Abstract
Aims
Because an increased risk of amputation with canagliflozin was reported in the CANVAS trials, there has been a concern about the safety of sodium–glucose cotransporter 2 inhibitors in patients with peripheral artery disease (PAD) who are at higher risk of amputation.
Methods and results
A patient-level pooled analysis of the DAPA-HF and DELIVER trials, which evaluated the efficacy and safety of dapagliflozin in patients with heart failure (HF) with reduced, mildly reduced/preserved ejection fraction, respectively, was conducted. In both trials, the primary outcome was the composite of worsening HF or cardiovascular death, and amputation was a prespecified safety outcome. Peripheral artery disease history was available for 11 005 of the total 11 007 patients. Peripheral artery disease was reported in 809 of the 11 005 patients (7.4%). Median follow-up was 22 months (interquartile range 17–30). The rate of the primary outcome (per 100 person-years) was higher in PAD patients than that in non-PAD patients: 15.1 [95% confidence interval (CI) 13.1–17.3) vs. 10.6 (10.2–11.1]; adjusted hazard ratio 1.23 (95% CI 1.06–1.43). The benefit of dapagliflozin on the primary outcome was consistent in patients with [hazard ratio 0.71 (95% CI 0.54–0.94)] and without PAD [0.80 (95% CI 0.73–0.88)] (Pinteraction = 0.39). Amputations, while more frequent in PAD patients, were not more common with dapagliflozin, compared with placebo, irrespective of PAD status (PAD, placebo 4.2% vs. dapagliflozin 3.7%; no PAD, placebo 0.4% vs. dapagliflozin 0.4%) (Pinteraction = 1.00). Infection rather than ischaemia was the main trigger for amputation, even in patients with PAD.
Conclusion
The risk of worsening HF or cardiovascular death was higher in patients with PAD, as was the risk of amputation. The benefits of dapagliflozin were consistent in patients with and without PAD, and dapagliflozin did not increase the risk of amputation.
Keywords: Heart failure with reduced ejection fraction, Heart failure with preserved ejection fraction, Peripheral artery disease, Amputation, Clinical trial
Structured Graphical Abstract
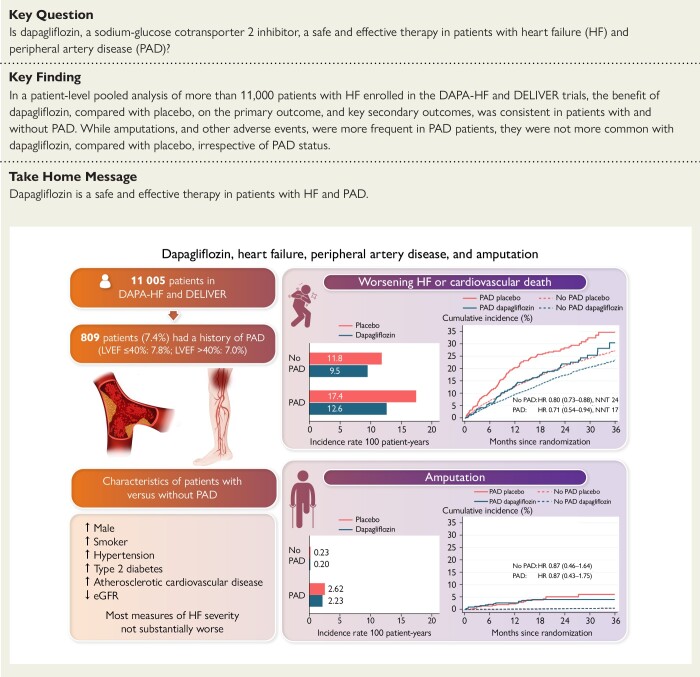
Structured Graphical Abstract.
The upper part of the figure shows the incidence rate, cumulative incidence, and the risk of the primary outcome (worsening HF or cardiovascular death) with dapagliflozin compared with placebo according to PAD status. The lower part of the figure shows the incidence rate, cumulative incidence, and the risk of amputations with dapagliflozin compared with placebo according to PAD status. eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; PAD, peripheral artery disease.
Introduction
Since the Canagliflozin Cardiovascular Assessment Study (CANVAS) trials reported a higher rate of lower extremity amputations in the canagliflozin group, compared to the placebo group, there has been a concern about the safety of sodium–glucose cotransporter 2 (SGLT2) inhibitors in patients with peripheral artery disease (PAD) who have an inherently higher risk of amputation compared to individuals without PAD.1–3 Although the findings in CANVAS have not been replicated with other SGLT2 inhibitors or in other populations,4–14 some believe that this concern remains, including in people with heart failure (HF). This concern may, in part, be influenced by the notion that diuretics, ubiquitous in patients with HF,15 have also been associated with a heightened risk of amputation, possibly because these agents cause volume depletion and increased blood viscosity, thereby further impairing perfusion through an already compromised circulation.16–18 If this hypothesis is correct, then adding an SGLT2 inhibitor to a conventional diuretic could exacerbate this risk, since an SGLT2 inhibitor also causes diuresis (at least initially) and raises haematocrit. However, if these safety concerns were ill-founded, withholding SGLT2 inhibitors in HF patients with concomitant PAD would deprive a particularly high-risk group of patients of beneficial therapy. We and others have previously reported that HF patients with PAD are at up to a two-fold higher risk of death and a higher risk of hospital admission than HF patients without PAD.19–25 To address this question further, we examined the safety and efficacy of the SGLT2 inhibitor dapagliflozin in over 11 000 patients with HF, 809 of whom had a history of PAD, enrolled in two placebo-controlled randomized trials, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) and Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure trial (DELIVER).7,9
Methods
This was a post hoc analysis of two Phase 3 clinical trials. DAPA-HF and DELIVER were randomized, double-blind, controlled trials in patients with symptomatic HF and elevated natriuretic peptides, comparing the efficacy and safety of dapagliflozin 10 mg once daily with a matching placebo. The principal difference between the trials was that patients with a left ventricular ejection fraction (LVEF) ≤40% were randomized in DAPA-HF and those with a LVEF >40% in DELIVER. The design and primary results of both trials are published.7,9,26–29 The trial protocols were approved by Ethics Committees at all participating institutions, and all patients provided written informed consent.
Trial patients
Ambulatory patients in New York Heart Association (NYHA) functional Classes II to IV, with a LVEF ≤40% and elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP), were eligible for DAPA-HF.26 Participants were required to receive guideline-recommended treatments for HF with a reduced ejection fraction (HFrEF). Key exclusion criteria were a history of Type 1 diabetes, symptomatic hypotension or a systolic blood pressure <95 mmHg, and an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2.26
Ambulatory and hospitalized patients in NYHA functional Classes II–IV, with a LVEF >40% and elevated NT-proBNP, were eligible for DELIVER.28 Participants were required to have evidence of structural heart disease (left atrial enlargement or left ventricular hypertrophy). All patients had to be receiving at least intermittent diuretic therapy. The main exclusion criteria were similar to those in DAPA-HF, although the eGFR threshold was lower in DELIVER (25 mL/min/1.73 m2).28
History of peripheral artery disease
In both trials, data on medical and surgical history were investigator-reported and retrieved from the trial electronic case report forms (eCRF). The following eCRF variables were used to identify PAD: history of peripheral arterial occlusive disease, prior revascularization of a peripheral artery, and prior stent insertion in a peripheral artery. In a sensitivity analysis, the definition of PAD was expanded to include prior surgical amputation (any).
Trial outcomes
The primary outcome in both trials was the composite of a worsening HF event or cardiovascular death. In the present study, we also examined each of the components of the primary outcome; the composite of HF hospitalization or cardiovascular death; total HF hospitalizations (first and repeat HF hospitalizations) and cardiovascular death; the composite of myocardial infarction, stroke, or cardiovascular death; all-cause death; and change from baseline to 8 months in the Kansas City Cardiomyopathy Questionnaire (KCCQ) total symptom score (KCCQ-TSS), overall symptom score (KCCQ-OSS), and clinical summary score (KCCQ-CSS). The definition of death from cardiovascular causes included deaths of undetermined causes, following the prespecified statistical analysis plan. Worsening HF events and cause of death were adjudicated by an independent clinical events committee in both trials. Data on myocardial infarction and stroke during follow-up were collected systemically in both trials, although these were only adjudicated in DAPA-HF and not in DELIVER. In both trials, amputation was a prespecified safety outcome.
All the efficacy analyses were performed according to the intention-to-treat principle. The safety analysis was performed in patients who had undergone randomization and received at least one dose of dapagliflozin or placebo. A total of 18 randomized patients were excluded from the safety analysis.
Statistical analyses
Baseline characteristics according to PAD status were summarized as frequencies with percentages, means with standard deviation, or medians with interquartile ranges (IQRs). Differences in baseline characteristics were tested using the chi-square test for binary or categorical variables and the Wilcoxon test and two-sample t-test for nonnormal and normally distributed continuous variables, respectively.
Regardless of treatment allocation, time-to-event data were evaluated using the Kaplan–Meier estimator (all-cause death), the Aalen-Johansen estimator (all outcomes except all-cause death), and Cox proportional hazard models, stratified according to Type 2 diabetes status and trial and adjusted for treatment assignment and history of HF hospitalization (except in the analysis of all-cause death), and hazard ratios (HRs) with 95% confidence intervals (CIs) were reported. Total events were evaluated with semiparametric proportional rate models,30 stratified according to Type 2 diabetes status and trial and adjusted for treatment assignment and history of HF hospitalization, and rate ratios (RRs) with 95% CIs were reported. In addition, HRs and RRs, stratified according to Type 2 diabetes status and trial and adjusted for treatment assignment, a history of HF hospitalization, age, sex, geographical region, systolic blood pressure, heart rate, body mass index, log of NT-proBNP, eGFR, LVEF, NYHA functional class, a history of myocardial infarction, stroke, atrial fibrillation, and hypertension, were reported.
To compare the effects of dapagliflozin vs. placebo on clinical outcomes, time-to-event data and total events were evaluated with Cox proportional hazard models and semiparametric proportional rate models, respectively, and these models were stratified according to Type 2 diabetes status and trial and adjusted for a history of HF hospitalization (except in the analysis of all-cause death and amputation). The difference between treatment groups in the change in KCCQ scores from baseline to 8 months was analysed using mixed-effect models for repeated measurements, adjusted for baseline value, visit (months 4 and 8), treatment assignment, interaction of treatment and visit, and trial. The least-squares mean differences with 95% CI between treatment groups were reported.
The Wald test was used to test for interaction between the treatment effect of dapagliflozin and PAD status for all efficacy endpoints and for amputation, and the respective models included treatment assignment, PAD status, and their interaction as covariates, in addition to those described above. For the other safety outcomes, the Wald test was used to test for interaction between the treatment effect of dapagliflozin and PAD status in a logistic regression model, which included treatment assignment, PAD status, and their interaction as covariates.
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and STATA version 17.0 (College Station, TX, USA).
Results
Of the 11 007 patients randomized in DAPA-HF and DELIVER, 2 were excluded due to missing history related to PAD. Median follow-up was 22 months (IQR 17–30 months).
Patient characteristics
A total of 809 patients (7.4%) had a history of PAD at baseline. The prevalence was 7.8% and 7.0% in patients with a LVEF ≤40% and >40%, respectively. The prevalence in men was 8.6% compared with 5.0% in women and 9.3% in patients with Type 2 diabetes compared to 5.9% in those without.
Patients with PAD were more often men and White and slightly older than patients without PAD (Table 1). They were more likely to have a history of smoking, hypertension, Type 2 diabetes, coronary heart disease, and a prior stroke but less likely to have a history of atrial fibrillation. Patients with PAD also had lower eGFR and body mass index. Although patients with PAD had a longer duration of HF, they had a similar average LVEF and NYHA class distribution, no difference in the rate of prior HF hospitalization, and only marginal differences in KCCQ scores and NT-proBNP levels. Patients with PAD more often had HFrEF compared to patients without PAD.
Table 1.
Baseline characteristics according to a history of PAD
| No PAD | PAD | P-value | |
|---|---|---|---|
| n = 10 196 | n = 809 | ||
| Age (years), mean (SD) | 69.3 ± 10.6 | 70.7 ± 8.8 | <0.001 |
| Sex, n (%) | <0.001 | ||
| Women | 3663 (35.9) | 193 (23.9) | |
| Men | 6533 (64.1) | 616 (76.1) | |
| Race, n (%) | <0.001 | ||
| White | 7126 (69.9) | 644 (79.6) | |
| Asian | 2273 (22.3) | 117 (14.5) | |
| Black or African American | 358 (3.5) | 27 (3.3) | |
| Other | 439 (4.3) | 21 (2.6) | |
| Geographic region, n (%) | <0.001 | ||
| Europe and Saudi Arabia | 4743 (46.5) | 414 (51.2) | |
| North America | 1350 (13.2) | 178 (22.0) | |
| South America | 1892 (18.6) | 106 (13.1) | |
| Asia/Pacific | 2211 (21.7) | 111 (13.7) | |
| Physiological measures | |||
| Systolic blood pressure (mmHg), mean (SD) | 125.2 ± 16.1 | 128.2 ± 16.1 | <0.001 |
| Diastolic blood pressure (mmHg), mean (SD) | 73.8 ± 10.4 | 73.4 ± 10.1 | 0.30 |
| Heart rate (b.p.m.), mean (SD) | 71.6 ± 11.8 | 70.0 ± 11.2 | <0.001 |
| Body mass index (kg/m2), mean (SD) | 29.2 ± 6.1 | 28.6 ± 5.5 | 0.017 |
| Body mass index (kg/m2), n (%) | 0.041 | ||
| <18.5 | 133 (1.3) | 8 (1.0) | |
| 18.5–24.9 | 2410 (23.7) | 194 (24.0) | |
| 25.0–29.9 | 3482 (34.2) | 312 (38.6) | |
| 30–34.9 | 2404 (23.6) | 182 (22.5) | |
| ≥ 35.0 | 1759 (17.3) | 113 (14.0) | |
| NT-proBNP (pg/mL), median (IQR) | 1172 (703–2107) | 1269 (683–2336) | 0.12 |
| Atrial fibrillation/flutter on ECG | 1531 (1025–2484) | 1710 (1135–3056) | 0.006 |
| No atrial fibrillation/flutter on ECG | 959 (564–1815) | 1077 (578–2060) | 0.073 |
| Haemoglobin A1c (%), mean (SD) | 6.5 ± 1.4 | 6.8 ± 1.5 | <0.001 |
| Creatinine (μmol/L), mean (SD) | 102.8 ± 30.6 | 110.4 ± 32.0 | <0.001 |
| eGFR (mL/min/1.73m2), mean (SD) | 63.4 ± 19.5 | 59.4 ± 18.3 | <0.001 |
| Smoking status, n (%) | <0.001 | ||
| Current | 1042 (10.2) | 135 (16.7) | |
| Former | 3939 (38.6) | 413 (51.1) | |
| Never | 5215 (51.1) | 261 (32.3) | |
| Duration of HF, n (%) | 0.008 | ||
| 0–3 months | 680 (6.7) | 38 (4.7) | |
| >3–6 months | 916 (9.0) | 69 (8.5) | |
| >6–12 months | 1301 (12.8) | 94 (11.6) | |
| >1–2 years | 1571 (15.4) | 110 (13.6) | |
| >2–5 years | 2482 (24.4) | 192 (23.7) | |
| >5 years | 3241 (31.8) | 306 (37.8) | |
| LVEF (%), mean (SD) | 44.2 ± 14.0 | 43.6 ± 13.1 | 0.23 |
| LVEF (%), n (%) | 0.040 | ||
| ≤ 40 | 4375 (42.9) | 372 (46.0) | |
| 41–49 | 1947 (19.1) | 166 (20.5) | |
| ≥ 50 | 3874 (38.0) | 271 (33.5) | |
| NYHA class, n (%) | 0.11 | ||
| II | 7353 (72.1)a | 562 (69.5) | |
| III/IV | 2843 (27.9) | 247 (30.5) | |
| KCCQ-TSS, mean (SD) | 71.8 ± 22.0 | 69.0 ± 22.5 | <0.001 |
| KCCQ-CSS, mean (SD) | 69.8 ± 20.7 | 66.8 ± 21.2 | <0.001 |
| KCCQ-OSS, mean (SD) | 67.5 ± 20.4 | 64.9 ± 20.9 | <0.001 |
| Medical history, n (%) | |||
| Hospitalization for HF | 4430 (43.4) | 359 (44.4) | 0.61 |
| Time from last HF hospitalization, n (%) | 0.19 | ||
| No prior HF hospitalization | 5767 (56.6) | 450 (55.6) | |
| 0–3 months | 1286 (12.6) | 84 (10.4) | |
| 3–6 months | 655 (6.4) | 62 (7.7) | |
| 6–12 months | 782 (7.7) | 65 (8.0) | |
| >1 year | 1706 (16.7) | 148 (18.3) | |
| Atrial fibrillation | 4936 (48.4) | 347 (42.9) | 0.002 |
| Stroke | 924 (9.1) | 139 (17.2) | <0.001 |
| Myocardial infarction | 3318 (32.5) | 413 (51.1) | <0.001 |
| PCI or CABG | 3678 (36.1) | 512 (63.3) | <0.001 |
| Angina | 2327 (22.8) | 283 (35.0) | <0.001 |
| Hypertension | 8343 (81.8) | 731 (90.4) | <0.001 |
| Type 2 diabetes | 4345 (42.6) | 444 (54.9) | <0.001 |
| Treatment, n (%) | |||
| ACEi | 4559 (44.7) | 396 (48.9) | 0.020 |
| ARB | 3338 (32.7) | 240 (29.7) | 0.073 |
| ACEi/ARB | 7860 (77.1) | 633 (78.2) | 0.45 |
| ARNI | 746 (7.3) | 63 (7.8) | 0.62 |
| Beta-blocker | 9010 (88.4) | 724 (89.5) | 0.34 |
| MRA | 5634 (55.3) | 402 (49.7) | 0.002 |
| Loop diuretic | 7985 (78.3) | 649 (80.2) | 0.20 |
| Any diuretic | 9775 (95.9) | 779 (96.3) | 0.56 |
| Digoxin | 1118 (11.0) | 65 (8.0) | 0.010 |
| Statin | 6559 (64.3) | 656 (81.1) | <0.001 |
| Antiplatelet | 4656 (45.7) | 566 (70.0) | <0.001 |
| Anticoagulant | 4990 (48.9) | 361 (44.6) | 0.018 |
| CRT-P/CRT-D | 424 (4.2) | 30 (3.7) | 0.54 |
| ICD/CRT-D | 1279 (12.5) | 131 (16.2) | 0.003 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CABG, coronary artery bypass graft; CSS, clinical summary score; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide; OSS, overall summary score; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; SD, standard deviation; TSS, total symptom score.
1 additional patient was NYHA Class I.
Regarding pharmacological therapy, patients with PAD were more frequently treated with a statin and antiplatelet therapy but less likely to receive an MRA, than those without PAD. They were also more likely to have a cardiac defibrillator.
Outcomes according to a history of peripheral artery disease
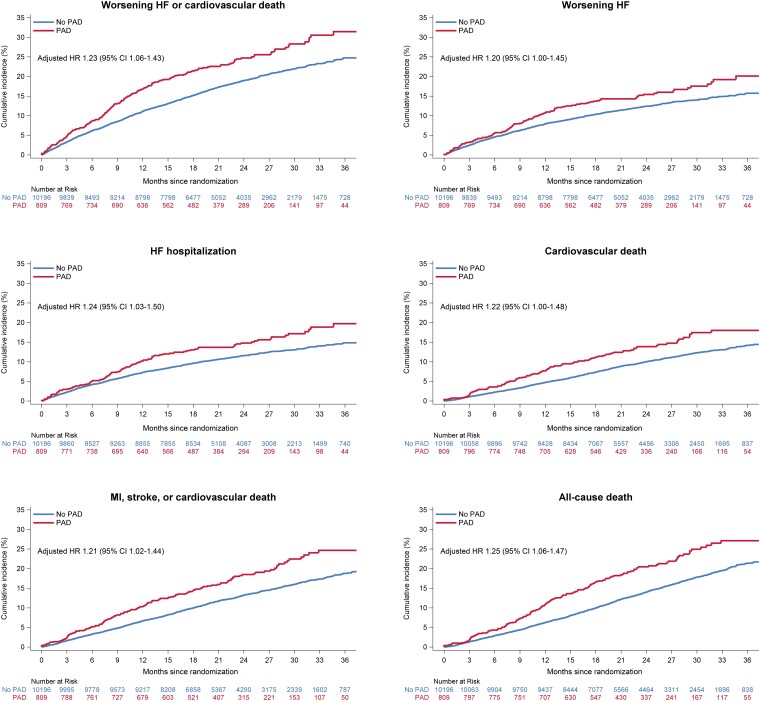
Patients with PAD had a higher risk of all clinical outcomes, compared to individuals without PAD (Figure 1 and Table 2). After adjustment for prognostic variables, including NT-proBNP, the relative risk in patients with PAD, compared to those without, was attenuated but remained significantly higher for all clinical outcomes, except the composite of total HF hospitalizations and cardiovascular death where the risk was numerically but not significantly higher (Table 2). For example, the unadjusted HR for the primary outcome in patients with PAD, compared to those without, was 1.34 (95% CI 1.16–1.55), and the adjusted HR was 1.23 (1.06–1.43). The corresponding HRs for all-cause mortality were 1.46 (1.25–1.72) and 1.25 (1.06–1.47), respectively.
Figure 1.
Outcomes in patients with and without a history of peripheral artery disease. This figure shows the cumulative incidence of outcomes according to peripheral artery disease status at baseline. The Cox models were stratified according to Type 2 diabetes status and trial and adjusted for treatment assignment, a history of heart failure hospitalization, age, sex, geographical region, systolic blood pressure, heart rate, body mass index, log of N-terminal pro-B-type natriuretic peptide, estimated glomerular filtration rate, left ventricular ejection fraction, New York Heart Association functional class, a history of myocardial infarction, stroke, atrial fibrillation, and hypertension. CI, confidence interval; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PAD, peripheral artery disease.
Table 2.
Outcomes according to a history of PAD
| No PAD | PAD | |
|---|---|---|
| n = 10 196 | n = 809 | |
| Worsening HF or cardiovascular death | ||
| No. of events (%) | 1918 (18.8) | 202 (25.0) |
| Event rate per 100 person-years (95% CI) | 10.6 (10.2–11.1) | 15.1 (13.1–17.3) |
| HR (95% CI)a | Reference | 1.34 (1.16–1.55) |
| HR (95% CI)b | Reference | 1.23 (1.06–1.43) |
| Worsening HF | ||
| No. of events (%) | 1.258 (12.3) | 127 (15.7) |
| Event rate per 100 person-years (95% CI) | 7.0 (6.6–7.4) | 9.5 (8.0–11.3) |
| HR (95% CI)a | Reference | 1.28 (1.06–1.53) |
| HR (95% CI)b | Reference | 1.20 (1.00–1.45) |
| HF hospitalization | ||
| No. of events (%) | 1173 (11.5) | 123 (15.2) |
| Event rate per 100 person-years (95% CI) | 6.5 (6.1–6.8) | 9.1 (7.6–10.9) |
| HR (95% CI)a | Reference | 1.32 (1.10–1.59) |
| HR (95% CI)b | Reference | 1.24 (1.03–1.50) |
| Cardiovascular death | ||
| No. of events (%) | 1018 (10.0) | 114 (14.1) |
| Event rate per 100 person-years (95% CI) | 5.3 (5.0–5.6) | 7.8 (6.5–9.3) |
| HR (95% CI)a | Reference | 1.41 (1.16–1.71) |
| HR (95% CI)b | Reference | 1.22 (1.00–1.48) |
| Myocardial infarction, stroke, or cardiovascular death | ||
| No. of events (%) | 1353 (13.3) | 150 (18.5) |
| Event rate per 100 person-years (95% CI) | 7.2 (6.8–7.6) | 10.6 (9.0–12.4) |
| HR (95% CI)a | Reference | 1.42 (1.20–1.68) |
| HR (95% CI)b | Reference | 1.21 (1.02–1.44) |
| All-cause death | ||
| No. of events (%) | 1460 (14.3) | 168 (20.8) |
| Event rate per 100 person-years (95% CI) | 7.6 (7.2–8.0) | 11.4 (9.8–13.3) |
| HR (95% CI)a | Reference | 1.46 (1.25–1.72) |
| HR (95% CI)b | Reference | 1.25 (1.06–1.47) |
| Total HF hospitalizations and cardiovascular death | ||
| No. of events | 2854 | 302 |
| RR (95% CI)a | Reference | 1.32 (1.11–1.56) |
| RR (95% CI)b | Reference | 1.17 (0.98–1.38) |
CI, confidence interval; HF, heart failure; HR, hazard ratio; RR, rate ratio.
Models were stratified by Type 2 diabetes status and trial and adjusted for treatment assignment and heart failure hospitalization (except in the analysis of all-cause death).
Models were stratified by Type 2 diabetes status and trial and adjusted for treatment assignment, a history of heart failure hospitalization (except in the analysis of all-cause death), age, sex, geographical region, systolic blood pressure, heart rate, body mass index, log of n-terminal pro-B-type natriuretic peptide, estimated glomerular filtration rate, left ventricular ejection fraction, New York Heart Association, a history or myocardial infarction, stroke, atrial fibrillation, and hypertension.
Efficacy of dapagliflozin on clinical outcomes according to a history of peripheral artery disease
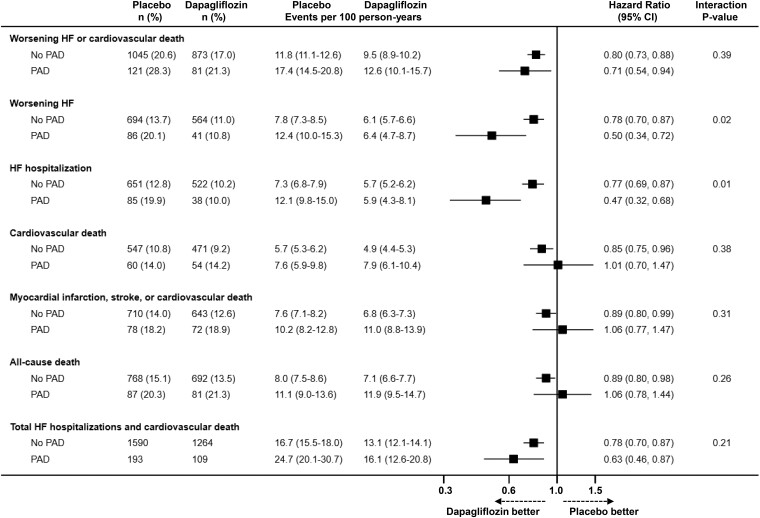
Dapagliflozin, compared with placebo, reduced the risk of worsening HF or cardiovascular death to the same extent in patients with [HR 0.71 (95% CI 0.54–0.94)] and without [0.80 (0.73–0.88)] PAD, with no interaction between PAD and effect of treatment (Pinteraction = 0.39) (Table 3 and Figure 2). The effect of dapagliflozin on the primary outcome was consistent in both LVEF groups (LVEF ≤40% vs. >40%) according to history of PAD (see Supplementary material online, Table S1).
Table 3.
Effects of dapagliflozin compared with placebo on outcomes according to a history of PAD
| No PAD n = 10 196 | PAD n = 809 | P-value for interaction | |||
|---|---|---|---|---|---|
| Placebo | Dapagliflozin | Placebo | Dapagliflozin | ||
| n = 5074 | n = 5122 | n = 428 | n = 381 | ||
| Worsening HF or cardiovascular death | 0.39 | ||||
| No. of events (%) | 1045 (20.6) | 873 (17.0) | 121 (28.3) | 81 (21.3) | |
| Event rate per 100 person-years (95% CI) | 11.8 (11.1–12.6) | 9.5 (8.9–10.2) | 17.4 (14.5–20.8) | 12.6 (10.1–15.7) | |
| HR (95% CI)a | 0.80 (0.73–0.88) | 0.71 (0.54–0.94) | |||
| Worsening HF | 0.02 | ||||
| No. of events (%) | 694 (13.7) | 564 (11.0) | 86 (20.1) | 41 (10.8) | |
| Event rate per 100 person-years (95% CI) | 7.8 (7.3–8.5) | 6.1 (5.7–6.6) | 12.4 (10.0–15.3) | 6.4 (4.7–8.7) | |
| HR (95% CI)a | 0.78 (0.70–0.87) | 0.50 (0.34–0.72) | |||
| HF hospitalization | 0.01 | ||||
| No. of events (%) | 651 (12.8) | 522 (10.2) | 85 (19.9) | 38 (10.0) | |
| Event rate per 100 person-years (95% CI) | 7.3 (6.8–7.9) | 5.7 (5.2–6.2) | 12.1 (9.8–15.0) | 5.9 (4.3–8.1) | |
| HR (95% CI)a | 0.77 (0.69–0.87) | 0.47 (0.32–0.68) | |||
| Cardiovascular death | 0.38 | ||||
| No. of events (%) | 547 (10.8) | 471 (9.2) | 60 (14.0) | 54 (14.2) | |
| Event rate per 100 person-years (95% CI) | 5.7 (5.3–6.2) | 4.9 (4.4–5.3) | 7.6 (5.9–9.8) | 7.9 (6.1–10.4) | |
| HR (95% CI)a | 0.85 (0.75–0.96) | 1.01 (0.70–1.47) | |||
| Myocardial infarction, stroke, or cardiovascular death | 0.31 | ||||
| No. of events (%) | 710 (14.0) | 643 (12.6) | 78 (18.2) | 72 (18.9) | |
| Event rate per 100 person-years (95% CI) | 7.6 (7.1–8.2) | 6.8 (6.3–7.3) | 10.2 (8.2–12.8) | 11.0 (8.8–13.9) | |
| HR (95% CI)a | 0.89 (0.80–0.99) | 1.06 (0.77–1.47) | |||
| All-cause death | 0.26 | ||||
| No. of events (%) | 768 (15.1) | 692 (13.5) | 87 (20.3) | 81 (21.3) | |
| Event rate per 100 person-years (95% CI) | 8.0 (7.5–8.6) | 7.1 (6.6–7.7) | 11.1 (9.0–13.6) | 11.9 (9.5–14.7) | |
| HR (95% CI)a | 0.89 (0.80–0.98) | 1.06 (0.78–1.44) | |||
| Total HF hospitalizations and cardiovascular death | 0.21 | ||||
| No. of events | 1590 | 1264 | 193 | 109 | |
| Event rate per 100 person-years (95% CI) | 16.7 (15.5–18.0) | 13.1 (12.1–14.1) | 24.7 (20.1–30.7) | 16.1 (12.6–20.8) | |
| RR (95% CI)a | 0.78 (0.70–0.87) | 0.63 (0.46–0.87) | |||
| KCCQ-TSS | 0.78 | ||||
| Change from baseline to 8 months (95% CI)b | 4.6 (4.1–5.1) | 7.0 (6.5–7.6) | 3.9 (1.9–5.9) | 6.7 (4.7–8.8) | |
| Placebo-corrected change at 8 months (95% CI)b | 2.4 (1.7–3.1) | 2.8 (0.0–5.7) | |||
| KCCQ-OSS | 0.61 | ||||
| Change from baseline to 8 months (95% CI)b | 4.5 (4.1–5.0) | 6.6 (6.1–7.1) | 3.9 (2.1–5.6) | 6.8 (5.0–8.7) | |
| Placebo-corrected change at 8 months (95% CI)b | 2.1 (1.4–2.7) | 3.0 (0.4–5.5) | |||
| KCCQ-CSS | 0.70 | ||||
| Change from baseline to 8 months (95% CI)b | 3.9 (3.5–4.4) | 6.2 (5.8–6.7) | 3.1 (1.3–5.0) | 6.1 (4.2–8.0) | |
| Placebo-corrected change at 8 months (95% CI)b | 2.3 (1.6–3.0) | 2.9 (0.3–5.6) | |||
CI, confidence interval; HF, heart failure; HR, hazard ratio; RR, rate ratio.
Models were stratified by Type 2 diabetes status and trial and adjusted for a history of HF hospitalization (except in the analysis of all-cause death).
Mixed-effect models for repeated measurements adjusted for baseline value, visit (months 4 and 8), randomized treatment, interaction of treatment and visit, and trial.
Cardiovascular death includes undetermined deaths.
Figure 2.
Effects of dapagliflozin compared with placebo on outcomes in patients with and without a history of peripheral artery disease. This figure shows the effect of dapagliflozin, compared with placebo, on outcomes according to peripheral artery disease status at baseline. The models were stratified according to Type 2 diabetes status and trial and adjusted for treatment assignment and history of heart failure hospitalization (except in the analysis of all-cause death). CI, confidence interval; HF, heart failure; PAD, peripheral artery disease.
The effect of dapagliflozin was also consistent, regardless of PAD history, for all the other clinical outcomes examined, except for worsening HF and HF hospitalization; dapagliflozin significantly reduced the risk of these outcomes in both patients with and without PAD, but the reduction appeared to be larger in those with PAD, with nominally significant interactions between PAD status and the effect of dapagliflozin on these outcomes (Table 3, Figure 2).
The mean increase in KCCQ scores from baseline to 8 months was greater with dapagliflozin compared with placebo in both patients with and without PAD (Pinteraction ≥ 0.61).
Absolute risk reduction according to the presence or absence of peripheral artery disease
Because the absolute risk was higher in patients with PAD, the absolute benefit was also greatest in those patients. Assuming a constant treatment effect size in each subgroup, the number of patients needed to treat (NNT) over the trial duration to prevent one participant from experiencing the primary endpoint was 17 (95% CI 13–26) for patients with PAD and 24 (18–36) for patients without PAD.
Safety and tolerability of dapagliflozin on clinical outcomes according to a history of peripheral artery disease
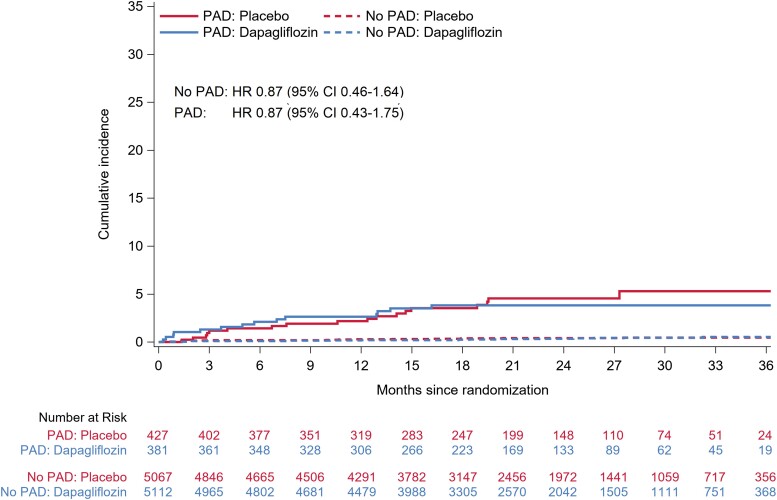
Examination of the placebo groups showed that adverse events were more common among patients with PAD compared to those without. However, there was no difference in the rate of these events between dapagliflozin and placebo among patients with or without PAD (Table 4). In particular, the number and rate of amputations were small and did not differ between treatments in patients with [HR 0.87 (95% CI 0.43–1.75)] or without PAD [0.87 (0.46–1.64)] (Table 5 and Figure 3). Infection rather than ischaemia was the main trigger for amputation, even in patients with PAD (see Supplementary material online, Table S2).
Table 4.
Adverse events of dapagliflozin compared with placebo according to a history of PAD
| No PAD n = 10 179 | PAD n = 808 | P-value for interaction | |||
|---|---|---|---|---|---|
| Placebo | Dapagliflozin | Placebo | Dapagliflozin | ||
| n = 5067 | n = 5112 | n = 427 | n = 381 | ||
| Discontinuation of study drug for any reason, n (%) | 623 (12.3) | 629 (12.3) | 76 (17.8) | 64 (16.8) | 0.72 |
| Discontinuation of study drug due to an adverse event, n (%) | 256 (5.1) | 262 (5.1) | 40 (9.4) | 32 (8.4) | 0.61 |
| Volume depletiona, n (%) | 178 (3.5) | 199 (3.9) | 21 (4.9) | 28 (7.3) | 0.31 |
| Renal adverse eventb, n (%) | 226 (4.5) | 202 (4.0) | 35 (8.2) | 35 (9.2) | 0.35 |
| Major hypoglycaemia, n (%) | 9 (0.2) | 12 (0.2) | 2 (0.5) | 0 (0.0) | N/A |
| Diabetic ketoacidosis, n (%) | 0 (0.0) | 3 (0.1) | 0 (0.0) | 2 (0.5) | N/A |
N/A, not applicable.
A total of 18 randomized patients were excluded from the safety analysis, as these were performed in patients who had undergone randomization and received at least one dose of dapagliflozin or placebo.
Any serious adverse event or adverse event that led to discontinuation of dapagliflozin or placebo that was suggestive of volume depletion in DELIVER.
Any renal serious adverse event or adverse event that led to discontinuation of dapagliflozin or placebo in DELIVER.
Table 5.
Amputations in patients randomized to dapagliflozin or placebo according to a history of PAD, overall and according to diuretic use at baseline and Type 2 diabetes
| No PAD | PAD | P-value for interaction | |||
|---|---|---|---|---|---|
| Placebo | Dapagliflozin | Placebo | Dapagliflozin | ||
| Full population | 1.00 | ||||
| No. of events (%) | 20 (0.39) | 18 (0.35) | 18 (4.22) | 14 (3.67) | |
| Event rate per 100 person-years (95% CI) | 0.23 (0.15–0.35) | 0.20 (0.12–0.31) | 2.62 (1.65–4.16) | 2.23 (1.32–3.76) | |
| HR (95% CI)a | 0.87 (0.46–1.64) | 0.87 (0.43–1.75) | |||
| No diuretics | N/A | ||||
| No. of events (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Event rate per 100 person-years (95% CI) | N/A | N/A | N/A | N/A | |
| HR (95% CI)a | N/A | N/A | |||
| Diuretics | 0.98 | ||||
| No. of events (%) | 20 (0.41) | 18 (0.37) | 18 (4.37) | 14 (3.83) | |
| Event rate per 100 person-years (95% CI) | 0.24 (0.15–0.37) | 0.20 (0.13–0.32) | 2.70 (1.70–4.29) | 2.31 (1.37–3.91) | |
| HR (95% CI)a | 0.86 (0.46–1.63) | 0.86 (0.43–1.73) | |||
| No Type 2 diabetes | 0.60 | ||||
| No. of events (%) | 3 (0.10) | 3 (0.10) | 4 (2.13) | 2 (1.14) | |
| Event rate per 100 person-years (95% CI) | 0.06 (0.02–0.18) | 0.06 (0.02–0.18) | 1.28 (0.48–3.42) | 0.69 (0.17–2.76) | |
| HR (95% CI)a | 0.96 (0.19–4.77) | 0.52 (0.09–2.83) | |||
| Type 2 diabetes | 0.81 | ||||
| No. of events (%) | 17 (0.79) | 15 (0.69) | 14 (5.86) | 12 (5.85) | |
| Event rate per 100 person-years (95% CI) | 0.46 (0.29–0.74) | 0.39 (0.23–0.64) | 3.73 (2.21–6.29) | 3.55 (2.01–6.24) | |
| HR (95% CI)a | 0.85 (0.42–1.69) | 0.97 (0.45–2.11) | |||
CI, confidence interval; HR, hazard ratio; N/A, not applicable.
A total of 18 randomized patients were excluded from the safety analysis, as these were performed in patients who had undergone randomization and received at least one dose of dapagliflozin or placebo.
Models were stratified by Type 2 diabetes status (except in the subgroup analysis of patients with and without Type 2 diabetes) and trial.
Figure 3.
Amputations in patients randomized to dapagliflozin or placebo according to a history of peripheral artery disease. This figure shows the cumulative incidence of amputations according to peripheral artery disease status at baseline. The Cox models were stratified according to Type 2 diabetes status and trial and adjusted for treatment assignment. CI, confidence interval; HF, heart failure; PAD, peripheral artery disease.
In high-risk subgroups (i.e. those with diuretic use at baseline and Type 2 diabetes), there was also no difference in the risk of amputation between dapagliflozin and placebo among patients with or without PAD (Table 5).
Sensitivity analysis including patients with a history of amputation
In a sensitivity analysis, the definition of PAD was expanded to include prior surgical amputation. With the expanded definition, 70 patients were reclassified from the no PAD group to the PAD group. Data on outcomes according to a history of PAD are shown in Supplementary material online, Table S3, and data on the effects of dapagliflozin, compared with placebo, on clinical outcomes and adverse events, are presented in Supplementary material online, Tables S4 and S5, respectively. These analyses yielded similar findings.
Discussion
There are three key findings of this post hoc analysis of the DAPA-HF and DELIVER trials. First, although relatively uncommon in patients with HF, concomitant PAD was associated with a higher risk of poor clinical outcomes, even after adjustment for known prognostic variables. Second, dapagliflozin had similar beneficial effects on clinical outcomes in patients with and without PAD. This was also the case in our sensitivity analysis which included patients with a history of amputation. Third, while patients with PAD experienced more adverse events, including amputations, the rates of these events were similar between dapagliflozin and placebo-treated patients with and without PAD (Structured Graphical Abstract).
The prevalence of clinically reported PAD among patients with HF in large registries is typically around 11% to 13%, whereas in prior trials, it ranged from 5% to 16% (e.g. ATMOSPHERE 5.1%, PARAGON-HF 5.4%, PARADIGM-HF 5.9%, HF-ACTION 6.8%, and BEST 16.4%),19,22,31–37 the variation likely reflecting the proportion of men, smokers, and geographical regions included.38,39 There are few reports of the prevalence according to LVEF phenotype, but one large German study documented a prevalence of 10.5% in patients with HFrEF compared to 7.6% in patients with HFpEF.40 In the two trials described here, we found an overall prevalence of 7.4%, with a prevalence of 7.8% and 7.0% in patients with a LVEF ≤40% and >40%, respectively, consistent with the aforementioned reports. It should be noted, however, that studies measuring ankle brachial arterial pressure index report a prevalence of PAD two to three times higher, although it is not clear how the haemodynamic derangement in HF affects the interpretation of this index.38,39,41,42
Also consistent with prior trials, we found that patients with PAD were more likely to be smokers, have Type 2 diabetes, and have other manifestations of atherosclerotic cardiovascular disease. Interestingly, the overall severity of HF, as reflected by NYHA class, LVEF, NT-proBNP level, history of HF hospitalization, etc., did not differ greatly between patients with and without PAD. However, patients with PAD were at greater risk of worsening HF than those without, although the elevation in risk was not as substantial as that for mortality. We were able to describe a broader range of outcomes than in prior studies and to adjust more comprehensively for other prognostic variables, including NT-proBNP.21,43 Despite covariate adjustment, PAD remained an independent predictor of a higher risk of most outcomes examined. Therefore, it was important to show that dapagliflozin was at least as effective in reducing the risk of worsening HF or cardiovascular death in these high-risk patients as it was in participants without PAD. Indeed, because patients with PAD were at higher absolute risk, their absolute benefit was greater, reflected in a smaller NNT (17 in patients with vs. 24 in those without PAD) even when conservatively calculated by applying the overall trial relative risk reduction to each subgroup. Similarly, dapagliflozin was as well tolerated in patients with PAD as in those without, although patients with PAD were more likely to experience adverse events overall (whether on dapagliflozin or placebo). While relatively few patients overall (n = 70) had an amputation, the rate was similar to that reported in other trials and substantially more common among patients with PAD (3.96%) than those without (0.37%).44 However, amputations were not more common with dapagliflozin than with placebo. Indeed, among patients with PAD, there were numerically fewer amputations in the dapagliflozin group. Interestingly, as reported in DECLARE-TIMI 58, infection rather than limb ischaemia was reported as the principal triggering event associated with amputation,44 emphasizing the importance of foot care in patients with HF and PAD, Type 2 diabetes, or both.
Study limitations
There are several limitations to this study. Patients enrolled in clinical trials are selected according to specific inclusion and exclusion criteria, and our results may not be generalizable to all patients with HF in the general population, including those with a systolic blood pressure <95 mmHg or an eGFR <30 mL/min/1.73 m2. Patients enrolled in trials are also usually better treated than those who are not. Although PAD and amputation were not exclusion criteria, investigators may have under-enrolled patients with these problems. Some degree of misclassification of PAD status cannot be precluded as PAD was investigator-reported, and no specific instructions as to how to define PAD were provided. Measurement of ankle brachial material pressure index was not required, and the prevalence of PAD reported in studies using this index is much higher than in studies based on a clinical diagnosis. It is also possible that functional limitations due to PAD may have influenced patient answers to the KCCQ. Finally, the large number of endpoints assessed and the post hoc nature of the present study may increase the risk of Type 1 errors.
Conclusions
In this post hoc analysis of two Phase 3 clinical trials, the risk of worsening HF or cardiovascular death was higher in HF patients with PAD compared to those without PAD. The benefit of dapagliflozin was consistent in patients with and without PAD, and dapagliflozin was safe and well tolerated in HF patients with PAD.
Supplementary Material
Acknowledgements
None.
Contributor Information
Jawad H Butt, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK; Department of Cardiology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark.
Toru Kondo, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK; Department of Cardiology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Mingming Yang, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Pardeep S Jhund, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Kieran F Docherty, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Muthiah Vaduganathan, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Brian L Claggett, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Adrian F Hernandez, Duke University Medical Center, Durham, NC, USA.
Carolyn S P Lam, National Heart Centre Singapore, Singapore; Duke-National University of Singapore, Singapore.
Silvio E Inzucchi, Yale School of Medicine, New Haven, CT, USA.
Felipe A Martinez, University of Cordoba, Cordoba, Argentina.
Rudolf A de Boer, Erasmus Medical Center, Rotterdam, The Netherlands.
Mikhail N Kosiborod, Saint Luke’s Mid America Heart Institute, Kansas City, MO, USA.
Akshay S Desai, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Lars Køber, Department of Cardiology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Piotr Ponikowski, Department of Heart Disease, Wroclaw Medical University, Wroclaw, Poland.
Marc S Sabatine, TIMI Study Group, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Sanjiv J Shah, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Natalia Zaozerska, Late-Stage Development, Cardiovascular, Renal, and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Ulrica Wilderäng, Late-Stage Development, Cardiovascular, Renal, and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Olof Bengtsson, Late-Stage Development, Cardiovascular, Renal, and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Scott D Solomon, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
John J V McMurray, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, 126 University Place, Glasgow G12 8TA, UK.
Supplementary data
Supplementary data are available at European Heart Journal online.
Pre-registered clinical trial number
The pre-registered clinical trial numbers are NCT03036124 and NCT03619213.
Ethical approval
The trial protocols were approved by Ethics Committees at all participating institutions, and all patients provided written informed consent.
Data availability
Trial data will be made available by the sponsor, AstraZeneca, in accordance with their data sharing policy.
Funding
The DAPA-HF and DELIVER trials were funded by AstraZeneca. T.K. receives grants from and the Uehara Memorial Foundation and the Japanese Heart Failure Society Tsuchiya Foundation. J.J.V.M. and P.S.J. are supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217 and the Vera Melrose Heart Failure Research Fund.
References
- 1. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 2. Matthews DR, Li Q, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, et al. Effects of canagliflozin on amputation risk in type 2 diabetes: the CANVAS program. Diabetologia 2019;62:926–938. 10.1007/s00125-019-4839-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnott C, Huang Y, Neuen BL, Di Tanna GL, Cannon CP, Oh R, et al. The effect of canagliflozin on amputation risk in the CANVAS program and the CREDENCE trial. Diabetes Obes Metab 2020;22:1753–1766. 10.1111/dom.14091 [DOI] [PubMed] [Google Scholar]
- 4. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 5. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 6. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 7. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 8. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 9. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med 2022;387:1089–1098. 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 10. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–1461. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 11. Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 12. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020;383:1425–1435. 10.1056/NEJMoa2004967 [DOI] [PubMed] [Google Scholar]
- 13. Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117–127. 10.1056/NEJMoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paul SK, Bhatt DL, Montvida O. The association of amputations and peripheral artery disease in patients with type 2 diabetes mellitus receiving sodium-glucose cotransporter type-2 inhibitors: real-world study. Eur Heart J 2021;42:1728–1738. 10.1093/eurheartj/ehaa956 [DOI] [PubMed] [Google Scholar]
- 15. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 16. Potier L, Roussel R, Velho G, Saulnier PJ, Bumbu A, Matar O, et al. Lower limb events in individuals with type 2 diabetes: evidence for an increased risk associated with diuretic use. Diabetologia 2019;62:939–947. 10.1007/s00125-019-4835-z [DOI] [PubMed] [Google Scholar]
- 17. Alshnbari A, Alkharaiji M, Anyanwagu U, Idris I. Diuretics and risk of lower extremity amputation amongst patients with insulin-treated type 2 diabetes—exploring the mechanism of possible sodium glucose co-transporter 2 inhibitor induced risk of lower extremity amputations. Curr Med Res Opin 2020;36:1985–1989. 10.1080/03007995.2020.1840340 [DOI] [PubMed] [Google Scholar]
- 18. Potier L, Mohammedi K, Velho G, Roussel R. SGLT2 Inhibitors and lower limb complications: the diuretic-induced hypovolemia hypothesis. Cardiovasc Diabetol 2021;20:107. 10.1186/s12933-021-01301-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmed MI, Aronow WS, Criqui MH, Aban I, Love TE, Eichhorn EJ, et al. Effects of peripheral arterial disease on outcomes in advanced chronic systolic heart failure: a propensity-matched study. Circ Heart Fail 2010;3:118–124. 10.1161/CIRCHEARTFAILURE.109.866558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sandesara PB, Hammadah M, Samman-Tahhan A, Kelli HM, O'Neal WT. Peripheral artery disease and risk of adverse outcomes in heart failure with preserved ejection fraction. Clin Cardiol 2017;40:692–696. 10.1002/clc.22716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei B, Qian C, Fang Q, Wang Y. The prognostic value of peripheral artery disease in heart failure: insights from a meta-analysis. Heart Lung Circ 2016;25:1195–1202. 10.1016/j.hlc.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 22. Jones WS, Clare R, Ellis SJ, Mills JS, Fischman DL, Kraus WE, et al. Effect of peripheral arterial disease on functional and clinical outcomes in patients with heart failure (from HF-ACTION). Am J Cardiol 2011;108:380–384. 10.1016/j.amjcard.2011.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inglis SC, McMurray JJ, Bohm M, Schaufelberger M, van Veldhuisen DJ, Lindberg M, et al. Intermittent claudication as a predictor of outcome in patients with ischaemic systolic heart failure: analysis of the controlled rosuvastatin multinational trial in heart failure trial (CORONA). Eur J Heart Fail 2010;12:698–705. 10.1093/eurjhf/hfq070 [DOI] [PubMed] [Google Scholar]
- 24. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998–1005. 10.1016/j.jacc.2011.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260–269. 10.1056/NEJMoa051530 [DOI] [PubMed] [Google Scholar]
- 26. McMurray JJV, DeMets DL, Inzucchi SE, Kober L, Kosiborod MN, Langkilde AM, et al. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur J Heart Fail 2019;21:665–675. 10.1002/ejhf.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McMurray JJV, DeMets DL, Inzucchi SE, Kober L, Kosiborod MN, Langkilde AM, et al. The dapagliflozin and prevention of adverse-outcomes in heart failure (DAPA-HF) trial: baseline characteristics. Eur J Heart Fail 2019;21:1402–1411. 10.1002/ejhf.1548 [DOI] [PubMed] [Google Scholar]
- 28. Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail 2021;23:1217–1225. 10.1002/ejhf.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solomon SD, Vaduganathan M, Claggett BL, de Boer RA, DeMets D, Hernandez AF, et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Heart Fail 2022;10:184–197. 10.1016/j.jchf.2021.11.006 [DOI] [PubMed] [Google Scholar]
- 30. Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Series B 2000;62:711–730. 10.1111/1467-9868.00259 [DOI] [Google Scholar]
- 31. Klein L, Grau-Sepulveda MV, Bonow RO, Hernandez AF, Williams MV, Bhatt DL, et al. Quality of care and outcomes in women hospitalized for heart failure. Circ Heart Fail 2011;4:589–598. 10.1161/CIRCHEARTFAILURE.110.960484 [DOI] [PubMed] [Google Scholar]
- 32. Vaduganathan M, Fonarow GC, Greene SJ, DeVore AD, Kavati A, Sikirica S, et al. Contemporary treatment patterns and clinical outcomes of comorbid diabetes Mellitus and HFrEF: the CHAMP-HF registry. JACC Heart Fail 2020;8:469–480. 10.1016/j.jchf.2019.12.015 [DOI] [PubMed] [Google Scholar]
- 33. Canepa M, Straburzynska-Migaj E, Drozdz J, Fernandez-Vivancos C, Pinilla JMG, Nyolczas N, et al. Characteristics, treatments and 1-year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long-Term Registry. Eur J Heart Fail 2018;20:100–110. 10.1002/ejhf.964 [DOI] [PubMed] [Google Scholar]
- 34. Balsam P, Ozieranski K, Kaplon-Cieslicka A, Borodzicz S, Tyminska A, Peller M, et al. Differences in clinical characteristics and 1-year outcomes of hospitalized patients with heart failure in ESC-HF Pilot and ESC-HF-LT registries. Pol Arch Intern Med 2019;129:106–116. 10.20452/pamw.4418 [DOI] [PubMed] [Google Scholar]
- 35. Krum H, McMurray JJ, Abraham WT, Dickstein K, Kober L, Desai AS, et al. The Aliskiren Trial to Minimize OutcomeS in Patients with HEart failure trial (ATMOSPHERE): revised statistical analysis plan and baseline characteristics. Eur J Heart Fail 2015;17:1075–1083. 10.1002/ejhf.408 [DOI] [PubMed] [Google Scholar]
- 36. Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Heart Fail 2018;11:e004962. 10.1161/CIRCHEARTFAILURE.118.004962 [DOI] [PubMed] [Google Scholar]
- 37. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, et al. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Eur J Heart Fail 2014;16:817–825. 10.1002/ejhf.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keswani AN, White CJ. The impact of peripheral arterial disease on patients with congestive heart failure. Heart Fail Clin 2014;10:327–338. 10.1016/j.hfc.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 39. Inglis SC, Hermis A, Shehab S, Newton PJ, Lal S, Davidson PM. Peripheral arterial disease and chronic heart failure: a dangerous mix. Heart Fail Rev 2013;18:457–464. 10.1007/s10741-012-9331-1 [DOI] [PubMed] [Google Scholar]
- 40. Edelmann F, Stahrenberg R, Gelbrich G, Durstewitz K, Angermann CE, Dungen HD, et al. Contribution of comorbidities to functional impairment is higher in heart failure with preserved than with reduced ejection fraction. Clin Res Cardiol 2011;100:755–764. 10.1007/s00392-011-0305-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adesunloye BA, Valadri R, Mbaezue NM, Onwuanyi AE. Impact of peripheral arterial disease on functional limitation in congestive heart failure: results from the national health and nutrition examination survey (1999–2004). Cardiol Res Pract 2012;2012:306852. 10.1155/2012/306852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hebert K, Lopez B, Michael C, Franco E, Dias A, Trahan P, et al. The prevalence of peripheral arterial disease in patients with heart failure by race and ethnicity. Congest Heart Fail 2010;16:118–121. 10.1111/j.1751-7133.2010.00140.x [DOI] [PubMed] [Google Scholar]
- 43. Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: the European Stroke Organization (ESO)The task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. 10.1093/eurheartj/ehx095 [DOI] [PubMed] [Google Scholar]
- 44. Bonaca MP, Wiviott SD, Zelniker TA, Mosenzon O, Bhatt DL, Leiter LA, et al. Dapagliflozin and cardiac, kidney, and limb outcomes in patients with and without peripheral artery disease in DECLARE-TIMI 58. Circulation 2020;142:734–747. 10.1161/CIRCULATIONAHA.119.044775 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Trial data will be made available by the sponsor, AstraZeneca, in accordance with their data sharing policy.