Abstract
Introduction: Patients with acute heart failure (AHF) exacerbation are susceptible to complications in the setting of COVID-19 infection. Data regarding the clinical outcomes of COVID-19 in patients admitted with AHF is limited. Methods: We used the national inpatient sample database by utilizing ICD-10 codes to identify all hospitalizations with a diagnosis of AHF in 2020. We classified the sample into AHF with COVID-19 infection versus those without COVID-19. Primary outcome was in-hospital mortality. Secondary outcomes were acute myocardial infarction, need for pressors, mechanical cardiac support, cardiogenic shock, and cardiac arrest. Also, we evaluated for acute pulmonary embolism (PE), bacterial pneumonia, need for a ventilator, and acute kidney injury (AKI). Results: We identified a total of 694,920 of AHF hospitalizations, 660,463 (95.04%) patients without COVID-19 and 34,457 (4.96%) with COVID-19 infection. For baseline comorbidities, diabetes mellitus, chronic heart failure, ESRD, and coagulopathy were significantly higher among AHF patients with COVID-19 (P < .01). While CAD, prior MI, percutaneous coronary intervention, and coronary artery bypass graft, atrial fibrillation, chronic obstructive pulmonary disease, and peripheral vascular disease were higher among those without COVID-19. After adjustment for baseline comorbidities, in-hospital mortality (aOR 5.08 [4.81 to 5.36]), septic shock (aOR 2.54 [2.40 to 2.70]), PE (aOR 1.75 [1.57 to 1.94]), and AKI (aOR 1.33 [1.30 to 1.37]) were significantly higher among AHF with COVID-19 patients. The mean length of stay (5 vs 7 days, P < .01) and costs of hospitalization ($42,143 vs $60,251, P < .01) were higher among AHF patients with COVID-19 infection. Conclusion: COVID-19 infection in patients with AHF is associated with significantly higher in-hospital mortality, need for mechanical ventilation, septic shock, and AKI along with higher resource utilization. Predictors for mortality in AHF patients during the COVID-19 pandemic, COVID-19 infection, patients with end-stage heart failure, and atrial fibrillation. Studies on the impact of vaccination against COVID-19 in AHF patients are needed
Keywords: COVID-19, acute heart failure, in-hospital mortality, clinical outcomes, national inpatient sample
Introduction
The Coronavirus disease 2019 (COVID-19) 1 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus 2 and its variant has led to significant morbidity and mortality due to its effect on multiple organ systems including the cardiovascular system. 3 The virus can directly cause damage to the cardiac myocytes via angiotensin-converting enzyme-2 (ACE-2) receptors, 4 and presents in the form of myocarditis and acute myocardial infarction (MI) causing major acute cardiovascular events, including heart failure, arrhythmias, and sudden cardiac arrest. 5 Indirectly, it can induce severe inflammatory immune response, cytokine storm, and immune system dysfunction resulting in additional myocardial injury and arterial thrombosis. 5 Prior studies have demonstrated a higher morbidity or mortality from COVID-19 infection in chronic comorbidities such as heart failure.2,6
However, despite the various reports about its clinical impact on human health in general, there is scarce data regarding the trends of in-hospital mortality, clinical outcomes, and prognostic factors in patients hospitalized with acute heart failure (AHF) and a confirmed diagnosis of SARS-CoV-2 infection. 3 Therefore, we aim to utilize a contemporary database of inpatient hospitalizations in the US, the National Inpatient Sample 2020, to evaluate the trends and outcomes of AHF during the COVID-19 pandemic.
Methodology
Data Source
The National Inpatient Sample (NIS) is one of several databases managed by the Agency for Healthcare Research and Quality (AHRQ) through a Federal-State-Industry partnership called the Healthcare Cost and Utilization Project (HCUP). The NIS 2020 database contains administrative claims data from more than 7 million inpatient hospitalizations annually in 43 participating states plus the District of Columbia, representing more than 97% of the USA population. Since NIS data are compiled annually, the data can be used for the analysis of procedure trends over time using trend weights compiled by the HCUP. To calculate hospitalization cost which includes total expenses incurred to provide services, cost-to-charge ratio (CCR) files were used. The CCR files provide hospital-specific ratios to calculate hospitalization cost based on the specific hospitalization characteristics.
Ethical Consideration
Institutional Review Board approval and informed consent were not required for this study because NIS data are de-identified and publicly available. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. As per HCUP guidelines, observations with cell count <11 are reported as “<11”.
Study Sample and Patient Selection
We analyzed NIS data using the International Classification of Diseases, 10th Revision, and Clinical Modification (ICD-10-CM) claims codes. ICD-10-CM codes of I5021, I5023, I5031, I5033, I5041, I5043, I50811, and I5081 were used to identify patients hospitalized with AHF exacerbation. To identify COVID-19 cases, we used ICD-10 code of U071. All diagnosis and procedure fields were queried to select and categorize the study population, ICD-10 codes are reported in Table S1. Individuals under 18 years old and those who were admitted electively were excluded from the study. A detailed methods flowchart is presented in Figure S1.
Variables
Baseline patient characteristics included demographic variables (age, sex, and race), baseline comorbidities including hypertension, type 2 diabetes mellitus, coronary artery disease (CAD), prior MI, percutaneous coronary artery intervention (PCI), coronary artery bypass graft (CABG), valve surgery, pacemaker placement (PPM), and implantable cardioverter-defibrillator (ICD), atrial fibrillation, chronic heart failure, end-stage heart failure (ESHF), peripheral vascular disease (PVD), cerebrovascular accident (CVA), chronic obstructive pulmonary disease (COPD), pulmonary circulation disorders, chronic kidney disease (CKD), end-stage renal disease (ESRD), liver disease, coagulopathy, and obesity.
Study Outcomes
The primary study endpoint was in-hospital mortality. Secondary endpoints were clinical outcomes including (1) cardiovascular complications, such as acute MI, need for pressors, veno-arterial extracorporeal membrane oxygenation (VA-ECMO), impella, intra-aortic balloon pump (IABP), mechanical cardiac support, cardiogenic shock, and cardiac arrest, (2) pulmonary complications, such as acute pulmonary embolism (PE), bacterial pneumonia and need for ventilator, (3) septic shock and need for pressors, (4) acute kidney injury (AKI), (5) resource utilization (length of stay and cost of hospitalization). Associated procedures and complications were identified by using ICD-10-CM codes, Table S1.
Statistical Analysis
To estimate the effect of COVID-19 diagnosis on the outcomes of interest, we performed a 1:1 nearest-neighbor propensity-matching without replacement with a propensity score estimated using logistic regression. The matching accounted for confounding from all baseline variables. The matching yielded adequate balance as indicated by a caliper of 0.005 standard deviation, standardized mean differences for all the included covariates below 0.2, and low Kolmogorov–Smirnov statistics, Figure S2. The categorical variables were presented as counts and percentages and compared using Pearson's Chi-square. Continuous variables had a skewed distribution and were presented as median and interquartile range (IQR) and were compared using nonparametric Mann–Whitney U test. To determine independent predictors of in-hospital mortality, we conducted logistic regression analysis. Baseline variables associated with the outcome in a univariate analysis were included in a multivariate logistic regression model. Results of the logistic regression analysis were presented as odds ratios with 95% confidence intervals. The data were complete for all variables except for in-hospital mortality (193; 0.03%), sex (28; 0.004%), and race (14,087; 2.03%). We did not impute missing data because missing data of less than 5% were not likely to introduce bias. 7 The Joinpoint Regression software used t-tests to determine if monthly percent change (MPCs) and/or annual (APC [P-trend across the year of 2020]) were statistically significant from zero. For all analyses, a two-tailed P-value of ≤.05 was considered statistically significant. All statistical analyses were performed using R version 4.2.2 and SPSS version 26® (IBM Corp, Armonk, NY).
Results
Baseline Characteristics
A total of 694,920 cases of AHF hospitalizations, out of which 660,463 (95.04%) were patients without COVID-19 and 34,457 (4.96%) with COVID-19 infection. Patients with AHF and COVID-19 were older (median age 74 vs 73 years, P < .001), and the majority were male (53.7% vs 52.1%, P < .001). Figure S3 shows the age distribution. Majority of patients were Caucasian (67.2%), followed by African American (18.3%) and Hispanic (7.5%). Baseline comorbidities such as diabetes mellitus (32.1% vs 26.6%, P < .001), chronic heart failure (77.1% vs 67.7%, P < .001), ESRD (11.2% vs 9.2%, P < .001), coagulopathy (15.6% vs 9.7%, P < .001), and obesity (27.8% vs 26.5%, P < .001) were significantly higher among patients with COVID-19. Meanwhile, CAD (43.9% vs 49.0%, P < .001), prior MI (11.5% vs 14.4%, P < .001), PCI (10.7% vs 12.7%, P < .001), CABG (10.0% vs 11.1, P < .001), atrial fibrillation (29.1% vs 30.3%, P < .001), COPD (31.9% vs 35.2%, P < .001), and PVD (2.1% vs 3.4%, P < .001) were lower among those with COVID-19. The detailed baseline characteristics before and after propensity score matching analysis are shown in Table 1.
Table 1.
Baseline Characteristics of the AHF Population in Unmatched Cohort and Propensity-Score Matched Cohort.
| Unmatched Cohort | Propensity-Score Matched Cohort | |||||
|---|---|---|---|---|---|---|
| Variable n (%) | AHF Without COVID-19 (660,463) | AHF with COVID-19 (34,457) | P-Value | AHF Without COVID-19 (33,668) | AHF with COVID-19 (33,668) | P-Value |
| Age (Median, [IQR]) | 73 [62-82] | 74 [64-83] | <.001 | 72.9 (13.0) | 72.6 (13.1) | <.001 |
| Female | 316,070 (47.9) | 15,947 (46.3) | <.001 | 15,685 (46.6) | 15,581 (46.3) | .426 |
| Race | <.001 | <.001 | ||||
| White | 446,788 (69.0) | 20,278 (60.2) | 20,668 (61.4) | 20,278 (60.2) | ||
| Black | 120,048 (18.5) | 7402 (22.0) | 7351 (21.8) | 7402 (22.0) | ||
| Hispanic | 48,361 (7.5) | 4053 (12.0) | 3967 (11.8) | 4053 (12.0) | ||
| Asian or Pacific Islander | 13,449 (2.1) | 720 (2.1) | 642 (1.9) | 720 (2.1) | ||
| Native American | 3777 (0.6) | 279 (0.8) | 218 (0.6) | 279 (0.8) | ||
| Other | 14,742 (2.3) | 936 (2.8) | 822 (2.4) | 936 (2.8) | ||
| Diabetes mellitus | 175,933 (26.6) | 11,057 (32.1) | <.001 | 10,508 (31.2) | 10,788 (32.0) | .021 |
| Hypertension | 7750 (1.2) | 410 (1.2) | .782 | 352 (1.0) | 408 (1.2) | .045 |
| CAD | 323,713 (49.0) | 15,143 (43.9) | <.001 | 14,550 (43.2) | 14,810 (44.0) | .044 |
| Prior MI | 94,983 (14.4) | 3976 (11.5) | <.001 | 3711 (11.0) | 3896 (11.6) | .025 |
| Prior PCI | 83,646 (12.7) | 3697 (10.7) | <.001 | 3360 (10.0) | 3613 (10.7) | .001 |
| Prior CABG | 73,547 (11.1) | 3451 (10.0) | <.001 | 3160 (9.4) | 3376 (10.0) | .005 |
| Prior valve surgery | 30,725 (4.7) | 1285 (3.7) | <.001 | 1113 (3.3) | 1256 (3.7) | .003 |
| Prior PPM | 46,199 (7.0) | 2338 (6.8) | <.001 | 2276 (6.8) | 2290 (6.8) | .842 |
| Prior ICD | 45,060 (6.8) | 2103 (6.1) | <.001 | 1785 (5.3) | 2041 (6.1) | <.001 |
| Atrial fibrillation | 199,830 (30.3) | 10,041 (29.1) | <.001 | 9740 (28.9) | 9804 (29.1) | .593 |
| Chronic heart failure | 447,285 (67.7) | 26,562 (77.1) | <.001 | 26,170 (77.7) | 25,970 (77.1) | .067 |
| HFrEF | 104,659 (15.8) | 6968 (20.2) | 7040 (20.9) | 6968 (20.7) | ||
| HFpEF | 173,044 (26.2) | 12,899 (37.4) | 12,929 (38.4) | 12,899 (38.3) | ||
| HF, other | 169,582 (26.7) | 6677 (19.4) | 6201 (18.4) | 6103 (18.1) | ||
| End stage heart failure | 5516 (0.8) | 105 (0.3) | <.001 | 102 (0.3) | 103 (0.3) | 1.00 |
| PVD | 22,706 (3.4) | 716 (2.1) | <.001 | 637 (1.9) | 706 (2.1) | .061 |
| CVA | 44,321 (6.7) | 2154 (6.3) | <.001 | 1934 (5.7) | 2094 (6.2) | <.001 |
| COPD | 232,694 (35.2) | 10,982 (31.9) | <.001 | 10,943 (32.5) | 10,743 (31.9) | .101 |
| Pulmonary circulation disorder | 115,218 (17.4) | 3961 (11.5) | <.001 | 3775 (11.2) | 3869 (11.5) | .259 |
| CKD | 259,292 (39.3) | 13,028 (37.8) | <.001 | 12,903 (38.3) | 12,737 (37.8) | .19 |
| ESRD | 60,880 (9.2) | 3843 (11.2) | <.001 | 3606 (10.7) | 3752 (11.1) | .073 |
| Liver disease | 58,626 (8.9) | 2397 (7.0) | <.001 | 2100 (6.2) | 2326 (6.9) | <.001 |
| Coagulopathy | 64,212 (9.7) | 5378 (15.6) | <.001 | 4928 (14.6) | 5266 (15.6) | <.001 |
| Obesity | 174,754 (26.5) | 9586 (27.8) | <.001 | 9199 (27.3) | 9375 (27.8) | .131 |
Abbreviations: CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; ESRD, end-stage renal disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implanted cardioverter-defibrillator; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; PPM, pacemaker placement; PVD, peripheral vascular disease. As per HCUP regulations, observations with cell count <11 are reported as “<11”. Bold texts indicate statistical significance.
Clinical Outcomes
In-Hospital mortality
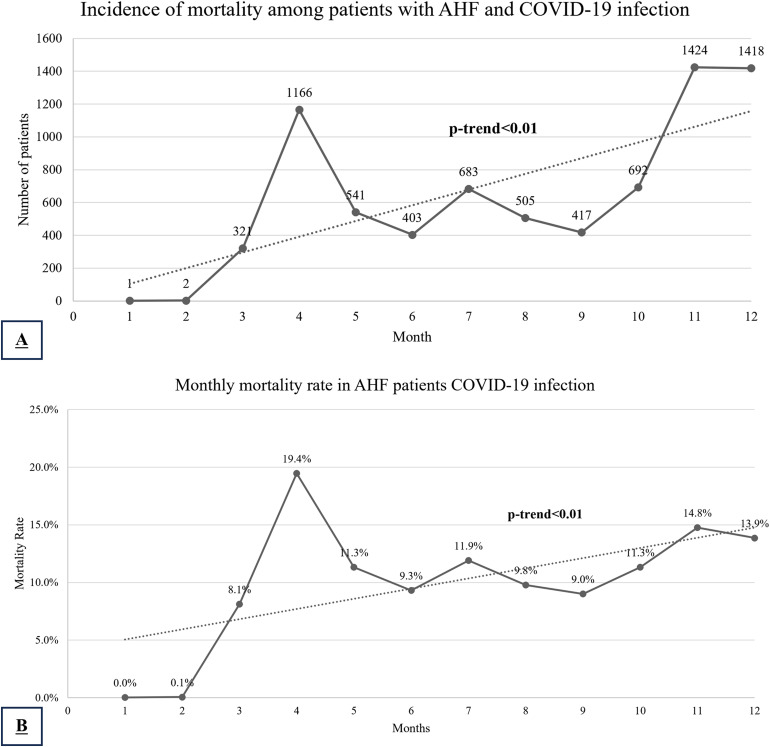
In-hospital mortality was significantly higher among patients with COVID-19 (22.0% vs 5.0%, P < .001). There was an overall significant increase in in-hospital crude mortality across the year of 2020 (APC 106.7, 95% CI [11.0 to 284.8], P-trend = .02), with the first peak in COVID-19 mortality in April (MPC 1296.7, 95% CI [329 to 4451], P < .01) followed by a decline (MPC −35.3, 95% CI [−96.5 to 1088], P = .05) and then a second peak in December (MPC 31.9, 95% CI [−15.5 to 105.7], P = .54), Figure 1A. Conversely, the mortality rates overall trended down during the study period (APC −3.6, 95% CI [−5.6 to −1.6], P < .01) with a peak in March with a monthly increase by 7.32% (95% CI [−2.2 to 17.8], P = .10), then trended down reaching a nadir in June with a monthly reduction by 16.1% (95% CI [−22.9 to −8.6], P < .01), and plateaued from June to December (MPC −0.4, 95% CI [−1.4 to 0.6], P = .33), Figure 1B.
Figure 1.
Mortality incidence (A) and rate (B) in AHF patients with COVID-19 patients across the year of 2020.
Clinical complications
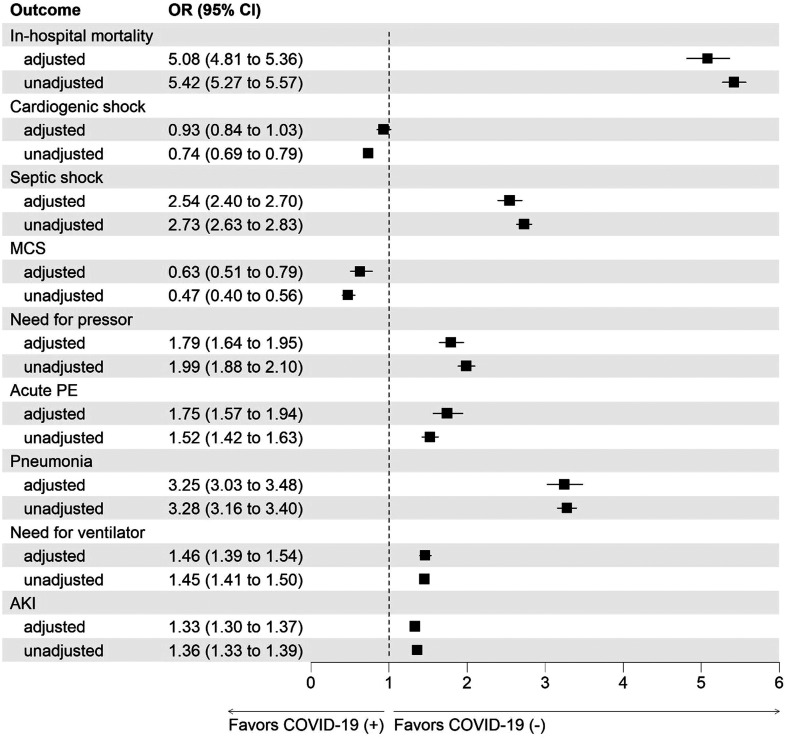
On propensity-matched analysis, cardiac arrest (4.2% vs 1.8%, P < .001), septic shock (11.2% vs 4.7%, P < .001), need for pressors (4.3% vs 2.5%, P < .001), acute PE (2.7% vs 1.6%, P < .001), bacterial pneumonia (9.9% vs 3.3%, P < .001), need for ventilator (11.6% vs 8.2%, P < .001), use of VA-ECMO (0.2% vs 0.1%, P = .02), and AKI (45.4% vs 38.4%, P < .001) were higher among AHF with COVID-19. Other complications such as acute MI (6.5% vs 5.4%, P < .001), use of mechanical cardiac support (0.6% vs 0.4%, P < .001), impella (0.2% vs 0.1%, P < .001), IABP (0.4% vs 0.2%), and need for blood transfusion (7.8% vs 6.5%, P < .001) were higher among AHF without COVID-19. On adjusted analysis propensity score-adjusted analysis, patients with COVID-19 and AHF were at higher odds of cardiac arrest (aOR 2.37 [2.15 to 2.61]), septic shock (aOR 2.54 [2.40 to 2.70]), need for pressors (aOR 1.79 [1.64 to 1.95]), acute PE (aOR 1.75 [1.57 to 1.94]), and need for ventilator (aOR 1.46 [1.39 to 1.54]) and AKI (aOR 1.33 [1.30 to 1.37]). Further detailed information regarding clinical outcomes before and after propensity score-based adjustment is listed in Table 2, Figure 2, and Table S2.
Table 2.
In-Hospital Complications and Clinical Outcomes on the Unmatched, Crude Analysis, and Following Propensity-Score Matching.
| Crude Analysis | Propensity-Score Matching analysis | |||||
|---|---|---|---|---|---|---|
| Variable n (%) | AHF Without COVID-19 (660,463) | AHF With COVID-19 (34,457) | P-Value | AHF Without COVID-19 (33,668) | AHF With COVID-19 (33,668) | P-Value |
| Died during hospitalization | 32,754 (5.0) | 7589 (22.0) | <.001 | 1768 (5.3) | 7393 (22.0) | <.001 |
| Acute myocardial infarction | 50,429 (7.6) | 1896 (5.5) | <.001 | 2172 (6.5) | 1823 (5.4) | <.001 |
| VA-ECMO | 792 (0.1) | 59 (0.2) | .010 | 33 (0.1) | 56 (0.2) | .02 |
| Impella | 1437 (0.2) | 17 (0.0) | <.001 | 51 (0.2) | 17 (0.1) | <.001 |
| IABP | 3754 (0.6) | 71 (0.2) | <.001 | 144 (0.4) | 65 (0.2) | <.001 |
| Mechanical cardiac support | 5429 (0.8) | 135 (0.4) | <.001 | 201 (0.6) | 127 (0.4) | <.001 |
| Cardiogenic shock | 20,932 (3.2) | 810 (2.4) | <.001 | 834 (2.5) | 777 (2.3) | .158 |
| Cardiac arrest | 11,736 (1.8) | 1431 (4.2) | <.001 | 604 (1.8) | 1399 (4.2) | <.001 |
| Septic shock | 29,161 (4.4) | 3858 (11.2) | <.001 | 1592 (4.7) | 3775 (11.2) | <.001 |
| Need for pressors | 14,789 (2.2) | 1502 (4.4) | <.001 | 830 (2.5) | 1459 (4.3) | <.001 |
| Need for blood transfusion | 44,570 (6.7) | 2256 (6.5) | .150 | 2612 (7.8) | 2203 (6.5) | <.001 |
| Acute pulmonary embolism | 11,858 (1.8) | 934 (2.7) | <.001 | 528 (1.6) | 911 (2.7) | <.001 |
| Bacterial pneumonia | 21,424 (3.2) | 3412 (9.9) | <.001 | 1102 (3.3) | 3332 (9.9) | <.001 |
| Need for a ventilator | 54,990 (8.3) | 4021 (11.7) | <.001 | 2773 (8.2) | 3910 (11.6) | <.001 |
| AKI | 250,433 (37.9) | 15,654 (45.4) | <.001 | 12,930 (38.4) | 15,287 (45.4) | <.001 |
| Resource utilization | ||||||
| Length of hospital stay (Median, [IQR]) | 5.00 [3.00, 8.00] | 7.00 [4.00, 13.00] | <.001 | 5.00 [3.00, 8.00] | 7.00 [4.00, 13.00] | <.001 |
| Median cost of hospitalization (Median, [IQR]) | 45,663.00 [25,241.00, 88,825.00] | 60,209.00 [31,814.00, 123,566.50] | <.001 | 42,143.50 [22,960.00, 83,562.50] | 60,251.00 [31,824.75, 123,762.50] | <.001 |
Abbreviations: AKI, acute kidney injury; IABP, intra-aortic balloon pump; IQR, interquartile range; VA-ECMO, veno-atrial extracorporeal membrane oxygenation. As per HCUP regulations, observations with cell count <11 are reported as “<11”. Bold texts indicate statistical significance.
Figure 2.
Adjusted and unadjusted analysis for the outcomes including in-hospital mortality, cardiogenic shock, septic shock, MCS, need for pressors, acute PE, pneumonia, need for ventilator, and AKI in patients with AHF and COVID-19 infection.
Resources Utilization
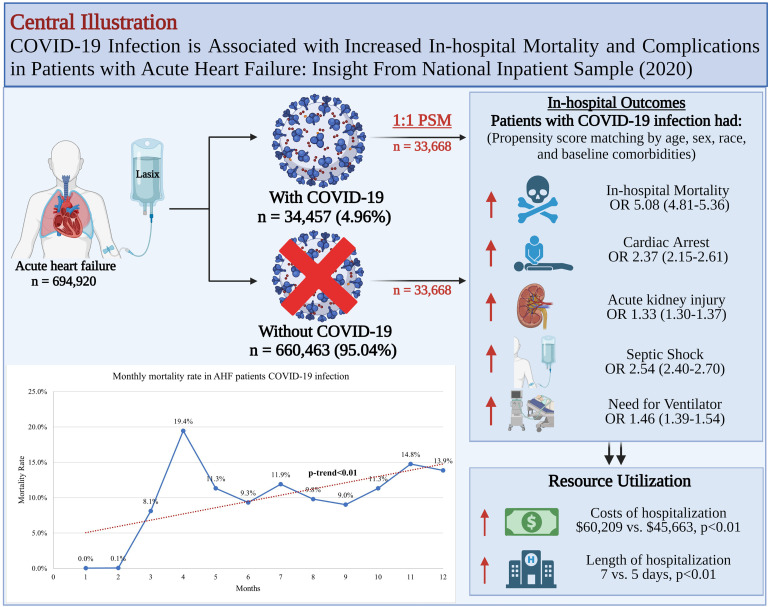
In terms of resource utilization, the length of hospitalization (LOS; median: 7 vs 5 days, P < .001) and the median costs of hospitalization ($60,209 vs $45,663, P < .001) for patients with AHF and COVID-19 infection were significantly higher, Table 2, Figure S4. Central illustration for our study is shown in Figure 3.
Figure 3.
Central illustration.
Mortality Predictors
In-hospital mortality varied across different clinical baseline characteristics, Table 3. On univariate analyses, in-hospital mortality was associated with variables such as COVID-19, age, gender, race, DM, HTN, CAD, prior MI, PCI, CABG, valve surgery, PPM, ICD, atrial fibrillation, chronic and end-stage heart failure, COPD, ESRD, liver disease, coagulopathy, and obesity.
Table 3.
Predictors of In-Hospital Mortality in Patients Diagnosed with AHF Exacerbation During Their Hospitalization.
| Variable n (%) | Univariate Analysis | Multivariate Analysis |
|---|---|---|
| COVID-19 diagnosis | 5.42 (5.27-5.57) | 5.67 (5.50-5.84) |
| Age (per year) | 1.0195 (1.0187-1.0204) | 1.026 (1.025-1.027) |
| Female | 0.90 (0.88-0.92) | 0.90 (0.88-0.92) |
| Race | ||
| White (reference) | 1 | 1 |
| Black | 0.82 (0.80-0.84) | 0.85 (0.83-0.88) |
| Hispanic | 1.07 (1.03-1.11) | 0.93 (0.90-0.97) |
| Asian or Pacific Islander | 1.20 (1.13-1.29) | 1.01 (0.94-1.08) |
| Native American | 1.20 (1.06-1.36) | 1.15 (1.01-1.31) |
| Other | 1.13 (1.06-1.21) | 1.03 (0.97-1.11) |
| Type 2 diabetes mellitus | 0.88 (0.86-0.90) | 0.97 (0.94-1.00) |
| HTN | 0.78 (0.70-0.86) | 0.81 (0.73-0.90) |
| CAD | 0.81 (0.79-0.82) | 0.85 (0.83-0.87) |
| Prior MI | 0.69 (0.67-0.71) | 0.83 (0.80-0.86) |
| Prior PCI | 0.59 (0.57-0.61) | 0.72 (0.69-0.75) |
| Prior CABG | 0.81 (0.79-0.84) | 0.92 (0.89-0.96) |
| Prior Valve surgery | 0.75 (0.71-0.79) | 0.77 (0.72-0.82) |
| Prior PPM | 0.74 (0.71-0.78) | 0.67 (0.64-0.70) |
| Prior ICD | 0.67 (0.63-0.70) | 0.69 (0.66-0.73) |
| Atrial fibrillation | 1.11 (1.09-1.14) | 1.04 (1.02-1.07) |
| Chronic HF | 0.73 (0.72-0.75) | 0.63 (0.62-0.65) |
| End-stage HF | 2.12 (1.95-2.30) | 2.04 (1.87-2.23) |
| PVD | 1.01 (0.96-1.07) | Not includeda |
| CVA | 0.96 (0.93-1.00) | Not includeda |
| COPD | 0.93 (0.91-0.95) | 1.03 (1.01-1.05) |
| Pulmonary circulation disorder | 1.02 (1.00-1.05) | Not includeda |
| CKD | 1.00 (0.97-1.01) | Not includeda |
| ESRD | 1.40 (1.36-1.44) | 1.57 (1.52-1.62) |
| Liver disease | 2.45 (2.38-2.52) | 2.41 (2.43-2.48) |
| Coagulopathy | 2.76 (2.69-2.83) | 2.19 (2.13-2.25) |
| Obesity | 0.61 (0.59-0.62) | 0.72 (0.70-0.74) |
Abbreviations: CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; DM, diabetes mellitus; ESRD, end-stage renal disease; HF, heart failure; ICD, implanted cardioverter-defibrillator; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; PPM, pacemaker placement; PVD, peripheral vascular disease. aThe statistically insignificant variables on univariate analysis were not included in the multivariate logistic regression model. Bold texts indicate statistical significance.
On multivariate logistic regression model, a diagnosis of COVID-19 (OR 5.67 [5.50 to 5.84]), liver disease (OR 2.41 [2.43 to 2.48]), coagulopathy (OR 2.19 [2.13 to 2.25]) and End-stage HF (OR 2.04 [1.87 to 2.23]) were independent predictors of in-hospital mortality. Interestingly, being a female (OR 0.90 [0.88 to 0.92]), history of hypertension (OR 0.81 [0.73 to 0.90]), CAD (OR 0.85 [0.83 to 0.87]), chronic heart failure (OR 0.63 [0.62 to 0.65]), and obesity (OR 0.72 [0.70 to 0.74]) were associated with lower odds of in-hospital mortality.
Discussion
To our knowledge, this is the largest study to analyze the outcomes of AHF admissions in the setting of COVID-19 infection, using a large national database. Our study shows that AHF in the setting of COVID-19 infection is associated with higher in-hospital mortality, as well as higher rates of AKI, need for pressors, and need for mechanical ventilation. Predictors of mortality in AHF include a diagnosis of COVID-19, liver disease, coagulopathy, end-stage HF, and COPD. We report significant variation in AHF-related mortality rate through the year 2020 with a peak in April 2020 followed by a significant decline throughout the rest of 2020.
Acute circulatory failure has been described in COVID-19 infection, usually septic in etiology. However, those with preexisting cardiomyopathy may be at risk of AHF with or without cardiogenic or mixed etiology shock state. Acute onset of cardiomyopathy leading to denovo AHF can also occur due to etiologies such as atrial tachyarrhythmias, stress cardiomyopathy, and probably myocarditis. A history of heart failure has been reported as a comorbidity in 3.3% to 21% of patients with COVID-19. 8 It is noteworthy that only a minority of those suffer AHF during COVID-19 infection. For example, chronic heart failure was only noted in 22% of patients with AHF during COVID-19. 3 However, few patients develop AHF in the absence of a prior diagnosis of heart failure. A cohort study of 3080 patients admitted with COVID-19 showed the incidence of AHF was 11.2% in patients with prior history of CHF, and 2.1% in patients without prior history of CHF. 3 Another study of 191 patients with COVID-19 showed the incidence of heart failure overall was 23% and was even higher in nonsurvivors (52% vs 12%). 9 Early studies from Wuhan, China, also showed this, in a study of 131 deceased patients who died from COVID-19 reported heart failure as a complication in 49%. 10 In contrast to prior studies, we report a high prevalence of chronic heart failure (77%) in those with AHF and COVID-19.
COVID-19 infection can result in increased mortality and higher complications in those with heart failure through multiple mechanisms. 11 One proposed mechanism is through the binding of SARS-CoV-2 virus to ACE-2 receptors, which are highly expressed in pericytes of adult human hearts. 4 The infection can then result in direct myocyte infiltration and inflammation, causing impaired cardiac function.4,11 ACE-2 receptors are usually upregulated in patients with heart failure making them more susceptible to SAR-CoV-2 virus-induced myocardial inflammation and necrosis. 2 SARS-CoV-2 virus can also cause damage to the endocardium by causing direct endothelial injury and micro-thrombosis.11–13 Additionally, COVID-19 infection causes excessive release of proinflammatory cytokines such as monocyte chemoattractant protein-1, interleukin-1β; interleukin-6; tumor necrosis factor-α. These proinflammatory cytokines can result in necrosis and death of myocardial cells.2,11,14 All of these, along with other yet unknown mechanisms, result in an increased risk of AHF in patients with COVID-19 infection. COVID-19 infection and AHF had a five-fold increased risk of in-hospital mortality compared to those without COVID-19. However, the mortality rate of 22% in our study is lower than previously reported in the cohort of AHF with COVID-19. Rey et al reported a high mortality rate of 46.8% in those who developed AHF during COVID-19 infection. 3 This is likely due to the difference in the study period between the two studies. The study was Rey et al was performed in the months of March and April of 2020, during the early phase of the pandemic. The mortality we report is for the entire calendar year of 2020, during which there was significant variation in mortality rates. We report a high mortality rate in similar months in the US as well. However, the mortality rate declined significantly in the US after the initial peak in April 2020. Berg et al reported a mortality rate of 43.8% in the AHF group with COVID-19 in a multicenter study from the US. 15 Meanwhile, the study only included patients admitted to the ICU, which is associated with increased mortality rate in COVID-19 infection. 16
Our study showed that patients with AHF and concomitant COVID-19 infection have an increased risk of developing AKI compared to heart failure patients without COVID-19 infection (aOR 1.33 [1.30 to 1.37]). Conversely, one could also hypothesize that AKI could also have resulted in AHF as a result of cardiorenal syndrome. A direct toxic effect of SARS-CoV2 virus resulting in acute tubular injury has been proposed. 17 The pathophysiological mechanism of acute tubular injury is similar to the mechanism of myocardial injury, which involves local inflammation and immune cell infiltration, cytokine storm, endothelial injury, podocyte injury, and microthrombi formation.18,19 Other nonspecific factors can contribute to development of AKI such as acute circulatory failure due to cardiogenic/mixed etiology shock,, hypoxia, and use of drugs with potential nephrotoxicity. 17
Although SARS-COV-2 virus has a wide range of impact on different body systems, it remains a predominantly respiratory illness. 20 Our study showed that patients with heart failure and concomitant COVID-19 infection have a higher risk of developing PE (aOR 1.75 [1.57 to 1.94]), pneumonia (aOR 3.25 [3.03 to 3.48]), and need for mechanical ventilation (aOR 1.46 [1.39 to 1.54]) compared to heart failure patients without COVID-19 infection. It has been reported that patients with HF were at over three times higher risk of mechanical ventilation. 21
We report a high crude mortality rate in AHF with COVID-19 in March and April of 2020. This is likely due to the COVID-19 surge that overwhelmed healthcare across the US. Further, the period of lockdown and COVID-19 transmission risk resulted in a barrier to timely access to care in patients with preexisting heart disease. To mitigate this, telemedicine was rapidly adopted by multiple institutions during the pandemic. The use of telemedicine in the heart failure population was associated with no increase in adverse outcomes. 22 We hypothesize that the decline in mortality during the latter months of 2020 could be due to improved systems of care from lessons learned from the early phase of the pandemic.
A Danish study reported improved outcomes in terms of all-cause and cardiovascular mortality in heart failure patients with influenza vaccination. 23 A similar benefit with use of vaccination against COVID-19 remains to be studied in heart failure population.
Strengths and Limitations
Our study provides a contemporary view of patients who were hospitalized with AHF in the US during the COVID-19 pandemic. We report hospitalization trends across the year, clinical outcomes, procedural interventions, and utilization of hospital resources from costs and lengths, which may aid in establishing or changing the current health care policies and interventions. However, we also recognize several limitations in our study including, but not limited to, the inherent shortcomings of the NIS database as it is based on administrative claims for billing purposes using the ICD codes that is subject to coding errors, although specificity likely remains high. 24 Also, NIS does not have information on laboratory, procedural or echocardiographic data. Imaging data such as indices of left ventricular systolic and diastolic function and cardiac biomarkers are not available.
Conclusions
COVID-19 infection in patients with AHF is associated with significantly higher in-hospital mortality, need for mechanical ventilation, septic shock, and AKI along with higher resource utilization. Predictors for mortality in AHF patients included COVID-19 infection, patients with end-stage HF, and atrial fibrillation. Further studies are needed to investigate the impact of vaccination and COVID-19 variants on outcomes.
Supplemental Material
Supplemental material, sj-docx-1-jic-10.1177_08850666231182380 for COVID-19 Infection Is Associated With Increased In-Hospital Mortality and Complications in Patients With Acute Heart Failure: Insight From National Inpatient Sample (2020) by Anas Hashem, Amani Khalouf, Mohamed Salah Mohamed, Tarek Nayfeh, Ahmed Elkhapery, Mohammad Elbahnasawy, Devesh Rai, Himanshu Deshwal, Scott Feitell and Sudarshan Balla in Journal of Intensive Care Medicine
Acknowledgments
Central illustration was created with BioRender.com.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Anas Hashem https://orcid.org/0000-0002-2008-7110
Tarek Nayfeh https://orcid.org/0000-0001-9052-5537
Himanshu Deshwal https://orcid.org/0000-0003-2086-1281
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Lai C-C, Shih T-P, Ko W-C, Tang H-J, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bader F, Manla Y, Atallah B, Starling RC. Heart failure and COVID-19. Heart Fail Rev. 2021;26(1):1–10. doi: 10.1007/s10741-020-10008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rey JR, Caro-Codón J, Rosillo SO, et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur J Heart Fail. 2020;22(12):2205-2215. doi: 10.1002/ejhf.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. May 1 2020;116(6):1097-1100. doi: 10.1093/cvr/cvaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, et al. COVID-19 and cardiac injury: clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti-Infect Ther. 2021;19(3):345-357. doi: 10.1080/14787210.2020.1822737 [DOI] [PubMed] [Google Scholar]
- 6.Panhwar MS, Kalra A, Gupta Tet al. et al. Effect of influenza on outcomes in patients with heart failure. ACC Heart Fail. 2019;7(2):112-117. doi: 10.1016/j.jchf.2018.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Dong Y, Peng C-YJ. Principled missing data methods for researchers. SpringerPlus. 2013;2(1):222. doi: 10.1186/2193-1801-2-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Italia L, Tomasoni D, Bisegna S, et al. COVID-19 and heart failure: from epidemiology during the pandemic to myocardial injury, myocarditis, and heart failure sequelae. Front Cardiovasc Med. 2021;8:713560. doi: 10.3389/fcvm.2021.713560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Br Med J. 2020;m1091:1-12. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adeghate EA, Eid N, Singh J. Mechanisms of COVID-19-induced heart failure: a short review. Heart Fail Rev. 2021;26(2):363-369. doi: 10.1007/s10741-020-10037-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60(16):1455-1469. doi: 10.1016/j.jacc.2011.11.082 [DOI] [PubMed] [Google Scholar]
- 13.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038-3044. doi: 10.1093/eurheartj/ehaa623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tufan A, Güler AA, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(9):620-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg DD, Alviar CL, Bhatt AS, et al. Epidemiology of acute heart failure in critically ill patients with COVID-19: an analysis from the critical care cardiology trials network. J Card Fail. 2022;28(4):675-681. doi: 10.1016/j.cardfail.2021.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Network Open. 2020;3(12):e2029058. doi: 10.1001/jamanetworkopen.2020.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legrand M, Bell S, Forni L, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nature Reviews Nephrology. 2021;17(11):751-764. doi: 10.1038/s41581-021-00452-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferlicot S, Jamme M, Gaillard F, et al. The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria. Nephrol Dial Transplant. 2021. doi: 10.1093/ndt/gfab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golmai P, Larsen CP, DeVita MV, et al. Histopathologic and ultrastructural findings in postmortem kidney biopsy material in 12 patients with AKI and COVID-19. J Am Soc Nephrol. 2020;31(9):1944-1947. doi: 10.1681/asn.2020050683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai AD, Lavelle M, Boursiquot BC, Wan EY. Long-term complications of COVID-19. Am J Physiol Cell Physiol. 2022;322(1):C1-C11. doi: 10.1152/ajpcell.00375.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez-Garcia J, Lee S, Gupta A, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76(20):2334-2348. doi: 10.1016/j.jacc.2020.09.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sammour Y, Spertus John A, Austin Bethany A, et al. Outpatient management of heart failure during the COVID-19 pandemic after adoption of a telehealth model. JACC: Heart Failure. 2021;9(12):916-924. doi: 10.1016/j.jchf.2021.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modin D, Jørgensen ME, Gislason G, et al. Influenza vaccine in heart failure. Circulation. 2019;139(5):575-586. doi: 10.1161/circulationaha.118.036788 [DOI] [PubMed] [Google Scholar]
- 24.Cooper LB, Psotka MA, Sinha S, et al. Specificity of administrative coding for older adults with acute heart failure hospitalizations. Am Heart J. 2020;223:1-2. doi: 10.1016/j.ahj.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jic-10.1177_08850666231182380 for COVID-19 Infection Is Associated With Increased In-Hospital Mortality and Complications in Patients With Acute Heart Failure: Insight From National Inpatient Sample (2020) by Anas Hashem, Amani Khalouf, Mohamed Salah Mohamed, Tarek Nayfeh, Ahmed Elkhapery, Mohammad Elbahnasawy, Devesh Rai, Himanshu Deshwal, Scott Feitell and Sudarshan Balla in Journal of Intensive Care Medicine