Abstract
Switch defective/sucrose nonfermentable (SWI/SNF) complexes are evolutionarily conserved multisubunit machines that play vital roles in chromatin architecture regulation for modulating gene expression via sliding or ejection of nucleosomes in eukaryotes. In plants, perturbations of SWI/SNF subunits often result in severe developmental disorders. However, the subunit composition, pathways of assembly, and genomic targeting of the plant SWI/SNF complexes are poorly understood. Here, we report the organization, genomic targeting, and assembly of 3 distinct SWI/SNF complexes in Arabidopsis thaliana: BRAHMA-Associated SWI/SNF complexes (BAS), SPLAYED-Associated SWI/SNF complexes (SAS), and MINUSCULE-Associated SWI/SNF complexes (MAS). We show that BAS complexes are equivalent to human ncBAF, whereas SAS and MAS complexes evolve in multiple subunits unique to plants, suggesting plant-specific functional evolution of SWI/SNF complexes. We further show overlapping and specific genomic targeting of the 3 plant SWI/SNF complexes on chromatin and reveal that SAS complexes are necessary for the correct genomic localization of the BAS complexes. Finally, we define the role of the core module subunit in the assembly of plant SWI/SNF complexes and highlight that ATPase module subunit is required for global complex stability and the interaction of core module subunits in Arabidopsis SAS and BAS complexes. Together, our work highlights the divergence of SWI/SNF chromatin remodelers during eukaryote evolution and provides a comprehensive landscape for understanding plant SWI/SNF complex organization, assembly, genomic targeting, and function.
Three Arabidopsis switch defective/sucrose non-fermentable (SWI/SNF) chromatin remodeling complexes have unique and shared roles and exhibit distinct organization, genomic targeting, and assembly.
Introduction
Switch defective/sucrose nonfermentable (SWI/SNF) complexes are chromatin remodelers that play essential roles in modulating chromatin architecture to enable DNA accessibility and gene expression in an ATP-hydrolysis-dependent manner (Clapier and Cairns 2009; Ho and Crabtree 2010; Hargreaves and Crabtree 2011; Ryan and Owen-Hughes 2011; Clapier et al. 2017). These complexes are multisubunit machineries, including one ATPase catalytic subunit and multiple additional regulatory core subunits, evolutionally conserved among yeasts, animals, and plants (Ho and Crabtree 2010; Sarnowska et al. 2016).
In Saccharomyces cerevisiae, there are 2 subfamilies of SWI/SNF remodelers, Swi/Snf and RSC (Remodeling the Structure of Chromatin). The yeast Swi/Snf complex consists of 12 proteins, which was the first chromatin remodeler discovered (Smith et al. 2003; Dutta et al. 2017), whereas the RSC complex is composed of 16 proteins (Wagner et al. 2020). The 2 subcomplexes contain 3 common subunits, ARP7, ARP9, and Rtt102, and the other subunits are specialized in the 2 subcomplexes (Dutta et al. 2017; Wagner et al. 2020). In humans, the subunits of SWI/SNF complexes are combinatorially assembled into 3 classes of complexes: canonical BAF (cBAF), polybromo-associated BAF (PBAF), and noncanonical BAF (ncBAF) (Gatchalian et al. 2018; Mashtalir et al. 2018). These 3 SWI/SNF subcomplexes share a set of core subunits, such as SMARCA2, SMARCA4, SMARCC1, SMARCC2, SMARCD, ACTL6A, ACTL6B, BCL7A, BCL7B, and BCL7C, but are distinguished by the inclusion of subtype-specific ones: DPF1, DPF2, ARID1A, and ARID1B for cBAF; PBRM1, PHF10, ARID2, and BRD7 for PBAF; and BRD9, GLTSCR1, and GLTSCR1L for ncBAF (Gatchalian et al. 2018; Mashtalir et al. 2018).
In the flowering plant Arabidopsis (Arabidopsis thaliana), genetic and molecular studies have identified a number of SWI/SNF subunits, including 4 ATPases (BRAHMA (BRM), SPLAYED (SYD), MINUSCULE 1 (MINU1), and MINU2); 4 SWI3 subunits (SWI3A–SWI3D); 2 SWI/SNF associated proteins 73 (SWP73A and SWP73B); 2 actin-related proteins (ARP4 and ARP7); a single SNF5 subunit called BUSHY (BSH); and an ARID paralog LEAF AND FLOWER RELATED (LFR) (Wagner and Meyerowitz 2002; Farrona et al. 2004; Hurtado et al. 2006; Mlynarova et al. 2007; Wang et al. 2009; Sang et al. 2012). In addition, we recently biochemically isolated several subunits of the BRM-containing SWI/SNF complexes in Arabidopsis, including 2 BRM-interacting proteins (BRIP1 and BRIP2) and 3 bromodomain-containing proteins, BRD1, BRD12, and BRD13 (Yu et al. 2020, 2021). More recently, analysis in Arabidopsis and rice (Oryza sativa) showed 3 types of complexes organized around BRM, SYD, MINU1, and MINU2 ATPase (Diego-Martin et al. 2022; Guo et al. 2022; Bieluszewski et al. 2023).
Plant SWI/SNF subunits serve critical roles in cell differentiation, development, and response to various environmental signals, and their mutations result in severe defects in leaf development, root stem cell maintenance, flower patterning and timing, embryo development, and vegetative to adult phase transition (Sarnowski et al. 2005; Ho and Crabtree 2010; Li et al. 2015; Wu et al. 2015; Yang et al. 2015; Zhao et al. 2015; Sarnowska et al. 2016; Xu et al. 2016). However, the mechanisms by which these mutations alter plant SWI/SNF complex function on chromatin and subsequently lead to impaired development and responses to environmental stimulus remain largely unknown. Particularly, whether and how the activity and genomic targeting of 1 SWI/SNF subtype are coordinated with the other 2 subtypes to regulate chromatin structure and gene expression are also poorly understood.
Another significant barrier to our mechanistic understanding of the functions of SWI/SNF complexes lies in the need for more information regarding the role of each subunit in complex assembly. Recent studies have begun to reveal the assembly steps of the subunits of SWI/SNF complexes. It was reported that mammalian SWI/SNF complex assembly is triggered by the formation of the initial BAF core that is composed of 2 SMARCC and 1 SMARCD subunits. This initial core then acts as a platform for independent docking of the subcomplex-specific subunits to form the core module. Finally, the core module recruits the ATPases SMARCA2 and SMARCA4 to finalize complex assembly (Gatchalian et al. 2018; Mashtalir et al. 2018). In line with this model, the removal of core module subunits SMARCCs resulted in the near-complete degradation of all 3 SWI/SNF complex components, whereas the removal of the ATPase module does not disrupt the formation of the core module (Mashtalir et al. 2018; Michel et al. 2018; Pan et al. 2019). Thus, the core module is required for assembly toward fully formed SWI/SNF complexes in mammals, but the ATPase module is the last to be incorporated into SWI/SNF complexes and, therefore, not necessary for the core module assembly in mammals (Mashtalir et al. 2018; Michel et al. 2018; Pan et al. 2019). In Arabidopsis, our recent studies showed that BRIP1, BRIP2, BRD1, BRD2, and BRD13, the homologs of human ncBAF core module subunits GLTSCR1, GLTSCR1L, and BRD9, are required for the assembly of SWI/SNF complexes (Yu et al. 2020). However, whether plant ATPases use the same strategy as their mammalian counterparts or a different one for complex assembly is completely unknown.
In this study, we examined the organization, genomic targeting, and assembly of the 3 nonredundant final form Arabidopsis SWI/SNF complexes: BRAHMA-Associated SWI/SNF complexes (BAS), SPLAYED-Associated SWI/SNF complexes (SAS) and MINUSCULE-Associated SWI/SNF complexes (MAS). BAS complexes are equivalent to human ncBAF, whereas SAS and MAS complexes evolve in multiple subunits unique to plants, suggesting a plant-specific functional evolution of SWI/SNF complexes. By focusing on the SAS and BAS complexes, we demonstrate a requirement of SAS complexes for the correct genomic localization of the BAS complexes. Finally, we define the role of core module subunit in the assembly of the plant SWI/SNF complexes and unexpectedly establish that ATPase module subunit (SYD and BRM) is required for the stability and interaction of core module subunits in SAS and BAS complexes in Arabidopsis. Together, these studies highlight the divergence of SWI/SNF chromatin remodelers during the eukaryote evolution and lay the groundwork for a comprehensive understanding of the plant SWI/SNF complex organization, assembly, genomic targeting, and function.
Results
Arabidopsis has 3 biochemically distinct SWI/SNF subcomplexes
To comprehensively investigate the plant SWI/SNF subcomplexes, including their subunit organization, coordination of genomic targeting, and assembly pathway, we started by performing immunopurification followed by mass spectrometry analysis (IP-MS) using stable transgenic Arabidopsis lines that expressed a green fluorescent protein (GFP)-tagged ATPase subunit, BRM (Li et al. 2016), SYD (Shu et al. 2021), or MINU2 (this study), driven by its native promoter in the corresponding null mutant background. This analysis revealed that the 3 ATPases were constituted into 3 distinct subassemblies (Fig. 1, A and B; Supplemental Fig. S1, A–C; Guo et al. 2022). Specifically, the BRM-containing complexes uniquely lack core, evolutionarily conserved ARID paralog, LFR, incorporate selective paralogs (that is, SWI3C but not SWI3A, SWI3B, SWI3D, and SWP73A) and contain a set of complex-specific subunits that are not shared by SYD- or MINU-complex, the GLTSCR1 or GLTSCR1L paralogs (BRIP1 and BRIP2) and BRD1, BRD2, and BRD13. In contrast, the SYD-containing complexes selectively contain paralog SWI3D but not SWI3A, SWI3B, and SWI3C, and include a complex-specific subunit, encoded by SYD subSWI/SNF Subunit 1/2/3 (SYS1, SYS2, or SYS3). Finally, consistent with the recent report (Diego-Martin et al. 2022), the MINU-containing complexes do not contain the GIF2 subunit (putative SS18 paralog) found in both BRM- and SYD-containing complexes, incorporate selective paralogs (that is, SWI3A and SWI3B but not SWI3C and SWI3D) and include a number of complex-specific subunits, including BSH, BRD5, SHH2, TPF1, TPF2, OPF1, OPF2, PSA1, and PSA2.
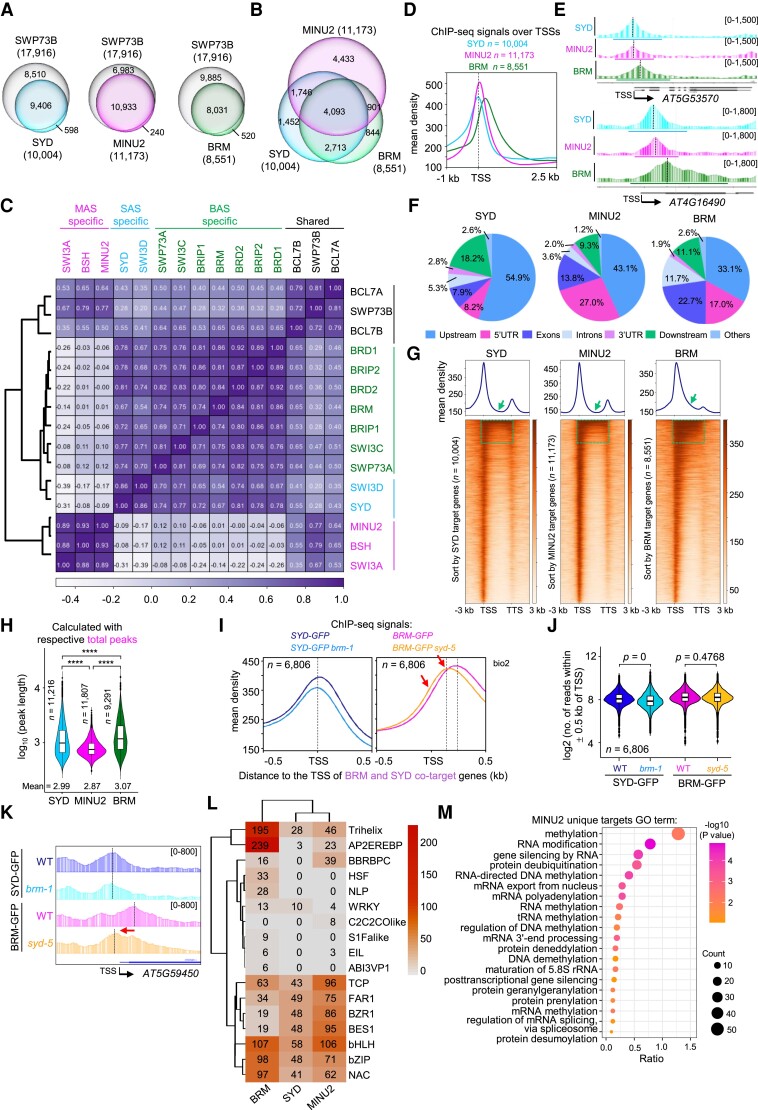
Figure 1.
Three distinct SWI/SNF subcomplexes in Arabidopsis. A) Volcano plots displaying SWI/SNF subunits that are enriched in GFP immunoprecipitations from BRM-GFP, SYD-GFP, and MINU2-GFP relative to GFP from 2 independent biological experiments. P-values were calculated by two-tailed Student's t-test. Subunits of the Arabidopsis SWI/SNF complexes are highlighted using green dots (BRM unique subunits), blue dots (SYD unique subunits), magenta dots (MINU unique subunits), and black dots (BRM, SYD, and MINU2 shared subunits). B) Heatmap showing the mean log2(PSM + 1) values (from 2 biological replications) of SWI/SNF complex subunits identified by IP-MS in BRM, SWI3C, BRIP2, BRD1, SWP73A, SYD-N, SWI3D, SYS1, SYS2, SYS3, MINU2, SWI3A, SWI3B, BSH, SWP73B, and BCL7B. C) Diagrams showing the organization of BAS, SAS, and MAS complexes, respectively. Sliver-stained gels of GFP immunoprecipitations from SWI3C-GFP, SWI3D-GFP, and SWI3A-GFP were shown. The specific subunits of each subcomplex were marked with different colors, and the common subunits of different subcomplexes were marked with gray.
To further confirm the 3 distinct SWI/SNF subcomplexes in Arabidopsis, we generated transgenic lines stably expressing GFP-tagged subunits specific to the BRM-complexes, the SYD-complexes, or the MINU-complexes, or subunits shared by the3 subcomplexes. We then identified proteins that co-purified with each subunit by mass spectrometry. Silver staining of proteins isolated from transgenic lines stably expressing GFP-tagged SWI3C, SWI3D, and SWI3A again demonstrated 3 distinct SWI/SNF subcomplexes in Arabidopsis (Fig. 1C; Supplemental Fig. S1D). Furthermore, mass spectrometry showed that SWI3C, BRIP2, BRD1, and SWP73A immunoprecipitation enriched the identical subunits immunoprecipitated by BRM, whereas none of the SYD-specific or MINU-specific subunits were co-immunoprecipitated, indicating that they are unique components of the BRM-containing complexes (Fig. 1B; Supplemental Fig. S1E). Likewise, the immunoprecipitation of SYD-specific or MINU-specific subunits did not catch subunits unique to the other 2 subcomplexes (Fig. 1B; Supplemental Fig. S1E). In contrast, SWP73B and BCL7B, the shared subunits by the 3 subcomplexes, immunoprecipitated all the subunits found in the 3 subcomplexes (Fig. 1B; Supplemental Fig. S1E).
To test whether the formation of the 3 subassemblies directly depends on DNA or RNA, we also added the Benzonase nuclease to remove the genomic DNA before the IP procedure. We used ProBRD1:BRD1-GFP, ProSYD:SYD-N-GFP, or ProSWI3B:SWI3B-GFP transgenic plants for each subcomplex. The Benzonase treatment efficiently removed the DNA and RNA (Supplemental Fig. S2A), whereas mass spectrometry showed that the subunits immunoprecipitated by BRD1, SYD-N, or SWI3B with Benzonase treatment were the same as those without the treatment (Supplemental Fig. S2B), indicating that the assembly of the Arabidopsis SWI/SNF complexes is DNA- or RNA-independent.
Together, our immunoprecipitation data are in line with the recent observation (Guo et al. 2022) and show the existence of 3 concurrently expressed plant SWI/SNF family subcomplexes that have specific subunits and are separately assembled on a mutually exclusive catalytic subunit, BRM, SYD, or MINUs. These 3 plant SWI/SNF subcomplexes were therefore termed as BAS, SAS, and MAS, respectively (Fig. 1C).
Comparison of SWI/SNF subcomplexes among yeast, human, and Arabidopsis
SWI/SNF complexes are multiprotein machineries evolutionally conserved among yeasts, animals, and plants (Ho and Crabtree 2010; Sarnowska et al. 2016). We compared the 3 distinct plant SWI/SNF complexes with the mammalian SWI/SNF complexes and identified several plant-specific properties in SWI/SNF complexes. First, in humans, the 3 different types of SWI/SNF subcomplexes, BAF, PBAF, and ncBAF, shared the same ATPases (BRM or BRG1) and initial BAF core subunits (2 SMARCCs and 1 SMARCD). However, in Arabidopsis, SAS, MAS, and BAS each used different ATPases and SMARCCs homologs. Specifically, the SAS complex contained SYD ATPase and SWI3D, the MAS complex included MINU1 or MINU2 ATPases and SWI3A and SWI3B, while BRM ATPase and SWI3C specifically presented in the BAS complex (Fig. 2A). Second, plant SWI/SNF complexes uniquely lacked core, metazoan-conserved subunits such as SMARCE1 and pBRM1, but selectively evolved in plant-specific subunits that are not found in metazoan and yeast, including PSA1, PSA2, SHH2, SYS1, SYS2, and SYS3 (Fig. 2A). Third, the BAS complexes are equivalent to the mammalian ncBAF because they share the identical putative paralog subunits (Yu et al. 2020, 2021). However, a comparison of the subunit compositions of the SAS and MAS subcomplexes with those of human BAF complexes suggested that the SAS and MAS are likely plant-specific. Indeed, SAS complexes did not contain any plant homeodomain (PHD) domain or bromodomain proteins that are signatures of mammalian PBAF and cBAF but had 3 plant-specific proteins, SYS1, SYS2, and SYS3, whose functions in the SAS remain to be investigated. In terms of MAS complexes, although they had PHD domain proteins TPF1, TPF2, OPF1, and OPF2 that are homologous to PHF10 subunits in PBAF and DPF1, DPF2, and DPF3 in cBAF and contained BRD5 protein that is homologous to the PBAF-specific subunit BRD7, they lacked homologous subunits of PBRM1 and SS18, which are specialized subunits in PBAF and cBAF, respectively. In contrast, the MAS complexes contained several subunits (SHH2, PSA1, and PSA2) that are specifically present in plants.
Figure 2.
Comparison of subunit composition of SWI/SNF complexes in S. cerevisiae, Homo sapiens, and A. thaliana. A) SWI/SNF subcomplex components in the 3 species. The SMARC names correspond to lineage-specific subunits previously identified (Hernandez-Garcia et al. 2022) and the characters in brackets represent different protein modules. B) Diagrams displaying the subunit composition of different SWI/SNF subcomplexes in eukaryotes. The specific subunits of each complex were highlighted by different colors, and the common subunits of different subcomplexes of the same species were marked with gray.
Based on these comparisons, we propose that the SWI/SNF subcomplexes in different kingdoms came from an ancestral BAF complex. This ancestor BAF complex first evolved into an ncBAF complex that has been preserved in 3 kingdoms by integrating the GLTSCR domain-containing subunits. Meanwhile, the ancestral BAF complex integrated different new subunits and discarded several original ones to evolve into the PBAF and cBAF subcomplexes in animals and fungi, as well as 2 plant-specific subcomplexes, SAS and MAS (Fig. 2B).
Differential localization of the Arabidopsis SWI/SNF subcomplexes, SAS, MAS, and BAS on chromatin
To further characterize the 3 distinct plant SWI/SNF assemblies and to determine whether the differences in their subunit compositions may result in differential genomic targeting, we performed a comprehensive genome-wide mapping of SAS, MAS, and BAS subcomplexes by performing chromatin immunoprecipitation-sequencing (ChIP-seq). To this end, we used the stable expression transgenic plants, including pan-plant-SWI/SNF subunits (SWP73B, BCL7A, and BCL7B) and complex specific subunits SYD and SWI3D for SAS, MINU2, SWI3A, and BSH for MAS, and BRM, SWP73A, and SWI3C for BAS (Supplemental Fig. S3A). The ChIP-seq data of other BAS-specific subunits BRIP1, BRIP2, BRD1, and BRD2 were previously described (Yu et al. 2020, 2021).
Consistent with biochemical results, subcomplex-specific subunits peaks comprised subsets of all pan-subunits peaks (Fig. 3A; Supplemental Fig. S3, A and B). The target genes of the 3 ATPases, SYD, MINU2, and BRM, showed significant overlaps (n = 4,093); however, they also exhibited specific binding sites, especially for MINU2 (n = 4,433), suggesting that the MAS complexes can bind to a set of genes that are not occupied by the SAS and BAS complexes (Fig. 3B). Similar results were shown for the other complex-specific subunits, such as SWI3D for SAS, SWI3A for MAS, and SWI3C for BAS (Supplemental Fig. S3C).
Figure 3.
SAS, MAS, and BAS subcomplexes show differential binding patterns in genome-wide. A) Venn diagrams displaying statistically significant overlaps among genes occupied by SYD, MINU2, or BRM with those by SWP73B. P-values were calculated by the hypergeometric test. The values in parentheses represent the total number of target genes for this protein. B) Venn diagram displaying statistically significant overlaps among genes occupied by SYD, MINU2, and BRM. P-values were calculated by the hypergeometric test. C) Heatmap representing correlation coefficients between normalized ChIP-seq reads over a merged set of all SWI/SNF subunit peaks. D) SYD, MINU2, and BRM complex ChIP-seq read density distribution over the TSS and 2.5 kb into the gene body at their target genes (biological replicate 1). E) IGV views of ChIP-seq signals of SYD, MINU2, and BRM at representative genes (AT5G53570 and AT4G16490). The dash lines indicated the peak summits (biological replicate 1). F) Pie charts showing the distribution of SYD, MINU2, and BRM peaks at genic and intergenic regions in the genome. G) Metagene plot and heatmap display the distribution of SYD, MINU2, and BRM peaks at genic and intergenic regions in the genome. The black arrow and dashed box indicated the gene body. H) Violin plots depicting the log10(peak length) of SYD, MINU2, or BRM at their corresponding peaks. P-values are from two-tailed Mann–Whitney U test. I) Metagene plot showing the mean occupancy of SYD or BRM at their co-target genes (biological replicate 2). The red arrow indicates the shift sites. J) Violin plots depicting the log2 (no. of mean reads) within ± 0.5 kb of TSS of BRM-GFP and SYD-GFP at their co-target genes, and P-values were determined by the two-tailed Mann–Whitney U test. K) IGV views of ChIP-seq signals of BRM and SYD at representative genes. The diagrams underneath indicate gene structure. The y axis scales represent shifted merged MACS2 tag counts for every 10-bp window. L) Heatmap of CentriMo-log adjusted P-values for top motifs returned by MEME-ChIP analysis for each ChIP-seq experiment. P-values were calculated using the binomial test. The sequence covering 250 bp (SYD and MINU2) or 400 bp (BRM) on either sides of each peak summit were used. M) Gene ontology analysis of MINU2-specific target genes.
Hierarchical clustering performed on ChIP-seq read density over the merged set of peaks across all ChIPs identified distinct, complex-specific enrichment on chromatin (Fig. 3C; Supplemental Fig. S3D). Indeed, SAS and MAS complexes were accumulated near transcription start sites (TSSs) relative to BAS complexes, which were substantially more enriched over gene bodies (Fig. 3, D and E; Supplemental Fig. S3E). Consistently, SYD and MINU2 located more frequently over the promoters of the target genes in comparison to BRM, which showed a preference for binding over exons and introns (Fig. 3, F and G), an observation that differed from the recent data suggesting that BAS tends to locate on genomic regions downstream to MAS-occupied regions (Guo et al. 2022).
When we examined the enrichment patterns of other complex-specific subunits, we found that they exhibited similar distributions to their corresponding ATPases. For instance, the BAS-specific subunits SWI3C, BRIP1, BRIP2, and SWP73A, like BRM, showed more enrichment over gene bodies compared with SWI3D and SWI3A (Supplemental Fig. S3G). Surprisingly, when comparing the binding summit of SWI3C with that of BRM, we found that the SWI3C summit was obviously shifted toward the TSSs, reflecting the different occupancies between SWI3C and the BRM ATPase within the BAS subcomplexes (Supplemental Fig. S3, H and I). In this regard, one possible explanation is that the BAS subcomplexes contain 3 BRDs (BRD1, BRD2, BRD13) in their core module that can recognize histone acetylation on chromatin and a BRM in their ATPase module that may bind DNA through its C-terminal AT-hook domain, respectively (Farrona et al. 2007; Zhao et al. 2018; Yu et al. 2021). Supporting this notion, we found that the width of BAS complexes peaks was significantly larger than that of SAS and MAS complexes (Fig. 3, E and H).
Next, we wanted to assess whether the 3 subcomplexes could mutually regulate their binding positions on chromatin. To this end, we examined the genomic occupancy of the BAS complexes upon the loss of the SAS and vice versa. We introduced the loss-of-function syd-5 mutant into the BRM-GFP transgenic line (ProBRM:BRM-GFP brm-1 syd-5) and the loss-of-function brm-1 mutant into the SYD-N-GFP transgenic line (ProSYD:SYD-N-GFP syd-5 brm-1) and then performed ChIP-seq assays. We found that the SYD occupancy density was decreased upon BRM mutation at the genes that are co-targeted by BRM and SYD, but the average occupancy intensity of BRM was almost the same in the syd-5 background compared with the wild type (WT) (Fig. 3, I–K). However, in the absence of SYD, the BRM binding position appeared to have a considerable shift (from gene body to TSS) at BRM-SYD co-target genes (Fig. 3, I and K), implying that SAS subcomplexes enable BAS subcomplexes to bind to chromatin accurately. The biological significance of the shifted BAS occupancy toward the TSS upon the loss of SAS is currently unclear and warrants future investigation.
Motif analyses revealed a significant central enrichment of the 3 subcomplexes over known transcription factor motifs, including NAC, bZIP, bHLH, BES1, BZR1, FAR1, TCP and BBRBPC. However, BRM preferentially localized to Trihelix and AP2EREBP and specifically localized to HSF, NLP, and ABI3VP1 motifs (Fig. 3L). In addition, MINU2 specifically localized to C2C2COlike (Fig. 3L). These results imply specialized roles for SAS, MAS, and BAS complexes at the corresponding motifs. Furthermore, Gene Ontology (GO) enrichment analysis of the 3 ATPases and core subunits (SWI3D, SWI3A, and SWI3C) showed enrichment of shoot system development, response to light stimulus, growth, and flower development (Supplemental Figs. S3J and S4). Of note, MAS subcomplex core members MINU2 and SWI3A were particularly enriched in many biological pathways different from BAS and SAS subcomplex, such as embryo development, mRNA processing, chromatin organization, and DNA methylation (Fig. 3M; Supplemental Figs. S3J and S4). When we further analyzed the enrichment signals of SAS, MAS, and BAS on the genes involved in DNA methylation regulation, we found that MINU2 and SWI3A, the MAS-specific subunits, were significantly enriched on these genes, while the SAS and BAS showed less or no enrichment (Supplemental Fig. S5). Collectively, these data suggested a potential specific role for the MAS subcomplexes in the regulation of DNA methylation.
We previously showed that BAS complex subunits BRM and BRDs preferentially occupied genomic sites with active histone modification markers (Zhao et al. 2018; Yu et al. 2021). In humans, all 3 BAF subcomplexes were localized to active enhancers and promoters (H3K27ac and H3K4me1) (Michel et al. 2018). When we analyzed the enrichment signals of the active markers (H3K9ac, H3K27ac, H4K5ac, H4K8ac, H3K4me2, H3K4me3, and H3K36me3) and the repressive markers (H3K27me3) at the peaks that are occupied by SYD, MINU2, or BRM, we found that the MINU2 and BRM binding peaks are enriched by the active histone marks but are depleted of the repressive one (Supplemental Fig. S6A). In contrast, the peaks center of SYD lacked H3K27ac, H3K4me2, H3K36me3, and H3K27me3 (Supplemental Fig. S6A). Moreover, SYD peaks displayed a weak Pol II enrichment relative to MINU2 and BRM peaks (Supplemental Fig. S6A). When we repeated this analysis using the subcomplexes co-binding peaks and their unique binding peaks, we observed that the active markers were significantly enriched on the co-binding peaks of SYD-MINU2-BRM, as well as on the MINU2 unique and BRM unique peaks, but were almost not enriched on the SYD unique peaks (Supplemental Fig. S6, B and C). However, there was an enrichment of H3K27me3 signals surrounding the SYD unique peaks (Supplemental Fig. S6, B and C). Moreover, the same results were obtained when we used the complex-specific subunit peaks for the analysis (Supplemental Fig. S7). Together, these results demonstrate plant SWI/SNF complex-specific chromatin localization and indicate a specialized enrichment of SAS over the repressive chromatin regions, which has not been observed in human SWI/SNF complexes.
SWI3D subunit acts in the SAS complexes to regulate genome-wide gene expression
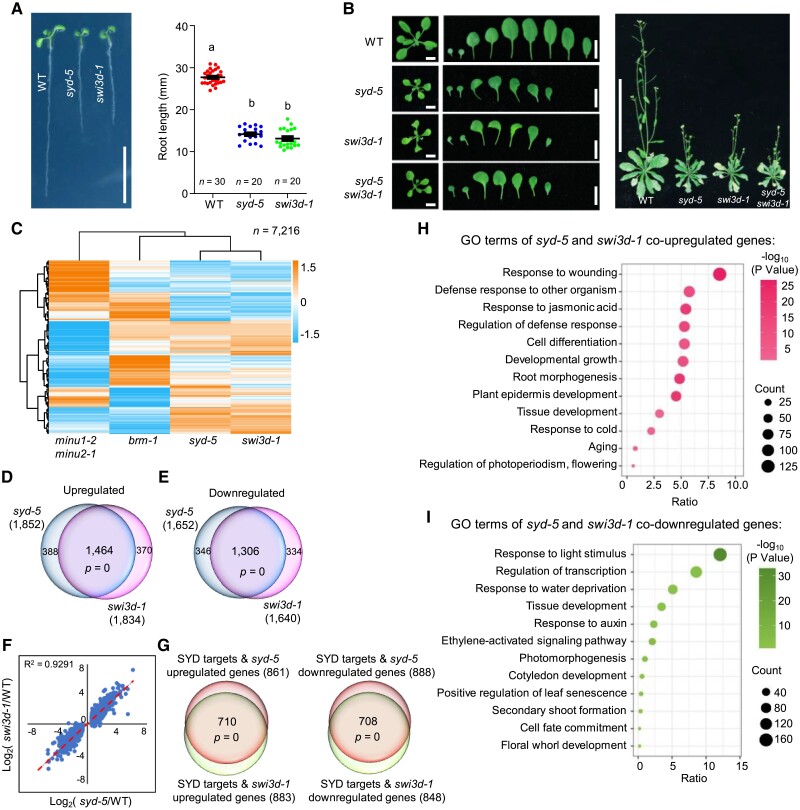
Our biochemical data above suggested that among the 4 SMARCC paralogs in plants, SWI3D is selectively incorporated into the SAS complexes but not the MAS and BAS complexes (Fig. 1). To determine whether SWI3D is functionally relevant to the SAS complexes, we compared the morphological phenotypes between syd-5 and swi3d-1 loss-of-function mutants and observed that the 2 mutant seedlings showed extraordinarily identical phenotypes, including root length, leaf shape, and plant growth status (Fig. 4, A and B; Sarnowski et al. 2005; Shu et al. 2021). Moreover, we introduced the swi3d-1 into the syd-5 and found that the swi3d-1 syd-5 double mutants displayed the same phenotypes as syd-5 and swi3d-1 single mutants, showing that loss of SWI3D did not enhance the phenotypes of the syd-5 null mutant. Thus, SWI3D functions selectively in the SAS complexes with SYD to regulate the plant development processes.
Figure 4.
SWI3D and SYD co-regulate gene expression. A) Root length of 7-d-old seedlings. Scale bars, 1 cm. Lowercase letters indicate significant differences between genetic backgrounds, as determined by the post hoc Tukey's HSD test. The number “n = ” indicates the number of plants that were used. Data are presented as mean ± Sd. B) Left, leaf phenotype of 21-d-old seedlings. Scale bars, 1 cm. Right, seedlings phenotype of 40-d-old seedlings. Scale bars, 10 cm. C) Heatmap showing hierarchical clustering of differentially expressed genes in different mutants (total mis-regulated genes, 7,216). D, E) Venn diagrams showing statistically significant overlaps between genes up- or down-regulated in syd-5 and those in swi3d-1. P = 0, hypergeometric test. F) Scatterplot of log2-fold change values over WT of syd-5 vs. swi3d-1 at genes that were differentially expressed in syd-5. The line of best fit is shown in red, with adjusted R-value indicated. Dots are mean values from 3 biologically independent experiments. G) Venn diagrams showing statistically significant overlaps between the mis-regulated genes targeted by SYD in swi3d-1 and SYD directs regulated genes. P-values were determined by a hypergeometric test. H, I) Gene ontology analysis of genes co-up- or co-down-regulated in syd-5 and swi3d-1.
To corroborate these findings, we analyzed RNA-sequencing (RNA-seq) data comparing the transcriptome of swi3d-1 mutants with that of syd-5, brm-1, and minu1-2 minu2-1 mutant seedlings. This global transcriptional profiling revealed similar effects on gene expression between swi3d-1 and syd-5 mutants, whereas loss of BRM or MINU1 and MINU2 (BAS- and MAS-ATPase, respectively) resulted in discordant transcriptional effects (Fig. 4C). Indeed, approximately 80% of the 1,834 up-regulated and 1,640 down-regulated genes in swi3d-1 (1,462 and 1,306, respectively) exhibited the same direction of mis-regulation in syd-5 mutants (Fig. 4, D and E). The transcriptome of swi3d-1 was strongly positively correlated with that of mutants syd-5 (correlation coefficient R2 = 0.9291) (Fig. 4F). Furthermore, genes mis-regulated in syd-5 or swi3d-1 significantly overlapped with SYD target genes (Supplemental Fig. S8). Moreover, the SYD target genes mis-regulated in swi3d-1 showed a very high overlap with those mis-regulated in syd-5 mutants (Fig. 4G). GO term analysis showed that genes regulated by SYD and SWI3D are involved in regulating the development of tissues and organs and responding to wounding and auxin (Fig. 4, H and I). Together, these data demonstrate that SWI3D and SYD are in the same complex to regulate gene expression in Arabidopsis.
SWI3D is required for the assembly and integrity of SAS subcomplexes
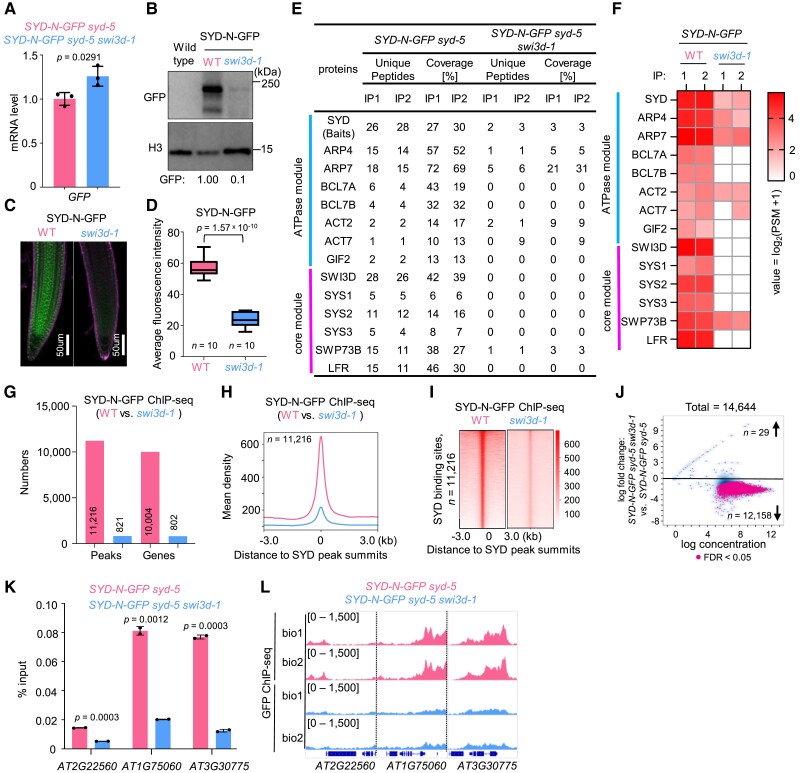
In mammals, the assembly of the SWI/SNF complexes is initiated by the formation of a “core module” that is constituted by 2 SMARCC and 1 SMARCD subunits. This initial trimer then acts as a platform for docking other subunits for assembly toward fully formed SWI/SNF complexes (Mashtalir et al. 2018). Because Arabidopsis SWI3D is a putative paralog of SMARCCs in mammals (Fig. 2A), we thought to biochemically evaluate the effects of SWI3D loss on SAS protein complex assembly and integrity. To this end, we introduced the SYD-GFP transgene into the swi3d-1 mutant background and assessed the abundance and integrity of SAS complexes using immunoprecipitation.
The loss of SWI3D did not reduce the messenger RNA levels of SYD-GFP (Fig. 5A) but resulted in a substantially reduced protein abundance of SYD-GFP (Fig. 5, B–D). Furthermore, immunoprecipitation of SYD-GFP followed by mass spectrometry analysis showed that loss of SWI3D resulted in significantly reduced peptides corresponding to SYD and near-complete degradation of SAS subcomplex components (Fig. 5, E and F). Notably, the protein level of BRM, the BAS-specific ATPase, showed no change in the swi3d-1 background compared to WT (Supplemental Fig. S9), confirming the specific down-regulation of the SAS integrity by SWI3D mutation. Together, these data demonstrate a crucial role for the core module subunit SWI3D in the stabilization of the SAS subcomplexes and imply that SWI3D may be a part of the core module that is also necessary for the assembly of SWI/SNF complexes in Arabidopsis.
Figure 5.
SWI3D is essential for maintaining SAS subcomplex integrity. A) The mRNA levels of SYD-N-GFP were determined by RT-qPCR in WT and swi3d-1 background. ACTIN2 was amplified as an internal control. Error bars are presented as mean values ± Sd from 3 biological replicates. B) Immunoblot analysis showing the relative protein levels of SYD-N-GFP in WT and swi3d-1. The numbers at the bottom represent amounts normalized to the loading control, histone H3 (average of 3 independent replicates, ± Sd). WT was used as a GFP-free control. C) Confocal images of root tips showing nuclear localization of the SYD-N-GFP fusion protein in a WT and a swi3d-1 background, respectively. The red fluorescent signal is derived from propidium iodide staining. D) Box plot showing the average fluorescence intensity of SYD-N-GFP in WT and swi3d-1 mutants. The “n = ” indicates the number of roots used. The boxes indicate the first and third quartiles, and the lines in the boxes indicate median values. Significant differences were determined by an unpaired, two-tailed Student's t-test. E) Unique peptide numbers of SAS subcomplex subunits identified by IP-MS in SYD-N-GFP under WT and swi3d-1 background. F) Heatmap showing the log2(PSM + 1) values of representative SAS subcomplex subunits identified by IP-MS in SYD-N-GFP under WT and swi3d-1 background. G) Number of SYD binding sites (number of peaks or genes) in the WT and swi3d-1 background. H, I) Metagene plot (H) and heatmap (I) represented the mean density of SYD occupancy at all SYD-occupied sites in swi3d-1 compared with WT. The average SYD binding signals within 3 kb genomic regions flanking SYD peak summits were shown (biological replicate 1). J) Fold change (log2) in SYD occupancy between WT and swi3d-1 background. Occupancy changes with a false discovery rate (FDR)of < 0.05 are highlighted. FDR values are multiple test-corrected Wilcoxon test P-values, 2 biological replicates. K) ChIP-qPCR validation of SYD occupancy at representative target genes using ChIP DNA samples independent from those used for ChIP-seq. The AT2G22560 was used as the negative control. Error bars are presented as mean values ± Sd from 2 biological replicates. Unpaired, two-tailed Student's t-test. L) IGV views of SYD occupancy at selected loci in the WT and swi3d-1 background. The y axis scales represent shifted merged MACS2 tag counts for every 10 bp window.
We next performed ChIP-seq assays comparing the genome-wide occupancy of SYD in WT with that in swi3d-1 to assess the impact of SWI3D loss on the genome-wide targeting of the SAS complexes. In line with our biochemical findings, we observed substantial attenuation in SAS complex occupancy on the genome. The numbers of SYD-associated genomic sites and corresponding genes were extremely decreased in swi3d-1 compared to WT (Fig. 5G). Consistently, the occupancy of SYD on the target genes was nearly abolished in swi3d-1 (Fig. 5, H and I). Furthermore, at more than 90% of SYD binding sites, we found a marked reduction in, or elimination of, SYD occupancy in the absence of SWI3D, while only 29 loci showed an increase (Fig. 5J). Independent ChIP-qPCR confirmed the reduction of SYD occupancy at individual loci in swi3d-1 (Fig. 5, K and L). These data indicate that SWI3D loss disrupts SYD occupancy on chromatin and target gene expression.
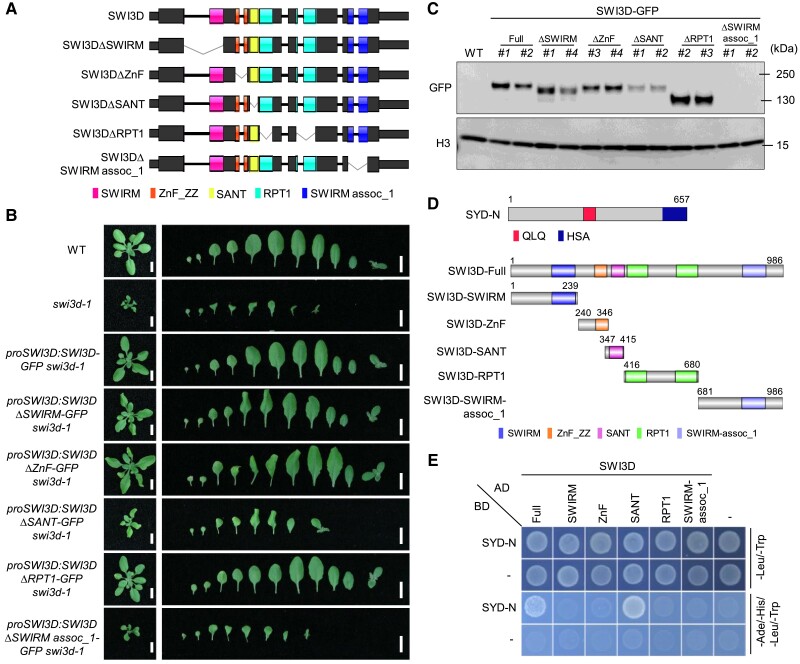
The SANT and SWIRM assoc_1 domains of SWI3D are required for the stability of SAS subcomplexes
We next sought to identify specific regions on SWI3D that uniquely underlie its function in SAS complex stability maintenance. SWI3D contains 5 conserved domains, including SWIRM, ZnF, SANT, RPT1, and SWIRM_assoc_1. We generated individual domain-truncated SWI3D fragments tagged by GFP and stably expressed them in the swi3d-1 mutant background (Fig. 6A). Deletion of the RPT1 domains did not affect the ability of SWI3D to rescue the swi3d-1 mutant phenotypes (Fig. 6B), indicating that the RPT1 domain is dispensable for the function of SWI3D, at least under our growth conditions. However, the loss of the SWIRM or ZnF domain partially compromised the function of SWI3D in rescuing the swi3d-1 mutant phenotypes (Fig. 6B), suggesting that the SWIRM and ZnF domains are necessary for the full function of SWI3D. Interestingly, previous studies in S. cerevisiae showed that mutation of the SWIRM domain of Swi3 (a yeast homolog of SWI3D) resulted in growth defects and impaired DNA-binding activity in vitro (Da et al. 2006), implying a conserved role for this domain in SWI/SNF complexes. In contrast, the deletion of SWIRM_assoc_1 caused a complete failure to restore the morphological defects of swi3d-1, while SWI3D truncation lacking the SANT domain only slightly recovered the phenotypes (Fig. 6B). These data highlight the essential function of the SANT and SWIRM_assoc_1 domains of SWI3D in SAS complexes.
Figure 6.
The SANT and SWIRM assoc-1 domains are required for maintaining the stability of SWI3D protein. A) Schematic illustration of the SWI3D protein and its truncated versions. B) Leaf phenotype of 21-d-old seedlings. Scale bars, 1 cm. C) Immunoblot analysis using an anti-GFP antibody shows the accumulation of SWI3D protein and its truncated versions. For each blot, the antibody used is indicated on the left, and the sizes of the protein markers are indicated on the right. H3 serves as a loading control. D) Schematic illustration of the N-terminal of SYD protein, SWI3D, and the different truncated versions of SWI3D. The number in the figures indicated the start or end sites of the proteins (SYD or SWI3D). SYD-N: the 1-657aa of SYD. E) Y2H assay to examine the direct interaction between SYD and SWI3D. Growth of transformed yeast on permissive SD−Ade-His-Leu-Trp medium indicates interaction.
To understand the role of SANT and SWIRM_assoc_1 in the SAS complexes, we examined the protein levels of the truncated SWI3D variants. We observed that the deletion of the SANT domain resulted in a reduction in the level of SWI3D protein, and the deletion of the SWIRM_assoc_1 domain led to a failure to accumulate SWI3D protein (Fig. 6C). Notably, the decrease in their protein contents was not caused by the reduction of their transcription levels (Supplemental Fig. S10A). Interestingly, the SANT and SWIRM assoc_1 domains are evolutionarily conserved in different eukaryotes, suggesting that their role in modulating SWI3D protein abundance could possibly be a conserved function (Supplemental Fig. S10B).
To further elaborate on this hypothesis, we performed yeast two-hybrid (Y2H) assays to test which domain(s) of SWI3D interacts with the SYD (Fig. 6D). We found that only the SANT domain, but not the other domains, is required for interaction with SYD ATPase and serves as a SAS binding domain (Fig. 6E). SANT domain deletion caused changes in gene expression in the same direction as swi3d-1 mutant, albeit the change was mostly smaller than that of swi3d-1 mutant, which was consistent with that deletion of SANT domain could marginally complement the phenotype of swi3d-1 mutant (Supplemental Fig. S10C). Together, these results demonstrate that the SWI3D SANT domain is a SAS-complex-binding domain that underlies the critical role of SWI3D in the assembly of the SAS complexes.
The SYD ATPase module is required for the stability of the core module of the SAS subcomplex
Our data so far showed that the core module subunit SWI3D of SAS complexes plays a vital role in regulating the integrity of the complex. In mammals, recent studies indicated that loss of ATPases results in a specific disruption of the ATPase module but has no effect on the assembly of the core module (Mashtalir et al. 2018; Pan et al. 2019). However, our above data showed that in Arabidopsis, disruption of SWI3D-SYD interaction by deleting the SWI3D SANT domain leads to a decrease in SWI3D protein abundance. We, therefore, speculated that in contrast to their human paralogs, ATPase subunits in plants might be required to stabilize the core module.
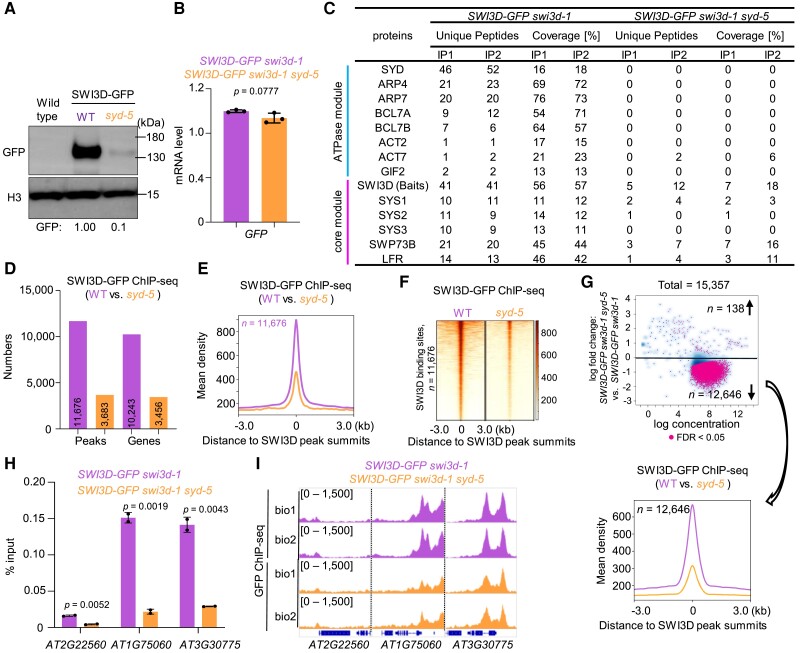
To test this possibility, we introduced the loss-of-function syd-5 mutant into the SWI3D-GFP transgenic line. Surprisingly, we found that the SWI3D-GFP protein levels were dramatically decreased in the absence of the SYD ATPase (Fig. 7A); however, the expression level of SWI3D-GFP mRNA did not change significantly (Fig. 7B). Furthermore, we conducted IP-MS experiments and found that in syd-5 mutants, the ATPase module subunits (such as ARP4, ARP7, BCL7A, and BCL7B) were not co-immunoprecipitated and less effectively immunoprecipitated than the core module subunits (such as SWP73B and LFR) (Fig. 7C). These results indicate that the ATPase in SAS subcomplexes plays an essential role in the stability of the core module.
Figure 7.
The ATPase module is required for the stability of the core module. A) Immunoblot analysis showing the relative protein levels of SWI3D-GFP in WT and syd-5 background. The numbers at the bottom represent amounts normalized to the loading control, histone H3. WT was used as a GFP-free control. B) The mRNA levels of SWI3D-GFP were determined by RT-qPCR in WT and syd-5 background. ACTIN2 was amplified as an internal control. Error bars are presented as mean values ± Sd from 3 biological replicates. C) Unique peptide numbers of SAS subcomplex subunits identified by IP-MS in SWI3D-GFP under WT and syd-5 background. D) Number of SWI3D binding sites (number of peaks or genes) in the WT and syd-5 background. E, F) Metagene plot (E) and heatmap (F) represented the mean density of SWI3D occupancy at all SWI3D-occupied sites in syd-5 compared with WT. The average SWI3D binding signals within 3 kb genomic regions flanking SWI3D peak summits were shown (biological replicate 1). G) Fold change (log2) in SWI3D occupancy between WT and syd-5 background. Occupancy changes with FDR of < 0.05 are highlighted. FDR values are multiple test-corrected Wilcoxon test P-values, 2 biological replicates per ChIP. H) ChIP-qPCR validation of SWI3D occupancy at representative target genes using ChIP DNA samples independent from those used for ChIP-seq. The AT2G22560 was used as the negative control. Error bars are presented as mean values ± Sd from 2 biological replicates. Unpaired, two-tailed Student's t-test. I) IGV views of SWI3D occupancy at selected loci in the WT and syd-5 background. The y axis scales represent shifted merged MACS2 tag counts for every 10 bp window.
To test whether the requirement of ATPase for the stability of the complex is not restricted to SAS complexes but a widespread phenomenon for plant SWI/SNF complexes, we examined the consequences of BRM loss on the stability of SWI3C core module subunit in BAS complexes. We introduced the loss-of-function brm-1 mutant into the SWI3C-GFP transgenic line and performed IP-MS assays. Upon the loss of BRM, SWI3C barely immunoprecipitated ATPase module subunits ARP4, ARP7, BCL7A, and BCL7B and less effectively immunoprecipitated the core subunits BRIP1, BRIP2, BRD2, BRD13, and SWP73B (Supplemental Fig. S11, A and B). Furthermore, we performed an immunoblotting assay, which again showed the reduced protein abundance of SWI3C-GFP (one of the core module subunits) in the absence of BRM (Supplemental Fig. S11C). However, the expression level of SWI3C-GFP did not change significantly (Supplemental Fig. S11D). Together, these data demonstrate that the ATPase module enables the full assembly of the core module of Arabidopsis SWI/SNF complexes, a plant-specific feature distinct from animal SWI/SNFs.
Finally, we tested the genomic binding of SWI3D in the syd-5 mutants. ChIP-seq results showed that compared with the enrichment signals of SWI3D in the wild-type background, the number and the signal intensity of SWI3D binding peaks in the genome decreased sharply in syd-5 (Fig. 7, D–G). Independent ChIP-qPCR confirmed the reduction of SWI3D occupancy at individual loci in syd-5 mutant (Fig. 7, H and I). However, a subset of genomic sites was still occupied by SWI3D in the absence of SYD ATPase (Fig. 7, D and E). Interestingly, we found that the SYD-independent SWI3D binding sites were more accessible than the SYD-dependent SWI3D binding sites (Supplemental Fig. S12), possibly underscoring the different dependencies on the SYD ATPase in SAS genomic targeting.
Discussion
In this study, we comprehensively investigated the 3 distinct Arabidopsis SWI/SNF subcomplexes (SAS, MAS, and BAS), each containing complex- and plant-specific subunits that probably define their identity (Figs. 1 and 2). We also systematically compared the chromatin binding profiles for the 3 subcomplexes relative to defined chromatin features (Fig. 3). The SAS, MAS, and BAS subcomplexes showed differential occupancy patterns on target genes, associated unique cis-motifs, and localized to chromatin with different histone modifications, possibly underlying complex-specific functions on chromatin. Consistent with these results, Guo et al. (2022) recently reported that the 3 Arabidopsis SWI/SNF subcomplexes bind to both overlapping and distinct genomic sites to regulate gene expression and chromatin accessibility.
In addition to those similar results, we further revealed that the loss of SAS complexes results in an unexpected shift of the BAS occupancy from gene body to TSSs, broadening our understanding of how the genomic targeting of different SWI/SNF subcomplexes is coordinated for chromatin structure regulation (Fig. 3, I and K). Moreover, we define the role of the core module subunits SWI3D in maintaining the stability/assembly of the SAS complexes, revealing the molecular function of these subunits in the complexes (Figs. 5 and 6). Finally, our work highlights that the ATPase module subunit is required for complex stability and the interaction of core module subunits in SAS and BAS complexes in Arabidopsis (Fig. 7). Together, our results and those reported by Guo et al. provide a critical foundation for further structural and functional characterization of this family of plant chromatin remodeling complexes that play crucial roles in regulating plant development and signaling responses through chromatin modulation. These results highlight and reinforce the power of examining organization, assembly, and genomic targeting to advance our mechanistic understanding of SWI/SNF-mediated chromatin remodeling in plants.
In the past two decades, numerous investigations on plant SWI/SNF complexes subunits have revealed various phenotypes caused by the perturbation of the subunits. However, due to the lack of information regarding the subunit composition of plant SWI/SNF complexes, our understanding of the mechanisms by which these subunits regulate plant development and responses to environmental stimulus has been severely affected. Moreover, in vitro protein–protein interaction assays may not identify endogenous, physiologically relevant complex-specific subunits. For example, previous Y2H assays showed the interaction between SWI3D and BRM (Bezhani et al. 2007). Here, our biochemical data demonstrated that SWI3C, but not SWI3D, is a BAS-specific subunit (Fig. 1), which explains the similar phenotypes between swi3c and brm mutants, rather than between swi3c and syd or minu mutants (Wagner and Meyerowitz 2002; Archacki et al. 2009; Sang et al. 2012). Consistently, we found that the phenotype and transcriptome of swi3d-1 are reminiscent of syd-5, in support of the notion that SWI3D and SYD are in the SAS complexes. Moreover, the swp73a single mutant showed no phenotypes compared with the wild-type, but the swp73b single mutation caused severe alterations in the development of vegetative and reproductive organs (Sacharowski et al. 2015; Huang et al. 2021). Our data showed that SWP73B exists in all 3 SWI/SNF subcomplex, but SWP73A belongs only to the BAS subcomplexes. Thus, in the swp73a mutant, SWP73B could act in the 3 subcomplexes to maintain plant growth and development. However, in the absence of SWP73B, both SAS and MAS subcomplexes were disintegrated, explaining the strong developmental disorders. Overall, our data underscore the importance of comprehensively defining the subunit composition of the complexes to illustrate subunit functions within the context of subcomplexes.
One particularly unexpected result is that the ATPase module (SYD or BRM) is required for global complex stability and the interaction of core module subunits in SAS and BAS complexes in Arabidopsis. Previous biochemical and structural studies in humans showed that the mammalian ATPase module is the last to be incorporated into SWI/SNF complexes and therefore not required for the core module assembly (Mashtalir et al. 2018; Michel et al. 2018; Pan et al. 2019). Thus, our studies suggest an alternative, plant-specific assembly pathway in SWI/SNF complexes, again highlighting the separation and divergence of SWI/SNF complexes in eukaryotes.
In humans, the subunits of SWI/SNF complexes are combinatorially assembled into 3 classes of complexes: cBAF, PBAF, and ncBAF (Gatchalian et al. 2018; Mashtalir et al. 2018). Previous studies have indicated that the cBAF complexes are more localized to distal enhancer regions to regulate chromatin accessibility, while ncBAF and PBAF complexes exhibited a promoter and gene body distribution, respectively, to enhance accessibility (Michel et al. 2018; Schick et al. 2021). We speculate that the 3 Arabidopsis SWI/SNF complexes (SAS, MAS, and BAS) may be localized at distinct genomic regions to shape local chromatin accessibility and ultimately regulate gene expression. In support of this speculation, it was recently reported that the SAS-dependent accessible regions are found at the distal promoter and intergenic regions, whereas the MAS- and BAS-dependent accessible regions are mainly observed near proximal promoter regions and 5′ untranslated region but are depleted at the distal promoter or intergenic regions (Guo et al. 2022). Moreover, given that SAS, MAS, and BAS complexes are localized to differential transcription factor motifs (Fig. 3L), the different sets of transcription factors may control chromatin accessibility through different classes of Arabidopsis SWI/SNF complexes.
Intriguingly, PSA1 (also termed MIS), PSA2 (also termed SSM), SHH2, and SYSs exist as conserved, plant-specific SWI/SNF subunits, suggesting an evolutionarily conserved function for those subunits that could not be appreciated by conventional sequence conservation analyses. The PSA1, PSA2, and SHH2 are specific subunits of the MAS complexes, which also contain 4 PHD proteins, TPF1 (also named PMS2B), TPF2 (also named PMS2A), OPF1 (also named PMS1A), and OPF2 (also named PMS1B) (Diego-Martin et al. 2022; Guo et al. 2022). It was recently reported that the PHD domain of TPF1 and TPF2 is similar to the well-characterized H3K4me3-binding PHD domains in the other proteins, and TPF1 can bind to H3K4me3 in vitro (Guo et al. 2022). In contrast, the OPF1 does not recognize H3K4me3 in vitro (Guo et al. 2022). Thus, it is tempting to speculate that, among 4 PHD domain proteins, only TPF1 and TPF2 may play a critical role in the binding of the MAS complex to target genes. Because PSA1 and PSA2 have no obviously recognizable domains, and TPF1 and TPF2 may function in genomic targeting, we envisage that PSA1 and PSA2 may contribute to maintaining the stability or activities of the MAS complexes.
Besides, compared with SAS and BAS, the MAS subcomplexes showed a preferential occupancy at genes involved in the regulation of DNA methylation (Fig. 3M; Supplemental Figs. S4 and S5). Previous studies have indicated that SWI3B, which we found to be a MAS-specific subunit, acts to promote DNA methylation at a subset of genomic regions (Yang et al. 2020). Moreover, the MAS complexes contain an SHH2 subunit, whose paralog, SHH1, functions as a core component of RNA-directed DNA methylation by assisting in the recruitment of Pol IV (Law et al. 2013; Zhang et al. 2013). Together, these observations imply that the MAS subcomplexes may play a specialized role in regulating DNA methylation, which warrants future investigation.
Interestingly, the SAS-specific subunits SYSs also have no known conserved domains. Given that we have demonstrated that the SAS complex-specific subunit SWI3D is essential to maintain the stability of the SAS complex, we propose that SYSs may be mainly responsible for the SAS complex to recognize and bind target genes. Future functional and mechanistic characterization of these plant-specific subunits would provide insight into the roles of plant SWI/SNF complexes in chromatin regulation.
A recent study demonstrated that the abundance of SWI/SNF complexes in human cells is not static but can be dynamically altered as a response to environmental changes (Tran et al. 2022). For example, the protein levels of PBAF-specific subunits ARID2 and PBRM1, and of ncBAF-specific subunit BRD9 are down-regulated in response to hypoxic stresses, while all cBAF-specific members are retained. Here, our GO analysis of the 3 plant SWI/SNF subcomplexes revealed varying degrees of enrichment in defense response to bacterium, response to salt stress, immune system process, response to light stimulus, and cellular response to phosphate starvation, and so on. Thus, we speculate that the abundances of the 3 plant SWI/SNF subcomplexes may be dynamic rather than static in response to multifarious environmental signals for timely and precise regulation of gene expression.
Finally, our proteomic analysis showed that some homologous subunits in SAS, MAS, or BAS are mutually exclusive in the complexes. For example, in SAS complexes, the 3 SYSs (SYS1, SYS2, SYS3) are separately incorporated into the SAS complexes (Fig. 1B). Similarly, TPF1, the MAS-specific subunit, did not exist in the TPF2-containing MAS complexes (Diego-Martin et al. 2022). Finally, BAS-specific subunit BRIP1 cannot co-immunoprecipitated with BRIP2, and BRD1 cannot co-immunoprecipitated with BRD2 and BRD13 (Fig. 1B). These observations suggest that the existence of multiple subunit paralogs across these 3 distinct SWI/SNF subcomplexes may result in further diversification. Based on the multiple subunit paralogs across the 3 SWI/SNF subcomplexes, we calculated the complete set of possible combinations (Supplemental Fig. S13), in which there are at least 36 possible combinations of SAS subcomplexes, 288 possible combinations of MAS subcomplexes, and 144 possible combinations of BAS subcomplexes in Arabidopsis. Investigation of these diverse subcomplexes will likely further enhance our understanding of the plant SWI/SNF-mediated chromatin structure and gene regulation.
Materials and methods
Plant materials and growth conditions
Transfer DNA insertion lines, minu2-1 (SALK_057856), swi3a-3 (SALK_068234), bsh (SALK_058513), syd-5 (SALK_023209), swi3d-1 (SALK_100310), swp73a-2 (SALK_083920), swp73b-1 (SALK_113834), sys2-1 (SALK_109947), sys3-1 (SALK_010574), bcl7a-1 (SALK_027934), and bcl7b-1 (SALK_029285) were obtained from the Arabidopsis Biological Resource Center (ABRC). Mutants brm-1 (SALK_030046), ProBRM:BRM-GFP brm-1 and ProSYD:SYD-N-GFP syd-5 transgenic plants were previously described (Li et al. 2016; Shu et al. 2021). Primers used for genotyping are listed in Supplemental Data Set 1.
For RT-qPCR/RNA-seq, ChIP-qPCR/ChIP-seq, and IP-MS assays, A. thaliana seeds were sterilized with 20% (v/v) sodium hypochlorite solution for 15 min, washed with sterile water 5 times, and then stratified at 4 °C in darkness for 3 d. Seeds were then sown on 1/2 Murashige and Skoog (MS) medium containing 1% (w/v) sucrose and 0.6% (w/v) agar. For phenotypic analysis, seeds were sown on a mixture of soil and vermiculite (1:1). Seedlings were grown under long-day conditions under cold white light (16 h light/8 h dark, ∼120 μmol m−2 s−1) at 22 °C.
Generation of transgenic plants
Full-length genomic regions of SWI3A, SWI3B, SWI3C, SWI3D, SWP73A, BSH, MINU2, SYS1, SYS2, SYS3, SWI3DΔSWIRM, SWI3DΔZnF, SWI3DΔSANT, SWI3DΔRPT1, and SWI3DΔSWIRM_assoc_1, driven by their native promoters, were cloned into pCAMBIA1302 (Abcam) vector by using ClonExpress Ultra One Step Cloning Kit (Vazyme, Cat. No. C115-01) and ClonExpress MultiS One Step Cloning Kit (Vazyme, Cat. No. C113-01). Full-length genomic regions of BCL7A, BCL7B, and SWP73B, driven by their native promoters were amplified from genomic DNA by PCR, then cloned into the pDONR221 vector by BP reaction (Invitrogen), and further subcloned into the destination plasmid pMDC107 (Curtis and Grossniklaus 2003) by LR reaction (Invitrogen). The constructs were introduced into Agrobacterium tumefaciens strain GV3101 (Chemically Competent Cell, Tsingke, Cat. No. TSC-A01) and were then used to transform corresponding single mutant or WT (e.g. SWI3B, SWI3C, and SYS1) plants using the floral dip method (Clough and Bent 1998). To obtain BRM-GFP brm-1 syd-5 and BRM-GFP brm-1 swi3d-1 transgenic plant, the BRM-GFP brm-1 transgenic plant was crossed with syd-5 and swi3d-1 single mutant. Similar to this, the SYD-N-GFP syd-5 transgenic plant was crossed with brm-1 single mutant to obtain SYD-N-GFP syd-5 brm-1 transgenic plant, the SYD-N-GFP syd-5 transgenic plant was crossed with swi3d-1 single mutant to obtain SYD-N-GFP syd-5 swi3d-1 transgenic plant, the SWI3C-GFP transgenic plant was crossed with brm-1 single mutant to obtain SWI3C-GFP brm-1 transgenic plant, and the SWI3D-GFP swi3d-1 transgenic plant was crossed with syd-5 single mutant to obtain SWI3D-GFP swi3d-1 syd-5 transgenic plant. Primers used for constructing are listed in Supplemental Data Set 1.
Y2H assay
The full-length or truncated coding regions of SYD (1–657aa) or SWI3D (full-length CDS, 1–239aa, 240–346aa, 347–415aa, 416–680aa, 681–986aa, respectively) were cloned into pGADT7 (AD) or pGBKT7 (BD) (Clontech) through the EcoRI cleavage site. Then the AD and BD plasmids were co-transformed into the yeast (S. cerevisiae) strain AH109 (preserved in our laboratory) and spread on the medium lacking leucine (Leu) and tryptophan (Trp) (SD-Leu/-Trp). Positive colonies on SD-Leu/-Trp medium were further picked up and dropped on the selection medium lacking adenine (Ade), histidine (His), Leu, and Trp (SD-Leu/-Trp/Ade/His). Primers used for constructing are listed in Supplemental Data Set 1.
Confocal microscopy
The GFP signals from the root tips of transgenic plants were observed using the LSM880 microscope. GFP was excited at 514 nm and detected at 517 to 557 nm with 50% intensity and 700 gains value under 20× objective. The average fluorescence intensity of the GFP signals was calculated by the Histo function of the LSM880 microscope. pProdidium iodide (PI) was used to display plasma membrane of the root tip. PI is detected under 535 to 615 nm.
Co-immunoprecipitation and mass spectrometry
For IP-MS, about 5 g of 14-d-old seedlings grown under long-day conditions were harvested and ground to a fine powder in liquid nitrogen. Then, the powder was collected and homogenized in 10 mL of lysis buffer (50 mM HEPES [pH 7.5], 300 mM NaCl, 10 mM EDTA, 1% [v/v] Triton X-100, 0.2% [v/v] NP-40, 10% [v/v] glycerol, 2 mM DTT, 1× Complete Protease Inhibitor Cocktail [Roche]) at 4 °C for 30 min. After centrifugation at 11,600 × g and 4 °C for 15 min (twice), the supernatant was diluted by equal volume dilution buffer (50 mM HEPES [pH 7.5], 10 mM EDTA, 10% [v/v] glycerol, 2 mM DTT, 1× Complete Protease Inhibitor Cocktail [Roche]) and then incubated with GFP_trap beads (Cat. No. KTSM1301) at 4 °C for 3 h with gentle rotation. Beads were then washed 3 times with washing buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10 mM EDTA, 0.2% [v/v] Triton X-100, 0.1% [v/v] NP-40, 10% [v/v] glycerol). Proteins were eluted in SDS loading buffer and incubated at 55 °C for 10 min, followed by immunoblotting or silver staining. For data shown in Supplemental Fig. S2, Benzonase (E1014, Sigma) was added into the protein samples (50 units per mL extract) and incubated for 1 h at 4 °C to remove DNA/RNA before immunoprecipitation.
For mass spectrometry, the immunoprecipitated proteins were eluted using 0.2 M glycine solution (pH 2.5), and then subjected to reduction with dithiothreitol, alkylation with iodoacetamide, and digested with trypsin (Thermo Fisher, Cat. No. 90057, MS grade). The samples were analyzed on a Thermo Scientific Q Exactive HF mass spectrometer. Spectral data were searched against the TAIR10 database using Protein Prospector 4.0. Two or three independent biological replicates were included in the IP-MS analysis. GFP-tagged transgenic seedlings were grown side by side and then randomly collected for experiments. This process was repeated one or two more times. Default settings for Label-free quantitation (LFQ) analysis using MaxQuant (Tyanova et al. 2016a) and Perseus (Tyanova et al. 2016b) software were applied to calculate the LFQ intensities with default settings.
Nuclear protein extraction
For nuclear protein extraction, 0.2 g of 14-d-old seedlings grown on 1/2 MS medium were ground to a fine powder in liquid nitrogen to extract the nucleoproteins. Nuclei were isolated following the ChIP protocol (Li et al. 2016) without tissue fixation. Briefly, nuclear proteins were released by incubating the nuclei preparation in 200 μL of lysis buffer (50 mM Tris-HCl, 10 mM EDTA, 1% [v/v] SDS, and 1× protease inhibitors) for 3 h at 4 °C.Then, the extract was diluted with an equal volume of ChIP dilution buffer (16.7 mM Tris-HCl [pH 8.0], 167 mM NaCl, and 1.1% [v/v] Triton X-100) and centrifuged at 15,000 × g for 10 min at 4 °C to remove debris, followed by immunoblotting.
Immunoblotting
Proteins were loaded onto 4% to 20% gradient protein gels (GenScript, SurePAGE, Cat. No. M00655) and 4% to 20% Precast Protein Plus Gel (Yeasen, Cat. No. 36256ES10) at 120 V for 2 h. A wet transformation was performed at 90 V for 90 min in an ice-cold transfer buffer. After that, the membranes were blocked in 5% (w/v) nonfat milk at room temperature for 1 h on a shaking table (100 g). Finally, the blocked membranes were incubated in the corresponding antibody solutions at room temperature for another 3 h. The following antibodies were used: anti-GFP (Abcam, Cat. No. ab290, 1:10,000 dilution) and anti-H3 (Proteintech, Cat. No. 17168-1-AP, 1:10,000 dilution). The intensities of blotting signals were quantified using ImageJ software (v.1.50i). Uncropped scans of immunoblotting results are shown in Supplemental Fig. S14.
Silver staining
For silver staining, samples were run on a 4% to 20% gradient protein gels (GenScript, SurePAGE, Cat. No. M00655) and stained with Fast Silver Stain Kit (Beyotime, Cat. No. P0017S) according to the manufacturer's instructions.
RNA isolation, RT-qPCR, and RNA-seq analyses
Total RNA was extracted from 14-d-old Arabidopsis seedlings grown under long-day conditions using the HiPure Universal RNA Mini Kit (MAGEN, Cat. No. R4130) according to the manufacturer's instructions. For RT-qPCR, 1 μg RNA was used for DNase digestion and reverse transcription using HiScript III 1st Strand cDNA Synthesis Kit (+gDNA wiper) (Vazyme, Cat. No. R312-01). The transcribed cDNA underwent qPCR assays using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Cat. No. Q711-02) in the StepOne Plus (Applied Biosystems). Results were repeated with 3 biological replicates. Wild-type and mutant seedlings were grown side by side and then randomly collected for experiments. This process was repeated two more times. Quantification was analyzed with the relative −ΔΔCt method (Livak and Schmittgen 2001). ACTIN2 was served as the control for mRNA analyses. The sequences of primers used are listed in Supplemental Data Set 1.
For RNA-seq analyses, RNA from 3 biological replicates was sequenced separately at Novogene (sequencing method: nova-seq PE150). Wild-type and mutant seedlings were grown side by side and then randomly collected for experiments. This process was repeated two more times. After the removal of adapters and low-quality reads, the clean reads were mapped to the TAIR10 Arabidopsis genome using TopHat (v2.1.1) with default settings (Kim et al. 2013), except that a minimum intron length of 20 bp and a maximum intron length of 4,000 bp were required. Mapped reads were then assembled according to the TAIR10 version of genome annotation using Cufflinks (v.2.1.1) with default settings (Trapnell et al. 2012). For analysis of differential expression, the assembled transcripts from 3 independent biological replicates in WT and other mutants (syd-5 and swi3d-1) were included and compared using Cuffdiff (v.2.1.1) with default settings (Trapnell et al. 2012). Finally, genes with at least 1.5-fold change in expression (P < 0.05) were considered differentially expressed (see Supplemental Data Set 2 for details). To calculate the significance of the overlap of 2 groups of genes drawn from the set of genes, the total number of genes in the Arabidopsis genome used was 34,218 (27,655 coding and 6,563 noncoding genes) according to EnsemblPlants (http://plants.ensembl.org/index.html).
ChIP and ChIP-seq analysis
ChIP experiments were performed as previously described (Gendrel et al. 2005; Li et al. 2016; Yu et al. 2021) with minor changes. In brief, 14-d-old seedlings (0.5 g for each biological replicate) grown on 1/2-strength MS medium under long-day conditions were fixed using 1% (v/v) formaldehyde under a vacuum for 15 min and then ground into a fine powder in liquid nitrogen. The chromatin was sonicated into 300 to 500 bp fragments using a Bioruptor sonicator with a 30/45-s on/off cycle (27 total on cycles). Immunoprecipitation was performed using 1 μL of anti-GFP (Abcam, Cat. No. ab290) plus 40 μL Agarose beads (Abcam, Cat. No. 16-157) at 4 °C overnight. Next day, the beads were washed subsequently with low-salt buffer, high-salt buffer, ChIP wash buffer, and TE buffer, and then the immunoprecipitated chromatin were eluted with Elution buffer. Finally, after the eluate was subsequently subjected to reverse crosslinks, RNase digestion, and Proteinase K digestion, the DNA was purified by phenol/chloroform/isoamyl. ChIP-qPCR was performed with 3 biological replicates, and the results were calculated as a percentage of input DNA according to the Champion ChIP-qPCR user manual (SABioscience). The primers used for ChIP-qPCR are listed in Supplemental Data Set 1.
For ChIP-seq, 1 g of seedlings was used (each biological replicate), and the DNA isolated from the ChIP was purified using the MinElute PCR purification kit (Qiagen, Cat. No. 28004). Libraries were constructed with 2 ng of DNA isolated from the ChIP using the VAHTS Universal DNA Library Prep Kit for Illumina V3 (Vazyme Biotech, Cat. No. ND607), VAHTSTM DNA Adapters set3-set6 for Illumina (Vazyme Biotech, Cat. No. N805), and VAHTS DNA Clean Beads (Vazyme Biotech, Cat. No. N411-02) according to the manufacturer’s protocol. High-throughput sequencing was performed at Novagene (sequencing method: NovaSeq-PE150).
ChIP-seq data analysis was performed as previously described (Yu et al. 2020, 2021). In brief, raw data were trimmed by fastp with the following parameters: “-g -q 5 -u 50 -n 15 -l 150.” The clean data were mapped to the A. thaliana reference genome (TAIR10) using Bowtie2 with the default settings (Langmead and Salzberg 2012). Only perfectly and uniquely mapped reads were used for further analysis. A summary of the number of reads and target genes for each sample were given in Supplemental Data Sets 3 and 4. MACS 2.0 (Feng et al. 2012) was used for peak calling with the following parameters: “gsize = 119,667,750, bw = 300, q = 0.05, nomodel, extsize = 200.” The aligned reads were converted to wiggle (wig) formats, and bigwig files were generated by bamCoverage with “-bs 10” and “-normalize using RPKM (reads per kilobase per million)” in deepTools. The data were imported into the Integrative Genomics Viewer (IGV) (Robinson et al. 2011) or Integrated Genome Browser (IGB) (Freese et al. 2016) for visualization. Only peaks that were present in both biological replicates (irreproducible discovery rate ≥ 0.05) were considered for further analysis. To annotate peaks to genes, ChIPseeker was used with default settings (Yu et al. 2015). Differential occupancy was determined using DiffBind with default settings (Ross-Innes et al. 2012). Venn diagrams were created using Venny (v.2.1) (https://bioinfogp.cnb.csic.es/tools/venny/index.html) to compare overlaps between different groups of genes. ComputeMatrix and plotProfile (Ramírez et al. 2016) were used to compare the mean occupancy density (details are shown in each corresponding figure legend).
To analyze read density and correlation between different ChIP-seq samples, we performed Spearman correlation analysis. Reads density was analyzed over the merged set of binding sites across all ChIPs using the multiBigwig-Summary function from deepTools. The heatmap of Spearman correlation was generated by the PlotCorrelation function in deepTools (Ramírez et al. 2016). Peak overlaps were analyzed by the Bedtools intersect function. The random regions were selected by using ShuffleBed with the default setting in DeepTools.
GO analysis
GO enrichment analysis was performed using the online tool DAVID Bioinformatics Resources (https://david.ncifcrf.gov/) with default setting and plotted at HIPLOT (hiplot-academic.com).
Phylogenetic analysis
The amino acid sequences of putative orthologs proteins of SWI3D in different species were downloaded from the UniProt database and used for phylogenetic analysis. The amino acid sequences were aligned by using the MUSCLE algorithm method. The phylogenetic tree was constructed with MEGA11 (Tamura et al. 2021) using the neighbor-joining method with 1,000 bootstrap replicates and the Poisson model. The protein sequences used are listed in Supplemental Data Set 5.
Statistical analysis
All statistics performed in this manuscript are detailed above, and statistical test methods, sample sizes, and P-values are indicated in the corresponding figure legends. Two-tailed Student's t-tests were conducted using Excel. P-values for the Venn diagram overlap analysis are based on hypergeometric tests on the online tool at http://nemates.org/MA/progs/overlap_stats.html with default setting. The post hoc Tukey’s honestly significant difference (HSD) test was performed with the online tool at https://astatsa.com/OneWay_Anova_with_TukeyHSD/. The post hoc Tukey’s HSD test and t-test results are listed in Supplemental Data Set 6.
Accession numbers
Accession numbers of genes reported in this study include: AT2G46020 (BRM), AT1G21700 (SWI3C), AT3G01890 (SWP73A), AT3G03460 (BRIP1), AT5G17510 (BRIP2), AT1G20670 (BRD1), AT1G76380 (BRD2), AT5G55040 (BRD13), AT2G28290 (SYD), AT4G34430 (SWI3D), AT5G07940 (SYS1), AT5G07970 (SYS2), AT5G07980 (SYS3), AT3G06010 (MINU1), AT5G19310 (MINU2), AT2G47620 (SWI3A), AT2G33610 (SWI3B), AT3G17590 (BSH), AT3G18380 (SHH2), AT1G58025 (BRD5), AT3G52100 (TPF1), AT3G08020 (TPF2), AT1G50620 (OPF1), AT3G20280 (OPF2), AT1G32730 (PSA1), AT1G06500 (PSA2), AT5G14170 (SWP73B), AT3G22990 (LFR), AT1G01160 (GIF2), AT1G18450 (ARP4), AT3G60830 (ARP7), AT4G22320 (BCL7A), AT5G55210 (BCL7B), AT3G18780 (ACTIN2), and AT5G09810 (ACTIN7). Full protein names are listed in Supplemental Data Set 7.
Supplementary Material
Acknowledgments
We thank the ABRC for seeds of T-DNA insertion lines.
Contributor Information
Wei Fu, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China.
Yaoguang Yu, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China.
Jie Shu, Key Laboratory of South China Agricultural Plant Molecular Analysis and Genetic Improvement & Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, Guangdong 510650, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Zewang Yu, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China.
Yixiong Zhong, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China.
Tao Zhu, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China.
Zhihao Zhang, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China.
Zhenwei Liang, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China.
Yuhai Cui, London Research and Development Centre, Agriculture and Agri-Food Canada, London, Ontario, Canada N5V 4T3.
Chen Chen, Key Laboratory of South China Agricultural Plant Molecular Analysis and Genetic Improvement & Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, Guangdong 510650, China; University of the Chinese Academy of Sciences, Beijing 100049, China.
Chenlong Li, State Key Laboratory of Biocontrol and Guangdong Key Laboratory of Plant Resources, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China.
Author contributions
C.L. conceived the project. W.F. and Y.Y. performed most of the experiments. J.S. generated SYD-GFP transgenic lines. W.F., Y.Y., Y.Z., and Z.Y. conducted bioinformatics analysis. W.F., Y.Y., J.S., Z.Y., Y.Z., T.Z., Z.Z., Z.L., Y.C., C.C., and C.L. analyzed data. C.L. wrote the manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1 . Immunopurification of 3 distinct SWI/SNF subcomplexes in Arabidopsis.
Supplemental Figure S2 . Immunopurification of BRD1, SYD, and SWI3B.
Supplemental Figure S3 . Differential binding of SAS, MAS, and BAS subcomplexes on chromatin.
Supplemental Figure S4 . Unique target genes bound by SWI3A.
Supplemental Figure S5 . The MAS subcomplex shows a preference for binding to genes involved in the regulation of DNA methylation.
Supplemental Figure S6 . The MAS and BAS subcomplexes, but not SAS subcomplex, show a significant overlap with activated histone modification markers.
Supplemental Figure S7 . Metagene plots displaying the ChIP-seq signals of different histone modifications at SWI3D, SWI3A, and SWI3C binding peaks.
Supplemental Figure S8 . Analysis of the overlaps between SYD target genes and mis-regulated genes in syd-5 or swi3d-1.
Supplemental Figure S9 . Loss of SWI3D does not affect BRM protein level.
Supplemental Figure S10 . The SANT and SWIRM assoc-1 domains of SWI3D are conserved in eukaryotes.
Supplemental Figure S11 . The ATPase of BAS subcomplex is essential to the stability of the core module.
Supplemental Figure S12 . The residual peaks of SWI3D enriched at high chromatin accessibility regions.
Supplemental Figure S13 . The possible combinations of the SAS, MAS, and BAS subcomplexes.
Supplemental Figure S14 . Gel source data.
Supplemental Data Set 1 . Summary of primers used for this study.
Supplemental Data Set 2 . List of genes mis-regulated in different mutants.
Supplemental Data Set 3 . Summary of mapped reads for ChIP-seq.
Supplemental Data Set 4 . List of occupied genes.
Supplemental Data Set 5 . Sequences used for phylogenetic analysis.
Supplemental Data Set 6 . ANOVA or t-test results.
Supplemental Data Set 7 . List of full names of proteins.
Funding
This work was supported by the National Natural Science Foundation of China to C.L. (32270322, 32070212, and 31870289) and to Y.Y. (32200279), the Guangdong Basic and Applied Basic Research Foundation to C.L. (2021A1515011286) and to Y.Y. (2021A1515110386), Postdoctoral Innovation Talents Support Program to Y.Y. (BX2021396), and the Fundamental Research Funds for the Central Universities to C.L. (18lgzd12).
Data availability
The ChIP-seq and RNA-seq data sets have been deposited in the Gene Expression Omnibus under accession nos GSE218841 and GSE218842, respectively. The mass spectrometry proteomics data have been deposited in the Integrated Proteome Resources under the dataset identifier IPX0005495000. The BRD1, BRD2, and BRD13 ChIP-seq data and brm-1 RNA-seq data were downloaded from GEO under accession no. GSE161595. BRIP1 and BRIP2 ChIP-seq data were downloaded from GEO under accession no. GSE142369. The H3K27me3 ChIP-seq data were downloaded from GEO under accession no. GSE145387. The H3K4me3 ChIP-seq data were downloaded from GEO under accession no. GSE183987. The Pol II and H3K4me2 ChIP-seq data were downloaded from DDBJ databases under the accession nos DRR235325 and DRA010413. The H3K36me3 ChIP-seq data and minu1-2 minu2-1 RNA-seq data were downloaded from GEO under accession no. GSE205112. The H3K9ac, H3K27ac, H4K5ac, and H4K8ac ChIP-seq data were downloaded from GEO under accession no. GSE183987.
References
- Archacki R, Sarnowski TJ, Halibart-Puzio J, Brzeska K, Buszewicz D, Prymakowska-Bosak M, Koncz C, Jerzmanowski A. Genetic analysis of functional redundancy of BRM ATPase and ATSWI3C subunits of Arabidopsis SWI/SNF chromatin remodelling complexes. Planta. 2009:229(6):1281–1292. 10.1007/s00425-009-0915-5 [DOI] [PubMed] [Google Scholar]
- Bezhani S, Winter C, Hershman S, Wagner JD, Kennedy JF, Kwon CS, Pfluger J, Su Y, Wagner D. Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED. Plant Cell. 2007:19(2):403–416. 10.1105/tpc.106.048272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieluszewski T, Prakash S, Roule T, Wagner D. The role and activity of SWI/SNF chromatin remodelers. Annu Rev Plant Biol. 2023. 10.1146/annurev-arplant-102820-093218 [DOI] [PubMed] [Google Scholar]
- Clapier CR, Cairns BR. The biology of chromatin remodeling complexes. Annu Rev Biochem. 2009:78(1):273–304. 10.1146/annurev.biochem.77.062706.153223 [DOI] [PubMed] [Google Scholar]
- Clapier CR, Iwasa J, Cairns BR, Peterson CL. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat Rev Mol Cell Biol. 2017:18(7):407–422. 10.1038/nrm.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998:16(6):735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003:133(2):462–469. 10.1104/pp.103.027979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da G, Lenkart J, Zhao K, Shiekhattar R, Cairns BR, Marmorstein R. Structure and function of the SWIRM domain, a conserved protein module found in chromatin regulatory complexes. Proc Natl Acad Sci U S A. 2006:103(7):2057–2062. 10.1073/pnas.0510949103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego-Martin B, Perez-Alemany J, Candela-Ferre J, Corbalan-Acedo A, Pereyra J, Alabadi D, Jami-Alahmadi Y, Wohlschlegel J, Gallego-Bartolome J. The TRIPLE PHD FINGERS proteins are required for SWI/SNF complex-mediated +1 nucleosome positioning and transcription start site determination in Arabidopsis. Nucleic Acids Res. 2022:50(18):10399–10417. 10.1093/nar/gkac826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, Sardiu M, Gogol M, Gilmore J, Zhang D, Florens L, Abmayr SM, Washburn MP, Workman JL. Composition and function of mutant SWI/SNF complexes. Cell Rep. 2017:18(9):2124–2134. 10.1016/j.celrep.2017.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrona S, Hurtado L, Bowman JL, Reyes JC. The Arabidopsis thaliana SNF2 homolog AtBRM controls shoot development and flowering. Development. 2004:131(20):4965–4975. 10.1242/dev.01363 [DOI] [PubMed] [Google Scholar]
- Farrona S, Hurtado L, Reyes JC. A nucleosome interaction module is required for normal function of Arabidopsis thaliana BRAHMA. J Mol Biol. 2007:373(2):240–250. 10.1016/j.jmb.2007.07.012 [DOI] [PubMed] [Google Scholar]
- Feng J, Liu T, Qin B, Zhang Y, Liu XS. Identifying ChIP-seq enrichment using MACS. Nat Protoc. 2012:7(9):1728–1740. 10.1038/nprot.2012.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese NH, Norris DC, Loraine AE. Integrated genome browser: visual analytics platform for genomics. Bioinformatics. 2016:32(14):2089–2095. 10.1093/bioinformatics/btw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchalian J, Malik S, Ho J, Lee DS, Kelso TWR, Shokhirev MN, Dixon JR, Hargreaves DC. A non-canonical BRD9-containing BAF chromatin remodeling complex regulates naive pluripotency in mouse embryonic stem cells. Nat Commun. 2018:9(1):5139. 10.1038/s41467-018-07528-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel AV, Lippman Z, Martienssen R, Colot V. Profiling histone modification patterns in plants using genomic tiling microarrays. Nat Methods. 2005:2(3):213–218. 10.1038/nmeth0305-213 [DOI] [PubMed] [Google Scholar]
- Guo J, Cai G, Li YQ, Zhang YX, Su YN, Yuan DY, Zhang ZC, Liu ZZ, Cai XW, Guo J, et al. Comprehensive characterization of three classes of Arabidopsis SWI/SNF chromatin remodelling complexes. Nat Plants. 2022:8(12):1423–1439. 10.1038/s41477-022-01282-z [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 2011:21(3):396–420. 10.1038/cr.2011.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Garcia J, Diego-Martin B, Kuo PH, Jami-Alahmadi Y, Vashisht AA, Wohlschlegel J, Jacobsen SE, Blazquez MA, Gallego-Bartolome J. Comprehensive identification of SWI/SNF complex subunits underpins deep eukaryotic ancestry and reveals new plant components. Commun Biol. 2022:5(1):549. 10.1038/s42003-022-03490-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010:463(7280):474–484. 10.1038/nature08911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Rangel DS, Qin X, Bui C, Li R, Jia Z, Cui X, Jin H. The chromatin-remodeling protein BAF60/SWP73A regulates the plant immune receptor NLRs. Cell Host Microbe. 2021:29(3):425–434. 10.1016/j.chom.2021.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado L, Farrona S, Reyes JC. The putative SWI/SNF complex subunit BRAHMA activates flower homeotic genes in Arabidopsis thaliana. Plant Mol Biol. 2006:62(1–2):291–304. 10.1007/s11103-006-9021-2 [DOI] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. Tophat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013:14(4):R36. 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with bowtie 2. Nat Methods. 2012:9(4):357–359. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JA, Du J, Hale CJ, Feng S, Krajewski K, Palanca AM, Strahl BD, Patel DJ, Jacobsen SE. Polymerase IV occupancy at RNA-directed DNA methylation sites requires SHH1. Nature. 2013:498(7454):385–389. 10.1038/nature12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen C, Gao L, Yang S, Nguyen V, Shi X, Siminovitch K, Kohalmi SE, Huang S, Wu K, et al. The Arabidopsis SWI2/SNF2 chromatin remodeler BRAHMA regulates polycomb function during vegetative development and directly activates the flowering repressor gene SVP. PLoS Genet. 2015:11(1):e1004944. 10.1371/journal.pgen.1004944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Gu L, Gao L, Chen C, Wei CQ, Qiu Q, Chien CW, Wang S, Jiang L, Ai LF, et al. Concerted genomic targeting of H3K27 demethylase REF6 and chromatin-remodeling ATPase BRM in Arabidopsis. Nat Genet. 2016:48(6):687–693. 10.1038/ng.3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001:25(4):402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Mashtalir N, D’Avino AR, Michel BC, Luo J, Pan J, Otto JE, Zullow HJ, McKenzie ZM, Kubiak RL, St Pierre R, et al. Modular organization and assembly of SWI/SNF family chromatin remodeling complexes. Cell. 2018:175(5):1272–1288. 10.1016/j.cell.2018.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel BC, D’Avino AR, Cassel SH, Mashtalir N, McKenzie ZM, McBride MJ, Valencia AM, Zhou Q, Bocker M, Soares LMM, et al. A non-canonical SWI/SNF complex is a synthetic lethal target in cancers driven by BAF complex perturbation. Nat Cell Biol. 2018:20(12):1410–1420. 10.1038/s41556-018-0221-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynarova L, Nap JP, Bisseling T. The SWI/SNF chromatin-remodeling gene AtCHR12 mediates temporary growth arrest in Arabidopsis thaliana upon perceiving environmental stress. Plant J. 2007:51(5):874–885. 10.1111/j.1365-313X.2007.03185.x [DOI] [PubMed] [Google Scholar]
- Pan J, McKenzie ZM, D’Avino AR, Mashtalir N, Lareau CA, St Pierre R, Wang L, Shilatifard A, Kadoch C. The ATPase module of mammalian SWI/SNF family complexes mediates subcomplex identity and catalytic activity-independent genomic targeting. Nat Genet. 2019:51(4):618–626. 10.1038/s41588-019-0363-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Ryan DP, Grüning B. Deeptools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016:44(W1):W160–W165. 10.1093/nar/gkw257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011:29(1):24–26. 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, Brown GD, Gojis O, Ellis IO, Green AR, et al. Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature. 2012:481(7381):389–393. 10.1038/nature10730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DP, Owen-Hughes T. Snf2-family proteins: chromatin remodellers for any occasion. Curr Opin Chem Biol. 2011:15(5):649–656. 10.1016/j.cbpa.2011.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacharowski SP, Gratkowska DM, Sarnowska EA, Kondrak P, Jancewicz I, Porri A, Bucior E, Rolicka AT, Franzen R, Kowalczyk J, et al. SWP73 subunits of Arabidopsis SWI/SNF chromatin remodeling complexes play distinct roles in leaf and flower development. Plant Cell. 2015:27(7):1889–1906. 10.1105/tpc.15.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y, Silva-Ortega CO, Wu S, Yamaguchi N, Wu MF, Pfluger J, Gillmor CS, Gallagher KL, Wagner D. Mutations in two non-canonical Arabidopsis SWI2/SNF2 chromatin remodeling ATPases cause embryogenesis and stem cell maintenance defects. Plant J. 2012:72(6):1000–1014. 10.1111/tpj.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnowska E, Gratkowska DM, Sacharowski SP, Cwiek P, Tohge T, Fernie AR, Siedlecki JA, Koncz C, Sarnowski TJ. The role of SWI/SNF chromatin remodeling complexes in hormone crosstalk. Trends Plant Sci. 2016:21(7):594–608. 10.1016/j.tplants.2016.01.017 [DOI] [PubMed] [Google Scholar]
- Sarnowski TJ, Rios G, Jasik J, Swiezewski S, Kaczanowski S, Li Y, Kwiatkowska A, Pawlikowska K, Kozbial M, Kozbial P, et al. SWI3 subunits of putative SWI/SNF chromatin-remodeling complexes play distinct roles during Arabidopsis development. Plant Cell. 2005:17(9):2454–2472. 10.1105/tpc.105.031203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick S, Grosche S, Kohl KE, Drpic D, Jaeger MG, Marella NC, Imrichova H, Lin JG, Hofstatter G, Schuster M, et al. Acute BAF perturbation causes immediate changes in chromatin accessibility. Nat Genet. 2021:53(3):269–278. 10.1038/s41588-021-00777-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu J, Chen C, Li C, Thapa RK, Song J, Xie X, Nguyen V, Bian S, Liu J, Kohalmi SE, et al. Genome-wide occupancy of Arabidopsis SWI/SNF chromatin remodeler SPLAYED provides insights into its interplay with its close homolog BRAHMA and polycomb proteins. Plant J. 2021:106(1):200–213. 10.1111/tpj.15159 [DOI] [PubMed] [Google Scholar]
- Smith CL, Horowitz-Scherer R, Flanagan JF, Woodcock CL, Peterson CL. Structural analysis of the yeast SWI/SNF chromatin remodeling complex. Nat Struct Biol. 2003:10(2):141–145. 10.1038/nsb888 [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021:38(7):3022–3027. 10.1093/molbev/msab120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KD, Chakravarty T, Garzon JL, Saraf A, Florens L, Washburn MP, Dutta A. Hypoxic response is driven by the BAF form of SWI/SNF. Biorxiv. 2022. 10.1101/2022.02.16.480689 [DOI] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012:7(3):562–578. 10.1038/nprot.2012.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyanova S, Temu T, Cox J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc. 2016a:11(12):2301–2319. 10.1038/nprot.2016.136 [DOI] [PubMed] [Google Scholar]
- Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, Mann M, Cox J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods. 2016b:13(9):731–740. 10.1038/nmeth.3901 [DOI] [PubMed] [Google Scholar]
- Wagner D, Meyerowitz EM. SPLAYED, a novel SWI/SNF ATPase homolog, controls reproductive development in Arabidopsis. Curr Biol. 2002:12(2):85–94. 10.1016/S0960-9822(01)00651-0 [DOI] [PubMed] [Google Scholar]
- Wagner FR, Dienemann C, Wang H, Stutzer A, Tegunov D, Urlaub H, Cramer P. Structure of SWI/SNF chromatin remodeller RSC bound to a nucleosome. Nature. 2020:579(7799):448–451. 10.1038/s41586-020-2088-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yuan T, Yuan C, Niu Y, Sun D, Cui S. LFR, which encodes a novel nuclear-localized Armadillo-repeat protein, affects multiple developmental processes in the aerial organs in Arabidopsis. Plant Mol Biol. 2009:69(1):121–131. 10.1007/s11103-008-9411-8 [DOI] [PubMed] [Google Scholar]
- Wu MF, Yamaguchi N, Xiao J, Bargmann B, Estelle M, Sang Y, Wagner D. Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate. Elife. 2015:4:e09269. 10.7554/eLife.09269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Guo C, Zhou B, Li C, Wang H, Zheng B, Ding H, Zhu Z, Peragine A, Cui Y, et al. Regulation of vegetative phase change by SWI2/SNF2 chromatin remodeling ATPase BRAHMA. Plant Physiol. 2016:172(4):2416–2428. 10.1104/pp.16.01588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yuan L, Yen MR, Zheng F, Ji R, Peng T, Gu D, Yang S, Cui Y, Chen PY, et al. SWI3B and HDA6 interact and are required for transposon silencing in Arabidopsis. Plant J. 2020:102(4):809–822. 10.1111/tpj.14666 [DOI] [PubMed] [Google Scholar]
- Yang S, Li C, Zhao L, Gao S, Lu J, Zhao M, Chen CY, Liu X, Luo M, Cui Y, et al. The Arabidopsis SWI2/SNF2 chromatin remodeling ATPase BRAHMA targets directly to PINs and is required for root stem cell niche maintenance. Plant Cell. 2015:27(6):1670–1680. 10.1105/tpc.15.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang LG, He QY. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015:31(14):2382–2383. 10.1093/bioinformatics/btv145 [DOI] [PubMed] [Google Scholar]
- Yu Y, Fu W, Xu J, Lei Y, Song X, Liang Z, Zhu T, Liang Y, Hao Y, Yuan L, et al. Bromodomain-containing proteins BRD1, BRD2, and BRD13 are core subunits of SWI/SNF complexes and vital for their genomic targeting in Arabidopsis. Mol Plant. 2021:14(6):888–904. 10.1016/j.molp.2021.03.018 [DOI] [PubMed] [Google Scholar]
- Yu Y, Liang Z, Song X, Fu W, Xu J, Lei Y, Yuan L, Ruan J, Chen C, Fu W, et al. BRAHMA-interacting proteins BRIP1 and BRIP2 are core subunits of Arabidopsis SWI/SNF complexes. Nat Plants. 2020:6(8):996–1007. 10.1038/s41477-020-0734-z [DOI] [PubMed] [Google Scholar]
- Zhang H, Ma ZY, Zeng L, Tanaka K, Zhang CJ, Ma J, Bai G, Wang P, Zhang SW, Liu ZW, et al. DTF1 is a core component of RNA-directed DNA methylation and may assist in the recruitment of Pol IV. Proc Natl Acad Sci U S A. 2013:110(20):8290–8295. 10.1073/pnas.1300585110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Yang S, Chen CY, Li C, Shan W, Lu W, Cui Y, Liu X, Wu K. Arabidopsis BREVIPEDICELLUS interacts with the SWI2/SNF2 chromatin remodeling ATPase BRAHMA to regulate KNAT2 and KNAT6 expression in control of inflorescence architecture. PLoS Genet. 2015:11(3):e1005125. 10.1371/journal.pgen.1005125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Zhang B, Yang M, Zhu J, Li H. Systematic profiling of histone readers in Arabidopsis thaliana. Cell Rep. 2018:22(4):1090–1102. 10.1016/j.celrep.2017.12.099 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ChIP-seq and RNA-seq data sets have been deposited in the Gene Expression Omnibus under accession nos GSE218841 and GSE218842, respectively. The mass spectrometry proteomics data have been deposited in the Integrated Proteome Resources under the dataset identifier IPX0005495000. The BRD1, BRD2, and BRD13 ChIP-seq data and brm-1 RNA-seq data were downloaded from GEO under accession no. GSE161595. BRIP1 and BRIP2 ChIP-seq data were downloaded from GEO under accession no. GSE142369. The H3K27me3 ChIP-seq data were downloaded from GEO under accession no. GSE145387. The H3K4me3 ChIP-seq data were downloaded from GEO under accession no. GSE183987. The Pol II and H3K4me2 ChIP-seq data were downloaded from DDBJ databases under the accession nos DRR235325 and DRA010413. The H3K36me3 ChIP-seq data and minu1-2 minu2-1 RNA-seq data were downloaded from GEO under accession no. GSE205112. The H3K9ac, H3K27ac, H4K5ac, and H4K8ac ChIP-seq data were downloaded from GEO under accession no. GSE183987.