Graphical abstract
Keywords: Cytokine storm, TNF-α, IFN-γ, IL-6, IL-1β, GM-CSF, G-CSF, mesenchymal stem cell therapy, therapeutic plasma exchange
Abstract
COVID-19 has claimed millions of lives during the last 3 years since initial cases were reported in Wuhan, China, in 2019. Patients with COVID-19 suffer from severe pneumonia, high fever, acute respiratory distress syndrome (ARDS), and multiple-organ dysfunction, which may also result in fatality in extreme cases. Cytokine storm (CS) is hyperactivation of the immune system, wherein the dysregulated production of proinflammatory cytokines could result in excessive immune cell infiltrations in the pulmonary tissues, resulting in tissue damage. The immune cell infiltration could also occur in other tissues and organs and result in multiple organs' dysfunction. The key cytokines implicated in the onset of disease severity include TNF-α, IFN-γ, IL-6, IL-1β, GM-CSF, and G-CSF. Controlling the CS is critical in treating COVID-19 disease. Therefore, different strategies are employed to mitigate the effects of CS. These include using monoclonal antibodies directed against soluble cytokines or the cytokine receptors, combination therapies, mesenchymal stem cell therapy, therapeutic plasma exchange, and some non-conventional treatment methods to improve patient immunity. The current review describes the role/s of critical cytokines in COVID-19-mediated CS and the respective treatment modalities.
1. Introduction
The Coronavirus Disease 2019 (COVID-19), initially reported in Wuhan, China, in December 2019 [1], [2], presented a significant risk to public health as well as communal stability. It was officially announced as a worldwide pandemic emergency on March 11, 2020, by the World Health Organization (WHO) [3]. The causative agent of COVID-19 is SARS-CoV-2, which is a component of the β-coronaviruses subfamily. Coronaviruses generally comprise enveloped, positive (+) sense, single-stranded RNA (ssRNA) viruses. The virus primarily spreads from an infected (symptomatic and/or asymptomatic) host through respiratory droplets via sneezing or coughing [4], [5], [6], [7]. Indeed, a significant number of people infected remain asymptomatic, and approximately 10% of the patients suffering from pneumonia require ICU admission with or without mechanical ventilation. The usual symptoms comprise fever, shortness of breath, dry cough, muscle pain, headaches, malaise, etc. In some cases, sore throat, hemoptysis, nausea, chest pain, and diarrhea occur. More severe symptoms include high fever, exhaustion, shock, diffused intravascular coagulation, acute respiratory distress syndrome (ARDS), and multi-organ failure. The unregulated Cytokine storm (CS) could also manifest death of the patient [7], [8], [9]. Within 7-14 days after the beginning of the symptomatic disease, the progression to pneumonia is ascertained using several radiological findings such as decreased oxygen saturation levels, weakening of blood gas, and multi-focal ground-glass opacities in the patient's CT scans. Furthermore, the patient's lung lesions may display patchy or segmental consolidations or vasodilation [9], [10], [11], [12].Table 1.
Table 1.
Therapeutics to treat COVID-19-mediated CS and related diseases
| Drug / Therapy | Target | Mechanism | Reference |
|---|---|---|---|
| AdalimumabInfliximab | TNF-α | Human monoclonal anti-TNFα antibody | [187] |
| Emapalumab | IFN-γ | Anti- IFN-γ antibody | [187] |
| Cankinumab | IL-1β | IL-1β antibody directed against the activity of IL-1β | [208] |
| Anakinra | IL-1R | IL-1α and IL-1β receptor antagonist | [200], [201] |
| TolicizumabSarilumab | Il-6R | Human anti-IL-6 receptor MAb | [187], [266], [214] |
| Siltuximab | IL-6 | Anti-IL-6 antibody | NCT04322188 |
| BarcitinibRuxolitinibTofacitinib | JAK-STAT | Non-selective JAK-STAT inhibition | [221], [222], [223] |
| MOR103LenzilumabOtilimab | GM-CSF | Anti-GM-CSF antibody | [236], [237], [238] |
| Mavrilimumab | GM-CSFRα | Human antibody directed against the GM-CSF receptor α (GM-CSFRα) | [232] |
| Colchicine | Non-selective | inhibit the inflammasome activation of NLRP3 and pyrin domain | [224], [267], [268] |
| Corticosteroids | Non-Selective | Inhibition of HAT and recruitment of HDAC activity to the inflammatory gene transcriptional complex to downregulate inflammatory genes | [269], [270] |
| Mesenchymal stem cells (MSCs) | Non-Selective | regulates the behaviour of adaptive as well as innate immune cells | [271] |
| Remedesivir | Non-selective | Broad spectrum antiviral adenosine analogue prodrug that inhibits viral RNA synthesis | [272] |
HAT: histone acetylase, HDACs: histone deacetylases, JAK-STAT: Janus kinase-Signal Transducer and Activator of Transcription, NLRP3: NLR Family Pyrin Domain Containing 3
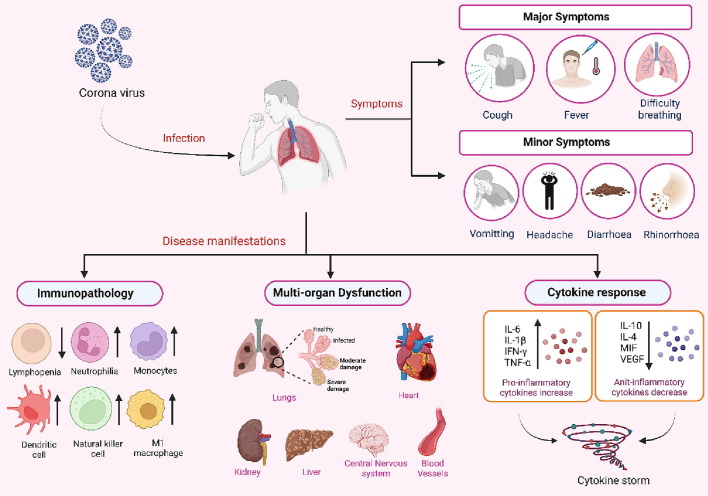
Evidence suggests that a subset of the population suffering from COVID-19 develops CS (Figure 1 ). CS, which has previously been documented for rheumatoid arthritis (RA) as well as in graft-versus-host disease, is a condition of hyperactivated immune response that is generally triggered as a result of various factors, including viral or bacterial infections, immunotherapies, and autoimmune diseases [8], [13], [14], [15]. When a cell is under a normal physiological condition, a homeostasis is maintained amid the concentrations of pro- and anti-inflammatory cytokines. This balance gets disrupted upon viral infection, leading to abnormal activation of different immune cells, including macrophages, T and B lymphocytes, dendritic cells or natural killer cells. This results in the abnormal activation of the immune cells, thereby producing extensive levels of proinflammatory cytokine/s and chemokines that further promote the activation of additional immune cells via a positive feedback loop. [8], [16]. Such an over-activated immune response helps clear off the viral titer and negatively affects the host. In COVID-19-associated CS, extensive pulmonary inflammation and damage to the lungs are observed [17]. It has been reported that almost 1/6th of patients suffering from the virus go on to develop ARDS, acute renal injury, and septic shock [9]. The situation is further worsened with the continued evolution of sub-variants of SARS-CoV-2 omicron, such as sublineages of BA.5, which include BQ.1 and BQ.1.1, BA.4.6 (a sub-variant of omicron B.1.1.529), BA.2.75.2 and BF.7 (also identified as BA.5.2.1.7). A recent report examined the sera from 3-dose vaccinated healthcare workers and found increased resistance to neutralization in all the new sub-variants suggesting a need to find new therapeutic options for COVID-19 cure [18]. In fact, Recently a new Covid variant (XBB.1.16 also referred as “Arcturus”) has been creating a new surge in Covid cases in India and it has been suggested to be similar to U.S. dominant XBB.1.5, which is considered as the most transmissible COVID variant yet [19](https://fortune.com/well/2023/03/31/arcturus-covid-variant-watch-who-world-health-organization-xbb116-omicron-wave/).
Figure 1.
Clinical manifestations of Covid-19: The causative agent of COVID-19 is SARS-CoV-2. The virus primarily spreads from an infected host through respiratory droplets via sneezing or coughing. The major symptoms comprise fever, shortness of breath, dry cough etc. In some cases headaches, sore throat, nausea, and diarrhoea may occur. The disease may manifest itself in the form of cytokine storm (CS) resulting in acute respiratory distress syndrome (ARDS), multi-organ failure or even death in severe cases. CS is a condition of hyper-cactivated immune response, which disrupts the normal physiological homeostasis maintained amid the concentrations of pro- and anti-inflammatory cytokines, leading to abnormal activation of different immune cells, including macrophages, T and B lymphocytes, dendritic cells or natural killer cells. This results in the production of extensive levels of pro-inflammatory cytokine/s (IFN-γ, TNF-α, IL-1, IL-2, IL-6, GM-CSF etc.) and chemokines that further promote the activation of additional immune cells. Such an over-activated immune response negatively affects the host.
The COVID-19-associated CS patients display elevated levels of several critical proinflammatory cytokines, for example, Interferon-gamma (IFN-γ), Tumor Necrosis Factor-alpha (TNF-α), Interleukin 1 (IL-1), Interleukin 2 (IL-2), Interleukin 6 (IL-6), IFN-γ-inducible protein 10 (IP-10), Monocyte Chemoattractant Protein-1 (MCP-1), Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF), and Interleukin 10 (IL-10). The levels of some of these cytokines were also found to correlate with the severity of the disease [20], [21], [22], [23], [24]. Therefore, several agents have been investigated for their potential to diminish the covid associated CS. Some drugs, such as Glucocorticoids and cytokine-specific inhibitors, have effectively reduced overall mortality rates, particularly in critically ill patients [25]. In the current review, we shall comprehensively describe the role of various cytokines in COVID-19-associated CS. We will particularly emphasize the role of GM-CSF and G-CSF cytokines and other emerging therapeutic strategies adopted to mitigate COVID-19.
2. Role of cytokines in CS
Cytokines are small proteins (∼5–25 kDa) involved in cell signaling, which play essential roles in regulating the development and activity of blood and immune system cells [26]. They are immunomodulating agents that are transiently expressed [27] and participate in various autocrine, paracrine, and endocrine signaling mechanisms. Cytokines play an important role in several biological processes, including tissue repair, cancer development, and progression, controlling cellular replication, and regulating cell death [28]. They are the fundamental mediators that establish communication among the immune system cells. When required, cytokines are rapidly secreted from the cell. Cytokines modulate the immune system functioning. The body requires a homeostatic balance of cytokine levels. However, excessive levels and perturbed cytokines homeostasis could harm the host system.
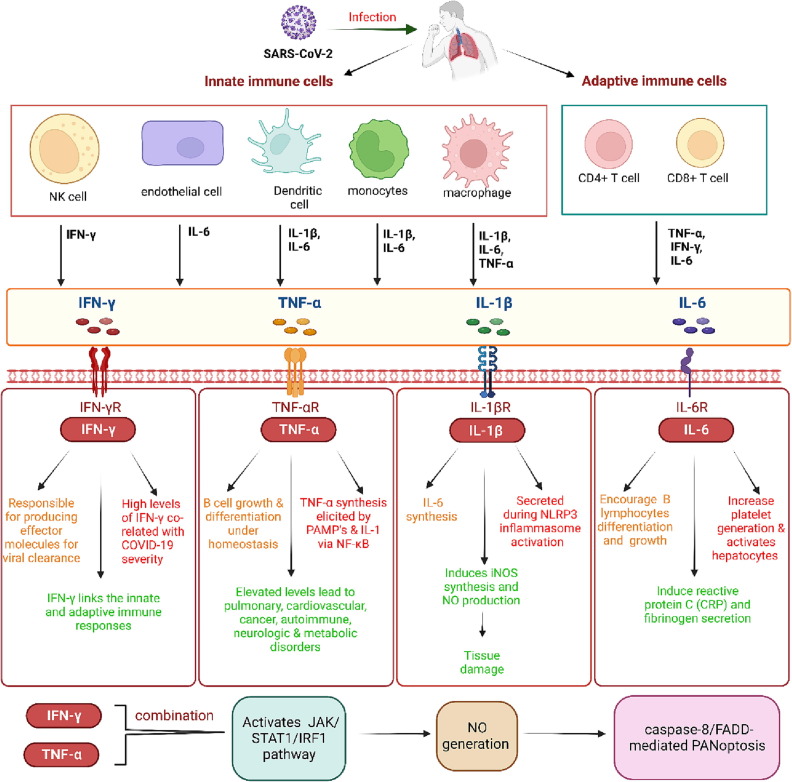
Interleukin 1β (IL-1β): IL-1β is a pleiotropic cytokine that is generated by monocytes/macrophages, dendritic cells, neutrophils, synovial fibroblasts, and B lymphocytes and is one of the most important cytokines engaged in COVID-19 mediated CS [29]. IL-1β has been shown to encourage the synthesis of IL-6 [30] and can induce the synthesis of cyclooxygenase and inducible nitric oxide synthase (iNOS) [31]. The nitric oxide produced by iNOS has been shown to contribute to tissue damage during airway inflammation [32]. IL-1β could also increase the expression of chemokines and adhesion molecules, particularly in endothelial as well as mesenchymal cells, which, in turn, promote the immunocompetent cells' infiltration within the injured tissues. Moreover, being a stimulant of bone marrow, it enhances the count of myeloid progenitor cells and neutrophils' release, resulting in neutrophilia [31]. IL-1β is found to be secreted during the activation of the Nod-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome. A study has suggested that the Viroporin 3a protein of SARS-CoV could activate the NLRP3 inflammasome in macrophages that have been primed by lipopolysaccharides (LPS) [33], which in turn stimulate the IL-1β production. In fact, studies have reported a positive correlation between the high IL-1β concentration in the blood and plasma levels of COVID-19 patients [20], [34] (Figure 2 ).
Figure 2.
Role of main cytokines in Covid-19: Cytokines are immunomodulating agents that are fundamental mediators for establishing communication amongst the immune system cells. When required, cytokines are rapidly secreted from the innate as well as adaptive immune cells. The body requires a homeostatic balance of cytokine levels, which if perturbed (such as in case of Covid-19 infection) could harm the host system. IL-1β is generated by monocytes/macrophages, dendritic cells, etc and is one of the most important cytokines engaged in Covid-19 mediated CS. IL-1β encourages the synthesis of IL-6 and can induce the synthesis of cyclooxygenase and inducible nitric oxide synthase (iNOS). The nitric oxide produced by iNOS contributes to tissue damage during airway inflammation. IL-1β is also secreted during the activation of the Nod-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome. IL-6 is expressed in different immune such as T and B cells, monocytes/macrophages, dendritic cells, and endothelial cells. The plasma concentration of IL-6 rises during Covid-19 infection. IL-6 encourages the differentiation and growth of B lymphocytes, increases platelet generation and induces reactive protein C (CRP) and fibrinogen secretion. Tumor necrosis factor (TNF-α) is a pro-inflammatory cytokine whose elevated plasma concentrations are in positive correlation with SARS-CoV-2 infection mediated CS. It can be produced by macrophages, T cells, and epithelial cells. TNF-α synthesis can be elicited by pathogen-associated molecular patterns and IL-1 through nuclear factor (NF-κB) activation. Under homeostasis conditions, TNF-α is related to B cells’ proliferation and differentiation. However, its unregulated levels are linked with several diseases, including pulmonary, cardiovascular, cancer, autoimmune, neurologic, and metabolic disorders. Interferon-gamma (IFN-γ) is an important pro-inflammatory cytokine implicated in immunity against intracellular pathogens and tumor control. IFN-γ is synthesized by natural killer and natural killer-T cells as part of the innate immune response. However, upon onset of adaptive immunity, IFN-γ can also be generated by the effector T cells, thus, linking the innate and adaptive immune responses. The combined increase in the levels of IFN-γ and TNF-α could activate the JAK/STAT1/IRF1 pathway, thereby inducing nitric oxide generation and ultimately resulting in caspase-8/FADD-interceded inflammatory cell death termed as PANoptosis.
IL-6: IL-6 is a glycoprotein and is also known as “hepatocyte stimulating factor” or “B cell stimulating factor” and is expressed in different immune cells that may include T and B lymphocytes, monocytes/macrophages, fibroblasts, dendritic cells, and endothelial cells. The plasma concentrations of IL-6 rise during various conditions, such as septic shocks, trauma, and burns [35]. IL-6 is a pleiotropic cytokine, which can encourage the differentiation as well as the growth of B lymphocytes and increase platelet generation. Furthermore, it can activate the hepatocytes and induce reactive protein C (CRP) and fibrinogen secretion [36], [37], [38]. According to several studies, the COVID-19 severity positively correlates with enhanced IL-6 and CRP serum levels [39], [40] (Figure 2).
TNF-α: Tumor necrosis factor (TNF-α), is part of the Tumor Necrosis Factor Superfamily (TNFSF) [41] and a potent proinflammatory cytokine whose elevated plasma concentrations are in positive correlation with SARS-CoV-2 infection mediated CS [20], [42], [43]. It can be produced by various cells, such as macrophages, T cells, mast cells, smooth muscle cells, and epithelial cells, and its synthesis can be elicited by pathogen-associated molecular patterns and IL-1 through nuclear factor (NF-κB) activation [16]. Although TNF-α is related to B cells’ proliferation and differentiation under homeostatic circumstances, it is also linked with a broad range of diseases, including pulmonary, cardiovascular, cancer, autoimmune, neurologic, and metabolic disorders [41] (Figure 2).
Interferon-gamma (IFN-γ): IFN-γ is an important proinflammatory cytokine implicated in immunity against intracellular pathogens and tumor control. However, deviation in the IFN-γ expression is related to several autoimmune and auto-inflammatory autoimmune diseases [44]. As part of the innate immune response, IFN-γ is synthesized by natural killer and natural killer-T cells. Upon onset of the antigen-specific adaptive immunity, IFN-γ can be generated by the effector T cells originating from Th1 CD4 cells as well as CD8 cytotoxic T lymphocytes (CTL) [44]. Upon viral infection, the CD8+ T cells differentiate into CTL [45], which in turn, are responsible for producing the effector molecules responsible for viral elimination; thus, IFN-γ somehow links the innate and adaptive immune responses. In individuals who died with COVID-19, high levels of IFN-γ were detected [46]. Interestingly, the combination of IFN-γ and TNF-α could be critical for inducing inflammatory cell death and results in pyroptosis (a programmed cell death pathway induced by proinflammatory cytokines, apoptosis, and necroptosis [47]. TNF-α and IFN-γ work synergistically and trigger inflammatory cell death [47]. The co-treatment of TNF-α and IFN-γ could activate the JAK/STAT1/IRF1 pathway, thereby inducing nitric oxide generation and ultimately resulting in caspase-8/FADD-interceded inflammatory cell death termed as PANoptosis. Mice treated with a combination of TNF-α and IFN-γ suffered from a fatal cytokine shock, which could mimic the inflammation and tissue injuries observed in COVID-19. Furthermore, PANoptosis inhibition could protect mice from such pathology and casualty [47]. Although studies have demonstrated the significance of IFN- and TNF- in reducing lung damage in COVID-19 patients, more studies are required to establish the precise function of these cytokines in mediating CS (Figure 2).
3. Role of GM-CSF
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a hematopoietic growth factor and immunomodulatory cytokine that aids in the clearance of respiratory microbes by stimulating alveolar macrophages. However, GM-CSF is also a key cytokine implicated during the hyper-inflammatory COVID-19 response. It has low or undetectable concentrations in healthy individuals, but several conditions can quickly raise its levels [48], [49]. Macrophages/monocytes, fibroblasts, activated B and T cells, and endothelial cells are some of the cells that create GM-CSF. Although it is produced locally, GM-CSF has a paracrine effect that can help attract lymphocytes, monocytes, and neutrophils to support host defense [48]. IL-1, IL-12, and prostaglandin E2 increase the synthesis of GM-CSF in human T cells [50], [51]. In cells like fibroblasts, chondrocytes, endothelial cells, etc., TNF-α and IL-1β stimulate the synthesis of GM-CSF [52].
It was previously shown that COVID-19 patients harbor greater circulating concentrations of GM-CSF than the healthy controls [20]. Previous reports have also suggested that the levels of GM-CSF were higher in the bronchoalveolar fluids of patients suffering from ARDS in comparison to healthy patient controls and that GM-CSF could be indirectly contributing to ARDS by suppressing the apoptosis of neutrophils [53], [54], since activated neutrophils are known to be involved in microvasculature and lung damages [55], [56]. It is speculated that excessive GM-CSF production may act as a factor in the unregulated immune reaction that occurs during critical COVID-19 in the later disease stages [57], where activated T cells target macrophages and neutrophils before IL-1 and IL-6 are released [58]. Moreover, GM-CSF is vital for the pathogenicity and differentiation of CD4(+) T cells; and an essential factor during the onset of various autoimmune or inflammatory disease conditions [58]. A study from China has reported that only the severely affected COVID-19 patients and not the less affected patients or healthy controls displayed the presence of a typical pathogenic T helper 1-cells expressing GM-CSF [59]. Indeed, such effects could be placed further upstream of other cytokines (TNF's, IL-1, IL-6, etc.) within an inflammatory cascade. Additionally, GM-CSF is believed to be a primary target for treating a number of immune-associated disorders, such as spondyloarthritis, giant-cell arteritis, and rheumatoid arthritis [60]. It is hypothesized that antagonists of GM-CSF may be beneficial in treating COVID-19 and other acute inflammatory diseases like sepsis or ARDS [61].
3.1. GM-CSF: From Growth Factor to a fundamental Tissue Inflammation Mediator
GM-CSF is encoded by a 2.5 kb mRNA and secreted in a monomeric form as a 23 kDa glycosylated protein. The mature human GM-CSF contains 127 residues and is derived from a signal peptide-containing precursor [48] . GM-CSF is found to be present in most tissues as well as serum; and it is also present as an integral membrane protein within the extracellular matrix [48], [62]. Although GM-CSF was initially recognized as a stimulating agent for the propagation of macrophages and granulocytes from the bone marrow precursor cells [63]. However, depending on its concentration, GM-CSF can stimulate the proliferation of multipotent progenitor cells into different cell types such as macrophages, granulocytes, eosinophils, megakaryocytes, etc. [64]. It also plays a key role in maintaining homeostasis in the innate immune system by promoting mature myeloid cell survival and activation [65] (Figure 3 ). Furthermore, it induces the proliferation of myeloid leukemia cells [66].Fig 4.
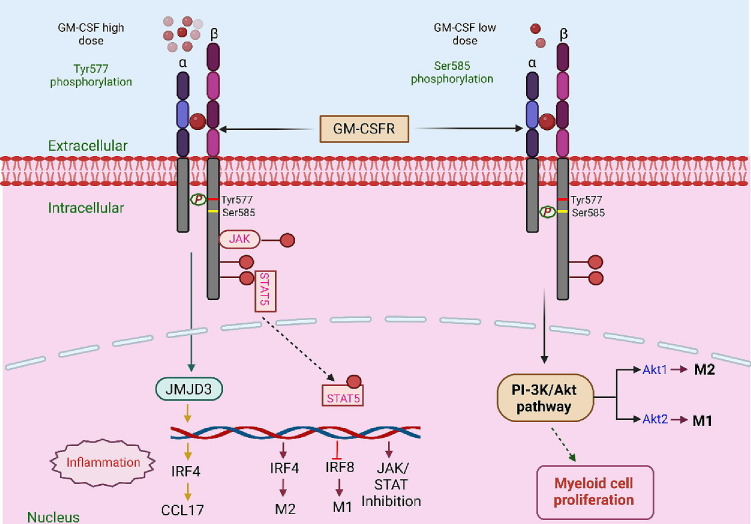
Figure 3.
Concentration dependent role of GM-CSF in cytokine storm: GM-CSF has the potential to function as both a regulatory cytokine and a proinflammatory cytokine depending on its concentration. Lower doses of GM-CSF may promote the tolerogenesis of myeloid cells, which is essential in maintaining the balance of regulatory T-cells. However, higher doses of GM-CSF induce myeloproliferation, resulting in long-lasting immunological responses. The biological activity and signaling of GM-CSF are mediated by means of attaching to the cell surface receptors of GM-CSF. The GM-CSF receptor (GM-CSF-R) represents a heterodimer comprising a GM-CSF-Rα-chain, involved in ligand binding, and a GM-CSF-Rβ -chain, involved in signal transduction. The receptor-cytokine binding could induce several cellular responses, including tyrosine phosphorylation of the β-chain and other intracellular substrates along with activation of Janus kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5), a mitogen-activated protein (MAP) kinase and RAS-Raf signaling pathways. The conserved motif in GM-CSF-R comprises a tyrosine (Tyr577) and serine (Ser585) residue. This motif serves as a binary switch and independently regulates multiple biological functions in a dose-dependent manner. At lower GM-CSF concentrations, signaling occurs through the Ser585 phosphorylation, which activates the PI-3 kinase pathway, thus resulting in the survival of myeloid cells. However, at higher GM-CSF levels, Tyr577 phosphorylation occurs, leading to cell survival, growth and differentiation and functional activation of signaling cascades such as JAK2/STAT5, RAS/MAPK and phosphoinositide 3-kinase (PI3K)-Akt pathway pathways. Both these processes are mutually exclusive and occur independently of each other. GM-CSF can also drive the production of CCL17 via up-regulating the expression of the IFN regulatory factor 4-dependent (IRF4-dependent) pathway by enhancing the activity of Jumonji domain-containing protein D3 (JMJD3) demethylase.
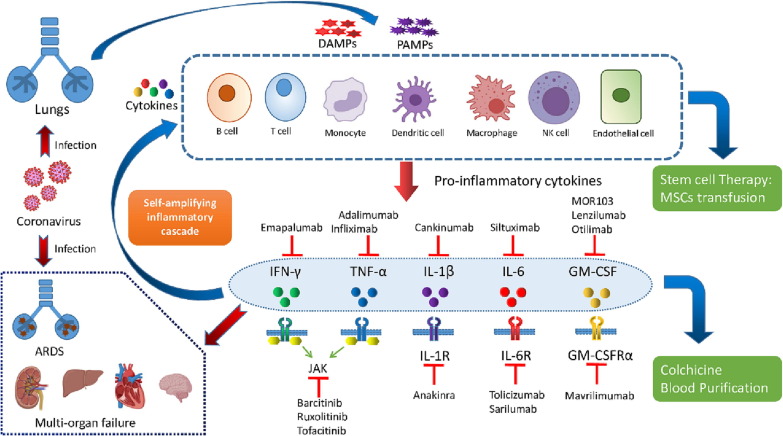
Figure 4.
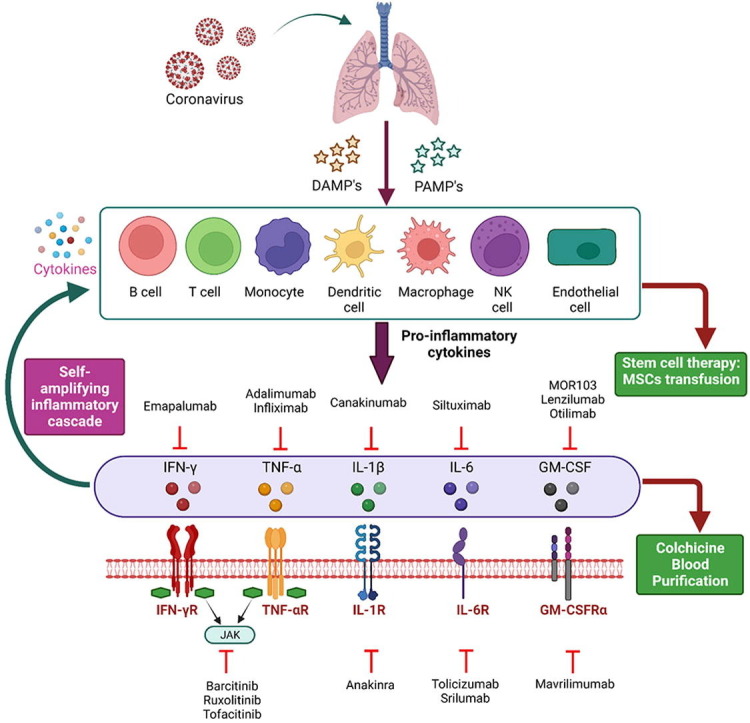
Therapeutic interventions for Covid-19 cytokine storm: Controlling the Covid-19 disease at early stages of infection with targeted therapies could be crucial for successful treatment of the disease. Different prospective treatment options for COVID-19-mediated CS include inhibitors of individual cytokines or their receptors (e.g., Anakinra, Tocilizumab, Emapalumab), targeting a combination of cytokines, inhibitors of JAK/STAT pathways (e.g., Baricitinib, Ruxolitinib), GM-CSF inhibitors (Mavrilimumab, Lenzilumab, Otilimab) etc. Apart from this, mesenchymal stem cells (MSCs) have potent immunomodulatory and anti-inflammatory properties, which help them, regulate the innate and adaptive immune systems. Thus, MSCs are considered an important therapeutic option for treating sepsis and COVID-19-associated CS. Therapeutic Plasma Exchange (TPE) works by efficiently clearing inflammatory cytokine/s from the blood, restoring oxygen levels, early CS resolution, and improving the overall survival rates. Additionally, traditional anti-inflammatory medicines, such as Colchicine and corticosteroids, are also being investigated for COVID-19-associated CS.
It is imperative to mention here that GM-CSF is known to be crucial for preserving the lung alveoli homeostasis, where GM-CSF is synthesized at low levels for the growth and long-lasting maintenance of alveolar macrophages [67], [68]. Pulmonary alveolar proteinosis (PAP) is a fatal disease of interstitial lungs in which the dysfunctional alveolar macrophages fail to clear off surfactants. PAP is caused due to a severe deficiency of GM-CSF. The vulnerability to opportunistic infections is greatly enhanced in individuals suffering from PAP because of impaired GM-CSF signaling that leads to defects in the antimicrobial function of basal circulating neutrophils and alveolar macrophages [68], [69]. This deficiency of GM-CSF may arise from alterations in the GM-CSF receptor genes CSF2RA (encodes GM-CSF-Rα chain) or CSF2RB (encodes GM-CSF-Rβ chain) [68], [70], or from high amounts of auto-antibodies directed against GM-CSF in an autoimmune disorder [71]. However, such mutations that could alter the function of the GM-CSF gene have not been found, which suggests that, unlike its receptor genes, the GM-CSF gene is highly conserved with a mutation resulting in its loss being fatal.
GM-CSF can be effectively induced by various bacterial endotoxins and inflammatory cytokines such as TNF-α, IL-6, and IL-1β, etc., which is also reflected in the enhanced mRNA expressions of these cytokines in macrophages and monocytes treated with GM-CSF [58], [72], [73]. Such findings suggest the possible role of GM-CSF in autoimmunity and inflammatory responses. IL-6 has been demonstrated to stimulate intestinal and splenic GM-CSF synthesis, promoting effects at systemic levels, including elevated splenic macrophage precursors [74]. GM-CSF can also boost the production of proinflammatory cytokines by up-regulating the expression of TLR2, TLR4, or CD14 [75], [76], [77], [78]. This leads to the polarization of the macrophage towards the M1-like phenotype, thereby enhancing the Th1-Th17 immune responses [79], [80], [81], [82] and also contributes to tissue injuries. In fact, various studies have suggested that GM-CSF is crucial for the Th17 cell pathogenesis [83], [84] and that the Th1/17 cells have been found to be present at the inflammatory sites in case of multiple sclerosis (MS), inflammatory bowel diseases (IBD) and juvenile idiopathic arthritis [85], [86], [87]. Additionally, GM-CSF controls the growth of resident CD8+ dendritic cells as well as the maturation of migratory CD103+CD11b+ dendritic cells [88], [89], [90].
Various studies have also demonstrated that GM-CSF overexpression is always followed by multiple pathological changes [91]. For instance, an analysis using transgenic mice carrying the overexpressed murine GM-CSF gene displayed macrophage accumulation, retinal damage, critical tissue damage at various sites, and increased inflammatory mediators and cytokines in these mice [92]. Similarly, the overexpression of GM-CSF inside the stomach may lead to autoimmune gastritis [93]. Similarly, in another study, murine bone marrow cells were treated with a recombinant retrovirus expressing GM-CSF and transplanted into irradiated mice. It resulted in the induction of a lethal myeloproliferative syndrome that was accompanied by extensive neutrophil and macrophage infiltration into various tissues, ultimately leading to death [94].
GM-CSF undoubtedly has the potential to function as both a regulatory cytokine and a proinflammatory cytokine [95]. It's interesting to note that it's still unclear what causes GM-pro-inflammatory CSF's and their immunomodulatory characteristics. These characteristics are thought to be influenced by the quantity and presence of other cytokines in an immune-responsive environment. Lower doses of GM-CSF may promote the tolerogenesis of myeloid cells, which is essential in maintaining the balance of regulatory T-cells [95]. However, higher doses of GM-CSF induces myeloproliferation, resulting in long-lasting immunological responses [72], [96] (Figure 3).
3.2. GM-CSF-dependent inflammatory pathways.
The biological activity and signaling of GM-CSF are mediated by means of attaching to the cell surface receptors of GM-CSF. There are two known components of the GM-CSF receptor (GM-CSF-R). The GM-CSF-R represents a heterodimer comprising a GM-CSF-Rα-chain, involved in Ligand binding, and a GM-CSF-Rβ -chain, involved in signal transduction. In fact, the GM-CSF-Rβ-chain (also referred to as βc subunit) is commonly present in the receptors of IL-3, IL-5, and GM-CSF [97], [98]. The receptor expression could be illustrated by high affinity (Kd = 20-100 pM) and low numbers (∼20 – 200 per cell) [99], [100]. The GM-CSF receptor α and β chains may be differentially expressed, depending on the cell types, as is suggested in some studies [101], [102]. In a study, it was found that the mouse GM-CSF-Rα-chain is polymorphic and spliced alternatively. The mice GM-CSF-Rα-chain is also suggested to be expressed explicitly by the myeloid cells [101]. However, in the case of endothelial cells, it was found that the GM-CSF-Rα-chain was minimally expressed, whereas the GM-CSF-Rβ chain was expressed at high levels [102]. Interestingly, both GM-CSF receptor α and β chains lack the catalytic domain for tyrosine kinase activity [103], [104]. Regardless, the receptor-cytokine binding could induce several cellular responses, including tyrosine phosphorylation of the β-chain and other intracellular substrates along with activation of Janus kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5), a mitogen-activated protein (MAP) kinase and RAS-Raf signaling pathways [103], [104], [105], [106], [107].
The pleiotropic behavior of the GM-CSF cytokine has been attributed to the presence of a conserved motif in GM-CSF-R comprising a tyrosine (Tyr577) and serine (Ser585) residue [108]. This motif serves as a binary switch and independently regulates multiple biological functions in a dose-dependent manner. At lower GM-CSF concentrations, signaling occurs through the phosphorylation of serine residue, which activates the PI-3 kinase pathway, thus resulting in the survival of myeloid cells. However, at higher GM-CSF levels, tyrosine phosphorylation occurs, leading to cell survival, growth and differentiation vis-a-vis functional activation of signaling cascades such as JAK2/STAT5, RAS/MAPK and phosphoinositide 3-kinase (PI3K)-Akt pathway pathways. Both these processes are mutually exclusive and occur independently of each other [108] (Figure 3). Another critical downstream signaling pathway of GM-CSF is the extracellular signal-regulated kinase (ERK) pathway. The activity of ERK has been implicated in the survival of GM-CSF-treated human proinflammatory monocytes [109]. Furthermore, the c-fps/fes protein-tyrosine kinase has been involved in the GM-CSF mediated receptor signaling pathways [110], [111].
Moreover, GM-CSF can also drive the production of CCL17 via up-regulating the expression of the IFN regulatory factor 4-dependent (IRF4-dependent) pathway within murine macrophages, in vivo mice, and human monocytes. GM-CSF could up-regulate the expression of IRF4 by enhancing the activity of Jumonji domain-containing protein D3 (JMJD3) demethylase [73]. The GM-CSF/IRF4/CCL17 axis is shown to be involved in tissue remodeling and inflammation [73], [112]. IRF4, a hemopoietic-specific transcription factor, was found to induce monocytic cell differentiation into dendritic cells [113], and thus it also controls the Th2 cell responses [114]. Moreover, the GM-CSF mediated upregulation of IRF4 signaling has also been associated with the elevated levels of major histocompatibility complex class II (MHCII) expression in macrophages and mice bone marrow cultures [115], [116], [117].
3.3. GM-CSF as a therapeutic target in autoimmunity
GM-CSF is found to play imperative roles in various inflammatory as well as autoimmune disease conditions, such as rheumatoid arthritis (RA), multiple sclerosis (MS), intestinal diseases, and COVID-19 (discussed above). We will succinctly discuss autoimmune diseases with respect to GM-CSF in this section.
3.3.1. Role of GM-CSF in rheumatoid arthritis (RA):
RA is an autoimmune condition with a chronic systemic pathology illustrated by erosive and persistent inflammatory polyarthritis. It damages joints and the extra-articular organs, including kidneys, heart, lungs, eye, skin, digestive and nervous systems [118], [119]. There are several clinical predictors of the presence of RA complications. These include male gender, severe joint disease, smoking, elevated levels of proinflammatory markers, increased titers of rheumatoid factor, and human leukocytic antigen (HLA)-associated shared epitopes [119]. Various studies have advocated the role of GM-CSF during RA pathogenesis [120], [121], [122]. Such studies have indicated elevated GM-CSF concentrations in RA patients' plasma and synovial fluids. In fact, the administration of GM-CSF led to exacerbated collagen-induced arthritis in a mouse model of RA infection [123].
On the contrary, the deficiency of GM-CSF in the collagen-induced arthritis model of mice failed to develop any disease [124]. One case study observed that recombinant GM-CSF administration could aggravate RA and induce IL-6 in-vivo. Furthermore, the flared-up arthritis symptoms were accompanied by the simultaneous release of high acute-phase protein levels, including C-reactive protein and serum amyloid A [125]. In addition, GM-CSF was also reported to be produced by human synovial fibroblasts and chondrocytes in response to IL-1 and TNF stimulus [126], [127]. GM-CSF was identified as a significant curative target in treating RA in light of these studies and other research on the involvement of GM-CSF in RA pathogenesis [128].
3.3.2. Role of GM-CSF in multiple sclerosis (MS):
MS represents a persistent inflammatory ailment of the central nervous system. The pathology of the disease can be illustrated via demyelination followed by axonal degeneration. The myelin sheath degeneration may result in the onset of some clinical manifestations such as muscle spasms, optic neuritis, paralysis, and neuropathic pain. The MS lesion pathology displays myelin sheath destruction, damage at the axonal sites, blood-brain barrier permeability, glial scar formation, and immune cell infiltration, especially lymphocytes, into the CNS [129]. Encephalomyelitis (EAE) represents an established model for studying experimental MS [130], [131]. The relapsing-remitting type patients of MS have been found to contain elevated levels of GM-CSF and TNF-α within the cerebrospinal fluids and not in serum [132]. T helper 1 (Th1) and T helper 17 (Th17) cells are known to mediate CNS autoimmunity in experimental MS and EAE [130]. A study has reported that the IL-23-mediated generation of GM-CSF in Th17 cells was indispensable for encephalitogenicity or encephalitogenic potential The study highlighted the importance of Th17 cells as a vital supplier of GM-CSF in autoimmunity [83]. A recent study showed that the combined scRNA/TCR-seq of >84000 Th17 cells could reveal extensive heterogeneity in Th17 cells with tissue-specific signatures. The stem-like SLAMF6+ and CXCR6+ Th17 pathogenic populations get induced during an autoimmune condition. This population gets trafficked to the intestine, where IL-23 derives the pathogenic production of the GM-CSF+ IFN-γ+ Th17 population. This, in turn, dictates the onset of extra-intestinal autoimmune diseases such as MS [133].
Another study by Dittel and group has demonstrated that the onset of EAE depends on the GM-CSF produced via Th1 cells. Such disease onset is driven by the activation of CNS-invading microglial cells in a GM-CSF-dependent process. The activated microglia could induce proinflammatory cytokine production, ultimately contributing to myelin sheath damage in the CNS. In fact, neutralizing GM-CSF or its deficit could prevent the onset of EAE disease [131]. In addition, recombinant GM-CSF administration in C57BL6 mice exacerbated microglial activity, which could be suppressed by injecting anti-GM-CSF antibody, implying that GM-CSF was critical for the microglial activity in CNS [134]. Such Reports suggest an essential role of GM-CSF in MS along with an indication that inhibiting GM-CSF could be a valuable approach for MS treatment.
3.3.3. Role of GM-CSF in intestinal diseases
Inflammatory bowel diseases (IBD) encompass the Crohn's disease (CD) along with ulcerative colitis (UC). Both of these are robustly involved in the progression of intestinal inflammatory lesions. Regulated macrophage activation is key to intestinal immunity. The macrophage activation is majorly regulated via GM-CSF within patients suffering from IBD and dextran sulfate sodium (DSS) mediated-colitis model of mice. During intestinal inflammation, the type 3 innate lymphoid cells (ILC3) are a significant source of GM-CSF [135], [136], [137]. The GM-CSF-dependent macrophage polarization drives a positive feedback loop leading to further activation of ILC3 and the activation of Th17 immune cells [136]. The extracellular matrix protein 1 (ECM1) expression has been considerably induced during the progression of IBD. Macrophage-specific ECM1 knockout impaired the polarization of M1 macrophage, which is crucial for inflammation control and repair of tissues within the intestine. The study suggested that ECM1 could regulate the polarization of M1 macrophages via the GM-CSF/STAT5 signaling pathway [138].
Generally, ILC3 has been implicated in maintaining steady-state intestinal homeostasis by protecting the intestinal mucosa from various pathogen infections. Such protection could be realized in different ways. For instance, secretion of IL-22, Il-17, and GM-CSF via ILC3 could trigger the production of antimicrobial peptides, including RegIIIβ and RegIIIγ, which in turn kill pathogens [139]. ILC3s are also found to regulate the CD4+ T-cell responses that are specific toward the commensal bacteria through the expression of MHCII [140], [141]. This microbiota-dependent crosstalk between ILC3 and macrophages is crucial for promoting intestinal homeostasis via ILC3-dependent GM-CSF secretion [142]. However, this homeostasis might quickly get disrupted by uncontrolled GM-CSF levels. In a recent study, elevated levels of ILC3 were observed in DSS induced-colitis mice and human IBD patients. Those ILC3s failed to express natural cytotoxicity receptors (NCR) and neutrophils. However, a co-culture of NCR- ILC3s with the neutrophils could help stimulate the neutrophils via enhanced GM-CSF production. Such enhanced GM-CSF production by neutrophils and NCR- ILC3s could exacerbate IBD, and inhibit the neutrophil activity via GM-CSF blockade could inhibit such disease progression [135].
Elevated levels of GM-CSF auto-antibodies could influence the mucosal integrity, pathogen clearance, and migration, proliferation, and survival of the neutrophils [58], [143]. A deficiency in GM-CSF resulted in reduced CD11c(+) DCs, delayed pathogen clearance, and more critical intestinal as well as systemic infection in mice suffering from enteric diseases [144]. Another study reported that the GM-CSF-/- mice displayed enhanced susceptibility towards acute DSS-induced colitis compared to wild-type [145]. Moreover, different studies and clinical trials have suggested a protective role of GM-CSF administration in IBD patients [146], [147], [148], [149].
4. Role of G-CSF
Granulocyte colony-stimulating factor (G-CSF) is an 18.8-kDa secreted glycoprotein encoded by the CSF3 gene [150]. G-CSF is released in an autocrine (hormones that act as ligands and bind to the receptors of cells that produce them) manner. The production of G-CSF in an endogenous setting could be stimulated via pathogenic infection and tissue damage. Although a variety of cells can produce G-CSF, it is mainly generated by cells such as macrophages, mesenchymal cells, fibroblasts, endothelial cells, and neuronal cells in response to proinflammatory stimuli, including IL-1, lipopolysaccharides, and TNF-α [150], [151], [152], [153]. G-CSF has been recognized to orchestrate the neutrophilic granulocytic colony formation in agar cultures of mouse bone marrow cells [154], [155]. In commercial settings, the recombinant G-CSF (rG-CSF) is retailed as Neupogen® (AMGEN®) (filgrastim). It was first launched into clinical trials during the mid-1980s, with a primary aim to restore neutrophil counts in patients subjected to chemotherapies [156] as G-CSF can mediate the differentiation as the proliferation of neutrophil progenitor cells. Moreover, it effectively treats neutropenia (a condition defined by a substantially reduced capacity to recruit or mount neutrophils in response to pathogenic infections) patients [150], [151]. The rG-CSF is administered via the subcutaneous or intravenous (i.v.) routes, and the serum saturation levels (∼40-50 ng/ml) could be attained within 2 to 8 hours [157]. Previous studies have reported that a deficiency of G-CSF in mice resulted in chronic neutropenia and a deficit in granulocyte and macrophage progenitor cell populations [158]. Besides, G-CSF is also considered to be a potent neuroprotectant and a potential candidate for treating neurological conditions in human patients [153], [159].
G-CSF must bind to its cognate receptor, G-CSFR, to carry out its biological activity. G-CSFR is a homo-oligo-dimer principally expressed on the exterior of the bone marrow precursor cells and neutrophils [155] and is a class I cytokine receptor superfamily [151], [160], [161]. A large part of G-CSFR is present in a glycosylated extracellular region. This region further comprises an approximately 200 amino acid-long-region named as cytokine receptor homology (CRH) domain, 3 fibronectin type III (FBN) domains, and an immunoglobulin (Ig)-like N-terminal domain [162]. The CRH domain comprises four conserved cysteine residues and a highly conserved WSxWS motif, which is implicated in ligand identification crucial for signal transduction [162]. On the other hand, the FBN and Ig domains are responsible for receptor stabilization. The membrane-proximal regions of the intracellular domains contain two conserved motifs, namely Box 1 and Box 2, and tyrosine residues (Y704, Y729, Y744, Y764) which are crucial for proliferative signaling, differentiation, and viability [163], [164], [165]. The distal regions contain a less conserved motif (Box 3) related to the trafficking of the receptor [163], [164]. G-CSF binding with its receptor further leads to the activation of various signaling pathways, including JAK/STAT, PI3K/AKT and MAPK that in turn promote survival, propagation, and differentiation of neuronal cells, mobilize the hematopoietic stem cells and progenitor cells [153], [166], [167], [168].
4.1. Role of G-CSF in COVID-19 and associated disease conditions
Neutropenia is a common condition that is observed in cancer patients due to the killing of several fast-growing cell types by chemotherapeutic agents. Neutrophils also die during chemotherapy leading to chemotherapy-induced Neutropenia. Filgrastim (rG-CSF) is routinely used to treat neutropenia in oncology patients [169]. Recently, an investigation was carried out to in a group of 379 cancer patients simultaneously suffering from COVID-19. The study explored the links between G-CSF administration and concurrent neutropenia on COVID-19-related respiratory failure and death. According to the study, G-CSF therapy to these COVID-19-positive neutropenic cancer patients may aggravate their clinical and respiratory conditions [169]. Similarly, another study (Chinese Clinical Trial Registry: ChiCTR2000030007) investigated the effects of rG-CSF administration on lymphopenia in COVID-19 patients. The preliminary results pointed toward reduced critical illness and morbidity counts in COVID-19 patients with lymphopenia. However, rG-CSF administration did not help to accelerate the clinical improvements in such patients [170]. Moreover, a meta-analysis reviewed the effects of rG-CSF treatment for patients with severe sepsis and septic shocks. Based on the data from extensive, randomized, double-blind studies, it was suggested that using G-CSF as an adjunct therapy does not significantly improve the clinical outcomes in severe sepsis patients [171].
It is imperative to mention here that, compared to GM-CSF, G-CSF is more predominantly released from lung cells in response to proinflammatory cytokines TNF-α and IL-1β [172]. Few reports have shown reduced neutrophil infiltration and neutrophil-driven inflammation in mouse models of infection and asthma following G-CSF receptor blockade [173], [174]. Another study demonstrated the presence of elevated G-CSF levels in bronchoalveolar lavage fluids of chronic obstructive pulmonary disease patients and suggested the significance of G-CSF in the pathogenesis of chronic obstructive pulmonary disease and its associated comorbidities [175]. Blocking G-CSF or its receptor (G-CSFR) could be an important strategy to cure such patients. Similarly, a case study reported the onset of ARDS in five patients that were administered G-CSF in combination with chemotherapy or a hematopoietic cell transplant [176]. All such reports indicate that giving G-CSF may worsen lung function, especially in the case of cytokine release syndrome, sepsis, or ARDS, which are also critical features of COVID-19 disease, thus warranting caution while using G-CSF in a therapeutic setting.
On the other hand, Matsushita and Arima [177] have reviewed the involvement of G-CSF during the propagation of mature T-cell leukemia (ATL) cells. In fact, for most of ATL patients, the primary ATL cells are believed to harbor G-CSFR on their cell surfaces. The ATL cells of several patients showed responsive proliferation to G-CSF ex-vivo, and for such patients, the ATL cell counts were significantly increased following G-CSF administration in-vivo, suggesting that care must be taken in routine G-CSF use for treating ATL [177]. In addition, different meta-analysis studies have addressed the effect of G-CSF in treating acute myocardial infarction in atherosclerosis patients. Interestingly, such studies show that G-CSF may be a safer remedial option for patients with minor side effects Taro et al., 2010; Ahmed et al., 2008; Lin et al., 2008; Ince et al., 2008; Sheng et al., 2007; Kasra et al., 2013;2013 [178], [179], [180], [181], [182], [183], [184]. Furthermore, it is suggested that as a mobilization agent of bone marrow stem cells [185], G-CSF could serve as a more convenient treatment option for atherosclerosis than stem cell transplantation [179].
5. Therapeutic interventions in CS
The COVID-19 disease progresses from an early to a pulmonary infection phase and quickly transforms into a hyper-inflammatory phase of infection. Controlling the disease at early infection stages with targeted therapies could be a key to successfully treating the disease [186]. A combination of antiviral drugs (that inhibit viral replication and transmission) and appropriate immunoregulatory therapies (to control the hyper-activated inflammatory immune response) might prove an important strategy to combat COVID-19 [8]. Different completed and ongoing clinical trials investigate the prospective treatment options for COVID-19-mediated CS. These include inhibitors of individual cytokines or their receptors (e.g., Anakinra, Tocilizumab, Emapalumab), targeting a combination of cytokines, inhibitors of JAK/STAT pathways (e.g., Baricitinib, Ruxolitinib), GM-CSF inhibitors (Mavrilimumab, Lenzilumab, Otilimab), Mesenchymal stem cell therapies and several other non-conventional therapies [8], [187]. Additionally, traditional anti-inflammatory medicines, such as Colchicine and corticosteroids, are also being investigated for COVID-19-associated CS [17]. Despite introducing a range of medications to minimize CS, no definite therapy for COVID-19 have been published yet [188].
5.1. Therapeutic interventions involving different cytokines
Treatments to decrease proinflammatory cytokine signaling could enhance clinical outcomes because cytokines play a central role in CS-related pathophysiology. Recently, treatments based on cytokine-specific monoclonal antibodies (MAbs) have gained wide acceptance for treating CS, due to high specificity and minimal side effects [189], [190]. Neutralizing cytokines that are critical components of a hyper-inflammatory pathway could effectively control CS, despite its low or normal circulating concentration [191], [192]. However, MAbs usage accompanies certain risks, including acute anaphylaxis, serum disorders and antibody generation. Such risks must be minimized to improve the overall therapeutic safety of MAbs [189].
5.1.1. Antibodies against TNF-α and IFN-γ
TNF-α and IFN-γ are important proinflammatory cytokines involved in CS and are regarded as potential targets for controlling COVID-19 [20], [47], [193]. The anti-TNF neutralizing antibodies were found to reduce pulmonary immune cell infiltration and overall disease pathology in mice infected with Respiratory syncytial virus (RSV) or influenza A virus (IAV) [194]. In a similar experiment, anti-TNF antibody treatment reduced the bronchoalveolar TNF-α levels and improved survival and gross pathology in IAV-infected mice. However, despite controlled TNF-α levels, it did not affect viral titers in the lungs; thus implying the role of TNF-α in modulating the pulmonary inflammation in IAV pneumonia rather than controlling the viral titers [195]. Unfortunately, such results could not be replicated in human subjects [196]. In addition, Emapalumab is an anti-IFN-γ antibody endorsed by the US FDA for treating patients suffering from relapsed HLH [197]. An earlier study reported that anti-IFN-γ neutralizing antibodies could improve survival and reduce serum levels of ferritin and proinflammatory cytokines in LPS-administered mice that overexpressed human IL-6 in a macrophage activation syndrome experimental model [198].
The IFN-γ and TNF-α synergy occurs upstream of inflammatory cell death inside human and murine macrophages, thereby causing a subsequent release of added cytokines and alarmins [47]. A study has reported that the simultaneous neutralization of both TNF-α and IFN-γ could extend mice survival by two days in IAV-infected mice co-administered with (SEB) [199]. Furthermore, Karki et al. demonstrated that co-treatment of IFN-γ in combination with TNF-α leads to the activation of the JAK/STAT1/IRF1 pathway via nitic oxide release, thus leading to caspase-8/FADD-driven PANoptosis. The combined neutralization of IFN-γ and TNF-α using antibodies could prevent mice death because of sepsis, HLH, cytokine shock, and COVID-19 as compared to blocking IFN-γ or TNF-α alone [47]. However, such studies need large-scale, randomized trials with human subjects to validate their efficacy in controlling COVID-19-mediated CS.
5.1.2. Blocking the IL-1 family
IL-1β is a component of the IL-1 family, which is also the most crucial cytokine implicated in COVID-19-mediated CS [29]. Anakinra (receptor antagonist of IL-1 that inhibits the activities of IL-1α as well as IL-1β) has been endorsed by the US FDA and European Drug Administration (EDA) for treating RA [200] and systemic-onset juvenile idiopathic arthritis [201]. Anakinra was also found to be safe and well tolerated by patients suffering from severe cryopyrin-associated autoinflammatory syndrome (NCT00069329) and RA in long-term safety clinical trials [202]. Anakinra treatment could also contain CS and CAR-T cell therapy-mediated neurotoxicity in humanized mice with elevated leukemia load [203]. Anakinra has also been suggested for use to treat patients experiencing COVID-19-associated CS [204]. In addition, primitive therapy using Anakinra (± dexamethasone) is also suggested as a possible remedy for sHLH disease [205]. Additionally, a recent retrospective cohort trial (NCT04324021) has shown that high doses of intravenous Anakinra are effective and safe for enhancing clinical outcomes in roughly 72% of patients with ARDS and COVID-19 [206].
Canakinumab is a Mab directed against IL-1β cytokine that was found to be beneficial and well tolerated in patients suffering from adult-onset Still's disease [207]. Furthermore, Canakinumab could help neutralize IL-1β levels in patients suffering from COVID-19-mediated CS [208]. Although, a recent clinical trial (NCT04362813), which tested the efficiency of Canakinumab in critically ill COVID-19 patients, has suggested that compared to placebo, canakinumab treatment failed to significantly enhance the patient survival without Invasive Mechanical Ventilation [209].
5.1.3. Anti-IL-6 receptor antagonists
The uncontrolled IL-6 release and unregulated IL-6 receptor signaling have been associated with robust proinflammatory responses [210]. Tocilizumab is a humanized MAb targeting the receptor of IL-6, which has been efficiently used in the treatment of multi-centric Castleman's disease [211], severe RA [210] and CAR-T cell-mediated CS [212]. Tolicizumab has been tested for its potential in COVID-19 in different studies (NCT04320615, NCT04372186) with variable outcomes [213]. Sarilumab is another IL-6 receptor antagonist being explored in COVID-19 infection [214], [215], with beneficial clinical outcomes during timely intervention using IL-6-regulatory therapies for COVID-19 [216]. Additionally, in a Phase IV, randomized, multi-factorial trial (NCT02735707), combined therapy utilizing tocilizumab and Sarilumab could meet its primary aim of improved outcomes with greater survival in significantly ill COVID-19 patients [217]. However, it is essential to mention here that such improved outcomes could also be due to the auxiliary organ support received by the ICU patients. Siltuximab is an anti-IL-6 MAb that could reduce mortality and cytokine-driven hyperinflammation in COVID-19 patients with respiratory failure requiring ventilatory (NCT04322188) [218].
5.1.4. JAK-STAT inhibitors and other therapies
JAK/STATs mediate multiple cytokine signaling pathways, including IFNs, G-CSF, GM-CSF, and interleukins. Fine regulation of these pathways is necessary for preventing immune dysfunctions [47], [219]. JAK inhibitors may be beneficial in fighting COVID-19, as is suggested in a recent study where Baricitinib (a JAK inhibitor) was employed in combination with Remdesivir (a broad-spectrum antiviral drug) for treating patients admitted due to COVID-19 disease. The drug combination effectively reduced recuperation time in patients and demonstrated superior activity compared to only Remdesivir (NCT04401579) [220]. Baricitinib mediates its antiviral effects via its affinity for AP2-linked protein AAK1, thus resulting in reduced SARS-CoV-2 endocytosis [221]. Ruxolitinib is another JAK1/2 inhibitor that could diminish clinical as well as laboratory symptoms for HLH in perforin-deficient (Prf1(-∕-)) mice models that were infected with lymphocytic choriomeningitis virus (LCMV) and sHLH [222]. Tofacitinib (NCT04332042) is a JAK1/3 inhibitor, which is being investigated for its potential in COVID-19 management, along with Barcitinib (NCT04321993) and Ruxolitinib (NCT04348695) [223].
Several other strategies are also employed to mitigate CS. Colchicine has been found to inhibit the inflammasome activation of NLRP3 (NLR Family Pyrin Domain Containing 3) and pyrin domain and is being investigated for its use in COVID-19 management (PMID: [224], PMID: 32732245, PMID: 32472681). Moreover, it is well established that the immune system facilitates the recurrent release and signaling of functionally redundant cytokines. This limits the overall efficacy of single cytokine targeting for CS control and implies the use of combination treatment. For example, the combined anti-cytokine antibodies directed against IFN-γ and TNF-α could improve disease pathology in infected mice [47]. Similarly, a treatment regimen directed against IL-6 and IL-1 could efficiently treat CAR-T cell-mediated neurotoxicity and CS [203]. However, further research is necessary to validate such observations.
5.2. Therapeutic interventions involving GM-CSF
GM-CSF might play a protective role in the early phases of virus-mediated injuries, as it is involved in pulmonary surfactant homeostasis [67], [68]. Therefore, two recombinant human GM-CSFs, Sargramostim and Molgramostim were tested for their potential in COVID-19-associated diseases [225], [226]. Sargramostim is the recombinant human GM-CSF, and was approved by the FDA in 1991 to speed up bone marrow recovery in varied cases of bone marrow failure and treat Neutropenia [225]. Sargramostim was tested for efficacy in patients dealing with acute lung injuries or ARDS. Significant differences could not be observed in the count of organ failure-free days, ventilator-free days, and death at 28 days between the hrGM-CSF treated groups versus placebo groups [227]. Similarly, an inhaled formulation of Sargramostim (25 μg twice daily for five days) is also being investigated in patients suffering from COVID-19-associated acute hypoxic respiratory malfunction in phase 4, open-label, randomized, controlled trial (NCT04326920). A randomized, phase II, placebo-controlled trial tested low-dose Molgramostim's efficiency in patients with critical sepsis and respiratory malfunction. The trial revealed that Molgramostim intervention could help improve gaseous exchange along with functionally activating the pulmonary macrophages but could not improve the 30-day survival rates [226]. Whereas a daily dose of 3 μg/kg Molgramostim for four days could reduce the infectious complications' rates and the duration of hospital admission in patients with bacterial and fungal abdominal sepsis [228].
On the contrary, at elevated levels, GM-CSF is also an important cytokine implicated in COVID-19-mediated complications. Therefore, a potential therapeutic strategy for the treatment of COVID-19 patients involves directly targeting GM-CSF or blocking the GM-CSF receptor [229]. Mavrilimumab is an entirely humanized antibody directed against GM-CSF-Rα. The phase I and phase II studies of mavrilimumab in RA patients demonstrated a good efficiency and safety profile for the drug on the whole, without any changes in pulmonary parameters [230], [231]. The treatment effects of mavrilimumab were studied in non-mechanically ventilated COVID-19 pneumonia patients with systemic hyperinflammation, and the outcome was compared to standard care [232]. As compared to the control group, patients administered with mavrilimumab showed clinical improvement, better survival, a lesser requirement of mechanical ventilation, and better fever resolution. The drug was tolerated well, and no infusion reactions were detected. However, the study is restricted by the short follow-up time, small sample size, and lack of randomization. Nevertheless, these results seem to be more favorable when compared with those observed in similarly designed studies for Tolicizumab [233], [234] or anakinra [206].
In a Phase Ib Clinical study for MS, the effectiveness of the humanized monoclonal antibody (MOR103), which binds to human GM-CSF, was examined [235]. MOR103 could be well-endured in both relapsing-remitting as well as secondary progressive MS with no indications of immunogenicity. MOR103 also displayed well-tolerability and preliminary efficacy data in a Phase Ib/IIa Clinical Trials for active RA patients [236]. Lenzilumab and Otilimab are monoclonal antibodies that directly bind and neutralize GM-CSF [237], [238]. Recently, a multicentre, phase 3, placebo-controlled clinical trial in COVID-19 patients showed that Lenzilumab could significantly improve patient survival without invasive mechanical ventilation at 28 days, and the patients displayed safety profiles similar to the placebo groups [237]. Lenzilumab has recently received approval from the FDA for considerate use during COVID-19 infection [239]. Additionally, the effects of Otilimab were studied in older patients aged more than 70 years. Interestingly, the Otilimab administration resulted in reduced inflammatory markers and acceptable safety profiles in patients (NCT04376684) [238]. In conclusion, GM-CSF targeting may be an excellent strategy to combat COVID-19-associated complications. However, more research is warranted to validate this.
5.3. CS treatment using Mesenchymal stem cells (MSCs) and therapeutic plasma exchange (TPE)
Mesenchymal stem cells (MSCs) represent a heterogenous population of pluripotent stem cells derived from early mesoderm as well as ectoderm [240], [241]. MSCs possess several advantages, such as abundant availability, self-renewal potential, multi-directional differentiation, and low immunogenicity [240]. Various cytokines, chemokines, and growth factors can manipulate the MSCs to adopt characteristics of a specific lineage or transform into a different lineage [242]. MSCs have potent immunomodulatory and anti-inflammatory properties, which help them regulate the innate and adaptive immune systems [243], [244]. Thus, MSCs are considered an important therapeutic option for treating sepsis and COVID-19-associated CS [245]. MSCs are known to evade immunity due to the deficiency of T cell costimulatory molecules (CD80 and CD86); and lower expression of the MHC-I as well as MHC-II proteins [246], [247], thus rendering them safe for use in allogeneic settings. In fact, MSCs from human induced pluripotent stem cells might offer a more effective treatment in an allogeneic transplant situation without the risk of immunological rejection [248].
The COVID-19-induced CS was improved by MSC-derived exosomes (ExoFlo), which also does not suppress host antiviral defense [249]. In addition, MSCs produce microvesicles (MV), which are biologically active molecules with healing properties. A recent study reported that pre-treatment of MSCs with siRNA of KGF protein diminished the regenerative effects of MSC-derived MVs, implying that KGF protein expression plays a vital role in MSC-based therapy [250]. Though MSC therapy is regarded as efficient and safe, it is crucial to note that there are some inadequacies in the current studies, primarily due to the small sample numbers and quick follow-up periods. Large-scale, multicenter clinical studies with extensive follow-ups are needed to prove the MSCs' safety and effectiveness in treating COVID-19.
TPE is a potential treatment strategy that has successfully treated severe COVID-19-mediated CS. Therapeutic Plasma Exchange (TPE) is a procedure that involves putting a patient's blood through an apheresis machine to filter out the plasma and discard it, then reinfusing red blood cells and replacing fluids like plasma or albumin in the patient, along with fluid replacement, including plasma or albumin in the patient [251]. It works by efficiently clearing inflammatory cytokine/s from the blood, restoring oxygen levels, early CS resolution, and improving the overall survival rates [252], [253], [254]. A recent report has demonstrated the efficacy of an artificial liver blood purification system in rapidly removing proinflammatory cytokines, balancing the fluids, electrolytes, and acid-base along with blocking CS, thus, resulting in improved treatment efficacy [255]. Moreover, a case study demonstrated the successful recuperation of a severe COVID-19 patient using extracorporeal blood purification therapy [256]. Despite TPE's ability to cure the CS associated with COVID-19, there are several limitations related to the mode of treatment, which include allergies, bleeding, thrombocytosis, air embolism, etc., which require prompt detection and management to guarantee a safe and effective course of action [255], [256], [257], [258], [259].
5.4. Non-conventional treatment strategies for COVID-19
Apart from the conventional therapeutic strategies employed to treat COVID-19-associated cytokine storm, several researchers have tried some potential non-conventional clinical therapeutic alternatives to resolve COVID-19-related illnesses. Kumar et al. recently demonstrated that vitamin D could ameliorate cytokine storm by increasing the counts of anti-inflammatory cytokines and simultaneously reducing proinflammatory cytokines for treating COVID-19-related cytokine storm [260]. Similarly, resveratrol and melatonin were found to enhance body immunity during SARS-CoV-2 disease and have been recommended as potential anti-SARS-CoV-2 inhibitory compounds [261], [262]. In addition, some natural remedies, especially those of plant-based origin, and supplements like vitamins, zinc, iron, caffeic acid, monochrome, and gallic acids have been suggested to cure COVID-19 effectively mediated CS by stimulating immunity [260], [263], [264]. Furthermore, steroids and tocilizumab could provide a safe and valuable alternative treatment for COVID-19 associated CS. However, the combined efficiency of such an anti-inflammatory treatment regime must be substantiated in randomized and controlled clinical trials [265].
6. Conclusion & Discussion
The delicate balance of the pro- and anti-inflammatory cytokine responses that mediate effective clearance of viral titters is crucial for the effective resolution of COVID-19. CS results from disrupting this equilibrium, leading to uncontrolled immune cell responses. Several clinical trials have examined how various cytokine inhibitors affected COVID-19 treatment. Despite all the efforts and some initial success, the COVID-19 treatment is still constrained by a number of factors. Small sample sizes and shorter follow-up times limit the overall efficacy of these trials across the various subgroups of the population and to various stages of the disease, as is the case with the majority of research. Furthermore, convalescent plasma and monoclonal antibodies are not only costly but are plagued with issues of availability. Additionally, some medications, like corticosteroids, carry a risk of severe side effects, such as high blood pressure, stomach ulcers, etc. Furthermore, underlying medical problems like cancer, diabetes, cardiovascular disease, and autoimmune conditions present in COVID-19 patients may impede the effectiveness of the treatment. Despite all the efforts put into developing a variety of pharmaceuticals, no firm therapeutic recommendations for COVID-19 have been delineated. To close this gap in our knowledge of COVID-19 treatment, more clinical studies with a larger population and a subset of the population is required.
Autho&rs’ contribution
SD conceptualized the study. RD, AKS and SD reviewed the literature and contributed to write the manuscript. RD prepared the figures. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Maharishi Markandeshwar (Deemed to be University), Mullana (Ambala) Haryana and CSIR-Institute of Microbial Technology, Chandigarh for providing the requisite platform to carry out this study. Figures were created with BioRender.com.
Data availability
No data was used for the research described in the article.
References:
- 1.Bogoch Isaac I., Alexander W., Andrea T.-B., Carmen H., Kraemer Moritz U.G., Kamran K. Potential for global spread of a novel coronavirus from China. J Travel Med. 2020:27(2). doi: 10.1093/jtm/taaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen W., Horby Peter W., Hayden Frederick G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Novel Coronavirus (2019-nCoV) SITUATION REPORT - 1. 2020.
- 4.Yan B., Lingsheng Y., Tao W., Fei T., Dong-Yan J., Lijuan C., et al. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Z., Zian Z., Jinjun R., Jiaer L., Guangpu Y., Lin Y., et al. The association between domestic train transportation and novel coronavirus (2019-nCoV) outbreak in China from 2019 to 2020: A data-driven correlational report. Travel Med Infect Dis. 2020;33 doi: 10.1016/j.tmaid.2020.101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X., Pan Z., Cheng Z. Association between 2019-nCoV transmission and N95 respirator use. J Hosp Infect. 2020;105(1):104–105. doi: 10.1016/j.jhin.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dina R., Haitham S.E., Mohamed T., Rasha K., Ramy S. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11(June):1–4. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang L.u., Yin Zhinan H.Y., Heng M. Controlling Cytokine Storm Is Vital in COVID-19. Front Immunol. 2020;11(November):1–14. doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanshan C., Min Z., Dong Xuan Q.u., Jieming G.F., Yang H., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Department of Error Lancet. 2020;395(10223):496 doi: 10.1016/S0140-6736(20)30252-X. [DOI] [Google Scholar]
- 11.Kanne Jeffrey P., Little Brent P., Chung Jonathan H., Elicker Brett M., Ketai L.H. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiology. 2020;296(2):E113–E114. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei Z., Zheng Z., Xie Xingzhi Y.u., Qizhi L.J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol. 2020;214(5):1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 13.Rudragouda C., Stanley P. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander S.-V., Philipp G., Subklewe Marion,Stemmler Hans Joachim, Schlößer Hans Anton, Schlaak Max,, et al. Cytokine release syndrome. J Immunother. Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrara J.L., Abhyankar S., Gilliland D.G. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc. 1993;25(1 Pt 2):1216–1217. [PubMed] [Google Scholar]
- 16.Dounia D., Ikram H., Ayyoub K., Imane E.I.S., Fadila G., Khadija A. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb Pathog. 2021;153 doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puja M., McAuley Daniel F., Michael B., Emilie S., Tattersall Rachel S., Manson Jessica J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panke Q.u., Evans John P., Faraone Julia N., Yi-Min Z., Claire C., Mirela A., et al. Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe. 2023;31(1):9–17.e3. doi: 10.1016/j.chom.2022.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prater Erin. ‘Everyone is kind of tired and has given up’ on COVID. But this new variant is ‘one to watch,’ the WHO says. Available at https://fortune.com/well/2023/03/31/arcturus-covid-variant-watch-who-world-health-organization-xbb116-omicron-wave/ 2023.
- 20.Chaolin H., Yeming W., Xingwang L.i., Lili R., Zhao Jianping H.u., Yi,, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J., et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 22.Zhe Z.u., Ting C., Lingyan F., Kehong L., Xin H.a., Zuoan H., et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marie D.V.D., Seunghee K.-S., Hsin-Hui H., Beckmann Noam D., Sharon N., Bo W., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiurong R., Kun Y., Wenxia W., Lingyu J., Jianxin S. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christian Z., Tatsiana R., Chiara M.A., Francesco F., Raffaele L.R., Giuseppe B., et al. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina (Kaunas) 2022;58(2) doi: 10.3390/medicina58020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelso A. Cytokines: principles and prospects. Immunol Cell Biol. 1998;76(4):300–317. doi: 10.1046/j.1440-1711.1998.00757.x. [DOI] [PubMed] [Google Scholar]
- 27.Takashi M., Osamu T. Post-transcriptional regulation of cytokine mRNA controls the initiation and resolution of inflammation. Biotechnol Genet Eng Rev. 2013;29:49–60. doi: 10.1080/02648725.2013.801236. [DOI] [PubMed] [Google Scholar]
- 28.Foster J.R. The functions of cytokines and their uses in toxicology. Int J Exp Pathol. 2001;82(3):171–192. doi: 10.1046/j.1365-2613.2001.iep0082-0171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 30.Sironi M., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., et al. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989;142(2):549–553. [PubMed] [Google Scholar]
- 31.Dinarello C.A. Interleukin-1beta. Crit Care Med. 2005;33(12 Suppl):S460–S462. doi: 10.1097/01.ccm.0000185500.11080.91. [DOI] [PubMed] [Google Scholar]
- 32.Warke T.J., Fitch P.S., Brown V., Taylor R., Lyons J.D.M., Ennis M., et al. Exhaled nitric oxide correlates with airway eosinophils in childhood asthma. Thorax. 2002;57(5):383–387. doi: 10.1136/thorax.57.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen I-Yin, Moriyama Miyu, Chang Ming-Fu, Ichinohe Takeshi. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jérôme H., Nader Y., Laura B., Aurélien C., Jeremy B., Nikaïa S., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones Simon A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18(12):773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 36.Kishimoto T. The biology of interleukin-6. Blood. 1989;74(1):1–10. [PubMed] [Google Scholar]
- 37.Heinrich P.C., Castell J.V., Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toshio T., Masashi N., Tadamitsu K. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10) doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiaohua C., Zhao Binghong Q.u., Yueming C.Y., Jie X., Yong F., et al. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated With Drastically Elevated Interleukin 6 Level in Critically Ill Patients With Coronavirus Disease 2019. Clin Infect Dis. 2020;71(8):1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobias H., Vindi J., Chiara A., Lipworth Brian J., Hellmuth Johannes C., von Bergwelt-Baildon Michael,, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aggarwal Bharat B., Gupta Subash C., Hye K.J. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119(3):651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuan Q., Zhou Luoqi H.u., Ziwei Z.S., Sheng Y., Tao Y.u., et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan. China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Guang W.u., Di G.W., Yong C., Da H., Hongwu W., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoenborn Jamie R., Wilson C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 45.Whitmire Jason K., Tan Joyce T., Lindsay W.J. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201(7):1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carolina G.A., de Castro Deus Marina, Telles Joao Paulo, Wind Rafael, Goes Marina, Garcia Charello Ossoski Roberta,, et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajendra K., Raj S.B., Shraddha T., Peter W.E., Lillian Z., Parimal S., et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell. 2021;184(1):149–168.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yufang S., Liu Catherine H., Roberts Arthur I., Das Jyoti X.u., Guangwu R.G., et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006;16(2):126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 49.Leavis Helen L., van de Veerdonk F.L., Srinivas M. Stimulating severe COVID-19: the potential role of GM-CSF antagonism. Lancet. Respir Med. 2022;10(3):223–224. doi: 10.1016/S2213-2600(21)00539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas D., Campbell D.J. IL-1β promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J Immunol. 2014;193(1):120–129. doi: 10.4049/jimmunol.1302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quill H., Gaur A., Phipps R.P. Prostaglandin E2-dependent induction of granulocyte-macrophage colony-stimulating factor secretion by cloned murine helper T cells. J Immunol. 1989;142(3):813–818. [PubMed] [Google Scholar]
- 52.Hamilton J.A. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23(8):403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 53.Aggarwal A., Baker C.S., Evans T.W., Haslam P.L. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur Respir J. 2000;15(5):895–901. doi: 10.1034/j.1399-3003.2000.15e14.x. [DOI] [PubMed] [Google Scholar]
- 54.Matute-Bello G., Liles W.C., Radella F., Steinberg K.P., Ruzinski J.T., Hudson L.D., et al. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28(1):1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Johan R., Semple John W., Rick K. The Pathogenic Involvement of Neutrophils in Acute Respiratory Distress Syndrome and Transfusion-Related Acute Lung Injury. Transfus Med Hemother. 2018;45(5):290–298. doi: 10.1159/000492950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonaventura Aldo, Montecucco Fabrizio, Dallegri Franco, Carbone Federico, Lüscher Thomas F, Camici Giovanni G, et al. Novel findings in neutrophil biology and their impact on cardiovascular disease. Cardiovasc Res 2019;115(8):1266–85. Doi: 10.1093/cvr/cvz084. [DOI] [PubMed]
- 57.Lang Frederick M, Lee Kevin M-C, Teijaro John R, Becher Burkhard, Hamilton John A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat Rev Immunol 2020;20(8):507–14. Doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed]
- 58.Aoi S., Takashi U. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Yonggang F.u., Binqing Z.X., Dongsheng W., Changcheng Z., Yingjie Q.i., et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crotti Chiara, Agape Elena, Becciolini Andrea, Biggioggero Martina, Favalli Ennio Giulio. Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects. Drugs 2019;79(16):1741–55. Doi: 10.1007/s40265-019-01192-z. [DOI] [PubMed]
- 61.van der Poll Tom, van de Veerdonk Frank L, Scicluna Brendon P, Netea Mihai G. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 62.Farrar W.L., Brini A.T., Harel-Bellan A., Korner M., Ferris D.K. Hematopoietic growth-factor signal transduction and regulation of gene expression. Immunol Ser. 1990;49:379–410. [PubMed] [Google Scholar]
- 63.Burgess A.W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977;252(6):1998–2003. [PubMed] [Google Scholar]
- 64.Burgess A.W., Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980;56(6):947–958. [PubMed] [Google Scholar]
- 65.Hamilton John A., Adrian A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34(2):81–89. doi: 10.1016/j.it.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Nicola N.A. Granulocyte colony-stimulating factor and differentiation-induction in myeloid leukemic cells. Int J Cell Cloning. 1987;5(1):1–15. doi: 10.1002/stem.5530050102. [DOI] [PubMed] [Google Scholar]
- 67.Guilliams Martin, De Kleer Ismé, Henri Sandrine, Post Sijranke, Vanhoutte Leen, De Prijck Sofie, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 2013;210(10):1977–92. Doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed]
- 68.Trapnell Bruce C, Nakata Koh, Bonella Francesco, Campo Ilaria, Griese Matthias, Hamilton John, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Prim 2019;5(1):16. Doi: 10.1038/s41572-019-0066-3. [DOI] [PubMed]
- 69.Kanji U., Beck David C., Takashi Y., Pierre-Yves B., Shuichi A., Staudt Margaret K., et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356(6):567–579. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- 70.Takuji S., Takuro S., Young Lisa R., Carey Brenna C., Wood Robert E., Maurizio L., et al. Hereditary pulmonary alveolar proteinosis: pathogenesis, presentation, diagnosis, and therapy. Am J Respir Crit Care Med. 2010;182(10):1292–1304. doi: 10.1164/rccm.201002-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anupam K., Basem A., Yoshikazu I., Culver D.A. Pulmonary alveolar proteinosis in adults: pathophysiology and clinical approach. Lancet. Respir Med. 2018;6(7):554–565. doi: 10.1016/S2213-2600(18)30043-2. [DOI] [PubMed] [Google Scholar]
- 72.Bonaventura Aldo, Vecchié Alessandra, Wang Tisha S, Lee Elinor, Cremer Paul C, Carey Brenna, et al. Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies. Front Immunol 2020;11:1625. Doi: 10.3389/fimmu.2020.01625. [DOI] [PMC free article] [PubMed]
- 73.Achuthan Adrian, Cook Andrew D, Lee Ming-Chin, Saleh Reem, Khiew Hsu-Wei, Chang Melody W N, et al. Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. J Clin Invest 2016;126(9):3453–66. Doi: 10.1172/JCI87828. [DOI] [PMC free article] [PubMed]
- 74.Rollwagen F M, Davis T A, Li Y-Y, Pacheco N D, Zhu X-L. Orally administered IL-6 induces elevated intestinal GM-CSF gene expression and splenic CFU-GM. Cytokine n.d.;27(4–5):107–12. Doi: 10.1016/j.cyto.2004.03.019. [DOI] [PubMed]
- 75.Berclaz Pierre-Yves, Carey Brenna, Fillipi Marie-Dominique, Wernke-Dollries Kara, Geraci Nick, Cush Stephanie, et al. GM-CSF regulates a PU.1-dependent transcriptional program determining the pulmonary response to LPS. Am J Respir Cell Mol Biol 2007;36(1):114–21. Doi: 10.1165/rcmb.2006-0174OC. [DOI] [PMC free article] [PubMed]
- 76.Shibata Y., Berclaz P.Y., Chroneos Z.C., Yoshida M., Whitsett J.A., Trapnell B.C. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15(4):557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 77.Bijay P., Yoshifumi S., Jun K., Yukiko D., Mariko N., Hideyuki T., et al. GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J Neuroinflammation. 2012;9:268. doi: 10.1186/1742-2094-9-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorgi Carlos Arterio, Rose Stephanie, Court Nathalie, Carlos Daniela, Paula-Silva Francisco Wanderley Garcia, Assis Patricia Aparecida, et al. GM-CSF priming drives bone marrow-derived macrophages to a pro-inflammatory pattern and downmodulates PGE2 in response to TLR2 ligands. PLoS One 2012;7(7):e40523. Doi: 10.1371/journal.pone.0040523. [DOI] [PMC free article] [PubMed]
- 79.Fleetwood Andrew J., Toby L., Hamilton John A., Cook A.D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol. 2007;178(8):5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 80.Thomas K., Katrina B., Tim S., Saba A., Helen L., Natasha S., et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12(3):231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 81.Verreck Frank A.W., de Boer Tjitske, Langenberg Dennis M L, Hoeve Marieke A, Kramer Matthijs, Vaisberg Elena,, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci U S A. 2004;101(13):4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dexi Z., Cheng H., Zhen L., Shuxiang Z., Lingna K., Chengbo F., et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26(2):192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Mohamed E.-B., Bogoljub C., Hong D., Yaping Y., Melissa C., Farinaz S., et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laura C., Gabor G., Vinko T., Lysann H., Adriano F., Laurent M., et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 85.Lorenzo C., Rolando C., Laura M., Veronica S., Manuela C., Francesco B., et al. Evidence of the transient nature of the Th17 phenotype of CD4+CD161+ T cells in the synovial fluid of patients with juvenile idiopathic arthritis. Arthritis Rheum. 2011;63(8):2504–2515. doi: 10.1002/art.30332. [DOI] [PubMed] [Google Scholar]
- 86.Piper Christopher, Pesenacker Anne M, Bending David, Thirugnanabalan Balathas, Varsani Hemlata, Wedderburn Lucy R, et al. T cell expression of granulocyte-macrophage colony-stimulating factor in juvenile arthritis is contingent upon Th17 plasticity. Arthritis Rheumatol (Hoboken, NJ) 2014;66(7):1955–60. Doi: 10.1002/art.38647. [DOI] [PMC free article] [PubMed]
- 87.Kebir Hania, Ifergan Igal, Alvarez Jorge Ivan, Bernard Monique, Poirier Josée, Arbour Nathalie, et al. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol 2009;66(3):390–402. Doi: 10.1002/ana.21748. [DOI] [PubMed]
- 88.King Irah L., Kroenke Mark A., Segal B.M. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med. 2010;207(5):953–961. doi: 10.1084/jem.20091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daro E., Pulendran B., Brasel K., Teepe M., Pettit D., Lynch D.H., et al. Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but notCD11b(low)CD11c(high) murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J Immunol. 2000;165(1):49–58. doi: 10.4049/jimmunol.165.1.49. [DOI] [PubMed] [Google Scholar]
- 90.Zhan Yifan, Carrington Emma M, van Nieuwenhuijze Annemarie, Bedoui Sammy, Seah Shirley, Xu Yuekang, et al. GM-CSF increases cross-presentation and CD103 expression by mouse CD8+ spleen dendritic cells. Eur J Immunol 2011;41(9):2585–95. Doi: 10.1002/eji.201141540. [DOI] [PubMed]
- 91.Hamilton John A., Anderson G.P. GM-CSF Biology. Growth Factors. 2004;22(4):225–231. doi: 10.1080/08977190412331279881. [DOI] [PubMed] [Google Scholar]
- 92.Lang R.A., Metcalf D., Cuthbertson R.A., Lyons I., Stanley E., Kelso A., et al. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- 93.Frank A., Mark B., Ban-Hock T. Organ-specific autoimmunity in granulocyte macrophage-colony stimulating factor (GM-CSF) deficient mice. Autoimmunity. 2002;35(1):67–73. doi: 10.1080/08916930290005954. [DOI] [PubMed] [Google Scholar]
- 94.Johnson G.R., Gonda T.J., Metcalf D., Hariharan I.K., Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989;8(2):441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palash B., Isadore B., Medha S., Muthusamy T., Khaled A., Hatem E., et al. Dual Role of GM-CSF as a Pro-Inflammatory and a Regulatory Cytokine: Implications for Immune Therapy. J Interferon Cytokine Res. 2015;35(8):585–599. doi: 10.1089/jir.2014.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nemunaitis John, Sterman Daniel, Jablons David, Smith John W, Fox Bernard, Maples Phil, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. J Natl Cancer Inst 2004;96(4):326–31. Doi: 10.1093/jnci/djh028. [DOI] [PubMed]
- 97.Miyajima A. Molecular structure of the IL-3, GM-CSF and IL-5 receptors. Int J Cell Cloning. 1992;10(3):126–134. doi: 10.1002/stem.5530100302. [DOI] [PubMed] [Google Scholar]
- 98.Martinez-Moczygemba Margarita, Huston David P. Biology of common beta receptor-signaling cytokines: IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol 2003;112(4):653–65; quiz 666. Doi: 10.1016/S0091. [DOI] [PubMed]
- 99.Onetto-Pothier N., Aumont N., Haman A., Bigras C., Wong G.G., Clark S.C., et al. Characterization of granulocyte-macrophage colony-stimulating factor receptor on the blast cells of acute myeloblastic leukemia. Blood. 1990;75(1):59–66. [PubMed] [Google Scholar]
- 100.Elliott M.J., Vadas M.A., Eglinton J.M., Park L.S., To L.B., Cleland L.G., et al. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989;74(7):2349–2359. [PubMed] [Google Scholar]
- 101.Marcela R., Siamon G., Taylor P.R. Characterisation of the expression and function of the GM-CSF receptor alpha-chain in mice. Eur J Immunol. 2007;37(9):2518–2528. doi: 10.1002/eji.200636892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colotta F., Bussolino F., Polentarutti N., Guglielmetti A., Sironi M., Bocchietto E., et al. Differential expression of the common beta and specific alpha chains of the receptors for GM-CSF, IL-3, and IL-5 in endothelial cells. Exp Cell Res. 1993;206(2):311–317. doi: 10.1006/excr.1993.1151. [DOI] [PubMed] [Google Scholar]
- 103.Mui A.L., Miyajima A. Cytokine receptors and signal transduction. Prog Growth Factor Res. 1994;5(1):15–35. doi: 10.1016/0955-2235(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 104.Jenkins B.J., Blake T.J., Gonda T.J. Saturation mutagenesis of the beta subunit of the human granulocyte-macrophage colony-stimulating factor receptor shows clustering of constitutive mutations, activation of ERK MAP kinase and STAT pathways, and differential beta subunit tyrosine phosphoryl. Blood. 1998;92(6):1989–2002. [PubMed] [Google Scholar]
- 105.Sakamaki K., Miyajima I., Kitamura T., Miyajima A. Critical cytoplasmic domains of the common beta subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992;11(10):3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duronio V., Clark-Lewis I., Federsppiel B., Wieler J.S., Schrader J.W. Tyrosine phosphorylation of receptor beta subunits and common substrates in response to interleukin-3 and granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1992;267(30):21856–21863. [PubMed] [Google Scholar]
- 107.Mui A.L., Wakao H., O’Farrell A.M., Harada N., Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995;14(6):1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guthridge Mark A., Powell Jason A., Barry Emma F., Stomski Frank C., McClure Barbara J., Hayley R., et al. Growth factor pleiotropy is controlled by a receptor Tyr/Ser motif that acts as a binary switch. EMBO J. 2006;25(3):479–489. doi: 10.1038/sj.emboj.7600948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Achuthan Adrian, Aslam Ahmad S M, Nguyen Quyen, Lam Pui-Yeng, Fleetwood Andrew J, Frye Ashlee T, et al. Glucocorticoids promote apoptosis of proinflammatory monocytes by inhibiting ERK activity. Cell Death Dis 2018;9(3):267. Doi: 10.1038/s41419-018-0332-4. [DOI] [PMC free article] [PubMed]
- 110.Hanazono Y., Chiba S., Sasaki K., Mano H., Yazaki Y., Hirai H. Erythropoietin induces tyrosine phosphorylation and kinase activity of the c-fps/fes proto-oncogene product in human erythropoietin-responsive cells. Blood. 1993;81(12):3193–3196. [PubMed] [Google Scholar]
- 111.Hanazono Y., Chiba S., Sasaki K., Mano H., Miyajima A., Arai K., et al. c-fps/fes protein-tyrosine kinase is implicated in a signaling pathway triggered by granulocyte-macrophage colony-stimulating factor and interleukin-3. EMBO J. 1993;12(4):1641–1646. doi: 10.1002/j.1460-2075.1993.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hamilton J.A. GM-CSF in inflammation. J Exp Med. 2020;217:1. doi: 10.1084/jem.20190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anne L., Ville V., Tuomas N., Riitta L., Leena K., Sampsa M., et al. Differential expression of IFN regulatory factor 4 gene in human monocyte-derived dendritic cells and macrophages. J Immunol. 2005;175(10):6570–6579. doi: 10.4049/jimmunol.175.10.6570. [DOI] [PubMed] [Google Scholar]
- 114.Gao Yan, Nish Simone A, Jiang Ruoyi, Hou Lin, Licona-Limón Paula, Weinstein Jason S, et al. Control of T helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity 2013;39(4):722–32. Doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed]
- 115.Shoichi S., Kiri H., Toshifumi M., Kazuo S., Kan T., Ichinose A., et al. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha- dendritic cell development. Proc Natl Acad Sci U S A. 2004;101(24):8981–8986. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Van der Borght Katrien, Scott Charlotte L, Martens Liesbet, Sichien Dorine, Van Isterdael Gert, Nindl Veronika, et al. Myocarditis Elicits Dendritic Cell and Monocyte Infiltration in the Heart and Self-Antigen Presentation by Conventional Type 2 Dendritic Cells. Front Immunol 2018;9:2714. Doi: 10.3389/fimmu.2018.02714. [DOI] [PMC free article] [PubMed]
- 117.Lee Ming-Chin, Lacey Derek C, Fleetwood Andrew J, Achuthan Adrian, Hamilton John A, Cook Andrew D. GM-CSF- and IRF4-Dependent Signaling Can Regulate Myeloid Cell Numbers and the Macrophage Phenotype during Inflammation. J Immunol 2019;202(10):3033–40. Doi: 10.4049/jimmunol.1801549. [DOI] [PubMed]
- 118.Andrei-Flavius R.d., Gabriela B.S. Management of Rheumatoid Arthritis: An Overview. Cells. 2021;10(11) doi: 10.3390/cells10112857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Conforti Alessandro, Di Cola Ilenia, Pavlych Viktoriya, Ruscitti Piero, Berardicurti Onorina, Ursini Francesco, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev 2021;20(2):102735. Doi: 10.1016/j.autrev.2020.102735. [DOI] [PubMed]
- 120.Mulherin D., Fitzgerald O., Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39(1):115–124. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 121.Xu W.D., Firestein G.S., Taetle R., Kaushansky K., Zvaifler N.J. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989;83(3):876–882. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fiehn C., Wermann M., Pezzutto A., Hüfner M., Heilig B. Plasma GM-CSF concentrations in rheumatoid arthritis, systemic lupus erythematosus and spondyloarthropathy. Z Rheumatol. 1992;51(3):121–126. [PubMed] [Google Scholar]
- 123.Campbell I.K., Bendele A., Smith D.A., Hamilton J.A. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Ann Rheum Dis. 1997;56(6):364–368. doi: 10.1136/ard.56.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Campbell I.K., Rich M.J., Bischof R.J., Dunn A.R., Grail D., Hamilton J.A. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1998;161(7):3639–3644. [PubMed] [Google Scholar]
- 125.Hazenberg B.P., Van Leeuwen M.A., Van Rijswijk M.H., Stern A.C., Vellenga E. Correction of granulocytopenia in Felty’s syndrome by granulocyte-macrophage colony-stimulating factor. Simultaneous induction of interleukin-6 release and flare-up of the arthritis. Blood. 1989;74(8):2769–2770. [PubMed] [Google Scholar]
- 126.Campbell I.K., Novak U., Cebon J., Layton J.E., Hamilton J.A. Human articular cartilage and chondrocytes produce hemopoietic colony-stimulating factors in culture in response to IL-1. J Immunol. 1991;147(4):1238–1246. [PubMed] [Google Scholar]
- 127.Leizer T., Cebon J., Layton J.E., Hamilton J.A. Cytokine regulation of colony-stimulating factor production in cultured human synovial fibroblasts: I. Induction of GM-CSF and G-CSF production by interleukin-1 and tumor necrosis factor. Blood. 1990;76(10):1989–1996. [PubMed] [Google Scholar]
- 128.Cornish Ann L., Campbell Ian K., McKenzie Brent S., Simon C., Wicks I.P. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):554–559. doi: 10.1038/nrrheum.2009.178. [DOI] [PubMed] [Google Scholar]
- 129.Hugo G., Rodrigo P. T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J Neuroinflammation. 2014;11:201. doi: 10.1186/s12974-014-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Joan G. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9(6):393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ponomarev Eugene D., Shriver Leah P., Katarzyna M., Joao P.-V., Daniela V., Dittel B.N. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178(1):39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 132.Carrieri P.B., Provitera V., De Rosa T., Tartaglia G., Gorga F., Perrella O. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing-remitting multiple sclerosis: a correlation with clinical activity. Immunopharmacol Immunotoxicol. 1998;20(3):373–382. doi: 10.3109/08923979809034820. [DOI] [PubMed] [Google Scholar]
- 133.Schnell Alexandra, Huang Linglin, Singer Meromit, Singaraju Anvita, Barilla Rocky M, Regan Brianna M L, et al. Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell 2021;184(26):6281-6298.e23. Doi: 10.1016/j.cell.2021.11.018. [DOI] [PMC free article] [PubMed]
- 134.Hemachandra R.P., Maria M., Wei Z., Kazuhiro N., Christopher B., Geoffrey Y., et al. Granulocyte-macrophage colony-stimulating factor antibody suppresses microglial activity: implications for anti-inflammatory effects in Alzheimer’s disease and multiple sclerosis. J Neurochem. 2009;111(6):1514–1528. doi: 10.1111/j.1471-4159.2009.06432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chang Yuna, Kim Ju Whi, Yang Siyoung, Chung Doo Hyun, Ko Jae Sung, Moon Jin Soo, et al. Increased GM-CSF-producing NCR- ILC3s and neutrophils in the intestinal mucosa exacerbate inflammatory bowel disease. Clin Transl Immunol 2021;10(7):e1311. Doi: 10.1002/cti2.1311. [DOI] [PMC free article] [PubMed]
- 136.Castro-Dopico Tomas, Fleming Aaron, Dennison Thomas W, Ferdinand John R, Harcourt Katherine, Stewart Benjamin J, et al. GM-CSF Calibrates Macrophage Defense and Wound Healing Programs during Intestinal Infection and Inflammation. Cell Rep 2020;32(1):107857. Doi: 10.1016/j.celrep.2020.107857. [DOI] [PMC free article] [PubMed]
- 137.Boning Z., Shengnan S., Gareth A., Changjiang D., Jing L., Feiyue X. ILC3 function as a double-edged sword in inflammatory bowel diseases. Cell Death Dis. 2019;10(4):315. doi: 10.1038/s41419-019-1540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yaguang Z., Xuezhen L.i., Zhongguang L., Liyan M.a., Songling Z.u., Zhishuo W., et al. ECM1 is an essential factor for the determination of M1 macrophage polarization in IBD in response to LPS stimulation. Proc Natl Acad Sci U S A. 2020;117(6):3083–3092. doi: 10.1073/pnas.1912774117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guo Xiaohuan, Qiu Ju, Tu Tony, Yang Xuanming, Deng Liufu, Anders Robert A, et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 2014;40(1):25–39. Doi: 10.1016/j.immuni.2013.10.021. [DOI] [PMC free article] [PubMed]
- 140.Hepworth Matthew R., Monticelli Laurel A., Fung Thomas C., Ziegler Carly G.K., Stephanie G., Rohini S., et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498(7452):113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hepworth Matthew R., Fung Thomas C., Masur Samuel H., Kelsen Judith R., McConnell Fiona M., Juan D., et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science. 2015;348(6238):1031–1035. doi: 10.1126/science.aaa4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mortha Arthur, Chudnovskiy Aleksey, Hashimoto Daigo, Bogunovic Milena, Spencer Sean P, Belkaid Yasmine, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014;343(6178):1249288. Doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed]
- 143.Jan D. Granulocyte macrophage colony-stimulating factor and the intestinal innate immune cell homeostasis in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2014;306(6):G455–G465. doi: 10.1152/ajpgi.00409.2013. [DOI] [PubMed] [Google Scholar]
- 144.Yoshihiro H., Laia E., Dann Sara M., Lars E., Kagnoff M.F. GM-CSF-facilitated dendritic cell recruitment and survival govern the intestinal mucosal response to a mouse enteric bacterial pathogen. Cell Host Microbe. 2010;7(2):151–163. doi: 10.1016/j.chom.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yinghua X.u., Hunt Nicholas H., Shisan B. The role of granulocyte macrophage-colony-stimulating factor in acute intestinal inflammation. Cell Res. 2008;18(12):1220–1229. doi: 10.1038/cr.2008.310. [DOI] [PubMed] [Google Scholar]
- 146.Sainathan Satheesh K., Hanna Eyad M., Qingqing G., Bishnupuri Kumar S., Qizhi L., Marco C., et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm Bowel Dis. 2008;14(1):88–99. doi: 10.1002/ibd.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dieckgraefe Brian K., Korzenik J.R. Treatment of active Crohn’s disease with recombinant human granulocyte-macrophage colony-stimulating factor. Lancet (London, England) 2002;360(9344):1478–1480. doi: 10.1016/S0140-6736(02)11437-1. [DOI] [PubMed] [Google Scholar]
- 148.Korzenik Joshua R., Dieckgraefe Brian K., Valentine John F., Hausman Diana F., Gilbert Mark J. Sargramostim in Crohn’s Disease Study Group. Sargramostim for active Crohn’s disease. N Engl J Med. 2005;352(21):2193–2201. doi: 10.1056/NEJMoa041109. [DOI] [PubMed] [Google Scholar]
- 149.Valentine J.F., Fedorak R.N., Feagan B., Fredlund P., Schmitt R., Ni P., et al. Steroid-sparing properties of sargramostim in patients with corticosteroid-dependent Crohn’s disease: a randomised, double-blind, placebo-controlled, phase 2 study. Gut. 2009;58(10):1354–1362. doi: 10.1136/gut.2008.165738. [DOI] [PubMed] [Google Scholar]
- 150.Roberts A.W. G-CSF: A key regulator of neutrophil production, but that’s not all! Growth Factors. 2005;23(1):33–41. doi: 10.1080/08977190500055836. [DOI] [PubMed] [Google Scholar]
- 151.Nagata S., Fukunaga R. Granulocyte colony-stimulating factor and its receptor. Prog Growth Factor Res. 1991;3(2):131–141. doi: 10.1016/s0955-2235(05)80004-3. [DOI] [PubMed] [Google Scholar]
- 152.Wright Craig R., Ward Alister C., Russell A.P. Granulocyte Colony-Stimulating Factor and Its Potential Application for Skeletal Muscle Repair and Regeneration. Mediators Inflamm. 2017;2017:7517350. doi: 10.1155/2017/7517350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vikrant R., Sumit J., Puneet K. Neuroprotection through G-CSF: recent advances and future viewpoints. Pharmacol Rep. 2021;73(2):372–385. doi: 10.1007/s43440-020-00201-3. [DOI] [PubMed] [Google Scholar]
- 154.Metcalf D., Nicola N.A. Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hemopoietic cells. J Cell Physiol. 1983;116(2):198–206. doi: 10.1002/jcp.1041160211. [DOI] [PubMed] [Google Scholar]
- 155.Lazarus Hillard M., Peter G.R. G-CSF and GM-CSF Are Different. Which One Is Better for COVID-19? Acta Haematol. 2021;144(4):355–359. doi: 10.1159/000510352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bronchud M.H., Scarffe J.H., Thatcher N., Crowther D., Souza L.M., Alton N.K., et al. Phase I/II study of recombinant human granulocyte colony-stimulating factor in patients receiving intensive chemotherapy for small cell lung cancer. Br J Cancer. 1987;56(6):809–813. doi: 10.1038/bjc.1987.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.de Haas M., Kerst J.M., van der Schoot C.E., Calafat J., Hack C.E., Nuijens J.H., et al. Granulocyte colony-stimulating factor administration to healthy volunteers: analysis of the immediate activating effects on circulating neutrophils. Blood. 1994;84(11):3885–3894. [PubMed] [Google Scholar]
- 158.Lieschke G.J., Grail D., Hodgson G., Metcalf D., Stanley E., Cheers C., et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84(6):1737–1746. [PubMed] [Google Scholar]
- 159.Maurer M.H., Schäbitz W.-R., Schneider A. Old friends in new constellations–the hematopoetic growth factors G-CSF, GM-CSF, and EPO for the treatment of neurological diseases. Curr Med Chem. 2008;15(14):1407–1411. doi: 10.2174/092986708784567671. [DOI] [PubMed] [Google Scholar]
- 160.Fukunaga R., Seto Y., Mizushima S., Nagata S. Three different mRNAs encoding human granulocyte colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1990;87(22):8702–8706. doi: 10.1073/pnas.87.22.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Layton Judith E., Hall N.E. The interaction of G-CSF with its receptor. Front Biosci. 2006;11:3181–3189. doi: 10.2741/2041. [DOI] [PubMed] [Google Scholar]
- 162.Yamasaki K., Naito S., Anaguchi H., Ohkubo T., Ota Y. Solution structure of an extracellular domain containing the WSxWS motif of the granulocyte colony-stimulating factor receptor and its interaction with ligand. Nat Struct Biol. 1997;4(6):498–504. doi: 10.1038/nsb0697-498. [DOI] [PubMed] [Google Scholar]
- 163.Clifford L., Craig W., Russell Aaron P., Ward A.C. Granulocyte colony-stimulating factor receptor: stimulating granulopoiesis and much more. Int J Biochem Cell Biol. 2009;41(12):2372–2375. doi: 10.1016/j.biocel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 164.Ward A.C., Hermans M.H., Smith L., van Aesch Y.M., Schelen A.M., Antonissen C., et al. Tyrosine-dependent and -independent mechanisms of STAT3 activation by the human granulocyte colony-stimulating factor (G-CSF) receptor are differentially utilized depending on G-CSF concentration. Blood. 1999;93(1):113–124. [PubMed] [Google Scholar]
- 165.Moran M.F., Koch C.A., Anderson D., Ellis C., England L., Martin G.S., et al. Src homology region 2 domains direct protein-protein interactions in signal transduction. Proc Natl Acad Sci U S A. 1990;87(21):8622–8626. doi: 10.1073/pnas.87.21.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jens M., Jan H., Jürgen W., Andreas R., Armin S., Wolf-Rüdiger S. Meta-analysis of the efficacy of granulocyte-colony stimulating factor in animal models of focal cerebral ischemia. Stroke. 2008;39(6):1855–1861. doi: 10.1161/STROKEAHA.107.506816. [DOI] [PubMed] [Google Scholar]
- 167.Schäbitz W.-R., Kollmar R., Schwaninger M., Juettler E., Bardutzky J., Schölzke M.N., et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34(3):745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- 168.Armin S., Carola K., Tobias S., Daniela W., Claudia P., Rico L., et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115(8):2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang Allen W., Sejal M., Anna K., Hohl Tobias M., Rekha P., Dhruvkumar P., et al. The Effect of Neutropenia and Filgrastim (G-CSF) on Cancer Patients With Coronavirus Disease 2019 (COVID-19) Infection. Clin Infect Dis. 2022;74(4):567–574. doi: 10.1093/cid/ciab534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lin-Ling C., Wei-Jie G., Chong-Yang D., Nuo-Fu Z., Lei Chun-Liang H.Y., et al. Effect of Recombinant Human Granulocyte Colony-Stimulating Factor for Patients With Coronavirus Disease 2019 (COVID-19) and Lymphopenia: A Randomized Clinical Trial. JAMA Intern Med. 2021;181(1):71–78. doi: 10.1001/jamainternmed.2020.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mohammad R.A. Use of granulocyte colony-stimulating factor in patients with severe sepsis or septic shock. Am J Health Syst Pharm. 2010;67(15):1238–1245. doi: 10.2146/ajhp090325. [DOI] [PubMed] [Google Scholar]
- 172.Dos Santos Cruz GJ, Loraine H, Zhang JG, Clark KL, Davis RJ. G-CSF and GM-CSF are differentially released from primary human lung cells in response to pro-inflammatory cytokines (abstract). n.d.
- 173.Wang Hao, FitzPatrick Meaghan, Wilson Nicholas J, Anthony Desiree, Reading Patrick C, Satzke Catherine, et al. CSF3R/CD114 mediates infection-dependent transition to severe asthma. J Allergy Clin Immunol 2019;143(2):785-788.e6. Doi: 10.1016/j.jaci.2018.10.001. [DOI] [PubMed]
- 174.Hao W., Christian A., Nick W., Steven B. G-CSFR antagonism reduces neutrophilic inflammation during pneumococcal and influenza respiratory infections without compromising clearance. Sci Rep. 2019;9(1):17732. doi: 10.1038/s41598-019-54053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tsantikos Evelyn, Lau Maverick, Castelino Cassandra Mn, Maxwell Mhairi J, Passey Samantha L, Hansen Michelle J, et al. Granulocyte-CSF links destructive inflammation and comorbidities in obstructive lung disease. J Clin Invest 2018;128(6):2406–18. Doi: 10.1172/JCI98224. [DOI] [PMC free article] [PubMed]
- 176.Hiroyuki T., Yoshinobu T., Ako M., Takahiro O., Akihisa K., Eizo K. Common features in the onset of ARDS after administration of granulocyte colony-stimulating factor. Chest. 2002;121(5):1716–1720. doi: 10.1378/chest.121.5.1716. [DOI] [PubMed] [Google Scholar]
- 177.Matsushita K., Arima N. Involvement of granulocyte colony-stimulating factor in proliferation of adult T-cell leukemia cells. Leuk Lymphoma. 1998;31(3–4):295–304. doi: 10.3109/10428199809059222. [DOI] [PubMed] [Google Scholar]
- 178.Taro M., Hideyuki W., Takahiro U., Akiko T., Ben H., Yoshiaki K., et al. Appropriate doses of granulocyte-colony stimulating factor reduced atherosclerotic plaque formation and increased plaque stability in cholesterol-fed rabbits. J Atheroscler Thromb. 2010;17(1):84–96. doi: 10.5551/jat.2279. [DOI] [PubMed] [Google Scholar]
- 179.Ahmed A.-L., Roberto B., Zuba-Surma Ewa K., Tleyjeh Imad M., Hornung Carlton A., Buddhadeb D. Granulocyte colony-stimulating factor therapy for cardiac repair after acute myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2008;156(2):216–226.e9. doi: 10.1016/j.ahj.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lin F., Lianglong C., Chen Xiangqi F.u., Fayuan. A meta-analysis of stem cell mobilization by granulocyte colony-stimulating factor in the treatment of acute myocardial infarction. Cardiovasc Drugs Ther. 2008;22(1):45–54. doi: 10.1007/s10557-007-6072-9. [DOI] [PubMed] [Google Scholar]
- 181.Ince H., Valgimigli M., Petzsch M., de Lezo J., Suarez K.F., Dunkelmann S., et al. Cardiovascular events and re-stenosis following administration of G-CSF in acute myocardial infarction: systematic review and meta-analysis. Heart. 2008;94(5):610–616. doi: 10.1136/hrt.2006.111385. [DOI] [PubMed] [Google Scholar]
- 182.Sheng K., Yuejin Y., Chong-Jian L.i., Runlin G. Effectiveness and tolerability of administration of granulocyte colony-stimulating factor on left ventricular function in patients with myocardial infarction: a meta-analysis of randomized controlled trials. Clin Ther. 2007;29(11):2406–2418. doi: 10.1016/j.clinthera.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 183.Moazzami Kasra, Roohi Aria, Moazzami Bobak. Granulocyte colony stimulating factor therapy for acute myocardial infarction. Cochrane Database Syst Rev 2013;2013(5):CD008844. Doi: 10.1002/14651858.CD008844.pub2. [DOI] [PMC free article] [PubMed]
- 184.Dietlind Z., Alban D., Tobias K., de Waha Antoinette, Ripa Rasmus Sejersten, Kastrup Jens,, et al. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol. 2008;51(15):1429–1437. doi: 10.1016/j.jacc.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 185.Elfenbein G.J. Granulocyte-colony stimulating factor primed bone marrow and granulocyte-colony stimulating factor mobilized peripheral blood stem cells are equivalent for engraftment: which to choose? Pediatr Transplant. 2005;9(Suppl 7):37–47. doi: 10.1111/j.1399-3046.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 186.Siddiqi Hasan K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rizk John G., Kamyar K.-Z., Mehra Mandeep R., Lavie Carl J., Youssef R. Forthal Donald N. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs. 2020;80(13):1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim Jae Seok, Lee Jun Young, Yang Jae Won, Lee Keum Hwa, Effenberger Maria, Szpirt Wladimir, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021;11(1):316–29. Doi: 10.7150/thno.49713. [DOI] [PMC free article] [PubMed]
- 189.Hansel Trevor T., Harald K., Thomas S., Mitchell Jane A., George Andrew J.T. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9(4):325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 190.Galván-Román José María, Rodríguez-García Sebastián C, Roy-Vallejo Emilia, Marcos-Jiménez Ana, Sánchez-Alonso Santiago, Fernández-Díaz Carlos, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study. J Allergy Clin Immunol 2021;147(1):72-80.e8. Doi: 10.1016/j.jaci.2020.09.018. [DOI] [PMC free article] [PubMed]
- 191.Marco F., Andrea F., Noemi B., Alessandro P., Chiara M., Luca B., et al. IL-1 Receptor Antagonist Anakinra in the Treatment of COVID-19 Acute Respiratory Distress Syndrome: A Retrospective. Observational Study. J Immunol. 2021;206(7):1569–1575. doi: 10.4049/jimmunol.2001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cauchois Raphaël, Koubi Marie, Delarbre David, Manet Cécile, Carvelli Julien, Blasco Valery Benjamin, et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A 2020;117(32):18951–3. Doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed]
- 193.Marc F., Maini Ravinder N., Woody James N., Holgate Stephen T., Gregory W., Matthew R., et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hussell T., Pennycook A., Openshaw P.J. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol. 2001;31(9):2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 195.Peper R.L., Van Campen H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb Pathog. 1995;19(3):175–183. doi: 10.1006/mpat.1995.0056. [DOI] [PubMed] [Google Scholar]
- 196.Chousterman Benjamin G., Swirski Filip K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39(5):517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 197.Locatelli Franco, Jordan Michael B, Allen Carl, Cesaro Simone, Rizzari Carmelo, Rao Anupama, et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N Engl J Med 2020;382(19):1811–22. Doi: 10.1056/NEJMoa1911326. [DOI] [PubMed]
- 198.Giusi P., Ivan C., Antonia P., Grom Alexei A., Claudia B., Laurence C., et al. Neutralization of IFN-γ reverts clinical and laboratory features in a mouse model of macrophage activation syndrome. J Allergy Clin Immunol. 2018;141(4):1439–1449. doi: 10.1016/j.jaci.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 199.Zhang W.J., Sarawar S., Nguyen P., Daly K., Rehg J.E., Doherty P.C., et al. Lethal synergism between influenza infection and staphylococcal enterotoxin B in mice. J Immunol. 1996;157(11):5049–5060. [PubMed] [Google Scholar]
- 200.Giulio C., Dinarello C.A. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatology (Oxford) 2015;54(12):2134–2144. doi: 10.1093/rheumatology/kev269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pierre Q., Florence A., Rolando C., Pascal P., Claude M., Christophe B., et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Ann Rheum Dis. 2011;70(5):747–754. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Torbjörn K., Malin L., Mika L., Raphaela G.-M., Hans O. Long-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromes. Rheumatology (Oxford) 2016;55(8):1499–1506. doi: 10.1093/rheumatology/kew208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Margherita N., Barbara C., Giulia B., Laura F., Ayurzana P., Marco G., et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 204.Kooistra Emma J., Waalders Nicole J.B., Inge G., Janssen Nico A.F., de Nooijer A.H., Netea Mihai G., et al. Anakinra treatment in critically ill COVID-19 patients: a prospective cohort study. Crit Care. 2020;24(1):688 doi: 10.1186/s13054-020-03364-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sakshi B., Anshul V., Danielle S., Mohamad B., Daniel C., Lipton Jeffrey M., et al. The use of anakinra in the treatment of secondary hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2020;67(11):e28581. doi: 10.1002/pbc.28581. [DOI] [PubMed] [Google Scholar]
- 206.Giulio C., Giacomo D.L., Corrado C., Emanuel D.-T., Marco R., Diana C., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet. Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Paolo S., Sara B., Andrea D., Eugen F., Paola G. Canakinumab for the treatment of adult-onset Still’s disease. Expert Rev Clin Immunol. 2020;16(2):129–138. doi: 10.1080/1744666X.2019.1707664. [DOI] [PubMed] [Google Scholar]
- 208.Daniele G., Giancarlo B., Fabio M., Antonio C., Sophie T., Laura R., et al. Canakinumab as treatment for COVID-19-related pneumonia: A prospective case-control study. Int J Infect Dis. 2021;104:433–440. doi: 10.1016/j.ijid.2020.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Roberto C., Antonio A., Ivan G., Jamie M., Hsue Priscilla Y., Tuhina N., et al. Effect of Canakinumab vs Placebo on Survival Without Invasive Mechanical Ventilation in Patients Hospitalized With Severe COVID-19: A Randomized Clinical Trial. JAMA. 2021;326(3):230–239. doi: 10.1001/jama.2021.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sujin K., Toshio T., Masashi N., Tadamitsu K. Targeting Interleukin-6 Signaling in Clinic. Immunity. 2019;50(4):1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 211.van Rhee Frits, Voorhees Peter, Dispenzieri Angela, Fosså Alexander, Srkalovic Gordan, Ide Makoto,, et al. International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease. Blood. 2018;132(20):2115–2124. doi: 10.1182/blood-2018-07-862334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chelsea K., David B., Teachey D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15(8):813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nasonov E., Samsonov M. The role of Interleukin 6 inhibitors in therapy of severe COVID-19. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Caballero Bermejo Antonio F., Belén R.-A., Ana F.C., Elena D.S., Alejandro C.D., Elena M.R., et al. Sarilumab versus standard of care for the early treatment of COVID-19 pneumonia in hospitalized patients: SARTRE: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):794 doi: 10.1186/s13063-020-04633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rosario G.-V., Francisco A.-S., Isidoro G.-A., Francisco R.-L., Sanz S.J. Subcutaneous Sarilumab in hospitalised patients with moderate-severe COVID-19 infection compared to the standard of care (SARCOVID): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):772 doi: 10.1186/s13063-020-04588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Vincenzo M., Roberto P., Chiara I., Antonella B., Elio M., Fiorentino F., et al. Outcomes and biomarker analyses among patients with COVID-19 treated with interleukin 6 (IL-6) receptor antagonist sarilumab at a single institution in Italy. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Investigators R.-E.-M.-A.-P.-C.-A.-P., Gordon Anthony C., Mouncey Paul R., Farah A.-B., Rowan Kathryn M., Nichol Alistair D., et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384(16):1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gritti G., Raimondi F., Ripamonti D., Riva I., Landi F., Alborghetti L. IL-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. MedRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.04.01.20048561. [DOI] [Google Scholar]
- 219.Stark George R., HyeonJoo C., Yuxin W. Responses to Cytokines and Interferons that Depend upon JAKs and STATs. 2018;10 doi: 10.1101/cshperspect.a028555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kalil Andre C., Patterson Thomas F., Mehta Aneesh K., Tomashek Kay M., Wolfe Cameron R., Varduhi G., et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Peter R., Ivan G., Catherine T., Dan S., Olly O., Anne P., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet (London, England) 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rupali D., Peng G., Leslee S., Katherine V., Paige T., Angel A.Q., et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood. 2016;127(13):1666–1675. doi: 10.1182/blood-2015-12-684399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Danielle P., William D., Brett K. The use of Janus kinase inhibitors in the time of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) J Am Acad Dermatol. 2020;82(6):e223–e226. doi: 10.1016/j.jaad.2020.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Deftereos Spyridon G., Georgios G., Vrachatis Dimitrios A., Siasos Gerasimos D., Giotaki Sotiria G., Panagiotis G., et al. Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw Open. 2020;3(6):e2013136. doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lazarus Hillard M, Ragsdale Carolyn E, Gale Robert Peter, Lyman Gary H. Sargramostim (rhu GM-CSF) as Cancer Therapy (Systematic Review) and An Immunomodulator. A Drug Before Its Time? Front Immunol 2021;12:706186. Doi: 10.3389/fimmu.2021.706186. [DOI] [PMC free article] [PubMed]
- 226.Presneill Jeffrey J., Trudi H., Stewart Alastair G., Cade John F., Wilson J.W. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med. 2002;166(2):138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 227.Robert P., Standiford Theodore J., Dechert Ronald E., Marc M., Martin Gregory S., Rosenberg Andrew L., et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med. 2012;40(1):90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Orozco Héctor, Arch Jorge, Medina-Franco Heriberto, Pantoja Juan P, González Quintín H, Vilatoba Mario, et al. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis: a randomized, double-blind, placebo-controlled clinical trial. Arch Surg 2006;141(2):150–3; discussion 154. Doi: 10.1001/archsurg.141.2.150. [DOI] [PubMed]
- 229.Giulio F.E., Roberto C. GM-CSF in the treatment of COVID-19: a new conductor in the pathogenesis of cytokine storm? Lancet. Rheumatol. 2020;2(8):e448–e449. doi: 10.1016/S2665-9913(20)30185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Burmester Gerd R., Weinblatt Michael E., McInnes Iain B., Duncan P., Olga B., Mykola V., et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann Rheum Dis. 2013;72(9):1445–1452. doi: 10.1136/annrheumdis-2012-202450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chiara C., Gabriella R.M., Andrea B., Martina B., Giulio F.E. Spotlight on mavrilimumab for the treatment of rheumatoid arthritis: evidence to date. Drug Des Devel Ther. 2017;11:211–223. doi: 10.2147/DDDT.S104233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Giacomo D.L., Giulio C., Corrado C., Emanuel D.-T., Piera A., Alessandro T., et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet. Rheumatol. 2020;2(8):e465–e473. doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Corrado C., Emanuel D.-T., Giulio C., Giacomo D.L., Marco R., Nicola B., et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Marta C., Laura B., Pietro V., Paolo S., Valentina Z., Fabio B., et al. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8(5) doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Constantinescu Cris S., Aliya A., Waldemar F., Wojciech K., Frank W., Jehan A., et al. Randomized phase 1b trial of MOR103, a human antibody to GM-CSF, in multiple sclerosis. Neurol Neuroimmunol Neuroinflammation. 2015;2(4):e117. doi: 10.1212/NXI.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Frank B., Tak Paul P., Mikkel Ø., Rumen S., Piotr W., Huizinga Thomas W., et al. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann Rheum Dis. 2015;74(6):1058–1064. doi: 10.1136/annrheumdis-2013-204816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zelalem T., Burger Charles D., Jason B., Christopher P., Libertin Claudia R., Kelley Colleen F., et al. Lenzilumab in hospitalised patients with COVID-19 pneumonia (LIVE-AIR): a phase 3, randomised, placebo-controlled trial. Lancet. Respir Med. 2022;10(3):237–246. doi: 10.1016/S2213-2600(21)00494-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jatin P., Damon B., Albertus B., Xavier B.R., Hatem B., Anthony C., et al. A randomised trial of anti-GM-CSF otilimab in severe COVID-19 pneumonia (OSCAR) Eur Respir J. 2023;61(2) doi: 10.1183/13993003.01870-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.FDA. FDA Approves Emergency IND Use of Humanigen’s Lenzilumab for Compassionate Use in COVID-19 Patients. Available at https://www.humanigen.com/press/FDA-Approves-Emergency-IND-Use-of-Humanigen’s-Lenzilumab-for-Compassionate-Use-in-COVID-19-Patients (accessed April 11, 2020) 2020.
- 240.Guojun S. The developmental basis of mesenchymal stem/stromal cells (MSCs) BMC Dev Biol. 2015;15:44. doi: 10.1186/s12861-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C., et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 242.Madhan J., Rajni R., Rakesh K., Arunabh A., Dushyant C., Shringeri A.S., et al. Cellular Therapy: Shafts of Light Emerging for COVID-19. Stem Cell Investig. 2020;7:11. doi: 10.21037/sci-2020-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jayavardini V., Narasimman G., Sheeja R., Vinoth S., Shivaani K., Thomas Edwin L., et al. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells. 2020;10(1) doi: 10.3390/cells10010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sébastien L.B., Cédric T., Laetitia B., Jean-Jacques L., Juliette P. Effect of Mesenchymal Stromal Cells on T Cells in a Septic Context: Immunosuppression or Immunostimulation? Stem Cells Dev. 2017;26(20):1477–1489. doi: 10.1089/scd.2016.0184. [DOI] [PubMed] [Google Scholar]
- 245.Wang L.u., Li Yun X.u., Moyan D.Z., Yan Z., Mengmeng Y., et al. Regulation of Inflammatory Cytokine Storms by Mesenchymal Stem Cells. Front Immunol. 2021:12(July). doi: 10.3389/fimmu.2021.726909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Elena K., Mosca Joseph D., Valentina Z., Majumdar Manas K., Beggs Kirstin J., Simonetti Donald W., et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12(1):47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 247.Keung C.W., Sik-Yin L.A., Chun-Bong L.J., Ka-Wai L.H., Lung L.u.Y., Chi-Fung C.G. MHC expression kinetics and immunogenicity of mesenchymal stromal cells after short-term IFN-gamma challenge. Exp Hematol. 2008;36(11):1545–1555. doi: 10.1016/j.exphem.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 248.Yue-Qi S., Yuelin Z., Xin L.i., Meng-Xia D., Wen-Xiang G., Yin Y., et al. Insensitivity of Human iPS Cells-Derived Mesenchymal Stem Cells to Interferon-γ-induced HLA Expression Potentiates Repair Efficiency of Hind Limb Ischemia in Immune Humanized NOD Scid Gamma Mice. Stem Cells. 2015;33(12):3452–3467. doi: 10.1002/stem.2094. [DOI] [PubMed] [Google Scholar]
- 249.Vikram S., Sascha S., Angel L., Peter W., Anna N., Nicholas B. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ying-Gang Z.u., Xiao-Mei F., Jason A., Xiao-Hui F., Qi H., Antoine M., et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32(1):116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bobati Shreedevi S., Ravishankar N.K. Therapeutic Plasma Exchange - An Emerging Treatment Modality in Patients with Neurologic and Non-Neurologic Diseases. J Clin Diagn Res. 2017;11(8):EC35–7 doi: 10.7860/JCDR/2017/27073.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gucyetmez Bulent, Atalan Hakan Korkut, Sertdemir Ibrahim, Cakir Ulkem, Telci Lutfi, COVID-19 Study Group Therapeutic plasma exchange in patients with COVID-19 pneumonia in intensive care unit: a retrospective study. Crit Care. 2020;24(1):492 doi: 10.1186/s13054-020-03215-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Larry G.W., Callahan Sean P., Brevetta Robert A., Stenbit Antine E., Smith Wesley M., Martin Julie C., et al. Efficacy of therapeutic plasma exchange in the treatment of penn class 3 and 4 cytokine release syndrome complicating COVID-19. Respir Med. 2020;175 doi: 10.1016/j.rmed.2020.106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mehmood K.S., Zill-E-Humayun M., Arshad N., Jahanzeb L., Imran F., Wasim A., et al. Therapeutic plasma exchange for coronavirus disease-2019 triggered cytokine release syndrome; a retrospective propensity matched control study. PLoS One. 2021;16(1):e0244853. doi: 10.1371/journal.pone.0244853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang Yimin Y.u., Liang T.L., Mengfei Z.u., Yanqi J., Zhouhan W., et al. A Promising Anti-Cytokine-Storm Targeted Therapy for COVID-19: The Artificial-Liver Blood-Purification System. Eng (Beijing, China) 2021;7(1):11–13. doi: 10.1016/j.eng.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wang Qiang H.u., Zhao. Successful recovery of severe COVID-19 with cytokine storm treating with extracorporeal blood purification. Int J Infect Dis. 2020;96:618–620. doi: 10.1016/j.ijid.2020.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kaijin X.u., Hongliu C., Yihong S., Qin N.i., Chen Y.u., Shaohua H.u., et al. Management of COVID-19: the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jie M.a., Peng X., Yangzhong Z., Zhengyin L., Xiang Z., Jinglan W., et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol. 2020;214 doi: 10.1016/j.clim.2020.108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Xiang-Hong Y., Ren-Hua S., Ming-Yan Z., Er-Zhen C., Jiao L., Hong-Liang W., et al. Expert recommendations on blood purification treatment protocol for patients with severe COVID-19. Chronic Dis Transl Med. 2020;6(2):106–114. doi: 10.1016/j.cdtm.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Raman K., Himani R., Afrozul H., Wimalawansa Sunil J., Alpana S. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. 2021;292 doi: 10.1016/j.virusres.2020.198235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.de Ligt Marlies, Hesselink Matthijs K C, Jorgensen Johanna, Hoebers Nicole, Blaak Ellen E, Goossens Gijs H. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte. 2021;10(1):408–411. doi: 10.1080/21623945.2021.1965315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Danilo I.A., Michele I.A., Roxana B.I., Giuseppina M., Edit X., Antonio S., et al. SARS-CoV-2 Disease Adjuvant Therapies and Supplements Breakthrough for the Infection Prevention. Microorganisms. 2021;9(3) doi: 10.3390/microorganisms9030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ajmal S.M., Azhar R., Rimsha Y., Muhammad H., Ishmal F.H., Ayesha H., et al. Combination of natural antivirals and potent immune invigorators: A natural remedy to combat COVID-19. Phytother Res. 2021;35(12):6530–6551. doi: 10.1002/ptr.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Fang L., Yuan Z.u., Jing Z., Yiming L.i., Zhiyong P. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10(7):e039519. doi: 10.1136/bmjopen-2020-039519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ameet D., Reema K., Zafer K., Danish M., Aparna K., Prashant P., et al. Combination therapy of Tocilizumab and steroid for management of COVID-19 associated cytokine release syndrome: A single center experience from Pune, Western India. Medicine (Baltimore) 2021;100(29):e26705. doi: 10.1097/MD.0000000000026705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lisa K., Itzhak R. Tocilizumab - a novel therapy for non-organ-specific autoimmune diseases. Best Pract Res Clin Rheumatol. 2012;26(1):157–165. doi: 10.1016/j.berh.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 267.Mirko S., Silvia P., Enrico C., Paolo A., Donata R., Marco M., et al. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79(10):1286–1289. doi: 10.1136/annrheumdis-2020-217712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Silvia P., Andrea P., Paola T., Roberto F., Franco F., Laura A., et al. Why not to use colchicine in COVID-19? An oldanti-inflammatory drug for a novel auto-inflammatory disease. Rheumatology (Oxford) 2020;59(7):1769–1770. doi: 10.1093/rheumatology/keaa217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Bram R., Oczkowski Simon J., Siemieniuk Reed A.C., Thomas A., Emilie B.-C., Frédérick D., et al. Corticosteroids in Sepsis: An Updated Systematic Review and Meta-Analysis. Crit Care Med. 2018;46(9):1411–1420. doi: 10.1097/CCM.0000000000003262. [DOI] [PubMed] [Google Scholar]
- 270.Ramamoorthy Sivapriya, Cidlowski John A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum Dis Clin North Am 2016;42(1):15–31, vii. Doi: 10.1016/j.rdc.2015.08.002. [DOI] [PMC free article] [PubMed]
- 271.Oren L., Rui K., Siren Erika M.J., Deepak B., Yuka M., Nabeel N., et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. 2020;6(30):eaba6884 doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gordon Calvin J., Tchesnokov Egor P., Emma W., Perry Jason K., Feng Joy Y., Porter Danielle P., et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295(20):6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.