Abstract
Co-operatively breeding mammals often exhibit a female reproductive skew and suppression of the subordinate non-breeding group members. According to evolutionary theory and the immunity–fertility axis, an inverse relationship between reproductive investment and survival (through immunocompetence) is expected. As such, this study investigated if a trade-off between immunocompetence and reproduction arises in two co-operatively breeding African mole-rat species, namely the Damaraland mole-rat (Fukomys damarensis) and common mole-rat (Cryptomys hottentotus hottentotus), which possess female reproductive division of labour. This study also attempted to investigate the relationship between the immune and endocrine systems in Damaraland mole-rats. There was no trade-off between reproduction and immunocompetence in co-operatively breeding African mole-rat species, and in the case of the Damaraland mole-rats, breeding females (BFs) possessed increased immunocompetence compared with non-breeding females (NBFs). Furthermore, the increased levels of progesterone possessed by Damaraland mole-rat BFs compared with NBFs appear to be correlated to increased immunocompetence. In comparison, BF and NBF common mole-rats possess similar immunocompetence. The species-specific differences in the immunity–fertility axis may be due to variations in the strengths of reproductive suppression in each species.
This article is part of the theme issue ‘Evolutionary ecology of inequality’.
Keywords: immunity–fertility axis, sociality, reproductive suppression, progesterone, innate immunity, adaptive immunity
1. Introduction
Mammals have evolved both behavioural and physiological adaptations to increase their individual fitness (how well a mammal can survive and reproduce in its environment) in their habitat despite biotic and abiotic constraints [1]. One adaptation that has garnered significant interest is group living and its ultimate causes and consequences. Mammals may form groups ranging from small pair-bonded units to large organized groups with highly complex social interactions that have been compared to those of eusocial insects, such as bees, ants and termites [2–4]. Compared with the so-called solitary mammalian species, which only come together to breed or when females have young, group-living or social mammalian species have a number of benefits that increase the individual fitness of group members, including, but not limited to, enhanced protection against predators, increased energy and water savings (through increased foraging efficiency and resource accessibility), access to potential mates, access to social information, and possibly the benefit of alloparental care (offspring receive care not only from their parents but also from other group members) [2,5,6]. However, individuals of a species that partake in a group-living strategy also face several consequences that are likely not experienced by their solitary-living counterparts; these include increased risk of exposure to diseases and parasites through intra-group transmission, and intraspecific competition for both resources and the opportunity to reproduce [7–9]. As a consequence of balancing the fitness benefits and costs of group living, a spectrum of sociality has evolved (see Clutton-Brock et al. [3] for review).
On the more extreme end of the spectrum of sociality is cooperative breeding, a social system whereby alloparental care is conducted; these groups of mammals often comprise a monogamous breeding pair and their offspring, with a fluctuating number of immigrants, and are often characterized by a reproductive skew (partitioning of reproduction among the same-sex individuals within social groups) [10]. The reproductive skew varies between species and can be associated with group size, with species forming larger groups often showing increased reproductive skew [11,12]. Interestingly, a high reproductive skew within a group is often accompanied by reproductive suppression (defined as when group members beyond the age of sexual maturity fail to raise their own offspring successfully [10,13,14]). Group members that are reproductively suppressed are often referred to as non-breeders or subordinates [10], and reproductive suppression is often more common in females in co-operatively breeding species. Reproductive suppression in females can range from infanticide of the subordinate's offspring, anovulation of female non-breeding subordinates and, in some extreme cases, a lack of follicular maturation in the ovaries of non-breeding female (NBF) subordinates, with NBF anovulation and lack of follicular maturation being more common in co-operatively breeding mammal species that show a high reproductive skew and suppression [15–18].
Reproduction in female mammals, including follicular development, ovulation, pregnancy and lactation, requires substantial energy and resource investment. Consequently, there is a disproportional investment into reproduction between breeding females (BFs) and NBFs in co-operatively breeding mammal species that show a high reproductive skew and suppression, with BFs investing more energy and resources into reproduction than NBFs. Investment into reproduction may divert energy and resources away from processes, pathways and systems that increase the health and life-span of an individual, ultimately affecting an individual's fitness [19]; this includes the immunocompetence (an individual's ability to mount an immune response to pathogenic invasion to provide strong resistance to disease and infection [20,21]) of an individual [22].
Widespread evidence has indicated reduced fertility in individuals under pathogenic challenges, while an increase in reproduction induces a drop in immune strength [23]. Accordingly, the immunity–fertility axis infers an inverse relationship between reproductive investment and immunocompetence [22]. Therefore, higher fertility and reproductive investment and output come with the costs of lower immunocompetence. Immunocompetence relies on both innate and adaptive immunity (see table 1). The innate, or non-specific, immune response (comprising neutrophils, monocytes, eosinophils and basophils (table 1)) is the first line of defence against non-self pathogens. The primary purpose of the innate immune response is to immediately prevent the spread and movement of foreign pathogens throughout the body [30]. An increased prevalence of innate immune system components would indicate increased ability to protect the body against foreign pathogens. The defence against non-self-pathogens is called the adaptive immune response. [30]. The adaptive immune system comprises T and B lymphocytes (table 1), to name only two components [31]. The B lymphocytes produce antibodies to attack invading bacteria, viruses and toxins [31]. The T lymphocytes destroy the body's cells that viruses have taken over or that have become cancerous [31]. Thus, humoral immunity depends on the B lymphocytes, while cell immunity depends on the T lymphocytes [31]. Lymphocytes can be either memory and effector T or B lymphocytes, with the effector cells being short-lived and produced in high numbers in response to exposure to a specific pathogen, while the memory cells persist in lower number in the body for many years, providing lifelong protection against reinfection by the same pathogen [31]. An increased prevalence of adaptive immune system components, particularly memory cells, would indicate an increased ability to protect the body against specific foreign pathogens. Furthermore, memory T and B lymphocytes not only are essential to immunity against microbial pathogens, but are also involved in autoimmunity and maternal–fetal tolerance [32]. Ratios of these leukocytic components have proven invaluable as prognostic tools for diagnosing the degree of inflammation, disease severity, mortality and tumorigenesis [33–38]. Lower neutrophil-to-lymphocyte ratio (NLR) is usually associated with favourable prognostic factors in every field of application, mirroring a preserved immune balance [24–29], while, a higher monocycte-to-lymphocyte ratio (MLR) is favourably associated with disease-free survival after infection or cancer [24–29].
Table 1.
Functions of the various innate immune strength proxies used in this study, with patterns indicating a strong innate immune system. Source material: [24–29].
| immune strength proxy (immune system) | function |
|---|---|
| neutrophils (innate) | Neutrophils are the first cells of the immune system to respond to invaders such as bacteria or viruses. Neutrophils help prevent infections by blocking, disabling, digesting or warding off invading particles and microorganisms. Neutrophils constantly search for signs of infection and quickly respond to trap and kill pathogens. |
| lymphocyte (adaptive) | Lymphocytes are responsible for antibody production (consequently destroying the pathogens), the direct cell-mediated killing of virus-infected and tumour cells, and regulation of the immune response. |
| monocytes (innate) | Monocytes surround and kill microorganisms (phagocytosis), ingest foreign material, remove dead cells, and boost immune response and the efficiency of other immune cell types. |
| eosinophils (innate) | Eosinophils move to inflamed areas, trap foreign substances, cause cell-mediated killing of virus-infected and tumour cells, have anti-parasitic and bactericidal activity, and participate in immediate allergic reactions and modulate inflammatory responses. |
| basophils (innate) | Basophils release histamine to mount a non-specific immune response. |
| neutrophil-to-lymphocyte ratio (NLR) | NLR is informative in identifying patients immunocompromised owing to infection and risk stratification uses. |
| monocycte-to-lymphocyte ratio (MLR) | MLR represents a pro-inflammatory immune microenvironment. |
Accordingly, it would be expected that a trade-off between reproductive investment and immunocompetence in co-operatively breeding mammal species would be observed, with the BF of a group expected to exhibit lower immunocompetence, which would be measured through the decreased bactericidal capacity of their blood and decreased proportions of the various components of the innate and adaptive immune system, compared with the NBFs, which do not ovulate, fall pregnant or nurse young. To test this prediction, this study investigated the immunocompetence of BFs and NBFs of two co-operatively breeding African mole-rat species, namely the Damaraland mole-rat (Fukomys damarensis) and common mole-rat (Cryptomys hottentotus hottentotus). The Damaraland and common mole-rats show an extreme reproductive skew and reproductive suppression that usually result in a single BF and breeding male in each group responsible for procreation, with the remaining colony members (often the offspring of the breeding pair) never achieving reproductive activation while in the confines of their natal colony [11]. Damaraland and common mole-rat NBFs show follicular growth, but are anovulatory in the presence of the breeding pair [11]. Furthermore, this study also attempted to investigate the relationship between the immune and endocrine systems using the endocrine profiles of BF and NBF Damaraland mole-rats. A comprehensive bidirectional relationship between the immune and endocrine systems has been well documented, with sex hormones, such as progesterone and testosterone (which have been recorded to be significantly higher in BFs compared with NBFs [39,40]), and hormones related to group maintenance, such as cortisol (which has been recorded to be higher in NBFs compared with BFs, but not significantly so [18,39,41]), being observed to possess both an immuno-enhancing and an immuno-suppressive role [42–46].
2. Methods
(a) . Study species
Twenty-five adult female Damaraland mole-rats, comprising nine BFs (144 ± 7.28 g) and 16 NBFs (130 ± 6.19 g) (Damaraland mole-rat body mass BF versus NBF: t = −1.34, p = 0.19), from 10 captive-bred colonies from the mole-rat laboratory at the University of Pretoria (25.7545° S, 28.2314° E), South Africa, were used in this study. In addition, a further 12, six BFs (87.7 ± 4.10 g) and six NBFs (66.3 ± 6.65 g) (common mole-rat body mass BF versus NBF: t = 2.78, p = 0.02), adult female common mole-rats, were captured using Hickman live traps, baited with a small piece of sweet potato [47], at Steinkopf (29.2602° S, 17.7340° E) and Kamieskroon (30.2195° S, 17.9236° E), in the Northern Cape, South Africa. The traps were positioned at the entrance of excavated burrows where tunnels were open. Traps were monitored for captures or blocking every 2–3 h for the day and left overnight, being checked first thing in the morning. All animals were grouped and housed in large polyurethane crates (1 × 0.5 × 0.5 m) with wood shavings and paper towelling for nesting material. Animals were maintained at approximately 25°C and 50% humidity with a 12L : 12D light cycle. Animals were fed fresh tubers and vegetables ad libitum.
All Damaraland mole-rat BFs had produced at least five litters prior to the onset of sampling. Furthermore, NBFs had not bred and were still housed within their natal colony prior to the start of the experiment. All BFs showed no visible signs of pregnancy during sampling. All common mole-rats were in captivity for approximately one month before the start of this study. For more details, see the electronic supplementary material.
(b) . Urine collection and hormonal analysis
Urine samples were collected from all 25 female Damaraland mole-rats and analysed for testosterone, progesterone and cortisol using a Coat-a-Count kit (Diagnostic Products Corporation, Los Angeles, California, USA). See electronic supplementary material for more details.
(c) . Blood collection
Whole blood was collected from all 25 female Damaraland mole-rats, 3 days after urine collection, and all 12 female common mole-rats. Before blood collection, a sterile bleed site was ensured by thoroughly swabbing the female hindfoot with Biotaine (0.5% chlorhexidine gluconate, 70% ethanol; Braun Medical, reg. no. 33/13.1/0526) and allowing it to dry. Next, the dorsal tarsal vein was pierced using a sterile 23G needle. Finally, an EDTA-coated microhaematocrit capillary tube was used to collect the whole blood into a sterile EDTA tube.
(i) . Complete blood count
Complete blood counts were performed on all 25 Damaraland mole-rat and 12 common mole-rat whole blood samples in an automated haematological analyser (ADVIA 21209(i) Siemens), designed for in vitro use at the Clinical Pathology unit at the Department of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria. Quantification of white cell count (×109 l−1) and leucocyte cell type count (segmented neutrophil (×109 l−1), lymphocyte (×109 l−1), eosinophil (×109 l−1), basophil (×109 l−1) and monocyte (×109 l−1)) was completed. BFs and NBFs of both species possessed similar white blood cell counts (t < 0.11, p > 0.91, for both species). Leucocyte percentages ((leucocyte cell type count (×109 l−1)/white cell count (×109 l−1)) × 100) were calculated (segmented neutrophil (%), lymphocyte (%), eosinophil (%), basophil (%) and monocyte (%)) and used for further analysis. From the leucocyte percentage, NLR and MLR were determined.
(ii) . Bacterial killing assay
The bacterial killing assay (BKA) was used to directly measure the ability of blood from a subset of 16 female Damaraland mole-rats, eight BFs and eight NBFs, and all 12 female common mole-rats to kill or prevent the growth of a laboratory strain of bacteria, in this case, Escherichia coli. The procedure used in this study followed the protocol demonstrated by Millet et al. [48] and DeRogatis et al. [49], with modifications (see electronic supplementary material for more details).
Data are presented as percentage (%) E. coli killed or prevented from growing.
(d) . Data analysis
Statistical analyses were performed with R 2022.02.0 and GraphPad Prism 8.4.3. Statistical significance is denoted by p ≤ 0.05, and data are presented as mean ± s.e.
Body mass was observed to not affect any measured biological parameters of Damaraland or common mole-rats (electronic supplementary material, table S1). Likewise, age did not affect any biological parameter of non-breeding female Damaraland mole-rats (electronic supplementary material, table S2). Therefore, age and body mass were not included in any subsequent statistical analysis. The distribution of each biological parameter was assessed, and the required model distribution was used (electronic supplementary material, table S3). Normality of each biological parameter was tested visually using QQ plots, Shapiro–Wilk tests and Levene's tests on model residuals. Data that were not normally distributed were log transformed and tested for normality. Linear models were used to analyse normally distributed variables, while generalized linear models were used to analyse non-normal variables. The authors want to highlight the complication of a two-species comparison, such as speciation between species, no genetic exchange and different environmental conditions (captive versus wild) [50]. These factors can all increase the likelihood of a type 1 error [50]. In the light of this problem, the response of each species is discussed and not directly compared. However, a multiple-species (6 or more) comparison would circumvent this problem and should be attempted in the future [50].
Differences between immune strength proxies (BKA, prevalence of neutrophils, lymphocytes, eosinophils, basophils and monocytes, and NLR and MLR) of BFs and NBFs of Damaraland mole-rat and common mole-rat, respectively, were investigated using either linear models or generalized linear models (electronic supplementary material, table S3). Furthermore, linear models were used to investigate differences between steroid hormones (testosterone, cortisol and progesterone) of BFs and NBFs of Damaraland mole-rats (electronic supplementary material, table S3). Generalized linear models, with beta logit-link (BKA and neutrophil and lymphocyte prevalence and NLR and MLR) or negative binominal (monocyte, eosinophil and basophil prevalence) distributions (electronic supplementary material, table S3) were used to investigate if the steroid hormones (testosterone, cortisol and progesterone) significantly affected any innate immune strength proxy (BKA, prevalence of neutrophils, lymphocytes, eosinophils, basophils and monocytes, and NLR and MLR) in female Damaraland mole-rats (BFs and NBFs combined).
3. Results
(a) . Reproductive status effects on immunocompetence
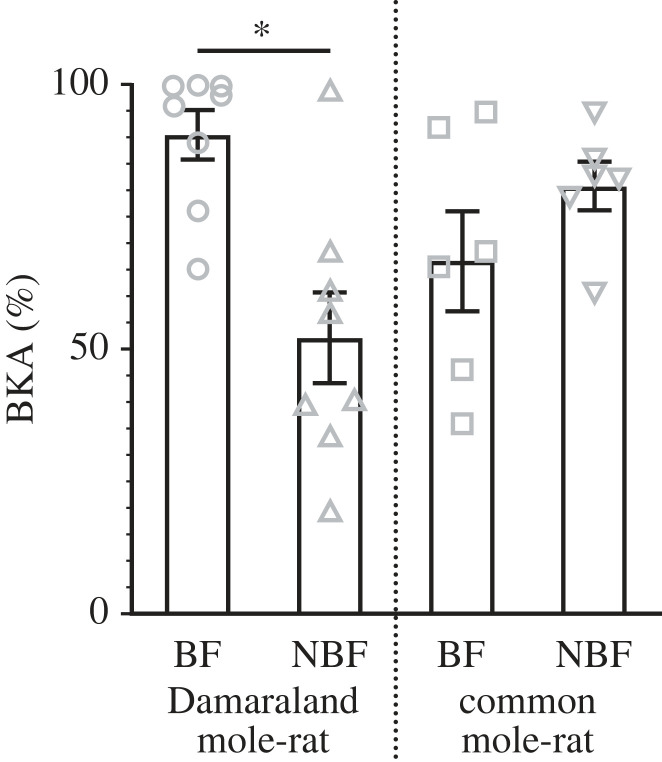
BF and NBF common mole-rats showed a similar bactericidal capacity of their blood (z = −1.07, p = 0.28; figure 1), suggesting BFs and NBFs have similar immune strengths. Furthermore, the similar NLR (z = −0.93, p = 0.40) and MLR (t = −0.13, p = 0.90) values, as well as the similar prevalence of neutrophils (z = −125, p = 0.21), lymphocytes (z = 0.90, p = 0.37), monocytes (z = 0.05, p = 0.96), eosinophils (z = 0.29, p = 0.77) and basophils (z = 0.32, p = 0.75) between common mole-rat BFs and NBFs (figure 2) further support this.
Figure 1.
The variation of the blood bactericidal capacity (bacterial killing assay (BKA) score (% of E. coli killed or prevented from growing)) of Damaraland mole rat (Fukomys damarensis) and common mole-rat (Cryptomys hottentotus hottentotus) breeding females (BFs, denoted by open circles in Damaraland mole-rats and open squares in common mole-rats) and non-breeding females (NBFs, denoted by open triangles in Damaraland mole-rats and open upside-down triangles in common mole-rats)). Statistical significance is denoted by an asterisk (*) p ≤ 0.05, and data are presented as mean ± s.e.
Figure 2.
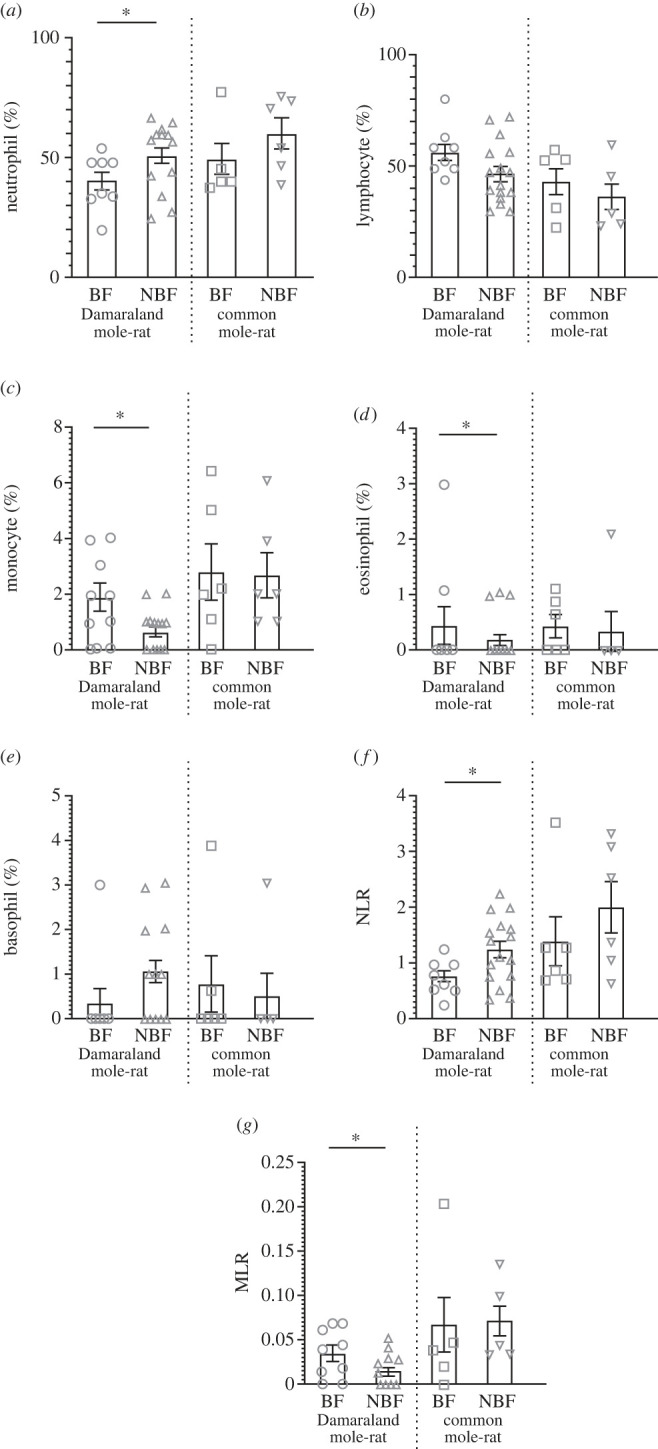
The variation of the prevalence of leucocytes ((a) neutrophils (%), (b) lymphocytes (%), (c) monocytes (%), (d) eosinophils (%), (e) basophils (%), and (f) neutrophil : lymphocyte ratio (NLR) and (g) monocycte : lymphocyte ratio (MLR)) of Damaraland mole-rat (Fukomys damarensis) and common mole-rat (Cryptomys hottentotus hottentotus) breeding females (BFs, denoted by open circles in Damaraland mole-rats and open squares in common mole-rats) and non-breeding females (NBFs, denoted by open triangles in Damaraland mole-rats and open upside-down triangles in common mole-rats). Statistical significance is denoted by an asterisk (*) p ≤ 0.05, and data are presented as mean ± s.e.
By contrast, a clear effect of reproductive status on immunocompetence was observable in Damaraland mole-rats, with BFs possessing a higher blood bactericidal capacity (z = −3.31, p = 0.001), increased monocyte (z = −2.86, p = 0.004), lymphocyte (not significant: z = −1.86, p = 0.06) and eosinophil (not significant: z = −0.90, p = 0.37) prevalence and MLR value (t = −2.25, p = 0.03), and decreased neutrophil (z = 2.10, p = 0.04) and basophil (not significant: z = 1.87, p = 0.06) prevalence and NLR value (z = 2.63, p = 0.02) (figures 1 and 2).
(b) . Hormonal parameters
(i) . Reproductive phenotype in hormonal parameters
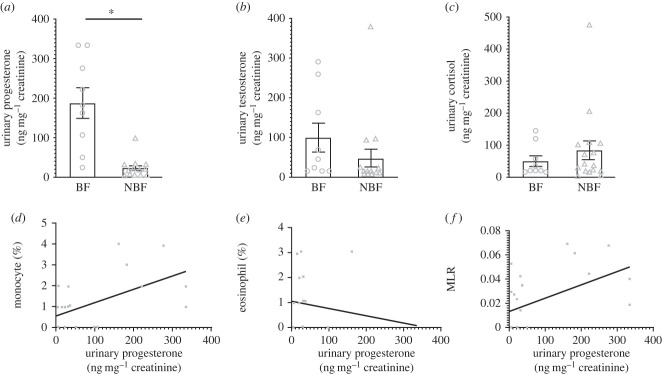
Damaraland mole-rat BFs possessed significantly higher levels of progesterone (t = −5.54, p = 0.00001) than their NBF counterparts (figure 3). By contrast, there were no differences in testosterone (t = −1.97, p = 0.06) or cortisol (t = 0.44, p = 0.67) concentrations between Damaraland mole-rat BFs and NBFs.
Figure 3.
The variation of the hormonal steroid profiles ((a) urinary testosterone (ng mg−1 creatinine), urinary corisol (µg mg−1 creatinine) and urinary progesterone (ng mg−1 creatinine)) of Damaraland mole rat (Fukomys damarensis) breeding females (BFs, denoted by open circles) and non-breeding females (NBFs, denoted by open triangles). The significant relationships between urinary progesterone (ng mg−1 creatinine) and the prevalence of (c) monocytes (%) and (d) eosinophils (%), and (e) monocycte : lymphocyte ratio (MLR) in female Damaraland mole-rats (BFs and NBFs combined) are also presented. Statistical significance is denoted by an asterisk (*) p ≤ 0.05, and data are presented as mean ± s.e.
(ii) . Effect on innate immune strength proxies
All three steroid hormones (testosterone, progesterone and cortisol) were observed not to affect the prevalence of neutrophils, lymphocytes and basophils and the BKA and NLR (electronic supplementary material, table S4) in female Damaraland mole-rats. Similarly, the testosterone and cortisol profiles of female Damaraland mole-rats did not affect the prevalence of monocytes and eosinophils and NLR, but in contrast, progesterone profiles did, in fact, affect the prevalence of monocytes and eosinophils and MLR (electronic supplementary material, table S4). In female Damaraland mole-rats with high progesterone levels, the prevalence of monocytes and MLR was high, while the eosinophil prevalence was low (figure 3).
4. Discussion
This study aimed to answer two fundamental, but crucial questions: (i) Is there a trade-off between reproduction and immunocompetence in co-operatively breeding African mole-rat species, and (ii) What is the relationship between immunocompetence and the endocrine systems in a co-operatively breeding African mole-rat species? This study successfully answered these questions; firstly, there was no trade-off between reproduction and immunocompetence in co-operatively breeding African mole-rat species, and in the case of the Damaraland mole-rats BFs actually possessed increased immunocompetence. Furthermore, the increased levels of progesterone possessed by Damaraland mole-rat BFs compared with NBFs appear to be correlated to increased immunocompetence, namely the prevalence of lymphocytes and monocytes and the MLR value.
Damaraland mole-rat BFs showed an increase in the bactericidal capacity of their blood (BKA scores) compared with NBFs, indicating BFs possess an increased ability to fight a bacterial infection and/or NBFs possess a reduced ability to fight a bacterial infection. Damaraland mole-rat NBFs showed below average (2–8%), even compared with other African mole-rat species [51], prevalence of monocytes, which could be a factor in the lowered bactericidal properties of their blood despite showing higher levels of neutrophils. Monocytes destroy foreign substances by phagocytosis and enhance the efficiency of the other immune cells (table 1). Furthermore, there is evidence that BFs possess a more robust adaptive immunity through higher lymphocyte (likely in the form of memory cells) prevalence, similar to other African mole-rat species [51]. An increased prevalence of adaptive immune system components, particularly memory cells, would indicate an increased ability to protect the body against specific foreign pathogens, such as E. coli, a common pathogen found in soils and on plant roots (mole-rats' main food source). Interestingly, the Damaraland mole-rat, particularly BF, immune system displays more similarities to those of the naked mole-rat (Heterocephalus glaber) and humans (Homo sapiens) rather than to those of other rodents [51,52], including increased prevalence of lymphocytes and monocytes in the peripheral blood. Reduced NLR and raised MLR (within the range of healthy [53]) of BFs compared with NBFs indicate that BFs possess a more balanced immune system compared with NBFs (table 1).
Increased levels of progesterone in BF Damaraland mole-rats compared with those of NBFs are correlated to increased immunocompetence, while testosterone and cortisol did not affect the immunocompetence of female Damaraland mole-rats. As BFs ovulate and fall pregnant, progesterone levels are higher in BFs than NBFs [18,39]. Human and animal studies' data demonstrate that progesterone influences most components of innate and adaptive immunity [54]. Evidence of these effects is found in the differences in immune responses between females and males, with females mounting a more vigorous B and T lymphocyte response than males [54]. Further, in many females, the hormone changes associated with pregnancy, including increases in progesterone, lessen the severity of disease [54]. In addition, progesterone positively affects human naive (or immature) T lymphocyte differentiation into memory and effector T lymphocytes [55], which ultimately increases the efficiency of the adaptive immune system. Increased progesterone has also been observed to increase the prevalence and function of monocytes [56,57]. While some studies have indicated progesterone's immuno-suppressive role, particularly on macrophage, antibody and NK cell activity, our study suggests an immuno-enhancing role of progesterone [43,58–62].
The common mole-rat's immune system displays more similarities to that of the Damaraland mole-rat, particularly NBFs, than to those of other rodents [51]. But, unlike the Damaraland mole-rat, common mole-rat BFs and NBFs show similar immunocompetence properties, including the bactericidal capacity of their blood and similar prevalence of neutrophils, lymphocytes and monocytes and similar MLR and NLR. As with the Damaraland mole-rat NBFs, common mole-rat NBFs are anovulatory in the presence of the breeding pair, and as such, it would be expected that sex hormones, such as progesterone, would be lower in NBFs compared with those of the BFs. Unfortunately, to our knowledge no study to date has compared progesterone concentrations of BF and NBF common mole-rats; however, it is speculated that, apart from periods of pregnancy or ovulation, common mole-rat BFs and NBFs may have similar levels of progesterone [63]. However, we refrain from further speculation as our current study did not compare progesterone concentrations of BF and NBF common mole-rats.
The difference in patterns of the relationship between immunocompetence and reproduction in the two co-operatively breeding African mole-rat species may be due to the Damaraland mole-rats being captive-bred and the common mole-rats being wild-caught, even after longer than a month in captivity [64–67]. However, captive-bred and wild-caught Norway rats (Rattus norvegicus) showed similar prevalence levels of neutrophils and lymphocytes, but differing levels in prevalence of monocytes, eosinophils and basophils [67]. Thus, the source of the animals (captive versus wild) may not be the primary reason for species-specific differences between immunocompetence in this study, but it cannot be discounted.
The Damaraland and common mole-rat share many similarities, but there are significant divergences in the social behaviour and reproductive systems between Damaraland and common mole-rats. The common mole-rat forms groups of up to 12 individuals with the female reproductive suppression strategy comprising primarily incest avoidance and/or aggressive behaviour from BFs directed towards NBFs, including BFs blocking mating attempts of their NBFs and/or the NBFs being the object of aggression (behavioural in nature) [12,68]. In contrast, Damaraland mole-rats form much larger groups, of up to 41 individuals, than common mole-rats. Consequently, the level of reproductive skew is likely greater in Damaraland mole-rats compared with the common mole-rat [69], resulting in the need for additional, possibly more potent, mechanisms of reproductive suppression (physiological in nature) to be required. In the Damaraland mole-rat, NBFs are both physiologically and behaviourally suppressed while in the natal group. Physiologically reproductive suppression in the NBFs presents itself in the form of NBFs exhibiting low circulating basal luteinizing hormone (LH) concentrations and a much-reduced response to an exogenous gonadotropin-releasing hormone (GnRH) challenge in comparison with BFs [70,71]. In addition, the varied expression of potent regulators of gonadotropin release, namely the RFamide peptides kisspeptin (Kiss1) and RFamide-related peptide-3 (RFRP-3), has been implicated in the reduced fertility and exhibiting of sexual behaviours of NBFs compared with BFs [72–75]. This ensures that if unrelated males enter a stable group (consisting of an established and breeding BF and breeding male pair) when ecological constraints are relaxed (such as good rainfall events), multiple BFs do not arise [76,77]. In marked contrast, common mole-rat NBFs have basal LH concentrations and responses to an exogenous GnRH challenge similar to their BF counterparts [68]. On occasion, multiple BFs can be found in the field in stable groups of the common mole-rat owing to the immigration of unrelated males into the group as there is only a behavioural inhibition of reproduction [78]. This pattern is not observed in stable and unmanipulated Damaraland groups in the wild [77,79]. Despite the disparate mechanisms of social suppression, the BFs in groups of both species exhibit induced ovulation [16,63]. Similarly, both species show an aversion to inbreeding and avoid this at all costs (behavioural inhibition of reproduction) [11].
Interestingly, several differences in behavioural and physiological traits between those species that possess larger group sizes, increased reproductive skew and behavioural and physiological mechanisms of reproductive suppression, such as the Damaraland mole-rat [70,71], and those species that possess smaller groups sizes, reduced reproductive skew and only behavioural mechanisms of reproductive suppression, such as the common mole-rat, have been observed. For example, in Damaraland mole-rats, the BFs are 50% less active than NBFs, which likely results in Damaraland mole-rat BFs possessing higher body condition and increased fat reserves compared with their NBFs [80,81]. By contrast, in Natal mole-rat (C. h. natalensis), with similar colony sizes and mechanisms of female reproductive suppression [82] to the common mole-rat, BFs and NBFs have similar activity levels [83], body condition [84] and body fat (D. M. Scantlebury 2023, unpublished results). Unsurprisingly, and similar to the patterns observed in common mole-rats, there was no difference in immunocompetence between breeding and non-breeding Natal mole-rats [85].
Our study has uncovered the natural inequality that arises in ecology and evolution. According to evolutionary theory, a balance must be struck between reproductive investment by the breeding individual and the breeding individual's survival. However, this study suggests that this is not always the case. Under conditions of extreme reproductive skew the breeders have the best of both worlds: increased reproductive investment and survival (as a result of increased immunocompetence). The physiological underpinning of how social living could cause inequality of evolutionary success, via reproduction and immunity, is currently not fully understood in mammals. But studying social African mole-rat species may be critical to address this dearth of knowledge of such naturally occurring inequalities.
Acknowledgements
Special thanks to Dr Yolandi Rautenbach, Carien Muller and the rest of the Clinical Pathology team at the Department of Companion Animal Clinical Studies, Faculty of Veterinary Science, University of Pretoria for conducting the haematological analysis for this project. Lastly, we would like to thank Philip Greef for guidance and assistance in microbial laboratory protocol.
Contributor Information
K. M. E. Wallace, Email: kyramew@gmail.com.
Daniel W. Hart, Email: u10022725@tuks.co.za.
Ethics
Experimental procedures were approved by the animal ethics committee of the University of Pretoria (NAS016/2021, NAS017-2021 and NAS022/2021). DAFF section 20 approval (SDAH-Epi-21021810222, SDAH-Epi-21021810221 and SDAH-Epi-21031811071) was obtained. Northern Cape (South Africa) animal capture permits TN 0558 2021 and TN 0559 2021 were obtained.
Data accessibility
The data are provided in electronic supplementary material [86].
Authors' contributions
K.M.E.W.: data curation, formal analysis, investigation, methodology, validation, writing—original draft; D.W.H.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, visualization, writing—original draft, writing—revision; F.V.: data curation, investigation, methodology, resources, supervision, validation, writing—original draft; A.K.J.v.V.: data curation, investigation, writing—original draft; N.C.B.: conceptualization, data curation, funding acquisition, methodology, project administration, resources, supervision, writing—original draft, writing—revision.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
The project was funded by the SARChI chair for Mammal Behavioural Ecology and Physiology, South Africa, no. 64796, for the DST-NRF awarded to N.C.B.
References
- 1.Ward D. 2009. Morphological, physiological, and behavioural adaptations of desert animals to the abiotic environment. In The biology of deserts (ed. Ward D), pp. 66-101. New York, NY: Oxford University Press. [Google Scholar]
- 2.Ebensperger LA, Hayes LD. 2016. Causes and evolution of group-living. In Sociobiology of caviomorph rodents: an integrative approach (eds LA Ebensperger, LD Hayes), pp. 173–200. Oxford, UK: Wiley-Blackwell.
- 3.Clutton-Brock T. 2021. Social evolution in mammals. Science 373, eabc9699. ( 10.1126/science.abc9699) [DOI] [PubMed] [Google Scholar]
- 4.Faulkes CG, Bennett NC. 2013. Plasticity and constraints on social evolution in African mole-rats: ultimate and proximate factors. Phil. Trans. R. Soc. B 368, 20120347. ( 10.1098/rstb.2012.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart DW, Bennett N, Oosthuizen MK, Waterman JM, Hambly C, Scantlebury DM. 2022. Energetics and water flux in the subterranean rodent family Bathyergidae. Front. Ecol. Evol. 10, 867350. ( 10.3389/fevo.2022.867350) [DOI] [Google Scholar]
- 6.Hart DW, Bennett NC. 2023. Seasonality of reproduction in African mole-rats (Rodentia: Bathyergidae) is a function of group size: a novel hypothesis. Lynx (New Series) 53, 53-64. ( 10.37520/lynx.2022.004) [DOI] [Google Scholar]
- 7.Majolo B, de Bortoli Vizioli A, Schino G. 2008. Costs and benefits of group living in primates: group size effects on behaviour and demography. Anim. Behav. 76, 1235-1247. ( 10.1016/j.anbehav.2008.06.008) [DOI] [Google Scholar]
- 8.Ezenwa VO, Ghai RR, McKay AF, Williams AE. 2016. Group living and pathogen infection revisited. Curr. Opin. Behav. Sci. 12, 66-72. ( 10.1016/j.cobeha.2016.09.006) [DOI] [Google Scholar]
- 9.Landry F, Li MF. 2019. Costs of group living. In Encyclopedia of animal cognition and behavior (eds Vonk J, Shackelford T), pp. 1-6. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 10.Montgomery TM, Pendleton EL, Smith JE. 2018. Physiological mechanisms mediating patterns of reproductive suppression and alloparental care in cooperatively breeding carnivores. Physiol. Behav. 193, 167-178. ( 10.1016/j.physbeh.2017.11.006) [DOI] [PubMed] [Google Scholar]
- 11.Bennett NC, Faulkes CG. 2000. African mole-rats: ecology and eusociality. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.Hager R, Jones CB. 2009. Reproductive skew in vertebrates: proximate and ultimate causes. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Creel S. 1995. Sociality, group size, and reproductive suppression among carnivores. Adv. Study Behav. 24, 203-257. ( 10.1016/S0065-3454(08)60395-2) [DOI] [Google Scholar]
- 14.Mustoe A. 2023. A tale of two hierarchies: hormonal and behavioral factors underlying sex differences in social dominance in cooperative breeding callitrichids. Horm. Behav. 147, 105293. ( 10.1016/j.yhbeh.2022.105293) [DOI] [PubMed] [Google Scholar]
- 15.Clarke FM, Miethe GH, Bennett NC. 2001. Reproductive suppression in female Damaraland mole-rats Cryptomys damarensis: dominant control or self-restraint? Phil. Trans. R. Soc. Lond. B 268, 899-909. ( 10.1098/rspb.2000.1426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voigt C, Medger K, Bennett NC. 2021. The oestrous cycle of the Damaraland mole-rat revisited: evidence for induced ovulation. J. Zool. 314, 85-95. ( 10.1111/jzo.12860) [DOI] [Google Scholar]
- 17.Bennett NC, Faulkes CG, Voigt C. 2022. Socially induced infertility in naked and Damaraland mole-rats: a tale of two mechanisms of social suppression. Animals 12, 3039. ( 10.3390/ani12213039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart DW, Bennett NC, Voigt C. 2022. Social stress is unlikely to play a major role in reproductive suppression of female subordinate naked mole-rats and Damaraland mole-rats. Biol. Lett. 18, 20220292. ( 10.1098/rsbl.2022.0292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkwood TBL. 1977. Evolution of ageing. Nature 270, 301-304. ( 10.1038/270301a0) [DOI] [PubMed] [Google Scholar]
- 20.French SS, Moore MC, Demas GE. 2009. Ecological immunology: the organism in context. Integr. Comp. Biol. 49, 246-253. ( 10.1093/icb/icp032) [DOI] [PubMed] [Google Scholar]
- 21.French SS, Neuman-lee LA. 2012. Improved ex vivo method for microbiocidal activity across vertebrate species. Biol. Open 1, 482-487. ( 10.1242/bio.2012919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheldon BC, Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317-321. ( 10.1126/science.328.5977.414-a) [DOI] [PubMed] [Google Scholar]
- 23.Naim N, Amrit FRG, McClendon TB, Yanowitz JL, Ghazi A. 2020. The molecular tug of war between immunity and fertility: emergence of conserved signaling pathways and regulatory mechanisms. Bioessays 42, 2000103. ( 10.1002/bies.202000103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naess A, Nilssen SS, Mo R, Eide GE, Sjursen H. 2017. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection 45, 299-307. ( 10.1007/s15010-016-0972-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buonacera A, Stancanelli B, Colaci M, Malatino L. 2022. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int. J. Mol. Sci. 23, 3636. ( 10.3390/ijms23073636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahorec R. 2021. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 122, 474-488. ( 10.4149/bll_2021_078) [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, et al. 2022. The monocyte to lymphocyte ratio not only at baseline but also at relapse predicts poor outcomes in patients with hepatocellular carcinoma receiving locoregional therapy. BMC Gastroenterol. 22, 98. ( 10.1186/s12876-022-02180-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Y-C, Jia Z-F, Cao D-H, Wu Y-H, Jiang J, Wen S-M, Zhao D, Zhang S-L, Cao X-Y. 2018. Preoperative lymphocyte-to-monocyte ratio (LMR) could independently predict overall survival of resectable gastric cancer patients. Medicine (Baltimore) 97, e13896. ( 10.1097/MD.0000000000013896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bain BJ. 2017. Structure and function of red and white blood cells. Medicine (Baltimore) 45, 187-193. ( 10.1016/j.mpmed.2017.01.011) [DOI] [Google Scholar]
- 30.Nowak J, Borkowska B, Pawlowski B. 2016. Leukocyte changes across menstruation, ovulation, and mid-luteal phase and association with sex hormone variation. Am. J. Hum. Biol. 28, 721-728. ( 10.1002/ajhb.22856) [DOI] [PubMed] [Google Scholar]
- 31.Anaya J-M, Shoenfeld Y, Rojas-Villarraga A, Levy RA, Cervera R. 2013. Autoimmunity: From Bench to Bedside. Bogota, Colombia: El Rosario University Press. See https://pubmed.ncbi.nlm.nih.gov/29087650/. [PubMed]
- 32.Ratajczak W, Niedźwiedzka-Rystwej P, Tokarz-Deptuła B, Deptuła W. 2018. Immunological memory cells. Centr. Eur. J. Immunol. 43, 194-203. ( 10.5114/ceji.2018.77390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Hu Q, Hu T. 2021. Association of lymphocyte to monocyte ratio and risk of in-hospital mortality in patients with cardiogenic shock: a propensity score matching study. Int. J. Gen. Med. 14, 4459-4468. ( 10.2147/IJGM.S325907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao K, Zhu W, Liu W, Li H, Yu W, Li Q, Cao Y. 2019. The predictive role of monocyte-to-lymphocyte ratio in osteoporosis patient. Medicine (Baltimore) 98, e16793. ( 10.1097/MD.0000000000016793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu P, Li P, Peng Z, Xiang Y, Xia C, Wu J, Yang B, He Z. 2020. Predictive value of the neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-neutrophil ratio, and neutrophil-to-monocyte ratio in lupus nephritis. Lupus 29, 1031-1039. ( 10.1177/0961203320929753) [DOI] [PubMed] [Google Scholar]
- 36.Fang W-F, et al. 2019. Incorporation of dynamic segmented neutrophil-to-monocyte ratio with leukocyte count for sepsis risk stratification. Scient. Rep. 9, 19756. ( 10.1038/s41598-019-56368-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stefaniuk P, Szymczyk A, Podhorecka M. 2020. The neutrophil to lymphocyte and lymphocyte to monocyte ratios as new prognostic factors in hematological malignancies – a narrative review. Cancer Manag. Res. 12, 2961-2977. ( 10.2147/CMAR.S245928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seyit M, Avci E, Nar R, Senol H, Yilmaz A, Ozen M, Oskay A, Aybek H. 2021. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am. J. Emerg. Med. 40, 110-114. ( 10.1016/j.ajem.2020.11.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace KME, Hart DW, Hagenah N, Ganswindt A, Bennett NC. 2023. A comprehensive profile of reproductive hormones in eusocial Damaraland mole-rats (Fukomys damarensis). Gen. Comp. Endocrinol. 333, 114194. ( 10.1016/j.ygcen.2022.114194) [DOI] [PubMed] [Google Scholar]
- 40.Jarvis JUM, O'Riain MJ, Bennett NC, Sherman PW. 1994. Mammalian eusociality - a family affair. Trends Ecol. Evol. 9, 47-51. ( 10.1016/0169-5347(94)90267-4) [DOI] [PubMed] [Google Scholar]
- 41.Vullioud P, Mendonça R, Glauser G, Bennett N, Zöttl M, Katlein N, Leal R, Fuerst R, Clutton-Brock T. 2021. Increases in glucocorticoids are sufficient but not necessary to increase cooperative burrowing in Damaraland mole-rats. Horm. Behav. 135, 105034. ( 10.1016/j.yhbeh.2021.105034) [DOI] [PubMed] [Google Scholar]
- 42.Wira CR, Sandoe CP. 1980. Hormonal regulation of immunoglobulins: influence of estradiol on immunoglobulins A and G in the rat uterus. Endocrinology 106, 1020-1026. ( 10.1210/endo-106-3-1020) [DOI] [PubMed] [Google Scholar]
- 43.Rai U. 1998. Sex steroid hormones modulate the activation of murine peritoneal macrophages: receptor mediated modulation. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 119, 199-204. ( 10.1016/S0742-8413(97)00207-7) [DOI] [PubMed] [Google Scholar]
- 44.Trumble BC, Blackwell AD, Stieglitz J, Thompson ME, Suarez IM, Kaplan H, Gurven M. 2016. Associations between male testosterone and immune function in a pathogenically stressed forager-horticultural population. Am. J. Phys. Anthropol. 161, 494-505. ( 10.1002/ajpa.23054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nowak J, Pawłowski B, Borkowska B, Augustyniak D, Drulis-Kawa Z. 2018. No evidence for the immunocompetence handicap hypothesis in male humans. Scient. Rep. 8, 7392. ( 10.1038/s41598-018-25694-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55-89. ( 10.1210/edrv.21.1.0389) [DOI] [PubMed] [Google Scholar]
- 47.Hickman GC. 1979. A live-trap and trapping technique for fossorial mammals. S. Afr. J. Zool. 14, 9-12. ( 10.1080/02541858.1979.11447641) [DOI] [Google Scholar]
- 48.Millet S, Bennett J, Lee KA, Hau M, Klasing KC. 2007. Quantifying and comparing constitutive immunity across avian species. Dev. Comp. Immunol. 31, 188-201. ( 10.1016/j.dci.2006.05.013) [DOI] [PubMed] [Google Scholar]
- 49.DeRogatis AM, Nguyen LV, Bandivadekar RR, Klasing KC, Tell LA. 2020. Anti-microbial activity of whole blood and plasma collected from Anna's hummingbirds (Calypte anna) against three different microbes. PLoS ONE 15, e0234239. ( 10.1371/journal.pone.0234239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garland T Jr, Adolph SC. 1994. Why not to do two-species comparative studies: limitations on inferring adaptation. Physiol. Zool. 67, 797-828. ( 10.1086/physzool.67.4.30163866) [DOI] [Google Scholar]
- 51.Bégay V, Cirovic B, Barker AJ, Klopfleisch R, Hart DW, Bennett NC, Lewin GR. 2022. Immune competence and spleen size scale with colony status in the naked mole-rat. Open Biol. 12, 210292. ( 10.1098/rsob.210292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pillay J, Tak T, Kamp VM, Koenderman L. 2013. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell. Mol. Life Sci. 70, 3813-3827. ( 10.1007/s00018-013-1286-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirna M, Schmutzler L, Topf A, Hoppe UC, Lichtenauer M. 2021. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Scient. Rep. 11, 18101. ( 10.1038/s41598-021-97678-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beagley KW, Gockel CM. 2003. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 38, 13-22. ( 10.1016/S0928-8244(03)00202-5) [DOI] [PubMed] [Google Scholar]
- 55.Lee JH, Ulrich B, Cho J, Park J, Kim CH. 2011. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J. Immunol. 187, 1778-1787. ( 10.4049/jimmunol.1003919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sominsky L, Younesi S, De Luca SN, Loone SM, Quinn KM, Spencer SJ. 2021. Ovarian follicles are resistant to monocyte perturbations—implications for ovarian health with immune disruption. Biol. Reprod. 105, 100-112. ( 10.1093/biolre/ioab049) [DOI] [PubMed] [Google Scholar]
- 57.Jain SK, Kannan K, Prouty L, Jain SK. 2004. Progesterone, but not 17β-estradiol, increases TNF-α secretion in U937 monocytes. Cytokine 26, 102-105. ( 10.1016/j.cyto.2004.01.002) [DOI] [PubMed] [Google Scholar]
- 58.Diodato MD, Knoferl MW, Schwacha MG, Bland KI, Chaudry IH. 2001. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine 14, 162-169. ( 10.1006/cyto.2001.0861) [DOI] [PubMed] [Google Scholar]
- 59.Baley JE, Schacter BZ. 1985. Mechanisms of diminished natural killer cell activity in pregnant women and neonates. J. Immunol. 134, 3042-3048. ( 10.4049/jimmunol.134.5.3042) [DOI] [PubMed] [Google Scholar]
- 60.Furukawa K, Itoh K, Okamura K, Kumagai K, Suzuki M. 1984. Changes in NK cell activity during the estrous cycle and pregnancy in mice. J. Reprod. Immunol. 6, 353-363. ( 10.1016/0165-0378(84)90045-7) [DOI] [PubMed] [Google Scholar]
- 61.Toder VL, Nebel LA, Elrad HA, Blank MI, Durdana AT, Gleicher NO. 1984. Studies of natural killer cells in pregnancy. II. The immunoregulatory effect of pregnancy substances. J. Clin. Lab. Immunol. 14, 129-133. [PubMed] [Google Scholar]
- 62.McKay LI, Cidlowski JA. 1999. Molecular control of immune/inflammatory responses: interactions between nuclear factor-κB and steroid receptor-signaling pathways. Endocr. Rev. 20, 435-459. ( 10.1210/edrv.20.4.0375) [DOI] [PubMed] [Google Scholar]
- 63.Spinks AC, Bennett NC, Jarvis JUM. 1999. Regulation of reproduction in female common mole-rats (Cryptomys hottentotus hottentotus): the effects of breeding season and reproductive status. J. Zool. 248, 161-168. ( 10.1017/S0952836999006032) [DOI] [Google Scholar]
- 64.Fagir DM, Bennett NC, Ueckermann EA, Howard A, Hart DW. 2021. Ectoparasitic community of the Mahali mole-rat, Cryptomys hottentotus mahali: potential host for vectors of medical importance in South Africa. Parasites Vectors 14, 24. ( 10.1186/s13071-020-04537-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Archer EK, Bennett NC, Junker K, Faulkes CG, Lutermann H. 2017. The distribution of gastrointestinal parasites in two populations of common mole-rats (Cryptomys hottentotus hottentotus). J. Parasitol. 103, 786-790. ( 10.1645/17-62) [DOI] [PubMed] [Google Scholar]
- 66.Archer EK, Bennett NC, Ueckermann EA, Lutermann H. 2014. Ectoparasite burdens of the common mole-rat (Cryptomys hottentotus hottentotus) from the Cape Provinces of South Africa. J. Parasitol. 100, 79-84. ( 10.1645/13-270.1) [DOI] [PubMed] [Google Scholar]
- 67.Kataranovski MV, Radović DL, Zolotarevski LD, Popov AD, Kataranovski DS. 2009. Immune-related health-relevant changes in natural populations of Norway rat (Rattus norvegicus Berkenhout, 1769): white blood cell counts, leukocyte activity, and peripheral organ infiltration. Arch. Biol. Sci. 61, 213-223. ( 10.2298/ABS0902213K) [DOI] [Google Scholar]
- 68.Spinks AC, Bennett NC, Faulkes CG, Jarvis JUM. 2000. Circulating LH levels and the response to exogenous GnRH in the common mole-rat: implications for reproductive regulation in this social, seasonal breeding species. Horm. Behav. 37, 221-228. ( 10.1006/hbeh.2000.1576) [DOI] [PubMed] [Google Scholar]
- 69.Faulkes CG, Bennett NC. 2009. Reproductive skew in African mole-rats: behavioural and physiological mechanisms to maintain high skew. In Reproductive skew in vertebrates: proximate and ultimate causes (eds R Hager, CB Jones), pp. 369–396. Cambridge, UK: Cambridge University Press.
- 70.Bennett NC, Jarvis JUM, Faulkes CG, Millar RP. 1993. LH responses to single doses of exogenous GnRH by freshly captured Damaraland mole-rats, Cryptomys damarensis. J. Reprod. Fertil. 99, 81-86. ( 10.1530/jrf.0.0990081) [DOI] [PubMed] [Google Scholar]
- 71.Bennett NC, Faulkes CG, Molteno AJ. 1996. Reproductive suppression in subordinate, non-breeding female Damaraland mole-rats: two components to a lifetime of socially induced infertility. Proc. R. Soc. Lond. B 263, 1599-1603. ( 10.1098/rspb.1996.0234) [DOI] [PubMed] [Google Scholar]
- 72.Voigt C, Bennett N. 2018. Reproductive status affects the expression of prolactin receptor mRNA in the brain of female Damaraland mole-rats. J. Chem. Neuroanat. 94, 1-7. ( 10.1016/j.jchemneu.2018.08.002) [DOI] [PubMed] [Google Scholar]
- 73.Voigt C, Bennett NC. 2018. Reproductive status-dependent kisspeptin and RF amide-related peptide (Rfrp) gene expression in female Damaraland mole-rats. J. Neuroendocrinol. 30, e12571. ( 10.1111/jne.12571) [DOI] [PubMed] [Google Scholar]
- 74.Hart DW, van Vuuren AKJ, Erasmus A, Süess T, Hagenah N, Ganswindt A, Bennett NC. 2022. The endocrine control of reproductive suppression in an aseasonally breeding social subterranean rodent, the Mahali mole-rat (Cryptomys hottentotus mahali). Horm. Behav. 142, 105155. ( 10.1016/j.yhbeh.2022.105155) [DOI] [PubMed] [Google Scholar]
- 75.Bennett NC, Ganswindt A, Ganswindt SB, Jarvis JUM, Zöttl M, Faulkes CG. 2018. Evidence for contrasting roles for prolactin in eusocial naked mole-rats, Heterocephalus glaber and Damaraland mole-rats, Fukomys damarensis. Biol. Lett. 14, 20180150. ( 10.1098/rsbl.2018.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rickard CA, Bennett NC. 1997. Recrudescence of sexual activity in a reproductively quiescent colony of the Damaraland mole-rat (Cryptomys damarensis), by the introduction of an unfamiliar and genetically unrelated male—a case of incest avoidance in ‘queenless’ colonies. J. Zool. 241, 185-202. ( 10.1111/j.1469-7998.1997.tb05508.x) [DOI] [Google Scholar]
- 77.Burland TM, Bennett NC, Jarvis JUM, Faulkes CG. 2002. Eusociality in African mole-rats: new insights from patterns of genetic relatedness in the Damaraland mole-rat (Cryptomys damarensis). Proc. R. Soc. Lond. B 269, 1025-1030. ( 10.1098/rspb.2002.1978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bishop JM, Jarvis JUM, Spinks AC, Bennett NC, O'Ryan C. 2004. Molecular insight into patterns of colony composition and paternity in the common mole-rat Cryptomys hottentotus hottentotus. Mol. Ecol. 13, 1217-1229. ( 10.1111/j.1365-294X.2004.02131.x) [DOI] [PubMed] [Google Scholar]
- 79.Burland TM, Bennett NC, Jarvis JUM, Faulkes CG. 2004. Colony structure and parentage in wild colonies of co-operatively breeding Damaraland mole-rats suggest incest avoidance alone may not maintain reproductive skew. Mol. Ecol. 13, 2371-2379. ( 10.1111/j.1365-294X.2004.02233.x) [DOI] [PubMed] [Google Scholar]
- 80.Francioli Y, Thorley J, Finn K, Clutton-Brock T, Zöttl M. 2020. Breeders are less active foragers than non-breeders in wild Damaraland mole-rats. Biol. Lett. 16, 20200475. ( 10.1098/rsbl.2020.0475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scantlebury M, Speakman JR, Oosthuizen MK, Roper TJ, Bennett NC. 2006. Energetics reveals physiologically distinct castes in a eusocial mammal. Nature 440, 795-797. ( 10.1038/nature04578) [DOI] [PubMed] [Google Scholar]
- 82.Oosthuizen MK, Bennett NC, Lutermann H, Coen CW. 2008. Reproductive suppression and the seasonality of reproduction in the social Natal mole-rat (Cryptomys hottentotus natalensis). Gen. Comp. Endocrinol. 159, 236-240. ( 10.1016/j.ygcen.2008.09.004) [DOI] [PubMed] [Google Scholar]
- 83.Finn KT, van Vuuren AKJ, Hart DW, Suess T, Zottl M, Bennett NC. 2022. Seasonal changes in locomotor activity patterns of wild social Natal mole-rats (Cryptomys hottentotus natalensis). Front. Ecol. Evol. 10, 819393. ( 10.3389/fevo.2022.819393) [DOI] [Google Scholar]
- 84.Jacobs P, Finn KT, van Vuuren AKJ, Suess T, Hart DW, Bennett NC. 2022. Defining the link between oxidative stress, behavioural reproductive suppression and heterothermy in the Natal mole-rat (Cryptomys hottentotus natalensis). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 261, 110753. ( 10.1016/j.cbpb.2022.110753) [DOI] [PubMed] [Google Scholar]
- 85.Lutermann H, Bennett NC. 2008. Strong immune function: a benefit promoting the evolution of sociality? J. Zool. 275, 26-32. ( 10.1111/j.1469-7998.2007.00403.x) [DOI] [Google Scholar]
- 86.Wallace KME, Hart DW, Venter F, van Vuuren AKJ, Bennett NC. 2023. The best of both worlds: no apparent trade-off between immunity and reproduction in two group-living African mole-rat species. Figshare. ( 10.6084/m9.figshare.c.6662602) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Wallace KME, Hart DW, Venter F, van Vuuren AKJ, Bennett NC. 2023. The best of both worlds: no apparent trade-off between immunity and reproduction in two group-living African mole-rat species. Figshare. ( 10.6084/m9.figshare.c.6662602) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are provided in electronic supplementary material [86].