Abstract
The extent of (in)equality is highly diverse across species of social mammals, but we have a poor understanding of the factors that produce or inhibit equitable social organizations. Here, we adopt a comparative evolutionary perspective to test whether the evolution of social dominance hierarchies, a measure of social inequality in animals, exhibits phylogenetic conservatism and whether interspecific variation in these traits can be explained by sex, age or captivity. We find that hierarchy steepness and directional consistency evolve rapidly without any apparent constraint from evolutionary history. Given this extraordinary variability, we next consider multiple factors that have evolved to mitigate social inequality. Social networks, coalitionary support and knowledge transfer advantage to privilege some individuals over others. Nutritional access and prenatal stressors can impact the development of offspring, generating health disparities with intergenerational consequences. Intergenerational transfer of material resources (e.g. stone tools, food stashes, territories) advantage those who receive. Nonetheless, many of the same social species that experience unequal access to food (survival) and mates (reproduction) engage in levelling mechanisms such as food sharing, adoption, revolutionary coalitions, forgiveness and inequity aversion. Taken together, mammals rely upon a suite of mechanisms of (in)equality to balance the costs and benefits of group living.
This article is part of the theme issue ‘Evolutionary ecology of inequality’.
Keywords: cooperation, coalition formation, dominance, food sharing, hierarchy, inequity aversion
1. Introduction
Wealth inequality is widespread, globally influencing patterns of health [1], reproduction [2] and lifespan [3] for humans. Unequal access to material resources accumulated in the environment, embodied differences (e.g. size, strength or knowledge) and social connections [4,5] exerts a powerful influence on the health [6] and opportunities [7] of individuals. The unequal distributions of wealth can shape divergent destinies [8,9], contributing to the economic concept of intergenerational wealth mobility [10–12], and even alter the evolutionary trajectories across human societies [13]. This imbalance [13,14] is featured in small-scale agricultural [5], pastoral [5] and large-scale modern [15] societies. Humans regularly challenge these sources of inequality through food sharing, peacekeeping, inequity aversion and forgiveness. Inequality also characterizes many animal societies from mole rats [16], hyaenas [17] and mongooses [18] to chimpanzees [19] across the tree of life [20–22], but the evolutionary forces shaping inequality across species have received far less attention.
The notion of natural systems as intrinsically unfair is widespread in western culture—Tennyson's widely quoted lines from In Memoriam [23] which paint a natural world filled with ‘evil dreams' (p. 34), 'at strife’ (p. 34) with God, and ‘red in tooth and claw’ (p. 36) offer but one example. This view contributes to the notion that ‘nearly all societies are structured by some type of dominance hierarchy’ [24, p. 2]. By contrast to these popular treatments of social organizations, social structures vary from egalitarian to those with strict dominance hierarchies [25,26]. At present, we have a poor understanding of the controls governing the evolution of fairness in animal societies. Comparative evolutionary approaches offer the potential both to characterize the extant diversity in social systems across the tree of life and reveal new insights into the evolutionary origins and underlying mechanisms promoting equality and inequality in animals [20,21,27].
2. (In)equality in mammalian societies
Inequality refers to the phenotypic variation shaped by social structures—reinforced within or across generations—that privileges some individuals over others. It is not simply phenotypic differences among individuals in resource access, well-being, survival and reproduction but rather inequality refers to differences imposed on individuals or classes of individuals by structural features of a social system. There are multiple axes of inequality that reflect social structures ranging from egalitarian to hierarchical. These in turn influence key phenomena from resource distributions to collective decision-making. Our emphasis here is on social processes pertaining to influence in a resource hierarchy (e.g. dominance rank; [28–30]) rather than in a decision-making (e.g. leadership; [31,32]) hierarchy. Specifically, we focus on systematic, socially driven inequality in wealth and the effects this has on differential power (social influence or control over conspecifics), well-being (health, stress, mortality, etc.), reproduction and ultimately fitness [33]. We define wealth as attributes or possessions that contribute to well-being or fitness, including material (e.g. food, territory), relational (e.g. social networks) and embodied (e.g. knowledge, skill) forms [5,20,21].
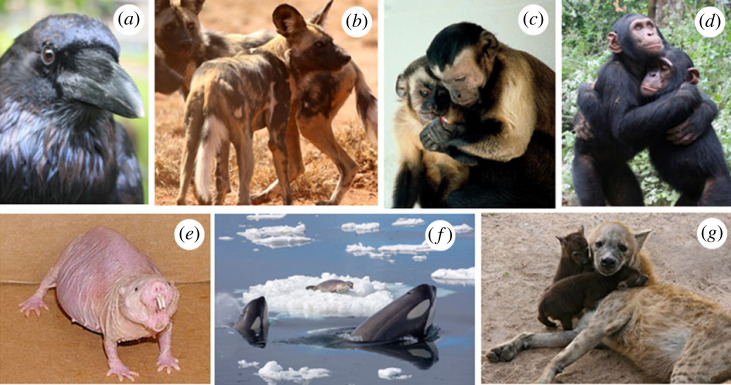
Here, we quantitatively test whether one measure of inequality (dominance) in animal societies is evolutionarily constrained, discuss evidence for developmental and social factors contributing to more or less equal societies and review mechanisms that have evolved to counter inequality in natural systems. We focus primarily on social mammals as these species share the same basic biological features as humans (e.g. lactation, gestation) but also consider examples from birds, a second tetrapod lineage showing multiple evolutions of highly structured social organization (figure 1).
Figure 1.
Mechanisms of equality and inequality in animals. Among the many examples in nature: (a) ravens co-feed with non-kin, are averse to inequity and reconcile after fights, (b) African wild dogs share food even with adults and adopt young born to others, (c) capuchins share food and are averse to inequity, (d) chimpanzees inherit tool sites and forgive each other after fights, (e) naked mole rat queens dominate reproduction within their unequal societies, (f) killer whales share food and knowledge, and (g) spotted hyaenas inherit social status and land within family lineages. Photographs reproduced via Creative Commons Licence or permission: (a) Stephan Dickson, (c) Frans de Waal, (d) (copyright: the Jane Goodall Institute)/by Fernando Turmo, (f) Callan Carpenter and (g) Bernard Dupont.
3. Strength and consistency of inequality diverse across mammals
Napoleon (the pig) asserted that ‘All animals are equal, but some animals are more equal than others’ [34, p. 75] to justify his political ambitions, but this statement is an accurate characterization of the diversity of fairness across animal species as well. In animals, dominance relationships contribute to patterns of inequality [24]. Social dominance can have important consequences for animals, contributing to unequal access to resources [35] and—in some cases—reproductive inequality (skew) [36–38], which varies within and among species (e.g. [39,40]). Because the diversity and evolution of reproductive inequality across humans and other mammals has recently been characterized elsewhere [40] and reproductive skew is discussed extensively in other contributions in this special issue [16,19,41], the first section of this paper focuses on social dominance as a measure of inequality. Social dominance reflects how individuals are organized into a dominance hierarchy based on the outcomes of pairwise agonistic interactions (e.g. wins, losses, and unsolicited appeasements) within a social group [28,42,43]. We sought to quantify the extent to which dominance structures vary within and among species as well as how these structures evolve.
Our initial goal was to investigate whether dominance structures are phylogenetically constrained across (non-domesticated) mammalian species. Drawing from the last century of research on dominance hierarchies, we analysed data that are publicly available from the R-package ‘DomArchive’ [44]. We focused on whether and how two major aspects of within-group social inequality (or lack thereof)—steepness and directional consistency (DC)—varied among and within species of non-human mammals. Steepness, the absolute slope of the straight line fitted to normalized David's scores (a dyadic dominance index) plotted against the subjects' ranks, reflects the degree to which individuals differ in their tendency to win agonistic encounters [45]. DC equals (H−L)/(H−L) based on the wins in the direction of higher (H) minus lower (L) frequency within each dyad and ranges from zero (complete symmetry) to one (completely asymmetric) [46,47]. Briefly, we asked whether steepness or DC was phylogenetically constrained after accounting for the age, sex and environmental context (naturalistic versus captive) for social mammals (see the electronic supplementary material for complete methods) [48].
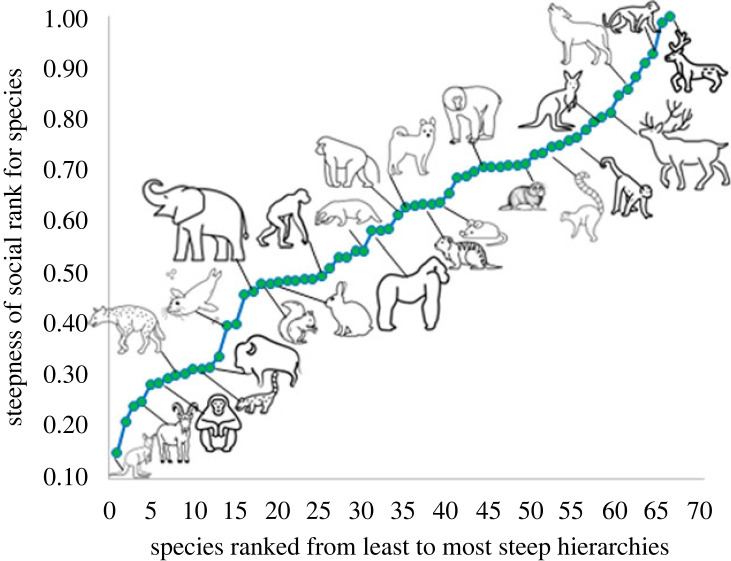
Our comparative analysis of mammalian species (electronic supplementary material, table S1) showed that hierarchies are highly variable across mammals (i.e. hierarchies are shallow in some groups but steep in others; electronic supplementary material, table S2 and figure S1); outcomes of agonistic interactions are consistent in some groups but not others (electronic supplementary material, table S3 and figure S1). Moreover, the degree of steepness or DC for a species is largely independent of phylogenetic constraints (see the electronic supplementary material for full results), and these traits vary considerably across mammalian species (e.g. steepness in figure 2), demonstrating the wide range of interspecific variation along a continuum. If dominance is tied to ecologically conservative traits, or if social structures evolved slowly within species, we would expect a strong pattern of phylogenetic conservatism such that closely related taxa should exhibit similar hierarchy properties. Our comparative analyses indicate quite the opposite, revealing that switches to divergent dominance social structures have occurred repeatedly across mammals (electronic supplementary material, figure S1), even after controlling for traits of the study groups (e.g. age or sex composition; electronic supplementary material, table S2). Hierarchies were significantly steeper for captive than non-captive groups and interactions were significantly more asymmetric within male-only groups (versus female-only groups) and in captivity (versus in naturalistic settings; electronic supplementary material, tables S2 and S3). Nonetheless, the random effect of species (or in some cases, subspecies) improved the fit of both models (steepness: X2 = 39.8 and DC: X2 = 46.2, p < 0.0001 for both), confirming that intraspecific dominance structures were generally similar across study groups.
Figure 2.
Steepness of dominance hierarchies across 66 species of mammals. For example, the following species are ranked from those with the most shallow to the steepest hierarchies: whiptail wallabies, mountain goats, chimpanzees, white-nosed coatis, bison, harbour seals, African elephant, Albert's squirrel, rabbit, bonobo, European badger, gorilla, chacma baboon, brown hyaena, dwarf mongoose, house mouse, Japanese macaque, Highveld mole rat, ring-tailed lemur, white-faced capuchin, eastern grey kangaroos, snub-nosed monkeys, wolves and sable antelopes (see the electronic supplementary material for the complete list). For visualization purposes only, data were pooled for subspecies or if multiple studies were conducted on the same species to present species-level averages. Domestic species were excluded from the dataset. Sources for species icons: thenounproject.com and Wikimedia commons.
The lack of evolutionary constraint on dominance structures is inconsistent with the idea that inequity within social systems is an inevitable evolutionary legacy, suggesting instead that diverse and divergent social organization strategies evolve quickly. A possible explanation of this pattern is that animal societies evolve rapidly by drift at a rate that erases the signature of the phylogeny. However, given the well-documented fitness consequences of sociality, this seems unlikely. Alternatively, social systems may evolve rapidly in response to local or shifting fitness optima associated with local resource characteristics. Future studies are required to consider the role of ecological conditions in explaining these patterns. For example, ecological theory predicts intense local competition for patchy, high-quality resources may favour steep and consistent fight outcomes; ecological conditions (e.g. defensibility of food or mates)—as well as the degree of relatedness within groups [49]—are expected to drive variation in hierarchy emergence, particularly in primates [50,51]. Differences between captive and natural settings may reflect limited opportunities for dispersive conflict resolution but an abundance of food in captivity. Additionally, a more nuanced approach that explicitly reveals the role of ecological context contributing to the dominance structures within our broad dataset would be valuable. This could further reveal the mechanisms contributing to (un)equal social structures, including the contexts in which sex differences in power over resources and mates are most likely to emerge [52,53]. Regardless of the drivers of this diversity, these patterns demonstrate the extraordinary breadth of social structures across mammals, the lack of a phylogenetic legacy of social dominance among mammals, and the variability of such structures even within members of the same species. Although social dominance is one major axis of inequality in that it reflects the social structures that contribute to resource access in animal groups, there are multiple mechanisms that contribute to differential access to resources and social support beyond an individual's ability to simply win contests. As discussed in the next section, developmental and social mechanisms often contribute to more or less equal societies (table 1).
Table 1.
Comparative data exemplifying mechanisms of (in)equality across well-studied social mammals.
| socioecology | mechanisms promoting intergenerational inequality |
mechanisms promoting equality |
||||||
|---|---|---|---|---|---|---|---|---|
| species | philopatry, social organization and trophic guild (defendable)a | social supportb, networks, knowledge (relational wealth) | maternal nutrition, stressors (embodied wealth) | resource (e.g. food, tools, land) transfer (material wealth) | food sharing and adoption of non-kinc | revolutionary coalitions and peacekeeping | fairness—inequity aversiond | conflict resolution (forgiveness/reconciliation)e |
| chimpanzee (Pan troglodytes spp.) | male philopatry; mixed sexes; omnivore (most food not defendable) | coalitions (N, R, CH) [54]; maternal presence positively influenced offspring muscle mass [55] and survival [56] | maternal rank and ‘stress’ during gestation correlated with offspring stress physiology [57] | intergenerational transfer of stone tools (hammers and anvils) [58] and natal community [59] | meat-sharing among kin and non-kin; some enforcement via aggression [60,61] | males join forces to overthrow the α male [62,63] | inequity aversion to unequal pay (food) paradigm [64] | post-conflict reconciliation [65–67] |
| bonobo (Pan paniscus) | male philopatry; mixed sexes; omnivore (most food not defendable) | coalitions (N, R) [54], but they also promote tolerance; maternal presences associated with increased mating, particularly by low and mid-ranking males [68] | mutual tolerance; co-feeding promotes social interactions [69–71] | females join forces to protect themselves from harassment by males [72] | inequity aversion to unequal pay (food) unclear [64] | post-conflict reconciliation [67] | ||
| chacma baboon (Papio ursinus) | both philopatric; mixed sexes; omnivore (most food not defendable) | coalitions (N,R) [73] | tolerated co-feeding limited to kin at playbacks [74] | post-conflict reconciliation [75] | ||||
| olive baboon (P. anubis) | females philopatric; mixed sexes; omnivore (most food not defendable) | coalitions (N) [54]; social skills in infants linked to maternal care [76]; maternal sociality predict infant growth and reproduction [77] | mothers experiencing more adversity had increased glucocorticoid levels and reduced offspring survival [78] | mutual feeding tolerance by males of males and females sexes of different ages [60] | cultural shift from competitive to peaceful troop [79] | post-conflict reconciliation [67,80] | ||
| yellow baboon (P. cynocephalus) | females philopatric; mixed sexes; omnivore (most food not defendable) | coalitions (N, R, CH) [54]; survival in future generations associated with maternal loss [56] | maternal status predicts son's glucocorticoid concentrations [81] | tolerance in food sharing present but varies with food type and availability [60] | low and mid-ranking males interrupt consorts by high-ranking males [82]; females join forces for protection against male harassment [83] | |||
| Barbary macaque (Macaca sylvanus) | females philopatric; mixed sexes; omnivore (most food not defendable) | coalitions (N, R, CH) [54] | low/mid-ranking males interrupt consorts by high-ranking males [84,85] | post-conflict reconciliation [67] | ||||
| Japanese macaque (M. fuscata) | females philopatric; mixed sexes; folivore/frugivore (food mostly not defendable) | coalitions (N, R, CH) [54] | co-feeding limited to kin [60] | inequity aversion to unequal food reward [86] | post-conflict reconciliation [67] | |||
| long-tailed macaque (M. fascicularis) | females philopatric; mixed sexes; frugivore (food is defendable) | coalitions (N, R) [54] | reciprocal exchange of food among non-kin [87] | inequity aversion to unequal pay (food) paradigm [64] | post-conflict reconciliation [67] | |||
| rhesus macaque (M. mulatta) | females philopatric; mixed sexes; folivore/frugivore (food mostly not defendable) | coalitions (N, R, CH) [54] | tolerated co-feeding between kin and non-kin [88] | inequity aversion to unequal food reward [64] | post-conflict reconciliation [67] | |||
| pig-tailed macaque (M. nemestrina) | females philopatric; mixed sexes; frugivore (food is defendable) | coalitions (N, R) [54] | post-conflict reconciliation [67] | |||||
| bonnet macaque (M. radiata) | females philopatric; mixed sexes; frugivore (food is defendable) | coalitions (N, R) [54] | post-conflict reconciliation [89] | |||||
| tonkean macaque (M. tonkeana) | females philopatric; mixed sexes; frugivore (food is defendable) | coalitions (N, R) [54] | post-conflict reconciliation [67] | |||||
| tufted (brown) capuchin monkey (Sapajus apella) | females philopatric; mixed sexes; omnivore (most food not defendable) | coalitions (N) [54] | co-feeding and food-sharing among kin and non-kin [60] | inequity aversion to unequal pay (food) paradigm [64] | post-conflict reconciliation [90]; grooming prevents conflict [91] | |||
| bearded capuchin monkey (Cebus libidinosus) | females philopatric; mixed sexes; folivore/frugivore (food mostly not defendable) | accumulation of stone tools and anvils [92] | ||||||
| grey wolf (Canis lupus) | females philopatric; mixed sexes; carnivore (food is defendable) | coalitions (N, R) [54]; | large size combined favours rapid neonatal growth [93] | territories passed from one generation to next along maternal line [94] | co-feeding tolerated within a closely related wolf pack [95] | post-conflict reconciliation [96,97] | ||
| African wild dog (Lycaon pictus) | neither sex philopatric; mixed sexes; carnivore (food is defendable) | coalitions (N, R) [54] | food sharing among closely related group members [60]; adoption common [98] | |||||
| African lion (Panthera leo leo) | females philopatric; mixed sexes; carnivore (food is defendable) | coalitions (N) [54] | food sharing among closely related group members [99] | females join forces to reduce infanticide [99] | ||||
| socioecology | mechanisms promoting intergenerational inequality | mechanisms promoting equality | ||||||
| spotted hyaena (Crocuta crocuta) | females philopatric; mixed sexes; carnivore (food is defendable) | coalitions (N, R, CH) [54], maternal rank-inheritance [100]; rank-based social network inheritance [101] | offspring born to high-ranking mothers with increased nutrition, grow faster, reproduce earlier, and have the best dispersal options [102] | territory inheritance by philopatric females and priority of access to high-ranking individuals [103] | rare cases of adoption at a single field site [104]; tolerance of co-feeding by unrelated close associates [105] | rank-changing revolutionary coalitions increase social mobility with intergenerational fitness benefits.[106] | post-conflict reconciliation [107] | |
| banded mongoose (Mungos mungo) | both or neither sex (varies by group) philopatric; mixed sexes; insectivore (food is not defendable) | coalitions (R) [73]; escorted pups heaviest at maturity and increased reproductive success [108] | supplemental food prenatally increased offspring lifespan and post-natally increased lifetime reproductive success [18] | tolerance of food sharing among close relatives [109]; equitably allocate care among group-mates when kinship masked [110] | dispersive conflict resolution via evictions from group [111] | |||
| wild horse (Equus caballus) | neither philopatric; mixed sexes; herbivore (food is not defendable) | coalitions (N) [54] | low and mid-ranking males interrupt consorts by high-ranking males [112] | post-conflict reconciliation [113] | ||||
| bottlenose dolphin (Tursiops truncatus) | both sexes philopatric; mixed sexes; piscivore (food is not defendable) | coalitions (N) [54] | post-conflict reconciliation [114] | |||||
| Indo-Pacific dolphin (T. aduncus) | both sexes philopatric; mixed sexes; piscivore (food is not defendable) | coalitions (N) [73] | low and mid-ranking males interrupt consorts by high-ranking males [115] | post-conflict reconciliation [116] | ||||
| killer whale (Orcinus orca) | both sexes philopatric; mixed sexes; piscivore /carnivore (most food is not defendable) | intergenerational transfer of ecological knowledge [117] increases survival of grandoffspring [118] | poor maternal nutrition negatively affects foetal brains [119] | tolerance of co-feeding by non-kin, including feeding by other pods [120,121] | post-conflict reconciliation [122] | |||
aPhilopatry scored as females (F), males, (M), both (B) or neither (N) sex remaining at home into adulthood; social organization reflects the sex composition of adults in the group, where ‘mixed’ refers to multiple adult males and females in the group; trophic guild reflects the typical diet for a species and whether that food is defendable (yes = defendable; no = non-defendable), for complete set of references, see [54]. For brevity, whenever possible, we cited early review papers.
bIntragroup coalition formation during which coalitionary supported was nepotistic (N; kin-biased) and whether coalitionary aggression was directed towards subordinate targets to reinforce (R) and/or towards dominant to targets to challenge (CH) the status quo [54,73].
cFood sharing [60].
dInequity aversion [64].
eReconciliation [67].
4. Developmental and social mechanisms contribute to inequality
Intergenerational transfer of embodied, material and relational wealth contribute to inequality in human and non-human societies [5,20,21]. Here, we focus on a diversity of mechanisms linked to promoting or inhibiting inequality across some of the best-studied examples of social mammals (table 1).
(a) . Maternal nutrition and stressors contribute to embodied wealth
In human societies around the world, the strongest predictor of undernutrition before, during and after pregnancy is poverty. Women without resources are less likely to have access to sufficient high-quality nutrition during these critical periods, compromising the immunological, neurological, metabolic and reproductive health throughout their, and perhaps their offspring's, lives [123–125]. Mounting evidence links high-quality maternal nutrition before and during human pregnancy to enhanced adult health outcomes in the developing fetus [126–128]. These include a lower incidence of diseases most linked to morbidity and mortality in humans. The quality of gestational nutrition also shapes pregnancy outcomes with superior nutrition linked to reduced risk of preterm delivery and forms of gestational hypertension. Additional research points to a linkage between quality preconception nutrition and long-term health benefits for the fetus through its life course [129].
Some mechanisms underlying these connections involve transgenerational epigenetic changes and the vertical transfer of beneficial microbiota during pregnancy and lactation. Transfer of immunities during and following pregnancy sets up a neonate not only for early resistance to pathogens but also shapes microbial endophenotypes increasingly linked to a range of consequential adult health effects [130–132]. Access to several nutrients, including fat, protein, vitamin B12, iron, zinc and iodine, impacts neonatal brain development and cognition. Humans whose mothers have greater access to diets rich in these and other nutrients appear to enjoy a number of neurological and cognitive benefits [130]. Undernutrition in utero disadvantages individuals whose low birth weights may be followed by stunted growth, shorter adult height and reduced economic outcomes [123,124,133].
It is well documented that maternal ‘stress’, reflected by high levels of glucocorticoids (GCs) during gestation, is linked to numerous effects on infant physiology that often extend into adulthood, and sometimes intergenerational effects for humans [134]. Similar effects have been shown for many other mammals as well. For example, maternal ‘stress’ experienced during gestation from snowshoe hares (Lepus americanus) [135] to monkeys [136] and apes [57] is often related to offspring stress physiology. Olive baboons (Papio anubis) with mothers who experienced more adversity, for example, also had increased GCs and reduced lifespans [78]. However, links between GCs and key traits (e.g. reproduction, health disparities) vary across and within species [137,138].
In some mammals, a parallel relationship exists between a female's nutritional resources and the health and reproductive success of her offspring. These differences in access can impose transgenerational effects on parental care [139], privileging some individuals over others. Evidence for such effects includes data from a mammalian carnivore, the spotted hyaena (Crocuta crocuta; figure 1). The quality of maternal nutrition, so often linked to a female's social rank and access to resources, places hyaena offspring at an advantage or disadvantage from its earliest days [103]. Young hyaenas born to high-ranking mothers are the beneficiaries of a nutritional ‘silver spoon effect’ during both gestation and lactation with long-lasting effects later in life [102]. Compared to offspring of low-ranking mothers, nutrition-advantaged offspring are weaned at earlier ages [140,141], enjoy enhanced immune systems [142] and grow faster [143]. Earlier consumption of meat may provide an early growth and size advantage for these cubs. Hyaena mothers with reduced access to food may also experience greater stress [144], exposing neonates of low-ranking mothers to elevated cortisol levels which influence the size, strength and immunocompetence of young cubs.
Do the offspring of stressed or malnourished mothers have any available mechanisms to cope with this form of disadvantage? Emerging evidence documents a great deal of behavioural and physiological plasticity associated with an animal's social rank [145]. A comparative study across mammals shows that offspring exposure to prenatal stress causes numerous effects in infant physiology that extend well into adulthood [146]. For instance, macaque offspring exposed to prenatal stress respond to reduced maternal investment through accelerated growth despite reduced motor skills and immune function [147]. Thus, across mammals, young may adjust—at least to some extent—to make the best of a challenging start [146].
Beyond these early life effects, many mammals often depend upon their mothers long after weaning. Comparative data across seven species of primates offer some insights into the intergenerational effects of maternal loss on offspring fitness [56]. Offspring survival for most species was lower in the years immediately prior to a mother's death despite mothers still being alive [56]. Beyond this, for several species of monkeys with social structures ranging from egalitarian to despotic, early maternal death experienced in one generation was also associated with reduced offspring survival in the next [56]. Similarly strong intergenerational effects may indeed be present in non-primate mammals, particularly others with slow life histories.
(b) . Resource inheritance contributes to material wealth
Parallels also exist in how resource distributions emerge in human and non-human societies. Links among social inequality, health and survival have been demonstrated for both human and non-human mammals [148]. Cultural and historical factors, including systemic (institutional) racism [15,149,150], contribute to inequality in human societies through forms of oppression, privileging some individuals over others. In other social mammals, wealth (resources) can be also acquired from the direct transfer of material wealth via non-genetic inheritance of material goods [151,152], such as a defended, high-quality real estate to perpetuate legacies of inequality across generations. Among others, wolves [94], lions [153], hyaenas [103] and chimpanzees [59] transfer territories from one generation to the next such that wealth accumulates in some family lines but less so for others.
Resource inheritance, when coupled with social learning and cultural traditions, can further perpetuate patterns of reproductive inequality [151]. Material transfer of resources and social knowledge of how to use materials (relational wealth, as discussed in the next section) can be transferred from one generation to the next. For example, Taï chimpanzees (Pan troglodytes versus) [58] and bearded capuchin monkeys (Cebus libidinosus) [92] inherit tools and social knowledge of how to use them. Individuals that inherit physical tools (e.g. stones) are advantaged over others who do not, and these beneficial effects are further compounded through the transfer of social information (e.g. traditions for how to use inherited materials) across generations. Still, some tools used by animals—from corvids to dolphins and apes—are ephemeral; the short-lived nature of ephemeral tools (i.e. sticks, sponges) can limit their intergenerational transfer [154], and tool use is not always socially learned [155]. Thus, although material inheritance when combined with social inheritance can strongly privilege some over others, the extent of these effects varies and warrants further study.
(c) . Intergenerational social support as a form of relational wealth
Coalitions and alliances are major forms of social support that influence an individual animal's social status [54,73,156,157]. Intragroup coalitions form when two or more individuals join forces to direct aggression towards another group member. This cooperative behaviour powerfully influences social structures of carnivores (e.g. spotted hyaenas [73,100,158,159], African wild dogs (Lycaon pictus) [160]), ungulates (e.g. fallow deer; Dama dama [161]) and primates (e.g. macaques [162], chimpanzees [163,164] and baboons [165]; table 1). Specifically, individuals benefit from intragroup coalitions that reinforce their own dominance and privilege their genetic relatives [73,166–168]. In the societies of spotted hyaenas [100], as well as those of yellow baboons (P. cynocephalus) [169], stumptail macaques (Macaca arctoides) [170] and vervet monkeys (Chlorocebus pygerythrus) [171,172]), young inherit status from mothers through maternal rank ‘inheritance,’ a non-genetic mechanism of socially learned status based on repeated maternal support. Thus, rank can be independent of an individual's size or fighting ability but flexible in that it is socially learned and these effects can be further ameliorated by differential social support into adulthood [73,106].
Group stability and associated dominance structures may be maintained or disrupted over time. For example, to compensate for rank instability owing to the loss of a keystone individual, baboons and macaques sometimes compensate through their proximity network [173] or policing [174], respectively. Among birds, ravens (Corvus corax) also maintain group stability through social interventions that prevent others from forming competitive alliances [175]. When social mammals inherit their social networks, this can reinforce systems of social support or isolation [101,176], but social mechanisms can also promote cultural shifts. In one baboon troop, the loss of dominant males contributed to a multigenerational shift from a competitive to a peaceful culture [79]. Yet, social networks are not always passed down from one generation to the next even in species with maternal rank inheritance (e.g. vervets) [177], further highlighting the breadth of ways social behaviour emerges to contribute to more or less equal societal structures. Taken together, these lines of evidence highlight the enormous amount of inter- and intra-specific variation in these emergent social processes, the limits of social inheritance and opportunities for behavioural flexibility across social mammals.
5. Mechanisms of fairness and equality in nature
A sense of fairness has long been believed to be a necessary precursor for the emergence of cooperation in particular and equality in general within human social groups. In this paper, we focus on four major mechanisms of fairness that promote equity within animal societies and highlight examples from social mammals (table 1). Levelling mechanisms include food sharing and adoption of non-kin, revolutionary coalitions, conflict resolution and inequity aversion.
(a) . Food sharing and adoption
Food sharing, defined as the unresisted transfer of food from one food-motivated individual to another, can promote equal access to limited food resources [60,178]. A comparative approach reveals that energetically costly food sharing among unrelated adults has evolved in a range of social species [60,179,180]. Food-sharing among unrelated primates is limited, but information on food sharing among chimpanzees and bonobos (Pan paniscus) is particularly well documented. Chimpanzees, for example, tolerate meat-sharing among community members [181–186], which can be enforced via aggression [61,187]. By contrast, bonobos share food in the absence of conflict [70] and their co-feeding among non-kin enhances social interactions [69,71]. Many baboons and social carnivores share food (table 1), particularly with close kin [166]; brown capuchins (Cebus apella) [188], ravens [189] and long-tailed macaques (M. fascicularis) [87] also share with non-kin. Moreover, mutual tolerance between males and females maintains social bonds in Guinea baboons (P. papio) [190] to domestic dogs (Canis lupus familiaris) [191]. Vampire bats (Desmodus rotundus) only feed on blood and will die after as little as 70 hours of fasting; roost-mates cooperate by regurgitating blood meals to kin and non-kin [192]. Killer whales (Orcinus orca) also share food with non-kin, including with whales belonging to other groups [121,193]. When cooperatively breeding banded mongooses (Mungos mungo) lack information regarding kinship they actively provide postnatal care to the most disadvantaged group-mates [110]. That is, only when knowledge about personal gains is masked, mongooses promote fairness, a finding consistent with the classic philosophical notion that a ‘veil of ignorance’ (hidden reward distributions) promotes fair, equitable outcomes in human societies.
Adoption occurs when adult animals allocate care to young individuals that are not their offspring, an altruistic act documented for multiple species of waterfowl [194] to social carnivores [104]. Snow geese (Anser caerulescens caerulescens), for example, regularly adopt eggs laid by conspecifics [195], and one study showed that adoption in African wild dogs occurred in at least one quarter of the packs [98]. For spotted hyaenas, only 13 cases of adoptions have been reported [104], all at a single study site despite their long-term study in multiple areas. Interestingly, postnatal maternal care by surrogate hyaena mothers levelled the playing field of previously orphaned cubs; the social rank of adopted offspring resembled that of their surrogate mothers and persisted into adulthood [104], elucidating how social learning can contribute to inequality in convention-based societies [106].
(b) . Revolutionary coalitions
The social lives of animals are by no means fixed but instead are often malleable, subject to cultural shifts based on the outcomes of coalitionary aggresssion or peacekeeping mechanisms from within. Revolutionary coalitions occur when both partners rank below their target. These can involve enormous immediate risks—but potentially high pay-offs—and often require an understanding of social dynamics [156,196,197]. Although revolutionary coalitions, also called all up coalitions, are always directed up the hierarchy towards dominants, they may either be levelling (i.e. change the pay-off distribution without shifting dominance rank relationships) or rank-changing (i.e. promote rank-reversals within a dominance hierarchy) coalitions [198].
Levelling coalitions permit at least one member of a coalition to obtain access to a mate without influencing the dominance structure [198]. For example, several species of macaques form levelling coalitions in which males selectively recruit allies that outrank themselves and their opponents [60,183]. Low and mid-ranking males may join forces against higher ranking males to gain mating opportunities (e.g. monkeys [82,84,85,199], horses [112], dolphins [115]). Low-ranking yellow baboons, for example, join forces against highest-ranking males [82,200–203] to increase their mating success. Females also join forces to blunt male power and offer protection from harassment or infanticide (e.g. bonobos [72], baboons [83] and lions [99]).
Rank-changing coalitions can improve an individual's position in its dominance hierarchy, affecting lifetime reproductive success, and restructure the group's dominance hierarchy through rank reversals [198]. Although rare, rank reversals contribute to revolutionary social change with major fitness consequences for the individuals involved and have compounding intergenerational effects [106]. For example, in spotted hyaenas, individuals that repeatedly form coalitions with top allies improve their upwards social mobility [106] (table 1). In several macaque species, although rare, mother–daughter reversals are achieved via coalitionary overthrows (e.g. [204,205]). Thus, coalitions can influence the distribution of resources to promote equality by dismantling current structures.
(c) . Conflict resolution
Forgiveness has profound consequences on multiple aspects of social life for humans, from family relationships to the political alliances among nations [206]. Other social mammals have also evolved a suite of behavioural mechanisms to mitigate conflict within groups and thereby reduce escalated aggression when conflicts of interest emerge [28,207,208]. For example, mountain gorillas (Gorilla beringei beringei) embrace [209], chimpanzees kiss [210] and bonobos massage genitals [211]. These gestures help to reduce immediate injury and promote sociopositive interactions, thereby increasing group cohesion. Because the strength of social bonds can predict future cooperation (e.g. [212]), repair of relationships can more broadly promote future opportunities for cooperation between former opponents. Evidence for reconciliation is now vast, occurring across many social mammals (table 1) as well as in some birds (e.g. ravens [213] and monk parakeets (Myiopsitta monachus) [214]). Reconciliation may be a common feature in groups of animals with repeated interactions.
(d) . Inequity aversion
A major requirement for equality within groups is the ability for conspecifics to share the pay-offs associated with cooperative behaviours. The evolutionary benefits of sensitivity to (in)equity include the ability to recognize when individuals receive less than their partners. Although a sense of fairness has long been considered an important aspect of economic decision-making in humans [215], with empathy for victims present in young human infants [216], understanding fairness—defined as the redistribution of resources to reduce initial inequalities [110]—in non-human animals is challenging. One major tool used to gain insights into the concept of fairness is to measure the behavioural reactions by animals exposed to situations in which one individual receives more or less than the other individual. Brosnan & de Waal [217] designed one such paradigm to show that brown capuchin monkeys refused a food reward associated with unfair offers (e.g. grapes versus cucumbers; table 1). Subsequent tests reveal that this form of inequity aversion is most pronounced in species that cooperate, especially those who do so outside of mating or kinship [64,218], such as occurs in chimpanzees [219,220] (but see: [221] for evidence of indifference to welfare of non-kin), long-tailed macaques [222] and corvids (e.g. crows, ravens) [223]. Moreover, social mammals from rats [224] to dogs [225] are also averse to inequity. Evidence for bonobos is equivocal [226] with data limited by small sample sizes [227]. However, there is little evidence for inequity aversion in non-cooperative species [228–230]. In summary, this trait coevolves with cooperation to reduce inequality in social mammals and has deep evolutionary roots.
6. Conclusion
Overall, we showed that multiple features characterizing dominance hierarchies (one measure of inequality in animals) are not evolutionary constrained, highlighting the enormous flexibility of social systems. We also documented that a diversity of mechanisms emerge across species to promote more or less equal social systems. These mechanisms may offer new ways to understand peacekeeping and conflict across mammalian societies and perhaps even help to offer insights into human inequality.
Acknowledgements
We acknowledge our positionality and privilege in having the opportunity to write this article. We are grateful to Christopher Schell and Ambika Kamath for thoughtful conversations and feedback that helped to shape the framing and content of this article.
Data accessibility
All data from the freely available online archive. For details, see [44].
Data and all R-code for statistical analyses is included in the electronic supplementary material [48].
Authors' contributions
J.E.S.: conceptualization, data curation, investigation, methodology, project administration, supervision, visualization, writing—original draft, writing—review and editing; B.N.-H.: investigation, writing—original draft; M.M.M.: data curation, investigation, writing—review and editing; M.E.A.: formal analysis, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This research was supported by a Student-Faculty Research Collaboration Grant to M.M.M. and J.E.S. from the Office of Research and Sponsored Programs at the University of Wisconsin - Eau Claire.
References
- 1.Subramanian SV, Kawachi I. 2004. Income inequality and health: what have we learned so far? Epidemiol. Rev. 26, 78-91. ( 10.1093/epirev/mxh003) [DOI] [PubMed] [Google Scholar]
- 2.Colleran H, Jasienska G, Nenko I, Galbarczyk A, Mace R. 2015. Fertility decline and the changing dynamics of wealth, status and inequality. Proc. R. Soc. B 282, 20150287. ( 10.1098/rspb.2015.0287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Raalte AA, Sasson I, Martikainen P. 2018. The case for monitoring life-span inequality. Science 362, 1002-1004. ( 10.1126/science.aau5811) [DOI] [PubMed] [Google Scholar]
- 4.Kaplan H. 1996. A theory of fertility and parental investment in traditional and modern human societies. Yearb. Phys. Anthropol. 101, 91-135. [Google Scholar]
- 5.Mulder MB, et al. 2009. Intergenerational wealth transmission and the dynamics of inequality in small-scale societies. Science 326, 682-688. ( 10.1126/science.1178336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deaton A. 2013. The great escape: health, wealth, and the origins of inequality. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Khan S. 2021. Privilege: the making of an adolescent elite at St. Paul's school. Princeton, NJ: Princeton University Press. [Google Scholar]
- 8.Kohler TA, et al. 2017. Greater post-Neolithic wealth disparities in Eurasia than in North America and Mesoamerica. Nature 551, 619-622. ( 10.1038/nature24646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flannery KV., Marcus J. 2012. The creation of inequality: how our prehistoric ancestors set the stage for monarchy, slavery, and empire. Cambridge, MA: Harvard University Press. [Google Scholar]
- 10.Corak M. 2013. Income inequality, equality of opportunity, and intergenerational mobility. J. Econ. Perspect. 27, 79-102. ( 10.1257/jep.27.3.79) [DOI] [Google Scholar]
- 11.Solon G. 1992. Intergenerational income mobility in the United States. Am. Econ. Rev. 82, 393-408. [Google Scholar]
- 12.Hendren N, et al. 2014. Where is the land of opportunity? The geography of intergenerational mobility in the United States. Q. J. Econ. 129, 1553-1623. ( 10.1093/qje/qju022) [DOI] [Google Scholar]
- 13.Smith EA, Borgerhoff Mulder M, Bowles S, Gurven M, Hertz T, Shenk MK. 2010. Production systems, inheritance, and inequality in premodern societies. Curr. Anthropol. 51, 85-94. ( 10.1086/649029) [DOI] [Google Scholar]
- 14.Bowles S, Smith EA, Mulder MB. 2010. The emergence and persistence of inequality in premodern societies: introduction to the special section. Curr. Anthropol. 51, 7-17. ( 10.1086/649206) [DOI] [Google Scholar]
- 15.Schell CJ, Dyson K, Fuentes TL, Des RS, Harris NC, Miller DS, Woelfle-Erskine CA, Lambert MR. 2020. The ecological and evolutionary consequences of systemic racism in urban environments. Science 369, eaay4497. ( 10.1126/science.aay4497) [DOI] [PubMed] [Google Scholar]
- 16.Wallace K, Hart D, Venter F, van Vuuren AJ, Bennett N. 2023. The best of both worlds: no apparent trade-off between immunity and reproduction in two group-living African mole-rat species. Phil. Trans. R. Soc. B 378, 20220310. ( 10.1098/rstb.2022.0310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strauss E. 2023. Demographic turnover can be a leading driver of hierarchy dynamics, and social inheritance modifies its effects. Phil. Trans. R. Soc. B 378, 20220308. ( 10.1098/rstb.2022.0308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitikainen E, et al. 2023. The social formation of fitness: lifetime consequences of prenatal nutrition and postnatal care in a wild mammal population. Phil. Trans. R. Soc. B 378, 20220309. ( 10.1098/rstb.2022.0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouginot M, et al. 2023. Reproductive inequality among males in the genus Pan. Phil. Trans. R. Soc. B 378, 20220301. ( 10.1098/rstb.2022.0301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JE, Natterson-Horowitz B, Alfaro ME. 2022. The nature of privilege: intergenerational wealth in animal societies. Behav. Ecol. 33, 1-6. ( 10.1093/beheco/arab137) [DOI] [Google Scholar]
- 21.Strauss ED, Shizuka D. 2022. The ecology of wealth inequality in animal societies. Proc. R. Soc. B 289, 20220500. ( 10.1098/rspb.2022.0500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith EA, Smith JE, Codding BF. 2023. Towards an evolutionary ecology of (in)equality. Phil. Trans. R. Soc. B 378, 20220287. ( 10.1098/rstb.2022.0287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tennyson A. 1850. In memoriam. London, UK: Bradbury and Evans. [Google Scholar]
- 24.Strauss ED, Curley JP, Shizuka D, Hobson EA. 2022. The centennial of the pecking order: current state and future prospects for the study of dominance hierarchies. Phil. Trans. R. Soc. B 377, 20200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silk JB, Kappeler PM. 2017. Sociality in primates. In Comparative social evolution (eds Rubenstein DR, Abbot P), pp. 253-283. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 26.Smith JE, Lacey EA, Hayes LD. 2017. Sociality in non-primate mammals. In Comparative social evolution (eds Rubenstein DR, Abbot P), pp. 284-319. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 27.Brosnan SF. 2018. Insights into human cooperation from comparative economics. Nat. Hum. Behav. 2, 432-434. ( 10.1038/s41562-018-0383-7) [DOI] [PubMed] [Google Scholar]
- 28.de Waal FBM. 1986. The integration of dominance and social bonding in primates. Q. Rev. Biol. 61, 459-479. ( 10.1086/415144) [DOI] [PubMed] [Google Scholar]
- 29.Hand JL. 1986. Resolution of social conflicts: dominance, egalitarianism, spheres of dominance, and game theory. Q. Rev. Biol. 61, 201-220. ( 10.1086/414899) [DOI] [Google Scholar]
- 30.Van Vugt M, Smith JE. 2019. A dual model of leadership and hierarchy: evolutionary synthesis. Trends Cogn. Sci. 23, 952-967. ( 10.1016/j.tics.2019.09.004) [DOI] [PubMed] [Google Scholar]
- 31.Smith JE, et al. 2016. Leadership in mammalian societies: emergence, distribution, power, and payoff. Trends Ecol. Evol. 31, 54-66. ( 10.1016/j.tree.2015.09.013) [DOI] [PubMed] [Google Scholar]
- 32.Smith JE, van Vugt M. 2020. Leadership and status in mammalian societies: context matters. Trends Cogn. Sci. 24, 263-264. ( 10.1016/j.tics.2020.01.003) [DOI] [PubMed] [Google Scholar]
- 33.Smith EA, Smith JE, Codding BF. 2023. Towards an evolutionary ecology of (in)equality. Phil. Trans. R. Soc. B 378, 20220287. ( 10.1098/rstb.2022.0287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orwell G. 2021. Animal farm. Oxford, UK: Oxford University Press. [Google Scholar]
- 35.Kaufmann JH. 1983. On the definitions and functions of dominance and territoriality. Biol. Rev. 58, 1-20. ( 10.1111/j.1469-185X.1983.tb00379.x) [DOI] [Google Scholar]
- 36.Cant MA. 1998. A model for the evolution of reproductive skew without reproductive suppression. Anim. Behav. 55, 163-169. ( 10.1006/anbe.1997.0589) [DOI] [PubMed] [Google Scholar]
- 37.Johnstone RA. 2000. Models of reproductive skew: a review and synthesis. Ethology 106, 5-26. ( 10.1046/j.1439-0310.2000.00529.x) [DOI] [Google Scholar]
- 38.Keller L, Reeve HK. 1994. Partitioning of reproduction in animal societies. Trends Ecol. Evol. 9, 98-102. ( 10.1016/0169-5347(94)90204-6) [DOI] [PubMed] [Google Scholar]
- 39.Ross CT, Jaeggi AV, Mulder MB, Smith JE, Smith EA, Gavrilets S, Hooper PL. 2020. The multinomial index: a robust measure of reproductive skew. Proc. R. Soc. B 287, 20202025. ( 10.1098/rspb.2020.2025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross CT, et al. 2023. Reproductive inequality in humans and other mammals. Proc. Natl Acad. Sci. USA 120, e2220124120. ( 10.1073/pnas.2220124120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bowles S, Hammerstein P. 2023. A biological employment model of reproductive skew. Phil. Trans. R. Soc. B 378, 20220289. ( 10.1098/rstb.2022.0289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowell TE. 1974. The concept of social dominance. Behav. Biol. 11, 131-154. ( 10.1016/S0091-6773(74)90289-2) [DOI] [PubMed] [Google Scholar]
- 43.Drews C. 1993. The concept and definition of dominance in animal behaviour. Behaviour 125, 283-313. ( 10.1163/156853993X00290) [DOI] [Google Scholar]
- 44.Strauss ED, Decasien AR, Galindo G, Hobson EA, Shizuka D, Curley JP. 2022. DomArchive: a century of published dominance data. Phil. Trans. R. Soc. B 377, 20200436. ( 10.1098/rstb.2020.0436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Vries H, Stevens JMG, Vervaecke H. 2006. Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 71, 585-592. ( 10.1016/j.anbehav.2005.05.015) [DOI] [Google Scholar]
- 46.Van Hooff JA, Wensing JA. 1987. Dominance and its behavioral measures in a captive wolf pack. In Man and wolf: advances, issues, and problems in captive wolf research (ed. Frank H), pp. 219-252. The Hague, The Netherlands: Dr W Junk Publishers. [Google Scholar]
- 47.de Vries H. 1993. The rowwise correlation between two proximity matrices and the partial rowwise correlation. Psychometrika 58, 53-69. ( 10.1007/BF02294470) [DOI] [Google Scholar]
- 48.Smith JE, Natterson-Horowitz B, Mueller MM, Alfaro ME. 2023. Mechanisms of equality and inequality in mammalian societies. Figshare. ( 10.6084/m9.figshare.c.6662596) [DOI] [PMC free article] [PubMed]
- 49.Vehrencamp SL. 1983. A model for the evolution of despotic versus egalitarian societies. Anim. Behav. 31, 667-682. ( 10.1016/S0003-3472(83)80222-X) [DOI] [Google Scholar]
- 50.Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour 75, 262-300. ( 10.1163/156853980X00447) [DOI] [Google Scholar]
- 51.Sterck EHM, Watts DP, van Schaik CP. 1997. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291-309. ( 10.1007/s002650050390) [DOI] [Google Scholar]
- 52.Davidian E, Surbeck M, Lukas D, Kappeler PM, Huchard E. 2023. The eco-evolutionary landscape of power relationships between males and females. Trends Ecol. Evol. 37, 706-718. ( 10.1016/J.TREE.2022.04.004) [DOI] [PubMed] [Google Scholar]
- 53.Smith JE, von Rueden CR, van Vugt M, Fichtel C, Kappeler PM. 2021. An evolutionary explanation for the female leadership paradox. Front. Ecol. Evol. 9, 468. [Google Scholar]
- 54.Smith JE, Jaeggi AV, Holmes RK, Silk JB. 2022. Sex differences in cooperative coalitions: a mammalian perspective. Phil. Trans. R. Soc. B 378, 20210426. ( 10.1098/rstb.2021.0426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuni L, Tkaczynski P, Deschner T, Löhrrich T, Wittig R, Crockford C. 2020. Maternal effects on offspring growth indicate post-weaning juvenile dependence in chimpanzees (Pan troglodytes verus). Front. Zool. 17, 1. ( 10.1186/s12983-019-0343-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zipple MN, et al. 2020. Maternal death and offspring fitness in multiple wild primates. Proc. Natl Acad. Sci. USA 118, e2015317118. ( 10.1073/pnas.2015317118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray C, Stanton M, Wellens K, Santymire R, Heintz M, Lonsdorf E. 2016. Maternal effects on offspring stress physiology in wild chimpanzees. Am. J. Primatol. 80, e22525. ( 10.1002/ajp.22525) [DOI] [PubMed] [Google Scholar]
- 58.Mercader J, Panger M, Boesch C. 2002. Excavation of a chimpanzee stone tool site in the African rainforest. Science 296, 1452-1455. ( 10.1126/science.1070268) [DOI] [PubMed] [Google Scholar]
- 59.Walker KK, Pusey AE. 2020. Inbreeding risk and maternal support have opposite effects on female chimpanzee dispersal. Curr. Biol. 30, R62-R63. ( 10.1016/j.cub.2019.11.081) [DOI] [PubMed] [Google Scholar]
- 60.Stevens JR, Gilby IC. 2004. A conceptual framework for nonkin food sharing: timing and currency of benefits. Anim. Behav. 67, 603-614. ( 10.1016/j.anbehav.2003.04.012) [DOI] [Google Scholar]
- 61.Gilby I. 2006. Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Anim. Behav. 71, 953-963. ( 10.1016/j.anbehav.2005.09.009) [DOI] [Google Scholar]
- 62.Nishida T. 1983. Alpha status and agonistic alliance in wild chimpanzees (Pan troglodytes schweinfurthii). Primates 24, 318-336. ( 10.1007/BF02381978) [DOI] [Google Scholar]
- 63.Nishida T, Hosaka K. 1996. Coalition strategies among adult male chimpanzees of the Mahale Mountains, Tanzania. In Great ape societies (eds McGrew WC, Marchant LF, Nishida T), pp. 114-134. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 64.Brosnan SF, De Waal FBM. 2014. Evolution of responses to (un)fairness. Science 346, 1251776. ( 10.1126/science.1251776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romero T, Castellanos MA, De Waal FBM. 2010. Consolation as possible expression of sympathetic concern among chimpanzees. Proc. Natl Acad. Sci. USA 107, 12 110-12 115. ( 10.1073/pnas.1006991107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romero T, de Waal FBM. 2010. Chimpanzee (Pan troglodytes) consolation: third-party identity as a window on possible function. J. Comp. Psychol. 124, 278-286. ( 10.1037/a0019144) [DOI] [PubMed] [Google Scholar]
- 67.Arnold K, Aureli F. 2007. Postconflict reconciliation. Primates Perspect. 2, 608-625. [Google Scholar]
- 68.Surbeck M, Mundry R, Hohmann G. 2011. Mothers matter! Maternal support, dominance status and mating success in male bonobos (Pan paniscus). Proc. R. Soc. B 278, 590-598. ( 10.1098/rspb.2010.1572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fruth B, Hohmann G. 2018. Food sharing across borders: first observation of intercommunity meat sharing by bonobos at LuiKotale, DRC. Hum. Nat. 29, 91-103. ( 10.1007/s12110-018-9311-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto S. 2015. Non-reciprocal be peaceful fruit sharing in wild banobos in Wamba. Behaviour 152, 335-357. ( 10.1163/1568539X-00003257) [DOI] [Google Scholar]
- 71.Tan J, Hare B. 2013. Bonobos share with strangers. PLoS ONE 8, 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furuichi T. 2011. Female contributions to the peaceful nature of bonobo society. Evol. Anthropol. 20, 131-142. ( 10.1002/evan.20308) [DOI] [PubMed] [Google Scholar]
- 73.Smith JE, Van Horn RC, Powning KS, Cole AR, Graham KE, Memenis SK, Holekamp KE. 2010. Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav. Ecol. 21, 284-303. ( 10.1093/beheco/arp181) [DOI] [Google Scholar]
- 74.Allan A, et al. 2022. Patterns of predation and meat-eating by chacma baboons in an afromontane environment. Folia Primatol. 94, 13-36. ( 10.1163/14219980-bja10004) [DOI] [Google Scholar]
- 75.Webb CE, Baniel A, Cowlishaw G, Huchard E. 2019. Friend or foe: reconciliation between males and females in wild chacma baboons. Anim. Behav. 151, 145-155. ( 10.1016/j.anbehav.2019.03.016) [DOI] [Google Scholar]
- 76.Most C, Strum S. 2020. Bringing up baby: maternal responsiveness, secondary attachments, and the development of infant social competence in wild olive baboons (Papio anubis). Dev. Psychobiol. 62, 963-978. ( 10.1002/dev.21973) [DOI] [PubMed] [Google Scholar]
- 77.Garcia C, Lee P, Rosetta L. 2009. Growth in colony living Anubis baboon infants and its relationship with maternal activity budgets and reproductive status. Am. J. Phys. Anthropol. 138, 123-135. ( 10.1002/ajpa.20909) [DOI] [PubMed] [Google Scholar]
- 78.Patterson S, Hinde K, Bond A, Trumble B, Strum S, Silk J. 2021. Effects of early life adversity on maternal effort and glucocorticoids in wild olive baboons. Behav. Ecol. Sociobiol. 75, 114. ( 10.1007/s00265-021-03056-7) [DOI] [Google Scholar]
- 79.Sapolsky RM, Share LJ. 2004. A Pacific culture among wild baboons: its emergence and transmission. PLoS Biol. 2, e106. ( 10.1371/journal.pbio.0020106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Castles DL, Whiten A. 1998. Post-conflict behaviour of wild olive baboons. II. Stress and self-directed behaviour. Ethology 104, 148-160. ( 10.1111/j.1439-0310.1998.tb00058.x) [DOI] [Google Scholar]
- 81.Onyango P, Gesquiere L, Wango E, Alberts S, Altmann J. 2008. Persistence of maternal effects in baboons: mother's dominance rank at son's conception predicts stress hormone levels in subadult males. Horm. Behav. 54, 319-324. ( 10.1016/j.yhbeh.2008.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noë R. 1992. Alliance formation among male baboons: shopping for profitable partners. In Coalitions and alliances in humans and other animals (eds de Waal FBM, Harcourt A), pp. 285-322. Oxford, UK: Oxford University Press. [Google Scholar]
- 83.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231-1234. ( 10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 84.Kuester J, Paul A. 1992. Influence of male competition and female mate choice on male mating success in Barbary macaques (Macaca sylvanus). Behaviour 120, 192-216. ( 10.1163/156853992X00606) [DOI] [Google Scholar]
- 85.Bissonnette A, Bischofberger N, van Schaik CP. 2011. Mating skew in Barbary macaque males: the role of female mating synchrony, female behavior, and male-male coalitions. Behav. Ecol. Sociobiol. 65, 167-182. ( 10.1007/s00265-010-1023-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sigmundson R, Stribos M, Hammer R, Herzele J, Pflüger L, Massen J. 2021. Exploring the cognitive capacities of Japanese macaques in a cooperation game. Animals 11, 1497. ( 10.3390/ani11061497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazumder J, Kaburu S. 2021. First report of food sharing among nicobar long-tailed macaques. Quat. Int. 603, 31-39. ( 10.1016/j.quaint.2020.11.049) [DOI] [Google Scholar]
- 88.Dubuc C, Hughes K, Cascio J, Santos L. 2012. Social tolerance in a despotic primate: co-feeding between consortship partners in rhesus macaques. Am. J. Phys. Anthropol. 148, 73-80. ( 10.1002/ajpa.22043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cooper MA, Aureli F, Singh M. 2007. Sex differences in reconciliation and post-conflict anxiety in bonnet macaques. Ethology 113, 26-38. [Google Scholar]
- 90.Daniel JR, Santos AJ, Cruz MG. 2009. Postconflict behaviour in brown capuchin monkeys (Cebus apella). Folia Primatol. 80, 329-340. ( 10.1159/000258647) [DOI] [PubMed] [Google Scholar]
- 91.di Sorrentino E P, Schino G, Visalberghi E, Aureli F. 2010. What time is it? Coping with expected feeding time in capuchin monkeys. Anim. Behav. 80, 117-123. ( 10.1016/j.anbehav.2010.04.008) [DOI] [Google Scholar]
- 92.Elisabetta V, Haslam M, Spagnoletti N, Fragaszy D. 2013. Use of stone hammer tools and anvils by bearded capuchin monkeys over time and space: construction of an archeological record of tool use. J. Archaeol. Sci. 40, 3222-3232. ( 10.1016/j.jas.2013.03.021) [DOI] [Google Scholar]
- 93.Stahler D, MacNulty D, Wayne R, vonHoldt B, Smith D. 2013. The adaptive value of morphological, behavioural and life-history traits in reproductive female wolves. J. Anim. Ecol. 82, 222-234. ( 10.1111/j.1365-2656.2012.02039.x) [DOI] [PubMed] [Google Scholar]
- 94.Mech LD, Boitani L. 2003. Wolf social ecology. In Wolves: behavior, ecology, and conservation (eds Mech LD, Boitani L), pp. 1-34. Chicago, IL, USA: University of Chicago Press. [Google Scholar]
- 95.Stahler D, Smith D, Guernsey D. 2006. Foraging and feeding ecology of the gray wolf (Canis lupus): lessons from Yellowstone National Park, Wyoming, USA. J. Nutr. 136, 1923S-1926S. ( 10.1093/jn/136.7.1923S) [DOI] [PubMed] [Google Scholar]
- 96.Cafazzo S, Marshall-Pescini S, Lazzaroni M, Virányi Z, Range F. 2018. The effect of domestication on post-conflict management: wolves reconcile while dogs avoid each other. R. Soc. Open Sci. 5, 171553. ( 10.1098/rsos.171553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cordoni G, Palagi E. 2008. Reconciliation in wolves (Canis lupus): new evidence for a comparative perspective. Ethology 114, 298-308. ( 10.1111/j.1439-0310.2008.01474.x) [DOI] [Google Scholar]
- 98.Mcnutt JW. 1996. Adoption in African wild dogs, Lycaon pictus. J. Zool. 240, 163-173. ( 10.1111/j.1469-7998.1996.tb05493.x) [DOI] [Google Scholar]
- 99.Schaller GB. 1972. The Serengetti lion: a study of predator-prey relations. Chicago, IL: University of Chicago Press. [Google Scholar]
- 100.Engh AL, Esch K, Smale L, Holekamp KE. 2000. Mechanisms of maternal rank ‘inheritance’ in the spotted hyaena, Crocuta crocuta. Anim. Behav. 60, 323-332. ( 10.1006/anbe.2000.1502) [DOI] [PubMed] [Google Scholar]
- 101.Ilany A, Holekamp KE, Akçay E. 2021. Rank-dependent social inheritance determines social network structure in spotted hyenas. Science 373, 348-352. ( 10.1126/science.abc1966) [DOI] [PubMed] [Google Scholar]
- 102.Höner OP, Wachter B, Hofer H, Wilhelm K, Thierer D, Trillmich F, Burke T, East ML. 2010. The fitness of dispersing spotted hyaena sons is influenced by maternal social status. Nat. Commun. 1, 1-7. ( 10.1038/ncomms1059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holekamp K, Smith JE, Strelioff CC, Van Horn RC, Watts HE. 2012. Society, demography and genetic structure in the spotted hyena. Mol. Ecol. 21, 613-632. ( 10.1111/j.1365-294X.2011.05240.x) [DOI] [PubMed] [Google Scholar]
- 104.East ML, Höner OP, Wachter B, Wilhelm K, Burke T, Hofer H. 2009. Maternal effects on offspring social status in spotted hyenas. Behav. Ecol. 20, 478-483. ( 10.1093/beheco/arp020) [DOI] [Google Scholar]
- 105.Smith JE, Memenis SK, Holekamp KE. 2007. Rank-related partner choice in the fission–fusion society of the spotted hyena (Crocuta crocuta). Behav. Ecol. Sociobiol. 61, 753-765. ( 10.1007/s00265-006-0305-y) [DOI] [Google Scholar]
- 106.Strauss ED, Holekamp KE. 2019. Social alliances improve rank and fitness in convention-based societies. Proc. Natl Acad. Sci. USA 116, 8919-8924. ( 10.1073/pnas.1810384116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wahaj SA, Guse KR, Holekamp KE. 2001. Reconciliation in the spotted hyena (Crocuta crocuta). Ethology 107, 1057-1074. ( 10.1046/j.1439-0310.2001.00717.x) [DOI] [Google Scholar]
- 108.Vitikainen E, Thompson F, Marshall H, Cant M. 2019. Live long and prosper: durable benefits of early-life care in banded mongooses. Phil. Trans. R. Soc. B 374, 20180114. ( 10.1098/rstb.2018.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rood J. 1975. Population dynamics and food habits of the banded mongoose. Afr. J. Ecol. 13, 89-111. ( 10.1111/j.1365-2028.1975.tb00125.x) [DOI] [Google Scholar]
- 110.Marshall HH, et al. 2021. A veil of ignorance can promote fairness in a mammal society. Nat. Commun. 12, 1-8. ( 10.1038/s41467-020-20314-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thompson FJ, Marshall HH, Vitikainen EIK, Young AJ, Cant MA. 2017. Individual and demographic consequences of mass eviction in cooperative banded mongooses. Anim. Behav. 134, 103-112. ( 10.1016/j.anbehav.2017.10.009) [DOI] [Google Scholar]
- 112.Feh C. 1999. Alliances and reproductive success in Camargue stallions. Anim. Behav. 1995, 705-713. ( 10.1006/anbe.1998.1009) [DOI] [PubMed] [Google Scholar]
- 113.Cozzi A, Sighieri C, Gazzano A, Nicol CJ, Baragli P. 2010. Post-conflict friendly reunion in a permanent group of horses (Equus caballus). Behav. Processes 85, 185-190. ( 10.1016/j.beproc.2010.07.007) [DOI] [PubMed] [Google Scholar]
- 114.Yamamoto C, Morisaka T, Furuta K, Ishibashi T, Yoshida A, Taki M, Mori Y, Amano M. 2015. Post-conflict affiliation as conflict management in captive bottlenose dolphins (Tursiops truncatus). Sci. Rep. 5, 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Connor RC, Smolker RA, Richards AF. 1992. Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc. Natl Acad. Sci. USA 89, 987-990. ( 10.1073/pnas.89.3.987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miller L. 2010. An examination of aggressive behavior in indo-pacific bottlenose dolphins (Tursiops aduncus). Master's thesis, Psychology Department, University of Southern Mississippi, Hattiesburg, Mississippi, USA. [Google Scholar]
- 117.Brent LJN, Franks DW, Foster EA, Balcomb KC, Cant MA, Croft DP. 2015. Ecological knowledge, leadership, and the evolution of menopause in killer whales. Curr. Biol. 25, 746-750. ( 10.1016/j.cub.2015.01.037) [DOI] [PubMed] [Google Scholar]
- 118.Nattrass S, et al. 2019. Postreproductive killer whale grandmothers improve the survival of their grandoffspring. Proc. Natl Acad. Sci. USA 116, 26 669-26 673. ( 10.1073/pnas.1903844116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wasser S, Lundin J, Ayres K, Seely E, Giles D, Balcomb K, Hempelmann J, Parsons K, Booth R. 2017. Population growth is limited by nutritional impacts on pregnancy success in endangered southern resident killer whales (Orcinus orca). PLoS ONE 12, e0179824. ( 10.1371/journal.pone.0179824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guinet C, Barrett-Lennard LG, Loyer B. 2000. Co-ordinated attack behavior and prey sharing by killer whales at Crozet Archipelago: strategies for feeding on negatively-buoyant prey. Mar. Mammal Sci. 16, 829-834. ( 10.1111/j.1748-7692.2000.tb00976.x) [DOI] [Google Scholar]
- 121.Wright B, Stredulinsky E, Ellis G, Ford J. 2016. Kin-directed food sharing promotes lifetime natal philopatry of both sexes in a population of fish-eating killer whales, Orcinus orca. Anim. Behav. 115, 81-95. ( 10.1016/j.anbehav.2016.02.025) [DOI] [Google Scholar]
- 122.Sánchez–Hernández P, Krasheninnikova A, Almunia J, Molina–Borja M. 2019. Social interaction analysis in captive orcas (Orcinus orca). Zoo Biol. 38, 323-333. ( 10.1002/zoo.21502) [DOI] [PubMed] [Google Scholar]
- 123.Baird J, Jacob C, Barker M, Fall C, Hanson M, Harvey N, Inskip H, Kumaran K, Cooper C. 2017. Developmental origins of health and disease: a lifecourse approach to the prevention of non-communicable diseases. Healthcare 5, 1-12. ( 10.3390/healthcare5010014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marshall N, et al. 2022. The importance of nutrition in pregnancy and lactation: lifelong consequences. Am. J. Obstet. Gynecol. 226, 607-632. ( 10.1016/j.ajog.2021.12.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Larson C. 2007. Poverty during pregnancy: its effects on child health outcomes. Paediatr. Child Health 12, 673-677. ( 10.1093/pch/12.8.673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodriguez A. 2010. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J. Child Psychol. Psychiatry 51, 134-143. ( 10.1111/j.1469-7610.2009.02133.x) [DOI] [PubMed] [Google Scholar]
- 127.Van Lieshout R, Taylor V, Boyle M. 2011. Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes. Rev. An Off. J. Int. Assoc. Study Obes. 12, e548-e559. ( 10.1111/j.1467-789X.2010.00850.x) [DOI] [PubMed] [Google Scholar]
- 128.Georgieff M, Ramel S, Cusick S. 2018. Nutritional influences on brain development. Acta Paediatr. 107, 1310-1321. ( 10.1111/apa.14287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stephenson J, et al. 2018. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet 391, 1830-1841. ( 10.1016/S0140-6736(18)30311-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mousa A, Naqash A, Lim S. 2019. Macronutrient and micronutrient intake during pregnancy: an overview of recent evidence. Nutrients 11, 443. ( 10.3390/nu11020443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Van den Elsen L, Garssen J, Burcelin R, Verhasselt V. 2019. Shaping the gut microbiota by breastfeeding: the gateway to allergy prevention? Front. Pediatr. 7, 47. ( 10.3389/fped.2019.00047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zaidi A, Moore S, Okala S. 2021. Impact of maternal nutritional supplementation during pregnancy and lactation on the infant gut or breastmilk microbiota: a systematic review. Nutrients 13, 1137. ( 10.3390/nu13041137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ford EL, Underwood MA, German JB. 2020. Helping mom help baby: nutrition-based support for the mother-infant dyad during lactation. Front. Nutr. 7, 1-10. ( 10.3389/fnut.2020.00001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bowers ME, Yehuda R. 2015. Intergenerational transmission of stress in humans. Neuropsychopharmacology 41, 232-244. ( 10.1038/npp.2015.247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sheriff MJ, Krebs CJ, Boonstra R. 2009. The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J. Anim. Ecol. 78, 1249-1258. ( 10.1111/j.1365-2656.2009.01552.x) [DOI] [PubMed] [Google Scholar]
- 136.Maestripieri D. 2005. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc. Natl Acad. Sci. USA 102, 9726-9729. ( 10.1073/pnas.0504122102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Montgomery TM, Pendleton EL, Smith JE. 2018. Physiological mechanisms mediating patterns of reproductive suppression and alloparental care in cooperatively breeding carnivores. Physiol. Behav. 193, 167-178. ( 10.1016/J.PHYSBEH.2017.11.006) [DOI] [PubMed] [Google Scholar]
- 138.Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308, 648-652. ( 10.1126/science.1106477) [DOI] [PubMed] [Google Scholar]
- 139.Champagne FA. 2008. Epigenetic mechanisms and the transgenerational effects of maternal care. Front. Neuroendocrinol. 29, 386-397. ( 10.1016/j.yfrne.2008.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Frank LG. 1986. Social organization of the spotted hyaena Crocuta crocuta. II. Dominance and reproduction. Anim. Behav. 34, 1510-1527. ( 10.1016/S0003-3472(86)80221-4) [DOI] [Google Scholar]
- 141.Holekamp KE, Smale L, Szykman M. 1996. Rank and reproduction in the female spotted hyaena. J. Reprod. Fertil. 108, 229-237. ( 10.1530/jrf.0.1080229) [DOI] [PubMed] [Google Scholar]
- 142.Flies AS, Mansfield LS, Flies EJ, Grant CK, Holekamp KE. 2016. Socioecological predictors of immune defences in wild spotted hyenas. Funct. Ecol. 30, 1549-1557. ( 10.1111/1365-2435.12638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Swanson EM, Dworkin I, Holekamp KE. 2011. Lifetime selection on a hypoallometric size trait in the spotted hyena. Proc. R. Soc. B 278, 3277-3285. ( 10.1098/rspb.2010.2512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goymann W, East ML, Wachter B, Höner OP, Möstl E, Van't Hof TJ, Hofer H. 2001. Social, state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyenas. Proc. R. Soc. B 268, 2453-2459. ( 10.1098/rspb.2001.1828) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Milewski TM, Lee W, Champagne FA, Curley JP. 2022. Behavioural and physiological plasticity in social hierarchies. Phil. Trans. R. Soc. B 377, 20200443. ( 10.1098/RSTB.2020.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Berghänel A, Heistermann M, Schülke O, Ostner J. 2017. Prenatal stress accelerates offspring growth to compensate for reduced maternal investment across mammals. Proc. Natl Acad. Sci. USA 114, E10658-E10666. ( 10.1073/pnas.1707152114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Berghänel A, Heistermann M, Schülke O, Ostner J. 2016. Prenatal stress effects in a wild, long-lived primate: predictive adaptive responses in an unpredictable environment. Proc. R. Soc. B 283, 20161304. ( 10.1098/rspb.2016.1304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Snyder-Mackler N, et al. 2020. Social determinants of health and survival in humans and other animals. Science 368, eaax9553. ( 10.1126/science.aax9553) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Feagin J, Bennefield Z. 2014. Systemic racism and U.S. health care. Soc. Sci. Med. 103, 7-14. ( 10.1016/j.socscimed.2013.09.006) [DOI] [PubMed] [Google Scholar]
- 150.Feagin JR. 2006. Systemic racism: a theory of oppression. New York, NY: Taylor and Francis. [Google Scholar]
- 151.Ragsdale JE. 1999. Reproductive skew theory extended: the effect of resource inheritance on social organization. Evol. Ecol. Res. 1, 859-874. [Google Scholar]
- 152.Hatchwell BJ. 2009. The evolution of cooperative breeding in birds: kinship, dispersal and life history. Phil. Trans. R. Soc. B 364, 3227. ( 10.1098/rstb.2009.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mosser A, Fryxell JM, Eberly L, Packer C. 2009. Serengeti real estate: density vs. fitness-based indicators of lion habitat quality. Ecol. Lett. 12, 1050-1060. ( 10.1111/j.1461-0248.2009.01359.x) [DOI] [PubMed] [Google Scholar]
- 154.Emery NJ, Clayton NS. 2004. The mentality of crows: convergent evolution of intelligence in corvids and apes. Science 306, 1903-1907. ( 10.1126/science.1098410) [DOI] [PubMed] [Google Scholar]
- 155.Tebbich S, Taborsky M, Fessl B, Dvorak M. 2002. The ecology of tool-use in the woodpecker finch (Cactospiza pallida). Ecol. Lett. 5, 656-664. ( 10.1046/j.1461-0248.2002.00370.x) [DOI] [Google Scholar]
- 156.Bissonnette A, Perry S, Barrett L, Mitani JC, Flinn M, Gavrilets S, de Waal FBM. 2015. Coalitions in theory and reality: a review of pertinent variables and processes. Behaviour 152, 1-56. ( 10.1163/1568539X-00003241) [DOI] [Google Scholar]
- 157.Harcourt AH, de Waal FBM. 1992. Coalitions and alliances in humans and other animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 158.Zabel CJ, Glickman SE, Frank LG, Woodmansee KB, Keppel G. 1992. Coalition formation in a colony of prepubertal spotted hyaenas. In Coalitions and alliances in humans and other animals (eds Harcourt AH, de Waal FBM), pp. 113-136. Oxford, UK: Oxford University Press. [Google Scholar]
- 159.Smale L, Laurence FG, Holekamp KE. 1993. Ontogeny of dominance in free-living spotted hyaenas: juvenile rank relations with adult females and immigrant males. Anim. Behav. 46, 467-477. ( 10.1006/anbe.1993.1215) [DOI] [Google Scholar]
- 160.de Villiers MS, Richardson PR, van Jaarsveld AS. 2003. Patterns of coalition formation and spatial association in a social carnivore, the African wild dog (Lycaon pictus). J. Zool. 260, 377-389. ( 10.1017/S0952836903003832) [DOI] [Google Scholar]
- 161.Jennings DJ, Carlin CM, Hayden TJ, Gammell MP. 2011. Third-party intervention behaviour during fallow deer fights: the role of dominance, age, fighting and body size. Anim. Behav. 81, 1217-1222. ( 10.1016/j.anbehav.2011.03.007) [DOI] [Google Scholar]
- 162.Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207-2210. ( 10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 163.Gilby IC, Brent LJN, Wroblewski EE, Rudicell RS, Hahn BH, Goodall J, Pusey AE. 2012. Fitness benefits of coalitionary aggression in male chimpanzees. Behav. Ecol. Sociobiol. 67, 373-381. ( 10.1007/s00265-012-1457-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Watts DP. 2002. Reciprocity and interchange in the social relationships of wild male chimpanzees. Behaviour 139, 343-370. ( 10.1163/156853902760102708) [DOI] [Google Scholar]
- 165.Silk JB, Alberts SC, Altmann J. 2004. Patterns of coalition formation by adult female baboons in Amboseli, Kenya. Anim. Behav. 67, 573-582. ( 10.1016/j.anbehav.2003.07.001) [DOI] [Google Scholar]
- 166.Smith JE. 2014. Hamilton's legacy: kinship, cooperation and social tolerance in mammalian groups. Anim. Behav. 92, 291-304. ( 10.1016/j.anbehav.2014.02.029) [DOI] [Google Scholar]
- 167.Silk JB. 2002. Kin selection in primate groups. Int. J. Primatol. 23, 849-875. ( 10.1023/A:1015581016205) [DOI] [Google Scholar]
- 168.Widdig A. 2007. Paternal kin discrimination: the evidence and likely mechanisms. Biol. Rev. 82, 319-334. ( 10.1111/j.1469-185X.2007.00011.x) [DOI] [PubMed] [Google Scholar]
- 169.Lee PC, Oliver JI. 1979. Competition, dominance and the acquisition of rank in juvenile yellow baboons (Papio cynocephalus). Anim. Behav. 27, 576-585. ( 10.1016/0003-3472(79)90193-3) [DOI] [Google Scholar]
- 170.Gouzoules H. 1975. Maternal rank and early social interactions of infant stumptail macaques, Macaca arctoides. Primates 16, 405-418. ( 10.1007/BF02382739) [DOI] [Google Scholar]
- 171.Fairbanks LA, McGuire MT. 1985. Relationships of vervet mothers with sons and daughters from one through three years of age. Anim. Behav. 33, 40-50. ( 10.1016/S0003-3472(85)80118-4) [DOI] [Google Scholar]
- 172.Horrocks J, Hunte W. 1983. Maternal rank and offspring rank in vervet monkeys: an appraisal of the mechanisms of rank acquisition. Anim. Behav. 31, 772-782. ( 10.1016/S0003-3472(83)80234-6) [DOI] [Google Scholar]
- 173.Barrett L, Peter Henzi S, Lusseau D. 2012. Taking sociality seriously: the structure of multi-dimensional social networks as a source of information for individuals. Phil. Trans. R. Soc. B 367, 2108-2118. ( 10.1098/rstb.2012.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Flack JC, Girvan M, De Waal FBM, Krakauer DC. 2006. Policing stabilizes construction of social niches in primates. Nature 439, 426-429. ( 10.1038/nature04326) [DOI] [PubMed] [Google Scholar]
- 175.Massen JJM, Szipl G, Spreafico M, Bugnyar T. 2014. Ravens intervene in others' bonding attempts. Curr. Biol. 24, 2733. ( 10.1016/j.cub.2014.09.073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ilany A, Akçay E. 2016. Social inheritance can explain the structure of animal social networks. Nat. Commun. 7, 1-10. ( 10.1038/ncomms12084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jarrett JD, Bonnell TR, Young C, Barrett L, Henzi SP. 2018. Network integration and limits to social inheritance in vervet monkeys. Proc. R. Soc. B 285, 20172668. ( 10.1098/rspb.2017.2668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jaeggi AV, Gurven M. 2013. Natural cooperators: food sharing in humans and other primates. Evol. Anthropol. Issues, News, Rev. 22, 186-195. ( 10.1002/evan.21364) [DOI] [PubMed] [Google Scholar]
- 179.Jaeggi AV, Gurven M. 2013. Reciprocity explains food sharing in humans and other primates independent of kin selection and tolerated scrounging: a phylogenetic meta-analysis. Proc. R. Soc. B 280, 20131615. ( 10.1098/rspb.2013.1615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jaeggi AV, Van Schaik CP. 2011. The evolution of food sharing in primates. Behav. Ecol. Sociobiol. 65, 2125-2140. ( 10.1007/s00265-011-1221-3) [DOI] [Google Scholar]
- 181.Boesch C, Boesch-Achermann H. 1989. Hunting behavior of wild chimpanzees in the Tai National Park. Am. J. Phys. Anthropol. 78, 547-573. ( 10.1002/ajpa.1330780410) [DOI] [PubMed] [Google Scholar]
- 182.Stanford C. 1998. Chimpanzee and red colobus: the ecology of predator and prey. Cambridge, MA: Harvard University Press. [Google Scholar]
- 183.Wrangham R. 1975. Behavioural ecology of chimpanzees in Gombe National Park, Tanzania. Cambridge, UK: University of Cambridge Press. [PubMed] [Google Scholar]
- 184.Goodall J. 1986. The chimpanzees of Gombe: patterns of behavior. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 185.Mitani J, Watts D. 1999. Demographic influences on the hunting behavior of chimpanzees. Am. J. Phys. Anthropol. 109, 439-454. () [DOI] [PubMed] [Google Scholar]
- 186.Mitani J, Watts D. 2001. Why do chimpanzees hunt and share meat? Anim. Behav. 61, 915-924. ( 10.1006/anbe.2000.1681) [DOI] [Google Scholar]
- 187.de Waal F. 1989. Food sharing and reciprocal obligations among chimpanzees. J. Hum. Evol. 18, 433-459. ( 10.1016/0047-2484(89)90074-2) [DOI] [Google Scholar]
- 188.de Waal F. 1997. Food transfers through mesh in brown capuchins. J. Comp. Psychol. 111, 370-378. ( 10.1037/0735-7036.111.4.370) [DOI] [PubMed] [Google Scholar]
- 189.Heinrich B. 1988. Winter foraging at carasses by three sympatric corvids, with emphasis on recruitment by the raven, Corvus corax. Behav. Ecol. Sociobiol. 23, 141-156. ( 10.1007/BF00300349) [DOI] [Google Scholar]
- 190.Goffe A, Fischer J. 2016. Meat sharing between male and female Guinea baboons (Papio papio). Primate Biol. 3, 1-8. ( 10.5194/pb-3-1-2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dale R, Marshall-Pescini S, Range F. 2017. Do females use their sexual status to gain resource access? Investigating food-for-sex in wolves and dogs. Curr. Zool. 63, 323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carter GG, Wilkinson GS. 2013. Food sharing in vampire bats: reciprocal help predicts donations more than relatedness or harassment. Proc. R. Soc. B 280, 20122573. ( 10.1098/rspb.2012.2573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hoelzel A. 1991. Killer whale predation on marine mammals at Punta-Norte, Argentina; food sharing, provisioning and foraging strategy. Behav. Ecol. Sociobiol. 29, 197-204. ( 10.1007/BF00166401) [DOI] [Google Scholar]
- 194.Kalmbach E. 2006. Why do goose parents adopt unrelated goslings? A review of hypotheses and empirical evidence, and new research questions. Ibis (Lond. 1859) 148, 66-78. ( 10.1111/j.1474-919X.2006.00496.x) [DOI] [Google Scholar]
- 195.Lank DB, Bousfield MA, Cooke F, Rockwell RF. 1991. Why do snow geese adopt eggs? Behav. Ecol. 2, 181-187. ( 10.1093/beheco/2.2.181) [DOI] [Google Scholar]
- 196.Pandit SA, Van Schaik CP. 2003. A model for leveling coalitions among primate males: toward a theory of egalitarianism. Behav. Ecol. Sociobiol. 55, 161-168. ( 10.1007/s00265-003-0692-2) [DOI] [Google Scholar]
- 197.Gygax L, Harley N, Kummer H. 1997. A matrilineal overthrow with destructive aggression in Macaca fascicularis. Primates 38, 149-158. ( 10.1007/BF02382005) [DOI] [Google Scholar]
- 198.van Schaik CP, Pandit SA, Vogel ER. 2006. Toward a general model for male-male coalitions in primate groups. In Cooperation in primates and humans (eds Kappeler PM, van Schaik CP), pp. 151-171. Berlin, Germany: Springer. [Google Scholar]
- 199.Toyoda A, Maruhashi T, Kawamoto Y, Matsudaira K, Matsuda I, Malaivijitnond S. 2022. Mating and reproductive success in free-ranging stump-tailed macaques: effectiveness of male–male coalition formation as a reproductive strategy. Front. Ecol. Evol. 10, 175. ( 10.3389/fevo.2022.802012) [DOI] [Google Scholar]
- 200.Packer C. 1977. Reciprocal altruism in Papio anubis. Nature 265, 441-443. ( 10.1038/265441a0) [DOI] [Google Scholar]
- 201.Smuts BB. 1985. Sex and friendship in baboons. New York, NY: Aldine. [Google Scholar]
- 202.Noë R. 1990. A veto game played by baboons: a challenge to the use of the Prisoner's Dilemma as a paradigm for reciprocity and cooperation. Anim. Behav. 39, 78-90. ( 10.1016/S0003-3472(05)80728-6) [DOI] [Google Scholar]
- 203.Bercovitch FB. 1988. Coalitions, cooperation and reproductive tactics among adult male baboons. Anim. Behav. 36, 1198-1209. ( 10.1016/S0003-3472(88)80079-4) [DOI] [Google Scholar]
- 204.Chikazawa D, Gordon TP, Bean CA, Bernstein IS. 1979. Mother-daughter dominance reversals in rhesus monkeys (Macaca mulatta). Primates 20, 301-305. ( 10.1007/BF02373382) [DOI] [Google Scholar]
- 205.Chapais B. 1985. An experimental analysis of a mother-daughter rank reversal in Japanese macaques (Macaca fuscata). Primates 26, 407-423. ( 10.1007/BF02382456/METRICS) [DOI] [Google Scholar]
- 206.Karremans JC, Van Lange PAM. 2008. Forgiveness in personal relationships: its malleability and powerful consequences. Eur. Rev. Soc. Psychol. 19, 202-241. ( 10.1080/10463280802402609) [DOI] [Google Scholar]
- 207.Aureli F, de Waal F. 2000. Natural conflict resolution. In Natural conflict resolution (eds Aureli F, de Waal FBM), pp. 586-590. Berkeley, CA: University of California Press. [Google Scholar]
- 208.Aureli F, Cords M, Van Schaik CP. 2002. Conflict resolution following aggression in gregarious animals: a predictive framework. Anim. Behav. 64, 325-343. ( 10.1006/anbe.2002.3071) [DOI] [Google Scholar]
- 209.Watts DP. 1995. Post-conflict social events in wild mountain gorillas. II. Redirection, side direction, and consolation. Ethology 100, 158-174. ( 10.1111/j.1439-0310.1995.tb00322.x) [DOI] [Google Scholar]
- 210.de Waal FBM, van Roosmalen A. 1979. Reconciliation and consolation among chimpanzees. Behav. Ecol. Sociobiol. 5, 55-66. ( 10.1007/BF00302695) [DOI] [Google Scholar]
- 211.Palagi E, Paoli T, Tarli SB. 2004. Reconciliation and consolation in captive bonobos (Pan paniscus). Am. J. Primatol. 62, 15-30. ( 10.1002/ajp.20000) [DOI] [PubMed] [Google Scholar]
- 212.Berghänel A, Ostner J, Schröder U, Schülke O. 2011. Social bonds predict future cooperation in male Barbary macaques, Macaca sylvanus. Anim. Behav. 81, 1109-1116. ( 10.1016/j.anbehav.2011.02.009) [DOI] [Google Scholar]
- 213.Fraser ON, Bugnyar T. 2011. Ravens reconcile after aggressive conflicts with valuable partners. PLoS One 6, e18118. ( 10.1371/journal.pone.0018118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Morrison L. 2009. Sociality and reconciliation in monk parakeets (Myiopsitta monachus). Masters thesis, University of Nebraska, Lincoln, Nebraska, USA. [Google Scholar]
- 215.Fehr E, Schmidt KM. 1999. A theory of fairness, competition, and cooperation. Q. J. Econ. 114, 817-868. ( 10.1162/003355399556151) [DOI] [Google Scholar]
- 216.Uzefovsky F, Paz Y, Davidov M. 2020. Young infants are pro-victims, but it depends on the context. Br. J. Psychol. 111, 322-334. ( 10.1111/bjop.12402) [DOI] [PubMed] [Google Scholar]
- 217.Brosnan SF, De Waal FBM. 2003. Monkeys reject unequal pay. Nature 425, 297-299. ( 10.1038/nature01963) [DOI] [PubMed] [Google Scholar]
- 218.Brosnan SF. 2011. A hypothesis of the co-evolution of cooperation and responses to inequity. Front. Neurosci. 5, 43. ( 10.3389/fnins.2011.00043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Brosnan SF, Schiff HC, De Waal FBM. 2005. Tolerance for inequity may increase with social closeness in chimpanzees. Proc. R. Soc. B 272, 253-258. ( 10.1098/rspb.2004.2947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brosnan SF, Talbot C, Ahlgren M, Lambeth SP, Schapiro SJ. 2010. Mechanisms underlying responses to inequitable outcomes in chimpanzees, Pan troglodytes. Anim. Behav. 79, 1229-1237. ( 10.1016/j.anbehav.2010.02.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Silk JB, Brosnan SF, Vonk J, Henrich J, Povinelli DJ, Richardson AS, Lambeth SP, Mascaro J, Schapiro SJ. 2005. Chimpanzees are indifferent to the welfare of unrelated group members. Nature 437, 1357-1359. ( 10.1038/nature04243) [DOI] [PubMed] [Google Scholar]
- 222.Massen JJM, Van Den Berg LM, Spruijt BM, Sterck EHM. 2012. Inequity aversion in relation to effort and relationship quality in long-tailed macaques (Macaca fascicularis). Am. J. Primatol. 74, 145-156. ( 10.1002/ajp.21014) [DOI] [PubMed] [Google Scholar]
- 223.Wascher CAF, Bugnyar T. 2013. Behavioral responses to inequity in reward distribution and working effort in crows and ravens. PLoS ONE 8, e56885. ( 10.1371/journal.pone.0056885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Oberliessen L, Hernandez-Lallement J, Schäble S, van Wingerden M, Seinstra M, Kalenscher T. 2016. Inequity aversion in rats, Rattus norvegicus. Anim. Behav. 115, 157-166. ( 10.1016/j.anbehav.2016.03.007) [DOI] [Google Scholar]
- 225.Range F, Horn L, Viranyi Z, Huber L. 2009. The absence of reward induces inequity aversion in dogs. Proc. Natl Acad. Sci. USA 106, 340-345. ( 10.1073/pnas.0810957105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bräuer J, Call J, Tomasello M. 2006. Are apes really inequity averse? Proc. R. Soc. B 273, 3123-3128. ( 10.1098/rspb.2006.3693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hare B, Melis AP, Woods V, Hastings S, Wrangham R. 2007. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 17, 619-623. ( 10.1016/j.cub.2007.02.040) [DOI] [PubMed] [Google Scholar]
- 228.Brosnan SF, Flemming T, Talbot CF, Mayo L, Stoinski T. 2011. Orangutans (Pongo pygmaeus) do not form expectations based on their partner's outcomes. Folia Primatol. 82, 56-70. ( 10.1159/000328142) [DOI] [PubMed] [Google Scholar]
- 229.Talbot CF, Freeman HD, Williams LE, Brosnan SF. 2011. Squirrel monkeys' response to inequitable outcomes indicates a behavioural convergence within the primates. Biol. Lett. 7, 680-682. ( 10.1098/rsbl.2011.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Freeman HD, Sullivan J, Hopper LM, Talbot CF, Holmes AN, Schultz-Darken N, Williams LE, Brosnan SF. 2013. Different responses to reward comparisons by three primate species. PLoS ONE 8, e76297. ( 10.1371/journal.pone.0076297) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Smith JE, Natterson-Horowitz B, Mueller MM, Alfaro ME. 2023. Mechanisms of equality and inequality in mammalian societies. Figshare. ( 10.6084/m9.figshare.c.6662596) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data from the freely available online archive. For details, see [44].
Data and all R-code for statistical analyses is included in the electronic supplementary material [48].