Abstract
Background
Although young women ( aged ≤ 55 years) are at higher risk than similarly aged men for hospital readmission within 1 year after an acute myocardial infarction (AMI), no risk prediction models have been developed for them. The present study developed and internally validated a risk prediction model of 1-year post-AMI hospital readmission among young women that considered demographic, clinical, and gender-related variables.
Methods
We used data from the US Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study (n = 2007 women), a prospective observational study of young patients hospitalized with AMI. Bayesian model averaging was used for model selection and bootstrapping for internal validation. Model calibration and discrimination were respectively assessed with calibration plots and area under the curve.
Results
Within 1-year post-AMI, 684 women (34.1%) were readmitted to the hospital at least once. The final model predictors included: any in-hospital complication, baseline perceived physical health, obstructive coronary artery disease, diabetes, history of congestive heart failure, low income ( < $30,000 US), depressive symptoms, length of hospital stay, and race (White vs Black). Of the 9 retained predictors, 3 were gender-related. The model was well calibrated and exhibited modest discrimination (area under the curve = 0.66).
Conclusions
Our female-specific risk model was developed and internally validated in a cohort of young female patients hospitalized with AMI and can be used to predict risk of readmission. Whereas clinical factors were the strongest predictors, the model included several gender-related variables (ie, perceived physical health, depression, income level). However, discrimination was modest, indicating that other unmeasured factors contribute to variability in hospital readmission risk among younger women.
Résumé
Contexte
Bien que les femmes jeunes (≤ 55 ans) présentent un risque plus élevé que les hommes du même âge de réadmission à l’hôpital dans l’année suivant un infarctus aigu du myocarde (IAM), il n’existe pas de modèle de prédiction des risques conçu spécialement pour elles. Dans le cadre de la présente étude, on a créé et validé à l’interne un modèle de prédiction des risques de réadmission à l’hôpital dans l’année suivant un IAM chez les femmes jeunes en tenant compte de variables démographiques, cliniques et associées au genre.
Méthodologie
Nous avons utilisé les données de l’étude américaine VIRGO (variation du rétablissement : le rôle du genre dans les résultats des jeunes patientes ayant subi un IAM) (n = 2007 femmes), une étude observationnelle prospective menée auprès de jeunes patientes hospitalisées pour un IAM. Un modèle bayésien d’établissement de la moyenne a été utilisé pour la sélection du modèle et la méthode bootstrap a été utilisée pour la validation interne. L’étalonnage et la discrimination du modèle ont été évalués respectivement au moyen des courbes d’étalonnage et de la surface sous la courbe.
Résultats
Dans l’année suivant l’IAM, 684 femmes (34,1 %) ont été réadmises à l’hôpital au moins une fois. Les facteurs prédictifs finaux du modèle sont notamment : toute complication survenue à l’hôpital, l’état de santé physique perçu au départ, la coronaropathie obstructive, le diabète, les antécédents d’insuffisance cardiaque congestive, le faible revenu (< 30 000 $ US), les symptômes dépressifs, la durée du séjour à l’hôpital et l’ethnie (blanc par rapport à noir). Parmi les neuf facteurs prédictifs retenus, trois sont associés au genre. Le modèle est bien étalonné et présente une discrimination modeste (surface sous la courbe = 0,66).
Conclusions
Notre modèle de risque propre aux femmes a été conçu et validé à l’interne auprès d’une cohorte de femmes jeunes hospitalisées pour un IAM et peut être utilisé pour prédire le risque de réadmission. Bien que les facteurs cliniques soient les facteurs prédictifs les plus puissants, le modèle inclut plusieurs variables liées au genre (p. ex., état de santé physique perçu, dépression, revenu). Cependant, la discrimination étant modeste, d’autres facteurs non mesurés contribuent à la variabilité du risque de réadmission à l’hôpital chez les femmes plus jeunes.
Young women (aged ≤ 55 years) hospitalized for acute myocardial infarction (AMI) are more frequently readmitted than young men during the first year after discharge,1, 2, 3 reflecting poorer outcomes.4,5 Beyond sex-related (ie, biological) factors, gender-related factors (ie, psycho-socio-cultural) of young women are associated with worse outcomes after a first AMI.5, 6, 7 Gender is a complex construct consisting of various domains, including gender identity (eg, level of stress as a personality trait), gender roles (eg, household primary earner, employment, household chores), gender relations (eg, marital status, social support), and institutionalized gender (eg, socioeconomic status).8,9
Prior research has revealed that women of all ages have a higher risk of 1-year post-AMI hospital readmission after adjustment for demographics and clinical factors.2 However, after further adjustment for health status and gender-related factors, this association with female sex is attenuated.2 These results suggest that gender-related factors (eg, stress, social support) may better explain the differences in likelihood of readmission between young women and men than sex-related factors alone.
A major obstacle to the development of risk prediction models and effective interventions for young women with AMI is that gender-related factors have not been collected routinely in existing cohort studies. In fact, risk models for post-AMI hospital readmission have historically failed to incorporate these factors.10 In addition, existing AMI-specific readmission risk models do not measure risk separately in men and women, have unclear generalizability, and are often derived from older populations.10 A recent study developed a prediction model for risk of 1-year readmission among young adults hospitalized for AMI that highlighted female sex as a predictor.3 Because interventions for reducing readmission have previously failed in mitigating traditional risk factors, a step forward would be the development of a female-specific risk model that facilitates intervention on nontraditional modifiable factors for improving outcomes in this population.11,12
To address this gap in knowledge, our main objective was to develop and internally validate a risk prediction model of 1-year post-AMI hospital readmission among young women that considers both sex- and gender-related variables.13, 14, 15 We used data from the Variation In Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study,16 the largest prospective multicenter longitudinal study of young patients (aged ≤ 55 years) hospitalized for AMI. In addition to traditional variables (from prior AMI risk models), the VIRGO study has a broad range of gender-related factors as well as rigorously adjudicated readmissions.16 Because we suspect that gender-related factors may have a larger effect on female patients than on male patients, we see the need to develop this model in an all-female cohort. We hypothesize that the consideration of gender-related factors in a female cohort may yield a risk prediction model that includes such gender-related factors in addition to the traditional clinical factors.
Methods
Our study uses data gathered through the VIRGO study, which was designed to investigate factors associated with higher rates of adverse clinical outcomes in young women (aged ≤ 55 years) with AMI. The VIRGO study design has been described previously.16 In brief, participants aged between 18 and 55 years were prospectively recruited across 103 sites in the US, between August 2008 and May 2012, using a 2:1 enrollment ratio of women vs men. A total of 2985 US adults (67.2% women) hospitalized for AMI were enrolled. After excluding in-hospital deaths (n = 6), this process resulted in a final cohort of 2979 participants. Of those participants, the subcohort of 2007 women was used to develop and internally validate our risk prediction model of 1-year readmission. Institutional review board approval was obtained at each participating institution, and patients provided informed consent for their study participation, including baseline hospitalization and interviews.
All-cause hospital readmission was the primary outcome, defined as any hospital or observation stay lasting more than 24 hours within 1 year of post-AMI discharge. The readmission adjudication process has been described previously.3 Based on prior work in post-AMI readmission and interest in gender-related indicators, we initially selected 63 candidate variables for the risk prediction model (Supplemental Table S1).2,3,10,17 A combination of medical record abstraction and standardized in-person interviews administered by trained personnel was used to collect information at baseline and before discharge regarding demographics, baseline cardiac risk factors and comorbidities, and clinical and laboratory variables. Data for this study included US adults who self-reported as non-Hispanic Black (hereafter, Black), Hispanic or Latino, and non-Hispanic White (hereafter, White) individuals. We excluded individuals who self-reported being Asian or of other race and ethnicity (which included those who were American Indian or Alaska Native and Native Hawaiian or Other Pacific Islander). We used guidance from Flanagin et al.18 in reporting race and ethnicity in this study. We also considered additional care-related variables during index hospitalization, including receipt of percutaneous coronary intervention and late presentation to a hospital. Late presentation was defined as presenting more than 6 hours after symptom onset based on the American Heart Association/American College of Cardiology guidelines.19 In terms of gender-related variables (ie, psycho-socio-cultural), we used a framework to identify variables across the 4 domains of gender defined by the Canadian Institutes of Health Research (CIHR), which included the following: gender roles (household primary earner status, current employment/working status, support for household chores, and current presence of health insurance); gender identity (eg, level of depression, stress, quality of life); gender relations (eg, marital status, social support); and institutionalized gender (low income [personal income ≤ $30,000 US], which is a marker of low socioeconomic status; Supplemental Fig. S1). These variables capture elements of the psycho-social construct of gender, which is separate from the conceptualization of sex as a biological factor. Depression, stress, quality of life, and social support were assessed using validated patient-reported outcome measures in cardiac populations, including the following: the Patient Health Questionnaire-9 to measure depression,17 the 14-item global Perceived Stress Scale20 to measure perceived stress, the Seattle Angina Questionnaire to measure disease-specific quality of life,21,22 and the Short Form-12 Health Survey (SF-12) to measure general health-related quality of life.23 For the purposes of this study, the angina frequency, physical limitation, treatment satisfaction, and quality-of-life domains from the Seattle Angina Questionnaire were used, as well as the physical component score and mental component score of the SF-12. Lastly, we used the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Social Support ESSI-5 Instrument to measure perceived social support.24 For this study, we also used a question from the full-length 7-item scale to measure the burden of household chores.
Statistical methods
We generated descriptive statistics for the overall population and reported frequencies for categorical variables and means (standard deviations) or medians (interquartile ranges) for continuous and count variables. Differences in baseline characteristics between readmitted and non-readmitted women were evaluated with χ2 tests, t tests, and Wilcoxon rank-sum tests, as appropriate. Of the initial 63 candidate variables, 16 variables were ineligible based on the criteria outlined in Supplemental Figure S2, which included high levels of missingness, very high or very low prevalence, and unreliable interhospital assessment. For example, systolic and diastolic blood pressure exhibited 48% missingness and therefore could not be considered as candidate variables. This process of determining ineligibility resulted in 47 candidate variables with missingness generally < 3%. Data from angiograms at baseline exhibited the highest level of missingness at 10.1%, implying that presence of coronary artery disease (CAD; obstructive vs nonobstructive) could not be determined for those women. Data from the 14-item global Perceived Stress Scale were missing for 6.5%. Ejection fraction, SF-12 PCS and mental component score were missing < 5%, and no missingness was present in the outcome. Under the assumption that the data were missing-at-random, and based on maximum missingness from the angiograms, we generated 10 imputations using fully conditional specifications as implemented in the SAS procedure MI (multiple imputation).25
Our development and validation processes followed the practices outlined in the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement.26 Selection for the multivariable model was done using Bayesian model averaging (BMA), a selection approach used in the Comprehensive Evaluation of Risk Factors in Older Patients With Acute Myocardial Infarction (SILVER-AMI) study and described elsewhere.3,27 Because BMA was used for selection, rather than the corresponding P values, some terms retained in the model may not exhibit P values below 0.05. Finally, we separately fit logistic regression of readmission to each of the 10 imputations and used Firth penalized maximum likelihood to estimate the associations within each imputation-specific model.28 The coefficients from the imputation-specific models were subsequently combined using Rubin’s rules.25 The performance of the model was evaluated by assessing area under the curve (AUC) and calibration of the predicted risk. We considered good fit in each imputation as an AUC ≥ 65%, and plots of the mean observed probabilities with confidence intervals that overlap with the diagonal line were considered to represent perfect agreement, as recommended in the TRIPOD directives. Internal validation based on bootstrapping was employed to iteratively apply the coefficients derived from 100 bootstrapped samples to the full sample to estimate the optimism of the model’s AUC. Optimism indicates how much model fit can be expected to decrease when the model is applied to external datasets drawn from the same population. With the exception of BMA, as implemented using the R package “BMA,” (version 3.18.11; R Core Team, Vienna, Austria) all analyses were conducted using SAS version 9.4 with SAS/STAT 14.3 (SAS Institute, Cary, NC). Statistical significance was defined as a 2-sided P value < 0.05.
Finally, we constructed an integrated predictiveness curve (IPC) to identify meaningful intervention thresholds to aid in clinical decision-making and understanding of women’s predicted risk of hospital readmission.29 The IPC is a graphical representation of the distribution of predicted readmission risk. We plotted the average predicted risk of 1-year readmission within each decile of predicted risk in the development cohort using GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA). Using the observed rate of readmission as a reference, we calculated and plotted the average predicted risk of readmission among women with key protective and deleterious predictors in the model. The average predicted risk refers to the model-predicted 1-year risk of readmission among women sharing a particular risk factor in the sample.
Results
Baseline characteristics for the overall sample (N = 2007), stratified by hospital readmission status, are presented in Table 1. The mean age of women was 47.2 ± 6.3 years, and 26.0% were Black. Women who were readmitted within 1-year post-AMI were more likely to be older, Black, unmarried, not living with a partner, and with lower income. They also were less likely to be employed or to be the primary earner in the household, and tended to work fewer hours. They also had a more adverse clustering of cardiac risk factors and comorbidities, had longer hospital length of stay (LOS), were less likely to be discharged to another institution, and were more likely to experience in-hospital complications. Lastly, women who were readmitted tended to have lower levels of social support, a higher burden of stress and depression, poorer physical/mental health, and worse disease-specific quality of life (ie, greater angina frequency, more physical limitations, and lower treatment satisfaction level).
Table 1.
Baseline characteristics of young women hospitalized for acute myocardial infarction who were readmitted vs not readmitted within 1 year (comparison of 45 variables that passed 1st-stage selection, including missingness)
| Characteristic | All patients (N = 2007) | All patients (data missing) | Not readmitted (N = 1323) | Not readmitted (data missing) | Readmitted within 1 year (N = 684) | Readmitted within 1 year (data missing) | P |
|---|---|---|---|---|---|---|---|
| Sociodemographics/SES | |||||||
| Ethnicity: White∗ | 1485 (74.0) | 0 | 1009 (76.3) | 0 | 476 (69.6) | 0 | 0.0012 |
| Married or living with spouse | 1053 (52.5) | 0 | 725 (54.8) | 0 | 328 (48.0) | 0 | 0.0036 |
| Primary earner | 1484 (73.9) | 0 | 1002 (75.7) | 0 | 482 (70.5) | 0 | 0.0108 |
| Low income | 956 (47.6) | 0 | 573 (43.3) | 0 | 383 (56.0) | 0 | < 0.0001 |
| Working | 1128 (56.2) | 0 | 804 (60.8) | 0 | 324 (47.4) | 0 | < 0.0001 |
| Work hours per week | 21.7 ( ± 21.62) | 13 (0.6) | 23.6 ( ± 21.62) | 10 (0.8) | 18.0 (± 21.16) | 3 (0.4) | < 0.0001 |
| ESSI 7—Help with daily chores | 1255 (62.5) | 0 | 834 (63.0) | 0 | 421 (61.5) | 0 | 0.6278 |
| Cardiac risk factors | |||||||
| Diabetes | 799 (39.8) | 0 | 468 (35.4) | 0 | 331 (48.4) | 0 | < 0.0001 |
| Obesity (BMI 30 kg/m2) | 1107 (55.2) | 0 | 704 (53.2) | 0 | 403 (58.9) | 0 | 0.0171 |
| Hypertension | 1347 (67.1) | 0 | 847 (64.0) | 0 | 500 (73.1) | 0 | < 0.0001 |
| Dyslipidemia | 1679 (83.7) | 0 | 1085 (82.0) | 0 | 594 (86.8) | 0 | 0.0055 |
| Currently smoking | 601 (29.9) | 0 | 394 (29.8) | 0 | 207 (30.3) | 0 | 0.8230 |
| Family history of CVD | 1350 (67.3) | 0 | 882 (66.7) | 0 | 468 (68.4) | 0 | 0.5112 |
| Inactivity | 751 (37.4) | 0 | 454 (34.3) | 0 | 297 (43.4) | 0 | < 0.0001 |
| Medical history | |||||||
| Previous MI | 413 (20.6) | 0 | 231 (17.5) | 0 | 182 (26.6) | 0 | < 0.0001 |
| History of renal disease | 254 (12.7) | 0 | 144 (10.9) | 0 | 110 (16.1) | 0 | 0.0009 |
| History of COPD | 284 (14.2) | 0 | 159 (12.0) | 0 | 125 (18.3) | 0 | 0.0001 |
| History of heart failure | 115 (5.7) | 0 | 48 (3.6) | 0 | 67 (9.8) | 0 | < 0.0001 |
| Presentation characteristics | |||||||
| Ejection fraction < 40% | 211 (10.5) | 0 | 133 (10.1) | 0 | 78 (11.4) | 0 | 0.3442 |
| Angiogram | 203 (10.1) | 126 (9.5) | 77 (11.3) | 0.0028 | |||
| Nonobstructive CAD < 50% | 232 (11.6) | 174 (13.2) | 58 (8.5) | ||||
| Obstructive CAD 50% | 1572 (78.3) | 1023 (77.3) | 549 (80.3) | ||||
| Peak Troponin (ng/mL) | 5.9 (1.3–23.0) | 26 (1.3) | 5.9 (1.4–23.6) | 18 (1.4) | 5.8 (1.3–22.1) | 8 (1.2) | 0.4146 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 88.1 ( ± 25.74) | 8 (0.4) | 89.2 ( ± 23.84) | 6 (0.5) | 86.0 ( ± 28.96) | 2 (0.3) | 0.0143 |
| First white blood cell count (103/µL) | 10.8 ( ± 3.89) | 8 (0.4) | 10.7 ( ± 3.74) | 4 (0.3) | 10.8 ( ± 4.16) | 4 (0.6) | 0.7522 |
| First hematocrit (%) | 39.7 ( ± 4.95) | 9 (0.4) | 39.9 ( ± 4.64) | 5 (0.4) | 39.2 ( ± 5.47) | 4 (0.6) | 0.0015 |
| Chest pain as primary symptom | 1733 (86.3) | 0 | 1149 (86.8) | 0 | 584 (85.4) | 0 | 0.3640 |
| Type of myocardial infarction | 0 | 0 | 0 | 0.2358 | |||
| STEMI | 920 (45.8) | 619 (46.8) | 301 (44.0) | ||||
| NSTEMI | 1087 (54.2) | 704 (53.2) | 383 (56.0) | ||||
| Total length of stay in days | 3.0 (2.0–5.0) | 10 (0.5) | 3.0 (2.0–4.0) | 6 (0.5) | 3.0 (2.0–6.0) | 4 (0.6) | < 0.0001 |
| Discharge counseling | |||||||
| Recommended counseling (cardiac+diet+smoking) | 631 (31.4) | 0 | 418 (31.6) | 0 | 213 (31.1) | 0 | 0.8353 |
| Exercise counseling | 1845 (91.9) | 0 | 1215 (91.8) | 0 | 630 (92.1) | 0 | 0.8342 |
| Discharge medication | |||||||
| Clopidogrel/thienopyridines | 1355 (67.5) | 0 | 889 (67.2) | 0 | 466 (68.1) | 0 | 0.6723 |
| Statins | 1814 (90.4) | 0 | 1188 (89.8) | 0 | 626 (91.5) | 0 | 0.2142 |
| Dual antiplatelet therapy (DAPT) | 1289 (64.2) | 0 | 848 (64.1) | 0 | 441 (64.5) | 0 | 0.8674 |
| ACEI/ARBs | 1229 (61.2) | 0 | 797 (60.2) | 0 | 432 (63.2) | 0 | 0.2038 |
| Beta-blockers | 1798 (89.6) | 0 | 1188 (89.8) | 0 | 610 (89.2) | 0 | 0.6692 |
| Calcium-channel blocker | 122 (6.1) | 0 | 77 (5.8) | 0 | 45 (6.6) | 0 | 0.5001 |
| Gender psychosocial factors | |||||||
| Social support (ESSI 5) | 27.0 (23.0–30.0) | 39 (1.9) | 27.0 (23.0–30.0) | 19 (1.4) | 27.0 (22.0–30.0) | 20 (2.9) | 0.1396 |
| Depression (PHQ-9) | 8.0 (3.0–13.0) | 82 (4.1) | 7.0 (3.0–12.0) | 44 (3.3) | 9.0 (4.0–15.0) | 38 (5.6) | < 0.0001 |
| Stress (PSS-14) | 27.0 (21.0–33.0) | 131 (6.5) | 27.0 (20.0–33.0) | 75 (5.7) | 28.5 (22.0–35.0) | 56 (8.2) | < 0.0001 |
| Physical limitation (SAQ) | 91.7 (58.3–100.0) | 56 (2.8) | 94.4 (66.7–100.0) | 32 (2.4) | 80.6 (47.2–100.0) | 24 (3.5) | < 0.0001 |
| Anginal frequency (SAQ) | 90.0 (70.0–100.0) | 7 (0.3) | 90.0 (70.0–100.0) | 5 (0.4) | 90.0 (60.0–100.0) | 2 (0.3) | < 0.0001 |
| Treatment satisfaction (SAQ) | 100.0 (87.5–100.0) | 18 (0.9) | 100.0 (87.5–100.0) | 13 (1.0) | 100.0 (81.25–100.0) | 5 (0.7) | 0.0056 |
| Quality of life (SAQ) | 58.3 (41.7–75.0) | 14 (0.7) | 58.3 (41.7–75.0) | 10 (0.8) | 50.0 (33.3–66.7) | 4 (0.6) | < 0.0001 |
| General health (SF-12 PCS) | 42.9 (32.5–42.9) | 92 (4.6) | 45.1 (35.2–53.5) | 58 (4.4) | 38.8 (28.6–48.4) | 34 (5.0) | < 0.0001 |
| General health (SF-12 MCS) | 44.9 (34.9–54.4) | 92 (4.6) | 46.4 (36.2–54.7) | 58 (4.4) | 43.2 (32.7–53.4) | 34 (5.0) | 0.0003 |
Values are n (%), median (interquartile range), or mean ( ± standard deviation), unless otherwise indicated.
ACEI/ARB, angiotensin-converting enzyme inhibitors/angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; ESSI, Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Social Support Instrument; MCS, mental component score; MI, myocardial infarction; NSTEMI, non-ST elevation MI; ; PCS, physical component score; PHQ-9, Patient Health Questionnaire-9; PSS-14, Perceived Stress Scale; SAQ, Seattle Angina Questionnaire; SF-12 short form-12 health survey; SES, socioeconomic status; STEMI, ST-elevation MI.
Data for this study included US adults who self-reported as non-Hispanic Black (hereafter, Black), Hispanic or Latino, and non-Hispanic White (hereafter, White) individuals. We excluded individuals who self-reported being Asian or of other race and ethnicity (which included those who were American Indian or Alaska Native and Native Hawaiian or Other Pacific Islander). We used guidance from Flanagin et al. (2021) in reporting race and ethnicity in this study.18
A total of 1293 hospital readmissions occurred within 1 year of discharge with AMI, and approximately 34.1% of women were readmitted at least once. The median time to first readmission was 71.5 days (interquartile range: 20.0-188.0). The majority of readmissions were for cardiac-related reasons, most commonly stable or unstable angina (33.4%). Approximately 42.2% were non-cardiac-related, and 97 readmissions (7.5%) were for recurrent AMI (Supplemental Table S2). Of the 2007 women, up to 418 (20.8%) were readmitted once, 128 (6.4%) were readmitted twice, and 138 (6.9%) were readmitted 3 or more times. Women who were readmitted 3 or more times were younger (mean age 46.6 years), more likely to be White (62.3%), and presented with primarily cardiac-related complaints (53.3%).
The following total of 9 predictors were selected using BMA (Fig. 1): congestive heart failure (CHF; odds ratio [OR] = 1.65, 95% confidence interval [CI] 1.10- 2.49); diabetes (OR = 1.29, 95% CI 1.05-1.58); any in-hospital complication (OR = 1.25, 95% CI 0.98-1.60); increasing hospital LOS (OR = 1.03, 95% CI 1.01-1.06); obstructive CAD (OR = 1.30, 95% CI 0.96-1.78); low income (OR = 1.17, 95% CI 0.95-1.43); depressive symptoms at baseline (OR = 1.03, 95% CI 1.01-1.04); White race (OR = 0.76, 95% CI 0.61-0.95); and better physical health at baseline (OR = 0.98, 95% CI 0.97-0.99). With regard to magnitude of the model coefficients, the strongest predictors of hospital readmission within 1 year were CHF and obstructive CAD. Of the 9 predictors, 3 were gender-related: physical health, low income, and depressive symptoms. Only 2 predictors, specifically better physical health and White race, were protective. The final model had excellent calibration and modest discrimination (AUC [95% CI] = 0.655 [0.641-0.669]) across the 10 imputed datasets (Fig. 2). Internal validation via bootstrapping calculated an optimism (95% CI for the AUC of 1.2% [0.08%-1.22%]), suggesting comparable model performance in external datasets drawn from the same population. An individual’s probability of readmission within 1 year is easily calculated using the model coefficients and that patient’s predictor values, as illustrated in Supplemental Figure S3.
Figure 1.
Forest plot for risk model for 1-year hospital readmission among young women (aged ≤ 55 years) hospitalized for acute myocardial infarction. Associations between model predictors and readmission are displayed as odds ratios (OR; black squares) with 95% confidence intervals (CI; horizontal lines). CAD, coronary artery disease; PHQ-9, Patient Health Questionnaire–9; SF-12, 12-item short form health survey.
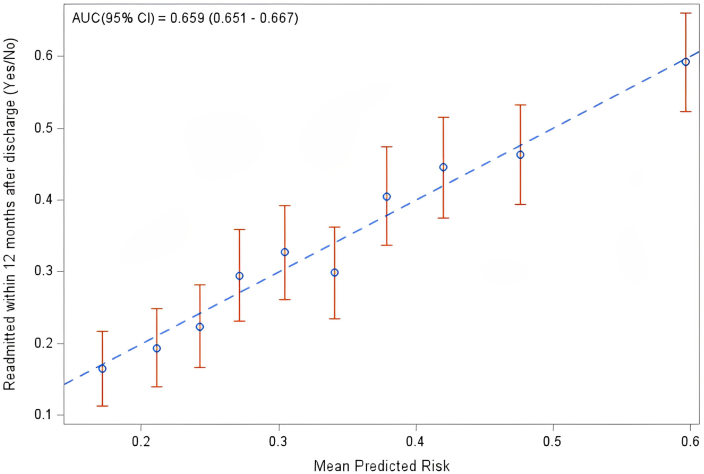
Figure 2.
Calibration plot of observed vs predicted risk from the 9-predictor risk model of all-cause readmission within 1-year of hospitalization for acute myocardial infarction among young women. This calibration plot demonstrates how well the deciles of observed and predicted probabilities of 1-year readmission (blue circles) agree over the entire range of predicted risk with 95% confidence intervals (CIs; vertical red lines). The diagonal blue line represents perfect agreement. AUC, area under the curve.
The 1-year observed rate of hospital readmission in the derivation cohort was 34%. For the IPC, we calculated the average predicted risk of 1-year readmission among subgroups of the women with the 2 most deleterious predictors and the most protective predictor (Supplemental Fig. S4). The average predicted risks among the women with these deleterious and protective factors can be compared to the observed rate. The 1-year average predicted risks of readmission among women with CHF and women with any in-hospital complications were 58% and 41%, respectively. Notably, the average predicted risk among women with CHF corresponds to the highest decile. Further, the 1-year average predicted risk of readmission among women in the top quartile of the SF-12 physical component score was 23%.
Discussion
In this study of US young women hospitalized for AMI, over one third experienced at least one hospital readmission within 1 year of follow-up. Clinical factors, including history of CHF and diabetes, were the strongest predictors of readmission. Further, women with obstructive CAD and longer hospital stays, the latter an indicator of poor overall health, were more likely to be readmitted. In contrast, White race was associated with lower odds of readmission. Several of the predictors were gender-related (ie, physical health, low income, and depression), indicating that those factors were important complements to the dominant clinical and acute care factors. This finding is distinct from traditional prediction models, which tend to include primarily indicators of AMI severity.10 The model demonstrated excellent calibration and modest discrimination in a cohort of young women hospitalized with AMI in the US and can be used to evaluate the risk of readmission during the first year of recovery.
This study provides several novel contributions to the field. This study is the first to consider gender-related variables in its development of a risk prediction model for 1-year hospital readmission that is specific to young women hospitalized for AMI. Apart from a recently published study,3 most of the existing AMI-specific readmission risk models have been developed in older populations.10 The few studies with participants aged < 50 years did not conduct age-based subanalyses to identify predictors of readmission specific to younger adults.3,10 Moreover, prior models did not stratify analyses based on sex,10 generally relied on single-centre study designs, and were developed using data from administrative, electronic medical record and clinical databases that did not benefit from the expertly adjudicated readmission characteristic of the VIRGO study. Lastly, previous studies have focused largely on readmission within 30 days.
Our group recently published a study focused on the development and validation of a 1-year hospital readmission risk model in the complete VIRGO study cohort (women and men).3 Compared to the model derived from the complete VIRGO study cohort, the female-only model included the following 3 unique predictors: history of CHF, obstructive CAD, and race. Notably, the strongest predictor of increased readmission risk in the women-only model—history of CHF—was not retained in the model derived in the complete VIRGO study cohort. The calibration and discrimination of the models were nearly identical when validated in their respective study samples. Our findings suggest that, in contrast to predictors of readmission for men, factors related to pre-event cardiac conditions such as CHF and obstructive CAD may be particularly important predictors of post-AMI readmission among younger women. Previous studies have shown that participating in either in-hospital or home-based cardiac rehabilitation programs that focus on physical activity, diet, and other lifestyle factors is associated with reduced risk of readmission among patients with CHF.30,31 Further, one study using data from TriNetX, a large multi-national electronic health record network, found that exercise-based cardiac rehabilitation was associated with lower odds of 18-month readmission among patients with chronic coronary syndrome, compared to the odds among patients who underwent percutaneous coronary intervention.32 Cardiac rehabilitation has been shown to be underutilized in women, and interventions to improve access and uptake, such as improving physician referrals, offering home-based programs or transportation to in-hospital programs, and creating programs tailored to young women, may be especially important to reduce the number of post-AMI readmission in this population.33
Our model also included race, by contrast to the model derived from the mixed-sex VIRGO study cohort. Specifically, White race was a strong predictor of protection against hospital readmission. A systematic review of post-AMI risk prediction models found that the addition of race improved indicators of model performance in one model that was derived using Centers for Medicare and Medicare Services administrative data.10 Another single-centre study developed 3 separate 30-day readmission risk prediction models among patients hospitalized for AMI, CHF, and pneumonia. Race (White vs Black) was only retained as a final predictor in the model derived in patients who were initially hospitalized for AMI.34 The inclusion of race in our women-only model is consistent with previous risk prediction models of post-AMI readmission. Further, young women represent a notably underrepresented group, as a majority of risk prediction models of post-AMI readmission are developed in middle-aged and older adults. Women with multiple socially disadvantaged identities (ie, Black race) have been shown to be at increased risk for post-AMI readmission.35,36 Previous studies posit that race serves as a marker for several indicators of health affected by socioeconomic measures, which may explain why factors such as employment and health insurance status were not retained in the model.37 Interventions that address structural barriers, such as improving the utilization of readmission-reduction strategies in under-resourced hospitals, and implementing care coordination, may reduce racial disparities in post-AMI readmission.38
Emerging evidence indicates that gender-related factors (ie, social norms and expectations assigned to women)8,9 can differentially impact health behaviours and disease burden.6 Notably, our model includes several gender-related factors and relatively few clinical factors.6 This model suggests that even after adjustment for clinical confounders, factors associated with gender identity, roles, and relations, and institutionalized gender, may be important predictors of hospital readmission among younger women with AMI. In constrast to previous studies, measures of disease severity and presentation characteristics were considered, but they were not retained in our final model.10 Of note, depressive symptoms and low income were predictors of readmission. Depression is an independent risk factor for cardiac morbidity and mortality,39 and it has also been shown to be associated with increased risk of readmission.40 Depression is hypothesized to negatively impact care-seeking behaviour, medication adherence, and health behaviours.39 Very few prior risk prediction models for post-AMI readmission have included patient-level indicators of income.10 Young adults are more likely to have unmet medical needs, compared to older adults, and previous studies suggest that the lack of financial resources serves as a barrier to accessing healthcare.41 A previous study among non-elderly adults (aged 18-64 years) with CAD found that women were more likely to report financial hardship from medical bills than men.42 This suggests that the uneven distribution of wealth and of access to health-promoting resources may serve to perpetuate sex-related disparities in AMI recovery.
Furthermore, experiencing any in-hospital complication and having a longer hospital LOS, both proxies of overall health status, were associated with increased risk of readmission. Women have been found to be more prone to complications during hospitalization than men,5 predominantly CHF.10 Suboptimal AMI care may explain some of the variation in risk for in-hospital events and subsequent readmission. Indeed, patients who were less likely to receive recommended diagnostic imaging and percutaneous coronary intervention were at higher risk for 30-day readmission post-AMI.43 Although diabetes, cerebrovascular disease, and cardiac dysrhythmia have been associated with an extended hospital LOS in patients with AMI,44,45 the LOS was not associated with 7- or 30-day readmission.45 In contrast, our findings indicate that longer LOS is an important predictor of longer-term readmission among young women.
Limitations
Some limitations should be considered in the interpretation of our findings. First, important measures of baseline risk and disease severity (eg, Global Registry of Acute Coronary Events [GRACE] score and Killip class) were excluded from analysis based on inconsistencies in how they were measured and reported at different study sites. Second, important gender-related variables, such as caregiver burden, personality traits, and social roles, were not available. The presence of feminine traits and social norms are important determinants of health-behaviours.6 Given the importance of these additional gender-related factors, their availability in a composite measure of gender7,46 might have led to deeper insight into factors associated with readmission. Third, our findings may not be generalizable to women of some racial minority groups (eg, American Indian, Alaska Native, Asian, Pacific Islander, East Indian) or to Hispanic women, who were underrepresented in the study. Nevertheless, an important point to note is that the VIRGO study cohort is the largest and most racially diverse cohort of young AMI survivors in the US. Perhaps the primary limitation is that the AUC of our model indicated only modest discrimination, at 0.66. Notwithstanding this limitation, this value is in the upper range of existing risk prediction models (0.53- 0.79).3,10 Further, we considered only patient-level predictors as candidate variables for the model. The modest discrimination of our final model indicated that healthcare system-level factors may also contribute to variation in readmission risk. Hospital readmission as an outcome is challenging to predict because it is influenced by complex interactions between individual- and system-level factors, as well as by individualized decision-making among providers of healthcare. Lastly, we acknowledge that because this model is based on data from women only and was not validated in an external dataset, it may have limited generalizability.
Conclusion
CHF, diabetes, and obstructive CAD were the strongest positive predictors of 1-year hospital readmission among younger women hospitalized for AMI. However, gender-related factors, including income level, depressive symptoms, and patient-reported physical health, were important complements. This model demonstrated excellent calibration and modest discrimination and can be used to predict the risk of 1-year readmission following hospitalization for AMI among younger women.
Acknowledgments
Ethics Statement
Institutional review board approval was obtained at each participating institution, and patients provided informed consent for their study participation, including baseline hospitalization and interviews.
Funding Sources
The VIRGO study was supported by a 4-year National Heart, Lung, and Blood Institute grant (No. 5R01HL081153). R.P.D. was supported by an American Heart Association (AHA) Transformational Project Award (#19TPA34830013). This project was additionally supported by a Canadian Institutes of Health Research project grant (PJT-159508). Lastly, T.E.M. is supported by the Yale Claude D. Pepper Older Americans Independence Centre (P30AG021342).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Received for publication August 7, 2022. Accepted December 13, 2022.
See page 342 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2022.12.004.
Supplementary Material
References
- 1.Dreyer R.P., Ranasinghe I., Wang Y., et al. Sex differences in the rate, timing, and principal diagnoses of 30-day readmissions in younger patients with acute myocardial infarction. Circulation. 2015;132:158–166. doi: 10.1161/CIRCULATIONAHA.114.014776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dreyer R.P., Dharmarajan K., Kennedy K.F., et al. Sex differences in 1-year all-cause rehospitalization in patients after acute myocardial infarction: a prospective observational study. Circulation. 2017;135:521–531. doi: 10.1161/CIRCULATIONAHA.116.024993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreyer R.P., Raparelli V., Tsang S.W., et al. Development and validation of a risk prediction model for 1-year readmission among young adults hospitalized for acute myocardial infarction. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.021047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreyer R.P., Sciria C., Spatz E.S., et al. Young women with acute myocardial infarction: current perspectives. Circ Cardiovasc Qual Outcomes. 2017;10 doi: 10.1161/CIRCOUTCOMES.116.003480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta L.S., Beckie T.M., DeVon H.A., et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 6.Pelletier R., Khan N.A., Cox J., et al. Sex versus gender-related characteristics: Which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol. 2016;67:127–135. doi: 10.1016/j.jacc.2015.10.067. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier R., Ditto B., Pilote L. A composite measure of gender and its association with risk factors in patients with premature acute coronary syndrome. Psychosom Med. 2015;77:517–526. doi: 10.1097/PSY.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 8.Johnson J.L., Greaves L., Repta R. Better science with sex and gender: facilitating the use of a sex and gender-based analysis in health research. Int J Equity Health. 2009;8:1–11. doi: 10.1186/1475-9276-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raparelli V., Norris C.M., Bender U., et al. Identification and inclusion of gender factors in retrospective cohort studies: the GOING-FWD framework. BMJ Glob Health. 2021;6 doi: 10.1136/bmjgh-2021-005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith L.N., Makam A.N., Darden D., et al. Acute myocardial infarction readmission risk prediction models: a systematic review of model performance. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.117.003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amarasingham R., Moore B.J., Tabak Y.P., et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Med Care. 2010;48:981–988. doi: 10.1097/MLR.0b013e3181ef60d9. [DOI] [PubMed] [Google Scholar]
- 12.Amarasingham R., Patel P.C., Toto K., et al. Allocating scarce resources in real-time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf. 2013;22:998–1005. doi: 10.1136/bmjqs-2013-001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson J., Greaves L., Repta R. Better science with sex and gender: a primer for health research. http://bccewh.bc.ca/wp-content/uploads/2012/05/2007_BetterSciencewithSexandGenderPrimerforHealthResearch.pdf Available at: [DOI] [PMC free article] [PubMed]
- 14.Raparelli V., Proietti M., Basili S. Explanatory power of gender relations in cardiovascular outcomes: the missing piece of the puzzle. Heart. 2018;104:1900–1901. doi: 10.1136/heartjnl-2018-313469. [DOI] [PubMed] [Google Scholar]
- 15.Canadian Institutes of Health Research Online training modules: integrating sex & gender in health research. http://www.cihr-irsc.gc.ca/e/49347.html Available at:
- 16.Lichtman J.H., Lorenze N.P., D'Onofrio G., et al. Variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–693. doi: 10.1161/CIRCOUTCOMES.109.928713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagin A., Frey T., Christiansen S.L., AMA Manual of Style Committee Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA. 2021;326:621–627. doi: 10.1001/jama.2021.13304. [DOI] [PubMed] [Google Scholar]
- 19.O’Gara P.T., Kushner F.G., Ascheim D.D., et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. doi: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 21.Spertus J.A., Winder J.A., Dewhurst T.A., et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 22.Spertus J.A., Winder J.A., Dewhurst T.A., Deyo R.A., Fihn S.D. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 23.Ware J.J., Kosinski M., Keller S.D. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 24.ENRICHD Investigators Enhancing recovery in coronary heart disease (ENRICHD) study intervention: rationale and design. Psychosom Med. 2001;63:747–755. [PubMed] [Google Scholar]
- 25.Rubin D.B. John Wiley & Sons; New York: 2004. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 26.Moons K.G., Altman D.G., Reitsma J.B., et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 27.Murphy T.E., Tsang S.W., Leo-Summers L.S., et al. Bayesian model averaging for selection of a risk prediction model for death within thirty days of discharge: the SILVER-AMI study. Int J Stat Med Res. 2019;8:1–7. doi: 10.6000/1929-6029.2019.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 29.Pepe M.S., Feng Z., Huang Y., et al. Integrating the predictiveness of a marker with its performance as a classifier. Am J Epidemiol. 2008;167:362–368. doi: 10.1093/aje/kwm305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y.-W., Wang C.-Y., Lai Y.-H., et al. Home-based cardiac rehabilitation improves quality of life, aerobic capacity, and readmission rates in patients with chronic heart failure. Med (Baltimore) 2018;97:e9629. doi: 10.1097/MD.0000000000009629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scalvini S., Grossetti F., Paganoni A.M., et al. Impact of in-hospital cardiac rehabilitation on mortality and readmissions in heart failure: a population study in Lombardy, Italy, from 2005 to 2012. Eur J Prev Cardiol. 2019;26:808–817. doi: 10.1177/2047487319833512. [DOI] [PubMed] [Google Scholar]
- 32.Buckley B.J., de Koning I.A., Harrison S.L., et al. Exercise-based cardiac rehabilitation vs. percutaneous coronary intervention for chronic coronary syndrome: impact on morbidity and mortality. Eur J Prev Cardiol. 2022;29:1074–1080. doi: 10.1093/eurjpc/zwab191. [DOI] [PubMed] [Google Scholar]
- 33.Khadanga S., Gaalema D.E., Savage P., Ades P.A. Underutilization of cardiac rehabilitation in women: barriers and solutions. J Cardiopulm Rehabil Prev. 2021;41:207–213. doi: 10.1097/HCR.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebert C., Shivade C., Foraker R., et al. Diagnosis-specific readmission risk prediction using electronic health data: a retrospective cohort study. BMC Med Inform Decis Mak. 2014;14:65. doi: 10.1186/1472-6947-14-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandey A., Keshvani N., Khera R., et al. Temporal trends in racial differences in 30-day readmission and mortality rates after acute myocardial infarction among Medicare beneficiaries. JAMA Cardiol. 2020;5:136–145. doi: 10.1001/jamacardio.2019.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damiani G., Salvatori E., Silvestrini G., et al. Influence of socioeconomic factors on hospital readmissions for heart failure and acute myocardial infarction in patients 65 years and older: evidence from a systematic review. Clin Interv Aging. 2015;10:237. doi: 10.2147/CIA.S71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham G.N., Jones P.G., Chan P.S., et al. Racial disparities in patient characteristics and survival after acute myocardial infarction. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Figueroa J.F., Joynt K.E., Zhou X., Orav E.J., Jha A.K. Safety-net hospitals face more barriers yet use fewer strategies to reduce readmissions. Med Care. 2017;55:229–235. doi: 10.1097/MLR.0000000000000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reese R.L., Freedland K.E., Steinmeyer B.C., et al. Depression and rehospitalization following acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4:626–633. doi: 10.1161/CIRCOUTCOMES.111.961896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lett H.S., Blumenthal J.A., Babyak M.A., et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305–315. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 41.Marshall E.G. Do young adults have unmet healthcare needs? J Adolesc Health. 2011;49:490–497. doi: 10.1016/j.jadohealth.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Mszar R., Grandhi G.R., Valero-Elizondo J., et al. Cumulative burden of financial hardship from medical bills across the spectrum of diabetes mellitus and atherosclerotic cardiovascular disease among non-elderly adults in the United States. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwok C.S., Wong C.W., Shufflebotham H., et al. Early readmissions after acute myocardial infarction. Am J Cardiol. 2017;120:723–728. doi: 10.1016/j.amjcard.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 44.Magalhaes T., Lopes S., Gomes J., Seixo F. The predictive factors on extended hospital length of stay in patients with AMI: laboratory and administrative data. J Med Syst. 2016;40:2. doi: 10.1007/s10916-015-0363-7. [DOI] [PubMed] [Google Scholar]
- 45.Saczynski J.S., Lessard D., Spencer F.A., et al. Declining length of stay for patients hospitalized with AMI: impact on mortality and readmissions. Am J Med. 2010;123:1007–1015. doi: 10.1016/j.amjmed.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelletier R., Humphries K.H., Shimony A., et al. Sex-related differences in access to care among patients with premature acute coronary syndrome. CMAJ. 2014;186:497–504. doi: 10.1503/cmaj.131450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.