Abstract
High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) is a standard of care for selected patients with refractory/relapsed Hodgkin’s lymphoma (HL) or non-Hodgkin’s lymphoma (NHL), and it is also used as first-line clinical consolidation option for some aggressive NHL subtypes. Conditioning regimen prior to ASCT is one of the essential factors related with clinical outcomes post transplant. The conditioning regimen of carmustine, etoposide, cytarabine, and melphalan (BEAM) traditionally is considered the standard of care for patients with lymphoma who are eligible for transplantation. Replacement of carmustine with bendamustine (BeEAM) was described as an alternative conditioning regimen in the autograft setting for patients with lymphoma. Several studies have reported inconsistent clinical outcomes comparing BeEAM and BEAM. Therefore, in the lack of well-designed prospective comparative studies, the comparison of BeEAM versus BEAM is based on retrospective trials. To compare the clinical outcomes between BeEAM and BEAM, we performed a meta-analysis of 10 studies which compared the outcomes between BeEAM and BEAM in patients autografted for lymphoma disease (HL or NHL). We searched article titles and compared transplantation with BeEAM versus BEAM in MEDLINE (PubMed), Cochrane library, and EMBASE database. Here, we report the results of nine main endpoints in our meta-analysis comparing BeEAM and BEAM, including neutrophil engraftment (NE), platelet engraftment (PE), overall survival (OS), progression free survival (PFS), non-relapse mortality (NRM), relapse rate (RR), grade 3 mucositis, renal toxicity, and cardiotoxicity. We discovered that the BeEAM regimen was associated with a slightly better PFS [pooled odds ratio (OR) of 0.70, 95% confidence interval (CI), 0.52–0.94, P = 0.02], lower RR (0.49, 95% CI, 0.31–0.76, P = 0.002), higher mucositis (3.43, 95% CI, 2.29–5.16, P = 0.001), renal toxicity (4.49, 95% CI, 2.68–7.51, P = 0.001), and cardiotoxicity (1.88, 95% CI, 1.03–3.40, P = 0.03). We also discovered that the two groups had equivalent NE (pooled WMD –0.64, 95% CI, –1.46 to 0.18, P = 0.13), PE (pooled WMD –0.3, 95% CI, –1.68 to 2.28, P = 0.77), OS (0.73, 95% CI, 0.52–1.01, P = 0.07), and NRM (1.51, 95% CI, 0.76–2.98, P = 0.24). The results of this meta-analysis show that the BeEAM regimen is a viable alternative to BEAM. More prospective comparisons between BeEAM and BEAM are required.
Keywords: autologous stem cell transplantation, conditioning regimen, lymphoma, BeEAM, BEAM, meta-analysis
Introduction
High-dose therapy (HDT) with autologous stem cell transplantation (ASCT) has been a standard component of therapy for patients with chemosensitive relapsed/refractory Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL) for decades 1 . The therapeutic rationale for HDT with ASCT depends on increased cytotoxicity brought about by the administration of myeloablative chemotherapeutic doses or total body irradiation. Traditional criteria for selecting an HDT regimen have included institutional experience, disease categories, disease status at transplantation, and comorbidities. Several regimens are regarded as standard and are often used for patients with all histologies of lymphoma 2 . The first use of HDT followed by autologous bone marrow cell infusion for lymphomas was described in the 1960s 3 . In 1978, investigators at the National Cancer Institute reported successful treatment of resistant malignant lymphoma and Burkitt lymphoma with HDT and ASCT4,5. The use of HDT-ASCT as an effective therapy for individuals with recurrent HL was originally described in the 1980s 6 . Subsequent trials have demonstrated HDT-ASCT as the standard of care for management of R/R NHL7,8, upfront consolidation of a first remission for mantle cell lymphoma (MCL), or as first-line consolidation option for high-risk diffuse large B-cell lymphoma (DLBCL) and T-cell lymphoma9–11.
Each conditioning program used before ASCT has pros and cons in terms of effectiveness and toxicity. A topic of major interest is conditioning regimen and its effect on results 12 . In the past, BEAM (carmustine, etoposide, cytarabine, and melphalan) was often utilized all over the world 13 . Other alternative effective regimens include CBV (cyclophosphamide, carmustine, and etoposide), BuCyE (busulfan, cyclophosphamide, etoposide), BEAC (carmustine, etoposide, cytarabine, cyclophosphamide), SEAM (semustine, etoposide, cytarabine, cyclophosphamide) and total body irradiation (TBI)-containing regimens14–18. In recent years, researchers from all over the world have replaced carmustine with alternatives such as bendamustine (BeEAM) due to its limited availability, high cost, and potential toxicity 19 . BeEAM is a novel HDT regimen, hence efficacy and safety need to be examined further. It is essential to evaluate the clinical outcomes of the BeEAM and traditional BEAM regimens to optimize the conditioning regimen before ASCT. Here, we want to summarize the most recent research on the effectiveness of BeEAM for ASCT lymphoma patients as compared with BEAM. Our primary endpoints are non-hematologic toxicity, overall survival (OS), progression free survival (PFS), non-relapse mortality (NRM), platelet engraftment (PE), neutrophil engraftment (NE), and relapse rate (RR).
Materials and Methods
Search Strategy
We used the phrases autologous stem cell transplant/transplantation, BeEAM, BEAM, and conditioning regimen to search the MEDLINE (PubMed), EMBASE, and Cochrane Registry of Controlled Trials databases (updated December 2022). The PubMed and EMBASE searches were restricted to humans and English language articles. We permitted solely comparative clinical research publications. To select acceptable research, the titles, abstracts, and reference lists were examined, and blatantly irrelevant papers were deleted. Furthermore, the reference lists of pertinent research and reviews were examined to uncover other possibly qualifying studies.
Study Selection and Data Extraction
All published clinical trials on BeEAM and BEAM conditioning regimens with survival results were included. Studies were considered if they met the following criteria: patients with hematologic malignancies, and prospective or retrospective trials with more than 10 patients undergoing BeEAM and BEAM in each group. The data from the selected studies were extracted separately by two authors. The following data were retrieved from the included studies: the first author’s name, the year of publication, the number of cases, the percentage of male, the age of patients, the research design, the kind of disease, the type of transplantation, the number of participants, the type of conditioning regimen, the length of follow-up, and so on. Engraftment (NE, PE), OS, PFS, NRM, RR, and non-hematologic toxicity (grade 3 mucositis, renal toxicity, cardiotoxicity) were the primary endpoints for data synthesis.
Data Synthesis
STATA (version 12.0) software was used for the meta-analysis. The significance level was set at P < 0.05. To evaluate publication bias, Egger’s test, Begg’s test, and funnel plots were utilized. To measure statistical heterogeneity, the I2 statistic was utilized, with I2 > 50% selected as the cut-off to indicate considerable result heterogeneity. Each research provided hazard ratios (HRs) and 95% confidence intervals (CIs). When HRs and CIs were not provided in the publication, values were computed using Tierney et al.’s 20 technique. Using binary variable fixed-effects analysis, a forest plot with combined odds ratio (OR) (with 95% CIs) for OS, PFS, NRM, RR, grade 3 mucositis, renal toxicity, and cardiotoxicity advantages of BeEAM versus BEAM was generated. The approach of Luo et al. 21 and Wan et al. 22 was used to transform NE and PE time (median, range) to a continuous variable (mean, standard deviation). Using continuous variable random-effects analysis, a forest plot with combined Weighted Mean Difference (WMD) (with 95% CIs) for NE and PE advantages of BeEAM versus BEAM was created.
Results
General Description of Included Studies
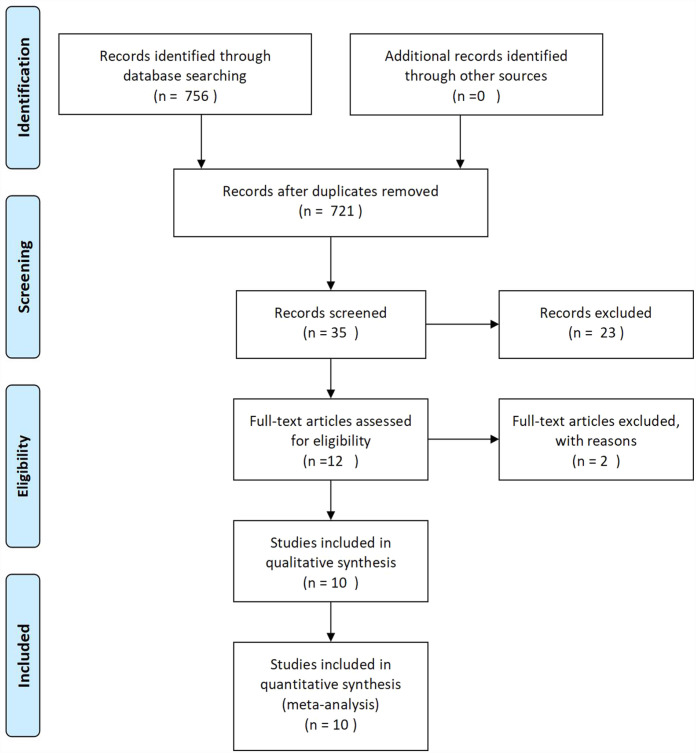
The initial search generated 756 articles, 721 of which were eliminated from the title and abstract review as they were unrelated to our topic. A total of 35 studies were reviewed in full; 23 were eliminated because they exclusively examined patients who received either BeEAM or BEAM, and there were no direct comparison findings; and 2 articles were excluded owing to inadequate data. Ten retrospective cohort studies23–32 were included in the final analysis (Fig. 1). This includes 1,304 patients who had BeEAM (467 patients) or BEAM (837 patients) ASCT. Table 1 outlines the features of the 10 studies that were considered. The median sample size was 114 patients (range = 60–237). The diseases treated with ASCT were mostly HL or all types of NHL. One study enrolled only HL patients 32 , only MCL patients were enrolled in one study 26 , another study enrolled mainly HL 29 , and the remnant seen studies enrolled mainly NHL patients23–25,27,28,30,31. The majority of patients got the standard BEAM treatment. ASCT was used to treat first-line CR, primary refractory, or relapse illnesses. The disease status at ASCT of one study was totally complete remission (CR) 24 , four studies were mainly CR26,30–32, and two studies were mainly partial remission (PR)/stable disease (SD)/progressive disease (PD)28,29. Patients in two studies were much younger than that of the other eight studies.
Figure 1.
PRISMA flow diagram of meta-analysis.
Table 1.
Clinical Characteristics of Patients.
| Study | n | Age, median (range), year | Male, % | Disease type | Disease status at ASCT | Follow-up, median (range), months |
|---|---|---|---|---|---|---|
| Lachance et al. 30 | ||||||
| BeEAM | 131 | 56 (47–65) | 64 | HD 14%, NHL 86% | CR 76%, PR/SD/PD 24% | 48 |
| BEAM/BEAC | 96 | 51 (39–63) | 55 | HD 25%, NHL 75% | CR 64%, PR/SD/PD 36% | 60 |
| Hueso et al. 26 | ||||||
| BeEAM | 60 | 59 (36–70) | 78 | MCL | CR 82%, PR/SD/PD 18% | 37 (1–49) |
| BEAM | 108 | 58 (31–69) | 84 | MCL | CR 60%, PR/SD/PD 40% | 36 (1–154) |
| Glover et al. 28 | ||||||
| BeEAM | 49 | 59 (23–71) | 73 | HD 20%, NHL 80% | PR/SD/PD 100% | 12.8 |
| BEAM | 98 | 59 (23–69) | 62 | HD 20%, NHL 80% | PR/SD/PD 100% | 41.2 |
| AlJohani et al. 29 | ||||||
| BeEAM | 17 | 31 (21–60) | 53 | HD 76%, NHL 24% | CR 6%, PR/SD 94% | 14.62 (1.61–19.45) |
| BEAM | 54 | 23.69 (15.05–59.06) | 52 | HD 72%, NHL 28% | CR 13%, PR/SD 87% | 29.88 (0.76–127.11) |
| Lucijanic et al. 25 | ||||||
| BeEAM | 17 | 50 (39–56) | 47 | HD 13%, NHL 87% | NA | NA |
| BEAM | 43 | 52 (38.5–59) | 56 | HD 14%, NHL 86% | NA | NA |
| Frankiewicz et al. 27 | ||||||
| BeEAM | 63 | 45 (21.5–72.8) | 59 | HD 40%, NHL 60% | CR 53%, PR/SD/PD 47% | 9 |
| BEAM | 174 | 46.5 (19.5–69.4) | 60 | HD 49%, NHL 51% | CR 59%, PR/SD/PD 41% | 29 |
| Hahn et al. 23 | ||||||
| BeEAM | 41 | 55 (43–67) | 76 | HD 17%, NHL 83% | NA | NA |
| BEAM | 86 | 51 (38–64) | 58 | HD 33%, NHL 67% | NA | NA |
| Saleh et al. 31 | ||||||
| BeEAM | 34 | 49 (21–68) | 62 | HD 26%, NHL 74% | CR 62%, PR 38% | 18 (2–28) |
| BEAM | 68 | 52.5 (21–71) | 62 | HD 26%, NHL 74% | CR 60%, PR 40% | 45 (2–126) |
| Tsang et al. 32 | ||||||
| BeEAM | 26 | 29 (16–66) | 54 | HD | CR/PR 73%, SD/PD 27% | 43.2 (10–96) |
| BEAM | 52 | 32 (17–62) | 60 | HD | CR/PR 73%, SD/PD 27% | 46.8 (13.2–120) |
| Garciaz et al. 24 | ||||||
| BeEAM | 29 | 58 (19–68) | 62 | DLBCL 62%, MCL 38% | CR 100% | NA |
| BEAM | 58 | 54 (25–65) | 69 | DLBCL 69%, MCL 31% | CR 100% | NA |
ASCT: autologous stem cell transplantation; BEAC: carmustine, etoposide, cytarabine, cyclophosphamide; BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; DLBCL: diffuse large B-cell lymphoma; MCL: mantle cell lymphoma; NHL: non-Hodgkin’s lymphoma.
Meta-Analysis Results
We assessed for publication bias prior to meta-analysis using Egger’s test, Begg’s test, and funnel plot approach. Except for renal toxicity, all trials evaluating outcomes such as NE, PE, OS, PFS, NRM, RR, and mucositis were not statistically significant (Table 2). The funnel plot likewise indicated that no publication bias existed for these seven endpoints.
Table 2.
Results of Publication Bias Test.
| Endpoints | Publication bias, Begg’s P | Publication bias, Egger’s P (95% CI) |
|---|---|---|
| NE | 0.322 | 0.777 (–2.336 to 2.978) |
| PE | 0.624 | 0.205 (–2.191 to 6.695) |
| OS | 0.297 | 0.508 (–2.236 to 4.104) |
| PFS | 0.652 | 0.467 (–3.816 to 7.177) |
| NRM | 0.211 | 0.071 (–0.187 to 3.536) |
| RR | 1 | 0.878 (–10.817 to 12.012) |
| Grade 3 mucositis | 0.142 | 0.141 (–1.597 to 6.193) |
| Renal toxicity | 0.039 | 0.355 (–1.422 to 3.140) |
CI: confidence interval; NE: neutrophil engraftment; NRM: non-relapse mortality; OS: overall survival; PE: platelet engraftment; PFS: progression free survival; RR: relapse rate.
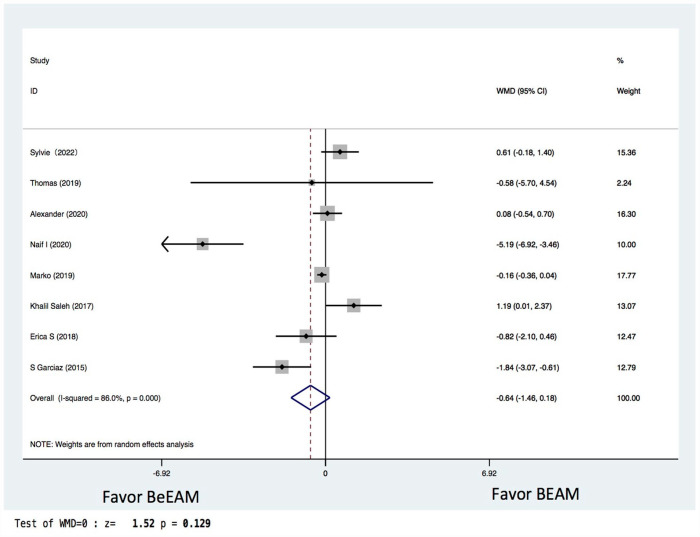
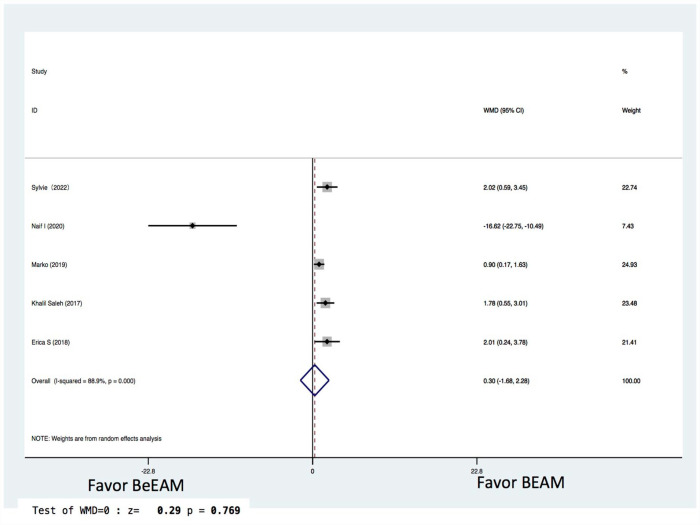
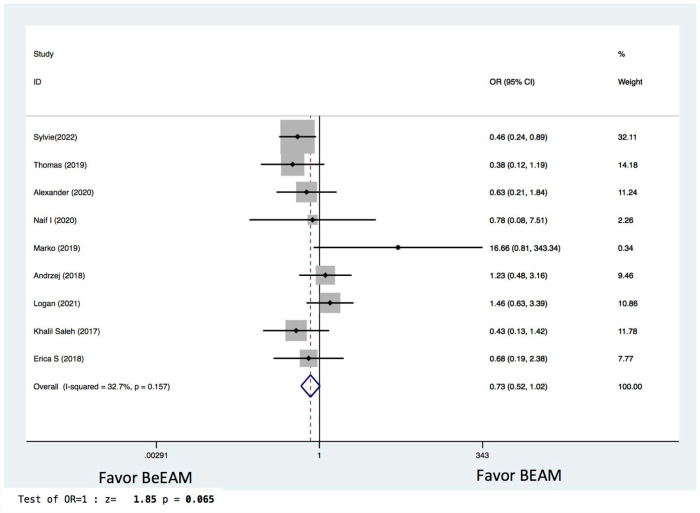
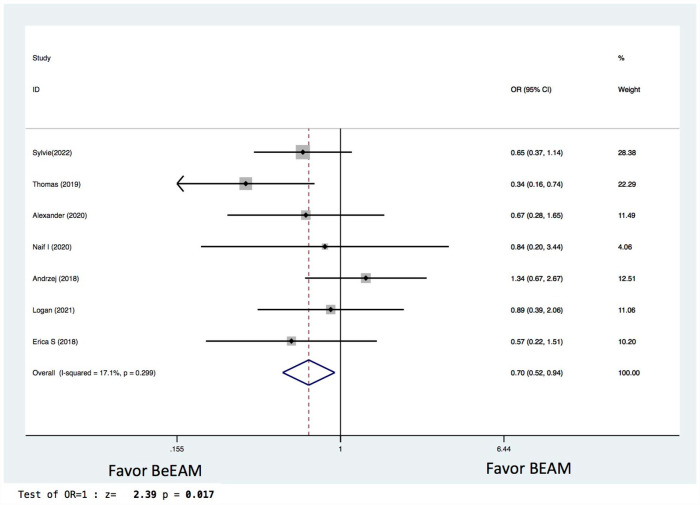
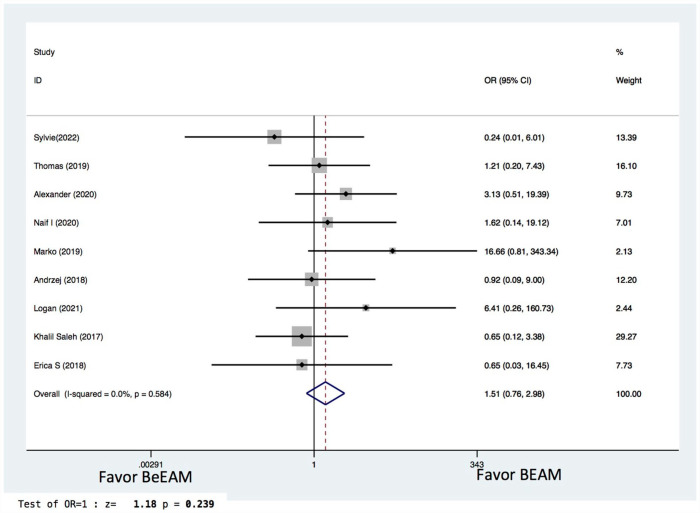
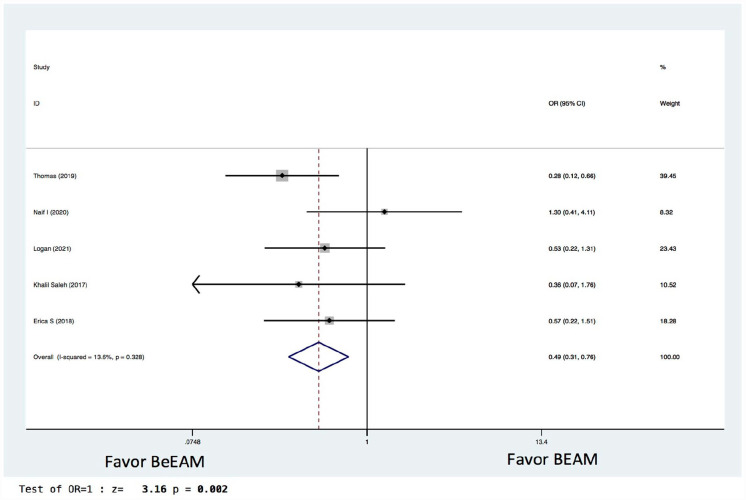
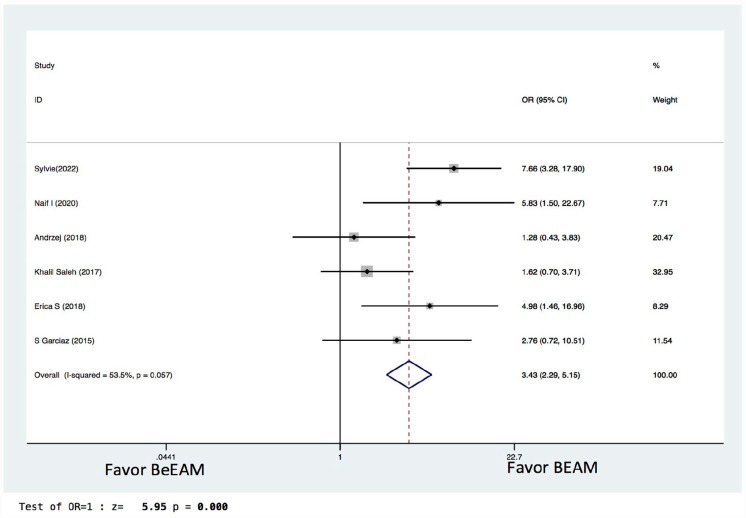
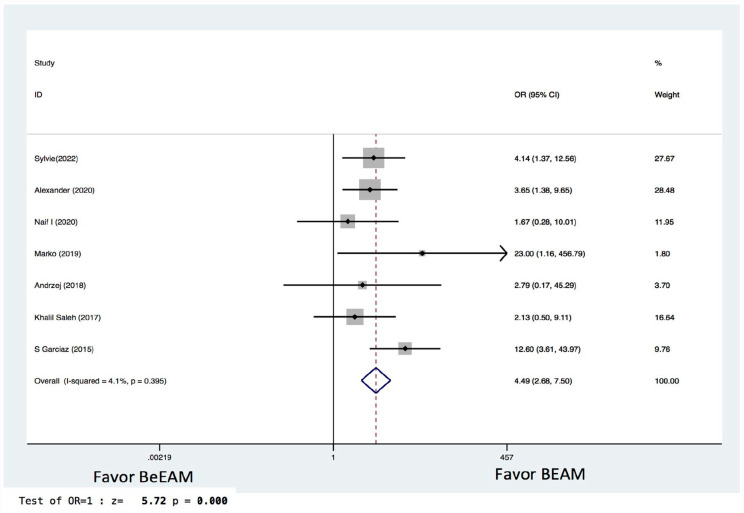
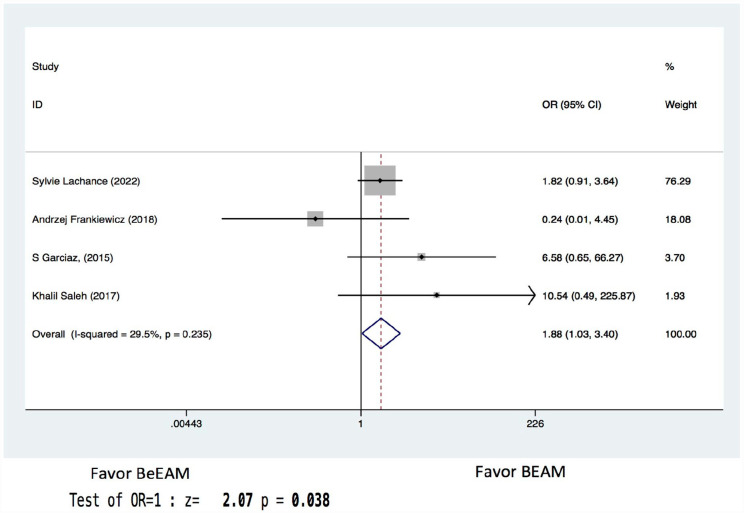
There were eight studies providing NE data, and the inter-study heterogeneity was considerable (P = 0.000), with I2 = 86%. The combined WMD for NE in a random-effects forest plot was pooled WMD of –0.64, 95% CI, –1.46 to 0.18, P = 0.13, demonstrating that BeEAM was associated with comparable NE result as BEAM (Fig. 2). PE data could be analyzed for five trials, and the inter-study heterogeneity was considerable (P = 0.000), with I2 = 88.9%. The combined WMD for PE in a random-effects forest plot was pooled WMD of –0.3, 95% CI, –1.68 to 2.28, P = 0.77, demonstrating that BeEAM was linked with identical PE result as BEAM (Fig. 3). There were nine studies containing OS data, and the inter-study heterogeneity was non-significant (P = 0.167), with I2 = 32.7%. The combined OR for OS in a fixed-effects forest plot was pooled OR of 0.73, 95% CI, 0.52–1.02, P = 0.06, demonstrating that the OS of the two groups was comparable (Fig. 4). PFS data for seven trials were available; inter-study heterogeneity was non-significant (P = 0.3), with I2 = 17.1%. In a fixed-effects forest plot, the combined OR for PFS was pooled OR of 0.70, 95% CI, 0.52–0.94, P = 0.02, indicating that BeEAM might modestly improve patients’ PFS when compared with BEAM (Fig. 5). NRM data for nine trials were evaluable; inter-study heterogeneity was non-significant (P = 0.6), with I2 = 0%. The combined OR for NRM in a fixed-effects forest plot was pooled OR of 1.51, 95% CI, 0.76–2.98, P = 0.24, indicating that the NRM of the two groups was comparable (Fig. 6). There were five studies having RR data, and the inter-study heterogeneity was non-significant (P = 0.33), with I2 = 13.6%. The combined OR for RR in a fixed-effects forest plot was pooled OR of 0.49, 95% CI, 0.31–0.76, P = 0.002, demonstrating that BeEAM was associated with a reduced RR result when compared with BEAM (Fig. 7). Data on grade 3 mucositis were available for six research, with inter-study heterogeneity being non-significant (P = 0.06) and I2 = 53.5%. In a fixed-effects forest plot, the combined OR for grade 3 mucositis was pooled OR of 3.43, 95% CI, 2.29–5.16, P = 0.001, indicating that BeEAM may increase grade 3 mucositis in patients when compared with BEAM (Fig. 8). Data on renal toxicity were available for seven research; inter-study heterogeneity was non-significant (P = 0.39), with I2 = 4.1%. The combined OR for renal toxicity in a fixed-effects forest plot was pooled OR of 4.49, 95% CI, 2.68–7.51, P = 0.001, demonstrating that BeEAM might increase renal toxicity in patients compared with BEAM (Fig. 9). Data on cardiotoxicity were evaluable for four studies; inter-study heterogeneity was non-significant (P = 0.23), with I2 = 29.5%. The combined OR for cardiotoxicity in a fixed-effects forest plot was pooled OR of 1.88, 95% CI, 1.03–3.40, P = 0.03, demonstrating that BeEAM might increase cardiotoxicity in patients compared with BEAM (Fig. 10).
Figure 2.
Meta-analysis result of neutrophil engraftment. BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; CI: confidence interval.
Figure 3.
Meta-analysis result of platelet engraftment. BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; CI: confidence interval.
Figure 4.
Meta-analysis result of overall survival. BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; CI: confidence interval; OR: odds ratio.
Figure 5.
Meta-analysis result of disease free survival. BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; CI: confidence interval; OR: odds ratio.
Figure 6.
Meta-analysis result of non-relapse mortality. BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; CI: confidence interval; OR: odds ratio.
Figure 7.
Meta-analysis result of relapse rate. BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; CI: confidence interval; OR: odds ratio.
Figure 8.
Meta-analysis result of grade 3 mucositis. BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; CI: confidence interval; OR: odds ratio.
Figure 9.
Meta-analysis result of renal toxicity. BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; CI: confidence interval; OR: odds ratio.
Figure 10.
Meta-analysis result of cardiotoxicity. BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; CI: confidence interval; OR: odds ratio.
We also performed subgroup meta-analysis including different disease type and disease status at ASCT. Two studies of mainly HL and eight studies of mainly NHL were analyzed. Compared with BEAM, in HL-dominated studies, BeEAM cohort shared comparable OS, PFS, NRM, and RR except an increased rate of grade 3 mucositis; while in NHL-dominated studies, BeEAM cohort shared comparable OS and NRM, decreased RR and increased PFS, and increased rate of grade 3 mucositis and renal toxicity. In addition, five studies of mainly CR status at ASCT and two studies of mainly PR/SD/PD were also analyzed. In CR-dominated studies, BeEAM cohort shared better OS, PFS, and RR, and increased rate of grade 3 mucositis and renal toxicity; while in PR/SD/PD dominated studies, BeEAM cohort shared comparable OS, PFS, NRM, and RR, and increased rate of grade 3 mucositis and renal toxicity (Table 3).
Table 3.
Outcomes of BeEAM Compared With BEAM According to Disease Type or Disease Status at ASCT.
| Endpoint | Disease | Disease status at ASCT | ||||||
|---|---|---|---|---|---|---|---|---|
| HL majoratity | NHL majoratity | CR majoratity | PR/SD/PD majoratity | |||||
| Pooled OR (95% CI) | P | Pooled OR (95% CI) | P | Pooled OR (95% CI) | P | Pooled OR (95% CI) | P | |
| OS | 0.70 (0.23–2.11) | 0.53 | 0.73 (0.51–1.04) | 0.08 | 0.46 (0.29–0.75) | 0.002 | 0.65 (0.25–1.73) | 0.39 |
| PFS | 0.65 (0.29–1.45) | 0.29 | 0.71 (0.51–0.97) | 0.03 | 0.52 (0.35–0.79) | 0.002 | 0.72 (0.34–1.53) | 0.38 |
| NRM | 1.11 (0.16–7.74) | 0.91 | 1.57 (0.76–3.27) | 0.22 | 0.70 (0.24–2.03) | 0.52 | 2.5 (0.59–10.55) | 0.68 |
| RR | 0.80 (0.38–1.67) | 0.55 | 0.38 (0.21–0.66) | 0.001 | 0.37 (0.21–0.67) | 0.002 | 1.29 (0.41–4.11) | 0.66 |
| Grade 3 mucositis | 5.38 (2.16–13.42) | 0.001 | 3.06 (1.94–4.83) | 0.001 | 3.79 (2.35–6.12) | 0.001 | 5.83 (1.50–22.67) | 0.01 |
| Renal toxicity | 1.66 (0.28–10.01) | 0.58 | 4.87 (2.82–8.39) | 0.001 | 5.05 (2.48–10.27) | 0.001 | 3.06 (1.32–7.10) | 0.009 |
ASCT: autologous stem cell transplantation; BEAM: carmustine, etoposide, cytarabine, melphalan; BeEAM: bendamustine, etoposide, cytarabine, melphalan; CI: confidence interval; DLBCL: diffuse large B-cell lymphoma; HL: Hodgkin’s lymphoma; NHL: non-Hodgkin’s lymphoma; NRM: non-relapse mortality; OR: odds ratio; OS: overall survival; PFS: progression free survival; RR: relapse rate.
Discussion
High-dose chemotherapy followed by ASCT is the mainstay of treatment in patients with relapsed or refractory classical HL or aggressive NHL7,8,33,34. It is also crucial to highlight that ASCT is a viable upfront consolidation therapy option for certain histological aggressive NHL, such as high-risk DLBCL, MCL, or T-cell lymphoma, as suggested by the European Group for Blood and Marrow Transplantation (EBMT) or American Society for Transplantation and Cellular Therapy (ASTCT) guidelines9,10. The fundamental component of the ASCT technique is a conditioning regimen consisting of high-dose chemotherapy. A good conditioning regimen should be both high effective and less toxic. Since the 1980s, several conditioning programs have been tried with little evidence of apparent advantage of one regimen over another. BEAM is one of the most regularly used conditioning procedures for both HL and NHL, with a high effectiveness and an acceptable safety profile, which gives rise to 3-year OS (79%), 3-year PFS (62%) for HL, and 3-year OS (64%), 3-year PFS (51%) for NHL, respectively. A relatively lower 1-year mortality of 4% for all lymphoma underwent ASCT13,35,36. However, there are concerns regarding carmustine’s rising cost, decreased availability, and high toxicity profile, notably in second malignancies and interstitial pneumonitis37,38. These reservations indicate the necessity for a different agent or regimen.
Bendamustine’s molecular structure is unusual in that it combines the alkylating characteristics of nitrogen mustard with the antimetabolite activity of purine analogs. Bendamustine is now a standard therapy for indolent B-cell lymphoma 39 . The optimal alternate conditioning regimen for individuals with hematologic malignancies who require ASCT but lack carmustine remains unknown. In terms of efficacy and tolerance, the various types of alternate regimens have distinct advantages and disadvantages. Visani et al. 19 first designed a phase 1 to 2 study to assess the safety and efficacy of increasing doses of bendamustine (160 mg/m2, 180 mg/m2, and 200 mg/m2 given on days –7 and –6) in combination with fixed doses of etoposide, cytarabine, and melphalan (BeEAM regimen) as the conditioning regimen for ASCT in R/R lymphoma patients. A total of 43 individuals with NHL (n = 28) or HL (n = 15) were treated consecutively. All patients engrafted, with a 10-day median time to absolute neutrophil count >0.5× 109/L. There was no transplant-related death after 100 days. After a median of 18 months of follow-up, 35 of 43 patients (81%) were in full remission, whereas 6 of 43 relapsed and 2 did not respond. Gilli et al. 40 analyzed 39 lymphoma patients receiving BeEAM conditioning. The median neutrophil recovery was 11 days, and 15 days for platelet recovery. The most common grade 3/4 non-hematologic toxicities comprised mucosal side effects (27 pts.). Pulmonary toxicity was observed in one patient (2.5%). The CR rate increased from 33% to 74% 100 days after ASCT, 2 years PFS and OS were 69% and 72%. To further improve clinical outcomes of BeEAM regimen and reduce relapse post ASCT, Stoffel et al. 41 reported 12 DLBCL patients received polatuzumab vedotin (PV, targeting CD79b) plus BeEAM conditioning regimen and found this novel combination was feasible and safe, but the limited cohort prevents definite conclusions regarding efficacy. Another group documented 12 CD30+ lymphoma patients received a single BV dose at three dose levels (DL) (0.9/1.2/1.8 mg/kg b.w.) prior to standard BeEAM regimen, and they found addition of brentuximab to standard BeEAM High-Dose Chemotherapy (HDCT) seemed to be safe with CR rate of 75% post-ASCT in a highly pretreated population 42 . Nevertheless, in 474 patients who received ASCT, the Lymphoma Study Association (LYSA) from France reported observed toxicities related with bendamustine conditioning. Bendamustine was administered at a median dosage of 196 mg/m2/day on days –7 and –6. The study found a rate of all-grade acute renal failure of 27.9%, the admission rate to the intensive care unit (ICU) for patients treated with BeEAM conditioning was high (24%) 43 , but these results were argued by Isidori et al. 44 based upon following reasons: (1) The French experience was not a multicenter study, but rather retrospective data collection made from at least 22 French sites. Every transplant site had a different policy in terms of hydration, prophylaxis of infections, supportive care, and management of post-transplant complications. (2) High rate of grade ≥ III Acute Renal Failure (ARF) was not seen in other controlled phase II studies40,45. (3) Cytarabine and etoposide administration method showed discrepancy in different centers. (4) ICU admission rates could not be considered as an interesting endpoint and a reliable indicator in the context of a retrospective study.
Although some retrospective studies compared BeEAM and BEAM, fewer prospective trials were available. A recent randomized prospective study directly comparing BeEAM with BEAM was shown as a presentation at the 2022 American Society of Hematology (ASH) conference by Keil et al. 46 The author drew the conclusion that BeEAM was a feasible, well-tolerated regimen resulting in similar results as observed with the BEAM regimen. Because there had been no published meta-analysis comparing clinical results of patients who received ASCT using BeEAM or BEAM, we did this meta-analysis to examine clinical outcomes of the BeEAM or BEAM regimen. In our meta-analysis, we discovered that the BeEAM group was equivalent to BEAM in terms of four endpoints: NE/PE, OS, and NRM. Interestingly, the recurrence rate in BeEAM was somewhat lower than in BEAM, resulting in a longer PFS, although one issue to note here was that there were more CR status patients in BeEAM groups, which might generate bias. Non-hematologic toxicities, such as grade 3 mucositis, renal toxicity, and cardiotoxicity may compromise patient outcomes and negatively affect life quality; therefore, we examined these three endpoints and discovered that increased rate of grade 3 mucositis, renal toxicity, and cardiotoxicity could be seen in the BeEAM cohort, which were controllable and did not result in increased NRM. Apart from the conditioning regimen, the disease type and disease status prior to ASCT have a considerable influence on transplant outcome in terms of OS, PFS, and recurrence incidence. Therefore, we performed subgroup meta-analysis according to different disease type and disease status at ASCT. Compared with BEAM, in HL-dominated studies, BeEAM cohort shared comparable OS, PFS, NRM, and RR except an increased rate of grade 3 mucositis; while in NHL-dominated studies, BeEAM cohort shared comparable OS and NRM, decreased RR and increased PFS, and increased rate of grade 3 mucositis and renal toxicity. In addition, in CR-dominated studies, BeEAM cohort had better OS, PFS, and RR, and increased rate of grade 3 mucositis and renal toxicity; while in PR/SD/PD-dominated studies, BeEAM cohort had comparable OS, PFS, NRM, and RR, and increased rate of grade 3 mucositis and renal toxicity. These findings from subgroup meta-analysis suggest that both regimens are viable options for HL or NHL patients, but that the BeEAM regimen may be preferable for CR patients. There are some limitations to our study: (1) All the studies included were retrospective clinical trials, which may have influenced the objectivity and accuracy of the meta-analysis. (2) Our meta-analysis is limited by the heterogeneity between different studies, which stems from different indications for transplant, disease types, pretransplant comorbidities, disease status at transplant, and bendamustine dosage (ranging from 160 to 200 mg/m2). Subgroup analysis for parameters such as bendamustine dosage level should be undertaken. Furthermore, several studies lacked detailed information or outcomes related to various diseases types and clinical status at transplant. This makes subgroup analysis harder. (3) Variable follow-up period in various studies may affect meta-analysis results, which is a typical problem in all meta-analyses.
Conclusion
The findings of this meta-analysis show that the BeEAM conditioning regimen provides equivalent clinical results to BEAM in terms of NE, PE, OS, and transplant-related mortality (TRM), but is somewhat superior in terms of lowered RR and longer PFS despite higher grade 3 mucositis, renal damage, and cardiotoxicity. BeEAM is a viable choice for patients who are deficient in carmustine. More large-scale, multi-center, prospective, controlled trials are needed to compare the long-term effects of BeEAM against BEAM, which will give clinicians unambiguous information to help them choose the optimum conditioning regimen for ASCT patients.
Acknowledgments
The authors would like to thank all members of the Department of Hematology, Shanghai Ninth People’s Hospital Affiliated to Shanghai Jiaotong University School of Medicine, for their support.
Footnotes
Authors’ Contributions: LM made substantial contributions to the design of the present study. RW and LM performed the data collection and data analysis. RW and LM wrote the manuscript. All authors read and approved the final manuscript.
Ethical Approval: Ethical approval is not applicable to this study.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: Not applicable because this study did not directly involve human subjects.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Shanghai Ninth People’s Hospital clinical trial grant provided support for the research, authorship, and/or publication of this article.
ORCID iD: Liyuan Ma  https://orcid.org/0000-0003-1589-7300
https://orcid.org/0000-0003-1589-7300
References
- 1.Moskowitz AJ, Moskowitz CH. Controversies in the treatment of lymphoma with autologous transplantation. Oncologist. 2009;14(9):921–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez HF, Escalón MP, Pereira D, Lazarus HM. Autotransplant conditioning regimens for aggressive lymphoma: are we on the right road? Bone Marrow Transplant. 2007;40(6):505–13. [DOI] [PubMed] [Google Scholar]
- 3.Mc FW, Granville NB, Dameshek W. Autologous bone marrow infusion as an adjunct in therapy of malignant disease. Blood. 1959;14(5):503–21. [PubMed] [Google Scholar]
- 4.Appelbaum FR, Deisseroth AB, Graw RG, Jr, Herzig GP, Levine AS, Magrath IT, Pizzo PA, Poplack DG, Ziegler JL. Prolonged complete remission following high dose chemotherapy of Burkitt’s lymphoma in relapse. Cancer. 1978;41(3):1059–63. [DOI] [PubMed] [Google Scholar]
- 5.Appelbaum FR, Herzig GP, Ziegler JL, Graw RG, Levine AS, Deisseroth AB. Successful engraftment of cryopreserved autologous bone marrow in patients with malignant lymphoma. Blood. 1978;52(1):85–95. [PubMed] [Google Scholar]
- 6.Phillips GL, Wolff SN, Herzig RH, Lazarus HM, Fay JW, Lin HS, Shina DC, Glasgow GP, Griffith RC, Lamb CW. Treatment of progressive Hodgkin’s disease with intensive chemoradiotherapy and autologous bone marrow transplantation. Blood. 1989;73(8):2086–92. [PubMed] [Google Scholar]
- 7.Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, Link BK, Hay A, Cerhan JR, Zhu L, Boussetta S, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, Sonneveld P, Gisselbrecht C, Cahn JY, Harousseau JL, Coiffier B, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540–45. [DOI] [PubMed] [Google Scholar]
- 9.Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, Dolstra H, Duarte RF, Glass B, Greco R, Lankester AC, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022;57(8):1217–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanate AS, Majhail NS, Savani BN, Bredeson C, Champlin RE, Crawford S, Giralt SA, LeMaistre CF, Marks DI, Omel JL, Orchard PJ, et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020;26(7):1247–56. [DOI] [PubMed] [Google Scholar]
- 11.Stiff PJ, Unger JM, Cook JR, Constine LS, Couban S, Stewart DA, Shea TC, Porcu P, Winter JN, Kahl BS, Miller TP, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369(18):1681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zahid U, Akbar F, Amaraneni A, Husnain M, Chan O, Riaz IB, McBride A, Iftikhar A, Anwer F. A review of autologous stem cell transplantation in lymphoma. Curr Hematol Malig Rep. 2017;12(3):217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You B, Salles G, Bachy E, Casasnovas O, Tilly H, Ribrag V, Sebban C, Hénin E, Guitton J, Tod M, Freyer G. Etoposide pharmacokinetics impact the outcomes of lymphoma patients treated with BEAM regimen and ASCT: a multicenter study of the LYmphoma Study Association (LYSA). Cancer Chemother Pharmacol. 2015;76(5):939–48. [DOI] [PubMed] [Google Scholar]
- 14.El-Najjar I, Boumendil A, Luan JJ, Bouabdallah R, Thomson K, Mohty M, Colombat P, Biron P, Tilly H, Pfreundschuh M, Cordonnier C, et al. The impact of total body irradiation on the outcome of patients with follicular lymphoma treated with autologous stem-cell transplantation in the modern era: a retrospective study of the EBMT Lymphoma Working Party. Ann Oncol. 2014;25(11):2224–29. [DOI] [PubMed] [Google Scholar]
- 15.Flowers CR, Costa LJ, Pasquini MC, Le-Rademacher J, Lill M, Shore TB, Vaughan W, Craig M, Freytes CO, Shea TC, Horwitz ME, et al. Efficacy of pharmacokinetics-directed busulfan, cyclophosphamide, and etoposide conditioning and autologous stem cell transplantation for lymphoma: comparison of a multicenter phase II study and CIBMTR outcomes. Biol Blood Marrow Transplant. 2016;22(7):1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jantunen E, Kuittinen T, Nousiainen T. BEAC or BEAM for high-dose therapy in patients with non-Hodgkin’s lymphoma? A single centre analysis on toxicity and efficacy. Leuk Lymphoma. 2003;44(7):1151–58. [DOI] [PubMed] [Google Scholar]
- 17.Puig N, de la Rubia J, Remigia MJ, Jarque I, Martín G, Cupelli L, Sanz GF, Lorenzo I, Sanz J, Martínez JA, Jiménez C, et al. Morbidity and transplant-related mortality of CBV and BEAM preparative regimens for patients with lymphoid malignancies undergoing autologous stem-cell transplantation. Leuk Lymphoma. 2006;47(8):1488–94. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Yang H, Qian C, Zhou J, Zhu Q, Jiang Y, Liu S, Chen X, Xu T, Qu C, Li C, et al. Efficacy and toxicity of SEAM (semustine, etoposide, cytarabine, and melphalan) conditioning regimen followed by autologous stem cell transplantation in lymphoma. Hematology. 2022;27(1):404–11. [DOI] [PubMed] [Google Scholar]
- 19.Visani G, Malerba L, Stefani PM, Capria S, Galieni P, Gaudio F, Specchia G, Meloni G, Gherlinzoni F, Giardini C, Falcioni S, et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood. 2011;118(12):3419–25. [DOI] [PubMed] [Google Scholar]
- 20.Tierney JFSL, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805. [DOI] [PubMed] [Google Scholar]
- 22.Wan XWW, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn L, Lim H, Dusyk T, Sabry W, Elemary M, Stakiw J, Danyluk P, Bosch M. BeEAM conditioning regimen is a safe, efficacious and economical alternative to BEAM chemotherapy. Sci Rep. 2021;11(1):14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garciaz S, Coso D, Schiano de, Collela JM, Broussais F, Stoppa AM, Aurran T, Chabannon C, Helvig A, Xerri L, Blaise D, Bouabdallah R. Bendamustine-based conditioning for non-Hodgkin lymphoma autologous transplantation: an increasing risk of renal toxicity. Bone Marrow Transplant. 2016;51(2):319–21. [DOI] [PubMed] [Google Scholar]
- 25.Lucijanic M, Prka Z, Jaksic O, Mitrovic Z, Vrkljan A, Pejsa V. Bendamustine-based conditioning prior to autologous stem cell transplantation is associated with high rate of febrile neutropenia and higher mortality. Am J Hematol. 2019;94(2):E42–43. [DOI] [PubMed] [Google Scholar]
- 26.Hueso T, Gastinne T, Garciaz S, Tchernonog E, Delette C, Casasnovas RO, Durot E, Houot R, Tessoulin B, Tournilhac O, Malak S, et al. Bendamustine-EAM versus BEAM regimen in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in the frontline setting: a multicenter retrospective study from Lymphoma Study Association (LYSA) centers. Bone Marrow Transplant. 2020;55(6):1076–84. [DOI] [PubMed] [Google Scholar]
- 27.Frankiewicz A, Saduś-Wojciechowska M, Najda J, Czerw T, Mendrek W, Sobczyk-Kruszelnicka M, Soska K, Ociepa M, Hołowiecki J, Giebel S. Comparable safety profile of BeEAM (bendamustine, etoposide, cytarabine, melphalan) and BEAM (carmustine, etoposide, cytarabine, melphalan) as conditioning before autologous haematopoietic cell transplantation. Contemp Oncol. 2018;22(2):113–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glover A, Fox CP, McMillan A, Beech A, Martinez-Calle N, Griffiths C, Richardson F, Russell N, Bishton MJ. A comparison of bendamustine-EAM versus BEAM conditioning for autogolous stem cell transplant in lymphoma. Leuk Lymphoma. 2020;61(14):3529–31. [DOI] [PubMed] [Google Scholar]
- 29.AlJohani NI, Nasani M, Ahmed HE, Ur Rehman J, Nawaz A, Alzahrani Z, Albeirouti B. Dose-adjusted bendamustine as a replacement for carmustine in autologous stem cell transplant conditioning for patients with relapsed lymphoma: a retrospective single-center study. Hematol Oncol Stem Cell Ther. 2021;14(4):327–35. [DOI] [PubMed] [Google Scholar]
- 30.Lachance S, Bourguignon A, Boisjoly JA, Bouchard P, Ahmad I, Bambace N, Bernard L, Cohen S, Delisle JS, Fleury I, Kiss T, et al. Impact of implementing a bendamustine-based conditioning regimen on outcomes of autologous stem cell transplantation in lymphoma while novel cellular therapies emerge. Transplant Cell Ther. 2023;29(1):34.e1–34.e7. [DOI] [PubMed] [Google Scholar]
- 31.Saleh K, Danu A, Koscielny S, Legoupil C, Pilorge S, Castilla-Llorente C, Ghez D, Lazarovici J, Michot JM, Khalife-Saleh N, Lapierre V, et al. A retrospective, matched paired analysis comparing bendamustine containing BeEAM versus BEAM conditioning regimen: results from a single center experience. Leuk Lymphoma. 2018;59(11):2580–87. [DOI] [PubMed] [Google Scholar]
- 32.Tsang ES, Villa D, Loscocco F, Visani G, Power M, Guiducci B, Clissa C, Song K, Toze C, Abou Mourad Y, Sutherland H, et al. High-dose Benda-EAM versus BEAM in patients with relapsed/refractory classical Hodgkin lymphoma undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2019;54(3):481–84. [DOI] [PubMed] [Google Scholar]
- 33.Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, Chopra R, Milligan D, Hudson GV. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–54. [DOI] [PubMed] [Google Scholar]
- 34.Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H, Ma D, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milpied N, Deconinck E, Gaillard F, Delwail V, Foussard C, Berthou C, Gressin R, Lucas V, Colombat P, Harousseau J-L; Groupe Ouest-Est des Leucémies et des Autres Maladies du Sang. Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. N Engl J Med. 2004;350(13):1287–95. [DOI] [PubMed] [Google Scholar]
- 36.Chen YB, Lane AA, Logan B, Zhu X, Akpek G, Aljurf M, Artz A, Bredeson CN, Cooke KR, Ho VT, Lazarus HM, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(6):1046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fagnoni P, Milpied N, Limat S, Deconinck E, Nerich V, Foussard C, Colombat P, Harousseau JL, Woronoff-Lemsi MC.Groupe Ouest-Est des Leucémies et des Autres Maladies du Sang. Cost effectiveness of high-dose chemotherapy with autologous stem cell support as initial treatment of aggressive non-Hodgkin’s lymphoma. Pharmacoeconomics. 2009;27(1):55–68. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia S, Robison LL, Francisco L, Carter A, Liu Y, Grant M, Baker KS, Fung H, Gurney JG, McGlave PB, Nademanee A, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mondello P, Steiner N, Willenbacher W, Wasle I, Zaja F, Zambello R, Visentin A, Mauro E, Ferrero S, Ghione P, Pitini V, et al. Bendamustine plus rituximab versus R-CHOP as first-line treatment for patients with indolent non-Hodgkin’s lymphoma: evidence from a multicenter, retrospective study. Ann Hematol. 2016;95(7):1107–14. [DOI] [PubMed] [Google Scholar]
- 40.Gilli S, Novak U, Taleghani BM, Baerlocher GM, Leibundgut K, Banz Y, Zander T, Betticher D, Egger T, Rauch D, Pabst T. BeEAM conditioning with bendamustine-replacing BCNU before autologous transplantation is safe and effective in lymphoma patients. Ann Hematol. 2017;96(3):421–29. [DOI] [PubMed] [Google Scholar]
- 41.Stoffel T, Bacher U, Banz Y, Daskalakis M, Novak U, Pabst T. BeEAM high-dose chemotherapy with polatuzumab (pola-BeEAM) before ASCT in patients with DLBCL—a pilot study. J Clin Med. 2022;11(13):3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rausch C, Bacher U, Rabaglio M, Vorburger C, Klingenberg A, Banz Y, Daskalakis M, Pabst T. Combining BeEAM with brentuximab vedotin for high-dose therapy in CD30 positive lymphomas before autologous transplantation—a phase I study. J Clin Med. 2022;11(18):5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chantepie SP, Garciaz S, Tchernonog E, Peyrade F, Larcher MV, Diouf M, Fornecker LM, Houot R, Gastinne T, Soussain C, Malak S, et al. Bendamustine-based conditioning prior to autologous stem cell transplantation (ASCT): results of a French multicenter study of 474 patients from LYmphoma Study Association (LYSA) centers. Am J Hematol. 2018;93(6):729–35. [DOI] [PubMed] [Google Scholar]
- 44.Isidori A, Loscocco F, Guiducci B, Visani G. Benda-EAM prior to ASCT and renal toxicity: much ado about nothing. Am J Hematol. 2019;94(4):E104–105. [DOI] [PubMed] [Google Scholar]
- 45.Noesslinger T, Panny M, Simanek R, Moestl M, Boehm A, Menschel E, Koller E, Keil F. High-dose bendamustine-EAM followed by autologous stem cell rescue results in long-term remission rates in lymphoma patients, without renal toxicity. Eur J Haematol. 2018;101(3):326–31. [DOI] [PubMed] [Google Scholar]
- 46.Keil F, Müller AMS, Berghold A, Buxhofer-Ausch V, Schuster J, Riedl R, Vorburger C, Boehm A, Panny M, Noesslinger T, Greil R, et al. A randomized phase II trial comparing BeEAM with BEAM as conditioning regimen for autologous stem cell transplantation in lymphoma patients. Blood. 2022;140(Suppl 1):1180–82. [Google Scholar]