Abstract
The social behavior network (SBN) has provided a framework for understanding the neural control of social behavior. The original SBN hypothesis proposed this network modulates social behavior and should exhibit distinct patterns of neural activity across nodes, which correspond to distinct social contexts. Despite its tremendous impact on the field of social neuroscience, no study has directly tested this hypothesis. Thus, we assessed Fos responses across the SBN of male prairie voles (Microtus ochrogaster). Virgin/non-bonded and pair bonded subjects were exposed to a sibling cagemate or pair bonded partner, novel female, novel male, novel meadow vole, novel object, or no stimulus. Inconsistent with the original SBN hypothesis, we did not find profoundly different patterns of neural responses across the SBN for different contexts, but instead found that the SBN generated significantly different patterns of activity in response to social novelty in pair bonded, but not non-bonded males. These findings suggest that non-bonded male prairie voles may perceive social novelty differently from pair bonded males or that SBN functionality undergoes substantial changes after pair bonding. This study reveals novel information about bond-dependent, context-specific neural responsivity in male prairie voles and suggests that the SBN may be particularly important for processing social salience. Further, our study suggests there is a need to reconceptualize the framework of how the SBN modulates social behavior.
Introduction
Displaying context-appropriate social behavior requires the integration of sensory stimuli, assessment of the social and internal state, evaluation of that information to estimate the best possible output, and finally action in the form of complex motor outputs. Needless to say, this is a computationally complex process. During different social encounters, an animal’s response is rarely attributable to a single region of interest or the result of a simple brain region to behavior linear relationships. Rather, the computational load to process such information relies on a distributed network of brain structures communicating in concert to cohesively modulate the interplay of neural activity across the brain.
The Social Behavior Network (SBN) was originally proposed by Newman (1999) as a framework for how a collection of brain areas could orchestrate rodent social behavior across varying social contexts. The SBN hypothesis proposed that different patterns of neural activity would result from exposure to distinct social contexts. At the core of the SBN are seven key brain regions: the medial extended amygdala (medial amygdala (MeA); bed nucleus of the stria terminalis (BST)), the lateral septum (LS), the preoptic area (POA), the anterior hypothalamus (AH), the ventromedial hypothalamus (VMH), and the periaqueductal gray (PAG). These nodes were chosen based on three specific criteria: reciprocal anatomical connectivity, presence of gonadal hormone receptors, and a priori demonstration of being involved in more than one social behavior (Newman, 1999). Together, these nodes were hypothesized to modulate social behavior by collectively exhibiting distinct “neural landscapes” across different social contexts (Newman, 1999). For example, an animal interacting in a mating context might have increased LS and POA activity to allow for prosocial and sexual behavior but decreased AH and MeA activity to suppress aggression (Newman, 1999; Goodson, 2005). Conversely, in a territorial context, the LS and POA might exhibit low activity whereas the AH and MeA may increase activity (Newman, 1999; Goodson, 2005).
Goodson (2005) advanced the definition of the network to account for additional considerations, including: (a) refocusing the SBN as a core network of nodes integral for social behavior processing, but incorporating support from cortical and reward areas, (b) broadening social behaviors to include responses to social stress and vocal communication, and (c) centering the presence of peptidergic neurons and neuropeptide receptors as criteria for inclusion in the network. The SBN has since provided a valuable framework for understanding the social brain across social domains in an array of taxa (Crews, 2003; Forlano et al., 2005; Goodson, 2005; Goodson et al., 2005b; Crews, 2008; Kelly and Goodson, 2013; Zheng et al., 2013; Kabelik et al., 2018; Reppucci et al., 2018; Butler et al., 2020; Duque-Wilckens et al., 2020; Tripp et al., 2020; Grieb et al., 2021). Furthermore, the SBN has been integrated with other brain networks hypothesized to modulate other core functions such as reward (O’Connell and Hofmann, 2011), aggression (Lischinsky and Lin, 2020), social salience (Johnson et al., 2017), pair bonding (Walum and Young, 2018), and socio-spatial memory (Ophir, 2017). Arguably the most popular expansion of the SBN is the social decision making network (SDMN), which proposed that animals regulate complex, adaptive behavior via interactions between the SBN and the mesolimbic reward system and that these circuits together form a larger network (O’Connell and Hofmann, 2011).
A few studies have examined how social context influences neural activity patterning across the social decision making network (SDMN) – an expansion of the SBN that includes reward circuitry. For example, zebrafish in varying agonistic contexts exhibit differential patterning depending on whether they lost, won, or had an unclear outcome of a fight (Teles et al., 2015). Additionally, zebrafish that won or lost a fight generally exhibit greater neural activity across SDMN nodes compared to zebrafish that did not engage in a social interaction, and network centrality analysis revealed both shared and sex-specific hubs for distinct social conditions (i.e., win, lose, control), suggesting that context and sex elicit differential activation patterns of the SDMN (Scaia et al., 2022). Notably, these studies test subjects in agonistic interactions, and thus it remains unclear whether the SDMN may exhibit differential patterns in non-agonistic social contexts. Further, the SDMN is an expansion of the SBN, and we believe that it is important to test the foundation in which hypotheses have been built upon. To our knowledge, the original assumption that the SBN should produce distinct patterns of neural activation in response to different social contexts (positive, neutral, and negative) has never been directly tested. Researchers have compared patterns of activity across nodes of the SBN, but these studies were limited to comparing only two social contexts (Maney et al., 2008; Petersen et al., 2021) or comparing species within a single social context (Goodson et al., 2005a). Although studies such as these have provided highly valuable insights into the roles of SBN nodes, questions persist about the collective functioning of the SBN across social (and non-social) contexts. Here we explicitly test the hypothesis that differential patterns of neural responses across the nodes of the SBN are associated with different social contexts. Specifically, we compare SBN neural responses across five ecologically-relevant social contexts and two nonsocial contexts in male prairie voles (Microtus ochrogaster).
Prairie voles have emerged as a model system for studying the neural mechanisms of social behavior (Carter, 1998; Tabbaa et al., 2016; Bosch and Young, 2018; Potretzke and Ryabinin, 2019). Much of this progress has been built upon a strong understating of the natural history and behavioral ecology of this socially monogamous and biparental rodent species (Madrid et al., 2020). Because prairie voles often form long-lasting pair bonds between male-female partners (Getz et al., 1981) and exhibit neural changes and selective aggression toward novel conspecifics after pair bonding (Carter et al., 1995; Wang et al., 2013; Lopez-Gutierrez et al., 2021), we examined whether SBN neural responsivity differs in non-bonded (i.e., virgin) and pair bonded male prairie voles. To specifically test the hypothesis that patterns of neural activity across the SBN differ based on social context, we used a relatively simple multivariate (multi-region) classification approach followed by random permutation testing to assess statistical significance.
Methods
Animals
Prairie voles used in this study were obtained from the Ophir Lab breeding colony at Cornell University. All subjects (PND 100–200) were from breeding pairs that were offspring of wild caught animals we captured in Champagne County, Illinois, USA. Animals were pair housed in standard polycarbonate rodent cages (29 × 18 × 13cm) lined with Sani-chip bedding and provided nesting material. Animals were kept on a 14L:10D cycle and were provided with rodent chow (Laboratory Rodent Diet 5001, LabDiet, St. Louis, MO, USA) and water ad libitum. Ambient temperature was maintained at 20±2°C. Sex was assessed and assigned at weaning based on differences in external genitalia. All procedures were approved by the Institutional Animal Care and Use Committee of Cornell University (2013–0102).
Experimental design
Our study aimed to assess context-dependent neural responses across the SBN in adult male prairie voles that were either pair bonded or non-bonded (i.e., Group). Female partners were moved into separate cages and were primed with soiled bedding of future male mates for 2 days prior to pairing. Males in the Pair Bonded group were then placed into the home cages of the females and allowed to cohabitate for 1 week prior to testing. Males in the Non-bonded group remained housed with a same-sex sibling; any additional same-sex siblings were removed from the subjects’ cages and rehoused so that all subjects were housed with one conspecific.
To examine neural responses across the SBN to varying contexts, we assessed expression of the immediate early gene (IEG) c-Fos in subjects after exposing them to i) a familiar conspecific which was either their pair bond partner or their same-sex sibling cagemate, ii) an age-matched novel female conspecific, iii) an age-matched novel male conspecific, iv) a novel heterospecific meadow vole (Microtus pennsylvanicus; serving as an ecologically-relevant social stimulus), v) a novel object (a rubber duck), or vi) no stimulus (i.e., isolation). These different exposure conditions are referred to as Context. We placed all subjects into a novel standard rodent cage and exposed males to one of the aforementioned stimulus conditions. Due to the experimental design, only pair bonded subjects could be exposed to a familiar pair bonded partner, whereas only non-bonded subjects could be exposed to a familiar same-sex cagemate. Sample sizes for each group were n = 12 non-bonded males and n = 12 pair bonded males, totaling n = 144 males.
All tests were video recorded with a camera positioned above the cage for subsequent behavioral scoring. Stimulus animals received a zip-tie collar the day prior to testing for ease of identification in video analysis. To begin, subjects were transferred into a novel cage containing clean Sani-chip bedding and were allowed to habituate for 30 min. Because this experiment sought to measure IEG neural responses, we included this habituation time to reduce the likelihood that neural responses would be attributable to the stress of handling and/or investigating a novel environment. After the habituation phase, the stimulus was placed inside the test cage for 30 min. During the 30 min test period, the subject and stimulus were able to freely interact, and we assessed the latency to approach the stimulus as well as prosocial and aggressive behavior. Next, the stimulus was removed from the test cage. The subject remained in the test cage for an additional 60 minutes. Subjects were then immediately perfused such that perfusion occurred 90 min after the introduction of the stimulus. Brains were extracted to quantify Fos expression (see below).
Behavioral quantification
Behavior was scored using Observer XT (Noldus) by an observer blind to subject Group and Context. The first 10 min of interactions with the stimulus were scored because this time is the most representative window for behavior that relates to Fos expression. We recorded the latency to first approach the stimulus, prosocial behavior (any positive physical contact that included passive bodily contact, allogrooming, positive investigation, or huddling) and aggression (attacks, chases, lunges, bites, and defensive/offensive upright posture). Note that we also scored behavior with the novel object; behaviors exhibited toward the novel object included positive investigation and lunges or bites, and thus we report prosocial and aggressive interaction data in voles that were assigned to the novel object context.
Histology and immunohistochemistry
Neural responses were quantified using the IEG cFos (assessed via its immunohistochemically labeled protein, Fos). Fos functions by rapidly altering gene expression, either positively or negatively, in response to cell surface signals (Hoffman et al., 1993) and is rapidly induced in neurons, with its protein product reaching a maximum 60–90 min after stimulation.
To visualize Fos, subjects were euthanized by isoflurane overdose and transcardially perfused with 0.1M phosphate buffered saline (PBS) followed by 4% paraformaldehyde dissolved in 0.1M borate buffer (pH 9.5). Brains were extracted, post-fixed overnight in 4% paraformaldehyde dissolved in 0.1M borate buffer (pH 9.5) before cryoprotection in 30% sucrose dissolved in PBS for 48 h. Tissue was sectioned into three 40μm series. One series of tissue was immunofluorescently stained for Fos. Tissue was rinsed 5x for 10 min in 0.1M PBS (pH 7.4), incubated for 1 h in block (PBS + 10% normal donkey serum + 0.03% Triton-X-100), and then incubated for approximately 48 h in primary antibodies diluted in PBS containing 5% normal donkey serum + 0.03% Triton-X-100. The primary antibody used was rabbit anti-Fos (5:1000; Santa Cruz Biotechnology, Santa Cruz, CA). Notably, the Fos antibody used for this study was obtained in 2014 prior to the temporary shutdown of Santa Cruz Biotechnology. Batches of the Santa Cruz rabbit anti-Fos antibody prior to 2014 were specific and widely used with success, whereas subsequent batches post-2017 have been reported to be non-specific. This particular antibody was previously validated with preadsorption controls to verify specificity (Kelly et al., 2017). The primary incubation was followed by two 30 min rinses in PBS. Tissue was then incubated for 2 h at room temperature in a donkey anti-rabbit secondary conjugated to Alexa Fluor 594 (5:1000). The secondary antibody was diluted in PBS containing 5% normal donkey serum + 0.03% Trion-X-100. Alexa Fluor conjugates were obtained from ThermoFisher Scientific (Waltham, MA). Following two 30 min rinses in PBS, sections were mounted on microscope slides and cover-slipped with Prolong Gold antifade containing a DAPI nuclear stain (ThermoFisher Scientific).
Neural quantification
To perform cell counts, images were obtained using an Aperio ScanScope at 40X (Leica BioSystems, USA). Tissue sections containing the following brain regions were imaged: LS, BST, POA, AH, VMH, MeA, and PAG. A unique ROI for each brain region was applied to images to capture the center of the brain region of interest in each hemisphere. This was done to account for any individual differences in brain size across subjects. The same ROIs were used for all individuals. Cell counts were obtained from two consecutive brain tissue sections for each brain region and an average was used for analyses. An observer blind to subject information conducted cell counts in Photoshop CS6 (Adobe Systems, San Jose, CA) and Image J (National Institutes of Health, Bethesda, MD) as previously described (Kelly et al., 2017).
Statistical analysis
We first conducted analyses to examine behavioral- and brain region-specific responses to exposure condition. We analyzed behavioral and neural (Fos expression) data using general linear models (GLMs) with multiple comparisons and Bonferroni correction. GLMs were analyzed in SPSS 28 (IBM Analytics, USA). Unless specified, all GLMs included Group and Context as fixed factors. Reported GLM results include degrees of freedom, r2, F, p-values, and partial eta squared (η2) effect sizes. If appropriate, post-hoc pairwise comparisons were conducted within the GLM, the absolute values of the mean difference, the p value, and Cohen’s d effect size were reported. Figures of GLMs and post hoc comparisons were made using PRISM 9 (GraphPad, USA).
We then employed a less conventional, but more direct, analysis to specifically test Newman’s hypothesis that distinct social contexts will elicit distinct patterns of neural activity across the SBN. A leave-one-out nearest-neighbor classification approach was used to estimate the extent to which a vole’s pattern of Fos expression across the 7 brain regions of the SBN contained information about the vole’s stimulus condition or mating status (i.e., pair bonded or non-bonded). This approach is similar to one used previously for firing rate data from multiple neurons (Manns et al., 2007; Manns and Eichenbaum, 2009). Each subject’s Fos-ir+ cell counts were represented as a single point in a seven-dimensional space in which each axis represented the cell count for one of the seven brain regions analyzed. The distance between any two points accordingly reflected the similarity of the cell count patterns for those two voles, with shorter distances indicating greater similarity. The specific distance metric used here was Mahalanobis distance, a commonly used metric that accounts for within-region variability and between-region correlations of cell counts. To ask if cell count patterns distinguished between voles in two different stimulus conditions (yet from the same mating status), the data for each of the voles in those two stimulus conditions (e.g., pair bonded voles in the novel object context vs. pair bonded voles in the novel female context) were entered one at a time into a nearest-neighbor classifier. Specifically, the stimulus condition label for each vole was temporarily “left out” of the two-condition data subset and was guessed based on the stimulus condition label for the vole whose data point was closest in the seven-dimensional space. The classification accuracy was based on the proportion of those guesses that correctly identified the actual stimulus condition (i.e., Context) label, with 0.5 representing chance levels and 1.0 representing perfect classification. This approach was conducted for all 15 possible pairings of two conditions for the voles in the Pair bonded group and, separately, for all 15 possible pairings of two conditions for voles in the Non-bonded group. Similarly, to ask if cell count patterns distinguished between voles in matching stimulus conditions between the Pair bonded and Non-bonded groups (e.g., pair bonded voles in the novel female context vs. non-bonded voles in the novel female context), a similar leave-one-out nearest neighbor classifier was used for all six of the pairings between matching stimulus conditions.
A random shuffling procedure was used for each pairwise classification (e.g., pair bonded voles in the novel object context vs. pair bonded voles in the novel female context) to estimate the likelihood that the observed classification accuracy could have been due to chance. Specifically, in each of 10,000 iterations, the stimulus condition labels for the two sets of voles were randomly shuffled across those voles, and the leave-one-out nearest-neighbor classification was repeated on that randomly shuffled data. The distribution of 10,000 classification accuracy values, thus, reflected the sampling distribution under the null hypothesis, and the 9,500th highest value represented the cutoff for the upper 5th percentile of the null distribution (i.e., statistically significant at the uncorrected 0.05 level). The 9,967th highest value represented the cutoff for statistical significance at the 0.05/15 (0.0033) level, correcting for the 15 comparisons within each group. A similar procedure was used to evaluate the statistical significance of classification accuracy of matching stimulus conditions between groups (e.g., pair bonded voles in the novel female context vs. non-bonded voles in the novel female context), with the exception that a cutoff of 0.05/6 (0.0083) was used to correct for the six comparisons. A one-tailed null distribution was used in each case because only above-chance classification accuracies were considered to be meaningful. Classification and permutation testing approaches were conducted in MATLAB (MathWorks, USA).
Results
Context-dependent effects on behavior
We exposed adult male prairie voles to a range of social and nonsocial contexts to test the hypothesis that different patterns of neural activity across the SBN are associated with different social contexts. We also sought to determine whether the status of being pair bonded or non-bonded (i.e., virgin males) influenced SBN neural activity. The first 10 minutes of the IEG test was video recorded and we quantified latency to approach the stimulus, prosocial behavior, and aggressive behavior. Statistics for all behavior can be found in Table S1.
Latencies to approach stimuli differed by Context (exposure stimulus: F(5,112) = 5.87, P < 0.01, η2 = 0.21; Fig. 1A), but not based on being pair bonded or non-bonded (i.e., Group: F(1,112) = 3.34, P = 0.07, η2 = 0.29). Bonferroni-corrected post hoc analyses showed that subjects were slower to approach a heterospecific meadow vole than all other social stimuli (all P’s < 0.01, all d’s > 3.35). The latency to approach a meadow vole was the same as the novel object (P = 0.79, d = 1.81). Subjects did not differ in their time to approach a partner, cagemate, novel male, novel female, or novel object (all P’s > 0.26, all d’s < 2.32; Fig. 1A). We found no interaction between Group and Context (F(3,112) = 2.42, P = 0.07, η2 = 0.06).
Figure 1: Behavior in the IEG test.
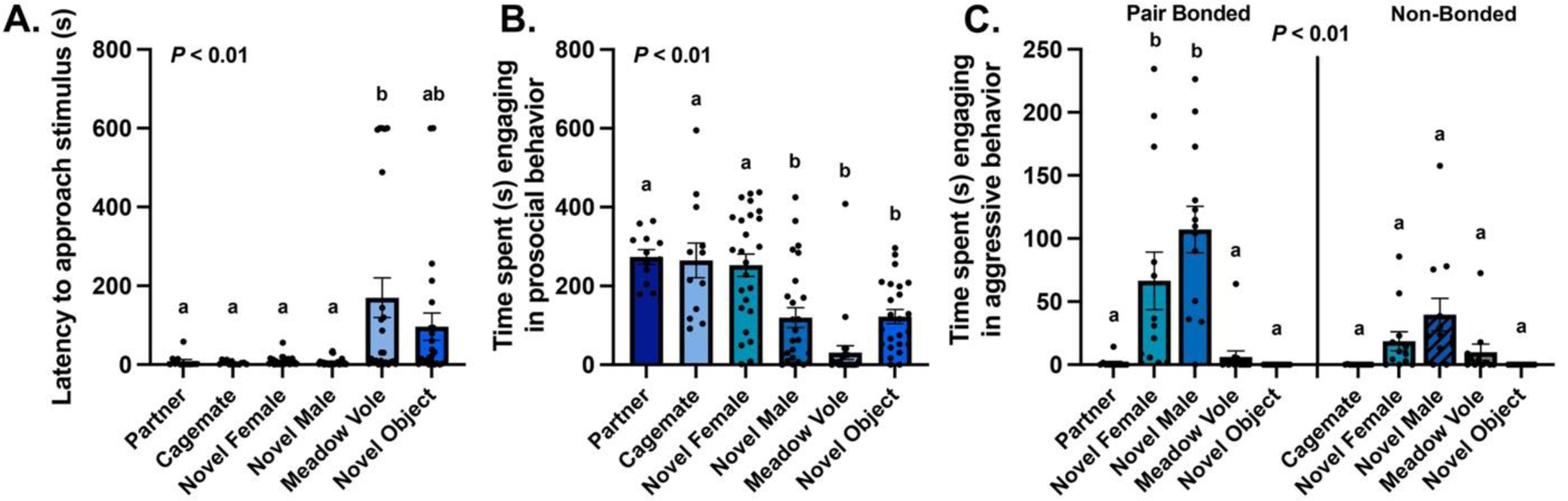
(A) Mean ± SEM time (seconds, s) to approach the stimulus for all subjects combined. (B) Mean ± SEM time spent engaging in prosocial behavior with the stimulus for all subjects combined. (C) Mean ± SEM time spent engaging in aggressive behavior with the stimulus, which differed between pair bonded and non-bonded males. Dots represent individual data points. Shared letters over bars represent statistical similarity.
Non-bonded males generally exhibited more prosocial behavior than pair bonded males (Group: F(1,112) = 6.46, P = 0.012, η2 = 0.05), and subjects across contexts differed in their prosocial behavior (Context: F(5,112) = 15.39, P < 0.01, η2 = 0.41; Fig. 1B). Bonferroni-corrected post hoc analyses between contexts showed that subjects exposed to a partner, cagemate, and novel female exhibited significantly more prosocial behavior than subjects exposed to the novel male, the meadow vole, and the novel object (all P’s < 0.01, all d’s > 5.92; Fig. 1B). Within these two distinct groups though, subjects did not differ in prosocial behavior when exposed to the partner, cagemate, or novel female (all P’s = 1.00, all d’s < 0.51), and likewise, subjects exposed to the novel male, the meadow vole, and the novel object also did not differ in prosocial behavior (all P’s > 0.09, all d’s < 2.75). We observed no significant Group × Context interaction (F(3,112) = 0.35, P = 0.79, η2 = 0.01).
Pair bonded males were generally more aggressive than non-bonded males (Group: F(1,112) = 12.30, P < 0.01, η2 = 0.10), and aggression differed across contexts (Context: F(5,112) = 13.70, P < 0.01, η2 = 0.38). Further, we observed a significant Group × Context interaction (F(3,112) = 4.95, P < 0.01, η2 = 0.12; Fig. 1C). Bonferroni-corrected post hoc analyses showed that pair bonded subjects exhibited more aggression than non-bonded subjects when exposed to the novel female (P = 0.003, d = 3.13) and novel male (P < 0.001, d = 4.47), but not the meadow vole (P = 0.813, d = 0.19) or novel object (P = 1.00, d < 0.01). When specifically comparing just pair bonded subjects across contexts, pair bonded subjects were indeed more aggressive towards the novel male and novel female than the other stimuli (all P’s < 0.021, all d’s > 4.00; Fig. 1C, left). Conversely, when comparing just non-bonded subjects, aggressive behavior did not significantly differ across contexts (all P > 0.15, all d’s < 2.44; Fig. 1C, right).
Differential neural responses to social context in SBN nuclei
We first broadly examined how individual nodes of the SBN respond to different social and nonsocial contexts among pair bonded and non-bonded male prairie voles. We descriptively characterized the degree of Fos activation by visualizing average Fos expression per region (z-scored) in each context. Our results suggest that exposure to a novel male and exposure to a meadow vole generally elicited the highest Fos responses, whereas exposure to a novel object, familiar conspecific (partner or cagemate), or no stimulus elicited the lowest Fos responses (Fig. 2). The MeA and BST showed the greatest variation in Fos expression across contexts, ranging from relatively low to relatively high mean Fos counts (Fig. 2). Lastly, pair bonded and non-bonded subjects appeared to qualitatively differ in neural activation most when exposed to novel females, and to a lesser extent to novel males (Fig. 2).
Figure 2. Fos expression across social contexts.
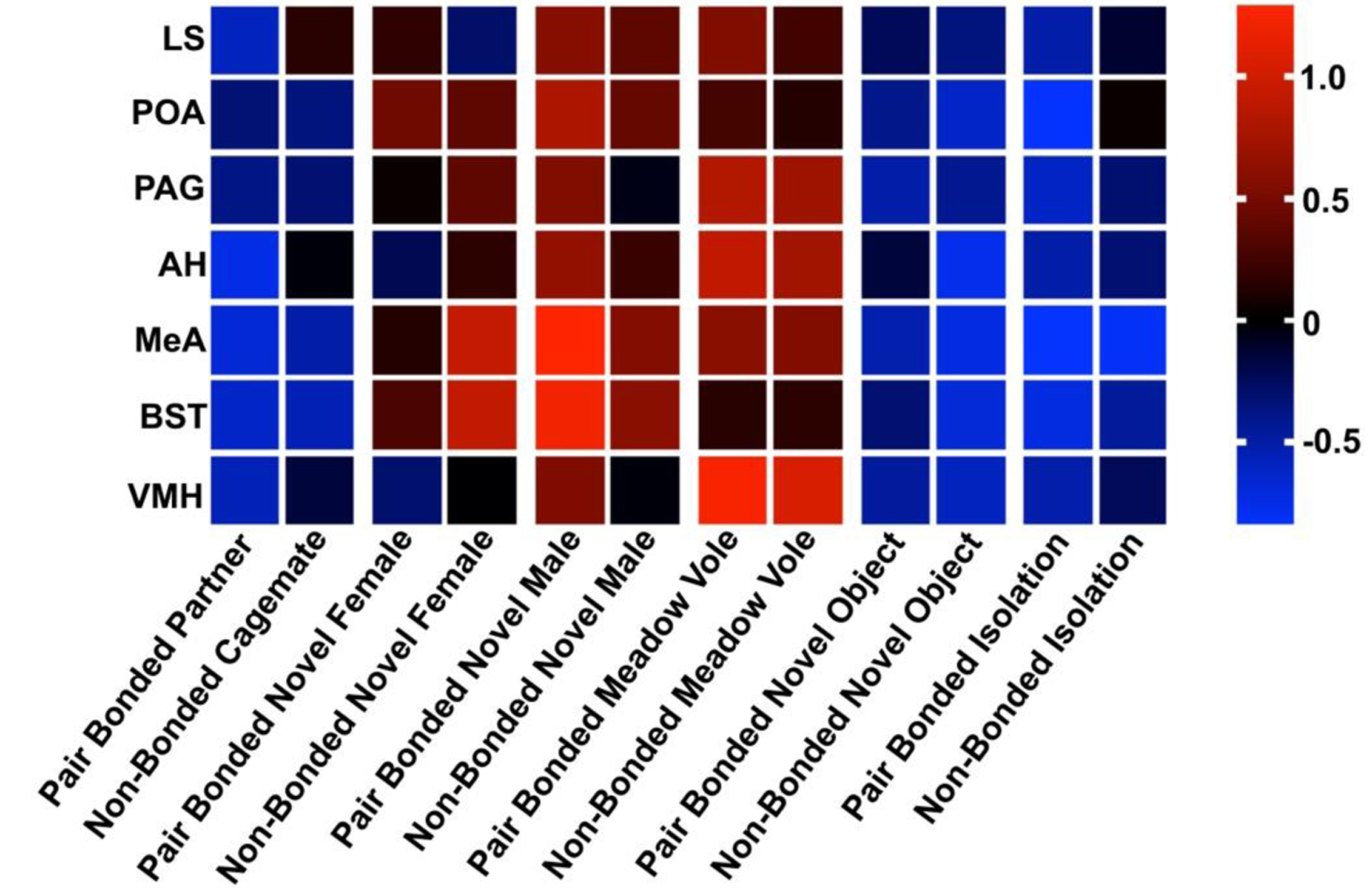
Visualization of average z-scored Fos-ir expression for all subjects, excluding the Pair Bonded Partner context (which included data from partnered animals only) and Non-Bonded Cagemate context (non-bonded animals only).
We next tested for statistical differences in Fos expression (number of Fos-ir cells) for each node of the SBN across contexts. Statistics for Fos expression in individual brain nuclei can be found in Table S2. Fos counts were analyzed using a unique ROI for each brain region within the SBN; the same ROIs were used across all subjects. ROIs were used instead of gross brain structure to account for any individual differences in brain size.
Lateral septum -
Pair bonded subjects exhibited greater Fos expression in the LS than non-bonded subjects across all contexts combined (Group: F(1,134) = 13.76, P < 0.01, η2 = 0.09). Similarly LS Fos differed across context (Context: F(6,134) = 5.75, P < 0.01, η2 = 0.20; Fig. 3A). Bonferroni-corrected post hoc analyses showed that subjects exposed to the novel male exhibited significantly higher Fos expression than subjects exposed to the partner (P = 0.005, d = 3.63), novel object (P = 0.014, d = 3.47), or isolation (P = 0.019, d = 3.40). Additionally, subjects exposed to the meadow vole exhibited more Fos expression than subjects exposed to the partner (P = 0.015, d = 3.32) and novel object (P = 0.046, d = 3.29). We did not observe a significant interaction between Group × Context (F(4,134) = 1.60, P = 0.18, η2 = 0.05).
Figure 3. GLM analysis of brain region-specific Fos expression.
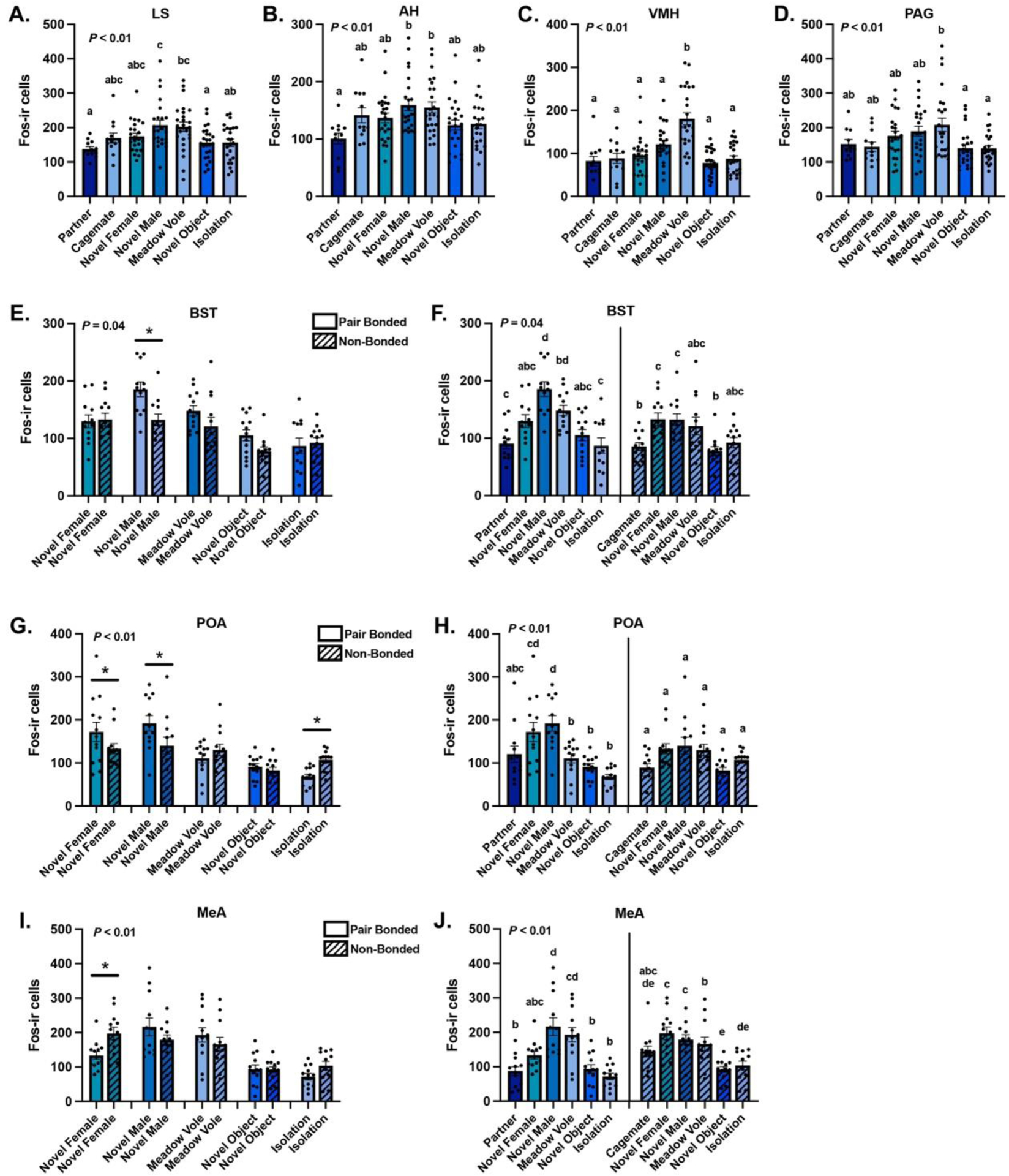
Mean ± SEM Fos-ir expression across contexts in the (A) lateral septum (LS), (B) anterior hypothalamus (AH), (C) ventromedial hypothalamus (VMH), and (D) periaqueductal gray (PAG). Mean ± SEM Fos-ir expression interactions between Group and Context in the (E-F) bed nucleus of the stria terminalis (BST), (G-H) preoptic area (POA), and (I-J) medial amygdala (MeA). Dots represent individual data points. Asterisks indicate statistical significance. Shared letters over bars represent statistical similarity whereas different letters over bars indicate statistically significant differences.
Bed nucleus of the stria terminalis -
Like the LS, pair bonded subjects exhibited greater Fos expression in the BST than non-bonded males across all contexts combined (Group: F(1,134) = 8.78, P < 0.01, η2 = 0.06). BST Fos expression also differed across context (Context: F(6,134) = 13.38, P < 0.01, η2 = 0.38), with a significant Group × Context interaction (F(4,134) = 2.62, P = 0.04, η2 = 0.07). Between groups (i.e., pair bonded vs. non-bonded), Bonferroni-corrected post hoc analyses showed that BST Fos expression during the novel male exposure was significantly greater in pair bonded subjects than non-bonded subjects (P < 0.01, d = 3.53; Fig. 3E). Within a group (i.e., pair bonded or non-bonded), pair bonded subjects exposed to the novel male exhibited higher Fos expression in the BST compared to pair bonded subjects exposed to the partner, novel female, novel object, and isolation (all P’s < 0.001, all d’s > 3.67; Fig. 3F). Similarly, pair bonded subjects exposed to the meadow vole exhibited more Fos expression than those exposed to the partner (P = 0.003, d = 3.93) and isolation (P = 0.001, d = 4.29). On the other hand, non-bonded males exposed to the novel female and novel male exhibited significantly more Fos expression in the BST than non-bonded subjects exposed to the cagemate (both P’s = 0.03, both d = 3.13) and novel object (both P’s = 0.06, both d =3.60; Fig. 3F).
Preoptic area -
Fos expression in the POA differed across contexts (Context: F(6,134) = 10.00, P < 0.01, η2 = 0.35), but did not differ between pair bonded and non-bonded subjects (Group: F(1,134) = 0.90, P = 0.35, η2 = 0.01). We observed a significant Group × Context interaction for Fos expression within the POA (F(4,134) = 3.85, P < 0.01, η2 = 0.13). Bonferroni-corrected post hoc analyses showed that POA Fos expression was greater in pair bonded subjects than non-bonded subjects after exposure to the novel male (P= 0.009, d = 2.83) and novel female (P = 0.043, d = 2.61), whereas non-bonded subjects exhibited higher Fos expression than pair bonded subjects following isolation (P = 0.048, d = 2.17) (Fig. 3G). Within pair bonded subjects, those exposed to the novel male exhibited higher Fos expression in the POA than those exposed to the partner, meadow vole, novel object, and isolation (all P’s < 0.006, all d > 4.44; Fig. 3H). Similarly, pair bonded subjects exposed to the novel female exhibited greater POA Fos expression than bonded subjects exposed to the meadow vole, novel object, and isolation (all P’s < 0.02, all d > 3.83). Conversely, POA Fos expression did not differ across contexts within the Non-bonded group (all P’s < 0.06, all d < 3.16; Fig. 3H).
Anterior hypothalamus -
Like the POA, Fos expression in the AH differed across social contexts (Context: F(6,134) = 3.92, P < 0.01, η2 = 0.15; Fig. 3B) but did not differ between pair bonded and non-bonded subjects (Group: F(1,134) = 1.21, P = 0.27, η2 = 0.01). Bonferroni-corrected post hoc analyses showed that subjects exposed to the partner exhibited significantly lower Fos expression than subjects exposed to the novel male (P = 0.005, d = 3.93) and meadow vole (P = 0.013, d = 3.60). We did not find a significant Group × Context interaction (F(4,134) = 0.64, P = 0.63, η2 = 0.02).
Ventromedial hypothalamus -
Pair bonded subjects exhibited greater VMH Fos expression than Non-bonded males across contexts (Group: F(1,134) = 4.84, P = 0.03, η2 = 0.04) and differed across social contexts (Context: F(6,134) = 16.12, P < 0.01, η2 = 0.42; Fig. 3C). Bonferroni-corrected post hoc analyses showed that males exposed to the meadow vole exhibited significantly more VMH Fos expression than in all other contexts (all P’s < 0.01, all d’s > 4.83). We did not find a significant Group × Context interaction (F(4,134) = 1.96, P = 0.10, η2 = 0.05).
Medial amygdala -
Fos expression in the MeA differed by context (Context: F(6,134) = 15.14, P < 0.01, η2 = 0.40), but not between pair bonded and non-bonded subjects (Group: F(1,134) = 0.36, P = 0.55, η2 < 0.01). We did observe a significant Group × Context interaction (F(4,134) = 3.51, P < 0.01, η2 = 0.05). Bonferroni-corrected post hoc analyses showed that MeA Fos expression was greater in non-bonded subjects than pair bonded subjects only after exposure to the novel female (P < 0.01, d = 2.87; Fig. S3I). Within Group, pair bonded subjects exposed to the novel male exhibited higher Fos expression in the MeA than those exposed to the partner, novel female, novel object, and isolation (all P’s < 0.01, all d > 3.91; Fig. 3J). Similarly, pair bonded subjects exposed to the meadow vole exhibited higher MeA Fos expression than those exposed to the partner, novel object, and isolation (all P’s < 0.01, all d > 4.50). In contrast, non-bonded subjects exposed to the novel female, novel male, and meadow vole exhibited significantly more MeA Fos-ir cells than non-bonded subjects exposed to the novel object and isolation (all P’s < 0.02, all d > 2.81; Fig. 3J).
Periaqueductal grey -
Finally, PAG Fos significantly differed across context (Context: F(6,134) = 3.82, P < 0.01, η2 = 0.15; Fig. 3D), but not between pair bonded and non-bonded subjects (Group: F(1,134) = 0.63, P = 0.43, η2 < 0.01). Bonferroni-corrected post hoc analyses showed that males exposed to the meadow vole exhibited significantly greater Fos expression compared to males exposed to the novel object (P = 0.008, d = 3.72) and isolation (P = 0.009, d = 3.72). We did not find a significant Group × Context interaction (F(4,134) = 0.36, P = 0.84, η2 = 0.01).
Effects of context on Fos expression patterns across the SBN
For visualization purposes, we created topographical heat maps of mean Fos-ir cell counts for each stimulus condition for both non-bonded and pair bonded prairie voles in the style Newman (1999) and Goodson (2005) used to hypothetically represent patterns of activity across the SBN (Fig. 4). This allows for a whole network view of neural responsivity.
Figure 4. Mean Fos-ir+ cell counts across the SBN.
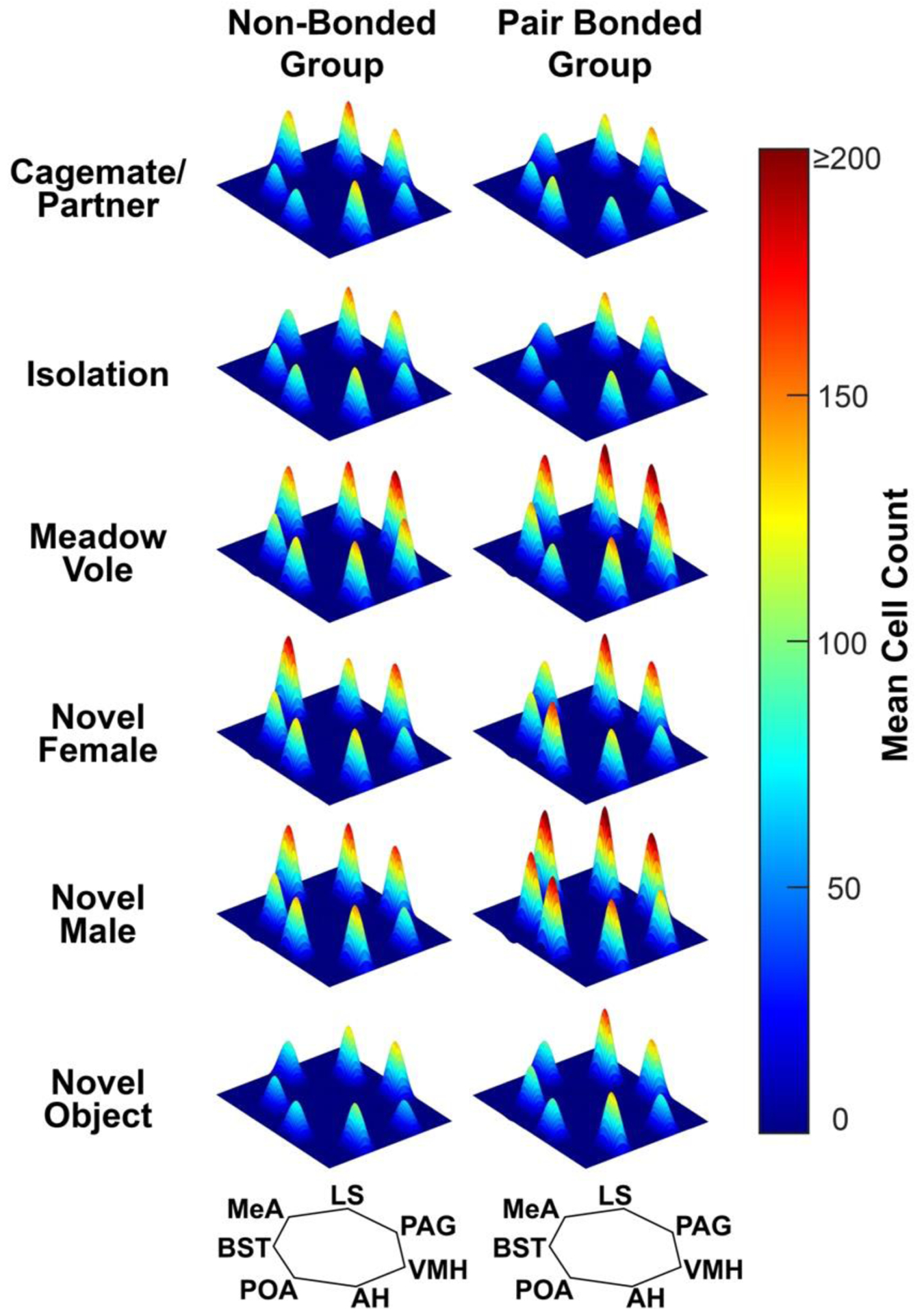
Mean ± SEM Fos-ir expression across SBN nodes in non-bonded (left) and pair bonded (right) male prairie voles across contexts. Color gradation represents mean Fos-ir cell counts with dark blue representing 0 cells and dark red representing greater than 200 cells. Abbreviations: anterior hypothalamus (AH), bed nucleus of the stria terminalis (BST), lateral septum (LS), medial amygdala (MeA), periaqueductal gray (PAG), preoptic area (POA), ventromedial hypothalamus (VMH).
We used classification accuracy and permutation testing approaches to explore whether patterns of neural responses across the SBN varied in relation to the social or nonsocial context to which an animal was exposed. To test the hypothesis that distinct social contexts produce different patterns of activity across the SBN, a leave-one-out nearest-neighbor classification approach was used to estimate the extent to which a vole’s pattern of Fos expression across the seven brain regions of the SBN contained information about the vole’s stimulus Context condition or mating Group status (i.e., pair bonded or non-bonded; see Statistical analysis section). This relatively simple type of supervised learning classifier uses proximity of individual prairie voles’ data points in a multi-dimensional space (in this case, a 7-dimensional space reflecting the seven brain regions) to ask if patterns of brain activity from a subject in one Context could be used to guess accurately the Context of the subject with the most similar pattern of brain activity, for example. A random shuffling procedure was used for each pairwise classification to estimate the likelihood that the observed classification accuracy could have been due to chance or was statistically greater than chance. Because we observed significant differences in Fos expression in individual brain regions based on mating status (see GLM analyses above), this approach was conducted for all 15 possible pairings of two conditions for the subjects in the Pair Bonded group and, separately, for all 15 possible pairings of two conditions for subjects in the Non-Bonded group. After correction for multiple comparisons, the significance threshold was 0.0033. Similarly, to ask if SBN Fos expression patterns are distinguishable between subjects in matching stimulus conditions between the Pair Bonded and Non-Bonded groups, a similar leave-one-out nearest neighbor classifier was used for all six of the pairings between matching stimulus conditions. After correction for multiple comparisons, the significance threshold was 0.0083.
For non-bonded male prairie voles, prior to correction for multiple comparisons, patterns of SBN Fos co-expression significantly differed between males exposed to a meadow vole compared to those exposed to the novel object or a novel female. Additionally, males exposed to the novel female exhibited significantly different patterns compared to males exposed to the isolation condition or the novel object (Fig. 5A). However, these differences did not survive corrections for multiple comparisons (all P > 0.0033), suggesting that patterns of neural activity (at least as assessed via Fos expression) did not differ based on social/nonsocial context in non-bonded male prairie voles. Alternatively, we may lack statistical power to detect any potential differences.
Figure 5. Classification accuracy matrices.
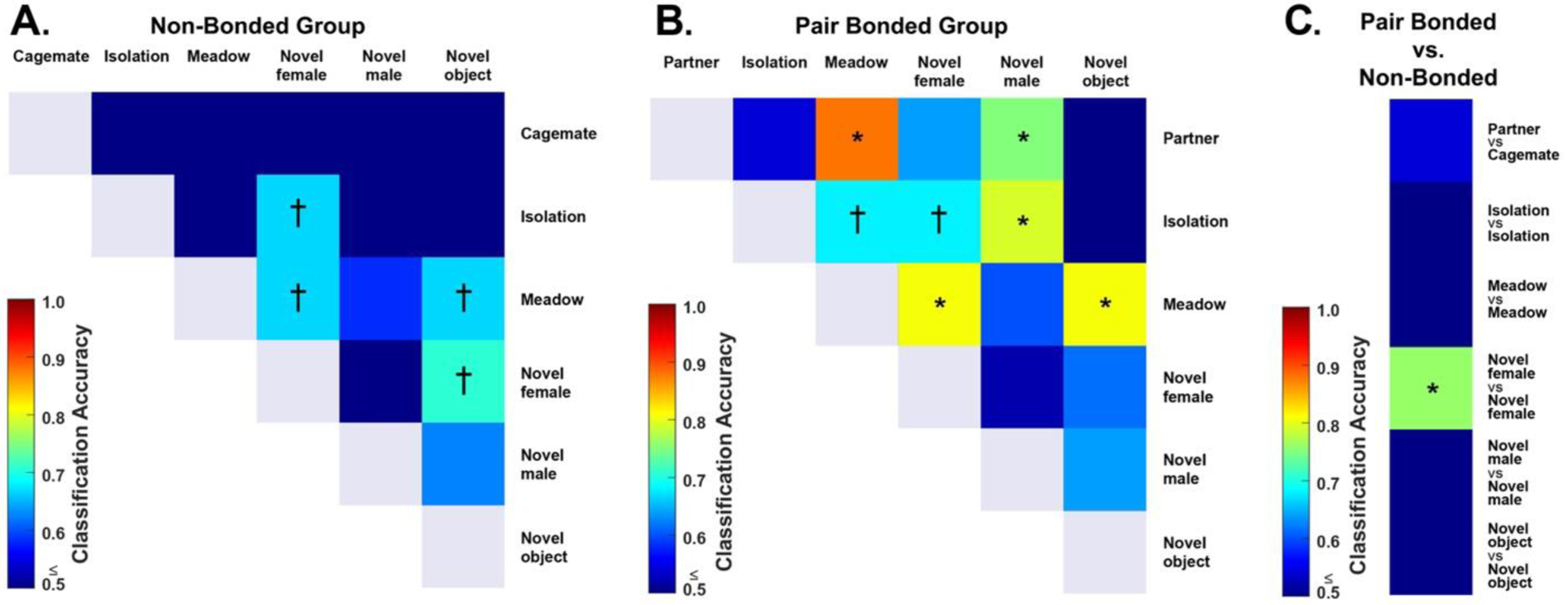
Classification accuracies based on pairwise comparisons of patterns of Fos expression across the SBN of (A) non-bonded and (B) pair bonded male prairie voles between two stimulus Context conditions. (C) Classification accuracies and pairwise comparisons of patterns of Fos expression across the SBN between non-bonded and pair bonded vole Groups exposed to the same stimulus condition. Color gradation represents classification accuracy with dark blue representing a less than or equal to accuracy of 50% and dark red representing 100% accuracy of correct classification. A dagger represents statistical significance before correction for multiple comparisons whereas an asterisk represents statistical significance after correction for multiple comparisons.
In contrast to non-bonded males, several pairwise comparisons remained significant after correction for multiple comparisons for male prairie voles in the Pair Bonded group (Fig. 5B). Pair bonded males exposed to a meadow vole exhibited significantly different patterns of activity across the SBN compared to males exposed to their partner (P = 0.0001), a novel female (P = 0.0011), or a novel object (P = 0.0013). Further, males exposed to a novel male exhibited significantly different patterns compared to males exposed to their partner (P = 0.0009) or the isolation condition (P = 0.0007).
Lastly, when comparing SBN patterns of activity between males in the Pair Bonded and Non-Bonded groups exposed to the same stimulus condition, we found that pair bonded and non-bonded males had significantly different patterns of activity in response to exposure to a novel female (P = 0.0031). Together these findings suggest that pair bonding significantly alters how the brain responds to social stimuli.
Discussion
The SBN framework has enabled comparative and integrative neurobiologists to understand the neural control and modulation of social behavior. Although the SBN was never meant to be considered an exhaustive set of nuclei that control social behavior, the early identification of node membership enabled a heightened attention to the regions that have now been identified as central hubs of social behavior. In addition to identifying these nuclei, the SBN shifted focus of brain-behavior relationships away from single regions of interests and toward an emergent network perspective. Unequivocally, this emphasis on the relational nature of these nodes has advanced our understanding of the ways the brain processes and shapes social behavior (Goodson, 2005; O’Connell and Hofmann, 2011; Tremblay et al., 2017; Prounis and Ophir, 2020).
The original hypothesis promoted by Newman (1999) was that different social contexts would produce unique and varied signatures of neural activation across the network. Yet, despite the value that the SBN has provided, the field has not explicitly or comprehensively tested differential network activity across different social contexts, a priori. In the present study, we compared neural responses in nodes of the SBN across five social and two nonsocial contexts to assess if neural responsivity patterns among pair bonded and non-bonded male prairie voles were different across corresponding contexts. In order to fully support Newman’s hypothesis, we would have expected to find that each social context produced a significantly different pattern of activation, and perhaps especially when compared to nonsocial contexts (i.e., novel object or isolation). However, our results did not identify different unique neural landscapes for each social (or asocial) context to the degree that Newman’s hypothesis originally expected. Rather, only some contexts produced distinct neural landscapes, and when taking conservative statistical approaches and correcting for multiple comparisons, patterns of activity only varied in relation to context in pair bonded, and not non-bonded, male prairie voles. Notably, comparisons of two contexts that produced significantly different network activity profiles were typically between highly salient or presumably intensely valenced (positive or negative) contexts and familiar or nonsocial contexts. For example, a novel male and a meadow vole (both likely seen as intruders) produced different SBN responses compared to exposure to the pair bond partner. Yet, exposure to a pair bond partner did not produce significantly different responses when compared to males that experienced isolation or interacted with a novel object. Although the profound shifts in SBN activation that Newman envisioned were not observed, our findings suggest that the SBN may be particularly responsive to socially salient stimuli, and surprisingly that the partner’s salience is not reflected by this brain network. We also acknowledge that methodological and experimental design limitations might have masked our ability to fully capture the variability in SBN function that could exist. We expand below on recommended directions and methodologies for future assessment of the SBN, but we first highlight more specific observations from our experiment, which i) recapitulated the roles of specific SBN nodes in modulating social behavior, ii), identified the importance of social novelty as a driver of contextual responses, and iii) emphasized the influence of pair bonding in reshaping functionality of the prairie vole SBN.
Consistencies in SBN node function for brain regions involved in defensive/aggressive behaviors
Our study was intended to assess the collective neural activation of the core nodes of the SBN in response to varied social contexts. However, many of our observations for specific nodes of the SBN were consistent with prior studies that focused on a particular node or subset of nodes within the SBN. For example, we found that the MeA was significantly more responsive in subjects exposed to social novelty (i.e., novel female, novel male, novel meadow vole) compared to other contexts. The socially novel contexts were also those in which male prairie voles displayed the most aggression. Prior Fos studies have also implicated the MeA in the processing of threatful stimuli (Dielenberg et al., 2001; McGregor et al., 2004; Choi et al., 2005; Martinez et al., 2011), and excitotoxic lesions of the MeA reduce freezing and defensive behaviors in rats exposed to live cats (Martinez et al., 2011). Additionally, the MeA is implicated in avoidance of unfamiliar conspecifics in male and female prairie voles after social defeat (Tickerhoof et al., 2020). Together these results suggest that the MeA plays an important role in assessing social salience, and it may regulate defensive behaviors in male prairie voles.
Similarly, the BST was significantly more responsive in all subjects exposed to some form of social novelty compared to all other contexts. The BST regulates fear to unpredictable threat signals (Goode et al., 2019), and oxytocin receptors and neurons in the BST promote stress-induced social avoidance in California mice (Duque-Wilckens et al., 2018; Duque-Wilckens et al., 2020). Moreover, sexually experienced prairie vole males often respond to interactions with a novel same-sex conspecific with aggression (Winslow et al., 1993). Indeed, a novel meadow vole is a wholly unknown social stimulus that is sure to evoke some degree of uncertainty to a prairie vole subject. Finally, even though a novel female presents the potential for a mating opportunity, initial interactions between male and female prairie voles typically results in some amount of aggression (Lee et al., 2019). These studies suggest that the greater BST activation that we observed in response to social novelty could reflect the uncertainty of how a novel social interaction will progress.
We also found that AH neural responses were significantly higher in response to interactions with a novel male compared to interactions with a pair bond partner. The AH has been implicated in flank marking and overt aggression in hamsters (Albers, 2012), and selective aggression displayed by pair bonded male prairie voles toward novel males is closely associated with higher densities of vasopressin receptors and increased levels of vasopressin release within the AH (Gobrogge et al., 2009). These studies are consistent with our finding in pair bonded male prairie voles and, again, suggests a role for the AH in selective aggression. Interestingly, although non-bonded males directed aggression toward novel males, we did not find a significant difference in AH neural responses between non-bonded male voles exposed to their cagemate and the novel male. This likely reflects the lack of onset of selective aggression given that the non-bonded males had never formed a pair bond and suggests a re-shaping of AH functionality after pair bonding.
The VMH was the only node of the SBN that uniquely responded most strongly after exposure to the heterospecific meadow vole. Indeed, Fos expression in the VMH was significantly greater following exposure to the meadow vole context than every other context. The VMH is particularly important for regulating and even promoting aggression in rodents (Lin et al., 2011; Hashikawa et al., 2017). However, VMH sub-populations projecting to the PAG can propagate inflexible immobility (i.e., freezing) and sub-populations projecting to the AH can induce defensive avoidance (Wang et al., 2015). Our behavioral data showed little to no aggression among animals that interacted with a meadow vole, and the latency to approach the meadow vole was greater than all other contexts except for the novel object context. Thus, relatively high VMH activation following meadow vole exposure might have induced social hesitancy, reflected by an immobility and defensive response, that might be expected from facing an unfamiliar heterospecific. Notably, meadow and prairie voles are sympatric, and aggression for the purpose of competitive exclusion can occur between the two species (Getz, 1962; Klatt, 2015). Because of the known heterogeneity within the VMH and the one-to-many configurations that produce different agonistic outcomes (Wang et al., 2015), we believe that the heightened activation of the VMH in the meadow vole context might represent a context-specific response pattern for heterospecific agonistic behavior.
Significant influence of social novelty
A striking pattern that emerged from our investigation was that novel social context generally elicited greater neural activation across SBN nodes compared to nonsocial contexts and social contexts involving familiar conspecifics. Stated in another way, the SBN appeared to be strongly sensitive to social novelty. Social novelty is a crucial axis of variation in social interactions. Indeed, the ability to distinguish between novel and familiar individuals is necessary to determine when behaviors such as aggression, trust, and prosociality are appropriate, and it is these behaviors that serve as a cornerstone for the social-salience hypothesis. Indeed, the social salience hypothesis emphasizes that ingroup-outgroup preferences and memory of previously encountered social cues can mediate aggression, trust, and prosociality with the help of oxytocin acting on the social brain (Shamay-Tsoory and Abu-akel, 2015). The presence of a novel individual can have dramatic consequences for prairie vole males. For example, a pair bonded male may engage aggressively after encountering a potential rival male encroaching on his territory (Lee et al., 2019). A virgin and/or non-bonded male encountering a novel female may instead take advantage of a reproductive opportunity (Ophir et al., 2008b). This is to say, the ability to evaluate the context and discriminate between the specific features of social novelty can have profoundly different behavioral outcomes, each with associated costs and benefits.
The relative neural activation of the SBN, as measured by Fos, captured this important ecological dimension of social novelty. Neural activation was relatively low across all nodes of the SBN following exposure to a novel (non-social) object, social isolation, or familiar conspecific (i.e., pair bond partner or sibling cagemate). On the other hand, Fos responses tended to be greater across the SBN following exposure to novel animals (male or female conspecifics or a heterospecific). Specifically, the MeA responded significantly more to novel conspecifics or heterospecific stimuli, and this pattern was also seen to a lesser extent in the BST. Notably, the novel object did not generate the same degree of Fos responsivity as the novel social contexts did, indicating it was specifically social novelty that produced this outcome. These results highlight the sensitivity of the extended amygdala (BST and MeA) to the perception and processing of social novelty (Maney et al., 2008; Kenkel et al., 2012; Maruska et al., 2013; Kelly et al., 2017; Kabelik et al., 2018; Otsuka et al., 2020; Petersen et al., 2021). Further, Fos responses in the AH, MeA, VMH, and PAG were the most elevated following exposure to the novel meadow vole and novel male contexts, although these elevated responses were not always significantly greater than other contexts.
When examining differences in SBN patterns of neural activity across contexts, we consistently found significant differences between a socially novel context and either a familiar social or nonsocial context. In pair bonded males, exposure to a meadow vole or a novel male produced distinct patterns of activity compared to males exposed to their familiar, pair bond partner. Yet, patterns of activity did not significantly differ between male prairie voles exposed to the meadow vole compared to those exposed to the novel male (two socially novel contexts). Interestingly, although patterns of activity did not differ between pair bonded males in the novel female and novel male conditions (again, two socially novel contexts), SBN activity did differ between males exposed to the novel female and the meadow vole. Although these are two novel social contexts, a novel female conspecific could provide an extrapair mating opportunity (i.e., possessing a positive valence) whereas encountering a novel heterospecific meadow vole would likely never be beneficial (i.e., possessing a negative valence). This suggests that the SBN may not only be responsive to social novelty, but that the SBN may be especially sensitive to negatively valenced novel, social stimuli and prime an animal to exhibit context-appropriate behavior that will aid in survival.
Strong role of pair bonding on SBN neural activity
As mentioned above, the assessment of a novel individual is greatly influenced by the pair bond status of the focal individual. More generally, in this socially monogamous species, pair bonding induces dramatic changes in prairie vole brain, body, and behavior. Several studies have shown that neural (Wang et al., 2013; Scribner et al., 2020; Lopez-Gutierrez et al., 2021) and cognitive (Blocker and Ophir, 2015) changes occur when prairie voles form pair bonds. In addition to searching the SBN for pattern shifts across social contexts, manipulating the bonding status of male voles allowed us to identify social contexts for which the relevance might change after an individual has transitioned to a pair bonded state.
Like studies before (Williams et al., 1992; Winslow et al., 1993; Young et al., 2008), we found that non-bonded males were more prosocial across all contexts than pair bonded males, whereas pair bonded males were more aggressive. This aggression by the pair bonded males was not generalized, and instead it was directed towards novel males and novel females significantly more than other stimuli. Similarly, we observed that pair bonded males were more aggressive with novel conspecifics compared to non-bonded males. Such behavior is a hallmark of the prairie vole pair bond; once individuals are pair bonded they exhibit selective aggression toward novel conspecifics – behavior that contributes toward the maintenance and fidelity of the bond (Getz et al., 1981; Aragona and Wang, 2004; Gobrogge et al., 2009).
Additionally, several studies have shown that neural changes occur when prairie voles form pair bonds (Wang et al., 2013; Scribner et al., 2020; Lopez-Gutierrez et al., 2021). Therefore, it is not surprising that we observed neural differences in some brain regions in response to novel conspecifics in pair bonded and non-bonded males. For example, pair bonded males exhibited greater Fos responses to novel males in the BST compared to non-bonded males. This result is consistent with the hypothesis that the BST plays an important role in regulating fear to unpredictable threat signals. If true, bonded males should be more sensitive to assessing the threat presented by unfamiliar same-sex rivals, and this difference might be reflected by BST Fos activation.
Our data indicate that POA Fos expression was particularly impacted by being bonded. Specifically, the POA exhibited more Fos in response to a novel female and novel male, but less Fos expression in response to social isolation when males were pair bonded compared to when they were non-bonded. Because the POA is crucial for modulating sexual behavior (Dominguez and Hull, 2005), this may reflect neural changes related to sexual experience. Further, the differential POA response to social isolation might also indicate that separation from a partner is more impactful on POA function than separation from a same-sex sibling cagemate. Partner loss in prairie voles results in a suite of neurobiological changes (Sun et al., 2014; Scribner et al., 2020), supporting this hypothesis.
MeA Fos expression exhibited fewer Fos-positive cells in pair bonded males exposed to novel females compared to non-bonded males. This is inconsistent with commonly observed functions of the MeA (i.e., to facilitate defensive behaviors, see above). It is also a peculiar result considering that the pair bonded males in our study were more aggressive toward a novel female than non-bonded males. It is worth noting that our study did not examine Fos responses specifically in excitatory or inhibitory cells. In mice, the MeA contains large numbers of GABA neurons (Baleisyte et al., 2022). Therefore, it is possible that the Fos expression patterns we observed in the MeA indicate that fewer inhibitory neurons were recruited in the MeA during an interaction with a novel female, thereby allowing for a higher expression of aggression. Lastly, studies in mice have demonstrated that sexual experience triggers long-term changes in MeA neural activity during conspecific interactions (Li et al., 2017) and our findings may not necessarily reflect a simple direct relationship between brain and [aggressive] behavior.
Arguably one of the most striking findings in our study is that patterns of neural activity across the SBN significantly varied by context in pair bonded, but not non-bonded, male prairie voles. Considering that establishing and maintaining a bond represents an important life history event, with important implications for reproductive success for males (e.g., (Ophir et al., 2008a; Blocker and Ophir, 2016; Madrid et al., 2020), drastic shifts in neural activity patterning in pair bonded males might reflect a change in neural processing to facilitate and/or preserve recognition for and interaction with a pair bond partner (Young et al., 2008). Our data suggest that the SBN of pair bonded male prairie voles demonstrates different degrees of neural responsiveness to novel, social stimuli than non-bonded males. If the SBN is primarily serving to direct attention toward and/or modulate behavior appropriately in response to social novelty, the lack of significant differences in SBN neural patterning in non-bonded voles may reflect that single, virgin male prairie voles are more amenable to novel social interactions, which would presumably increase the chances of finding a mate and forming a pair bond. Therefore, a lack of statistically significant differences between contexts in non-bonded males may be due to non-bonded males being at a life history stage where they do not yet perceive social novelty as negative and/or threatening. If true, this would suggest that the SBN exhibits plasticity in responsivity to correspond to life history stage (i.e., a social niche, (Saltz et al., 2016)). It would be interesting to see whether similar patterns would hold for a species that does not form pair bonds and undergo a major life history change related to reproduction in adulthood. Ultimately, it remains unclear whether shifting patterns of activity across the SBN were simply undetected in non-bonded males a) due to insufficient power or due to fundamental differences in degrees of perception of social novelty in non-bonded vs. pair bonded male prairie voles or b) if the brain essentially undergoes a substantial “functional re-wiring” after pair bonding.
Methodological constraints and prospects
We acknowledge that methodological issues could explain why we failed to demonstrate large-scale pattern differences in SBN neural activation across all social contexts. This could be the result of issues associated with identifying and defining functions of brain networks (reviewed by (Kelly, 2022). Similarly, it is important to consider that examining neural activity via Fos expression (as was originally proposed by Newman and others (Newman, 1999; Goodson, 2005) may be insufficient to detect differences in neural patterning across interconnected brain regions. Indeed, Fos expression lacks temporal specificity, and it cannot distinguish the directionality of regulation (Kovacs, 1998; Hudson, 2018), which may be especially important if one is aiming to identify subtle differences in activity across a collection of brain regions. Additionally, we quantified Fos expression by counting all Fos-immunoreactive cells. However, Fos is not expressed exclusively in neurons, and other cell types that express Fos, including astrocytes and glia (Zhang et al., 2014), are presumably included in our analyses. The potential role of microglia in pair bond formation has been highlighted in prairie voles as a critical avenue of further investigation (Loth and Donaldson, 2021), and differences in neural responses between pair bonded and non-bonded males reported here may, at least partially, be the result of changes at the level of microglia. Future studies using electrophysiological methods targeting multiple brain regions (i.e., neuropixel) have the potential to obtain more temporally precise measurements of neural activity underlying specific types of social behaviors in distinct contexts. Such methods could potentially identify distinct patterns of activity across SBN nodes in response to distinct social contexts in any animal, regardless of pair bonding status.
Conclusions
Our data suggest that the nodes of the SBN are indeed critical modulators of social behavior, particularly for social novelty. However, we did not consistently observe distinct patterns of neural responses across the SBN in relation to different social contexts, and therefore do not fully support Newman’s (1999) original hypothesis that the SBN functions as a cohesive network to modulate social behavior by producing signature patterns of neural activation that map to specific social contexts. Several possible explanations for this are possible. For example, it is possible that the SBN does not respond to social contexts as predicted by Newman. If true, it could be that the SBN serves a more general function than originally hypothesized (e.g., a social novelty detector, see above). It is also possible that the SBN could fulfill the function that Newman and others have hypothesized, but it accomplishes this through nuanced variations of neural activation that only subtly differ from a general theme. Another plausible explanation for our failure to find the profound shifts in SBN function under different social contexts could relate to the methods employed by our study, and further testing using refined and modern approaches might be necessary. Nevertheless, despite not finding full support for Newman’s SBN hypothesis, our study does indicate that the SBN appears to modulate aspects of social behavior by collectively responding to some social contexts differently, particularly among bonded male prarie voles. The relationship between life-history stage and SBN responsivity to social context underscores the need to consider the full scope of phenotypic plasticity under ecologically relevant conditions. Notably our study also reveals a new perspective – that the SBN may play a particularly important role in processing social salience, and that the SBN may be especially sensitive to negatively valenced novel, social stimuli and prime an animal to exhibit context-appropriate behavior that will aid in survival and reproduction. The critical role of SBN nodes and their evolutionary conservation has been well-documented and has rightly directed research efforts in the field of behavioral neuroendocrinology. However, our data suggest that we should question whether our conceptualization of how these nodes interact, in the form of a landscape with shifting patterns corresponding to shifting contexts, is appropriate. We urge researchers to use increasingly accessible technological advances and statistical tools such as network analyses, graph theory, and modeling to assess the SBN framework from an unbiased approach (Kelly, 2022). Such future investigations will help contextualize the present study, in which we uncovered distinct patterns of neural activity across the SBN in some contexts, specifically in pair bonded, but not non-bonded, prairie voles.
Supplementary Material
Highlights.
Neural response patterns across the SBN did not differ by context
SBN brain regions increase responsivity to social novelty
Pair bonding alters functional connectivity within the SBN
Acknowledgements
The authors would like to thank Rebecca Horowitz for scoring behavioral videos and all members of the Kelly & Ophir Labs for advice and feedback.
Funding
The authors acknowledge the support from the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development HD081959 to AMK and HD079573 to AGO) and the National Science Foundation (Postdoctoral Research Fellowship in Biology 2011001 to KJW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
Primary data files and MATLAB code are available on Dryad.
REFERENCES
- Albers HE, 2012. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm. Behav 61, 283–292. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z, 2004. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. ILAR journal / National Research Council, Institute of Laboratory Animal Resources 45, 35–45. [DOI] [PubMed] [Google Scholar]
- Baleisyte A, Schneggenburger R, Kochubey O, 2022. Stimulation of medial amygdala GABA neurons with kinetically different channelrhodopsins yields opposite behavioral outcomes. Cell Rep 39, 110850. [DOI] [PubMed] [Google Scholar]
- Blocker TD, Ophir AG, 2015. Social recognition in paired but not single male prairie voles. Anim Behav 108, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blocker TD, Ophir AG, 2016. A preference to bond? Male prairie voles form pair bonds even in the presence of multiple receptive females. Anim Behav 122, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Young LJ, 2018. Oxytocin and Social Relationships: From Attachment to Bond Disruption. Curr Top Behav Neurosci 35, 97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM, Herath EM, Rimal A, Whitlow SM, Maruska KP, 2020. Galanin neuron activation in feeding, parental care, and infanticide in a mouthbrooding African cichlid fish. Horm. Behav 126, 104870. [DOI] [PubMed] [Google Scholar]
- Carter CS, 1998. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinol. 23, 779–818. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL, 1995. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci. Biobehav. Rev 19, 303–314. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ, 2005. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron 46, 647–660. [DOI] [PubMed] [Google Scholar]
- Crews D, 2003. The development of phenotypic plasticity: where biology and psychology meet. Dev Psychobiol 43, 1–10. [DOI] [PubMed] [Google Scholar]
- Crews D, 2008. Epigenetics and its implications for behavioral neuroendocrinology. Front. Neuroendocrinol 29, 344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS, 2001. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience 104, 1085–1097. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM, 2005. Dopamine, the medial preoptic area, and male sexual behavior. Physiol. Behav 86, 356–368. [DOI] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, Laredo SA, Hao R, Perkeybile AM, Minie VA, Tan PB, Bales KL, Trainor BC, 2018. Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice. Biol Psychiatry 83, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Torres LY, Yokoyama S, Minie VA, Tran AM, Petkova SP, Hao R, Ramos-Maciel S, Rios RA, Jackson K, Flores-Ramirez FJ, Garcia-Carachure I, Pesavento PA, Iniguez SD, Grinevich V, Trainor BC, 2020. Extrahypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc Natl Acad Sci U S A 117, 26406–26413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Deitcher DL, Bass AH, 2005. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J. Comp. Neurol 483, 91–113. [DOI] [PubMed] [Google Scholar]
- Getz LL, 1962. Aggressive behavior of the meadow and prairie voles. J. Mammal 43, 351–358. [Google Scholar]
- Getz LL, Carter CS, Gavish L, 1981. The mating system of the prairie vole Microtus ochrogaster: field and laboratory evidence for pair bonding. Behav. Ecol. Sociobiol 8, 184–194. [Google Scholar]
- Gobrogge KL, Liu Y, Young LJ, Wang Z, 2009. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc. Natl. Acad. Sci. USA 106, 19144–19149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Ressler RL, Acca GM, Miles OW, Maren S, 2019. Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav 48, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD, 2005a. Neuro-evolutionary patterning of sociality. Proc. Biol. Sci 272, 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Soma KK, 2005b. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport 16, 1719–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb ZA, Ross AP, McCann KE, Lee S, Welch M, Gomez MG, Norvelle A, Michopoulos V, Huhman KL, Albers HE, 2021. Sex-dependent effects of social status on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT), and serotonin (5-HT) 1A receptor binding and aggression in Syrian hamsters (Mesocricetus auratus). Horm. Behav 127, 104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, Piper WT, Lee H, Rudy B, Lin D, 2017. Esr1(+) cells in the ventromedial hypothalamus control female aggression. Nat Neurosci 20, 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG, 1993. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocrinol 14, 173–213. [DOI] [PubMed] [Google Scholar]
- Hudson AE, 2018. Genetic Reporters of Neuronal Activity: c-Fos and G-CaMP6. Methods Enzymol 603, 197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Xiao Y, Riefkohl PC, Young LJ, 2017. Oxytocin receptors modulate a social salience neural network in male prairie voles. Horm. Behav 87, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelik D, Weitekamp CA, Choudhury SC, Hartline JT, Smith AN, Hofmann HA, 2018. Neural activity in the social decision-making network of the brown anole during reproductive and agonistic encounters. Horm. Behav 106, 178–188. [DOI] [PubMed] [Google Scholar]
- Kelly AM, 2022. A consideration of brain networks modulating social behavior. Horm. Behav 141, 105138. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Goodson JL, 2013. Functional significance of a phylogenetically widespread sexual dimorphism in vasotocin/vasopressin production. Horm. Behav 64, 840–846. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hiura LC, Saunders AG, Ophir AG, 2017. Oxytocin neurons exhibit extensive functional plasticity due to offspring age in mothers and fathers. Integr. Comp. Biol 57, 603–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS, 2012. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J. Neuroendocrinol 24, 874–886. [DOI] [PubMed] [Google Scholar]
- Klatt BJ, 2015. Interspecific Interactions and Habitat Use by Prairie Voles (Microtus ochrogaster) and Meadow Voles (M. pennsylvanicus). The American Midland Naturalist 173, 241–252. [Google Scholar]
- Kovacs KJ, 1998. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 33, 287–297. [DOI] [PubMed] [Google Scholar]
- Lee NS, Goodwin NL, Freitas KE, Beery AK, 2019. Affiliation, Aggression, and Selectivity of Peer Relationships in Meadow and Prairie Voles. Front. Behav. Neurosci 13, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, Murthy VN, Dulac C, 2017. Neuronal Representation of Social Information in the Medial Amygdala of Awake Behaving Mice. Cell 171, 1176–1190 e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ, 2011. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischinsky JE, Lin D, 2020. Neural mechanisms of aggression across species. Nat Neurosci 23, 1317–1328. [DOI] [PubMed] [Google Scholar]
- Lopez-Gutierrez MF, Gracia-Tabuenca Z, Ortiz JJ, Camacho FJ, Young LJ, Paredes RG, Diaz NF, Portillo W, Alcauter S, 2021. Brain functional networks associated with social bonding in monogamous voles. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loth MK, Donaldson ZR, 2021. Oxytocin, Dopamine, and Opioid Interactions Underlying Pair Bonding: Highlighting a Potential Role for Microglia. Endocrinology 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid JE, Parker KJ, Ophir AG, 2020. Variation, plasticity, and alternative mating tactics: Revisiting what we know about the socially monogamous prairie vole. Adv. Stud. Behav 52, 203–242. [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL, 2008. Estradiol modulates neural responses to song in a seasonal songbird. J. Comp. Neurol 511, 173–186. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H, 2009. A cognitive map for object memory in the hippocampus. Learn Mem 16, 616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H, 2007. Gradual changes in hippocampal activity support remembering the order of events. Neuron 56, 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez RC, Carvalho-Netto EF, Ribeiro-Barbosa ER, Baldo MV, Canteras NS, 2011. Amygdalar roles during exposure to a live predator and to a predator-associated context. Neuroscience 172, 314–328. [DOI] [PubMed] [Google Scholar]
- Maruska KP, Becker L, Neboori A, Fernald RD, 2013. Social descent with territory loss causes rapid behavioral, endocrine and transcriptional changes in the brain. J. Exp. Biol 216, 3656–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE, 2004. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J. Neurosci 24, 4134–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW, 1999. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci 877, 242–257. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA, 2011. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol 519, 3599–3639. [DOI] [PubMed] [Google Scholar]
- Ophir AG, 2017. Navigating Monogamy: Nonapeptide Sensitivity in a Memory Neural Circuit May Shape Social Behavior and Mating Decisions. Front Neurosci 11, 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO, 2008a. Social but not genetic monogamy is associated with higher reproductive success in prairie voles. Anim. Behav 75, 1143–1154. [Google Scholar]
- Ophir AG, Wolff JO, Phelps SM, 2008b. Variation in neural V1aR predicts sexual fidelity and space use among male prairie voles in semi-natural settings. Proc. Natl. Acad. Sci. USA 105, 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka A, Nomura C, Miura K, Honda A, Kagawa N, 2020. Immediate Early Gene Expression in Brain Regions Associated with the Social Behavioral Network After Male Competition in Medaka Fish. Zoolog Sci 37, 391–398. [DOI] [PubMed] [Google Scholar]
- Petersen CL, Davis SED, Patel B, Hurley LM, 2021. Social Experience Interacts with Serotonin to Affect Functional Connectivity in the Social Behavior Network following Playback of Social Vocalizations in Mice. eNeuro 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potretzke S, Ryabinin AE, 2019. The Prairie Vole Model of Pair-Bonding and Its Sensitivity to Addictive Substances. Front Psychol 10, 2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prounis GS, Ophir AG, 2020. One cranium, two brains not yet introduced: Distinct but complementary views of the social brain. Neurosci Biobehav Rev 108, 231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppucci CJ, Gergely CK, Veenema AH, 2018. Activation patterns of vasopressinergic and oxytocinergic brain regions following social play exposure in juvenile male and female rats. J Neuroendocrinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltz JB, Geiger AP, Anderson R, Johnson B, Marren R, 2016. What, if anything, is a social niche? Evol. Ecol 30, 349–364. [Google Scholar]
- Scaia MF, Akinrinade I, Petri G, Oliveira RF, 2022. Sex Differences in Aggression Are Paralleled by Differential Activation of the Brain Social Decision-Making Network in Zebrafish. Front. Behav. Neurosci 16, 784835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribner JL, Vance EA, Protter DSW, Sheeran WM, Saslow E, Cameron RT, Klein EM, Jimenez JC, Kheirbek MA, Donaldson ZR, 2020. A neuronal signature for monogamous reunion. Proc Natl Acad Sci U S A 117, 11076–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-akel A, 2015. The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194–202. [DOI] [PubMed] [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, Wang Z, 2014. Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behav. Brain Res 265, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbaa M, Paedae B, Liu Y, Wang Z, 2016. Neuropeptide Regulation of Social Attachment: The Prairie Vole Model. Compr Physiol 7, 81–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles MC, Almeida O, Lopes JS, Oliveira RF, 2015. Social interactions elicit rapid shifts in functional connectivity in the social decision-making network of zebrafish. Proc. Biol. Sci 282, 20151099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickerhoof MC, Hale LH, Butler MJ, Smith AS, 2020. Regulation of defeat-induced social avoidance by medial amygdala DRD1 in male and female prairie voles. Psychoneuroendocrinol. 113, 104542. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Sharika KM, Platt ML, 2017. Social Decision-Making and the Brain: A Comparative Perspective. Trends Cogn Sci 21, 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp JA, Salas-Allende I, Makowski A, Bass AH, 2020. Mating Behavioral Function of Preoptic Galanin Neurons Is Shared between Fish with Alternative Male Reproductive Tactics and Tetrapods. J. Neurosci 40, 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, Young LJ, 2018. The neural mechanisms and circuitry of the pair bond. Nat Rev Neurosci 19, 643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Duclot F, Liu Y, Wang Z, Kabbaj M, 2013. Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nat Neurosci 16, 919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen IZ, Lin D, 2015. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron 85, 1344–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T, 1992. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann N Y Acad Sci 652, 487–489. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Wang Z, 2008. The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP 148, 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ, 2014. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng DJ, Larsson B, Phelps SM, Ophir AG, 2013. Female alternative mating tactics, reproductive success and nonapeptide receptor expression in the social decision-making network. Behav. Brain Res 246, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Primary data files and MATLAB code are available on Dryad.


