Abstract
Reproductive inequality, or reproductive skew, drives natural selection, but has been difficult to assess, particularly for males in species with promiscuous mating and slow life histories, such as bonobos (Pan paniscus) and chimpanzees (Pan troglodytes). Although bonobos are often portrayed as more egalitarian than chimpanzees, genetic studies have found high male reproductive skew in bonobos. Here, we discuss mechanisms likely to affect male reproductive skew in Pan, then re-examine skew patterns using paternity data from published work and new data from the Kokolopori Bonobo Reserve, Democratic Republic of Congo and Gombe National Park, Tanzania. Using the multinomial index (M), we found considerable overlap in skew between the species, but the highest skew occurred among bonobos. Additionally, for two of three bonobo communities, but no chimpanzee communities, the highest ranking male had greater siring success than predicted by priority-of-access. Thus, an expanded dataset covering a broader demographic range confirms that bonobos have high male reproductive skew. Detailed comparison of data from Pan highlights that reproductive skew models should consider male–male dynamics including the effect of between-group competition on incentives for reproductive concessions, but also female grouping patterns and factors related to male–female dynamics including the expression of female choice.
This article is part of the theme issue ‘Evolutionary ecology of inequality’.
Keywords: bonobo, chimpanzee, reproductive skew, multinomial index, priority-of-access model
1. Introduction
Given that natural selection results from differential reproductive success, factors affecting the distribution of reproduction constitute a central issue for understanding social evolution [1]. Disparities in individual physical condition, access to resources, social status and social relationships can lead to inequality in reproduction, also known as reproductive skew [2]. Across societies, the degree of inequality in lifetime reproductive success varies from low, such as in cooperatively breeding greater ani (Crotophaga major) [3], to high, such as in eusocial naked-mole rats (Heterocephalus glaber) [4] and mound-building termites (Macrotermes) [5], in which many individuals assist the reproduction of one fertile pair. Efforts to establish a unified theory of reproductive skew depend on long-term field data from species living in a broad range of societies. Such data have been difficult to obtain, however, particularly for males in species with internal fertilization, promiscuous mating, and slow life histories, including our closest living relatives, chimpanzees (Pan troglodytes) and bonobos (Pan paniscus).
In a strictly promiscuous mating system, where males and females mate indiscriminately, equal access to reproductive opportunities can theoretically occur. In practice, however, the distribution of paternities often deviates from an equal distribution owing to competition and conflicting interests within and between the sexes [6–10], as observed in a broad range of species, including rock iguanas (Cyclura nubila caymanensis) [11], acorn woodpeckers (Melanerpes formicivorus) [12], tree sloths (Bradypus variegatus and Choloepus hoffmanni) [13], buffy flower bats (Erophylla sezekorni) [14], spotted hyaenas (Crocuta crocuta) [15], white-faced capuchins (Cebus capucinus) [16], Barbary macaques (Macaca sylvanus) [17] and yellow baboons (Papio cynocephalus) [18]. This in turn shapes male and female reproductive strategies [19]. On one hand, males seek to increase reproductive success through strategies including contest competition with other males, sperm competition, efforts to constrain female mating behaviour with sexual coercion, and infanticides [20–22]. On the other hand, females compete among themselves for resources to enhance offspring survival while trying to obtain benefits from males such as good genes, paternal care, and help in resource acquisition [20–22]. The interplay of these strategies makes it difficult to assess the relative importance of different factors affecting male reproductive success across species [6,17,23].
The various models proposed to explain male reproductive skew generally focus on the ability of males to monopolize reproductive opportunities with females [24,25] and to control reproductive activities of other males [1,7,26–30]. The priority-of-access (PoA) model [31] provides a useful heuristic for the expected distribution of paternities in a given multi-male, multi-female group. This model assumes that males queue for reproductive opportunities based on their competitive ability and the availability of fertile females at a given point in time. Deviations from this expected distribution can arise when dominant males cannot control reproductive access to sexually receptive females [27] or when they permit reproduction by other males [32]. Explaining such deviations require theoretical models that focus on the ultimate reasons for group formation, including fitness benefits that males may gain from group-living even if low-ranking [33].
The limited-control and concession models seek to explain variation in male reproductive skew across species, considering the ultimate reasons for low-ranking males to stay in a group. Under the limited-control model, the highest ranking male does not have complete control over the reproductive activities of other males and females, which may result in low-ranking males gaining reproductive opportunities [7,26–29]. Empirical evidence supports this model across taxa including acorn woodpeckers [12], American crows (Corvus brachyrhynchos) [34], meerkats (Suricata suricatta) [35], spotted hyaenas [15], mandrills (Mandrillus sphinx) [36], rhesus macaques (Macaca mulatta) [17] and mountain gorillas (Gorilla gorilla beringei) [37].
Alternatively, the concession model assumes that the highest ranking male controls reproduction in the group and permits reproduction by lower ranking males in exchange for social benefits [1,7,26,27,30]. This model was originally developed to explain reproductive skew in eusocial insects, where dominant queens control reproduction by destroying eggs laid by subordinate queens [32]. Cooperatively breeding species such as dwarf mongooses (Helogale parvula) provide some empirical evidence to support this model, as dominant individuals apparently concede a share of reproduction to subordinates to entice their cooperation in offspring rearing [38]. Nevertheless, in many group-living species, it may rarely be the case that top-ranking males sufficiently control reproductive access to females that concessions need to be invoked. As noted above, top-ranking males appear to lack complete control over reproductive opportunities in a broad range of species. However, even without complete control, these males may still benefit by conceding some of the reproduction which they can control in exchange for other benefits [33,39]. The reproductive concession and the limited-control models can, therefore, be difficult to distinguish.
Understanding male reproductive skew in our two closest living relatives, chimpanzees and bonobos, is challenging owing to their long lives and low reproductive rates. Both species live in multi-male, multi-female communities [40–44] with: (i) fission–fusion dynamics, in which individuals travel in subgroups (parties) that can vary in size and composition throughout the day [41,42,45]; (ii) female-biased dispersal [41,44,46,47]; (iii) promiscuous mating [41,46,48]; and (iv) female signalling of fertility though sexual swellings [49–51]. Bonobos are commonly considered to be more peaceful and egalitarian than chimpanzees [42,52,53]. Consistent with this notion, one study based on mating behaviour found bonobos to have low reproductive skew [23]. However, studies using genetic paternity data revealed that bonobos at LuiKotale [54,55] and Wamba [56] have high reproductive skew, even higher than that reported for both eastern (Pan troglodytes schweinfurthii: Gombe, Mahale, Kibale and Budongo) and western (Pan troglodytes verus: Taï) chimpanzees [54]. However, the sample reported for bonobos remains small (12 population-years in bonobos compared to 41 population-years in chimpanzees) [54].
In this review, we will first consider potential mechanisms underlying reproductive skew in Pan. Second, we re-examine reproductive skew in Pan using existing and new data for bonobos (n = 27 population-years) and chimpanzees (n = 91 population-years). Third, we re-evaluate the mechanisms underlying reproductive skew in Pan and consider the broader consequences of our findings.
2. Mechanisms underlying reproductive skew in Pan
In many species, including fig wasps [57], wire-tailed manakins (Pipra filicauda) [58], greater sage-grouse (Centrocercus urophasianus) [59], Weddell seals (Leptonychotes weddellii) [60], elephant seals (Mirounga leonine) [61], feral horses (Equus ferus caballus), plains zebras (Equus quagga) [62] and red deer (Cervus elaphus) [63], high male reproductive skew results from intense contest competition between males. Contrary to the common portrayal of bonobos as peaceful, in both bonobos [55,64–67] and chimpanzees [68–74], more aggressive, high-ranking males obtain more mating success than less aggressive, low-ranking males. Nonetheless, dominance status does not completely predict male reproductive success in Pan, requiring the consideration of additional mechanisms [28,75,76].
(a) . Mechanisms operating under reproductive concession
(i) . Importance of male coalitionary aggression and opportunities for extra-community paternities
High-ranking males can gain fitness benefits from reproductive concessions when their reproductive success depends on the support of coalition members, as in wire-tailed manakins (Pi. filicauda) [58], bottlenose dolphins (Tursiops aduncus) [77] and lions (Panthera leo) [78]. Male chimpanzees defend a group territory, which serves as a feeding territory for themselves, their mates and their offspring; females reproduce faster when territory size is larger [79]. Males depend on support from other males to patrol territory boundaries and exclude outside males from mating with resident females [76,80,81], and to attack and kill extra-community members [82–86]. Within communities, males may also rely on support from allies to achieve high status and gain mating opportunities [28,75,87,88]. With their strictly hostile inter-community interactions and strong cooperative outgroup defence, male chimpanzees reduce opportunities for within-community females to reproduce with extra-community males.
Unlike male chimpanzees, male bonobos rarely provide agonistic support to other males [89], and mostly rely on individualistic strategies during conflict within [66,67,89] and between communities [90,91]. Thus, high-ranking male bonobos may have little motivation to concede reproductive opportunities, which should increase reproductive skew (table 1). Moreover, without strong coalitions among males, interactions among neighbouring bonobo communities are more relaxed; males and females can spend many hours associating with extra-community individuals [52,90,92–94], creating opportunities for males to sire offspring in other communities. Such opportunities can result in further inequalities in lifetime reproductive success, particularly if they correlate with dominance rank (table 1).
Table 1.
Predicted effects of different mechanisms that may drive variation in male reproductive skew in Pan.
| model | mechanism | type of relationship affected | predicted effect on skew | which species expected to have higher skew |
|---|---|---|---|---|
| reproductive concessions | reduced importance of male coalitionary aggression decreases the need to reward allies with reproductive concessions | male–male | increase | bonobos |
| with little cooperative outgroup defence, increased opportunities for extra- community paternities | male–male | unclear | unclear | |
| limited-control | increased social cohesion and female gregariousness increase monopolizability of females by top-ranking male | female–female | increase | bonobos |
| lower number of females per community increases monopolizability of females by top-ranking male | female–female | increase | bonobos | |
| more apparent female reproductive synchrony | female–female | decrease | chimpanzees | |
| reduced infanticide risk reduces female need to distribute paternity certainty across many males | male–female | increase | bonobos | |
| additional cues of ovulation (e.g. odour) improves male ability to detect fertile females and control reproduction | male–female | increase | unclear | |
| increased importance of female alliances reduces males' ability to control reproduction | female–female; male–female | unclear | unclear | |
| reduced male sexual coercion | male–female | unclear | unclear | |
| increased potential for expression of female mate preference | male–female | unclear | unclear | |
| increased influence of mothers on sons’ reproductive behaviour | male–female | unclear | unclear | |
| low number of males per community increases the ability of high-ranking males to monitor the activities of low-ranking males | male–male | increase | bonobos | |
| within-community relatedness between sexes and inbreeding avoidance | male–female | decrease | unclear |
(b) . Mechanisms operating under limited-control
(i) . Social cohesion and female gregariousness
Although variation in social cohesion occurs across chimpanzee communities (Budongo [95]; Gombe: [96–98]; Kanyawara: [99,100]; Mahale:[101]; Ngogo [102,103]; Taï [69,104,105]), bonobos generally appear to travel in larger [106–111] and more cohesive parties [104,112] than chimpanzees, with females being more gregarious in the former [106,109,112]. The mean bonobo party size contains 27–51% of all community members (n = 2 communities), compared to only 9–20% for chimpanzees (n = 8 communities) [106]. Increased female social cohesion may allow male bonobos to monitor the reproductive status and activity of maximally tumescent females more effectively, potentially leading to higher male reproductive skew in bonobos than chimpanzees (table 1).
In chimpanzees, the more dispersed ranging patterns of females may provide opportunities for consortships whereby a male and female travel together, away from others [71,76,113,114]. Insofar as lower ranking males use consortships to evade control by higher ranking males, consortships should reduce reproductive skew (table 1).
(ii) . Female reproductive synchrony
Female reproductive synchrony can hinder reproductive monopolization by high-ranking males [23,25,115], decreasing male reproductive skew. In primates, sexual swellings appear to have evolved as a female strategy to attract mating effort from many different males, thereby confusing paternity and reducing infanticide risk [115–118]. While both bonobos and chimpanzees exhibit sexual swellings, the extent to which females synchronously display full tumescence differs between the two species. Compared to chimpanzees, female bonobos: (i) resume swelling sooner after giving birth [49,51,64,119–122]; (ii) have longer maximally tumescent phases and greater variation in their duration; and (iii) therefore, spend a larger proportion of their interbirth intervals maximally tumescent [49,116,119,120,123]. Hence, for a given number of females, at any given time, more females display full tumescence in bonobos than chimpanzees [52,124]. Because bonobo swellings signal ovulation less reliably, monitoring and mate-guarding females seem more difficult and costly for male bonobos, reducing male reproductive skew compared to chimpanzees [55] (table 1).
(iii) . Additional cues of ovulation
In mammals, males also rely on other signals to infer female reproductive status and fecundability. This includes the use of olfactory cues by males in Djungarian hamsters (Phodopus campbelli) [125], ring-tailed lemurs (Lemur catta) [126] and cotton-top tamarins (Saguinus oedipus) [127]; the use of female vocal signals in grey mouse lemurs (Microcebus murinus) [128] and Barbary macaques [129]; and detection of female behavioural changes in rhesus macaques [130] and long-tailed macaques (Macaca fasciularis) [131]. Reproductive skew may then be increased, insofar as high-ranking males are better able to act on this information than low-ranking males. Chimpanzees in captivity use olfactory cues to obtain information about social relationships [132], suggesting they may be able to use them to detect ovulation [133], but whether either species of Pan does, in fact, do so remains untested.
(iv) . Importance of female alliance
In some species, females may cooperate with kin to combat male aggression and harassment (rhesus macaques: [134,135]; olive baboons: [136]; also review by Smuts [137]). Strikingly, in bonobos, females form coalitions with unrelated females; this strategy may have evolved to counter sexual coercion and/or infanticide by males [52,138,139]. One of the key behaviours facilitating female social bonding in bonobos is genito-genital rubbing, which often involves maximally tumescent females [140,141]. Having a full swelling, therefore, may increase a female's value as a social partner [104,142]. Thus, although sexual swellings presumably originated as a reproductive signal to males [99], they may also serve as a signal to attract affiliation and coalitionary support among females. This may in turn restrict males' reproductive control and increase females’ ability to express their mate preferences; the resulting effect on male reproductive skew would depend on female preferences (see below).
(v) . Male sexual coercion and female mate preference
While males commonly seek to control female reproductive activities, females have their own goals and preferences. Females can seek out preferred mating partners and avoid or resist advances by non-preferred males. Among chimpanzees, females face the risk of sexual coercion by males [69,74,76,143–147], and the extent to which females can exercise mate choice remains disputed [116,146,148,149]. However, whether sexual coercion increases male reproductive skew depends on which males use this strategy. While high-ranking male chimpanzees may coerce females to restrict their mating activity [74,114,145], lower ranking males also coerce females, including during consortships, when males threaten and attack females if they do not follow [71,76,114].
By contrast, female bonobos commonly outrank even the highest ranking males [52,139] and they do not appear to experience sexual coercion by males [67,139,150]. Thus, female bonobos probably have a greater capacity to act on their preferences than do female chimpanzees [151,152].
Factors that guide female preferences may affect male reproductive skew differently. Females can use male dominance status as a cue of genetic quality [153–155], in which case increased female choice would increase male reproductive skew, such as in Verreaux's sifakas [156] and white-faced capuchins [16], reaching an extreme in species with a lek mating system [157], such as in black grouse (Lyrurus tetrix) [158] and wire-tailed manakins (Pi. filicauda) [58]. Alternatively, if females base their choice on male traits that do not necessarily depend on dominance rank, such as the provisioning of resources, paternal care or other services, increased expression of female choice could reduce male reproductive skew. In spotted hyaenas, where females are dominant over males, females can express their preferences and tend to mate with immigrant males that are typically lower ranking than natal resident males, potentially to ensure genetic diversity in their offspring [15]. In bonobos, females both affiliate and copulate more with high-ranking than low-ranking males [66], suggesting that female preferences combine with competition among males to increase male bonobo reproductive skew [146] (table 1).
(vi) . Influence of mothers
Mothers can assist the reproductive success of their sons [159], as reported in orcas (Orcinus orca) [160] and northern muriquis (Brachyteles hypoxanthus) [161]. Although in chimpanzees, sons with mothers present have a higher rank and reproductive success [96,162–166], the impact of mothers appears stronger and more direct in bonobos [89,167,168]. Bonobo mothers may support their sons by interrupting copulations between females and other males [168], providing agonistic support to their sons during conflict with other males [52,139]. Males may also take advantage of their mother's social ties, to monitor maximally tumescent females more effectively, and to form close associations and copulate with them [169]. Because high-ranking females often occupy high-quality food patches [170], other females potentially mate with the sons of high-ranking females in exchange for better access to food. The effect of mothers on male reproductive skew probably depends on whether it is high- or low-ranking sons that have a living mother to provide support (table 1).
(vii) . Number of males
The number of males per group probably impacts the potential for male reproductive control [18,23,115,171–173]. In the Kasekela chimpanzee community at Gombe, low-ranking males sometimes do manage to sire offspring, despite PoA expectations, perhaps because males are too numerous and dispersed to be monitored effectively by the highest ranking male [76]. In this situation, the highest ranking male may focus on monitoring males that are close to him in rank [76]. Overall, we should expect male reproductive skew to be lower in communities with more males (table 1).
(viii) . Relatedness
Within-community relatedness may also influence the ability of high-ranking males to control female reproduction and thus the degree of male reproductive skew, owing to female efforts to avoid inbreeding [174]. Even when they remain in their natal community, female chimpanzees frequently reject mating attempts by close male kin and generally avoid conceiving offspring with them [174]. As in spotted hyaenas [15] and white-faced capuchins [175], females with high-ranking male relatives may willingly participate in consortships and mate preferentially with lower ranking, unrelated males to avoid costs of inbreeding [71,76,113,114]. High relatedness within communities may thus reduce male reproductive skew, but only insofar as females have high-ranking male kin, because low-ranking male kin may already be excluded from mating opportunities (table 1).
3. Existing data on reproductive skew in bonobos and chimpanzees
Among the 13 mechanisms reviewed above, which potentially affect variation in male reproductive skew in Pan, five predict higher reproductive skew for bonobos compared to chimpanzees, whereas only one mechanism predicts higher reproductive skew for chimpanzees (table 1). The relative importance of these different mechanisms remains to be assessed. Nonetheless, while a previous analysis based on mating behaviour inferred that bonobos should have low reproductive skew [23], the introduction of genetic paternity data revealed that high-ranking males bonobos sire more offspring [55]. For example, the most successful male in the Bompusa bonobo community sired 62% of the offspring during a 7 year period, in contrast with chimpanzees, in which the most successful male sired a mean of 26% of offspring (range: 7–56% for n = 5 communities) [54]. Similarly, genetic paternity data from Wamba bonobos revealed that the most successful male sired 86% and 75% of the offspring during a 4 year period for communities E1 and PE, respectively [56]. However, recent data from a small chimpanzee community at Bulindi found that the most successful male sired 88% of the offspring over a 4 year period [176], indicating that under suitable circumstances, chimpanzees can also exhibit high male reproductive skew. Thus, the extent to which male reproductive skew differs consistently between bonobos and chimpanzees warrants further investigation.
In long-lived, slowly reproducing taxa such as primates, it takes many years to obtain a clear understanding of factors affecting reproductive success [33]. For example, early studies of baboons (Papio spp. [18,177–183]) reported conflicting findings regarding the relationship between male rank and reproductive success. Only after many group-years of genetic paternity data had been obtained did it become clear that higher ranking males generally sire more offspring, but that over shorter time-scales factors such as demographic composition can disrupt the relationship between male rank and reproductive success [18,184]. Similarly, among mountain gorillas, researchers initially assumed the top-ranking male sired all offspring, but genetic paternity data eventually revealed reproduction by other males in groups with multiple males [17,37,184,185]. Accumulating data from different bonobo and chimpanzee populations now provide new opportunities for understanding male reproductive skew in Pan. We re-examine male reproductive skew in Pan by collating published data from Surbeck et al. [54], Ishizuka et al. [56] and McCarthy et al. [176] with new data from two chimpanzee communities in Gombe National Park, Tanzania, and two additional bonobo communities at the Kokolopori Bonobo Reserve, Democratic Republic of Congo. This results in a total of 91 population-years for chimpanzees and 27 population-years for bonobos, which provides greater statistical power than previous studies.
4. Methods to re-evaluate male reproductive skew in Pan
The PoA model uses a simple queuing model to provide a baseline of expected patterns of reproductive skew for a given number of males and fertile females. We therefore first examined the extent to which bonobos and chimpanzees followed or deviated from PoA predictions. Then we characterized and compared patterns of skew between the two species, using the multinomial index (M), the most successful sire's share, and the total number of within-community and extra-community offspring sired by identified males per year. We included only males old enough to potentially sire offspring (i.e. ≥10 years old) in all analyses, including only offspring with assigned fathers. We considered extra-community offspring as those with an identified within-community mother and an identified extra-community father. To calculate PoA, M, and the most successful sire's share, we considered only within-community offspring. Finally, we examined the effect of male demography on reproductive skew by conducting linear mixed models with M or the most successful sire's share as the dependent variable and the mean number of males per conception during the time period as the independent variable (electronic supplementary material).
To calculate skew, we included bonobo communities from three sites in the Democratic Republic of Congo: Bompusa from Luikotale [54] (n = 13 paternities), E1 from Wamba (n = 7 paternities) [56], and Ekalakala and Kokoalongo from Kokolopori (n = 20 paternities) (this study). For PoA, M, and the most successful sire's share, we included all individuals that were conceived between 2016 and 2019 in Ekalakala (n = 5 paternities) and Kokoalongo (n = 8 paternities). For the total number of offspring sired by each male per year, we included data from 2013 to 2019 for Ekalakala (n = 7) and included five extra-community paternities (Ekalakala: n= 4; Kokoalongo: n = 1). For the most successful sire's share, we also included PE from Wamba (n = 4 paternities) [56].
For chimpanzees, we included six communities for eastern chimpanzees (Pan t. schweinfurthii): Ngogo, Kibale National Park, Uganda (n = 109 paternities); Sonso, Budongo Forest, Uganda (n = 13 paternities); Bulindi, Uganda (n = 7 paternities) [176]; M-group, Mahale National Park, Tanzania (n = 11 paternities); and Kasekela and Mitumba from Gombe National Park, Tanzania (n = 75 paternities) (this study); and one community of western chimpanzees: North, Taï, Côte d'Ivoire (n = 13 paternities) [54] (electronic supplementary material, table S2).
For Gombe chimpanzees, we included genotyped individuals born between 1985 and 2017 (n = 62 paternities) in Kasekela and between 2004 and 2013 (n = 13 paternities) in Mitumba.
(a) . Priority-of-access model
Using data on female reproductive states and demography, we calculated the expected distribution of paternities (n = 75 for chimpanzees, n = 13 for bonobos) based on male dominance rank and the number of maximally tumescent females available at a given time in two chimpanzee communities (Kasekela and Mitumba) and two bonobo communities (Ekalakala and Kokoalongo), with 95% confidence intervals (CIs) for each rank. We also included PoA calculations from Surbeck et al. [54] for the Bompusa bonobo community, and Taï North and Sonso chimpanzee communities [59]. Deviations from PoA expectations imply the operation of other mechanisms besides male dominance rank and female reproductive synchrony.
(b) . Comparing bonobo and chimpanzee reproductive skew
(i) . The multinomial index
Many studies have used Nonac's binomial index (B) to measure reproductive skew [16,17,54,56,58,186–188]. However, this measure is sensitive to factors including community size (e.g. number of potential sires), productivity (e.g. number of paternities) and the time of presence of each sire during the period of observation [189]. To overcome these limitations, Ross et al. [189] introduced the multinomial index, M, which is insensitive to community size, sample size, mean reproductive success, and age-structure, allowing for better comparisons of skew across populations and species. M can be calculated using Nonac's B and remains robust to the factors previously cited through the conversion [189]. We report M below and Nonac's B in the electronic supplementary material.
Because apes reproduce slowly, and changes in male rank and demography each year can affect estimates of skew, we calculated M for each community using an overlapping set of time windows, moving at 1 year increments. We chose 7-year windows when possible, to maximize comparability with previous studies [54]. However, for four communities, only 4 years of data were available. For communities with several periods, we calculated the mean M across all overlapping 7-year periods.
To examine species differences in skew, we calculate 95% CIs for M for chimpanzees for a given mean number of males per conception using a parametric bootstrap. Since we did not have sufficient data to calculate these intervals for bonobos, we inferred species differences by visually inspecting whether estimates of M for bonobos fell outside the 95% CIs for chimpanzees. We used linear mixed models with M as the dependent variable, and the mean number of males per conception as the independent variable, with a random intercept for the community and a random slope for the mean number of males within each community. Ideally, we would also control for the number of maximally tumescent females, but these data were not available for seven communities. From the results of these models, we generated 100 bootstrap samples to obtain the 95% CIs (electronic supplementary material).
(ii) . Most successful male's share of reproduction
As an additional measure of male reproductive skew, we examined the proportion of offspring sired by the most successful male in the community. This measure is independent of differences in size and productivity between communities and is not affected by the absence of unidentified/ungenotyped males who did not reproduce [54]. Along with the data used to calculate M, we added data from the PE bonobo community where information about the most successful sire's share of reproduction was available (chimpanzees: n = 228 conceptions; bonobos: n = 37 conceptions; electronic supplementary material, table S2). As with M, we generated 95% confidence intervals for a given mean number of males per conception in chimpanzees (figure 2b; electronic supplementary material).
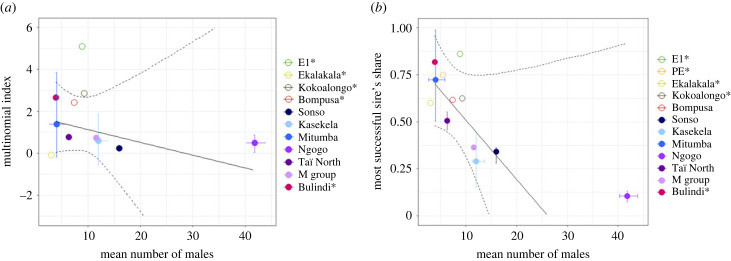
Figure 2.
Male reproductive skew in bonobos and chimpanzees. (a) Relationship between the mean number of males per conception and male reproductive skew measured by the multinomial index, M. (b) Relationship between the mean number of males per conception and the most successful sire's share of reproduction. Circles indicate the mean for a given community; open circles represent chimpanzees and filled circles represent bonobos. Vertical and horizontal error bars indicate the 2.5% and 97.5%. The solid and dotted lines represent the model estimate and 95% confidence intervals, respectively, for the effect of number of males in chimpanzees. Asterisks indicate communities with only one 4-year period (electronic supplementary material, table S3).
(iii) . Total number of offspring sired by each male per year
The striking differences in inter-community relationships between chimpanzees and bonobos can strongly affect male reproductive opportunities. Specifically, the hostile relationships between chimpanzee communities limit opportunities for males to sire offspring with extra-community females, whereas the more tolerant inter-community interactions of bonobos provide opportunities for extra-community mating. Therefore, we examined the number of within- and extra-community paternities obtained by each individual male in the Kokolopori bonobo communities and the Gombe and Bulindi chimpanzee communities. We could assign extra-community paternities because we obtained genetic samples from multiple communities (electronic supplementary material).
Since calculation of the most successful sire's share of reproduction does not require information about the number of males present in the community, we extended the study period for Ekalakala from 2016–2019 to 2013–2019, before the community was fully habituated. Doing so enabled us to include all successfully assigned paternities, whether the paternity was within-community (Ekalakala: n = 7; Kokoalongo: n = 8; Bulindi: n = 8; Kasekela: n = 62; Mitumba: n = 13) or extra-community (Ekalakala: n = 4; Kokoalongo: n = 1). The number of extra-community paternities may be underestimated as males may have sired offspring in other unfollowed and ungenotyped neighbouring communities.
To facilitate between-community comparison, we calculated the mean number of offspring each male sired per year, by dividing the total number of paternities for each male by the total number of years this male was observed during the respective study period.
5. New results on male reproductive skew in Pan
(a) . Priority-of-access model
For both bonobos and chimpanzees, the highest ranking males sired the highest proportion of offspring compared to other males in the community (electronic supplementary material, figure S2). For all four chimpanzee communities but only one of the three bonobo communities, the observed proportion fell within the 95% CI of that expected by the PoA model (figure 1).
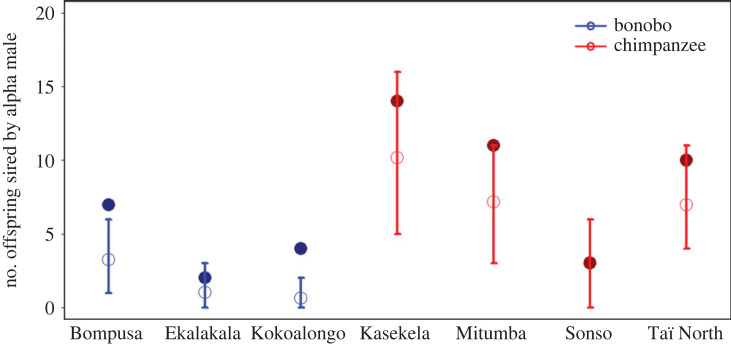
Figure 1.
Observed (filled circles) versus expected (open circles) number of offspring sired by the highest ranking male in three bonobo (blue) and four chimpanzee (red) communities. The observed number is based on all infants sired by the current alpha male, rather than the number sired by any particular male. The expected total number is based on the PoA model. Error bars represent the 95% confidence intervals obtained from resampling the expected number of offspring sired by a given male 10 000 times.
As previously reported [76], low-ranking Kasekela males (ranks 10, 12, 13 and 15 of 15) obtained more offspring than expected by the PoA model (electronic supplementary material, figure S2). Although low-ranking males also obtained more paternities than expected in Sonso (ranks 7 and 12 of 17) and Taï North (rank 8 of 8), the distribution of male reproduction more closely aligned with patterns predicted by the PoA model (electronic supplementary material, figure S2; [54,80,81]).
(b) . Comparing bonobo and chimpanzee reproductive skew
(i) . The multinomial index
As indicated by the multinomial index, M, bonobos had higher male reproductive skew (mean ± s.d.: M = 2.57 ± 2.12; n = 4 bonobo communities) than chimpanzees (M = 0.979 ± 0.819; n = 7 chimpanzee communities; electronic supplementary material, table S2). Also, bonobos exhibited greater variation in M than chimpanzees (electronic supplementary material, figure S3a). Estimates of M for two bonobo communities, Kokoalongo and E1, exceeded the 95% CIs calculated for chimpanzees, whereas two other bonobo communities, Ekalakala and Bompusa, were within the chimpanzee range (figure 2a).
(ii) . Most successful male's share of reproduction
The most successful male sired more offspring in bonobos (mean ± s.d.: 0.69 ± 0.11) than in chimpanzees (0.44 ± 0.25) (electronic supplementary material, table S3). However, only in one of the four bonobo communities (E1) did this exceed the 95% CI for chimpanzees (figure 2b).
(iii) . Total number of offspring sired by each male per year
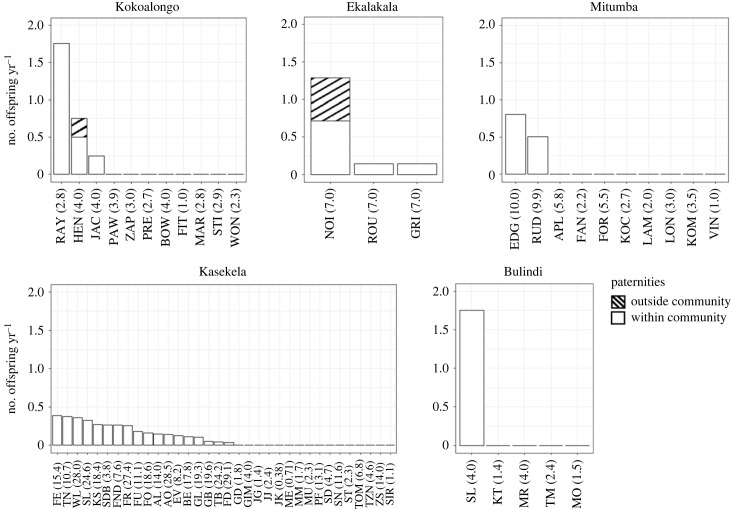
Two male bonobos sired offspring in other communities: NOI, in Ekalakala, sired three offspring in Kokoalongo and one in Fekako (a habituated neighbouring community with insufficient data to be included in this study), and HEN, in Kokoalongo, sired one offspring in Ekalakala. The distribution of male reproduction appeared more skewed in Ekalakala when we considered both within- and extra-community paternities (figure 3), as the most successful male was NOI, whose four extra-community paternities gave him a total of nine offspring (electronic supplementary material, table S2). Measures that did not include extra-community paternities—M and the most successful sire's share of reproduction—thus underestimated the degree of reproductive skew in this population. The only two males who sired extra-community offspring both had their mother present in the community at the time of siring (NOI and HEN).
Figure 3.
Number of paternities per year per male for two bonobo communities (Ekalakala and Kokoalongo) and three chimpanzee communities (Bulindi, Kasekela and Mitumba). Values in parentheses represent the number of years each male was present in the community throughout the study period.
6. Discussion
Based on data accumulating from long-term field studies, we found considerable variation in reproductive skew within and between communities of both Pan species. Overall, bonobos exhibited greater variation in M, an improved measure of reproductive skew, than chimpanzees. The estimates for M exceeded the 95% CI for chimpanzees for two out of three bonobo communities, and the estimate for the most successful sire's share exceeded the 95% CI for chimpanzees for one of five bonobo communities. However, we also found that the two species overlapped in all three measures of male reproductive skew (M, the most successful sire's share, and the total number of paternities per male per year). As has been found for other species [17,37,184,185], longer term data thus provide a more nuanced picture of male reproductive skew in Pan.
Despite the common perception of bonobos as more peaceful than chimpanzees [42,52,53], several studies report that males of both species compete over access to maximally tumescent females (bonobos: [55,64–67,90]; chimpanzees: [68–74]). Aggression among males thus drives reproductive inequality in both species.
The number of males in the community may also affect male–male competitive dynamics and thus reproductive skew. Although the number of males did not have a statistically significant effect on skew in our dataset, we note that among chimpanzees, skew was the highest in communities with few males, and lower in communities with many males (figure 2; electronic supplementary material). More data are needed, even for chimpanzees, to ensure a sufficient sample size to assess demographic effects with confidence.
The hypothesis that high reproductive synchrony among female bonobos should result in low reproductive skew [55] is supported by the quantitative PoA predictions (electronic supplementary material, figure S2). However, the highest ranking male achieved more paternities than expected in two of the three bonobo communities. We, therefore, must consider additional mechanisms to explain male reproductive skew in bonobos. One such mechanism involves higher gregariousness among females, which allows male bonobos to monitor and monopolize females more effectively. Additionally, male bonobos appear to have fewer incentives to concede reproduction than male chimpanzees, given the reduced importance of male coalitions for intergroup competition in bonobos. Supporting this view, in chimpanzees, but not bonobos, low-ranking males sire more offspring than expected. However, this pattern could also result from the more limited control possible in the larger, more dispersed communities of chimpanzees; distinguishing among these alternatives poses a formidable challenge [28].
While distinguishing between limited control and concessions remains challenging, confirmation that bonobos have a relatively high reproductive skew supports the view that reproductive concessions may relate to the intensity of between-group competition. In humans, researchers have proposed that ‘reproductive levelling’ (i.e. low male reproductive skew) evolved because intense between-group aggression selected for more cooperation within groups [190,191]. The contrast between bonobos and chimpanzees is consistent with this hypothesis, though additional mechanisms should also be considered. For example, Chapais [192] has argued that polygyny declined in hominins after the invention of deadly weapons increased the costs of competition among males and decreased variance in competitive ability [192].
Differences in male–female dynamics between the two species may additionally influence reproductive skew. When considering the physiological mechanisms underlying reproductive competition between males [193], bonobos and chimpanzees show different patterns of associated changes in testosterone levels [66,194]. Male chimpanzees have higher aggression rates and testosterone levels in the presence of maximally tumescent females [194]. By contrast, high-ranking male bonobos, who are more aggressive to other males but also more affiliative towards females, do not have higher testosterone levels in the presence of maximally tumescent females [66]. As heightened testosterone levels during male mate competition generally indicate the relevance of aggression in securing reproductive success [193,195], other factors besides male–male aggression, such as male–female affiliative interactions that are potentially suppressed by high testosterone levels [66], may affect male reproductive skew in bonobos.
Male reproductive skew depends not just on competition among males, but also on relationships between males and females, and female choice. Studies of other species have found that males can gain mating opportunities by forming close associations with females [181,196,197] and by caring for offspring [181,196–199]. Female bonobos probably have more agency in the expression of mate choice owing to their high status [52,139] and strong female alliances [52,138,139]. Because female bonobos tend to affiliate and mate with high-ranking rather than low-ranking males, female preferences probably contribute to higher skew in bonobos than chimpanzees. Female choice can also explain the occurrence of extra-community paternities in bonobos and other species including superb fairy-wrens (Malurus cyaneus) [200], blue tits (Parus caeruleus) [201], dwarf mongooses [202], black howler monkeys (Alouatta caraya) [203], ring-tailed lemurs [204], rhesus macaques [17], long-tailed macaques [131] and toque macaques (Macaca sinica) [205].
In bonobos, the strongest relationship between males and females involves mothers and sons. Interestingly, both males that sired extra-community offspring had a living mother present in their community. Bonobo mothers actively support their son's mating attempts with extra-community females during inter-community encounters (L. Cheng 2018, personal observation). However, the actual mechanism by which maternal presence positively influences a male's reproductive success within and across communities needs further investigation.
Our results confirm that substantial reproductive skew can emerge among males in species with promiscuous mating. While bonobos have long been characterized as peaceful and egalitarian [42,52,53], with lower reproductive skew [23,206] than chimpanzees, the accumulation of additional paternity data provides a more nuanced view of the competitive dynamics in reproductive contexts in Pan.
In summary, bonobo males have higher reproductive skew than expected based on degree of reproductive synchrony among females. Detailed comparison of factors promoting reproductive skew in Pan highlights that models of reproductive skew should consider the effect of between-group competition on incentives for reproductive concessions, as well as factors beyond those related to male–male dynamics, such as female grouping patterns and factors related to male–female dynamics including the expression of female choice.
Acknowledgements
We thank the Institut Congolais pour la Conservations de la Nature and the Ministry of Scientific Research and Technology in the Democratic Republic of the Congo for their support and permission to work in the Kokolopori Bonobo Reserve, Democratic Republic of Congo, as well as the Bonobo Conservation Initiative and Vie Sauvage, especially Sally Coxe and Albert Lotana Lokasola, and the Jane Goodall Institute, for supporting our work at the Kokolopori Bonobo Reserve and the Gombe National Park. We are grateful to the Tanzania Commission for Science and Technology, the Tanzania Wildlife Research Institute and the Tanzania National Parks, for their support and permission to work at Gombe. We thank all the field staff and visiting researchers at the Kokolopori Bonobo Research Project and the Gombe Stream Research Centre for contributing to the long-term database. We also thank Nisarg Desai for providing statistical advice.
Contributor Information
Maud Mouginot, Email: mougi002@umn.edu.
Leveda Cheng, Email: levedacheng@g.harvard.edu.
Ethics
All methods used in this study were non-invasive. All aspects of this study complied with the ethical standards of the American Society of Primatologists Principles for the Ethical Treatment of Non Human Primates for the use of animals in research.
Data accessibility
New data used in this paper (for chimpanzees at the Gombe National Park and for bonobos at the Kokolopori Bonobo Reserve) are available in the electronic supplementary material [207]. Published data used in this paper are available in Surbeck et al. [54], Ishizuka et al. [56] and McCarthy et al. [176].
Authors' contributions
M.M.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, writing—original draft, writing—review and editing; L.C.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, writing—original draft, writing—review and editing; M.S.: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—review and editing; M.L.W.: conceptualization, funding acquisition, investigation, methodology, resources, supervision, writing—review and editing; J.T.F.: writing—review and editing; V.S.: data curation, formal analysis, methodology, writing—review and editing; E.E.W.: data curation, formal analysis, methodology, resources, writing—review and editing; L.V.: resources, writing—review and editing; B.H.H.: formal analysis, methodology, writing—review and editing; Y.L.: formal analysis and methodology; I.C.G.: resources, writing—review and editing; A.E.P.: resources, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We are grateful to the following funders for making this research possible: Harvard University, Duke University, Franklin and Marshall College, George Washington University, the University of Minnesota, the Max Planck Society, the Institute for Advanced Study Toulouse (IAST) funding from the French National Research Agency (ANR) under grant no. ANR-17-EURE-0010 (Investissements d'Avenir program), the Leakey Foundation, the National Institutes of Health (grant nos. R00 HD057992, R01 AI 091595, R01 AI050529 and R01 AI120810), the National Science Foundation (grant nos. DBS-9021946, SBR-9319909, BCS-0452315, IOS-1052693, IOS-1457260, BCS-0648481, BCS-1753437 and BCS-1743506), the Arcus Foundation, Carnegie Corporation, the Leo S. Guthman Foundation, Margo Marsh, Mazuri (AAZV), the Morris Animal Foundation, the National Geographic Society, the Harris Steel Group, the Wilkie Foundation, the William T. Grant Foundation, the Windibrow Foundation, the Jane Goodall Institute, and the Alexander von Humboldt Foundation (Feodor Lynen Return Fellowship).
References
- 1.Vehrencamp SL. 1983. A model for the evolution of despotic versus egalitarian societies. Anim. Behav. 31, 667-682. ( 10.1016/S0003-3472(83)80222-X) [DOI] [Google Scholar]
- 2.Strauss ED, Shizuka D. 2022. The ecology of wealth inequality in animal societies. Proc. R. Soc. B 289, 20220500. ( 10.1098/rspb.2022.0500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riehl CP. 2012. Mating system and reproductive skew in a communally breeding cuckoo: hard-working males do not sire more young. Anim. Behav. 84, 707-714. ( 10.1016/j.anbehav.2012.06.028) [DOI] [Google Scholar]
- 4.Faulkes CG, Bennett NC. 2009. Reproductive skew in African mole-rats: behavioral and physiological mechanisms to maintain high skew. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Jones CB, Hager R), pp. 369-396. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Sherman PW, Lacey EA, Reeve HK, Keller L. 1995. Forum: the eusociality continuum. Behav. Ecol. 6, 102-108. ( 10.1093/beheco/6.1.102) [DOI] [Google Scholar]
- 6.Hager R, Jones CB. 2009. Reproductive skew in vertebrates: proximate and ultimate causes. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 7.Clutton-Brock TH. 1988. Reproductive success: studies of individual variation in contrasting breeding systems. Chicago, IL: University of Chicago Press. [Google Scholar]
- 8.Magrath RD, Heinsohn R, Johnstone RA. 2004. Reproductive skew. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 9.Magrath RD, Heinsohn RG. 2000. Reproductive skew in birds: models, problems and prospects. J. Avian Biol. 31, 247-258. ( 10.1034/j.1600-048X.2000.310217.x) [DOI] [Google Scholar]
- 10.Reeve HK, Keller L. 2001. Tests of reproductive-skew models in social insects. Annu. Rev. Entomol. 46, 347-385. ( 10.1146/annurev.ento.46.1.347) [DOI] [PubMed] [Google Scholar]
- 11.Moss JB, Gerber GP, Schwirian A, Jackson AC, Welch ME. 2019. Evidence for dominant males but not choosy females in an insular rock iguana. Behav. Ecol. 30, 181-193. ( 10.1093/beheco/ary131) [DOI] [Google Scholar]
- 12.Haydock J, Koenig WD. 2002. Reproductive skew in the polygynandrous acorn woodpecker. Proc. Natl Acad. Sci. USA 99, 7178-7183. ( 10.1073/pnas.102624199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcés-Restrepo MF, Peery MZ, Reid B, Pauli JN. 2017. Individual reproductive strategies shape the mating system of tree sloths. J. Mammal. 98, 1417-1425. ( 10.1093/jmammal/gyx094) [DOI] [Google Scholar]
- 14.Murray KL, Fleming TH. 2008. Social structure and mating system of the buffy flower bat, Erophylla sezekorni (Chiroptera, Phyllostomidae). J. Mammal. 89, 1391-1400. ( 10.1644/08-MAMM-S-068.1) [DOI] [Google Scholar]
- 15.Engh AL, Funk SM, Horn RCV, Scribner KT, Bruford MW, Libants S, Szykman M, Smale L, Holekamp KE. 2002. Reproductive skew among males in a female-dominated mammalian society. Behav. Ecol. 13, 193-200. ( 10.1093/beheco/13.2.193) [DOI] [Google Scholar]
- 16.Muniz L, Perry S, Manson JH, Gilkenson H, Gros-Louis J, Vigilant L. 2010. Male dominance and reproductive success in wild white-faced capuchins (Cebus capucinus) at Lomas Barbudal, Costa Rica. Am. J. Primatol. 72, 1118-1130. ( 10.1002/ajp.20876) [DOI] [PubMed] [Google Scholar]
- 17.Widdig A, Bercovitch FB, Streich WJ, Sauermann U, Nürnberg P, Krawczak M. 2004. A longitudinal analysis of reproductive skew in male rhesus macaques. Proc. R. Soc. B 271, 819-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberts SC, Watts HE, Altmann J. 2003. Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Anim. Behav. 65, 821-840. ( 10.1006/anbe.2003.2106) [DOI] [Google Scholar]
- 19.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of Man (ed. B Campbell), pp. 136-179. Aldine, Chicago, IL: Routledge. [Google Scholar]
- 20.Clutton-Brock T. 2007. Sexual selection in males and females. Science 318, 1882-1885. ( 10.1126/science.1133311) [DOI] [PubMed] [Google Scholar]
- 21.Kappeler PM, van Schaik CP. 2002. Evolution of primate social systems. Int. J. Primatol. 23, 707-740. ( 10.1023/A:1015520830318) [DOI] [Google Scholar]
- 22.Palombit RA. 2015. Infanticide as sexual conflict: coevolution of male strategies and female counterstrategies. Cold Spring Harb. Perspect. Biol. 7, a017640. ( 10.1101/cshperspect.a017640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kutsukake N, Nunn CL. 2006. Comparative tests of reproductive skew in male primates: the roles of demographic factors and incomplete control. Behav. Ecol. Sociobiol. 60, 695-706. ( 10.1007/s00265-006-0213-1) [DOI] [Google Scholar]
- 24.Clutton-Brock TH, Parker GA. 1995. Sexual coercion in animal societies. Anim. Behav. 49, 1345-1365. ( 10.1006/anbe.1995.0166) [DOI] [Google Scholar]
- 25.Ostner J, Nunn CL, Schülke O. 2008. Female reproductive synchrony predicts skewed paternity across primates. Behav. Ecol. 19, 1150-1158. ( 10.1093/beheco/arn093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnstone RA. 2000. Models of reproductive skew: a review and synthesis (Invited Article). Ethology 106, 5-26. ( 10.1046/j.1439-0310.2000.00529.x) [DOI] [Google Scholar]
- 27.Reeve HK, Emlen ST, Keller L. 1998. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders? Behav. Ecol. 9, 267-278. ( 10.1093/beheco/9.3.267) [DOI] [Google Scholar]
- 28.Bray J, Pusey AE, Gilby IC. 2016. Incomplete control and concessions explain mating skew in male chimpanzees. Proc. R. Soc. B 283, 20162071. ( 10.1098/rspb.2016.2071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cant MA. 1998. A model for the evolution of reproductive skew without reproductive suppression. Anim. Behav. 55, 163-169. ( 10.1006/anbe.1997.0589) [DOI] [PubMed] [Google Scholar]
- 30.Buston PM, Reeve HK, Cant MA, Vehrencamp SL, Emlen ST. 2007. Reproductive skew and the evolution of group dissolution tactics: a synthesis of concession and restraint models. Anim. Behav. 74, 1643-1654. ( 10.1016/j.anbehav.2007.03.003) [DOI] [Google Scholar]
- 31.Altmann SA. 1962. A field study of the sociobiology of rhesus monkeys, Macaca mulatta. Ann. N Y Acad. Sci. 102, 338-435. ( 10.1111/j.1749-6632.1962.tb13650.x) [DOI] [PubMed] [Google Scholar]
- 32.Reeve HK, Ratnieks FLW. 1993. Queen-queen conflict in polygynous societies: mutual tolerance and reproductive skew. In Queen number and sociality in insects (ed. L Keller), pp. 45-85. Oxford, UK: Oxford University Press. [Google Scholar]
- 33.Port M, Kappeler PM. 2010. The utility of reproductive skew models in the study of male primates, a critical evaluation. Evol. Anthropol. 19, 46-56. ( 10.1002/evan.20243) [DOI] [Google Scholar]
- 34.Townsend AK, Clark AB, McGowan KJ, Lovette IJ. 2009. Reproductive partitioning and the assumptions of reproductive skew models in the cooperatively breeding American crow. Anim. Behav. 77, 503. ( 10.1016/j.anbehav.2008.10.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clutton-Brock TH, et al. 2001. Cooperation, control, and concession in meerkat groups. Science 291, 478-481. ( 10.1126/science.291.5503.478) [DOI] [PubMed] [Google Scholar]
- 36.Charpentier M, Peignot P, Hossaert-McKey M, Gimenez O, Setchell JM, Wickings EJ. 2005. Constraints on control: factors influencing reproductive success in male mandrills (Mandrillus sphinx). Behav. Ecol. 16, 614-623. ( 10.1093/beheco/ari034) [DOI] [Google Scholar]
- 37.Bradley BJ, Robbins MM, Williamson EA, Steklis HD, Steklis NG, Eckhardt N, Boesch C, Vigilant L. 2005. Mountain gorilla tug-of-war: silverbacks have limited control over reproduction in multimale groups. Proc. Natl Acad. Sci. USA 102, 9418-9423. ( 10.1073/pnas.0502019102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cant MA, Reeve HK. 2002. Female control of the distribution of paternity in cooperative breeders. Am. Nat. 160, 602-611. ( 10.1086/342820) [DOI] [PubMed] [Google Scholar]
- 39.Port M, Schülke O, Ostner J. 2018. Reproductive tolerance in male primates: old paradigms and new evidence. Evol. Anthropol. 27, 107-120. ( 10.1002/evan.21586) [DOI] [PubMed] [Google Scholar]
- 40.Nishida T. 1968. The social group of wild chimpanzees in the Mahali Mountains. Primates 9, 167-224. ( 10.1007/BF01730971) [DOI] [Google Scholar]
- 41.Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior. Cambridge, MA & London, UK: The Belknap Press of Harvard University Press. [Google Scholar]
- 42.Kano T. 1992. The last ape: pygmy chimpanzee behavior and ecology, p. 248. Stanford, CA: Stanford Univeristy Press. [Google Scholar]
- 43.Hohmann G, Fruth B. 2002. Dynamics in social organization of bonobos (Pan paniscus). In Behavioural diversity in chimpanzees and bonobos, (eds C Boesch, L Marchant, G Hohmann), pp. 138-154. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 44.Hanamura S. 2015. Fission-fusion grouping. In Mahale chimpanzees: 50 years of research (eds M Nakamura, K Hosaka, N Itoh, K Zamma), pp. 106-118. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 45.Mitani JC. 2009. Cooperation and competition in chimpanzees: current understanding and future challenges. Evol. Anthropol. 18, 215-227. ( 10.1002/evan.20229) [DOI] [Google Scholar]
- 46.Furuichi T. 1989. Social interactions and the life history of female Pan paniscus in Wamba, Zaire. Int. J. Primatol. 10, 173-197. ( 10.1007/BF02735199) [DOI] [Google Scholar]
- 47.Emery Thompson M. 2013. Reproductive ecology of female chimpanzees. Am. J. Primatol. 75, 222-237. ( 10.1002/ajp.22084) [DOI] [PubMed] [Google Scholar]
- 48.Nishida T. 1972. Inter-unit-group relationships among wild chimpanzees of the Mahali Mountains. Kyoto Univ. Afr. Stu. 7, 131-169. [Google Scholar]
- 49.Furuichi T. 1987. Sexual swelling, receptivity, and grouping of wild pygmy chimpanzee females at Wamba, Zaïre. Primates 28, 309-318. ( 10.1007/BF02381014) [DOI] [Google Scholar]
- 50.Tutin CE. 1980. Reproductive behaviour of wild chimpanzees in the Gombe National Park, Tanzania. J. Reprod. Fertil. Suppl. 28, 43-57. [PubMed] [Google Scholar]
- 51.Wrangham RW. 1993. The evolution of sexuality in chimpanzees and bonobos. Hum. Nat. 4, 47-79. ( 10.1007/BF02734089) [DOI] [PubMed] [Google Scholar]
- 52.Furuichi T. 2011. Female contributions to the peaceful nature of bonobo society. Evol. Anthropol. 20, 131-142. ( 10.1002/evan.20308) [DOI] [PubMed] [Google Scholar]
- 53.de Waal FBM, Lanting F. 1998. Bonobo: the forgotten ape. Berkeley, CA: University of California Press. [Google Scholar]
- 54.Surbeck M, Langergraber KE, Fruth B, Vigilant L, Hohmann G. 2017. Male reproductive skew is higher in bonobos than chimpanzees. Curr. Biol. 27, R640-R641. ( 10.1016/j.cub.2017.05.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerloff U, Hartung B, Fruth B, Hohmann G, Tautz D. 1999. Intracommunity relationships, dispersal pattern and paternity success in a wild living community of bonobos (Pan paniscus) determined from DNA analysis of faecal samples. Proc. R. Soc. Lond. B 266, 1189-1195. ( 10.1098/rspb.1999.0762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishizuka S, Kawamoto Y, Sakamaki T, Tokuyama N, Toda K, Okamura H, Furuichi T. 2018. Paternity and kin structure among neighbouring groups in wild bonobos at Wamba. R. Soc. Open Sci. 5, 171006. ( 10.1098/rsos.171006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray MG. 1990. Comparative morphology and mate competition of flightless male fig wasps. Anim. Behav. 39, 434-443. ( 10.1016/S0003-3472(05)80406-3) [DOI] [Google Scholar]
- 58.Ryder TB, Parker PG, Blake JG, Loiselle BA. 2009. It takes two to tango: reproductive skew and social correlates of male mating success in a lek-breeding bird. Proc. R. Soc. B 276, 2377-2384. ( 10.1098/rspb.2009.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jahner JP, Gibson D, Weitzman CL, Blomberg EJ, Sedinger JS, Parchman TL. 2016. Fine-scale genetic structure among greater sage-grouse leks in central Nevada. BMC Evol. Biol. 16, 127. ( 10.1186/s12862-016-0702-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harcourt RG, Kingston JJ, Cameron MF, Waas JR, Hindell MA. 2007. Paternity analysis shows experience, not age, enhances mating success in an aquatically mating pinniped, the Weddell seal (Leptonychotes weddellii). Behav. Ecol. Sociobiol. 61, 643-652. ( 10.1007/s00265-006-0294-x) [DOI] [Google Scholar]
- 61.Fabiani A, Galimberti F, Sanvito S, Hoelzel AR. 2004. Extreme polygyny among southern elephant seals on Sea Lion Island, Falkland Islands. Behav. Ecol. 15, 961-969. ( 10.1093/beheco/arh112) [DOI] [Google Scholar]
- 62.Rubenstein DI, Nuñez CM. 2009. Sociality and reproductive skew in horses and zebras. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Jones CB, Hager R), pp. 196-226. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 63.Clutton-Brock TH, Guinness FE, Albon SD. 1982. Red deer: behavior and ecology of two sexes. Chicago, IL: University of Chicago Press. See https://press.uchicago.edu/ucp/books/book/chicago/R/bo24325870.html. [Google Scholar]
- 64.Hashimoto C, Ryu H, Mouri K, Shimizu K, Sakamaki T, Furuichi T. 2022. Physical, behavioral, and hormonal changes in the resumption of sexual receptivity during postpartum infertility in female bonobos at Wamba. Primates 63, 109-121. ( 10.1007/s10329-021-00968-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Surbeck M, Deschner T, Weltring A, Hohmann G. 2012. Social correlates of variation in urinary cortisol in wild male bonobos (Pan paniscus). Horm. Behav. 62, 27-35. ( 10.1016/j.yhbeh.2012.04.013) [DOI] [PubMed] [Google Scholar]
- 66.Surbeck M, Deschner T, Schubert G, Weltring A, Hohmann G. 2012. Mate competition, testosterone and intersexual relationships in bonobos, Pan paniscus. Anim. Behav. 83, 659-669. ( 10.1016/j.anbehav.2011.12.010) [DOI] [Google Scholar]
- 67.Mouginot M, Surbeck M, Wilson ML, Desai N. In preparation. Differences in expression of male aggression between wild bonobos and chimpanzees. [DOI] [PMC free article] [PubMed]
- 68.Wilson ML, et al. 2014. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414-417. ( 10.1038/nature13727) [DOI] [PubMed] [Google Scholar]
- 69.Boesch C, Boesch-Achermann H. 2000. The chimpanzees of the Taï forest: behavioural ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 70.Bygott J. 1979. Agonistic behaviour, dominance, and social structure in wild chimpanzees of the Gombe National Park. In The great apes (eds DA Hamburg, ER McCown), pp. 405-428. Menlo Park, CA: Benjamin. [Google Scholar]
- 71.Van Lawick-Goodall J. 1968. The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim. Behav. Monogr. 1, 161-IN12. ( 10.1016/S0066-1856(68)80003-2) [DOI] [Google Scholar]
- 72.Muller MN. 2002. Agonistic relations among Kanyawara chimpanzees. In Behavioural diversity in chimpanzees and bonobos (eds C. Boesch, L. Marchant, G. Hohmann), pp. 112-124. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 73.Muller MN, Mitani JC. 2005. Conflict and cooperation in wild chimpanzees. In Advances in the study of behavior (eds PJB. Slater, J Rosenblatt, C Snowdon, T Roper, M Naguib), pp. 275-331. New York, NY: Academic Press. [Google Scholar]
- 74.Watts DP. 2022. Male chimpanzee sexual coercion and mating success at Ngogo. Am. J. Primatol. 84, e23361. ( 10.1002/ajp.23361) [DOI] [PubMed] [Google Scholar]
- 75.Feldblum JT, Krupenye C, Bray J, Pusey AE, Gilby IC. 2021. Social bonds provide multiple pathways to reproductive success in wild male chimpanzees. iScience 24, 102864. ( 10.1016/j.isci.2021.102864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wroblewski EE, Murray C, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. 2009. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 77, 873-885. ( 10.1016/j.anbehav.2008.12.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiszniewski J, Corrigan S, Beheregaray LB, Möller LM. 2012. Male reproductive success increases with alliance size in Indo-Pacific bottlenose dolphins (Tursiops aduncus). J. Anim. Ecol. 81, 423-431. ( 10.1111/j.1365-2656.2011.01910.x) [DOI] [PubMed] [Google Scholar]
- 78.Packer C, Gilbert DA, Pusey AE, O'Brieni SJ. 1991. A molecular genetic analysis of kinship and cooperation in African lions. Nature 351, 562-565. ( 10.1038/351562a0) [DOI] [Google Scholar]
- 79.Williams JM, Oehlert GW, Carlis JV, Pusey AE. 2004. Why do male chimpanzees defend a group range? Anim. Behav. 68, 523-532. ( 10.1016/j.anbehav.2003.09.015) [DOI] [Google Scholar]
- 80.Newton-Fisher NE, Thompson ME, Reynolds V, Boesch C, Vigilant L. 2010. Paternity and social rank in wild chimpanzees (Pan troglodytes) from the Budongo Forest, Uganda. Am. J. Phys. Anthropol. 142, 417-428. ( 10.1002/ajpa.21241) [DOI] [PubMed] [Google Scholar]
- 81.Boesch C, Kohou G, Néné H, Vigilant L. 2006. Male competition and paternity in wild chimpanzees of the Taï forest. Am. J. Phys. Anthropol. 130, 103-115. ( 10.1002/ajpa.20341) [DOI] [PubMed] [Google Scholar]
- 82.Langergraber KE, Watts DP, Vigilant L, Mitani JC. 2017. Group augmentation, collective action, and territorial boundary patrols by male chimpanzees. Proc. Natl Acad. Sci. USA 114, 7337-7342. ( 10.1073/pnas.1701582114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Massaro AP, Gilby IC, Desai N, Weiss A, Feldblum JT, Pusey AE, Wilson ML. 2022. Correlates of individual participation in boundary patrols by male chimpanzees. Phil. Trans. R. Soc. B 377, 20210151. ( 10.1098/rstb.2021.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Samuni L, Crockford C, Wittig RM. 2021. Group-level cooperation in chimpanzees is shaped by strong social ties. Nat. Commun. 12, 539. ( 10.1038/s41467-020-20709-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watts D, Mitani J. 2001. Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour 138, 299-327. ( 10.1163/15685390152032488) [DOI] [Google Scholar]
- 86.Wilson ML, Wrangham RW. 2003. Intergroup relations in chimpanzees. Ann. Rev. Anthropol. 32, 363-392. ( 10.1146/annurev.anthro.32.061002.120046) [DOI] [Google Scholar]
- 87.Duffy KG, Wrangham RW, Silk JB. 2007. Male chimpanzees exchange political support for mating opportunities. Curr. Biol. 17, R586-R587. ( 10.1016/j.cub.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 88.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE. 2013. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373-381. ( 10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Surbeck M, Hohmann G. 2017. Affiliations, aggressions and an adoption: male-male relationships in wild bonobos. In Bonobos: unique in mind, brain, and behavior (eds B Hare, S Yamamoto), pp. 35-46. Oxford, UK: Oxford University Press. [Google Scholar]
- 90.Cheng L, Samuni L, Lucchesi S, Deschner T, Surbeck M. 2022. Love thy neighbour: behavioural and endocrine correlates of male strategies during intergroup encounters in bonobos. Anim. Behav. 187, 319-330. ( 10.1016/j.anbehav.2022.02.014) [DOI] [Google Scholar]
- 91.Tokuyama N, Sakamaki T, Furuichi T. 2019. Inter-group aggressive interaction patterns indicate male mate defense and female cooperation across bonobo groups at Wamba, Democratic Republic of the Congo. Am. J. Phys. Anthropol. 170, 535-550. ( 10.1002/ajpa.23929) [DOI] [PubMed] [Google Scholar]
- 92.Lucchesi S, Cheng L, Janmaat K, Mundry R, Pisor A, Surbeck M. 2020. Beyond the group: how food, mates, and group size influence intergroup encounters in wild bonobos. Behav. Ecol. 31, 519-532. ( 10.1093/beheco/arz214) [DOI] [Google Scholar]
- 93.Moscovice LR, Hohmann G, Trumble BC, Fruth B, Jaeggi AV. 2022. Dominance or tolerance? Causes and consequences of a period of increased intercommunity encounters among bonobos (Pan paniscus) at LuiKotale. Int. J. Primatol. 43, 434-459. ( 10.1007/s10764-022-00286-y) [DOI] [Google Scholar]
- 94.Furuichi T. 2020. Variation in intergroup relationships among species and among and within local populations of African apes. Int. J. Primatol. 41, 203-223. ( 10.1007/s10764-020-00134-x) [DOI] [Google Scholar]
- 95.Emery Thompson M, Wrangham RW. 2008. Male mating interest varies with female fecundity in Pan troglodytes schweinfurthii of Kanyawara, Kibale National Park. Int. J. Primatol. 29, 885-905. ( 10.1007/s10764-008-9286-1) [DOI] [Google Scholar]
- 96.Williams JM, Pusey AE, Carlis JV, Farm BP, Goodall J. 2002. Female competition and male territorial behaviour influence female chimpanzees' ranging patterns. Anim. Behav. 63, 347-360. ( 10.1006/anbe.2001.1916) [DOI] [Google Scholar]
- 97.Wrangham RW, Smuts BB. 1980. Sex differences in the behavioural ecology of chimpanzees in the Gombe National Park, Tanzania. J. Reprod. Fertil. Suppl. 28, 13-31. [PubMed] [Google Scholar]
- 98.Murray CM, Wroblewski E, Pusey AE. 2007. New case of intragroup infanticide in the chimpanzees of Gombe National Park. Int. J. Primatol. 28, 23-37. ( 10.1007/s10764-006-9111-7) [DOI] [Google Scholar]
- 99.Wrangham RW. 2002. The cost of sexual attraction: is there a trade-off in female Pan between sex appeal and received coercion. In Behavioural diversity in chimpanzees and bonobos (eds C Boesch, G Hohmann, L Marchant), pp. 204-2019. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 100.Wrangham RW. 1996. Social ecology of the Kanyawara chimpanzees: implications for understanding the cost of great apes groups. In Great apes societies (eds WC McGrew, L Marchant, T Nishida), pp. 45-57. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 101.Hasegawa T. 1989. Sexual behavior of immigrant and resident female Pan troglodytes at Mahale. In Understanding chimpanzees (eds P Heltne, LA Marquadt), pp. 90-103. Cambridge, MA: Harvard University Press. [Google Scholar]
- 102.Wakefield ML. 2008. Grouping patterns and competition among female Pan troglodytes schweinfurthii at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. 29, 907. ( 10.1007/s10764-008-9280-7) [DOI] [Google Scholar]
- 103.Watts DP. 2012. Long-term research on chimpanzee behavioral ecology in Kibale National Park, Uganda. In Long-term field studies of primates (eds Kappeler PM, Watts DP), pp. 313-338. Berlin, Germany: Springer. [Google Scholar]
- 104.Surbeck M, Girard-Buttoz C, Samuni L, Boesch C, Fruth B, Crockford C, Wittig RM, Hohmann G. 2021. Attractiveness of female sexual signaling predicts differences in female grouping patterns between bonobos and chimpanzees. Commun. Biol. 4, 1-11. ( 10.1038/s42003-021-02641-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lehmann J, Boesch C. 2008. Sexual differences in chimpanzee sociality. Int. J. Primatol. 29, 65-81. ( 10.1007/s10764-007-9230-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Furuichi T. 2009. Factors underlying party size differences between chimpanzees and bonobos: a review and hypotheses for future study. Primates 50, 197-209. ( 10.1007/s10329-009-0141-6) [DOI] [PubMed] [Google Scholar]
- 107.Malenky RK, Kuroda S, Vineberg EO, Wrangham RW. 1996. The significance of terrestrial herbaceous foods for bonobos, chimpanzees, and gorillas. In Chimpanzee culture (eds RW Wrangham, WC McGrew, FBM de Waal, PG Heltne), pp. 59-76. Cambridge, MA: Harvard University Press. [Google Scholar]
- 108.Mulavwa M, et al. 2008. Seasonal changes in fruit production and party size of bonobos at Wamba. In The bonobos: behavior, ecology, and conservation (eds T Furuichi, J Thompson), pp. 121-134. New York, NY: Springer; ( 10.1007/978-0-387-74787-3_7). [DOI] [Google Scholar]
- 109.White FJ. 1988. Party composition and dynamics in Pan paniscus. Int. J. Primatol. 9, 179-193. ( 10.1007/BF02737400) [DOI] [Google Scholar]
- 110.White FJ, Wrangham RW. 1988. Feeding competition and patch size in the chimpanzee species Pan paniscus and Pan troglodytes. Behaviour 105, 148-164. [Google Scholar]
- 111.Malenky RK, Wrangham RW. 1994. A quantitative comparison of terrestrial herbaceous food consumption by Pan paniscus in the Lomako Forest, Zaire, and Pan troglodytes in the Kibale Forest, Uganda. Am. J. Primatol. 32, 1-12. ( 10.1002/ajp.1350320102) [DOI] [PubMed] [Google Scholar]
- 112.Chapman CA, White FJ, Wrangham RW. 1996. Party size in chimpanzees and bonobos. In Chimpanzee culture (eds RW Wrangham, WC McGrew, FBM de Waal, PG Heltne), pp. 41-58. Cambridge, MA: Harvard University Press. [Google Scholar]
- 113.Constable JL, Ashley MV, Goodall J, Pusey AE. 2001. Noninvasive paternity assignment in Gombe chimpanzees. Mol. Ecol. 10, 1279-1300. ( 10.1046/j.1365-294x.2001.01262.x) [DOI] [PubMed] [Google Scholar]
- 114.Tutin CEG. 1979. Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. Sociobiol. 6, 29-38. ( 10.1007/BF00293242) [DOI] [Google Scholar]
- 115.Nunn CL. 1999. The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim. Behav. 58, 229-246. ( 10.1006/anbe.1999.1159) [DOI] [PubMed] [Google Scholar]
- 116.Matsumoto-Oda A. 1999. Mahale chimpanzees: grouping patterns and cycling females. Am. J. Primatol. 47, 197-207. () [DOI] [PubMed] [Google Scholar]
- 117.Hrdy SB. 1981. The women that never evolved. Cambridge, MA: Harvard University Press. [Google Scholar]
- 118.Hrdy SB, Whitten PL. 1987. Patterning of sexual activity. In Primate societies (eds B Smuts, D Cheney, R Seyfarth, RW Wrangham, T Struhsaker), pp. 370-384. Chicago, IL: University of Chicago Press. [Google Scholar]
- 119.Douglas PH, Hohmann G, Murtagh R, Thiessen-Bock R, Deschner T. 2016. Mixed messages: wild female bonobos show high variability in the timing of ovulation in relation to sexual swelling patterns. BMC Evol. Biol. 16, 140. ( 10.1186/s12862-016-0691-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deschner T, Heistermann M, Hodges K, Boesch C. 2003. Timing and probability of ovulation in relation to sex skin swelling in wild West African chimpanzees, Pan troglodytes verus. Anim. Behav. 66, 551-560. ( 10.1006/anbe.2003.2210) [DOI] [Google Scholar]
- 121.Reichert KE, Heistermann M, Hodges JK, Boesch C, Hohmann G. 2002. What females tell males about their reproductive status: are morphological and behavioural cues reliable signals of ovulation in bonobos (Pan paniscus)? Ethology 108, 583-600. ( 10.1046/j.1439-0310.2002.00798.x) [DOI] [Google Scholar]
- 122.Furuichi T. 1992. The prolonged estrus of females and factors influencing mating in a wild group of bonobos (Pan paniscus) in Wamba, Zaire. In Topics in primatology, vol. 2: behavior, ecology, and conservation (eds N Itoigawa, Y Sugiyama, G Sackett, R Thompson), pp. 179-190. Tokyo, Japan: University of Tokyo Press. [Google Scholar]
- 123.Tutin CEG, McGinnis PR. 1981. Chimpanzee reproduction in the wild. In Reproductive biology of the great apes: comparative and biomedical perspectives (ed. CE Graham), pp. 239-264. New York, NY: Academic Press. [Google Scholar]
- 124.Jaeggi AV, Boose KJ, White FJ, Gurven M. 2016. Obstacles and catalysts of cooperation in humans, bonobos, and chimpanzees: behavioural reaction norms can help explain variation in sex roles, inequality, war and peace. Behaviour 153, 1015-1051. ( 10.1163/1568539X-00003347) [DOI] [Google Scholar]
- 125.Lai SC, Yu N, Johnston V, Johnston RE. 1996. Odors providing sexual information in djungarian hamsters: evidence for an across-odor code. Horm. Behav. 30, 26-36. [DOI] [PubMed] [Google Scholar]
- 126.Scordato ES, Drea CM. 2007. Scents and sensibility: information content of olfactory signals in the ringtailed lemur, Lemur catta. Anim. Behav. 73, 301-314. ( 10.1016/j.anbehav.2006.08.006) [DOI] [Google Scholar]
- 127.Ziegler TE, Epple G, Snowdon CT, Porter TA, Belcher AM, Küderling I. 1993. Detection of the chemical signals of ovulation in the cotton-top tamarin, Saguinus oedipus. Anim. Behav. 45, 313-322. ( 10.1006/anbe.1993.1036) [DOI] [Google Scholar]
- 128.Buesching CD, Heistermann M, Hodges JK, Zimmermann E. 1998. Multimodal oestrus advertisement in a small nocturnal prosimian, Microcebus murinus. Folia Primatol. 69, 295-308. ( 10.1159/000052718) [DOI] [Google Scholar]
- 129.Semple S, McComb K. 2000. Perception of female reproductive state from vocal cues in a mammal species. Proc. R. Soc. Lond. B 267, 707-712. ( 10.1098/rspb.2000.1060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wallen K, Winston LA, Gaventa S, Davis-DaSilva M, Collins DC. 1984. Periovulatory changes in female sexual behavior and patterns of ovarian steroid secretion in group-living rhesus monkeys. Horm. Behav. 18, 431-450. ( 10.1016/0018-506X(84)90028-X) [DOI] [PubMed] [Google Scholar]
- 131.Engelhardt A, Hodges JK, Niemitz C, Heistermann M. 2005. Female sexual behavior, but not sex skin swelling, reliably indicates the timing of the fertile phase in wild long-tailed macaques (Macaca fascicularis). Horm. Behav. 47, 195-204. ( 10.1016/j.yhbeh.2004.09.007) [DOI] [PubMed] [Google Scholar]
- 132.Henkel S, Setchell JM. 2018. Group and kin recognition via olfactory cues in chimpanzees (Pan troglodytes). Proc. R. Soc. B 285, 20181527. ( 10.1098/rspb.2018.1527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Emery Thompson M. 2005. Endocrinology and ecology of wild female chimpanzee reproduction. PhD thesis, Harvard University, Cambridge, MA, USA. See https://www.proquest.com/docview/305002716/abstract/73F062EFD1D64039PQ/1. [Google Scholar]
- 134.Bernstein IS, Ehardt CL. 1985. Agonistic aiding: kinship, rank, age, and sex influences. Am. J. Primatol. 8, 37-52. ( 10.1002/ajp.1350080105) [DOI] [PubMed] [Google Scholar]
- 135.Kaplan JR. 1977. Patterns of fight interference in free-ranging rhesus monkeys. Am. J. Phys. Anthropol. 47, 279-287. ( 10.1002/ajpa.1330470208) [DOI] [Google Scholar]
- 136.Smuts B. 1987. Gender, aggression, and influence. In Primate societies (eds BB Smuts, DL Cheney, RM Seyfarth, RW Wrangham, TT Struhsaker), pp. 386-400. Chicago, IL: University of Chicago Press. [Google Scholar]
- 137.Smuts B. 1992. Male aggression against women: an evolutionary perspective. Hum. Nat. 3, 1-44. ( 10.1007/BF02692265) [DOI] [PubMed] [Google Scholar]
- 138.Tokuyama N, Furuichi T. 2016. Do friends help each other? Patterns of female coalition formation in wild bonobos at Wamba. Anim. Behav. 119, 27-35. ( 10.1016/j.anbehav.2016.06.021) [DOI] [Google Scholar]
- 139.Surbeck M, Hohmann G. 2013. Intersexual dominance relationships and the influence of leverage on the outcome of conflicts in wild bonobos (Pan paniscus). Behav. Ecol. Sociobiol. 67, 1767-1780. ( 10.1007/s00265-013-1584-8) [DOI] [Google Scholar]
- 140.Fruth B, Hohmann G. 2006. Social grease for females? Same-sex genital contacts in wild bonobos. In Homosexual behaviour in animals: an evolutionary perspective (eds Sommer V, Vasey PL), pp. 294-315. New York, NY: Cambridge University Press. [Google Scholar]
- 141.Moscovice LR, Surbeck M, Fruth B, Hohmann G, Jaeggi AV, Deschner T. 2019. The cooperative sex: sexual interactions among female bonobos are linked to increases in oxytocin, proximity and coalitions. Horm. Behav. 116, 104581. ( 10.1016/j.yhbeh.2019.104581) [DOI] [PubMed] [Google Scholar]
- 142.Ryu H, Hill DA, Furuichi T. 2015. Prolonged maximal sexual swelling in wild bonobos facilitates affiliative interactions between females. Behaviour 152, 285-311. ( 10.1163/1568539X-00003212) [DOI] [Google Scholar]
- 143.Kaburu SSK, Newton-Fisher NE. 2015. Trading or coercion? Variation in male mating strategies between two communities of East African chimpanzees. Behav. Ecol. Sociobiol. 69, 1039-1052. ( 10.1007/s00265-015-1917-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Reddy RB, Langergraber KE, Sandel AA, Vigilant L, Mitani JC. 2021. The development of affiliative and coercive reproductive tactics in male chimpanzees. Proc. R. Soc. B 288, 20202679. ( 10.1098/rspb.2020.2679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Feldblum JT, Wroblewski EE, Rudicell RS, Hahn BH, Paiva T, Cetinkaya-Rundel M, Pusey AE, Gilby IC. 2014. Sexually coercive male chimpanzees sire more offspring. Curr. Biol. 24, 2855-2860. ( 10.1016/j.cub.2014.10.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Muller MN, Thompson ME, Kahlenberg SM, Wrangham RW. 2011. Sexual coercion by male chimpanzees shows that female choice may be more apparent than real. Behav. Ecol. Sociobiol. 65, 921-933. ( 10.1007/s00265-010-1093-y) [DOI] [Google Scholar]
- 147.Enigk DK, Emery Thompson M, Machanda ZP, Wrangham RW, Muller MN. 2021. Female-directed aggression by adolescent male chimpanzees primarily constitutes dominance striving, not sexual coercion. Am. J. Phys. Anthropol. 176, 66-79. ( 10.1002/ajpa.24296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stumpf RM, Boesch C. 2006. The efficacy of female choice in chimpanzees of the Taï Forest, Côte d'Ivoire. Behav. Ecol. Sociobiol. 60, 749-765. ( 10.1007/s00265-006-0219-8) [DOI] [Google Scholar]
- 149.Pieta K. 2008. Female mate preferences among Pan troglodytes schweinfurthii of Kanyawara, Kibale National Park, Uganda. Int. J. Primatol. 29, 845-864. ( 10.1007/s10764-008-9282-5) [DOI] [Google Scholar]
- 150.Surbeck M, et al. 2017. Sex-specific association patterns in bonobos and chimpanzees reflect species differences in cooperation. R. Soc. Open Sci. 4, 161081. ( 10.1098/rsos.161081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paoli T. 2009. The absence of sexual coercion in bonobos. In Sexual coercion in primates and humans (eds MN Muller, RW Wrangham), pp. 410-423. Cambridge, MA: Harvard University Press. [Google Scholar]
- 152.Furuichi T, Hashimoto C. 2004. Sex differences in copulation attempts in wild bonobos at Wamba. Primates 45, 59-62. ( 10.1007/s10329-003-0055-7) [DOI] [PubMed] [Google Scholar]
- 153.Mays HL, Hill GE. 2004. Choosing mates: good genes versus genes that are a good fit. Trends Ecol. Evol. 19, 554-559. ( 10.1016/j.tree.2004.07.018) [DOI] [PubMed] [Google Scholar]
- 154.Møller APM, Alatalo RV. 1999. Good-genes effects in sexual selection. Proc. R. Soc. Lond. B 266, 85. ( 10.1098/rspb.1999.0607) [DOI] [Google Scholar]
- 155.Clutton-Brock T, McAuliffe K. 2009. Female mate choice in mammals. Q. Rev. Biol. 84, 3-27. ( 10.1086/596461) [DOI] [PubMed] [Google Scholar]
- 156.Kappeler PM, Schäffler L. 2008. The lemur syndrome unresolved: extreme male reproductive skew in sifakas (Propithecus verreauxi), a sexually monomorphic primate with female dominance. Behav. Ecol. Sociobiol. 62, 1007-1015. ( 10.1007/s00265-007-0528-6) [DOI] [Google Scholar]
- 157.Wiley RH. 1991. Lekking in birds and mammals: behavioral and evolutionary issues. In Advances in the study of behavior (eds PJB. Slater, JS Rosenblatt, C Beer, M Milinski), pp. 201-291. New York, NY: Academic Press. [Google Scholar]
- 158.Alatalo RV, Höglund J, Lundberg A, Sutherland WJ. 1992. Evolution of black grouse leks: female preferences benefit males in larger leks. Behav. Ecol. 3, 53-59. ( 10.1093/beheco/3.1.53) [DOI] [Google Scholar]
- 159.Clutton-Brock T. 2016. Mammal societies. New York, NY: John Wiley & Sons. [Google Scholar]
- 160.Foster EA, Franks DW, Mazzi S, Darden SK, Balcomb KC, Ford JKB, Croft DP. 2012. Adaptive prolonged postreproductive life span in killer whales. Science 337, 1313-1313. ( 10.1126/science.1224198) [DOI] [PubMed] [Google Scholar]
- 161.Strier KB, Chaves PB, Mendes SL, Fagundes V, Di Fiore A. 2011. Low paternity skew and the influence of maternal kin in an egalitarian, patrilocal primate. Proc. Natl Acad. Sci. USA 108, 18 915-18 919. ( 10.1073/pnas.1116737108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Crockford C, Samuni L, Vigilant L, Wittig RM. 2020. Postweaning maternal care increases male chimpanzee reproductive success. Sci. Adv. 6, eaaz5746. ( 10.1126/sciadv.aaz5746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pusey AE, Schroepfer-Walker K. 2013. Female competition in chimpanzees. Phil. Trans. R. Soc. B 368, 20130077. ( 10.1098/rstb.2013.0077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pusey A, Williams J, Goodall J. 1997. The influence of dominance rank on the reproductive success of female chimpanzees. Science 277, 828-831. ( 10.1126/science.277.5327.828) [DOI] [PubMed] [Google Scholar]
- 165.Murray CM, Eberly LE, Pusey AE. 2006. Foraging strategies as a function of season and rank among wild female chimpanzees (Pan troglodytes). Behav. Ecol. 17, 1020-1028. ( 10.1093/beheco/arl042) [DOI] [Google Scholar]
- 166.Wittig RM, Boesch C. 2003. Food competition and linear dominance hierarchy among female chimpanzees of the Taï National Park. Int. J. Primatol. 24, 847-867. ( 10.1023/A:1024632923180) [DOI] [Google Scholar]
- 167.Surbeck M, et al. 2019. Males with a mother living in their group have higher paternity success in bonobos but not chimpanzees. Curr. Biol. 29, R354-R355. ( 10.1016/j.cub.2019.03.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Surbeck M, Mundry R, Hohmann G. 2011. Mothers matter! Maternal support, dominance status and mating success in male bonobos (Pan paniscus). Proc. R. Soc. B 278, 590-598. ( 10.1098/rspb.2010.1572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yokoyama T, Furuichi T. 2022. Why bonobos show a high reproductive skew towards high-ranking males: analyses for association and mating patterns concerning female sexual states. Primates 63, 483-494. ( 10.1007/s10329-022-01004-1) [DOI] [PubMed] [Google Scholar]
- 170.Nurmi NO, Hohmann G, Goldstone LG, Deschner T, Schülke O. 2018. The ‘tolerant chimpanzee’—towards the costs and benefits of sociality in female bonobos. Behav. Ecol. 29, 1325-1339. ( 10.1093/beheco/ary118) [DOI] [Google Scholar]
- 171.Cowlishaw G, Dunbar RIM. 1991. Dominance rank and mating success in male primates. Anim. Behav. 41, 1045-1056. ( 10.1016/S0003-3472(05)80642-6) [DOI] [Google Scholar]
- 172.Mitani JC, Gros-Louis J, Manson JH. 1996. Number of males in primate groups: comparative tests of competing hypotheses. Am. J. Primatol. 38, 315-332. () [DOI] [PubMed] [Google Scholar]
- 173.Mitani JC, Watts DP, Pepper JW, Merriwether DA. 2002. Demographic and social constraints on male chimpanzee behaviour. Anim. Behav. 64, 727-737. ( 10.1006/anbe.2002.4014) [DOI] [Google Scholar]
- 174.Walker KK, Rudicell RS, Li Y, Hahn BH, Wroblewski E, Pusey AE. 2017. Chimpanzees breed with genetically dissimilar mates. R. Soc. Open Sci. 4, 160422. ( 10.1098/rsos.160422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wikberg EC, Jack KM, Fedigan LM, Campos FA, Yashima AS, Bergstrom ML, Hiwatashi T, Kawamura S. 2017. Inbreeding avoidance and female mate choice shape reproductive skew in capuchin monkeys (Cebus capucinus imitator). Mol. Ecol. 26, 653-667. ( 10.1111/mec.13898) [DOI] [PubMed] [Google Scholar]
- 176.McCarthy MS, Lester JD, Cibot M, Vigilant L, McLennan MR. 2020. Atypically high reproductive skew in a small wild chimpanzee community in a human-dominated landscape. FPR 91, 688-696. ( 10.1159/000508609) [DOI] [PubMed] [Google Scholar]
- 177.DeVore I. 1965. Male dominance and mating behavior in baboons. In Sex and behavior (ed. FA Beach), pp. 266-289. New York, NY: John Wiley & Sons. [Google Scholar]
- 178.Hausfater G. 1975. Dominance and reproduction in baboons (Papio cynocephalus). Contrib. Primatol. 7, 1-150. [PubMed] [Google Scholar]
- 179.Packer C. 1979. Male dominance and reproductive activity in Papio anubis. Anim. Behav. 27, 37-45. ( 10.1016/0003-3472(79)90127-1) [DOI] [PubMed] [Google Scholar]
- 180.Noë R, Sluijter AA. 1990. Reproductive tactics of male savanna baboons. Behaviour 113, 117-170. [Google Scholar]
- 181.Smuts BB. 1985. Sex and friendship in baboons. New York, NY: Routledge. [Google Scholar]
- 182.Bercovitch FB. 1986. Male rank and reproductive activity in savanna baboons. Int. J. Primatol. 7, 533-550. ( 10.1007/BF02736660) [DOI] [Google Scholar]
- 183.Strum SC. 1982. Agonistic dominance in male baboons: an alternative view. Int. J. Primatol. 3, 175-202. ( 10.1007/BF02693494) [DOI] [Google Scholar]
- 184.Altmann J, Alberts SC. 2003. Variability in reproductive success viewed from a life-history perspective in baboons. Am. J. Hum. Biol. 15, 401-409. ( 10.1002/ajhb.10157) [DOI] [PubMed] [Google Scholar]
- 185.Vigilant L, Roy J, Bradley BJ, Stoneking CJ, Robbins MM, Stoinski TS. 2015. Reproductive competition and inbreeding avoidance in a primate species with habitual female dispersal. Behav. Ecol. Sociobiol. 69, 1163-1172. ( 10.1007/s00265-015-1930-0) [DOI] [Google Scholar]
- 186.Williams DA. 2004. Female control of reproductive skew in cooperatively breeding brown jays (Cyanocorax morio). Behav. Ecol. Sociobiol. 55, 370-380. ( 10.1007/s00265-003-0728-7) [DOI] [Google Scholar]
- 187.Rossiter SJ, Ransome RD, Faulkes CG, Dawson DA, Jones G. 2006. Long-term paternity skew and the opportunity for selection in a mammal with reversed sexual size dimorphism. Mol. Ecol. 15, 3035-3043. ( 10.1111/j.1365-294X.2006.02987.x) [DOI] [PubMed] [Google Scholar]
- 188.Dugdale HL, Macdonald DW, Pope LC, Johnson PJ, Burke T. 2008. Reproductive skew and relatedness in social groups of European badgers, Meles meles. Mol. Ecol. 17, 1815-1827. ( 10.1111/j.1365-294X.2008.03708.x) [DOI] [PubMed] [Google Scholar]
- 189.Ross CT, Jaeggi AV, Borgerhoff Mulder M, Smith JE, Smith EA, Gavrilets S, Hooper PL. 2020. The multinomial index: a robust measure of reproductive skew. Proc. R. Soc. B 287, 20202025. ( 10.1098/rspb.2020.2025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bowles S. 2006. Group competition, reproductive leveling, and the evolution of human altruism. Science 314, 1569-1572. ( 10.1126/science.1134829) [DOI] [PubMed] [Google Scholar]
- 191.Alexander RD. 1987. The biology of moral systems. New York, NY: Aledine De Gruyter. [Google Scholar]
- 192.Chapais B. 2010. The deep structure of human society: primate origins and evolution. In Mind the gap: tracing the origins of human universals (eds Kappeler PM, Silk J), pp. 19-51. Berlin, Germany: Springer. [Google Scholar]
- 193.Wingfield JC, Hegner RE, Dufty AM, Ball GF. 1990. The ‘challenge hypothesis': theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829-846. [Google Scholar]
- 194.Muller MN, Wrangham RW. 2004. Dominance, aggression and testosterone in wild chimpanzees: a test of the ‘challenge hypothesis’. Anim. Behav. 67, 113-123. ( 10.1016/j.anbehav.2003.03.013) [DOI] [Google Scholar]
- 195.Wingfield JC. 1984. Androgens and mating systems: testosterone-induced polygyny in normally monogamous birds. Auk 101, 665-671. ( 10.2307/4086893) [DOI] [Google Scholar]
- 196.Nguyen N, Van Horn RC, Alberts SC, Altmann J. 2009. ‘Friendships’ between new mothers and adult males: adaptive benefits and determinants in wild baboons (Papio cynocephalus). Behav. Ecol. Sociobiol. 63, 1331-1344. ( 10.1007/s00265-009-0786-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Städele V, Roberts ER, Barrett BJ, Strum SC, Vigilant L, Silk JB. 2019. Male–female relationships in olive baboons (Papio anubis): parenting or mating effort? J. Hum. Evol. 127, 81-92. ( 10.1016/j.jhevol.2018.09.003) [DOI] [PubMed] [Google Scholar]
- 198.Finkenwirth C, Burkart JM. 2018. Why help? Relationship quality, not strategic grooming predicts infant-care in group-living marmosets. Physiol. Behav. 193, 108-116. ( 10.1016/j.physbeh.2018.02.050) [DOI] [PubMed] [Google Scholar]
- 199.Paul A, Kuester J, Arnemann J. 1996. The sociobiology of male–infant interactions in Barbary macaques, Macaca sylvanus. Anim. Behav. 51, 155-170. ( 10.1006/anbe.1996.0013) [DOI] [Google Scholar]
- 200.Dunn PO, Cockburn A. 1998. Costs and benefits of extra-group paternity in superb fairy-wrens. Ornithol. Monogr. 147-161. (https://www.jstor.org/stable/40166722) [Google Scholar]
- 201.Kempenaers B, Verheyen GR, Dhondi AA. 1997. Extrapair paternity in the blue tit (Parus caeruleus): female choice, male characteristics, and offspring quality. Behav. Ecol. 8, 481-492. ( 10.1093/beheco/8.5.481) [DOI] [Google Scholar]
- 202.Nichols HJ, Cant MA, Sanderson JL. 2015. Adjustment of costly extra-group paternity according to inbreeding risk in a cooperative mammal. Behav. Ecol. 26, 1486-1494. ( 10.1093/beheco/arv095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Van Belle S, Estrada A, Ziegler TE, Strier KB. 2009. Sexual behavior across ovarian cycles in wild black howler monkeys (Alouatta pigra): male mate guarding and female mate choice. Am. J. Primatol. 71, 153-164. ( 10.1002/ajp.20635) [DOI] [PubMed] [Google Scholar]
- 204.Parga JA, Sauther ML, Cuozzo FP, Youssouf Jacky IA, Lawler RR, Sussman RW, Gould L, Pastorini J. 2016. Paternity in wild ring-tailed lemurs (Lemur catta): implications for male mating strategies. Am J. Primatol. 78, 1316-1325. ( 10.1002/ajp.22584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Keane B, Dittus WPJ, Melnick DJ. 1997. Paternity assessment in wild groups of toque macaques Macaca sinica at Polonnaruwa, Sri Lanka using molecular markers. Mol. Ecol. 6, 267-282. ( 10.1046/j.1365-294X.1997.00178.x) [DOI] [PubMed] [Google Scholar]
- 206.Holekamp KE, Engh AL. 2009. Reproductive skew in female-dominated mammalian societies. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Jones CB, Hager R), pp. 53-83. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 207.Mouginot M, et al. 2023. Reproductive inequality among males in the genus Pan. Figshare. ( 10.6084/m9.figshare.c.6662632) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mouginot M, et al. 2023. Reproductive inequality among males in the genus Pan. Figshare. ( 10.6084/m9.figshare.c.6662632) [DOI] [PMC free article] [PubMed]
Data Availability Statement
New data used in this paper (for chimpanzees at the Gombe National Park and for bonobos at the Kokolopori Bonobo Reserve) are available in the electronic supplementary material [207]. Published data used in this paper are available in Surbeck et al. [54], Ishizuka et al. [56] and McCarthy et al. [176].