Abstract
The aim of the present study is to explore the anti-cancer, anti-oxidant, and anti-obesity potential of saffron petal extract (SPE) prepared through the hydro-alcoholic extraction method. Further partitioning was done with a series of polar and non-polar solvents to find out the most potent fraction of SPE against HCC. Organoleptic characterization depicted the color, odor, taste, and texture of the sub-fractions of SPE. Phytochemical, and pharmacognostic screening of these fractions revealed the presence of alkaloids, flavonoids, carbohydrates, glycosides, and phenols. The quantitative assessment demonstrated that the n-butanol fraction showed maximum phenolic (60.8 mg GAE eq./mg EW), and flavonoid (23.3 mg kaempferol eq./mg EW) content. The anti-oxidant study revealed that the n-butanol fraction exhibited the highest radical scavenging activity, as assessed through DPPH and FRAP assay. The results of the comparative cytotoxic potential also showed n-butanol as the best against liver cancer cells (Huh-7), as it has the least IC50 value (462.8 µg/ml). While other extracts viz., chloroform, n-hexane, ethyl acetate, and aqueous fractions have IC50 values as 1088, 733.9, 1043, and 1245 µg/ml, respectively. Additionally, the n-butanol fraction exerted the highest inhibitory potential against α-amylase (92.5%) and pancreatic lipase enzymes (78%), indicating its anti-adipogenesis property. Based on the current finding, we can deduce that the n-butanol fraction of SPE has better cytotoxic, anti-oxidant, and anti-obesity potential than the other fractions.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-023-03669-x.
Keywords: Liver cancer, Pancreatic lipase activity, Partitioning fractionation, Phytochemical screening, Reactive oxygen species (ROS)
Introduction
Saffron is used as a food additive and colorant for centuries (Patel et al. 2017). It is widely cultivated in many parts of the world, especially in Kashmir (India), Iran, and Spain (Sumaiya et al. 2020). Saffron has been used in traditional Islamic-Persian medicine as a stomach pain reliever, antispasmodic, digestion aid, renal colic pain reliever, anti-depressant, and appetizer (Milajerdi et al. 2016; Namgyal and Sarwat 2020). The saffron stigma is the widely used part of the plant. One kg of the dried stigma is produced from 78 kg of the freshly harvested saffron flower. During the process of harvesting saffron stigma from its flower, petals are obtained in large quantities, these are not generally used and are discarded as a waste product or byproduct (Hosseini et al. 2018). The petals have several active components including anthocyanins, flavonoids, mineral matter, starch, amino acids, and vitamins (riboflavin and thiamine) (Shakeri et al. 2020). Owing to these metabolites, they show diverse biological activities including anti-oxidant, anti-inflammatory, hepatoprotective, neuroprotective, cardioprotective, and anti-microbial properties (Mohaqiq et al. 2020; Mohamadpour et al. 2020; Omidi et al. 2014; Chen et al. 2017; Moshfegh et al. 2022). Therefore, it is worth considering saffron petals as a treatment option for various ailments or diseases including cancer.
Liver cancer is the most common malignancy and the second leading cause of cancer-associated mortality worldwide (Gupta et al. 2020, 2022a). It accounts for about 90% of cases globally (Ringelhan et al. 2018). The American Cancer Society estimated that 28,600 men and 12,660 women were diagnosed with liver cancer; of which 30,520 patients have died (American Cancer Society 2022). Risk factors include unhealthy diet, alcohol consumption, air pollution, and physical inactivity (Behrens et al. 2018; Gupta et al. 2022b; Sumaiya et al. 2023). Several molecular mechanisms are involved in the progression of this disease but disruption of redox hemostasis is one of the most important mechanisms underlying cancer development in human cells (Aggarwal et al. 2019). Recent literature reports the correlation between reactive oxygen species (ROS) and adipogenesis with liver cancer progression (Sharma et al. 2021). This impairment in the redox system is induced by the generation of unstable radicals called reactive oxygen species (ROS) (Ahmad et al. 2008; Misra et al. 2009). These molecules impair cellular metabolism, mitochondrial functioning, peroxisome activity, cellular receptor signaling, and oncogene activity (Namgyal et al. 2021; Sharma et al. 2021). Additionally, elevated ROS levels during or following cancer therapies enhance resistance to existing therapies and therefore, contributes to cancer development and metastasis (Perillo et al. 2020).
Adipogenesis is the process of converting pre-adipocytes into mature adipocytes which plays a vital role in tumor microenvironments (Ali et al. 2013). Adipocyte secretes several adipokines that are associated with cancer development, metastasis, and chemo-resistance through various signaling pathways (Rajesh and Sarkar 2021). Additionally, elevated ROS and adipokines levels during or following cancer therapies enhance resistance to existing therapies and contribute to cancer development and metastasis (Perillo et al. 2020). In spite of recent advances in diagnostics and treatment, the clinical outcome of patients with liver cancer remains unsatisfactory (Anwanwan et al. 2020). Additionally, patients may experience social, psychological, and physical problems leading to depression and poor quality of life (Niedzwiedz et al. 2019). Therefore, the development of a novel therapeutic agent with low toxicity and more favorable outcomes is urgently required. In this regard, scientists have extensively evaluated the potential therapeutic effect of natural compounds in the treatment of various cancers. Currently, saffron (Crocus sativus L.) is gaining widespread attention from researchers in treating liver cancer (Gupta et al. 2020). Thus, the present study was designed to investigate and compare the organoleptic, phytochemical, and pharmacognostic parameters, total flavonoid and phenolic content, effect on the α-amylase and pancreatic lipase enzymes, anti-oxidant, and cytotoxic activity of sub-fractions of hydro-alcoholic extract of saffron petals using different solvents.
Materials and methods
Chemicals and reagents
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), sodium phosphate monobasic, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) was purchased from Central Drug House Pvt. Ltd. (Pune, India) and di-nitrosalicylic acid, p-nitrophenyl butyrate, α-amylase and ascorbic acid was obtained from Sisco Research Laboratory Pvt. Ltd. (Pune, India). Dimethyl sulphoxide (cell-culture grade), and phenol solution were obtained from HiMedia Laboratories Pvt. Ltd. (Mumbai, India). Glucose, Sulphuric acid, Starch, Fetal Bovine Serum (FBS), Dulbecco’s Modified Eagle Medium (DMEM), Penicillin–Streptomycin (Penstrep) antibiotic solution, Trypsin EDTA, Epidermal Growth Factor (EGF), ferric chloride, acetonitrile, chloroform, potassium ferricyanide, and trichloroacetic acid were purchased from Thermo Fisher Scientific (Whattman, MA, USA). Sodium carbonate, glacial acetic acid and acetic acid was obtained from Merck Specialities Pvt. Ltd (Darmstadt, Germany). Mayer’s reagent and sodium hydroxide was bought from Central Drug House (CDH) Ltd. (New Delhi, India).
Plant material and preparation of plant extract
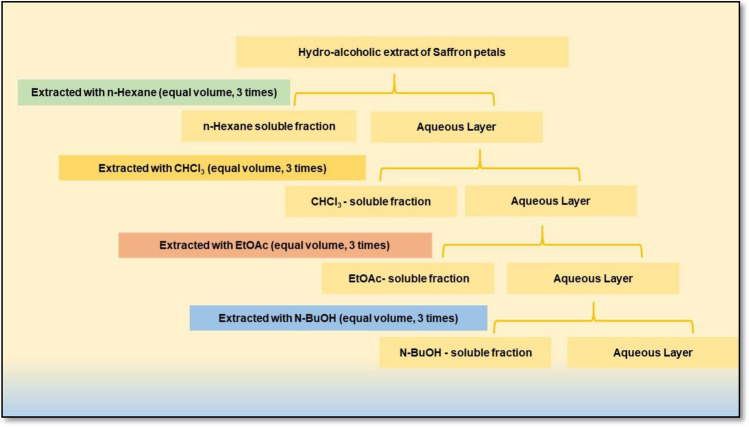
Fresh saffron flowers were collected from the farms of Kashmir in the month of Oct–Nov. The saffron petals were manually separated and air-dried to be used for extraction. For the preparation of hydro-alcoholic extract (SPE), the petals were pulverized in an electric grinder. Extraction was carried out by dissolving 50 g of dried petal powder in 1000 ml of ethanol (80% v/v), followed by shaking for 24 h at room temperature. The extract was then filtered using Whatman No. 1 filter paper. The filtrate was stored at 4 ℃ in a dark bottle till further use. For partitioning, the SPE was further subjected to sequential extraction with 100 ml of n-hexane (SHE), n-butanol (SBE), chloroform (SCE), ethyl acetate (SEE) and water (SAE) (Fig. 1). After extraction, the contents were filtered through Whatman No. 1 filter paper. The filtrates were vacuum-dried with the help of a rotary evaporator and stored at 4 ℃ till further use.
Fig. 1.
Schematic diagram representing the methodology of fractional separation of the hydro-alcoholic extract of saffron petals through partitioning using different solvents
Pharmacognostical and phytochemical evaluation
Organoleptic evaluation
The organoleptic evaluation is a qualitative method to evaluate the characteristic features of crude drugs. This property characterizes any drug on the basis of its color, odor, taste, and texture. These properties were evaluated by the method of Wallis (2004). To determine odor, a small amount of sub-fractions of SPE was placed in a beaker and slowly inhaled. The impression of odor (rancid, aromatic, moldy, mushy, fruity, etc.) was noted. The taste was recorded as per its classification (pungent, bitter, sweet, sour, mucilaginous, or astringent). Additionally, samples were placed between the thumb and index finger to determine a texture, and the observations were recorded by rubbing it.
Fluorescence analysis
1–2 mg of each sub-fractions of SPE was taken, kept on a microscopic slide and observed in daylight and as well as in short-wave (254 nm) and long-wave UV light (366 nm). The sub-fractions were further treated with various reagents including 1N sodium hydroxide (aqueous), 1N sodium hydroxide (alcoholic), 1N hydrochloric acid, ammonia, 5% iodine, acetic acid, 5% ferric chloride, 1N nitric acid and 1N sulphuric acid and the results were recorded.
Preliminary phytochemical screening
Qualitative analyses were carried out for the phytochemical screening of different extracts. Standard procedures (Supplementary Table 1) were followed to check the presence or absence of saponins, alkaloids, quinones, glycosides, cardiac glycosides, terpenoids, coumarins, phytosterols, phlobatannins, and anthraquinones. The positive ( +) sign is indicating the presence of these phytochemicals and the negative (−) sign is indicating their absence.
Quantitative phytochemical analysis
Sample processing
For the determination of the total phenolic (TPC), flavonoid (TFC), and soluble sugar (TSSC), the sub-fractions were resuspended in distilled water. The extracts were filtered through a membrane filter, aliquoted, and stored at − 20 °C away from light.
Determination of total phenolic content (TPC) and total flavonoid content (TFC)
The TPC of reconstituted solutions of the n-hexane (SHE), n-butanol (SBE), chloroform (SCE), ethyl acetate (SEE), and aqueous (SAE) extracts of saffron petals was evaluated as follows: 2 ml of distilled water is added to 1 ml of each sub-fraction (SHE, SBE, SCE, SEE, and SAE) followed by the addition of a few drops of 10% ferric chloride. The appearance of green or blue color indicates the presence of phenols. The results were expressed as Gallic acid equivalent (GAE)/mg Extractive weight (EW). For the evaluation of TFC, 1 ml of 2N sodium hydroxide was added to 2 ml of each sub-fraction. The appearance of yellow color depicts the presence of flavonoids. The results were expressed kaempferol equivalent/mg EW.
Determination of total soluble sugar content (TSSC)
The colorimetric method was used to determine the concentration of carbohydrates in extracts. Initially, 100 mg of glucose was taken in a test tube and 5 ml of 2.5N HCl was added into it. The test tube was boiled in a water bath to hydrolyze the sugar. The reaction mixture was allowed to cool at room temperature. Further, a sufficient quantity of sodium carbonate was added to the test tube until the effervescence stopped. The content was filtered and the volume was made up to 100 ml. From the stock, the working solution was prepared (0.2, 0.4, 0.6, 0.8, and 1 ml). The blank was prepared with all the reagents without the test sample. Further, 1 ml of phenol solution was added to each test tube and shaken well. 5 ml of sulphuric acid was added and the reaction mixture was incubated for the next 10 min. The test tubes were placed at 25–30 ℃ in the water bath for 20 min. The test samples were prepared in a similar fashion. Finally, the absorbance was recorded at 490 nm in a UV spectrophotometer.
Evaluation of anti-oxidant activity
2,2-Diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical scavenging activity
The free radical scavenging activity of all the sub-fractions of SPE was evaluated using DPPH as per the standard protocol of our lab (Gupta et al. 2023). Discoloration of these solvent fractions was measured after incubating it for 10 min in the dark at 517 nm. The percentage of inhibition was evaluated by using a formula:
where A0 is the absorbance of the blank and A1 is the absorbance of the test sample.
Ferric reducing anti-oxidant power (FRAP) assay
The reduction of ferric ions by the different solvent extracts was evaluated using a spectrophotometer. 1 ml of sub-fractions of SPE at different concentrations (0.05, 0.1, 0.25, 0.55, and 1 mg/ml) were mixed with 2.5 ml of potassium buffer (0.2 M) and 2.5 ml of 1% potassium ferricyanide solution in a test tube. The reaction mixture was further incubated at 50 °C for 20 min for the reaction to occur. Further, 2.5 ml of 10% trichloroacetic acid solution was added to the test tubes and each reaction mixture was centrifuged at 3000 rpm for 10 min. From the supernatant, 2.5 ml was taken and mixed with 2.5 ml distilled water, and 0.5 ml of 0.1% ferric chloride solution was added to it. The absorbance was measured at 700 nm against blank.
α-amylase inhibition assay
The α-amylase inhibition assay was performed as per the method developed in our lab (Walia and Sarwat 2020). The sub-fractions (250 µl) of SPE (10 mg/ml) were dissolved in 250 µl of sodium phosphate buffer (0.02 M, pH 6.9) containing α-amylase solution (0.5 mg/ml). This reaction mixture was incubated for 10 min at 25 ℃. Further, 250 µl of 1% starch solution prepared in sodium phosphate buffer was added and incubated for the next 10 min. The dinitrosalicylic acid (DNS) reagent was used to terminate the reaction. These tubes were incubated in boiling water for 5 min and cooled to room temperature. Finally, the mixture was diluted with distilled water (5 ml) and the absorbance was recorded at 540 nm. A similar method was followed for the preparation of control where the sub-fractions were replaced by distilled water. Acarbose (10 mg/ml) was used as a standard. The α-amylase inhibitory activity was expressed as a percent inhibition and calculated using the following equation:
Pancreatic lipase inhibition assay
The inhibitory activity of the sub-fractions of SPE against pancreatic lipase enzyme was evaluated using p-Nitrophenylbutyrate (PNPB) as a substrate as per our standardized protocol (Walia and Sarwat 2020). The PNPB working solution was prepared with 8.403 µl of PNPB stock solution and the volume was made up to 10 ml using acetonitrile. The sub-fractions were weighed and further prepared in phosphate-buffered saline (1 mg/ml) using maceration at 37 ℃ for 4 h followed by centrifugation at 4000 g for 10 min. The supernatant thus collected was used as a stock solution and was serially diluted with phosphate buffer saline to achieve a concentration of 10 µg/ml. Orlistat was used as a standard and was prepared by dissolving the content of one capsule in 12 ml of DMSO. 25 µl of the sub-fractions and standard solution were incubated with 50 µl of the pancreatic lipase enzyme solution in each test tube. Further, 100 µl of the buffer solution and 25 µl of the PNPB solution were incorporated into this solution. This reaction mixture was allowed to incubate at 37 ℃ for 30 min. The amount of p-nitrophenol released by the hydrolysis of PNPB with the help of lipase enzyme was measured using an ELISA plate reader at 400 nm. The inhibition of the lipase activity was calculated using the formula:
Cell lines and cell culture
The adherent hepatic cancer cell lines (Huh-7) were a kind gift from Prof. Subhrajit Biswas (Amity Institute of Molecular Medicine and Stem Cell Research, Amity University, Uttar Pradesh). Huh-7 were grown in DMEM with 10% FBS and 1% Penstrep solution in a humidified 5% CO2 incubator at 37 ℃. Cells were cultured in T-25 mm2 culture flasks (Nunclon, Denmark). Cells were routinely renewed with fresh medium every 2–3 days. After attaining 80–90% confluence, cells were counted on a hemocytometer and seeded for further experimentation.
Cell viability assay
Cell viability was determined with the help of an MTT assay as per the standard protocol of our lab (Aggarwal et al. 2022; Gupta et al. 2022a, b). Cells were seeded in 96 well plates at a density of 5 × 103 cells/well. After 24 h of incubation, cells were treated with various concentrations (400–3200 µg/ml) of each sub-fraction for 24 h. Further, the medium was removed and the cells were treated with MTT after the exposure period and the plate was allowed to incubate at 37 ℃ for 4 h. Further, 100 µl of DMSO was added to the plate and the absorbance was recorded at 570 nm.
Statistical analysis
Data were analyzed using GraphPad Prism version 5.01. All experiments were done in triplicates and the results were demonstrated as Mean ± SD. The data were analyzed using ANOVA. p < 0.05 was considered statistically significant.
Results and discussions
Indigenous herbal medicines are an integral part of the primary healthcare sector. However, their scientific evaluation is necessary for their proper utilization (Sen and Chakraborty 2017). Plants produce various active constituents to guard themselves against harmful microbes and insects (Kaur et al. 2022). These phytochemicals consist of different classes of compounds including terpenoids, phytosterols, flavonoids, alkaloids, carotenoids, phenols, organic acids, proteases inhibitors, etc. These compounds possess medicinal properties and thus can be used as the best template for future development. Notably, a wide variety of techniques are being used for the extraction of phytochemicals among which solvent extraction is the most popular one (Kumar et al. 2023). Due to the different polarity and other physicochemical characteristics of the solvents, the amount and yield of secondary metabolites vary depending upon the kind of solvents. The polar solvents promote the extraction of phenolic compounds and their glycosidic derivatives and saponins, while non-polar solvents are typically used to isolate steroids and fatty acid (Dirar et al. 2019). Different and effective bioactive compounds were extracted using different solvents and further compared on the basis of their biological properties. Therefore, sub-fractions of SPE prepared in different polarity solvents were analyzed through different evaluation parameters. To the best of our knowledge, we have performed this study for the first time with the sub-fractions of SPE.
Organoleptic evaluation
Organoleptic (sensory) evaluation helps in the identification of crude natural or herbal drugs. Physical and morphological evaluation provides easy, quick, and valuable information on crude drugs’ characteristics and recognition (Selvam 2015). The sub-fractions of SPE showed characteristic taste, odor, and mushy appearance. The color and texture of all the sub-fractions are mentioned in Table 1.
Table 1.
Organoleptic characteristics of sub-fractions of SPE
| S. No. | Sub-fraction | Organoleptic properties | ||||
|---|---|---|---|---|---|---|
| Appearance | Color | Taste | Odor | Texture | ||
| 1 | n-Hexane | Mushy | Pale yellow | Characteristic | Characteristic | Sticky |
| 2 | Chloroform | Mushy | Dark brown | Characteristic | Characteristic | Sticky |
| 3 | Butanol | Mushy | Brown | Characteristic | Characteristic | Sticky |
| 4 | Ethyl acetate | Mushy | Mustard | Characteristic | Characteristic | Sticky |
| 5 | Aqueous | Mushy | Purple | Characteristic | Characteristic | Gritty |
Preliminary phytochemical screening
In the current study, phytochemical screening of sub-fractions of SPE was performed with different reagents, and a comparison was made. The results observed were summarized in Table 2. These results demonstrated that phytochemicals like carbohydrates, flavonoids, alkaloids, glycosides, cardiac glycosides, and phenols were present in all the sub-fractions whereas tannins, terpenoids, phlobatannins, and coumarins were present in few. Additionally, the saponins were not observed in any of the extracts. The results of phytochemical tests confirmed that the petals of saffron are rich in various bioactive compounds such as flavonoids, alkaloids, glycosides, phenols, steroids, and essential oil contents. The presence of various phytochemicals was noticed qualitatively in all five different extracts, and the maximum number of phytochemicals was identified in the n-butanol extract.
Table 2.
Screening the presence of secondary metabolites in different sub-fractions of SPE
| S. No. | Phytochemical | Sub-fractions | ||||
|---|---|---|---|---|---|---|
| n-Hexane | n-Butanol | Ethyl acetate | Chloroform | Aqueous | ||
| 1 | Carbohydrates | + | + | + | + | + |
| 2 | Tannins | + | − | + | + | − |
| 3 | Saponins | − | − | − | − | − |
| 4 | Flavonoids | + | + | + | + | + |
| 5 | Alkaloids | − | + | + | − | − |
| 6 | Quinones | − | + | + | + | − |
| 7 | Glycosides | + | + | + | + | + |
| 8 | Cardiac glycosides | + | + | + | + | + |
| 9 | Terpenoids | − | + | + | − | − |
| 10 | Phenols | + | + | + | + | + |
| 11 | Coumarin | − | − | − | − | − |
| 12 | Steroids | − | + | + | + | − |
| 13 | Phlobatannins | − | + | − | + | − |
| 14 | Anthraquinones | − | − | − | − | − |
Fluorescence analysis
Fluorescence analysis is also an important parameter for standardizing plant extracts. Every compound has its own specific color of fluorescence. But, if the components do not produce fluorescence, they should be treated with different chemical reagents to achieve it (Majid et al. 2016). Each sub-fraction of SPE exhibited a characteristic fluorescence after treatment with various chemicals. Similar experiments were performed by Govindarajan and team on the Trikatu Churna, where they subjected it to pharmacognostic and phytochemical evaluation (Govindarajan et al. 2016). Fluorescence analysis of the sub-fractions of SPE with different chemical reagents showed variation in colors (Supplementary Table 2).
Total phenolic and flavonoid content
Phenolic compounds are the secondary metabolites consisting of the hydroxyl group responsible for scavenging free radicals. Flavonoids are considered highly effective scavengers of most oxidizing molecules, including singlet oxygen, and various free radicals. Plant parts which are rich in phenols and flavonoids are quite popular in the food industry because they retard the oxidative degradation of lipids and thus enhance the nutritional value of food. (Serra et al. 2021). The total phenolic and flavonoid content (TPC and TFC) in the sub-fractions of SPE is presented in Table 3. In the tested sub-fractions, the amount of phenols varied from 28.9 to 60.8 mg GAE eq./mg EW. The n-butanol sub-fraction was found to show the highest phenolic level (60.8 mg GAE eq./mg EW) whereas the aqueous extract of saffron petals showed the lowest phenolic content (28.9 mg GAE eq./mg EW). The compounds showed a similar pattern of results when tested for flavonoid content. The n-butanol sub-fraction showed the highest flavonoid content (23.3 mg kaempferol eq./mg EW) whereas the aqueous fraction showed the lowest flavonoid content (5.0 mg kaempferol eq./mg EW) (Table 3). Similar results were seen in D. ramosissimum and Butea monosperma flower, where the n-butanol fraction showed the highest level of total phenolic and flavonoid content (Ezealigo et al. 2020; Subramaniyan et al. 2016). Jing et al. 2015 also showed high phenol and flavonoid content and high anti-oxidant potential of the n-butanol fraction of Rhododendron anthopogonoides. In another study (Lachguer et al. 2022), the stamens and petals (saffron flower byproducts) were extracted with different solvents, and the diethyl ether fraction performed better than butanol in terms of polyphenol and flavonoid content. It might be because of the presence of stamens in the study material, which is different from our research, consisting only of saffron petals.
Table 3.
Anti-oxidant potential analysis of various sub-fractions of SPE
| Components | Total phenolic content (mg GAE eq./mg EW) | Total flavonoid content (mg kaempferol eq./mg EW) |
|---|---|---|
| n-Hexane extract | 40.8 ± 0.7 | 7.6 ± 0.33 |
| Chloroform extract | 44.1 ± 0.85 | 10.5 ± 0.25 |
| n-Butanol extract | 60.83 ± 0.63 | 23.3 ± 0.42 |
| Ethyl acetate extract | 45.2 ± 0.88 | 19.9 ± 0.39 |
| Aqueous extract | 28.9 ± 0.46 | 5.0 ± 0.15 |
Total soluble sugar estimation
The total carbohydrate content of the sub-fractions was evaluated using the phenol–sulphuric method. The results demonstrated that the n-butanol fraction showed maximum absorbance (2.9283) at 5 µg/ml whereas the aqueous sub-fraction had the lowest absorbance (0.1048) at the same concentration. These findings suggested that n-butanol sub-fraction contains the maximum sugar content. The absorbance of the sub-fractions of SPE is listed in Table 4.
Table 4.
Absorbance recorded for estimating the sugar content in various sub-fractions of SPE
| S. No. | Sub-fractions | Absorbance recorded at 490 nm | |
|---|---|---|---|
| 1 | n-Butanol | 1.5290 | 2.9283 |
| 2 | n-Hexane | 0.1621 | 0.1926 |
| 3 | Chloroform | 0.6728 | 0.8167 |
| 4 | Ethyl acetate | 1.1419 | 1.1932 |
| 5 | Aqueous | 0.0997 | 0.1048 |
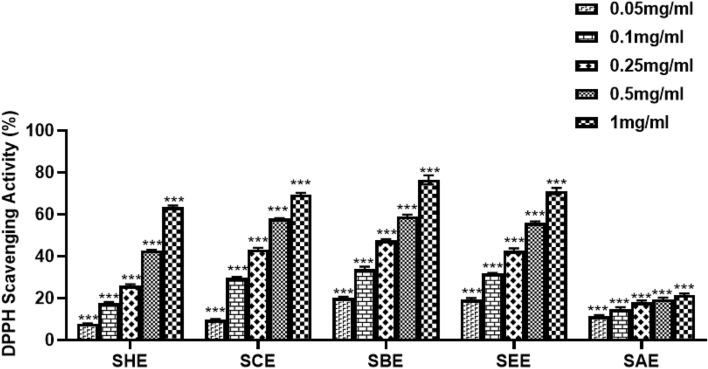
DPPH free radical scavenging activity
DPPH solution helps in evaluating the electron-donating ability of natural products. These herbal compounds works by decolorizing the DPPH solution as the change in color depicts their anti-oxidant activity. In the current study, different sub-fractions were evaluated for their anti-oxidant potential by the DPPH method at various concentrations (0.05, 0.1, 0.25, 0.5 and 1 mg/ml). Ascorbic acid was used as a positive control. The n-butanol fraction showed 47% scavenging activity at a concentration of 0.25 mg/ml whereas ascorbic acid at the same concentration showed 79.5% scavenging. The n-butanol sub-fraction was observed to exhibit maximum scavenging activity among all the tested sub-fractions. The activity of the n-hexane fraction and ascorbic acid was comparable at a concentration of 1 mg/ml and 0.05 mg/ml, respectively. A similar pattern of results was observed for the remaining sub-fractions. The chloroform extract showed 70% scavenging at 1 mg/ml whereas ascorbic acid showed 74.2% scavenging at a concentration of 0.1 mg/ml. It was observed that the DPPH free radical scavenging activity of all the tested sub-fractions increased with an increase in concentration. Ethyl acetate fraction showed a linear increase in scavenging activity with concentration and exhibited 56.4% scavenging at 0.5 mg/ml. The aqueous extract was the least active among all the tested sub-fractions as shown in Fig. 2.
Fig. 2.
The percentage radical scavenging activity of various sub-fractions of SPE as assessed by DPPH assay at 517 nm. A dose-dependent effect was observed in all sub-fractions of SPE. The butanol extract showed the best activity among all the fractions tested. SHE Saffron hexane extract, SCE Saffron chloroform extract, SEE Saffron ethyl acetate extract, SBE Saffron butanol extract, SAE Saffron aqueous extract
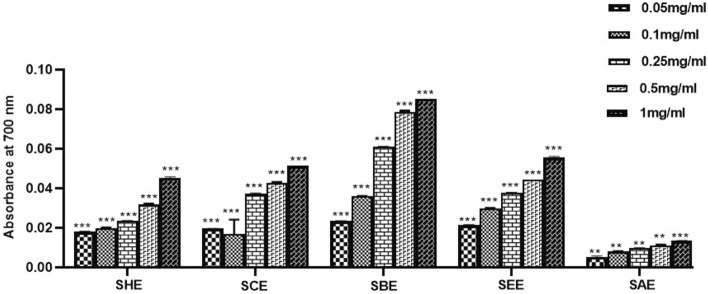
FRAP assay
The ferric-reducing anti-oxidant power assay measures the anti-oxidant ability to reduce Fe3+ to Fe2+ ions. The reducing ability of all the tested components was analyzed by plotting a graph between the concentrations v/s absorbance. An increase in absorbance is directly proportional to the anti-oxidant activity of the tested components. The results demonstrated that sub-fractions of SPE possess anti-oxidant potential but n-butanol extract showed the highest reducing capacity among all the tested sub-fractions (Fig. 3).
Fig. 3.
The ferric ion reducing potential of various sub-fractions of SPE as evaluated by FRAP assay at 700 nm. A dose-dependent effect was observed in all sub-fractions of SPE. The butanol extract showed the best activity among all the fractions tested. SHE Saffron hexane extract, SCE Saffron chloroform extract, SEE Saffron ethyl acetate extract, SBE Saffron butanol extract, SAE Saffron aqueous extract
In vitro α-amylase inhibitory assay
The hepatic α-amylase is a key enzyme responsible for the digestion of starch and carbohydrates that causes blood glucose to rise (Peyrot et al. 2016). Due to elevated levels of blood glucose, the adipose tissues in the liver produce inflammation, liver cirrhosis, fibrosis and further contribute to hepatocarcinogenesis (Sun and Karin 2012). The inhibition of this enzyme is recommended as a strategy to treat obesity and associated diseases including cancer. In the present study, we evaluated the effect of sub-fractions of SPE on the inhibition of α-amylase. The result demonstrated that the n-butanol fraction showed the highest inhibitory enzymatic activity (92.5%) whereas the aqueous sub-fraction showed the lowest percentage inhibition (13.9%). Additionally, the standard drug acarbose showed a percent inhibition of 84.2%. The percentage inhibition of each sub-fractions of SPE is shown in Fig. 4.
Fig. 4.
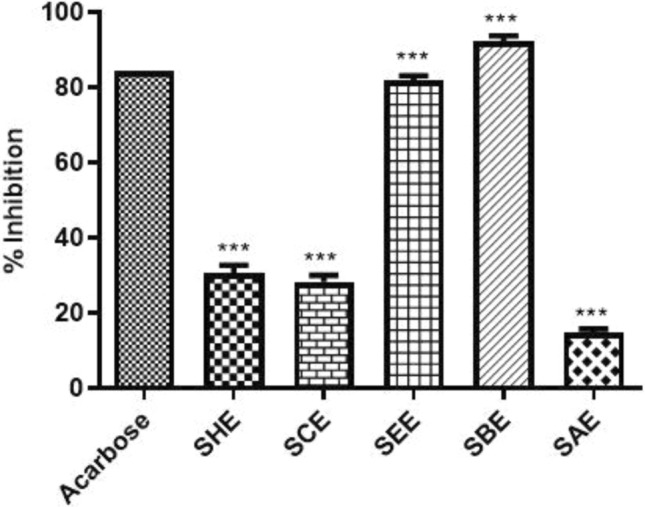
Inhibitory effects of different sub-fractions of SPE and Acarbose (standard) at 10 mg/ml concentration against α-amylase enzyme. The n-butanol fraction showed the best enzymatic percentage inhibition among all the compounds tested including standard. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the standard. All data are representative of 3 independent experiments (Mean ± SD). SHE Saffron hexane extract, SCE Saffron chloroform extract, SEE Saffron ethyl acetate extract, SBE Saffron butanol extract, SAE Saffron aqueous extract
In vitro pancreatic lipase inhibitory assay
Pancreatic lipase is an enzyme secreted by the pancreas that contributes in the digestion of dietary triglycerides (Paul et al. 2022). It has been documented that higher levels of triglycerides are associated with liver cancer (Kim et al. 2016). Therefore, in the present study, we investigated the inhibitory effect of the different sub-fractions of SPE on the pancreatic lipase enzyme. The results showed that the n-butanol fraction inhibited the lipase activity by 78% whereas the aqueous sub-fraction appeared to possess weak inhibition power as the inhibitory percentage was found to be 22%. The percentage inhibition of the remaining sub-fractions i.e., chloroform, n-hexane and ethyl acetate was 49%, 38% and 36%, respectively. The n-butanol fraction showed better inhibitory potential than orlistat which was 74.1% as depicted in Fig. 5. Other researchers have also shown inhibitory lipase activity by the extract of Ephedra altissima (Bouafia et al. 2022), Monotheca buxifolia (Ali et al. 2022), fermented and non-fermented oats (Cai et al. 2012).
Fig. 5.
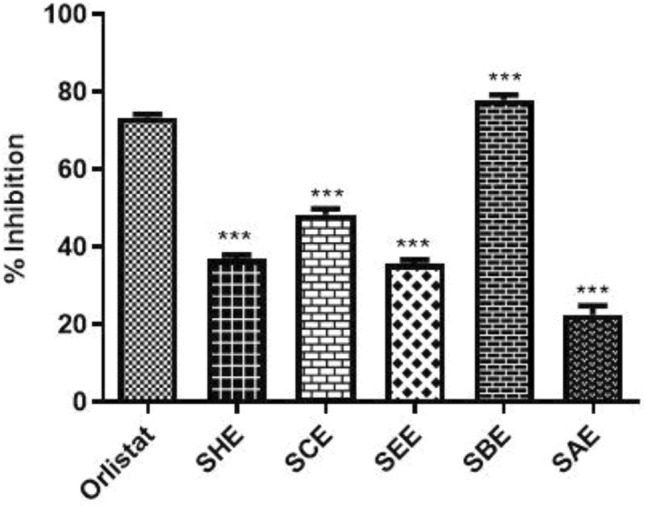
Inhibitory effect of different sub-fractions of SPE and Orlistat (standard drug) against pancreatic lipase enzyme. The n-butanol fraction exerted a maximum inhibitory effect on the lipase enzyme whereas the aqueous sub-fraction appeared to show weak inhibition. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the standard. All data are representative of 3 independent experiments (Mean ± SD). SHE Saffron hexane extract, SCE Saffron chloroform extract, SEE Saffron ethyl acetate extract, SBE Saffron butanol extract, SAE Saffron aqueous extract
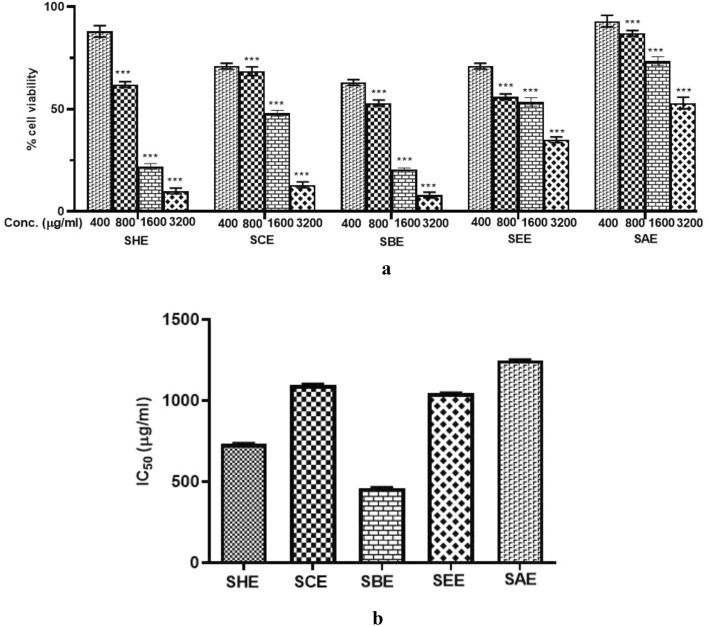
Cytotoxic potential of sub-fractions of SPE
An effective anti-cancer therapy should kill the tumor cells without affecting the healthy ones. In this study, the IC50 value of sub-fractions of SPE was evaluated using MTT assay. Huh-7 cells were treated with different concentrations (400–3200 µg/ml) of each sub-fraction for 24 h to assess their effect on cancer cell growth. A significant dose-dependent reduction was observed in the viability of liver cancer cells as compared to the vehicle-treated cells. The IC50 values observed are shown in Fig. 6 and Table 5. Among all the sub-fractions tested, the lowest IC50 value was observed for the n-butanol extract of SPE, indicating a better cytotoxic profile. As, anti-cancer activity is also mediated by the anti-oxidant potential of herbal drugs, we can further confirm that the n-butanol fraction of SPE contains the most promising phytoconstituents responsible for its medicinal properties.
Fig. 6.
Effect of different sub-fractions on the viability of liver cancer cells. Huh-7 cells were incubated with different concentrations (400–3200 µg/ml) of sub-fractions at 24 h. a Sub-fractions of SPE exerted an inhibitory effect on the viability of Huh-7 cells, measured by MTT assay, b Bar graph showing the IC50 values of the different sub-fractions of SPE. All data are representative of 3 independent experiments. SHE Saffron hexane extract, SCE Saffron chloroform extract, SEE Saffron ethyl acetate extract, SBE Saffron butanol extract, SAE Saffron aqueous extract
Table 5.
IC50 values of sub-fractions of SPE of human liver cancer cell line (Huh-7)
| S. No. | Name of the sub-fraction | IC50 value (µg/ml) |
|---|---|---|
| 1 | n-Hexane | 733.9 |
| 2 | Chloroform | 1088 |
| 3 | Butanol | 462.8 |
| 4 | Ethyl acetate | 1043 |
| 5 | Aqueous | 1245 |
Conclusions
The current research compared various sub-fractions of SPE based on their physicochemical parameters and anti-oxidant potential. It was found that n-butanol extract contained the maximum number of phytochemicals and the highest phenolic and flavonoid content indicating its anti-oxidant potential. Additionally, it showed the best cytotoxic activity against liver cancer cells among all the compounds tested. Therefore, the n-butanol sub-fraction of SPE could be seen as a potential candidate in the management of various diseases including cancer. In order to further investigate the molecular mechanism behind the anti-cancer and anti-obesity potential of the extract, the identification of phytoconstituents should be carried out through various chemical identification techniques like MS, HPLC and FTIR.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization: NS, SB, and MS; methodology: NS, MS, and SB; investigation and data curation: NS and MG; formal analysis: NS and MG; project administration: MS, SB, and SA; resources: MS, SB, and GN; writing—original draft: NS; writing—review and editing: NS, MS, and MG.
Funding
None.
Declarations
Conflict of interest
The author declares no conflict of interest in the publication.
Contributor Information
Nidhi Sharma, Email: nidhi13995@gmail.com, https://scholar.google.com/citations?view_op=list_works&hl=en&authuser=2&user=UA44pjMAAAAJ.
Meenakshi Gupta, Email: meenakshigupta231294@gmail.com, https://www.researchgate.net/profile/Meenakshi-Gupta-13.
Gowher Nabi, Email: Gowher.nabi@gmail.com, https://www.researchgate.net/profile/Gowher-Nabi.
Subhrajit Biswas, Email: sbiswas2@amity.edu, https://www.researchgate.net/scientific-contributions/Subhrajit-Biswas-2195155281.
Sher Ali, Email: profsali55@gmail.com.
Maryam Sarwat, Email: msarwat@amity.edu, Email: maryam21_7@yahoo.com, https://www.researchgate.net/profile/Maryam-Sarwat.
References
- Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan MA, Sethi G. Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules. 2019;13:735–761. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal K, Madan S, Sarwat M. Herbal Medicines. Amsterdam: Elsevier; 2022. Traditional nutritional and health practices to tackle the lifestyle diseases; pp. 253–268. [Google Scholar]
- Ahmad P, Sarwat M, Sharma S. Reactive oxygen species, anti-oxidants and signaling in plants. J Plant Biol. 2008;51:167–173. doi: 10.1007/bf03030694. [DOI] [Google Scholar]
- Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol. 2013;92:229–236. doi: 10.1016/j.ejcb.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Ali JS, Riaz N, Mannan A, Tabassum S, Zia M. Antioxidative, antimicrobial, enzyme inhibition, and cytotoxicity-based fractionation and isolation of active components from Monotheca buxifolia (Falc.) A. DC. stem extracts. ACS Omega. 2022;7:3407–3423. doi: 10.1021/acsomega.1c05647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society (2022) Epidimiology of liver cancer. https://www.cancer.org/cancer/liver-cancer/about/what-is-key-statistics.html. Assessed 5 Mar 2023
- Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314–188338. doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens G, Gredner T, Stock C, Leitzmann MF, Brenner H, Mons U. Cancers due to excess weight, low physical activity, and unhealthy diet: estimation of the attributable cancer burden in Germany. Dtsch Arztebl Int. 2018;115:578–585. doi: 10.3238/arztebl.2018.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouafia W, Hamdi A, Mouffouk S, Arcos RR, Araujo AJ, Bejarano RG, Haba H. Phenolic composition, in vitro alpha-amylase and pancreatic lipase inhibitory effects, anti-inflammatory and anti-oxidant activities of Ephedra altissima. Indian J Pharm Sci. 2022;84:890–901. doi: 10.36468/pharmaceutical-sciences.984. [DOI] [Google Scholar]
- Cai S, Wang O, Wang M, He J, Wang Y, Zhang D, Zhou F, Ji B. In vitro inhibitory effect on pancreatic lipase activity of subfractions from ethanol extracts of fermented oats (Avena sativa L.) and synergistic effect of three phenolic acids. J Agric Food Chem. 2012;60:7245–7251. doi: 10.1021/jf3009958. [DOI] [PubMed] [Google Scholar]
- Chen K, Wang XM, Chen F, Bai J. In vitro antimicrobial and free radical scavenging activities of the total flavonoid in petal and stamen of Crocus sativus. Indian J Pharm Sci. 2017;79:482–487. doi: 10.4172/pharmaceutical-sciences.1000254. [DOI] [Google Scholar]
- Dirar AI, Alsaadi DH, Wada M, Mohamed MA, Watanabe T, Devkota HP. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S Afr J Bot. 2019;120:261–267. doi: 10.1016/j.sajb.2018.07.003. [DOI] [Google Scholar]
- Ezealigo US, Joshua PE, Ononiwu CP, Agbo MO, Asomadu RO, Ogugua VN. Total phenolic and flavonoid content and in vitro anti-oxidant activity of methanol extract and solvent fractions of Desmodium ramosissimum G. Don. MDPI. 2020;2:1–6. doi: 10.3390/CAHD2020-08594. [DOI] [Google Scholar]
- Govindarajan N, Chinnapillai A, Balasundaram M, Narasimhaji CV, Ganji K, Raju I. Pharmacognostical and phytochemical evaluation of a polyherbal ayurvedic formulation Trikatu churna. J Ayu Med Sci. 2016;1:1–8. doi: 10.5530/jams.2016.1.5. [DOI] [Google Scholar]
- Gupta M, Chandan K, Sarwat M. Role of microRNA and long non-coding RNA in hepatocellular carcinoma. Curr Pharm Des. 2020;26:415–428. doi: 10.2174/1381612826666200115093835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M, Chandan K, Sarwat M. Natural products and their derivatives as immune checkpoint inhibitors: targeting cytokine/chemokine signaling in cancer. Semin Cancer Biol. 2022;86:242–232. doi: 10.1016/j.semcancer.2022.06.009. [DOI] [PubMed] [Google Scholar]
- Gupta M, Yadav V, Sarwat M. Pharmacological importance of the active molecule “guggulsterone” in overall human health. Her Med. 2022 doi: 10.1016/B978-0-323-90572-5.00016-0. [DOI] [Google Scholar]
- Gupta M, Sumaiya S, Ali S, Naved T, Sharma A, Ahmad A, Sikander M, Sarwat M. Pharmacognostical and phytochemical evaluation of an unani polyherbal formulation: Dawa ul Kurkum by HPTLC. Separations. 2023;10:89–100. doi: 10.3390/separations10020089. [DOI] [Google Scholar]
- Hosseini A, Razavi BM, Hosseinzadeh H. Saffron (Crocus sativus) petal as a new pharmacological target: a review. Iran J Basic Med Sci. 2018;21:1091–1099. doi: 10.22038/IJBMS.2018.31243.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Ma H, Fan P, Gao R, Jia Z. Anti-oxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement Altern Med. 2015;15:1–2. doi: 10.1186/s12906-015-0820-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Samota MK, Choudhary M, Choudhary M, Pandey AK, Sharma A, Thakur J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol Mol Biol. 2022;28:485–504. doi: 10.1007/s12298-022-01146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GN, Shin MR, Shin SH, Lee AR, Lee JY, Seo BI, Kim MY, Kim TH, Noh JS, Rhee MH, Roh SS. Study of antiobesity effect through inhibition of pancreatic lipase activity of Diospyros kaki fruit and Citrus unshiu peel. Biomed Res Int. 2016;2016:1–7. doi: 10.1155/2016/1723042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kumar M, Jose A, Tomer V, Oz E, Proestos C, Zeng M, Elobeid T, Oz F. Major phytochemicals: recent advances in health benefits and extraction method. Molecules. 2023;28:887–928. doi: 10.3390/molecules28020887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachguer K, El Merzougui S, Boudadi I, Laktib A, Ben El Caid M, Ramdan B, Boubaker H, Serghini MA. Major phytochemical compounds, in vitro anti-oxidant, antibacterial, and antifungal activities of six aqueous and organic extracts of Crocus sativus L. flower waste. Waste Biomass Valorization. 2022;17:1–7. doi: 10.1007/s12649-022-01964-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid N, Nissar S, Raja WY, Nawchoo IA, Bhat ZA. Pharmacognostic standardization of Aralia cachemirica: a comparative study. Future J Pharm Sci. 2016;7:1–8. doi: 10.1186/s43094-021-00181-y. [DOI] [Google Scholar]
- Milajerdi A, Djafarian K, Hosseini B. The toxicity of saffron (Crocus sativus L.) and its constituents against normal and cancer cells. J Nutr Intermed Metab. 2016;3:23–32. doi: 10.1016/j.jnim.2015.12.332. [DOI] [Google Scholar]
- Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N (2009) Oxidative stress and ischemic myocardial syndromes. Med Sci Monit 15:209–219. http://www.medscimonit.com/fulltxt.php?ICID=878204 [PubMed]
- Mohamadpour M, Shahmir P, Asadi M, Asadi S, Amraei M. Investigating the effect of saffron petal extract on anti-oxidant activity and inflammatory markers in hypercholesterolemic rats. Entomol Appl Sci Lett. 2020;7:13–22. [Google Scholar]
- Mohaqiq Z, Moossavi M, Hemmati M, Kazemi T, Mehrpour O. Anti-oxidant properties of saffron stigma and petals: a potential therapeutic approach for insulin resistance through an insulin-sensitizing adipocytokine in high-calorie diet rats. Int J Prev Med. 2020;184:11–17. doi: 10.4103/ijpvm.IJPVM_275_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfegh F, Balanejad SZ, Shahrokhabady K, Attaranzadeh A. Crocus sativus (saffron) petals extract and its active ingredient, anthocyanin improves ovarian dysfunction, regulation of inflammatory genes and anti-oxidant factors in testosterone-induced PCOS mice. J Ethnopharmacol. 2022;282:114594–114606. doi: 10.1016/j.jep.2021.114594. [DOI] [PubMed] [Google Scholar]
- Namgyal D, Sarwat M. Saffron. Amsterdam: Elsevier; 2020. Saffron as a neuroprotective agent; pp. 93–102. [Google Scholar]
- Namgyal D, Ali S, Hussain MD, Kazi M, Ahmad A, Sarwat M. Curcumin ameliorates the Cd-induced anxiety-like behavior in mice by regulating oxidative stress and neuro-inflammatory proteins in the prefrontal cortex region of the brain. Anti-Oxidants. 2021;10:1710–1725. doi: 10.3390/antiox10111710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiedz CL, Knifton L, Robb KA, Katikireddi SV, Smith DJ. Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer. 2019;19:1–8. doi: 10.1186/s12885-019-6181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidi A, Riahinia N, Torbati MB, Behdani MA. Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicen J Phytomed. 2014;4:330–337. [PMC free article] [PubMed] [Google Scholar]
- Patel S, Sarwat M, Khan TH. Mechanism behind the anti-tumour potential of saffron (Crocus sativus L.): the molecular perspective. Crit Rev Oncol Hematol. 2017;115:27–35. doi: 10.1016/j.critrevonc.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Paul B, Lewinska M, Andersen JB. Lipid alterations in chronic liver disease and liver cancer. JHEP Reports. 2022;26:100479–1004807. doi: 10.1016/j.jhepr.2022.100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrot D, Gachons C, Breslin PA. Salivary amylase: digestion and metabolic syndrome. Curr Diab Rep. 2016;16:1–7. doi: 10.1007/s11892-016-0794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh Y, Sarkar D. Association of adipose tissue and adipokines with development of obesity-induced liver cancer. Int J Mol Sci. 2021;22:2163–2191. doi: 10.3390/ijms22042163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringelhan M, Pfister D, O’Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222–232. doi: 10.1038/s41590-018-0044-z. [DOI] [PubMed] [Google Scholar]
- Selvam AB. Standardization of organoleptic terminology with reference to description of vegetable crude drugs. Int J Pharm Tech. 2015;7:3282–3289. [Google Scholar]
- Sen S, Chakraborty R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: importance, challenges and future. J Tradit Complement Med. 2017;7:234–244. doi: 10.1016/j.jtcme.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra V, Salvatori G, Pastorelli G. Dietary polyphenol supplementation in food producing animals: effects on the quality of derived products. Animals. 2021;2:401–446. doi: 10.3390/ani11020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeri M, Tayer AH, Shakeri H, Jahromi AS, Moradzadeh M, Hojjat-Farsangi M. Toxicity of saffron extracts on cancer and normal cells: a review article. Asian Pac J Cancer Prev. 2020;21:1867–1875. doi: 10.31557/APJCP.2020.21.7.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Biswas S, Al-Dayan N, Alhegaili AS, Sarwat M. Anti-oxidant role of kaempferol in prevention of hepatocellular carcinoma. Anti-Oxidants. 2021;10:1419–1436. doi: 10.3390/antiox10091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniyan B, Polachi N, Mathan G. Isocoreopsin: an active constituent of n-butanol extract of Butea monosperma flowers against colorectal cancer (CRC) J Pharm Anal. 2016;6:318–325. doi: 10.1016/j.jpha.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumaiya S, Naved T, Sharma A, Sarwat M. Saffron: the age old panacea in new light. Amsterdam: Elsevier; 2020. Amelioration of liver ailments by saffron (Crocus sativus) and its secondary metabolites; pp. 1–20. [Google Scholar]
- Sumaiya S, Gupta M, Sharma A, Naved T, Akhtar J, Sarwat M (2023) A Comprehensive Review on Medicinal Properties of Dawa-ul-Kurkum and its Ingredients against Liver Ailments in the Light of Ancient Wisdom and Modern Research. In: Ethnic Knowledge on Biodiversity, Nutrition and Health Security. Taylor and Francis, Europe
- Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia R, Sarwat M (2020) A novel herbal composition for the treatment of obesity. Indian Patent. Patent Application No 201911014033
- Wallis TE. Text Book of Pharmacognosy. New Delhi, India: CBS Publishers and Distributors; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.