Abstract
SASH1 is a scaffold protein with context-dependent biological functions in cell adhesion, tumor metastasis, lung development, and pigmentation. As a member of the SLy protein family, it contains the conserved SLY, SH3, and SAM domains. The 19 kDa SLY domain harbors over 70% of the SASH1 variants associated with pigmentation disorders. However, its solution structure or dynamics have not been investigated yet, and its exact position in the sequence is not clearly defined. Based on the bioinformatic and experimental evidence, we propose renaming this region to the SLy Proteins Associated Disordered Region (SPIDER) and defining the exact position to be amino acids 400–554 of SASH1. We have previously identified a variant in this region linked to a pigmentation disorder, S519N. Here, we used a novel deuteration technique, a suite of TROSY-based 3D NMR experiments, and a high-quality HNN to obtain near complete solution backbone assignment of SASH1’s SPIDER. A comparison with the chemical shifts of non-variant (S519) SPIDER shows that the S519N substitution does not alter the free form solution structural propensities of SPIDER. This assignment is the first step to characterize the role of SPIDER in SASH1-mediated cellular functions and provides a model for the future study of sister SPIDER domains in the SLy protein family.
Keywords: SLy3, SLy-family, Pigmentation, Protein domains, SSP
Biological context
SASH1 is a member of the SLy scaffold protein family and is important in many cellular and developmental functions, including cytoskeletal reorganization (Martini et al. 2011), regulation of cell migration and tumor metastasis (Martini et al. 2011; Lin et al. 2012; Franke et al. 2019; Chen et al. 2020), atherosclerosis (Weidmann et al. 2015), human skin pigmentation(Zhou et al. 2013, 2017; Courcet et al. 2015; Shellman et al. 2015; Wang et al. 2017; Zhong et al. 2019; Cui et al. 2020; Araki et al. 2021; Jaufmann et al. 2021), and mouse lung development (Coulombe et al. 2019). Downregulation of SASH1 expression is a significant negative prognostic, predictive factor in colon, breast, and liver cancer (Zeller et al. 2003; Martini et al. 2011; Jaufmann et al. 2021). Additionally, 19 variants are known to be linked to pigmentation disorders (Zhou et al. 2013, 2017; Courcet et al. 2015; Shellman et al. 2015; Wang et al. 2017; Zhong et al. 2019; Cui et al. 2020; Araki et al. 2021; Jaufmann et al. 2021) (Fig. 1). Despite these multiple critical biological functions, to date only one peer-reviewed structural study has been published(Clements et al. 2022) and two structural data sets have been deposited in the PDB (2EBP and 2DL0) regarding the structural and biochemical properties of SASH1. The SLy family consists of three members: SASH1/SLy3, SASH3/SLy1 and SAMSN1/SLy2 (Jaufmann et al. 2021). All three contain a conserved tri-domain region, which consists of a “SLY”, SH3, and SAM domain (Zhong et al. 2019; Araki et al. 2021; Jaufmann et al. 2021) (Fig. 1). Note that SLy is used to refer to a domain and a protein, which is confusing. This “SLY” domain/region is conserved and predicted to be a disordered region. In SASH1, the “SLY” region has important functions in regulating cell migration and it harbors multiple variants that are associated with pigmentation disorders (Martini et al. 2011; Weidmann et al. 2015; Araki et al. 2021; Jaufmann et al. 2021). However, this domain/region has not been well defined. In this study, we provide bioinformatic evidence and rationale to propose renaming this conserved region to the “SLy Proteins Associated Disordered Region” (SPIDER) and defining the exact position to be amino acids 400–554 of SASH1. This is a unique acronym and is not found in any of the major gene or protein databases. In addition, we present the near complete 1H-15N backbone assignment for the S519N variant of SPIDER in SASH1, which we previously reported as associated with a lentiginous pigmentation disorder (Shellman et al. 2015). These data constitute the first step in defining the biochemical role of these regions across the SLy protein family and will provide the basis for the future study of SPIDERs across the SLy protein family.
Fig. 1.

Domain architecture of the SLy protein family showing the conserved SLy Proteins Associated Disordered Regions (SPIDER; formally known as SLY domain), SH3, and SAM domains. The 19 known substitution variants associated with pigmentation disorders are listed with arrows displaying the domain location across the protein. Over 70% of them are in SPIDER/“SLY”. S519N, the variant of interest in this assignment, is shown in red
Methods and experiments
Bioinformatic analyses and definition of region under study
SPIDER is a conserved region in all three members of the SLy family and spans residues 400–554 in SASH1. A Protein BLAST of this region in the family members revealed homologous regions with a sequence identity between 28 and 35% in SAMSN1 (20–163) and SASH3 (20–173) (Fig. 2). However, the literature referencing this region as the “SLY” domain has conflicting and ambiguous definitions. The current definition in the InterPro databases (PF12485) is unclear and contradictory as to whether “SLY” is a domain or full protein (as of March 9, 2023). Specifically, InterPro describes SLY as a eukaryotic domain family from 144 to 156 amino acids long, containing an LGKK motif, and being found associated with an SH3 and SAM domain. It then states that “SLY contains an [SH3] domain and [SAM]”. For future studies of this region and these proteins, an unambiguous consensus on its name and definition needs to be reached. We propose to refer to this region as “SLy Proteins Associated Disordered Region” (SPIDER). This name is unique and not associated with any other protein family or acronyms, and it signifies the family that contains this region and its disordered characteristics. We have conducted sequence alignment of all three family members (Fig. 2) and the adjacent SH3 domain. Based on these data, we propose that spider be defined as spanning residues 400–554 in SASH1, 20–163 in SAMSN1, and 20–173 in SASH3. Specifically, the starting point (residue 400 in SASH1) marks the beginning of the conserved sequence of this region, while the end point (residue 554 in SASH1) is a proline, which is abundant in disordered regions. Additionally, residue 555 marks the start of the SH3 domain as reported in PDB: 2EBP. Based on our analysis here (Fig. 2) and findings by others, we propose SPIDERs should be defined as a conserved sequence spanning 143 to 154 amino acids in length, containing a nuclear localization sequence (NLS) (Uchida et al. 2001; Beer et al. 2005; Franke et al. 2020; Jaufmann et al. 2021), and a conserved LGKK motif of unknown function. Thus, SPIDER of SASH1 spans residues 400–554; SAMSN1-SPIDER spans 20–163, and SASH3-SPIDER spans 20–173.
Fig. 2.
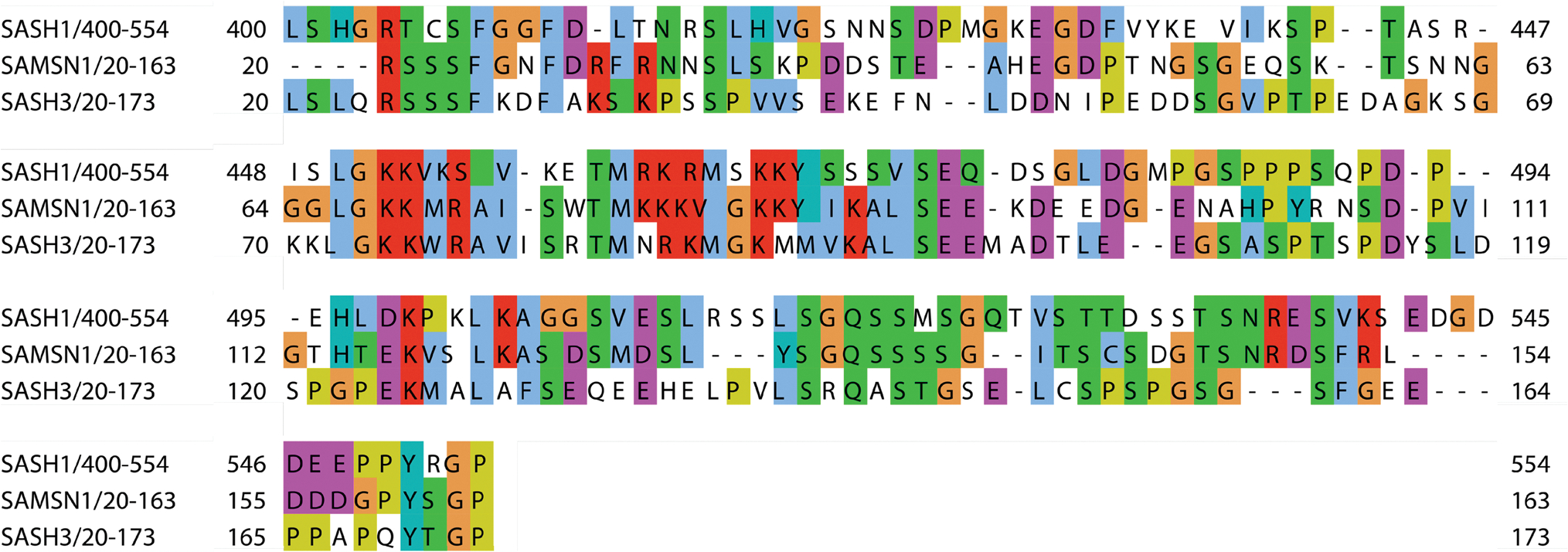
Sequence alignment between SPIDERs of the three members of the SLy protein family, which span the conserved disordered region immediately N-terminal of the SH3 domains. These regions are only present in the SLy/SASH1 family of scaffolding proteins and exhibit 28–35% sequence similarity. Residue colors indicate side-chain chemistry: yellow, proline; blue, hydrophobic; red, positively charged; magenta, negatively charged; green, polar uncharged; orange, glycine; cyan, aromatic
Protein expression and purification
We designed a construct that comprises residues 396–564 in SASH1. This covers the SASH1 SPIDER (400–554) and the N-terminus of the SH3 domain (555–564). The construct which has a substitution at position 519 (S519N) was cloned into the expression vector pET-28a(+) with an N-terminal His-tag coupled to a thrombin cleavage site. The plasmid was transformed into E. coli strain BL21 (LEMO21-DE3). The expression of the triple labeled protein was conducted using the protocol published recently (Li and Byrd 2022) to ensure proper bacterial adaptation to the deuterated minimal media. The deuterated M9 media was formulated with 1 g/L 15N-ammonium chloride and 2 g/L 13C-7D-glucose. The culture was induced with 0.4 mM isopropyl-1-thio-d-galactopyranoside once the A600 reached 0.7 and shaken overnight at 20 °C. Cells were harvested by centrifugation at 4 °C for 10 min at 5000 × g. The cells were lysed using low-imidazole binding buffer (20 mM HEPES, 200 mM NaCl, 1 mM EGTA, 1 mM MgCl2, 1 mM N aN3, 20 mM imidazole, pH 7.3), disrupted by sonication and cleared by centrifugation at 30,900×g. The lysate was purified using a HisTrap FF column (Cytiva), where the protein was eluted using high-imidazole buffer (20 mM HEPES, 200 mM NaCl, 1 mM EGTA, 1 mM MgCl2, 1 mM NaN3, 200 mM imidazole, pH 7.3). The eluted protein was concentrated to a 4 ml volume. Further purification was completed on a size-exclusion HiLoad 16/600 Superdex 75 pg (Cytiva) in NMR buffer (20 mM NaP, 100 mM NaCl, 1 mM DTT, 0.02 % N aN3, pH 7.0). The relevant fractions were concentrated using a 3000 MWCO concentrator to a concentration of 800 μM before NMR measurements.
NMR spectroscopy
2H–,13C–, and 15N-labeled NMR sample of SPIDER was prepared in the NMR buffer at a concentration of 800 μM and measured in a 5 mm Shigemi tube. The spectra were recorded on BRUKER Avance NEO 600 MHz triple-resonance cryoprobe spectrometers at 5 °C. Backbone assignment was achieved using 1H-15N HSQC, 3D HNCACB–, HN(co) CACB–, HNCO–, and HN(ca)CO-TROSY (Ikura et al. 1990; Cavanagh 2007), and HNN (Weisemann et al. 1993). The 3D spectra were acquired with a nonuniform sampling (NUS) scheme generated by the NUS@HMS scheme generator (Hyberts et al. 2012) employing 2048 complex data points in the direct dimension and 25–30% sampling of the original 256 and 92 points in the indirect 13C and 15N dimensions, respectively. For all experiments, the spectral widths were 8196 Hz (1H), 2129 Hz (15N), 2113 Hz (13C=O), and 12076 Hz (13Cα/13Cβ), the number of scans was 16 and 32 for 3D experiments and 2D HSQC, respectively, and the interscan delay was 1.0 s. The 3D NUS spectra were reconstructed using the hmsIST software (Hyberts et al. 2012), and the linearly acquired 2D spectra were subject to NUS zero-filling as an alternative to linear prediction. A solvent subtraction function was applied in the direct dimension. Further data processing and visualization were performed using NMRpipe/NMRDraw (Delaglio et al. 1995) and NMRFAM Sparky (Lee et al. 2015). Resonance assignment was performed using the CCPNmr analysis software v 2.5.1 (Vranken et al. 2005).
Extent of assignment and data deposition
The 1H-15N HSQC of the SPIDER showed a narrow dispersion of peaks characteristic of a disordered protein (Fig. 3), confirming the prediction. Due to the relatively large protein size, disordered nature and the presence of multiple redundant charged regions, severe peak overlap only enabled us to assign ~ 50% of the backbone without deuteration. Pursuing protein deuteration following a recently introduced optimized protocol (Li and Byrd 2022) in combination with TROSY versions of the 3D backbone assignment experiments supported by a high-quality HNN experiment yielded a signal separation that allowed us to obtain near-to-complete backbone assignment (97.4%) (Table 1; Fig. 3). Of note, lowering the temperature to 5 °C improved the line shapes significantly. The backbone assignment for SPIDER has been deposited in the BMRB with accession code 51747.
Fig. 3.

Deuteration combined with 3D TROSY and HNN experiments allowed for the assignment of 97.4% of the S519N variant of SPIDER. Left: F3-F2 plane showing 1H–15N correlations through the 3D HNN spectrum at the F1–15N chemical shift of 123.2 ppm (orange) overlaid with the 2D 1H–15N HSQC spectrum (teal). Off-diagonal peaks (marked with squares and connected by a line) indicate the 15N chemical shifts of the residues preceding and succeeding the residue represented by the diagonal peak (marked with a circle; L418). Right: 1H–15N HSQC spectrum illustrating peak assignment.
Table 1.
Backbone assignment statistics of SPIDER
| Construct | Total number of relevant residues* | Total number of relevant non-proline residues | % Backbone resonances assigned (number of backbone atoms assigned) |
|---|---|---|---|
|
| |||
| SASH1 396–564 (Linker: 396–399; SPIDER: 400–554; SH3: 555–564) | 169 | 158 | 97.4% (153 15N, 161 Cα, 143 Cβ, 156 CO) |
The construct has 14 additional non-relevant residues from cloning (thrombin cleavage site; His-tag).
Chemical shift analysis
An overlay of 1H–15N HSQC spectra of the S519N variant with the wild-type indicates that while minor peak shifts occur, the overall fingerprint remains unchanged. This indicates that the mutation does not alter the protein’s overall structural and dynamic properties (Fig. 4). Thus, the assignment can also be used for wild-type constructs in future studies of SASH1-SPIDER.
Fig. 4.

Overlay of 1H–15N HSQC spectra of 15N-labeled wild-type (W.T.) SPIDER and S519N variant. While minor peak shifts occur, the overall fingerprint remains mostly unchanged, indicating that the mutation preserves the structural and dynamic properties. Both S519 (unpublished data) and N519 are marked.
To assess the presence of residual secondary structure, we computed the residue-specific Secondary Structure Propensity (SSP) score (Marsh et al. 2006) from the HN, N, Cα, Cβ, and CO chemical shifts (Fig. 5). The SSP score ranges from +1 for a fully formed helix to −1 for a fully formed β-sheet and is ~ 0 for loops and disordered residues. Despite of the overall disordered nature of SPIDER, there are many regions with some β-strand propensity separated by more disordered linkers. Considering SASH1’s established role as a scaffolding protein, an intriguing avenue for further investigation is whether these strands act as nucleation sites for oligomerization events or participate in specific binding interactions. Notably, residue 519 is situated at the N-terminus of one of these transient β-strands (519–529), potentially linking the mutation to disorders associated with this domain.
Fig. 5.

Secondary structure propensities derived from chemical shift values of 1HN, 15N, Cα, Cβ, and CO. An SSP score of +1 indicates a fully formed α-helix, while −1 indicates a fully formed β-sheet. Multiple regions show some strand propensity and are separated by more disordered parts.
Acknowledgements
The authors thank David Jones (University of Colorado, Denver) for his help and support.
Funding
The project was supported by NIH grants R01 AR074420 to YGS, R01 GM130694 to BV, and 1R21 AI171827 to MAH, University of Colorado Cancer Center Support Grant P30 CA046934, and NIH Biomedical Research Support Shared Grant S10 OD025020-01.
Footnotes
Competing interests The authors declare no competing interests.
Ethical approval Not applicable.
Consent of publication All authors have agreed to the publication of the manuscript.
Data availability
The chemical shift assignment of the S519N variant of SPIDER (BMRB 51747) has been deposited in the Biological Magnetic Resonance Data Bank.
References
- Araki A, Okamura K, Saito T (2021) Five novel mutations in SASH1 contribute to lentiginous phenotypes in Japanese families. Pigment Cell Melanoma Res 34:174–178 [DOI] [PubMed] [Google Scholar]
- Beer S, Scheikl T, Reis B et al. (2005) Impaired immune responses and prolonged allograft survival in sly1 mutant mice. Mol Cell Biol 25:9646–9660. 10.1128/mcb.25.21.9646-9660.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J (2007) Protein NMR spectroscopy: principles and practice. Academic Press, Cambridge [Google Scholar]
- Chen S-Z, Zhang Y, Lei S-Y, Zhou F-Q (2020) SASH1 Suppresses the proliferation and invasion of human skin squamous cell carcinoma cells via inhibiting Akt cascade. OncoTarget Ther 30:4617–4625. 10.2147/OTT.S234667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CM, Vögeli B, Shellman YG, Henen MA (2022) SAM1 domain of SASH1 harbors distinctive structural heterogeneity. J Struct Biol. 10.1016/j.jsb.2022.107914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P, Paliouras GN, Clayton A et al. (2019) Endothelial Sash1 is required for lung maturation through nitric oxide signaling. Cell Rep 27:1769–1780. 10.1016/j.celrep.2019.04.039 [DOI] [PubMed] [Google Scholar]
- Courcet JB, Elalaoui SC, Duplomb L et al. (2015) Autosomal-recessive SASH1 variants associated with a new genodermatosis with pigmentation defects, palmoplantar keratoderma and skin carcinoma. Eur J Hum Genet 23:957–962. 10.1038/ejhg.2014.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Guo S, He H et al. (2020) SASH1 promotes melanin synthesis and migration via suppression of TGF-β1 secretion in melanocytes resulting in pathologic hyperpigmentation. Int J Biol Sci 16:1264–1273. 10.7150/ijbs.38415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW et al. (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293 [DOI] [PubMed] [Google Scholar]
- Franke FC, Müller J, Abal M et al. (2019) The tumor suppressor SASH1 Interacts with the signal adaptor CRKL to inhibit epithelial–mesenchymal transition and metastasis in colorectal cancer. Cell Mol Gastroenterol Heptol 7:33–53. 10.1016/j.jcmgh.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke FC, Slusarenko BO, Engleitner T et al. (2020) Novel role for CRK adaptor proteins as essential components of SRC/FAK signaling for epithelial-mesenchymal transition and colorectal cancer aggressiveness. 10.1002/ijc.32955 [DOI] [PubMed] [Google Scholar]
- Hyberts SG, Milbradt AG, Wagner AB et al. (2012) Application of iterative soft thresholding for fast reconstruction of NMR data non-uniformly sampled with multidimensional Poisson Gap scheduling. J Biomol NMR 52:315–327. 10.1007/s10858-012-9611-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikura M, Marion D, Lewis, et al. (1990) Heteronuclear 3D NMR and isotopic labeling of calmodulin: towards the complete assignment of the ‘H NMR spectrum. Bio Chem Pharm 40(1):153–160 [DOI] [PubMed] [Google Scholar]
- Jaufmann J, Franke FC, Sperlich A et al. (2021) The emerging and diverse roles of the SLy/SASH1-protein family in health and disease—overview of three multifunctional proteins. FASEB J. 10.1096/fj.202002495r [DOI] [PubMed] [Google Scholar]
- Lee W, Tonelli M, Markley JL (2015) NMRFAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics 31:1325–1327. 10.1093/bioinformatics/btu830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Byrd RA (2022) A simple protocol for the production of highly deuterated proteins for biophysical studies. J Biol Chem. 10.1016/j.jbc.2022.102253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Zhang J, Xu J et al. (2012) Effects of SASH1 on melanoma cell proliferation and apoptosis in vitro. Mol Med Rep 6:1243–1248. 10.3892/mmr.2012.1099 [DOI] [PubMed] [Google Scholar]
- Marsh JA, Singh VK, Jia Z, Forman-Kay JD (2006) Sensitivity of secondary structure propensities to sequence differences between α- and γ-synuclein: implications for fibrillation. Protein Sci 15:2795–2804. 10.1110/ps.062465306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini M, Gnann A, Scheikl D et al. (2011) The candidate tumor suppressor SASH1 interacts with the actin cytoskeleton and stimulates cell-matrix adhesion. Int J Biochem Cell Biol 43:1630–1640. 10.1016/j.biocel.2011.07.012 [DOI] [PubMed] [Google Scholar]
- Shellman YG, Lambert KA, Brauweiler A et al. (2015) SASH1 is involved in an autosomal dominant lentiginous phenotype. J Invest Dermatol 135:3192–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Nakao A, Nakano N et al. (2001) Identification of Nash1, a novel protein containing a nuclear localization signal, a sterile α motif, and an SH3 domain preferentially expressed in mast cells. Biochem Biophys Res Commun 288:137–141. 10.1006/bbrc.2001.5722 [DOI] [PubMed] [Google Scholar]
- Vranken WF, Boucher W, Stevens TJ et al. (2005) The CCPN data model for NMR spectroscopy: development of a software pipeline. Proteins: Struct Function Genet 59:687–696. 10.1002/prot.20449 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang J, Li X et al. (2017) A novel de novo mutation of the SASH1 gene in a chinese family with multiple lentigines. Acta Derm Venereol 97:530–531 [DOI] [PubMed] [Google Scholar]
- Weidmann H (2015) SASH1, a new potential linkbetween smoking andatherosclerosis. Doctor of physiology and physiopathology. University Pierre et Marie Curie, Paris [Google Scholar]
- Weidmann H, Touat-Hamici Z, Durand H et al. (2015) SASH1, a new potential link between smoking and atherosclerosis. Atherosclerosis 242:571–579. 10.1016/j.atherosclerosis.2015.08.013 [DOI] [PubMed] [Google Scholar]
- Weisemann R, Ruterjans H, Bermel W (1993) 3D Triple-resonance NMR techniques for the sequential assignment of NH and 15N resonances in 15N- and 13C-labelled proteins. J Biomol NMR 3:113–120. 10.1007/BF00242479 [DOI] [PubMed] [Google Scholar]
- Zeller C, Hinzmann B, Seitz S et al. (2003) SASH1: a candidate tumor suppressor gene on chromosome 6q24.3 is downregulated in breast cancer. Oncogene 22:2972–2983. 10.1038/sj.onc.1206474 [DOI] [PubMed] [Google Scholar]
- Zhong WL, Wang HJ, Lin ZM, Yang Y (2019) Novel mutations in SASH1 associated with dyschromatosis universalis hereditaria. Indian J Dermatol Venereol Leprol 85:440. [DOI] [PubMed] [Google Scholar]
- Zhou D, Wei Z, Deng S et al. (2013) SASH1 regulates melanocyte transepithelial migration through a novel Gαs-SASH1-IQGAP1-E-Cadherin dependent pathway. Cell Signal 25:1526–1538. 10.1016/j.cellsig.2012.12.025 [DOI] [PubMed] [Google Scholar]
- Zhou D, Wei Z, Kuang Z et al. (2017) A novel P53/POMC/Gαs/SASH1 autoregulatory feedback loop activates mutated SASH1 to cause pathologic hyperpigmentation. J Cell Mol Med 21:802–815. 10.1111/jcmm.13022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The chemical shift assignment of the S519N variant of SPIDER (BMRB 51747) has been deposited in the Biological Magnetic Resonance Data Bank.


