Abstract
Few reliable or easily obtainable biomarkers to predict long-term outcome in infants with hypoxic-ischemic encephalopathy (HIE) have been identified. We previously showed that mattress temperature (MT), as proxy for disturbed temperature regulation during therapeutic hypothermia (TH), predicts injury on early MRI and holds promise as physiologic biomarker. To determine whether MT in neonates treated with TH for moderate-severe HIE is associated with long-term outcome at 18–22 months, we performed a secondary analysis of the Optimizing Cooling trial using MT data from 167 infants treated at a core temperature of 33.5°C. Median MTs from four time-epochs (0–6 h, 6–24 h, 24–48 h, and 48–72 h of TH) were used to predict death or moderate-severe neurodevelopmental impairment (NDI), using epoch-specific derived and validated MT cutoffs. Median MT of infants who died or survived with NDI was consistently 1.5–3.0°C higher throughout TH. Infants requiring a median MT above the derived cut-offs had a significantly increased odds of death or NDI, most notably at 0–6 h (aOR 17.0, 95%CI 4.3–67.4). By contrast, infants who remained below cut-offs across all epochs had 100% NDI-free survival. MTs in neonates with moderate-severe HIE during TH are highly predictive of long-term outcome and can be used as physiologic biomarker.
Keywords: Hypoxic-ischemic encephalopathy, therapeutic hypothermia, thermoregulation, biomarker, neurodevelopmental outcome
Introduction
Hypoxic-ischemic encephalopathy (HIE) is the most common cause of acute neonatal encephalopathy, affecting 1–4 per 1,000 live births in high resource countries.1,2 Therapeutic hypothermia (TH, 72 h at 33.5°C), initiated within 6 hours of birth, remains the only therapy that improves outcomes in infants with moderate-severe HIE.3–8 Predicting the severity of HIE injury and its impact on outcomes is difficult, particularly prior to completion of TH treatment. Due to the poor prognostic performance of traditional clinical markers such as Apgar score and day of life 1 Sarnat exam or Thompson score, significant research has been conducted to identify markers of injury with predictive value on outcome.9–11 A multitude of candidates have been identified, but so far no single easily obtainable and interpretable marker has been consistently reliable.12–16 To date, electroencephalography (EEG) background pattern has been the most reliable marker for long-term outcome. In particular, correlation with a normal outcome is high when the EEG background is normal within the first six hours and stays normal. 17 Similarly, highly abnormal resistive indices or cerebral blood flow measured on transcranial doppler prior to initiation of hypothermia may be able to predict death or severe disability. 18 However, both EEG and transcranial doppler technology still require expensive additional equipment and trained staff to read and accurately interpret the recordings.
Asphyxiated newborns and uncooled infants with moderate-severe HIE demonstrate disturbed temperature regulation.19,20 Previously, our group used the cooling device water output temperature, from now on referred to as mattress temperature (MT), during servo-controlled cooling as a proxy for impaired thermoregulation and found an association with increased MRI injury and unfavorable short-term outcome, concluding that thermoregulatory assessment offers promise as a physiologic biomarker for predicting outcome by reflecting severity of injury. 21 Based on our prior work, we hypothesized that infants with a worse long-term outcome would show a greater degree of temperature dysregulation and therefore require a higher MT (less active cooling) during TH to maintain a core temperature around 33.5°C. We also postulated that MT cut-offs could be derived to predict long-term outcome as early as 6 hours into the treatment course.
Methods
We performed a secondary analysis of data from infants enrolled in the Optimizing Cooling (OC) trial, which randomized 364 infants with moderate-severe HIE to variations in length (72 vs 120 hours) and depth (33.5°C vs 32.0°C) of TH. We obtained written permission from the Data and Specimen Hub (DASH) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to obtain and use the publicly available data from the OC trial for re-analysis and publication. The institutional review boards at the University of Washington (UW) and Children’s Hospital of Philadelphia (CHOP) determined that the study protocol (UW IRB ID: STUDY00011104; CHOP IRB 21-018646) was exempt from review.
We analyzed the first 72 hours of temperature data from infants randomized to a target esophageal core temperature of 33.5°C for either 72 hours (n = 95) or 120 hours (n = 96). Infants treated at 32°C were excluded. Infants were cooled using either the Blanketrol II or the Blanketrol III device (Cincinnati Sub-Zero Product LLC, Gentherm, Cincinnati, OH). Mattress and esophageal core temperatures were recorded every 15 minutes for the first 4 hours of cooling, hourly for the next 8 hours, and every 4 hours for the remaining duration of TH.
To understand the relationship between MT and injury most accurately, outcomes were analyzed by the intervention provided rather than by intention-to-treat. For instance, if a subject was randomized to 32.0°C but treatment occurred at 33.5°C, the subject was included in this study and vice versa. Subjects were excluded if they were treated with extracorporeal membrane oxygenation (ECMO), if less than 50% of the expected temperature data points were available, if they were re-warmed prior to 72 hours of TH, or if no outcome data were available.
HIE severity based on the Sarnat examination at randomization was re-scored according to contemporary criteria. The Sarnat exam used at the time this trial was conducted considered hypotonia and hypertonia equally as signs of moderate encephalopathy; however, further evolution of the Sarnat Exam now classifies hypertonia as a feature of mild encephalopathy. 22 The dataset was therefore adjusted accordingly to reflect the more contemporary descriptions of the Sarnat stages for mild, moderate, and severe encephalopathy. Two subjects were adjusted from severe to moderate, 6 from moderate to severe, and 6 from moderate to mild based on the data provided.
An organ dysfunction score was also derived to examine evidence of peripheral injury that might confound thermoregulation and outcome. End-organ involvement as well as documented clinical and/or EEG seizures were abstracted from the original dataset. The following parameters were included: cardiac dysfunction, defined as diagnosis by echocardiography and/or hypotension, respiratory failure requiring mechanical ventilation or continuous positive pressure ventilation, disseminated intravascular coagulopathy and/or thrombocytopenia, renal dysfunction defined as oliguria and/or anuria, hypoglycemia defined as blood glucose <50 mg/dL, and hepatic dysfunction defined as alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) > 100 IU/L; end-organ involvement was categorized from 0–6 based on the number of systems involved.
Our primary outcome was the composite of death or moderate-severe neurodevelopmental impairment (NDI) at 18–22 months, as defined in the original trial (supplemental Table s1). Detailed study descriptions of the OC trial have been published previously.23,24
Statistical analysis
Descriptive statistics were used to characterize the selected cohort compared to the original trial cohort, including maternal and infant factors as well as incidence of the primary outcome. For ultimate statistical inference, generalized estimating equations (GEE) with robust standard errors were used to appropriately account for potential clustering of outcomes by study site, as performed in the original trial outcome analyses. 24
Graphical illustrations were used to display MTs over time for the included cohort, stratified by primary and secondary outcomes (death and NDI separately). Average MT over time by outcome group was depicted graphically using the LOESS (locally estimated scatterplot smoothing) method. Epochs of TH were determined based on visual examination of MT trajectories, and median MT was derived for each infant within each epoch. Infants who had <50% of the expected temperature measurements within an epoch recorded were not included in the analyses for that epoch (n = 4–6 infants excluded per epoch).
As multiple clinical variables could potentially confound the relationship between MT and outcome, medication exposure and severity of illness parameters were compared by outcome group (NDI-free survival vs moderate or severe NDI vs death; Table 2). Medication exposure and severity of illness parameters that were significantly different across groups were included as covariates in later models examining MT by outcome as well as to predict outcome using MT. As a result, all GEE models examining MT and/or outcome groups were adjusted for TH treatment duration (72 vs 120 hours), severity of encephalopathy at enrollment (severe vs moderate), documented seizures, exposure to phenobarbital, morphine, or inotropes (yes vs no), and degree of end-organ dysfunction on a 0–6 continuous scale.
Table 2.
Temperature, encephalopathy, and organ dysfunction by outcome.
|
n (% of available data)/Median (IQR) |
p-value vs no NDI | ||
|---|---|---|---|
| Outcome group (n=) | NDI-free survival (n = 112) | Death/NDI (n = 55) | |
| Encephalopathy parameters | |||
| Severe Sarnat at randomization | 16 (14.3) | 23 (41.8) | 0.0001 |
| Documented seizures | 46 (41.4) | 40 (72.7) | 0.0001 |
| Esophageal temperature at TH initiation (°C) | 34.5 (33.5–36.1) | 33.5 (32.7–36.0) | 0.007 |
| Anticonvulsant medications | |||
| Phenobarbital | 39 (34.8) | 37 (67.3) | 0.0002 |
| Lorazepam (as anticonvulsant) | 14 (12.5) | 8 (14.5) | 0.70 |
| Levetiracetam | 6 (5.4) | 7 (12.7) | 0.11 |
| Other anticonvulsant | 8 (7.1) | 6 (10.9) | 0.39 |
| Analgesic medications | |||
| Morphine | 39 (34.8) | 33 (60.0) | 0.002 |
| Fentanyl | 21 (18.8) | 10 (18.2) | 0.90 |
| Midazolam | 33 (29.5) | 15 (27.3) | 0.77 |
| Other analgesic | 15 (13.4) | 9 (16.4) | 0.62 |
| Inotrope exposure | 45 (40.2) | 33 (60.0) | 0.016 |
| Median mattress temperature by epoch | |||
| 0–6 h | 33.0 (29.0–35.0) | 34.5 (33.8–36.0) | <0.0001 |
| 6–24 h | 32.0 (30.0–34.5) | 33.5 (32.0–36.2) | <0.0001 |
| 24–48 h | 30.5 (28.0–32.5) | 33.0 (31.0–34.5) | <0.0001 |
| 48–72 h | 32.0 (29.0–34.0) | 33.0 (32.0–34.5) | 0.002 |
| Organ involvement | |||
| Oliguria/anuria | 47 (42.0) | 44 (80.0) | <0.0001 |
| Liver dysfunction | 69 (61.6) | 43 (78.2) | 0.038 |
| DIC | 38 (33.9) | 31 (56.4) | 0.005 |
| Hypoglycemia | 30 (26.8) | 20 (36.4) | 0.17 |
| Hypotension | 36 (32.1) | 26 (47.3) | 0.056 |
| Respiratory support | 96 (85.7) | 54 (98.2) | 0.025 |
| Total organ involvement score | 3 (2–4) | 4 (3–5) | <0.0001 |
| Sarnat after rewarming | |||
| Mild | 72 (66.1) | 6 (10.9) | <0.0001 |
| Moderate | 34 (31.2) | 26 (47.3) | |
| Severe | 3 (2.8) | 15 (27.3) | |
| Missing/Dieda | 3 (2.8) | 8 (14.5) | – |
Comparison of average mattress temperatures (MT) by epoch of therapeutic hypothermia (TH) as well as evidence of encephalopathy and organ dysfunction by long-term outcome. Infants who died or survived with moderate-severe neurodevelopmental impairment (NDI) were more likely to have severe encephalopathy at randomization, documented seizures, evidence of end-organ dysfunction, a higher likelihood of being exposed to anticonvulsant medications, particularly phenobarbital, as well as morphine and inotropes. Infants who died or survived with NDI also had significantly higher average MTs across all time epochs, though only a trend was seen between survivors at 48–72 h. Bolded values indicate p-value <0.05 compared to the NDI-free survival group after adjustment for duration of cooling, where those that survived with NDI or died were considered as separate factors within the same regression models. Binary outcomes were compared with generalized estimated equations (GEE) logistic regression, and continuous outcomes by GEE linear regression. All comparisons are unadjusted except for TH treatment duration.
aSarnat exam was not documented after rewarming in 11 infants: 3 without NDI, 2 with NDI, and 5 who died. MT: mattress temperature; IQR: interquartile range; NDI: neurodevelopmental impairment; ALT: alanine aminotransferase; AST: aspartate aminotransferase; DIC: disseminated intravascular coagulopathy.
Within each epoch, differences in mean adjusted MT (with 95% confidence interval, CI) by outcome were determined using GEE linear regression models. To determine predictive MT cut-offs within each epoch, a modified 5-fold cross-validation approach was used. The data were randomly split into five folds such that each infant appeared in four training folds and one validation fold. Within each epoch, MT cut-offs were determined using the cutpointr library in R, optimizing for the sum of sensitivity and specificity to predict primary outcome (death or NDI) in unadjusted models. The sensitivity and specificity of the cut-off in the validation fold was then examined. To select a final cut-off for each epoch for outcome prediction, the cut-off identified in the most folds was selected. In the 24–48 h epoch, two different MT cut-offs were selected in the same number of training folds (n = 2 each), so the cut-off that appeared to have higher sensitivity for predicting the primary outcome in the validation folds was selected. Using GEE logistic regression models, adjusted odds ratios (aOR) with 95% confidence intervals (CI) for primary outcome based on being above the derived cut-off within an epoch were derived in the validation datasets.
Finally, exploratory analyses were then performed using a combination of the derived cut-offs across the entire dataset. Infants were combined into trajectory groups based on whether they were above or below cut-offs within multiple epochs, and aORs for the primary outcome were determined within those trajectory groups for both the cohort overall and those surviving to discharge, as well as when split by severity of encephalopathy. A sensitivity analysis was performed by applying the same trajectory analyses to the entire cohort of 191 infants cooled to 33.5°C as presented in the OC dataset in an intention-to-treat manner without further selection or adjustment of participants based on reassessment of severity of encephalopathy or availability of MT data.
Analyses were conducted in RStudio using the R statistical package (Version 4.1.2, Foundation for Statistical Computing, Vienna, Austria). 25 P-values <0.05 were considered significant.
Results
Patient characteristics
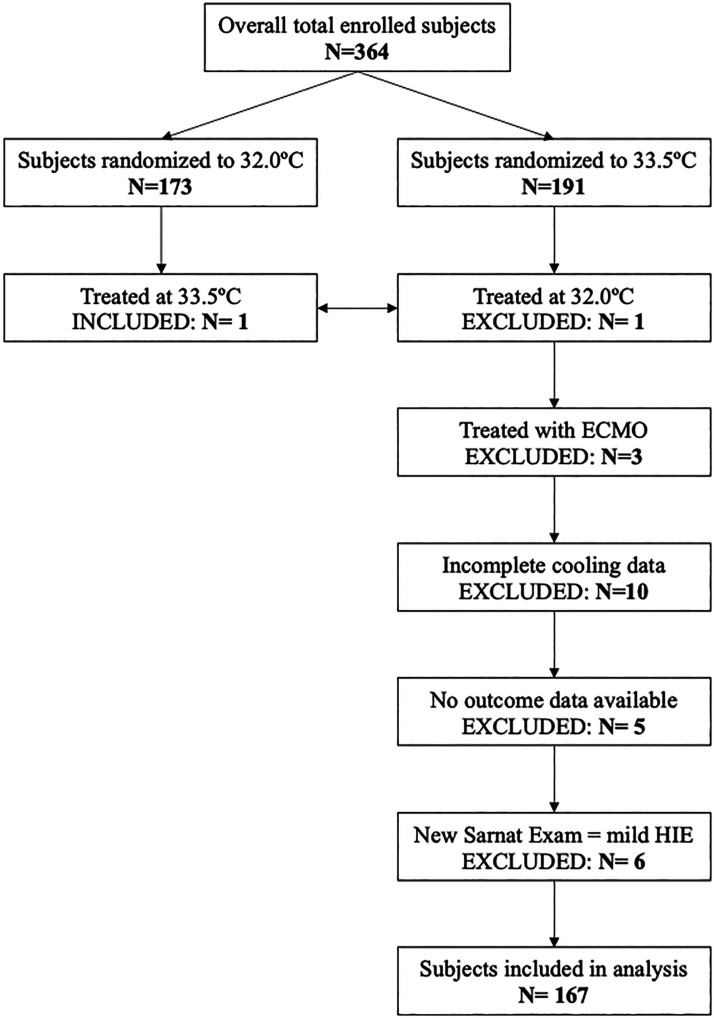
Characteristics of the 167 included infants are displayed in Table 1, including comparison to the overall cohort of the original trial. Final cohort selection information and CONSORT diagram are shown in Figure 1. Of the 364 patients enrolled in the OC trial, 191 were randomized to receive TH to a target core temperature of 33.5°C. After applying our inclusion and exclusion criteria and excluding a further 6 subjects with mild encephalopathy according to contemporary Sarnat staging, 167 infants remained for analysis (Figure 1). The infants included in our analysis appeared to closely reflect the overall OC cohort; the only detectable difference between the final sub-cohort and the original OC cohort was a more balanced distribution of infant sex (51.5% vs 58.2% males, p = 0.001) and a slightly lower incidence of histologic chorioamnionitis (12.6% vs 17.6%, p = 0.02).
Table 1.
Demographics of included infants compared to all infants in the original trial cohort.
|
n (%) / median (IQR) |
p-Value | ||
|---|---|---|---|
| Current cohort | Original trial cohort | ||
| Total number of included infants | 167 | 364 | |
| Maternal characteristics | |||
| Age | 29.0 (23.0–33.5) | 28.0 (22.0–33.0) | 0.15 |
| Pregnancy complications | |||
| Diabetes | 20 (12.0) | 46 (12.6) | 0.54 |
| Hypertension/preeclampsia | 31 (18.6) | 73 (20.1) | 0.55 |
| Mode of delivery | |||
| Vaginal delivery | 54 (32.3) | 117 (32.1) | 0.93 |
| Cesarean section | 113 (67.7) | 247 (67.9) | |
| Labor complications | |||
| Decelerations/loss of fetal heart tones | 135 (80.8) | 282 (77.5) | 0.07 |
| Cord accident | 26 (15.6) | 49 (13.5) | 0.24 |
| Antepartum hemorrhage | 20 (12.0) | 40 (11.0) | 0.45 |
| Maternal hemorrhage | 29 (17.4) | 55 (15.1) | 0.16 |
| Shoulder dystocia | 11 (6.6) | 29 (8.0) | 0.18 |
| Clinical chorioamnionitis | 11 (6.6) | 28 (7.7) | 0.40 |
| Placental problems | 38 (22.8) | 80 (22.0) | 0.67 |
| Uterine rupture | 8 (4.8) | 22 (6.0) | 0.23 |
| Placenta | |||
| Placenta pathology performed | 80 (47.9) | 188 (51.6) | 0.07 |
| Histologic chorioamnionitis | 21 (12.6) | 64 (17.6) | 0.02* |
| Infant characteristics | |||
| Inborn | 56 (33.5) | 130 (35.7) | 0.18 |
| Gestational age (weeks) | 39 (37–40) | 39 (38–40) | 0.84 |
| Male sex | 86 (51.5) | 212 (58.2) | 0.001* |
| Birth weight (grams) | 3,270 (2,891–3,788) | 3,310 (2,949–3,784) | 0.33 |
| Apgar | |||
| 1 minutes | 1 (0–2) | 1 (0–2) | 0.47 |
| 5 minutes | 2 (1–3) | 2 (1–3) | 0.33 |
| 10 minutes | 4 (2–6) | 4 (3–6) | 0.46 |
| Resuscitation | |||
| Positive pressure ventilation | 161 (96.4) | 343 (94.2) | 0.15 |
| Intubation | 132 (79.0) | 286 (78.6) | 0.68 |
| Chest compressions | 75 (44.9) | 160 (44.0) | 0.71 |
| Continued resuscitation at 10 min | 146 (87.4) | 315 (86.5) | 0.71 |
| Cord blood | |||
| pH | 6.9 (6.8–7.0) | 6.9 (6.8–7.1) | 0.24 |
| Base deficit | 16 (11–20) | 16 (11–20) | 0.83 |
| Cord or first blood gas within 1 hour of life | |||
| pH ≤7.0 | 118 (70.7) | 264 (72.5) | 0.23 |
| Base deficit ≥16 | 113 (67.7) | 255 (70.1) | 0.28 |
| Sarnat stage at enrollment | |||
| Moderate | 133 (79.6) | 280 (76.9) | 0.15 |
| Severe | 34 (20.4) | 84 (23.1) | |
| Clinical seizures present at randomization | 53 (31.7) | 105 (28.8) | 0.11 |
| Infant sedated during qualifying exam | 49 (29.3) | 98 (26.9) | 0.48 |
| Age cooling started (hours) | 4.1 (3.1–5.0) | 4.1 (3.1–5.0) | 0.71 |
| Outcomes | |||
| Survival to discharge | 147 (88.0) | 315 (86.5) | 0.40 |
| Outcomes at 18–22 months | |||
| Death | 23 (13.8) | 56 (15.4) | 0.33 |
| Moderate-severe disability | 32 (19.2) | 54 (14.8) | 0.20 |
| Disability-free survival | 112 (67.1) | 237 (65.1) | 0.72 |
*Indicates a significant difference between cohorts. IQR: interquartile range.
Figure 1.
Patient inclusion and exclusion criteria. One subject was not treated as intended (therapeutic hypothermia at 32.0°C instead of 33.5°C) and excluded from this analysis, and one subject from the 32.0°C group was treated at 33.5°C and was included. ECMO = extracorporeal membrane oxygenation, HIE: hypoxic-ischemic encephalopathy.
Primary analyses by dichotomous primary outcome
Mattress temperature over time by primary outcome
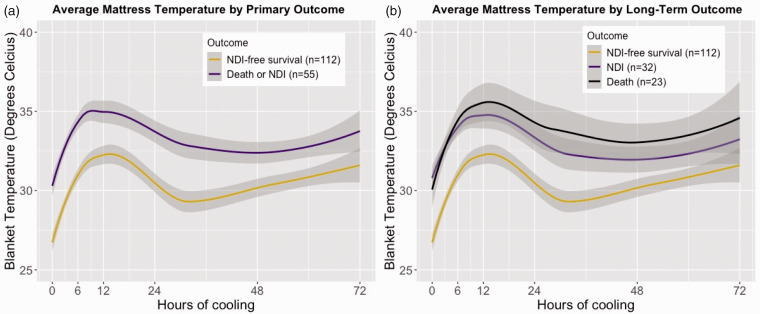
Of the included infants, n = 55 (33%) either died (n = 23, 14%) or survived with NDI (n = 32, 19%). Of the 23 deaths, 20 (87%) occurred during the initial hospitalization at a median age of 134 hours (IQR 89, 208), and 3 (13%) died after discharge. Infants with the composite primary outcome of death or NDI required a significantly higher MT to maintain a core body temperature of 33.5°C compared to infants who survived without NDI. Visual examination of MTs over time showed that average MT trajectories during TH followed a similar pattern with three distinct phases: 1) an increase over the first 6–12 h, 2) a decrease in the period around 12–30 h, and 3) a slow increase over time until 72 h (Figure 2). The average MT of infants who died or survived with NDI was consistently 2–3°C higher throughout the entire 72 h of TH (Figure 2(a)). While infants who died followed a similar trajectory as those who survived with NDI for the first 6 h of cooling, after 24 h the MT of those who died remained approximately 1°C higher compared to neonates who survived with NDI (Figure 2(b)).
Figure 2.
Average mattress temperature with 95% CI by combined primary outcome death or neurodevelopmental impairment (a) and split primary outcome (b) over the first 72 h of cooling, determined using the LOESS (locally estimated scatterplot smoothing) method.
Selection of independent confounding outcome predictors
Based on the temporal changes in average MT that were seen and the expected physiologic changes occurring in HIE, the 72 h of TH were divided into four distinct epochs. A median MT was determined for each infant within each TH epoch: 0–6 h, 6–24 h, 24–48 h, and 48–72 h. Infants who died or survived with NDI had significantly lower esophageal temperatures at initiation of TH as well as higher average MTs across all epochs. They were also more likely to have severe encephalopathy at randomization, documented seizures, evidence of end-organ dysfunction, and a higher likelihood of being exposed to anticonvulsant medications, particularly phenobarbital, inotropes, and morphine (Table 2). As many of these clinical factors may confound the association between MT and outcome, later analyses were adjusted for severity of encephalopathy, organ dysfunction score, documented seizures, exposure to phenobarbital, morphine, and inotropes, and treatment duration of TH.
Mattress temperature by outcome across cooling time epochs
Among infants who died or survived with NDI, adjusted average MTs were significantly higher across all epochs compared to the NDI-free survival group (supplemental Table s2). In the 0–6 h period, average adjusted MT was 3.4°C higher in the death/NDI group (95% CI 1.5–5.3°C, p < 0.001), and remained 1.5–2.1°C higher in the later epochs. Full model outputs for adjusted average MT within each epoch including the coefficients for all covariates are shown in supplemental Table s2.
Secondary analyses by temperature trajectories and outcomes
Epoch-specific cut-offs for outcome prediction
Epoch-specific final optimal MT cut-offs including the sensitivity and specificity for predicting outcome in the validation fold is shown in Table 3. In the 24–48 h epoch, two different MT cut-offs were selected in the same number of training folds, so the cut-off of ≥31.0°C, which had the higher sensitivity for predicting outcome in the associated validation fold (80% and 85% vs 29% and 45%), was selected. The final cut-offs for each epoch were ≥33.0°C (0–6 h), >31.0°C (6–24 h), >31.0°C (24–48 h), and ≥32.0°C (48–72 h). In fully adjusted models using the entire cohort, having a median MT above the selected cut-off in the 0–6 h, 24–48 h, and 48–72 h epochs was associated with a significantly increased aOR (95%CI) for death or NDI; 0–6 h: 17.0 (4.3–67.4); 24–48 h: 3.1 (1.3–7.5), and 48–72 h: 3.3 (3.3–8.4) (Table 4). Full model outputs including aORs for all variables in each model are displayed in supplemental Table s3.
Table 3.
Epoch-specific cut-ff cross-validation.
|
0–6 h |
6–24 h |
24–48 h |
48–72 h |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fold | Cut-off | Sens | Spec | Cut-off | Sens | Spec | Cut-off | Sens | Spec | Cut-off | Sens | Spec |
| (80% Training) | (20% Validation) | (80% Training) | (20% Validation) | (80% Training) | (20% Validation) | (80% Training) | (20% Validation) | |||||
| 1 | 33.0°C | 92% | 50% | 31.0°C | 75% | 27% | 32.5°C | 45% | 59% | 32.0°C | 64% | 50% |
| 2 | 33.0°C | 83% | 30% | 35.0°C | 25% | 76% | 29.0°C | 83% | 24% | 32.0°C | 64% | 36% |
| 3 | 33.0°C | 88% | 45% | 31.0°C | 100% | 29% | 32.5°C | 29% | 75% | 31.5°C | 100% | 45% |
| 4 | 33.0°C | 100% | 57% | 31.0°C | 100% | 38% | 31.0°C | 80% | 57% | 32.0°C | 90% | 57% |
| 5 | 33.0°C | 100% | 58% | 31.0°C | 100% | 0% | 31.0°C | 85% | 50% | 31.5°C | 85% | 38% |
| Cut-off | Sens | Spec | Cut-off | Sens | Spec | Cut-off | Sens | Spec | Cut-off | Sens | Spec | |
| Overall | 33.0°C | 93% | 49% | 31.0°C | 91% | 30% | 31.0°C | 77% | 54% | 32.0°C | 79% | 48% |
Epoch-specific cut-offs to predict the primary outcome were developed using 5-fold cross-validation. The data were randomly split into five folds such that each infant for whom at least 50% of expected MTs were available in that epoch appeared in four training folds and one validation fold. Within each fold in each epoch, average MT cut-offs were selected optimizing for the sum of sensitivity and specificity to predict outcome in unadjusted models. For each of the five training folds, the optimal MT cut-off and the sensitivity and specificity for predicting outcome in the validation fold is shown. To select a final cut-off for each epoch for outcome prediction, the cut-off identified in the most folds was selected. In the 24–48 h epoch two different MT cut-offs were selected in the same number of training folds (≥32.5°C and ≥31°C, n = 2 each), so the cut-off of ≥31°C was selected as it had a higher sensitivity for predicting the primary outcome in the associated validation folds. Overall sensitivity and specificity for the cut-offs across the entire cohort are also shown.
Table 4.
Epoch-specific mattress temperature (MT) cut-offs to predict primary outcome.
|
Optimal cut-off |
Infants above cut-point (all data) |
||
|---|---|---|---|
| Time epoch | Temperature (°C) | n = (%) | OR for death or NDI (95% CI) |
| 0–6 h | ≥33.0 | 107 (65.2) | 17.0 (4.3–67.4) |
| 6–24 h | ≥31.0 | 104 (77.0) | 3.3 (0.9–12.3) |
| 24–48 h | ≥31.0 | 92 (56.4) | 3.1 (1.3–7.5) |
| 48–72 h | ≥32.0 | 99 (60.7) | 3.3 (1.3–8.4) |
Epoch-specific cut-offs of average MT to predict primary outcome were determined within each epoch separately due to the fluctuating nature of average MT over time (Figure 2). Cut-offs were determined using the cutpointr library in R with five-fold cross validation. Adjusted odds ratios (aOR) with 95% CI are provided for the whole dataset after adjusting for severity of encephalopathy at enrollment, length of TH treatment, exposure to phenobarbital, morphine, or inotropes, and degree of organ dysfunction. aORs with the confidence intervals that does not cross 1 are highlighted in bold. NDI: neurodevelopmental impairment.
Trajectories of mattress temperature over time and long-term outcome
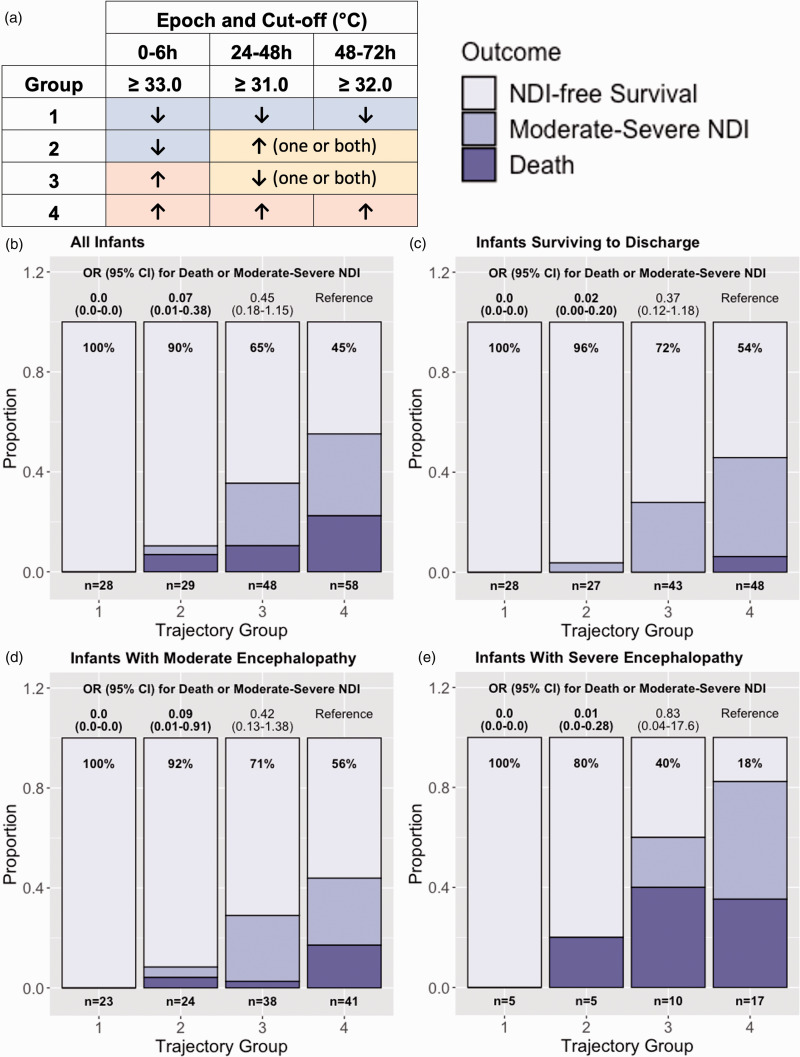
In an exploratory attempt to predict outcome based on MT trajectories, we considered whether being above or below target cut-offs at multiple time points might provide greater granularity in outcome prediction. Infants were thus categorized based on being above or below the cut-offs within the 0–6 h, 24–48 h, and 48–72 h epochs. Infants were classified into four groups based on whether they were: 1) below the cut-off at all three time points (n = 28), 2) below the cut-off at 0–6 h but above one or both of the 24–48 h and 48–72 h cut-offs (n = 29), 3) above the 0–6 h cut-off but below at least one of the 24–48 h or 48–72 h cut-offs (n = 48), and 4) above all three cut-offs (n = 58) (Figure 3(a)). Those in Group 1 had 100% NDI-free survival, with increasing risk of death or NDI across each subsequent group (Figure 3(b)). Using Group 4 as the reference, Group 1 had a significantly lower aOR for death or NDI (0.0, 95% CI 0.0–0.0) as did Group 2 (0.06; 0.01–0.38), with a non-significantly decreased aOR in Group 3 (0.45; 0.18–1.15).
Figure 3.
(a) Infants were classified into four groups: 1) those that were below the cut-off at all three time points, 2) below the cut-off at 0–6 h but above one or both the 24–48 h and 48–72 h epochs, 3) above the 0–6 h cut-off but below at least one of the 24–48 h or 48–72 h cut-offs, and 4) above all three cut-offs. Adjusted odds ratio (aOR) for death or moderate-severe neurodevelopmental impairment (NDI) by 0–6 h, 24–48 h, and 48–72 h temperature cut-offs in all infants (b), in those surviving to discharge (c), and separately in those with moderate (d) and severe (e) encephalopathy. Percentages indicate those with NDI-free survival within each Continued.trajectory group. Using Group 4 as the reference group, Groups 1 and 2 both had significantly reduced odds for death or NDI in all infants as well as those surviving to discharge, with a nonsignificant decrease in odds in Group 3. Regardless of severity of encephalopathy, being below all three cut-offs (Group 1) was associated with 100% NDI-free survival. All models were adjusted for exposure to phenobarbital, inotropes, and morphine, documented seizures, severity of encephalopathy (except D and E), duration of therapeutic hypothermia treatment, and degree of organ dysfunction.
Similar results were seen in the 146 infants surviving to discharge (Figure 3(c)). Three infants died after discharge, all of whom were in Group 4, meaning that they were all above all three MT cut-offs. When examining trajectory group and outcome by level of encephalopathy (Figure 3(d) to (e)), infants who were below all three cut-points had 100% NDI-free survival regardless of level of encephalopathy. The ability of trajectory group to separately predict outcome in infants with moderate encephalopathy (n = 129, Figure 3(d)) and severe encephalopathy (n = 37, Figure 3(e)) mirrored that of the cohort as a whole.
A sensitivity analysis including all 191 infants treated at 33.5°C, adjusting for level of encephalopathy in the original trial documentation, did not alter the results (supplemental Figure s1): using Group 4 as the reference, 100% NDI-free survival was seen in Group 1, a significant 92% reduction in odds of death/NDI was seen in group 2 (aOR 0.08; 95% CI 0.01–0.44), and a non-significant 56% reduction in odds of death/NDI was seen in Group 3 (aOR 0.44; 95% CI 0.17–1.13).
Full model outputs including coefficients for all covariates from the results presented in Figure 3 and supplemental Figure s1 are presented in supplemental Table s4.
Discussion
This secondary analysis of the OC trial adds further evidence to the emerging literature supporting temperature dysregulation as a strong biomarker of outcomes for newborns with HIE. Infants with a combined outcome of death or moderate-severe NDI required less active cooling and therefore a significantly higher MT during TH compared to those who survived without NDI. This association was detectable and strongly predictive of outcome in fully adjusted multivariable models within the first 6 h of cooling. Infants who required a MT of 33.0°C or higher during the first 6 h of TH had an aOR of 17.0 for death or moderate-severe NDI even after adjusting for severity of overall illness including additional end-organ involvement, highlighting that MT may provide a novel biomarker that is strongly predictive of outcome without requiring any additional measurements or sample collection. Importantly, however, it must be acknowledged that this secondary analysis of a convenience dataset using older cooling technologies is not intended to provide a comment on the exact MT cut-offs that may predict outcome in all infants undergoing TH in modern NICUs. Instead, it provides greater support for the development of thermoregulatory biomarkers using modern equipment in future appropriately designed clinical trials.
We have previously shown in a smaller cohort that MT during TH correlates with MRI injury and short-term outcome; however, correlation with standardized long-term outcome was not available. 21 Instead, the Weeke MRI injury score, which correlates with neurodevelopmental outcome at 2 years of age, was used as a surrogate.21,26,27 To validate our findings, we applied the same hypothesis to a larger dataset with standardized long-term outcome data and were able to confirm our initial findings. We then examined the cohort with an unfavorable outcome in more detail to better understand whether the higher MT trajectory was mainly driven by infants who died. Our analysis showed that while infants who died required the highest MTs, newborns who survived with moderate-severe NDI also required significantly higher MTs compared to those who survived NDI-free. Similarly, when predicting death or NDI in infants with moderate or severe encephalopathy separately, MT temperature trajectory remained predictive of outcome regardless of grade of encephalopathy, particularly in those whose MT remained below the cut-offs throughout the entire 72 h period who had 100% NDI-free survival.
There is strong biologic plausibility that temperature dysregulation reflects the degree of brain injury and thus by proxy could predict later outcome, as thermoregulatory circuits are located adjacent to the central deep grey matter, an area that is commonly affected in severe HIE.28–30 In human infants with perinatal asphyxia, a spontaneous decrease in core temperature within a few hours after birth followed by a slow recovery period was first described by Burnard and Cross in 1958. 19 This observation was confirmed in a more recent HIE cohort by Enweronu-Laryea et al., with a greater degree of spontaneous hypothermia in neonates with an abnormal outcome, and even greater disturbances in infants who died. 20 Similar relationships between spontaneous hypothermia and degree of brain injury had been shown in animal models. For instance, rat pups exposed to hypoxic-ischemic brain injury had a spontaneous decrease of their core body temperature 1 h after the insult that slowly recovered within 24 h, and their degree of hypothermia was associated with neuropathological injury on day 7. 31
Despite the supporting evidence, the exact physiologic mechanisms that drive the differences in MT by outcome group are not entirely clear. Healthy infants underdoing the fetal-neonatal transition maintain body temperature after exposure to the cold outside environment in large part by activating non-shivering thermogenesis (NST) via the activation of brown adipose tissue (BAT). 32 Heat production by BAT requires oxygen in order for the electron transport chain to pump protons into the intermembrane space before they can be dissipated by uncoupling proteins, which generates heat. 33 A loss of core body temperature early after asphyxia may therefore be explained by initial hypoxia. However, later disruptions in thermoregulation in infants with HIE are likely due to some combination of direct injury to the thermoregulatory centers integral to the hypothalamic-pituitary-adrenal axis, loss of sympathetic tone to drive NST (through noradrenaline), and loss of muscle tone and capacity for shivering. 34 Others have also shown that more severe encephalopathy is associated with higher central brain temperatures, which may result in a reduction in the temperature set-point in the hypothalamus. 35 However, MTs were not provided in the brain temperature studies, therefore it remains unclear if the higher brain temperatures in infants with more severe encephalopathy is due to endogenous (patho)physiology or because the cooling mattress the infants were lying on had a higher temperature, as would be expected based on our data.
Another important area to consider when examining the relationship between thermoregulatory parameters (or their proxies, such as MT) and outcome in infants with HIE are medications administered to infants with encephalopathy, particularly anticonvulsants. Perhaps the best example of this is phenobarbital, which was by far the most common anticonvulsant used in the dataset. In animal models, low doses of GABA agonists such as barbiturates (e.g. phenobarbital) and benzodiazepines (e.g. diazepam) produce increases in body temperature, with decreases seen at higher doses. 36 As first-line anticonvulsant drugs are often GABA-agonists, this may be an ongoing confounder with respect to the relationship between MT and outcome unless it is appropriately accounted for. Treatment with phenobarbital has also been associated with more rapid cooling during the induction phase, but even after adjusting for phenobarbital exposure in all final MT models, MT was still strongly predictive of outcome. 37
Though spontaneous hypothermia appears to correlate with degree of injury in infants with HIE, it is difficult to routinely measure this in the modern era of TH in which core temperature is actively controlled as soon as possible after birth. Instead, the MT required to achieve a standardized TH temperature can be used as an indirect measurement of these physiologic disturbances. Due to the evolving nature of the injury, the MT required to achieve a core temperature of 33.5°C also evolves over the TH period, and the cut-offs derived to predict abnormal outcome changed as well. In our work, the time epoch between 6–24 h appeared to be the least clear with respect to outcome prediction, which may be due to inter-individual differences in timing of onset of secondary injury phases.21,38–40 The distinction became more robust with a stronger predictive signal during the 24–48 h and 48–72 h epochs, when infants with a MT above the cut-offs had a significantly higher aOR of 3.1 and 3.3, respectively, for an unfavorable outcome. While both the MT difference by outcome and aOR for death or NDI were most notable in the 0–6 h epoch when applied to the entire cohort, the predictive signal in the later time epochs was still remarkably strong relative to other studies of biomarkers in neonates with HIE. 41
In addition to discrete cut-offs within epochs, we observed that MT trajectory over time was strongly predictive of outcome. Infants who maintained MTs below the cut-offs throughout all time epochs had a universally favorable outcome, which is a rarity in studies of HIE biomarkers. In fact, this meant that we were unable to use this group as the reference group in our trajectory analyses, as it is impossible to calculate an aOR with a denominator of zero. By comparison, infants who maintained their MTs above all cut-offs had a more than 50% chance of adverse outcome. Infants who had MTs both above and below various cut-offs had intermediate risk, though the 0–6 h cut-off appeared to have the greatest predictive ability with those above that cut-off having a worse outcome than those below it regardless of later temperatures. This observation highlights the weight of critical time periods following an acute perinatal event, emphasizes the significant role the first hours of TH play, and potentially directs future investigations toward this time-period for additional adjunct neuroprotective interventions.
Our results are in line with a recent study that posed a similar question, utilizing data from the original NICHD Hypothermia trial combined with a smaller subset of OC trial participants to demonstrate an association between blanket temperatures during TH and outcome at 18–22 months by analyzing outcomes based on number of MT readings above 33.5°C during the maintenance phase of TH only. 42 Critically, this replication of findings is important if MT is to be developed as a prognostic marker in clinical practice. Though there is some overlap in the data used, our approach was much more stringent as we opted to only include infants from one trial to minimize the effect of changing care practice over time, and we chose a different analytic approach. 43 This included: 1) taking into account the entire duration of TH, 2) including all MT values from all infants, 3) developing and validating epoch specific cut-offs based on the natural evolution of MTs and injury over time, and 4) evaluating the prognostic value of simple combinatorial trajectories. We also found our most notable predictive signal during the 0–6 h cooling period that was excluded in the other analysis. We therefore believe that this is the first analysis demonstrating the strong ability of MTs to predict long-term outcome as early as 6 hours into the treatment course, highlighting its potential as a biomarker of injury.
The ideal biomarker should be quantifiable, reliable, reproducible, easy to obtain and interpret with minimal training, and in the case of neonates with HIE provide an early window into severity of injury and long-term outcome. The search for early biomarkers in neonates with HIE has been the subject of numerous studies, but accurate biomarkers have been notoriously difficult to obtain, are too non-specific, or have poor reproducibility across different studies.10,44 EEG characteristics have emerged as promising physiologic biomarkers, particularly EEG background pattern during TH which when normal at 6 h and throughout the first 24 h of life is universally associated with a good outcome, whereas a lack of normal background by 72 h is highly associated with an unfavorable outcome.17,45,46 Chalak et al. studied the impact of seizures during rewarming on long-term outcome in a sub-cohort of the OC trial and found that infants with seizures during rewarming were more likely to have an abnormal outcome at 18–22 months compared to those who did not (46% vs 25%) with a risk ratio of 1.72. 47 Similarly, the Lactate to N-Acetyl Aspartate ratio measured by magnetic resonance spectroscopy has shown promising results as a predictor of outcome. 48 However, both, EEG monitoring and MRS require additional equipment and expert interpretation, which limits their use as biomarkers.
Our work shows strong and early predictive ability using MT as a physiologic biomarker. It is also inexpensive and easily interpretable by non-experts, and therefore applicable in lower-resource settings. Using MT alone at single time points is clearly not going to be adequate for predicting long-term outcome; however, when coupled with simple clinical variables and modeled as temperature trajectories over time, predictive accuracy improved. For example, a recent study showed that adding measures of lactate dehydrogenase (LDH) provided additional prognostic ability when combined with MRI data. 49 While not all necessary information may be available in the first 0–6 h of cooling, a risk calculator including other clinical variables could be updated with MTs in real time, providing useful prognostic information within the standard TH period.
This secondary analysis has several limitations. This dataset is from a trial in a different era of TH during which MRI was not performed in all patients, EEG recording throughout cooling was not standard of care, and outcome was only available at 18–22 months of age. Furthermore, while a large number of confounding factors could be taken into account as having an effect on temperature regulation and perfusion, the dataset did not provide hemodynamic information that is now more accessible, such as serial assessments of cardiac function by bedside echocardiography. In addition, MT recording intervals in this dataset were protocolized to capture overcooling within the first few hours of TH rather than to track physiologic changes in the neonate. This resulted in more sampling during the first 24 hours, particularly the first 6 hours, which could have potentially favored predictive ability in the earlier time window due to a greater density of available data. Lastly, the devices used during the OC trial have been largely replaced by more contemporary technology which now allows for shorter feedback loops and tighter temperature control. The longer feedback loops and larger temperature swings observed historically could have confounded the derived cut-points to a certain degree, especially during the later stages of TH when temperatures where only recorded every 4 hours. However, given that we were able to replicate findings obtained from a smaller cohort using continuous patient and MT recordings, the findings shown in this study provide robust evidence for use of MT measurement as an indirect physiologic biomarker and strong predictor of long-term outcomes.
Conclusion
This study confirms the value and utility of cooling MT as an early prognostic physiologic biomarker in neonates undergoing TH with moderate-severe HIE, providing a free and easily accessible metric to assist clinical decision making and longer-term prognostication for clinicians and families.
Supplemental Material
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231162174 for Temperature dysregulation during therapeutic hypothermia predicts long-term outcome in neonates with HIE by Ulrike Mietzsch, John J Flibotte, Janessa B Law, Mihai Puia-Dumitrescu, Sandra E Juul and Thomas R Wood in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
We would like to thank Seetha Shankaran, the principal investigator of the Optimizing Cooling Trial, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network for designing, sponsoring, and executing the Optimizing Cooling trial, NCT01192776. We acknowledge NICHD DASH for providing the Optimizing Cooling Strategies at <6 Hours of Age for Neonatal Hypoxic-Ischemic Encephalopathy data that was used for this research.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: UM and TW developed the concept and designed of the project, acquired, analyzed, and interpreted the data, drafted and critically revised the article for important intellectual content, and approved the final.
JF contributed to the concept of the project, data acquisition and interpretation, critically revised the article for important intellectual content, and approved the final version.
JL and MPD contributed interpreting the data and critically revised the article for important intellectual content and approved the final version.
SJ critically revised the article for important intellectual content and approved the final version.
Supplementary material: Supplemental material for this article is available online.
Data availability
Data from the Optimizing Cooling Trial are available through the Eunice Kennedy Shriver National Institute of Child Health and Human Development Data and Specimen Hub (https://dash.nichd.nih.gov/study/228264).
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N.Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 2010; 86: 329–338. [DOI] [PubMed] [Google Scholar]
- 2.Lee AC, Kozuki N, Blencowe H, et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res 2013; 74Suppl 1: 50–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005; 353: 1574–1584. [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009; 361: 1349–1358. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005; 365: 663–670. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013; 1: CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thoresen M, Tooley J, Liu X, et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 2013; 104: 228–233. [DOI] [PubMed] [Google Scholar]
- 8.Laptook AR, Shankaran S, Tyson JE, et al. Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with Hypoxic-Ischemic encephalopathy: a randomized clinical trial. JAMA 2017; 318: 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankaran S, Laptook AR, Tyson JE, et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2012; 160: 567–572 e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalak L, Hellstrom-Westas L, Bonifacio S, et al. Bedside and laboratory neuromonitoring in neonatal encephalopathy. Semin Fetal Neonatal Med 2021; 26: 101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendler MR, Mendler I, Hassan MA, et al. Predictive value of Thompson-score for long-term neurological and cognitive outcome in term newborns with perinatal asphyxia and Hypoxic-Ischemic encephalopathy undergoing controlled hypothermia treatment. Neonatology 2018; 114: 341–347. [DOI] [PubMed] [Google Scholar]
- 12.Massaro AN, Wu YW, Bammler TK, et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J Pediatr 2018; 194: 67–75 e61. [DOI] [PubMed] [Google Scholar]
- 13.Lv H, Wang Q, Wu S, et al. Neonatal hypoxic ischemic encephalopathy-related biomarkers in serum and cerebrospinal fluid. Clin Chim Acta 2015; 450: 282–297. [DOI] [PubMed] [Google Scholar]
- 14.Ahearne CE, Boylan GB, Murray DM.Short and long term prognosis in perinatal asphyxia: an update. World J Clin Pediatr 2016; 5: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walas W, Wilińska M, Bekiesińska-Figatowska M, et al. Methods for assessing the severity of perinatal asphyxia and early prognostic tools in neonates with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia. Adv Clin Exp Med 2020; 29: 1011–1016. [DOI] [PubMed] [Google Scholar]
- 16.Murray DM.Biomarkers in neonatal hypoxic-ischemic encephalopathy – review of the literature to date and future directions for research. Handb Clin Neurol 2019; 162: 281–293. [DOI] [PubMed] [Google Scholar]
- 17.Murray DM, Boylan GB, Ryan CA, et al. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics 2009; 124: e459–467. [DOI] [PubMed] [Google Scholar]
- 18.Rath C, Rao S, Suryawanshi P, et al. Does abnormal doppler on cranial ultrasound predict disability in infants with hypoxic-ischaemic encephalopathy? A systematic review. Dev Med Child Neurol 2022; 64: 1202–1213. [DOI] [PubMed] [Google Scholar]
- 19.Burnard ED, Cross KW.Rectal temperature in the newborn after birth asphyxia. Br Med J 1958; 2: 1197–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enweronu-Laryea C, Martinello KA, Rose M, et al. Core temperature after birth in babies with neonatal encephalopathy in a Sub-Saharan African hospital setting. J Physiol 2019; 597: 4013–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mietzsch U, Radhakrishnan R, Boyle FA, et al. Active cooling temperature required to achieve therapeutic hypothermia correlates with short-term outcome in neonatal hypoxic-ischaemic encephalopathy. J Physiol 2020; 598: 415–424. [DOI] [PubMed] [Google Scholar]
- 22.Wu YW, Mathur AM, Chang T, et al. High-dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics 2016; 137: e2016-0191. [DOI] [PubMed] [Google Scholar]
- 23.Shankaran S, Laptook AR, Pappas A, et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA 2014; 312: 2629–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shankaran S, Laptook AR, Pappas A, et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with Hypoxic-Ischemic encephalopathy: a randomized clinical trial. JAMA 2017; 318: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. R version 4.1.2 (accessed 01 November 2021).
- 26.Weeke LC, Groenendaal F, Mudigonda K, et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J Pediatr 2018; 192: 33–40 e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szakmar E, Meunier H, El-Dib M, et al. Interobserver reliability of an MR imaging scoring system in infants with hypoxic-ischemic encephalopathy. AJNR Am J Neuroradiol 2021; 42: 969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayasinghe D.Innate hypothermia after hypoxic ischaemic delivery. Neonatology 2015; 107: 220–223. [DOI] [PubMed] [Google Scholar]
- 29.Wu TW, Tamrazi B, Hsu KH, et al. Cerebral lactate concentration in neonatal hypoxic-ischemic encephalopathy: in relation to time, characteristic of injury, and serum lactate concentration. Front Neurol 2018; 9: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massaro AN, Kadom N, Chang T, et al. Quantitative analysis of magnetic resonance images and neurological outcome in encephalopathic neonates treated with whole-body hypothermia. J Perinatol 2010; 30: 596–603. [DOI] [PubMed] [Google Scholar]
- 31.Wood T, Hobbs C, Falck M, et al. Rectal temperature in the first five hours after hypoxia-ischemia critically affects neuropathological outcomes in neonatal rats. Pediatr Res 2018; 83: 536–544. [DOI] [PubMed] [Google Scholar]
- 32.Aherne W, Hull D.Brown adipose tissue and heat production in the newborn infant. J Pathol Bacteriol 1966; 91: 223–234. [DOI] [PubMed] [Google Scholar]
- 33.Shamsi F, Wang CH, Tseng YH.The evolving view of thermogenic adipocytes – ontogeny, niche and function. Nat Rev Endocrinol 2021; 17: 726–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kooijman S, van den Heuvel JK, Rensen PCN.Neuronal control of brown fat activity. Trends Endocrinol Metab 2015; 26: 657–668. [DOI] [PubMed] [Google Scholar]
- 35.Wu TW, McLean C, Friedlich P, et al. Brain temperature in neonates with hypoxic-ischemic encephalopathy during therapeutic hypothermia. J Pediatr 2014; 165: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 36.Olds ME, Gardner M.Effects of diazepam and phenobarbital on self-stimulation in posterior hypothalamic and preoptic regions and on the thermoregulatory responses to rewarding brain stimulation. Neuropharmacology 1976; 15: 103–115. [DOI] [PubMed] [Google Scholar]
- 37.Sant'Anna G, Laptook AR, Shankaran S, et al. Phenobarbital and temperature profile during hypothermia for hypoxic-ischemic encephalopathy. J Child Neurol 2012; 27: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denihan NM, Boylan GB, Murray DM.Metabolomic profiling in perinatal asphyxia: a promising new field. Biomed Res Int 2015; 2015: 254076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wassink G, Davidson JO, Lear CA, et al. A working model for hypothermic neuroprotection. J Physiol 2018; 596: 5641–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferriero DM.Neonatal brain injury. N Engl J Med 2004; 351: 1985–1995. [DOI] [PubMed] [Google Scholar]
- 41.Bona E, Andersson AL, Blomgren K, et al. Chemokine and inflammatory cell response to hypoxia-ischemia in immature rats. Pediatr Res 1999; 45: 500–509. [DOI] [PubMed] [Google Scholar]
- 42.Flibotte J, Laptook AR, Shankaran S, et al. Blanket temperature during therapeutic hypothermia and outcomes in hypoxic ischemic encephalopathy. J Perinatol 2022; 42: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonifacio SL, McDonald SA, Chock VY, et al. Differences in patient characteristics and care practices between two trials of therapeutic hypothermia. Pediatr Res 2019; 85: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietrick B, Molloy E, Massaro AN, et al. Plasma and cerebrospinal fluid candidate biomarkers of neonatal encephalopathy severity and neurodevelopmental outcomes. J Pediatr 2020; 226: 71–79.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sewell EK, Vezina G, Chang T, et al. Evolution of Amplitude-Integrated electroencephalogram as a predictor of outcome in term encephalopathic neonates receiving therapeutic hypothermia. Am J Perinatol 2018; 35: 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goswami I, Guillot M, Tam EWY.Predictors of long-term neurodevelopmental outcome of hypoxic-ischemic encephalopathy treated with therapeutic hypothermia. Semin Neurol 2020; 40: 322–334. [DOI] [PubMed] [Google Scholar]
- 47.Chalak LF, Pappas A, Tan S, et al. Association of increased seizures during rewarming with abnormal neurodevelopmental outcomes at 2-year follow-up: a nested multisite cohort study. JAMA Neurol 2021; 78: 1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lally PJ, Montaldo P, Oliveira V, et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol 2019; 18: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thoresen M, Jary S, Walloe L, et al. MRI combined with early clinical variables are excellent outcome predictors for newborn infants undergoing therapeutic hypothermia after perinatal asphyxia. EClinicalMedicine 2021; 36: 100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jcb-10.1177_0271678X231162174 for Temperature dysregulation during therapeutic hypothermia predicts long-term outcome in neonates with HIE by Ulrike Mietzsch, John J Flibotte, Janessa B Law, Mihai Puia-Dumitrescu, Sandra E Juul and Thomas R Wood in Journal of Cerebral Blood Flow & Metabolism
Data Availability Statement
Data from the Optimizing Cooling Trial are available through the Eunice Kennedy Shriver National Institute of Child Health and Human Development Data and Specimen Hub (https://dash.nichd.nih.gov/study/228264).