Abstract
Recent progress in pain research indicates distinct mechanisms underlying the pathogenesis and resolution of pain. Specialized pro-resolving mediators (SPMs), including resolvins, protectins, and maresins, are endogenous lipid mediators that are synthesized from omega-3 polyunsaturated fatty acids during the acute phase or resolution phase of inflammation. SPMs possess broad safety profiles and exhibit potent actions in resolving inflammation in preclinical and clinical models. Accumulating evidence in the past decade has demonstrated powerful analgesia of exogenous SPMs in rodent models of inflammatory, post-operative, neuropathic, and cancer pain. Furthermore, endogenous SPMs are produced by sham surgery and neuromodulation such as vagus nerve stimulation, spinal cord stimulation, and electroacupuncture. SPMs produce their beneficial actions through multiple GPCRs, expressed by immune cells, glial cells, and neurons. Notably, loss of SPM receptors such as GPR37 impairs the resolution of inflammation and pain. I also highlight the emerging role of SPMs in the control of itch. Pharmacological targeting of SPMs or SPM receptors has the potential to lead to novel therapeutics for pain and itch as emerging approaches in “resolution pharmacology”.
Keywords: Neuroimmune modulation, nociceptive sensory neurons, neuroprotectin, omega-3 polyunsaturated fatty acids, resolvins, TRP channels
INTRODUCTION
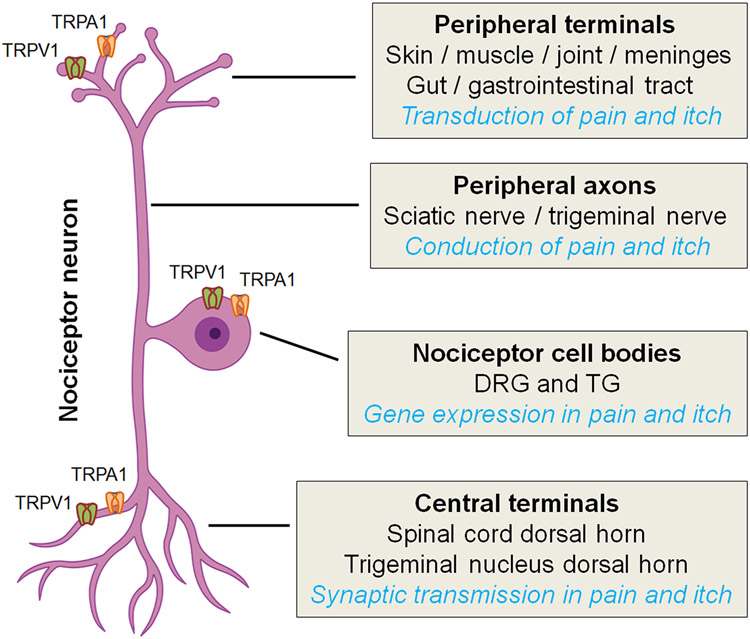
Molecular and cellular mechanisms underlying the pathogenesis of pain have been extensively studied in the last two decades (1, 2). Pain is sensed by nociceptive sensory neurons (nociceptors), with cell bodies located in the dorsal root ganglion (DRG) and trigeminal ganglion, peripheral axons terminated in the skin, muscle, joint, and gut, and central axons terminated in the spinal cord and trigeminal nucleus (3) (Figure 1). (Nociceptors can be activated by physical stimuli, such as noxious thermal and mechanical stimuli through specific molecular sensors, such as transient receptor potential subtype V1, V4, A1, and M8 (TRPV1, TRPV4, TRPA1, TRPM8) and Piezo for sensing heat, cold, and mechanical pain, respectively (4-8). Nociceptors are also activated by chemical stimuli, including various inflammatory mediators (IFMs) (9). Pain is one of five cardinal symptoms of inflammation: rubor (redness), tumor (swelling), calor (increased heat), dolor (pain), and functio laesa (loss of function).
Figure 1.
Schematic of nociceptor neurons and their functions. Nociceptor cell bodies reside in dorsal root ganglia (DRG) and trigeminal ganglia (TG). Nociceptor neurons have two axons: peripheral axons terminate in the skin, muscle, and gut and central axons terminate in the spinal cord and trigeminal nucleus dorsal horn. A subset of nociceptor neurons can also sense itch as pruriceptors. TRPV1 and TRPA1 ion channels are critical for the transduction and transmission of both pain and itch.
IFMs activate nociceptors through receptive receptors for prostaglandin E2, proinflammatory cytokines and chemokines (e.g., IL-1β, TNF, IL-17, and CCL2), and ATP (1, 10-12). Furthermore, nociceptors express pattern recognition receptors such as toll-like receptors (TLRs) and STING (stimulator of interferon-gene) that can sense danger signals, such as RNAs and DNAs to elicit pain, itch, and analgesia (13, 14). IFMs directly activate nociceptors to produce spontaneous pain and further increase the sensitivity of nociceptors, leading to peripheral sensitization via modulation of ion channels that include voltage-gated sodium channels (Nav1.7, Nav1.8, Nav1.9) and TRP channels (e.g., TRPA1 and TRPV1, Figure 1), Peripheral sensitization also requires the activation of protein kinases, such as mitogen-activated protein kinases (MAPK) (15-18).
Central sensitization occurs in the central nervous system (CNS) and is triggered by the activation of NMDA receptors and extracellular signal-regulated kinase (ERK), a MAPK family member (19, 20). Central sensitization regulates chronic pain and widespread pain (21, 22). Loss of inhibitory synaptic transmission (disinhibition) is a critical feature of central sensitization. Peripheral sensitization typically precedes central sensitization, but central sensitization may send descending signals to sustain peripheral sensitization (22, 23). Recent studies suggest that neuroinflammation plays a crucial role in sustaining chronic pain (10). In addition to infiltration of immune cells to the peripheral nervous system (PNS) and CNS, neuroinflammation is characterized by activation of glial cells, such as satellite glial cells and Schwann cells in the PNS and microglia and astrocytes in the CNS (10, 24). Painful insults such as nerve injury, surgeries, arthritis, and cancer result in reactive changes in glial cells (25). Following activation, glia produce and release many powerful neuromodulators, such as TNF, IL-1β, IL-17, CCL2, CXCL1, and BDNF, in part through activation of MAPK signaling pathways (e.g., ERK, p38, and JNK pathways) and modulation of TRP channel function, as TRPV1 and TRPA1 are also expressed at presynaptic terminals of nociceptors (Figure 1). Glia-produced cytokines and BDNF potentiate excitatory synaptic transmission and further suppress inhibitory synaptic transmission in the pain neurocircuit to drive central sensitization and pathological pain (26-28).
A major advance in inflammation research is the realization that the resolution of acute inflammation is an active biochemical process, and accordingly, “resolution biology” and “resolution pharmacology” represent a new therapeutic frontier for the control of inflammation (29, 30). In 2011, emerging roles of resolvins in the resolution of inflammation and pain were proposed (31). Interestingly, certain anti-inflammatory treatments, such as inhibitors of cyclooxygenases, may delay the resolution of inflammation (29, 32). Different types of immune cells have been implicated in the resolution of pain. For example, macrophages resolve inflammatory pain and infection-induced pain via phagocytosis and production of anti-inflammatory cytokines (IL-10 and TGF-β1) (33, 34). CD8+ T cells have been shown to resolve neuropathic pain after chemotherapy via IL-10 production (35). Bone marrow stromal cells resolve chronic arthritic pain and neuropathic pain via opioid receptor and TGF-β1 signaling (36, 37). A failure in the resolution process can lead to a transition from acute pain to chronic pain (31, 38).
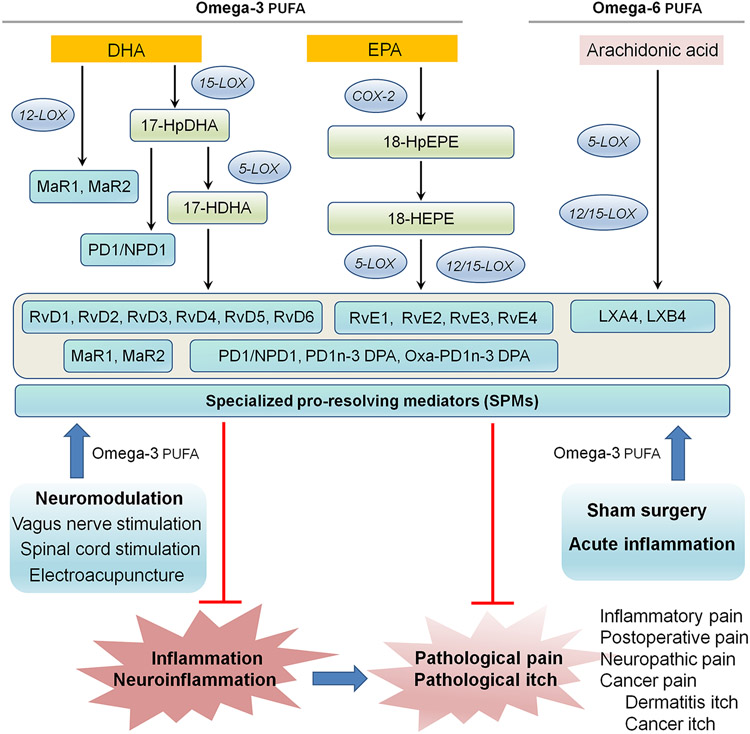
Work by Serhan and collaborators has demonstrated that resolution of acute inflammation is an active process and requires the production of lipid-derived specialized pro-resolving mediators (SPMs), which are generated during the resolution phase of inflammation and contribute to the resolution process (39, 40). SPMs belong to a rapidly expanding family of molecules that are derived from ω-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The SPM family includes resolvins (RvD1-RvD6), protectins/neuroprotectins (PD1/NPD1) and maresins (MaR1, MaR2, and eMaR), cysteinyl-SPMs (MCTR1-R3, PCTR1-R3, and RCTR1-R3), as well as n-3 docosapentaenoic acid (DPA)-derived SPMs (PD1n-3 DPA). Additionally, SPMs consist of ω-6 arachidonic acid-derived lipoxins, such as lipoxin A4 and lipoxin B4 (LXA4 and LXB4) (41) (Figure 2). SPMs exert remarkable protective effects in animal models of certain human diseases, such as arthritis, kidney injury, infection, sepsis, and cancer, as well as periodontal, dry eye, and Alzheimer’s diseases (31, 41-45). Notably, sham surgery produces acute pain and increases serum levels of RvD1 in the recovery phase (46). In this article, I review an increasing body of evidence demonstrating the potent biological actions of SPMs in the control of pain and itch (Table 1). I also highlight immune / glial modulation (Figure 3) and neuromodulation (Figure 4) as underlying mechanisms by which SPMs regulate pain and itch. Special emphasis has also been on pharmacological targeting of SPMs or SPM receptors for the development of novel therapeutics for pain and itch.
Figure 2.
Schematic of SPM biosynthesis and the actions of SPMs in the control of inflammation, neuroinflammation, pathological pain and itch. SPMs (resolvins, protectins, maresins, and lipoxins) are primarily biosynthesized from omega-3 poly-unsaturated fatty acids (Omega-3 PUFA, including EPA and DHA) and Omega-6 PUFA (arachidonic acid) via different lipoxygenase (LOX) and cyclooxygenase (COX) enzymes. SPM production is facilitated by neuromodulation, such as vagus nerve stimulation, spinal cord stimulation, and electroacupuncture, sham surgery, and acute inflammation.
Table-1.
Beneficial effects of synthetic SPMs in the control of pain, itch, and inflammatory diseases. SPMs reduce pathological pain and itch in animal disease models and human diseases. SPMs also control disease progression in other inflammatory disorders in animal models. Abbreviations: IT, intrathecal, IP, intraperitoneal, IPL, intraplantar, IV, intravenous.
| Pain/itch models | SPMs | Species/Route | Effects | References |
|---|---|---|---|---|
| Inflammatory pain | ||||
| Capsaicin {TRPV1) | RvE1, RvD2/D3, MaR1, NPD1 | mice, IPL | Spontaneous pain ↓ | 55, 58, 59 |
| Mustard oil (TPA1) | RvD1, RvD2 | mice, IPL | Spontaneous pain ↓ | 57, 58, |
| Formalin | RvE1, NPD1, RvD5 | mice, IT | Spontaneous pain ↓ | 55, 59, 84 |
| Carrageenan | RvD1, RvE1, LXA4, LXB4 | mice/rats IT, IV | Heat and mechanical pain ↓ | 55, 68 |
| CFA | RvD1, RvD2, RvE1, NPD1 | mice, IT | Heat hyperalgesia ↓ | 55, 58, 59 |
| Visceral pain | RvD2 | mice, rats, IP | Visceral pain ↓ | 60 |
| Bladder pain | RvD2 | rats, IT | Mechanical pain ↓ | 71 |
| Low back pain | LXA4, MaR1 | rats, IT | Mechanical pain ↓ | 72, 73 |
| Vulvodynia | MaR1 | mice, topical | Mechanical pain ↓ | 65 |
| Osteoarthris | 17{R)-HDHA, AT-RvD1 | rats / IP | Spontaneous & mechanical pain ↓ | 41, 61 |
| Rheumatoid arthritis | MaR1, AT-RvD1 | mice/rats, IP | Mechanical pain ↓ | 63, 64 |
| Neuropathic pain | ||||
| Nerve injury (CCI) | RvE1, MaR1, NPD1 | mice, IT | Mechanical and heat pain ↓ | 83, 87. 88 |
| Spinal cord injury | LXA4 | mice, IT | Mechanical allodynia ↓ | 86 |
| Chemotherapy | RvD1, RvD2, MaR1 | mice, IT | Mechanical allodynia ↓ | 84 |
| Diabetic neuropathy | 3-oxa-PD1n-3 DPA | mice, IT | Mechanical allodynia ↓ | 85 |
| Post-operative pain | ||||
| Muscle retraction | RvD1, RvE1 | rats, IT | Mechanical allodynia ↓ | 78 |
| Thoracotomy | RvD1, RvD2 | rats, IT | Mechanical and nocifensive pain ↓ | 79 |
| Tibial bone fracture | RvD1, RvD2, MaR1 | mice, IV, IT | Mechanical pain ↓ | 46 |
| Cancer pain | ||||
| Oral cancer pain | RvD2 | mice, IP, | Mechanical $ spontaneous pain ↓ | 96 |
| Bone cancer pain | RvD1, RvE1 | mice, IT | Mechanical and thermal pain ↓ | 95 |
| Dermatitis and itch | ||||
| Eczema | LXA4 | human, topic | Infantile eczema severely ↓ | 128 |
| Psoriasiform itch | RvD3 | mice, topic | scratching ↓ | 124 |
| Cancer itch | 3-oxa-PD1n-3 DPA | mice, IT | scratching ↓ | 85 |
| Inflammatory diseases | SPMs | Species/Route | Effects | References |
|---|---|---|---|---|
| Allergy | RvE1, RvD1 | mice, IP | Decreased allergic airway inflammation | 31 |
| Colitis | RvE1, RvD5 | mice, IP | Reduced neutrophil recruitment & increased survival | 31, 41 |
| Lung injury | RvE1 | mice, IP | Inhibited acute lung injury & bacterial pneumonia | 31, 41 |
| Peritonitis | RvD1/D2 | mice, IP | Reduced neutrophil recruitment & alveolar bone loss | 31, 41 |
| Infection/sepsis | RvD2, NPD1, MaR1 | mice, IP | Inhibited sepsis and increased survival | 31, 34, 41 |
| Kidney injury | RvD1 | mice, IP | Increased Treg cells & alleviated renal tubular injury | 31, 41 |
| Alzheimer’s disease | RvE1, LXA4 | mice, IP | Inhibited glial activation and neuroinflammation | 41, 146 |
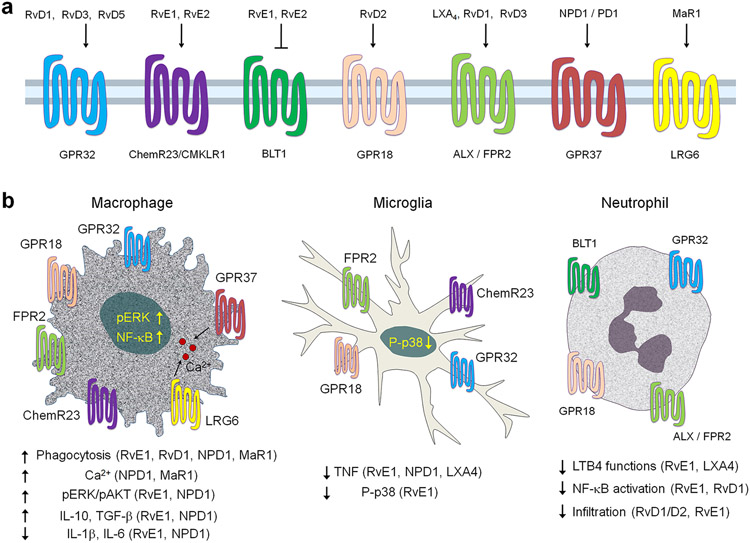
Figure 3.
SPM receptors and SPM actions on macrophages, microglia, and neutrophils. (a) SPMs receptors for resolvins, protectins, maresins, and lipoxins. SPMs signal through GPCRs and multiple SPMs share the same receptors. (b) SPMs activate their respective receptors (GPCRs) to regulate the function of macrophages, microglia, and neutrophils and produce anti-inflammatory and pre-resolving effects. Abbreviation: ERK, extracellular signal-regulated kinase.
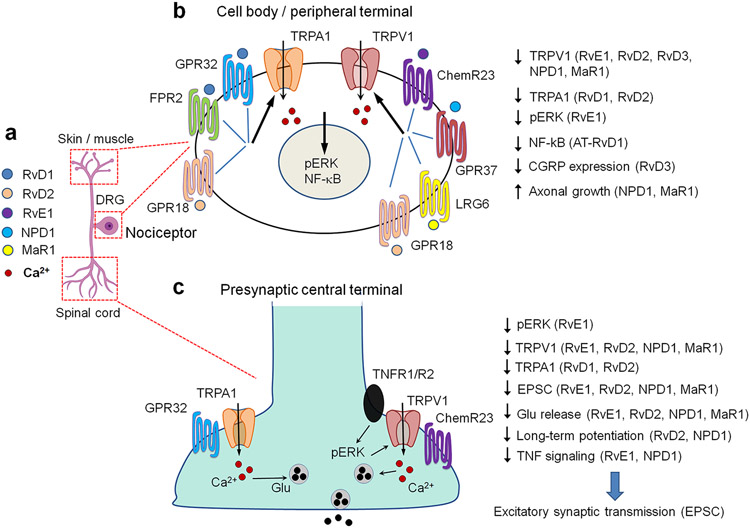
Figure 4.
SPMs act on nociceptive sensory neurons to regulate ion channel function, synaptic transmission, and axonal growth. (a) Location of nociceptor cell body in DRG, peripheral axons terminals in skin and muscle, and central axonal terminals in the spinal cord. (b) SPM receptor signaling in nociceptor cell body and peripheral terminal, leading to reduced activity of TRPV1 and TRPA1 and increased axonal growth. (c) SPM receptor signaling in nociceptor central terminal, leading to reductions in TRPV1 and TRPA1 activity, glutamate release, excitatory synaptic transmission, and spinal cord pain transmission. Abbreviations: EPSC, excitatory postsynaptic current, ERK, extracellular signal-regulated kinase, Glu, glutamate.
ROLE OF EXOGENOUS SPMs IN PAIN CONTROL
Local and systemic application of exogenous SPMs have been shown to alleviate pathological pain following tissue and nerve injuries via peripheral and central actions. These injuries are associated with inflammatory, postoperative, neuropathic, and cancer pain (Table-1).
Inflammatory Pain
Inflammatory pain manifests as spontaneous pain (licking, lifting, and biting the affected paw in experimental animals) and evoked pain, including mechanical hyperalgesia (increased response to painful stimuli), mechanical allodynia (painful response induced by previously innocuous stimuli), and thermal pain (heat hyperalgesia). Inflammatory pain is typically treated using nonsteroid anti-inflammatory drugs (NSAIDs), such as inhibitors of cyclooxygenases (COX1/2). While COX-1 is constitutively expressed, COX-2 is inducible after inflammation and drives inflammatory pain through peripheral and central modulation (47-50). COX-2 inhibitors are relatively safe but can produce gastrointestinal side effects (51). Using a rat model of pleurisy, Gilroy and coworkers demonstrated the paradoxical effects of the COX-2 inhibitor NS-398: it reduced inflammation in the early phase (~2 hours) but enhanced inflammation in the late-phase (~48 hours) (29). COX-2 drives acute inflammation via recruiting leucocytes but promotes inflammatory resolution through mononuclear cells such as macrophages (29, 52). Importantly, COX-2 is required for thebiosynthesis of SPMs and resolution of inflammation (53, 54).
Many lines of evidence from different labs have demonstrated that SPMs are able to potently and effectively control inflammatory pain (31) (Table-1). Intraplantar (i.e., within the sole of the foot) injection of carrageenan induces signs of inflammation in rodents, such as neutrophil infiltration, edema, and upregulation of proinflammatory cytokines and chemokines (e.g., TNF, IL-1β, IL-6, and CCL2), as well as inflammatory pain (heat and mechanical hyperalgesia) for >24 hours. Intraplantar pretreatment of mice with very low doses of RvE1 or RvD1 (20 ng ~ 60 pmol) completely prevented inflammatory pain development and reduced the signatures of inflammation, including neutrophil infiltration, paw edema, and down-regulated the expression of pain-inducing IFMs (IL-1β, IL-6, TNF-α, and CCL2) (55).
Capsaicin and allyl isothiocyanate (AITC), known as mustard oil, are natural compounds and induce intense pain via activation of TRPV1 and TRPA1 receptors, respectively (5, 56) (Figure 1). Strikingly, capsaicin-induced spontaneous pain is blocked by RvE1 (55). Furthermore, capsaicin-induced pain is suppressed by MaR1, RvD2, NPD1 but not RvD1, whereas mustard oil-induced pain is inhibited by RvD1 and RvD2 but not RvE1, suggesting distinct modulation of inflammatory pain by SPMs (32, 57-59).
SPMs were shown to reduce different types of inflammatory pain (Table-1), including colitis or bacteria induced pelvic pain by RvD2 (1500 ng/rat, 300 ng/mouse, IP) (60), osteoarthritis-induced pain by intraperitoneal (IP) treatment of D series resolving precursor 17(R)-HDoHE (17(R)-HDHA, 300 ng/mouse) (61), and arthritis-induced joint stiffness by aspirin-triggered-RvD1 (AT-RvD1, 300 ng/rat, IP) (62, 63). Reduction of adjuvant-induced arthritic pain is much more efficacious by an RvD1 analogue (AT-RvD1, 1.5 mg/kg, 72 % pain inhibition) than opioid (morphine, 0.5 mg/kg, 34% pain inhibition), steroid (dexamethasone, 5 mg/kg, 12% pain inhibition), or COX-2 inhibitor (indomethacin, 5 mg/kg, 35% inhibition) (63). In a mouse model of rheumatoid arthritis, persistent arthritic pain is correlated with decreased levels of MaR1 but not with joint swelling. Systemic MaR1 administration caused sustained reversal of mechanical pain and reduced inflammatory macrophage infiltration in the DRG (64). Vulvodynia is an inflammatory condition and a common cause of pain in premenopausal women (65). In a murine vulvar pain model, topical treatment of MaR1 not only reduced PGE2 levels but also alleviated mechanical pain (65). MaR1 may also modulate trigeminal pain associated with the temporomandibular joint by modulation of inflammation-induced synaptic plasticity in the trigeminal nucleus (66). Lipoxins, such as lipoxin A4 (LXA4) and lipoxin B4 (LXA4), are biosynthesized from arachidonic acid and require lipoxygenases (e.g., LOX-5, LOX-12, and LOX-15) (67) (Figure 2). Intravenous (10 μg/kg) of LXA4 and LXB4 was found to inhibit carrageenan-induced inflammatory pain in rats (68).
The intrathecal route has been extensively used to deliver drugs to the spinal cord via an implanted catheter or direct lumbar puncture (69, 70). The intrathecal route can also deliver reagents (e.g., proteins, RNAs, DNAs) to the DRGs, which are partially covered by the meninges (14). Intrathecal injection of either RvE1 or RvD1 reduced adjuvant-induced heat and mechanical hypersensitivity within 10 min (62). These rapid effects of RvD1 and RvE1 suggest transcription-independent modifications by SPMs. Intrathecal pre-treatment of RvE1 (0.3 and 1 ng) also blocked the formalin-induced 2nd phase spontaneous pain through inhibition of central sensitization (55). Remarkably, the RvE1 dosage for inhibiting the formalin-induced 2nd phase pain is 100 times lower than that of morphine, highlighting the potency of SPMs (55). Intrathecal RvD2, NPD1, and MaR1 (1-10 ng) also alleviated persistent inflammatory pain following adjuvant injection without altering motor function in mice (58, 59). Interstitial cystitis syndrome is characterized by hyperalgesia and bladder overactivity. Intrathecal RvD2 not only reduced pelvic pain but also improved bladder function (71). Low-back pain is one of the most common chronic pain conditions. Intrathecal administration of MaR1 (10 or 100 ng) alleviated nucleus pulposus-induced mechanical and thermal hyperalgesia for several days and reduced the activation of NLRP3-mediated inflammasome in rats (72). In a rat model of lumbar disc herniation, intrathecal LXA4 reduced pain hypersensitivity, downregulated TNF-α and IL-1β expression, and upregulated TGF-β1 and IL-10 expression in the spinal cord (73).
Postoperative Pain
Postoperative pain is a main focus of anesthesiology research and was traditionally managed by opioids, which not only produce side effects but also delays recovery (74). In rodents, postoperative pain is induced by plantar incision, skin-muscle retraction, and bone fracture (75). While acute postoperative pain is mostly reminiscent of inflammatory pain, chronic postoperative pain after amputation and thoracotomy has characteristics of neuropathic pain (76). Prolonged muscle retraction produces postoperative pain for 3-4 weeks in humans and rodents (77). Intrathecal pre-treatment of RvE1 and RvD1 prevented the muscle incision-induced postoperative pain in rats (78). Intrathecal RvD1 and RvD2 also prevented or delayed the development of post-thoracotomy pain (79). Post-treatment of RvD1 at later time points is also effective, producing transient pain relief in the thoracotomy model (78). Tibial bone fracture induces postoperative pain for several weeks, intrathecal post-treatment of RvD1 and RvD5, but not RvD3 and RvD4, can alleviate postoperative pain including mechanical and cold hypersensitivity (46). Postoperative pain after bone fracture is also associated with cognitive decline, a comorbidity of chronic pain (80). Intriguingly, systemic treatment of AT-RvD1 in mice was able to prevent cognitive decline by control of neuroinflammation (81).
Neuropathic Pain
Neuropathic pain is induced by nerve injury, spinal cord injury, diabetes, and chemotherapy in animal models so as to resemble neuropathic pain in clinical settings (82). SPMs have demonstrated effectiveness in reducing neuropathic pain in animal models of those settings (83-86). Intrathecal administration of RvE1 and MaR1 reduced mechanical and thermal pain hypersensitivity after nerve injury by chronic constriction injury (CCI) of the sciatic nerve (83, 87). Peri-operative application of NPD1 (300 ng/mouse) on the sciatic nerve around the ligatures prevented the development of mechanical allodynia. This pre-treatment also alleviated stump pain and autotomy, a self-mutilation behavior that occurs after nerve transection, an animal model of amputation (88). Mechanistically, RvE1 or NPD1 inhibited nerve trauma-induced glial activation and neuroinflammation in the mouse spinal cord (83, 88). Spinal NPD1 treatment also blocked long-term potentiation (LTP), a cellular mechanism of chronic pain (88, 89). Post-treatment of NPD1 via the intrathecal route, 2 weeks after nerve trauma, also inhibited mechanical allodynia, with an efficacy similar to gabapentin (a widely prescribed drug for the management of neuropathic pain), but with a potency 500 times higher than gabapentin (88). Repeated injections of NPD1 produced no side effects or signs of analgesic tolerance (88), whereas gabapentin is known to impair cognition. After spinal nerve ligation, intrathecal MaR1 administration inhibited neuropathic pain and neuroinflammation in rats, as evidenced by the downregulation of NF-κB p65 nuclear translocation and protein levels of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6). Moreover, this treatment maintained synaptic integrity by restoring the expression of synaptic proteins (PSD95 and synapsin II) in rat spinal cord with nerve injury (90). In a mouse model of diabetic neuropathy, intrathecal NPD1 also relieved streptozotocin-induced mechanical allodynia (85). Chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting side effect of chemotherapy drugs, such as paclitaxel (91, 92). In mice, intrathecal post-treatment of RvD1, RvD2, and RvD5, but not RvD3 and RvD4, alleviated chemotherapy-induced neuropathic pain (84). 3-oxa-PD1n-3DPA is a novel analogue of PD1/NPD1 (85). Intrathecal administration of 3-oxa-PD1n-3 DPA, PD1, or PD1n-3 DPA (30-100 ng) each significantly relieved streptozotocin-induced neuropathic pain (mechanical allodynia) in mice. However, at a low dose (10 ng), only 3-oxa PD1n-3 DPA was able to alleviate diabetic neuropathic pain, due to increased metabolic stability of 3-oxa-PD1n-3 DPA when compared to PD1 and PD1n-3 DPA (85). Spinal cord injury (SCI) results in central neuropathic pain, which is driven by glial activation and neuroinflammation (93). Intrathecal LXA4 treatment (300 pmol) at 4 and 24 hours after spinal cord hemisection produced marked reductions in SCI-induced mechanical pain, microgliosis, and pro-inflammatory cytokines (86). In primary culture of microglia, LXA4 inhibited TNF-α release through microglial ALX/FPR2 receptors (86).
Cancer Pain
Cancer pain produced by bone cancer is often severe and difficult to treat (94). In a mouse model of bone cancer produced by implantation of osteolytic fibrosarcoma, RvD1 and RvE1 exhibited antinociceptive potencies in reducing heat and mechanical pain following intrathecal administration (95). Interestingly, RvD1 increased levels of the endocannabinoids anandamide and 2-Arachidonoylglycerol in the spinal cord, suggesting a connection between SPM and endocannabinoid signaling (95). In mouse models of oral squamous cell carcinoma, daily IP injections of 200 ng RvD2 decreased tongue tumor size. (96). This treatment also attenuated cancer-induced thermal, mechanical, and spontaneous pain in mice (96).
Furthermore, resolution failure may cause carcinogenesis (97). Thus, resolvins have been shown to suppress tumor growth and enhance cancer therapy (44). Traditional cancer therapies, such as chemotherapy and radiotherapy, reduce tumor burden by killing tumor cells. Notably, tumor cell debris can trigger inflammation to promote tumor growth. SPMs, such as RvD2, RvD2, and RvE1 each markedly reduced debris-stimulated tumors. Mechanistically, SPMs stimulate macrophage phagocytosis in local tumor environment and inhibit the release of inflammatory cytokines and chemokines, such as TNFα, IL-6, IL-8, CCL4, and CCL5. SPM-mediated endogenous clearance of tumor cell debris through macrophage phagocytosis may offer a new therapeutic strategy (44). In a mouse model of lung cancer, induced by inoculation of Lewis lung carcinoma (LLC) cells, aspirin-triggered SPMs, such as AT-RvD1, AT-RvD3, and AT-LXA4 (600 ng/kg/day) inhibited primary tumor growth through macrophage phagocytosis of therapy-generated tumor cell debris (98). Metastasis of cancer to the bone cavity produces bone destruction and severe bone cancer pain (99, 100). Immunotherapies such as monoclonal antibodies against PD-1 (program death protein 1) and agonists of STING (stimulator of interferon gene) were able to alleviate bone cancer pain through inhibition of osteoclast formation and prevention of bone destruction (99, 100). Thus, it is of great interest to investigate the role of SPMs in bone destruction in cancer pain.
Physiological Pain
Physiological pain is important for the maintenance of homeostasis in the nervous system and immune system (2). Analgesics such as opioids and local anesthetics suppress physiological pain, making animals and humans insensitive to pain. Suppression of physiological pain can be detrimental and even lethal, in the case of heart attacks without pain or congenital insensitivity to pain in individuals with mutations in SCN9A, which encodes the sodium channel subtype Nav1.7 (101). Importantly, SPMs do not interfere with normal pain perception: local or systemic injection of resolvins has no effects on thermal or mechanical pain sensitivity in rats and mice under steady-state conditions (58, 62). NPD1 and RvD2 do not change basal synaptic transmission in the spinal cord pain circuit in physiological conditions, although they potentially abolish inflammation-induced synaptic plasticity (58, 59). Other studies confirmed that RvD1 and RvE1 show no effects on acute nociception or motor function in naïve mice (95). Thus, SPMs serve to restore the homeostatic balance of pain without suppressing physiological pain. Similarly, in the immune system SPMs control excessive inflammation but are not immune suppressants (53).
Distinct Analgesic Potency of DHA and DHA-derived SPMs
Numerous DHA-derived SPMs have been identified, including RvD1-RvD6, NPD1, MaR1, MaR2, etc. Notably, SPMs and their precursor DHA exhibit distinct analgesic properties. DHA appears to be effective in pain relief following pre-treatment at much higher doses (100-1000 fold of that of SPMs), presumably via the DHA receptor GRP120 (102), whereas SPMs show efficacy and potency in both pre- and post-treatment (46). Pre-treatment with DHA reduces neuropathic and postoperative pain (46, 88). In contrast, intrathecal DHA post-treatment does not reduce neuropathic pain after nerve injury or post-operative pain after bone fracture, even at high doses (500 μg, 1000-fold of SPMs) (46, 88). Thus, giving more SPM precursor (e.g., DHA) cannot substitute for the efficacy of SPMs in chronic disease conditions, because DHA cannot be effectively converted to SPMs, most likely due to disruption of SPM biosynthesis.
Sex Differences in SPM Regulation of Pain
The majority of preclinical studies in pain research, including SPM-related studies, are conducted in male animals, even though females have a higher incidence of chronic pain such as migraine and fibromyalgia (103, 104). Recent studies have also shown sex dimorphism in microglia and macrophage regulations of pain (12, 105). Intriguingly, intrathecal RvD5 inhibited inflammatory and neuropathic pain in male mice but not in female mice. In contrast, intrathecal RvD1, RvD2, NPD1, MaR1 reduced chemotherapy-induced mechanical allodynia in both sexes (84). Based on the sex dimorphism of microglial and macrophage signaling in pain (12, 105), intrathecal RvD5 may act on microglia and/or macrophages to mediate male-specific pain relief. Future studies that investigate the role of sex hormones in regulating the production and analgesic actions of SPMs may thus be of interest.
SPMS CONTROL PAIN VIA GPCR SIGNALING AND NEUROIMMUNE MODULATION
SPMs exert their actions by activating G protein-coupled receptors (GPCRs)(106). Multiple SPM receptors have been identified: including ChemR23 (ERV1) for RvE1 and RvE2, GPR32 (DRV1) for RvD1 and RvD5, and GPR18 (DRV2) for RvD2 (107-109). SPM receptors are expressed by macrophages, neutrophils, nociceptive sensory neurons and glial cells (Figure 3, Figure 4). Spinal astrocytes and microglia express ALX (FPR2) to mediate the analgesic effects of LXA4 (68, 86). RNAseq analysis reveals that microglia express ChemR23, GPR32, and GPR18 (106, 110).
RvE1/ChemR23 Axis in Pain Resolution
Arita et al. screened receptors for RvE1 and identified ChemR23 (ERV-1, CMKLR1) and confirmed specific binding of this receptor to RvE1 using [3H]-labeled RvE1. ChemR23 is required to mediate RvE1’s regulation of NF-κB signaling and IL-12 expression in dendritic cells (111). Further studies revealed that RvE1 also binds leukotriene B4 receptor BLT1 in polymorphonuclear leukocytes and serves as a local inhibitor of BLT2 signaling on leukocytes (112). ChemR23 is expressed at high levels in monocytes, but at lower levels in neutrophils and T lymphocytes (111). Xu et al. demonstrated that RvE1’s analgesic actions were mediated by ChemR23, as knockdown of ChemR23 expression in the DRG and spinal cord by intrathecal injection of specific siRNA was sufficient to block RvE1-induced antinociception in mice. Furthermore, chemerin, a natural peptide ligand for ChemR23 could produce similar analgesic effect as RvE1 (55). Endogenous chemerin and ChemR23 also contribute to the resolution of inflammatory pain (113). Microglia express ChemR23 (106, 114), and RvE1 inhibits TNF-α production and release in spinal microglial cultures (83). Chronic arthritis is associated with down-regulation of Chemr23 mRNA levels in rat osteoarthritis (OA) joint tissue (61). Moreover, systemic treatment of 17(R)-HDHA) not only reversed arthritic pain but also increased ChemR23 expression in the spinal cord (61). Due to the short half-lives of RvE1 and chemerin, a 9-amino acid-tethered chemerin fragment was developed as a stable chemerin analog. This membrane-anchored ChemR23 agonist reduced neuropathic pain for more than 24 hours after a single intrathecal injection (115). Together, these findings support an active role of ChemR23 in the resolution of pain.
NPD1/GPR37 Axis in Pain Resolution
Recently, Bang et al. demonstrated several lines of evidence that NPD1 acts on macrophage-expressing GPR37 for the resolution of inflammatory pain and infection-induced pain (33, 34). First, NPD1 activation of GPR37 induced macrophage phagocytosis of zymosan particles via increasing intracellular Ca2+ and activation of ERK signaling (33). NPD1 also triggered GPR37-dependent phagocytosis of listeria bacteria by macrophages (34). Second, intraplantar injection of zymosan, which can activate TLR2, elicited inflammatory pain that normally resolves in several days. However, mice lacking Gpr37 failed to resolve this inflammatory pain (33). Intraplantar injection of listeria elicited severe inflammatory pain that normally resolves in 2 weeks but this resolution process is disrupted in Gpr37 knockout mice (34). Third, depletion of macrophages impaired the resolution of zymosan-induced inflammatory pain (33), whereas adoptive transfer of wild-type macrophages, treated with GPR37 agonist, was sufficient to reverse listeria-induced inflammatory pain (34). Additionally, GPR37 regulates the macrophage phenotype: a lack of GPR37 results in a switch from a M2-like to M1-like macrophages, which have an upregulation of pro-inflammatory cytokines (IL-1β, IL-β, IL-6) and the downregulation of anti-inflammatory cytokines (IL-10, TGF-β1) (33, 34, 116). Single-cell RNA sequencing has revealed GPR37 expression in DRG neurons of rodents and non-human primates (117, 118), but this expression is downregulated in mouse DRG neurons after nerve injury (117). Thus, loss of GPR37 in both macrophages and sensory neurons may contribute to the pathogenesis of chronic pain.
Receptor Signaling for RvD2 and MaR1
Specific binding of RvD2 to recombinant GPR18 was confirmed using a 3H-labeled-RvD2. RvD2 limited PMN infiltration and enhanced phagocyte clearance of bacteria, but these effects are lost in Gpr18-deficient mice (108). Interestingly, oral cancer pain is correlated with the downregulation of mRNA levels for Gpr18 and Gpr32 in cancer tissue (96). DRG nociceptive neurons express GPR18, but bladder inflammation resulted in a marked reduction of GPR18 in DRG neurons. Functionally, the GPR18 antagonist O-1918 blocked the therapeutic effects of RvD2 (71). Leucine-rich repeat containing G-protein-coupled receptor 6 (LGR6) is a marker of osteoprogenitor cells and is dynamically expressed during the differentiation of bone in mouse and human mesenchymal stem cells. Recently, LGR6 was identified as a MaR1 receptor, its actions were significantly amplified with LGR6 overexpression but diminished by Lrg6 gene silencing in phagocytes (119). MaR1 stimulates LGR6-mediated cAMP activity during osteogenesis (120). Additionally, MaR1 was shown to activate orphan nuclear receptor RORα (retinoic acid-related orphan receptor α) to regulate M1/M2 polarization in hepatic residential Kupffer cells and control the pathogenesis of nonalcoholic steatohepatitis (NASH) (121).
Regulation of TRP Channels and Synaptic Plasticity by SPMs
SPMs are potent inhibitors of TRPA1 and TRPV1 and have distinct effects on TRPA1- and TRPV1 -mediated pain (32, 55, 57, 58, 122). Consistent with those observations, SPMs also differentially inhibit the function of TRPA1 and TRPV1 in cultured DRG neurons (57-59). Interestingly, RvD2 potently inhibits the function of both TRPV1 (IC50 = 0.1 nM) and TRPA1 (IC50 = 2 nM) in mouse DRG neurons. (58). MaR1 also potently inhibited TRPV1 function in trigeminal sensory neurons collected from animals with inflammatory pain in the temporomandibular joint (66). Bladder inflammation resulted in upregulation of TRPV1 and RvD2 receptor GPR18 in the same DRG neurons, and RvD2 inhibited capsaicin-induced calcium influx in DRG neurons from bladder-inflamed rats. This effect of RvD2 was blocked by the GPR18 antagonist O-1918, supporting the involvement of GPR18 in regulating the RvD2’s inhibition of TRPV1 (71). RvD2 also reduced the capsaicin-induced Ca2+ response of rectal submucosal neurons from patients with inflammatory bowel syndrome, suggesting the clinical relevance of the RvD2/TRPV1 axis (60). PSB-KK-1415, an agonist of GPR18, has anti-inflammatory and anti-nociceptive activities in animal models of intestinal inflammation and inflammatory pain (123). Additionally, RvD3 inhibited TRPV1 signaling in mouse and human DRG neurons (124). It is generally believed that SPMs inhibit TRP channel via Gi-mediated GPCR signaling in sensory neurons (59, 60, 66), but SPMs at high concentrations may also alter TRP channel activity via lipid raft modification at the cell membrane (125). Interestingly, long-term potentiation (LTP), a cellular mechanism of learning and memory identified in hippocampal neurons, was found in the pain circuit and contributes to persistent pain (126). Spinal cord administration of NPD1 and RvD1 was able to reverse C-fiber stimulation-evoked spinal cord LTP in intact animals (59, 89). Spinal treatment with NPD1 blocked TNF-induced synaptic plasticity and pain hypersensitivity (59). Thus, apart from immune/glial modulation, SPMs control pain via neuromodulation in the PNS and CNS (Figure 4).
SPMs CONTROL ACUTE AND CHRONIC ITCH
Although pain and itch are highly related, itch (pruritus) is a distinct sensation, triggering scratching responses following activation of pruriceptors in primary sensory neurons, which are a subset of nociceptors (127). Acute itch is typically defined as histamine-dependent itch, induced by histamine and compound 48/80, and histamine-independent itch, induced by chloroquine (127). Notably, TRPV1 and TRPA1 are also expressed by pruriceptors (Figure 1) and play distinct roles in regulating histamine-dependent and histamine-independent itch. While pain is known to suppress itch, scratching-induced skin injury may exacerbate itch. Chronic itch involves skin injury and dermatitis. SPMs have been shown to alleviate itch by reducing skin inflammation. Eczema is commonly associated with persistent pruritus. In a double-blinded, placebo-controlled, randomized study with 60 patients, topic treatment of LXA4 cream significantly relieved the severity of infantile eczema and improved the quality of life, however, pruritus was not directly quantified in this study (128). LXA4 controlled skin inflammation in an animal model of psoriasis by downregulating TLR4 signaling (129). RvE1 attenuates murine psoriatic dermatitis by inhibiting migration of cutaneous dendritic cells and γδ T cells (130). Systemic RvD3 treatment prevented the development of psoriasiform itch and skin inflammation. Mechanistically, RvD3 was shown to inhibit TRPV1 signaling in mouse and human DRG neurons, which may account for the acute anti-itch effects of RvD3 (124). Cutaneous T-cell lymphoma (CTCL) is associated with chronic itch in patients and animals (131). Intrathecal administration of PD-1, PD1n-3 DPA, and the new analog 3-oxa-PD1n-3 DPA significantly reduced scratching for several hours (85). Taken together, SPMs may alleviate chronic itch via effective control of skin inflammation and inhibit acute pruritus via regulation of TRP channel function (Figures 1, 2, 4). A recent study suggests that spinal glial cells play an important role in driving chronic itch (80). It will thus be of interest to investigate how SPMs regulate glial activation and neuroinflammation in chronic itch.
MRGPR receptors are a family of mas-related GPCRs and MrgprA3 has been identified as an itch receptor in mice (132). Recently, the structures of MRGPRX2 and MRGPRX4 (the proposed human itch receptors) were solved (133, 134), which should accelerate the structure-guided discovery of therapeutic agents for pain and itch. Although the structures of SPM receptors remain to be solved, it is of great interest to investigate possible interactions between SPM receptors and MRGPRs.
CONCLUSIONS AND FUTURE DIRECTIONS
The resolution phase of inflammation and pain is now widely recognized as an active biological process, governed by a superfamily of endogenous chemical mediators that stimulate resolution of inflammatory responses, namely SPMs (41). As a natural response for the resolution of inflammation and pain, SPMs have gained great attention in the past decade as a newly recognized class of pain killers (31). Many laboratories worldwide have confirmed potent analgesic actions of SPMs in various animal models (Table 1). However, no SPM-related treatment has been as-yet approved by the FDA. SPMs are derived from omega-3 PUFAs, which have shown benefits in humans with various pain-related conditions, such as chronic headaches, migraines, joint discomfort, sickle cell disease, diabetic neuropathy, and rheumatic diseases (135, 136). In December 2019, FDA approved Vascepa (Icosapent Ethyl, an omega-3 fish oil medication that contains EPA) for reduction of the risk for heart attack and stroke. In a randomized trial, Ramsdon and collaborators have shown that dietary omega-3 fatty acids may alleviate headache by producing SPM precursors (136, 137). Notably, SPMs have wide safety profile, and so far no neurotoxicity or cellular toxicity of SPMs has been reported. Since SPMs are produced naturally in humans, SPM-containing softgels are sold in the marker as nutritional and dietary supplement.
SPMs have been tested in several human trials. An early clinical trial on infantile eczema (a condition associated with itch) demonstrated the first successful treatment of the disease with a topical LXA4 analog (128). Methyl ester-benzo-lipoxin A4 (BLXA4), an oral rinse containing a LXA4 mimetic, is well tolerated by patients, and once-daily rinsing with BLXA4 for 28-days decreased gingival inflammation and increased serum levels of SPMs (138). Additionally, RX-10045, a derivative of RvE1 in topical eye drops, has progressed to clinical trials to evaluate ocular inflammation and pain in patients with cataract surgery (NCT02329743).
A major limitation of SPMs is that they are metabolically unstable and can be rapidly inactivated in vivo. It is noteworthy that 19-pf-RvE1, a metabolically stable form of RvE1 (139), extended the anti-hyperalgesic effect of RvE1 from two hours to 6 hours (55). The pro-resolving effects of AT-RvD1 was also prolonged by constructing RvD1-containing nanoparticles (140). Furthermore, 3-oxa PD1n-3 DPA, a novel analogue of PD1/NPD1, displays increased metabolic stability (compared to other PD1 analogues) and greater analgesic efficacy at a low dose (93). Hence, improving the PK of SPMs, using different analogs or unique delivery system (e.g., degradable polymeric vehicle) (141), could prolong and enhance the analgesic and pro-resolving benefits of SPMs. Although high doses of Omega-3 PUFA may bolster the biosynthesis of SPMs, this normal biosynthetic process can be disrupted by diseases. Our body’s ability to produce SPMs may be compromised in clinical settings of chronic pain that is associated with arthritis, diabetes, obesity, and aging. However, SPMs are still produced in many disease conditions (41), which may contribute to the resolution of chronic diseases in some patients.
An alternative approach is to boost endogenous production of SPMs by neuromodulation. For example, vagus nerve stimulation (VNS) can powerfully regulate inflammation and neuroinflammation, in part via increasing the SPM production (142, 143) (Figure 2). Conversely, vagotomy reduced local production of SPMs (144). Human vagus produces SPM and VNS increased SPMs, meanwhile decreasing pro-inflammatory prostaglandins and leukotrienes (142, 145). Auricular VNS via electroacupuncture significantly increased RvD1 level in DRGs of animals with chemotherapy (142). Furthermore, spinal cord stimulation, an effective clinical treatment for chronic pain, significantly increased the level of RvD1 in the cerebrospinal fluid (146). Studying the synergistic actions between a healthy diet containing Omaga-3 PUFA and neuromodulation may prove useful for the prevention and reversal of pain through regulation of inflammation and neuroinflammation (143).
Lastly, it is of great importance to investigate how endogenous SPM signaling regulates the homeostasis, initiation, chronification (a pain that can last more than 3 months), and resolution of pain. Several receptors of SPMs have been identified and knockout mice lacking these SPM receptors can be utilized to study their functions. It is worth characterizing the host receptors and their signaling pathways that mediate the biological functions of different SPMs in immune cells, glial cells, and neurons. Given the ongoing opioid epidemic in the United States, there is an urgent need to develop non-opioid analgesics (147). The combination of analgesic, anti-inflammatory, and pro-resolving properties of SPMs, as a resolution pharmacology (148), offers a great potential for the control of acute and chronic pain and itch. SPMs have also demonstrated efficacy in other inflammatory conditions, such as allergy, colitis, lung injury, peritonitis, bacterial infection, and Alzheimer’s disease (31, 41, 149) (Table 1). Thus, the scope of the SPM-based resolution pharmacology is beyond pain and itch control and should cover many inflammation-related diseases.
ACKNOWLEDGMENTS
The work was supported by Duke University Research Funds, NIH R01 grants NS67686 and NS87988, and DoD grant W81XWH2110885.
Footnotes
DISCLOSURE STATEMENT
Ru-Rong Ji is a consultant of Boston Scientific and received a grant from the company. This activity is not related to study manuscript.
LITERATURE CITED
- 1.Basbaum AI, Bautista DM, Scherrer G, Julius D. 2009. Cellular and molecular mechanisms of pain. Cell 139:267–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji RR, Chamessian A, Zhang YQ. 2016. Pain regulation by non-neuronal cells and inflammation. Science 354:572–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woolf CJ, Ma Q. 2007. Nociceptors--noxious stimulus detectors. Neuron 55:353–64 [DOI] [PubMed] [Google Scholar]
- 4.Ji RR, Lee SY. 2021. Molecular Sensors of Temperature, Pressure, and Pain with Special Focus on TRPV1, TRPM8, and PIEZO2 Ion Channels. Neurosci Bull [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. 1997. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–24 [DOI] [PubMed] [Google Scholar]
- 6.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, et al. 2007. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448:204–8 [DOI] [PubMed] [Google Scholar]
- 7.Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. 2007. TRPM8 is required for cold sensation in mice. Neuron 54:371–8 [DOI] [PubMed] [Google Scholar]
- 8.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. 2012. The role of Drosophila Piezo in mechanical nociception. Nature 483:209–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold MS, Gebhart GF. 2010. Nociceptor sensitization in pain pathogenesis. Nat.Med 16:1248–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji RR, Xu ZZ, Gao YJ. 2014. Emerging targets in neuroinflammation-driven chronic pain. Nat.Rev.Drug Discov 13:533–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White FA, Sun J, Waters SM, Ma C, Ren D, et al. 2005. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc.Natl.Acad.Sci.U.S.A 102:14092–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo X, Chen O, Wang Z, Bang S, Ji J, et al. 2021. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 109:2691–706 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly CR, Chen O, Ji RR. 2020. How Do Sensory Neurons Sense Danger Signals? Trends Neurosci 43:822–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly CR, Jiang C, Andriessen AS, Wang K, Wang Z, et al. 2021. STING controls nociception via type I interferon signalling in sensory neurons. Nature [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummins TR, Dib-Hajj SD, Black JA, Waxman SG. 2000. Sodium channels and the molecular pathophysiology of pain. Prog.Brain Res 129:3–19 [DOI] [PubMed] [Google Scholar]
- 16.Moore C, Gupta R, Jordt SE, Chen Y, Liedtke WB. 2018. Regulation of Pain and Itch by TRP Channels. Neurosci Bull 34:120–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hucho T, Levine JD. 2007. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 55:365–76 [DOI] [PubMed] [Google Scholar]
- 18.Ji RR, Gereau RW, Malcangio M, Strichartz GR. 2009. MAP kinase and pain. Brain Res.Rev 60:135–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latremoliere A, Woolf CJ. 2009. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain 10:895–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji RR, Kohno T, Moore KA, Woolf CJ. 2003. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 26:696–705 [DOI] [PubMed] [Google Scholar]
- 21.Woolf CJ. 2011. Central sensitization: implications for the diagnosis and treatment of pain. Pain 152:S2–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. 2018. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q, Wu Z, Hadden JK, Odem MA, Zuo Y, et al. 2014. Persistent pain after spinal cord injury is maintained by primary afferent activity. J Neurosci 34:10765–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellis A, Bennett DL. 2013. Neuroinflammation and the generation of neuropathic pain. Br.J Anaesth 111:26–37 [DOI] [PubMed] [Google Scholar]
- 25.Ji RR, Berta T, Nedergaard M. 2013. Glia and pain: Is chronic pain a gliopathy? Pain [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, et al. 2005. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438:1017–21 [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki Y, Zhang L, Cheng JK, Ji RR. 2008. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J.Neurosci 28:5189–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo H, Liu HZ, Zhang WW, Matsuda M, Lv N, et al. 2019. Interleukin-17 Regulates Neuron-Glial Communications, Synaptic Transmission, and Neuropathic Pain after Chemotherapy. Cell Rep 29:2384–97 e5 [DOI] [PubMed] [Google Scholar]
- 29.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. 1999. Inducible cyclooxygenase may have anti-inflammatory properties. Nat.Med 5:698–701 [DOI] [PubMed] [Google Scholar]
- 30.Fullerton JN, Gilroy DW. 2016. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov 15:551–67 [DOI] [PubMed] [Google Scholar]
- 31.Ji RR, Xu ZZ, Strichartz G, Serhan CN. 2011. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, et al. 2012. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 26:1755–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ, Ji RR. 2018. GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J Clin Invest 128:3568–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bang S, Donnelly CR, Luo X, Toro-Moreno M, Tao X, et al. 2021. Activation of GPR37 in macrophages confers protection against infection-induced sepsis and pain-like behaviour in mice. Nat Commun 12:1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krukowski K, Eijkelkamp N, Laumet G, Hack CE, Li Y, et al. 2016. CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci 36:11074–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen G, Park CK, Xie RG, Ji RR. 2015. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-beta secretion. J Clin.Invest 125:3226–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo W, Wang H, Zou S, Gu M, Watanabe M, et al. 2011. Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells 29:1294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price TJ, Basbaum AI, Bresnahan J, Chambers JF, De Koninck Y, et al. 2018. Transition to chronic pain: opportunities for novel therapeutics. Nat Rev Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckley CD, Gilroy DW, Serhan CN. 2014. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40:315–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bannenberg G, Serhan CN. 2010. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim.Biophys.Acta 1801:1260–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang N, Serhan CN. 2020. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem 64:443–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, et al. 2005. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J.Clin.Invest 115:2774–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spite M, Norling LV, Summers L, Yang R, Cooper D, et al. 2009. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461:1287–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulciner ML, Serhan CN, Gilligan MM, Mudge DK, Chang J, et al. 2018. Resolvins suppress tumor growth and enhance cancer therapy. J Exp Med 215:115–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, et al. 2006. Resolvin D series and protectin D1 mitigate acute kidney injury. J.Immunol 177:5902–11 [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Terrando N, Xu ZZ, Bang S, Jordt SE, et al. 2018. Distinct Analgesic Actions of DHA and DHA-Derived Specialized Pro-Resolving Mediators on Post-operative Pain After Bone Fracture in Mice. Front Pharmacol 9:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, et al. 2001. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 410:471–5 [DOI] [PubMed] [Google Scholar]
- 48.Yaksh TL. 1999. Spinal systems and pain processing: development of novel analgesic drugs with mechanistically defined models. Trends Pharmacol.Sci 20:329–37 [DOI] [PubMed] [Google Scholar]
- 49.Malmberg AB, Yaksh TL. 1995. Cyclooxygenase inhibition and the spinal release of prostaglandin E2 and amino acids evoked by paw formalin injection: a microdialysis study in unanesthetized rats. J Neurosci 15:2768–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brigham NC, Ji RR, Becker ML. 2021. Degradable polymeric vehicles for postoperative pain management. Nat Commun 12:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brigham NC, Nofsinger R, Luo X, Dreger NZ, Abel AK, et al. 2021. Controlled release of etoricoxib from poly(ester urea) films for post-operative pain management. J Control Release 329:316–27 [DOI] [PubMed] [Google Scholar]
- 52.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. 2001. Lipid mediator class switching during acute inflammation: signals in resolution. Nat.Immunol 2:612–9 [DOI] [PubMed] [Google Scholar]
- 53.Serhan CN, Chiang N, Van Dyke TE. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat.Rev.Immunol 8:349–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Serhan CN. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, et al. 2010. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat.Med 16:592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patapoutian A, Tate S, Woolf CJ. 2009. Transient receptor potential channels: targeting pain at the source. Nat.Rev.Drug Discov 8:55–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. 2010. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br.J.Pharmacol 161:707–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park CK, Xu ZZ, Liu T, Lu N, Serhan CN, Ji RR. 2011. Resolvin d2 is a potent endogenous inhibitor for transient receptor potential subtype v1/a1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin d1, d2, and e1. J.Neurosci 31:18433–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park CK, Lu N, Xu ZZ, Liu T, Serhan CN, Ji RR. 2011. Resolving TRPV1- and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci 31:15072–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perna E, Aguilera-Lizarraga J, Florens MV, Jain P, Theofanous SA, et al. 2021. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut 70:1275–86 [DOI] [PubMed] [Google Scholar]
- 61.Huang J, Burston JJ, Li L, Ashraf S, Mapp PI, et al. 2017. Targeting the D Series Resolvin Receptor System for the Treatment of Osteoarthritis Pain. Arthritis Rheumatol 69:996–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu ZZ, Ji RR. 2011. Resolvins are potent analgesics for arthritic pain. Br.J.Pharmacol 164:274–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lima-Garcia JF, Dutra RC, da SK, Motta EM, Campos MM, Calixto JB. 2011. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br.J.Pharmacol 164:278–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allen BL, Montague-Cardoso K, Simeoli R, Colas RA, Oggero S, et al. 2020. Imbalance of pro-resolving lipid mediators in persistent allodynia dissociated from signs of clinical arthritis. Pain [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Falsetta ML, Wood RW, Linder MA, Bonham AD, Honn KV, et al. 2021. Specialized Pro-resolving Mediators Reduce Pro-nociceptive Inflammatory Mediator Production in Models of Localized Provoked Vulvodynia. J Pain 22:1195–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park CK. 2015. Maresin 1 Inhibits TRPV1 in Temporomandibular Joint-Related Trigeminal Nociceptive Neurons and TMJ Inflammation-Induced Synaptic Plasticity in the Trigeminal Nucleus. Mediators Inflamm 2015:275126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serhan CN, Hamberg M, Samuelsson B. 1984. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A 81:5335–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Svensson CI, Zattoni M, Serhan CN. 2007. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J.Exp.Med 204:245–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yaksh TL, Rudy TA. 1976. Analgesia mediated by a direct spinal action of narcotics. Science 192:1357–8 [DOI] [PubMed] [Google Scholar]
- 70.Hylden JL, Wilcox GL. 1980. Intrathecal morphine in mice: a new technique. Eur.J.Pharmacol 67:313–6 [DOI] [PubMed] [Google Scholar]
- 71.Lu Q, Yang Y, Zhang H, Chen C, Zhao J, et al. 2021. Activation of GPR18 by Resolvin D2 Relieves Pain and Improves Bladder Function in Cyclophosphamide-Induced Cystitis Through Inhibiting TRPV1. Drug Des Devel Ther 15:4687–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang YH, Li Y, Wang JN, Zhao QX, Jin J, et al. 2020. Maresin 1 Attenuates Radicular Pain Through the Inhibition of NLRP3 Inflammasome-Induced Pyroptosis via NF-kappaB Signaling. Front Neurosci 14:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miao GS, Liu ZH, Wei SX, Luo JG, Fu ZJ, Sun T. 2015. Lipoxin A4 attenuates radicular pain possibly by inhibiting spinal ERK, JNK and NF-kappaB/p65 and cytokine signals, but not p38, in a rat model of non-compressive lumbar disc herniation. Neuroscience 300:10–8 [DOI] [PubMed] [Google Scholar]
- 74.Kharasch ED, Clark JD, Adams JM. 2022. Opioids and Public Health: The Prescription Opioid Ecosystem and Need for Improved Management. Anesthesiology 136:10–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brennan TJ. 2002. Frontiers in translational research: the etiology of incisional and postoperative pain. Anesthesiology 97:535–7 [DOI] [PubMed] [Google Scholar]
- 76.Kehlet H, Jensen TS, Woolf CJ. 2006. Persistent postsurgical pain: risk factors and prevention. Lancet 367:1618–25 [DOI] [PubMed] [Google Scholar]
- 77.Flatters SJ. 2008. Characterization of a model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR). Pain 135:119–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang L, Wang CF, Serhan CN, Strichartz G. 2011. Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. Pain 152:557–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang JC, Strichartz GR. 2017. Prevention of Chronic Post-Thoracotomy Pain in Rats By Intrathecal Resolvin D1 and D2: Effectiveness of Perioperative and Delayed Drug Delivery. J Pain [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ji RR, Donnelly CR, Nedergaard M. 2019. Astrocytes in chronic pain and itch. Nat Rev Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Terrando N, Gomez-Galan M, Yang T, Carlstrom M, Gustavsson D, et al. 2013. Aspirin-triggered resolvin D1 prevents surgery-induced cognitive decline. FASEB J 27:3564–71 [DOI] [PubMed] [Google Scholar]
- 82.Costigan M, Scholz J, Woolf CJ. 2009. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu.Rev.Neurosci 32:1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu ZZ, Berta T, Ji RR. 2013. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J Neuroimmune Pharmacol. 8:37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luo X, Gu Y, Tao X, Serhan CN, Ji RR. 2019. Resolvin D5 Inhibits Neuropathic and Inflammatory Pain in Male But Not Female Mice: Distinct Actions of D-Series Resolvins in Chemotherapy-Induced Peripheral Neuropathy. Front Pharmacol 10:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nesman JI, Chen O, Luo X, Ji RR, Serhan CN, Hansen TV. 2021. A new synthetic protectin D1 analog 3-oxa-PD1n-3 DPA reduces neuropathic pain and chronic itch in mice. Org Biomol Chem 19:2744–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martini AC, Berta T, Forner S, Chen G, Bento AF, et al. 2016. Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. J Neuroinflammation 13:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei J, Su W, Zhao Y, Wei Z, Hua Y, et al. 2022. Maresin 1 promotes nerve regeneration and alleviates neuropathic pain after nerve injury. J Neuroinflammation 19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu ZZ, Liu XJ, Berta T, Park CK, Lu N, et al. 2013. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann Neurol 74:490–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruscheweyh R, Wilder-Smith O, Drdla R, Liu XG, Sandkuhler J. 2011. Long-term potentiation in spinal nociceptive pathways as a novel target for pain therapy. Mol.Pain 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao J, Tang C, Tai LW, Ouyang Y, Li N, et al. 2018. Pro-resolving mediator maresin 1 ameliorates pain hypersensitivity in a rat spinal nerve ligation model of neuropathic pain. J Pain Res 11:1511–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. 2014. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain 15:712–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flatters SJ, Bennett GJ. 2004. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 109:150–61 [DOI] [PubMed] [Google Scholar]
- 93.Gwak YS, Kang J, Unabia GC, Hulsebosch CE. 2012. Spatial and temporal activation of spinal glial cells: role of gliopathy in central neuropathic pain following spinal cord injury in rats. Exp.Neurol 234:362–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmidt BL. 2014. The neurobiology of cancer pain. Neuroscientist. 20:546–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khasabova IA, Golovko MY, Golovko SA, Simone DA, Khasabov SG. 2020. Intrathecal Administration of Resolvin D1 and E1 Decreases Hyperalgesia in Mice with Bone Cancer Pain: Involvement of Endocannabinoid Signaling. Prostaglandins Other Lipid Mediat:106479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ye Y, Scheff NN, Bernabe D, Salvo E, Ono K, et al. 2018. Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology 139:182–93 [DOI] [PubMed] [Google Scholar]
- 97.Fishbein A, Hammock BD, Serhan CN, Panigrahy D. 2021. Carcinogenesis: Failure of resolution of inflammation? Pharmacol Ther 218:107670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gilligan MM, Gartung A, Sulciner ML, Norris PC, Sukhatme VP, et al. 2019. Aspirin-triggered proresolving mediators stimulate resolution in cancer. Proc Natl Acad Sci U S A 116:6292–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang K, Gu Y, Liao Y, Bang S, Donnelly CR, et al. 2020. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J Clin Invest [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang K, Donnelly CR, Jiang C, Liao Y, Luo X, et al. 2021. STING suppresses bone cancer pain via immune and neuronal modulation. Nat Commun 12:4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bennett DL, Woods CG. 2014. Painful and painless channelopathies. Lancet Neurol 13:587–99 [DOI] [PubMed] [Google Scholar]
- 102.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, et al. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142:687–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mogil JS. 2020. Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat Rev Neurosci 21:353–65 [DOI] [PubMed] [Google Scholar]
- 104.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd. 2009. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 10:447–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, et al. 2015. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat.Neurosci 18:1081–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. 2018. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 100:1292–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, et al. 2012. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484:524–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chiang N, Dalli J, Colas RA, Serhan CN. 2015. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med 212:1203–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, et al. 2010. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc.Natl.Acad.Sci.U.S.A 107:1660–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thion MS, Low D, Silvin A, Chen J, Grisel P, et al. 2018. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell 172:500–16 e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, et al. 2005. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J.Exp.Med 201:713–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. 2007. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J.Immunol 178:3912–7 [DOI] [PubMed] [Google Scholar]
- 113.Xie YK, Luo H, Qiu XY, Xu ZZ. 2021. Resolution of Inflammatory Pain by Endogenous Chemerin and G Protein-Coupled Receptor ChemR23. Neurosci Bull 37:1351–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, et al. 2007. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat.Med 13:868–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Doyle JR, Krishnaji ST, Zhu G, Xu ZZ, Heller D, et al. 2014. Development of a membrane-anchored chemerin receptor agonist as a novel modulator of allergic airway inflammation and neuropathic pain. J Biol Chem 289:13385–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qu L, Caterina MJ. 2018. Accelerating the reversal of inflammatory pain with NPD1 and its receptor GPR37. J Clin Invest 128:3246–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Renthal W, Tochitsky I, Yang L, Cheng YC, Li E, et al. 2020. Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Neuron 108:128–44 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kupari J, Usoskin D, Parisien M, Lou D, Hu Y, et al. 2021. Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nat Commun 12:1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN. 2019. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest 129:5294–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khedgikar V, Charles JF, Lehoczky JA. 2022. Mouse LGR6 regulates osteogenesis in vitro and in vivo through differential ligand use. Bone 155:116267. [DOI] [PubMed] [Google Scholar]
- 121.Han YH, Shin KO, Kim JY, Khadka DB, Kim HJ, et al. 2019. A maresin 1/RORalpha/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis. J Clin Invest 129:1684–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, et al. 2012. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 26:1755–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fabisiak A, Fabisiak N, Mokrowiecka A, Malecka-Panas E, Jacenik D, et al. 2021. Novel selective agonist of GPR18, PSB-KK-1415 exerts potent anti-inflammatory and anti-nociceptive activities in animal models of intestinal inflammation and inflammatory pain. Neurogastroenterol Motil 33:e14003. [DOI] [PubMed] [Google Scholar]
- 124.Lee SH, Tonello R, Im ST, Jeon H, Park J, et al. 2020. Resolvin D3 controls mouse and human TRPV1-positive neurons and preclinical progression of psoriasis. Theranostics 10:12111–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Payrits M, Horvath A, Biro-Suto T, Erostyak J, Makkai G, et al. 2020. Resolvin D1 and D2 Inhibit Transient Receptor Potential Vanilloid 1 and Ankyrin 1 Ion Channel Activation on Sensory Neurons via Lipid Raft Modification. Int J Mol Sci 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. 2003. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 299:1237–40 [DOI] [PubMed] [Google Scholar]
- 127.LaMotte RH, Dong X, Ringkamp M. 2014. Sensory neurons and circuits mediating itch. Nat.Rev.Neurosci 15:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. 2013. Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br J Dermatol 168:172–8 [DOI] [PubMed] [Google Scholar]
- 129.Liu X, Wang X, Duan X, Poorun D, Xu J, et al. 2017. Lipoxin A4 and its analog suppress inflammation by modulating HMGB1 translocation and expression in psoriasis. Sci Rep 7:7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sawada Y, Honda T, Nakamizo S, Otsuka A, Ogawa N, et al. 2018. Resolvin E1 attenuates murine psoriatic dermatitis. Sci Rep 8:11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Han Q, Liu D, Convertino M, Wang Z, Jiang C, et al. 2018. miRNA-711 Binds and Activates TRPA1 Extracellularly to Evoke Acute and Chronic Pruritus. Neuron 99:449–63 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 139(7):1353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cao C, Kang HJ, Singh I, Chen H, Zhang C, et al. 2021. Structure, function and pharmacology of human itch GPCRs. Nature. 600:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang F, Guo L, Li Y, Wang G, Wang J, et al. 2021. Structure, function and pharmacology of human itch receptor complexes. Nature, 600:164–169 [DOI] [PubMed] [Google Scholar]
- 135.Chavez-Castillo M, Ortega A, Cudris-Torres L, Duran P, Rojas M, et al. 2021. Specialized Pro-Resolving Lipid Mediators: The Future of Chronic Pain Therapy? Int J Mol Sci 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ramsden CE, Faurot KR, Zamora D, Suchindran CM, Macintosh BA, et al. 2013. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain 154:2441–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ramsden CE, Faurot KR, Zamora D, Palsson OS, MacIntosh BA, et al. 2015. Targeted alterations in dietary n-3 and n-6 fatty acids improve life functioning and reduce psychological distress among patients with chronic headache: a secondary analysis of a randomized trial. Pain 156:587–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hasturk H, Schulte F, Martins M, Sherzai H, Floros C, et al. 2021. Safety and Preliminary Efficacy of a Novel Host-Modulatory Therapy for Reducing Gingival Inflammation. Front Immunol 12:704163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arita M, Oh SF, Chonan T, Hong S, Elangovan S, et al. 2006. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J.Biol.Chem 281:22847–54 [DOI] [PubMed] [Google Scholar]
- 140.Norling LV, Spite M, Yang R, Flower RJ, Perretti M, Serhan CN. 2011. Cutting edge: humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J.Immunol 186:5543–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brigham NC, Ji RR, Becker ML. 2021. Degradable polymeric vehicles for postoperative pain management. Nat Commun 12:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Serhan CN, de la Rosa X, Jouvene C. 2019. Novel mediators and mechanisms in the resolution of infectious inflammation: evidence for vagus regulation. J Intern Med 286:240–58 [DOI] [PubMed] [Google Scholar]
- 143.Tao X, Lee MS, Donnelly CR, Ji RR 2020. Neuromodulation, specialized pro-resolving mediators, and resolution of pain Neurotherapeutics [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. 2014. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med 211:1037–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Serhan CN, de la Rosa X, Jouvene CC. 2018. Cutting Edge: Human Vagus Produces Specialized Proresolving Mediators of Inflammation with Electrical Stimulation Reducing Proinflammatory Eicosanoids. J Immunol 201:3161–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tao X, Luo X, Zhang T, Hershey B, Esteller R, Ji RR. 2021. Spinal Cord Stimulation Attenuates Mechanical Allodynia and Increases Central Resolvin D1 Levels in Rats With Spared Nerve Injury. Front Physiol 12:687046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Volkow ND, Collins FS. 2017. The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377:391–4 [DOI] [PubMed] [Google Scholar]
- 148.Perretti M, Leroy X, Bland EJ, Montero-Melendez T, 2015. Resolution pharmacology: opportunities for therapeutic innovation in inflammation. Trends Pharmacol Sci 36:737–55. [DOI] [PubMed] [Google Scholar]
- 149.Kantarci A, Aytan N, Palaska I, Stephens D, Crabtree L, Benincasa C, et al. 2018. Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp Neurol. 2018,300:111–20. [DOI] [PubMed] [Google Scholar]