Abstract
Objective:
To determine whether implementation of antimicrobial stewardship (AMS) interventions improve management of bacteriuria in hospitalized adults.
Data Sources:
EMBASE, MEDLINE, CINAHL, and Clinical Trials Registries via Cochrane CENTRAL were searched from inception through May 2021. Reference lists of included studies were searched, and Scopus was used to retrieve articles that cited included references.
Study Selection and Data Extraction:
Randomized and nonrandomized trials, controlled before-after studies, interrupted time-series studies, and repeated measures studies evaluating AMS interventions for hospitalized adult inpatients with bacteriuria were included. Risk of bias was assessed independently by 3 team members and compared. Results were summarized descriptively.
Data Synthesis:
The search yielded 5509 articles, of which 13 met inclusion criteria. Most common interventions included education (N = 8) and audit and feedback (N = 5) alone or in combination with other interventions. Where assessed, resource and antimicrobial use primarily decreased and appropriateness of antimicrobial use improved; however, impact on guideline adherence was variable. All studies were rated as having unclear or serious risk of bias. This review summarizes and assesses the quality of evidence for AMS interventions to improve the management of bacteriuria. Results provide guidance to both AMS teams and researchers aiming to develop and/or evaluate AMS interventions for management of bacteriuria.
Conclusions:
This review demonstrated benefit of AMS interventions on management of bacteriuria. However, most studies had some risk of bias, and an overall effect across studies is unclear due to heterogeneity in outcome measures.
Keywords: antimicrobial stewardship, bacteriuria, antimicrobial resistance, systematic review
Introduction and Objectives
Bacteriuria is a common occurrence in many populations. Bacteriuria is defined as the presence of bacteria in the urine in quantitative counts ≥105 colony-forming units/mL. 1 Individuals with bacteriuria may develop symptoms of urinary tract infection (UTI); however, many individuals remain asymptomatic. While treatment with antimicrobial agents is recommended for UTIs, current guidelines recommend against antimicrobial therapy for asymptomatic bacteriuria (ASB) except in specific populations, including pregnant individuals and those undergoing endoscopic urologic procedures associated with mucosal trauma.1,2 The Choosing Wisely® and Choosing Wisely Canada® campaigns also recommend against treating bacteriuria unless symptoms are present.3,4 Despite clear evidence-based recommendations for managing individuals with bacteriuria, suboptimal treatment for UTIs and inappropriate prescribing for ASB have been widely reported in the literature.5-7
Unnecessary use of antimicrobial agents is considered a major driver of antimicrobial resistance, 8 one of the greatest global public health challenges today. 9 In particular, high rates of resistance have been observed in bacteria that cause UTIs. 10 In hospitalized patients, UTIs are one of the most common reasons for prescribing antibiotics, accounting for up to 17% of antimicrobial use.11,12 Detection of bacteriuria has been shown to lead to unnecessary therapy in 30% to 50% of ASB patients.13,14 and treatment of ASB has been found to account for half of all unnecessary fluoroquinolone regimens in hospitalized patients. 15 To minimize the negative consequences of antimicrobial use, including antimicrobial resistance, antibiotics must be used judiciously. 16
As a result of growing rates of antimicrobial resistance, antimicrobial stewardship (AMS) initiatives are recommended to improve the use of antibiotics.17,18 Antimicrobial stewardship is defined as “coordinated interventions designed to improve and measure the appropriate use of [antibiotic] agents by promoting the selection of the optimal [antibiotic] drug regimen including dosing, duration of therapy, and route of administration.” 17 A Cochrane review of AMS interventions has shown improved compliance with antibiotic policy and reduced duration of antimicrobial use. 19 The review however, did not focus on AMS interventions specifically for patients with bacteriuria. We aimed to determine whether implementation of AMS interventions improves management of bacteriuria (UTIs and ASB) in hospitalized adults, as compared to usual care.
Methods
This systematic review was completed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) methods 20 (http://www.prisma-statement.org/). The protocol was registered with PROSPERO 2020 CRD42020159051. 21
Data Sources and Search Strategy
We systematically searched EMBASE (Elsevier), MEDLINE (Ovid), and Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO). ClinicalTrials.gov and World Health Organization (WHO)’s International Clinical Trials Registry Platform were both searched through the Cochrane Central Register of Controlled Trials (CENTRAL). In keeping with the Cochrane Effective Practice and Organization of Care (EPOC) recommendations, 22 eligible study types were randomized and nonrandomized trials, controlled before-after studies, interrupted time series (ITS) studies, and repeated measures studies. An EPOC MEDLINE method filter was used in the search process to limit the results to the eligible study types.22,23 Reference lists from the 13 included studies were reviewed to identify additional studies and Scopus was searched to retrieve any citing articles of these included studies. The search was developed and performed by one of our authors (MH) who is a librarian. The search was peer reviewed by a second librarian.
The searches were conducted on July 12, 2019 and updated on May 29, 2021. Search results were imported into Covidence® software and duplicates were identified and removed. Our search appendix and search data are available at https://doi.org/10.5683/SP3/D9XYSU.
Study Selection and Data Extraction
Two authors or research assistants (MH, HM, EB, HN, TR, and/or MS) independently reviewed the title/abstract of all eligible studies and completed full-text review of all included studies in Covidence software. A third author (EB or HN) resolved disagreements independently. Studies were included if they (1) described an AMS intervention delivered by any healthcare provider to adult inpatients 18 years of age and older with symptomatic UTIs and/or ASB and (2) reported the impact of the intervention. Studies that were exclusively completed in outpatient settings, long-term care homes, intensive care units, and emergency departments were excluded. Studies published in languages other than English were excluded.
Data were extracted from included studies by a member of the research team (GM, MH, and HM) using a standardized data extraction tool adapted from the EPOC guidelines. 22 All data extracted were reviewed by a second member of the research team. Study data were extracted as well as detailed information on interventions, which were compared to other interventions or usual care.
We classified intervention components according to the EPOC taxonomy. 22 Intervention components were also categorized as outlined by Davey et al 19 (education, persuasion, enablement, restriction, and environmental restructuring). Outcomes of interest as recommended by EPOC included any patient outcomes (including health behavior[s] and health status), utilization of services, quality of care, adverse effects or harms, and resource use outcomes. 22 Main outcome measures are listed in Table 1.
Table 1.
Main Outcome Measures.
| Patient | Utilization of services | Quality of care | Adverse effects or harms | Resource use |
|---|---|---|---|---|
| • Mortality | • Length of hospital stay | • Appropriateness of antimicrobial use and guideline adherence | • Medication associated adverse effects | • Cost |
| • Resolution of infection including resolution of symptoms (if applicable) | • Admission to intensive care units | • Quality of antimicrobial use (appropriate antimicrobial use, adherence to guidelines, de-escalation, switch from IV to PO) | • C. difficile infection | • Human resources, time |
| • Recurrence of infection | • Re-admission rates | • Antimicrobial resistance | • Microbiologic testing | |
| • Complications of infection (bacteremia, pyelonephritis) | • Colonization with multidrug-resistant strains | • Antimicrobial use metrics |
Risk of bias was assessed independently by 3 members of the research team (EB, HN, and GM or MH) using the Cochrane risk of bias (RoB 2) assessment tool for randomized trials 24 and the risk of bias in nonrandomized studies of interventions (ROBINS-I) tool. 25 Disagreements were resolved by consensus. We had intended to complete a meta-analysis on main outcome measures; however, there were many differences in the populations, interventions, comparisons, and methods. As a result of heterogeneity, we conducted a descriptive synthesis of the findings.
Results
Data Synthesis
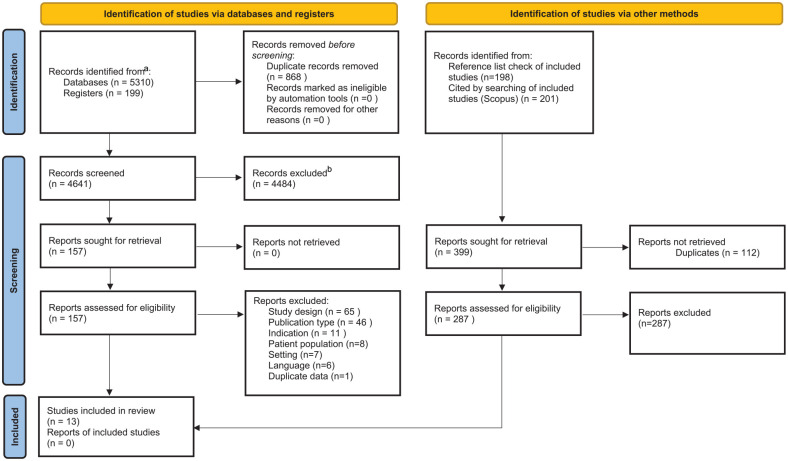
A total of 3539 studies were screened in July 2019 by titles and abstracts after duplicates were removed. In May 2020, 144 references of included studies were reviewed, and Scopus was used to identify, 143 articles that had cited the included studies after duplicates were removed. As well a total of 142 Clinical Trials were screened by titles and abstracts after duplicates were removed. The literature search including clinical trials search was updated in May 2021 and an additional 1017 studies were screened after duplicates were removed. Full-texts were reviewed for 157 studies and 13 studies met inclusion criteria (Figure 1) and were included in the analysis.
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers, and other sources.
Source. Page et al 20 . For more information, visit: http://www.prisma-statement.org/.
aConsider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers).
bIf automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
A summary of the included studies is provided in Table 2. There were 10 nonrandomised studies (NRSs),26-35 2 randomized controlled trials (RCTs),36,37 and 1 randomized trial with no control. 38 Of the NRS, 6 were controlled studies,26-30,35 3 were ITS analyses,31,32,34 and 1 was a before-and-after study with a secondary analysis of main outcomes by ITS analysis. 33 All studies were completed in high-income countries as defined by the World Bank Atlas method. 39 Seven were carried out in the United States,27,30-34,37 3 in Canada,28,29,36 1 in France, 26 1 in the Netherlands, 38 and 1 in New Zealand. 35 Most were undertaken at tertiary and/or teaching hospitals (N = 10)26-32,34,36,37 or a combination of teaching and nonteaching hospitals (N = 3).33,35,38 Two studies included hospitalized and nonhospitalized individuals.30,38
Table 2.
Comprehensive Summary of Results.
| Reference | Study design and study period | Study aim | Patients and setting | Country | AMS team | Intervention target | Intervention and comparator (who provided, how, where, when and how much) | Intervention subcategory (EPOC taxonomy) | Intervention function (education, persuasion, restriction, environmental restructuring, enablement) | Outcomes | Bias assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pavese et al 26 | Controlled before-after study Preintervention: February 2005 Postintervention: June 2005 |
To assess the effectiveness of an education session aimed to reduce inappropriate antibiotic use for patients with positive urine culture results | Adult inpatients with positive urine culture results Control group: Preintervention (N = 57), Postintervention (N = 80) Intervention group: Preintervention (N = 58), Postintervention (N = 57) One university affiliated tertiary teaching hospital |
France | Yes (hospital’s multidisciplinary antimicrobial stewardship team) |
Physicians | Educational session for physicians conducted by an infectious diseases physician (1-hour didactic session followed by group discussion, review and presentation of guidelines, and comment on 1-page report on inappropriate antibiotic use for UTIs) compared to dissemination of guideline and 1-page report on inappropriate antibiotic use for UTIs. | Educational meetings | Education (educational meetings) | Preintervention vs postintervention • Inappropriate antibiotic therapy ◼ I: 65.5% vs 29.8% ◼ C: 45.6% vs 43.8% ◼ P = .007 (for first-order interaction between study group and period) |
Serious |
| Linares et al 27 | Quasi-experimental controlled study Convenience sample of urine samples collected 2-3 days per week (duration NR) |
To determine whether a standardized memorandum added to the electronic medical record could result in a decrease in mean antimicrobial days for ASB, asymptomatic candiduria, and culture-negative pyuria | Inpatients with a urine culture or urinalysis that could trigger antimicrobial use for possible UTI (urine cultures with any growth and urinalyses with a report of pyuria, leukocyte esterase, or a nitrate) Control group (N = 30) Intervention group (N = 24) A tertiary Veterans Affairs teaching hospital |
USA | NR | Prescribers | Educational memorandum reminding physicians of evidence-based guidelines against treating ASB and culture-negative pyuria was placed in charts of patients receiving systemic antimicrobials (this was done within 48 hours of urinalysis or urine culture collection if any of the following criteria were met: no documented UTI-related symptoms, presence of < 105 CFU/mL of a single pathogen on urine culture, or no pyuria) compared to usual care. | Reminders | Enablement (circumstantial reminders) | Intervention vs control • Mean number of antimicrobial-days for ASB ◼ 2.2 +/- 3.06 vs 6.3 +/− 4.2 (absolute mean reduction = 4.1 days; relative reduction = 65%; unpaired t-test: P < .001) |
Serious |
| Leis et al 28 | Controlled before-after study January/June 2013 baseline period February/July 2013 intervention period |
To evaluate impact of modified reporting for positive urine cultures | Noncatheterized inpatients compared to catheterized patients (control group) on medical and surgical units Control group: Preintervention (N = 28) Postintervention (N = 49) Intervention group: Preintervention (N = 37) Postintervention (N = 37) One acute care teaching hospital |
Canada | NR | Clinicians | Positive urine cultures were not automatically released to the unit, instead the lab released a standard statement and recommended if clinicians strongly suspected a UTI to call the microbiology laboratory. Results were only released if any clinician providing care to the patient called the lab Culture results for catheterized patients (control) were released automatically |
The use of information and communication technology | Restriction (selective reporting of laboratory cultures) | Baseline vs post-intervention—rate of ASB treatment • I: 48% vs 12%, P = .002 • C: 42% vs 41% • Control rate of ASB treatment remained significantly above Intervention group ( P = .01) No clinical signs of UTI or sepsis in the intervention arm 72 hours after urine specimen collection |
Serious |
| Irfan et al 29 | Controlled before-after study (time series data) January 30, 2012—April 17, 2012, baseline period January 30th—April 30th, 2012, intervention period |
To identify risk factors for unnecessary treatment and to assess the impact of an educational intervention focused on these risk factors on treatment of ABU | Hospitalized patients with positive urine cultures on general internal medicine teaching units. Two academic, tertiary acute care centers Baseline N = 160 patients total with ASB Intervention period - Control group (N = 29) - Intervention group (N = 24 patients) |
Canada | If the senior author was not available, another infectious diseases physician delivered the session. | Prescribers | Initial education on ASB at Medical Grand Rounds for all residents and staff physicians in general internal medicine. As well, 15-minute educational sessions were offered as part of rounds on the clinical teaching units every 4 weeks for residents. The educational sessions included: an algorithm on management of UTI that emphasized nontreatment for ASB, and verbal feedback on baseline findings and recently encountered patients that were inappropriately managed. Comparator: control site and preintervention at the study site. |
Educational meetings, educational materials, and audit and feedback | Education (educational meetings, dissemination of educational materials), Enablement (audit and feedback) |
Intervention vs control site: • Inappropriate antibiotic use in ASB patients ◼ Baseline: intervention 8/19 (42%) vs control 10/15 (67%) (OR 0.4, 95% CI 0.1-1.5; P = .15) ◼ Postintervention: intervention 2/24 (8%) vs control 14/29 (48%) (OR 0.1, 0.02-0.5; P < .001) • Positive urine cultures ordered unnecessarily: intervention 24/93 (25.8%) vs control 29/62 (46.7%) (OR 0.4, 0.2-0.8; P = .007) Preintervention vs postintervention: • Odds of inappropriate treatment with antibiotics compared to baseline ◼ Intervention OR 0.1 (95% CI, 0.02-0.7; P < .01) ◼ Control OR 0.5 (95% CI, 0.1-1.7; P = .25) |
Serious |
| Trautner et al 30 | Controlled before-after study July 2010 to June 2013 |
To evaluate the effectiveness and sustainability of an intervention to reduce urine culture ordering and antimicrobial prescribing for catheter-associated ASB compared with standard quality improvement methods | Patients with urinary catheters on acute medicine wards and long-term care units (total 289 754 bed-days). Intervention targeted the health care professionals who order urine cultures and prescribe antimicrobials. Two tertiary Veterans Affairs teaching hospitals: the intervention site and comparison site |
USA | Health care providers who participated in the Kicking-CAUTI project, previously and included three infectious diseases physicians |
Prescribers treating patients with ASB or CAUTI (medical residents, staff physicians, nurses, physician assistants) | Email distribution of guidelines, algorithm pocket card distribution, and internal medicine grand rounds. Internal medicine team-based audit and feedback, in-service workshops with long-term care personnel, and Kicking CAUTI surveys. CAUTI working group established by a champion physician. Comparator: standard quality improvement methods (email distribution of guidelines, algorithm pocket card distribution, internal medicine grand rounds, and didactic overview of guidelines). |
Educational materials, reminders, and audit and feedback | Education (dissemination of educational materials), environmental restructuring (reminders; pocket-size summaries), Enablement (audit and feedback) |
Baseline vs intervention vs maintenance • Total number of urine cultures ordered and reported by the microbiology lab per 1000 bed-days ◼ I: 41.2 vs 23.3 (P < .001) vs 12.0 (P < .001) ◼ C: 49.3 vs 54.4 vs 46.6 (NS) • Urine cultures ordered per month over time between the two sites ◼ Decreased (P < .001) • Incidence rates of overtreatment of ASB per 1000 bed-days ◼ I: 1.6 vs 0.6 (P < .001) vs 0.4 (P < .001) ◼ C: 0.6 vs 0.6 vs 0.5 (NS) • Rate of CAUTI undertreatment ◼ Similar in all 3 periods at both sites. |
No Information |
| O’Brien et al 201531 | ITS January 1, 2006, to December 31, 2012 |
To evaluate the impact of stewardship initiated antimicrobial restriction on empirical use of ciprofloxacin on the nonsusceptibility of E. coli urinary isolates to ciprofloxacin | Hospitalized patients with positive urine cultures containing E. coli isolates. N = 3714 urine cultures during the study period A tertiary and quaternary academic medical center |
USA | Yes and an on call infectious disease physician were available. | Prescribers treating patients with positive urine cultures containing E. coli | Formulary restriction on empirical use of ciprofloxacin (if the intended use of ciprofloxacin did not meet the indications listed on the restriction the prescriber was advised to consult with the antimicrobial stewardship team or on call ID physician for approval of the agent). Comparator: no formulary restrictions. |
Local consensus process | Restriction (formulary restriction) | Preintervention vs postintervention • Ciprofloxacin use (DDD/1000 patient-days) ◼ 141.1 vs 39.8 (P = NR) Over the entire 7-year period • E. coli urinary isolates nonsusceptible to ciprofloxacin ◼ increased from 20.7% to 32.8% (P = .025) • After the introduction of ciprofloxacin restriction E. coli urinary isolates nonsusceptible to ciprofloxacin ◼ decreased from 41.5% to 32.8% (P = NR) |
Serious |
| Keller et al 32 | ITS Baseline: September 2014 to June 2015 Post-intervention: September 2015 to June 2016 |
To design a multifaceted intervention to reduce unnecessary urinalysis and urine culture orders and treatment of ASB and investigate its impact | Hospitalized adult patients 18 years of age or older (Sample size NR) Large tertiary medical center |
USA | NR | Department of medicine clinicians, institution wide health care providers | Multifaceted intervention (provider education and passive electronic clinical decision support) compared to usual care. Materials were disseminated through hospital-wide computer workstation screensavers and a 1-page e-mailed newsletter. CDS tool included simple informational messages recommending against urine testing without symptoms and against treating ASB; these messages accompanied electronic health record, orders for urinalysis, UC, and antibiotics commonly used within the institution to treat UTI. The information was displayed automatically when orders for these tests and antibiotics were selected. |
Educational materials, reminders | Education Enablement (decision support through computerized systems or through circumstantial reminders) |
Preintervention vs postintervention percentage of monthly admissions • Total urinalysis ◼ 70.5% vs 60.3% (P = .24) • Total urine cultures ◼ 18.2% to 11.8% (P < .001) • Urinalysis followed by antibiotic within 1-24 hours ◼ 4.4 to 3.9% (P = .021) • Urine culture results followed by antibiotic within 24 hours ◼ 1.7% to 1.5% (P = .036) |
No information |
| Jenkins et al 33 | Before and after study (secondary analysis of main outcomes using ITS analysis) Baseline: January 1, 2014 to December 31, 2014 Postintervention: July 1, 2015 to December 31, 2015 |
To assess effects of the collaborative on prespecified performance metrics | Adults 18 years of age or older with UTIs* admitted to hospital (N = 1530 baseline, N = 2530 postintervention) 26 teaching and nonteaching tertiary and community hospitals that were part of the Colorado Hospital Association |
USA | Team lead at each hospital was identified and asked to organize a multidisciplinary team to carry out the intervention; the team lead was either an infectious diseases (ID) physician or pharmacist, when possible. Half of teams included an ID physician or pharmacist. 11 hospitals with established antibiotic stewardship program (ASP) 15 hospitals Considering an ASP or ASP in development |
NR | Implementation of evidence-based guidelines for diagnosis and treatment of UTIs among adult inpatients. Hospitals were provided with guidance to promote uptake of guidelines using strategies feasible and appropriate at each site, for example, through education, prospective audit and feedback, or incorporation of recommendations into order sets. Colorado Hospital Association provided a number of services to support teams throughout the intervention period. This included quarterly performance reports, monthly webinars with pertinent antibiotic stewardship educational content, twice-monthly coaching newsletters, optional site visits, access to local and national antibiotic stewardship experts, and 3 in-person educational meetings. |
Clinical practice guidelines, local consensus processes (guideline implementation), educational materials, educational meetings | Education (educational meetings, dissemination of educational materials) | Baseline vs intervention periods • Significant decrease trend of fluoroquinolone use ◼ Decreased (P = .03) • Trends for proportion of cases meeting IDSA criteria for symptomatic UTI ◼ NS (P = .10) • Duration of therapy ◼ NS (P = .99) |
Serious |
| Hecker et al 34 | Quasi-experimental, ITS analysis January 1, 2008 through 2016 |
To determine the impact of stewardship interventions on UTI syndromes and fluoroquinolone use | Inpatients with a positive urine cultures (Sample size NR) Academic urban level 1 trauma center |
USA | Yes (a formal antimicrobial stewardship program was implemented) |
Prescribers treating patients with ASB or UTIs | Grand rounds and prescriber education, electronic medical record modifications (displaying previous urine cultures and links to ASB, uncomplicated UTI, and complicated UTI guidelines), and audit and feedback interventions were implemented in areas that had frequent nonadherence to guidelines. Grand rounds and small group educational sessions were provided. Educational sessions included data collected from the baseline evaluation as well as information on diagnosis and treatment of UTI, ASB, and appropriate use of urinary catheters. |
Local consensus process, reminders, educational meetings, and audit and feedback | Education (educational meetings), environmental restructuring (EHR modifications including displaying previous urine cultures and links to ASB, uncomplicated UTI, and complicated UTI guidelines) Enablement (audit and feedback) |
Over the 2-year intervention period • Inpatient fluoroquinolone use ◼ Rate ratio = .91 (adjusted P < .01) ◼ Change in slope of quarterly DDD/1000 patient days = −21.3 (adjusted P < .01) |
Serious |
| Yoon et al 35 | Controlled before-after study Baseline period: January 1, 2016—May 31, 2016 Intervention period: June 1, 2016 to August 31, 2016 |
To test the hypothesis that the introduction of the SCRIPT app would increase prescriber adherence to guidelines | Adult patients (>18 years) with UTI who had been admitted for ≥4 hours Control group: One Tertiary care teaching hospital; 1 teaching community hospital, and 1 non-teaching community hospital Preintervention (N = 422) Postintervention (N = 407) Intervention group: Tertiary care hospital Preintervention (N = 209) Postintervention (N = 211) |
New Zealand | Yes | Prescribers | Development and implementation of a mobile phone app (SCRIPT) which provided the ACH antibiotic guidelines in a user-friendly, decision-making process format compared to usual care. The existing ACH antibiotic guidelines were directly mapped into decision trees that branched out to the eventual antibiotic treatment recommendations. |
Local consensus processes (guideline implementation) | Enablement (decision support through computerized systems) | Baseline vs intervention • Guideline adherence ◼ I : 47% vs 50% (P = .49) ◼ C (Site 1): 45% vs 40% (P = .28) ◼ C (Site 2/3): 24% vs 29% (P = .25) |
Serious |
| Spoorenberg et al 38 | Randomized trial Baseline period: February to November 2009 Intervention Implementation: April 15 to October 15 2010 Post-intervention: From 6 months after the intervention was implemented |
To assess the effectiveness, measured as the before-and-after intervention performance on quality indicators, of 2 improvement strategies | Adults (≥ 16 years) who were referred to the hospital (inpatient/outpatient clinic) and diagnosed by an internist or urologist with a complicated UTI (including catheter-associated UTIs) as main diagnosis and treated as such. Baseline (N = 1964) Group 1 (N = 963) Group 2 (N = 1064) Internal Medicine and Urology departments of 19 teaching and nonteaching hospitals |
The Netherlands | No | Prescribers |
Group 1: Multi-faceted strategy (MFS) consisting of 3 phases: Phase I: feedback report (plenary meeting and MFS feedback report 1) Phase II: Standardized improvement activities initiated by the QUANTI trial team (standardized LOC meeting; kick-off meeting; improvement plan; Feedback report 2) Phase III: Optional and additional improvement activities (extra LOC meeting[s]; pocket (reminder) card; reminders; additional improvement actions) Group 2: Competitive feedback strategy (CFS): providing individual feedback to professionals by nonanonymously ranking the various departments. Competitive feedback reports contained, for each QI, a list of all 38 departments’ performance scores, in which the names of the MFS departments were blinded but the others were visible. |
Educational sessions, reminders ("Critical-care pathway"-reminder pocket cards; reminder phone calls), and audit and feedback | Education (educational meetings), environmental restructuring (reminders) Enablement (audit and feedback) |
Preintervention vs postintervention • Perform a urine culture (T1-T0) ◼ MFS: +7.4% (P = .01) ◼ CFS: +6.6% (P = .008) • Prescribe according to national guideline (T1-T0) ◼ MFS: +1.4% (P = .83) ◼ CFS: +3.2% (P = .32) • Switch from IV to oral therapy within 72 hours (T1-T0) ◼ MFS: −2.9% (P = .47) ◼ CFS: +4.5% (P = .48) • Tailor antibiotic treatment based on culture result (T1-T0) ◼ MFS: +3.8% (P = .46) ◼ CFS: +6.8% (P = .03) • Treatment duration should be at least 10 days (T1-T0) ◼ MFS: −0.1% (P = .78) ◼ CFS: −0.1% (P = .97) • Treat UTI in men according to national guideline (T1-T0) ◼ MFS: +3.1% (P = .23) ◼ CFS: +5.1% (P = .047) • Total QI set performance (mean of all patients [T1-T0]) ◼ MFS: +3.3% (P = .043) ◼ CFS: +3.9% (P = .010) |
Some concerns |
| Daley et al 36 | Randomized, parallel, superiority trial January 3, 2017 to March 27, 2017 |
To compare two different methods of reporting positive urine cultures under the hypothesis that modified reporting of positive urine cultures among inpatients would reduce treatment of ASB without increasing untreated UTI, pyelonephritis, bacteraemia, or death | Inpatients aged 18 or older, with positive urine cultures (N = 55 urine cultures in the standard arm and N = 55 urine cultures in the intervention arm). Two tertiary care academic hospitals |
Canada | NR | Prescribers | Modified report of positive urine cultures that informed the physician that significant bacterial growth was detected and unless requested, bacterial identification and susceptibility information was not provided compared to standard report (control) which included bacterial count, identification, and antibiotic susceptibility information along with drug dosages and cost. | The use of information and communication technology | Restriction (selective reporting of laboratory cultures) | Intervention vs comparator • Proportion of appropriate antibiotic prescribed ◼ 80.0% vs 52.7% (ITT analysis, P = .002) • Bacteremia rate (cases) ◼ 1 vs 2 • Untreated UTI ◼ 2/20 vs 2/14 (P = .37) • Death(s) ◼ 2 vs 1 • Cost savings (mean ± SD) ◼ $19.84 ± $64.88 vs $35.78 ± $109.77 (P = .37) |
Some concerns |
| Ridgway et al 37 | Randomized controlled trial July 1, 2015, to June 30, 2018 |
To investigate the impact of WISCA utilization during active antimicrobial stewardship surveillance | RCT with crossover design Adult patients (N = 1803 patients with UTIs enrolled in the study) diagnosed with UTI during an inpatient hospitalization at four community and tertiary teaching hospitals |
USA | Yes | Primary providers | WISCA (an electronic clinical decision support tool for inpatient antimicrobial stewardship) was utilized by the ASP physician to determine the most appropriate antibiotic regimen for patients with ABI or UTI. Intervention group: audit and feedback performed by the ASP physician to the primary provider via page or phone call and via written documentation in the electronic health record. Control group: the ASP physician recorded the recommended antibiotic in the study database but did not communicate the recommendation to the patient’s provider. |
Reminders, educational outreach through review of individual patients and recommendations for change | Education (educational outreach through review of individual patients with recommendation for change) Enablement (decision support; educational outreach by review and recommend change) |
Intervention vs comparator • Mean LOS (days [SD]) ◼ 4.50 (4.39) vs 4.54 (4.42) (P = .6899) ◼ Subgroup: multivariable linear regression model coefficient estimate for UTI = .144 (P = .4809) • 30-day mortality ◼ 5.49% vs 5.37% (P = .8730) ◼ Subgroup: multivariable linear regression model adjusted OR for UTI = 1.494 (P = .1284) |
High |
Quality of care: Appropriateness of antimicrobial use, guideline adherence. Patient outcomes: Mortality, resolution of infection (including resolution of symptoms, if applicable), complications of infection, recurrence of infection, bacteremia. Utilization of services: Length of hospital stay, admission to intensive care units, re-admission rates. Adverse effects or harms: Medication associated adverse effects, antimicrobial resistance, colonization with multidrug resistant strains, C. difficile infection. Resource use: Cost, human resources, time, microbiologic testing, antimicrobial use metrics.
Abbreviations: ASB, asymptomatic bacteriuria; ASP, Antimicrobial stewardship physician; B, baseline; C, control; I, intervention; M, maintenance; FU, follow-up; CAUTI, catheter-associated urinary tract infection; CDI, C. difficile infection; CDS, computer decision support; CFU, colony-forming units; DDD, defined daily doses; IDSA, Infectious Diseases Society of America; IRR, incidence rate ratios; ITS, interrupted time series; ITT, intention-to-treat; LOS, length of stay; QI, quality indicator; RCT, randomized controlled trial; RR, rate ratio; UTI, urinary tract infection; WISCA, weighted incidence syndromic combination antibiogram; NR, not reported.
Results of the intervention for patients with skin and soft tissue infections reported separately and not included in this review.
All of the NRS were rated as having serious risk of bias26-29,31,33-35or lacked sufficient detail to assess.30-32 Of the 3 randomized trials, 2 were considered to have some concern36,38 and 1 RCT had a high risk of bias 37 (Table 2). Common reasons for a rating of “some concern” in a bias domain included lack of blinding (both recipients and individuals delivering the interventions) or no information on prespecified analysis plan of data. Ridgeway et al 37 was assessed as having a high risk of bias due to baseline differences between intervention groups, suggestive of a problem with randomization.
Intervention types
Many studies assessed multifaceted interventions.29,30,32-34,37,38 The most common intervention described was provider education alone or in combination with other interventions (N = 8)26,27,29,30,32,34,38 followed by audit and feedback (N = 4).29,30,24,28 A fifth study by Ridgeway et al 37 also described a feedback intervention that did not meet the EPOC definition of “a summary of health workers’ performance over a specified period of time.” The intervention in this study was educational outreach consisting of review and recommended changes. Jenkins et al 30 also suggested participating study sites use audit and feedback as one strategy to promote uptake of implemented guidelines; however, the number of sites that used this strategy is unknown. Details of all identified interventions targeted at healthcare workers classified using EPOC taxonomy subcategories are outlined in Table 2.
Outcomes
Quality of care
Six studies assessed quality-of-care outcomes as defined by appropriateness of antimicrobial use26,28-30,36,38 or local guideline adherence 35 as a primary outcome. The studies used a variety of interventions; most used education either alone 26 or combined with audit and feedback.29,30,38 Six studies demonstrated a statistically significant change in target AMS practice compared to baseline or usual care after intervention delivery, including decrease in inappropriate antibiotic therapy26,28-30,36 or improvement in overall quality indicator sum score. 37 Spoorenberg et al 38 was the only study to also compare the effectiveness of 2 strategies: they compared a multifaceted strategy including feedback, education, and reminders to a less time-consuming competitive audit and feedback strategy that included individual feedback with nonanonymous ranking of departments and found no difference between the 2 arms in quality of care measures. Only 2 studies assessed the effectiveness of a modified reporting intervention on appropriateness of antimicrobial use. These studies assessed modified reporting of positive urine cultures and showed statistically significant improvement of appropriate treatment of ASB compared to control.28,35 One study that assessed implementation of an electronic phone app failed to show improvement in adherence to guidelines compared to usual care. 35
Resource use and utilization of services
Resource use was reported frequently, with 9 studies assessing the impact of AMS interventions on resource use29-34,36,38 or utilization of services33,37 as a primary or secondary outcome. Most of these studies were multifaceted and included education with audit and feedback, reminders, clinical decision support, or guideline implementation.29,30,32-34,37,38 Other studies assessed formulary restriction 31 and modified reporting of urine cultures.28,36 The impact of these interventions on resource use primarily resulted in less microbiologic testing29,30,32 and a decline in antibiotic utilization.28,33,34 Cost savings of modified culture reporting was assessed by Daley et al 36 who found no significant benefit. One study assessed the impact of electronic decision support on hospital length of stay and found no significant decrease compared to control groups. 37
Adverse effects or harms
One study reported adverse effects or harms as outcomes. 31 O’Brien et al 31 evaluated restriction of fluoroquinolones and reported a decline in antimicrobial resistance after the intervention was implemented, with the proportion of E. coli isolates nonsusceptible to ciprofloxacin decreasing from 41.5% to 32.8%, and there were no other infection control policies or procedures that may have impacted resistance patterns implemented concurrently.
Patient Outcomes
Three studies reported patient outcomes. Daley et al 36 reported a similar rate of bacteremia between intervention and control groups when modified urine culture reporting was implemented. Leis et al 28 also reported no patients with clinical signs of a UTI or sepsis with modified urine culture reporting. Bacteremia, sepsis, and clinical signs of a UTI were categorized as a patient outcome, but could equally be considered an adverse effect or harm as the negative consequence of an AMS intervention. As well. Ridgway et al 37 assessed the odds of 30-day mortality as a secondary outcome. They implemented an electronic clinical decision support tool and educational outreach through review of individual patients and recommendations for change. This intervention did not result in a statistically significant decrease in mortality.
Interpretation of the Data
This systematic review is the first to summarize the results of AMS interventions that specifically aimed to improve management of bacteriuria in adults admitted to hospital. Most of the included studies implemented interventions that demonstrated improved quality of care and/or a decrease in use of antimicrobials and microbiologic testing.
Our findings are consistent with a large Cochrane review by Davey et al 19 that evaluated AMS interventions for all infectious syndromes and found many restrictive and enabling interventions were successful in reducing unnecessary antibiotic use in hospitals. Davey et al 19 highlighted that while both enablement and restriction were independently associated with increased effectiveness of interventions, including an enabling component further enhanced the effect of restrictive interventions. Interventions that showed the greatest benefit in the Cochrane review were those that included the addition of feedback to enabling interventions. 19
A systematic review of pharmacist-led education-based AMS interventions also reported consistent improvement in antimicrobial use. Forty-five of 52 studies in their review included an educational intervention. The authors additionally reported that combined interventions, particularly with audit and feedback, were more effective than single educational interventions. 40 Consistent with these findings, 5 studies in our review reported use of some form of audit and feedback as a component of multifaceted interventions. Where reported, this intervention led to improved quality of care and decreased antimicrobial use.29,30,34,38
Strengths and Limitations
Our review has several strengths that should be considered. We completed a systematic review using a standardized approach as outlined by EPOC 22 and PRISMA. 20 This systematic review was authored by an interdisciplinary team of clinicians (pharmacists and a physician) with infectious disease expertise and researchers with experience performing systematic reviews. Our search was designed by members of the team with expertise in library services and was peer reviewed. In addition, risk of bias was assessed independently by 3 members of our research team.
Despite these strengths, several limitations should be noted. While we completed a comprehensive search, for practical reasons we only included studies published in English and relevant studies in other languages may have been missed. Only 3 randomized trials met inclusion criteria. In additional, the quality of included studies was generally low, with all NRS at serious risk of bias or receiving a rating of no information. This is, however, consistent with the quality of evidence in the general AMS literature. A systematic review by Schweitzer and colleagues from 2019 reported generally low quality of evidence for studies evaluating AMS interventions, which did not change over time. 41 Finally, differences in context, patient population, study design, interventions implemented, and outcome measures made it difficult to compare the effectiveness of interventions across studies, such that we were unable to perform a meta-analysis to identify which intervention(s) are more effective.
Generalizability is also limited as all studies were completed in high-income countries primarily in North America. Furthermore, studies were mainly completed at teaching or tertiary care hospitals and only 3 studies included community hospitals.33,35,38 Where described, most sites had a formal AMS team or pharmacists with infectious diseases and/or microbiology expertise. An established network of clinicians delivering AMS may influence acceptability or practicability of implementing interventions at other institutions.
Relevance to Patient Care and Clinical Practice
This review adds to the literature that is focused on delivery of AMS interventions for individuals with bacteriuria. When designing interventions for inpatients with bacteriuria, institutions may consider implementation of an intervention that includes audit and feedback in combination with education or other stewardship strategies. Where assessed in this systematic review, studies that incorporated audit and feedback into their intervention consistently demonstrated improved antimicrobial use. Further evidence is needed to determine the best format for audit and feedback; however, limited results from this study suggest a less time consuming competitive feedback strategy may result in similar benefit to anonymous feedback in conjunction with a multifaceted intervention. 37 Greatest benefit may also be observed for indications or on hospital units where baseline adherence to best practice is low. 30 For sites aiming to see more judicious use of microbiology testing, implementation of a multifaceted intervention as described by Keller and colleagues that includes education combined with clinical decision support may be considered. 32
When multifaceted interventions that are resource intensive may not be feasible, another strategy consistently resulting in positive outcomes that could be considered is modified culture reporting. As identified by Leis et al 28 and Daley et al, 36 this strategy may decrease treatment of asymptomatic bacteriuria with antibiotics.
To develop an AMS intervention at our institution, results of this review will be considered in conjunction with practical considerations and previous qualitative research that our team completed on local antimicrobial use and stewardship. Health care providers in Nova Scotia have reported audit and feedback in conjunction with other initiatives such as education as possible facilitators to improving antimicrobial use.42,43 Antimicrobial stewardship teams at other institutions may also consider this approach to develop a tailored intervention that meets local needs.
Conclusion
While findings from our review provide evidence to support some AMS interventions, future studies should consider study designs that limit the risk of bias such as randomized controlled trials or stepped wedge designs. 44 Pragmatic clinical trials and strong observational designs including studies that use ITS analysis, a control site, or repeated measures may also further contribute to the literature on effectiveness of implementing AMS initiatives for hospitalized adults with bacteriuria.
Supplemental Material
Supplemental material, sj-docx-1-aop-10.1177_10600280221134539 for A Systematic Review of Antimicrobial Stewardship Interventions to Improve Management of Bacteriuria in Hospitalized Adults by Mari Humphrey, Gemma MacDonald, Heather Neville, Melissa Helwig, Tasha Ramsey, Holly MacKinnon, Ingrid Sketris, Lynn Johnston and Emily K. Black in Annals of Pharmacotherapy
Acknowledgments
The authors of this review would like to acknowledge Robin Parker for their peer review of the literature search. They would also like to acknowledge Morgan Sproul for assistant with title and abstract screening.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: GM, MH, and HM received salary support for their work on this review from Research Nova Scotia. EB has received research funding from Research Nova Scotia, Dalhousie Pharmacy Endowment Fund, Dalhousie University Faculty of Health, and the Drug Evaluation Alliance of Nova Scotia. She has also received speaker/consulting fees from Dalhousie Continuing Pharmacy Education, Ontario Pharmacists Association, and Pear Healthcare Solutions and Prime Event Partners dba Prime Strategies Inc., a division of MGME.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by an Establishment grant from Research Nova Scotia.
ORCID iDs: Melissa Helwig  https://orcid.org/0000-0001-9915-3928
https://orcid.org/0000-0001-9915-3928
Emily K. Black  https://orcid.org/0000-0002-8230-9807
https://orcid.org/0000-0002-8230-9807
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):e83-e110. doi: 10.1093/cid/ciy1121. [DOI] [PubMed] [Google Scholar]
- 2.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 3.Choosing Wisely. Don’t use antimicrobials to treat bacteriuria in older adults unless specific urinary tract symptoms are present. Published February 21, 2013. Accessed July 17, 2021. https://www.choosingwisely.org/clinician-lists/american-geriatrics-society-antimicrobials-to-treat-bacteriuria-in-older-adults/.
- 4.Canadian Geriatrics Society. Five things physicians and patients should question. Choosing Wisely Canada. Date unknown. Accessed July 17,2021. https://choosingwiselycanada.org/geriatrics/.
- 5.Kiyatkin D, Bessman E, McKenzie R. Impact of antibiotic choices made in the emergency department on appropriateness of antibiotic treatment of urinary tract infections in hospitalized patients. J Hosp Med. 2016;11(3):181-184. doi: 10.1002/jhm.2508. [DOI] [PubMed] [Google Scholar]
- 6.Lee MJ, Kim M, Kim NH, et al. Why is asymptomatic bacteriuria overtreated? a tertiary care institutional survey of resident physicians. BMC Infect Dis. 2015;15:289. doi: 10.1186/s12879-015-1044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black E, Neville H, Losier M, et al. antimicrobial use at acute care hospitals in Nova Scotia: a point prevalence survey. Can J Hosp Pharm. 2018;71(4):234-242. doi: 10.4212/cjhp.v71i4.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176-187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Published 2019. Accessed October 14, 2022. https://stacks.cdc.gov/view/cdc/82532.
- 10.World Health Organization. Global antimicrobial resistance and use surveillance system (GLASS) report: 2021. Published 2021. Accessed August 22, 2021. https://www.who.int/publications-detail-redirect/9789240027336.
- 11.Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi: 10.1001/jama.2014.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control. Point prevalence survey of healthcare-associated infections and antimicrobial use in European Acute Care Hospitals 2011-2012. Published 2013. Accessed July 25, 2021. https://www.ecdc.europa.eu/en/publications-data/point-prevalence-survey-healthcare-associated-infections-and-antimicrobial-use-0.
- 13.Lin E, Bhusal Y, Horwitz D, Shelburne SA, Trautner BW. Overtreatment of enterococcal bacteriuria. Arch Intern Med. 2012;172(1):33. doi: 10.1001/archinternmed.2011.565. [DOI] [PubMed] [Google Scholar]
- 14.Leis JA, Gold WL, Daneman N, Shojania K, McGeer A. Downstream impact of urine cultures ordered without indication at two acute care teaching hospitals. Infect Control Hosp Epidemiol. 2013;34(10):1113-1114. doi: 10.1086/673151. [DOI] [PubMed] [Google Scholar]
- 15.Werner NL, Hecker MT, Sethi AK, Donskey CJ. Unnecessary use of fluoroquinolone antibiotics in hospitalized patients. BMC Infect Dis. 2011;11:187. doi: 10.1186/1471-2334-11-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308. doi: 10.1001/jamainternmed.2017.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Society for Healthcare Epidemiology of America, Infectious Diseases Society of America, Pediatric Infectious Diseases Society. Policy statement on antimicrobial stewardship by the Society for Healthcare Epidemiology of America (SHEA), the Infectious Diseases Society of America (IDSA), and the Pediatric Infectious Diseases Society (PIDS). Infect Control Hosp Epidemiol. 2012;33(4):322-327. doi: 10.1086/665010. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Core elements of hospital antibiotic stewardship programs. Published 2019. Accessed August 22, 2021. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf. [DOI] [PMC free article] [PubMed]
- 19.Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017;2:CD003543. doi: 10.1002/14651858.CD003543.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black E, Neville H, Ramsey T, et al. A systematic review of antimicrobial stewardship interventions to improve management of bacteriuria in hospitalized adults. PROSPERO 2020 CRD42020159051. Date unknown. Accessed October 14, 2022. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020159051. [DOI] [PMC free article] [PubMed]
- 22.Cochrane Effective Practice and Organisation of Care. EPOC resources for review authors. Published 2017. Accessed December 31, 2021. https://epoc.cochrane.org/resources/epoc-resources-review-authors.
- 23.Cochrane Effective Practice and Organisation of Care. How to develop a search strategy for an intervention review Published July 2021. Accessed October 14, 2022. https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/how_to_develop_a_search_strategy.pdf.
- 24.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavese P, Saurel N, Labarère J, et al. Does an educational session with an infectious diseases physician reduce the use of inappropriate antibiotic therapy for inpatients with positive urine culture results? a controlled before-and-after study. Infect Control Hosp Epidemiol. 2009;30(6):596-599. doi: 10.1086/597514. [DOI] [PubMed] [Google Scholar]
- 27.Linares LA, Thornton DJ, Strymish J, Baker E, Gupta K. Electronic memorandum decreases unnecessary antimicrobial use for asymptomatic bacteriuria and culture-negative pyuria. Infect Control Hosp Epidemiol. 2011;32(7):644-648. doi: 10.1086/660764. [DOI] [PubMed] [Google Scholar]
- 28.Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980-983. doi: 10.1093/cid/ciu010. [DOI] [PubMed] [Google Scholar]
- 29.Irfan N, Brooks A, Mithoowani S, Celetti SJ, Main C, Mertz D. A controlled quasi-experimental study of an educational intervention to reduce the unnecessary use of antimicrobials for asymptomatic bacteriuria. PLoS ONE. 2015;10(7):e0132071. doi: 10.1371/journal.pone.0132071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an antimicrobial stewardship approach for urinary catheter-associated asymptomatic bacteriuria. JAMA Intern Med. 2015;175(7):1120-1127. doi: 10.1001/jamainternmed.2015.1878. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien KA, Zhang J, Mauldin PD, et al. Impact of a stewardship-initiated restriction on empirical use of ciprofloxacin on nonsusceptibility of Escherichia coli urinary isolates to ciprofloxacin. Pharmacotherapy. 2015;35(5):464-469. doi: 10.1002/phar.1590. [DOI] [PubMed] [Google Scholar]
- 32.Keller SC, Feldman L, Smith J, Pahwa A, Cosgrove SE, Chida N. The use of clinical decision support in reducing diagnosis of and treatment of asymptomatic bacteriuria. J Hosp Med. 2018;13(6):392-395. doi: 10.12788/jhm.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins TC, Hulett T, Knepper BC, et al. A statewide antibiotic stewardship collaborative to improve the diagnosis and treatment of urinary tract and skin and soft tissue infections. Clin Infect Dis. 2018;67(10):1550-1558. doi: 10.1093/cid/ciy268. [DOI] [PubMed] [Google Scholar]
- 34.Hecker MT, Son AH, Murphy NN, et al. Impact of syndrome-specific antimicrobial stewardship interventions on use of and resistance to fluoroquinolones: an interrupted time series analysis. Am J Infect Control. 2019;47(8):869-875. doi: 10.1016/j.ajic.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 35.Yoon CH, Ritchie SR, Duffy EJ, et al. Impact of a smartphone app on prescriber adherence to antibiotic guidelines in adult patients with community acquired pneumonia or urinary tract infections. PLoS ONE. 2019;14(1):e0211157. doi: 10.1371/journal.pone.0211157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daley P, Garcia D, Inayatullah R, Penney C, Boyd S. Modified reporting of positive urine cultures to reduce inappropriate treatment of asymptomatic bacteriuria among nonpregnant, noncatheterized inpatients: a randomized controlled trial. Infect Control Hosp Epidemiol. 2018;39(7):814-819. doi: 10.1017/ice.2018.100. [DOI] [PubMed] [Google Scholar]
- 37.Ridgway JP, Robicsek A, Shah N, et al. A randomized controlled trial of an electronic clinical decision support tool for inpatient antimicrobial stewardship. Clin Infect Dis. 2021;72(9):e265-e271. doi: 10.1093/cid/ciaa1048. [DOI] [PubMed] [Google Scholar]
- 38.Spoorenberg V, Hulscher ME, Geskus RB, et al. A cluster-randomized trial of two strategies to improve antibiotic use for patients with a complicated urinary tract infection. PLoS ONE. 2015;10(12):e0142672. doi: 10.1371/journal.pone.0142672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The World Bank. World bank country and lending groups. Date unknown. Accessed May 8, 2022. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519#High_income.
- 40.Monmaturapoj T, Scott J, Smith P, Abutheraa N, Watson MC. Pharmacist-led education-based antimicrobial stewardship interventions and their effect on antimicrobial use in hospital inpatients: a systematic review and narrative synthesis. J Hosp Infect. 2021;115:93-116. doi: 10.1016/j.jhin.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Schweitzer VA, van Heijl I, van Werkhoven CH, et al. The quality of studies evaluating antimicrobial stewardship interventions: a systematic review. Clin Microbiol Infect. 2019;25(5):555-561. doi: 10.1016/j.cmi.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Black EK, MacDonald L, Neville HL, et al. Health care providers’ perceptions of antimicrobial use and stewardship at acute care hospitals in Nova Scotia. Can J Hosp Pharm. 2019;72(4):263-270. [PMC free article] [PubMed] [Google Scholar]
- 43.Black E, MacLean D, Neville H, et al. A qualitative study evaluating barriers and enablers to improving antimicrobial use for bacteriuria in hospitalized adults (abstract). JAMMI. 2022;7(suppl 1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review. BMC Med Res Methodol. 2006;6(1):54. doi: 10.1186/1471-2288-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-aop-10.1177_10600280221134539 for A Systematic Review of Antimicrobial Stewardship Interventions to Improve Management of Bacteriuria in Hospitalized Adults by Mari Humphrey, Gemma MacDonald, Heather Neville, Melissa Helwig, Tasha Ramsey, Holly MacKinnon, Ingrid Sketris, Lynn Johnston and Emily K. Black in Annals of Pharmacotherapy