in HF with reduced, mildly reduced, and preserved ejection fractions, the importance of neurohormonal activation is predominantly established in patients with a lower left ventricular ejection fraction (LVEF). Nevertheless, there was no treatment effect modification by underlying LVEF of acetazolamide when added to treatment with loop diuretics.10 Therefore, ADVOR is the first randomized clinical trial in AHF to effectively demonstrate that a combination of diuretic agents, i.e. acetazolamide with standardized loop diuretic therapy, can lead to an appreciable reduction in the proportion of patients discharged with residual signs of congestion across a wide range of LVEF categories.
To improve natriuresis
As the objective of diuretic therapy is to get rid of excessive sodium (and accompanying water), the measurement of urinary sodium content has experienced a renewed interest as a better indicator for diuretic response.11–14 Therefore, a sodium-based strategy might allow the clinician to determine the loop diuretic response in a systematic and timely fashion, allowing for more timely adjustments in therapy.2 The use of urinary sodium following the first administration of a loop diuretic to guide diuretic therapy has now been incorporated into the European guidelines for the diagnosis and treatment of HF.1 However, during consecutive days of loop diuretic therapy, increasingly hypotonic urine is produced, which probably explains why current decongestive strategies perform poorly to achieve a durable reduction in plasma volume.15
Acetazolamide inhibits carbonic anhydrase, resulting in diminished sodium–hydrogen exchanger 3 (NHE3)-mediated proximal tubular sodium reabsorption.2,16 In both healthy conditions and HF, the proximal nephron is responsible for the largest proportion of glomerular filtered sodium reabsorption.17 While this is ∼60%–65% in healthy individuals, proximal sodium reabsorption in HF may rise to ∼75%–85%.17,18 The results of a pre-specified analysis from the ADVOR trial clearly indicate a strong natriuretic effect of acetazolamide when provided on top of high-dose loop diuretics in AHF (Verbrugge FH, unpublished data). Moreover, treatment with acetazolamide kept the sodium content of the urine high, whereas this decreased rapidly in patients allocated loop diuretics only (Verbrugge FH, unpublished data). This probably explains the incremental benefit of acetazolamide after the randomized treatment period ended (NNT 8.5) towards discharge (NNT only 6) and suggests that a practice-relevant aspect of the use of acetazolamide is that it should be used upfront rather than as a rescue therapy for diuretic resistance.6 Also, greater natriuretic response to diuretics boosted by acetazolamide was associated with faster and more complete symptomatic relief from congestion and with a significant reduction in the rate of the combined clinical endpoint of all-cause mortality and HF readmission during the 90-day follow-up period (Verbrugge FH, unpublished data).
To avoid (loop) diuretic resistance
More than half of the patients presenting with AHF in the DOSE-HF, ROSE-AHF, and CARRESS-HF trials had elevated HCO3− levels, and treatment with loop diuretics resulted in further increases in HCO3−.19,20 This feature is incorrectly termed as ‘contraction alkalosis’.21 The term contraction alkalosis stems from the observation that in healthy individuals, a reduction in effective circulating volume (ECV) leads to neurohormonal activation. This results in an increase in the filtration fraction, which drives proximal sodium and HCO3− reabsorption.18 Additionally, angiotensin II stimulates proximal nephron Na+/HCO3− retention via the luminal NHE3 transporter and the basolateral Na+/HCO3− cotransporter, resulting in increased sodium retention but also further generation of HCO3− (three HCO3− molecules per one sodium molecule for the basolateral Na+/HCO3− cotransporter).22,23 As such, rather than being a sign of a contracted ECV, the elevation of HCO3− is merely a reflection of neurohormonal activation in AHF. A recent subanalysis of ADVOR demonstrated that almost half of the patients presented with elevated HCO3−, which was linked to more pronounced clinical signs of volume overload, indicating a general lack of volume depletion (Martens P, unpublished data). Additionally, the increase in HCO3− during decongestion —almost exclusively seen in the loop diuretic therapy-only group —was not caused by a contraction of the ECV but was related to worsening of (intra-renal) neurohormonal activation during decongestion, further promoting proximal sodium reabsorption (Martens P, unpublished data).
Importantly, acetazolamide improved decongestive effectiveness over the entire range of baseline HCO3−, and patients with an elevated HCO3− exhibited a more pronounced response to acetazolamide, which was mediated by the prevention of loop diuretic resistance (Martens P, unpublished data). These data strongly corroborate physiological reasoning that increased HCO3−, both at baseline and during monotherapy with loop diuretics, is the result of increased neurohormonal stimulation with more proximal sodium reabsorption, which can be specifically counteracted by the addition of acetazolamide.
To avoid side effects of other diuretic agents
Improving decongestion without causing harm is important when selecting the most appropriate diuretic approach. The addition of acetazolamide to loop diuretic therapy was not associated with an increased incidence of adverse events, and the higher incidence of successful decongestion was associated with a shorter duration of hospital stay.6 Furthermore, there was no increase in the rates of metabolic acidosis, renal endpoints, hypokalaemia, or hypotension in the acetazolamide-treated patients in the ADVOR trial.6
Thiazides, which work in the distal nephron, can induce significant kaliuresis, since especially in high aldosterone states like AHF, each excreted sodium ion is associated with the excretion of two to three ions of potassium.24,25 In a propensity-matched analysis of the real-world use of thiazides combined with lower-dose loop diuretics vs. high-dose loop diuretics in HF patients, thiazides, not high-dose loop diuretics, were independent predictors of the occurrence of hyponatraemia and hypokalaemia with a strong trend towards a higher risk for all-cause mortality.26 This adverse effect of thiazides on potassium levels was further corroborated by the CLOROTIC trial, where a very high prevalence of hypokalaemia defined as potassium ≤3.0 mmol/L was noted within 5 days of 25 mg daily of HCTZ administration (40.6% vs. 16.1%, P < .001).9 Additionally, the modest effect on weight loss was countered by a marked increased rate of impaired renal function (46.5% vs. 17.2%).9 Hence, the side effect profile augments the greater efficacy on decongestion such that acetazolamide should be favoured over thiazide-like agents.
To improve outcomes
As stated previously, the use of acetazolamide did lead to better decongestion and more pronounced natriuresis and prevented the occurrence of diuretic resistance, all of which are associated with improved long-term outcomes. Moreover, the use of acetazolamide was associated with at least 1 day less in the hospital. Finally, acetazolamide has an excellent safety profile that can be traced back to >70 years of HF treatment and has been proved again in the ADVOR trial. Finally, the drug is very easy to use, widely available, and inexpensive.
As with previous therapies employed in patients admitted with AHF, the benefits of improved decongestion seen with acetazolamide did not translate into improvements in long-term outcomes such as death or HF rehospitalization. However, ADVOR was not powered to be definitive on patient-oriented endpoints, especially when one considers that the risk of death or HF rehospitalization (27.8% vs. 29.7% at 3 months) was considerably lower than that in the DOSE trial (50% at 60 days) and in the CARRESS-HF trial (40% at 60 days).5,27 This feature, which is seen despite the fact that patients enrolled in ADVOR had many coexisting conditions and advanced age, might be the result of the higher incidence of decongestion at discharge and the increase in the dose of neurohumoral blockers during the remainder of the hospital stay.6,28 Nevertheless, it is worth noting that the higher age, the pronounced clinical signs of volume overload, and the markedly elevated levels of natriuretic peptides (6173 pg/mL) of the ADVOR population included are more reflective of a real-world HF population than previous diuretic trials in AHF (see Supplementary data online, Table S1).28 As such, the results of ADVOR do provide contemporary and novel insights into the diuretic treatment of patients with AHF and fluid overload.28 However, more and larger trials of diuretic agents are required to elucidate the complex relationships between the degree of decongestion, quality of life, and outcomes in patients with AHF.28
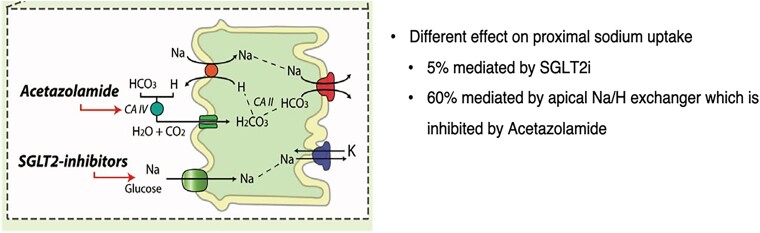
While chronic (long-term) use of SGLT2i has been associated with improved outcomes, these agents were not indicated and had not been approved as drugs to treat HF during most of the ADVOR trial period. To avoid confounding by any imbalance in their use between the trial groups, the trial design excluded their use. Although SGLT2i and acetazolamide both exert natriuretic and diuretic effects on the proximal tubules, their mode of action and potency differ substantially.2 Only 5% of proximal sodium uptake is mediated by SGLT2, whereas 60% is mediated by the apical Na/H exchanger, i.e. inhibited by acetazolamide17,29–31 (Figure 2). As a result, SGLT2i do not lead to a significant increase in natriuresis.32 Therefore, there is no physiological reason to assume that the effect of acetazolamide should be attenuated by SLGT2i or that there is any risk related to the concurrent use of both agents. Moreover, acetazolamide should be seen as a diuretic agent to be used temporarily to treat extracellular volume overload, whereas SGLT2i are disease-modifying agents to be used chronically, which should be either started within 3 days of admission with AHF or, if already being prescribed, continued through the hospitalization and beyond.
Figure 2.
Different effect of natriuresis of acetazolamide and SLGT2i (adapted from Mullens et al.2).
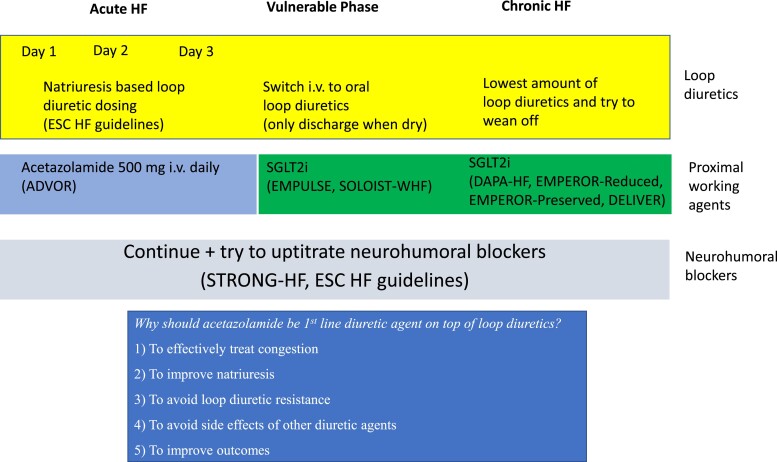
Based upon the aforementioned reasons, i.v. acetazolamide on top of loop diuretics should be recommended for all patients with AHF admitted with signs/symptoms of fluid overload who were previously treated with oral loop diuretics to improve the incidence of decongestion (Figure 3).
Figure 3.
Medical approach during acute heart failure in all left ventricular ejection fraction categories. ESC, European Society of Cardiology; HF, heart failure; i.v., intravenous; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
Supplementary data
Supplementary data is available at European Heart Journal online.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. . The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:137–155. 10.1002/ejhf.1369 [DOI] [PubMed] [Google Scholar]
- 3. Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, et al. . Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Hear Fail 2012;5:54–62. 10.1161/CIRCHEARTFAILURE.111.963413 [DOI] [PubMed] [Google Scholar]
- 4. Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Silwa K, et al. . Acute heart failure. Nat Rev Dis Prim 2020;6:16. 10.1038/s41572-020-0151-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. . Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797–805. 10.1056/NEJMoa1005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. . Acetazolamide In acute decompensated heart failure with volume overload. N Engl J Med 2022;387:1185–1195. 10.1056/NEJMoa2203094 [DOI] [PubMed] [Google Scholar]
- 7. Mullens W, Verbrugge FH, Nijst P, Martens P, Tartaglia K, Theunissen E, et al. . Rationale and design of the ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial. Eur J Heart Fail 2018;20:1591–1600. 10.1002/ejhf.1307 [DOI] [PubMed] [Google Scholar]
- 8. Testani JM, Brisco MA, Kociol RD, Jacoby D, Bellumkonda L, Parikh CR, et al. . Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment. Am J Med 2015;128:776–783. 10.1016/j.amjmed.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trullàs JC, Morales-Rull JL, Casado J, Carrera-Izquierdo M, Sánchez-Marteles M, Conde-Martel A, et al. . CLOROTIC Trial Investigators. Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J 2023;44:411–421. 10.1093/eurheartj/ehac689 [DOI] [PubMed] [Google Scholar]
- 10. Martens P, Verbrugge FH, Dauw J, Nijst P, Meekers E, Augusto SN Jr, et al. . Decongestion with acetazolamide in acute decompensated heart failure across the spectrum of left ventricular ejection fraction: a pre-specified analysis from the ADVOR trial. Circulation 2023;147:201–211. 10.1161/CIRCULATIONAHA.122.062486 [DOI] [PubMed] [Google Scholar]
- 11. Verbrugge FH, Nijst P, Dupont M, Reynders C, Penders J, Tang WHW, et al. . Prognostic value of glomerular filtration changes versus natriuretic response in decompensated heart failure with reduced ejection. J Card Fail 2014;20:817–824. 10.1016/j.cardfail.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 12. Ferreira JP, Girerd N, Bettencourt MP, Ricardo MB, Almeida T, Rola A, et al. . Lack of diuretic efficiency (but not low diuresis) early in an acutely decompensated heart failure episode is associated with increased 180-day mortality. Cardiorenal Med 2017;7:137–149. 10.1159/000455903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brinkley DMJ, Burpee LJ, Chaudhry SP, Samllwood JA, Lindenfeld JA, Lakdawala NK, et al. . Spot urine sodium as triage for effective diuretic infusion in an ambulatory heart failure unit. J Card Fail 2018;24:349–354. 10.1016/j.cardfail.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 14. Singh D, Shrestha K, Testani JM, Verbrugge FH, Dupont M, Mullens W, et al. . Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J Card Fail 2014;20:392–399. 10.1016/j.cardfail.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verbrugge FH, Nijst P, Dupont M, Penders J, Tang WH, Mullens W. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ Heart Fail 2014;7:766–772. 10.1161/CIRCHEARTFAILURE.114.001377 [DOI] [PubMed] [Google Scholar]
- 16. Verbrugge FH, Martens P, Ameloot K, Haemels V, Penders J, Dupont M, et al. . Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur J Heart Fail 2019;21:1415–1422. 10.1002/ejhf.1478 [DOI] [PubMed] [Google Scholar]
- 17. Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol 2015;10:676–687. 10.2215/CJN.12391213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mullens W, Martens P, Testani JM, Tang WHW, Skouri H, Verbrugge FH, et al. . Renal effects of guideline-directed medical therapies in heart failure: a consensus document from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2022;24:603–619. 10.1002/ejhf.2471 [DOI] [PubMed] [Google Scholar]
- 19. Cooper LB, Mentz RJ, Gallup D, Lala A, DeVore AD, Vader JM, et al. . Serum bicarbonate in acute heart failure: relationship to treatment strategies and clinical outcomes. J Card Fail 2016;22:738–742. 10.1016/j.cardfail.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vader JM, LaRue SJ, Stevens SR, Mentz RJ, DeVore AD, Lala A, et al. . Timing and causes of readmission after acute heart failure hospitalization-insights from the heart failure network trials. J Card Fail 2016;22:875–883. 10.1016/j.cardfail.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alpern RJ, Cogan MG, Rector FC Jr. Effects of extracellular fluid volume and plasma bicarbonate concentration on proximal acidification in the rat. J Clin Invest 1983;71:736–746. 10.1172/JCI110821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He P, Klein J, Yun CC. Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 2010;285:27869–27878. 10.1074/jbc.M110.133066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na+-H+ exchange and Na+/HCO3− cotransport in the rabbit proximal tubule. Proc Natl Acad Sci U S A 1990;87:7917–7920. 10.1073/pnas.87.20.7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, et al. . Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 2013;83:811–824. 10.1038/ki.2013.14 [DOI] [PubMed] [Google Scholar]
- 25. Hoorn EJ, Nelson JH, McCormick JA, Ellison DH. The WNK kinase network regulating sodium, potassium, and blood pressure. J Am Soc Nephrol 2011;22:605–614. 10.1681/ASN.2010080827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brisco-Bacik MA, Ter Maaten JM, Houser SR, Vedage NA, Rao V, Ahmad T, et al. . Outcomes associated with a strategy of adjuvant metolazone or high-dose loop diuretics in acute decompensated heart failure: a propensity analysis. J Am Heart Assoc 2018;7:e009149. 10.1161/JAHA.118.009149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, et al. . Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–2304. 10.1056/NEJMoa1210357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mullens W, Dauw J, Martens P, Meekers E, Nijst P, Verbrugge FH, et al. . Acetazolamide In Decompensated Heart Failure with Volume Overload trial (ADVOR): baseline characteristics. Eur J Heart Fail 2022;24:1601–1610. 10.1002/ejhf.2587 [DOI] [PubMed] [Google Scholar]
- 29. Frömter E, Rumrich G, Ullrich KJ. Phenomenologic description of Na+, Cl− and HCO3− absorption from proximal tubules of rat kidney. Pflugers Arch 1973;343:189–220. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 30. Aronson PS. Role of ion exchangers in mediating NaCl transport in the proximal tubule. Kidney Int 1996;49:1665–1670. 10.1038/ki.1996.243 [DOI] [PubMed] [Google Scholar]
- 31. Beloto-Silva O, Machado UF, Oliveira- Souza M. Glucose-induced regulation of NHEs activity and SGLTs expression involves the PKA signaling pathway. J Membr Biol 2011;239:157–165. 10.1007/s00232-010-9334-6 [DOI] [PubMed] [Google Scholar]
- 32. Boorsma E, Beusekamp J, Ter Maaten J, Figarska SM, Danser AHJ, Van Veldhuisen DJ, et al. . Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail 2021;23:68–78. 10.1002/ejhf.2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
Contra
P. Christian Schulze, Julian Westphal, Jürgen Bogoviku
Chronic HF and acute decompensated HF belong to the most common hospital admission diagnoses in the Western world.1 Following an initial cardiac injury and development of myocardial damage, subsequent cardiac decompensations are associated with increased myocardial load responsible for the structural and functional deterioration of the failing myocardium.1,2 Clinical hallmarks of acute decompensated HF are congestion, volume overload, and systemic hypoperfusion.1
Decongestive strategies in acute heart failure
Decongestion through enhanced diuresis is one of the most important early treatment goals in patients with acute decompensated HF. Diuretic therapies are primarily based on loop diuretics along with additional pharmacotherapeutic options for increasing urine output. This includes primarily loop diuretics but also other pharmacotherapies such as acetazolamide (a carbonic anhydrase inhibitor acting in the proximal tubule of the kidney) and HCTZ (a competitor for the sodium–chloride cotransporter in the distal convoluted tubule, leading to increased natriuresis and urine production).
It has been decades since the introduction of new therapies for acute decompensated HF. Several trials testing in patients with acute decompensated HF failed to show the benefit of novel pharmacotherapies on post-discharge outcomes, thus highlighting a critical unmet need.3–6 Administration of natriuretic peptides (nesiritide or ularitide),4,6 vasopressin antagonism,5,7 and ultrafiltration8,9 in addition to standard diuretic regimens have not shown consistent clinical benefits in patients with acute decompensated HF.
Acetazolamide is good but not very good for diuresis
The recent ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) trial attempted to increase diuresis using loop diuretics in combination with acetazolamide (500 mg once daily) in comparison to placebo.10 The study included patients with acute decompensated HF randomized to i.v. bolus of acetazolamide compared with standard diuretic treatment only followed for 3 days during the acute in-hospital treatment phase.10 Successful decongestion occurred more frequently in the acetazolamide group (42.2%) compared with placebo (30.5%) along with better diuresis and natriuresis without differences in major clinical events that included WRF, hypokalaemia, and hypotension. The duration of hospital stay was shorter in the acetazolamide group (8.8 vs. 9.9 days, P = .016). Absolute diuretic effects were clinically of marginal significance with an increase of only 0.5 L at 48 h after the randomization day (Day 3 of the study protocol) in the acetazolamide group compared with placebo. While these differences reached statistical significance, it is doubtful whether these effects justify a change in clinical treatment algorithms. All-cause mortality or rehospitalization for HF during the 3-month follow-up was not statistically significant [29.7% vs. 27.8%; with death from any cause 39 (15.2%) acetazolamide vs. 31 (12.0%) placebo] with a trend towards more frequent renal safety endpoint events in the acetazolamide group (2.7% vs. 0.8%, P = .10).
Thiazide diuretics outperform acetazolamide on diuretic response
Another recent study in this field was the CLOROTIC trial that had a comparable study design and tested the hypothesis that oral HCTZ on top of a standard diuretic regimen leads to an increase in diuresis in patients with acute decompensated HF.11 Over the course of 3 days, the additional treatment with HCTZ led to an increase in urine production of ∼375 mL per 24 h and better weight loss but no changes in diuretic efficiency (millilitre urine production per milligram furosemide) in the HCTZ group compared with placebo. While patients showed more pronounced weight loss, the authors noted a lack of improvement in clinical symptoms (dyspnoea score) in the HCTZ group. Finally, the HCTZ group showed a higher frequency of WRF compared with placebo. Thus, it is unlikely that the results of the CLOROTIC trial will have a major clinical impact on the treatment algorithms of patients with acute decompensated HF.
SGLT2 inhibition enhances diuresis and has a proven long-term impact in heart failure
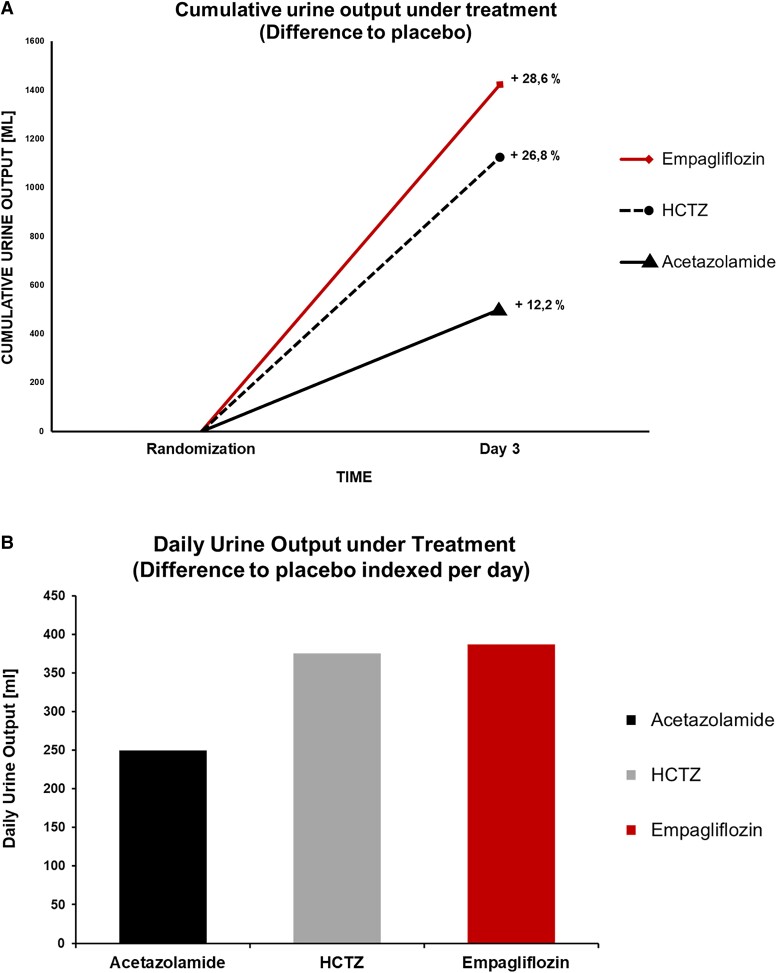
The recent introduction of SGLT2i expanded the treatment option for patients with HF, both with reduced and preserved ejection fractions.12–17 The SGLT2i act through inhibition of the sodium–glucose cotransporter 2 in the proximal renal tubule, leading to increased glucosuria and diuresis.18 These effects are noted in prior studies on patients with chronic stable and acute decompensated HF14,15,18,19 but also in patients hospitalized with acute decompensated HF.19,20 Of note, SGLT2i do not increase the sympathetic or neurohumoral drive in patients with HF and enhance the effects of loop diuretics. The recent EMPAG-HF (Empagliflozin in Acute Decompensated Heart Failure) study tested the hypothesis of whether early initiation of SGLT2i in acute decompensated HF would lead to an increase in diuresis without affecting renal function.20 EMPAG-HF randomized patients to empagliflozin 25 mg or placebo within 12 h of hospital presentation. The study showed an increase in urine production of 2.2 L compared with placebo over the course of 5 days without changes in renal function or other clinical events. This underlines the beneficial safety profile of empagliflozin with nephroprotective effects in patients at risk for and with HF.21 One might even speculate that this might be of particular value in patients with HF and concomitant renal dysfunction, many of whom may not be on an inhibitor of the renin–angiotensin system or MRA.12,21 Further, patients showed a more pronounced decrease in body weight and N-terminal pro-B-type natriuretic peptide as well as furosemide-sparing effects with higher diuretic efficiency (millilitre urine per milligram furosemide) and lower doses of furosemide equivalent per day in the empagliflozin-treated patients. Cumulative urine output on Day 3 post-randomization is higher in EMPAG-HF and CLOROTIC compared with ADVOR (Figure 4).
Figure 4.
(A) Comparison of cumulative urine output in patients with acute decompensated heart failure in ADVOR, CLOROTIC, and EMPAG-HF. Cumulative urine output on Day 3 (48 h post-randomization) in each group is calculated based on published data.10,11,20 Per cent change describes group differences compared with placebo in each individual study. (B) Daily urine output under treatment (difference to placebo indexed per day). HCTZ, hydrochlorothiazide.
The trial was different from another recent study, the EMPULSE trial.19 This study randomized patients hospitalized with acute decompensated HF randomized after 24 h (mean 3 days) following hospital admission followed for up to 90 days. EMPULSE showed significant clinical effects of 10 mg of empagliflozin compared with placebo based on a win ratio analysis. In comparison, EMPAG-HF utilized 25 mg of empagliflozin based on the hypothesis that the glucosuric and diuretic effects are more pronounced under the higher dose compared with only 10 mg of empagliflozin.12,13,15,16,19,21 The study approach and patient cohort of EMPULSE are comparable to the currently ongoing DICTATE-AHF (Efficacy and Safety of Dapagliflozin in Acute Heart Failure) study, i.e. testing the hypothesis that the addition of 10 mg of dapagliflozin in stabilized patients admitted with acute decompensated HF will change clinical outcomes.22 The study also requires initial stabilization and treatment and will include patients only after 24 h of hospitalization. Both studies exclude the clinically relevant early treatment time where therapies are initiated and the outcome is defined.
Conclusion
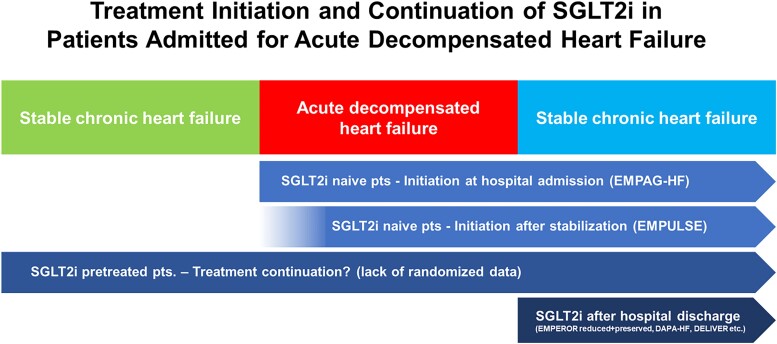
In conclusion, the current clinical evidence does not support a broad role for acetazolamide in the treatment algorithms for patients with acute decompensated HF due to the lack of clear clinical effects. In contrast, growing evidence supports the early introduction or even continuation of SGLT2i in patients with acute decompensated HF with comparable diuretic effects, similar safety profiles, and overwhelming evidence for medium- and long-term benefits in HF patients with both reduced and preserved ejection fractions (Figure 5).
Figure 5.
Treatment initiation and continuation of sodium–glucose cotransporter 2 inhibitors (SGLT2i) in patients admitted for acute decompensated heart failure.
Supplementary Material
Contributor Information
Wilfried Mullens, Ziekenhuis Oost-Limburg, Genk, Belgium and Hasselt University, Diepenbeek/Hasselt, Belgium.
Paul Christian Schulze, Department of Internal Medicine I, Cardiology, Angiology and Intensive Medical Care, University Hospital Jena, Jena, Germany.
Julian Westphal, Department of Internal Medicine I, Cardiology, Angiology and Intensive Medical Care, University Hospital Jena, Jena, Germany.
Jürgen Bogoviku, Department of Internal Medicine I, Cardiology, Angiology and Intensive Medical Care, University Hospital Jena, Jena, Germany.
Johann Bauersachs, Department of Cardiology and Angiology, Hannover Medical School, Hannover, Germany.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2. Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart: myth, magic, or molecular target? J Am Coll Cardiol 2012;60:2465–2472. 10.1016/j.jacc.2012.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Metra M, Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, et al. . Effects of serelaxin in patients with acute heart failure. N Engl J Med 2019;381:716–726. 10.1056/NEJMoa1801291 [DOI] [PubMed] [Google Scholar]
- 4. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. . Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43. 10.1056/NEJMoa1100171 [DOI] [PubMed] [Google Scholar]
- 5. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. . Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA 2007;297:1319–1331. 10.1001/jama.297.12.1319 [DOI] [PubMed] [Google Scholar]
- 6. Packer M, O'Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, et al. . Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 2017;376:1956–1964. 10.1056/NEJMoa1601895 [DOI] [PubMed] [Google Scholar]
- 7. Matsue Y, Suzuki M, Nagahori W, Yoshida K, Onishi Y, Satoh Y, et al. . Clinical effectiveness of tolvaptan in patients with acute decompensated heart failure and renal failure: design and rationale of the AQUAMARINE study. Cardiovasc Drugs Ther 2014;28:73–77. 10.1007/s10557-013-6491-8 [DOI] [PubMed] [Google Scholar]
- 8. Costanzo MR, Negoianu D, Jaski BE, Bart BA, Heywood JT, Anand IS, et al. . Aquapheresis versus intravenous diuretics and hospitalizations for heart failure. JACC Heart Fail 2016;4:95–105. 10.1016/j.jchf.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 9. Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, et al. . Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–683. 10.1016/j.jacc.2006.07.073 [DOI] [PubMed] [Google Scholar]
- 10. Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. . Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med 2022;387:1185–1195. 10.1056/NEJMoa2203094 [DOI] [PubMed] [Google Scholar]
- 11. Trullas JC, Morales-Rull JL, Casado J, Carrera-Izquierdo M, Sanchez-Marteles M, Conde-Martel A, et al. . Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J 2023;44:411–421. 10.1093/eurheartj/ehac689 [DOI] [PubMed] [Google Scholar]
- 12. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 13. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 14. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlavek J, et al. . Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020;323:1353–1368. 10.1001/jama.2020.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, et al. . Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-Preserved trial. Circulation 2021;144:1284–1294. 10.1161/CIRCULATIONAHA.121.056824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 17. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. . Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–1098. 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 18. Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation 2017;136:1643–1658. 10.1161/CIRCULATIONAHA.117.030012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. . The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022;28:568–574. 10.1038/s41591-021-01659-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schulze PC, Bogoviku J, Westphal J, Aftanski P, Haertel F, Grund S, et al. . Effects of early empagliflozin initiation on diuresis and kidney function in patients with acute decompensated heart failure (EMPAG-HF). Circulation 2022;146:289–298. 10.1161/CIRCULATIONAHA.122.059038 [DOI] [PubMed] [Google Scholar]
- 21. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. . Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023; 388:117–127. 10.1056/NEJMoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cox ZL, Collins SP, Aaron M, Hernandez GA, Iii ATM, Davidson BT, et al. . Efficacy and safety of dapagliflozin in acute heart failure: rationale and design of the DICTATE-AHF trial. Am Heart J 2021;232:116–124. 10.1016/j.ahj.2020.10.071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data were generated or analysed for or in support of this paper.