Abstract
Behavioral neuroscience has long since relied on in vivo electrophysiology to provide spatially and temporally precise answers to complex questions related to the neural dynamics underlying sensory processing and action execution. Investigating the neural correlates of behavior can be challenging in freely behaving animals, especially when making inferences related to internal states that are temporally or conceptually ambiguous, such as decision-making or motivation. This necessitates careful creation of appropriate and rigorous controls, and awareness of the many potential confounds when attributing neural signals to animal behavior. This article discusses some fundamental considerations related to the optimal design and interpretation of rodent in vivo electrophysiological recording experiments and focuses on the different optimization strategies required when investigating neural encoding of external stimuli versus free behavior. The first protocol offers suggestions specific to intracranial surgical implantation of multielectrode arrays. The second protocol delves into optimization strategies and tips useful for both the design and interpretation of recording experiments conducted in freely behaving rodents.
Basic Protocol 1: Surgical implantation of multielectrode array
Basic Protocol 2: Optimizing experimental design and parameters
Keywords: electrophysiology, rodent behavior, single unit recording
INTRODUCTION
Electrophysiological recording experiments involving freely behaving animals are often designed to assess dynamic neural signaling in response to stimuli or events, during behavior, or both. Two important considerations are 1) designing experimental protocols and tasks in an optimal way to parse and interpret neural responses and 2) selecting parameters for subsequent analyses to draw accurate conclusions from the data. Aspects of these considerations can differ substantially, however, depending on the neural correlate under investigation, such as neural responses elicited by an external stimulus (e.g., an auditory cue) versus neural activity during free behavior (e.g., choice).
Here, relevant mouse surgical procedures for microelectrode array implantation (Basic Protocol 1) and optimization of experimental and behavioral parameters (Basic Protocol 2) are described. Protocols and concepts should translate to most behavioral assays and applications.
NOTE: Approval from appropriate institutional and other review boards should be obtained prior to carrying out procedures described here.
BASIC PROTOCOL 1: SURGICAL IMPLANTATION OF MULTIELECTRODE ARRAY
Several published protocols exist for implantation of chronic multielectrode arrays, many of which incorporate in-lab fabricated microdrives (e.g., Brunetti et al., 2014; Liang et al., 2017; Mohapatra et al., 2022). Here, descriptions of surgical procedures are purposefully general and relevant to implantation of pre-fabricated or custom microelectrode arrays.
STRATEGIC PLANNING
Timing of surgery
For behavioral tasks requiring substantial training, such as ethanol-rewarded operant conditioning (Halladay et al., 2017, 2020), whether to conduct surgical procedures prior to the start of training or after meeting certain training criteria may depend on several factors including task training time and difficulty, whether food- or water-deprivation is required for the task, or whether a substantial gap in training and testing would alter behavioral outcomes. For example, for more complex behavioral tasks, one may choose to wait until criteria are met rather than risk implanting electrodes an in an animal that never reaches criteria. However, as a 7+ day post-surgery recovery time is typical, animals may need to be retrained following surgery, and in some cases may never return to presurgery performance rates. Practical considerations of surgical timing may also include facility resources and space since mice with chronic implants are typically single-housed to protect the implanted array. Similarly, prolonged social isolation and/or the sudden switch from group housing to social isolation may have unintended consequences on behavioral outcomes, and thus should be considered when interpreting results, especially in comparison to datasets from nonimplanted, socially-housed cohorts.
Materials
Adult mice (or rats)
Inhalation anesthesia system (VetEquip, Inc., Item #901806)
Isoflurane, USP (Allivet, Item #50562–1)
Stereotaxic instrument (Kopf Instruments, model 942)
- Sterile surgical tools:
- Fine forceps (0.3×0.15mm tip; Fine Science Tools, Item #11923–13
- Micro scissors (12cm L, 41mm edge; Fine Science Tools, Item #14002–12)
- Scalpels (Fine Science Tools, Item #10000–10)
Warming pad (Amazon, Item #B08D6QNKPV)
Multielectrode array (fixed or fabricated; Innovative Neurophysiology)
Alcohol and iodine antiseptic prep pads
Puralube ophthalmic ointment (Allivet, Item #26870)
Cotton-tipped applicators
Ultra-fine tip surgical marker (Viscot Medical, LLC, Item #1456XLSR)
Micro drill (Harvard Apparatus, Item #72–6065)
Gorilla super glue (Amazon, Item #B01M9GPMWV)
Bone screws (Antrin Miniature Specialties, Item #00–90 × 1/16 SL BIND MS SS)
Silver conductive epoxy (MG Chemicals, Item #8331)
Dental cement (black/dark-colored if using in conjunction with optogenetics; Lang Dental, Item #Ortho-Jet)
Post-operative analgesic and anti-inflammatory medications
Anesthetize the mouse with 1–2% isoflurane in oxygen (0.5 L/min flow) and place it securely in the stereotaxic instrument, with warming pad below to prevent hypothermia.
Confirm that the mouse is completely anesthetized by the absence of motor response to foot pinch, and that the mouse is breathing normally.
Apply ophthalmic ointment to prevent eyes from drying.
Use fine-tipped forceps to pluck hair from the scalp prior to cleaning the scalp area with 3x alternations of iodine antiseptic prep pad and alcohol prep pad application.
Grasp the midline scalp with forceps, creating a tent. Cut through the skin with micro scissors, removing enough tissue to fully expose an area of the skull that spans reference points bregma and lambda, as well as sites for bone screws and the target coordinate for the microelectrode array.
Clean the skull surface with a sterile cotton-tipped applicator.
Level the skull using bregma and lambda as references, prior to marking coordinates of interest with an ultra-fine tip surgical marker.
Gently drill burr holes of appropriate size to accommodate the multielectrode array and bone screws. In some cases, a hand drill may be preferable for this step.
Record the X, Y, and Z stereotaxic coordinates for the ventromedial-most point of the multielectrode array contacting bregma (i.e., “zero” the array to bregma) prior to completing the next steps, which may obscure or alter bregma’s coordinates.
Scrape the skull with a scalpel in two different directions to create a crosshatched pattern, which will provide more surface area for super glue to adhere to (see next step).
Using the non-cotton-tipped end of an applicator (or other appropriate tool), apply a thin layer of Gorilla super glue onto all exposed areas of the skull, avoiding the exposed tissue below burr holes, and allow the glue to harden before proceeding to the next step.
Secure bone screws in the skull, leaving a small gap between the skull and bottom of the screw head for dental cement to adhere. The screws should be secured tightly; if needed, use a tiny amount of super glue to fully adhere the screws to the skull.
Slowly lower the multielectrode array into its final DV coordinate.
Wrap the electrode array’s ground wire firmly around a skull screw, and cover the wire and screw with a small amount of silver conductive epoxy.
Apply dental cement to fully cover the skull, sides of the microelectrode array, bone screws, and any excess ground wire. Ensure that there are no gaps between the scalp and dental cement.
Once the dental cement has hardened (approx. 10 min after application), remove the mouse from the stereotaxic instrument and administer post-operative medications. Place the mouse in a clean cage with fresh food and water, and monitor until the mouse is fully recovered.
BASIC PROTOCOL 2: OPTIMIZING EXPERIMENTAL DESIGN AND PARAMETERS
The focus of Basic Protocol 2 is optimization of experimental parameters to reduce confounds and improve experimental validity. This section does not cover procedures specific to post-processing of data or relevant statistical analyses, but several previously published protocols can be found on these topics, especially those related to general recommendations for analyses and assessing recording quality (Eichenbaum and Davis, 1998; Journal of Neuroscience, 2018; Friend et al., 2015; Harris et al., 2017; Mohapatra et al., 2022; Rieke at al., 1999; Zhao et al., 2014).
STRATEGIC PLANNING
Modifications to behavioral arenas, as well as some procedures, may be required to optimize recordings (Figure 1). The chief modification when recording from tethered animals is clearance for the tether to connect to an overhead commutator. This can be done by simply removing the ceiling of enclosures, or by creating a hole in the top of an enclosure for the tether to feed through. If opting for the latter, ensure the hole is large enough for the animal to move freely around the behavioral enclosure; too much tension and/or too much slack can distract animals from tasks and stimuli. Likewise, entry ports and reward receptacles often will not accommodate added height of implanted headstages, so purchasing or fabricating modified receptacles will be required.
Figure 1. Example in vivo electrophysiology rig and behavioral equipment.

A) Benchtop utility shelving rack allows overhead mounting of cameras, commutators, and other experimental equipment (e.g., lasers). The rig is large enough to accommodate a variety of behavioral apparatuses (e.g., rectangular arena, as shown). B) In vivo recording studies may require some modifications to behavioral arenas to accommodate tethers (e.g., open top and heightened walls, as shown).
Careful design of arenas and selection of stimuli can improve the quality of recordings through reduction in noise from external sources. For example, inadequately sized or poorly designed arenas may lead to the subject’s headstage coming into contact with walls or other objects, which can introduce artifacts that occlude neural signals. Likewise, chewing is a common source of noise in recordings; it is recommended to use soft foods or liquids in studies incorporating rewards.
Recording stimuli-evoked responses
Many behavioral experiments utilize stimuli like discrete cues to measure aspects of learning, motivation, reward, and aversion. These may include auditory, visual, or somatosensory cues such as tones, lights, and footshocks. Interpreting neural responses to discrete cues is somewhat simpler than inferring neural correlates of free behavior (discussed in section 2.2) because in most cases, experimental stimuli have distinct temporal boundaries (e.g., ON versus OFF). This temporal precision offers a clear time window of interest upon which neural firing rates can be compared to the “baseline” period to determine whether neurons are responsive to that stimulus.
Defining baseline periods
Continuous single unit recordings will reveal fluctuations in firing rates across the recording session (Stringer et al., 2019). To assert that a fluctuation in the firing rate of a single unit is stimulus evoked, its neural activity during the timepoint of interest (e.g., stimulus onset) is compared to its baseline, typically defined as the firing rate of that single unit during some other time window when the stimulus is not present. Optimizing the timing and duration of the baseline period is crucial for accurately interpreting data, however there are no strict guidelines per se for defining the baseline period aside from the requirement that the baseline period take place during the same recording session.
When assessing stimuli-evoked neural responses, a common practice is to define the baseline period as the time immediately prior to stimulus onset (see Figure 1). For example, one may compare single unit firing rates during a 1-sec tone cue presentation with corresponding firing rates during a 1-sec window occurring just prior to cue onset (i.e., pre- and post-stimulus time periods). This is preferable to using the behavioral baseline period of a task (e.g., the first 3 min of a cued fear conditioning session, prior to any stimuli presentations) because basal firing rates may change over long periods of time due to internal and external factors, while instantaneous fluctuations in firing rates that correspond with a discrete event or stimulus more convincingly demonstrate neural responsivity attributable to that event or stimulus (i.e., not due to random firing rate changes).
The duration of the baseline period chosen should vary to suit each research question, in part to ensure that neural events recorded during the baseline period correspond with times when no meaningful stimuli are present. For instance, designating a 10-sec pre-stimulus period to serve as the baseline for a 10-sec tone cue presentation period would not suffice if the interval between cue presentations is shorter than the baseline period window, as there would be overlap between the baseline window and prior stimuli presentations. To prevent the baseline period from overlapping with stimuli presentations, its duration could be shortened, or the behavioral task could be modified to increase the duration of the interstimulus interval.
Quantity of observations
Determining whether single units are responsive to stimuli necessitates recording over multiple trials to ensure sufficient statistical power. Some behavioral protocols can present challenges, however, if the number of stimuli presentations are limited, such as tone stimuli presented during auditory fear conditioning procedures (e.g., in the case of acquisition sessions with 1–5 tone-shock pairings). This limits statistical power and may lead to false negatives (or false positives if coincidental random neural firing takes place during a stimulus or event). In some of these occasions, adjustments to the stimuli can greatly improve statistical power. For example, the long-duration continuous tone cues often used during cued fear acquisition may be substituted with an equal duration train of noise pips (e.g., 500-ms pips delivered at 1 Hz), which will increase the number of stimuli presentations (i.e., observations, or “trials”) by ~10- to 30-fold depending on stimulus duration, providing much greater statistical power without interfering with behavioral outcomes (Glover et al., 2020; Gunduz-Cinar et al., 2019; Halladay and Blair, 2015).
Interpreting data: controls
Interpreting the information encoded by single units can be challenging without proper controls. For example, when analyzing neural correlates of cued fear conditioning, pervasive assumptions often made are that neural responses to an auditory stimulus that was paired with an aversive shock evidence neural encoding of a “fear” cue, and that units responsive to freezing behavior encode “fear”. To make these assertions, proper controls must be employed; analyzing neural responses evoked by the auditory cue prior to any tone-shock pairings will determine whether units are responsive to auditory cues in general rather than valance-specific cues. Likewise, neurons responsive to freezing behavior may simply be responsive to body movement or movement speed (Halladay and Blair, 2015); analysis of locomotion evoked responses will reveal whether activity of single units is contingent upon movement speed regardless of emotional state.
Interpreting data: confounds
Single unit recordings can be subject to noise from various sources, which can be misinterpreted as neural data, or occlude neural signals altogether. Stimuli and hardware used in behavioral tasks are not always compatible with recording electrical signals in the brain, and thus should be controlled for or removed. One major issue associated with fear conditioning tasks, for instance, is that shocks delivered through metal grid floors introduce noise that occludes neural signals when the animal is in contact with the grid floor (i.e., waveforms cannot be sorted properly in post-processing), which can lead to misinterpretations of data. Potential workarounds for this include programming shock hardware to shut off at all times other than during shock delivery, or delivering shock through an implanted electrode to the dorsal body (Zambetti et al., 2022) or eyelid (Tarpley et al., 2009, 2010; Halladay and Blair, 2012, 2017). Electrical artifacts can also arise from various hardware such as commercially available lickometers, which can be alleviated with some modifications (e.g., see Hayar et al., 2006; Schoenbaum et al., 2001). Among other sources of potential noise that can interfere with recording clean signals are fluorescent bulbs and alternating DC current near recording equipment (Friend et al., 2015). It is recommended to consider these factors when deciding which experimental rooms and locations to conduct recording studies. Relatedly, noise in recordings due to static build up on apparatus surfaces can be reduced with the use of anti-static spray.
Recording behavior-evoked responses
Unlike stimuli-evoked responses, behavior-evoked responses (e.g., choice) are usually not temporally discrete. Hardware may accurately track behavioral actions, such as lever presses or screen touches, but the precise time at which internal events happen, such as decision-making, can be ambiguous and temporally inconsistent (e.g., how long before a lever-press does an animal decide to initiate the response?). Since it is not possible to determine exactly when internal states change, when decisions are made, or when planning begins, caution should be taken when designing protocols and interpreting data when investigating the neural correlates of free behavior.
Defining baseline periods
The ideal baseline period for assessing behavior-evoked responses will vary across behavioral tasks, but ultimately, should include a window of time when the test subject is clearly not engaged in the behavior being evaluated. Unlike baseline periods for stimuli-evoked responses, it may not be optimal to use a time window occurring immediately before the defined behavioral event (see Figures 1–2). Presumably, behavioral actions require a sequence of internal events from choice to action. To ensure the chosen baseline period does not overlap with the relevant behavior period (e.g., during choice preceding an action), it may be optimal to use a baseline time window not occurring immediately before the behavioral action window, such as using a time window preceding the event window by a few seconds, assuming that the subject is not engaged in another meaningful action at the time (Halladay et al., 2020). This can become challenging in tasks that allow a subject to perform rapid actions (e.g., bouts of lever presses in a small time window), discussed further below.
Figure 2. Example single unit activity evoked by stimulus presentations or free behavior.
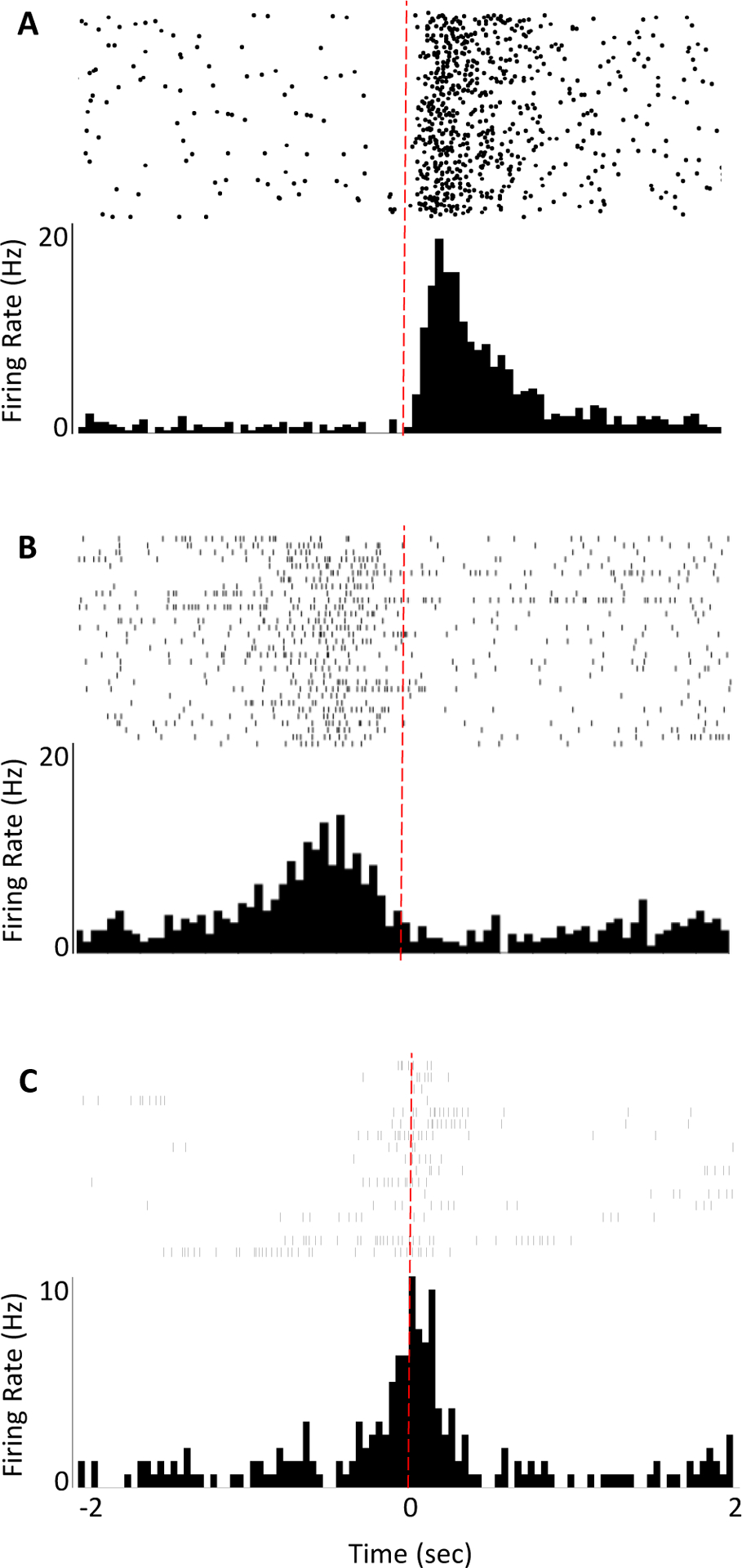
Selection of baseline periods and time windows of interest will vary depending on research question, behavior of interest, and whether firing rates encode external events or internally driven behaviors. A) Peri-stimulus time histogram (PSTH) of a single unit recorded during presentations of an auditory cue (red dotted line indicates cue onset). Unit activity just prior to the cue onset would serve as an appropriate baseline period to determine whether the unit’s firing rate changes significantly in response to the cue. B,C) Peri-event time histograms (PETH) of two single units recorded during an operant reinforcement task (red dotted line indicates execution of a lever press). In these cases, one would want to designate a baseline period occurring during an earlier time window when the test subject is not engaged in behaviors related to the eventual execution of an action.
Quantity of observations
Similar to stimuli-evoked neural responses, in general, greater observations will provide more statistical power. However with the case of behavior-evoked recordings, this can be problematic if a subject does not perform the behavior of interest often, such as responding on a lever that was previously paired with shock. In these cases, it may be necessary to determine criteria for including or excluding behavioral sessions from the data if too few trials are performed. Consideration of experimental parameters may help increase behavioral observations in some cases, such as decreasing the shock intensity in punishment tasks, or increasing session duration. Or, one may shift the research question, perhaps investigating the neural correlates of choosing to abort certain behavioral responses (Halladay et al., 2020).
Interpreting data: controls and confounds
Controls for behavioral tasks designed to assess behavior-evoked neural activity will be task specific, but since these interpretations are often based on subjects’ internal states (e.g., decisions), it is imperative to ensure that neural activity assumed to be evoked by behavior are not actually a result of other factors. For example, when recording choice behavior in an operant task, assessing neural correlates of locomotion can support (or negate) interpretations that unit responses are choice-related. Additionally, dummy stimuli (e.g., an inactive lever) should be included to assess whether neural responses are related to expectation of reward (or punishment) or are simply responses to an action irrespective of outcome.
In some cases, configuration of hardware can help control for overlapping behavioral responses, which otherwise can present challenges when determining baseline periods. For example, levers, nose pokes, or touchscreens can be placed on walls opposite reward ports to provide greater temporal separation between choice and reward collection. Increasing the size of behavioral arenas can also allow greater flexibility in parsing behavioral events from each other.
COMMENTARY
Background Information
In vivo electrophysiology is an essential tool for investigating neural correlates of behavior, and is often considered the gold-standard for capturing temporally precise neural dynamics underlying behavior (Buzsaki, 2004). It can provide insight to neural encoding of a wide range of behaviors including both sensory processing and action execution. Often in vivo electrophysiology is used in conjunction with causative methods, to provide information about the intact system prior to manipulating neural activity through tests of necessity and/or tests of sufficiency (e.g., drug application, genetic manipulation, opto- or chemogenetics, etc.). However, dissecting neural correlates of free behavior can be nuanced due to several important factors including experimental design and behavioral paradigms, and requires rigorous controls and cognizance of potential confounds.
Critical Parameters and Troubleshooting
Compatibility of tasks and apparatuses
In vivo electrophysiology recordings can be conducted during most behavioral tasks, but may require adjustments to hardware to accommodate tethered animals and headstages, or refinements to procedures to optimize precise and discrete observations of behavioral events. Wireless, untethered recoding systems are now available, but often are constrained to shorter recording sessions due to weight of batteries or other hardware requirements. Procedural refinements, some of which are suggested above, may include maximizing the number of possible observations of events or behaviors (e.g., using trains of noise pips rather than continuous tones to increase observations of “cue ON” events), or modifying other parameters such as reinforcement schedules or reward size used in operant tasks (to increase voluntary responses) or aversive stimulus intensity (to prevent floor effects in punished reward-seeking tasks). Careful piloting and early assessment of whether task adjustments are needed will help ensure optimal behavioral observations while reducing potential time and/or animals lost.
Tracking and scoring behavior
Analysis of neural correlates of free behavioral events necessitates high temporal precision alongside clear operational definitions for behaviors of interest. Most commercially available recording systems provide synced video and spike timestamp recordings. Simple behaviors such as movement speed, location, and zone entries may be scored using automated algorithms, but many behaviors may require hand scoring, especially in the case of behavioral events not otherwise recorded by hardware inputs (e.g., aborted lever presses). This is also usually true for studies on social behavior, when one would want to distinguish behaviors of one animal versus the other or record interaction types separately (e.g., sniffing versus contact or nose-to-nose versus nose-to-tail contact). It can be useful to record behaviors from multiple angles (whether or not additional cameras are also synced to the neural acquisition system) to enable experimenters to more accurately score behaviors that may be hard to detect (e.g., maternal licking) or hard to distinguish with only an overhead or front-facing camera (e.g., food consumption). It is highly suggested that strict operational definitions be determined prior to hand-scoring behavior, which will increase inter-rater reliability and maximize consistency in aligning neural data (i.e., the theoretical peak of neural activity is narrowest if consistently aligned n timestamps away from the behavior of interest).
Prior to conducting any recordings during behavioral training or testing, it is imperative to habituate the test animal to the recording tether, which can restrict voluntary movement. Habituation to tethers and cables should be done over the course of several days prior to experimentation, and after the animal has completely recovered from surgery. Ensure that the length of the recording tether allows the test subject to easily traverse all areas of the behavioral arena without having so much slack that the tether is heavy or can be chewed. It is also recommended to use an electrical commutator that accounts for angled turning, to both alleviate tension on the tether and to prevent it from shortening due to twists or kinks in the cable.
Understanding Results
Even after taking into account the above suggestions for isolating genuine neural correlates of behaviors, it would be prudent to ensure that stimuli- and behavior-evoked neural signaling is consistent across test subjects. Robust conclusions related to neural correlates of behavior necessitate convergent evidence across test subjects, so it is recommended to use caution when the number of units recorded across subjects varies substantially (i.e., non-equivalent weights), as well as considering individual subjects as covariates in analyses.
Time Considerations
Surgical implantation of microelectrode arrays typically takes 1–2 hours, depending on proficiency of the experimenter. A post-operative period of at least 7 days is standard to ensure full recovery. Experimentation will vary based on behavioral tasks, but generally, implanted microelectrode arrays can last for many weeks to months with proper care and monitoring for signs of infection. Processing of spike and behavioral data can be quite time consuming depending on the number of units and sessions recorded, session length, and whether behaviors are scored automatically or by hand.
Figure 3. Example population unit coding of behavioral events.
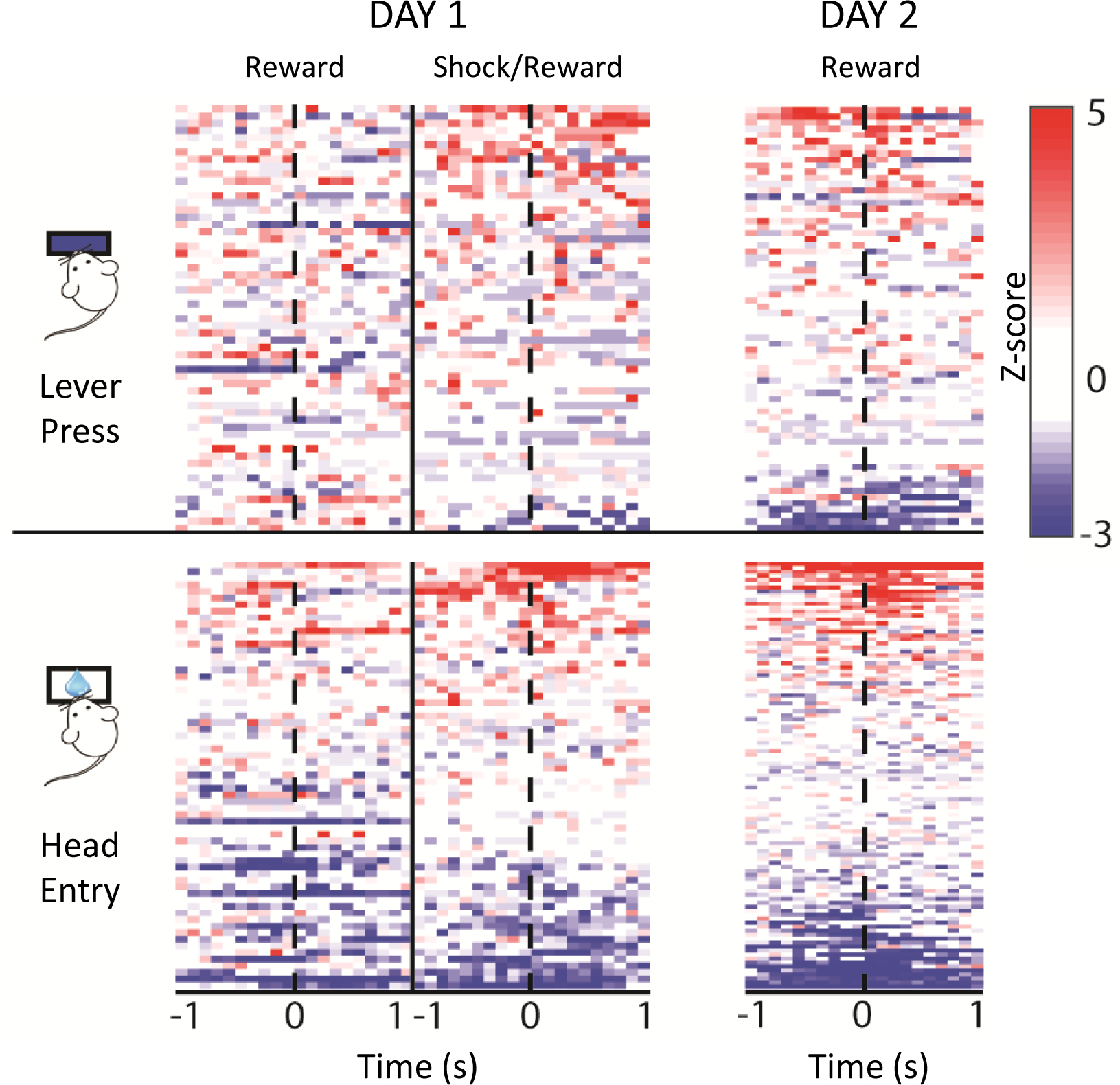
Heatmaps provide a quick look at population data by showing the magnitude of each unit’s response to a particular event, represented by color in two dimensions. Here, data for individual units (each represented as a row on the Y axis) were first z-score normalized to each unit’s baseline, and color-coded to illustrate change in z-score across time (100ms bins; each event takes place at time zero.) Positive versus negative z-scores are shown in red or blue, respectively. Data shown were recorded during a punished reward-seeking task that took place over two days. On day 1, trained mice pressed a lever during two periods, the first of which was rewarded, and the second was either rewarded or punished with a mild footshock. One day later, mice were tested in a rewarded probe session. Heatmaps allow a quick comparison of population unit activity across each recording session.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Mental Health of the National Institutes of Health award R15MH127514, and the Brain & Behavior Research Foundation NARSAD Young Investigator Grant (Walder Family Investigator). Content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health or the Brain & Behavior Research Foundation.
Footnotes
CONFLICTS OF INTEREST
The author has no conflict of interest to declare.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
LITERATURE CITED
- Brunetti PM, Wimmer RD, Liang L, Siegle JH, Voigts J, Wilson M, & Halassa MM (2014). Design and fabrication of ultralight weight, adjustable multi-electrode probes for electrophysiological recordings in mice. Journal of visualized experiments : JoVE, (91), e51675. 10.3791/51675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G (2004). Large-scale recording of neuronal ensembles. Nature Neuroscience, 7(5), 446–451. 10.1038/nn1233 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H and Davis JL (1998). Neuronal Ensembles: Strategies for Recording and Decoding. John Wiley & Sons, New York. [Google Scholar]
- Friend DM, Kemere C, and Kravitz AV (2015). Quantifying Recording Quality in In Vivo Striatal Recordings. Curr. Protoc. Neurosci 70:6.28.1–6.28.9. doi: 10.1002/0471142301.ns0628s70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover LR, McFadden KM, Bjorni M, Smith SR, Rovero NG, Oreizi-Esfahani S, Yoshida T, Postle AF, Nonaka M, Halladay LR, & Holmes A (2020). A prefrontal-bed nucleus of the stria terminalis circuit limits fear to uncertain threat. eLife, 9, e60812. 10.7554/eLife.60812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Cinar O, Brockway E, Lederle L, Wilcox T, Halladay LR, Ding Y, Oh H, Busch EF, Kaugars K, Flynn S, Limoges A, Bukalo O, MacPherson KP, Masneuf S, Pinard C, Sibille E, Chesler EJ, & Holmes A (2019). Identification of a novel gene regulating amygdalamediated fear extinction. Molecular Psychiatry, 24(4), 601–612. 10.1038/s41380-017-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay LR, & Blair HT (2015). Distinct ensembles of medial prefrontal cortex neurons are activated by threatening stimuli that elicit excitation vs. inhibition of movement. Journal of neurophysiology, 114(2), 793–807. 10.1152/jn.00656.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay LR, & Blair HT (2017). Prefrontal infralimbic cortex mediates competition between excitation and inhibition of body movements during pavlovian fear conditioning. Journal of Neuroscience Research, 95(3), 853–862. 10.1002/jnr.23736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay LR, & Blair HT (2012). The role of mu-opioid receptor signaling in the dorsolateral periaqueductal gray on conditional and unconditional responding to threatening and aversive stimuli. Neuroscience, 216, 82–93. 10.1016/j.neuroscience.2012.04.045. [DOI] [PubMed] [Google Scholar]
- Halladay LR, Kocharian A, & Holmes A (2017). Mouse strain differences in punished ethanol selfadministration. Alcohol (Fayetteville, N.Y.), 58, 83–92. 10.1016/j.alcohol.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay LR, Kocharian A, Piantadosi PT, Authement ME, Lieberman AG, Spitz NA, Coden K, Glover LR, Costa VD, Alvarez VA, & Holmes A (2020). Prefrontal Regulation of Punished Ethanol Self-administration. Biological Psychiatry, 87(11), 967–978. 10.1016/j.biopsych.2019.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Golder D, & Likhtik E (2017). Multisite Electrophysiology Recordings in Mice to Study Cross-Regional Communication During Anxiety. Current Protocols in Neuroscience, 80, 8.40.1–8.40.21. 10.1002/cpns.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Bryant JL, Boughter JD, & Heck DH (2006). A low-cost solution to measure mouse licking in an electrophysiological setup with a standard analog-to-digital converter. Journal of Neuroscience Methods, 153(2), 203–207. 10.1016/j.jneumeth.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Oline SN, Kirk JC, Schmitt LI, Komorowski RW, Remondes M, & Halassa MM (2017). Scalable, Lightweight, Integrated and Quick-to-Assemble (SLIQ) Hyperdrives for Functional Circuit Dissection. Frontiers in neural circuits, 11, 8. 10.3389/fncir.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra AN, Netser S, & Wagner S (2022). Modular electrode array for multi-site extracellular recordings from brains of freely moving rodents. Current Protocols, 2, e399. doi: 10.1002/cpz1.399 [DOI] [PubMed] [Google Scholar]
- Recommendations for the Design and Analysis of In Vivo Electrophysiology Studies. (2018). The Journal of Neuroscience : the official journal of the Society for Neuroscience, 38(26), 5837–5839. 10.1523/JNEUROSCI.1480-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Warland D, and Bialek W 1999. Spikes: Exploring the Neural Code. MIT Press, Cambridge, Mass. [Google Scholar]
- Schoenbaum G, Garmon JW, & Setlow B (2001). A novel method for detecting licking behavior during recording of electrophysiological signals from the brain. Journal of Neuroscience Methods, 106(2), 139–146. 10.1016/s0165-0270(01)00341-7. [DOI] [PubMed] [Google Scholar]
- Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, & Harris KD (2019). Spontaneous behaviors drive multidimensional, brainwide activity. Science (New York, N.Y.), 364(6437), 255. 10.1126/science.aav7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpley JW, Shlifer IG, Birnbaum MS, Halladay LR, & Blair HT (2009). Bilateral phosphorylation of ERK in the lateral and centrolateral amygdala during unilateral storage of fear memories. Neuroscience, 164(3), 908–917. 10.1016/j.neuroscience.2009.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpley JW, Shlifer IG, Halladay LR, & Blair HT (2010). Conditioned turning behavior: a Pavlovian fear response expressed during the post-encounter period following aversive stimulation. Neuroscience, 169(4), 1689–1704. 10.1016/j.neuroscience.2010.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti PR, Schuessler BP, Lecamp BE, Shin A, Kim EJ, & Kim JJ (2022). Ecological analysis of Pavlovian fear conditioning in rats. Communications biology, 5(1), 830. 10.1038/s42003-022-03802-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao FY, Jeggo R, Wei H, Whyment A, Fang X, & Spanswick D (2014). In vivo electrophysiological recording techniques for the study of neuropathic pain in rodent models. Current Protocols in Pharmacology, 66, 11.15.1–11.15.26. 10.1002/0471141755.ph1115s66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


