Abstract
Ultrasound-guided ablation procedures have been growing in popularity and offer many advantages compared with traditional surgery for thyroid nodules. Many technologies are available, with thermal ablative techniques being the most popular currently though other nonthermal techniques, such as cryoablation and electroporation, are gaining interest. The objective of the present review is to provide an overview of each of the currently available ablative therapies and their applications in various clinical indications.
Keywords: thyroid nodules, thermal ablation, radiofrequency ablation, microwave ablation, laser ablation, ethanol ablation
The use of ultrasound-guided ablation procedures to safely treat both benign and malignant diseases of the thyroid was introduced over 3 decades ago and has been growing in popularity in the past decade. Many technologies have been developed, harnessing the power of extreme temperatures, chemicals, and electricity. These procedures eliminate the need for general anesthesia, surgical incision, and removal of the thyroid gland and can be attractive nonoperative alternatives for patients.
Thermal ablation is the most widely used, and is based upon the principle of inducing irreversible tissue damage by generating an increase in local tissue temperature [1]. Thermal ablation techniques are differentiated based on the method or energy used to achieve the desired temperature within the target tissue. Radiofrequency ablation (RFA) and laser ablation (LA) are currently the most widely used treatments for solid nodules, though microwave ablation (MWA) and high-intensity focused ultrasound (HIFU) have gained traction in recent years.
Nonthermal ablative therapies consist of chemical ablation, cryoablation, and irreversible electroporation. The primary chemical ablation modality is ethanol ablation and is considered the treatment of choice for simple cystic nodules [2]. The mechanism of cell death is via cellular dehydration, leading to protein denaturation and induction of coagulation necrosis, and damage to the vascular endothelium and platelet aggregation, leading to vascular thrombosis and tissue ischemia [3]. Cryoablation and irreversible electroporation have been used in clinical applications for tumors throughout the body, but are less well studied in the thyroid, though their use for thyroid nodules is being explored. Cryoablation uses circulating cooled fluids such as nitrogen or argon, which rapidly expand into gas, creating freezing temperatures in tissues, causing physical damage to cellular membranes as well as disrupting metabolic functions and resulting in cell necrosis [4]. Finally, irreversible electroporation uses high electric field pulses to disrupt and permeate cell membranes causing permanent cell damage [5].
Indications for Ablation
Thyroid nodules are very common, with a prevalence of up to 50% to 60% in the general population [6]. Most nodules are benign and asymptomatic, though some may cause compressive symptoms, cosmetic concerns, or subclinical or overt hyperthyroidism. Malignant nodules harbor various forms of thyroid cancer.
For benign thyroid nodules, clinical manifestations are due to nodule growth and symptoms are not necessarily correlated with just the size of the nodule. The severity of symptoms often depends on other factors such as neck anatomy and location of the nodule within the thyroid gland. However, the presence of pressure or cosmetic symptoms are considered appropriate indications for intervention. Autonomously functioning thyroid nodules can also cause subclinical or overt hyperthyroidism, and may be targeted with ablative treatments. However, the success of achieving and maintaining euthyroidism is less predictable than surgery or radioactive iodine (RAI) ablation, especially for larger volume nodules [7, 8].
Thyroid nodules may also harbor malignancies. Prior to any therapeutic ablative intervention, fine needle aspiration biopsy is indicated to rule out the possibility of malignancy, either with 2 benign cytologic results or 1 cytologic result with concordant benign ultrasonographic features. However, when the cytologic result is indeterminate, diagnostic lobectomy is indicated to achieve definitive diagnosis and to prevent progression of disease [9]. Because ablation does not accomplish either 1 of these objectives, and there are few data on the short- and long-term safety of ablative procedure for these nodules, it is not currently recommended as first-line treatment for indeterminate nodules [2]. Furthermore, postablative changes within the nodule may confound future attempts at diagnosis. When the diagnosis of malignancy is confirmed, surgery is considered the gold standard of treatment for both primary and recurrent well-differentiated thyroid cancer. However, there are increasing data to suggest that ablative therapies may be safe alternative for the primary treatment of small indolent papillary thyroid microcarcinoma (PTMC) and recurrent cancers, especially when patient comorbidities or anatomy preclude surgical management.
Procedural Considerations
Preprocedure
For all ablative techniques, preprocedural ultrasound is vital in assessing the target lesion [2]. Important characteristics to evaluate include nodule volume and dimensions, proportion of solid and cystic components, vascularity, and anatomic landmarks for technical safety. It is also important to perform a voice and laryngeal assessment prior to intervention. Laryngoscopy is recommended in patients with hoarseness, prior neck surgery, or those with nodules close to critical structures [10].
Intraprocedure
The procedural setup is similar for all the ablative techniques, usually in an outpatient setting under local analgesia (with the exception of HIFU, which is performed under general anesthesia). The use of local anesthesia is also advantageous over general anesthesia in that it allows the patient to communicate the onset of any pain or discomfort, which may represent thermal or chemical propagation beyond the thyroid gland, and facilitates the monitoring of any potential complications such as voice changes or ptosis. A mild sedative may be used to help relax the patient during the procedure to minimize head movements and frequent swallowing. The patient is positioned supine in mild cervical extension. A local anesthetic solution is infiltrated in the skin puncture site and around the thyroid capsule to anesthetize the soft tissue and sensory nerves in the capsule. Additional local analgesia or 5% dextrose in water (D5W) solution may be used for hydrodissection to separate the thyroid from any adjacent vulnerable structures and to provide a heat sink to protect these structures. The appropriate probe is then inserted or applied, and energy is delivered while the treatment effect is monitored in real-time under ultrasound guidance. Each ablative technique is described separately in further detail.
Postprocedure
Clinical and ultrasound evaluation is performed immediately following the procedure to define the ablated area and to detect potential complications [2]. Cold compresses and anti-inflammatory analgesics are often used for any postprocedural pain and discomfort. Patients with difficulty swallowing, breathing, or speaking should be observed until symptom resolution or further evaluated with additional imaging. Finally, early- (eg, 3 months), intermediate- (eg, 6 and 12 months), and long-term (eg, every 1-2 years) clinical, biochemical, and ultrasound follow-up is recommended [2, 10]. Symptom scores may be used to document symptom resolution, laboratory analyses may be performed for functional nodules (eg, thyroid function tests) and malignancy (eg, thyroglobulin), and ultrasonography may be used to evaluate nodule volume reduction, potential regrowth, and, in rare cases, diagnose missed cancers or progression of malignant disease [2, 10].
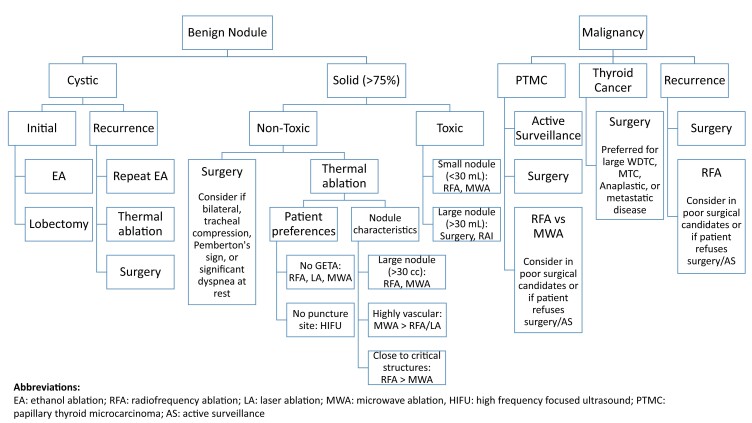
The objective of the present review is to provide an overview of each of the currently available ablative therapies and their applications and outcomes in various clinical indications. Table 1 summarizes all ablative techniques as well as potential indications and any specific procedural considerations. Table 2 summarizes the published clinical outcomes associated with each of the thermal ablative techniques. Finally, Fig. 1 outlines some basic considerations for technique selection and procedural planning though ultimate clinical decision making in technique will be highly dependent on individual nodule characteristics, interventionalist skill and preferences, and local environment and resources available.
Table 1.
Summary of ablation indication, technique, and procedural considerations
| Indications | Technique | Procedural considerations | |
|---|---|---|---|
| Thermal ablation | |||
| Radiofrequency ablation Uses high frequency alternating electric current to generate heat; temperature can reach up to 100 °C |
Symptomatic benign nonfunctioning nodules Recurrent thyroid cancer when risk of surgery is prohibitively elevated May be considered for small autonomously functioning nodules and PTMC Minimal data exist for indeterminate nodules |
Transisthmic approach Moving shot technique, treating ablative units from deepest to most superficial portions |
Efficacy limited by tissue carbonization and heat sink effect from cystic component or adjacent blood flow |
| Laser ablation Delivers focused light energy through optical fibers, which is converted into heat through photon scatter; temperature can reach up to 100 °C |
Symptomatic benign nonfunctioning nodules May be considered for small autonomously functioning nodules and PTMC Minimal data exist for indeterminate nodules and recurrent thyroid cancer |
Transisthmic approach Flexible optical fibers are inserted into the target lesion and then withdrawn incrementally while administering energy pulses |
Fixed application of energy |
| Microwave ablation Uses electromagnetic field to cause oscillation of polarized particles to generate heat through particle collision |
Symptomatic benign nonfunctioning nodules May be considered for PTMC Minimal data exist for autonomously functioning nodules, indeterminate nodules and recurrent thyroid cancer |
Transisthmic approach Moving shot technique, treating ablative units with 5-10 seconds of microwave pulses |
Results in high tissue temperature not impeded by char or heat sink Potential greater risk of injury near critical structures |
| High-intensity focused ultrasound Focuses high-intensity sound waves from numerous sources onto the same target, causing tissue vibration and frictional heat; generates temperatures up to 85 °C |
Symptomatic benign nonfunctioning nodules Minimal data exist for autonomously functioning nodules, indeterminate nodules, PTMC, and recurrent thyroid cancer |
A probe is positioned on the skin and the treatment area is mapped by the device HIFU pulses are delivered followed by cooling time |
Completely noninvasive technique without needle puncture Efficacy limited by motion |
| Chemical ablation | |||
| Ethanol ablation Ethanol causes direct tissue death by cellular dehydration and coagulation necrosis and tissue necrosis by vascular thrombosis |
Cystic nodules | Cyst contents are aspirated and then irrigated with saline followed by ethanol solution | Less predictable area of tissue destruction in solid nodules |
| Other ablation techniques | |||
| Cryoablation Uses circulating cooled fluids which rapidly expand into gas; creates temperatures as low as –190 °C |
Minimal data exist for cryoablation in thyroid nodules | A cryoprobe is placed within the target lesion and freezing occurs until the “iceball” covers the entire lesion under ultrasound guidance | More favorable profile near nerves, vessels, and airways |
| Irreversible electroporation Uses microsecond-long high electric field pulses to cause permeabilization of cell membrane |
No data exist for irreversible electroporation in thyroid nodules | Only affects the cell membrane and spares the extracellular matrix, allowing for its use near sensitive structures | |
Abbreviation: PTMC, papillary thyroid microcarcinoma.
Table 2.
Summary of clinical outcomes for thermal ablation techniques
| Ablation technique and indications | Volume reduction rate | Other outcomes | Complications | Considerations | References |
|---|---|---|---|---|---|
| Radiofrequency ablation | |||||
| Benign nonfunctioning thyroid nodules | 64.5-76.1% at 6 months 80.3% at 3 years |
Better health-related quality of life and patient satisfaction compared with surgery | 4.6% overall complications 1.3% major complications |
Most effective for nodule volumes <10 mL Most important parameter is total energy delivered |
[11-14, 15, 16] |
| Autonomously functioning nodules | 69.4-76.4% at 12 months | 50-90.9% thyroid function normalization at 12 months Higher satisfaction with cosmetic outcome, but lower satisfaction with symptom resolution compared with surgery |
1.0% complication rate, lower than surgery (6.0%, P = .002) No incidence of hypothyroidism, lower than surgery (71.5%) |
Thyroid function normalization generally achieved with volume reduction by 80% Thyroid function normalization higher for smaller nodules |
[7, 12, 17, 18-20] |
| Papillary thyroid microcarcinoma | 99.3-99.9% at 12 months Complete resolution in 65.2-95.8% of ablated tumors |
0.01% recurrence rate No difference in tumor progression, lymph node metastasis at 4 years and 5 years compared with surgery |
0-2.6% overall complications 0% major complications Lower complications compared with surgery (none reported in RFA group) |
[21-26] | |
| Recurrent thyroid cancer | 99.5% at 6+ years Complete disappearance in 68-93% tumors |
No difference in recurrence-free survival rates compared with surgery | Lower overall complications (10.2% vs 41.6%) and major complications (3.1% vs 31.2%) compared with surgery | [2, 27-28] | |
| Laser ablation | |||||
| Benign nonfunctioning thyroid nodules | 48.3-49.9% at 6 months 45.9-57% at 3 years |
Pressure symptom improvement of 48% Cosmetic symptom improvement of 86% Improved ThyPRO scores at 6 months |
2.4% overall complication rate 1.8% major complications |
More effective in spongiform and mixed nodules compared with solid nodules | [11, 12, 29-32] |
| Autonomously functional nodules | 44% at 6 months (vs 47% in RAI group, P < .001) | 50% thyroid function normalization at 6 months (vs 100% in RAI group, P = .0025) | No reported complications in LA group, compared with 2 patients who developed hypothyroidism in RAI group | Small nodule size and large volume reduction are predictive of thyroid function normalization | [7, 18, 33-34] |
| Papillary thyroid microcarcinoma | 88.6% at 12 months Complete resolution in 48.7-100% of ablated tumors |
0.6-0.92% overall complication rate 0% major complication rate |
N/A | [23-24, 35] | |
| Recurrent thyroid cancer | N/A | N/A | N/A | N/A | |
| Microwave ablation | |||||
| Benign nonfunctioning thyroid nodules | 88.6-92.4% at 12 months | Better general health and mental health scores at 6 months and 12 months compared with surgery Better cosmetic scores compared with surgery |
52.4% overall complication rate 4.8% major complication rate |
N/A | |
| Autonomously functional nodules | N/A | N/A | N/A | Can consider combined approach of MWA with RAI to reduce RAI dose and rapidly control hyperthyroidism | [36] |
| Papillary thyroid microcarcinoma | 95.3% at 12 months Complete resolution in 56.5% of ablated tumors |
0.85% recurrence rate | 5.1-6.0% overall complication rate 2.5% major complication rate |
N/A | |
| Recurrent thyroid cancer | N/A | N/A | N/A | N/A | |
| High-intensity focused ultrasound | |||||
| Benign nonfunctioning thyroid nodules | 48.8-55% at 3 months 48.7-51.7% at 6 months 68.9% at 12 months |
Improved pressure symptoms at 12 months | No major complications Lower incidence of hypothyroidism compared with surgery |
Nodule volume predictive of ablation success (>50% volume reduction) | [37, 38-39] |
| Autonomously functional nodules | Similar nodule volume reduction compared with RAI | Lower thyroid function normalization (27%) compared with RAI (82%, P = .0008) | N/A | N/A | [40] |
| Papillary thyroid microcarcinoma | N/A | N/A | N/A | N/A | |
| Recurrent thyroid cancer | N/A | N/A | N/A | N/A | |
Figure 1.
Summary and considerations for management of thyroid nodules.
Radiofrequency Ablation
RFA is a thermal ablative technique that uses high-frequency alternating electric current to generate heat. As radiofrequency waves agitate tissue ions, their motion under the influence of alternating current produces friction and heat. The temperature can reach up to 100 °C, dehydrating cells and denaturing proteins leading to coagulation necrosis [41]. Heat is generated in the tissue within a few millimeters of the electrode tip and heat conduction from the ablated area leads to additional thermal damage to tissue further from the electrode. As such, RFA efficacy may be limited by tissue carbonization and heat sink effect from adjacent blood flow or cystic components in the target lesion [42].
Technique
The RFA system consists of a radiofrequency generator and an internally cooled electrode needle. The RFA probe is introduced in the midline through the isthmus from a medial to lateral direction to the target lesion. The transisthmic approach minimizes risk to vulnerable structures adjacent to the thyroid and prevents leakage of hot ablated fluid to the perithyroidal areas [2]. A moving shot technique is utilized where the operator moves the probe back and forth in the target lesion under ultrasound guidance [43]. The nodule is divided into multiple small conceptual ablation units which are treated from the deepest and most caudal portion to the most superficial and cranial portion of the nodule. Heat is generated in the tissue within a few millimeters of the electrode tip and causes tissue necrosis, which may be visualized as hyperechoic changes under ultrasound. Care is taken to avoid the posterior and lateral capsule to prevent injury to nearby structures, especially the recurrent laryngeal nerve, which lies in the “danger triangle” along the trachea in the posteromedial aspect of the thyroid. To combat incomplete ablation due to heat sinks from surrounding vasculature, vascular ablation has been introduced [2]. The artery-first technique identifies the nodule's main arterial supply by Doppler ultrasound and ablates it prior to the nodule to reduce edema and heat-sink effect. Marginal vein ablation technique targets the draining veins, which may also contribute to the heat sink effect. A mean power of 35 to 60 W is delivered and the procedure usually requires 15 to 40 minutes [10].
Outcomes
Benign nonfunctioning thyroid nodules
Many studies have demonstrated the efficacy of RFA for reducing the volume of benign nodules. A meta-analysis by Ha et al showed a pooled volume reduction of 76.1% at 6 months [11]. Long-term studies demonstrated rapid volume reduction at 12 months, a plateau from 12 to 36 months, and further volume reduction after 36 months [12]. A meta-analysis of long-term outcomes reported a pooled volume reduction of 80.3% at 3 years. The overall complication rate was low at 4.6% with 1.3% major complications, including voice change, laryngeal nerve injury, and brachial nerve injury [12]. Generally, RFA is most effective for smaller nodules (volumes less than 10 mL) with larger nodules requiring more than 1 treatment [13]. The most important technical parameter associated with volume reduction is the total energy delivered [14].
Compared with surgery, RFA is associated with fewer complications, better health-related quality of life, and preservation of thyroid function [17, 44, 45]. However, it can take longer to achieve the desired volume reduction. A meta-analysis comparing thermal ablation with surgery found no difference in symptom improvement but significantly lower incidence of pain, hoarseness, and hypothyroidism, better cosmetic outcomes, and shorter hospitalization with thermal ablation [46]. In a telephone survey of 126 patients treated with RFA and 84 patient treated with surgery, there was no difference in the overall satisfaction, but more patients were fully satisfied with the resolution of nodule-related symptoms in the surgery group (96% vs 81%) and more patients were fully satisfied with the cometic results in the RFA group (92% vs 69%) [15].
Autonomously functioning nodules
Outcomes following RFA for benign autonomously functioning thyroid nodules tend to be more variable than nonfunctioning nodules, with studies reporting rates of thyroid function normalization ranging 24% to 86% [47]. Bernardi et al demonstrated that 33% of patients achieved euthyroidism at 3 months, 43% at 6 months, and 50% at 12 months after a single RFA treatment. Patients went into remission when their nodules were reduced by 80% after 12 months [7]. A second study from the same group showed that thyroid function normalization was significantly higher in small nodules than medium-sized nodules (86% vs 45%) [18]. A meta-analysis reported thyrotropin normalization in 71.2% and volume reduction of 69.4% at 12 months [19].
Compared with surgery, RFA is associated with lower rates of hypothyroidism and complications as well as shorter hospital stay [17]. However, compared with surgery, fewer patients were fully satisfied with symptom resolution with RFA (76% vs 100%), though more patients were satisfied with the cosmetic results (97% vs 71%) [15]. Compared with RAI, RFA demonstrated greater volume reduction at 12 months (76.4% vs 68.4%) and higher rates of euthyroidism (90.9% vs 72.0%) [20].
Papillary thyroid microcarcinoma
Surgery is considered first-line treatment for PTMC. However, due to the indolent nature of these small lesions, nonsurgical options with fewer complications, and better quality of life and cosmetic outcomes are desirable. On the other hand, these options must also be weighed against active surveillance, which has also been established as a satisfactory management strategy for PTMC.
Ding et al treated 38 PTMCs in 37 patients and achieved complete ablation in all lesions with a mean volume reduction of 99.3% without any complications observed [21]. Similarly, Zhang et al demonstrated resolution of 41.7% and 95.8% of ablated PTMC at 6 months and 12 months, respectively. There was no evidence of tumor on ultrasound or core needle biopsy and there was no recurrence or lymph node metastasis [22]. A meta-analysis of 11 studies of ablation of PTMC found a mean pooled volume reduction rate of 99.3% and pooled complete disappearance of 65.2% of treated lesions. RFA also had significantly higher volume reduction than LA (88.6%) and MWA (95.3%) [23]. Another meta-analysis found a pooled complete disappearance rate of 76.2% and 0.01% recurrence rate, which was lower for RFA (0.01%) than LA (1.87%) and MWA (0.85%) though not statistically significant [24].
A long-term follow-up study comparing RFA with lobectomy found no difference in local tumor progression, lymph node metastasis, and persistent lesion at 4 years [25]. This finding was confirmed by Zhang et al at 5-year follow-up. Further, compared with RFA, surgery was associated with longer procedure time, longer hospitalization, more complications, and lower thyroid-related quality of life [26].
Recurrent thyroid cancer
Revision surgery after thyroidectomy for recurrent or residual cancer is challenging due to scarring in the thyroid bed, and there is a higher risk of injury to the recurrent laryngeal nerves and parathyroid glands. As such, many studies have evaluated the use of RFA in these settings with complete disappearance ranging from 68% to 93% [2]. Choi et al compared RFA with repeat surgery in patients with locally recurrent thyroid cancers and found no difference in recurrence-free survival rates. Immediate postprocedural complications were similar but overall (10.2% RFA vs 41.6% surgery, P < .001) and major (10.2% vs 41.6% surgery, P < .001) complications were more frequent in the surgery group [27]. Longer term outcomes are emerging and also promising. Chung et al reviewed patients who underwent RFA for recurrent PTC with at least 5 years of follow-up (mean follow-up duration was 6.7 years) and found a mean volume reduction of 99.5% with 91.3% complete disappearance rate by the final evaluation [28].
Laser Ablation
In LA, a beam of focused light energy is delivered into the target lesion through an optical fiber. The light energy is converted into heat due to photon scatter and can raise local temperature to 100 °C instantaneously, causing coagulation necrosis. The most commonly used configuration for thyroid nodules is the Nd:YAG or Diode laser with an emission wavelength of 1064 nm, though other laser sources and optical fibers are available [42]. Relative to the other thermal ablation modalities, LA delivers the lowest total energy among the thermal ablative techniques, which may confer greater safety and control in critical areas [10].
Technique
Under ultrasound guidance, 300-μm flexible optical fiber(s) are inserted into the target lesion through the sheath of a 21-gauge introducer needle, either along the longitudinal axis or via a transisthmic approach. Depending on the volume of the target nodule, 1 to 4 optical fibers may be used and placed at 10 mm distance from each other forming an ellipsoid shape matching the nodule [42]. Once the fibers are appropriately positioned, an introducer needle is then withdrawn to expose 5 mm of bare fiber. The laser energy is delivered, starting in the deepest part of the nodule. The sheath and fiber are then pulled back incrementally, and the energy is administered repeatedly to ablate the entire nodule until the superficial margin of the nodule is reached. A highly echogenic region will develop from heating and vaporization as the nodule is ablated and may be followed in real-time with ultrasonography. The number of fibers, energy deliveries, and total amount of energy delivered is tailored to the nodule volume and shape. The mean power delivered is usually 3 to 7 W per fiber and the procedure requires 15 to 30 minutes [10].
Outcomes
Benign nonfunctioning nodules
Several single-center randomized controlled trials have demonstrated the short- and long-term efficacy of LA. A meta-analysis of 5 randomized controlled trials and 2 prospective observational studies showed a pooled volume reduction of 49.9% at 6 months [11]. These results are confirmed by a multicenter trial of 200 patients randomized to a single LA treatment vs follow-up, which found 57% volume reduction at 3 years and more than 50% volume reduction in 67.3% of cases [29]. Similarly, a large multicenter study of 1534 laser-treated nodules demonstrated a mean volume reduction of 72% at 12 months and few complications (0.9%) [30]. A more recent meta-analysis with long-term follow-up reported a pooled volume reduction of 48.3%, 52.3%, 45.5%, and 45.9% at 6 months, 1 year, 2 years, and 3 years [12]. Pooled overall complication rate was 2.4%, with 1.8% major complications, which included voice change, laryngeal nerve injury, and pseudocyst formation [12]. Factors influencing volume reduction have not been systemically studied, but LA is more effective in spongiform and mixed nodules than completely solid nodules [31].
In addition to volume reduction, LA is effective in improving symptom and cosmetic concerns. The multicenter randomized controlled trial by Papini et al showed a reduction of pressure complaints from 38% at baseline to 8% at final evaluation [29]. Similarly, Pacella et al found a pressure and cosmetic symptom relief of 48% and 86%, respectively [30]. One study using the validated 13-scale ThyPRO questionnaire found improvement at 6 months [32].
Autonomously functioning nodules
A randomized study compared single LA treatment with RAI in 30 patients with subclinical or mild hyperthyroidism from a solitary hot nodule and found no difference in mean volume reduction, but RAI achieved thyrotropin normalization in 100% of patients compared with 50% of RFA patients [33]. Similar results have been reported in other noncontrolled series as well, and thyrotropin normalization often requires multiple LA sessions [7]. Small nodule size and large reduction in volume may be predictive factors in restoring euthyroidism [7, 18, 34].
Although current international guidelines do not recommend RFA as primary treatment for autonomously functioning thyroid nodules, a pilot study raises the possibility of using LA in combination with RAI to achieve faster and greater improvement than RAI alone [48].
Papillary thyroid microcarcinoma
The use of LA for PTMC was first reported by Papini et al in 2011 in a patient at high risk for surgery and demonstrated no recurrence or metastases at 2 years [49]. Valcavi et al performed LA in 3 patients with PTMC followed by standard total thyroidectomy and demonstrated complete PTMC destruction on histology [50]. A meta-analysis found a mean pooled volume reduction rate of 88.6% and pooled complete disappearance of 48.7% of treated lesions [23]. Another meta-analysis demonstrated similar pooled complete disappearance rate of 57.3% with a recurrence rate of 1.87%. The overall pooled proportions of complications following LA was 0.92%, which was lower than RFA (1.7%) and MWA (6.0%) though not statistically significant [24]. A 10-year long-term follow-up study of LA showed 100% disappearance rate after 12 months and the only subsequently found foci of PTMC were in untreated areas [35].
Microwave Ablation
MWA uses electromagnetic field to cause oscillation of polarized particles, generating heat as the particles collide converting kinetic energy into heat [51]. With continuous high-frequency microwaves, extensive thermal energy can be generated in a short period of time, resulting in a higher final tissue temperature that is not impeded by char or heat sink [51]. Although this may be valuable in treating larger tumors in other organs, these characteristics may also represent disadvantages in the compact anatomy of the thyroid and central neck where critical structures can lie very close to the target lesion [2].
Technique
The microwave system consists of a microwave generator and an internally cooled shaft antenna. A small 1- to 2-mm incision in made in the skin to accommodate the 14- or 16-gauge antenna and the antenna is inserted into the target lesion under ultrasound guidance [2, 16]. Similar to RFA, a transisthmic moving shot technique is used. The nodule is divided into smaller conceptual ablation units and treated sequentially with 5 to 10 seconds of microwave fire per unit until the entire nodule is hyperechoic on ultrasound. Approximately 30 to 50 W are delivered over 10 to 20 minutes for an average procedure [10].
Outcomes
Benign nonfunctioning nodules
A meta-analysis of MWA in benign thyroid nodules found a pooled volume reduction of 54.3%, 73.5%, and 88.6% at 3-month, 6-month, and 12-month follow-up, respectively [52]. However, this was associated with significant complication rate of 52.4% with 4.8% major complications (transient voice changes, Horner's syndrome, thyroid dysfunction, and nodule rupture) and 48.3% minor complications (pain, burns, and bleeding). Cooled MWA has significantly lower minor and overall complications than uncooled MWA [52].
A prospective randomized trial of 52 patients demonstrated volume reductions of 72.3%, 84.5%, and 92.4% at 3-month, 6-month, and 12-month follow-up, respectively. Compared with surgery, MWA had fewer complications, less pain, shorter operative time and hospitalization, and lower total costs. Those who underwent MWA also had better general health and mental health scores at 6 months and 12 months [53]. Similar results were found in a larger propensity score matched study comparing MWA to surgery, where MWA was associated with better cosmetic scores, less blood loss and shorter operative time and hospitalization [54].
Autonomously functioning nodules
MWA in functional nodules has not been studied. However, a combined approach of MWA with RAI may be used to reduce the dose of RAI needed and rapidly control of hyperthyroidism. A pilot study of combined MWA and RAI showed that a significant reduction in size of thyroid nodule was associated restoration of euthyroidism and reduced dose of RAI [36].
Papillary thyroid microcarcinoma
A meta-analysis found a mean pooled volume reduction rate of 95.3% and pooled complete disappearance of 56.5% of treated lesions. MWA had a pooled 5.1% complication rate with 2.5% major complications [23]. Tong et al performed a meta-analysis which showed similar pooled complete disappearance proportion of 62.9% after MWA and recurrence of 0.85%. The overall pooled proportions of complications following MWA was 6.0% [24].
High-Intensity Focused Ultrasound
HIFU is a completely noninvasive technique uses sound waves to target a lesion without the need for even a needle puncture. Energy is delivered to a small area by focusing high-intensity sound waves from numerous sources onto the same target. The conversion of energy causes tissue vibration and frictional heat which generates high temperatures over 85 °C in seconds leading to tissue vaporization, microbubble expansion and collapse, and cell death [55]. Tissues beyond the focused zone experience low density of acoustic energy and remains unharmed.
Technique
The HIFU system consists of an energy generator, a cooled probe, which is also an ultrasound diagnostic receiver, and a computer/monitor. The probe is positioned on the skin to view the targeted nodule. The treatment area is mapped by the device and may also be manually adjusted by the operator. Once the target area is contoured, repeated HIFU pulses are delivered followed by cooling time. Each pulse can create temperatures up to 60 to 80 °C. The cooling device circulates 10 °C liquid through a balloon at the probe, cooling the skin during the procedure [2]. HIFU generally delivered 30 to 40 W and is the most time-consuming of the thermal ablation techniques, lasting 45 to 60 minutes and sometimes requiring multiple treatments [10].
Outcomes
Benign nonfunctioning nodules
The first clinical study treated 25 patients with HIFU for benign multinodular goiter, followed by thyroidectomy. Histological examination showed that all treatment effects were confirmed to the targeted area without affecting nearby structures and the extent of nodule destruction ranged from 2% to 80% [56]. A systematic review found volume reduction of 48.8% to 68.9% after a single treatment [37]. Similar to other thermal alation techniques, an inverse correlation between nodule volume and volume reduction has been reported [38, 57]. No major complications were reported in the studies, but minor side effects included pain, skin redness, and subcutaneous swelling [37].
HIFU ablation was associated with significantly improved pressure symptoms at 12 months compared with baseline [38]. Compared with surgery, HIFU ablation was associated with shorter length of stay, less subclinical hypothyroidism, fewer complications, greater symptom improvement, and lower cost [39].
Autonomously functioning nodules
A retrospective study comparing single session HIFU ablation and RAI for toxic thyroid nodules found similar volume reduction in both groups but significantly improved scintigraphy response (94% vs 53%, P = .024) and resolution of hyperthyroidism in the RAI group (82% vs 27%, P = .0008) [40].
Ethanol Ablation
Ethanol ablation is a chemical ablation technique that is primarily used in predominantly cystic nodules. Ethanol causes direct tissue death by cellular dehydration and coagulation necrosis. When it enters the local circulation, ethanol also causes damage to the vascular endothelium and platelet aggregation leading to vascular thrombosis and tissue ischemia [2]. Unfortunately, there is variability in ethanol diffusion from the injection site, ablation efficacy is often unpredictable and leakage out of the thyroid into cervical tissue may lead to pain and late fibrosis. As such, ethanol ablation is more successful in small, encapsulated lesions that provide a barrier to diffusion and is most effective in cystic nodules. However, by the mechanism of vascular thrombosis, ethanol ablation has been described in vascular lesions and may play an adjuvant role with thermal therapies.
Technique
Under ultrasound guidance, a needle is inserted into the center of the cystic nodule—choice of needle gauge is dependent on the viscosity of the cyst content. Like in the other modalities, a transisthmic approach allows for stabilization of the needle and prevents perithyroidal leakage of ethanol. The cyst contents are aspirated and sterile saline solution is used to irrigate the cyst to remove any residual debris and cyst contents. Once the cyst contents are adequately removed 95% to 99% ethanol is injected into the cystic space. The ethanol is allowed to sit within the cyst to allow time to react with the cells [58]. There is no consensus on whether the ethanol should be retained or aspirated and studies have not shown a difference in success or complications [2].
Outcomes
Ethanol ablation is the recommended first-line treatment for benign cystic or predominantly cystic nodules. Volume reduction following ethanol ablation ranges from 46% to 97% [59-62]. At 5 years post-treatment, greater than 75% volume reduction persisted in 86.2% of cystic nodules and local symptom improvement in 91.4% [63]. However, due to the less predictable area of tissue destruction with ethanol ablation, it is not recommended for use in solid thyroid nodules, though it can be combined with other ablative techniques in the management of mixed cystic and solid nodules [2].
Cryoablation
Cryoablation uses circulating cooled fluids such as nitrogen or argon, which rapidly expand into gas, creating temperatures as low as −190 °C. Ice crystals form at temperatures below −20 °C, causing physical damage to cellular membranes as well as disrupting metabolic functions, resulting in cell necrosis [4]. Cryoablation is more commonly used to treat tumors of the liver, prostate, kidney, lung, breast, and bone.
Technique
The cryoablation system includes a cryoprobe and a software platform. The cryoprobe is placed within the target lesion and the freezing started. Ice growth is observed in real-time with ultrasound until the iceball had covered the entire tumor with a 2-mm margin. Once the iceball coverage goal is achieved, freezing is continued for a total of 10 minutes for a single freeze cycle [64].
Outcomes
Cryoablation has a more favorable profile near nerves, vessels, and airways. However, there are few reports on the application of cryoablation in the thyroid. One retrospective study evaluated cryoablation in 10 patients with recurrent PTC in whom reoperation was contraindicated due to inseparable scarring to tracheal neural and/or vascular structures. Technical success, as defined by complete tumor coverage plus 2-mm margin, was achieved in all cases. Mean tumor volume reduction was 88% with 60% achieving complete involution during the study period (2019-2021). Complications included voice change, Horner syndrome, and intraprocedural stridor and hypertension, which all resolved at 6 months [64]. Another study compared cryoablation with ethanol ablation in patients with cervical lymph node metastasis. Cryoablation was associated with fewer recurrences than ethanol ablation (0% vs 14.9%) but this was not significant. Ethanol ablation also required significantly more treatments [65].
Irreversible Electroporation
Electroporation is the permeabilization of cell membrane with microsecond-long high electric field pulses, which can be transient (reversible) or permanent (irreversible). Reversible electroporation is commonly used for gene transfer during genetic engineering. The theoretical advantage of electroporation is that it only affect the cell membrane and spare the extracellular matrix within and around the treated area, allowing for its use near sensitive structures such as the esophagus and nerves [5].
Outcomes
Although irreversible electroporation has been used to treat hepatic, pancreatic, and prostate cancer for almost a decade, it has not be systematically studied in thyroid cancer. A proof of concept study in rats demonstrated complete follicle ablation in the treated area without affecting the nearby trachea [5]. A case report described the use of irreversible electroporation in a patient with a third recurrence of follicular thyroid carcinoma who had already underwent total thyroidectomy followed by partial tracheal resection for the first recurrence and total laryngectomy, partial pharyngectomy, and bilateral neck lymph node dissections for the second recurrence, as well as RAI and external beam radiation therapy (EBRT). A new paratracheal recurrence was found. Two monopolar needle electrodes were placed against the mass under computed tomography fluoroscopy guidance and 80 treatment pulses were delivered. Postablation computed tomography demonstrated a nonenhancing ablation zone. The patient tolerated the procedure well without any complications, and 7-month follow-up computed tomography did not show any signs of residual disease [66].
Future Directions
The increasing popularity of ablative techniques in management of thyroid nodules has led to the rapid growth of the number of studies evaluating the efficacy of these technique across a variety of indications. However, further investigation is needed to further expand the knowledge base of this evolving field. Specifically, RFA has been on the forefront of thermal ablation techniques but the other techniques, including LA, MWA, and HIFU, have demonstrated valuable advantages. More data are needed for these other thermal ablation techniques across all indications and specifically comparative studies against RFA and surgery. Despite the wealth of data available the role of RFA in benign thyroid nodules, its role in cytologically indeterminate nodules and primary malignancy is still poorly understood. Finally, thermal ablation techniques are still limited by the risk of thermal spread and injury to adjacent structures. Thus, there is increasing interest in investigating the role of nonthermal ablation techniques, such as cryoablation and irreversible electroporation, that negate this concern, and have been adopted in other organ systems. More robust studies evaluating the role of these nonthermal techniques are still needed.
Conclusions
The use of ultrasound-guided ablation procedures offers many advantages compared with thyroidectomy: minimally invasive, lower complication profile, avoidance of surgery and general anesthesia, and greater chances of preserving thyroid hormone function. When performed appropriately, they offer a safe nonoperative alternative for many patients. The growth and adoption of these thermal techniques are quickly changing the algorithms used to treat thyroid disease.
Abbreviations
- HIFU
high-intensity focused ultrasound
- LA
laser ablation
- MWA
microwave ablation
- PTMC
papillary thyroid microcarcinoma
- RAI
radioactive iodine
- RFA
radiofrequency ablation
Contributor Information
Q Lina Hu, Email: qlh2000@cumc.columbia.edu, Division of GI/Endocrine Surgery, Columbia University, New York, NY 10032, USA.
Jennifer H Kuo, Division of GI/Endocrine Surgery, Columbia University, New York, NY 10032, USA.
Disclosures
The authors have no conflict of interest.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13(2):129‐147. [DOI] [PubMed] [Google Scholar]
- 2. Orloff LA, Noel JE, Stack BC Jr, et al. Radiofrequency ablation and related ultrasound-guided ablation technologies for treatment of benign and malignant thyroid disease: an international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck. 2022;44(3):633‐660. [DOI] [PubMed] [Google Scholar]
- 3. Shiina S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991;68(7):1524‐1530. [DOI] [PubMed] [Google Scholar]
- 4. Kwak K, Yu B, Lewandowski RJ, Kim DH. Recent progress in cryoablation cancer therapy and nanoparticles mediated cryoablation. Theranostics. 2022;12(5):2175‐2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lv Y, Zhang Y, Huang J, Wang Y, Rubinsky B. A study on nonthermal irreversible electroporation of the thyroid. Technol Cancer Res Treat. 2019;18:1533033819876307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College Of Endocrinology, and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016;22(5):622‐639. [DOI] [PubMed] [Google Scholar]
- 7. Bernardi S, Stacul F, Michelli A, et al. 12-Month efficacy of a single radiofrequency ablation on autonomously functioning thyroid nodules. Endocrine. 2017;57(3):402‐408. [DOI] [PubMed] [Google Scholar]
- 8. Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol. 2008;34(5):784‐791. [DOI] [PubMed] [Google Scholar]
- 9. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papini E, Monpeyssen H, Frasoldati A, Hegedüs L. 2020 European Thyroid Association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ha EJ, Baek JH, Kim KW, et al. Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and Bayesian network meta-analysis. J Clin Endocrinol Metab. 2015;100(5):1903‐1911. [DOI] [PubMed] [Google Scholar]
- 12. Cho SJ, Baek JH, Chung SR, Choi YJ, Lee JH. Long-Term results of thermal ablation of benign thyroid nodules: a systematic review and meta-analysis. Endocrinol Metab. 2020;35(2):339‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol. 2013;23(4):1044‐1049. [DOI] [PubMed] [Google Scholar]
- 14. Trimboli P, Deandrea M. Treating thyroid nodules by radiofrequency: is the delivered energy correlated with the volume reduction rate? A pilot study. Endocrine. 2020;69(3):682‐687. [DOI] [PubMed] [Google Scholar]
- 15. Bernardi S, Dobrinja C, Carere A, et al. Patient satisfaction after thyroid RFA versus surgery for benign thyroid nodules: a telephone survey. Int J Hyperthermia. 2018;35(1):150‐158. [DOI] [PubMed] [Google Scholar]
- 16. Yue WW, Wang SR, Lu F, et al. Radiofrequency ablation vs. microwave ablation for patients with benign thyroid nodules: a propensity score matching study. Endocrine. 2017;55(2):485‐495. [DOI] [PubMed] [Google Scholar]
- 17. Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36(7):1321‐1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cesareo R, Naciu AM, Iozzino M, et al. Nodule size as predictive factor of efficacy of radiofrequency ablation in treating autonomously functioning thyroid nodules. Int J Hyperthermia. 2018;34(5):617‐623. [DOI] [PubMed] [Google Scholar]
- 19. Kim HJ, Cho SJ, Baek JH, Suh CH. Efficacy and safety of thermal ablation for autonomously functioning thyroid nodules: a systematic review and meta-analysis. Eur Radiol. 2021;31(2):605‐615. [DOI] [PubMed] [Google Scholar]
- 20. Cervelli R, Mazzeo S, Boni G, et al. Comparison between radioiodine therapy and single-session radiofrequency ablation of autonomously functioning thyroid nodules: a retrospective study. Clin Endocrinol (Oxf). 2019;90(4):608‐616. [DOI] [PubMed] [Google Scholar]
- 21. Ding M, Tang X, Cui D, et al. Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol. 2019;74(9):712‐717. [DOI] [PubMed] [Google Scholar]
- 22. Zhang M, Luo Y, Zhang Y, Tang J. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581‐1587. [DOI] [PubMed] [Google Scholar]
- 23. Choi Y, Jung SL. Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid. 2020;30(5):720‐731. [DOI] [PubMed] [Google Scholar]
- 24. Tong M, Li S, Li Y, Li Y, Feng Y, Che Y. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36(1):1278‐1286. [DOI] [PubMed] [Google Scholar]
- 25. Yan L, Zhang M, Song Q, Luo Y. Ultrasound-Guided radiofrequency ablation versus thyroid lobectomy for low-risk papillary thyroid microcarcinoma: a propensity-matched cohort study of 884 patients. Thyroid. 2021;31(11):1662‐1672. [DOI] [PubMed] [Google Scholar]
- 26. Zhang M, Tufano RP, Russell JO, et al. Ultrasound-guided radiofrequency ablation versus surgery for low-risk papillary thyroid microcarcinoma: results of over 5 years' follow-up. Thyroid. 2020;30(3):408‐417. [DOI] [PubMed] [Google Scholar]
- 27. Choi Y, Jung SL, Bae JS, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia. 2019;36(1):359‐367. [DOI] [PubMed] [Google Scholar]
- 28. Chung SR, Baek JH, Choi YJ, Lee JH. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol. 2019;29(9):4897‐4903. [DOI] [PubMed] [Google Scholar]
- 29. Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab. 2014;99(10):3653‐3659. [DOI] [PubMed] [Google Scholar]
- 30. Pacella CM, Mauri G, Achille G, et al. Outcomes and risk factors for complications of Laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab. 2015;100(10):3903‐3910. [DOI] [PubMed] [Google Scholar]
- 31. Negro R, Salem TM, Greco G. Laser ablation is more effective for spongiform than solid thyroid nodules. A 4-year retrospective follow-up study. Int J Hyperthermia. 2016;32(7):822‐828. [DOI] [PubMed] [Google Scholar]
- 32. Oddo S, Felix E, Mussap M, Giusti M. Quality of life in patients treated with percutaneous laser ablation for non-functioning benign thyroid nodules: a prospective single-center study. Korean J Radiol. 2018;19(1):175‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Døssing H, Bennedbaek FN, Bonnema SJ, Grupe P, Hegedüs L. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol. 2007;157(1):95‐100. [DOI] [PubMed] [Google Scholar]
- 34. Pacella CM, Mauri G. Is there a role for minimally invasive thermal ablations in the treatment of autonomously functioning thyroid nodules? Int J Hyperthermia. 2018;34(5):636‐638. [DOI] [PubMed] [Google Scholar]
- 35. Kim HJ, Chung SM, Kim H, et al. Long-term efficacy of ultrasound-guided laser ablation for papillary thyroid microcarcinoma: results of a 10-year retrospective study. Thyroid. 2021;31(11):1723‐1729. [DOI] [PubMed] [Google Scholar]
- 36. Korkusuz H, Happel C, Koch DA, Gruenwald F. Combination of ultrasound-guided percutaneous microwave ablation and radioiodine therapy in benign thyroid disease: a 3-month follow-up study. Rofo. 2016;188(1):60‐68. [DOI] [PubMed] [Google Scholar]
- 37. Lang BH, Wu ALH. High intensity focused ultrasound (HIFU) ablation of benign thyroid nodules—a systematic review. J Ther Ultrasound. 2017;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lang BH, Woo YC, Wong CKH. High-intensity focused ultrasound for treatment of symptomatic benign thyroid nodules: a prospective study. Radiology. 2017;284(3):897‐906. [DOI] [PubMed] [Google Scholar]
- 39. Lang BHH, Wong CKH, Ma EPM. Single-session high intensity focussed ablation (HIFU) versus open cervical hemithyroidectomy for benign thyroid nodule: analysis on early efficacy, safety and voice quality. Int J Hyperthermia. 2017;33(8):868‐874. [DOI] [PubMed] [Google Scholar]
- 40. Giovanella L, Piccardo A, Pezzoli C, et al. Comparison of high intensity focused ultrasound and radioiodine for treating toxic thyroid nodules. Clin Endocrinol (Oxf). 2018;89(2):219‐225. [DOI] [PubMed] [Google Scholar]
- 41. Fuller CW, Nguyen SA, Lohia S, Gillespie MB. Radiofrequency ablation for treatment of benign thyroid nodules: systematic review. Laryngoscope. 2014;124(1):346‐353. [DOI] [PubMed] [Google Scholar]
- 42. Baek JH, Lee JH, Valcavi R, Pacella CM, Rhim H, Na DG. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol. 2011;12(5):525‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ha EJ, Baek JH, Lee JH. Moving-shot versus fixed electrode techniques for radiofrequency ablation: comparison in an ex-vivo bovine liver tissue model. Korean J Radiol. 2014;15(6):836‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol. 2014;2014:934595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yue WW, Wang SR, Li XL, et al. Quality of life and cost-effectiveness of radiofrequency ablation versus open surgery for benign thyroid nodules: a retrospective cohort study. Sci Rep. 2016;6:37838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guan SH, Wang H, Teng DK. Comparison of ultrasound-guided thermal ablation and conventional thyroidectomy for benign thyroid nodules: a systematic review and meta-analysis. Int J Hyperthermia. 2020;37(1):442‐449. [DOI] [PubMed] [Google Scholar]
- 47. Cesareo R, Palermo A, Pasqualini V, et al. Radiofrequency ablation on autonomously functioning thyroid nodules: a critical appraisal and review of the literature. Front Endocrinol (Lausanne). 2020;11:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chianelli M, Bizzarri G, Todino V, et al. Laser ablation and 131-iodine: a 24-month pilot study of combined treatment for large toxic nodular goiter. J Clin Endocrinol Metab. 2014;99(7):E1283‐6. [DOI] [PubMed] [Google Scholar]
- 49. Papini E, Guglielmi R, Gharib H, et al. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid. 2011;21(8):917‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Valcavi R, Piana S, Bortolan GS, Lai R, Barbieri V, Negro R. Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid. 2013;23(12):1578‐1582. [DOI] [PubMed] [Google Scholar]
- 51. Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(Suppl 1):S69‐S83. [DOI] [PubMed] [Google Scholar]
- 52. Zheng BW, Wang JF, Ju JX, Wu T, Tong G, Ren J. Efficacy and safety of cooled and uncooled microwave ablation for the treatment of benign thyroid nodules: a systematic review and meta-analysis. Endocrine. 2018;62(2):307‐317. [DOI] [PubMed] [Google Scholar]
- 53. Zhi X, Zhao N, Liu Y, Liu JB, Teng C, Qian L. Microwave ablation compared to thyroidectomy to treat benign thyroid nodules. Int J Hyperthermia. 2018;34(5):644‐652. [DOI] [PubMed] [Google Scholar]
- 54. Jin H, Fan J, Liao K, He Z, Li W, Cui M. A propensity score matching study between ultrasound-guided percutaneous microwave ablation and conventional thyroidectomy for benign thyroid nodules treatment. Int J Hyperthermia. 2018;35(1):232‐238. [DOI] [PubMed] [Google Scholar]
- 55. Esnault O, Franc B, Monteil JP, Chapelon JY. High-intensity focused ultrasound for localized thyroid-tissue ablation: preliminary experimental animal study. Thyroid. 2004;14(12):1072‐1076. [DOI] [PubMed] [Google Scholar]
- 56. Esnault O, Franc B, Ménégaux F, et al. High-intensity focused ultrasound ablation of thyroid nodules: first human feasibility study. Thyroid. 2011;21(9):965‐973. [DOI] [PubMed] [Google Scholar]
- 57. Sennert M, Happel C, Korkusuz Y, Grünwald F, Polenz B, Gröner D. Further investigation on high-intensity focused ultrasound (HIFU) treatment for thyroid nodules: effectiveness related to baseline volumes. Acad Radiol. 2018;25(1):88‐94. [DOI] [PubMed] [Google Scholar]
- 58. Kim YJ, Baek JH, Ha EJ, et al. Cystic versus predominantly cystic thyroid nodules: efficacy of ethanol ablation and analysis of related factors. Eur Radiol. 2012;22(7):1573‐1578. [DOI] [PubMed] [Google Scholar]
- 59. Bennedbaek FN, Hegedüs L. Percutaneous ethanol injection therapy in benign solitary solid cold thyroid nodules: a randomized trial comparing one injection with three injections. Thyroid. 1999;9(3):225‐233. [DOI] [PubMed] [Google Scholar]
- 60. Ferreira MC, Piaia C, Cadore AC. Percutaneous ethanol injection versus conservative treatment for benign cystic and mixed thyroid nodules. Arch Endocrinol Metab. 2016;60(3):211‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Valcavi R, Frasoldati A. Ultrasound-guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocr Pract. 2004;10(3):269‐275. [DOI] [PubMed] [Google Scholar]
- 62. Sung JY, Baek JH, Kim KS, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013;269(1):293‐300. [DOI] [PubMed] [Google Scholar]
- 63. Guglielmi R, Pacella CM, Bianchini A, et al. Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid. 2004;14(2):125‐131. [DOI] [PubMed] [Google Scholar]
- 64. Sag AA, Perkins JM, Kazaure HS, et al. Salvage cryoablation for local recurrences of thyroid cancer inseparable from the trachea and neurovascular structures. J Vasc Interv Radiol. 2023;34(1):54‐62. [DOI] [PubMed] [Google Scholar]
- 65. Young S, Chen T, Golzarian J, Sanghvi T. Ablation of cervical lymph nodes in patients with thyroid cancer: a comparison between cryoablation and percutaneous ethanol injection. J Vasc Interv Radiol. 2022;33(6):S121‐S122. [DOI] [PubMed] [Google Scholar]
- 66. Meijerink MR, Scheffer HJ, de Bree R, Sedee RJ. Percutaneous irreversible electroporation for recurrent thyroid cancer – a case report. J Vasc Interv Radiol. 2015;26(8):1180‐1182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.