Abstract
Objective
Advances in computer vision make it possible to combine low-cost cameras with algorithms, enabling biomechanical measures of body function and rehabilitation programs to be performed anywhere. We evaluated a computer vision system's accuracy and concurrent validity for estimating clinically relevant biomechanical measures.
Design
Cross-sectional study.
Setting
Laboratory.
Participants
Thirty-one healthy participants and 31 patients with axial spondyloarthropathy.
Intervention
A series of clinical functional tests (including the gold standard Bath Ankylosing Spondylitis Metrology Index tests). Each test was performed twice: the first performance was recorded with a camera, and a computer vision algorithm was used to estimate variables. During the second performance, a clinician measured the same variables manually.
Main measures
Joint angles and inter-limb distances. Clinician measures were compared with computer vision estimates.
Results
For all tests, clinician and computer vision estimates were correlated (r2 values: 0.360–0.768). There were no significant mean differences between methods for shoulder flexion (left: 2 ± 14° (mean ± standard deviation), t = 0.99, p < 0.33; right: 3 ± 15°, t = 1.57, p < 0.12), side flexion (left: − 0.5 ± 3.1 cm, t = −1.34, p = 0.19; right: 0.5 ± 3.4 cm, t = 1.05, p = 0.30) and lumbar flexion ( − 1.1 ± 8.2 cm, t = −1.05, p = 0.30). For all other movements, significant differences were observed, but could be corrected using a systematic offset.
Conclusion
We present a computer vision approach that estimates distances and angles from clinical movements recorded with a phone or webcam. In the future, this approach could be used to monitor functional capacity and support physical therapy management remotely.
Keywords: Artificial intelligence, physiotherapy, clinical test, telerehabilitation, remote monitoring, computer vision
Introduction
Clinical testing of body function plays an important role in both diagnosis and rehabilitation of common musculoskeletal conditions and is usually performed in dedicated facilities such as hospitals and community clinics. Some musculoskeletal conditions, such as axial spondyloarthropathy, require ongoing monitoring to guide self-management, so access to regular testing must be flexible and convenient. However, the need for appropriate facilities and trained staff means that testing is hindered by time and financial costs. 1 This was further compounded by the Covid-19 pandemic, which limited peoples’ ability to travel and reduced access to musculoskeletal services, placing further strain on healthcare systems. 2
Recent developments in the field of artificial intelligence, particularly computer vision, offer potential solutions to the difficulties of administering functional tests. For example, OpenPose 3,4 is an open-source algorithm trained to detect human anatomical landmarks in images, and has been used to track complex human movements from data collected with low-cost cameras. 5 Nowadays almost all mobile phones include a camera, and mobile phone usage and literacy are extremely high, even among older adults. 6 Numerous clinical interventions have also reported that patient acceptance of mobile phones in research is high. 7 By combining the use of low-cost phone cameras with computer vision algorithms, it is possible to perform functional tests anywhere, and to obtain quantitative results automatically. 8 Such a system could allow individuals to perform the tests in their own homes and without the need for direct supervision, reducing the associated costs of testing, and removing the need for dedicated facilities. This would be especially valuable for those living with chronic conditions such as axial spondyloarthropathy, who attend regular clinic appointments to measure posture and movement with a standardised test, the Bath Ankylosing Spondylitis Metrology Index (BASMI). 9
The purpose of this study was to demonstrate the accuracy and concurrent validity of a semi-automated computer vision system that allows distance and angle parameters to be assessed from a range of common functional tests (including the modified Bath Ankylosing Spondylitis tests) recorded with a mobile phone or webcam. Accuracy was assessed by imaging objects with known dimensions using different camera positions and settings. For concurrent validity, distance/angle parameters estimated with the computer vision system were compared to the gold standard of a trained clinician.
Methods
This study was part of a larger clinical study that aimed to examine the validity of a computer vision system to assess movement in various functional tests (ClinicalTrials.gov Identifier: NCT04895826). The reader is referred to a protocol paper for additional details. 10 Note that fewer tests are included in the present study than in the protocol paper because during pilot testing it became clear that some test variables could not reliably be estimated using computer vision (specifically: chest expansion, standing posture and the modified Schober test). The study was approved by the Oxford Brookes University institutional ethical committee (UREC Registration No: 201429) and was conducted in accordance with the Declaration of Helsinki. The test protocol included two stages presented in detail below. Firstly, the accuracy of the computer vision system was tested using different camera positions and objects with known dimensions. The second stage involved concurrent validity testing in two participant groups, whereby the results generated by our computer vision system were compared to those of a trained clinician.
Participants
A group of 31 healthy participants (11 males, age 35 ± 8, body mass index 27.57 ± 3.57; 20 females, age 36 ± 11, body mass index 25.00 ± 3.48) and a group of 31 participants with axial spondyloarthropathy (18 males, age 53 ± 12, body mass index 27.34 ± 3.24; 13 females, age 55 ± 14, body mass index 27.37 ± 7.43) were recruited via social media and the National Axial Spondyloarthritis Society between the 1st of March and the 25th of June 2021.
A minimum of 17 participants were required per group, assuming 1-beta = 0.90, alpha = 0.05 and effect size |ρ| = 0.50. Inclusion criteria for the healthy group were aged 18 or above, and healthy with no long-standing back pain. Exclusion criteria were surgery within 6 months of registration; inability to stand independently; inability to answer screening questions about participation in physical activity; serious neurological condition preventing normal movement or walking ability; and any severe medical conditions, such as cardiovascular disease.
Computer vision system accuracy
To confirm the accuracy of our computer vision approach, we first performed three tests in a controlled setting: (1) with a camera-object distance of 2 m, we imaged a series of objects with known dimensions. Each object was also imaged at three different heights (low, mid, high) to determine whether perspective distortion affected dimension estimates. (2) We imaged the same objects at camera-object distances of 2, 3 and 4 m to determine whether this variable affected computer vision estimates of object dimensions. For tests 1 and 2, prior to each test, a calibration checkerboard was held parallel to the camera and at the same distance from the camera at which the test was to be performed. Python's OpenCV package was used to automatically detect the corners of the checkerboard, and this information combined with the known distances between checkerboard squares was used to scale all distance values from pixels to cm. (3) Since one aim of our system is to enable users to perform tests without supervision, we examined the effects of misalignment of the calibration checkerboard on computer vision estimates of object dimensions. To do this, the calibration frame was first held parallel to the camera, and then rotated in increments of 5° with respect to the camera axis up to 35°. We then quantified the effect of each calibration orientation on estimates of a known distance.
Concurrent validity
Participants performed a series of functional tests, including a modified version of the BASMI (Table 1; Supplemental video 1), whilst standing or sitting at a fixed distance of 1.5 m from the camera. For all except one of the tests, no time limit was given and participants performed two repetitions per test. For the sit-to-stand test, they were instructed to perform five repetitions as fast as possible. In addition, for all tests except the sit-to-stand, participants repeated the movement a second time, during which an experienced certified physiotherapist (blinded to group to reduce risk of bias) manually measured variables of interest (defined in Table 1) so that these values could later be compared to the results obtained with computer vision. For a description of how the tasks were performed, see Hannink et al. 10
Table 1.
Description of the functional tests that were examined and the variables that were computed manually by a clinician and with our computer vision approach.
| Test | Camera position | Measured variable(s) – computer vision | Measured variable(s) – manual |
|---|---|---|---|
| Tragus to wall | Side | Minimum horizontal distance between tragus and ipsilateral shoulder | Minimum horizontal distance between tragus and wall |
| Cervical rotation | Front | Change in distance between the nose and corresponding ear from the head forward position to the point of maximal cervical rotation | Peak change in the position of the tragus relative to the sternum |
| Shoulder flexion | Side | Peak angle between the upper arm and torso (shoulder-hip axis) | Same as for computer vision |
| Side flexion | Front | Minimum vertical distance between the wrist and ipsilateral knee | Same as for computer vision |
| Lumbar flexion | Side | Minimum vertical distance between the wrist and ipsilateral ankle | Same as for computer vision |
| Hip abduction | Front | Peak angle of thigh with respect to pelvis | Same as for computer vision |
| Hip internal rotation | Front | Peak horizontal distance between the left and right ankles | Same as for computer vision |
| Sit-to-stand | Side | Number of completed repetitions | Same as for computer vision |
Analysis
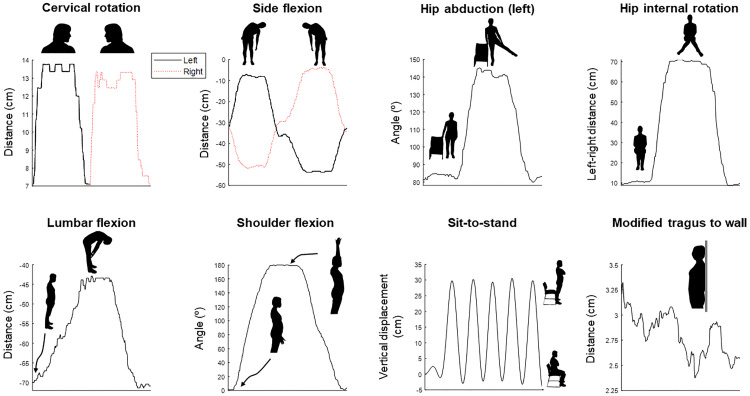
OpenPose 3,4 is a computer vision algorithm trained to detect key landmarks on the human body in camera images. Because it has been trained on many thousands of images, it is sufficiently robust to be used in almost any setting. For a given frame of image/video data, OpenPose returns predicted x,y coordinates for each body part and each human detected in the image. We used the x,y coordinates to compute joint angles and distances between two body parts for each test (Table 1) using Python software (Python Software Foundation, v3.8). Angles were computed using the atan2 function applied to the coordinates of two body segments. Distances were computed as either the absolute distance in the horizontal or vertical direction or using the distance formula. In both cases, distances were first computed in pixels and then translated into real-world values using the calibration procedure outlined above. For this purpose, a research assistant held up a calibration checkerboard in front of the camera prior to each test. Computer vision analysis and comparisons between methods were performed post-hoc and independently to minimise any risk of researcher bias. Typical examples of the output of our approach are shown for a single participant in Figure 1.
Figure 1.
Typical output traces from the computer vision system for each test for a single participant. Movement phases are identified on each panel.
Statistics
To determine computer vision system accuracy, errors between computer vision estimates and known dimensions/angles were calculated and reported as % difference. For all functional tests where scoring was continuous, manual and computer vision-derived scores were compared using Bland-Altman plots and 95% confidence intervals. 11 Data were first tested for normality with a Shapiro-Wilk test, and data that were not normally distributed were log transformed before computing Bland-Altman plots and 95% confidence intervals, as suggested by Giavarina. 12 Missing value analysis confirmed that missing data were randomly distributed and excluded from the comparisons. One-sample t-tests were used to determine whether differences between methods were significantly different to 0 (where p < 0.05 denotes statistical significance). Pearson's correlations between the two methods were also computed. The Pearson's r values were squared and effect size was interpreted as small (r2 > = 0.01), medium (r2 > = 0.09) or large (r2 > = 0.25). 13 For the sit-to-stand test, where the outcome was discrete (number of repetitions), Mann–Whitney U was used to compare methods.
Results
Computer vision system accuracy
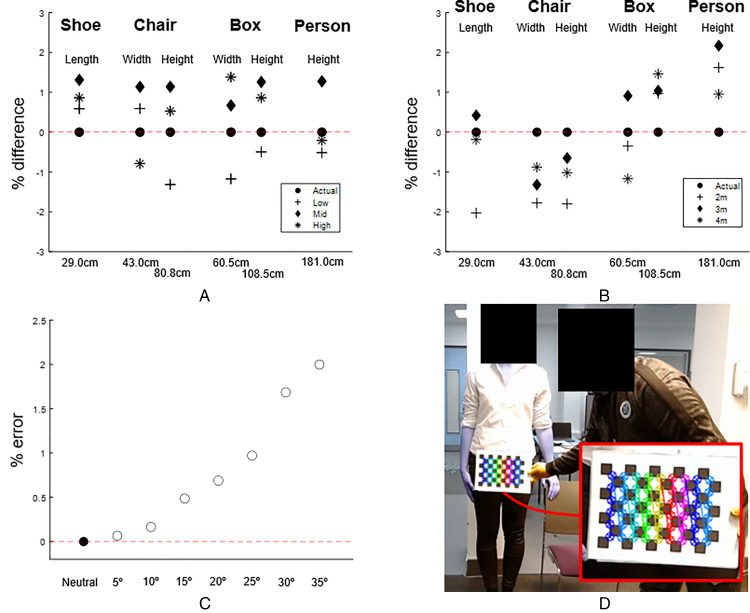
With our computer vision approach we were able to estimate the dimensions of different sized objects to within 2% of the known dimensions, including when the objects were placed at different heights relative to the camera (Figure 2A). When examining the same objects from different distances, errors were again typically 2% or less, and there was no systematic effect of camera-object distance on the measured dimensions (Figure 2B). Scaling factors obtained from the calibration checkerboard were surprisingly robust to rotation of the checkerboard with respect to the camera (Figure 2C). Even when the checkerboard was rotated by 35°, the error for the estimation of object dimensions was only around 2%. An example of the output of automatic calibration with Python's OpenCV module is shown in Figure 2D.
Figure 2.
Accuracy test results. (A) Using computer vision to determine the length/width of objects with known dimensions. Positive values denote where computer vision overestimates the correct value. The correct dimensions for each object are shown below the x-axis. Low, Mid and High are trials where the object was positioned at different heights relative to the camera (for the person this was achieved by standing on blocks). (B) The effects of computing dimensions when the camera was placed at different distances from the object. (C) Effect of rotating the calibration checkerboard in relation to the camera on measured object dimensions. (D) Screenshot of OpenCV's processing of a calibration checkerboard, showing the automatically detected corners (enlarged in the inset).
Comparison of clinician and computer vision results
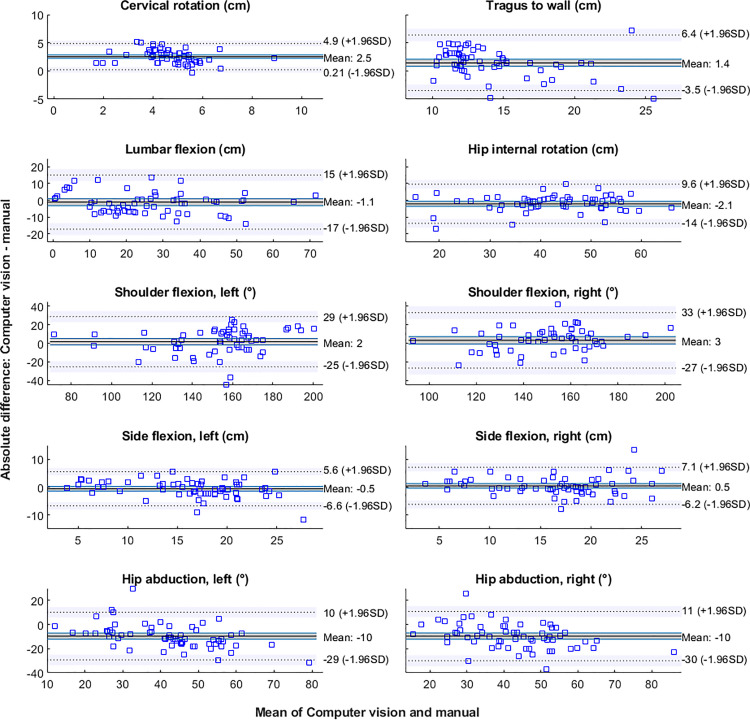
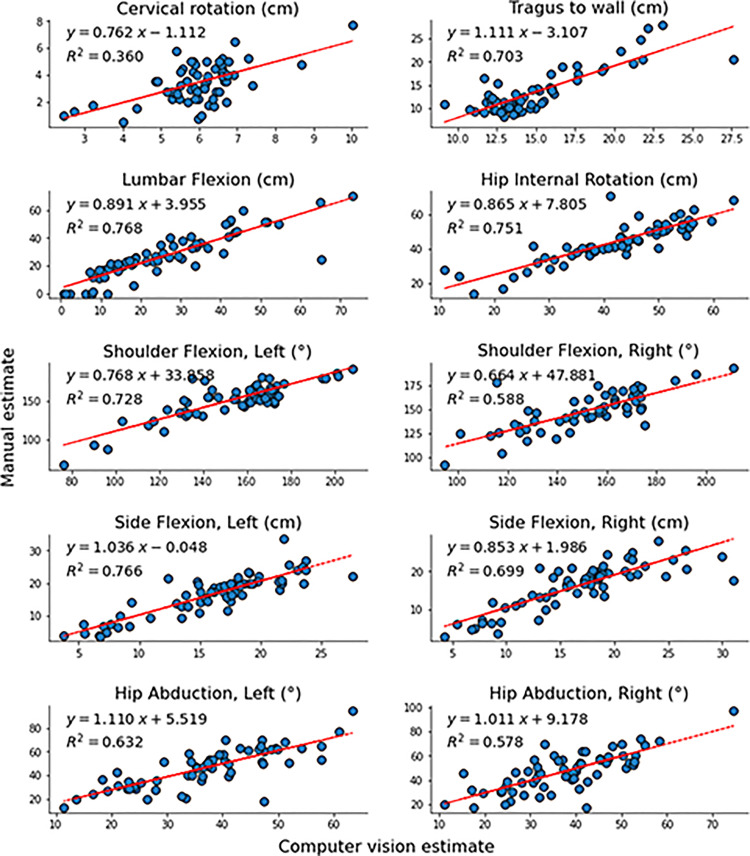
Mean differences between methods and 95% confidence intervals are shown for all tests in Table 2. Mean differences between methods were not significant for shoulder flexion (left: 2 ± 14°, t = 0.99, p < 0.33; right: 3 ± 15°, t = 1.57, p < 0.12), side flexion (left: − 0.5 ± 3.1 cm, t = −1.34, p = 0.19; right: 0.5 ± 3.4 cm, t = 1.05, p = 0.30) and lumbar flexion ( − 1.1 ± 8.2 cm, t = −1.05, p = 0.30). This is also evident from the Bland-Altman plots in Figure 3, where the confidence interval of the mean difference between measures always overlaps 0 for the above-mentioned tests. On the contrary, there were significant mean differences for tragus-to-wall (1.4 ± 2.5 cm, t = 4.52, p < 0.001), hip internal rotation ( − 2.1 ± 6 cm, t = −2.78, p = 0.007), cervical rotation (2.5 ± 1.2 cm, t = 16.81, p < 0.001), and hip abduction (left: − 10 ± 10 °, t = −7.51, p < 0.001; right: − 10 ± 10 °, t = −7.26, p < 0.001), and the confidence interval of the mean difference did not overlap 0 in the Bland-Altman plots. However, for all tests, the correlations between the estimates of the two methods represented large effect sizes (Figure 4), that is, all r2 values were >0.25. 13 For the sit-to-stand test, there was no statistical difference between methods in the number of repetitions performed (Mann–Whitney U (n = 60) = 3601, z = −0.432, p = 0.666).
Table 2.
Mean differences between methods and 95% confidence intervals for each test (except sit-to-stand where the outcome was discrete). Positive mean values denote a larger mean estimate by the computer vision system compared to clinician measures, and vice versa for negative values. One sample t-test results are also shown for each test, where p values < 0.05 denote a significant difference between the computer vision and clinician-derived means.
| Test (unit) | Mean difference | SD of difference | 95% CI | t statistic | p value |
|---|---|---|---|---|---|
| Tragus to wall (cm) | 1.4 | 2.5 | 0.6–2.3 | 4.52 | < 0.001 |
| Cervical rotation (cm) | 2.5 | 1.2 | 2.1–3.0 | 16.81 | < 0.001 |
| Shoulder flexion, left (°) | 2 | 14 | −3–7 | 0.99 | 0.33 |
| Shoulder flexion, right (°) | 3 | 15 | −2–9 | 1.57 | 0.12 |
| Side flexion, left (cm) | −0.5 | 3.1 | −1.6–0.6 | −1.34 | 0.19 |
| Side flexion, right (cm) | 0.5 | 3.4 | −0.7–1.7 | 1.05 | 0.30 |
| Lumbar flexion (cm) | −1.1 | 8.2 | −4.0–1.8 | −1.05 | 0.30 |
| Hip abduction, left (°) | −10 | 10 | −13–6 | −7.51 | < 0.001 |
| Hip abduction, right (°) | −10 | 10 | −13–6 | −7.26 | < 0.001 |
| Hip internal rotation (cm) | −2.1 | 6.0 | −4.2–0.0 | −2.78 | 0.007 |
Figure 3.
Bland-Altman plots for each test comparing manual scores to computer vision estimates. Note that the y-axis scale for each panel on the right is the same as for the corresponding left panel. Dotted horizontal lines show the upper and lower limits of agreement, and the surrounding shaded regions represent 95% confidence intervals of these limits. The shaded region around the mean represents the confidence interval of the mean difference between computer vision and manual estimates.
Figure 4.
Correlation plots for each test comparing manual scores (y-axes) to computer vision estimates (x-axes).
Discussion
We examined accuracy and concurrent validity of a computer vision system for estimating angle and distance measures from the modified BASMI and other measures of body function. We found that the system could estimate known object dimensions to within 2% error, and was robust to changes in object height, object-camera distance and large rotations (up to 35°) of the calibration checkerboard relative to the camera. When estimating angle/distance values, the computer vision system gave statistically similar estimates to the current gold standard (manual measurements by a clinician) for shoulder flexion, side flexion, lumbar flexion and sit-to-stand. For the other tests, there were significant differences between the two methods, but the measures were still well correlated (large effect size), allowing the offset to be corrected for. These results highlight the potential of low-cost computer vision approaches for administering remote functional tests, including the modified BASMI.
Several studies have used computer vision to examine human movement in one or more tasks (for review see reference 8 ). Stenum et al. 14 used OpenPose to perform gait analysis, and reported mean absolute errors in sagittal plane hip, knee and ankle angles between motion capture and OpenPose of 4.0°, 5.6° and 7.4° respectively. Similarly, when detecting specific joint locations, Nakano et al. 15 reported that of all the mean absolute errors calculated between OpenPose and 3D motion analysis, approximately 47% were <20 mm, and 80% were <30 mm. However, 10% were >40 mm, largely due to incorrect tracking by OpenPose. Kidsinski et al. 5 used OpenPose to compute joint kinematics and subsequently used this information to predict gait parameters such as walking speed with high accuracy. Schmitz et al. 16 estimated sagittal and frontal plane joint angles with a single Kinect camera and reported agreement with inclinometer measurements to within 0.5°. Our results are generally consistent with previous work, and add to the literature by examining several functional tests including those from the BASMI, as well as the inclusion of an axial spondyloarthropathy group of patients. Furthermore, we examined tests that isolate the upper body (e.g. shoulder flexion), lower body (e.g. hip abduction) and whole body movements (e.g. sit-to-stand).
Based on the mean comparisons, our computer vision estimates were correlated with those made by a clinician for all tests. However, the Bland-Altman plots allowed us to also examine individual data points, and this analysis revealed that differences between methods were sometimes large. For example, for lumbar flexion, the mean difference between estimates was 1.1 cm, but the standard deviation was 8.2 cm, and the Bland-Altman limits of agreement ranged between − 17 and 15 cm. Similarly, for shoulder flexion, mean differences were 2° (left) and 3° (right), but standard deviations were 14° and 15° respectively, leading to limits of agreement ranging between − 25 and 29° (left) and − 27 and 33° (right). For all of these movements, the relatively large limits of agreement were due to a small number of extreme data points, but their existence demonstrates the need to further develop the computer vision approach (note that we deliberately did not test for or remove outliers, since this could have exaggerated the accuracy of our method). Researchers aiming to use computer vision instead of manual assessment would need to define acceptable limits of agreement a priori, depending on the research question and precision requirements. An additional consideration when using computer vision is the tradeoff between measurement accuracy and real-world practicality. For example, with computer vision we estimated cervical rotation differently to the clinician (see definitions in Table 1) because a single camera positioned in front of the participant cannot accurately capture the angle of rotation. This likely contributed to the significant mean difference between methods (2.5 cm), which represented about 74% of the mean manually measured distance. This could be solved by mounting a camera above the participant, or performing the test lying down, but neither of these would be practical for remote testing. Tests such as those in the BASMI were not designed for remote measurement, and this may restrict the use of fully remote systems for clinical testing to simpler, more planar tests or modified versions of common tests, as used here.
In spite of the promising computer vision results, it is logical that there was not perfect overlap between computer vision and clinician estimates. Computer vision estimates may have been influenced by lens distortion, which we did not correct for, and/or errors in the tracking of the three anatomical points needed to compute an angle. Also, OpenPose does not detect anatomical landmarks such as bones, but has been trained to detect general landmarks, which were labelled by non-experts. 17 This likely causes some differences with respect to manual measures, where bony landmarks are often used as reference points. As already noted above, it was also not always possible to estimate parameters in the exact same way that the physiotherapist did (see Table 1 for a description of how each variable was estimated). Moreover, the physiotherapist results reported here were the average of two repetitions, whereas the computer vision system only returned the peak value from the two repetitions. Manual measures also include inherent variation and error, which could be caused by inexact placement of a goniometer or tape measure. 18 For joint angle measures, Reissner et al. 19 have shown that this error can be up to 7° with respect to the actual angle. It should be noted that computer vision and clinician estimates were taken from separate trials, so inter-trial variability (and clinician error) may have affected our results. Previous evidence from a repeated seated reaching task performed by healthy and low back pain participants suggests that within-session reliability is moderate, with an intraclass correlation coefficient of 0.60–0.73. 20 Similarly, lumbar displacement is highly repeatable during flexion and extension in the absence of fatigue. 21 It therefore seems likely that inter-trial variability had only minimal effects on our findings, although we were not able to quantify this. Finally, human movement is 3-dimensional but our approach uses a single camera and is 2-dimensional, so any out-of-plane rotation would not be accounted for by the computer vision estimate. We chose movements that primarily occur in a single plane to minimise this source of error, but it cannot be completely avoided.
One challenge in this study was the need to hold up the calibration checkerboard before each trial. Although this process was quite robust, we did lose some data due to reflections on the checkerboard that made it impossible to detect the checkerboard pattern automatically, or because the checkerboard was otherwise obscured. It should be noted that this stage is only needed for distance estimates, whereas joint angles can be accurately estimated without calibration information. We did not implement our approach in real-time so that it could be used on a standard central processing unit, but real-time tracking is possible with sufficient hardware, using OpenPose or other markerless methods such as DeepLabCut.22,23 It is also likely that the performance of our computer vision system could be improved by imposing stricter test conditions, for example, by ensuring that the participant stays in the correct plane to minimise the effect of rotation errors on angle estimates.
Conclusions
We demonstrate that for a range of common clinical tests of body function (including the modified BASMI) performed by populations with and without axial spondyloarthropathy, a markerless computer vision approach provides estimates of distance and angle measures that are generally statistically comparable to manual measures performed by a trained clinician. In the future, this kind of approach could be used to monitor functional capacity and to manage physical therapy remotely. Our approach is built on top of an open-source algorithm, and thus lowers potential barriers to implementing accurate movement analysis outside of a lab environment.
Clinical messages.
Computer vision estimates of angle/distance values were not statistically different to manual estimates by a clinician for shoulder flexion, side flexion, sit-to-stand and lumbar flexion.
For all other tests, there were significant differences between manual and computer vision estimates, but they were still well correlated (large effect size), allowing the offset to be corrected for.
In the future, low-cost computer vision approaches could be used to administer functional tests remotely, including the modified BASMI.
Supplemental Material
Supplemental material, sj-docx-1-cre-10.1177_02692155221150133 for Accuracy of a computer vision system for estimating biomechanical measures of body function in axial spondyloarthropathy patients and healthy subjects by Neil J Cronin, Maedeh Mansoubi, Erin Hannink, Benjamin Waller and Helen Dawes in Clinical Rehabilitation
Acknowledgements
This study was supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre and National Institute for Health and Care Research Exeter Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The research team are grateful to the National Axial Spondyloarthritis Society for their support and assistance with participant recruitment, as well as to all our participants who attended the lab despite their difficulties.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: NC developed the computer vision approach as a paid consultant of Good boost wellbeing but was not directly involved in data collection. All statistical comparisons between methods were performed independently in the Oxford Brookes lab. BW was a paid consultant, and EH was a paid employee of Good boost wellbeing during the project but were not involved in data analysis or interpretation.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from Innovate UK, The IUK project number 79429, (CRN: 11495760). National Institute for Health and Care Research Exeter Biomedical Research Centre and National Institute for Health and Care Research Exeter Clinical Research Facility support the research of Professor Helen Dawes and Dr Maedeh Mansoubi. The views expressed are those of the authors and not necessarily those of the NIHR, NHS or the UK Department of Health and Social Care.
ORCID iDs: Maedeh Mansoubi https://orcid.org/0000-0002-8829-2217
Erin Hannink https://orcid.org/0000-0002-1422-4625
Supplemental material: Supplemental material for this article is available online.
References
- 1.Kairy D, Lehoux P, Vincent Cet al. et al. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil Rehabil [Internet] 2009; 31: 427–447. [cited 2021 Oct 28] Available from:https://pubmed.ncbi.nlm.nih.gov/18720118/. [DOI] [PubMed] [Google Scholar]
- 2.Iyengar K, Bahl S, Vaishya Ret al. et al. Challenges and solutions in meeting up the urgent requirement of ventilators for COVID-19 patients. Diabetes Metab Syndr [Internet] 2020; 14: 499. [cited 2021 Oct 28] Available from: /pmc/articles/PMC7198404/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Z, Simon T, Wei S-Eet al. et al. Realtime multi-person 2D pose estimation using part affinity fields [Internet]. In: CVPR. 2017. p. 1302–1310. [cited 2020 Nov 5] Available from:https://youtu.be/pW6nZXeWlGM.
- 4.Cao Z, Hidalgo Martinez G, Simon Tet al. et al. Realtime multi-person 2D pose estimation using part affinity fields. IEEE Trans Pattern Anal Mach Intell. 2021; 43(1): 172–186. 10.1109/TPAMI.2019.2929257 [DOI] [PubMed] [Google Scholar]
- 5.Kidziński Ł, Yang B, Hicks JLet al. et al. Deep neural networks enable quantitative movement analysis using single-camera videos. Nat Commun [Internet] 2020; 11: 1–10. [cited 2021 Mar 3]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zickuhr K, Madden M. Main Report Pew Research Center [Internet]. 2012 [cited 2021 May 27]. Available from:https://www.pewresearch.org/internet/2012/06/06/main-report-15/.
- 7.Joe J, Demiris G. Older adults and mobile phones for health: a review [internet]. J Biomed Inform 2013; 46: 947–954. [cited 2021 May 27] Available from:https://pubmed.ncbi.nlm.nih.gov/23810858/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellsten T, Karlsson J, Shamsuzzaman Met al. et al. The potential of computer vision-based marker-less human motion analysis for rehabilitation. Rehabil Process Outcome [Internet] 2021; 10: 1–12. [cited 2021 Aug 20] Available from:https://journals.sagepub.com/doi/full/10.1177/11795727211022330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkinson T, Mallorie P, Whitelock Het al. et al. Defining spinal mobility in ankylosing spondylitis (AS). The bath AS metrology index. J Rheumatol [Internet] 1994; 21: 1694–1698. [cited 2021 Dec 30] Available from:https://pubmed.ncbi.nlm.nih.gov/7799351/. [PubMed] [Google Scholar]
- 10.Hannink E, Mansoubi M, Cronin Net al. et al. Computer-vision aided functional movement measurement in people with and without axial spondyloarthritis – validation and feasibility study protocol. OSF [Internet] 2021. [cited 2021 Nov 19]; Available from:https://osf.io/hsv7p/. [Google Scholar]
- 11.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England) [Internet] 1986; 1: 307–310. [cited 2016 Sep 30] Available from:http://www.ncbi.nlm.nih.gov/pubmed/2868172. [PubMed] [Google Scholar]
- 12.Giavarina D. Understanding Bland Altman analysis. Biochem Medica [Internet] 2015; 25: 141–151. [cited 2021 Oct 28] Available from:https://pubmed.ncbi.nlm.nih.gov/26110027/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen J. A power primer. Psychol Bull [Internet] 1992; 112: 155–159. [cited 2021 Dec 30] Available from:. [DOI] [PubMed] [Google Scholar]
- 14.Stenum J, Rossi C, Roemmich RT. Two-dimensional video-based analysis of human gait using pose estimation. PLOS Comput Biol [Internet] 2021; 17: e1008935. [cited 2021 Oct 28] Available from:https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1008935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakano N, Sakura T, Ueda K, et al. Evaluation of 3D markerless motion capture accuracy using OpenPose with multiple video cameras. Front Sport Act Living [Internet] 2020; 2: 50. [cited 2020 Jun 11] Available from:https://www.frontiersin.org/article/10.3389/fspor.2020.00050/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz A, Ye M, Shapiro Ret al. et al. Accuracy and repeatability of joint angles measured using a single camera markerless motion capture system. J Biomech [Internet] 2014; 47: 587–591. [cited 2021 Aug 20] Available from:https://pubmed.ncbi.nlm.nih.gov/24315287/. [DOI] [PubMed] [Google Scholar]
- 17.Cronin NJ. Using deep neural networks for kinematic analysis: challenges and opportunities. J Biomech 2021; 123: 110460. [DOI] [PubMed] [Google Scholar]
- 18.Mohsin F, McGarry A, Bowers R. The reliability of a video analysis system (PnO clinical movement data) and the universal goniometer in the measurement of hip, knee, and ankle sagittal plane motion among healthy subjects. J Prosthetics Orthot [Internet] 2018; 30: 145–151. [cited 2021 Oct 28] Available from:https://journals.lww.com/jpojournal/Fulltext/2018/07000/The_Reliability_of_a_Video_Analysis_System__PnO.8.aspx. [Google Scholar]
- 19.Reissner L, Fischer G, List Ret al. et al et al. Minimal detectable difference of the finger and wrist range of motion: comparison of goniometry and 3D motion analysis. J Orthop Surg Res 201914173. 10.1186/s13018-019-1177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wildenbeest MH, Kiers H, Tuijt Met al. et al. Reliability of measures to characterize lumbar movement patterns, in repeated seated reaching, in a mixed group of participants with and without low-back pain: a test-retest, within- and between session. J Biomech 2021; 121: 110435. [DOI] [PubMed] [Google Scholar]
- 21.Bauer CM, Rast FM, Ernst MJ, et al. The effect of muscle fatigue and low back pain on lumbar movement variability and complexity. J Electromyogr Kinesiol 2017; 33: 94–102. [DOI] [PubMed] [Google Scholar]
- 22.Kane G, Lopes G, Saunders JLet al. et al. Real-time, low-latency closed-loop feedback using markerless posture tracking. bioRxiv [Internet] 2020. [cited 2020 Nov 5]; 2020.08.04.236422. Available from: 10.1101/2020.08.04.236422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathis A, Mamidanna P, Cury KM, et al. Deeplabcut: markerless pose estimation of user-defined body parts with deep learning. Nat Neurosci [Internet] 2018; 21: 1281–1289. [cited 2018 Nov 15] Available from:http://www.nature.com/articles/s41593-018-0209-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cre-10.1177_02692155221150133 for Accuracy of a computer vision system for estimating biomechanical measures of body function in axial spondyloarthropathy patients and healthy subjects by Neil J Cronin, Maedeh Mansoubi, Erin Hannink, Benjamin Waller and Helen Dawes in Clinical Rehabilitation