Abstract
Background
Identifying the preoperative risk factors for lymph node upstaging could contribute to the development of individualized perioperative treatment for patients with non‐small cell lung cancer (NSCLC). The current study aimed to evaluate the risk factors for lymph node upstaging, including gene mutation and programmed death ligand‐1 expression in patients with resectable NSCLC.
Methods
Data on the clinicopathological characteristics of patients who underwent lobectomy for clinical N0 NSCLC at our institution were collected. The clinicopathological findings of the pathological N0 and lymph node upstaging groups were then analyzed. Univariate and multivariate analyses were performed to examine the predictive factors for nodal upstaging.
Results
Of 291 patients, 40 had postoperative nodal upstaging (n = 25, N1; n = 15, N2). Large tumor size and high maximum standardized uptake value were significantly associated with nodal upstaging. The nodal upstaging group had a higher proportion of patients with solid adenocarcinoma and lymphatic, vascular, and pleural invasion than the pathological N0 group. Further, the nodal upstaging group had a higher proportion of patients with positive programmed death ligand‐1 expression than the pathological N0 group. Univariate and multivariate analyses showed that tumor size and positive programmed death ligand‐1 expression were associated with nodal upstaging.
Conclusion
The appropriate therapeutic strategy including preoperative treatment and resection should be cautiously considered preoperatively in patients with clinical N0 NSCLC who have large tumors and positive programmed death ligand‐1 expression.
Keywords: non‐small cell lung cancer, occult lymph node metastasis, PD‐L1 expression
The relationship between programmed death ligand‐1 (PD‐L1) expression in lung cancer and lymph node upstaging is unclear. Data on the 291 patients who underwent lobectomy for clinical N0 non‐small cell lung cancer were collected. Positive PD‐L1 expression are associated with an increased risk of nodal upstaging. Treatment strategy for positive PD‐L1 should be cautiously considered preoperatively.

INTRODUCTION
Recent surgical strategies for lung cancer have developed significantly. In early‐stage lung cancer, several studies including JCOG0802/WJOG4607L and CALGB140503 have shown the benefits of sublobar resection. 1 , 2 Patients with advanced stage or recurrent lung cancer who have positive programmed death ligand‐1 (PD‐L1) expression, epidermal growth factor receptor (EGFR) mutation, and anaplastic lymphoma kinase and c‐ros oncogene 1 rearrangement have more treatment options, which can significantly improve prognosis. In recent years, different studies have assessed the effect of tyrosine kinase inhibitors and immunotherapeutic agents when used as a perioperative treatment for resectable lung cancer. 3 , 4 Therefore, the clinical and oncological characteristics of patients with resectable lung cancer should be identified to establish a more effective treatment strategy.
Patients with clinical N0 lung cancer occasionally present with lymph node metastasis, which requires additional postoperative treatment on postoperative pathological examination. Based on recent studies, in patients with clinical N0 status, the risk factors for pathological lymph node upstaging (pathological N1 or N2) are maximum standardized uptake value (SUVmax) on positron emission tomography–computed tomography (PET/CT) scan, tumor invasion diameter, lymphatic and vascular invasion, and presence of micropapillary structures. 5 , 6 However, only a few reports have examined the association between nodal upstaging and PD‐L1 expression or genetic alteration. Identifying the risk factors for nodal upstaging, including gene mutation and PD‐L1 expression, can contribute to the development of individualized treatment options. Further, patients who will benefit from preoperative and surgical treatment can be identified appropriately. Therefore, the current study aimed to evaluate the risk factors for nodal upstaging, including gene mutation and PD‐L1 expression in patients who received surgical treatment.
METHODS
This was a single center, retrospective study, and the protocol was established in accordance with the principles of the Declaration of Helsinki. Moreover, the research was approved by the Clinical Research Ethics Committee of Kobe University Graduate School of Medicine (#B220098).
We collected information on the clinicopathological characteristics of patients (n = 552) who underwent lobectomy for clinical N0 NSCLC between August 2016 and November 2021 at our institution. The inclusion criteria were as follows: patients with medical details that could be obtained from the medical records and those with positive EGFR gene mutation and PD‐L1 expression. The exclusion criteria were as follows: patients with missing data on preoperative peripheral blood analysis and pathological factors and those who received neoadjuvant chemotherapy and radiotherapy. The evaluation methods included physical examination, medical history taking, CT scan, fluorodeoxyglucose (FDG)‐PET/CT scan, and peripheral blood analysis. The assessment of tumor marker levels was routinely performed within 1 month before surgery. CT scan was conducted within 1 month before surgery, and FDG‐PET/CT scan was performed within 3 months before surgery. The standard cutoff values of carcinoembryonic antigen (CEA) and cytokeratin antigen were 5.0 and 3.5 ng/mL, respectively, based on the manufacturer's recommendation. Information on TNM staging based on the eighth edition of the American Joint Committee on Cancer Staging Manual and the Revised International System for staging lung cancer was recorded. Tumor location and size were assessed preoperatively via chest CT scan. Chest and abdominal CT scan, FDG‐PET/CT scan, and brain magnetic resonance imaging or CT scan were performed to validate the presence of metastatic lesion. In our institution, FDG‐PET/CT scan was performed to evaluate SUVmax. In another institution, FDG‐PET/CT scan was performed to evaluate positive or negative results. All patients underwent lobectomy or bilobectomy and systematic dissection of all hilar and mediastinal lymph nodes.
Clinical lymph node staging
Preoperative lymph node staging was assessed via contrast‐enhanced chest CT scan and FDG‐PET/CT scan. Clinical N0 was diagnosed by the thoracic surgeons, physicians, and radiologists of the cancer board. The N0 criteria were as follows: (1) Patients without enlargement of the hilar or mediastinal lymph nodes (minimum axial diameter of <10 mm) and without significant FDG uptake in lymph nodes other than the primary tumor; (2) patients with enlargement of hilar or mediastinal lymph nodes (minimum axial diameter of >10 mm) but without pathological metastasis under endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA); and (3) patients with significant FDG uptake in the lymph nodes and suspected lymph node metastasis but without pathological metastasis under EBUS‐TBNA.
Sample analysis
All pathological data were retrospectively reviewed. Pathological tumor factors including lymph node metastasis and lymphatic, vascular invasion, and pleural invasion were evaluated. In patients with metastatic lymph node, the maximum axial diameter of total and tumor invasion was also assessed. In patients with multiple lymph node metastasis, the largest lymph node was evaluated. Tumor‐to‐lymph node ratio was defined as the ratio of the maximum axial diameter of tumor on lymph node to the maximum axial diameter of lymph node. Formalin‐fixed paraffin embedded samples obtained via biopsy and surgical resection were used for the immunohistochemical analysis of PD‐L1 expression and genetic alteration tests. PD‐L1 22C3 antibody was used in staining. PD‐L1 expression was assessed using the tumor proportion score (TPS). Positive mutation status was defined as EGFR point mutations in Ex18 (G719X) or Ex21 (L858R, L861Q), deletion in Ex19, and insert in Ex 20. The genetic alteration tests were performed using the PNA‐LNA PCR clamp test and the Oncomine Dx Target Test Multi‐CDx System based on Thermo Fisher's Ion AmpliSeq technology.
Statistical analysis
The clinicopathological characteristics of the N upstaging (pathological N1 or N2 lymph node metastasis) and pathological N0 (pN0) groups were compared. The demographic characteristics of patients are expressed as mean and standard deviation for continuous variables and as frequency and proportion for categorical variables. Multivariate analysis was performed to identify the independent predictors of N upstaging. The prediction model was established via multivariate logistic regression analysis. Only the SUVmax of 238 patients from our hospital were used with consideration of differences in imaging conditions among facilities. Then, the SUVmax was not included in the multivariate analysis. Tumor marker levels, tumor size, consolidation tumor ratio (CTR), presence of EGFR mutation, and PD‐L1 expression were used as covariates in the multivariate analysis. All statistical analyses were conducted with EZR version 1.40 (Saitama, Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface of R (The R Foundation for Statistical Computing, Vienna, Austria). 7 A p‐value of <0.05 was considered statistically significant.
RESULTS
Of 552 patients, 291 who met the inclusion criteria were enrolled in this study (Figure 1). Among them, 40 (13.7%) had postoperative nodal upstaging (n = 25, N1; n = 15, N2). Table 1 depicts the association between nodal upstaging and clinical characteristics. There were 157 male and 134 female participants, with a mean age of 69 years. In total, 171 (58.8%) patients presented with a smoking history. No significant difference was observed in terms of age, sex, and smoking history, tumor marker between the N upstaging and pN0 groups. Moreover, tumor location and CTR did not significantly differ between the two groups. The N upstaging group had a significantly larger tumor than the pN0 group. The association between SUVmax on FDG‐PET/CT scan and lymph node metastasis was evaluated in 30 patients in the N upstaging group and 208 in the pN0 group. Results showed that a high SUVmax was significantly associated with N upstaging.
FIGURE 1.
Flow chart of patient recruitment.
TABLE 1.
Patient characteristics.
| Factors | All patients | pN1/N2 | pN0 | p‐value |
|---|---|---|---|---|
| n = 291 | n = 40 | n = 251 | ||
| Age | ||||
| Mean (± SD) | 69.1 ± 9.4 | 70.4 ± 9.8 | 68.9 ± 9.4 | 0.348 |
| Sex | ||||
| Male | 157 (54.0) | 20 (50) | 137 (54.6) | 0.612 |
| Female | 134 (46.0) | 20 (50) | 114 (45.4) | |
| Smoking history | ||||
| Yes | 171 (58.8) | 20 (50) | 151 (60.2) | 0.299 |
| pack year | ||||
| Mean (± SD) | 25.4 ± 29.0 | 19.8 ± 24.6 | 26.3 ± 29.6 | 0.236 |
| Clinical T stage | ||||
| Tis | 2 (0.6) | 0 | 2 (0.8) | 0.046 |
| T1 | 201 (69.1) | 21 (52.5) | 180 (71.7) | |
| T2 | 68 (23.4) | 17 (42.5) | 51 (20.3) | |
| T3 | 14 (4.8) | 2 (5) | 12 (4.8) | |
| T4 | 6 (2.1) | 0 | 6 (2.4) | |
| Clinical stage | ||||
| 0 | 2 (0.6) | 0 | 2 (0.8) | 0.110 |
| IA | 200 (68.7) | 22 (55) | 178 (70.9) | |
| IB | 54 (18.6) | 14 (35) | 40 (15.9) | |
| IIA | 13 (4.5) | 2 (5) | 11 (4.4) | |
| IIB | 15 (5.2) | 2 (5) | 13 (5.2) | |
| IIIA | 7 (2.4) | 0 | 7 (2.8) | |
| Tumor location | ||||
| Right upper | 101 (34.7) | 16 (40) | 85 (33.9) | 0.738 |
| Right middle | 24 (8.2) | 2 (5) | 22 (8.8) | |
| Right lower | 59 (20.3) | 8 (20) | 51 (20.3) | |
| Left upper | 59 (20.3) | 9 (22.5) | 50 (19.9) | |
| Left lower | 48 (16.5) | 5 (12.5) | 43 (17.1) | |
| Surgical procedure | ||||
| VATS | 190 (65.3) | 31 (77.5) | 159 (63.3) | 0.225 |
| RATS | 97 (33.3) | 9 (22.5) | 88 (35.0) | |
| Thoracotomy | 4 (13.7) | 0 | 4 (0.4) | |
| Tumor size (mm) | ||||
| Mean (± SD) | 26.8 ± 13.0 | 28.9 ± 8.7 | 26.4 ± 13.6 | 0.008 |
| CTR | ||||
| >0.5 | 264 (90.7) | 39 (97.5) | 225 (89.6) | 0.145 |
| ≤0.5 | 27 (9.3) | 1 (2.5) | 26 (10.4) | |
| SUVmax | ||||
| Mean (± SD) | 6.5 ± 5.3 | 9.9 ± 4.7 | 6.0 ± 5.2 | <0.001 |
| CEA (ng/mL) | ||||
| Mean (± SD) | 5.14 ± 11.5 | 4.67 ± 3.69 | 5.22 ± 12.3 | 0.076 |
| CYFRA (ng/mL) | ||||
| Mean (± SD) | 1.32 ± 1.55 | 1.27 ± 0.91 | 1.32 ± 1.63 | 0.250 |
| Tumor marker | ||||
| Positive | 73 (25.1) | 11 (27.5) | 62 (24.7) | 0.855 |
Note: Values for categorical variables were presented as n (%) and were assessed using the Fisher's exact test. Variables for continuous variables were expressed as mean and range and were examined using the Wilcoxon rank‐sum test. Tumor marker positive: CEA (>5.0 ng/mL) or CYFRA (>3.5 ng/mL).
Abbreviations: CEA, carcinoembryonic antigen; CTR, consolidation tumor ratio; CYFRA, cytokeratin antigen; RATS, robot‐assisted thoracoscopic surgery; SD, standard deviation; SUVmax, maximum standardized uptake value; VATS, video‐assisted thoracoscopic surgery.
Table 2 depicts the association between N upstaging and pathological characteristics. The N upstaging group had a significantly higher proportion of patients with solid adenocarcinoma and lymphatic, vascular, and pleural invasion than the pN0 group. The N upstaging group had a significantly higher tumor and invasion diameter than the pN0 group. There was no significant difference in terms of proportion of patients with EGFR mutation between the two groups. However, the N upstaging group had a significantly higher proportion of patients with positive PD‐L1 expression than the pN0 group.
TABLE 2.
Tumor characteristics of the patients.
| Factors | All patients | pN1/N2 | pN0 | p‐value |
|---|---|---|---|---|
| n = 291 | n = 40 | n = 251 | ||
| Histology | ||||
| Adenocarcinoma | 247 | 34 | 213 | 1.000 |
| Lepidic | 19 | 1 | 18 | 0.488 |
| Acinar | 23 | 3 | 20 | 1.000 |
| Papillary | 152 | 21 | 131 | 1.000 |
| Micropapillary | 2 | 1 | 1 | 0.256 |
| Solid | 19 | 7 | 12 | 0.007 |
| Others | 32 | 1 | 31 | 0.097 |
| Squamous cell carcinoma | 33 | 4 | 29 | 1.000 |
| Other | 11 | 2 | 9 | 0.652 |
| Tumor diameter (mm) | ||||
| Mean (± SD) | 27.9 ± 15.7 | 30.6 ± 10.3 | 27.5 ± 16.3 | 0.004 |
| Invasive diameter (mm) | ||||
| Mean (± SD) | 25.3 ± 16.9 | 29.0 ± 10.4 | 24.7 ± 17.6 | <0.001 |
| Pathological T stage | ||||
| Tis | 2 (0.7) | 0 | 2 (0.8) | <0.001 |
| T1 | 170 (58.4) | 12 (30) | 158 (62.9) | |
| T2 | 87 (29.9) | 22 (55) | 65 (25.9) | |
| T3 | 20 (6.9) | 4 (10) | 16 (6.4) | |
| T4 | 12 (4.1) | 2 (5) | 10 (4.0) | |
| Pathological N stage | ||||
| N1 | 25 (8.6) | 25 (62.5) | 0 | <0.001 |
| N2 | 15 (5.2) | 15 (37.5) | 0 | |
| Pathological stage | ||||
| 0 | 3 (1.0) | 0 | 3 (1.2) | <0.001 |
| IA | 155 (53.3) | 0 | 155 (61.8) | |
| IB | 51 (17.5) | 0 | 51 (20.3) | |
| IIA | 12 (4.1) | 0 | 12 (4.8) | |
| IIB | 42 (14.4) | 23 (57.5) | 19 (7.6) | |
| IIIA | 23 (7.9) | 12 (30) | 11 (4.4) | |
| IIIB | 5 (1.7) | 5 (12.5) | 0 | |
| Lymphatic invasion | ||||
| Positive | 70 (24.1) | 26 (65) | 44 (17.5) | <0.001 |
| Vascular invasion | ||||
| Positive | 76 (26.1) | 21 (52.5) | 55 (21.9) | <0.001 |
| Pleural invasion | ||||
| Positive | 63 (21.6) | 16 (40) | 47 (18.7) | 0.005 |
| EGFR mutation | ||||
| Ex18 (G719X) | 7 (2.4) | 3 (7.5) | 4 (1.4) | 0.180 |
| Ex19del | 51 (17.5) | 9 (22.5) | 42 (16.7) | |
| Ex20ins | 1 (0.3) | 0 | 1 (0.4) | |
| Ex21 (L858R, L861Q) | 59 (20.3) | 7 (17.5) | 52 (20.7) | |
| Negative | 173 (59.5) | 21 (52.5) | 152 (60.6) | |
| PD‐L1 expression | ||||
| TPS of ≥1% | 146 (50.2) | 29 (72.5) | 117 (46.6) | 0.004 |
Note: Values are presented as n (%) and were assessed using the Fisher's exact test. Variables for continuous variables are expressed as mean and range and were examined using the Wilcoxon rank‐sum test.
Abbreviations: EGFR, epidermal growth factor receptor; PD‐L1, programmed death‐ligand‐1; SD, standard deviation; TPS, tumor proportion score.
Table 3 shows the correlation between PD‐L1 expression and the pathological characteristics of lymph nodes. In total, 146 patients with NSCLC, including 108 patients with a PD‐L1 TPS of 1%–49% and 38 patients with a TPS of ≥50%, had positive PD‐L1 expression. A higher PD‐L1 expression was associated with pathological nodal upstaging and multiple lymph node metastasis. The total lymph node diameter, tumor invasive diameter, and tumor‐to‐lymph node ratio did not differ in patients with metastatic lymph node who presented with positive PD‐L1 expression. The SUVmax and PD‐L1 TPS were more likely to increase with a higher pathological N stage in patients diagnosed with cN0 NSCLC (Figure 2).
TABLE 3.
PD‐L1 and LN pathological characteristics.
| Outcomes | All patients | TPS <1% | TPS 1–49% | TPS ≥50% | p‐value |
|---|---|---|---|---|---|
| n = 291 | n = 145 | n = 108 | n = 38 | ||
| Pathological N stage | |||||
| pN1/2 | 40 (13.7) | 11 (7.6) | 18 (16.7) | 11 (28.9) | 0.002 |
| pN0 | 251 (86.3) | 134 (92.4) | 90 (83.3) | 27 (71.1) | |
| Lymph node metastasis | |||||
| Multiple | 16 (5.5) | 5 (3.4) | 4 (3.7) | 7 (18.4) | <0.001 |
| Single | 24 (8.2) | 6 (4.1) | 13 (12.0) | 5 (13.2) | |
| None | 251 (86.3) | 134 (92.4) | 91 (84.3) | 26 (68.4) |
| N upstaging group outcomes | All patients | TPS <1% | TPS 1–49% | TPS ≥50% | p‐value |
|---|---|---|---|---|---|
| n = 40 | n = 11 | n = 18 | n = 11 | ||
| Maximum axial diameter (mm) | |||||
| Mean (± SD) | 7.2 ± 3.0 | 6.3 ± 1.6 | 6.8 ± 2.4 | 8.7 ± 4.2 | 0.346 |
| Tumor invasive diameter (mm) | |||||
| Mean (± SD) | 4.6 ± 2.8 | 3.6 ± 2.0 | 4.4 ± 2.0 | 5.9 ± 4.0 | 0.309 |
| Tumor‐to‐lymph node ratio | |||||
| Mean (± SD) | 63.4 ± 24.0 | 57.1 ± 26.1 | 67.0 ± 25.7 | 64.2 ± 19.9 | 0.602 |
Note: Values for categorical variables were presented as n (%) and assessed with the Fisher's exact test. Variables for continuous variables were expressed as mean and range and were examined using the Kruskal‐Wallis test.
Abbreviations: PD‐L1, programmed death‐ligand‐1; SD, standard deviation; TPS, Tumor Proportion Score.
FIGURE 2.
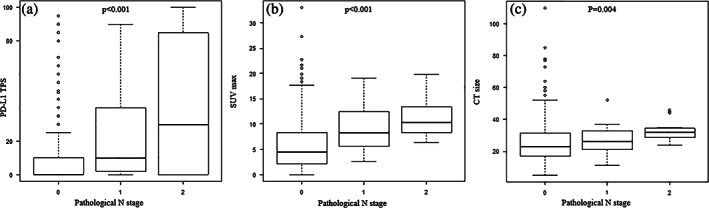
Lymph node status according to PD‐L1 TPS (a), SUVmax (b), and tumor size (c). The scatter plot showing that tumor size, SUVmax, and PD‐L1 TPS were more likely to increase with a higher pathological N stage in patients diagnosed with cN0 non‐small cell lung cancer. PD‐L1, programmed death ligand‐1; SUVmax, maximum standardized uptake; TPS, tumor proportion score.
Based on the univariate analysis, tumor size (>20 mm) and PD‐L1 expression with a cutoff value of 1% were associated with nodal upstaging. Multivariate analysis was performed to assess the independent variables associated with nodal upstaging. Results showed that tumor size and PD‐L1 expression remained significant predictors of nodal upstaging (Table 4).
TABLE 4.
Univariate and multivariate analysis of clinicopathological features for N upstaging.
| Outcomes | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p‐value | OR | 95% CI | p‐value | |
| Tumor marker (positive) | 1.16 | 0.55–2.45 | 0.855 | 1.040 | 0.47–2.28 | 0.922 |
| Tumor size (>20 mm) | 3.34 | 1.35–8.24 | 0.007 | 3.210 | 1.28–8.07 | 0.013 |
| CTR (>0.5) | 4.51 | 0.60–34.2 | 0.145 | 2.520 | 0.31–20.40 | 0.386 |
| EGFR (positive) | 1.39 | 0.71–2.72 | 0.507 | 1.760 | 0.86–3.58 | 0.121 |
| PD‐L1 (positive) | 3.01 | 1.39–6.98 | 0.004 | 3.03 | 1.40–6.58 | 0.005 |
| Age (>80 years) | 1.27 | 0.27–6.01 | 0.673 | |||
| Sex (male) | 1.20 | 0.62–2.34 | 0.712 | |||
| Smoking history | 0.66 | 0.34–1.29 | 0.299 | |||
Note: Tumor marker: CEA (>5 ng/mL) or CYFRA (>3.5 ng/mL). PD‐L1, programmed death‐ligand‐1.
Abbreviations: CI: confidence interval; CTR, consolidation tumor ratio; EGFR, epidermal growth factor receptor; OR, odds ratio.
DISCUSSION
The risk factors for pathological lymph node upstaging including gene mutation and PD‐L1 expression were assessed via multivariate analysis. Results showed that tumor size and PD‐L1 expression were independent nodal upstaging factors.
Previous studies reported that several factors could be predictors of lymph node metastasis. 5 , 8 In patients with clinical T1a‐bN0M0 adenocarcinoma, preoperative serum CEA levels, solid part tumor diameter, and CTR of ≥0.5 on chest CT scan were significant predictive factors associated with nodal upstaging. Moreover, patients had a worse survival. 8 The current study did not find any association between tumor marker levels and occult lymph node metastasis. However, this may be attributed to the fact that most patients with high tumor marker levels are diagnosed with clinical nodal metastasis preoperatively because PET/CT scan was routinely performed as a preoperative examination in this study. There was no difference in terms of CTR because most patients had a CTR of ≥0.5. A retrospective study of 350 patients with clinical N0 lung adenocarcinoma showed that the risk of occult lymph node metastasis increases with a higher SUVmax, presence of lymphatic invasion, vascular invasion, and micropapillary component. 5 The N upstaging group had a high proportion of patients with lymphatic, vascular, and pleural invasion, and they could be predictive factors based on our study. However, they were not included as covariates in the multivariate analysis because they are challenging to evaluate preoperatively.
EGFR mutation and PD‐L1 expression are more prevalent in patients with advanced‐stage disease regardless of cancer type. Some reports have shown that EGFR mutations are involved in brain metastasis, dissemination, and skip N2 in NSCLC. 9 , 10 , 11 A large retrospective study of the association between brain metastases and EGFR mutations in East Asian patients with lung cancer found that patients with EGFR‐mutant NSCLC were more likely to develop brain metastasis. However, it may be less associated with distant metastases to the adrenal glands and lymph node. 9 Enomoto et al. performed a retrospective analysis of 95 patients with stage IV lung cancer and found that the presence of EGFR mutations was correlated with lower lymph node stage and a greater number of lung and brain metastatic lesions in patients with lung adenocarcinoma. 10 A retrospective study evaluated data collected from the Japanese Joint Committee of Lung Cancer Registry on patients with resectable lung cancer. Results revealed that patients with EGFR‐mutant NSCLC have a low incidence of lymphatic permeation and vessel invasion. 12 EGFR‐positive lung cancer is more likely to metastasize to other organs than lymph nodes. Hence, EGFR‐positive lung cancer may not be a risk factor of occult lymph node metastasis in this study.
By contrast, in a meta‐analysis of the association between PD‐L1 expression and clinicopathological features in lung cancer, PD‐L1 expression was associated with positive lymph node metastasis and poor prognosis. 13 Other multicenter studies showed that lymph node metastasis and pleural and lymphovascular invasion were more likely to be observed in patients with PDL‐1‐positive lung cancer. 14 In addition, we microscopically examined occult lymph node metastasis lesions, and a higher PD‐L1 expression was associated with multiple lymph node metastasis. Recently, a study showed that an epigenetically instilled tumor‐intrinsic interferon response program can enhance LN metastatic potential by increasing the expression of interferon responsive genes such as MHC class I and PD‐L1, allowing tumor cells to evade NK cells, and promoting LN colonization. 15 Based on this finding, we considered that the PD‐1/PD‐L1 pathway evading immune recognition for cancer cell is associated with a higher incidence of lymph node metastasis in PDL‐1‐expressing lung cancer, and PDL‐1 expression was a risk factor of occult lymph node metastasis in this study.
The amount of 18F‐FDG uptake is associated with molecules relevant to glucose metabolism, hypoxia, angiogenesis, and phosphoinositide 3‐kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway in patients with primary lung cancer. 16 Some reports have shown that the SUVmax on preoperative 18F‐FDG‐PET/CT was significantly higher in patients with NSCLC who have positive PD‐L1 expression. 17 , 18 The PI3K/AKT/mTOR pathway is considered a related factor and is a signal transduction pathway involved in the regulation of multiple cellular functions, including cell proliferation, survival, differentiation, adhesion, motility, and invasion. 19 Moreover, the activation of the PI3K/AKT/mTOR pathway increases PD‐L1 protein expression in NSCLC. 20 Therefore, the correlation between high FDG uptake and PD‐L1 protein expression might be affected via the activation of the AKT/mTOR pathway. Considering the abovementioned relationship, a high SUVmax may be a good indicator of PD‐L1 expression even in cases in which preoperative PD‐L1 is challenging to measure.
The current study had some limitations. First, it was retrospective and conducted at a single center. In addition, the exclusion criteria of the current study included cases with unmeasured PD‐L1 expression and EGFR gene mutation. In particular, early‐stage lung cancers such as pstage0 and pstage1A1 are not often evaluated. However, a positive PD‐L1 expression is common in advanced‐stage cancer, and we do not believe that including such cases in the study could significantly affect the results of this study. Second, we did not collect information on long‐term prognosis because the cases were collected between 2016 and 2021. Based on recent studies, the number of patients with positive PD‐L1 expression may increase in the future. Thus, we are going to increase our samples and conduct a larger prospective study. Finally, in our study, PD‐L1 expression was examined in specimens collected preoperatively from bronchoscopy and CT‐guided biopsy in 92 cases. In the remaining cases, we aimed to collecting good‐quality postoperative surgical specimens from patients with positive PD‐L1 expression and genetic mutations. A previous study showed that the concordance of PD‐L1 TPS between bronchoscopy samples and surgical materials was high. 21 The meta‐analyses on PD‐L1 IHC on cytological samples in patients with lung cancer analysis confirmed the feasibility of PD‐L1 testing for lung cancer cytology specimens and existence of discrepancies between cytology and surgical specimens, which may be attributed to the intratumoral heterogeneity of PD‐L1 expression, cellularity, and presence of more three‐dimensional cell clusters in cytology samples. Further discussion and studies to improve the accuracy of PD‐L1 IHC in lung cytology specimens are warranted. 22
In conclusion, large tumor size (>20 mm) and positive PD‐L1 expression with a cutoff value of 1% are associated with an increased risk of occult lymph node metastasis in patients with resectable cN0 NSCLC. There are several ongoing clinical trials on PD‐1 or PD‐L1. Thus, the indication for preoperative treatment could expand, and the frequency of preoperative tests for PD‐L1 will increase in the future. To further improve treatment outcome, our findings can be used for identifying the degree of lymph node dissection, extent of pulmonary resection, and appropriate preoperative treatments with immune checkpoint inhibitors.
AUTHOR CONTRIBUTIONS
SM and YT worked on the dataset, designed the analysis, and developed the study concept. SM and YT drafted the manuscript. NJ, TD, ST, DH, and YM critically revised the manuscript. The authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare there are no conflicts of interest.
Mitsui S, Tanaka Y, Jimbo N, Doi T, Tane S, Hokka D, et al. Programmed death ligand‐1 expression and occult lymph node metastasis in non‐small cell lung cancer. Thorac Cancer. 2023;14(18):1774–1781. 10.1111/1759-7714.14922
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, et al. Segmentectomy versus lobectomy in small‐sizzed peripheral non‐small‐cell lung cancer (JCOG0802/WJOG4607L): a multicenter, open‐label, phase 3, randomized controlled, non‐inferiority trial. Lancet. 2022;399:1610–7. [DOI] [PubMed] [Google Scholar]
- 2. Altorki NK, Wang X, Kozono D, Watt C, Landrenau R, Wigle D, et al. Lobar or sublobar resection for peripheral stage IA non‐small‐cell lung cancer. N Engl J Med. 2023;388:489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD‐1 blockade in resectable lung cancer. N Engl J Med. 2018;378:1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun L, Guo YJ, Song J, Wang YR, Zhang SL, Huang LT, et al. Neoadjuvant EGFR‐TKI therapy for EGFR‐mutant NSCLC: a systematic review and pooled analysis of five prospective clinical trials. Front Oncol. 2020;10:586596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moon Y, Kim KS, Lee KY, Sung SW, Kim YK, Park JK. Clinicopathologic factors associated with occult lymph node metastasis in patients with clinically diagnosed n0 lung adenocarcinoma. Ann Thorac Surg. 2016;101:1928–35. [DOI] [PubMed] [Google Scholar]
- 6. Moon Y, Choi SY, Park JK, Lee KY. Risk factors for occult lymph node metastasis in peripheral non‐small cell lung cancer with invasive component size 3 cm or less. World J Surg. 2020;44:1658–65. [DOI] [PubMed] [Google Scholar]
- 7. Kanda Y. Investigation of the freely available easy‐to‐use software “EZR” (easy R) for medical statistics. Bone Marrow Transplant. 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai TM, Liu CY, Lin MW, Hsu HH, Chen JS. Factors associated with nodal upstaging in clinical T1a‐bN0M0 non‐small cell lung cancers. Cancer. 2022;14:1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iuchi T, Shingyoji M, Itakura M, Yokoi S, Moriya Y, Tamura H, et al. Frequency of brain metastases in non‐small‐cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol. 2015;20:674–9. [DOI] [PubMed] [Google Scholar]
- 10. Enomoto Y, Takada K, Hagiwara E, Kojima E. Distinct features of distant metastasis and lymph node stage in lung adenocarcinoma patients with epidermal growth factor receptor gene mutations. Respir Investig. 2013;51:153–7. [DOI] [PubMed] [Google Scholar]
- 11. Guerrera F, Renaud S, Tabbó F, Voegeli’ AC, Filosso PL, Legrain M, et al. Epidermal growth factor receptor mutations are linked to skip N2 lymph node metastasis in resected non‐small‐cell lung cancer adenocarcinomas. Eur J Cardiothorac Surg. 2017;51:680–8. [DOI] [PubMed] [Google Scholar]
- 12. Suda K, Mitsudomi T, Shintani Y, Okami J, Ito H, Ohtsuka T, et al. Clinical impacts of EGFR mutation status: analysis of 5780 surgically resected lung cancer cases. Ann Thorac Surg. 2021;111:269–76. [DOI] [PubMed] [Google Scholar]
- 13. Li H, Xu Y, Wan B, Song Y, Zhan P, Hu Y, et al. The clinicopathological and prognostic significance of PD‐L1 expression assessed by immunohistochemistry in lung cancer: a meta‐analysis of 50 studies with 11,383 patients. Transl Lung Cancer Res. 2019;8:429–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zheng Q, Huang Y, Zeng X, Chen X, Shao S, Jin Y, et al. Clinicopathological and molecular characteristics associated with PD‐L1 expression in non‐small cell lung cancer: a large‐scale, multi‐center, real‐world study in China. J Cancer Res Clin Oncol. 2021;147:1547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reticker‐Flynn NE, Zhang W, Belk JA, Basto PA, Escalante NK, Pilarowski GOW, et al. Lymph node colonization induces tumor‐immune tolerance to promote distant metastasis. Cell. 2022;185:1924–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaira K, Serizawa M, Koh Y, Takahashi T, Yamaguchi A, Hanaoka H, et al. Biological significance of 18F‐FDG uptake on PET in patients with non‐small‐cell lung cancer. Lung Cancer. 2014;83:197–204. [DOI] [PubMed] [Google Scholar]
- 17. Takada K, Toyokawa G, Tagawa T, Kohashi K, Akamine T, Takamori S, et al. Association between PD‐L1 expression and metabolic activity on 18F‐FDG PET/CT in patients with small‐sized lung cancer. Anticancer Res. 2017;37:7073–82. [DOI] [PubMed] [Google Scholar]
- 18. Zarogoulidis P, Christakidis V, Petridis D, Sapalidis K, Kosmidis C, Vagionas A, et al. Connection between PD‐L1 expression and standardized uptake value in NSCLC: an early prognostic treatment combination. Expert Rev Respir Med. 2021;15:675–9. [DOI] [PubMed] [Google Scholar]
- 19. Fumarola C, Bonelli MA, Petronini PG, Alfieri RR. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem Pharmacol. 2014;90:197–207. [DOI] [PubMed] [Google Scholar]
- 20. Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of PD‐L1 expression by oncogenic activation of the AKT–mTOR pathway in non‐small cell lung cancer. Cancer Res. 2016;76:227–38. [DOI] [PubMed] [Google Scholar]
- 21. Tsunoda A, Morikawa K, Inoue T, Miyazawa T, Hoshikawa M, Takagi M, et al. A prospective observational study to assess PD‐L1 expression in small biopsy samples for non‐small‐cell lung cancer. BMC Cancer. 2019;19:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Satturwar S, Girolami I, Munari E, Ciompi F, Eccher A, Pantanowitz L. Program death ligand‐1 immunocytochemistry in lung cancer cytological samples: a systematic review. Diagn Cytopathol. 2022;50:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.


