Abstract
Despite the global spread of intensive agriculture, many populations retained foraging or mixed subsistence strategies until well into the twentieth century. Understanding why has been a longstanding puzzle. One explanation, called the marginal habitat hypothesis, is that foraging persisted because foragers tended to live in marginal habitats generally not suited to agriculture. However, recent empirical studies have not supported this view. The alternative but untested oasis hypothesis of agricultural intensification claims that intensive agriculture developed in areas with low biodiversity and a reliable water source not reliant on local rainfall. We test both the marginal habitat and oasis hypotheses using a cross-cultural sample drawn from the 'Ethnographic atlas' (Murdock 1967 Ethnology 6, 109–236). Our analyses provide support for both hypotheses. We found that intensive agriculture was unlikely in areas with high rainfall. Further, high biodiversity, including pathogens associated with high rainfall, appears to have limited the development of intensive agriculture. Our analyses of African societies show that tsetse flies, elephants and malaria are negatively associated with intensive agriculture, but only the effect of tsetse flies reached significance. Our results suggest that in certain ecologies intensive agriculture may be difficult or impossible to develop but that generally lower rainfall and biodiversity is favourable for its emergence.
This article is part of the theme issue ‘Evolutionary ecology of inequality’.
Keywords: foragers, marginal habitat hypothesis, cultural evolution
1. Introduction
During the Neolithic transition, societies across the globe transitioned from foraging to horticulture and over time many of them developed intensive agriculture. However, well into the twentieth and twenty-first centuries some regions never adopted intensive agriculture, instead maintaining foraging, horticulture, or a mix of both. Understanding why foraging and horticulture persisted well after the development of intensive agriculture has been a major puzzle. Intensive agriculture has profoundly altered human societies, providing phenomenal abundance, but is also associated with high levels of inequality both within and between human societies. Small-scale subsistence populations, such as foragers and horticulturalists, typically have less inequality—both in economic differentiation, and also in social and political capital [1,2]. Because of this, there is long-standing interest in using small-scale subsistence societies as models to better understand human social organization prior to the development of intensive agriculture, as well as the factors that may inhibit or promote inegalitarian social structures.
Unilinear evolutionist thought, which has long fallen out of favour, proposed that human societies that were not on the path to industrialization were primitive, and with sufficient time they would develop intensive food production [3–5]. More recently, multi-linear evolutionists have argued that the mode of subsistence of a population is generally dependent on the local ecology [6–8]. This framework is the starting point for the marginal habitat hypothesis, which proposes that foraging continued (or persisted) in environments that were not suitable for agriculture because they were environmentally marginal [9,10].
Hunter–gatherers or foragers are people who acquire their food through hunting, gathering or fishing [11], depending on wild foods not domesticated or cultivated by humans [12]. Even though many foragers were relatively egalitarian within sexes and age groups, a few hunting and gathering societies had food storage and high social stratification, the societies of the American Pacific Northwest being a well-known example [11,13]. Today, there are no pure foragers, and those who retain some aspects of their foraging lifeway are highly interdependent with their non-foraging neighbours [11]. We use the term ‘agricultural societies' to refer to those societies with a high dependence on domesticates––plant or animal species that are under human selection [12]. By the term ‘agriculture’, we refer to non-foragers, including horticulturalists, pastoralists, and intensive agriculturalists [14,15]. Horticulturalists are described as small-scale farmers who plant gardens or use swidden plots while they may continue to get a significant portion of their diets from foraging [12]. Pastoralists have a high dependence on animal husbandry, though usually supplemented with agricultural or foraged products [14]. Murdock defined intensive agriculture as farming ‘on permanent fields, utilizing fertilization by compost or animal manure, crop rotation or other techniques so that fallowing is either unnecessary or is confined to relatively short periods' [16, p. 159;17].
There have only been two quantitative tests of the marginal habitat hypothesis, both of which used net primary productivity (NPP) to assess habitat quality [14,15]. NPP is often used as a proxy to evaluate how suitable a habitat is for agriculture—with higher values considered more suitable. NPP is calculated based on the amount of new plant growth annually in an area, excluding the plant's own metabolic needs. NPP is therefore a measure of the energy available to support life in a specified area per year beyond the maintenance costs of the flora [14,18,19]. Porter & Marlowe [15] attempted to test the marginal habitat hypothesis, comparing the NPP of foragers (those with <10% dependence on plant cultivation or animal husbandry) to agriculturalists using the standard cross-cultural sample (SCCS) consisting of 186 societies designed to capture a globally representative sample of human societies for cross-cultural analysis. They found that the difference in NPP between the foragers and agriculturalists was not significant, which led them to reject the marginal habitat hypothesis, concluding that foragers ‘living in marginal habitats [compared with agriculturalists] is not a reason that need concern us' [15. p. 67].
Cunningham et al. [14] also tested the marginal habitat hypothesis, using several different measurements of NPP as well as the population density of human communities. They came to similar conclusions to Porter & Marlowe [15], rejecting the marginal habitat hypothesis. However, they found that NPP predicted the population density of foragers but there were unexpected NPP–population density relationships among pastoralists, horticulturalists, and intensive agriculturalists. Intensive agriculturalists and pastoralists could achieve medium to high population density at low NPP while horticulturalists had intermediate population density at high NPP.
Despite their controls, such as excluding cold weather foragers, both studies found no differences in habitat quality between foragers and agriculturalists [14,15]. But, as Cunningham et al. [14] argue, NPP is a poor measure of habitat quality. It measures only non-metabolic plant production, yet the equatorial rainforests have extremely high NPP, but much of it is non-edible (leaves, woody tissues) or difficult to forage (high in the canopy) [20]. Further, many areas that had foragers or horticulturalists until recently now have intensive agriculture, demonstrating that these habitats are in fact suitable for agriculture or can be modified to be suitable for agriculture.
A more promising approach to understanding the relationships between environment and subsistence is demonstrated by Tallavaara et al. [21], who study how ecological factors including biodiversity, pathogens and NPP predict the population density of non-industrial foragers. While these authors do not assess how these factors impact the retention of foraging and horticulture, they show that biodiversity and pathogens are important forces shaping the distribution of foraging populations. Their results suggest that the pathway between NPP and agriculture may require considering the impact of biodiversity and pathogens.
The roles that specific kinds of biodiversity or pathogens may have had in shaping human subsistence have generally been overlooked, with some exceptions. Diamond [6], for example, notes that certain kinds of biodiversity could improve intensive agriculture, such as through providing pathways to the domestication of draft animals. The converse may also be true: the types of biodiversity, the prevalence of disease, or even high levels of rainfall may be inimical for intensive agriculture in regions with high NPP. For example, the Mbuti, who are central African rainforest foragers, inhabit an area with extremely high rainfall [22]. While the groups that neighbour the Mbuti practise horticulture and raise goats and chickens, they are unable to raise cattle for food or ploughing, in part owing to the high prevalence of tsetse fly in the region, which negatively affects cattle [23].
At high rainfall, pathogens and biodiversity are not the only challenge for intensive agriculture. Holden et al. [24] report that when the government of Indonesia moved people from the overpopulated inner islands of Indonesia to the rainforest-covered outer islands, farming failed among these recently moved people. They attributed the failure of farming to pests such as wild pigs, rodents, weeds and insects, as well as waterlogging during periods of high rainfall, which can reduce crop yields by killing seedlings [24].
Elephants, monkeys, birds and other animals have also been shown to negatively impact agriculture in Africa [25]. Elephants in particular can devastate farms [26–29]. While the Asian elephant has been used as an aid in agriculture for centuries, the larger and more aggressive African elephant species (Loxodonta africana and Loxodonta cyclotis [30]) have not been domesticated to the point where they can be used in agriculture [31]. The North African elephant that was used in wars in ancient Egypt has been extinct for a few hundred years, therefore only Sub-Saharan elephants are relevant for our analyses [31]. While elephants in Africa today still can have devastating impacts on farming, their impact historically was likely to have been much larger prior to the introduction of firearms and widespread poaching, which has decimated African elephant populations. The carrying capacity of elephants prior to the introduction of guns around 1810 has been calculated to be around 27 million in Sub-Saharan Africa compared with an estimated 2016 population of 415 428 [32,33]. Thus, the effect of elephants on crops in the past was likely extremely significant.
Pathogens affect both humans and their domesticates, which negatively impacts intensive agriculture [23]. Human labour is required for intensification by plough, and animals can be used for draught power. Malaria has been shown to affect the productivity of farmers [34–37]. The sickle cell trait is mostly found in descendants of yam farmers in West Africa because clearing the land for farming helped mosquitoes thrive in mud puddles [36]. Likewise, Alsan [23] demonstrated the negative impacts of the tsetse fly on African development. Tsetse not only can affect people but has much more profound effects on livestock, rendering areas with high numbers of tsetse fly unsuitable for cattle. Alsan [23] argues that historic Zimbabwe became transiently successful because it was in a highland area which had low tsetse fly suitability that likely allowed some success from cattle rearing.
The marginal habitat hypothesis is not the only hypothesis used to explain why agriculture was not universally adopted after the Neolithic transition. The alternative oasis hypothesis [38] argues that the domestication of plants and animals occurred around reliable ‘water sources as the climate dried out at the end of the last ice age’ [39, p. 1390]. This hypothesis was formulated to explain the ideal environments for domestication to occur; however, we posit that these environmental conditions were also critical for progression to intensive agriculture after domestication. In this paper, we present a modified version of the oasis hypothesis: namely, the oasis hypothesis of agriculture intensification. Unlike the original version, which referred to a literal oasis, we interpret an oasis more broadly as a place with low to moderate rainfall, a water source not solely reliant on local rainfall (such as a river), and low to moderate biodiversity, including pathogens. We propose that the intensification of agriculture was more likely in places that approximated these oasis conditions. Therefore, we expect intensive agriculture to have been more likely at low or moderate rainfall than at high rainfall, and in areas with low to moderate biodiversity. This study is the first to our knowledge to quantitatively test the oasis hypothesis.
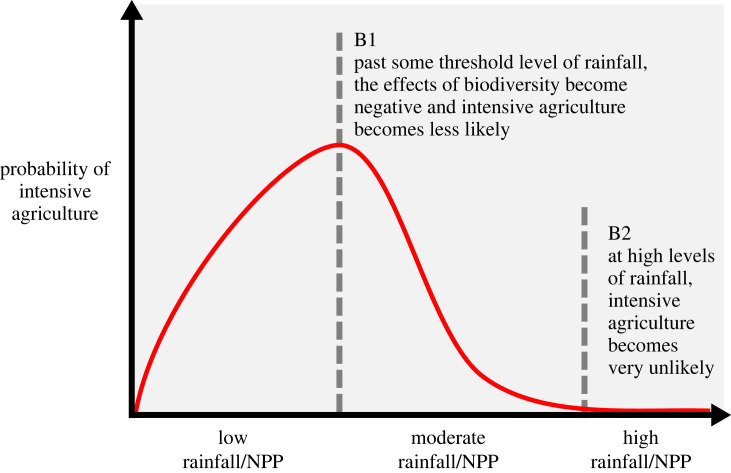
The relationship between the environment, foraging persistence and the development of intensive agriculture is expected to be complex, depending on factors such as the amount and intensity of rainfall, biodiversity, and pathogens. Increased rainfall should be associated with greater NPP and biodiversity, which at moderate levels may facilitate intensive agriculture. But as rainfall continues to increase, it may adversely affect the likelihood of intensive agriculture, either through deleterious effects of excessive rain, or through by-products such as increased biodiversity and pathogens. We expect that initially more rainfall will lead to a greater likelihood of intensive agriculture, but past a certain threshold the relationship will become negative. Our expectations are outlined in figure 1, which is a hypothetical probability density plot for two variables, with biodiversity as a third variable that stratifies the plot.
Figure 1.
The expected relationships between rainfall, biodiversity and intensive agriculture. As rainfall increases, biodiversity increases. B1 is the hypothesized point where the density of intensive agriculture societies starts to decline owing to increased rainfall and biodiversity. B2 is the point where the frequency of agricultural intensification approaches zero because the levels of rainfall and biodiversity are prohibitive. Beyond this point, biodiversity must be decoupled from rainfall for intensive agriculture to occur.
To understand why foraging, horticulture and pastoralism persisted well into the twentieth century, we use a global sample of pre-industrial societies to investigate how rainfall, NPP and biodiversity including pathogens, separately and in combination affect the degree of agricultural intensification. We then use a restricted sample of African societies to evaluate the effects of specific kinds of biodiversity and pathogens on agriculture intensity, focusing on elephants, malaria, and tsetse flies. We hypothesize that foraging, horticulture, and pastoralism persisted in areas where the environment limited or prohibited intensive agriculture in different ways.
2. Materials
Data were obtained from the 'Ethnographic atlas' (EA), accessed through the D-PLACE database [16,17], to examine the relationship between agriculture intensity and various ecological variables. The EA database contains 1291 societies from across the globe, representing a range of socio-political systems [40]. We excluded any societies without a numerical code for our main dependent variable of agricultural intensity (EA variable ID EA028), resulting in a sample of 1188 societies. We also used a restricted sample limited to the African societies (including Madagascar) present in the EA (n = 497 societies).
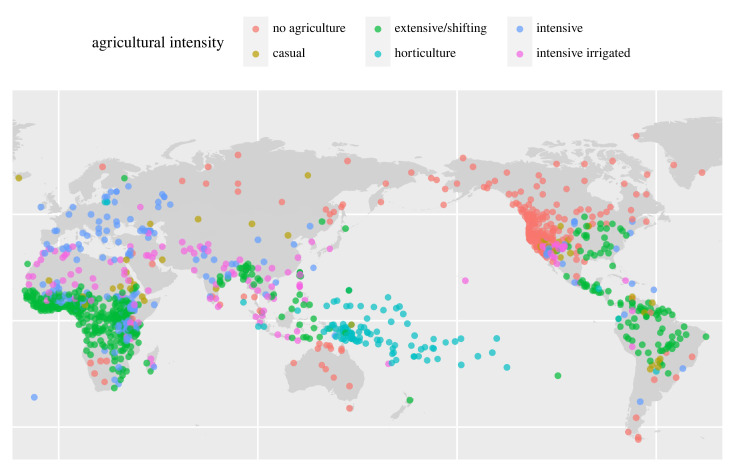
Our main dependent variable (EA028) categorizes societies on a scale from 1 to 6 based on their degree of agricultural intensification, with Level 1 being no agriculture and Level 6 being intensive irrigated agriculture (figure 2). Intensive agriculture (Level 5) is defined as growing crops on ‘permanent fields, utilizing fertilization by compost or animal manure, crop rotation or other techniques so that fallowing is either unnecessary or is confined to relatively short periods', while intensive irrigated agriculture (Level 6) was where intensive agriculture mainly relied on irrigation [16, p. 159;17].
Figure 2.
Societies from the 'Ethnographic atlas' [16] used to evaluate the relationships among agricultural intensity and rainfall, NPP, pathogens and biodiversity (N = 1188). Our focus is on comparing intensive and intensive irrigated agriculture with non-intensive forms of subsistence.
3. Methods
The EA is vulnerable to phylogenetic autocorrelation, which is the inflation of spurious association owing to shared ancestry [41,42]. We overcome this problem by controlling for phylogeny in regression analyses and repeating non-regression analysis using the SCCS, which is a subset of the EA created to control for phylogeny as well as diffusion from geographical proximity [43] (but see [44,45]).
To investigate the ecological determinates of agricultural intensity, we analysed the effects of rainfall (monthly mean precipitation in ml m−2 month−1), NPP (monthly mean net primary production), and several biodiversity variables, including plant vascular richness, bird richness, mammal richness, amphibian richness, malaria index (MI), tsetse suitability index (TSI) and elephant presence [17,46,47]. The MI and TSI were extracted from an existing data repository created by Alsan [48]. For our African sample, we manually coded the presence or absence of elephants in the late precolonial era based on the society's geographical location and the predicted historical range of elephants based on Wall et al.'s estimates [49]. All the other variables were found in D-PLACE [17]. Data were matched at the society level using society codes and manual identification.
For basic hypotheses tests, we categorized societies by whether they had intensive agriculture (Levels 5 and 6) or not (Levels 1–4) and compared these two groups in terms of rainfall, NPP and plant vascular richness, as well as bird, amphibian and mammal richness. For plant vascular, bird and mammal richness these tests were done for all the EA societies and for each continent—Eurasia, Africa, South America, North America, Australia and Papunesia (a macro-area referring to Insular South East Asia, Papua New Guinea and all of Oceania except Australia [50]).
We also visually inspected the probability density plots for intensive agriculture to identify the inflecion points at which intensive agriculture becomes less likely (B1) and extremely unlikely (B2) from our hypothetical model in figure 1. Our regression analyses included generalized linear models (GLMs) and Bayesian regression models. We used a binomial regression model to predict the probability of a society having intensive or intensive-irrigated agriculture according to rainfall to test our hypothetical model presented in figure 1. The rainfall variable was scaled to approximate a normal distribution centred around 0, and a nonlinear (quadratic) term was added to the model. To control for historical relatedness of cultures, a random intercept was added for each language family that the society belonged to. The model parameters were estimated using Bayesian estimation in the R package brms [51].
We then used Bayesian regression estimation to perform two separate path analyses—one for all EA societies and one for just African EA societies—of the relationship between rainfall, biodiversity variables and the presence of intensive agriculture. The biodiversity variables used for the EA path analysis were all four species richness variables. For the Africa path analysis, we used the four biodiversity variables as well as tsetse flies, malaria and elephants. Some of the biodiversity variables had a handful of missing data points which limited the sample size. For African societies, these values were interpolated spatially in a general additive model. This creates a model of how the variable varies across space, using smooth splines between observed data to estimate missing points. The model was highly significant and fitted the data almost linearly (it explained 92% of the deviance). The four biodiversity variables were then combined into a single composite variable using geographically weighted principal components analysis (PCA) using the R package GWmodel [52,53]. This variable explained 73% of the variance in the underlying variables and was positively correlated with each. The PCA was only done for the Africa analysis to reduce the number of variables that could cause collinearity problems, as the all-EA analysis had fewer variables. Finally, we used basic hypothesis tests and Bayesian models to directly test the marginal habitat hypothesis.
The path analysis used the structure shown in electronic supplementary material, figure S5, which reflects the hypothesized causal relationships between the variables. Agricultural intensity was predicted in an ordinal regression by (nonlinear) rainfall, malaria, tsetse flies, the first component of the biodiversity PCA and the presence of elephants. A random effect for language family was included to control for the historical relatedness of societies. Each of the dependent variables was itself predicted by rainfall. Parameters were estimated simultaneously in a Markov chain Monte Carlo (MCMC) framework using the R package brms [51]. The full model equation is provided in electronic supplementary material, §S4.
4. Results
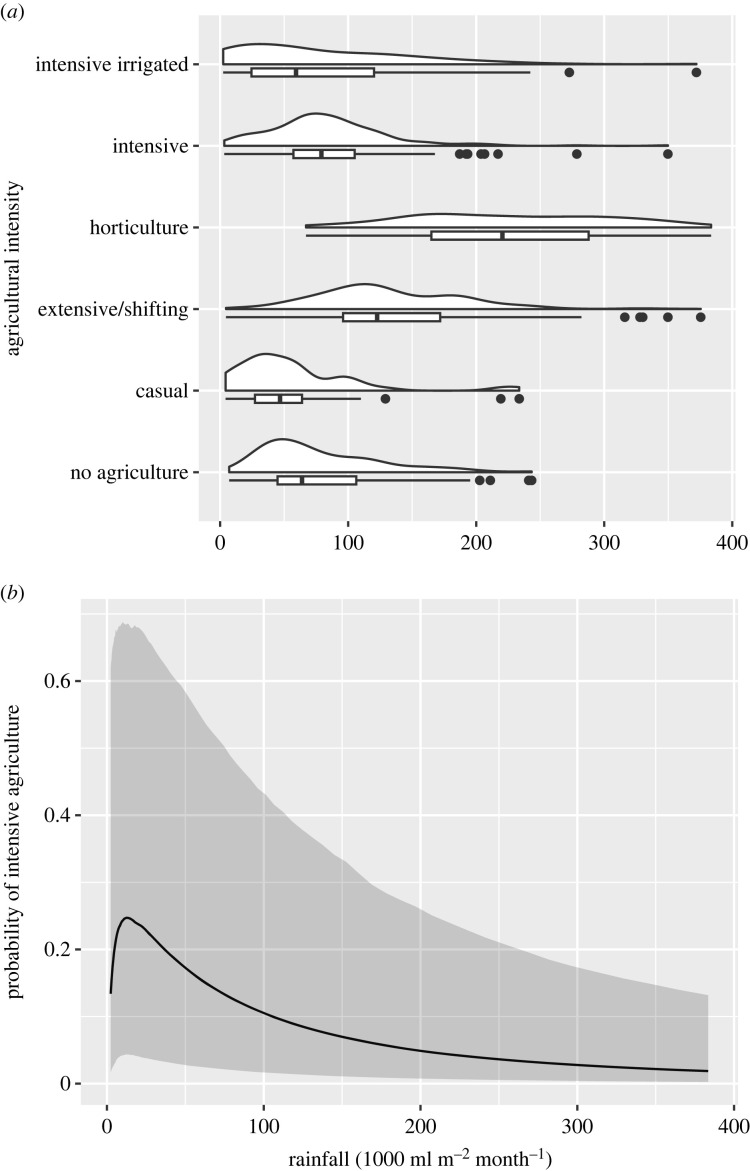
Figure 3a demonstrates that the relationship between rainfall and agriculture is parabolic, not linear. Initially more rainfall is associated with greater agricultural intensity, but at some threshold the relationship between rainfall and agricultural intensity becomes negative. Intensive agriculture occurred at a lower rainfall than horticulture, as shown in figure 3a. We found similar trends for NPP (electronic supplementary material, figure S1). We also repeated the plots, restricting our sample to the SCCS (electronic supplementary material, figures S2 and S3) and the SCCS with modifications by Worthington & Cunningham, who used EA004 to separate pastoralists [54] (electronic supplementary material, figure S4) and found the same trends.
Figure 3.
Rainfall and agriculture intensity. (a) Boxplot and density plots of agricultural intensity and rainfall. The relationship is nonlinear. Intensive and intensive irrigated agriculture occurred at lower rainfall than expected. (b) A Bayesian binomial regression model controlling for phylogeny to predict the probability of intensive agriculture by rainfall for all EA societies (electronic supplementary material, §S3). Intensive agriculture was significantly unlikely at high rainfall compared to other types of agriculture which supports our modified version of the oasis hypothesis.
To test our model (figure 1), we used a binomial regression model to estimate the relationship between subsistence types and annual rainfall. The results showed that intensive agriculture was very rare at high rainfall and there was a significant parabolic relationship between probability of intensive agriculture and rainfall (figure 3b). Furthermore, in the raw data, the mean rainfall for societies with horticulture and extensive/shifting subsistence was higher than for societies with intensive agriculture (electronic supplementary material, table S5). The result was still significant even after removing horticulture societies because many of these societies were clustered in Papunesia and were likely highly related (electronic supplementary material, table S6).
Additional analyses are available in the electronic supplementary material. Of note from our results is that agricultural intensification happened at significantly lower mean rainfall, NPP, plant vascular richness, bird richness and mammal richness for all EA societies when compared with societies with no intensification on t-tests and GLMs. However, some of these tests did not reach significance when repeated for the subset of SCCS societies. Given that foragers and intensive agriculturalists are found at low rainfall and NPP, we wanted to discern if they inhabit similar productivity areas by comparing their mean NPP values. For this comparison we used the Worthington & Cunningham [54] sample drawn from the SCCS, which separated pastoralists from foragers. This comparison is not testing the marginal habitat hypothesis because it is not comparing foragers to all agricultural groups, only foragers with intensive agriculturalists. We found that foragers and intensive agriculturalists had indistinguishable productivity levels (electronic supplementary material, table S4).
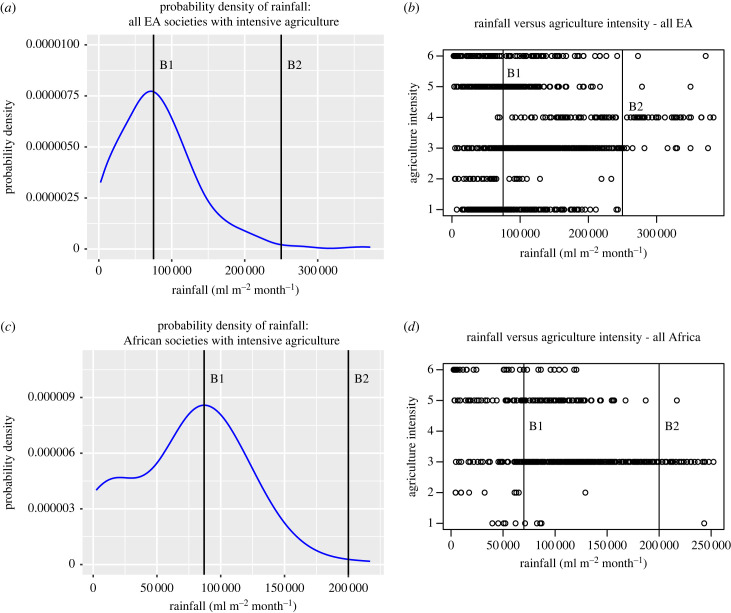
In figure 4a,b we compare actual probability density plots from the data with our hypothetical probability density plot (figure 1) to find B1 and B2 for EA and EA African societies. In figure 4b, we include lines at B1 and B2 on a scatter plot for all EA societies, showing the different agricultural intensities of the societies. The plot confirms that past B2 intensive agriculture was rare but horticulture and extensive agriculture were not rare. Interestingly there were only four societies with intensive agriculture past B2 for the entire EA and they were all highland farmers (electronic supplementary material, table S3). The point at which intensive agriculture approaches zero (B2) for Africa EA societies is much lower than the B2 for all EA and SCCS societies. This suggests that the negative effects of biodiversity became prohibitive for intensive agriculture at much lower rainfall in Africa than in other areas. B1 and B2 values with associated scatterplots for Eurasia, Africa, South America, North America, Australia and Papunesia are provided in the electronic supplementary material, figures S5–S10.
Figure 4.
Probability density plots of intensive agriculture and scatter plots for EA societies (a,b) and African societies (c,d). (a) The probability density for intensive agriculture (AI 5 and 6 combined) for all EA societies. B1 and B2 rainfall values are marked with straight lines. (b) The values of B1 and B2 are marked on a scatterplot of all EA societies with lines. Past B2, agricultural intensity values of 5 and 6 are rare and all intensive agriculture societies were highland farmers.
To further explore the relationships among rainfall, biodiversity and intensive agriculture we ran Bayesian regression modelling path analyses. The model included an effect of rainfall on the biodiversity measures, the effect of biodiversity on agricultural intensity, and a direct effect of rainfall on agricultural intensity. The modelling of agricultural intensity included a random intercept for each language family as a control for phylogeny. For EA societies, rainfall had a significant positive effect on each biodiversity measure, but a significant negative direct effect on agriculture intensity (electronic supplementary material, figure S5a). The biodiversity variables gave mixed results for their individual effects on agriculture intensity for all EA societies. Vascular plant richness is the only measure of biodiversity with a significant effect on agriculture intensity and the only one with a positive coefficient.
For the path analysis for Africa, rainfall is significantly positively correlated to all biodiversity variables, as expected (electronic supplementary material, figure S5b). Rainfall had a negative direct effect on agriculture intensity, but this did not reach significance. Tsetse fly had a significant negative effect on agriculture intensity while other biodiversity variables were not significant. The effect of elephants was not significant, though we note the estimates were highly skewed towards being negative.
We tested the marginal habitat hypothesis using the MODIS variable mean NPP. Cunningham et al. [14] and Porter & Marlowe [15] distinguished agriculturalists from foragers by the extent of dependence on agriculture, with less than 10% dependence indicating a foraging society. Because the categories in the EA for dependence on agriculture (variable ID EA005) include ranges from 0 to 5% and 6 to 15%, we chose to use less than 16% dependence on agriculture as the cut-off for classifying a society as foragers. When it came to testing the marginal habitat hypothesis, we used three different methods to compare the mean NPP of foragers with that of agriculturalists. Firstly, using a t-test with the EA dataset, we found that foragers had a significantly lower NPP than agriculturalists using our cut-off value of less than 16% reliance on agriculture (t655.21 = 11.41, p < 0.01). We repeated our analysis with SCCS societies to control for autocorrelation, and the results were still significant (t122.35 = 4.52, p < 0.01). Finally, we used linear mixed effects models to test the relationship between EA foragers and agriculturalists regarding NPP while controlling for language family and continent. The first model predicts NPP but only includes the control variables. The second model adds the subsistence type variable, and the fit of the two models is compared. Adding subsistence type significantly improves the fit of the model (log likelihood difference = 9.067, p < 0.001); therefore, NPP is lower for foraging societies than for agricultural societies.
5. Discussion
We have explored how features of the ecology, including rainfall, NPP and biodiversity including elephants and pathogens, are associated with the development of intensive agriculture. Our analysis suggests that in certain ecologies intensive agriculture may be difficult or impossible to develop. Intensive agriculture differs from foraging and horticulture in that it requires larger amounts of labour input and human capital, especially if requiring irrigation or ploughing. A high abundance of pathogens, such as malaria or tsetse fly-borne pathogens, may reduce available human and animal capital. Biodiversity may also create potential obstacles to intensive agriculture. Elephants, for instance, can decimate farms, rendering intensive agriculture an especially vulnerable subsistence strategy.
Many regions in Africa that recently had foraging or horticulture now have intensive agriculture. However, these changes have only come about through technologies generally not available to pre-industrial societies that compensate for erratic and low rainfall with irrigation systems. Such irrigation systems often use water from boreholes drilled using gasoline-operated technology. Similarly, pathogens such as tsetse are managed by mass eradication campaigns that rely on chemical mechanisms. The effect of elephants has been similarly reduced both through declines in elephant populations and the utilization of electric fences.
But even within our sample of largely pre-state societies there were a few notable exceptions where intensive agriculture developed in regions with high rainfall, including the Inca, Muisca, Sherpa and Kakoli of New Guinea—all of whom were highland farmers [55–58]. The facts that intensification is rare at high rainfall and that the four exceptions were highland populations supports the hypothesis that biodiversity limits agriculture intensification. This is likely because in highlands, rainfall water is more likely to run off [59], potentially reducing plant and animal biodiversity compared with a region in lowlands with similar rainfall. The lower temperatures at high altitude are also likely to contribute to the reduction in biodiversity. Terracing is usually required to support plant cultivation to overcome run-off on steep terrain [57,59]. We also hypothesize that terracing limits competition from native plants. This is supported by work from Inbar & Llerena [60] which found that the natural vegetation at the highest elevations of the mountainous farming region of Peru varied altitudinally and was limited to xerophytic plants, shrubs, cactus and grass, with no deep-rooted vegetation because the soils at high elevation were shallow and prone to run-off. They also found that there was little natural vegetation on abandoned terraces because the process of terrace creation cleared natural vegetation, which did not return even after terrace abandonment [60]. Thus, highland farming is essentially ‘oasis’ farming because the oasis conditions of water access with reduced biodiversity are met.
The results of our path analyses (electronic supplementary material, figure S5) support our model but also include some unexpected findings. The negative relationship between rainfall and agricultural intensity and the positive correlation between rainfall and all the biodiversity variables are consistent with our hypothesis that as rainfall increases biodiversity also increases, but beyond a certain point both rainfall and biodiversity have a negative effect on intensive agriculture. That the effect of some of the variables did not reach significance or were not in the direction expected could be due to data quality, collinearity in the models, or lack of specificity of the composite variables like mammal biodiversity, which encompasses some mammals that are positive for intensification (e.g. horses), and those that are deleterious for intensification (e.g. primates that may raid crops). Additionally, the biodiversity data in our analyses were collected amidst the rapid decline in species caused by globalization and thus may not match the pre-industrial levels, especially if the decline was not uniform across our sample of societies. We hypothesized that elephants would have inhibited agricultural intensification, and although the results were trending towards significance, they did not reach statistical significance.
Many of our variables were highly correlated with rainfall and may cause collinearity problems that affect the model's estimates. However, the unexpected results might provide clues to the mechanisms of how biodiversity affected agricultural intensity. Some aspects of biodiversity can be positive for agricultural intensification while others may be neutral or negative [6]. Thus, composite variables such as those we use may give unreliable results. For biodiversity effects, both the type and the amount of biodiversity are likely to influence agriculture intensity; therefore, models should use more-specific variables such as elephants instead of mammals, a crop-eating bird species instead of bird richness, or a difficult-to-clear plant instead of plant vascular richness.
(a) . Oasis theory and marginal habitat hypothesis
Our results tentatively support the oasis theory of agricultural intensification—modified from the version Childe put forth, which focused on the emergence of domestication [38]. We found that intensive agriculture was more successful in low to moderate rainfall areas (figure 3b). With high rainfall likely came increased biodiversity which made some areas marginal for agricultural intensification. If agricultural intensification was initially favourable in ‘oasis’ conditions, it follows that it was not initially favourable where these conditions were not met, i.e. in environments ‘marginal’ to agricultural intensification. We also found support for the marginal habitat hypothesis directly using a different cut-off of dependence on agriculture for categorizing foraging societies from that used in the previous quantitative tests for the marginal habitat hypothesis (16% rather than 10%) [14,15]. However, we remain sceptical that NPP provides a suitable test of the marginal habitat hypothesis.
While we did not directly test the proximity to rivers for societies with intensive agriculture, the outliers in our data are instructive, tentatively providing further support for our modified oasis hypothesis. In our sample of societies from the EA, the Pokomo of Kenya had intensive irrigated agriculture at the lowest rainfall for all EA societies with intensive agriculture. Their proximity to a reliable water source is likely the reason why they developed intensive agriculture. ‘The Pokomo … [live] along the banks of the Tana, Kenya's largest river. The area is semi-desert, with scant and irregular rainfall, especially in the north …. The Pokomo cultivate the banks of the river over the last 400 km of its course’ [61, p. 386]. The Sonjo of Tanzania had the second-lowest level of rainfall for intensive agriculture and also lived in a semi-arid region with two main sources of perennial water decoupled from local rainfall: springs from the foot of the hills, and nearby rivers [62]. This contrasts with many low rainfall foragers and pastoralists who inhabited arid regions with very limited permanent water sources [8].
We propose that the environments of foragers and intensive agriculturalists were often similar in terms of productivity and biodiversity given their similar NPP (electronic supplementary material, table S4). However, the key difference was that intensive agriculturalists typically had access to a perennial water supply not related to the local precipitation, usually in the form of rivers. Without such a water source, arid terrain leads to low agriculture intensity, but with a perennial water source it enables intensive irrigated agriculture. It follows from this that the closer a society is to ideal ‘oasis' conditions, the more likely agriculture intensification was.
We propose the following as oasis conditions that are favourable for agricultural intensification:
-
(1)
Generally low biodiversity favours more intensive agriculture. In areas with high rainfall, factors such as terracing or high altitude are necessary to decouple rainfall from biodiversity.
-
(2)
Access to a reliable perennial water source such as a river favours intensification. If no such water source existed, then rainfall itself was likely to be a major contributor to agricultural intensification at low to moderate levels but not at high levels.
-
(3)
Agricultural suitability indices (such as soil suitability, slope of the terrain, etc.) should be favourable to intensification insofar as they can be extrapolated to historical conditions [63].
(b) . Population density, productivity and marginality
Our results also suggest that in contrast to the Porter & Marlowe [15] and Cunningham et al. [14] studies, NPP alone is not a reliable determinant of how marginal an environment is for agriculture. Cunningham et al. [14] questioned how intensive agriculturalists and pastoralists could achieve high population densities at low NPP but foragers were constrained to low population densities at similar NPP ranges. We propose that at low rainfall and resulting low NPP, intensive agriculturalists generally had access to perennial water which in turn substantially boosted crop productivity. Foragers in low rainfall areas relied on a larger suite of resources than agriculturalists, and many of these resources were not amenable to productivity increases, even if perennial water sources were present. For intensive agriculturalists the perennial water source in areas without the high biodiversity that comes with high rainfall facilitated increased food production in ways that led to much higher population densities than what foragers at the same NPP, or horticulturalists encumbered by high biodiversity at high NPP, could achieve.
Tallavaara et al. [21] evaluated the effects of NPP, biodiversity and pathogen stress on a dataset of pre-industrial hunter–gatherers. Prior studies have suggested positive relationships between primary and secondary productivity and hunter–gatherer population density [64–67]. Tallavaara et al. [21] found that productivity affects human population density but local ecological conditions were more influential than productivity. At low productivity, forager population density was more correlated with biodiversity, while at high productivity, pathogens were the most significant driver of population density [21]. Our findings that tsetse-borne pathogens and malaria negatively affected agricultural intensity support this conclusion because these pathogens are highly correlated with rainfall and hence most problematic at high rainfall, a proxy for high productivity.
Freeman et al. [68] extended the study by Tallavaara et al. [21] by including agriculturalists and industrialists in addition to foragers. They found that population densities were stratified by technological level, with the most technologically advanced societies having higher population densities. For each respective productive technology group, increasing NPP led to higher population density, but species richness and pathogen load tempered the relationship. Specifically, the ‘highest human population densities occur in settings with high NPP, moderate levels of species richness and moderate to low pathogen loads. At lower levels of NPP, higher species richness increases population density, and at high levels of NPP, higher levels of species richness lead to lower population densities' [68, p. 1]. Their findings are in line with our predictions from the oasis theory of agriculture intensification and our findings.
Our study suggests that NPP alone should not be used to evaluate marginality to agriculture (food production). We plotted the subsistence types from the Worthington & Cunningham [54] dataset against rainfall (electronic supplementary material, figure S4). The plot shows probability density lines for the frequency of SCCS societies of each subsistence type at different rainfall levels. Because rainfall can be used as a proxy for productivity and agriculture intensity can be a proxy for population density the figure can help us evaluate the relationships between multiple variables. The probability density lines show that foragers, pastoralists and intensive agriculturalists were more frequent at low rainfall while horticultural societies had high frequency at moderate to high rainfall and productivity. This figure suggests that agriculture (food production) was possible at all rainfall levels and NPP levels: intensive agriculturalists and pastoralists clustered at low levels of NPP and horticulturalists clustered at high levels of NPP. The relative absence of intensive agriculture at high rainfall and NPP indicates that some environments are marginal to agricultural intensification. This is why we advocate determining marginality to agricultural intensification and not marginality to agriculture (food production).
(c) . The middle ground between foraging and agriculture
Were there some environmental conditions that could make foraging as compelling or more compelling than agriculture even after the Neolithic transition? Denham & Donohue [69] argue that the transition to agriculture was not all-or-nothing and often involved a middle ground (or mixed strategy) between the two. They argue that the middle ground was geographical because there ‘are clear geographical clusters in terms of middle-ground societies in which there is more than 15% dependence on each of gathering and cultivation, including several areas of wet tropical rainforest and two regions within North America, the Pacific Southwest and the Mississippi Basin’ [69, pp. 6–8]. We argue that the middle ground was not only geographical, it was also ecological. The persistence of foraging alongside agriculture, which encompasses casual farmers, pastoralists and horticulturalists that retained some foraging, can be explained by rainfall distribution and its relationship to biodiversity. Denham & Donohue note that some foragers in North America incorporated maize cultivation. From the D-PLACE precipitation predictability map, we were able to ascertain that the region of North America they pointed out, the Southwest, had the lowest rainfall predictability in North America [16,17]; therefore, there was great risk in fully abandoning foraging for rain-fed maize. Given the erratic rainfall without an alternative reliable water source, a middle-ground subsistence strategy between foraging and intensive agriculture was more reliable than becoming fully agrarian. Additionally, mixing foraging and maize agriculture in the Southwest were favoured owing to the lack of a domesticated protein source until domesticated turkeys were imported from Mexico around AD 1100 [70].
The middle ground in the wet tropics is in a very high rainfall belt that goes from South America to Central Africa and to the Pacific Islands [69]. Very few societies in this belt had intensive agriculture. We attribute their middle-ground status to high biodiversity and rainfall. This abundance likely had benefits and drawbacks. Some of this naturally abundant biodiversity made foraging a compelling way of life even after the Neolithic transition because there were many animals and plants to eat. This explains some of the high rainfall foragers in the tropics that persisted until the twentieth century. The biodiversity also made agriculture a front-end-heavy enterprise, with high costs and labour required to clear the biodiversity to make room for domesticates, and more costs to set up infrastructure to keep out some of the biodiversity that preys on or competes with crops.
If a society at high rainfall adopted farming, the biodiversity likely posed risks to agriculture. Risk management would have taken many forms, which included not fully abandoning foraging so that if pests or pathogens destroyed agricultural investments, horticulturalists could supplement their diets with foraged food. Another way to manage risk may have been keeping food production at the family level so that the family could diversify the products it produced, increasing resilience to risks posed by biodiversity and environmental conditions owing to erratic rainfall. Such societies might be fully agrarian but never intensify because intensification in any one food source might increase vulnerability to starvation.
In conclusion, the distribution of rainfall and its relationship to biodiversity can explain the persistence of foragers, horticulturalists, pastoralists and middle-ground societies. Low rainfall foragers were in areas with low rainfall and no perennial water source. High rainfall foragers were in high rainfall environments where the high biodiversity provided abundant food such that the incentive to adopt agriculture was low or the high biodiversity made agriculture risky. Horticulturalists were in areas where the rainfall was too low for intensification, with frequent droughts, or too high for intensification owing to abundant biodiversity or the harsh effects of water on plants, like waterlogging. Middle-ground societies mixed foraging with agriculture to take advantage of biodiversity or to mitigate the risks due to drought or abundant biodiversity.
(d) . Implications for cultural evolution
Many anthropologists are of the view that there is a link between surplus food production and an increase in inequality and sociopolitical complexity [71,72]. Surplus food production can lead to inequality among individuals or families through differences in access to or ownership of land for farming, resources such as water, and the ability to control the labour of others (e.g. serfdom, slavery), among other kinds of inequality [73,74]. The trajectory towards individual economic specialization within a society (division of labour at the population level rather than the family level) can be traced back to surplus, whether from intensive agriculture or foraging an abundant resource like fish [13,75]. It is this population level division of labour that can lead to rapid technological advances. If living with elephants or other aspects of biodiversity that limited agriculture intensification required a family to diversify food sources with small-scale farming or by mixing foraging with subsistence agriculture, this could inhibit a progressive increase of surplus, greatly delaying or curtailing a population-level division of labour. Diversification of food sources for each family or band likely provided more resilience than specializing in one food type in the face of risks like crop decimation by elephants or pathogens. We thus argue that, if managing the risks posed by biodiversity, drought, or both required family food source diversification, retaining foraging and/or horticulture would be the most adaptive subsistence strategy for the local ecology. In such circumstances, we should not expect to see labour specialization, high population densities or significant social inequality—and the absence of these things cannot be viewed as a failure of any kind.
6. Conclusion
Low to moderate levels of rainfall and biodiversity made some environments ideal oases for intensive agriculture in regions with a perennial water source. However, in environments where rainfall was low, without a perennial water source, or too high, especially alongside high biodiversity including pathogens, intensive agriculture was not likely. Intensive agriculture was rare at very high rainfall unless the terrain decoupled rainfall from biodiversity, as in the case of highland farmers. Our work is the first to our knowledge to provide quantitative support for the oasis theory of agricultural intensification. We propose focusing on marginality to agricultural intensification instead of the lack of suitability for agriculture because agriculture can be adopted at the lowest rainfall or NPP if there is a perennial water source like a river.
Our work has implications for the possible cultural evolutionary trajectories that human societies could take. Where there were few or no limitations on agricultural intensification, surplus likely created the conditions for economic specialization and increased sociopolitical complexity. However, if rainfall was too low or erratic for agricultural intensification, or biodiversity otherwise limited intensification, a flexible subsistence strategy that was resilient against ecological conditions would be favoured. This strategy was not economic specialization but diversification at the family or band level. Such diversification is resilient against ecological stresses but curtails the development of a social division of labour, therefore avoiding or delaying increased sociopolitical complexity and inequality. Diversification-oriented societies were seen as simple by unilineal evolutionists who failed to recognize that the lack of economic specialization represented an effective cultural adaption to risk. With industrial technology and globalization, most areas that were not suitable for intensive agriculture can now have intensive agriculture using boreholes, electric fences and chemicals to eradicate pathogens. However, the front-end costs are not always affordable to inhabitants of those regions, and challenges like drought continue to limit intensification in some regions today.
Acknowledgements
We also thank Eric Alden Smith for his very helpful editorial insight and guidance.
Contributor Information
Dithapelo Medupe, Email: dzm5868@psu.edu.
Luke Glowacki, Email: laglow@bu.edu.
Data accessibility
Data and code are hosted on OSF: https://osf.io/t75jh/ [76].
Additional analyses are presented in the electronic supplementary material [77].
Authors' contributions
D.M.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, visualization, writing—original draft, writing—review and editing; S.G.R.: formal analysis, methodology, visualization, writing—original draft, writing—review and editing; M.K.S.: conceptualization, supervision, writing—review and editing; L.G.: conceptualization, investigation, methodology, project administration, supervision, writing—original draft, writing—review and editing.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Carol Ember and all the participants of the NSF HRAF Summer Institute on Cross Cultural Anthropological Research 2022 provided stimulating discussions and training relating to this work (NSF award numbers 2020156, 2022). S.G.R. was supported by an AHRC grant (AH/T006927/1).
References
- 1.Bowles S, Smith EA, Borgerhoff Mulder M. 2010. The emergence and persistence of inequality in premodern societies. Curr. Anthropol. 51, 7-17. ( 10.1086/649206) [DOI] [Google Scholar]
- 2.Smith EA, Borgerhoff Mulder M, Bowles S, Gurven M, Hertz T, Shenk MK. 2010. Production systems, inheritance, and inequality in premodern societies. Curr. Anthropol. 51, 85-94. ( 10.1086/649029) [DOI] [Google Scholar]
- 3.Morgan LH. 1877. Ancient society. New York, NY: Henry Holt and Co. [Google Scholar]
- 4.Spencer H. 1907. Essays, scientific, political, and speculative, vols 1–3. New York, NY: D. Appleton. [Google Scholar]
- 5.Tylor EB. 1883. Primitive culture: researches into the development of mythology, philosophy, religion, language, art and custom. London, UK: John Murray.
- 6.Diamond JM. 1997. Guns, germs, and steel: the fates of human societies, 1st edn. New York, NY: W.W. Norton & Co. [Google Scholar]
- 7.Service ER, White LA, Sahlins M. 1988. Evolution and culture. Ann Arbor, MI: University of Michigan Press. [Google Scholar]
- 8.Steward JH. 1972. Theory of culture change: the methodology of multilinear evolution. Chicago, IL: University of Illinois Press. [Google Scholar]
- 9.Bigelow R. 1975. The role of competition and cooperation in human evolution. In War, its causes and correlates (eds MA Nettleship, RD Givens, A Nettleship), pp. 235–262. Paris: Mouton.
- 10.Lee RB, DeVore I. 2017. Man the hunter: the first intensive survey of a single, crucial stage of human development : man's once universal hunting way of life, 1st edn. London, UK: Taylor and Francis. [Google Scholar]
- 11.Kelly RL. 2013. The lifeways of hunter-gatherers: the foraging spectrum. New York, NY: Cambridge University Press. [Google Scholar]
- 12.Winterhalder B, Kennett DJ. 2019. Behavioral ecology and the transition from hunting and gathering to agriculture. In Behavioral ecology and the transition to agriculture (eds Kennett DJ, Winterhalder B), pp. 1-21. Berkeley, CA: University of California Press. [Google Scholar]
- 13.Bettinger RL. 2015. Orderly anarchy: sociopolitical evolution in aboriginal California. Berkeley, CA: University of California Press. See http://ebookcentral.proquest.com/lib/pensu/detail.action?docID=1747547. [Google Scholar]
- 14.Cunningham AJ, Worthington S, Venkataraman VV, Wrangham RW. 2019. Do modern hunter-gatherers live in marginal habitats? J. Archaeol. Sci. Rep. 25, 584-599. ( 10.1016/j.jasrep.2019.05.028) [DOI] [Google Scholar]
- 15.Porter CC, Marlowe FW. 2007. How marginal are forager habitats? J. Archaeol Sci. 34, 59-68. ( 10.1016/j.jas.2006.03.014) [DOI] [Google Scholar]
- 16.Murdock GP. 1967. Ethnographic atlas, a summary. Ethnology 6, 109-236. [Google Scholar]
- 17.Kirby KR, et al. 2016. D-PLACE: a global database of cultural, linguistic and environmental diversity. PLoS ONE 11, e0158391. ( 10.1371/journal.pone.0158391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odum EP, Barrett GW. 1971. Fundamentals of ecology, vol. 3. Philadelphia, PA: Saunders. [Google Scholar]
- 19.Roxburgh SH, Berry SL, Buckley TN, Barnes B, Roderick ML. 2005. What is NPP? Inconsistent accounting of respiratory fluxes in the definition of net primary production. Funct. Ecol. 19, 378-382. ( 10.1111/j.1365-2435.2005.00983.x) [DOI] [Google Scholar]
- 20.Singh M, Glowacki L. 2022. Human social organization during the Late Pleistocene: beyond the nomadic-egalitarian model. Evol. Hum. Behav. 43, 418-431. ( 10.1016/j.evolhumbehav.2022.07.003) [DOI] [Google Scholar]
- 21.Tallavaara M, Eronen JT, Luoto M. 2018. Productivity, biodiversity, and pathogens influence the global hunter-gatherer population density. Proc. Natl Acad. Sci. USA 115, 1232-1237. ( 10.1073/pnas.1715638115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turnbull CM. 1965. The Mbuti Pygmies: an ethnographic survey. Anthropol. Pap. Am. Mus. Nat. Hist. 50, 139-282. [Google Scholar]
- 23.Alsan M. 2015. The effect of the tsetse fly on African development. Am. Econ. Rev. 105, 382-410. ( 10.1257/aer.20130604) [DOI] [Google Scholar]
- 24.Holden S, Hvoslef H, Simanjuntak R. 1995. Transmigration settlements in Seberida, Sumatra: deterioration of farming systems in a rain forest environment. Agric. Syst. 49, 237-258. ( 10.1016/0308-521X(94)00046-T) [DOI] [Google Scholar]
- 25.Balmford A, Moore JL, Brooks T, Burgess N, Hansen LA, Williams P, Rahbek C. 2001. Conservation conflicts across Africa. Science 291, 2616-2619. ( 10.1126/science.291.5513.2616) [DOI] [PubMed] [Google Scholar]
- 26.Chiyo PI, Cochrane EP. 2005. Population structure and behaviour of crop-raiding elephants in Kibale National Park, Uganda. Afr. J. Ecol. 43, 233-241. ( 10.1111/j.1365-2028.2005.00577.x) [DOI] [Google Scholar]
- 27.Darkoh MBK, Mbaiwa JE. 2009. Land-use and resource conflicts in the Okavango Delta, Botswana. Afr. J. Ecol. 47, 161-165. ( 10.1111/j.1365-2028.2008.01064.x) [DOI] [Google Scholar]
- 28.Gunaryadi D, Sugiyo, Hedges S. 2017. Community-based human–elephant conflict mitigation: the value of an evidence-based approach in promoting the uptake of effective methods. PLoS ONE 12, e0173742. ( 10.1371/journal.pone.0173742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mbaiwa JE, Stronza A, Kreuter U. 2011. From collaboration to conservation: insights from the Okavango Delta, Botswana. Soc. Nat. Resour. 24, 400-411. ( 10.1080/08941921003716745) [DOI] [Google Scholar]
- 30.Shoshani J. 2005. Mammal species of the world: a taxonomic and geographic reference, 3rd edn, p. 2. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 31.Baker CMA, Manwell C. 2010. Man and elephant: the ‘dare theory’ of domestication and the origin of breeds. Z. Tierz. Zücht. 100, 55-75. ( 10.1111/j.1439-0388.1983.tb00712.x) [DOI] [Google Scholar]
- 32.Milner-Gulland EJ, Beddington JR. 1993. The exploitation of elephants for the ivory trade: an historical perspective. Proc. R. Soc. Lond. B 252, 29-37. ( 10.1098/rspb.1993.0042) [DOI] [Google Scholar]
- 33.Thouless CR. 2016. African elephant status report 2016: an update from the African elephant database. Gland, Switzerland: IUCN. [Google Scholar]
- 34.Brown PJ. 1986. Cultural and genetic adaptations to malaria: problems of comparison. Hum. Ecol. Interdiscip. J. 14, 311-332. ( 10.1007/BF00889033) [DOI] [Google Scholar]
- 35.Mabe FN, Dafurika T. 2020. Averting expenditure on malaria: effects on labour productivity of maize farmers in Bunkpurugu-Nakpanduri District of Ghana. Malar. J. 19, 448. ( 10.1186/s12936-020-03521-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien MJ, Laland KN. 2012. Genes, culture, and agriculture: an example of human niche construction. Curr. Anthropol. 53, 434-470. ( 10.1086/666585) [DOI] [Google Scholar]
- 37.Sachs JD. 2002. A new global effort to control malaria. Science 298, 122-124. ( 10.1126/science.1077900) [DOI] [PubMed] [Google Scholar]
- 38.Childe VG. 1928. The most ancient east: the oriental prelude to European prehistory. London, UK: Kegan Paul, Trench, Trubner. [Google Scholar]
- 39.Patterson T. 2014. Childe, Vere Gordon (theory). In Encyclopedia of global archaeology (ed. C Smith), pp. 1389-1391. New York, NY: Springer. ( 10.1007/978-1-4419-0465-2_304) [DOI] [Google Scholar]
- 40.Bahrami-Rad D, Becker A, Henrich J. 2021. Tabulated nonsense? Testing the validity of the Ethnographic Atlas. Econ. Lett. 204, 109880. ( 10.1016/j.econlet.2021.109880) [DOI] [Google Scholar]
- 41.White D, Brudner-White L. 1988. The Murdock legacy: the Ethnographic Atlas and the search for a method. Behav. Sci. Res. 22, 59-81. ( 10.1177/106939718802200107) [DOI] [Google Scholar]
- 42.Bromham L, Hua X, Cardillo M, Schneemann H, Greenhill SJ. 2018. Parasites and politics: why cross-cultural studies must control for relatedness, proximity and covariation. R. Soc. Open Sci. 5, 181100. ( 10.1098/rsos.181100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murdock GP. 1966. Cross-cultural sampling. Ethnology 5, 97. ( 10.2307/3772903) [DOI] [Google Scholar]
- 44.Dow MM, Eff EA. 2008. Global, regional, and local network autocorrelation in the standard cross-cultural sample. Cross-Cult. Res. 42, 148-171. ( 10.1177/1069397107311186) [DOI] [Google Scholar]
- 45.Eff A. 2004. Does Mr. Galton still have a problem? Autocorrelation in the standard cross-cultural sample. World Cult. 15, 153-170. [Google Scholar]
- 46.Jenkins CN, Pimm SL, Joppa LN. 2013. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl Acad. Sci. USA 110, E2602-E2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreft H, Jetz W. 2007. Global patterns and determinants of vascular plant diversity. Proc. Natl Acad. Sci. USA 104, 5925-5930. ( 10.1073/pnas.0608361104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alsan M. 2015. Replication data for: the effect of the TseTse fly on African development. Nashville, TN: American Economic Association. See https://www.openicpsr.org/openicpsr/project/112921/version/V1/view.
- 49.Wall J, et al. 2021. Human footprint and protected areas shape elephant range across Africa. Curr. Biol. 31, 2437-2445.e4. ( 10.1016/j.cub.2021.03.042) [DOI] [PubMed] [Google Scholar]
- 50.Hammarström H, Donohue M. 2014. Some principles on the use of macro-areas in typological comparison. Lang. Dyn. Change 4, 167-187. ( 10.1163/22105832-00401001) [DOI] [Google Scholar]
- 51.Bürkner PC. 2021. Bayesian item response modeling in R with brms and Stan. J. Stat. Softw. 100, 1-54. ( 10.18637/jss.v100.i05) [DOI] [Google Scholar]
- 52.Gollini I, Lu B, Charlton M, Brunsdon C, Harris P. 2015. GWmodel: an R package for exploring spatial heterogeneity using geographically weighted models. J. Stat. Softw. 63, 1-50. ( 10.18637/jss.v063.i17) [DOI] [Google Scholar]
- 53.Lu B, Harris P, Charlton M, Brunsdon C. 2014. The GWmodel R package: further topics for exploring spatial heterogeneity using geographically weighted models. Geo-Spat. Inf. Sci. 17, 85-101. ( 10.1080/10095020.2014.917453) [DOI] [Google Scholar]
- 54.Worthington S, Cunningham A. 2018. R replication code and data for: Do modern hunter-gatherers live in marginal habitats? Zenodo. ( 10.5281/zenodo.835836) [DOI]
- 55.Bowers N. 1965. Permanent bachelorhood in the upper Kaugel Valley of Highland New Guinea. Oceania 36, 27-37. ( 10.1002/j.1834-4461.1965.tb00276.x) [DOI] [Google Scholar]
- 56.Glassner M. 1970. The Chibchas: a history and re-evaluation. Americas 26, 302-327. ( 10.2307/980080) [DOI] [Google Scholar]
- 57.Meseth E, Wang L, Chen S, Yu JCS, Buzinny M. 2015. Reconstructing agriculture in Vitcos Inca settlement, Peru. Irrig. Drain. 64, 340-352. ( 10.1002/ird.1891) [DOI] [Google Scholar]
- 58.Spoon J. 2011. The heterogeneity of Khumbu Sherpa ecological knowledge and understanding in Sagarmatha (Mount Everest) national park and buffer zone, Nepal. Hum. Ecol. Interdiscip J. 39, 657-672. ( 10.1007/s10745-011-9424-9) [DOI] [Google Scholar]
- 59.Brandolini P, Cevasco A, Capolongo D, Pepe G, Lovergine F, Del Monte M. 2018. Response of terraced slopes to a very intense rainfall event and relationships with land abandonment; a case study from Cinque Terre (Italy). Land Degrad. Dev. 29, 630-642. ( 10.1002/ldr.2672) [DOI] [Google Scholar]
- 60.Inbar M, Llerena CA. 2000. Erosion processes in high mountain agricultural terraces in Peru. Mount. Res. Dev. 20, 72-79. ( 10.1659/0276-4741(2000)020[0072:EPIHMA]2.0.CO;2) [DOI] [Google Scholar]
- 61.Townsend N. 1977. Age, descent and elders among the Pokomo. Africa 47, 386-397. ( 10.2307/1158344) [DOI] [Google Scholar]
- 62.Potkanski T, Adams WM. 1998. Water scarcity, property regimes and irrigation management in Sonjo, Tanzania. J. Dev. Stud. 34, 86-116. ( 10.1080/00220389808422530) [DOI] [Google Scholar]
- 63. FAO, IIASA. 2022 Global Agro Ecological Zones version 4 (GAEZ v4). Rome, Italy: Food and Agriculture Organization of the United Nations. See http://www.fao.org/gaez/ (accessed 1 February 2023).
- 64.Binford LR. 2001. Constructing frames of reference: an analytical method for archaeological theory building using hunter-gatherer and environmental data sets. Berkeley, CA: University of California Press. [Google Scholar]
- 65.Baumhoff MA. 1963. Ecological determinants of aboriginal California populations. Oakland, CA: University of California Press. [Google Scholar]
- 66.Keeley LH. 1988. Hunter-gatherer economic complexity and ‘population pressure’: a cross-cultural analysis. J. Anthropol. Archaeol. 7, 373-411. ( 10.1016/0278-4165(88)90003-7) [DOI] [Google Scholar]
- 67.Birdsell JB. 1953. Some environmental and cultural factors influencing the structuring of Australian aboriginal populations. Am. Nat. 87, 171-207. ( 10.1086/281776) [DOI] [Google Scholar]
- 68.Freeman J, Robinson E, Beckman NG, Bird D, Baggio JA, Anderies JM. 2020. The global ecology of human population density and interpreting changes in paleo-population density. J. Archaeol. Sci. 120, 105168. ( 10.1016/j.jas.2020.105168) [DOI] [Google Scholar]
- 69.Denham T, Donohue M. 2022. Mapping the middle ground between foragers and farmers. J. Anthropol. Archaeol. 65, 101390. ( 10.1016/j.jaa.2021.101390) [DOI] [Google Scholar]
- 70.Kohler TA, Glaude MP, Bocquet-Appel JP, Kemp BM. 2008. The Neolithic demographic transition in the U.S. Southwest. Am. Antiq. 73, 645-669. ( 10.1017/S000273160004734X) [DOI] [Google Scholar]
- 71.Brown JA, Kelly JE. 2015. Surplus labor, ceremonial feasting, and social inequality at Cahokia: a study in social process. In Surplus: the politics of production and the strategies of everyday life (eds Morehart CT, De Lucia K), pp. 221-238. Louisville, CO: University Press of Colorado. [Google Scholar]
- 72.Morehart CT, De Lucia K (eds). 2015. Surplus: the politics of production and the strategies of everyday life. Louisville, CO: University Press of Colorado. [Google Scholar]
- 73.Bolender DJ. 2015. From surplus land to surplus production in the Viking age settlement of Iceland. In Surplus: the politics of production and the strategies of everyday life (eds Morehart CT, De Lucia K), pp. 153-171. Louisville, CO: University Press of Colorado. [Google Scholar]
- 74.Norman NL. 2015. Surplus houses: palace politics in the Bight of Benin, West Africa, AD 1650–1727. In Surplus: the politics of production and the strategies of everyday life (eds Morehart CT, De Lucia K), pp. 203-216. Louisville, CO: University Press of Colorado. [Google Scholar]
- 75.Lucia KD, Morehart CT. 2015. Surplus and social change: the production of household and field in pre-Aztec Central Mexico. In Surplus: the politics of production and the strategies of everyday life (eds Morehart CT, De Lucia K), pp. 73-89. Louisville, CO: University Press of Colorado. [Google Scholar]
- 76.Medupe D, Roberts S, Glowacki L. 2023. Why did foraging, horticulture and pastoralism persist after the Neolithic transition? The oasis theory of agricultural intensification. OSF. (https://osf.io/t75jh/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Medupe D, Roberts SG, Shenk MK, Glowacki L. 2023. Why did foraging, horticulture and pastoralism persist after the Neolithic transition? The oasis theory of agricultural intensification. Figshare. ( 10.6084/m9.figshare.c.6662590) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Medupe D, Roberts SG, Shenk MK, Glowacki L. 2023. Why did foraging, horticulture and pastoralism persist after the Neolithic transition? The oasis theory of agricultural intensification. Figshare. ( 10.6084/m9.figshare.c.6662590) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data and code are hosted on OSF: https://osf.io/t75jh/ [76].
Additional analyses are presented in the electronic supplementary material [77].