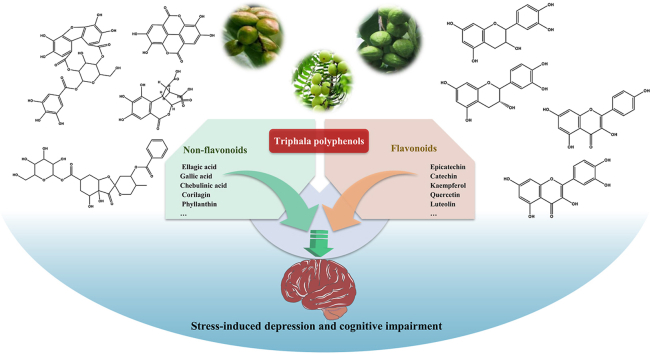
Abstract
In response to environmental challenges, stress is a common reaction, but dysregulation of the stress response can lead to neuropsychiatric disorders, including depression and cognitive impairment. Particularly, there is ample evidence that overexposure to mental stress can have lasting detrimental consequences for psychological health, cognitive function, and ultimately well-being. In fact, some individuals are resilient to the same stressor. A major benefit of enhancing stress resilience in at-risk groups is that it may help prevent the onset of stress-induced mental health problems. A potential therapeutic strategy for maintaining a healthy life is to address stress-induced health problems with botanicals or dietary supplements such as polyphenols. Triphala, also known as Zhe Busong decoction in Tibetan, is a well-recognized Ayurvedic polyherbal medicine comprising dried fruits from three different plant species. As a promising food-sourced phytotherapy, triphala polyphenols have been used throughout history to treat a variety of medical conditions, including brain health maintenance. Nevertheless, a comprehensive review is still lacking. Here, the primary objective of this review article is to provide an overview of the classification, safety, and pharmacokinetics of triphala polyphenols, as well as recommendations for the development of triphala polyphenols as a novel therapeutic strategy for promoting resilience in susceptible individuals. Additionally, we summarize recent advances demonstrating that triphala polyphenols are beneficial to cognitive and psychological resilience by regulating 5-hydroxytryptamine (5-HT) and brain-derived neurotrophic factor (BDNF) receptors, gut microbiota, and antioxidant-related signaling pathways. Overall, scientific exploration of triphala polyphenols is warranted to understand their therapeutic efficacy. In addition to providing novel insights into the mechanisms of triphala polyphenols for promoting stress resilience, blood brain barrier (BBB) permeability and systemic bioavailability of triphala polyphenols also need to be improved by the research community. Moreover, well-designed clinical trials are needed to increase the scientific validity of triphala polyphenols’ beneficial effects for preventing and treating cognitive impairment and psychological dysfunction.
Keywords: Triphala polyphenols, Resilience, Stress, Depression, Sleep deprivation, Cognitive impairment
Graphical abstract
Highlights
-
•
Triphala polyphenols, a promising phytotherapy for a variety of medical conditions, including brain health maintenance.
-
•
Triphala polyphenols promote resilience against stress-induced depression and cognitive impairment.
-
•
The mechanism is related to regulation of 5-HT and BDNF receptors, gut microbiota, and antioxidant signaling pathways.
1. Introduction
Changes in diet and lifestyle habits, as well as an increase in life expectancy, are contributing to an increase in chronic diseases, including psychiatric disorders, which have evolved into major global public health issues that seriously threaten human health and sustainable socioeconomic development (Sun et al., 2022). Especially, when chronic or improperly managed, stress is known to negatively affect people's health and well-being on a global scale (Cenat et al., 2021). The concept of resilience has been receiving increasing attention in recent years, the ability to maintain normal psychological and physical functioning and avoid serious mental illness despite extreme stress or trauma (Sher, 2019). However, evidence is mounting that overexposure to a wide range of stressors such as sleep deprivation induces a pathophysiological response in the body that may precipitate or worsen medical conditions of a wide variety (Lopresti, 2020). Indeed, 75%–90% of diseases are closely associated with chronic psychosocial stress, including depression, mood disorders, and a variety of other stress-related conditions (Liu et al., 2017). Resilience under stressful conditions is poorly understood (Unno et al., 2018), and it is imperative to identify and target the mechanisms that contribute to this resilience.
Intriguingly, natural medicines, especially traditional Chinese medicine, have long been used in clinical practice to prevent and treat myriad diseases such as neurological disorders (Sharma et al., 2022a, Sharma et al., 2022b). In addition, it is pertinent to note natural products or nutritional compounds that play an influential role in preventing or slowing cognitive decline, such as acetyl-L-carnitine and caffeine (Fisicaro et al., 2021; Pennisi et al., 2020). A recent study reported that daily consumption of mocha coffee was associated with better cognition and mood status in non-smokers subjects with mild vascular cognitive impairment (Fisicaro et al., 2022). Importantly, as a result of their extensive pharmacological effects, lower cost, higher bioavailability, and less toxicity, natural plant ingredients such as polyphenols are gaining increasing attention in the scientific community (Bernstein et al., 2021; Shah et al., 2021; W. Wang et al., 2023).
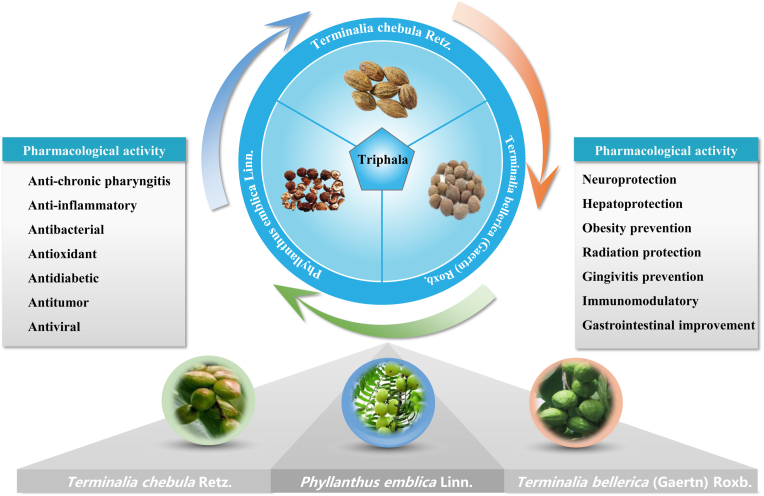
Triphala, also known as Zhe Busong decoction in Tibetan, is a well-recognized Ayurvedic polyherbal medicine comprising dried fruits from three different plant species in equal proportions, namely Phyllanthus emblica Linn. (also known as Emblica officinalis Gaertn. in the Ayurvedic pharmacopeia of India, Family Euphorbiaceae), Terminalia bellerica (Gaertn) Roxb. (Family Combretaceae), and Terminalia chebula Retz. (Family Combretaceae) (Peterson et al., 2017; Sharma et al, 2020; Sharma et al., 2019). Triphala originated in India, which was first recorded in the Ayurvedic text Charaka Samhita (X. Li et al., 2022). Thereafter, as a complementary and alternative medicine for chronic diseases, triphala was introduced to China via the Silk Road, and is now extensively used throughout East Asia as a complementary and alternative medicine (Tiwana et al., 2020). Nowadays, it is the most common basic prescription in Tibetan medicine. Moreover, triphala has been described by traditional healers as a powerful health tonic that purifies, rejuvenates, and balances the body's elements (Ahmed et al., 2021). As a multipurpose therapeutic drug, modern studies have revealed that triphala has many pharmacological activities, as shown in Fig. 1.
Fig. 1.
Overview of the composition and pharmacological action of triphala. Triphala is a well-recognized Ayurvedic polyherbal medicine that consists of dried fruits from three different plant species that possess many pharmacological activities, such as antioxidant, anti-inflammatory, and neuroprotective properties.
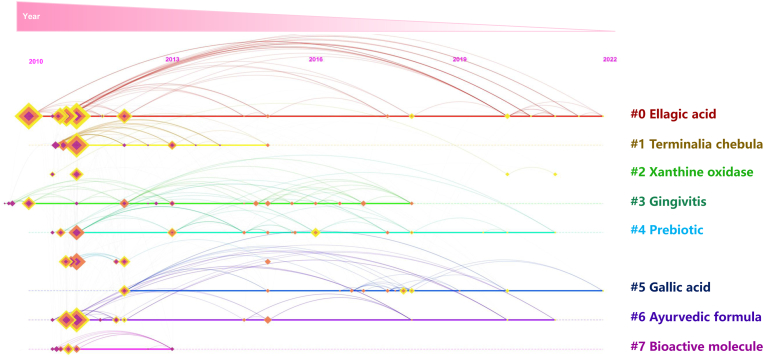
Among the many phytochemicals found in triphala are phenolic acids, ascorbic acid, flavonoids, alkaloids, and carbohydrates (Jantrapirom et al., 2021). In general, polyphenols are the main components of triphala. Interestingly, polyphenols have recently attracted major attention as one of the most attractive molecules for drug discovery and application in the pharmacological setting (Ullah et al., 2022; Wu et al., 2021). Furthermore, a growing number of scientific publications have been published about triphala in recent years. Researchers have been encouraged by the health benefits of triphala and their characteristic polyphenols for preventing diseases and promoting health, as evidenced by their publication record (Fig. 2). In spite of this, there has not been a comprehensive review of recent experimental studies and the mechanisms of action of triphala polyphenols toward the promotion of resilience against stress-induced depression and cognitive impairment. This review focuses on triphala, providing information on its classification, safety, pharmacokinetics, and health benefits, as well as providing a strategic framework for developing triphala polyphenols as therapeutic agents to therapeutically promote resilience and prevent psychological dysfunction and cognitive impairment.
Fig. 2.
Timeline and clustering view of triphala polyphenols, which visually shows the phased hotspots and development directions of research on triphala polyphenols from the time dimension.
2. Methodology
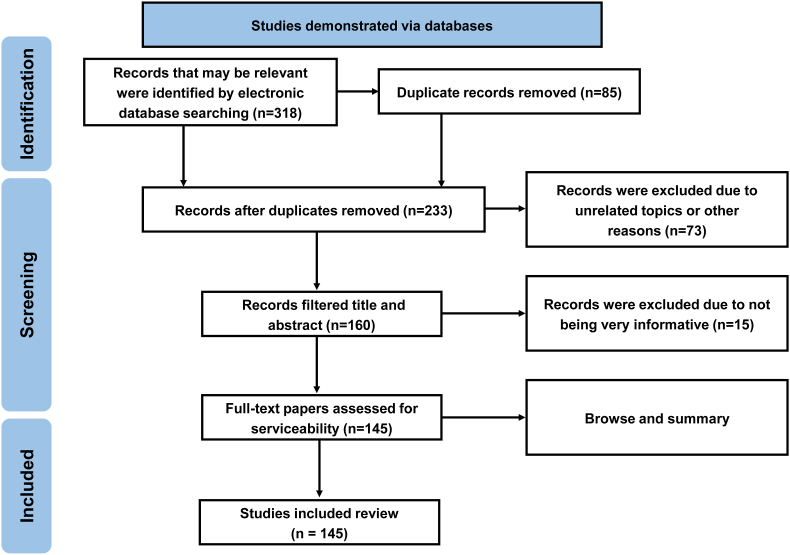
PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science (https://www.webofscience.com/), Embase (https://www.embase.com/), and Scopus (https://www.scopus.com/) databases were all used to perform this literature review. The following search terms were entered in data collection: triphala, polyphenols, flavonoids, non-flavonoids, tannins, phenolic acids, aromatic acids, resilience, stress, depression, cognitive impairment, pharmacological action, and molecular mechanism. In most cases, English-published research papers and original full-text articles were selected and analyzed until December 2022. As shown in Fig. 3, all steps involved in selecting the required literature were outlined in the flowchart.
Fig. 3.
The stages of selecting data for inclusion in existing research are illustrated in a flow chart (n = number of literature reports).
3. Safety of triphala polyphenols
Triphala is composed of three medicinal and food homologous herbs that have been extensively tested in animal experiments and clinical trials and have shown no adverse effects. In addition, polyphenols, the main components of triphala, are also recognized as safe natural ingredient agents. Acute and subacute oral toxicity studies of triphala on rats were conducted by some scholars, and the maximum tolerated dosage of 5000 mg/kg body weight was found to be safe, and importantly, this group conducted a clinical trial and indicated that a dose of 2500 mg/d of triphala for 28 d was considered to be safe when administered to 10 male and 10 female healthy volunteers (Arpornchayanon et al., 2022). Additionally, another clinical trial evaluating triphala ethanolic extract found no adverse effects in healthy volunteers taking 1050 mg/d orally for 14 d (Phetkate et al., 2012). All of the evidence suggested that the major polyphenols in triphala, like corilagin and ellagic acid, exert almost no toxicity on normal tissues. Notably, the amounts of ellagic acid in foods have been accepted as existing food additives in Japan, and it has primarily been accepted as an antioxidant with no toxicological data or limitations on its concentration utilization (W. Wang et al., 2022).
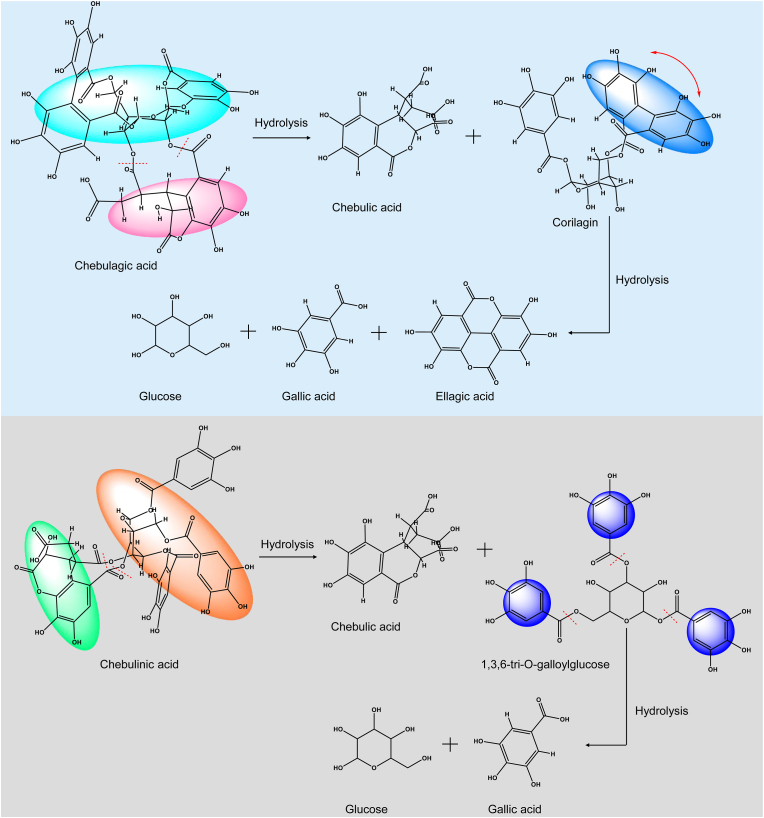
Industrially, triphala is processed using reflux extraction in accordance with current pharmaceutical production standards. Furthermore, it is well known that the types of preparation methods for triphala polyphenols affect the polyphenol composition. Particularly, the total polyphenol amounts may be affected by the extraction temperature. Triphala and related plants have previously been extracted using common hot and cold extraction methods, but the extraction temperature affects the chemical composition of the plant (Ran et al., 2021). Actually, macromolecular substances like chebulagic and chebulinic acid could be hydrolyzed into certain small molecules like gallic acid at higher temperatures (Fig. 4). For instance, heat- and reflux-mediated extractions of the Phyllanthus emblica Linn. component of triphala showed hydrolytic tannin conversion, such as gallic acid, suggesting that different polyphenols may be produced by different extraction techniques. Remarkably, a relatively low concentration of gallic acid is safe and effective for most cells, whereas a relatively high concentration is toxic (Borchers and Pieler, 2010; Tanaka et al., 2020). Therefore, although the existence of various preparation processes, the preferred extraction method should be selected based on the amounts of polyphenols to meet the consistency requirements and ensure the safety of triphala polyphenols.
Fig. 4.
Schematic diagram of the hydrolysis of macromolecular substances like chebulagic acid and chebulinic acid into certain small molecules like gallic acid.
4. Chemical properties and classification of triphala polyphenols
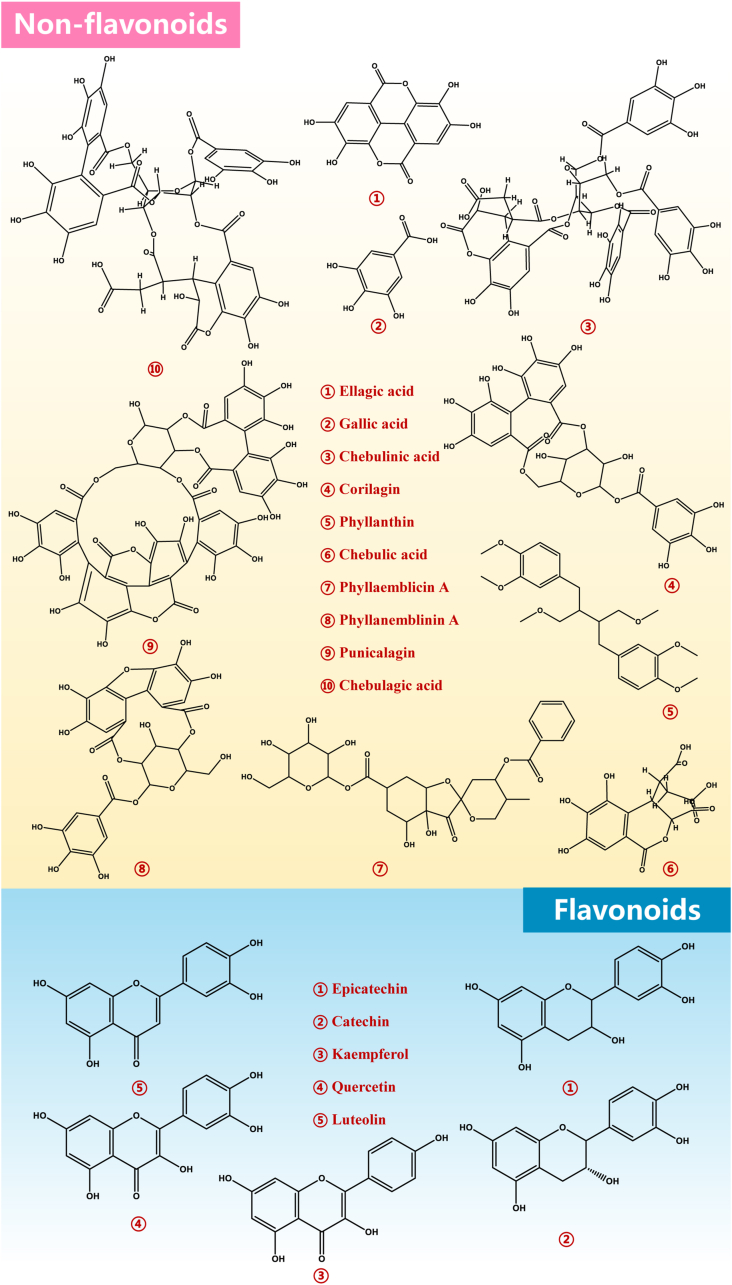
Triphala is rich in nutritious and functional ingredients. Particularly, many research groups, including our laboratory, focused on the main bioactive chemical components in triphala and some characteristic polyphenols were identified (Table 1). Several polyphenols were demonstrated in triphala, mainly including flavonoids, tannins, phenolic acids and glycosides (W. Wang et al., 2019). Additionally, other saponins, aromatic acids and carbohydrates, amino acids, and fatty acids are also abundant in triphala. In fact, polyphenols can conveniently be categorized as non-flavonoids and flavonoids based on the fact that they are characterized by at least two benzene rings and one or more hydroxyl substituents. Hence, non-flavonoids in triphala are tannins, phenolic acids, and aromatic acids. Some representative components of polyphenols in triphala are shown in Fig. 5. Among these triphala polyphenols, several of these compounds are widely distributed throughout the plant kingdom and have been reported to possess anti-oxidant, anti-apoptosis, anti-inflammatory, and neuroprotective properties (Oesterle et al., 2021).
Table 1.
Physicochemical and pharmacokinetic parameters of triphala polyphenols. The information is from TCMSP (https://old.tcmsp-e.com/tcmsp.php). Adapted from (Ru et al., 2014).
| Molecule Name | MW | AlogP | Hydrogen bond donor (Hdon) | Hydrogen bond acceptor (Hacc) | Oral bioavailability (OB, %) | Caco-2 permeability (Caco-2) | Blood-brain barrier (BBB) | Drug-likeness (DL) |
|---|---|---|---|---|---|---|---|---|
| Phyllanemblinin A | 616.47 | 1.42 | 9 | 17 | 72.44 | −2.28 | −3.14 | 0.33 |
| Chebulic acid | 356.26 | −0.26 | 6 | 11 | 72 | −1.4 | −1.75 | 0.32 |
| Quinic acid | 191.18 | −3.07 | 4 | 6 | 55.92 | −1.79 | −4.38 | 0.06 |
| (+)-catechin | 290.29 | 1.92 | 5 | 6 | 54.83 | −0.03 | −0.73 | 0.24 |
| Sennoside E | 524.5 | 3.91 | 6 | 9 | 50.69 | −0.74 | −1.56 | 0.61 |
| Quercetin | 302.25 | 1.5 | 5 | 7 | 46.43 | 0.05 | −0.77 | 0.28 |
| Phyllaemblicin A | 582.61 | −1.19 | 6 | 14 | 45.63 | −2.37 | −2.62 | 0.77 |
| Ellagic acid | 302.2 | 1.48 | 4 | 8 | 43.06 | −0.44 | −1.41 | 0.43 |
| Kaempferol | 286.25 | 1.77 | 4 | 6 | 41.88 | 0.26 | −0.55 | 0.24 |
| Teresautalic acid | 166.24 | 1.42 | 1 | 2 | 41.42 | 0.94 | 1.26 | 0.09 |
| Emblicanin B | 780.53 | 1.89 | 12 | 22 | 37.08 | −2.22 | −3.6 | 0.07 |
| β-sitosterol | 414.79 | 8.08 | 1 | 1 | 36.91 | 1.32 | 0.99 | 0.75 |
| Luteolin | 286.25 | 2.07 | 4 | 6 | 36.16 | 0.19 | −0.84 | 0.25 |
| Chebulinic acid | 956.72 | 1.83 | 13 | 27 | 33.48 | −3.24 | −4.28 | 0.13 |
| Phyllanthin | 418.58 | 4.11 | 0 | 6 | 33.31 | 1.06 | 0.57 | 0.42 |
| Gallic acid | 170.13 | 0.63 | 4 | 5 | 31.69 | −0.09 | −0.54 | 0.04 |
| (−)-epicatechin | 290.29 | 1.92 | 5 | 6 | 28.93 | −0.03 | −0.58 | 0.24 |
| Brevifolin | 196.22 | 1.27 | 1 | 4 | 27.19 | 0.82 | 0.77 | 0.06 |
| Progallin A | 198.19 | 1.23 | 3 | 5 | 25.61 | 0.33 | 0.11 | 0.06 |
| Epigallocatechin | 306.29 | 1.65 | 6 | 7 | 24.18 | −0.22 | −0.82 | 0.27 |
| Arjunolic acid | 488.78 | 4.36 | 4 | 5 | 23.22 | −0.32 | −1.01 | 0.72 |
| Phyllanemblinin D | 518.42 | −2.16 | 9 | 16 | 21.95 | −3.09 | −3.64 | 0.69 |
| Myristic acid | 228.42 | 5.46 | 1 | 2 | 21.18 | 1.07 | 0.99 | 0.07 |
| Palmitic acid | 256.48 | 6.37 | 1 | 2 | 19.3 | 1.09 | 1 | 0.1 |
| Punicalagin | 1084.75 | 3 | 17 | 30 | 18.17 | −3.44 | −4.46 | 0 |
| Maslinic acid | 472.78 | 5.46 | 3 | 4 | 15.54 | 0.1 | −0.55 | 0.74 |
| Phyllanemblinin E | 518.42 | −2.16 | 9 | 16 | 14.94 | −3.01 | −3.84 | 0.69 |
| Melissic acid | 452.9 | 12.75 | 1 | 2 | 13.22 | 1.31 | 0.9 | 0.49 |
| Emblicanin A | 782.55 | 2.21 | 12 | 22 | 12.36 | −2.4 | −3.17 | 0.22 |
| Phyllanemblinin C | 1254.94 | 1.17 | 19 | 36 | 12.08 | −4.34 | −4.98 | 0.04 |
| Phyllaemblicin B | 744.77 | −2.94 | 9 | 19 | 9.3 | −3.45 | −3.77 | 0.35 |
| Phyllanemblinin F | 518.42 | −2.16 | 9 | 16 | 4.11 | −2.94 | −3.51 | 0.69 |
| Sennoside C | 848.82 | 0.1 | 12 | 19 | 3.99 | −3.54 | −4.8 | 0.09 |
| Phyllaemblicin C | 876.9 | −4.18 | 11 | 23 | 3.04 | −4.48 | −5.03 | 0.16 |
| Punicafolin | 938.7 | 3.38 | 15 | 26 | 3.01 | −2.85 | −3.91 | 0.12 |
| Phyllanemblinin B | 634.49 | 0.9 | 11 | 18 | 3.01 | −2.36 | −3.31 | 0.47 |
| Pentagalloylglucose | 940.72 | 3.69 | 15 | 26 | 3.01 | −3.08 | −4.17 | 0.21 |
| Corilagin | 634.49 | 0.9 | 11 | 18 | 3.01 | −1.79 | −2.58 | 0.44 |
| Chebulagic acid | 954.7 | 1.52 | 13 | 27 | 3.01 | −3.79 | −4.44 | 0.03 |
| Gallocatechin | 306.29 | 1.65 | 6 | 7 | 2.26 | −0.27 | −1.11 | 0.27 |
Fig. 5.
Classification and some representative components of triphala polyphenols. Triphala polyphenols are primarily divided into flavonoids and non-flavonoids. Some representative components are shown in the figure.
5. Pharmacokinetics of triphala polyphenols in the body
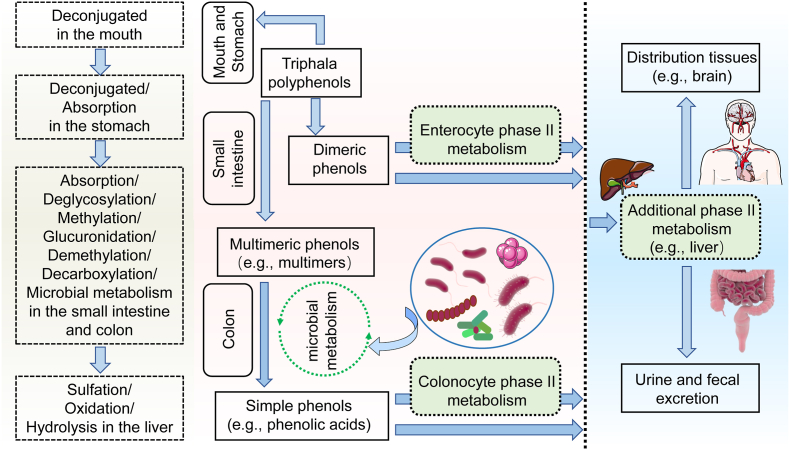
It is the chemical structure of triphala polyphenols that determines its pharmacokinetics, absorption capacity, and urinary excretion in humans. Generally, a small amount of digestion and absorption is carried out in the stomach after polyphenols are partially deconjugated in the mouth (Fig. 6) (Rajha et al., 2022). Meanwhile, an extensive amount of polyphenol is extensively metabolized in the intestine through deglycosylation, methylation, and glucuronidation etc. (Luca et al., 2020; Mithul Aravind, Wichienchot, Tsao, Ramakrishnan and Chakkaravarthi, 2021). Actually, a significant percentage of macromolecular polyphenols consumed cannot be absorbed by the upper gastrointestinal track (Luca et al., 2020). By contrast, these macromolecular polyphenols can be metabolized in the colon into phenolic metabolites and low molecular weight compounds by the gut microbiota (N. Zhou et al., 2020), which are more readily absorbed by the gastrointestinal epithelium (Fig. 6). Among the polyphenols in triphala, chebulagic acid, corilagin, and chebulinic acid monomers are relatively well-assimilated polyphenols, whereas a comparison is made between gallic acid and ellagic acid, which show poor absorption, low bioavailability, and easy saturation. There is evidence for chebulinic and chebulagic acids being present in tissues that are abundant in blood (Cmax, peak concentration) is 605.8 ± 35.6 ng/mL and 1327.1 ± 118.6 ng/mL, respectively), and the kidney had the highest concentrations (chebulinic acid, 462.6 ± 138.5 ng/g and chebulagic acid, 1651.7 ± 167.7 ng/g), followed by the heart, liver, spleen and lung (Lu et al., 2019). Furthermore, the gastrointestinal tract absorbs a significant amount of gallic acid, which is then distributed primarily in the kidneys, followed by other organs (X. Chen et al., 2018). An LC-MS/MS study on plasma pharmacokinetics of oral gallic acid in rats found that the parameters of the mean time to peak concentration (Tmax, peak time), Cmax, and terminal elimination half-life (T1/2) are 1.5 h, 0.83 μg/mL, and 2.56 h, respectively (Yu et al., 2018). There is also evidence that the gut microbiome modulates the bioconversion and bioavailability of bioactive phenolic metabolites obtained from oral consumption of polyphenol-rich botanical supplements, particularly phenolic acids such as ellagic acid (D'Amico et al., 2021). There is a very small amount of ellagic acid in the peripheral tissues and systemic circulation, and the Cmax value ranges from 0.1 μmol/L to 0.4 μmol/L, whereas its metabolites urolithins and conjugates can reach concentrations that are as high as micromolar in the human body (Gupta et al., 2021). Additionally, a metabolic hydrolysis of macromolecules ellagitannin-rich triphala like punicalagin in the gastrointestinal tract could result in the release of free ellagic acid that can either be absorbed in the body or undergo a massive metabolic process to produce urolithins. It should also be mentioned that the blood brain barrier (BBB) prevents many potential therapeutic macromolecular agents from entering the brain, and is a major obstacle to drug delivery into the brain (Mignani et al., 2021). But happily, certain small molecules of triphala polyphenols like epicatechin can cross the BBB and reach the central nervous system (Shimazu et al., 2021). Detailed pharmacokinetics of triphala polyphenols information such as oral bioavailability, drug-likeness, intestinal epithelial permeability was obtained from Traditional Chinese Medicine Systems Pharmacology (TCMSP) (https://old.tcmsp-e.com/tcmsp.php) (Ru et al., 2014), as outlined in Table 1.
Fig. 6.
Digestion and absorption of triphala polyphenols in the human body.
6. Triphala polyphenols toward the promotion of resilience against stress-induced depression
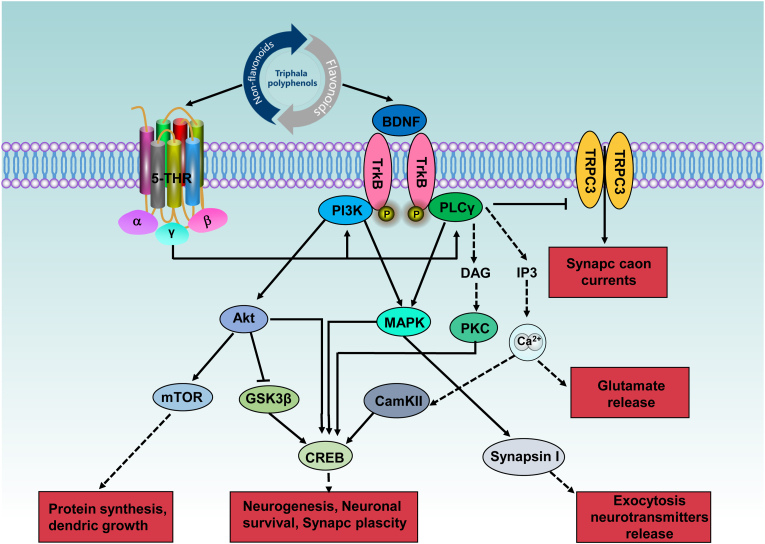
As one of the major mental disorders, depression is characterized by a triad of symptoms, including low mood, anhedonia, and low energy levels (McCarron et al., 2021). There is no doubt that depression disorders are highly chronic illnesses that have a significant adverse impact on an individual's functioning and well-being as well as on their family (Monroe and Harkness, 2022). In addition, depression has become a public health concern due to its high prevalence, mortality, suicide rates, and recurrence rates, and it has become a significant economic burden on society as well (Disease, G. B. D. et al., 2018). The problem is that there is no effective proven strategy to prevent depression caused by stress. In fact, despite the fact that a large number of antidepressant drugs that act on the central monoaminergic system are available for the treatment of depression, in particular 5-hydroxytryptamine (5-HT) and noradrenergic synaptic neurotransmissions, such as benzodiazepines, and these drugs provide improvement in the clinical condition of patients, their slow onset of action and adverse effects are major concerns (Sharma et al., 2018). Henceforth, there is an urgent need for new, more selective therapeutics that target stress-induced depression's underlying mechanisms. Additionally, in view of the fact that stress-related anxiety and depression often coexist in patients (Dolan et al., 2022), it would be beneficial to develop drugs that act as both anti-depressants and anti-anxiety agents. Hence, the promotion of resilience against stress-induced depression and anxiety is a promising line of research using bioactive and bioavailable triphala polyphenols. As a matter of fact, there is compelling clinical evidence to suggest that polyphenols, such as luteolin, gallic acid, and quercetin, are effective in relieving and treating depression and anxiety in patients (Ferianec et al., 2020; Taliou et al., 2013). Exploration of the recent advances in the use of triphala polyphenols in stress-induced depression and anxiety has revealed several common mechanisms, including the receptors of 5-HT and brain-derived neurotrophic factor (BDNF), that alter neuronal and synaptic functions (Fig. 7, Table 2).
Fig. 7.
Triphala polyphenols improve stress-induced depression and anxiety by activating the receptors of 5-HT and BDNF, which in turn alter neuronal and synaptic functions. 5-HT, 5-hydroxytryptamine; Akt, protein kinase B; BDNF, brain-derived neurotrophic factor; CamKII, Calcium/Calmodulin Dependent Protein Kinase II; CREB, cAMP responsive element binding protein; DAG, diacylglycerol; GSK3β, glycogen synthase kinase 3β; 5-HTR, 5-hydroxytryptamine receptor; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide-3-kinase; PLCγ, phospholipase C γ; TrkB, tyrosine receptor kinase B; TRPC3, transient receptor potential canonical subfamily 3.
Table 2.
Animal behavioral tests representing triphala polyphenols toward the promotion of resilience against stress-induced depression.
| Triphala polyphenols | Animal model | Behavioral tests | Molecular mechanisms | Reference |
|---|---|---|---|---|
| Non-flavonoids | ||||
| Gallic acid | UCMS in male Swiss albino mice | FST and SPT | ↓MAO-A; ↓MDA; ↑CAT; ↓plasma corticosterone | Chhillar and Dhingra (2013) |
| Gallic acid | CRS in male BALB/c mice | EPM and OFT | ↓Serum and brain MDA; ↑total antioxidant capacity | Salehi et al. (2018) |
| Ellagic acid | UCMS in male C57BL/6 mice | SPT, TST, and FST | ↑BDNF; ↑5-HT; inflammatory cytokines levels (↓TNF-α, ↓IL-1β, ↑IL-10) | Huang et al. (2020) |
| Flavonoids | ||||
| Quercetin | UCMS in male Swiss albino mice | TST, FST, and OFT | ↑5 HT; ↑SOD; ↑GSH; ↓Glutamate; ↓TNF-α; ↓IL-6 | Khan et al. (2019) |
| Quercetin | UCMS in male Swiss albino mice | EPM, OFT, SPT, and PAST | ↓TBARS; ↓NO; ↑total | (V. Mehta et al., 2017) |
| Thiol; ↑CAT; ↓IL-6; ↓TNF-α; ↓IL-1β; ↓COX-2 | ||||
| Quercetin | UCMS in male Sprague-Dawley rats | SPT | antioxidant enzymes (↑Cu–Zn, ↑SOD,↑CAT,↑GPx); ↓MAO; ↑GSH; ↓TNF-α; ↓IL-1β | (Guan et al., 2021a, Guan et al., 2021b) |
| Quercetin | UCMS in male ICR mice | SPT, OFT, and TST | Promoting adult hippocampal neurogenesis via FoxG1/CREB/BDNF signaling pathway | (Z. X. Ma et al., 2021) |
| Quercetin | UCMS in male Swiss albino mice | TST, FST, and PAST | Modulating hippocampal insulin | (V. Mehta et al., 2017) |
| signaling and neurogenesis; | ||||
| ↓insulin; ↓IR; ↑GLUT-4; ↑DCX | ||||
| Quercetin | Immobilization stress in male Swiss albino mice | Light-dark activity test, EPM, FST, MWM | Antioxidant enzymes (↑SOD,↑CAT,↑GPx); ↓AChE; ↑Ach; | Samad et al. (2018) |
| ↑5-HT; ↑5-HIAA | ||||
| Quercetin | UCMS in male Kunming mice | OFT, SPT, and FST | Upregulation of hippocampal PI3K/Akt/Nrf2/HO-1 signaling pathway | (Guan et al., 2021a, Guan et al., 2021b) |
| Quercetin | CSDS in male mice | OFT, SPT, FST, and EPM | Inhibiting astrocyte reactivation | (J. Zhang et al., 2020) |
| Luteolin | Noise in male Kunming mice | SPT, OFT, TST, and FST | Improving neuroinflammation and synaptic Plasticity Impairments | Cheng et al. (2022) |
| Kaempferol | Contextual fear conditioning in Wistar rats | EPM | Inhibition of fatty-acid amide hydrolase | Ahmad et al. (2020) |
| Epicatechin | Chronic mild stress in male C57BL/6 mice | SPT and OFT | ↑Muscle kynurenine aminotransferases | Martinez-Damas et al. (2021) |
| Epicatechin | Stress in male C57BL/6 mice | OFT and EPM | ↑Hippocampal monoamine and BDNF | Stringer et al. (2015) |
| Catechin | UCMS in male Sprague Dawley rats | SPT and FST | ↑CAT; ↑GSH; ↑SOD | Rai et al. (2019) |
5-HIAA, 5-hydroxyindole acetic acid; 5-HT, 5-hydroxytryptamine; ACh, acetylcholine; AChE, acetylcholine esterase; Akt, protein kinase B; BDNF, brain-derived neurotrophic factor; CAT, catalase; CRS, chronic restraint stress; CSDS, chronic social defeat stress; COX-2, cyclooxygenase-2; CREB, cAMP responsive element binding protein; DCX, doublecortin; EPM, elevated plus maze; FST, forced swim test; FoxG1, forkhead box G1; GLUT-4, glucose transporter 4; GPx, glutathione peroxidase; GSH, glutathione; HO-1, heme oxygenase 1; IL-10, interleukin-10; IL-1β, interleukin-1β; IL-6, interleukin-6; IR, insulin receptor; MAO, monoamine oxidase; MAO-A, monoamine oxidase-A; MDA, malondialdehyde; MWM, Morris water maze; NO, nitric oxide; Nrf2, nuclear factor-E2-related factor 2; OFT, open filed test; PAST, passive avoidance step-through task; PI3K, phosphatidylinositol3-kinase; SPT, sucrose preference test; SOD, superoxide dismutase; TST, tail suspension test; TBARS, thiobarbituric acid-reactive substances; TNF-α, tumor necrosis factor α; UCMS, unpredictable chronic mild stress.
6.1. Non-flavonoids
Gallic acid is not only the most abundant in triphala, but also one of the most abundant phenolic acids in the plant kingdom (Ahmed et al., 2021; Bai et al., 2021; Pereira et al., 2020). Actually, gallic acid is nontoxic to mammals at pharmacological doses and exhibits potent neuroprotective effects (Al Zahrani, El-Shishtawy and Asiri, 2020). A study based on histopathology in rats found that gallic acid administration ameliorated anxiety and depression in the behavioral tests, as well as altered cellular loss in the CA1, CA2, CA3 (CA, cornu ammonis) and dentate gyrus (DG) hippocampal subdivisions (Moghadas et al., 2016). Ulteriorly, Samad and colleagues suggested that gallic acid has protective effects on anxiety and depression-like behaviors in male rats via its antioxidant potential (Samad et al., 2019). Mechanistically, Salehi et al. found that gallic acid treatment significantly reduced elevated levels of serum and brain malondialdehyde (MDA) and increased total antioxidant capacity to ameliorate anxiety-like behaviors induced by chronic restraint stress (Salehi et al., 2018). Homoplastically, Chhillar and Dhingra examined gallic acid treatment in Swiss young male albino mice subjected to chronic unpredictable mild stress, showing that gallic acid demonstrated antidepressant-like effects in stressed mice as a result of its antioxidant activity and its ability to reduce oxidative-nitrosative stress and inhibit monoamine oxidase-A (MAO-A), as well as decreased hypothalamic-pituitary-adrenal axis hyperactivity as indicated by a decrease in plasma corticosterone levels (Chhillar and Dhingra, 2013). Interestingly, a new study by Chinese scientists showed that gallic acid inhibits the expression of the P2X7 receptor in the hippocampus, spinal cord, and dorsal root ganglion, which can alleviate comorbid visceral pain and depression (Wen et al., 2022). Additionally, a preclinical study has demonstrated that gallic acid increases levels of both serotonin and catecholamine in synaptic clefts of the central nervous system in a dual antidepressant way, and the effects of gallic acid are further mediated by the modulation of alpha adrenergic receptors, 5-HT2A/2C and 5-HT3 serotonergic receptors, and dopaminergic receptors D1, D2, and D3 (Can et al., 2017).
Corilagin, as a gallotannin, is a major active component of Phyllanthus emblica Linn. in triphala (W. Wang et al., 2019). It is also isolated as one of the main polyphenols in certain ethnopharmacological plants, such as Phyllanthus amarus Schumm & Thonn. (Family Euphorbiaceae) and Phyllanthus tenellus Roxb. (Family Phyllanthaceae) (Chopade and Sayyad, 2015; Hou et al., 2020). Scientists from Brazil discovered that corilagin acts as selective inhibitors of the enzymes prolyl oligopeptidase (POP) and acetyl cholinesterase (AChE), particularly, a dose-dependent inhibitor of POP (IC50 value is 19.7 ± 2.6 μg/mL) (Dos Santos et al., 2021). Additionally, this study also found that corilagin had a moderate ability to pass through the phospholipid membrane (presenting effective permeability of 1.26 × 10−7 cm/s and membrane retention of 1.8%), which indicates that corilagin can cross the BBB and reach the central nervous system in order to act directly against brain POP. Analogously, there is also evidence that corilagin has potential clinical applications in the treatment of anxiety, and the mechanism of action may be due to its interaction with the γ-aminobutyric acid (GABA) type A receptor (Chopade et al., 2021).
Consistent with the above observations of gallic acid and corilagin, ellagic acid has a very strong effect on improving stress-induced depression and anxiety. Using the forced swimming test and tail suspension test, Girish et al. found that ellagic acid exhibited an antidepressant effect in mice, but no effect on locomotor activity, suggesting that ellagic acid interacts with the monoaminergic system rather than the opioid system to produce its effects (Girish et al., 2012). Subsequently, this research team further verified that ellagic acid plays an anxiolytic effect by involving in the GABAergic neurotransmitter system (Girish et al., 2013). In addition, a possible role for excitatory amino acids in depression pathophysiology has also been proposed. Increased hippocampal glutamatergic activity via N-Methyl-D-aspartic acid (NMDA) receptors has been implicated in the pathophysiology of depression and affects hippocampus plasticity. It was determined that ellagic acid has an antidepressant-like effect in mice through mediating the NMDA-nitric oxide (NO) pathway (Lorigooini et al., 2019). There is a correlation between depression and a reduction in brain and plasma concentrations of BDNF, which may be used to treat mental disorders such as depression. There is evidence that ellagic acid's antidepressant-like effects are mediated by an increase in BDNF levels in the hippocampus of mice (Bedel et al., 2018). Actually, there has been a proliferation of research showing that the pathophysiology of human depression goes beyond neuroendocrine function to include inflammation and oxidative stress injury as well. A recent study based on serum metabolomics found that ellagic acid attenuated chronic unpredictable mild stress (CUMS)-induced depression by regulating the neurotransmitter levels like BDNF and 5-HT, suppressing the inflammation by inhibiting tumor necrosis factor α (TNF-α) and iterleukin (IL)-1β or promoting IL-10 expression in the serum, and protecting the integrity of the hippocampal tissue (Huang et al., 2020). Mechanistically, Ferreres et al. verified the anti-depressant effect of ellagic acid and its derivatives in vitro experiments and found they have strong anti-oxidant and that anti-cholinesterase (AChE, butyrylcholinesterase and MAO-A) activities (Ferreres et al., 2013).
In fact, phyllathin, chebulagic acid, chebulic acid, and chebulinic acid are also widely enriched in triphala (Akter et al., 2022), possessing multiple biological benefits that might contribute to the promotion of resilience against stress-induced depression. According to Tao et al., phyllathin mitigates epileptic convulsions and kindling associated post-ictal depression in mice by balancing excitatory glutamate and inhibitory GABA molecules in the brain as well as by inhibiting the activation of the nuclear factor kappa-B (NF-kB)/Toll-like receptor 4 (TLR4) pathway in the brain (Tao et al., 2020). Song and coworkers demonstrated that chebulinic acid is a potent neuroprotectant via inhibiting reactive oxygen species (ROS) production, Ca2+ influx, and phosphorylation of mitogen-activated protein kinases (MAPKs), as well as reducing the ratio of Bcl-2-assaciated X protein (Bax) to B-cell lymphoma-2 (Bcl-2), which contribute to glutamate-induced neuronal cell death and oxidative stress injury (Song et al., 2018). Coincidentally, chebulagic acid and chebulinic acid have been shown to have the ability to suppress ferroptosis by regular antioxidant pathways, including iron chelation and ROS scavenging (Yang et al., 2021). An in vitro-study was performed to determine the neuroprotective properties of chebulagic acid, and results indicated that this compound increased autophagy in human neuroblastoma SH-SY5Y cells via the main control factors of Adenosine 5‘-monophosphate (AMP)-activated protein kinase (AMPK), mammalian target of rapamycin, Beclin-1, and microtubule-associated protein 1 light chain 3 (LC3) (Kim et al., 2014).
6.2. Flavonoids
Luteolin, also called 3’,4’,5,7-Tetrahydroxyflavone, belongs to flavonoids which play an extremely potent neuroprotective activity (Kou et al., 2022). Studies have confirmed that luteolin has strong anxiolytic and antidepressant effects (Achour et al., 2021; Gadotti and Zamponi, 2019). A previous study has shown that luteolin has antidepressant-like effects in mice, partly due to the suppression of endoplasmic reticulum stress (Ishisaka et al., 2011). Similarly, a latest study also indicated that luteolin ameliorates depression-like behaviors by preventing endoplasmic reticulum stress to suppress microglial activation in the brain (Tana and Nakagawa, 2022). According to Cheng and colleagues, luteolin administration protects against noise-induced depression and other mental disorders by improving synaptic plasticity and neuroinflammation in the brain (Cheng et al., 2022). Like the flavone luteolin, its glycoside luteolin-7-O-glucuronide also elicits anti-stress and antidepressant effects. Recently, a team from Korea reported that luteolin-7-O-glucuronide mitigates depression-like behavior and stress coping in a sleep deprivation stress model by activating the BDNF signaling pathway (Ryu et al., 2022).
One of the most common plant flavonoids, quercetin (3,3′,4′,5,7-pentahydroxyflavone), has been found to reduce anxiety and depressive behavior in rodents. In a study carried out on mice, quercetin was found to prevent the impairment of antioxidant enzymes, regulate 5-HT and cholinergic neurotransmission, produce an antianxiety effect, antidepressant effect, and enhance memory following a 2-h immobilization period (Samad et al., 2018). The antidepressant effects of quercetin were also studied by Khan et al. using a mouse model of CUMS-induced depression. They concluded quercetin had antidepressant-like effects by enhancing antioxidant, anti-inflammatory, excitotoxic (glutamate) levels and enhancing 5-HT levels, resulting in antidepressant-like effects (Khan et al., 2019). In the same study, Guan et al. found quercetin to be antidepressant in a rat model of depression induced by UCMS, and that quercetin regulates serum elements through the inhibition of inflammation, reduction of oxidative stress, and regulation of neurotransmitter systems (Guan et al., 2021a, Guan et al., 2021b). A great deal of information regarding the mechanisms leading to the development of stress-mediated neurological complications remains unresolved. The comorbidity of oxidative stress and neuroinflammation in the brain, however, has been demonstrated. Specifically, oxidative stress and neuroinflammation interactions in the hippocampus impair neuronal plasticity to a significant degree. The study conducted by Mehta et al. revealed that quercetin alleviates the oxidative and inflammatory stress in the hippocampal area of mice inflicted with CUMS to prevent depressive-like behavior (Mehta et al., 2017a, Mehta et al., 2017b, Mehta et al., 2017c). Meanwhile, this team further found that quercetin treatment was effective in alleviating stress-mediated behavioral dysfunction by modulating hippocampal insulin signaling (lowered insulin and insulin receptor expression and significantly enhanced glucose transporter 4) and neurogenesis (enhanced doublecortin expression) in the brain (Mehta et al., 2017a, Mehta et al., 2017b, Mehta et al., 2017c). According to Guan et al., quercetin has been shown to reduce depression in CUMS mice by upregulating hippocampal nuclear factor-E2-related factor 2 (Nrf2) and inhibiting inducible nitric oxide synthase, thus restoring balance between oxidative and antioxidant activity and controlling the inflammatory response in the central nervous system (Guan et al., 2021a, Guan et al., 2021b). Prominently, the activated microglia of the brain play an instrumental role in the pathogenesis of depression, which release inflammatory cytokines into the extracellular milieu, carry out neurotoxic effects on surrounding neurons, and secrete inflammatory cytokines into the extracellular milieu. Researchers have studied the mechanisms underlying quercetin's antidepressant activity using electrophysiological and magnetic-activated cell sorting techniques, and quercetin was shown to enhance neuronal activity in the medial prefrontal cortex and hippocampus and improve behavioral performance by inhibiting microglial and astrocyte responses to stress by improving neuronal activity (J. Zhang et al., 2020). Ulteriorly, the latest research has demonstrated quercetin to protect neurons by inhibiting mtROS-mediated activation of the NOD-like receptor protein 3 (NLRP3) inflammasome in microglia through mitophagy, hence providing a potential new therapeutic intervention for stress-induced depression (Han et al., 2021). Intriguingly, neurogenesis has been proposed as a potential mechanism of antidepressant action. A study in vivo suggested that quercetin exerts its antidepressant effects by promoting adult hippocampal neurogenesis through the forkhead box G1 (FoxG1)/cAMP responsive element binding protein (CREB)/BDNF signaling pathway (Z. X. Ma et al., 2021). Additionally, an estrogen-mediated hormone regulator, estrogen receptor of some type, plays a crucial role in protecting against depression. In an article made by Wang et al., the authors proposed quercetin had significant improved estrogen receptor α−/−-induced hippocampal dysfunction antidepressant effects via BDNF/tyrosine receptor kinase B (TrkB)/protein kinase B (AKT)/extracellular regulated protein kinases 1/2 (ERK1/2) (G. Wang et al., 2021).
It is of note that kaempferol, known chemically as 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, is capable of exerting beneficial effects on depression and anxiety. Some studies have demonstrated that kaempferol and other flavonoids like quercetin have shown some anxiolytic activity in studies conducted on male C57BL/6 mice or Swiss albino mice (Aguirre-Hernandez et al., 2010; Grundmann et al., 2009; Vissiennon et al., 2012). Anxiolytic properties of kaempferol have been demonstrated in vivo and in vitro by Ahmad and collaborators. Therefore, in vivo results demonstrated that kaempferol facilitated the extinction of aversive memories along with a reduction of anxiety induced by stress using contextual fear conditioning and elevated plus maze. The in-vitro test revealed that kaempferol can inhibit fatty acid amide hydrolase (IC50: 1.064 μM), thus regulating the action time of the endocannabinoid molecular system, regulating complex circuits to take an influential part in anxiety-like states (Ahmad et al., 2020).
Catechin also widely derived from natural sources belongs to a class of flavanols that has been proven to have extensive biological activities including antidepression and antianxiety (Mehta et al., 2021). CUMS-induced depression in rats was studied in India by a research team, and catechin was found to reverse depression by reducing oxidative stress (Rai et al., 2019). Catechin administration improved depression and anxiety-like behaviors in rats by modulating the central noradrenergic system, according to another study from the Republic of Korea (Lee et al., 2013). In the depression model of mice plasma, Geng et al. found catechin to be metabolized primarily by glucuronidation and methylated glucuronidation, and suggested that catechin may be an effective antidepressant candidate targeting the melatonin receptors (Geng et al., 2019).
Epicatechin is an isomer of catechin, which is collectively referred to as catechin compounds together with catechin gallate, epicatechin gallate, epigallocatechin, and epigallocatechin gallate (EGCG) (Borges, Ottaviani, van der Hooft, Schroeter and Crozier, 2018). It has been demonstrated that epicatechin is capable of crossing the BBB and may have a direct effect on neurons and supporting structures (Shimazu et al., 2021). Evidence has implicated that epicatechin mitigates depression and anxiety (Bernatova, 2018). In a murine model exposed to mild chronic stress, Mexican researchers found that epicatechin treatment can induce resilience to depression-like behavior (Martinez-Damas et al., 2021). Importantly, it was indicated that chronic epicatechin administration reduces anxiety in mice by increasing hippocampal monoamine oxidase-A levels and BDNF levels, but does not affect pattern separation in mice (Stringer et al., 2015). EGCG is a polyphenolic catechin. EGCG has been demonstrated to alleviate depressive symptoms in rats by increasing 5-HT levels in the hippocampus and is helpful in regaining lost body weight in depressed rats by protecting intestinal structure (G. Li et al., 2020). Particularly, a pilot study conducted by a Spanish research team suggested that EGCG and coconut oil alleviate multiple sclerosis patients’ state of anxiety and functional abilities (Platero et al., 2020). Abdelmeguid et al. recently investigated the antidepressant action of EGCG in mice exposed to CUMS, and demonstrated that the effect of EGCG on depression is related to down-regulation of serum IL-1, up-regulation of BDNF mRNA in the hippocampus, and reduction of lesions in CA3 neurons (Abdelmeguid et al., 2022).
7. Triphala polyphenols toward the promotion of resilience against stress/sleep deprivation-induced cognitive impairment
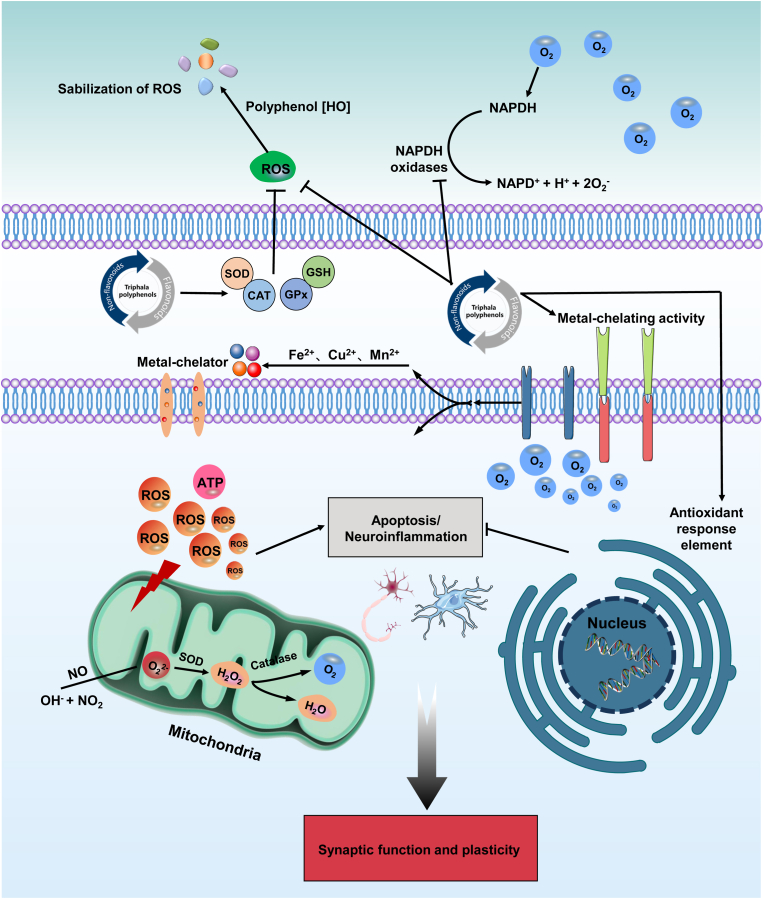
Sleep is a state of immobility and hypo-responsiveness that renders animals immobile (Figorilli et al., 2021). Sleep facilitates the elimination of metabolic waste products from the brain that accumulate during wakefulness, as demonstrated by countless clinical and experimental evidence (Anafi et al., 2019). In reality, sleep functions as a resilient plastic state that consolidates previously acquired information and prioritizes network activity for optimal brain function, regardless of pathological conditions (Lanza et al., 2022). Unfortunately, insufficient sleep or sleep deprivation deleteriously affects the health of individuals, especially cognitive performance (Mason et al., 2021). There is a noticeable decline in cognitive performance even after just one night of sleep deprivation, defined as less than five uninterrupted hours of sleep (Stout et al., 2021). The prevalence of sleep deprivation in the American adult population has recently been declared a common condition (Gonzalez and Tyminski, 2020). Furthermore, shift workers, including medical professionals, are highly susceptible to sleep deprivation (Shriane et al., 2020). Sleep deprivation or loss impairs cognitive functions, including attention, executive functioning, and risk-taking behaviors (Y. Ma et al., 2020). However, the biological basis for these effects is not entirely understood. Until now, despite considerable advances in pharmacotherapy, more effective therapeutic interventions exist for preventing, halting or healing cognitive impairment caused by sleep deprivation remain limited. Consequently, it is vital to investigate the molecular and cellular effects of sleep deprivation in an effort to find more effective treatments or measures to counteract these impacts. Polyphenols are widely regarded as potential agents that can improve cognitive impairment especially through modulating signaling pathways related to inflammation, oxidative damage, autophagy, and apoptosis (Arruda et al., 2020). More precisely, recent advances in the application of triphala polyphenols in stress/sleep deprivation-induced cognitive impairment revealed the main common mechanism of antioxidant-related signaling pathways (Fig. 8).
Fig. 8.
Triphala polyphenols play a key role in the promotion of resilience against stress-induced cognitive impairment through the activation of antioxidant-related signaling pathways. ATP, adenosine-triphosphate; CAT, catalase; GPx, glutathione peroxidase; GSH, glutathione; ROS, reactive oxygen species; SOD, superoxide dismutase.
7.1. Non-flavonoids
Derived from natural phytochemicals have been found to be robust efficacious and non-toxic in treating stress and sleep-related disorders (Hu et al., 2018). Prevailingly, it has been demonstrated that polyphenols have various biological activities related to physiological resilience to cognitive deficits, including modulation of synaptic plasticity, inhibition of oxidative stress injury and inflammatory pathways, as well as promotion of neurogenesis (Caruso et al., 2022; Sharma and Padwad, 2020a, Sharma and Padwad, 2020b). Polyphenolic compounds are increasing in popularity for their potential to attenuate cognitive deterioration and prevent the occurrence of detrimental neuropathology in the brain (Caruso et al., 2022). The promotion of resilience against sleep deprivation-induced cognitive impairment is another promising line of investigation using bioactive and bioavailable triphala polyphenols. Alagan and collaborators have isolated bioactive compounds from phyllanthus amarus, including corilagin, ellagic acid, gallic acid, and phyllanthin, for which the rodents were effectively protected from memory deficits (Alagan et al., 2019). Specifically, a great deal of natural products and enriched foods containing corilagin and ellagic acid are available as nutraceuticals and are used to prevent cognitive decline owing to their excellent biological activities in neurodegenerative disorders (W. Wang et al., 2022) For example, previous reports showed that both corilagin and ellagic acid can improve cognitive function in rodents (Farbood et al., 2019; Tong et al., 2016). Studies from our laboratory have shown that corilagin and ellagic acid derived from triphala provide effective protection against sleep deprivation-induced hippocampus-dependent memory impairment, as assessed by novel object recognition, novel object location, and morris water maze tests. Moreover, corilagin and ellagic acid improved memory consolidation and was associated with an increase in dendritic spine density and the number of hippocampal neurons, as well as a decrease in oxidative stress damage and inflammation. As one of the triphala polyphenols, the macromolecule ellagitannin punicalagin also showed strong neuroprotective effects. It was confirmed that punicalagin could ameliorate spatial learning and memory impairments by activating AMPK/peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α)/Nrf2 signaling pathway (Cao et al., 2020). Furtherly, Nasehi and collaborators found that punicalagin showed a tendency of restoring the memory impairment following 24 h of total sleep deprivation using the passive avoidance test in male rats (Nasehi, Mohammadi-Mahdiabadi-Hasani, Zarrindast and Zarrabian, 2021).
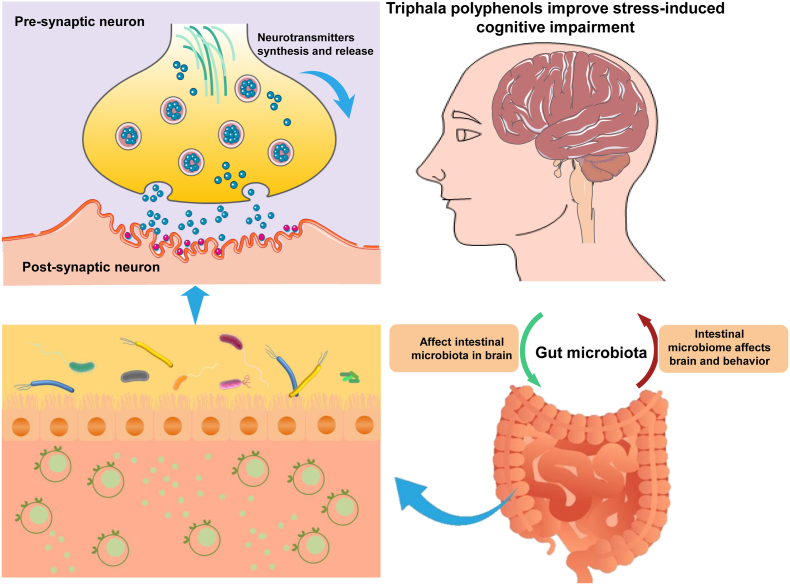
Further, polyphenols’ biological characteristics are determined largely by their bioavailability, which is heavily influenced by the gut microbiota (Sharma and Padwad, 2020a, Sharma and Padwad, 2020b). Macromolecule polyphenols are therefore broken down by the gut microbiota into low-molecular-weight phenolic metabolites and modified polyphenol conjugates and phenolic acids that can be more readily absorbed (Sorrenti et al., 2020). Increasingly, researchers generally agree that the gut microbiota plays a vital part in promoting the effects of polyphenol-rich diets on cognitive function and resilience (Horn et al., 2022). A team of researchers from the United States has confirmed the existence of bioavailable phenolic metabolites that can restore cognitive function impaired by sleep deprivation (Frolinger et al., 2019). What seems certain is that triphala-derived polyphenols like chebulinic acid and ellagic acid can be converted into bioactive metabolites such as urolithins by the human microbiota (Olennikov et al., 2015; Tarasiuk et al., 2018). Importantly, Punicalagin is also an abundant bioactive compound in triphala. Empirical studies have shown that punicalagin can improve cognitive decline or stress-induced cognitive impairment and anxiety (Cao et al., 2020; Xu et al., 2022). Mohammad Nasehi et al. found that punicalagin has a tendency of restoring the cognitive impairment following 24 h of sleep deprivation in male wistar rats by the passive avoidance test (Nasehi et al., 2021). As a hydrolyzable tannin, punicalagin is the precursor of ellagitannin, which is capable of being hydrolyzed spontaneously into ellagic acid in living organisms. In turn, ellagic acid may be converted into urolithins by the gut microbiota. Furthermore, studies have shown that urolithins can also further ameliorate memory deficits. Some recent studies have shown that urolithin A and urolithin B effectively improve cognitive deficits by inhibiting apoptosis, promoting the survival of neurons, and triggering neurogenesis (P. Chen et al., 2021; Gong et al., 2019). Taken together, these evidences were exciting especially in view of the fact that triphala polyphenols may play a key role in the promotion of resilience against stress/sleep deprivation-induced cognitive impairment through gut microbiota (Fig. 9).
Fig. 9.
Triphala polyphenols play a key role in the promotion of resilience against stress-induced cognitive impairment through gut microbiota.
7.2. Flavonoids
There is evidence that stress impairs neuronal functioning, alters insulin signaling, and negatively affects behavioral functioning, which are closely associated with cognitive impairment. According to Vineet Mehta and colleagues, quercetin ameliorates stress-induced memory impairment by reducing insulin resistance and increasing hippocampal glucose transporter 4 levels independently of insulin receptor expression, thus preventing neuronal damage to the regions of the brain responsible for memory (Mehta et al., 2017a, Mehta et al., 2017b, Mehta et al., 2017c). Several other researchers have confirmed quercetin's ability to improve cognitive impairment caused by stress, arguing that quercetin works by clearing senescent cells to alleviate chronic unpredictable stress-induced cognitive deficits (Lin et al., 2021). In fact, many scholars have carried out corresponding research on quercetin or its derivatives to improve cognitive impairment caused by sleep deprivation. Brazilian scientists identified quercetin's protective mechanism as its ability to reduce oxidative stress caused by sleep deprivation (Kanazawa et al., 2016). In a recently published study, quercetin was found to suppress the TLR4/myeloid differentiation factor 88 (MyD88)/NF-kB signaling that mediates abnormal microglia activation in the hippocampus in a paradoxical sleep deprivation-related memory impairment model (Y. Zhang et al., 2022). Quercetin-3-O-glucuronide, a quercetin derivative, has shown a significant reduction in sleep deprivation-induced cognitive impairment in mice by activating the CREB pathway (Zhao et al., 2015). In addition, in a recent study by Hairui Zhou and colleagues, it has been demonstrated that isoquercetin improves the learning and cognition of sleep-deprived mice through the suppression of NLRP3-induced pyroptosis of hippocampal neurons (H. Zhou et al., 2022).
Interestingly, it is clear from preclinical, clinical, and epidemiological studies that consuming other specific flavanols, such as epicatechin, kaempferol, and catechin, can help improve cognitive function (Alemany-Gonzalez et al., 2020; Lei et al., 2012; Navarrete-Yanez et al., 2020). An in-vivo experiment suggested that kaempferol protects against cognitive impairment through attenuation of oxidative stress, in addition to the stimulation of BDNF/TrkB/CREB signaling (Yan et al., 2019). Luteolin permeated the BBB in vivo and attenuated scopolamine-induced amnesia in rats (Ionita et al., 2018). Forouzanfar and collaborators evaluated the effects of catechin and EGCG on sleep deprivation-induced spatial memory impairments using male Wistar rats, and the authors observed that catechin and EGCG could prevent sleep deprivation-induced cognitive deficits in rats through normalizing the hippocampal antioxidant thiol defense system (Forouzanfar et al., 2021).
8. Conclusion and future perspectives
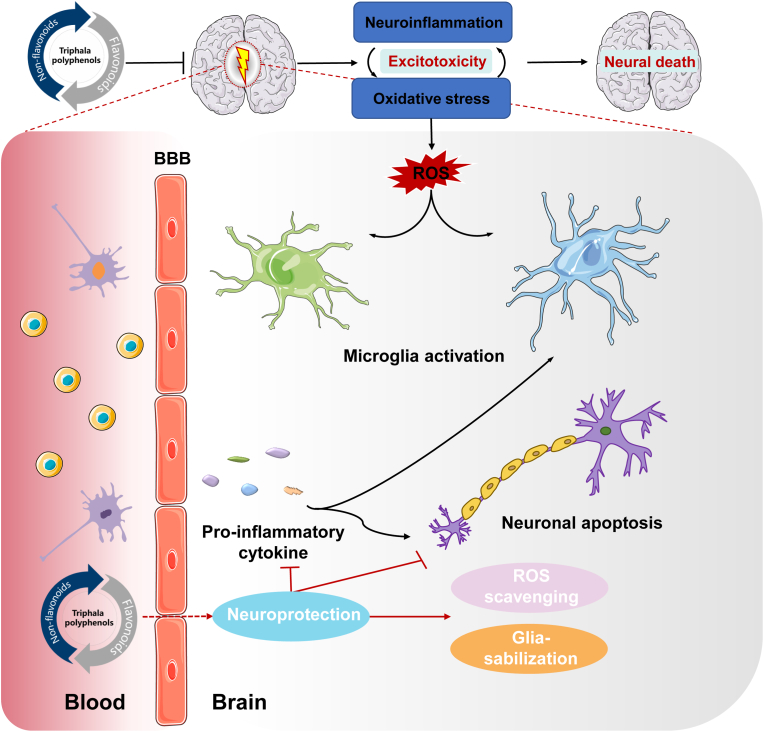
Pharmaceutical and medical industries have made considerable progress and efforts to develop therapeutics for the prevention and treatment of mood disorders, such as anxiety and depression, and sleep deprivation-induced cognitive impairments. The currently available approaches, however, still have a lot of side effects and adverse drug reactions, as well as undesirable interactions between drugs and foods. There is therefore an urgent need to demonstrate the efficacy and safety of novel phytotherapy in order to overcome the prevailing and failing treatment by the pharmaceutical industry. The exciting news is that triphala polyphenols are capable of promoting resilience against depression and cognitive impairment caused by stress. Aside from this, triphala polyphenols are recognized as safe natural ingredient agents that have been extensively tested in animal experiments and clinical trials and have not been found to cause adverse effects. Exploration of the recent advances in the use of triphala polyphenols in stress-induced depression and cognitive impairment has demonstrated related mechanisms including the regulation of 5-HT and BDNF receptors, gut microbiota, and activation of antioxidant-related signaling pathways. Additionally, several triphala polyphenols like corilagin and epicatechin can cross the BBB and promote resilience against stress-induced depression and cognitive impairment (Fig. 10). Of concern, since most triphala polyphenols are commonly administered orally, no clear evidence exists that all of these triphala polyphenols are capable of reaching the central nervous system from the systemic circulation. The BBB permeability and systemic bioavailability of triphala polyphenols need to be improved, therefore future research should focus on improving these aspects.
Fig. 10.
Some triphala polyphenols can cross the blood brain barrier (BBB) and promote resilience against stress-induced depression and cognitive impairment.
Clinical trials need to be thoroughly investigated on the basis of preclinical discussions, as they are an essential component of medical research. Although triphala polyphenols have been studied in animals, fewer clinical studies have been conducted on the effect of triphala polyphenols on stress-induced depression and cognitive impairment in humans. For example, a pilot study confirmed that a supplement containing polyphenols, especially ellagic acid and gallic acid, is effective in improving fatigue, mood disorders, and insomnia in patients suffering from high levels of oxidative stress (Belcaro et al., 2018). Nevertheless, a small sample size clinical study or pilot study has limitations in terms of methodological validity, so there is no guarantee that conclusions will be completely conclusive. Therefore, it is necessary to conduct more rigorous clinical studies, including the use of better study design types, large sample sizes, high quality outcome measurements, and the selection of the correct endpoint, to overcome these methodological constraints. Most importantly, for the purpose of determining whether triphala polyphenols, whether single or mixed, promote resilience to stress-induced depression and cognitive impairment, a comprehensive understanding of the biological mechanisms of triphala polyphenols along with large multicenter clinical trials is also needed.
CRediT authorship contribution statement
Wenjun Wang: Investigation, Conceptualization, Methodology, Formal analysis, Writing – original draft, Visualization. Olufola Oladoyin Ige: Investigation, Conceptualization, Methodology. Yi Ding: Writing – review & editing, Visualization. Mengshan He: Investigation, Writing – original draft. Pan Long: Investigation, Visualization. Shaohui Wang: Writing – review & editing, Visualization. Yi Zhang: Conceptualization, Methodology, Supervision. Xudong Wen: Conceptualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (82204761 and 82274313), Sichuan Provincial Science and Technology Department (2021YJ0198), and the National Key Research and Development Program of China (2017YFC1703904).
Handling Editor: Professor A.G. Marangoni
Contributor Information
Wenjun Wang, Email: wenjunwang018@163.com.
Yi Zhang, Email: zhangyi0238@163.com.
Xudong Wen, Email: xudongwen@cdutcm.edu.cn.
Abbreviations
- 5-HT
5-hydroxytryptamine
- AChE
acetylcholinesterase
- AKT
protein kinase B
- AMPK
Adenosine 5‘-monophosphate (AMP)-activated protein kinase
- Bax
Bcl-2-assaciated X protein
- Bcl-2
B-cell lymphoma-2
- BBB
blood brain barrier
- BDNF
brain-derived neurotrophic factor
- CA
cornu ammonis
- Cmax
peak concentration
- CUMS
chronic unpredictable mild stress
- CREB
cAMP-response element-binding protein
- EGCG
epigallocatechin gallate
- ERK1/2
extracellular regulated protein kinases 1/2
- DG
dentate gyrus
- GABA
γ-aminobutyric acid
- IL
iterleukin
- iNOS
inducible nitric oxide synthase
- LC3
microtubule-associated protein 1 light chain 3
- MAO-A
monoamine oxidase-A
- MAPKs
mitogen-activated protein kinases
- MDA
malondialdehyde
- MyD88
myeloid differentiation factor 88
- NF-kB
nuclear factor kappa-B
- NLRP3
NOD-like receptor protein 3
- NMDA
N-Methyl-D-aspartic acid
- NO
nitric oxide
- Nrf2
nuclear factor-E2-related factor 2
- PGC-1α
peroxisome proliferator-activated receptor-gamma coactivator-1alpha
- POP
prolyl oligopeptidase
- ROS
reactive oxygen species
- T1/2
half-life
- Tmax
peak time
- TLR4
Toll-like receptor 4
- TNF-α
tumor necrosis factor α
- TrkB
tyrosine receptor kinase B
Data availability
Data will be made available on request.
References
- Abdelmeguid N.E., Hammad T.M., Abdel-Moneim A.M., Salam S.A. Effect of epigallocatechin-3-gallate on stress-induced depression in a mouse model: role of interleukin-1beta and brain-derived neurotrophic factor. Neurochem. Res. 2022;47(11):3464–3475. doi: 10.1007/s11064-022-03707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achour M., Ferdousi F., Sasaki K., Isoda H. Luteolin modulates neural stem cells fate determination: in vitro study on human neural stem cells, and in vivo study on LPS-induced depression mice model. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.753279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre-Hernandez E., Gonzalez-Trujano M.E., Martinez A.L., Moreno J., Kite G., Terrazas T., Soto-Hernandez M. HPLC/MS analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids from Tilia americana var. mexicana. J. Ethnopharmacol. 2010;127(1):91–97. doi: 10.1016/j.jep.2009.09.044. [DOI] [PubMed] [Google Scholar]
- Ahmad H., Rauf K., Zada W., McCarthy M., Abbas G., Anwar F., Shah A.J. Kaempferol facilitated extinction learning in contextual fear conditioned rats via inhibition of fatty-acid amide hydrolase. Molecules. 2020;25(20) doi: 10.3390/molecules25204683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Ding X., Sharma A. Exploring scientific validation of Triphala Rasayana in ayurveda as a source of rejuvenation for contemporary healthcare: an update. J. Ethnopharmacol. 2021;273 doi: 10.1016/j.jep.2021.113829. [DOI] [PubMed] [Google Scholar]
- Akter R., Khan S.S., Kabir M.T., Halder S. GC-MS-employed phytochemical characterization, synergistic antioxidant, and cytotoxic potential of Triphala methanol extract at non-equivalent ratios of its constituents. Saudi J. Biol. Sci. 2022;29(6) doi: 10.1016/j.sjbs.2022.103287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Zahrani N.A., El-Shishtawy R.M., Asiri A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: a review. Eur. J. Med. Chem. 2020;204 doi: 10.1016/j.ejmech.2020.112609. [DOI] [PubMed] [Google Scholar]
- Alagan A., Jantan I., Kumolosasi E., Ogawa S., Abdullah M.A., Azmi N. Protective effects of Phyllanthus amarus against lipopolysaccharide-induced neuroinflammation and cognitive impairment in rats. Front. Pharmacol. 2019;10:632. doi: 10.3389/fphar.2019.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany-Gonzalez M., Gener T., Nebot P., Vilademunt M., Dierssen M., Puig M.V. Prefrontal-hippocampal functional connectivity encodes recognition memory and is impaired in intellectual disability. Proc. Natl. Acad. Sci. U. S. A. 2020;117(21):11788–11798. doi: 10.1073/pnas.1921314117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anafi R.C., Kayser M.S., Raizen D.M. Exploring phylogeny to find the function of sleep. Nat. Rev. Neurosci. 2019;20(2):109–116. doi: 10.1038/s41583-018-0098-9. [DOI] [PubMed] [Google Scholar]
- Arpornchayanon W., Subhawa S., Jaijoy K., Lertprasertsuk N., Soonthornchareonnon N., Sireeratawong S. Safety of the oral triphala recipe from acute and chronic toxicity tests in sprague-dawley rats. Toxics. 2022;10(9) doi: 10.3390/toxics10090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda H.S., Neri-Numa I.A., Kido L.A., Maróstica Júnior M.R., Pastore G.M. Recent advances and possibilities for the use of plant phenolic compounds to manage ageing-related diseases. J. Funct.Foods. 2020;75 doi: 10.1016/j.jff.2020.104203. [DOI] [Google Scholar]
- Bai J., Zhang Y., Tang C., Hou Y., Ai X., Chen X.…Meng X. Gallic acid: pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110985. [DOI] [PubMed] [Google Scholar]
- Bedel H.A., Kencebay Manas C., Ozbey G., Usta C. The antidepressant-like activity of ellagic acid and its effect on hippocampal brain derived neurotrophic factor levels in mouse depression models. Nat. Prod. Res. 2018;32(24):2932–2935. doi: 10.1080/14786419.2017.1385021. [DOI] [PubMed] [Google Scholar]
- Belcaro G., Saggino A., Cornelli U., Luzzi R., Dugall M., Hosoi M.…Cesarone M.R. Improvement in mood, oxidative stress, fatigue, and insomnia following supplementary management with Robuvit(R) J. Neurosurg. Sci. 2018;62(4):423–427. doi: 10.23736/S0390-5616.18.04384-9. [DOI] [PubMed] [Google Scholar]
- Bernatova I. Biological activities of (-)-epicatechin and (-)-epicatechin-containing foods: focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018;36(3):666–681. doi: 10.1016/j.biotechadv.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Bernstein N., Akram M., Yaniv-Bachrach Z., Daniyal M. Is it safe to consume traditional medicinal plants during pregnancy? Phytother Res. 2021;35(4):1908–1924. doi: 10.1002/ptr.6935. [DOI] [PubMed] [Google Scholar]
- Borchers A., Pieler T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes. 2010;1(3):413–426. doi: 10.3390/genes1030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges G., Ottaviani J.I., van der Hooft J.J.J., Schroeter H., Crozier A. Absorption, metabolism, distribution and excretion of (-)-epicatechin: a review of recent findings. Mol. Aspect. Med. 2018;61:18–30. doi: 10.1016/j.mam.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Can O.D., Turan N., Demir Ozkay U., Ozturk Y. Antidepressant-like effect of gallic acid in mice: dual involvement of serotonergic and catecholaminergic systems. Life Sci. 2017;190:110–117. doi: 10.1016/j.lfs.2017.09.023. [DOI] [PubMed] [Google Scholar]
- Cao K., Lv W., Hu S., Gao J., Liu J., Feng Z. Punicalagin activates AMPK/PGC-1alpha/Nrf2 cascade in mice: the potential protective effect against prenatal stress. Mol. Nutr. Food Res. 2020;64(14) doi: 10.1002/mnfr.202000312. [DOI] [PubMed] [Google Scholar]
- Caruso G., Godos J., Privitera A., Lanza G., Castellano S., Chillemi A.…Grosso G. Phenolic acids and prevention of cognitive decline: polyphenols with a neuroprotective role in cognitive disorders and alzheimer's disease. Nutrients. 2022;14(4) doi: 10.3390/nu14040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenat J.M., Blais-Rochette C., Kokou-Kpolou C.K., Noorishad P.G., Mukunzi J.N., McIntee S.E.…Labelle P.R. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: a systematic review and meta-analysis. Psychiatr. Res. 2021;295 doi: 10.1016/j.psychres.2020.113599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Chen F., Lei J., Wang G., Zhou B. The gut microbiota metabolite urolithin B improves cognitive deficits by inhibiting cyt C-mediated apoptosis and promoting the survival of neurons through the PI3K pathway in aging mice. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.768097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhu P., Liu B., Wei L., Xu Y. Simultaneous determination of fourteen compounds of Hedyotis diffusa Willd extract in rats by UHPLC-MS/MS method: application to pharmacokinetics and tissue distribution study. J. Pharm. Biomed. Anal. 2018;159:490–512. doi: 10.1016/j.jpba.2018.07.023. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Wang X., Yu Y., Gu J., Zhao M., Fu Q.…Liu Y. Noise induced depression-like behavior, neuroinflammation and synaptic plasticity impairments: the protective effects of luteolin. Neurochem. Res. 2022;47(11):3318–3330. doi: 10.1007/s11064-022-03683-0. [DOI] [PubMed] [Google Scholar]
- Chhillar R., Dhingra D. Antidepressant-like activity of gallic acid in mice subjected to unpredictable chronic mild stress. Fundam. Clin. Pharmacol. 2013;27(4):409–418. doi: 10.1111/j.1472-8206.2012.01040.x. [DOI] [PubMed] [Google Scholar]
- Chopade A.R., Pol R.P., Patil P.A., Dharanguttikar V.R., Naikwade N.S., Dias R.J., Mali S.N. An insight into the anxiolytic effects of lignans (phyllanthin and hypophyllanthin) and tannin (corilagin) rich extracts of Phyllanthus amarus : an in-silico and in-vivo approaches. Comb. Chem. High Throughput Screen. 2021;24(3):415–422. doi: 10.2174/1386207323666200605150915. [DOI] [PubMed] [Google Scholar]
- Chopade A.R., Sayyad F.J. Pain modulation by lignans (phyllanthin and hypophyllanthin) and tannin (corilagin) rich extracts of Phyllanthus amarus in carrageenan-induced thermal and mechanical chronic muscle hyperalgesia. Phytother Res. 2015;29(8):1202–1210. doi: 10.1002/ptr.5366. [DOI] [PubMed] [Google Scholar]
- D'Amico D., Andreux P.A., Valdes P., Singh A., Rinsch C., Auwerx J. Impact of the natural compound urolithin A on health, disease, and aging. Trends Mol. Med. 2021;27(7):687–699. doi: 10.1016/j.molmed.2021.04.009. [DOI] [PubMed] [Google Scholar]
- Disease, G. B. D. Injury, I. Prevalence, C Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M., Jin L., Sharma R., Weiss N.H., Contractor A.A. The relationship between number of trauma types, resilience, and psychological symptoms in ex-military personnel from India. Psychol Trauma. 2022;14(3):437–445. doi: 10.1037/tra0001050. [DOI] [PubMed] [Google Scholar]
- Dos Santos M.Z., de Avila J., Morel A., Canto-Dorow T.S., Mostardeiro M.A., Dalcol Evaluation of prolyl oligopeptidase and acetylcholinesterase inhibition by Phyllanthus tenellus Roxb. from Brazil. Nat. Prod. Res. 2021;35(11):1840–1846. doi: 10.1080/14786419.2019.1637869. [DOI] [PubMed] [Google Scholar]
- Farbood Y., Rashno M., Ghaderi S., Khoshnam S.E., Sarkaki A., Rashidi K.…Badavi M. Ellagic acid protects against diabetes-associated behavioral deficits in rats: possible involved mechanisms. Life Sci. 2019;225:8–19. doi: 10.1016/j.lfs.2019.03.078. [DOI] [PubMed] [Google Scholar]
- Ferianec V., Fulop M., Jezovicova M., Radosinska J., Husseinova M., Feriancova M.…Durackova Z. The oak-wood extract robuvit((R)) improves recovery and oxidative stress after hysterectomy: a randomized, double-blind, placebo-controlled pilot study. Nutrients. 2020;12(4) doi: 10.3390/nu12040913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreres F., Grosso C., Gil-Izquierdo A., Valentao P., Andrade P.B. Ellagic acid and derivatives from Cochlospermum angolensis Welw. Extracts: HPLC-DAD-ESI/MS(n) profiling, quantification and in vitro anti-depressant, anti-cholinesterase and anti-oxidant activities. Phytochem. Anal. 2013;24(6):534–540. doi: 10.1002/pca.2429. [DOI] [PubMed] [Google Scholar]
- Figorilli M., Lanza G., Congiu P., Lecca R., Casaglia E., Mogavero M.P.…Ferri R. Neurophysiological aspects of REM sleep behavior disorder (RBD): a narrative review. Brain Sci. 2021;11(12) doi: 10.3390/brainsci11121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisicaro F., Lanza G., Pennisi M., Vagli C., Cantone M., Falzone L.…Bella R. Daily mocha coffee intake and psycho-cognitive status in non-demented non-smokers subjects with subcortical ischaemic vascular disease. Int. J. Food Sci. Nutr. 2022;73(6):821–828. doi: 10.1080/09637486.2022.2050999. [DOI] [PubMed] [Google Scholar]
- Fisicaro F., Lanza G., Pennisi M., Vagli C., Cantone M., Pennisi G.…Bella R. Moderate mocha coffee consumption is associated with higher cognitive and mood status in a non-demented elderly population with subcortical ischemic vascular disease. Nutrients. 2021;13(2) doi: 10.3390/nu13020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzanfar F., Gholami J., Foroughnia M., Payvar B., Nemati S., Khodadadegan M.A.…Hajali V. The beneficial effects of green tea on sleep deprivation-induced cognitive deficits in rats: the involvement of hippocampal antioxidant defense. Heliyon. 2021;7(11) doi: 10.1016/j.heliyon.2021.e08336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolinger T., Sims S., Smith C., Wang J., Cheng H., Faith J.…Pasinetti G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019;9(1):3546. doi: 10.1038/s41598-019-39994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadotti V.M., Zamponi G.W. Anxiolytic effects of the flavonoid luteolin in a mouse model of acute colitis. Mol. Brain. 2019;12(1):114. doi: 10.1186/s13041-019-0539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng C.A., Yang T.H., Huang X.Y., Ma Y.B., Zhang X.M., Chen J.J. Antidepressant potential of Uncaria rhynchophylla and its active flavanol, catechin, targeting melatonin receptors. J. Ethnopharmacol. 2019;232:39–46. doi: 10.1016/j.jep.2018.12.013. [DOI] [PubMed] [Google Scholar]
- Girish C., Raj V., Arya J., Balakrishnan S. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur. J. Pharmacol. 2012;682(1–3):118–125. doi: 10.1016/j.ejphar.2012.02.034. [DOI] [PubMed] [Google Scholar]
- Girish C., Raj V., Arya J., Balakrishnan S. Involvement of the GABAergic system in the anxiolytic-like effect of the flavonoid ellagic acid in mice. Eur. J. Pharmacol. 2013;710(1–3):49–58. doi: 10.1016/j.ejphar.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Gong Z., Huang J., Xu B., Ou Z., Zhang L., Lin X., Xuan A. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J. Neuroinflammation. 2019;16(1):62. doi: 10.1186/s12974-019-1450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Tyminski Q. Sleep deprivation in an American homeless population. Sleep Health. 2020;6(4):489–494. doi: 10.1016/j.sleh.2020.01.002. [DOI] [PubMed] [Google Scholar]
- Grundmann O., Nakajima J., Kamata K., Seo S., Butterweck V. Kaempferol from the leaves of Apocynum venetum possesses anxiolytic activities in the elevated plus maze test in mice. Phytomedicine. 2009;16(4):295–302. doi: 10.1016/j.phymed.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Guan T., Cao C., Hou Y., Li Y., Wei X., Li S.…Zhao X. Effects of quercetin on the alterations of serum elements in chronic unpredictable mild stress-induced depressed rats. Biometals. 2021;34(3):589–602. doi: 10.1007/s10534-021-00298-w. [DOI] [PubMed] [Google Scholar]
- Guan Y., Wang J., Wu X., Song L., Wang Y., Gong M., Li B. Quercetin reverses chronic unpredictable mild stress-induced depression-like behavior in vivo by involving nuclear factor-E2-related factor 2. Brain Res. 2021;1772 doi: 10.1016/j.brainres.2021.147661. [DOI] [PubMed] [Google Scholar]
- Gupta A., Singh A.K., Kumar R., Jamieson S., Pandey A.K., Bishayee A. Neuroprotective potential of ellagic acid: a critical review. Adv. Nutr. 2021;12(4):1211–1238. doi: 10.1093/advances/nmab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Xu T., Fang Q., Zhang H., Yue L., Hu G., Sun L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021;44 doi: 10.1016/j.redox.2021.102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J., Mayer D.E., Chen S., Mayer E.A. Role of diet and its effects on the gut microbiome in the pathophysiology of mental disorders. Transl. Psychiatry. 2022;12(1):164. doi: 10.1038/s41398-022-01922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Cheng Z., Wang J. Preparative purification of corilagin from Phyllanthus by combining ionic liquid extraction, prep-HPLC, and precipitation. Anal. Methods. 2020;12(26):3382–3389. doi: 10.1039/d0ay00860e. [DOI] [PubMed] [Google Scholar]
- Hu Z., Oh S., Ha T.W., Hong J.T., Oh K.W. Sleep-aids derived from natural products. Biomol Ther (Seoul) 2018;26(4):343–349. doi: 10.4062/biomolther.2018.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Li W., You B., Tang W., Gan T., Feng C.…Yang R. Serum metabonomic study on the antidepressant-like effects of ellagic acid in a chronic unpredictable mild stress-induced mouse model. J. Agric. Food Chem. 2020;68(35):9546–9556. doi: 10.1021/acs.jafc.0c02895. [DOI] [PubMed] [Google Scholar]
- Ionita R., Postu P.A., Mihasan M., Gorgan D.L., Hancianu M., Cioanca O., Hritcu L. Ameliorative effects of Matricaria chamomilla L. hydroalcoholic extract on scopolamine-induced memory impairment in rats: a behavioral and molecular study. Phytomedicine. 2018;47:113–120. doi: 10.1016/j.phymed.2018.04.049. [DOI] [PubMed] [Google Scholar]
- Ishisaka M., Kakefuda K., Yamauchi M., Tsuruma K., Shimazawa M., Tsuruta A., Hara H. Luteolin shows an antidepressant-like effect via suppressing endoplasmic reticulum stress. Biol. Pharm. Bull. 2011;34(9):1481–1486. doi: 10.1248/bpb.34.1481. [DOI] [PubMed] [Google Scholar]
- Jantrapirom S., Hirunsatitpron P., Potikanond S., Nimlamool W., Hanprasertpong N. Pharmacological benefits of triphala: a perspective for allergic rhinitis. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.628198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa L.K.S., Vecchia D.D., Wendler E.M., Hocayen P.A.S., Dos Reis Livero F.A., Stipp M.C.…Andreatini R. Quercetin reduces manic-like behavior and brain oxidative stress induced by paradoxical sleep deprivation in mice. Free Radic. Biol. Med. 2016;99:79–86. doi: 10.1016/j.freeradbiomed.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Khan K., Najmi A.K., Akhtar M. A natural phenolic compound quercetin showed the usefulness by targeting inflammatory, oxidative stress markers and augment 5-HT levels in one of the animal models of depression in mice. Drug Res. 2019;69(7):392–400. doi: 10.1055/a-0748-5518. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Kim J., Kang K.S., Lee K.T., Yang H.O. Neuroprotective effect of chebulagic acid via autophagy induction in SH-SY5Y cells. Biomol Ther (Seoul) 2014;22(4):275–281. doi: 10.4062/biomolther.2014.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou J.J., Shi J.Z., He Y.Y., Hao J.J., Zhang H.Y., Luo D.M.…Pang X.B. Luteolin alleviates cognitive impairment in Alzheimer's disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2022;43(4):840–849. doi: 10.1038/s41401-021-00702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza G., DelRosso L.M., Ferri R. Sleep and homeostatic control of plasticity. Handb. Clin. Neurol. 2022;184:53–72. doi: 10.1016/B978-0-12-819410-2.00004-7. [DOI] [PubMed] [Google Scholar]
- Lee B., Sur B., Kwon S., Yeom M., Shim I., Lee H., Hahm D.H. Chronic administration of catechin decreases depression and anxiety-like behaviors in a rat model using chronic corticosterone injections. Biomol Ther (Seoul) 2013;21(4):313–322. doi: 10.4062/biomolther.2013.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Chen J., Zhang W., Fu W., Wu G., Wei H.…Ruan J. In vivo investigation on the potential of galangin, kaempferol and myricetin for protection of D-galactose-induced cognitive impairment. Food Chem. 2012;135(4):2702–2707. doi: 10.1016/j.foodchem.2012.07.043. [DOI] [PubMed] [Google Scholar]
- Li G., Yang J., Wang X., Zhou C., Zheng X., Lin W. Effects of EGCG on depression-related behavior and serotonin concentration in a rat model of chronic unpredictable mild stress. Food Funct. 2020;11(10):8780–8787. doi: 10.1039/d0fo00524j. [DOI] [PubMed] [Google Scholar]
- Li X., Wu L., Wu R., Sun M., Fu K., Kuang T., Wang Z. Comparison of medicinal preparations of Ayurveda in India and five traditional medicines in China. J. Ethnopharmacol. 2022;284 doi: 10.1016/j.jep.2021.114775. [DOI] [PubMed] [Google Scholar]
- Lin Y.F., Wang L.Y., Chen C.S., Li C.C., Hsiao Y.H. Cellular senescence as a driver of cognitive decline triggered by chronic unpredictable stress. Neurobiol Stress. 2021;15 doi: 10.1016/j.ynstr.2021.100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.Z., Wang Y.X., Jiang C.L. Inflammation: the common pathway of stress-related diseases. Front. Hum. Neurosci. 2017;11:316. doi: 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopresti A.L. The effects of psychological and environmental stress on micronutrient concentrations in the body: a review of the evidence. Adv. Nutr. 2020;11(1):103–112. doi: 10.1093/advances/nmz082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorigooini Z., Salimi N., Soltani A., Amini-Khoei H. Implication of NMDA-NO pathway in the antidepressant-like effect of ellagic acid in male mice. Neuropeptides. 2019;76 doi: 10.1016/j.npep.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Lu Y., Yan H., Teng S., Yang X. A liquid chromatography-tandem mass spectrometry method for preclinical pharmacokinetics and tissue distribution of hydrolyzable tannins chebulinic acid and chebulagic acid in rats. Biomed. Chromatogr. 2019;33(3):e4425. doi: 10.1002/bmc.4425. [DOI] [PubMed] [Google Scholar]
- Luca S.V., Macovei I., Bujor A., Miron A., Skalicka-Wozniak K., Aprotosoaie A.C., Trifan A. Bioactivity of dietary polyphenols: the role of metabolites. Crit. Rev. Food Sci. Nutr. 2020;60(4):626–659. doi: 10.1080/10408398.2018.1546669. [DOI] [PubMed] [Google Scholar]
- Ma Y., Liang L., Zheng F., Shi L., Zhong B., Xie W. Association between sleep duration and cognitive decline. JAMA Netw. Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.X., Zhang R.Y., Rui W.J., Wang Z.Q., Feng X. Quercetin alleviates chronic unpredictable mild stress-induced depressive-like behaviors by promoting adult hippocampal neurogenesis via FoxG1/CREB/BDNF signaling pathway. Behav. Brain Res. 2021;406 doi: 10.1016/j.bbr.2021.113245. [DOI] [PubMed] [Google Scholar]
- Martinez-Damas M.G., Genis-Mendoza A.D., Perez-de la Cruz V., Canela-Tellez G.D., Jimenez-Estrada I., Nicolini-Sanchez J.H.…Coral-Vazquez R.M. Epicatechin treatment generates resilience to chronic mild stress-induced depression in a murine model. Physiol. Behav. 2021;238 doi: 10.1016/j.physbeh.2021.113466. [DOI] [PubMed] [Google Scholar]
- Mason G.M., Lokhandwala S., Riggins T., Spencer R.M.C. Sleep and human cognitive development. Sleep Med. Rev. 2021;57 doi: 10.1016/j.smrv.2021.101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron R.M., Shapiro B., Rawles J., Luo J. Depression. Ann. Intern. Med. 2021;174(5):ITC65–ITC80. doi: 10.7326/AITC202105180. [DOI] [PubMed] [Google Scholar]
- Mehta R., Bhandari R., Kuhad A. Effects of catechin on a rodent model of autism spectrum disorder: implications for the role of nitric oxide in neuroinflammatory pathway. Psychopharmacology (Berl) 2021;238(11):3249–3271. doi: 10.1007/s00213-021-05941-5. [DOI] [PubMed] [Google Scholar]
- Mehta V., Parashar A., Sharma A., Singh T.R., Udayabanu M. Quercetin ameliorates chronic unpredicted stress-mediated memory dysfunction in male Swiss albino mice by attenuating insulin resistance and elevating hippocampal GLUT4 levels independent of insulin receptor expression. Horm. Behav. 2017;89:13–22. doi: 10.1016/j.yhbeh.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Mehta V., Parashar A., Udayabanu M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol. Behav. 2017;171:69–78. doi: 10.1016/j.physbeh.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Mehta V., Singh T.R., Udayabanu M. Quercetin ameliorates chronic unpredicted stress-induced behavioral dysfunction in male Swiss albino mice by modulating hippocampal insulin signaling pathway. Physiol. Behav. 2017;182:10–16. doi: 10.1016/j.physbeh.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Mignani S., Shi X., Karpus A., Majoral J.P. Non-invasive intranasal administration route directly to the brain using dendrimer nanoplatforms: an opportunity to develop new CNS drugs. Eur. J. Med. Chem. 2021;209 doi: 10.1016/j.ejmech.2020.112905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithul Aravind S., Wichienchot S., Tsao R., Ramakrishnan S., Chakkaravarthi S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021;142 doi: 10.1016/j.foodres.2021.110189. [DOI] [PubMed] [Google Scholar]
- Moghadas M., Edalatmanesh M.A., Robati R. Histopathological analysis from gallic acid administration on hippocampal cell density, depression, and anxiety related behaviors in A trimethyltin intoxication model. Cell J. 2016;17(4):659–667. doi: 10.22074/cellj.2016.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S.M., Harkness K.L. Major depression and its recurrences: life course matters. Annu. Rev. Clin. Psychol. 2022;18:329–357. doi: 10.1146/annurev-clinpsy-072220-021440. [DOI] [PubMed] [Google Scholar]
- Nasehi M., Mohammadi-Mahdiabadi-Hasani M.H., Zarrindast M.R., Zarrabian S. Punicalagin effect on total sleep deprivation memory deficit in male Wistar rats. J. Integr. Neurosci. 2021;20(1):87–93. doi: 10.31083/j.jin.2021.01.378. [DOI] [PubMed] [Google Scholar]
- Navarrete-Yanez V., Garate-Carrillo A., Rodriguez A., Mendoza-Lorenzo P., Ceballos G., Calzada-Mendoza C.…Ramirez-Sanchez I. Effects of (-)-epicatechin on neuroinflammation and hyperphosphorylation of tau in the hippocampus of aged mice. Food Funct. 2020;11(12):10351–10361. doi: 10.1039/d0fo02438d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle I., Braun D., Berry D., Wisgrill L., Rompel A., Warth B. Polyphenol exposure, metabolism, and analysis: a global exposomics perspective. Annu. Rev. Food Sci. Technol. 2021;12:461–484. doi: 10.1146/annurev-food-062220-090807. [DOI] [PubMed] [Google Scholar]
- Olennikov D.N., Kashchenko N.I., Chirikova N.K. In vitro bioaccessibility, human gut microbiota metabolites and hepatoprotective potential of chebulic ellagitannins: a case of padma hepaten(R) formulation. Nutrients. 2015;7(10):8456–8477. doi: 10.3390/nu7105406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi M., Lanza G., Cantone M., D'Amico E., Fisicaro F., Puglisi V.…Malaguarnera G. Acetyl-L-carnitine in dementia and other cognitive disorders: a critical update. Nutrients. 2020;12(5) doi: 10.3390/nu12051389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira G.A., Arruda H.S., de Morais D.R., Peixoto Araujo N.M., Pastore G.M. Mutamba (Guazuma ulmifolia Lam.) fruit as a novel source of dietary fibre and phenolic compounds. Food Chem. 2020;310 doi: 10.1016/j.foodchem.2019.125857. [DOI] [PubMed] [Google Scholar]
- Peterson C.T., Denniston K., Chopra D. Therapeutic uses of triphala in ayurvedic medicine. J. Alternative Compl. Med. 2017;23(8):607–614. doi: 10.1089/acm.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phetkate P., Kummalue T., Y U.P., Kietinun S. Significant increase in cytotoxic T lymphocytes and natural killer cells by triphala: a clinical phase I study. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/239856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platero J.L., Cuerda-Ballester M., Ibanez V., Sancho D., Lopez-Rodriguez M.M., Drehmer E., Orti J.E.R. The impact of coconut oil and epigallocatechin gallate on the levels of IL-6, anxiety and disability in multiple sclerosis patients. Nutrients. 2020;12(2) doi: 10.3390/nu12020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai A., Gill M., Kinra M., Shetty R., Krishnadas N., Rao C.M.…Kumar N. Catechin ameliorates depressive symptoms in Sprague Dawley rats subjected to chronic unpredictable mild stress by decreasing oxidative stress. Biomed Rep. 2019;11(2):79–84. doi: 10.3892/br.2019.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajha H.N., Paule A., Aragones G., Barbosa M., Caddeo C., Debs E.…Edeas M. Recent advances in research on polyphenols: effects on microbiota, metabolism, and health. Mol. Nutr. Food Res. 2022;66(1) doi: 10.1002/mnfr.202100670. [DOI] [PubMed] [Google Scholar]
- Ran F., Han X., Deng X., Wu Z., Huang H., Qiu M.…Han L. High or low temperature extraction, which is more conducive to Triphala against chronic pharyngitis? Biomed. Pharmacother. 2021;140 doi: 10.1016/j.biopha.2021.111787. [DOI] [PubMed] [Google Scholar]
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C.…Yang L. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminf. 2014;6:13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu D., Jee H.J., Kim S.Y., Hwang S.H., Pil G.B., Jung Y.S. Luteolin-7-O-Glucuronide improves depression-like and stress coping behaviors in sleep deprivation stress model by activation of the BDNF signaling. Nutrients. 2022;14(16) doi: 10.3390/nu14163314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi A., Rabiei Z., Setorki M. Effect of gallic acid on chronic restraint stress-induced anxiety and memory loss in male BALB/c mice. Iran J Basic Med Sci. 2018;21(12):1232–1237. doi: 10.22038/ijbms.2018.31230.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad N., Jabeen S., Imran I., Zulfiqar I., Bilal K. Protective effect of gallic acid against arsenic-induced anxiety-/depression- like behaviors and memory impairment in male rats. Metab. Brain Dis. 2019;34(4):1091–1102. doi: 10.1007/s11011-019-00432-1. [DOI] [PubMed] [Google Scholar]
- Samad N., Saleem A., Yasmin F., Shehzad M.A. Quercetin protects against stress-induced anxiety- and depression-like behavior and improves memory in male mice. Physiol. Res. 2018;67(5):795–808. doi: 10.33549/physiolres.933776. [DOI] [PubMed] [Google Scholar]
- Shah D., Gandhi M., Kumar A., Cruz-Martins N., Sharma R., Nair S. Current insights into epigenetics, noncoding RNA interactome and clinical pharmacokinetics of dietary polyphenols in cancer chemoprevention. Crit. Rev. Food Sci. Nutr. 2021:1–37. doi: 10.1080/10408398.2021.1968786. [DOI] [PubMed] [Google Scholar]
- Sharma R., Jadhav M., Choudhary N., Kumar A., Rauf A., Gundamaraju R.…Shen B. Deciphering the impact and mechanism of Trikatu, a spices-based formulation on alcoholic liver disease employing network pharmacology analysis and in vivo validation. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1063118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Kabra A., Rao M.M., Prajapati P.K. Herbal and holistic solutions for neurodegenerative and depressive disorders: leads from ayurveda. Curr. Pharmaceut. Des. 2018;24(22):2597–2608. doi: 10.2174/1381612824666180821165741. [DOI] [PubMed] [Google Scholar]
- Sharma R., Martins N. Telomeres, DNA damage and ageing: potential leads from ayurvedic rasayana (Anti-Ageing) drugs. J. Clin. Med. 2020;9(8) doi: 10.3390/jcm9082544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Martins N., Kuca K., Chaudhary A., Kabra A., Rao M.M., Prajapati P.K. Chyawanprash: a traditional Indian bioactive health supplement. Biomolecules. 2019;9(5) doi: 10.3390/biom9050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Padwad Y. Plant-polyphenols based second-generation synbiotics: emerging concepts, challenges, and opportunities. Nutrition. 2020;77 doi: 10.1016/j.nut.2020.110785. [DOI] [PubMed] [Google Scholar]
- Sharma R., Padwad Y. Probiotic bacteria as modulators of cellular senescence: emerging concepts and opportunities. Gut Microb. 2020;11(3):335–349. doi: 10.1080/19490976.2019.1697148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R., Singla R.K., Banerjee S., Sinha B., Shen B., Sharma R. Role of Shankhpushpi (Convolvulus pluricaulis) in neurological disorders: an umbrella review covering evidence from ethnopharmacology to clinical studies. Neurosci. Biobehav. Rev. 2022;140 doi: 10.1016/j.neubiorev.2022.104795. [DOI] [PubMed] [Google Scholar]
- Sher L. Resilience as a focus of suicide research and prevention. Acta Psychiatr. Scand. 2019;140(2):169–180. doi: 10.1111/acps.13059. [DOI] [PubMed] [Google Scholar]
- Shimazu R., Anada M., Miyaguchi A., Nomi Y., Matsumoto H. Evaluation of blood-brain barrier permeability of polyphenols, anthocyanins, and their metabolites. J. Agric. Food Chem. 2021;69(39):11676–11686. doi: 10.1021/acs.jafc.1c02898. [DOI] [PubMed] [Google Scholar]
- Shriane A.E., Ferguson S.A., Jay S.M., Vincent G.E. Sleep hygiene in shift workers: a systematic literature review. Sleep Med. Rev. 2020;53 doi: 10.1016/j.smrv.2020.101336. [DOI] [PubMed] [Google Scholar]
- Song J.H., Shin M.S., Hwang G.S., Oh S.T., Hwang J.J., Kang K.S. Chebulinic acid attenuates glutamate-induced HT22 cell death by inhibiting oxidative stress, calcium influx and MAPKs phosphorylation. Bioorg. Med. Chem. Lett. 2018;28(3):249–253. doi: 10.1016/j.bmcl.2017.12.062. [DOI] [PubMed] [Google Scholar]
- Sorrenti V., Ali S., Mancin L., Davinelli S., Paoli A., Scapagnini G. Cocoa polyphenols and gut microbiota interplay: bioavailability, prebiotic effect, and impact on human health. Nutrients. 2020;12(7) doi: 10.3390/nu12071908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout J.W., Beidel D.C., Brush D., Bowers C. Sleep disturbance and cognitive functioning among firefighters. J. Health Psychol. 2021;26(12):2248–2259. doi: 10.1177/1359105320909861. [DOI] [PubMed] [Google Scholar]
- Stringer T.P., Guerrieri D., Vivar C., van Praag H. Plant-derived flavanol (-)epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice. Transl. Psychiatry. 2015;5:e493. doi: 10.1038/tp.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Yu D., Fan J., Yu C., Guo Y., Pei P. Healthy lifestyle and life expectancy at age 30 years in the Chinese population: an observational study. Lancet Public Health. 2022 doi: 10.1016/S2468-2667(22)00110-4. China Kadoorie Biobank Collaborative, G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliou A., Zintzaras E., Lykouras L., Francis K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin. Therapeut. 2013;35(5):592–602. doi: 10.1016/j.clinthera.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Tana, Nakagawa T. Luteolin ameliorates depression-like behaviors by suppressing ER stress in a mouse model of Alzheimer's disease. Biochem. Biophys. Res. Commun. 2022;588:168–174. doi: 10.1016/j.bbrc.2021.12.074. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Sato A., Kishimoto Y., Mabashi-Asazuma H., Kondo K., Iida K. Gallic acid inhibits lipid accumulation via AMPK pathway and suppresses apoptosis and macrophage-mediated inflammation in hepatocytes. Nutrients. 2020;12(5) doi: 10.3390/nu12051479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z., Chun-Yan H., Hua P., Bin-Bin Y., Xiaoping T. Phyllathin from Phyllanthus amarus ameliorates epileptic convulsion and kindling associated post-ictal depression in mice via inhibition of NF-kappaB/TLR-4 pathway. Dose Response. 2020;18(3) doi: 10.1177/1559325820946914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasiuk A., Mosinska P., Fichna J. Triphala: current applications and new perspectives on the treatment of functional gastrointestinal disorders. Chin. Med. 2018;13:39. doi: 10.1186/s13020-018-0197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwana G., Cock I.E., White A., Cheesman M.J. Use of specific combinations of the triphala plant component extracts to potentiate the inhibition of gastrointestinal bacterial growth. J. Ethnopharmacol. 2020;260 doi: 10.1016/j.jep.2020.112937. [DOI] [PubMed] [Google Scholar]
- Tong F., Zhang J., Liu L., Gao X., Cai Q., Wei C.…Dong X. Corilagin attenuates radiation-induced brain injury in mice. Mol. Neurobiol. 2016;53(10):6982–6996. doi: 10.1007/s12035-015-9591-6. [DOI] [PubMed] [Google Scholar]
- Ullah H., Khan A., Riccioni C., Di Minno A., Tantipongpiradet A., Buccato D.G.…Daglia M. Polyphenols as possible alternative agents in chronic fatigue: a review. Phytochemistry Rev. 2022 doi: 10.1007/s11101-022-09838-9. [DOI] [Google Scholar]
- Unno K., Furushima D., Hamamoto S., Iguchi K., Yamada H., Morita A.…Nakamura Y. Stress-reducing function of matcha green tea in animal experiments and clinical trials. Nutrients. 2018;10(10) doi: 10.3390/nu10101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissiennon C., Nieber K., Kelber O., Butterweck V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin--are they prodrugs? J. Nutr. Biochem. 2012;23(7):733–740. doi: 10.1016/j.jnutbio.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Wang G., Li Y., Lei C., Lei X., Zhu X., Yang L., Zhang R. Quercetin exerts antidepressant and cardioprotective effects in estrogen receptor alpha-deficient female mice via BDNF-AKT/ERK1/2 signaling. J. Steroid Biochem. Mol. Biol. 2021;206 doi: 10.1016/j.jsbmb.2020.105795. [DOI] [PubMed] [Google Scholar]
- Wang W., Liu T., Ding Y., Zhang Y. Effects of polyphenol-rich interventions on sleep disorders: a systematic review and meta-analysis. Curr. Res. Food Sci. 2023;6 doi: 10.1016/j.crfs.2023.100462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Liu T., Yang L., Ma Y., Dou F., Shi L.…Ding Y. Study on the multi-targets mechanism of triphala on cardio-cerebral vascular diseases based on network pharmacology. Biomed. Pharmacother. 2019;116 doi: 10.1016/j.biopha.2019.108994. [DOI] [PubMed] [Google Scholar]
- Wang W., Wang S., Liu Y., Wang X., Nie J., Meng X., Zhang Y. Ellagic acid: a dietary-derived phenolic compound for drug discovery in mild cognitive impairment. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.925855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L., Tang L., Zhang M., Wang C., Li S., Wen Y.…Gao Y. Gallic acid alleviates visceral pain and depression via inhibition of P2X7 receptor. Int. J. Mol. Sci. 2022;23(11) doi: 10.3390/ijms23116159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Zhou J., Creyer M.N., Yim W., Chen Z., Messersmith P.B., Jokerst J.V. Phenolic-enabled nanotechnology: versatile particle engineering for biomedicine. Chem. Soc. Rev. 2021;50(7):4432–4483. doi: 10.1039/d0cs00908c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Xu L., Huang S., Li D., Liu Y., Guo H.…Zhong S. Analysis of the molecular mechanism of punicalagin in the treatment of alzheimer's disease by computer-aided drug research Technology. ACS Omega. 2022;7(7):6121–6132. doi: 10.1021/acsomega.1c06565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T., He B., Xu M., Wu B., Xiao F., Bi K., Jia Y. Kaempferide prevents cognitive decline via attenuation of oxidative stress and enhancement of brain-derived neurotrophic factor/tropomyosin receptor kinase B/cAMP response element-binding signaling pathway. Phytother Res. 2019;33(4):1065–1073. doi: 10.1002/ptr.6300. [DOI] [PubMed] [Google Scholar]
- Yang L., Liu Y., Zhang W., Hua Y., Chen B., Wu Q.…Li X. Ferroptosis-inhibitory difference between chebulagic acid and chebulinic acid indicates beneficial role of HHDP. Molecules. 2021;26(14) doi: 10.3390/molecules26144300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.A., Teye Azietaku J., Li J., Wang H., Zheng F., Hao J., Chang Y.X. Simultaneous quantification of gallic acid, bergenin, epicatechin, epicatechin gallate, isoquercitrin, and quercetin-3-rhamnoside in rat plasma by LC-MS/MS method and its application to pharmacokinetics after oral administration of Ardisia japonica extract. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/4964291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Ning L., Wang J. Dietary quercetin attenuates depressive-like behaviors by inhibiting astrocyte reactivation in response to stress. Biochem. Biophys. Res. Commun. 2020;533(4):1338–1346. doi: 10.1016/j.bbrc.2020.10.016. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xie Y., Cheng Z., Xi K., Huang X., Kuang F., Wu S. Quercetin ameliorates memory impairment by inhibiting abnormal microglial activation in a mouse model of paradoxical sleep deprivation. Biochem. Biophys. Res. Commun. 2022;632:10–16. doi: 10.1016/j.bbrc.2022.09.088. [DOI] [PubMed] [Google Scholar]
- Zhao W., Wang J., Bi W., Ferruzzi M., Yemul S., Freire D., Pasinetti G.M. Novel application of brain-targeting polyphenol compounds in sleep deprivation-induced cognitive dysfunction. Neurochem. Int. 2015;89:191–197. doi: 10.1016/j.neuint.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Wu J., Gong Y., Zhou Z., Wang J. Isoquercetin alleviates sleep deprivation dependent hippocampal neurons damage by suppressing NLRP3-induced pyroptosis. Immunopharmacol. Immunotoxicol. 2022;44(5):766–772. doi: 10.1080/08923973.2022.2082976. [DOI] [PubMed] [Google Scholar]
- Zhou N., Gu X., Zhuang T., Xu Y., Yang L., Zhou M. Gut microbiota: a pivotal hub for polyphenols as antidepressants. J. Agric. Food Chem. 2020;68(22):6007–6020. doi: 10.1021/acs.jafc.0c01461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.