Abstract
Three-dimensional (3D) chromatin organization is highly dynamic during development and seems to play a crucial role in regulating gene expression. Self-interacting domains, commonly called topologically associating domains (TADs) or compartment domains (CDs), have been proposed as the basic structural units of chromatin organization. Surprisingly, although these units have been found in several plant species, they escaped detection in Arabidopsis (Arabidopsis thaliana). Here, we show that the Arabidopsis genome is partitioned into contiguous CDs with different epigenetic features, which are required to maintain appropriate intra-CD and long-range interactions. Consistent with this notion, the histone-modifying Polycomb group machinery is involved in 3D chromatin organization. Yet, while it is clear that Polycomb repressive complex 2 (PRC2)-mediated trimethylation of histone H3 on lysine 27 (H3K27me3) helps establish local and long-range chromatin interactions in plants, the implications of PRC1-mediated histone H2A monoubiquitination on lysine 121 (H2AK121ub) are unclear. We found that PRC1, together with PRC2, maintains intra-CD interactions, but it also hinders the formation of H3K4me3-enriched local chromatin loops when acting independently of PRC2. Moreover, the loss of PRC1 or PRC2 activity differentially affects long-range chromatin interactions, and these 3D changes differentially affect gene expression. Our results suggest that H2AK121ub helps prevent the formation of transposable element/H3K27me1-rich long loops and serves as a docking point for H3K27me3 incorporation.
Polycomb repressive complex 1 maintains intra-compartment domain interactions and hampers certain local and long-range interactions in the Arabidopsis genome to help shape three-dimensional chromatin organization.
IN A NUTSHELL.
Background: How chromatin is organized in three dimensions (3D) within the nucleus has been proposed to be important for controlling biological processes. Post-translational histone modifications within chromatin, such as those mediated by the Polycomb Group machinery, seem to be involved in shaping this 3D organization, but their exact role is not clear. Recent studies have shown that the genome of animals and many plant species is partitioned into blocks of chromatin that self-interact. These topologically associating domains (TADs) or compartment domains (CDs) are usually enriched in specific histone modifications. Although Arabidopsis (Arabidopsis thaliana) chromatin is also decorated with histone modifications such as histone H2A monoubiquitination on lysine 121 (H2AK121ub), TADs or CDs have not been detected.
Question: What is the role of Polycomb repressive complex 1 (PRC1)-mediated H2AK121ub in Arabidopsis 3D chromatin organization? Are TADs or CDs really absent in Arabidopsis?
Findings: Our results showed that TADs or CDs are present in the Arabidopsis genome. We identified CDs dominated by specific histone marks, like trimethylation of histone H3 on lysine 27 (H3K27me3), which is mediated by Polycomb repressive complex 2 (PRC2), H3K27me1 or H3K4me3. Interestingly, PRC1 mediated H2AK121ub could be found in combination with other modifications, displaying different roles. We found that H2AK121ub acting together with H3K27me3 is required to maintain the interactions inside H3K27me3 enriched CDs. In addition, PRC1 and H2AK121ub when acting independently of PRC2 prevent the formation of chromatin loops marked with H3K4me3. Likewise, we found that PRC1 affects interactions between distant CDs, participating in a higher order of chromatin organization. Our results reveal a versatile role of PRC1 activity in shaping 3D chromatin configuration in Arabidopsis.
Next steps: An interesting path to continue our findings would be to explore whether the 3D interactions observed to be dependent on PRC1 are key in defining the 3D structure genome associated with developmental phase transitions and environmental responses.
Introduction
Genome topology is emerging as a key regulator of gene expression (Bourbousse et al. 2019; Zheng and Xie 2019). The presence of chromatin interacting domains has been described in animals and plants (Szabo et al. 2019). These contact domains are commonly known as topologically associating domains (TADs). However, they appear to have specific characteristics in terms of size, structure, and the proteins involved in their formation. In mammals, TAD formation relies on CTCF (CCCTC-binding factor) and cohesin (Szabo et al. 2019; Zheng and Xie 2019), which mediate chromatin loop formation by strongly anchoring TAD borders (Sanborn et al. 2015; Fudenberg et al. 2016). Conversely, Drosophila (Drosophila melanogaster) and plants with large genomes lack the hotspots indicative of highly localized loop anchor elements. Instead, interacting domains appear to be well correlated with epigenetic states and thus have been termed compartment domains (CDs) (Ramírez et al. 2018; Dong et al. 2020).
There are four main types of CDs in Drosophila: (1) transcriptionally active CDs associated with histone modifications such as trimethylation of histone H3 lysine 4 (H3K4me3); (2) Polycomb-repressed CDs enriched in trimethylation of histone H3 on lysine 27 (H3K27me3); (3) heterochromatic-repressed CDs enriched in dimethylation of histone H3 on lysine 9 (H3K9me2), and (4) a CD type devoid of known specific features (Szabo et al. 2019). Nevertheless, recent studies using higher mapping resolution showed that Drosophila CDs and inter-domain regions are subdivided into smaller domains. Moreover, pairs of insulator proteins co-localize at the majority of their borders. Therefore, even though the epigenetic state may influence higher-order interactions, insulator proteins could play a definitive role in domain formation (Wang et al. 2018). The same four types of CDs were found in plants with large genomes such as rice (Oryza sativa) (Dong et al. 2017, 2020; Rowley et al. 2017; Ouyang et al. 2020). Plants do not have CTCF-like genes, and domain borders were proposed to overlap with active gene islands (Dong et al. 2017). However, recent analyses in rice revealed an enrichment of TEOSINTE BRANCHED 1, CYCLOIDEA, PCF1 (TCP) and BASIC LEUCINE ZIPPER (bZIP) transcription factor binding motifs at CD borders (Liu et al. 2017; Doğan and Liu 2018), pointing to a potential role of these proteins in chromatin insulation, dividing chromatin into relatively independent units.
Unexpectedly, TAD-like structures or CDs have not been found in the Arabidopsis (Arabidopsis thaliana) genome. Instead, Arabidopsis was proposed to harbor few compacted domains enriched in H3K9me2 or H3K27me3 repressive marks (called compacted structural domains), which are separated by unstructured inter-domain regions rich in active H3K4me3- and H3K9ac-marked chromatin (Feng et al. 2014; Grob et al. 2014; Wang et al. 2015). Nevertheless, both H3K27me3- and H3K9ac-rich regions were recently shown to be involved in short- and long-range interactions in Arabidopsis (Liu et al. 2016; Nützmann et al. 2020; Huang et al. 2021), suggesting the presence of different types of domains.
Nonetheless, H3K27me3 has been shown to contribute to the establishment of local (within the same domain) and long-range (between noncontinuous distant domains) chromatin interactions in different organisms, including Arabidopsis (Liu et al. 2016; Cheutin and Cavalli 2019; Pachano et al. 2019; Nützmann et al. 2020; Huang et al. 2021). H3K27me3 incorporation is mediated by Polycomb Repressive Complex 2 (PRC2). In both animals and plants, PRC2 acts together with a second Polycomb group complex, PRC1, to maintain gene repression (Baile et al. 2022). PRC1 is also a histone-modifying complex, but it functions as an E3 monoubiquitin ligase toward H2A (Baile et al. 2022). Nevertheless, even though the enzymatic activities of PRC2 and PRC1 are conserved between animals and plants (Makarevich et al. 2006; Bratzel et al. 2010, 2012; Yang et al. 2013; Mozgova and Hennig 2015), there are important differences in terms of complex composition and distribution of H2A monoubiquitination and H3K27me3 in the genome (Baile et al. 2022).
In Arabidopsis, PRC1 comprises RING1A or RING1B and one of the three Polycomb ring finger (PCGF) proteins, BMI1A, B or C, which constitute the H2A E3 monoubiquitin ligase module that incorporates histone H2A monoubiquitination on lysine 121 (H2AK121ub) (Bratzel et al. 2010; Chen et al. 2010; Yang et al. 2013; Li et al. 2017). Similarly, vertebrate PRC1 contains a E3 monoubiquitin ligase module consisting one of the two RING1 and one of the six PCGF proteins, but it is also associated with other nonenzymatic activities involved in chromatin compaction (Wang et al. 2015; Cheutin and Cavalli 2019; Pachano et al. 2019; Huang et al. 2021). While PRC1 chromatin compaction activity seems to be essential in establishing 3D chromatin interactions in animals, H2A monoubiquitination has been proposed to be dispensable for this process (Guo et al. 2021).
Plant PRC2 core components are well conserved with their animal counterparts (Mozgova and Hennig 2015; Xiao and Wagner 2015). In Arabidopsis, CURLY LEAF (CLF), MEDEA (MEA), and SWINGER (SWN) are homologs of the animal catalytic subunit Enhancer of zeste (E(z)) (Goodrich et al. 1997; Grossniklaus et al. 1998; Chanvivattana et al. 2004). FERTILIZATION INDEPENDENT SEED 2 (FIS2), EMBRYONIC FLOWER 2 (EMF2), and VERNALIZATION 2 (VRN2) are homologs of the scaffold protein Suppressor of zeste 12 (Su(z)12) (Luo et al. 1999; Gendall et al. 2001; Yoshida et al. 2001). FERTILIZATION INDEPENDENT ENDOSPERM (FIE) is the unique homolog of the H3K27me3 binding protein extra sex combs (Esc) (Ohad et al. 1999), and MULTIPLE SUPPRESSOR OF IRA 1 (MSI1) is homolog of the nucleosome-remodeling factor Nurf55 (Köhler et al. 2003). However, in contrast to animals, the activities involved in chromatin compaction are associated with PRC2 in Arabidopsis (Derkacheva et al. 2013; Bloomer et al. 2020; Baile et al. 2022). Regarding the distribution of Polycomb-mediated histone marks, while in animals H2A monoubiquitination and H3K27me3 co-localize over large genomic regions (Blackledge et al. 2014), they partially overlap at gene regions in Arabidopsis (Zhou et al. 2017). Furthermore, H2AK121ub can be found at a considerable number of genes devoid of H3K27me3 in Arabidopsis (Zhou et al. 2017). These two important differences are advantageous to investigations of the possible role of H2AK121ub in 3D chromatin organization in plants, as it could be analyzed independently of H3K27me3 and without the interference of chromatin compaction activities.
Three-dimensional genome conformation is highly dynamic during development in eukaryotes (Bourbousse et al. 2019; Zheng and Xie 2019). In Arabidopsis, the strong bmi1a bmi1b bmi1c (bmi1abc) triple mutant, which is impaired in H2A monoubiquitination activity, is characterized by its inability to switch from embryo-to-vegetative development after germination (Yang et al. 2013). By contrast, the strong clf swn double mutant, which lacks virtually all H3K27me3 during sporophyte development (Lafos et al. 2011), can initiate embryo-to-seedling phase transition, but immediately after, it experiences a loss of differentiation (Bouyer et al. 2011; Yang et al. 2013). These data point to a role for H2AK121ub in initiating the onset of changes required to undergo seedling development and a role for H3K27me3 in maintaining these changes (Yang et al. 2013; Zhou et al. 2017), which presumably affect chromatin topology.
Therefore, to investigate the possible role of PRC1 in shaping chromatin organization, we carried out in situ chromosome conformation capture followed by high-throughput sequencing (Hi-C) in wild type, bmi1abc and clf swn plants early in development and integrated these data with the levels of different histone modifications and transcriptome data in the three genotypes. Our analyses revealed that (1) the Arabidopsis genome is partitioned into CDs enriched in different histone modifications and (2) BMI1 binding and H2AK121ub incorporation indeed play roles in shaping chromatin interactions. We found that PRC1, when acting together with PRC2, is required to maintain intra-CD interactions, but when acting independently of PRC2 hampers certain local and long-range interactions. These results represent a step forward toward understanding the roles of Polycomb complexes in establishing higher-order chromatin folding in plants.
Results
The Arabidopsis genome is partitioned into compartment domains enriched in different histone modifications
The transition from embryonic to vegetative development involves important changes in chromatin topology (Simon and Probst 2023) in which, according to previous results (Yang et al. 2013), PRC1 activity might play a role. Therefore, to explore the possible role of PRC1 in shaping chromatin interactions, we carried out Hi-C in wild-type Col-0 (WT), bmi1abc and clf swn Arabidopsis seedlings at 10 d after germination. Although Hi-C data for WT and clf swn plants have been already published (Feng et al. 2014), we generated a new Hi-C dataset under our experimental conditions.
Hi-C was performed in two biological replicates. Total reads were filtered to retain high-quality Hi-C read-pairs representative of true interactions (Supplemental Table S1). As the two biological replicates demonstrated high correlation and showed the same pattern (Supplemental Figs. S1, A–C and S2), we combined them and obtained 481, 339, and 236 million high-quality read-pairs for WT, bmi1abc, and clf swn, respectively. Since these Hi-C data showed very high sequencing depth, to understand the effect of the loss of PRC1 and PRC2 function on chromatin interactions, we first re-evaluated the presence of possible interacting domains in WT seedlings using our data.
We identified significant intra-chromosomal interactions using the nonparametric spline method implemented in Fit-Hi-C (Ay et al. 2014). To identify regions enriched with significant intra-chromosomal interactions, we applied the negative binomial distributions and Wilcoxson rank-sum tests developed in the toolbox HiCExplorer (Ramírez et al. 2018). These significant interactions were corroborated through visual inspection. For this, Hi-C data were represented in a contact matrix and visualized as heatmaps in which the frequency of interactions are represented in a red scale (Supplemental Fig. S3). We used the Knight–Ruiz (KR) algorithm (Knight and Ruiz 2013) to perform matrix normalization. KR normalization has been shown to outperform most available methods with respect to the reproducibility of TAD or interacting domain identification (Lyu et al. 2020). Individual chromosome Hi-C matrices in WT zoomed in to 25 to 50 kb resolution (Supplemental Fig. S3A) showed strong interactions at pericentromeric regions and long-range intra-arm interactions, such as those termed KNOT established between KNOT ENGAGED ELEMENTs (KEEs) or Interactive Heterochromatic Islands (IHIs), as previously reported (Feng et al. 2014; Grob et al. 2014). Interestingly, when we examined Hi-C matrices of different chromosome regions zoomed in to 1 kb resolution (Supplemental Fig. S3A), we found numerous interacting domains (squares along the diagonal) partitioning the Arabidopsis genome. We also observed long-range interactions between noncontinuous distant domains (squares or rectangles far from the diagonal). Such long-range interactions could involve two or multiple domains (Supplemental Fig. S3A).
To determine the number of interacting domains, we used the insulation score (IS) as the calling method (Crane et al. 2015). IS calculates for each bin the average of interaction frequencies crossing over it. This method assumes that positions with minimal IS or interaction frequencies separate and define regions enriched in interactions, allowing domain borders to be defined by identifying local minimums (Supplemental Fig. S3, B and C). To verify its correct functioning (Zufferey et al. 2018), we explored standard and different parameter values, resulting in the identification of essentially the same interacting domains. In total, we identified 4,794 interacting domains with strong insulation at their borders, whose median length was 17 kb and median number of genes was 5. Together, these domains covered nearly all of the Arabidopsis genome (∼90%) and contained most of the genes (95%). Therefore, these data indicate that the Arabidopsis genome is partitioned into interacting domains.
To characterize Arabidopsis interacting domains, we aligned Hi-C contact matrices and observed over expected (observed/expected) signal ratio matrices to account for the genomic distance effect (Lieberman-Aiden et al. 2009), with the distribution of the active mark H3K4me3, Polycomb repressive marks (H3K27me3 and H2AK121ub), and the repressive mark monomethylation of histone H3 on lysine 27 (H3K27me1) (Fig. 1, A and B). For this, we used previously published Chromatin Immunoprecipitation sequencing (ChIP-seq) datasets for H3K27me3, H2AK121ub, and H3K27me1 in WT seedlings of similar ages (Zhou et al. 2017; Antunez-Sanchez et al. 2020), and generated a dataset for H3K4me3. It is important to note that H3K27me1 marks in Arabidopsis can be incorporated by ARABIDOPSIS TRITHORAX-RELATED PROTEIN 5/6 (ATRX5/6) and are associated with the repression of transposable elements (TEs) (Jacob et al. 2009), or they can be generated by RELATIVE EARLY FLOWERING (REF6) demethylase activity after removing two methyl groups from PRC2-mediated H3K27me3 (Lu et al. 2011; Antunez-Sanchez et al. 2020). Therefore, to differentiate their origins, we included H3K27me3 ChIP-seq data from the ref6-5 mutant (Antunez-Sanchez et al. 2020), since H3K27me1-marked regions resulting from REF6 activity in WT appear as H3K27me3 marked in ref6-5.
Figure 1.
Arabidopsis interacting domains are enriched in different histone modifications. A) Hi-C contact matrices showing a pericentromeric (left) and an arm (right) region of chromosome 1 aligned to the levels of H3K27me1, H3K27me3, H2AK121ub, and H3K4me3 in WT, and H3K27me3 in ref6. The IS profile is also included. The upper heatmaps show the positions of CDs. The color intensity represents the frequency of contact. The lower heatmaps show observed over expected signal ratio matrices, where the depletion of interactions is shown in blue and enrichment in red. B) A detailed view of a chromosome 5 arm showing the positions of different types of CDs and the significance of observed contacts within these CDs. The levels of the different modifications in WT, H3K27me3 levels in ref6, gene regions, and IS profile (positive values are indicated in blue and negative values in pink) are included. Pink arrowheads indicate CD borders. Black rectangles highlight the absence of H3K27me3, H2AK121ub, and H3K4me3 at TE and IRs marked with REF6-independent H3K27me1, similar to what happens at pericentromeric H3K27me1 CDs (A). C) Length in kilobase of the different CDs. D) Boxplot showing expression levels in fragments per kilobase of transcript per million mapped reads (FPKM) of genes included in different types of CDs. In the boxplots, the median (middle line), upper and lower quartiles (boxes), and minimum and maximum values (whiskers) are indicated. P-values of differences according to one-sided Mann–Whitney–Wilcoxon test are indicated.
To determine whether an interacting domain was enriched for a given histone modification, we first determined the proportion of the domain length that overlapped with peaks of the different histone marks. If the proportion of a specific mark was greater than or equal to two times the proportion for the other marks, the domain was considered to be enriched in this mark. The largest interacting domains were located at pericentromeric regions (Fig. 1, A and C). We identified 240 pericentromeric domains with a median number of eight genes (mostly TEs). These interacting domains were enriched in H3K27me1 and depleted of H3K27me3, H2AK121ub, and H3K4me3 marks (Fig. 1A; Supplemental Fig. S4, A and B). Since H3K9me2 co-localizes with H3K27me1 at these regions (Lindroth et al. 2004; Jacob et al. 2009; Feng et al. 2014), these domains most likely correspond to previously reported compact structural domains (Feng et al. 2014).
We identified different types of interacting domains at chromosome arms (Fig. 1A). We found 425 H3K27me3, 336 H3K27me1, and 1,450 H3K4me3 enriched domains (Fig. 1B; Supplemental Fig. S4, A and B). H3K27me3 domains were longer (median number of genes = 7) than those enriched in H3K27me1 (median number of genes = 5), and those enriched in H3K4me3 (median number of genes = 3) (Fig. 1C). Using transcriptome data from WT seedlings (Yin et al. 2021), we found that average expression levels of genes within H3K27me3 and H3K27me1 domains were lower than that of genes within H3K4me3 domains (Fig. 1D). This fits with the role of these modifications in transcriptional regulation and supports our classification of the different domains. Nevertheless, 48.66% of the identified interacting domains did not show a clear enrichment of any of these histone marks over the others (Supplemental Fig. S4). This is most likely a consequence of their different epigenetic states in different cell types. Given that the ChIP-seq data were obtained from whole seedlings, they provide average levels from a mixture of cells in which chromatin may be differently marked. Therefore, these regions will not show a clear enrichment of one modification over the others.
To further validate the existence of H3K27me3, H3K27me1, and H3K4me3 enriched domains at chromosome arms, we obtain their average IS profile (Fig. 2A). The average IS at domain borders was low in all three types of domains, indicating that they are all insulated from neighbor domains (Fig. 2A). We next determined the number of significant interactions per kb (filtered with a q-value < 0.05) within the different domains, as determined by the Fit-Hi-C methods (Ay et al. 2014). Even though the number of significant interactions was lower in H3K4me3 enriched domains than in the other two types of domains (Fig. 2B), the three domains showed significant interactions. Moreover, the three types of domains could be visually detected in Hi-C contact matrices and observed/expected signal ratio matrices (Fig. 2F). Consistent with this finding, a previous report showed that transcribed genes display weaker interactions in the 3D space than repressed genes (Liu et al. 2016). Therefore, these results indicate that, similar to Drosophila and other plants (Dong et al. 2020), the Arabidopsis genome is partitioned into compartment domains with different epigenetic features, and thus, we will refer to them as CDs hereafter.
Figure 2.
Arabidopsis CD borders co-localize with accessible regions depleted of histone modifications. A) Metaplots showing average IS along the different CDs. The upstream (downstream) 5 kb region of the left (right) border is included. B) Boxplot showing significant intra-CD interactions per kilobase in H3K27me3, H3K27me1, and H3K4me3 CDs. The median (middle line), upper and lower quartiles (boxes), and minimum and maximum values (whiskers) are indicated. P-values of differences according to one-sided Mann–Whitney–Wilcoxon test are indicated. C) Metaplots showing the average levels of H3K27me1, H3K27me3 H2AK121ub, H3K4me3, and accessibility in all WT CDs at chromosome arms. The upstream (downstream) 5 kb region of the left (right) border is included. D) Scatter plot showing the relationship between IS and accessibility. The correlation coefficient (r) and P-value according to F-test are indicated. E) Distribution of CD borders relative to gene features (upper panel). Motifs enriched at the region spanning from 250 bp up- and downstream of CD borders. Transcription factors recognizing the two first ranked motifs and the P-values are indicated (bottom panel). F) Hi-C contact matrix (upper panel, where color intensity represents the frequency of contact) and observed over expected signal ratio matrix (bottom panel, where depletion of interaction is shown in blue and enrichment in red) of a chromosome 5 region zoomed in to 1 kb resolution. The IS profile (positive values are indicated in blue and negative values in pink), as well as the levels of H3K27me3 in ref6, the levels of H3K27me1, H3K27me3, H2AK121ub, and H3K4me3 in WT, transcript levels, and gene regions are included. Dashed triangles indicate representative H3K27me3 and H3K4me3 CDs. Rectangles indicate representative H3K27me1 marked regions depleted of H3K27me3, H2AK121ub and H3K4me3. Right panel shows a detailed view of self-interacting genes within H3K4me3 enriched CD.
We then investigated whether any epigenetic feature was correlated with CD borders. For this, we obtained average profiles of H3K4me3, H3K27me1, H3K27me3, H2AK121ub, and accessibility coverage using our previously published Assay for Transposase-Accessible Chromatin-sequencing (ATAC-seq) data (Yin et al. 2021) at all CDs and surrounding regions (Fig. 2C; Supplemental Fig. S4A). Interestingly, CD borders co-localized with a peak of accessibility (Fig. 2C; Supplemental Fig. S4A), which agreed with the negative correlation observed between IS and accessibility (Fig. 2D). In addition, CD borders were flanked by peaks of H3K4me3 and H2AK121ub (Fig. 2C), which are modifications usually located at the transcriptional start site (TSS) (Zhang et al. 2009; Zhou et al. 2017). Next, we analyzed the distribution of CD borders relative to gene features (Fig. 2E), finding that 81.76% of them were located at promoter regions. All together, these data indicate that CD borders in Arabidopsis associate with accessible regions.
Since studies in the rice genome showed that CD borders were enriched in motifs recognized by TCP and bZIP transcription factors (Liu et al. 2017; Doğan and Liu 2018), we conducted a motif analysis at the region spanning from 250 bp upstream to 250 bp downstream of CD borders in Arabidopsis. Similar to rice, we found a significant enrichment of these motifs (Fig. 2E), suggesting their possible role in CD formation in plants. Nonetheless, even though TCP1 is also enriched at TAD borders in the liverwort Marchantia polymorpha, the tcp1 mutant does not exhibit drastic changes in domain distribution (Karaaslan et al. 2020). Therefore, more work is required to determine whether TCP, bZIP or any other transcription factor contributes to TAD or CD formation in the green lineage.
BMI1 and H2AK121ub hamper the formation of local chromatin loops
Interestingly, we did not find an H2AK121ub-enriched CD type. Except for the case of TEs and several intergenic regions (IRs), which are marked with H3K27me1 and devoid of H3K27me3, H2AK121ub, and H3K4me3, as occurs in pericentromeric regions, this modification could be detected along with the other marks (Figs. 1B and 2F; Supplemental Fig. S4). H2AK121ub has been shown to co-localize with H3K27me3 at gene regions (Zhou et al. 2017; Kralemann et al. 2020; Yin et al. 2021). In addition, our data showed co-localization of H2AK121ub with H3K4me3 and H3K27me1 at 9,560 and 3,252 genes, respectively (Supplemental Fig. S4C). To investigate the possible role of H2AK121ub acting in combination with these marks, we analyzed the expression levels of double marked subsets of genes. H2AK121ub/H3K27me3 and H2AK121ub/H3K27me1 marked genes (5,311 and 3,252, respectively) displayed low average expression levels, and H2AK121ub/H3K4me3 genes showed significantly lower expression levels than genes marked only with H3K4me3 (6,992) (Fig. 3A), correlating H2AK121ub and gene repression.
Figure 3.
BMI1 binding and H2AK121ub deposition reduce the strength of chromatin loops enriched in H3K4me3 activation marks. A) Expression levels in FPKM of genes marked with different combinations of histone modifications. The median (middle line), upper and lower quartiles (boxes), and minimum and maximum values (whiskers) are indicated. P-values of differences according to one-sided Mann–Whitney–Wilcoxon test are indicated. B) Metagene plots of BMI1B-FLAG and H2AK121ub coverage at BMI1B target genes. C) Integrated genomics viewer (IGV) screenshot showing H3K27me3, H2AK121ub, and BMI1B-FLAG peaks. The localizations of VAL1-GFP-binding regions and genes are indicated (upper panel). First ranked motif enriched at BMI1 binding sites (bottom panel). D) Pie chart showing the proportions of BMI1/H2AK121ub_loops, only H3K4me3_loops, and other loops. Table indicating the number of ≥6 kb and ≤6 kb chromatin loops and gene loops in the different types of loops (bottom panel). E) Heatmap showing the enrichment of BMI1, H3K4me3, H2AK121ub, and H3K27me3, at H2AK121ub/H3K27me3 marked genes, BMI1/H2AK121ub_loops, only H3K4me3_loops and other loops. F) Boxplot showing the strength of the different types of chromatin loops in WT. The median (middle line), upper and lower quartiles (boxes), and minimum and maximum values (whiskers) are indicated. P-values of differences according to one-sided Mann–Whitney–Wilcoxon test are indicated.
A previous study in Arabidopsis showed that the chromatin of transcriptionally active genes tends to form small chromatin loops (≤6 kb) and that gene loops are more likely to occur in highly expressed genes, suggesting a role for gene looping in promoting transcription (Liu et al. 2016). Consistent with this finding, we observed that transcribed genes within H3K4me3 enriched CDs frequently self-interacted (Fig. 2F). Thus, we wondered whether PRC1-mediated H2AK121ub, when co-localizing with H3K4me3-marked genes, could affect chromatin looping. To investigate this notion, we integrated BMI1B-FLAG, H2AK121ub, and H3K4me3 ChIP-seq data with chromatin loop data from euchromatic chromosome arms (Liu et al. 2016).
For this, we first analyzed the genome-wide binding of BMI1B-FLAG in the Arabidopsis bmi1b single mutant (Supplemental Fig. S5). We found 5,547 genes targeted by BMI1B-FLAG (Supplemental Dataset 1). Sixty-five percent of these genes were marked with H2AK121ub (Supplemental Fig. S5C), indicating that not all BMI1B-FLAG targets are marked with H2AK121ub. Nevertheless, this percentage is similar to that of genes bound by the PRC2 component FIE and marked with H3K27me3 (Xiao et al. 2017). When we analyzed the distribution of BMI1B-FLAG at target genes, we found an enrichment of BMI1B-FLAG peaks at the region upstream of the TSS and downstream of the transcriptional termination site (Fig. 3B; Supplemental Fig. S5B). Interestingly, 80% of BMI1B-FLAG target genes were also bound by the transcriptional repressors VIVIPAROUS1/ABI3-LIKE1 (VAL1) and VAL2 (Yuan et al. 2021) (Fig. 3C; Supplemental Fig. S5D). Accordingly, there was a significant enrichment of RY elements (VAL1/2 binding motif) under BMI1B-FLAG peaks (Fig. 3C). These results support the notion that VAL factors participate in BMI1 recruitment (Yang et al. 2013; Qüesta et al. 2016; Baile et al. 2021).
Next, we transferred previously published chromatin loop data (Liu et al. 2016) to 1 kb resolution format and identified 18,441 loops, including 6,239 that were ≥6 kb and 12,202 that were ≤6 kb (Fig. 3D). After verifying that similar chromatin loops could be called from our Hi-C data (Supplemental Fig. S5E), we used the statistically significant dataset from Liu et al. (2016) to determine the percentage of loops that were bound by BMI1B-FLAG and marked with H2AK121ub (BMI1/H2AK121ub_loops). These regions accounted for 5.18% of all loops (Fig. 3D). As BMI1/H2AK121ub_loops were also enriched in H3K4me3 (Fig. 3E), we determined the number of H3K4me3_loops that were depleted of BMI1 binding and H2AK121ub (only H3K4me3_loops), representing 13.66% of all loops (Fig. 3D). We then compared the strength of BMI1/H2AK121ub_loops, only H3K4me3_loops, and all loops (Fig. 3F), finding that the strongest loops were those marked only with H3K4me3. Remarkably, even though BMI1/H2AK121ub_loops contained high levels of H3K4me3 (Fig. 3F), their strength was lower than that of only H3K4me3_loops. The same was true for the loops containing only BMI1 (BMI1_loops) or H2AK121ub (H2AK121ub_loops) (Fig. 3F). Moreover, while 32.6% of only H3K4me3_loops corresponded to gene loops (823/2519), the percentage of gene loops within BMI1/H2AK121ub_loops was considerably lower (7.6%, 73/955) (Fig. 3D). All together, these results suggest that both BMI1 and H2AK121ub hinder the formation of local chromatin loops.
PRC1 and PRC2 activities are required to maintain intra-CD interactions
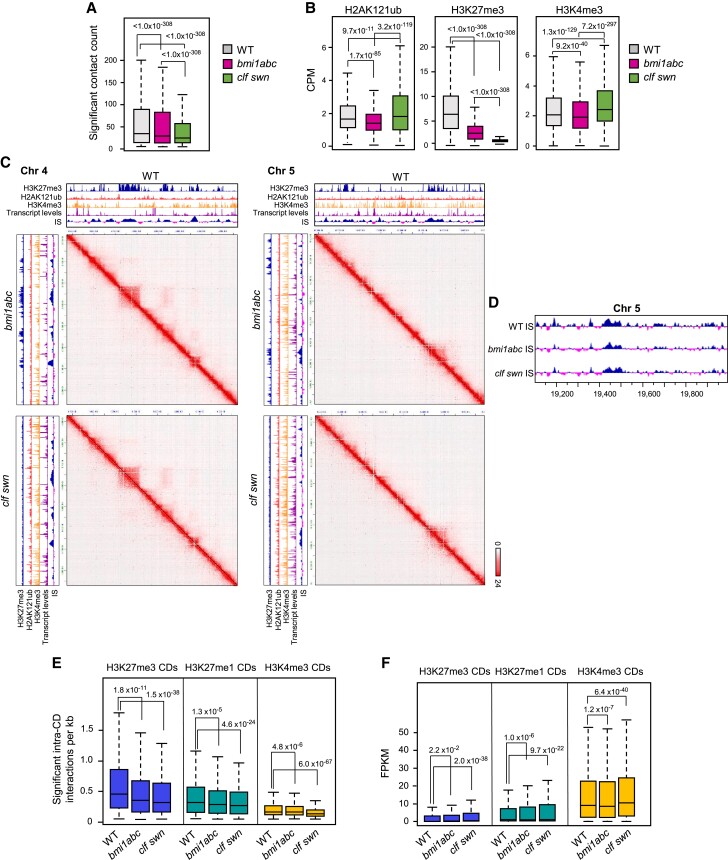
To further analyze the effect of PRC1 activity on 3D chromatin organization, either in combination with PRC2 activity or by itself, we compared chromatin interactions in WT, bmi1abc, and clf swn. We used the KR algorithm (Knight and Ruiz 2013) to perform matrix normalization to remove biases originating from different library sizes, ruling out the influence of varying sequencing depths among the three genotypes (Supplemental Fig. S6). We detected a global decrease in chromatin interactions in the two mutants compared to WT, but especially in clf swn (Fig. 4A). To determine whether this decrease in interactions was accompanied by a change in the number and/or position of CDs, we identified CD borders in bmi1abc and clf swn using the IS. Interestingly, albeit the levels of H2AK121ub, H3K27me3, and H3K4me3 were differentially altered in the mutants (Fig. 4B), the positions of CD borders and the number of CDs (4,794 in WT, 4,887 in bmi1abc, and 4,981 in clf swn) were similar in the three genotypes (Fig. 4, C and D). These results support the notion that histone modifications are not decisive for CD establishment in Arabidopsis.
Figure 4.
PRC1 and PRC2 activities are required to maintain intra-CD interactions. A) Boxplots showing significant contact counts in WT, bmi1abc, and clf swn. B) Boxplots showing levels of H3K27me3, H2AK121ub, and H3K4me3 in WT, bmi1abc, and clf swn. C) Hi-C contact matrices of single regions of chromosomes 4 and 5 in WT, bmi1abc, and clf swn zoomed in to 1 kb resolution. Color intensity represents the frequency of contact. In the IS profiles (positive values are indicated in blue and negative values in pink), H3K27me3, H2AK121ub, H3K4me3, and transcript levels are indicated in each case. D) IS profile of a region of chromosome 5 in WT, bmi1abc, clf swn. Positive values are indicated in blue and negative values in pink. E) Boxplot showing significant intra-CD interactions in WT, bmi1abc, and clf swn. F) Boxplot showing the expression levels of genes included in the different CDs in WT, bmi1abc, and clf swn. In all boxplots, the median (middle line), upper and lower quartiles (boxes) and minimum and maximum values (whiskers) are indicated. P-values of differences according to one-sided Wilcoxon signed rank test are indicated.
Nevertheless, as bmi1abc and clf swn showed a global decrease in chromatin interactions, we investigated if this was reflected at the intra-CD interactions level. Thus, we compared the number of significant intra-CD interactions in H3K27me3, H3K27me1, and H3K4me3 CDs as defined in WT in the three genotypes (Fig. 4E). We found that the three types of CDs displayed a significant decrease in interactions in the two mutants compared to WT, indicating that PRC1 and PRC2 activities are required to maintain the appropriate intra-CD interactions. However, whereas intra-CD interactions were markedly reduced only within H3K27me3 CDs in bmi1abc, these interactions were markedly reduced in all three types of CDs in clf swn (Fig. 4E). Since H3K27me3 levels are reduced in bmi1abc and undetectable in clf swn (Zhou et al. 2017) (Fig. 4B), it was not surprising to find a consistent decrease in intra-CD interactions in H3K27me3 CDs in these mutants. Indeed, previous Hi-C data showed that interactions within H3K27me3 domains were strongly reduced in clf swn compared to WT (Feng et al. 2014). In addition, as REF6-dependent H3K27me1 is connected to PRC2 activity (Antunez-Sanchez et al. 2020), interactions within H3K27me1 CDs might also be affected in the mutants. However, the observation that clf swn showed a notable decrease in H3K4me3 CDs intra-domain interactions indicates that the loss of PRC2 function affects interactions outside of H3K27me3 domains.
Since changes in chromatin interactions are thought to affect gene expression (Kumar et al. 2021), we investigated if altered intra-CD interactions could lead to transcriptional changes (Fig. 4F). The average expression levels of genes within H3K27me3 and H3K27me1 CDs were higher in both mutants compared to WT, but especially in clf swn, which is consistent with the repressive roles of PRC1 and PRC2 (Supplemental Fig. S7A). However, the average expression levels of the genes within H3K4me3 CDs were altered in bmi1abc and clf swn (Fig. 4F). Although these results seem surprising, they fit with the finding that most misregulated genes in bmi1abc and clf swn are not marked with H3K27me3 in WT (Supplemental Fig. S7B). Interestingly, the average expression levels of the genes within H3K4me3 CDs were lower in bmi1abc and higher in clf swn compared to the WT (Fig. 4F). Accordingly, the number of downregulated genes was higher in bmi1abc than in clf swn (Supplemental Fig. S7B). All together, these results suggest that the loss of PRC1 and PRC2 activities affect a higher-order level of 3D chromatin organization that influences gene expression outside of H3K27me3 CDs in a different manner.
Loss-of-function of PRC1 and PRC2 differentially affects long-range chromatin interactions
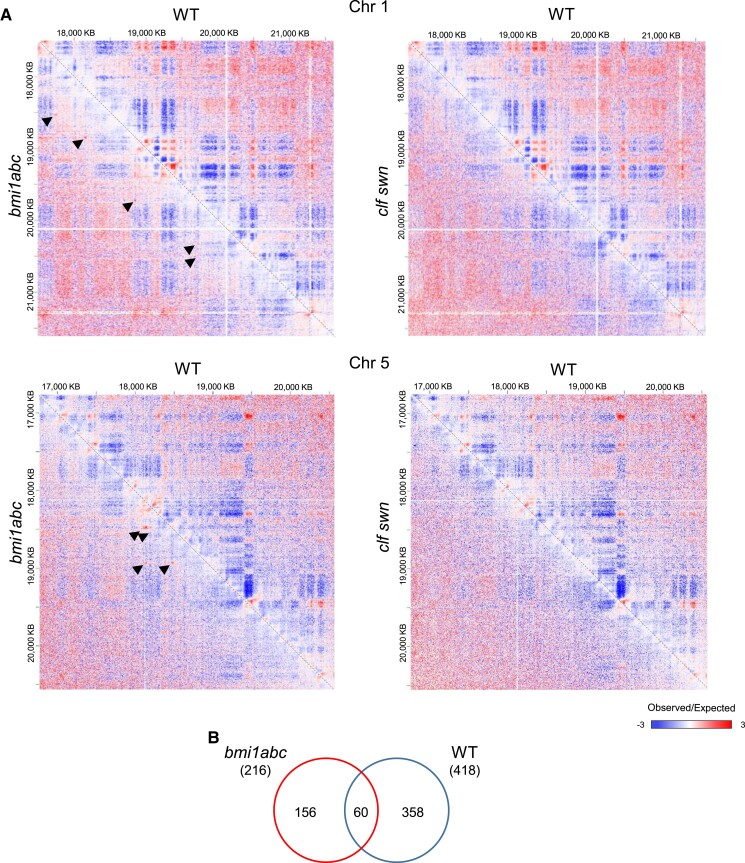
Hence, we investigated whether long-range interactions were altered in the mutants compared to WT. Most prominent long-range interactions in WT were established among H3K27me3 CDs, although long-range interactions among other CDs were also observed (Supplemental Fig. S8A). This is consistent with previously reported interactions among H3K27me3 repressed domains or H3K9ac active domains (Feng et al. 2014; Wang et al. 2015; Huang et al. 2021). Comparison of observed/expected signal ratio matrices between WT and mutants showed that long-range interactions were reduced in bmi1abc and even more so in clf swn (Fig. 5A). Unexpectedly, bmi1abc showed an enrichment in long-range interactions or loops that was not observed in WT or clf swn (Fig. 5A). We identified 156 of these enriched long-range loops (Fig. 5B). The same results were obtained when analyzing independent replicates (Supplemental Fig. S8B).
Figure 5.
Long-range interactions are altered in mutants compared to WT. A) Observed over expected signal ratio matrices showing a region of chromosome 1 (upper panel) and 5 (bottom panel) in WT, bmi1abc, and clf swn zoomed in to 5 kb resolution. Red (blue) indicate regions exhibiting much greater (lower) interaction strength than expected by chance. Arrowheads indicate enriched long-range interactions detected in bmi1abc. B) Venn diagram showing overlap between the numbers of long-range interactions (loops) identified in bmi1abc and WT. Total numbers are shown in parentheses.
To investigate the nature of these interactions, we aligned Hi-C matrices in WT, bmi1abc, and clf swn to H2AK121ub, H3K27me3, H3K4me3 and transcript levels in the corresponding genotypes and to the positions of H3K27me1 peaks in WT (Fig. 6A). Based on the levels of H3K27me3 in the mutants, H3K27me3 CD long-range interactions were reduced in bmi1abc and severely affected in clf swn (Fig. 6, A and B). Remarkably, bmi1abc-enriched long-range interactions were established between regions marked with H3K27me1 in WT (Fig. 6A). Accordingly, the percentage of significant long-range interactions between H3K27me1 CDs was higher in bmi1abc than WT or clf swn (Fig. 6B). We also detected a considerable decrease in these and all other types of long-range interactions in clf swn compared to WT.
Figure 6.
bmi1abc Displays enrichment in long-range interactions among TE-rich/H3K27me1 marked regions. A) Observed over expected signal ratio matrices showing a region of chromosome 5 in WT, bmi1abc, and clf swn aligned with the positions of H3K27me1 peaks in WT; H3K27me3, H2AK121ub, and H3K4me3 levels, transcript levels, and gene regions in each genotype are indicated. Green triangles indicate interacting regions in bmi1abc and black arrowheads indicate long-range interactions between these regions. A detailed view of interacting regions in bmi1abc, with the levels of different histone marks in WT and ref6 shown below. Brown triangles indicate TE genes. B) Percentage of significant interactions among the different types of CDs in WT, bmi1abc, and clf swn. Color code also refers to the percentage of interactions. The percentage of H3K27me1 CD–CD interactions in bmi1abc is highlighted in red. C) Boxplot showing H3K27me1 levels in WT and ref6-5 at bmi1abc TE-rich/H3K27me1 interacting domains. The median (middle line), upper and lower quartiles (boxes), and minimum and maximum values (whiskers) are indicated. “ns” indicates not significant difference according to one-sided Mann–Whitney–Wilcoxon test.
Interestingly, the H3K27me1-interacting domains in bmi1abc were enriched in TEs (Fig. 6A; enrichment: 1.86, P-value: 1.0 × 10−5), suggesting that H3K27me1 marks at these regions might be mostly REF6-independent. Consistent with this notion, H3K27me1 levels at interacting regions were not significantly affected in ref6-5 compared to WT (Fig. 6, A and C). TE-rich regions have been shown to participate in long-range interactions in plants, as is the case of KEEs or IHIs (Feng et al. 2014; Grob et al. 2014). Interestingly, KEEs are enriched in both H3K27me1 and H3K9me2; however, KNOT remains intact in suvh4 suvh5 suvh6, ddm1 and met1 mutants, which are affected in H3K9me2 and/or DNA methylation (Feng et al. 2014). Since H3K9me2 and DNA methylation occur independently of H3K27me1 (Jacob et al. 2009), perhaps this modification plays a role in mediating the long-range interactions observed in bmi1abc and among KEEs.
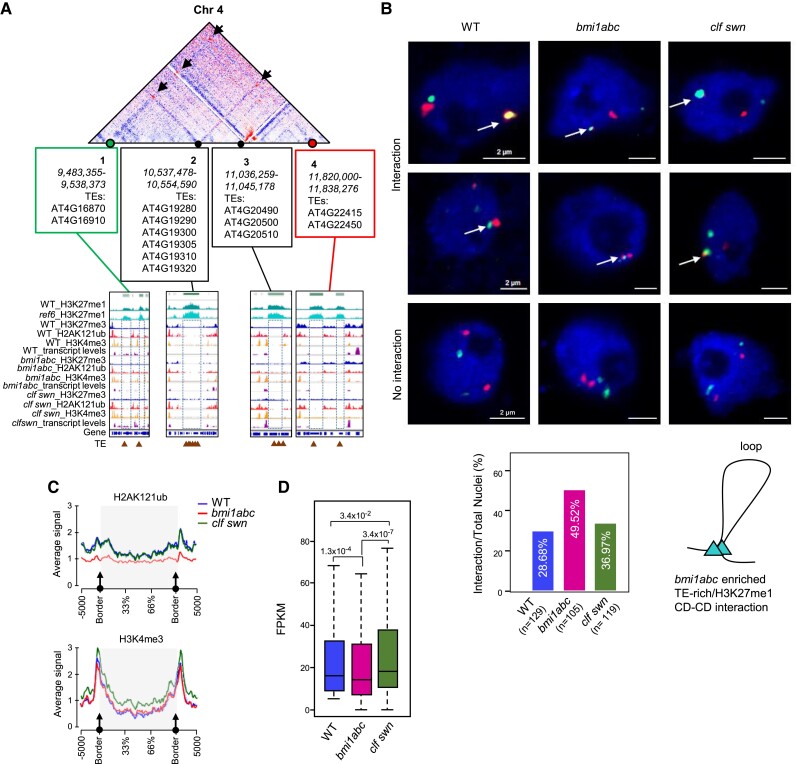
To confirm the enriched long-range interactions in bmi1abc, we performed 3D DNA fluorescence in situ hybridization (FISH) using BAC-based probes located at two interacting regions of chromosome 4 (Fig. 7A). Indeed, the percentage of nuclei showing interactions between these regions was higher in bmi1abc than in WT or clf swn (Fig. 7B). Since these regions were depleted of H3K27me3, H2AK121ub, and H3K4me3, and the deposition of H3K27me1 occurred independently of REF6 (Figs. 6A and 7A; Supplemental Fig. S9), we wondered why these interactions were not enriched in WT or clf swn. Thus, we compared the epigenetic states of the chromatin surrounding TE-rich/H3K27me1 interacting regions in the three genotypes. The only common feature between WT and clf swn that contrasted with bmi1abc was the presence of H2AK121ub (Fig. 7, A and C; Supplemental Fig. S9). This suggests that H2AK121ub might help prevent the formation of long-range interactions with H3K27me1 regions. Reduced levels of H2AK121ub in bmi1abc may allow the formation of long-range TE-rich/H3K27me1 interactions but impede the appropriate incorporation of H3K27me3, and therefore, long-range interactions of H3K27me3 chromatin are also reduced. On the other hand, H2AK121ub in WT and clf swn may prevent long-range TE-rich/H3K27me1 interactions, and the lack of H3K27me3 in clf swn might impede the formation of long-range interactions of H3K27me3 chromatin.
Figure 7.
BMI1-mediated H2AK121ub incorporation hampers the formation of long-range interactions among TE-rich/H3K27me1-enriched regions. A) A region of chromosome 4 showing the positions of H3K27me1 CDs (1 to 4) involved in bmi1abc-enriched long-range interactions (red dots indicated by black arrows). TEs included in these CDs are indicated. The levels of H3K27me1 in WT and ref6-5 as well as the levels of H3K27me3, H2AK121ub, H3K4me3 and transcription in the different genotypes are shown. Dotted rectangles highlight the absence of H3K27me3, H2AK121ub, H3K4me3 modifications at these regions. Brown triangles indicate TE genes. B) Representative examples of 3D FISH visualization of long-range interactions enriched in bmi1abc predicted by HI-C. BAC probes are located at position 1 (A, green) and position 4 (A, red) of chromosome 4. Scale bars are 2 µm. Bar plot (below) shows the percentage of nuclei showing interactions between these regions in WT, bmi1abc, and clf swn. Data were collected from three independent biological replicates. The schematic drawing depicts the loop created by the interaction of two TE-rich/H3K27me1 CDs. C) Metaplots showing average levels of H2AK121ub (top panel) and H3K4me3 (bottom panel) at H3K27me1 CDs and surrounding regions in WT clf swn and bmi1abc. D) Boxplot showing the expression levels of genes included in bmi1abc loops in WT, bmi1abc and clf swn. For comparison, we used genes that in WT showed transcript levels > 5 FPKM. The median (middle line), upper and lower quartiles (boxes), and minimum and maximum values (whiskers) are indicated. P-values of differences according to one-sided Mann–Whitney–Wilcoxon test are indicated.
Nevertheless, the FISH results showed a higher percentage of interactions between the tested regions in clf swn than in WT, which suggest that H3K27me3 might help prevent TE-rich/H3K27me1 interactions as well. This appears to be in contrast to the finding of a reduced percentage of significant long-range interactions of H3K27me1 CDs in clf swn compared with WT (Fig. 6B). However, this percentage includes interactions between REF6-dependent H3K27me1 marked regions, and the levels of this modification are reduced in clf swn (Antunez-Sanchez et al. 2020). Altogether, these results are consistent with the global decrease in long-range interactions found in clf swn. Interestingly, the average expression levels of genes contained within the loops formed by TE-rich/H3K27me1 regions in bmi1abc were lower in bmi1abc than in WT or clf swn (Fig. 7D), suggesting that these long-range interactions might promote gene repression.
Discussion
The presence of chromatin-interacting domains has been revealed in both animal and plant genomes using Hi-C approaches. These domains are named TADs or CDs according to their specific characteristics (Szabo et al. 2019). However, previous Hi-C studies led to the proposal that TAD-like structures or CDs were not present in Arabidopsis, which was attributed to its small genome size (Feng et al. 2014; Grob et al. 2014; Wang et al. 2015). In its place, Arabidopsis was proposed to harbor a few compacted domains enriched in H3K9me2 or H3K27me3 repressive marks, which are separated by unstructured inter-domain regions rich in active chromatin modifications and transcriptional activity (Feng et al. 2014; Grob et al. 2014; Wang et al. 2015). However, these studies were performed at low resolution considering their sequencing depth and/or the size of the restriction fragments (Feng et al. 2014; Grob et al. 2014; Wang et al. 2015), which are major determinants of resolution (Lajoie et al. 2015). More recently, an analysis of Arabidopsis chromatin interaction patterns at gene resolution level was performed by reducing the length of restriction fragments. The results of this study led to the proposal that gene loops might constitute a widespread phenomenon in Arabidopsis and that the preferred units of chromatin packaging are gene bodies (Liu et al. 2016). Nevertheless, in the current study, by increasing sequencing depth and using small restriction fragments, we showed that Arabidopsis genome is partitioned into CDs with different epigenetic features. These results are similar to what has been found in Drosophila (Wang et al. 2018), whose genome size is comparable to that of Arabidopsis (∼180 Mb and ∼135 Mb, respectively).
Our findings suggest that the epigenetic state does not play a primary role in CD establishment, since altered levels of different histone modifications, such as in those Polycomb mutants, did not lead to major changes in the genomic distribution of CDs. Instead, these findings are consistent with a role of histone modifications in creating the appropriate intra-CD interactions, which are necessary for a higher-order level of 3D chromatin organization in which Polycomb complexes play a crucial role.
The role of Polycomb marks in regulating gene expression has been extensively studied in Arabidopsis (Baile et al. 2022); however, little is known about the role of these marks in establishing a higher-order chromatin network, especially for H2AK121ub. Several studies in plants and animals showed that H3K27me3 helps establish local and long-range chromatin interactions (Feng et al. 2014; Wang et al. 2015; Liu et al. 2016; Nützmann et al. 2020; Huang et al. 2021). H3K27me3-mediated interactions may lead to chromatin compaction at H3K27me3 CDs, thus reducing chromatin accessibility and promoting the repression of the genes included in these CDs. However, it is unknown whether long-range H3K27me3 interactions influence gene expression outside of H3K27me3-marked regions. On the other hand, little known about the participation of H2AK121ub in 3D chromatin organization in plants. In animals, PRC1-mediated H2A monoubiquitination is important for regulating gene expression but seems to be dispensable for mediating chromatin interactions (Boyle et al. 2020). Animal H2A monoubiquitination is mainly established by PRC1 variant complexes, while canonical PRC1 has lower catalytic activity and associates with subunits that alter chromatin structure (Kassis et al. 2017; Cheutin and Cavalli 2019; Guo et al. 2021). Conversely, Arabidopsis functional homologs of the subunits that alter chromatin structure are linked to PRC2 (Baile et al. 2022).
In agreement with the role of H2AK121ub in recruiting H3K27me3 for gene repression, we found a significant decrease in intra-CD interactions in H3K27me3-enriched CDs in bmi1abc, indicating that the combined activity of PRC1 and PRC2 is required to maintain the appropriate intra-CD interactions (Fig. 8A). However, this result does not clarify a specific role for H2AK121ub in regulating chromatin organization. In Arabidopsis, BMI1 and H2AK121ub can also be present at H3K4me3 CDs. Our results show that H3K4me3 CDs are smaller and display weaker intra-CD interactions than the other types of CDs, which is in line with previous results (Liu et al. 2016). Interestingly, small chromatin loops are enriched in H3K4me3-marked regions; however, the presence of BMI1/H2AK121ub significantly decreases the strength of these loops. These results suggest a role for BMI1/H2AK121ub in hindering the formation of chromatin loops at H3K4me3 marked regions. Moreover, self-loop structures involving 5′ and 3′ regions are more likely to occur in transcribed vs. untranscribed genes (Liu et al. 2016); these structures are marked with H3K4me3. However, the percentage of gene loops within BMI1/H2AK121ub_loops was very low, even though they contain high levels of H3K4me3. Interestingly, BMI1B binds predominantly to the 5′ and 3′ regions of genes, and H2AK121ub/H3K4me3-marked genes display lower average expression levels than genes only marked with H3K4me3. An intriguing possibility is that the binding of BMI1 and H2AK121ub incorporation dampen gene loop formation to modulate gene transcription (Fig. 8B), possibly by reducing chromatin accessibility (Yin et al. 2021).
Figure 8.
Model for the different roles of PRC1-mediated H2AK121ub in shaping local and long-range interactions. A) The combined activity of PRC1 and PRC2 is required to maintain the appropriate intra-CD interactions. B) The binding of BMI1 and the incorporation of H2AK121ub at H3K4me3-marked genes dampen gene loop formation to modulate gene transcription. C) The incorporation of H2AK121ub helps change the 3D organization by preventing TE rich/H3K27me1 loop interactions and serving as a docking point for H3K27me3 incorporation. Subsequently, interactions among H3K27me3 CDs shape the 3D chromatin organization required to regulate gene expression.
Our results also show that the loss of PRC1 and PRC2 activity differentially affects long-range chromatin interactions, which seems to influence gene expression outside H3K27me3-marked domains in a different manner. Previous and current findings show that the loss of PRC2 activity induces a global reconfiguration of chromatin architecture (Feng et al. 2014; Nützmann et al. 2020; Huang et al. 2021). In addition, a previous study showed that the loss of H3K27me3 induces the destabilization of repressive domains and that the genes within these domains tend to be upregulated (Huang et al. 2021). Thus, the loss of H3K27me3 at H3K27me3 CDs might enable the incorporation of H3K4me3 and thus a transcriptionally permissive state consistent with gene upregulation within these CDs. However, we also found that genes outside H3K27me3 CDs, such as those in H3K4me3 CDs, displayed increased average expression levels in clf swn vs. the WT, suggesting that long-range interactions are part of a more complex regulatory mechanism.
Moreover, our data indicate that PRC1-mediated H2AK121ub also influences long-range chromatin interactions in Arabidopsis, which differs from what has been reported in animals (Kassis et al. 2017; Cheutin and Cavalli 2019). Interestingly, long-range interactions and gene expression outputs were different in bmi1abc and clf swn. Since reduced levels of H2AK121ub affect H3K27me3 levels, we found a consistent reduction in long-range H3K27me3 CD interactions in bmi1abc. However, bmi1abc showed an enrichment in long-range interactions involving TE-rich/H3K27me1 marked regions, pointing to a role for H2AK121ub in preventing these interactions. Although further work is needed to understand this role of H2AK121ub, perhaps BMI1/H2AK121ub hampers the establishment of these interactions, which appears to be the case for local chromatin loop interactions. Remarkably, the average expression levels of genes included within these long-range loops were lower in bmi1abc than in WT or clf swn, suggesting that these interactions influence gene expression.
The 3D genome conformation is highly dynamic in response to developmental and exogenous signals (Bourbousse et al. 2019; Zheng and Xie 2019; Nützmann et al. 2020; Huang et al. 2021; Yadav et al. 2021). PRC1 activity is required for the initial switch from embryo-to-vegetative development, and PRC2 activity is required to maintain vegetative development (Bouyer et al. 2011; Yang et al. 2013). Since the bmi1abc mutant remains in an embryo maturation phase after germination, we speculate that its 3D chromatin organization might be reminiscent of that of seeds before germination, in which chromatin becomes condensed and transcriptionally inactive (van Zanten et al. 2011). An interesting possibility is that the interactions observed among TE-rich/H3K27me1 regions might help organize chromatin in a condensed conformation that is not conducive to transcription. As TE-rich/H3K27me1 CDs are interspersed in euchromatin, it is possible that these regions help shape chromatin organization under other developmental stages or conditions. In support of this notion, it was recently reported that compact and loose structural domains showed a different distribution of genes and TEs in Arabidopsis leaves and endosperm (Yadav et al. 2021). We propose that the incorporation of H2AK121ub helps alter the 3D organization of chromatin by preventing TE rich/H3K27me1 loop interactions and serving as a docking point for H3K27me3 incorporation. Subsequently, interactions among H3K27me3 CDs may shape the 3D chromatin organization required to regulate gene expression during vegetative development (Fig. 8C).
Methods
Plant materials and growth conditions
Arabidopsis Col-0, bmi1abc (Yang et al. 2013), and clf swn (Lafos et al. 2011) mutants and BMI1B-FLAG bmi1b transgenic plants (Wang et al. 2014) were grown in a growth chamber (CU36L5, Percival Scientific) under cool white light (Philips, ∼100 μmol m−2 s−1) in long-day conditions (16 h of light/8 h of dark) at 22 °C on half-strength MS agar plates containing 1% sucrose and 0.7% agar.
In situ Hi-C
One gram of 10-d-old seedling tissue was used for in situ Hi-C. Tissue fixation, nuclei isolation, chromatin digestion, ligation, and DNA purification were performed as previously described (Wang et al. 2015) using DpnII (New England Biolabs, R0543S) as the restriction enzyme for chromatin digestion. DNA libraries were constructed using a NEBNext Ultra II DNA Library Prep Kit for Illumina (New England Biolabs, E7645S). Dynabeads MyOne Streptavidin C1 beads (Invitrogen, 65001) were used to enrich an aliquot of proximally ligated DNA. Two biological replicates, which were independent since seedling tissue collection, were processed for next-generation sequencing library preparation. Libraries were prepared by PCR amplification using NEBNext Multiplex Oligos for Illumina (New England Biolabs, E7335) with 10 cycles. The libraries were purified using VAHTS DNA Clean Beads (Vazyme, N411). The libraries were sequenced on the Illumina HiSeq-Xten PE150 platform by generating 2 × 150-bp paired-end reads.
Hi-C data analysis
Two independent biological replicates were used to generate Hi-C data for each genotype: WT, bmi1abc, and clf swn. Once both replicates were found to be highly concordant, the raw data were merged to obtain higher coverages. Hi-C raw data were processed using the HiC-Pro pipeline version 3.1.0 (Servant et al. 2015) with default parameters using TAIR10 as the reference genome for Arabidopsis. Read mapping, filtering, and quality checks were performed with this pipeline in order to generate valid read pairs and interactions matrices. The hicpro2juicebox script from the HiC-Pro toolbox was applied to generate.hic files, which were imported into Juicebox (Durand et al. 2016) for visualization. KR balancing was used for contact matrix visualization (Knight and Ruiz 2013). TAD/interacting domain calling was performed based on the IS computed using HiCExplorer (Ramírez et al. 2018). The hicpro2Fit-Hi-C.py script from the HiC-Pro toolbox was used to generate contact matrices compatible with Fit-Hi-C (Ay et al. 2014). Fit-Hi-C provides a computational method to model the distance decay and account for technical biases in order to find statistically significant intra-chromosomal Hi-C contacts. This toolbox was used to identify significant contacts in the three different genotypes filtered with a q-value < 0.05.
A custom R script based on the library GenomicRanges was used to count intra- and inter-CD interactions. Specifically, intra-CD interactions were computed by counting the number of significant interactions identified previously by Fit-Hi-C with coordinates located inside the same CD. Two nonconsecutive CDs were assumed to exhibit long-range interactions when at least two significant interactions were identified by Fit-Hi-C with coordinates contained in the two different CDs. Loop interactions in the WT and mutants were detected using hicDetectLoops and hicMergeLoops from HiCExplorer (Ramírez et al. 2018), and bedtools (Quinlan 2014) was used to intersect WT and mutant loops. Plots representing the average IS over CDs were generated with the statistical programming language R using the function featureAlignedSignal from the Bioconductor package ChIPpeakAnno (Zhu et al. 2010). Annotation of CD boundaries with respect to gene features was performed with the Bioconductor R package ChIPseeker (Yu et al. 2015). DNA motif enrichment analysis was carried out using HOMER (Heinz et al. 2010). Significance analysis between global distributions represented using boxplots was performed using the nonparametric Mann–Whitney–Wilcoxon (or Wilcoxon rank-sum) test to compare two independent samples and Wilcoxon signed-rank test to analyze paired samples. Linear regression analysis between accessibility and IS was performed using the lm function in R.
ChIP-seq and data analysis
The H3K4me3 or BMI1B-FLAG ChIP-seq experiments were performed using 0.5 g or 2.5 g of 10-d-old seedling tissue. The ChIP-seq protocol was performed as previously described (Yin et al. 2021) using anti-H3K4me3 (Millipore, 17-614) or anti-FLAG antibodies (Sigma, F7425). Two independent biological replicates were processed for next-generation sequencing library preparation. DNA libraries were constructed using the Ovation Ultralow Library Systems (NuGEN). Libraries were prepared by PCR amplification with 14 cycles. DNA of a size range between 200 and 600 bp was selected using VAHTS DNA Clean Beads. The libraries were sequenced on the Illumina HiSeq-Xten PE150 platform by generating 2 × 150-bp paired-end reads.
Files in bigwig (bw) format for H2AK121ub and H3K27me3 ChIP-seq data for bmi1abc, clf swn, and WT, H3K27me1 data for ref6-5 and WT, and H3K27me3 data for ref6-5 were previously generated (Zhou et al. 2017; Antunez-Sanchez et al. 2020). H3K4me3 for bmi1abc, clf swn, and WT data BMI1B-FLAG ChIP-seq data were generated, processed and analyzed in this study. Bowtie (Langmead et al. 2009) was used for read mapping to the TAIR10 Arabidopsis reference genome. CPM (counts per million) normalized bw files were generated using the utility bamCoverage from deepTools (Ramírez et al. 2014). H3K4me3 peaks were determined with MACS2 (Zhang et al. 2008) using default parameters, and BMI1B-FLAG peaks were determined with HOMER (Heinz et al. 2010). HOMER was also used to perform BMI1B motif analysis. H3K4me3- or BMI1B-marked genes were identified using the bioconductor R package ChIPpeakAnno (Zhu et al. 2010) when an overlap was detected with either the gene body or promoter (defined as the region 1 kb upstream from the TSS). Profiles of histone modification on BMI1B-marked gene were generated using SeqPlots (Stempor and Ahringer 2016). The statistical significance of differences in epigenetic mark boxplots were assessed using the nonparametric Mann–Whitney–Wilcoxon test implemented in the wilcox.test function in R.
Analysis of chromatin loops
Over 20,000 chromatin loops from the euchromatic chromosome arms were identified previously (Liu et al. 2016). For analysis purposes, these loops were transferred to 1 kb resolution. Pgltools (Greenwald et al. 2017) were used to distinguish loops with BMI1B binding sites. Loop strength was calculated using GENOVA (van der Weide et al. 2021). Histone modification enrichment analysis was performed using bedmap (Neph et al. 2012). Modification levels on specific loop anchors were compared using multiBigwigSummary from deepTools (Ramírez et al. 2016). The statistical significance of differences in gene resolution loop-related boxplots were assessed using the nonparametric Mann–Whitney–Wilcoxon test implemented in the ggpubr R package.
Fluorescence in situ hybridization
The FISH experiment was performed as previously described with minor modifications (Bi et al. 2017; Nützmann et al. 2020). Ten-day-old seedlings were used for nucleus isolation. Digoxigenin-11-dUTP (Roche, 11175033910) labeled probes were prepared by nick translation of BAC JAtY53E16 (Chr. 4:9483355-9538373), and dinitrophenol-11-dUTP (PerkinElmer, NEL551001EA) labeled probes were prepared by nick translation of BAC JAtY78G16 (Chr. 4:11820000-11838276). For detection, mouse antidigoxin antibody (Sigma, D-8156) (1:500) and goat antimouse antibody coupled to Alexa Fluor 488 (Invitrogen, A11017) (1:150) were used to detect digoxigenin-11-dUTP-labeled probes. Rabbit antidinitrophenyl antibody (Invitrogen, A6430) (1:300) and goat antirabbit antibody coupled to Alexa Fluor 546 (Invitrogen, A-11071) (1:150) were used to detect dinitrophenol-11-dUTP-labeled probes. The samples were mounted on slides with SlowFade Diamond Antifade Mountant with DAPI (Invitrogen, S36964). FISH images were obtained with a Zeiss LSM 900 Airyscan 2 system using a 63X oil objective. The images were processed using ZEN Microscopy Software. Interacting nuclei were defined as two partially or completely overlapping signal spots.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL libraries under the following accession numbers: Hi-C, BMI1B-FLAG (AT1G06770) and H3K4me3 ChIP-seq data sets generated in this study have been deposited in the European Nucleotide Archive (ENA) under accession code PRJEB52473. Previously generated ChIP-seq and RNA-seq data for WT, bmi1abc, and clf swn Arabidopsis (Zhou et al. 2017; Yin et al. 2021) are available in the Gene Expression Omnibus (GEO) under accession numbers GSE89358 and GSE155378, respectively. ChIP-seq data of H3K27me1 in WT and ref6-5 and H3K27me3 in ref6-5 (Antunez-Sanchez et al. 2020) are available at ENA under accession code PRJEB36508.
Supplementary Material
Acknowledgments
We thank Dr. Yuling Jiao (Peking University, China) for sharing the BACs, Drs. Yan He (China Agricultural University, China) and Siyang Huang (Yangzhou University, China) for providing technical support for the FISH experiment, and Dr. Yuehui He (Peking University, China) for sharing seeds of BMI1B-FLAG/bmi1b transgenic plants. This work was carried out at the Peking University High Performance Computing Platform, and the calculations were performed using CLS-HPC.
Contributor Information
Xiaochang Yin, State Key Laboratory of Protein and Plant Gene Research, School of Advanced Agricultural Sciences, Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China.
Francisco J Romero-Campero, Institute of Plant Biochemistry and Photosynthesis (IBVF-CSIC), Avenida Américo Vespucio 49, 41092 Seville, Spain; Department of Computer Science and Artificial Intelligence, University of Sevilla, Avenida Reina Mercedes s/n, Seville 41012, Spain.
Minqi Yang, State Key Laboratory of Protein and Plant Gene Research, School of Advanced Agricultural Sciences, Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China.
Fernando Baile, Institute of Plant Biochemistry and Photosynthesis (IBVF-CSIC), Avenida Américo Vespucio 49, 41092 Seville, Spain.
Yuxin Cao, State Key Laboratory of Protein and Plant Gene Research, School of Advanced Agricultural Sciences, Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China.
Jiayue Shu, State Key Laboratory of Protein and Plant Gene Research, School of Advanced Agricultural Sciences, Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China; Academy for Advanced Interdisciplinary Studies, Peking University, Beijing 100871, China.
Lingxiao Luo, State Key Laboratory of Protein and Plant Gene Research, School of Advanced Agricultural Sciences, Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China.
Dingyue Wang, State Key Laboratory of Protein and Plant Gene Research, School of Advanced Agricultural Sciences, Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China; Academy for Advanced Interdisciplinary Studies, Peking University, Beijing 100871, China.
Shang Sun, Key Laboratory of Plant Functional Genomics of the Ministry of Education/Jiangsu Key Laboratory of Crop Genomics and Molecular Breeding, Agricultural College of Yangzhou University, Yangzhou 225009, China.
Peng Yan, State Key Laboratory of Protein and Plant Gene Research, School of Advanced Agricultural Sciences, Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China; State Key Laboratory of Plant Physiology and Biochemistry, College of Life Science, Zhejiang University, Hangzhou 310058, China.
Zhiyun Gong, Key Laboratory of Plant Functional Genomics of the Ministry of Education/Jiangsu Key Laboratory of Crop Genomics and Molecular Breeding, Agricultural College of Yangzhou University, Yangzhou 225009, China.
Xiaorong Mo, State Key Laboratory of Plant Physiology and Biochemistry, College of Life Science, Zhejiang University, Hangzhou 310058, China.
Genji Qin, State Key Laboratory of Protein and Plant Gene Research, School of Life Sciences, Peking University, Beijing 100871, China.
Myriam Calonje, Institute of Plant Biochemistry and Photosynthesis (IBVF-CSIC), Avenida Américo Vespucio 49, 41092 Seville, Spain.
Yue Zhou, State Key Laboratory of Protein and Plant Gene Research, School of Advanced Agricultural Sciences, Peking-Tsinghua Center for Life Sciences, Peking University, Beijing 100871, China.
Author contributions
X.Y., P.Y., and X.M. performed the Hi-C experiment, X.Y. and Z.G. performed the FISH experiments. X.Y. performed H3K4me3 ChIP in clf swn, and M.Y. performed the BMI1B-FLAG ChIP experiments. F.B. performed the H3K4me3 ChIP experiments of WT and bmi1abc and analyzed sequencing data. M.Y. performed the BMI1B-FLAG ChIP-seq and loop analyses. FJRC analyzed Hi-C, ChIP-seq, RNA-seq and ATAC-seq data. M.C. and Y.Z. designed the study. M.C. interpreted the data and wrote the manuscript. All authors read and approved the final manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Quality control of Hi-C data (supports Figs. 1 and 4).
Supplemental Figure S2. Hi-C contact matrix of the Arabidopsis genome in WT, bmi1abc, and clf swn separated and combined replicates (supports Figs. 1 and 4).
Supplemental Figure S3. The Arabidopsis genome is partitioned into self-interacting domains (supports Fig. 1).
Supplemental Figure S4. Levels of different histone modifications and accessibility in Arabidopsis CDs (supports Figs. 1–3).
Supplemental Figure S5. Genome wide binding of BMI1B-FLAG in bmi1b mutant background (supports Fig. 3).
Supplemental Figure S6. Chromatin interactions in WT, bmi1abc, and clf swn (supports Fig. 4).
Supplemental Figure S7. Most of the genes misregulated in bmi1abc and clf swn are not marked with H3K27me3 in WT (supports Fig. 4).
Supplemental Figure S8. Long-range interactions in Arabidopsis (supports Fig. 5).
Supplemental Figure S9. bmi1abc enriched loop interactions among TE-rich/H3K27me1 CDs (supports Figs. 6 and 7).
Supplemental Table S1. Hi-C read-pairs representative of true interactions in separate and combined replicates.
Supplemental Data Set 1. List of BMI1B-FLAG target genes.
Funding
This work was supported by grant number 31970532 (Y.Z.) from the National Natural Science Foundation of China; grant numbers PID2019-106664GB-I00 (M.C.) and BIO2017-84066-R (F.J.R.-C.) from the Spanish Ministry of Science and Innovation; grant number 2021T140019 from the China Postdoctoral Science Foundation (X.Y.); startup funds from the State Key Laboratory for Protein and Plant Gene Research, the School of Advanced Agricultural Sciences, and the Peking-Tsinghua Center for Life Sciences at Peking University (Y.Z.).
References
- Antunez-Sanchez J, Naish M, Ramirez-Prado JS, Ohno S, Huang Y, Dawson A, Opassathian K, Manza-Mianza D, Ariel F, Raynaud C, et al. A new role for histone demethylases in the maintenance of plant genome integrity. Elife. 2020:9:e58533. 10.7554/eLife.58533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay F, Bailey TL, Noble WS. Statistical confidence estimation for Hi-C data reveals regulatory chromatin contacts. Genome Res. 2014:24(6):999–1011. 10.1101/gr.160374.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baile F, Gómez-Zambrano Á, Calonje M. Roles of polycomb complexes in regulating gene expression and chromatin structure in plants. Plant Commun. 2022:3(1):100267. 10.1016/j.xplc.2021.100267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baile F, Merini W, Hidalgo I, Calonje M. EAR domain-containing transcription factors trigger PRC2-mediated chromatin marking in Arabidopsis. Plant Cell. 2021:33(8):2701–2715. 10.1093/plcell/koab139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X, Cheng Y-J, Hu B, Ma X, Wu R, Wang J-W, Liu C. Nonrandom domain organization of the Arabidopsis genome at the nuclear periphery. Genome Res. 2017:27(7):1162–1173. 10.1101/gr.215186.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackledge NP, Farcas AM, Kondo T, King HW, McGouran JF, Hanssen LL, Ito S, Cooper S, Kondo K, Koseki Y, et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell. 2014:157(6):1445–1459. 10.1016/j.cell.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer RH, Hutchison CE, Bäurle I, Walker J, Fang X, Perera P, Velanis CN, Gümüs S, Spanos C, Rappsilber J, et al. The Arabidopsis epigenetic regulator ICU11 as an accessory protein of polycomb repressive complex 2. Proc Natl Acad Sci USA. 2020:117(28):16660–16666. 10.1073/pnas.1920621117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbousse C, Barneche F, Laloi C. Plant chromatin catches the sun. Front Plant Sci. 2019:10:1728. 10.3389/fpls.2019.01728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouyer D, Roudier F, Heese M, Andersen ED, Gey D, Nowack MK, Goodrich J, Renou J-P, Grini PE, Colot V, et al. Polycomb repressive complex 2 controls the embryo-to-seedling phase transition. PLoS Genet. 2011:7(3):e1002014. 10.1371/journal.pgen.1002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S, Flyamer IM, Williamson I, Sengupta D, Bickmore WA, Illingworth RS. A central role for canonical PRC1 in shaping the 3D nuclear landscape. Genes Dev. 2020:34(13–14):931–949. 10.1101/gad.336487.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratzel F, López-Torrejón G, Koch M, Del Pozo JC, Calonje M. Keeping cell identity in Arabidopsis requires PRC1 RING-finger homologs that catalyze H2A monoubiquitination. Curr Biol. 2010:20(20):1853–1859. 10.1016/j.cub.2010.09.046 [DOI] [PubMed] [Google Scholar]
- Bratzel F, Yang C, Angelova A, López-Torrejón G, Koch M, del Pozo JC, Calonje M. Regulation of the new Arabidopsis imprinted gene AtBMI1C requires the interplay of different epigenetic mechanisms. Mol Plant. 2012:5(1):260–269. 10.1093/mp/ssr078 [DOI] [PubMed] [Google Scholar]
- Chanvivattana Y, Bishopp A, Schubert D, Stock C, Moon Y-H, Sung ZR, Goodrich J. Interaction of polycomb-group proteins controlling flowering in Arabidopsis. Development. 2004:131(21):5263–5276. 10.1242/dev.01400 [DOI] [PubMed] [Google Scholar]
- Chen D, Molitor A, Liu C, Shen W-H. The Arabidopsis PRC1-like ring-finger proteins are necessary for repression of embryonic traits during vegetative growth. Cell Res. 2010:20(12):1332–1344. 10.1038/cr.2010.151 [DOI] [PubMed] [Google Scholar]
- Cheutin T, Cavalli G. The multiscale effects of polycomb mechanisms on 3D chromatin folding. Crit Rev Biochem Mol Biol. 2019:54(5):399–417. 10.1080/10409238.2019.1679082 [DOI] [PubMed] [Google Scholar]
- Crane E, Bian Q, McCord RP, Lajoie BR, Wheeler BS, Ralston EJ, Uzawa S, Dekker J, Meyer BJ. Condensin-driven remodelling of X chromosome topology during dosage compensation. Nature. 2015:523(7559):240–244. 10.1038/nature14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkacheva M, Steinbach Y, Wildhaber T, Mozgová I, Mahrez W, Nanni P, Bischof S, Gruissem W, Hennig L. Arabidopsis MSI1 connects LHP1 to PRC2 complexes. EMBO J. 2013:32(14):2073–2085. 10.1038/emboj.2013.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doğan ES, Liu C. Three-dimensional chromatin packing and positioning of plant genomes. Nat Plants. 2018:4(8):521–529. 10.1038/s41477-018-0199-5 [DOI] [PubMed] [Google Scholar]
- Dong P, Tu X, Chu P-Y, Lü P, Zhu N, Grierson D, Du B, Li P, Zhong S. 3D chromatin architecture of large plant genomes determined by local A/B compartments. Mol Plant. 2017:10(12):1497–1509. 10.1016/j.molp.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Dong QL, Wang JB, Li XC, Gong L. Progresses in the plant 3D chromatin architecture. Yi Chuan. 2020:42(1):73–86. 10.16288/j.yczz.19-326 [DOI] [PubMed] [Google Scholar]
- Durand NC, Robinson JT, Shamim MS, Machol I, Mesirov JP, Lander ES, Aiden EL. Juicebox provides a visualization system for Hi-C contact maps with unlimited zoom. Cell Syst. 2016:3(1):99–101. 10.1016/j.cels.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Cokus SJ, Schubert V, Zhai J, Pellegrini M, Jacobsen SE. Genome-wide Hi-C analyses in wild-type and mutants reveal high-resolution chromatin interactions in Arabidopsis. Mol Cell. 2014:55(5):694–707. 10.1016/j.molcel.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudenberg G, Imakaev M, Lu C, Goloborodko A, Abdennur N, Mirny LA. Formation of chromosomal domains by loop extrusion. Cell Rep. 2016:15(9):2038–2049. 10.1016/j.celrep.2016.04.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendall AR, Levy YY, Wilson A, Dean C. The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell. 2001:107(4):525–535. 10.1016/S0092-8674(01)00573-6 [DOI] [PubMed] [Google Scholar]
- Goodrich J, Puangsomlee P, Martin M, Long D, Meyerowitz EM, Coupland G. A polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature. 1997:386(6620):44–51. 10.1038/386044a0 [DOI] [PubMed] [Google Scholar]
- Greenwald WW, Li H, Smith EN, Benaglio P, Nariai N, Frazer KA. Pgltools: a genomic arithmetic tool suite for manipulation of Hi-C peak and other chromatin interaction data. BMC Bioinformatics. 2017:18(1):207. 10.1186/s12859-017-1621-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob S, Schmid MW, Grossniklaus U. Hi-C analysis in Arabidopsis identifies the KNOT, a structure with similarities to the flamenco locus of Drosophila. Mol Cell. 2014:55(5):678–693. 10.1016/j.molcel.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB. Maternal control of embryogenesis by MEDEA, a polycomb group gene in Arabidopsis. Science. 1998:280(5362):446–450. 10.1126/science.280.5362.446 [DOI] [PubMed] [Google Scholar]
- Guo Y, Zhao S, Wang GG. Polycomb gene silencing mechanisms: pRC2 chromatin targeting, H3K27me3 “readout”, and phase separation-based compaction. Trends Genet. 2021:37(6):547–565. 10.1016/j.tig.2020.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010:38(4):576–589. 10.1016/j.molcel.2010.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Sicar S, Ramirez-Prado JS, Manza-Mianza D, Antunez-Sanchez J, Brik-Chaouche R, Rodriguez-Granados NY, An J, Bergounioux C, Mahfouz MM, et al. Polycomb-dependent differential chromatin compartmentalization determines gene coregulation in Arabidopsis. Genome Res. 2021:31(7):1230–1244. 10.1101/gr.273771.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob Y, Feng S, LeBlanc CA, Bernatavichute YV, Stroud H, Cokus S, Johnson LM, Pellegrini M, Jacobsen SE, Michaels SD. ATXR5 and ATXR6 are H3K27 monomethyltransferases required for chromatin structure and gene silencing. Nat Struct Mol Biol. 2009:16(7):763–768. 10.1038/nsmb.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaaslan ES, Wang N, Faiß N, Liang Y, Montgomery SA, Laubinger S, Berendzen KW, Berger F, Breuninger H, Liu C. Marchantia TCP transcription factor activity correlates with three-dimensional chromatin structure. Nat Plants. 2020:6(10):1250–1261. 10.1038/s41477-020-00766-0 [DOI] [PubMed] [Google Scholar]
- Kassis JA, Kennison JA, Tamkun JW. Polycomb and trithorax group genes in Drosophila. Genetics. 2017:206(4):1699–1725. 10.1534/genetics.115.185116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight PA, Ruiz D. A fast algorithm for matrix balancing. IMA Journal of Numerical Analysis. 2013:33(3):1029–1047. 10.1093/imanum/drs019 [DOI] [Google Scholar]
- Köhler C, Hennig L, Bouveret R, Gheyselinck J, Grossniklaus U, Gruissem W. Arabidopsis MSI1 is a component of the MEA/FIE polycomb group complex and required for seed development. EMBO J. 2003:22(18):4804–4814. 10.1093/emboj/cdg444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralemann LEM, Liu S, Trejo-Arellano MS, Muñoz-Viana R, Köhler C, Hennig L. Removal of H2Aub1 by ubiquitin-specific proteases 12 and 13 is required for stable polycomb-mediated gene repression in Arabidopsis. Genome Biol. 2020:21(1):144. 10.1186/s13059-020-02062-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kaur S, Seem K, Kumar S, Mohapatra T. Understanding 3D genome organization and its effect on transcriptional gene regulation under environmental stress in plant: a chromatin perspective. Front Cell Dev Biol. 2021:9:1562. 10.3389/fcell.2021.774719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafos M, Kroll P, Hohenstatt ML, Thorpe FL, Clarenz O, Schubert D. Dynamic regulation of H3K27 trimethylation during Arabidopsis differentiation. PLoS Genet. 2011:7(4):e1002040. 10.1371/journal.pgen.1002040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie BR, Dekker J, Kaplan N. The Hitchhiker's Guide to Hi-C analysis: practical guidelines. Methods. 2015:72:65–75. 10.1016/j.ymeth.2014.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009:10(3):R25. 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang Z, Hu Y, Cao Y, Ma L. Polycomb group proteins RING1A and RING1B regulate the vegetative phase transition in Arabidopsis. Front Plant Sci. 2017:8:867. 10.3389/fpls.2017.00867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman-Aiden E, Van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009:326(5950):289–293. 10.1126/science.1181369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroth AM, Shultis D, Jasencakova Z, Fuchs J, Johnson L, Schubert D, Patnaik D, Pradhan S, Goodrich J, Schubert I, et al. Dual histone H3 methylation marks at lysines 9 and 27 required for interaction with CHROMOMETHYLASE3. EMBO J. 2004:23(21):4286–4296. 10.1038/sj.emboj.7600430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Cheng Y-J, Wang J-W, Weigel D. Prominent topologically associated domains differentiate global chromatin packing in rice from Arabidopsis. Nat Plants. 2017:3(9):742–748. 10.1038/s41477-017-0005-9 [DOI] [PubMed] [Google Scholar]
- Liu C, Wang C, Wang G, Becker C, Zaidem M, Weigel D. Genome-wide analysis of chromatin packing in Arabidopsis thaliana at single-gene resolution. Genome Res. 2016:26(8):1057–1068. 10.1101/gr.204032.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Cui X, Zhang S, Jenuwein T, Cao X. Arabidopsis REF6 is a histone H3 lysine 27 demethylase. Nat Genet. 2011:43(7):715–719. 10.1038/ng.854 [DOI] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Koltunow A, Dennis ES, Peacock WJ, Chaudhury AM. Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1999:96(1):296–301. 10.1073/pnas.96.1.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu H, Liu E, Wu Z. Comparison of normalization methods for Hi-C data. BioTechniques. 2020:68(2):56–64. 10.2144/btn-2019-0105 [DOI] [PubMed] [Google Scholar]
- Makarevich G, Leroy O, Akinci U, Schubert D, Clarenz O, Goodrich J, Grossniklaus U, Köhler C. Different polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep. 2006:7(9):947–952. 10.1038/sj.embor.7400760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozgova I, Hennig L. The polycomb group protein regulatory network. Annu Rev Plant Biol. 2015:66(1):269–296. 10.1146/annurev-arplant-043014-115627 [DOI] [PubMed] [Google Scholar]
- Neph S, Kuehn MS, Reynolds AP, Haugen E, Thurman RE, Johnson AK, Rynes E, Maurano MT, Vierstra J, Thomas S, et al. BEDOPS: high-performance genomic feature operations. Bioinformatics. 2012:28(14):1919–1920. 10.1093/bioinformatics/bts277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nützmann H-W, Doerr D, Ramírez-Colmenero A, Sotelo-Fonseca JE, Wegel E, Di Stefano M, Wingett SW, Fraser P, Hurst L, Fernandez-Valverde SL, et al. Active and repressed biosynthetic gene clusters have spatially distinct chromosome states. Proc Natl Acad Sci USA. 2020:117(24):13800–13809. 10.1073/pnas.1920474117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad N, Yadegari R, Margossian L, Hannon M, Michaeli D, Harada JJ, Goldberg RB, Fischer RL. Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell. 1999:11(3):407–416. 10.1105/tpc.11.3.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Xiong D, Li G, Li X. Unraveling the 3D genome architecture in plants: present and future. Mol Plant. 202013(12):1676–1693 10.1016/j.molp.2020.10.002 [DOI] [PubMed] [Google Scholar]
- Pachano T, Crispatzu G, Rada-Iglesias A. Polycomb proteins as organizers of 3D genome architecture in embryonic stem cells. Brief Funct Genomics. 2019:18(6):358–366 10.1093/bfgp/elz022 [DOI] [PubMed] [Google Scholar]
- Qüesta JI, Song J, Geraldo N, An H, Dean C. Arabidopsis transcriptional repressor VAL1 triggers polycomb silencing at FLC during vernalization. Science. 2016:353(6298):485–488. 10.1126/science.aaf7354 [DOI] [PubMed] [Google Scholar]
- Quinlan AR. BEDTools: the Swiss-army tool for genome feature analysis. Curr Protoc Bioinformatics. 2014:47(1):11.12.1-34. 10.1002/0471250953.bi1112s47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Bhardwaj V, Arrigoni L, Lam KC, Grüning BA, Villaveces J, Habermann B, Akhtar A, Manke T. High-resolution TADs reveal DNA sequences underlying genome organization in flies. Nat Commun. 2018:9(1):189. 10.1038/s41467-017-02525-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Dündar F, Diehl S, Grüning BA, Manke T. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014:42(W1):W187–W191. 10.1093/nar/gku365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Ryan DP, Grüning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Dündar F, Manke T. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016:44(W1):W160–W165. 10.1093/nar/gkw257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley MJ, Nichols MH, Lyu X, Ando-Kuri M, Rivera ISM, Hermetz K, Wang P, Ruan Y, Corces VG. Evolutionarily conserved principles predict 3D chromatin organization. Mol Cell. 2017:67(5):837–852.e7. 10.1016/j.molcel.2017.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanborn AL, Rao SS, Huang SC, Durand NC, Huntley MH, Jewett AI, Bochkov ID, Chinnappan D, Cutkosky A, Li J, et al. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc Natl Acad Sci USA. 2015:112(47):E6456-6465. 10.1073/pnas.1518552112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant N, Varoquaux N, Lajoie BR, Viara E, Chen C-J, Vert J-P, Heard E, Dekker J, Barillot E. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 2015:16(1):259. 10.1186/s13059-015-0831-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Probst AV. Maintenance and dynamic reprogramming of chromatin organization during development. Plant J. 2023. 10.1111/tpj.16119 [DOI] [PubMed] [Google Scholar]
- Stempor P, Ahringer J. SeqPlots – interactive software for exploratory data analyses, pattern discovery and visualization in genomics. Wellcome Open Res. 2016:1:14. 10.12688/wellcomeopenres.10004.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo Q, Bantignies F, Cavalli G. Principles of genome folding into topologically associating domains. Sci Adv. 2019:5(4):eaaw1668. 10.1126/sciadv.aaw1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weide RH, van den Brand T, Haarhuis JHI, Teunissen H, Rowland BD, de Wit E. Hi-C analyses with GENOVA: a case study with cohesin variants. NAR Genom Bioinform. 2021:3(2):lqab040. 10.1093/nargab/lqab040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zanten M, Koini MA, Geyer R, Liu Y, Brambilla V, Bartels D, Koornneef M, Fransz P, Soppe WJJ. Seed maturation in Arabidopsis thaliana is characterized by nuclear size reduction and increased chromatin condensation. Proc Natl Acad Sci USA. 2011:108(50):20219–20224. 10.1073/pnas.1117726108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gu X, Yuan W, Schmitz RJ, He Y. Photoperiodic control of the floral transition through a distinct polycomb repressive complex. Dev Cell. 2014:28(6):727–736. 10.1016/j.devcel.2014.01.029 [DOI] [PubMed] [Google Scholar]
- Wang C, Liu C, Roqueiro D, Grimm D, Schwab R, Becker C, Lanz C, Weigel D. Genome-wide analysis of local chromatin packing in Arabidopsis thaliana. Genome Res. 2015:25(2):246–256. 10.1101/gr.170332.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sun Q, Czajkowsky DM, Shao Z. Sub-kb Hi-C in D. Melanogaster reveals conserved characteristics of TADs between insect and mammalian cells. Nat Commun. 2018:9(1):188. 10.1038/s41467-017-02526-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Jin R, Yu X, Shen M, Wagner JD, Pai A, Song C, Zhuang M, Klasfeld S, He C, et al. Cis and trans determinants of epigenetic silencing by polycomb repressive complex 2 in Arabidopsis. Nat Genet. 2017:49(10):1546–1552. 10.1038/ng.3937 [DOI] [PubMed] [Google Scholar]
- Xiao J, Wagner D. Polycomb repression in the regulation of growth and development in Arabidopsis. Curr Opin Plant Biol. 2015:23:15–24. 10.1016/j.pbi.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Yadav VK, Santos-González J, Köhler C. INT-Hi-C reveals distinct chromatin architecture in endosperm and leaf tissues of Arabidopsis. Nucleic Acids Res. 2021:49(8):4371–4385. 10.1093/nar/gkab191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Bratzel F, Hohmann N, Koch M, Turck F, Calonje M. VAL- and AtBMI1-mediated H2Aub initiate the switch from embryonic to postgerminative growth in Arabidopsis. Curr Biol. 2013:23(14):1324–1329. 10.1016/j.cub.2013.05.050 [DOI] [PubMed] [Google Scholar]
- Yin X, Romero-Campero FJ, de Los Reyes P, Yan P, Yang J, Tian G, Yang X, Mo X, Zhao S, Calonje M, et al. H2AK121ub in Arabidopsis associates with a less accessible chromatin state at transcriptional regulation hotspots. Nat Commun. 2021:12(1):315. 10.1038/s41467-020-20614-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida N, Yanai Y, Chen L, Kato Y, Hiratsuka J, Miwa T, Sung ZR, Takahashi S. EMBRYONIC FLOWER2, a novel polycomb group protein homolog, mediates shoot development and flowering in Arabidopsis. Plant Cell. 2001:13(11):2471–2481. 10.1105/tpc.010227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Wang L-G, He Q-Y. ChIPseeker: an R/bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015:31(14):2382–2383. 10.1093/bioinformatics/btv145 [DOI] [PubMed] [Google Scholar]
- Yuan L, Song X, Zhang L, Yu Y, Liang Z, Lei Y, Ruan J, Tan B, Liu J, Li C. The transcriptional repressors VAL1 and VAL2 recruit PRC2 for genome-wide polycomb silencing in Arabidopsis. Nucleic Acids Res. 2021:49(1):98–113. 10.1093/nar/gkaa1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bernatavichute YV, Cokus S, Pellegrini M, Jacobsen SE. Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol. 2009:10(6):R62. 10.1186/gb-2009-10-6-r62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. Model-based analysis of ChIP-seq (MACS). Genome Biol. 2008:9(9):R137. 10.1186/gb-2008-9-9-r137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Xie W. The role of 3D genome organization in development and cell differentiation. Nat Rev Mol Cell Biol. 2019:20(9):535–550. 10.1038/s41580-019-0132-4 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Romero-Campero FJ, Gómez-Zambrano Á, Turck F, Calonje M. H2A monoubiquitination in Arabidopsis thaliana is generally independent of LHP1 and PRC2 activity. Genome Biol. 2017:18(1):69. 10.1186/s13059-017-1197-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LJ, Gazin C, Lawson ND, Pagès H, Lin SM, Lapointe DS, Green MR. ChIPpeakAnno: a bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinformatics. 2010:11(1):237. 10.1186/1471-2105-11-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey M, Tavernari D, Oricchio E, Ciriello G. Comparison of computational methods for the identification of topologically associating domains. Genome Biol. 2018:19(1):217. 10.1186/s13059-018-1596-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.