Abstract
Ultrasound is the most widely used medical imaging modality worldwide. It is abundant, extremely safe, portable and inexpensive. In this review, we consider some of the current development trends for ultrasound imaging, which build upon its current strength and the popularity it experiences among medical imaging professional users.
Ultrasound has rapidly expanded beyond traditional radiology departments and cardiology practices. Computing power and data processing capabilities of commonly available electronics put ultrasound systems in a labcoat pocket or on a user’s mobile phone. Taking advantage of new contributions and discoveries in ultrasound physics, signal processing algorithms, and electronics, the performance of ultrasound systems and transducers have progressed in terms of them becoming smaller, with higher imaging performance, and having lower cost. Ultrasound operates in real time, now at ultrafast speeds: KHz frame rates are already achieved by many systems.
Ultrasound has progressed beyond anatomical imaging and monitoring blood flow in large vessels. With clinical approval of ultrasound contrast agents (gas-filled microbubbles) that are administered in the bloodstream, tissue perfusion studies are now routine. Through the use of modern ultrasound pulse sequences, individual microbubbles, with sub-picogram mass, can be detected and observed in real time, many centimeters deep in the body. Ultrasound imaging has broken the wavelength barrier: by tracking positions of microbubbles within the vasculature, super-resolution imaging has been made possible. Ultrasound can now trace the smallest vessels and capillaries, and obtain blood velocity data in those vessels.
Molecular ultrasound imaging has now moved closer to clinic: the use of microbubbles with a specific affinity to endothelial biomarkers allows selective accumulation and retention of ultrasound contrast in the areas of ischemic injury, inflammation, or neoangiogenesis. This will aid in non-invasive molecular imaging, and may provide additional help with real-time guidance of biopsy, surgery, and ablation procedures.
The ultrasound field can be tightly focused inside the body, many centimeters deep, with millimeter precision, and ablate lesions by energy deposition, with thermal or mechanical bioeffects. Some of such treatments are already in clinical use, with more indications progressing through the clinical trial stage. In conjunction with intravascular microbubbles, focused ultrasound can be used for tissue-specific drug delivery: localized triggered release of sequestered drugs from particles in the bloodstream may take time to get to clinic. A combination of intravascular microbubbles with circulating drug and low power ultrasound allows transient opening of vascular endothelial barriers, including blood brain barrier; this approach has reached clinical trial stage. Therefore, the drugs that normally would not be getting to the target tissue in the brain will now have an opportunity to produce therapeutic efficacy.
Overall, medical ultrasound is developing at a brisk rate, even in an environment where other imaging modalities are also advancing rapidly and may be considered more lucrative. With all the current advances that we discuss, and many more to come, ultrasound may help solve many problems that modern medicine is facing.
Keywords: Ultrasound, Focused ultrasound, Therapeutic ultrasound, Perfusion, Ultrasound Contrast, Microbubbles, Super-resolution ultrasound, Transducer, Drug Delivery, Ultrafast imaging
1. Introduction
Ultrasound imaging is sometimes viewed, incorrectly, as being limited in terms of complexity and signal quality when compared with peer medical imaging modalities. At its most basic level, ultrasound involves generating and receiving very short acoustic pressure pulses and mapping the time of arrival of echoes arising from acoustic impedance discontinuities in the body to their associated depths and angles according to the time and angle, respectively, of the received signal. Thus, it appears as a scaled down version of sonar or a close relative to ultrasound-based nondestructive examination. This simple description may still be applicable to a degree for basic B-Mode imaging. However, in the past several decades there has been a rapid growth in all aspects of signal generation, processing and imaging reconstruction/analysis. Some of these areas of rapid development are discussed in this review. However, the authors found it impossible to do justice to all the domains of development. Some areas that have the greatest potential are still in the formative stages. For example, artificial intelligence undoubtedly will have an enormous role in ultrasound imaging just as it should in the other modalities. In fact, it may be possible to make the case that the very advanced fundamental signal analysis methods now being explored have the greatest potential impact in ultrasound, versus other modalities, because the starting point with raw ultrasound image data is so noisy and artifact prone. In essence, because it starts from a lower baseline, there is greater scope for improvement until reconstructed images converge to something near ideal in terms of contrast and spatial qualities.
In common with many signal processing systems, and especially in the case of medical imaging, overall performance is dominated by the performance of the weakest link in a serial processing chain. Historically, signal processing bandwidth (electronic hardware) was a limiting consideration but rapid developments in all aspects of the signal processing chain have placed progressively greater pressure on transducer signal sensitivity and bandwidth. Thus, the review will start with recent innovations in the transducer field. Thereafter, we have taken a selective approach and address a few of the major technological shifts that are ongoing. Due to space and time considerations, we have had to omit the important topics of Doppler processing and elastography. Each of these topics are worthy of their own reviews.
Ultrasound as a medical imaging modality has come a very long way since its introduction in the middle of twentieth century1. Equipment technology progressed from purely analog signal generation and detection at a single element (or line) level (A-mode), to two-dimensional (B-mode) scanning in real time. With the advent of digital signal processing technologies capable of handling data at high bandwidth, 3D imaging became possible at useful frame rates. Current imaging systems involve a signal processing chain from the echo signals sensed by the transducer, through several stages of amplification, digitization, beamforming, filtering and display of image mapping, at 2D and 3D. In the context of lowering the cost of imaging hardware, this places emphasis on transducer conversion efficiency and signal bandwidth. Rapid advancement of computing power in accordance with Moore’s law helped make medical ultrasound the most widespread imaging tool, build pocket-size imaging systems, and achieve availability of extremely fast, convenient, and inexpensive devices. Limitation on the level of transmitted energy is also an important consideration to assure safety, which has an almost-impeccable track record for ultrasound imaging, as it lacks the use of ionizing radiation and does not require strong magnetic fields. The use of ultrasound imaging as a real-time enabling guidance method in medical procedures, such as surgery, biopsy, and ablation, is critically important. In addition to the progress of the equipment, the progress of contrast agents for ultrasound imaging has been observed – but the latter was much slower, due to the injectable nature of contrast materials, which are currently treated by regulatory agencies as drug formulations, not as micro- or nanodevices. We will discuss the general development trends and successful advances. As it is not possible to cover a field of such magnitude and review all the literature, we focus only on some observed important trends. We admit that the selection of topics is highly subjective and open to critique.
2. Transducer
In diagnostic ultrasound imaging, the transducer performs both the conversion of electrical pulses to ultrasonic pulses (in the transmitter signal path) and conversion of ultrasonic pulses to electrical pulses (in the receiver signal path). One might very logically consider optimizing two separate transducers for each mode of operation. However, separate transducers are very rarely used in practice. Placing transmit and receive transducers side by side results in a critical near field blind spot and placing transmit and receive transducers one over the other creates a technical challenge because the elements are necessarily mechanically coupled and therefore acoustically interfere with each other. Fortunately, the design of diagnostic transducers for roundtrip pulse-echo performance is readily tractable. Several technical attributes feed into transducer performance. These include electrical matching, acoustic matching and energy conversion efficiency. As a practical matter, because transducer elements are necessarily very small to achieve the desired fine spatial sampling of the acoustic field, individual element electrical impedance must be relatively high. This in turns motivates the choice of materials with low electrical impedance per unit volume. The low acoustic impedance of human tissue drives a choice towards materials with as low acoustic impedance as possible (i.e. low density and stiffness). The conversion efficiency of most current transducer materials is sufficiently high that electrical and acoustic matching are the dominant factors determining net transducer signal performance.
Piezoelectric-based Transducers
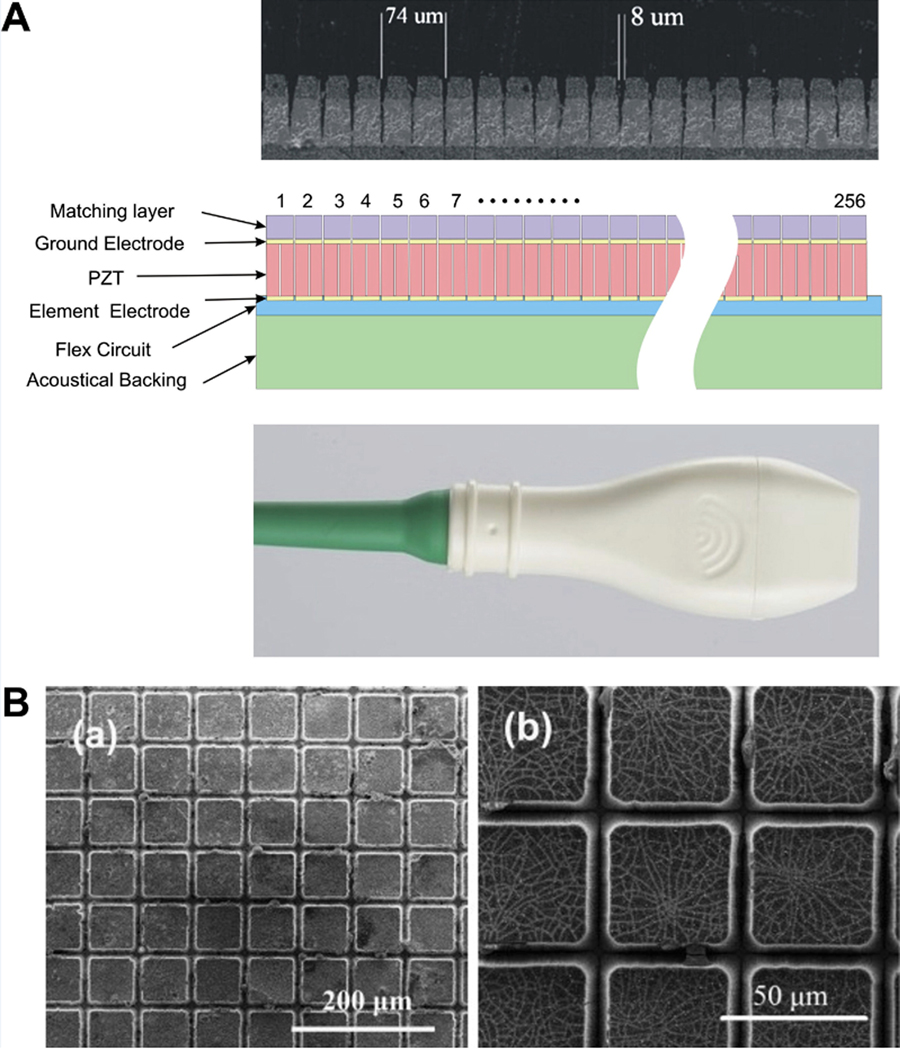
Piezoelectric ceramics, comprising lead zirconate titanate (Pb,Zr,Ti)O3 - i.e. PZT) ceramics, have been a mainstay in the field since the beginning of the rapid evolution of ultrasound diagnostic imaging in the late 1970s and 1980s. As a ferroelectric ceramic, the material is characterized by very high dielectric constant and consequently a lower electrical impedance than would otherwise be possible. The tradeoff involving optimal electrical characteristics versus non-optimal acoustic performance (due to the high density and stiffness) of the material is readily apparent. Using a highly optimized design (comprising multiple matching layers and an appropriate backing layer), designers have achieved −6 dB fractional bandwidths in the range 75% - 80%. The general principles of design, using a circuit model, are described in a classic paper by Desilets2. However, in recent decades a number of technical developments have placed greater priority on yet higher signal bandwidths. As an example, contrast agent imaging frequently uses nonlinear, harmonic, signals to differentiate contrast agent origin receive signals from tissue origin signals. This motivates interest in transducers possessing signal bandwidths of 100% and more. An important PZT-based innovation that has addressed this need involves the use of PZT/polymer composite materials3 and these materials remain a cornerstone of many current commercial designs. There are many instances of advances in PZT-based transducers that encompass dual layer designs4, 1D and 2D array5 and high frequency applications6. As one example of adapting this technologically mature piezoelectric material to a very challenging application, a 30 MHz linear array has been fabricated using an excimer laser element to form isolation trenches that are only 5–10 μm wide7 (Figure 1A).
Figure 1.
(A) Construction of a 30-MHz high-frequency linear array7. Copyright (2009), World Federation for Ultrasound in Medicine & Biology, reprinted with permission.
(B) SEM surface morphologies of (left) PZT after etching for 7 h and (right) PMN-PT after etching for 3 h10. Copyright (2015), 2015 Elsevier Ltd and Techna Group S.r.l., reprinted with permission.
In recent decades, considerable progress has been made in the field of single crystal piezoelectric materials8 as an improvement over the highly mature piezoceramic technology. The primary technical advantage that these materials offer in practical medical ultrasound imaging is a high electromechanical coupling coefficient (k33 ~ 0.9 vs. k33 ~ 0.7) for piezoceramic. The materials possess a number of other qualities that make them attractive to varying degrees according to the application. For example, the materials can be formulated to possess high Curie temperatures and high permittivity that translates into lower electrical impedance in practical medical imaging designs. Using these materials has enabled significant performance improvements over PZT performance. As an example, the materials have been used to achieve 35 MHz center frequency designs with 100% −6 dB fractional bandwidth9. Additionally, there have been elegant innovations in device machining – such as the use of deep reactive ion etching (DRIE) to yield high aspect ratio designs with extremely fine inter-post kerfs10 (Figure 1B). Additionally, there has been progress in the field of lead-free piezoelectric materials, including single crystal materials, to address progressively tightening environmental related regulations (ROHS)11.
cMUT
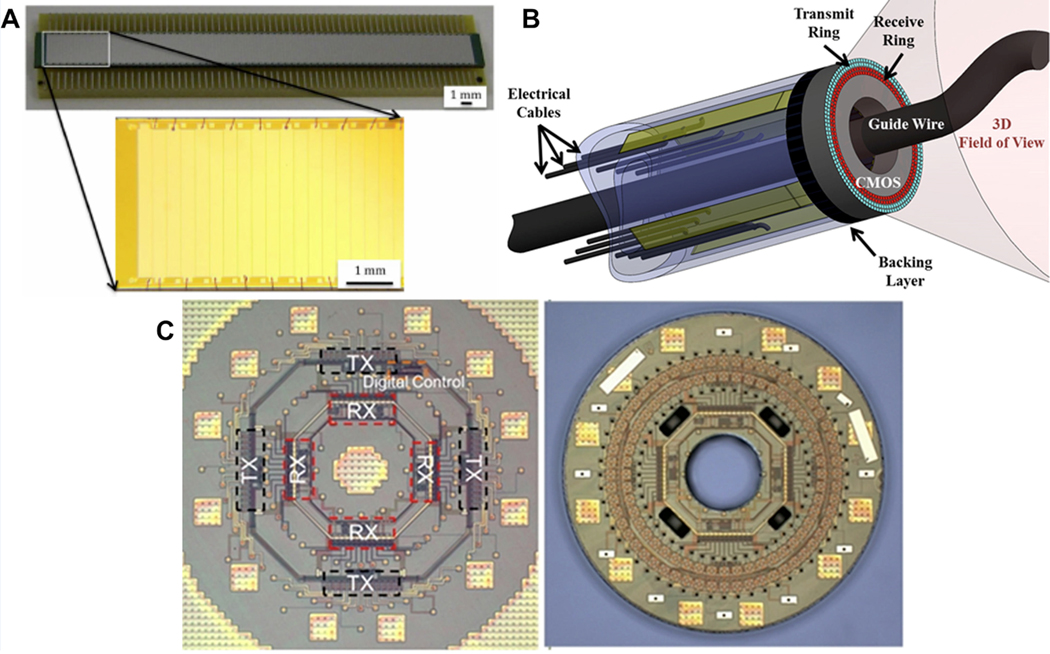
The capacitive ultrasound micromachined transducer (cMUT) represents a revolution in transducer design12,13. The underlying mechanism of actuation and detection are completely different from the piezoelectric-based devices in the preceding sections. The devices are physically comprised of an electrode micromachined thin membrane suspended, and under considerable tension, above a conducting, typically silicon wafer-based substrate. The moving upper membrane is actuated by application of an ultrasonic frequency voltage pulse that is superimposed on a static bias voltage. In the receive mode of operation, changes in capacitance are sensed as the plate gap dimension changes in response to impinging acoustic waves. Several generations of design have occurred since the first descriptions of these devices were presented by Haller12. The low acoustic impedance of the thin membrane allows for very high intrinsic signal bandwidth when applied to medical ultrasound applications. However, cMUTs are not without a few challenges. These include an inherently nonlinear transmitting mode of operation because of the voltage squared dependence of the electrostatically induced force in the transmit mode but also because of the challenge of operating the device as close as possible to “collapse”. In fact, some modes of operation purposely make use of collapse-mode14. Another technical challenge with cMUTs is their high potential for problematic inter element crosstalk that may result in artifacts in the acquired ultrasound image15 although significant progress has been made in resolving crosstalk problems16. There are also some development cost considerations that need to be considered with cMUTs. The cost of microlithography mask and access to a high capital cost microfabrication facility need to be borne in mind and make iterative new design expensive. This, of course, motivates the use of very advanced finite element models to more fully predict performance apriori17. Among the most compelling aspects of a cMUT design is the potential to place associated front-end electronics in the immediate vicinity of the transducer element. In fact, this quality mitigates the otherwise extremely challenging electrical interconnect problem posed by high density transducer element designs such as those encountered in 2D arrays and in high frequency applications – including catheter-based cMUT designs18,19 (Figure 2). Thus, at this time, the bulk of applications of cMUT that are at or near commercial usage involve either 2D arrays or catheter-based designs where the high product volume and small device design are well matched to the realities of cMUT design and fabrication.
Figure 2.
(A) A 132-element 1-D CMUT array fabricated using the simple wafer bonding process19. Copyright (2013), SPIE, reprinted with permission. (B) Schematic of forward looking IVUS catheter with cMUT array monolithically integrated with the complete front-end CMOS IC18. Copyright (2012), IEEE, reprinted with permission.
(C) Micrograph of the IC with receive and transmit electronics and the digital control circuitry (left). Monolithically fabricated single chip dual ring CMUT-on-CMOS array (right) 18. Copyright (2012), IEEE, reprinted with permission.
3. Ultrafast Imaging
Conventional ultrasound imaging is based on sequential insonification with narrowly focused transmit beams and sequential reconstruction of each receive line20. Ultrafast imaging describes image acquisition technologies with very high frame rate, typically in kilohertz range, with two major components: wide-field beam transmission and parallel receive beamforming21 (Figure 3A). Delannoy et al. completed the pioneering work on ultrafast imaging in 1979 by using an analog-based parallel processing approach to produce entire frames from a single pulse22. The parallel processing approach was then implemented for phased-array23 and later for fast volumetric imaging24,25. Another approach for high frame rate imaging was proposed by Lu et al. using non-diffracting beams26–28. The use of spatial compounding with limited diffraction beams coherently or incoherently was also reported29,30. Parallel to the previous work, Fink’s group demonstrated that the combination of plane wave illumination with parallel receive beamforming could achieve ultrafast imaging with kilohertz frame rate31–33, which was used in transient elastography and led to the first in vivo clinical study using ultrafast imaging for breast tumor detection34. Additionally, coherent plane wave compounding was reported to achieve better image quality without compromising ultrafast frame rate35. Parallel to the ultrafast imaging work, Jensen’s groups performed studies on synthetic aperture imaging (usually single element aperture)36–38. To increase the frame rate, sparse synthetic aperture beamforming39,40 with virtual ultrasound sources41 were reported. Recently, fast 3D imaging was implemented on a handheld device42. In addition, with divergent or plane wave from a sparse virtual array, 3D ultrafast imaging was demonstrated in humans in vivo43.
Figure 3.
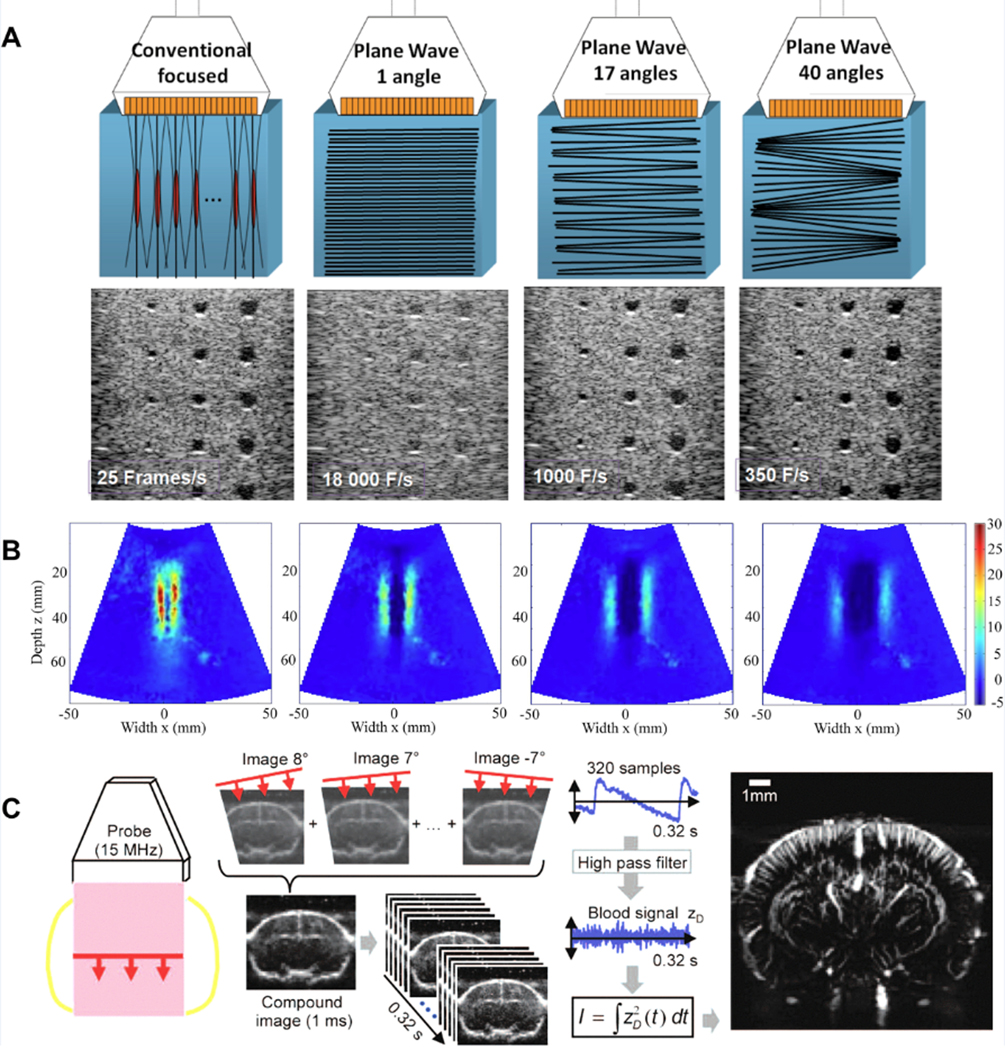
(A) Conventional focused and ultrafast ultrasound imaging sequences for a typical medical imaging setup21. Copyright (2014), IEEE, reprinted with permission.
(B) Displacement field of shear wave at successive times49. Copyright (2009), World Federation for Ultrasound in Medicine & Biology, reprinted with permission.
(C) μDoppler mode based on ultrafast imaging for functional ultrasound imaging69. Copyright (2013), IEEE, reprinted with permission.
Emerging Applications
Shear wave elastography is one of the early clinical applications of ultrafast imaging. Real-time tracking of shear waves induced by mechanical vibration from the surface of the body can only be achieved by ultrafast imaging in transient elastography32–34. In 2004, Bercoff et al. introduced supersonic shear imaging (SSI) which is the combination of remote palpation induced by acoustic radiation force and ultrafast imaging-based transient elastography44 (Figure 3B). SSI has been studied in multiple clinical applications, including breast lesion imaging45–47, liver fibrosis evaluation48,49, musculoskeletal imaging50,51, arterial rigidity52 and cardiac stiffness assessments53,54.
Another application of ultrafast methods is to form images of the natural or intrinsic waves propagating through the organs. Examples of these include electromechanical waves and pulse waves. Konofagou’s group performed studies on cardiac electromechanical wave imaging (EWI) using conventional ultrasound scanners by reconstructing the ultrafast tracking frames from multiple cardiac cycles55–59. The electromechanical waves propagating in human biceps have been studied using ultrafast plane wave imaging60. In addition, multiple research groups have demonstrated the potential value of using ultrafast imaging for arterial pulse wave imaging, including the estimation of pulse wave velocity (PWV)61–64.
Ultrafast imaging can be used for Doppler flow imaging. Due to the limitations of conventional ultrasound scanners with line-by-line acquisitions, Doppler flow imaging is typically categorized in two separate modes: Spectral Doppler, including continuous wave (CW) and pulsed wave (PW) Doppler, for continuous (i.e. long) acquisitions at a narrow position; and Color Doppler, including Power Doppler, for shorten acquisitions over an extended area21. Ultrafast Doppler imaging provides a new solution to achieve PW Doppler data for the entire image frame, including both flow and tissue motion65, within a limited number of cardiac cycles (e.g. one or two)66. In addition, vector Doppler imaging can be achieved for complex flow dynamics67. In neuroscience, ultrafast Doppler imaging with very high sensitivity can be used for functional imaging of brain activity (fUltrasound)68,69 (Figure 3C).
Finally, ultrafast imaging has several important applications for contrast-enhanced ultrasound (CEUS). It can be used for contrast enhancement in perfusion imaging70, contrast plane wave imaging71, monitoring of drug delivery72, cavitation assessment73,74, super-resolution imaging75, and enhanced molecular imaging76.
Software-based Platforms
Conventional hardware-based platforms were initially designed to process one receive line at a time. To further increase frame rate, most current hardware-based platforms have multiline processing capabilities: simultaneous computation of several receive lines (i.e. multiline, typically from 2 to 32 and limited by hardware) can be achieved for each transmit beam77. In comparison, software-based platforms are able to compute unlimited number of receive lines in parallel so that a full frame of image can be reconstructed from each insonification. With such platforms, the frame rate can reach kilohertz range and is physically limited by the time-of-flight for transmit beam propagation77. In addition, a software-based platform is flexible and fully configurable, allowing quick and easy implementation of advanced processing algorithms, such as adaptive beamforming and enhancement78. Thanks to the rapid growth in computational power nowadays, graphical processing unit (GPU)-based platforms which are capable of high-speed data transferring and powerful parallel computing have reached the satisfactory level of the software-based platform21,77. Indeed, many vendors of commercial ultrasound scanner have already released or plan to release their software-based platforms, for example, Aixplorer Ultimate with UltraFast technology (SuperSonic Imagine, Aix-en-Provence, France)79, Resona 7 with ZONE Sonography Technology (Mindray North America, Mahwah, NJ)80, and Vivid E95 with cSound platform (GE Healthcare, Wauwatosa, WI)81.
4. Portable Ultrasound
The past two decades have witnessed dramatic progress in the field of portable ultrasound. An early pioneer in the field, SonoSite, introduced handheld ultrasound imaging in the late 1990s. Since that time, a number of contributions from both academic institutions and industry have been made82–86. These imaging devices have the potential to become almost as ubiquitous as stethoscopes and be used for adjunctive information in a range of non-traditional medical imaging applications – such as for needle placement82 and epidural guidance86. Thus, while the impact on traditional medical imaging settings, based within medical center radiology and cardiology departments, is likely to be minimal, the impact in a range of new settings is impressive and may encompass: primary care medicine, the emergency room or remote settings such as ambulances, a battlefield or even space87. Perhaps the most impressive current version of portable ultrasound is the Butterfly IQ88,89. This device is based upon a current generation Apple iPhone for its display and related signal processing. Significantly, the design is also based on a highly integrated 2D cMUT array that yields the benefits of the cMUT approach as they apply to high product volume and the dense electrical integration of a high channel count 2D transducer array. This device appears to prove conclusively the role for cMUTs in a demanding, highly compact, high product volume, high channel count (9000) and low-cost application. While the ultimate extent of the portable ultrasound market remains unknown as of the time of writing, it is clear that handheld ultrasound is almost certain to have a profound impact on society’s most frequent encounters with medical imaging because the number of new imaging applications will almost certainly outweigh the number of traditional radiology department and cardiology department driven studies.
5. Vector Flow Imaging
Conventional Doppler flow imaging techniques, including color Doppler, power Doppler, CW Doppler, and PW Doppler, are limited to velocity estimation along beam direction, thus missing the velocity component perpendicular to the beam direction (i.e. transverse velocity)90,91. The goal of vector flow imaging is to achieve (1) angle-independent accurate estimation and (2) intuitive and dynamic visualization of velocity vectors. The velocity estimation methods of vector flow can be categorized into four major schemes92–94: (1) multi-angle Doppler, where the velocity vector is obtained from conventional Doppler method from multiple beam directions95; (2) transverse oscillation (TO), where the transverse velocity is achieved from oscillation perpendicular to the beam direction which is introduced by simultaneous transmissions of echoes from two sub-apertures96,97; (3) speckle tracking, where the velocity vector is derived from the displacements of blood speckles using motion tracking98,99; and (4) directional beamforming (DB), where the correct flow angle of each point is determined by cross-correlation analysis for all possible flow angles100,101. Another indirect velocity estimation method is color Doppler-based vector flow mapping93, where the transverse velocity component is computed from the model which requires conventional Doppler results and certain boundary conditions and assumptions102–104. These velocity estimation methods are implemented on conventional systems with sequential data acquisition93 and systems with parallel data acquisition94. There are a few methods to visualize the magnitude and direction of velocity vectors in real-time: (1) arrow displays, where the length of the arrow is associated with velocity magnitude and the pointing direction of the arrow is associated with velocity direction65,105,106; (2) color map displays, where multiple color palettes are used to encodes the velocity magnitude and direction107,108; and (3) dynamic displays, where arrows or short streamlines are used to dynamically update their positions and follow the blood flow92,109,110 (Figure 4A). Vector flow can be used for velocity flow estimation in the carotid artery111, ascending aorta112, pediatric cardiology99,110 (Figure 4B), and for volume flow estimation in arteriovenous fistulae113,114. It also can be used for complex flow visualization, for example, systolic backflow in ascending aorta and cardiac vortical flow115. In addition, 3D vector flow imaging has been developed for in vivo carotid artery imaging116,117 (Figure 4C).
Figure 4.
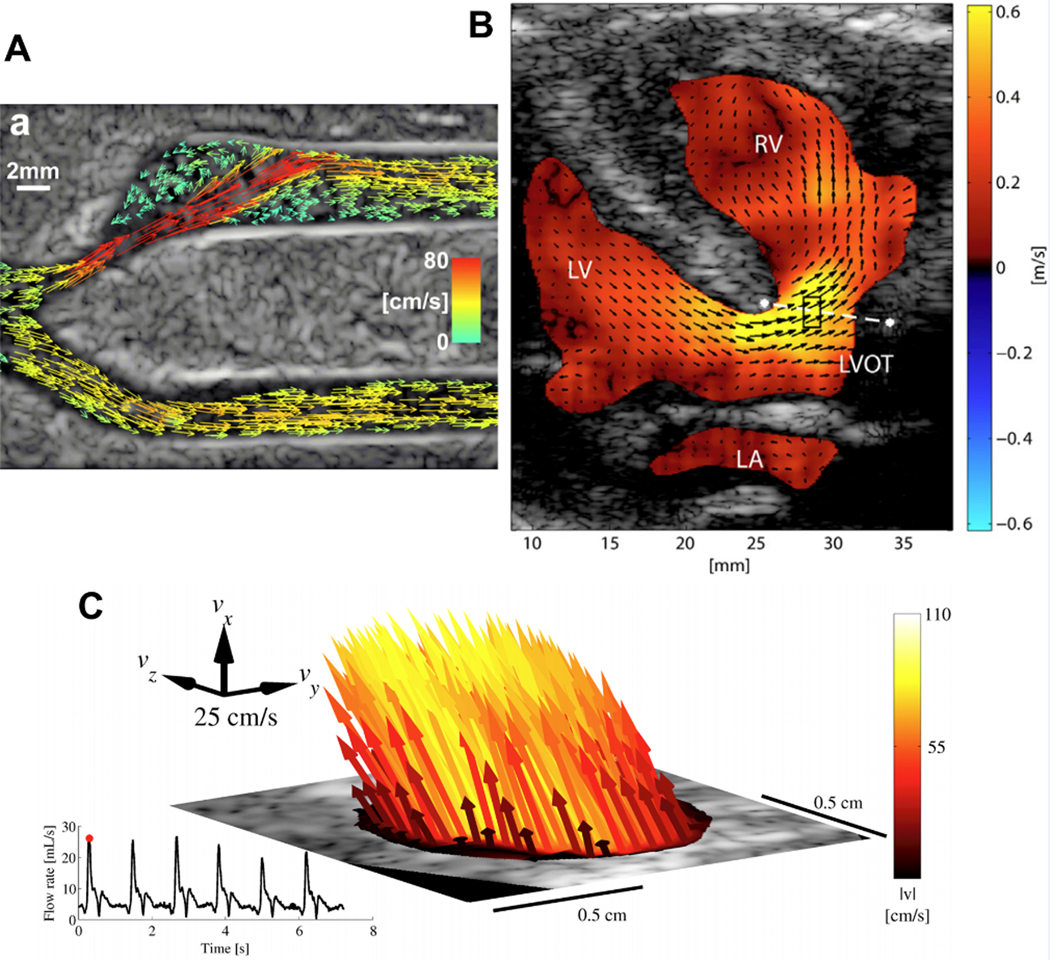
(A) Vector projectile imaging (VPI) showing peak systole92. Copyright (2014), World Federation for Ultrasound in Medicine & Biology, reprinted with permission.
(B) Vector flow image of a patient (8 days) indicating a ventricular septal defect from a parasternal long-axis view99. Copyright (2014), World Federation for Ultrasound in Medicine & Biology, reprinted with permission.
(C) 3-D vector flow from the common carotid artery in peak-systole116. Copyright (2017), IEEE, reprinted with permission.
6. Perfusion Imaging
Ultrasound Contrast Agents: Gas-filled Compressible Microbubbles
Ultrasound contrast materials were discovered in late 1960s, almost by accident, during M-mode ultrasound of the aortic root during left atrium injections of the solutions of indocyanine green, dextrose, and even saline118. As was conceivable those days, clinical trial result report followed rapidly119. Acoustic signal was eventually traced not to the actual solutions of the injected materials, but to small gas bubbles present in the injected media. Initial ultrasound contrast formulations had to be prepared and used immediately, as they were lacking a reasonable stabilizer shell, and would disappear as soon as they floated to the top of the aqueous dispersion. These early-generation gas bubbles were not observed in the arterial vasculature following intravenous administration: they were retained in lung capillaries, which limited the use of these agents to catheterization labs. Eventually, with the proper shell design and formulation optimization, “trans-pulmonary” intravenously injected ultrasound contrast agents appeared, with blood residence time sufficient to perform diagnostic imaging120.
A typical design of a modern clinical ultrasound contrast material is as follows121. It is a gas-filled microbubble, with an inert fluorinated gas (SF6, C3F8 or C4F10), and a thin stabilizer coat of a lipid, lipid/polymer or protein (human albumin). Typical mean particle size is several micrometers. There are several reasons for this particle design. The insoluble gas core provides a longer circulation time, because it reduces the rate of gas loss from the contrast agent particles. The upper limit of particle size (several micrometers) is given by the diameter of blood capillaries, e.g., in lungs, through which contrast agent particles have to pass. Lower size particles (known as nanobubbles) are possible, but then the acoustic response is orders of magnitude lower than for micrometer-size particles120; generally, higher acoustic pressure, i.e., destructive mode ultrasound imaging, may improve detection of small bubbles122. The resonance frequency of micrometer-size gas bubbles is within the range of several MHz, i.e., frequencies used by clinical ultrasound imaging systems; this coincidence may also be beneficial for ultrasound imaging of these particles. Gas-filled microbubbles, with typical micrometer diameter, stabilized with a thin flexible shell will easily compress and expand in the ultrasound field123. The shell has to be thin, to provide minimal interference with compression and expansion of the gas bubble, which is required for the efficient acoustic backscatter signal generation. If that condition is met, the gas-liquid interface of each bubble becomes a point source of ultrasound radiation in response to the passage of the ultrasound pressure wave. Medical ultrasound generates up to MPa levels of rapidly varying acoustic pressures, both positive and negative, so one can expect significant excursion of gas particle volume and size, at the frequency of the applied ultrasound, as well as harmonic124, subharmonic125 and superharmonic126 frequencies; those signals can also be applied for bubble-specific signal detection and imaging.
Perfusion Assessment with Ultrasound Contrast
Microbubbles are purely intravascular agents: due to their larger size they do not extravasate even in the leaky vasculature of tumors127. Therefore, they are quite useful as blood pool intravascular-specific contrast materials. With proper shell design and size distribution, these particles will not adhere nonspecifically in any vascular bed. This opens the possibilities to assess perfusion of tissues with blood in a quantitative manner. In the early days of microbubble contrast imaging, to image microbubbles in the tissue with reasonable levels of sensitivity, and to distinguish the signal from the microbubbles from the signal of background tissue, high-intensity (destructive) ultrasound pulses had to be used for imaging. Such high-pressure pulses would break and collapse the bubbles present in the insonated vessels, and microbubbles will be absent from the next several frames until brought in the imaging plane with the flow of blood. At that time, another high mechanical index imaging pulse can be delivered. Varying the interval between the imaging pulses, one can construct the destruction-replenishment curve to assess perfusion throughout the imaged tissue plane128. With the advent of low-power non-destructive ultrasound imaging modalities129, it is no longer necessary to destroy microbubbles every time imaging is performed: a destructive ultrasound pulse is followed with real-time imaging, and a replenishment rate image map (i.e., tissue perfusion visualization) can be generated from the analysis of the exponential curves at each region of interest and even each pixel. One does not even need to destroy microbubbles: monitoring the patterns and kinetics of particle influx into the tissue of interest following intravenous bolus administration is often sufficient to achieve diagnosis130 (see Supplemental Video file for the influx of microbubbles into the tissue). While the efforts to achieve microbubble contrast approval for a myocardial perfusion indication have not been successful so far131, perfusion assessment in other tissues, such as liver or tumors, is routine. This is enabled by the high sensitivity of ultrasound imaging, at the level of individual microbubble detection in real time122. The FDA approved the use of one of the ultrasound contrast microbubbles in a non-cardiac setting, not just in adults, but also for pediatric patients132, where use of other imaging modalities is complicated, and ionizing radiation use should be minimized.
7. Therapeutic Ultrasound
The review of ultrasound technology will not be complete without tackling the topic of therapeutic ultrasound. The history of the subject is significant: early visionaries, Frye et al developed focused ultrasound: they used the ability of ultrasound waves to penetrate deep in the body and provide tight focal zones of energy deposition133. Therapeutic interventions were attempted and initial successes were reported134. Unfortunately, at that time, imaging technology for real time guidance of therapeutic interventions (such as MRI) was not in existence, and clinical translation did not take hold. More recently, with the pioneering and highly systematic effort of Hynynen et al.135 as well as a number of industrial entities, practical use of focused ultrasound in clinic is spreading. While the initial therapeutic approval was for the treatment of uterine fibroids136, brain also became a target of clinical intervention with focused ultrasound, for treatment of essential tremor137. Non-invasive trans-skull thalamotomy intervention by ablative focused ultrasound hyperthermia is now approved for clinical use. First, by CT or MRI the shape and thickness of the skull is recorded, and used to control the delay of ultrasound pulses of the individual elements of the acoustic helmet to achieve focusing at the desired spot (thousands of individual elements assure tight focusing and control of focal zone location). MRI thermometry is used to map the temperature increase and focal spot location in relation to the desired treatment spot. Then, a minor but detectable temperature increase at the focal zone is achieved by focused ultrasound application, to assure precise location of the treatment focus. Finally, the ultrasound energy level is increased, and the patient can immediately recognize an improvement of their hand motor function (e.g., the ability to write or draw a spiral within predetermined borders). Such hyperthermia is obviously applicable as well for tumor therapy, within the brain and beyond, and clinical trials in that direction are ongoing; see, e.g., NCT00147056. While prostate cancer treatment indication is not yet approved by the FDA for clinical use, prostate tissue treatment is approved138. Likewise, treatment of breast tumors by focused ultrasound is possible, even under ultrasound guidance that would not require expensive and stationary MRI for guidance and thermometry139. Treatment is not necessarily limited to a hyperthermia approach; mechanical breakage of the tissue can be observed with very high acoustic pressures (tens of MPa peak negative acoustic pressure)140; a recent review can be found in the J. Ultrasound Med141.
Ablative treatment (both thermal and mechanical) may be accompanied by abscopal effects, where distant tumor nodes may shrink in response to the focal treatment of primary tumor142. Anti-cancer immune response has been reported following both mechanical (cavitational) and thermal ablation, so that reinoculation of tumor cells would not result in tumor growth143. From that idea, there is a clear path to immunotherapy144.
The use of dedicated novel micro- and nanoparticles loaded with drugs as ultrasound-sensitive triggered delivery system is an active area of research currently. Mild ultrasound hyperthermia (~40–42°C) of thermosensitive liposomes145 offers one approach that is currently in preclinical146 and clinical testing (e.g., NCT02181075). This thermosensitive nanoparticle formulation had been in clinical development well before focused ultrasound became an approved therapeutic modality. More common in current research is the use of microbubble- and superheated perfluorocarbon nanodroplet-based structures, where the ultrasound pressure wave is directly responsible for content release, as will be discussed next.
8. Drug Delivery
The general aim of drug delivery to particular tissues and disease sites with the use of ultrasound is obvious: the ability of ultrasound to focus tightly and deeply inside the body is highly beneficial. Drug delivery with ultrasound can be based on two complementary approaches. The first approach is to increase the delivery of the drug to the target zone, either by increasing the blood flow to the target via minor hyperthermia147, and/or by breaking endothelial barrier that restricts drug entry from the vasculature into the interstitial space148. This can be used to increase delivery in any tissue149, but is especially important for blood brain barrier opening (see below). The second approach is to have the drugs entrapped in a carrier micro- or nanoparticle, and have it sequestered in the particle as it is in the bloodstream, until the trigger by focused ultrasound; this will help create localized delivery of focally released drug at the target areas150–153. Nanoparticles on the microbubble surface that carry the drug may partially release it into the surrounding media, with improved extraction into the interstitial space. Some (or most) of the nanoparticles that decorate the bubble surface will be detached during microbubble destruction by ultrasound, and could be forced into the vessel wall and surrounding tissue154. Nucleic acid may also be delivered this way, where microbubbles may serve as a tool to open the vascular endothelial barrier for ease of transfection155 as well as a carrier/protector/transfection enhancer156–158.
The use of decorated microbubbles as drug and nucleic acid carriers might be scientifically interesting, but there are practical limitations to the usefulness of current designs. Bubble-based particles rapidly lose gas upon intravenous injection, which implies rapid loss of acoustic activation ability, within minutes. Especially in humans, this will imply that only a very small fraction of the intravenously administered carrier and drug material will have a chance to circulate through the tumor target, and an even lower amount will be deposited there. Even if successful as a concept, formulation complexity and development cost may slow clinical adoption. Therefore, the alternative of ultrasound-assisted drug delivery, which uses already approved drugs and drug carriers, in combination with approved or tested ultrasound contrast formulations, is the likely first candidate for success, and is already in clinical testing. The idea is to vibrate intravenous clinical microbubbles by moderate intensity ultrasound, to induce opening of the endothelial barrier, so that co-administered intravascular circulating drug would have a chance to exit the bloodstream and accumulate in the insonated tissue; there are suggestions of therapy efficacy improvement from early scale clinical testing159. While tumors are known to possess leaky vasculature160, further enhancement of drug extravasation is still needed. Following insonation, the endothelial barrier may stay open for hours, so recirculating drug or drug carrier in the bloodstream will have much better chance for therapy success, as was proven in animal models161. This is especially important for brain tumors, where opening of BBB has recently reached the clinical trial stage using this approach162, and may allow drug delivery and therapy for drugs that otherwise cannot reach the tumor cells.
9. Molecular Imaging
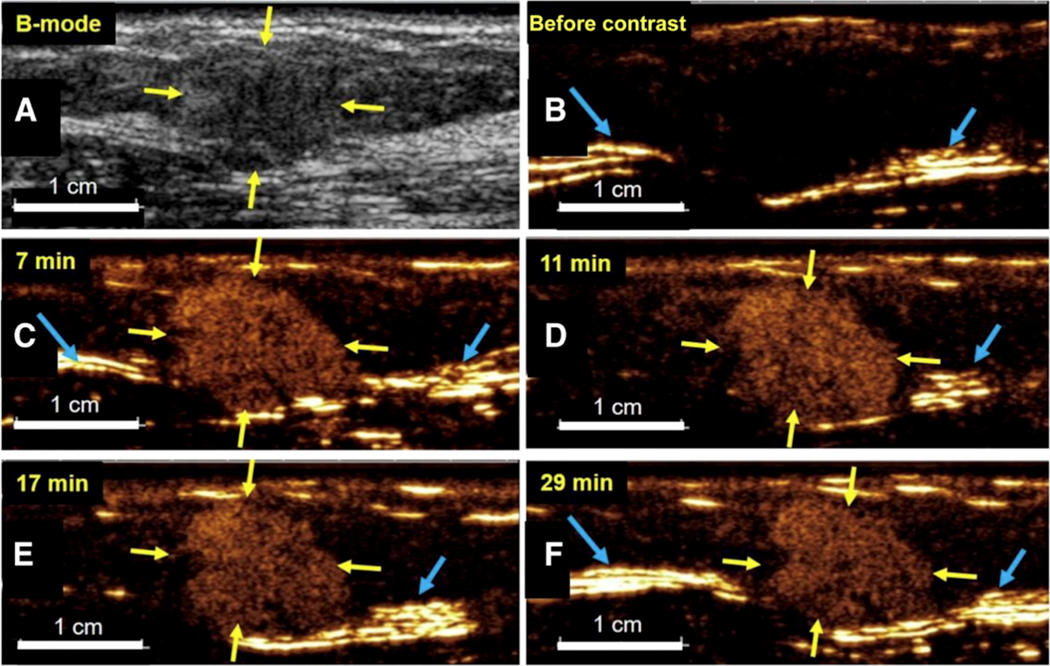
While initially, very early this century, it was assumed that ultrasound contrast agents were not suitable for molecular imaging applications163, eventually it became possible to change that opinion. The initial experiments were described prior to the term “molecular imaging” being coined164–166, as “targeted” ultrasound contrast agents. Initial experiments were carried out in fundamental imaging mode; even in relatively early studies it became possible to image individual microbubbles167, both in aqueous dispersion, and immobilized to surfaces. This level of sensitivity became the foundational success for the use of ultrasound contrast in molecular imaging: each microbubble contrast particle would have the mass of a fraction of a picogram, and carry ~105 – 106 of targeting ligand molecules on the surface. With the advent of contrast-specific pulse sequences168, it became possible to suppress the background signal from soft tissues completely and without microbubble destruction. At that point, molecular imaging of targeted bubbles was ripe for clinical testing. While initial preclinical studies focused on imaging inflammation169 and thrombus170, the main interest gradually turned to imaging cancer. As micrometer-size contrast agent particles would not extravasate due to their large size, despite the leakiness of tumor vascular endothelium, there is little possibility for microbubbles to target tumor cell biomarkers directly. Therefore, targeting has to be directed towards the molecular markers of endothelium in neovasculature, such as alphaVbeta3171, or markers of hypoxia, such as VEGFR2172. Clinical trials with a VEGFR2-targeted agent, a heterodimeric peptide attached to lipid microbubbles with perfluorobutane/nitrogen gas core, have demonstrated successful tumor node imaging in Phase 1–2 clinical trials in ovarian and breast cancers173 and prostate174 (Figure 5 demonstrates accumulation of VEGFR2-targeted microbubbles in a breast cancer node, with retention of contrast material in the tumor, but not in the surrounding tissues, for almost 30 minutes). This agent might find application beyond simple diagnostic imaging, in the areas of image-guided biopsy and ablation, to improve guidance precision and obtain information at the margin of the tumor and surrounding tissues. It might provide a tool to assess chemotherapy efficacy quickly, for instance with anti-angiogenesis therapy175, with effects observed much earlier than the usual tumor size monitoring by CT176.
Figure 5.
Duration of kinase insert domain receptor (KDR) –targeted ultrasound molecular imaging in a 59-year-old woman with ductal adenocarcinoma (yellow arrows) located in the upper external quadrant of the right breast. (A) Transverse B-mode image shows a 1.45-cm heterogeneous isoechoic solid lesion (yellow arrows) within the mammary gland. (B) On a contrast mode image obtained before contrast agent administration, there is no signal in the lesion or surrounding normal breast tissue. Note tissue leakage artifacts (caused by nonlinearities in the front-end electronics of ultrasound machines; blue arrows) as a result of high echo amplitudes at tissue interfaces. (C-F) Transverse contrast mode images obtained at four representative time points up to 29 minutes after intravenous administration of KDR-targeted contrast microbubbles (MBKDR) show strong and persistent targeted ultrasound image signal in breast cancer and low background signal. Scale bar is 1 cm. Copyright (2017), American Society of Clinical Oncology, reprinted with permission173.
The “opposite” approach for cancer imaging is already in clinic, specifically, for liver cancer imaging. Phosphatidylserine lipid shell perfluorobutane microbubbles are approved for clinical use and administered annually to tens of thousands of patients in Japan, Korea and Norway. As phosphatidylserine is the marker of apoptosis, these particles are actively taken up by Kupffer cells in normal liver parenchyma. Thus, intense uptake and prolonged retention of phosphatidylserine microbubbles lead to bright acoustic backscatter signal in normal, but not in cancer nodes, which lack phagocytic Kupffer cells177.
Molecular ultrasound imaging is currently under investigation as a tool for assessment of gene expression in experimental animal models. For several decades, it was used in bioluminescence (e.g., with luciferase) and fluorescence (with green or red fluorescent protein) imaging. Ultrasound contrast technology in this case is based on biosynthetic cylindrical or bi-conical hollow air-filled gas vesicles (GVs) that are naturally expressed in Archaea and certain bacteria. Expression of the necessary proteins and production of these GV nanostructures inside eukaryotic cells has recently become possible178, and may help assess gene expression in vivo. While clinical use of such structures is not likely, at least currently, ultrasound GV imaging opens up the possibility of monitoring gene expression deep inside the body, where optical imaging cannot penetrate easily. While GVs in the bloodstream are not very long lived179, their generation and location deep inside eukaryotic cells outside of the areas of rapid convection (i.e., vasculature) and rapid gas exchange (lungs) makes them much more stable and detectable in vivo.
10. Super-Resolution Imaging
The concept of super-resolution imaging was first introduced in optical microscopy to break the diffraction-based resolution limit180–183. Super-resolution fluorescence microscopy was implemented, which combines the detection and localization of individual sources (i.e. stochastic blinking of fluorescent sources), and accumulation of their center positions to form the super-resolved image184. Ultrasound super-resolution imaging was inspired by these principles using contrast agents (i.e. microbubbles) as sources. The feasibility of ultrasound super-resolution imaging was first demonstrated in 2011 by processing the centroid positions of detected individual microbubbles185,186. Following studies based on flow phantoms further quantified the resolution improvement by a factor of 2.2 to 13187–189. In 2015, Christensen-Jeffries at al. published the super-resolved images showing microvasculature of mouse ear with the resolution down to 19 μm190 (Figure 6A). Super-resolved velocity maps were also achieved by tracking the movement of individual microbubbles. In order to reduce the long acquisition times (hours per frame)190, ultrafast imaging with higher concentration of microbubbles21,187,191 was introduced for super-resolution imaging. Errico et al. further developed super-resolution imaging using an experimental imaging system with very high frame rate (500 Hz) to image mouse brain with short acquisition times (tens of seconds)75 (Figure 6B). The best resolution today can reach approximately 10 μm75. Super-resolution imaging has been applied in multiple pre-clinical models, including mouse ear190 and skeletal muscle192, rat brain75 and tumor193, and rabbit kidney194 and lymph node195, mostly for microvasculature mapping and assessment196,197. In 2017, Opacic et al. presented the first-in-patient study of super-resolution imaging in breast cancer and thyroid nodule198 (Figure 6C). Advanced microbubble tracking algorithms were developed to better fit clinical settings using commercial ultrasound scanners with regular frame rates (e.g. 50 Hz) and short acquisition times (e.g. 1 min)198,199. However, preliminary clinical data indicates that clinical translation is still challenging due to motion artifacts and unoptimized contrast dose and injection speed198.
Figure 6.
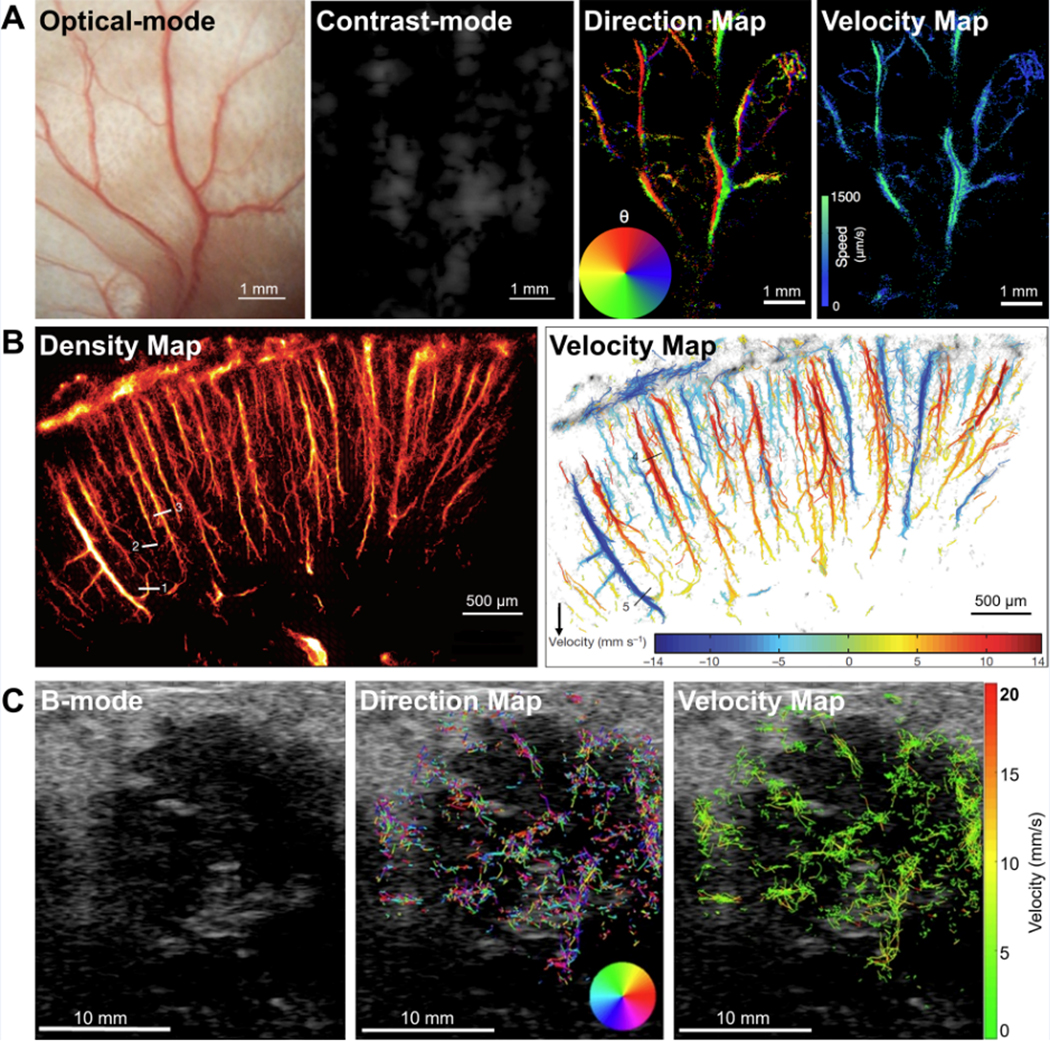
Super-resolution ultrasound imaging. (A) In vivo example of super-resolution imaging of microvasculature of mouse ear with spatial resolution of 19 μm190. Comparative images of optical image, regular contrast image (CPS mode), super-resolved blood flow direction map, and super-resolved blood velocity map. Copyright (2015), IEEE, reprinted with permission.
(B) In vivo example of super-resolution imaging of microvasculature of rat brain with spatial resolution of 8 μm × 10 μm75. Images showing the super-resolved microbubble density map and blood flow velocity map. Copyright (2015), Macmillan Publishers Limited, reprinted with permission.
(C) Preliminary super-resolution imaging results from breast cancer patients with the HER2 positive breast carcinoma198. Super-resolved blood flow direction map and velocity map are superimposed on the background B-mode images. Copyright (2018), Springer Nature, Creative Commons License, permission not required.
Challenges and Recent Developments
Recent developments focused on resolving the technical challenges which prevent the clinical translation of super-resolution imaging. High motion sensitivity is one of the major challenges which limits the achievable best resolution. Multiple motion compensation techniques (2D-based) were developed and adapted for super-resolution imaging, for example, two-stage (affine and nonrigid)-based200 and subwavelength phase correlation-based201 motion corrections. To better address the out-of-plane motion artifacts, 3D super-resolution imaging195,202–204 with 3D motion correction205 is under development. Long acquisition time is another major challenge which delays clinical adoption. The acquisition time is physiologically constrained by slow blood flow in capillaries which limits the number of individual microbubbles detected and used for image reconstruction206. Multiple approaches have been investigated to reduce the acquisition time down to minutes with dense microbubble concentrations, including ultrafast imaging with advanced spatial-temporal filtering75,191,194,207, advanced microbubble pairing and tracking194,198,199, tracking of multiple (non-isolated) microbubbles199,208, sparsity-based recovery209,210, and deep learning-based localization211. In addition, other approaches were investigated to improve the sensitivity and resolution, including size-selected contrast agents192,212 and adaptive multi-focal imaging213,214.
11. Conclusions and Discussions
Over the past several decades, ultrasound has become the most widespread imaging modality. It is safe, inexpensive, portable and real-time. Advances in hardware (such as transducer designs, imaging and signal processing schemes) are rapidly adopted for clinical use. Ultrasound enables and guides numerous invasive interventions, such as targeted biopsy and tissue ablation. The ability to focus ultrasound deep within the body permits therapeutic ultrasound to effect both focal tissue ablation and targeted drug delivery. Unfortunately, the timeline for the regulatory approval process for injectable ultrasound contrast is slower than for equipment hardware. However, blood tracer ultrasound contrast agents are already routinely used for tissue perfusion assessment. Targeted microbubble contrast agents, enabling molecular ultrasound imaging, are currently in clinical testing. Super-resolution imaging breaks the wavelength barrier and may eventually allow ultrasound imaging of the smallest blood vessels in the clinic setting. We can expect that ultrasound will continue to expand in its acceptance and usefulness, despite the competition from other imaging modalities.
Supplementary Material
Acknowledgments
Sources of Support that require acknowledgment:
A. Klibanov acknowledges support in part via NIH R01 EB023055. J. Hossack acknowledges support in part via NIH R01 HL132395, HL141752. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Shiying Wang, Department of Biomedical Engineering, University of Virginia, Charlottesville, VA 22908, USA
John A. Hossack, Department of Biomedical Engineering and Electrical and Computer Engineering, University of Virginia, Charlottesville, VA 22908, USA.
Alexander L. Klibanov, Cardiovascular Division, Department of Medicine, Robert M Berne Cardiovascular Research Center, Department of Radiology, Department of Biomedical Engineering, University of Virginia Charlottesville VA 22908, USA.
12. References
- 1.Feigenbaum H. Diagnostic ultrasound. Ann. Intern. Med 1966;65(1):185–189. [DOI] [PubMed] [Google Scholar]
- 2.Desilets CS, Fraser JD, Kino GS. The design of efficient broad-band piezoelectric transducers. IEEE Trans. Sonics Ultrason 1978;25(3):115–125. [Google Scholar]
- 3.Gururaja TR, Cross LE, Newnham RE, et al. Piezoelectric composite materials for ultrasonic transducer applications. Part I: Resonant modes of vibration of PZT rod-polymer composites. IEEE Trans. Sonics Ultrason 1985;32(4):481–498. [Google Scholar]
- 4.Mills DM, Smith SW. Multi-layered PZT/polymer composites to increase signal-to-noise ratio and resolution for medical ultrasound transducers. II. Thick film technology. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002;49(7):1005–1014. [DOI] [PubMed] [Google Scholar]
- 5.Bureau J, Steichen W, Lebail G. A two-dimensional transducer array for real-time 3D medical ultrasound imaging. IEEE Ultrasonics Symposium Proceedings; 1998:1065–1068 vol.2. [Google Scholar]
- 6.Mamou J, Aristizába O, Silverman RH. A perspective on high-frequency ultrasound for medical applications. Int. Congr. Ultrason 2010;3(1):289–295. [Google Scholar]
- 7.Foster FS, Mehi J, Lukacs M, et al. A new 15–50 MHz array-based micro-ultrasound scanner for preclinical imaging. Ultrasound Med. Biol 2009;35(10):1700–1708. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Li F, Yu F, et al. Recent developments in piezoelectric crystals. J. Korean Ceram. Soc 2018;55(5):419–439. [Google Scholar]
- 9.Sun P, Wang G, Wu D, et al. High frequency PMN-PT 1–3 composite transducer for ultrasonic imaging application. Ferroelectrics. 2010;408(1):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Ren W, Jing X, et al. Deep reactive ion etching of PZT ceramics and PMN-PT single crystals for high frequency ultrasound transducers. Ceram. Int 2015;41:S656–S661. [Google Scholar]
- 11.Jiang M, Zhang J, Rao G, et al. Ultrahigh piezoelectric coefficient of a lead-free K0.5Na0.5NbO3-based single crystal fabricated by a simple seed-free solid-state growth method. J. Mater. Chem. C 2019;7(47):14845–14854. [Google Scholar]
- 12.Haller MI, Khuri-Yakub BT. A surface micromachined electrostatic ultrasonic air transducer. Proceedings of IEEE Ultrasonics Symposium; 1994:1241–1244 vol.2. [Google Scholar]
- 13.Khuri-Yakub BT, Oralkan Ö. Capacitive micromachined ultrasonic transducers for medical imaging and therapy. J. Micromechanics Microengineering 2011; 21(5):54004–54014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oralkan O, Bayram B, Yaralioglu GG, et al. Experimental characterization of collapse-mode CMUT operation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006;53(8):1513–1523. [DOI] [PubMed] [Google Scholar]
- 15.Oralkan O, Ergun AS, Johnson JA, et al. Capacitive micromachined ultrasonic transducers: next-generation arrays for acoustic imaging? IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002;49(11):1596–1610. [DOI] [PubMed] [Google Scholar]
- 16.Christiansen TL, Jensen JA, Thomsen EV. Acoustical cross-talk in row-column addressed 2-D transducer arrays for ultrasound imaging. Ultrasonics. 2015;63:174–178. [DOI] [PubMed] [Google Scholar]
- 17.Pirouz A, Magruder R, Harvey G, et al. Crosstalk study of large PMUT array using FEA and cloud HPC. IEEE International Ultrasonics Symposium.; 2019:788–791.
- 18.Tekes C, Zahorian J, Gurun G, et al. Volumetric imaging using single chip integrated CMUT-on-CMOS IVUS array. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS.; 2012:3195–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tekes C, Zahorian J, Xu T, et al. CMUT-based volumetric ultrasonic imaging array design for forward looking ICE and IVUS applications. Medical Imaging 2013: Ultrasonic Imaging, Tomography, and Therapy.Vol 8675. SPIE; 2013:86750B. [Google Scholar]
- 20.Szabo TL. Diagnostic Ultrasound Imaging: Inside Out; 2014.
- 21.Tanter M, Fink M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014;61(1):102–119. [DOI] [PubMed] [Google Scholar]
- 22.Delannoy B, Torguet R, Bruneel C, et al. Acoustical image reconstruction in parallel-processing analog electronic systems. J. Appl. Phys 1979;50(5):3153–3159. [Google Scholar]
- 23.Shattuck D, Weinshenker MD, Smith SW, et al. Explososcan: A parallel processing technique for high speed ultrasound imaging with linear phased arrays. J. Acoust. Soc. Am 1984;75(4):1273–1282. [DOI] [PubMed] [Google Scholar]
- 24.Smith SW, Pavy HG, von Ramm OT. High-speed ultrasound volumetric imaging system. I. Transducer design and beam steering. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1991;38(2):100–108. [DOI] [PubMed] [Google Scholar]
- 25.von Ramm OT, Pavy HG, Smith SW. High-speed ultrasound volumetric imaging system. II. Parallel processing and image display. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1991;38(2):109–115. [DOI] [PubMed] [Google Scholar]
- 26.Lu JY, Greenleaf JF. Ultrasonic nondiffracting transducer for medical imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1990;37(5):438–447. [DOI] [PubMed] [Google Scholar]
- 27.Lu JY, Greenleaf JF. Pulse-echo imaging using a nondiffracting beam transducer. Ultrasound Med. Biol 1991;17(3):265–281. [DOI] [PubMed] [Google Scholar]
- 28.Lu JY. 2D and 3D high frame rate imaging with limited diffraction beams. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1997;44(4):839–856. [DOI] [PubMed] [Google Scholar]
- 29.Lu JY. Experimental study of high frame rate imaging with limited diffraction beams. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998;45(1):84–97. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J, Lu JY. Extended high-frame rate imaging method with limited-diffraction beams. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006;53(5):880–899. [DOI] [PubMed] [Google Scholar]
- 31.Sandrin L, Catheline S, Tanter M, et al. Time-resolved pulsed elastography with ultrafast ultrasonic imaging. Ultrason. Imaging 1999;21(4):259–272. [DOI] [PubMed] [Google Scholar]
- 32.Tanter M, Bercoff J, Sandrin L, et al. Ultrafast compound imaging for 2-D motion vector estimation: Application to transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002;49(10):1363–1374. [DOI] [PubMed] [Google Scholar]
- 33.Sandrin L, Tanter M, Catheline S, et al. Shear modulus imaging with 2-D transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002;49(4):426–435. [DOI] [PubMed] [Google Scholar]
- 34.Bercoff J, Chaffai S, Tanter M, et al. In vivo breast tumor detection using transient elastography. Ultrasound Med. Biol 2003;29(10):1387–1396. [DOI] [PubMed] [Google Scholar]
- 35.Montaldo G, Tanter M, Bercoff J, et al. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009;56(3):489–506. [DOI] [PubMed] [Google Scholar]
- 36.Nikolov SI, Jensen JA. In-vivo synthetic aperture flow imaging in medical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2003;50(7):848–856. [DOI] [PubMed] [Google Scholar]
- 37.Jensen JA, Nikolov SI, Gammelmark KL, et al. Synthetic aperture ultrasound imaging. Ultrasonics. 2006;44(Suppl 1):e5–e15. [DOI] [PubMed] [Google Scholar]
- 38.Nikolov SI, Kortbek J, Jensen JA. Practical applications of synthetic aperture imaging. IEEE International Ultrasonics Symposium.; 2010:350–358.
- 39.Lockwood GR, Talman JR, Brunke SS. Real-time 3-D ultrasound imaging using sparse synthetic aperture beamforming. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998;45(4):980–988. [DOI] [PubMed] [Google Scholar]
- 40.Hazard CR, Lockwood GR. Theoretical assessment of a synthetic aperture beamformer for real-time 3-D imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1999;46(4):972–980. [DOI] [PubMed] [Google Scholar]
- 41.Nikolov S, Jensen JA. Virtual ultrasound sources in high-resolution ultrasound imaging. Ultrasonic Imaging and Signal Processing.; 2002:395–405. [Google Scholar]
- 42.Owen K, Fuller MI, Hossack JA. Application of X-Y separable 2-D array beamforming for increased frame rate and energy efficiency in handheld devices. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012;59(7):1332–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Provost J, Papadacci C, Arango JE, et al. 3D ultrafast ultrasound imaging in vivo. Phys. Med. Biol 2014;59(19):L1–L13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bercoff J, Tanter M, Fink M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004;51(4):396–409. [DOI] [PubMed] [Google Scholar]
- 45.Tanter M, Bercoff J, Athanasiou A, et al. Quantitative assessment of breast lesion viscoelasticity: Initial clinical results using supersonic shear imaging. Ultrasound Med. Biol 2008;34(9):1373–1386. [DOI] [PubMed] [Google Scholar]
- 46.Athanasiou A, Tardivon A, Tanter M, et al. Breast lesions: Quantitative elastography with supersonic shear imaging - Preliminary results. Radiology. 2010;256(1):297–303. [DOI] [PubMed] [Google Scholar]
- 47.Berg WA, Cosgrove DO, Doré CJ, et al. Shear-wave elastography improves the specificity of breast US: The BE1 multinational study of 939 masses. Radiology. 2012;262(2):435–449. [DOI] [PubMed] [Google Scholar]
- 48.Muller M, Gennisson JL, Deffieux T, et al. Quantitative viscoelasticity mapping of human liver using supersonic shear imaging: Preliminary in vivo feasability study. Ultrasound Med. Biol 2009;35(2):219–229. [DOI] [PubMed] [Google Scholar]
- 49.Bavu É, Gennisson JL, Couade M, et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: A clinical study on 113 Hepatitis C virus patients. Ultrasound Med. Biol 2011;37(9):1361–1373. [DOI] [PubMed] [Google Scholar]
- 50.Deffieux T, Gennisson JL, Tanter M, et al. Assessment of the mechanical properties of the musculoskeletal system using 2-D and 3-D very high frame rate ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008;55(10):2177–2190. [DOI] [PubMed] [Google Scholar]
- 51.Gennisson JL, Deffieux T, Macé E, et al. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med. Biol 2010;36(5):789–801. [DOI] [PubMed] [Google Scholar]
- 52.Couade M, Pernot M, Prada C, et al. Quantitative assessment of arterial wall biomechanical properties using shear wave imaging. Ultrasound Med. Biol 2010;36(10):1662–1676. [DOI] [PubMed] [Google Scholar]
- 53.Couade M, Pernot M, Messas E, et al. In vivo quantitative mapping of myocardial stiffening and transmural anisotropy during the cardiac cycle. IEEE Trans. Med. Imaging 2011;30(2):295–305. [DOI] [PubMed] [Google Scholar]
- 54.Pernot M, Couade M, Mateo P, et al. Real-time assessment of myocardial contractility using shear wave imaging. J. Am. Coll. Cardiol 2011;58(1):65–72. [DOI] [PubMed] [Google Scholar]
- 55.Pernot M, Fujikura K, Fung-Kee-Fung SD, et al. ECG-gated, mechanical and electromechanical wave imaging of cardiovascular tissues in vivo. Ultrasound Med. Biol 2007;33(7):1075–1085. [DOI] [PubMed] [Google Scholar]
- 56.Provost J, Lee WN, Fujikura K, et al. Electromechanical wave imaging of normal and ischemic hearts in vivo. IEEE Trans. Med. Imaging 2010;29(3):625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konofagou E, Lee W-N, Luo J, et al. Physiologic cardiovascular strain and intrinsic wave imaging. Annu. Rev. Biomed. Eng 2011;13(1):477–505. [DOI] [PubMed] [Google Scholar]
- 58.Provost J, Lee WN, Fujikura K, et al. Imaging the electromechanical activity of the heart in vivo. Proc. Natl. Acad. Sci. U. S. A 2011;108(21):8565–8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Provost J, Gambhir A, Vest J, et al. A clinical feasibility study of atrial and ventricular electromechanical wave imaging. Hear. Rhythm 2013;10(6):856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deffieux T, Gennisson JL, Tanter M, et al. Ultrafast imaging of in vivo muscle contraction using ultrasound. Appl. Phys. Lett 2006;89(18):184107. [Google Scholar]
- 61.Hasegawa H, Kanai H. Simultaneous imaging of artery-wall strain and blood flow by high frame rate acquisition of RF signals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008;55(12):2626–2639. [DOI] [PubMed] [Google Scholar]
- 62.Vappou J, Luo J, Konofagou EE. Pulse wave imaging for noninvasive and quantitative measurement of arterial stiffness in vivo. Am. J. Hypertens 2010;23(4):393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Couade M, Pernot M, Messas E, et al. Ultrafast imaging of the arterial pulse wave. IRBM. 2011;32(2):106–108. [Google Scholar]
- 64.Luo J, Li RX, Konofagou EE. Pulse wave imaging of the human carotid artery: An in vivo feasibility study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012;59(1):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ekroll IK, Swillens A, Segers P, et al. Simultaneous quantification of flow and tissue velocities based on multi-angle plane wave imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013;60(4):727–738. [DOI] [PubMed] [Google Scholar]
- 66.Bercoff J, Montaldo G, Loupas T, et al. Ultrafast compound Doppler imaging: Providing full blood flow characterization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011;58(1):134–147. [DOI] [PubMed] [Google Scholar]
- 67.Yiu BYS, Yu ACH. High-frame-rate ultrasound color-encoded speckle imaging of complex flow dynamics. Ultrasound Med. Biol 2013;39(6):1015–1025. [DOI] [PubMed] [Google Scholar]
- 68.Macé E, Montaldo G, Cohen I, et al. Functional ultrasound imaging of the brain. Nat. Methods 2011;8(8):662–664. [DOI] [PubMed] [Google Scholar]
- 69.Mace E, Montaldo G, Osmanski BF, et al. Functional ultrasound imaging of the brain: Theory and basic principles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013;60(3):492–506. [DOI] [PubMed] [Google Scholar]
- 70.Couture O, Bannouf S, Montaldo G, et al. Ultrafast imaging of ultrasound contrast agents. Ultrasound Med. Biol 2009;35(11):1908–1916. [DOI] [PubMed] [Google Scholar]
- 71.Couture O, Fink M, Tanter M. Ultrasound contrast plane wave imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012;59(12):2676–2683. [DOI] [PubMed] [Google Scholar]
- 72.Couture O, Urban A, Bretagne A, et al. In vivo targeted delivery of large payloads with an ultrasound clinical scanner. Med. Phys 2012;39(8):5229–5237. [DOI] [PubMed] [Google Scholar]
- 73.Gateau J, Aubry JF, Chauvet D, et al. In vivo bubble nucleation probability in sheep brain tissue. Phys. Med. Biol 2011;56(22):7001–7015. [DOI] [PubMed] [Google Scholar]
- 74.Gateau J, Aubry JF, Pernot M, et al. Combined passive detection and ultrafast active imaging of cavitation events induced by short pulses of high-intensity ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011;58(3):517–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Errico C, Pierre J, Pezet S, et al. Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature. 2015;527(7579):499–502. [DOI] [PubMed] [Google Scholar]
- 76.Couture O, Dransart E, Dehay S, et al. Tumor delivery of ultrasound contrast agents using shiga toxin B subunit. Mol. Imaging 2011;10(2):135–143. [PubMed] [Google Scholar]
- 77.Bercoff J. Ultrafast ultrasound imaging. Oleg Minin, ed. Ultrasound Imaging - Medical Applications. InTech; 2011:1–24. [Google Scholar]
- 78.GE Healthcare. cSound: A powerful, software-based beamformer image reconstruction platform. Available at: http://landing1.gehealthcare.com/rs/005-SHS-767/images/cSoundWP_EU_14815.pdf.
- 79.SuperSonic Imagine. Aixplorer Ultimate. Available at: https://www.supersonicimagine.com/Aixplorer-R/Aixplorer-Ultimate.
- 80.Mindray North America. Resona 7 Ultrasound System. Available at: https://www.mindraynorthamerica.com/ultrasound-systems/resona-7/.
- 81.GE Healthcare. Vivid E95 Premium 4D Cardiac Ultrasound System. Available at: https://www.gehealthcare.com/products/ultrasound/vivid/vivid-e95.
- 82.Fuller MI, Owen K, Blalock TN, et al. Real time imaging with the Sonic Window: A pocket-sized, C-scan, medical ultrasound device. IEEE International Ultrasonics Symposium.; 2009:196–199.
- 83.GE Healthcare. Vscan Family. Available at: https://www.gehealthcare.com/products/ultrasound/vscan-family.
- 84.Philips. Lumify. Available at: https://www.usa.philips.com/healthcare/sites/lumify.
- 85.Siemens Healthineers. ACUSON Freestyle Series Ultrasound Systems. Available at: https://www.siemens-healthineers.com/en-us/ultrasound/ultrasound-point-of-care/acuson-freestyle-ultrasound-machine.
- 86.Rivanna. Accuro. Available at: https://rivannamedical.com/why-accuro/.
- 87.Law J, Macbeth PB. Ultrasound: from Earth to space. McGill J. Med 2011;13(2):59. [PMC free article] [PubMed] [Google Scholar]
- 88.Butterfly. Butterfly Network. Available at: https://www.butterflynetwork.com/.
- 89.Thiele K. Commercializing CMUTs and Deep Learning to realize Worldwide Ultrasound Democratization. IEEE International Ultrasonics Symposium.; 2019. [Google Scholar]
- 90.Deane C. Estimation of blood velocities using ultrasound: A signal processing approach. Med. Eng. Phys 1997;19(2):200. [Google Scholar]
- 91.Hansen KL, Nielsen MB, Jensen JA. Vector velocity estimation of blood flow – A new application in medical ultrasound. Ultrasound. 2017;25(4):189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yiu BYS, Lai SSM, Yu ACH. Vector projectile imaging: Time-resolved dynamic visualization of complex flow patterns. Ultrasound Med. Biol 2014;40(9):2295–2309. [DOI] [PubMed] [Google Scholar]
- 93.Jensen JA, Nikolov SI, Yu ACH, et al. Ultrasound vector flow imaging-Part I: Sequential systems. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016;63(11):1704–1721. [DOI] [PubMed] [Google Scholar]
- 94.Jensen JA, Nikolov SI, Yu ACH, et al. Ultrasound vector flow imaging-Part II: Parallel systems. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016;63(11):1722–1732. [DOI] [PubMed] [Google Scholar]
- 95.Dunmire B, Beach KW, Labs KH, et al. Cross-beam vector Doppler ultrasound for angle-independent velocity measurements. Ultrasound Med. Biol 2000;26(8):1213–1235. [DOI] [PubMed] [Google Scholar]
- 96.Jensen JA, Munk P. A new method for estimation of velocity vectors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998;45(3):837–851. [DOI] [PubMed] [Google Scholar]
- 97.Andcrson ME. Multi-dimensional velocity estimation with ultrasound using spatial quadrature. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998;45(3):852–861. [DOI] [PubMed] [Google Scholar]
- 98.Trahey GE, Allison JW, Von Ramm OT. Angle independent ultrasonic detection of blood flow. IEEE Trans. Biomed. Eng 1987;BME-34(12):965–967. [DOI] [PubMed] [Google Scholar]
- 99.Fadnes S, Nyrnes SA, Torp H, et al. Shunt flow evaluation in congenital heart disease based on two-dimensional speckle tracking. Ultrasound Med. Biol 2014;40(10):2379–2391. [DOI] [PubMed] [Google Scholar]
- 100.Jensen JA. Directional velocity estimation using focusing along the flow direction I: Theory and simulation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2003;50(7):857–872. [DOI] [PubMed] [Google Scholar]
- 101.Jensen JA, Bjerngaard R. Directional velocity estimation using focusing along the flow direction II: Experimental investigation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2003;50(7):873–880. [DOI] [PubMed] [Google Scholar]
- 102.Garcia D, Juan JC, Tanné D, et al. Two-dimensional intraventricular flow mapping by digital processing conventional color-doppler echocardiography images. IEEE Trans. Med. Imaging 2010;29(10):1701–1713. [DOI] [PubMed] [Google Scholar]
- 103.Uejima T, Koike A, Sawada H, et al. A new echocardiographic method for identifying vortex flow in the left ventricle: Numerical validation. Ultrasound Med. Biol 2010;36(5):772–788. [DOI] [PubMed] [Google Scholar]
- 104.Tanaka T, Asami R, Kawabata K, et al. Intracardiac VFM technique using diagnostic ultrasound system. Hitachi Rev. 2015;64(8):488–492. [Google Scholar]
- 105.Maniatis TA, Cobbold RSC, Johnston KW. Two-dimensional velocity reconstruction strategies for color flow doppler ultrasound images. Ultrasound Med. Biol 1994;20(2):137–145. [DOI] [PubMed] [Google Scholar]
- 106.Maniatis TA, Cobbold RSC, Johnston KW. Flow imaging in an end-to-side anastomosis model using two-dimensional velocity vectors. Ultrasound Med. Biol 1994;20(6):559–569. [DOI] [PubMed] [Google Scholar]
- 107.Bohs LN, Trahey GE. A novel method for angle independent ultrasonic imaging of blood flow and tissue motion. IEEE Trans. Biomed. Eng 1991;38(3):280–286. [DOI] [PubMed] [Google Scholar]
- 108.Fei DY, Fu CT, Brewer WH, et al. Angle independent Doppler color imaging: Determination of accuracy and a method of display. Ultrasound Med. Biol 1994;20(2):147–155. [DOI] [PubMed] [Google Scholar]
- 109.Angelelli P, Snare SR, Nyrnes SA, et al. Live ultrasound-based particle visualization of blood flow in the heart. Proceedings - SCCG 2014: 30th Spring Conference on Computer Graphics. Association for Computing Machinery, Inc; 2014:13–20. [Google Scholar]
- 110.Fadnes S, Wigen MS, Nyrnes SA, et al. In vivo intracardiac vector flow imaging using phased array transducers for pediatric cardiology. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017;64(9):1318–1326. [DOI] [PubMed] [Google Scholar]
- 111.Tortoli P, Lenge M, Righi D, et al. Comparison of carotid artery blood velocity measurements by vector and standard doppler approaches. Ultrasound Med. Biol 2015;41(5):1354–1362,. [DOI] [PubMed] [Google Scholar]
- 112.Hansen KL, Møller-Sørensen H, Kjaergaard J, et al. Vector flow imaging compared with conventional Doppler ultrasound and thermodilution for estimation of blood flow in the ascending aorta. Ultrason. Imaging 2017;39(1):3–18. [DOI] [PubMed] [Google Scholar]
- 113.Brandt AH, Jensen J, Hansen KL, et al. Surveillance for hemodialysis access stenosis: Usefulness of ultrasound vector volume flow. J. Vasc. Access 2016;17(6):483–488. [DOI] [PubMed] [Google Scholar]
- 114.Hansen PM, Olesen JB, Pihl MJ, et al. Volume flow in arteriovenous fistulas using vector velocity ultrasound. Ultrasound Med. Biol 2014;40(11):2707–2714. [DOI] [PubMed] [Google Scholar]
- 115.Hansen KL, Pedersen MM, Møller-Sørensen H, et al. Intraoperative cardiac ultrasound examination using vector flow imaging. Ultrason. Imaging 2013;35(4):318–332. [DOI] [PubMed] [Google Scholar]
- 116.Holbek S, Ewertsen C, Bouzari H, et al. Ultrasonic 3-D vector flow method for quantitative in vivo peak velocity and flow rate estimation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017;64(3):544–554. [DOI] [PubMed] [Google Scholar]
- 117.Correia M, Provost J, Tanter M, et al. 4D ultrafast ultrasound flow imaging: In vivo quantification of arterial volumetric flow rate in a single heartbeat. Phys. Med. Biol 2016;61(23):L48–L61. [DOI] [PubMed] [Google Scholar]
- 118.Gramiak R, Shah PM. Echocardiography of the aortic root. Invest. Radiol 1968;3(5):356–366. [DOI] [PubMed] [Google Scholar]
- 119.Gramiak R, Shah PM, Kramer DH. Ultrasound cardiography: contrast studies in anatomy and function. Radiology. 1969;92(5):939–948. [DOI] [PubMed] [Google Scholar]
- 120.Galanti G, Jayaweera AR, Villanueva FS, et al. Transpulmonary transit of microbubbles during contrast echocardiography: Implications for estimating cardiac output and pulmonary blood volume. J. Am. Soc. Echocardiogr 1993;6(3):272–278. [DOI] [PubMed] [Google Scholar]
- 121.Klibanov AL. Molecular imaging with targeted ultrasound contrast microbubbles. Ernst Schering Res. Found. Workshop 2005;(49):171–191. [DOI] [PubMed]
- 122.Klibanov AL, Rasche PT, Hughes MS, et al. Detection of individual microbubbles of an ultrasound contrast agent: Fundamental and pulse inversion imaging. Academic Radiology. 2002;9(2):S279–S281. [DOI] [PubMed] [Google Scholar]
- 123.Patel D, Dayton P, Gut J, et al. Optical and acoustical interrogation of submicron contrast agents. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2002;49(12):1641–1651. [DOI] [PubMed] [Google Scholar]
- 124.Porter TR, Xie F. Transient myocardial contrast after initial exposure to diagnostic ultrasound pressures with minute doses of intravenously injected microbubbles: Demonstration and potential mechanisms. Circulation. 1995;92(9):2391–2395. [DOI] [PubMed] [Google Scholar]
- 125.Forsberg F, Shi WT, Goldberg BB. Subharmonic imaging of contrast agents. Ultrasonics. 2000;38(1):93–98. [DOI] [PubMed] [Google Scholar]
- 126.Cherin E, Yin J, Forbrich A, et al. In vitro superharmonic contrast imaging using a hybrid dual-frequency probe. Ultrasound Med. Biol 2019;45(9):2525–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan F, Dellian M, Fukumura D, et al. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995;55(17):3752–3756. [PubMed] [Google Scholar]
- 128.Wei K, Jayaweera AR, Firoozan S, et al. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97(5):473–483. [DOI] [PubMed] [Google Scholar]
- 129.Tiemann K, Lohmeier S, Kuntz S, et al. Real-time contrast echo assessment of myocardial perfusion at low emission power: First experimental and clinical results using power pulse inversion imaging. Echocardiography. 1999;16(8):799–810. [DOI] [PubMed] [Google Scholar]
- 130.Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall der Medizin. 2018;39(2):e2–e44. [DOI] [PubMed] [Google Scholar]
- 131.Senior R, Monaghan M, Main ML, et al. Detection of coronary artery disease with perfusion stress echocardiography using a novel ultrasound imaging agent: Two Phase 3 international trials in comparison with radionuclide perfusion imaging. Eur. J. Echocardiogr 2009;10(1):26–35. [DOI] [PubMed] [Google Scholar]
- 132.Hwang M, Piskunowicz M, Darge K. Advanced ultrasound techniques for pediatric imaging. Pediatrics. 2019;143(3): e20182609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fry WJ, Barnard JW, Fry FJ, et al. Ultrasonic lesions in the mammalian central nervous system. Science. 1955;122(3168):517–518. [PubMed] [Google Scholar]
- 134.FRY WJ MEYERS R. Ultrasonic method of modifying brain st ructures. Confin. Neurol 1962;22:315–327. [PubMed] [Google Scholar]
- 135.Hynynen K, Roemer R, Anhalt D, et al. A scanned, focused, multiple transducer ultrasonic system for localized hyperthermia treatments. Int. J. Hyperth 2010;26(1):1–11. [DOI] [PubMed] [Google Scholar]
- 136.Tempany CMC, Stewart EA, McDannold N, et al. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: A feasibility study. Radiology. 2003;226(3):897–905. [DOI] [PubMed] [Google Scholar]
- 137.Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med 2016;375(8):730–739. [DOI] [PubMed] [Google Scholar]
- 138.Bass R, Fleshner N, Finelli A, et al. Oncologic and functional outcomes of partial gland ablation with high intensity focused ultrasound for localized prostate cancer. J. Urol 2019;201(1):113–119. [DOI] [PubMed] [Google Scholar]
- 139.Peek MCL, Ahmed M, Douek M. High-intensity focused ultrasound for the treatment of fibroadenomata (HIFU-F) study. J. Ther. Ultrasound 2015;3(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Roberts WW, Hall TL, Ives K, et al. Pulsed cavitational ultrasound: A noninvasive technology for controlled tissue ablation (histotripsy) in the rabbit kidney. J. Urol 2006;175(2):734–738. [DOI] [PubMed] [Google Scholar]
- 141.Dubinsky TJ, Khokhlova TD, Khokhlova V, et al. Histotripsy: The next generation of high-intensity focused ultrasound for focal prostate cancer therapy. J. Ultrasound Med 2019. [DOI] [PubMed]
- 142.Qu S, Worlikar T, Felsted AE, et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J. Immunother. Cancer 2020;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hu Z, Yang XY, Liu Y, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J. Transl. Med 2007;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sheybani ND, Price RJ. Perspectives on recent progress in focused ultrasound immunotherapy. Theranostics. 2019;9(25):7749–7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Needham D, Anyarambhatla G, Kong G, et al. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000;60(5):1197–1201. [PubMed] [Google Scholar]
- 146.Besse HC, Barten-van Rijbroek AD, van der Wurff-Jacobs KMG, et al. Tumor Drug Distribution after Local Drug Delivery by Hyperthermia, In Vivo. Cancers (Basel). 2019;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li L, Ten Hagen TLM, Bolkestein M, et al. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J. Control. Release 2013;167(2):130–137. [DOI] [PubMed] [Google Scholar]
- 148.Price RJ, Skyba DM, Kaul S, et al. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98(13):1264–1267. [DOI] [PubMed] [Google Scholar]
- 149.Böhmer MR, Chlon CHT, Raju BI, et al. Focused ultrasound and microbubbles for enhanced extravasation. J. Control. Release 2010;148(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Klibanov AL, Shevchenko TI, Raju BI, et al. Ultrasound-triggered release of materials entrapped in microbubble-liposome constructs: A tool for targeted drug delivery. J. Control. Release 2010;148(1):13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yu FTH, Chen X, Wang J, et al. Low intensity ultrasound mediated liposomal doxorubicin delivery using polymer microbubbles. Mol. Pharm 2016;13(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yan F, Li L, Deng Z, et al. Paclitaxel-liposome-microbubble complexes as ultrasound-triggered therapeutic drug delivery carriers. J. Control. Release 2013;166(3):246–255. [DOI] [PubMed] [Google Scholar]
- 153.Geers B, Lentacker I, Cool S, et al. Ultrasound responsive doxorubicin-loaded microbubbles: Towards an easy applicable drug delivery platform. J. Control. Release 2010;148(1):e59–60. [DOI] [PubMed] [Google Scholar]
- 154.Roovers S, Deprez J, Priwitaningrum D, et al. Sonoprinting liposomes on tumor spheroids by microbubbles and ultrasound. J. Control. Release 2019;316:79–92. [DOI] [PubMed] [Google Scholar]
- 155.Burke CW, Suk JS, Kim AJ, et al. Markedly enhanced skeletal muscle transfection achieved by the ultrasound-targeted delivery of non-viral gene nanocarriers with microbubbles. J. Control. Release 2012;162(2):414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Unger EC, McCreery TP, Sweitzer RH. Ultrasound enhances gene expression of liposomal transfection. Invest. Radiol 1997;32(12):723–727. [DOI] [PubMed] [Google Scholar]
- 157.Christiansen JP, French BA, Klibanov AL, et al. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med. Biol 2003;29(12):1759–1767. [DOI] [PubMed] [Google Scholar]
- 158.Vandenbroucke RE, Lentacker I, Demeester J, et al. Ultrasound assisted siRNA delivery using PEG-siPlex loaded microbubbles. J. Control. Release 2008;126(3):265–273. [DOI] [PubMed] [Google Scholar]
- 159.Dimcevski G, Kotopoulis S, Bjånes T, et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 2016;243:172–181. [DOI] [PubMed] [Google Scholar]
- 160.Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol 2000;156(4):1363–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aryal M, Vykhodtseva N, Zhang YZ, et al. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J. Control. Release 2013;169(1–2):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mainprize T, Lipsman N, Huang Y, et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: A clinical safety and feasibility study. Sci. Rep 2019; 9(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weissleder R, Mahmood U. Molecular imaging. R adiology. 2001;219(2):316–333. [DOI] [PubMed] [Google Scholar]
- 164.Klibanov AL, Hughes MS, Marsh JN, et al. Targeting of ultrasound contrast material: An in vitro feasibility study. Acta Radiol. Suppl 1. 1997;38(412):s113–s120. [PubMed] [Google Scholar]
- 165.Klibanov AL, Hughes MS, Villanueva FS, et al. Targeting and ultrasound imaging of microbubble-based contrast agents. Magnetic Resonance Materials in Physics, Biology and Medicine; 1999; 8(3):177–184. [DOI] [PubMed] [Google Scholar]
- 166.Klibanov AL. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv. Drug Deliv. Rev 1999;37(1–3):139–157. [DOI] [PubMed] [Google Scholar]
- 167.Klibanov AL, Rasche PT, Hughes MS, et al. Detection of individual microbubbles of ultrasound contrast agents: imaging of free-floating and targeted bubbles. Invest. Radiol 2004;39(3):187–195. [DOI] [PubMed] [Google Scholar]
- 168.Phillips P, Gardner E. Contrast-agent detection and quantification. Eur. Radiol 2004;14(Suppl 8):4–10. [PubMed] [Google Scholar]
- 169.Lindner JR, Song J, Christiansen J, et al. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104(17):2107–2112. [DOI] [PubMed] [Google Scholar]
- 170.Unger EC, McCreery TP, Sweitzer RH, et al. In vitro studies of a new thrombus-specific ultrasound contrast agent. Am. J. Cardiol 1998;81(12A):58G–61G. [DOI] [PubMed] [Google Scholar]
- 171.Leong-Poi H, Christiansen J, Klibanov AL, et al. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation. 2003;107(3):455–460. [DOI] [PubMed] [Google Scholar]
- 172.Rychak JJ, Graba J, Cheung AMY, et al. Microultrasound molecular imaging of vascular endothelial growth factor receptor 2 in a mouse model of tumor angiogenesis. Mol. Imaging 2007;6(5):289–296. [PubMed] [Google Scholar]
- 173.Willmann JK, Bonomo L, Carla Testa A, et al. Ultrasound Molecular Imaging With BR55 in Patients With Breast and Ovarian Lesions: First-in-Human Results. J. Clin. Oncol 2017;35(19):2133–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smeenge M, Tranquart F, Mannaerts CK, et al. First-in-Human Ultrasound Molecular Imaging With a VEGFR2-Specific Ultrasound Molecular Contrast Agent (BR55) in Prostate Cancer: A Safety and Feasibility Pilot Study. Invest. Radiol 2017;52(7):419–427. [DOI] [PubMed] [Google Scholar]
- 175.Payen T, Dizeux A, Baldini C, et al. VEGFR2-Targeted Contrast-Enhanced Ultrasound to Distinguish between Two Anti-Angiogenic Treatments. Ultrasound Med. Biol 2015;41(8):2202–2211. [DOI] [PubMed] [Google Scholar]
- 176.Gehan EA, Tefft MC. Will there be resistance to the RECIST (Response Evaluation Criteria in Solid Tumors)? J. Natl. Cancer Inst 2000;92(3):179–181. [DOI] [PubMed] [Google Scholar]
- 177.Park JH, Park M-S, Lee SJ, et al. Contrast-enhanced US with Perfluorobutane for Hepatocellular Carcinoma Surveillance: A Multicenter Diagnostic Trial (SCAN). Radiology. 2019;292(3):638–646. [DOI] [PubMed] [Google Scholar]
- 178.Farhadi A, Ho GH, Sawyer DP, et al. Ultrasound imaging of gene expression in mammalian cells. Science. 2019;365(6460):1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shapiro MG, Goodwill PW, Neogy A, et al. Biogenic gas nanostructures as ultrasonic molecular reporters. Nat. Nanotechnol 2014;9(4):311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hell SW, Wichmann J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett 1994;19(11):780–782. [DOI] [PubMed] [Google Scholar]
- 181.Betzig E, Patterson GH, Sougrat R, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science (80-. ) 2006;313(5793):1642–1645. [DOI] [PubMed] [Google Scholar]
- 182.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006;3(10):793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hess ST, Girirajan TPK, Mason MD. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J 2006;91(11):4258–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang B, Babcock H, Zhuang X. Breaking the diffraction barrier super-resolution imaging of cells. Cell. 2010;143(7):1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Siepmann M, Schmitz G, Bzyl J, et al. Imaging tumor vascularity by tracing single microbubbles. IEEE International Ultrasonics Symposium.; 2011:1906–1908.
- 186.Couture O, Besson B, Montaldo G, et al. Microbubble ultrasound super-localization imaging (MUSLI). IEEE International Ultrasonics Symposium.; 2011.
- 187.Desailly Y, Couture O, Fink M, et al. Sono-activated ultrasound localization microscopy. Appl. Phys. Lett 2013;103(17):174107. [Google Scholar]
- 188.Viessmann OM, Eckersley RJ, Christensen-Jeffries K, et al. Acoustic super-resolution with ultrasound and microbubbles. Phys. Med. Biol 2013;58(18):6447–6458. [DOI] [PubMed] [Google Scholar]
- 189.O’Reilly MA, Hynynen K. A super-resolution ultrasound method for brain vascular mapping. Med. Phys 2013;40(11):110701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Christensen-Jeffries K, Browning RJ, Tang MX, et al. In vivo acoustic super-resolution and super-resolved velocity mapping using microbubbles. IEEE Trans. Med. Imaging 2015;34(2):433–440. [DOI] [PubMed] [Google Scholar]
- 191.Desailly Y, Pierre J, Couture O, et al. Resolution limits of ultrafast ultrasound localization microscopy. Phys. Med. Biol 2015;60(22):8723–8740. [DOI] [PubMed] [Google Scholar]
- 192.Ghosh D, Xiong F, Sirsi SR, et al. Toward optimization of in vivo super-resolution ultrasound imaging using size-selected microbubble contrast agents. Med. Phys 2017;44(12):6304–6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lin F, Shelton SE, Espíndola D, et al. 3-D ultrasound localization microscopy for identifying microvascular morphology features of tumor angiogenesis at a resolution beyond the diffraction limit of conventional ultrasound. Theranostics. 2017;7(1):196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Song P, Trzasko JD, Manduca A, et al. Improved super-resolution ultrasound microvessel imaging with spatiotemporal nonlocal means filtering and bipartite graph-based microbubble tracking. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018;65(2):149–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhu J, Rowland EM, Harput S, et al. 3D super-resolution us imaging of rabbit lymph node vasculature in vivo by using microbubbles. Radiology. 2019;291(3):642–650. [DOI] [PubMed] [Google Scholar]
- 196.Chen Q, Rush BM, Yu J, et al. Super-resolution ultrasound imaging for in vivo microvasculature assessment in acute kidney injury mouse model. J. Acoust. Soc. Am 2019;145(3):1859–1859. [Google Scholar]
- 197.Ghosh D, Peng J, Brown K, et al. Super-resolution ultrasound imaging of skeletal muscle microvascular dysfunction in an animal model of type 2 diabetes. J. Ultrasound Med 2019;38(10):2589–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Opacic T, Dencks S, Theek B, et al. Motion model ultrasound localization microscopy for preclinical and clinical multiparametric tumor characterization. Nat. Commun 2018; 9(1):1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ackermann D, Schmitz G. Detection and tracking of multiple microbubbles in ultrasound B-mode images. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016;63(1):72–82. [DOI] [PubMed] [Google Scholar]
- 200.Harput S, Christensen-Jeffries K, Brown J, et al. Two-stage motion correction for super-resolution ultrasound imaging in human lower limb. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018;65(5):803–814. [DOI] [PubMed] [Google Scholar]
- 201.Hingot V, Errico C, Tanter M, et al. Subwavelength motion-correction for ultrafast ultrasound localization microscopy. Ultrasonics. 2017;77:17–21. [DOI] [PubMed] [Google Scholar]
- 202.Christensen-Jeffries K, Brown J, Aljabar P, et al. 3-D in vitro acoustic super-resolution and super-resolved velocity mapping using microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017;64(10):1478–1486. [DOI] [PubMed] [Google Scholar]
- 203.Harput S, Christensen-Jeffries K, Ramalli A, et al. 3-D super-resolution ultrasound imaging with a 2-D sparse array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020;67(2):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jensen JA, Ommen ML, Oygard SH, et al. Three-dimensional super resolution imaging using a row-column array. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019. [DOI] [PubMed]
- 205.Harput S, Christensen-Jeffries K, Brown J, et al. 3-D motion correction for volumetric super-resolution ultrasound imaging. IEEE International Ultrasonics Symposium.; 2018. [DOI] [PMC free article] [PubMed]
- 206.Hingot V, Errico C, Heiles B, et al. Microvascular flow dictates the compromise between spatial resolution and acquisition time in ultrasound localization microscopy. Sci. Rep 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Desailly Y, Tissier AM, Correas JM, et al. Contrast enhanced ultrasound by real-time spatiotemporal filtering of ultrafast images. Phys. Med. Biol 2017;62(1):31–42. [DOI] [PubMed] [Google Scholar]
- 208.Harput S, Christensen-Jeffries K, Brown J, et al. Localisation of multiple non-isolated microbubbles with frequency decomposition in super-resolution imaging. IEEE International Ultrasonics Symposium.; 2017.
- 209.Bar-Zion A, Solomon O, Tremblay-Darveau C, et al. SUSHI: Sparsity-based ultrasound super-resolution hemodynamic imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018;65(12):2365–2380. [DOI] [PubMed] [Google Scholar]
- 210.Solomon O, Van Sloun RJG, Wijkstra H, et al. Exploiting flow dynamics for super-resolution in contrast-enhanced ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019;66(10):1573–1586. [DOI] [PubMed] [Google Scholar]
- 211.van Sloun RJG, Solomon O, Bruce M, et al. Super-resolution ultrasound localization microscopy through deep learning. arXiv. 2018. Available at: http://arxiv.org/abs/1804.07661. [DOI] [PubMed]
- 212.Lin F, Tsuruta JK, Rojas JD, et al. Optimizing sensitivity of ultrasound contrast-enhanced super-resolution Imaging by tailoring size distribution of microbubble contrast agent. Ultrasound Med. Biol 2017;43(10):2488–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Espindola D, Lin F, Soulioti DE, et al. Adaptive multifocus beamforming for contrast-enhanced-super-resolution ultrasound imaging in deep tissue. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018;65(12):2255–2263. [DOI] [PubMed] [Google Scholar]
- 214.Diamantis K, Greenaway AH, Anderson T, et al. Super-resolution axial localization of ultrasound scatter using multi-focal imaging. IEEE Trans. Biomed. Eng 2018;65(8):1840–1851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.