Abstract
Background
Effective regulation of complement activation may be crucial to preserving complement function during acute respiratory distress syndrome (ARDS). Factor H is the primary negative regulator of the alternative pathway of complement. We hypothesised that preserved factor H levels are associated with decreased complement activation and reduced mortality during ARDS.
Methods
Total alternative pathway function was measured by serum haemolytic assay (AH50) using available samples from the ARDSnet Lisofylline and Respiratory Management of Acute Lung Injury (LARMA) trial (n=218). Factor B and factor H levels were quantified using ELISA using samples from the ARDSnet LARMA and Statins for Acutely Injured Lungs from Sepsis (SAILS) (n=224) trials. Meta-analyses included previously quantified AH50, factor B and factor H values from an observational registry (Acute Lung Injury Registry and Biospecimen Repository (ALIR)). Complement C3, and complement activation products C3a and Ba plasma levels were measured in SAILS.
Results
AH50 greater than the median was associated with reduced mortality in meta-analysis of LARMA and ALIR (hazard ratio (HR) 0.66, 95% CI 0.45–0.96). In contrast, patients in the lowest AH50 quartile demonstrated relative deficiency of both factor B and factor H. Relative deficiency of factor B (HR 1.99, 95% CI 1.44–2.75) or factor H (HR 1.52, 95% CI 1.09–2.11) was associated with increased mortality in meta-analysis of LARMA, SAILS and ALIR. Relative factor H deficiency was associated with increased factor consumption, as evidenced by lower factor B and C3 levels and Ba:B and C3a:C3 ratios. Higher factor H levels associated with lower inflammatory markers.
Conclusions
Relative factor H deficiency, higher Ba:B and C3a:C3 ratios and lower factor B and C3 levels suggest a subset of ARDS with complement factor exhaustion, impaired alternative pathway function, and increased mortality, that may be amenable to therapeutic targeting.
Short abstract
Factor H, a key regulator of the alternative complement pathway (AP), associates with restrained AP activation and preserved AP factor levels and function in ARDS with improved survival. Alternative pathway regulation may be a therapeutic target in ARDS. https://bit.ly/3Mxs64F
Introduction
The complement system is a major component of the blood proteome [1], with key roles in host immunity including recognition and clearance of pathogens [2, 3]. Complement functions through intricate proteolytic cascades that require tight regulation to prevent exhaustion of complement factors and excessive inflammation [4]. In contrast to the target-based classical and lectin pathways of activation [3], the alternative complement pathway is unique, because it is constitutively active and it can amplify all complement activity, including its own, through a complement component 3 (C3) feedback loop [5]. Previously, we demonstrated in a heterogeneous single-centre prospective observational cohort that preserved alternative complement function is associated with improved survival during acute hypoxaemic respiratory failure [6]. However, two key knowledge gaps remain. First, it is unclear whether the association between preserved alternative pathway function and improved survival is generalisable to patients with the acute respiratory distress syndrome (ARDS). Second, the role of alternative pathway regulation in determining alternative pathway function during ARDS remains poorly characterised. Because ARDS is defined by life-threatening biological stress that may cause widespread complement activation, effective complement regulation may be crucial to prevent exhaustion of complement factors and thereby preserve complement function. Factor H is the primary negative regulator of the alternative pathway. Genetic deficiency of factor H protein or function is marked by exhaustion of complement factors including factor B and C3 and increased risk of infection and organ impairment [7, 8]. Therefore, we hypothesised that lower blood levels of factor H are associated with impaired alternative pathway function, lower levels of key alternative complement pathway proteins factor B and C3 and worse clinical outcomes in ARDS patients.
Methods
Patient biospecimens
Biospecimens were obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) [9]. Serum collected at the time of enrolment into the Lisofylline and Respiratory Management of Acute Lung Injury (LARMA) multicentre randomised clinical trial was provided by BioLINCC [10]. EDTA plasma collected at the time of enrolment into the Statins for Acutely Injured Lungs from Sepsis (SAILS) multicentre randomised clinical trial was provided by BioLINCC [11]. Notably, serum biospecimens were not available from SAILS. Previously quantified data using serum collected at the time of enrolment into the prospective observational University of Pittsburgh Acute Lung Injury Registry and Biospecimen Repository (ALIR) study were utilised in meta-analyses [6]. Only patients classified as ARDS (n=107) in ALIR were included in analyses. Factor H and factor B values were only available for a subset of patients with ARDS in ALIR (n=69). Inclusion and exclusion criteria have been described previously for the LARMA [10], SAILS [11] and ALIR [6] patient cohorts and are summarised in supplementary table S1. Only those patients for whom biospecimens were available are included herein. Informed patient consent was obtained by the relevant study teams prior to transfer of de-identified biospecimens and/or data.
Clinical characteristics
Patient clinical characteristics associated with de-identified biospecimens were provided by BioLINCC for the LARMA and SAILS cohorts and by study investigators for the ALIR cohort. Modified sequential organ failure assessment (mSOFA) scores were calculated in LARMA and SAILS using the unimputed score for the six components. Vasopressor dosage was not available in LARMA; therefore, a modified haemodynamics score was utilised for both SAILS and LARMA cohorts as follows: 0=mean arterial pressure (MAP) >70 mmHg, 1=MAP <70 mmHg not on vasopressors or MAP >70 mmHg on vasopressors, and 2=MAP <70 mmHg on vasopressors. The mSOFA in ALIR does not include the neurological component, providing a maximum score of 20 [6]. Ventilator-free days (VFD) and organ-support-free days (OSFD) were calculated to day 28.
Alternative complement pathway function
Alternative complement pathway function was quantified in serum from LARMA using a previously described microscaled haemolytic AH50 protocol [6]. Pooled serum from healthy adults (Complement Tech) was used as a reference control. Briefly, serially diluted subject serum was incubated in a 96-well plate with rabbit erythrocytes in the presence of gelatin-veronal buffer (GVB) with 5 mM magnesium chloride and EGTA. Samples were incubated for 60 min at 37°C with resuspension every 10 min. After incubation, plates were transferred immediately to ice, and ice-cold GVB-EDTA was added. The samples were centrifuged at 800×g for 3 min and the optical density (412 nm) of the supernatant was measured. A simple linear equation of the subject OD412 compared to a 100% lysis control was used to determine the AH50.
Factor B and Ba split-product
Factor B was quantified in plasma and serum using a sandwich ELISA kit (Abcam; ab137973). The Ba split-product was quantified in plasma by ELISA (Quidel; #A033).
Factor H
Factor H was quantified in plasma from the SAILS cohort using a sandwich ELISA kit (Abcam; ab137975). Factor H was quantified in serum from the LARMA cohort using a custom sandwich ELISA similar to prior studies [6]. A 96-well plate was coated with 1 µg·mL−1 anti-human factor H monoclonal antibody (Biolegend; #518402) in PBS overnight at 4°C then blocked with PBS with 3% bovine serum albumin for 2 h at 37°C. Varying dilutions of the patient samples or of purified factor H (0–3 ng·mL−1; Complement Tech; #A137) were added and the plate was then incubated for 1 h at 37°C. Anti-factor H polyclonal antibody (#5793) was used as a detection antibody with incubation at 37°C for 1 h followed by horseradish peroxidase-conjugated IgG. Finally, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) substrate and enhancer were added and the absorbance of each well at 405 nm was measured. The amount of factor H in the samples was calculated using a four-parameter model applied to the standard curve.
Complement C3 and C3a
Quantification of complement C3 and C3a in EDTA plasma from the SAILS cohort was performed by Exsera BioLabs (Aurora, CO, USA). C3 levels were quantified by nephelometry and C3a levels were quantified by ELISA (Quidel; #A301) per standard operating procedure.
Host biomarkers
Previously quantified interleukin (IL)-8 [12], plasminogen activator inhibitor (PAI)-1 [13], soluble tumour necrosis factor receptor (sTNFR)-1 [14] and surfactant protein-A [15] in LARMA were obtained from BioLINCC. Select host biomarkers previously quantified in ALIR using a customised Luminex assay were utilised [6, 16–18].
Statistical analysis and rigour
All assays were performed on de-identified biospecimens by personnel blinded to patient clinical data. The associations of AH50, factor B and factor H with survival were performed with Cox regression analysis after adjustment for age, gender and mSOFA. Meta-analyses were performed using fixed-effects inverse-variance models [19]. Kruskal–Wallis test with Dunn's test for multiple comparisons was applied to compare serum levels of factor B and factor H by AH50 quartile. We applied a mean-centring method to remove batch effect on factor H levels in the LARMA cohort. Incidence rate ratios for the association between factor B, factor H and VFD or OSFD were calculated by negative binomial regression after adjustment for age, gender and mSOFA. For plasma Ba concentrations greater than the upper limit of detection (6% of samples), we used the Ba distribution in all samples (shape and scale parameters of a γ-distribution) to re-assign an imputed value higher than the upper limit of detection. Correlations between complement factors and biomarkers were assessed using Spearman rank test. All analyses were performed after adjusting for confounders, which were selected among those variables considered plausible confounders.
Results
Patient clinical characteristics
Table 1 describes key clinical characteristics of patients included in the LARMA (n=218), SAILS (n=224) and ALIR (n=107) cohorts. Notably, patients with sepsis comprised a larger portion of the SAILS cohort (n=188, 84%), for which the presence of known or suspected infection was an inclusion criterion, than the more heterogeneous LARMA (n=76, 35%) cohort. Median (interquartile range (IQR)) VFD were higher in LARMA (19 days, 0–25 days) and SAILS (20 days, 0–25 days) than ALIR (6 days, 0–14 days). However, 28- and 60-day mortality were similar between LARMA (29% and 35%, respectively), SAILS (25% and 29%, respectively) and ALIR (30% and 37%, respectively) patient cohorts.
TABLE 1.
Clinical characteristics and outcomes of the Lisofylline and Respiratory Management of Acute Lung Injury (LARMA), Statins for Acutely Injured Lungs from Sepsis (SAILS) and the University of Pittsburgh Acute Lung Injury Registry and Biospecimen Repository (ALIR) cohorts
| LARMA | SAILS | ALIR | |
| Patients | 218 | 224 | 107 |
| Age years | 49 (37–66) | 57 (43–64) | 56 (44–65) |
| Male | 135 (62) | 107 (48) | 57 (53) |
| Self-reported race | |||
| White | 168 (77) | 171 (76) | 103 (96) |
| Black | 36 (17) | 38 (17) | 4 (4) |
| Other | 14 (6) | 11 (5) | 0 |
| Unknown | 0 | 4 (2) | 0 |
| Medical history | |||
| AIDS | 13 (6.0) | 7 (3.1) | |
| Immune suppression | 26 (11.9) | 41 (18.3) | 19 (17.8) |
| Cirrhosis | 4 (1.8) | 16 (7.2) | 10 (9.3) |
| Leukaemia | 2 (0.9) | 16 (7.1) | |
| Solid tumour malignancy | 3 (1.4) | 10 (4.5) | 4 (3.7) |
| Baseline severity of illness | |||
| Sepsis | 76 (35) | 188 (84) | 93 (87) |
| mSOFA score# | 8 (6–10) | 9 (7–11) | 8 (5–9) |
| Outcomes | |||
| VFD | 19 (0–25) | 20 (0–25) | 6 (0–14) |
| 28-day mortality | 61 (29) | 57 (25) | 32 (30) |
| 60-day mortality | 69 (35) | 66 (29) | 40 (37) |
Data are presented as n, median (interquartile range) or n (%). mSOFA: modified sequential organ failure assessment score; VFD: ventilator-free days. #: mSOFA in LARMA and SAILS reflects the unimputed score for the six components comprising the SOFA score. There were no data on vasopressor dosage in the LARMA cohort; therefore, a modified haemodynamics score (0=mean arterial pressure (MAP) >70 mmHg, 1=MAP <70 mmHg on no vasopressors or MAP >70 mmHg and on vasopressors, 2=MAP <70 mmHg and on vasopressors) was used for both LARMA and SAILS cohorts. The mSOFA score in ALIR does not include the neurological component; therefore, the maximum score is 20.
Preserved alternative complement pathway function is associated with reduced mortality in LARMA and ALIR cohorts
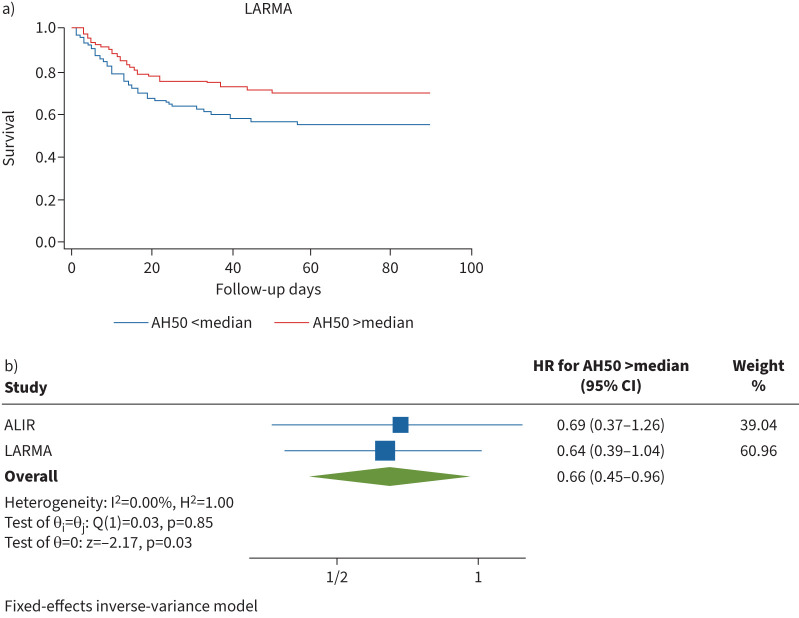
We quantified alternative complement pathway function in available LARMA serum samples (n=218) with a microscaled haemolytic AH50 assay [6]. We noted a broad distribution of AH50 values in LARMA with median 131 U·mL−1 (IQR 102–175 U·mL−1), which was comparable to AH50 values in the ALIR cohort (supplementary figure S1) despite ≥12 additional years of cryopreservation. Median AH50 in pooled reference serum from healthy adults was 187 U·mL−1 (IQR 180–197 U·mL−1). Patients with AH50 greater than the median in the LARMA cohort showed reduced hazard ratio (HR) for 90-day mortality (0.64, 95% CI 0.39–1.04) after adjustment for age, gender and mSOFA (figure 1a). Adjustment for randomisation to tidal volume strategy (i.e. 6–8 versus 10–12 mL·kg−1) was tested in LARMA, but was discarded as there was no change in results. Given the heterogeneity of the LARMA cohort, we performed a subanalysis for patients with sepsis, which strengthened the findings (HR 0.36, CI 0.14–0.89) compared to the entire cohort. Strikingly, the reduction in mortality with AH50 greater than the median in the entire LARMA cohort is nearly identical to that of patients with ARDS in ALIR (n=107; HR for 1-year mortality 0.69, 95% CI 0.37–1.26) [6]. Meta-analysis of the LARMA and ALIR cohorts demonstrated a consistent relationship between AH50 greater than the median and reduced mortality (HR 0.66, 95% CI 0.45–0.96, I2 for heterogeneity 0.00%) (figure 1b), which was replicated using a random-effects model for meta-analysis (supplementary table S2). We assessed serum levels of factor B and factor H in the LARMA cohort stratified by AH50 quartile to better understand the relationship between alternative pathway function and levels of key complement factors.
FIGURE 1.
Preserved alternative complement pathway function is associated with decreased mortality in acute respiratory distress syndrome. a) Patient survival in the Lisofylline and Respiratory Management of Acute Lung Injury (LARMA) cohort by AH50 stratified by relationship to median (hazard ratio (HR) 0.64, 95% CI 0.39–1.04). b) Meta-analysis of AH50 greater than the median and survival. Survival estimates in both cohorts are adjusted for age, sex and modified sequential organ failure assessment score and are presented as HR (95% CI). ALIR: University of Pittsburgh Acute Lung Injury Registry and Biospecimen Repository cohort.
Diminished alternative complement pathway function is associated with lower levels of factor B and factor H
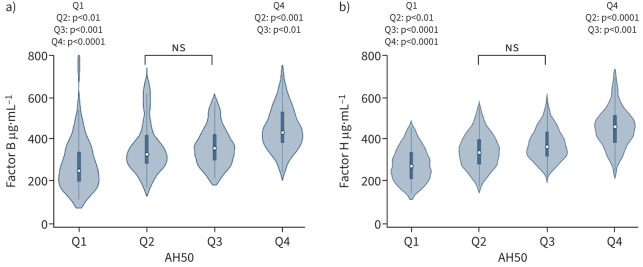
We found significantly decreased levels of both factor B (figure 2a) and Factor H (figure 2b) in the lowest AH50 quartile, which is similar to previously published results in the ALIR cohort [6]. Notably, the distributions of circulating levels of factor B and factor H were grossly similar across the LARMA, SAILS and ALIR cohorts (supplementary figure S2; factor B and factor H values were only available for 69 patients in ALIR). Functional complement assays such as AH50 are typically applied in clinical settings to assess for complement factor deficiencies [20, 21]. Therefore, we investigated whether relative deficiency of factor B or factor H is associated with increased mortality during ARDS.
FIGURE 2.
Diminished alternative pathway function is associated with relative deficiency of complement factor B and factor H. Sera from 218 patients in the Lisofylline and Respiratory Management of Acute Lung Injury (LARMA) cohort were characterised for a) factor B (Q1 median 249 µg·mL−1, interquartile range (IQR) 197–339 µg·mL−1; Q4 432 µg·mL−1, 382–531 µg·mL−1) and b) factor H (Q1 median 276 µg·mL−1, IQR 214–341 µg·mL−1; Q4 462 µg·mL−1, 384–515 µg·mL−1) stratified by quartile of AH50. p-values for interquartile post hoc comparisons are displayed in each figure panel. Statistical analysis was by Kruskal–Wallis test with Dunn's post hoc test for multiple comparisons. ns: nonsignificant.
Relative factor B deficiency is associated with increased mortality during ARDS
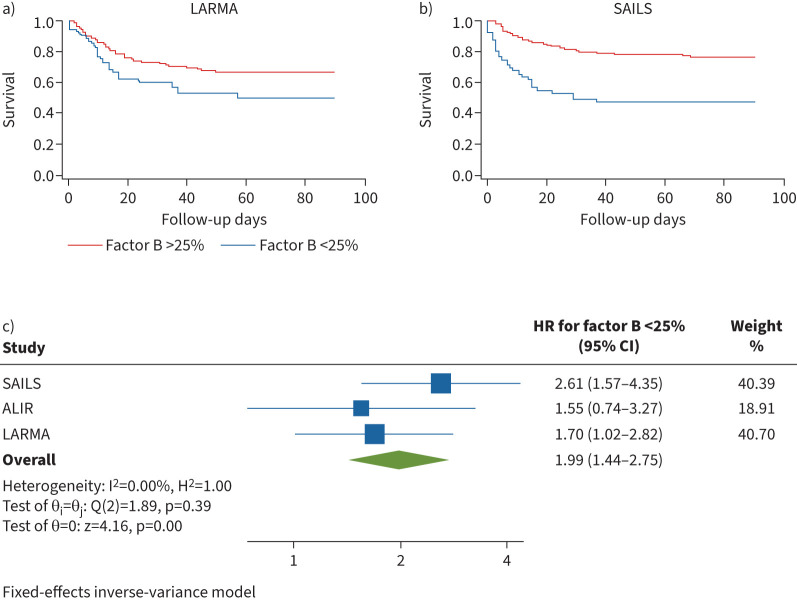
Patients with circulating factor B levels <25th percentile in LARMA were more likely to die (HR for 90-day mortality 1.70, 95% CI 1.02–2.82) after adjustment for age, sex and mSOFA (figure 3a). Similarly, in SAILS, patients with factor B <25th percentile were more likely to die (HR for 90-day mortality 2.61, 95% CI 1.57–4.35) after adjustment for age, sex and mSOFA (figure 3b). Meta-analysis of LARMA, SAILS and ALIR cohorts demonstrated a strong relationship with factor B <25th percentile and mortality (overall HR 1.99, 95% CI 1.44–2.75, I2 =0.0%) (figure 3c). Despite this relationship, we were unable to find an association between factor B <25th percentile and VFD in the LARMA (adjusted incidence rate ratio (IRR) 1.06, p=0.51) or SAILS (IRR 1.05, p=0.30) cohorts (table 2). Furthermore, we were unable to find an association between factor B <25th percentile and OSFD to day 28 (adjusted IRR 0.79, p=0.15) in the SAILS cohort (table 2). Given the strong association between relative factor B deficiency and deficits in alternative pathway function, we hypothesised that alternative pathway activation could lead to consumption of complement factors such as factor B.
FIGURE 3.
Patients in the lowest quartile of circulating factor B levels demonstrate increased mortality during acute respiratory distress syndrome. a) Patient survival at 90 days from intensive care unit (ICU) admission date by factor B grouped by relationship to 25th percentile (<25th percentile hazard ratio (HR) 1.70, 95% CI 1.02–2.82) in the Lisofylline and Respiratory Management of Acute Lung Injury (LARMA) cohort. b) Patient survival at 90 days from ICU admission date by factor B grouped by relationship to 25th percentile (<25th percentile HR 2.61, 95% CI 1.57–4.35) in the Statins for Acutely Injured Lungs from Sepsis (SAILS) cohort. c) Meta-analysis of factor B stratified by relationship to 25th percentile. Survival estimates in all cohorts are adjusted for age, sex and modified sequential organ failure assessment score and are presented as HR (95% CI). ALIR: University of Pittsburgh Acute Lung Injury Registry and Biospecimen Repository cohort.
TABLE 2.
Relative deficiency of circulating factor B or factor H levels does not associate with ventilator-free days (VFD) or organ-support-free days (OSFD)
| LARMA | SAILS | |||
|
Unadjusted
IRR (p-value) |
Adjusted
IRR (p-value) |
Unadjusted
IRR (p-value) |
Adjusted
IRR (p-value) |
|
| VFD | ||||
| Factor B <25% | 1.04 (0.59) | 1.06 (0.51) | 1.01 (0.80) | 1.05 (0.30) |
| Factor H <25% | 1.10 (0.24) | 1.10 (0.22) | 0.98 (0.68) | 1.01 (0.89) |
| OSFD | ||||
| Factor B <25% | N/A | N/A | 0.74 (0.06) | 0.79 (0.15) |
| Factor H <25% | N/A | N/A | 0.89 (0.45) | 0.93 (0.62) |
VFD and OSFD are calculated to day 28. OSFD are not available for the Lisofylline and Respiratory Management of Acute Lung Injury (LARMA) cohort. Incident rate ratios (IRRs) are displayed both before and after adjustment for age, sex and modified sequential organ failure assessment score. SAILS: Statins for Acutely Injured Lungs from Sepsis clinical trial cohort; N/A: not applicable.
Alternative complement pathway activation is higher in ARDS nonsurvivors and factor H levels inversely correlate with both alternative and total complement activation
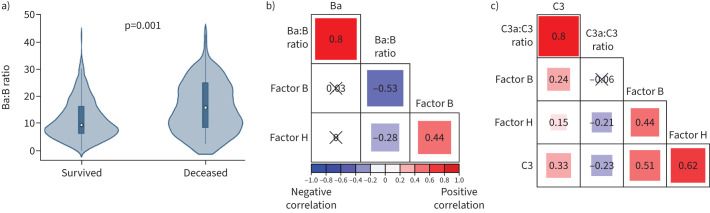
EDTA plasma quenches proteolytic complement reactions, which enables measurement of complement split-products such as Ba and C3a, which are produced by proteolysis of factor B and C3, respectively. We measured Ba, C3a and C3 in EDTA plasma from patients in the SAILS cohort (n=224). We calculated the Ba:B and C3a:C3 ratios as indices of alternative complement and total complement activation, respectively [22–25]. The Ba:B ratio was significantly higher in nonsurvivors compared to survivors (figure 4a). The C3a:C3 ratio was higher in nonsurvivors compared to survivors, but this result did not reach statistical significance. As factor H is the primary negative regulator of the alternative pathway, we hypothesised that factor H could restrain complement activation by preventing exhaustion of complement factors. Indeed, we found a weak inverse relationship between factor H levels and both the Ba:B (figure 4b; Spearman's ρ= −0.28, p=0.01) and C3a:C3 ratios (figure 4c; Spearman's ρ= −0.21, p<0.01). Moreover, restraint of alternative pathway activation by factor H would preserve key complement factors such as C3. Consistent with this notion, there was a moderate, positive association between C3 and factor H levels (figure 4c; Spearman's ρ=0.62, p<0.001). Because factor H restrains alternative pathway activation that preserves key complement factors such as factor B and C3, we hypothesised that relative factor H deficiency would be associated with increased mortality during ARDS.
FIGURE 4.
Alternative complement pathway activation is higher in acute respiratory distress syndrome nonsurvivors and factor H levels inversely correlate with both alternative and total complement activation. a) Ratio of factor Ba split-product to factor B levels, a marker of alternative complement pathway activation, is significantly higher in Statins for Acutely Injured Lungs from Sepsis (SAILS) nonsurvivors (n=57) compared to survivors (n=167). p=0.001. b) Correlogram of ratio of Ba:B, Ba, factor B and factor H in the SAILS cohort (n=224). c) Correlogram of ratio of C3a, C3, C3a:C3 ratio, factor B and factor H in the SAILS cohort (n=224). In panels b) and c), Spearman's ρ is displayed within each box and p≥0.05 is marked through with “×”. The size of the box corresponds to magnitude of correlation.
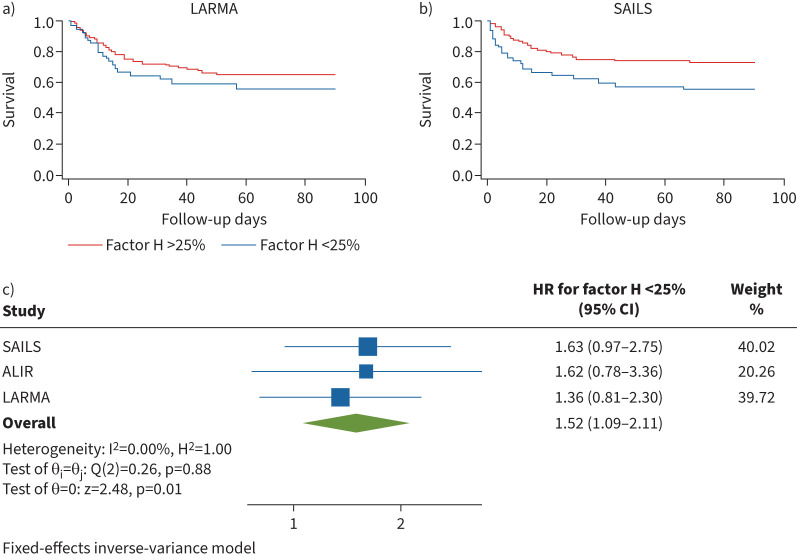
Relative factor H deficiency is associated with increased mortality during ARDS
Patients with circulating factor H levels lower than the 25th percentile showed a trend toward increased probability of death in both LARMA (HR for 90-day mortality 1.36, 95% CI 0.81–2.30) and SAILS (HR for 90-day mortality 1.62, 95% CI 0.97–2.75) (figure 5a, b). Similar to factor B, we noted a significantly increased risk of death for factor H lower than the 25th percentile (overall HR 1.52, 95% CI 1.09–2.11, I2 0.00%) by meta-analysis of LARMA, SAILS and ALIR cohorts (figure 5c). Congruent with factor B, we were unable to find an association between factor H lower than the 25th percentile and VFD in the LARMA (adjusted IRR 1.10, p=0.22) or SAILS cohorts (adjusted IRR 1.10, p=0.24), or OSFD to day 28 in SAILS (adjusted IRR 0.93, p=0.62) (table 2). Because overexuberant complement activation can lead to both exhaustion of complement factors and unrestrained inflammation, we investigated the relationship between circulating factor H levels and previously quantified biomarkers of host inflammatory response in the LARMA and ALIR cohorts.
FIGURE 5.
Patients in the lowest quartile of circulating factor H levels demonstrate increased mortality during acute respiratory distress syndrome. a) Patient survival at 90 days from intensive care unit (ICU) admission date by factor H grouped by relationship to 25th percentile (<25th percentile hazard ratio (HR) 1.36, 95% CI 0.81–2.30) in the Lisofylline and Respiratory Management of Acute Lung Injury (LARMA) cohort. b) Patient survival at 90 days from ICU admission date by factor H grouped by relationship to 25th percentile (<25th percentile HR 1.63, 95% CI 0.97–2.75) in the Statins for Acutely Injured Lungs from Sepsis (SAILS) cohort. c) Meta-analysis of factor H stratified by relationship to 25th percentile. Survival estimates in all cohorts are adjusted for age, sex and modified sequential organ failure assessment (mSOFA) score and are presented as HR (95% CI). ALIR: University of Pittsburgh Acute Lung Injury Registry and Biospecimen Repository cohort.
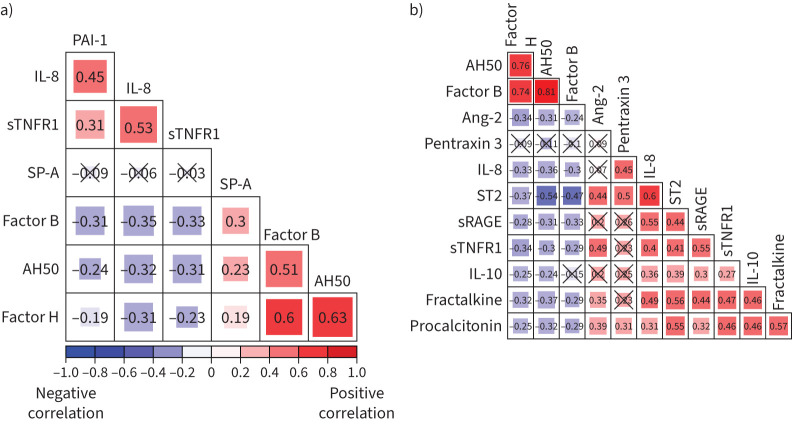
Lower circulating factor H levels are associated with higher circulating levels of inflammatory biomarkers
We noted weak, inverse correlations of factor H, as well as factor B and AH50, with circulating levels of PAI-1, IL-8 and sTNFR1 in LARMA (figure 6a). In ALIR, we found weak, inverse correlations between factor H and circulating levels of angiopoietin-2, IL-8, soluble suppressor of tumorigenicity-2, soluble receptor for advanced glycation end-products (sRAGE), sTNFR-1, IL-10, fractalkine and procalcitonin (figure 6b).
FIGURE 6.
Circulating factor H levels are associated with preserved complement function and inversely correlated with circulating markers of host inflammatory response during acute respiratory distress syndrome. a) Correlogram of plasminogen activator inhibitor (PAI)-1, interleukin (IL)-8, soluble tumour necrosis factor (sTNFR)1, surfactant protein (SP)-A, factor B, AH50 and factor H in the Lisofylline and Respiratory Management of Acute Lung Injury cohort (n=224). b) Correlogram of AH50, factor B, factor H, IL-10, IL-8, pentraxin 3, soluble suppressor of tumorigenicity (ST)2, angiopoietin (Ang)-2, procalcitonin, fractalkine, soluble receptor for advanced glycation end-products (sRAGE) and TNFR1 in the University of Pittsburgh Acute Lung Injury Registry and Biospecimen Repository cohort (n=107). Spearman's ρ is displayed in each box and p≥0.05 is marked through with “×”. The size of each box corresponds to magnitude of correlation.
Discussion
In two independent cohorts of patients with ARDS, we demonstrate that preserved circulating alternative complement pathway function is associated with reduced mortality. We note that impaired alternative pathway function is associated with lower levels of both factor B and factor H. Furthermore, relative deficiency of factor B is associated with increased mortality across three cohorts. Deficiency of factor H, the primary negative regulator of the alternative pathway, can lead to overconsumption and exhaustion of key complement factors such as factor B and C3 [7, 8]. Consistent with its regulatory role, we showed that a relative deficiency of factor H was weakly associated with both increased alternative pathway and total complement activation as measured by the Ba:B and C3a:C3 ratios. Furthermore, factor H levels were strongly associated with circulating levels of the central complement component, C3, and relative deficiency of factor H was associated with increased mortality. We also found a weak inverse relationship between factor H levels and biomarkers of host inflammatory response. Taken together, these data suggest a key role for factor H in restraint of overexuberant complement activation, which preserves C3 and factor B to sustain alternative complement function, limit inflammation and protect the host during ARDS.
Preserved alternative pathway function is protective in pre-clinical models of disseminated pneumonia [6] and is associated with improved survival across multiple human research cohorts. Because the alternative pathway is spontaneously and constitutively active and able to amplify all complement activity including its own, effective regulation of alternative pathway activity is crucial to limit exhaustion of complement factors and restrain excessive inflammation. Building upon this understanding of complement biology to highlight potential pathophysiology during ARDS, we propose that relative deficiency of factor H leads to inadequate alternative pathway regulation and exhaustion of the key complement factor C3, yielding a functional disarray or “complementopathy” of the alternative pathway [26]. Key findings in support of this model include the positive association between factor H levels and AH50, the inverse association between factor H levels and complement activation and the positive association between factor H levels and levels of C3. Conceptually, complementopathy during ARDS, which is most commonly caused by overwhelming infections such as pneumonia [27, 28], may be analogous to exhaustion of coagulation factors that can occur during the massive tissue injury of polytrauma [26, 29]. Coagulopathy during polytrauma can increase haemorrhage, whereas the potential consequences of complementopathy during ARDS may be wide-ranging, with implications for susceptibility to infection, host immune function and lung epithelial health [6, 30–33]. Therefore, we propose that adequate regulation of the alternative pathway by factor H is crucial to prevent exhaustion of complement factors such as C3 and factor B and preserve complement function during ARDS. One limitation of our study is that only baseline samples were available from the LARMA and SAILS cohorts, so the kinetics of alternative pathway function and factor H levels during ARDS remain unclear. Furthermore, we are unable to study the potential role of cell-membrane-bound regulators that may also influence complement function.
In conclusion, we demonstrate that relative deficiency of factor H is associated with increased complement activation and consumption of factor B and C3, impaired alternative complement function, probably due to complement factor exhaustion, and increased risk of mortality during ARDS. We interpret these data to suggest that factor H restraint of complement activation prevents complementopathy and preserves alternative pathway function to promote survival during ARDS. We speculate that alternative complement function may be a targetable biological pathway and we propose that a subset of ARDS patients with lower factor H levels, increased Ba:B and C3a:C3 ratios, and lower factor B and C3 levels may be a rational target for future therapeutic investigation. Further work is necessary to investigate whether factor H may be modulated for therapeutic benefit during ARDS.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00702-2022.SUPPLEMENT (173.1KB, pdf)
Acknowledgements
The authors wish to thank the patients and families that have participated in the LARMA, SAILS and ALIR research cohorts. They also thank the numerous research personnel that have conducted the LARMA, SAILS and ALIR research studies. They also thank Ashley Frazer-Abel, Julie Misayvahn and the staff of Exsera Biolabs (Aurora, CO, USA) for assistance with performing the C3 and C3a assays. They also thank Ella Meyler and Matthew Bittner (University of Pittsburgh Medical Center, Pittsburgh, PA, USA) for assistance with performing factor B and factor H ELISA assays.
Provenance: Submitted article, peer reviewed.
Author contributions: W. Bain conceived and designed the study, analysed and interpreted the data, performed the experiments, and wrote the manuscript. M. Tabary and X. An performed the experiments, analysed and interpreted the data, and revised the work for important intellectual content. S.R. Moore, G.D. Kitsios, B.J. McVerry, P. Ray, A. Ray, R.K. Mallampalli and V.P. Ferreira designed the study, interpreted the data and revised the work for important intellectual content. J.S. Lee conceived and designed the study, analysed and interpreted the data, and wrote the manuscript. S.M. Nouraie provided critical statistical expertise, conceived and designed the study, analysed and interpreted the data, and wrote the manuscript.
Conflict of interest: The authors declare no competing conflicts of interest with this manuscript.
Conflict of interest: B.J. McVerry discloses grant funding from Bayer Pharmaceuticals, and consulting fees from BioAegis, Boehringer Ingelheim and Synairgen Research, for work unrelated to this manuscript.
Conflict of interest: R.K. Mallampalli discloses stock ownership in Koutif Therapeutics, LLC, which is unrelated to this manuscript.
Conflict of interest: V.P. Ferreira discloses a pending patent (60126-US-PSP/D2018–26) as well as grant funding and consulting fees from Apellis Pharmaceuticals for work unrelated to this manuscript.
Support statement: This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award numbers R21HL148088 (J.S. Lee and S.M. Nouraie); P01HL114453 (B.J. McVerry, P. Ray, R.K. Mallampalli and J.S. Lee); R01HL112937 (V.P. Ferreira), and R01 HL136143, R01 HL142084 and K24 HL143285 (J.S. Lee); Career Development Award number IK2 BX004886 from the US Department of Veterans Affairs Biomedical Laboratory R&D Service (W. Bain); and the Competitive Medical Research Fund of the UPMC Health System (W. Bain). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Department of Veterans Affairs or any other sponsoring agency. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Tirumalai RS, Chan KC, Prieto DA, et al. Characterization of the low molecular weight human serum proteome. Mol Cell Proteomics 2003; 2: 1096–1103. doi: 10.1074/mcp.M300031-MCP200 [DOI] [PubMed] [Google Scholar]
- 2.Elvington M, Liszewski MK, Atkinson JP. Evolution of the complement system: from defense of the single cell to guardian of the intravascular space. Immunol Rev 2016; 274: 9–15. doi: 10.1111/imr.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Res 2010; 20: 34–50. doi: 10.1038/cr.2009.139 [DOI] [PubMed] [Google Scholar]
- 4.Liszewski MK, Atkinson JP. Complement regulators in human disease: lessons from modern genetics. J Intern Med 2015; 277: 294–305. doi: 10.1111/joim.12338 [DOI] [PubMed] [Google Scholar]
- 5.Blatt AZ, Pathan S, Ferreira VP. Properdin: a tightly regulated critical inflammatory modulator. Immunol Rev 2016; 274: 172–190. doi: 10.1111/imr.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bain W, Li H, van der Geest R, et al. Increased alternative complement pathway function and improved survival during critical illness. Am J Respir Crit Care Med 2020; 202: 230–240. doi: 10.1164/rccm.201910-2083OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reis ES, Falcão DA, Isaac L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand J Immunol 2006; 63: 155–168. doi: 10.1111/j.1365-3083.2006.01729.x [DOI] [PubMed] [Google Scholar]
- 8.Pickering MC, Cook HT. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin Exp Immunol 2008; 151: 210–230. doi: 10.1111/j.1365-2249.2007.03574.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giffen CA, Carroll LE, Adams JT, et al. Providing contemporary access to historical biospecimen collections: development of the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BIOLINCC). Biopreserv Biobank 2015; 13: 271–279. doi: 10.1089/bio.2014.0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The ARDS Clinical Trials Network, et al. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med 2002; 30: 1–6. doi: 10.1097/00003246-200201000-00001 [DOI] [PubMed] [Google Scholar]
- 11.Truwit JD, Bernard GR, Steingrub J, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 2014; 370: 2191–2200. doi: 10.1056/NEJMoa1401520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005; 33: 1–6. doi: 10.1097/01.CCM.0000149854.61192.DC [DOI] [PubMed] [Google Scholar]
- 13.Ware LB, Matthay MA, Parsons PE, et al. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med 2007; 35: 1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons PE, Matthay MA, Ware LB, et al. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 2005; 288: L426–L431. doi: 10.1152/ajplung.00302.2004 [DOI] [PubMed] [Google Scholar]
- 15.Eisner MD, Parsons P, Matthay MA, et al. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003; 58: 983–988. doi: 10.1136/thorax.58.11.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitsios GD, Yang L, Manatakis DV, et al. Host-response subphenotypes offer prognostic enrichment in patients with or at risk for acute respiratory distress syndrome. Crit Care Med 2019; 47: 1724–1734. doi: 10.1097/CCM.0000000000004018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drohan CM, Nouraie SM, Bain W, et al. Biomarker-based classification of patients with acute respiratory failure into inflammatory subphenotypes: a single-center exploratory study. Crit Care Explor 2021; 3: e0518. doi: 10.1097/CCE.0000000000000518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitsios GD, Yang H, Yang L, et al. Respiratory tract dysbiosis is associated with worse outcomes in mechanically ventilated patients. Am J Respir Crit Care Med 2020; 202: 1666–1677. doi: 10.1164/rccm.201912-2441OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rice K, Higgins JPT, Lumley T. A re-evaluation of fixed effect(s) meta-analysis. JR Stat Soc A 2018; 181: 205–227. doi: 10.1111/rssa.12275 [DOI] [Google Scholar]
- 20.Kirschfink M, Mollnes TE. Modern complement analysis. Clin Diagn Lab Immunol 2003; 10: 982–989. doi: 10.1128/cdli.10.6.982-989.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prohászka Z, Nilsson B, Frazer-Abel A, et al. Complement analysis 2016: clinical indications, laboratory diagnostics and quality control. Immunobiology 2016; 221: 1247–1258. doi: 10.1016/j.imbio.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 22.Zilow G, Sturm JA, Rother U, et al. Complement activation and the prognostic value of C3a in patients at risk of adult respiratory distress syndrome. Clin Exp Immunol 1990; 79: 151–157. doi: 10.1111/j.1365-2249.1990.tb05171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien ME, Fee L, Browne N, et al. Activation of complement component 3 is associated with airways disease and pulmonary emphysema in alpha-1 antitrypsin deficiency. Thorax 2020; 75: 321–330. doi: 10.1136/thoraxjnl-2019-214076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roumen RM, Redl H, Schlag G, et al. Inflammatory mediators in relation to the development of multiple organ failure in patients after severe blunt trauma. Crit Care Med 1995; 23: 474–480. doi: 10.1097/00003246-199503000-00010 [DOI] [PubMed] [Google Scholar]
- 25.Sinkovits G, Mező B, Réti M, et al. Complement overactivation and consumption predicts in-hospital mortality in SARS-CoV-2 infection. Front Immunol 2021; 12: 663187. doi: 10.3389/fimmu.2021.663187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burk A-M, Martin M, Flierl MA, et al. Early complementopathy after multiple injuries in humans. Shock 2012; 37: 348–354. doi: 10.1097/SHK.0b013e3182471795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent J-L, Akça S, De Mendonça A, et al. The epidemiology of acute respiratory failure in critically ill patients. Chest 2002; 121: 1602–1609. doi: 10.1378/chest.121.5.1602 [DOI] [PubMed] [Google Scholar]
- 28.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315: 788–800. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 29.Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med 2018; 379: 315–326. doi: 10.1056/NEJMoa1802345 [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni HS, Elvington ML, Perng Y-C, et al. Intracellular C3 protects human airway epithelial cells from stress-associated cell death. Am J Respir Cell Mol Biol 2019; 60: 144–157. doi: 10.1165/rcmb.2017-0405OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elvington M, Liszewski MK, Bertram P, et al. A C3(H2O) recycling pathway is a component of the intracellular complement system. J Clin Invest 2017; 127: 970–981. doi: 10.1172/JCI89412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liszewski MK, Kolev M, Le Friec G, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 2013; 39: 1143–1157. doi: 10.1016/j.immuni.2013.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arbore G, Kemper C, Kolev M. Intracellular complement – the complosome – in immune cell regulation. Mol Immunol 2017; 89: 2–9. doi: 10.1016/j.molimm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00702-2022.SUPPLEMENT (173.1KB, pdf)