Abstract
Background:
Neighborhood-level socioeconomic position has been shown to influence birth outcomes, including selected birth defects. This study examines the understudied association between neighborhood-level socioeconomic position during early pregnancy and risk of gastroschisis, an abdominal birth defect of increasing prevalence.
Methods:
We conducted a case–control study of 1,269 gastroschisis cases and 10,217 controls using data from the National Birth Defects Prevention Study (1997 – 2011). To characterize neighborhood-level socioeconomic position, we conducted principal component analysis to construct two indices – Neighborhood Deprivation Index (NDI) and Neighborhood Socioeconomic Position Index (nSEPI). We created neighborhood-level indices using census socioeconomic indicators corresponding to census tracts associated with addresses where mothers lived the longest during the periconceptional period. We used generalized estimating equations to estimate odds ratios (ORs) and 95% confidence intervals (CIs), with multiple imputation for missing data and adjustment for maternal race–ethnicity, household income, education, birth year, and duration of residence.
Results:
Mothers residing in moderate (NDI Tertile 2 aOR: 1.2; 95% CI: 1.0, 1.5 and nSEPI Tertile 2 aOR: 1.2; 95% CI: 1.0, 1.5) or low socioeconomic neighborhoods (NDI Tertile 3 aOR: 1.3; 95% CI: 1.01, 1.6 and nSEPI Tertile 3 aOR: 1.3, 95% CI: 1.1, 1.6) were more likely to deliver an infant with gastroschisis compared with mothers residing in high socioeconomic neighborhoods.
Conclusions:
Our findings suggest that lower neighborhood-level socioeconomic position during early pregnancy is associated with elevated odds of gastroschisis. Additional epidemiologic studies may aid in confirming this finding and evaluating potential mechanisms linking neighborhood-level socioeconomic factors and gastroschisis.
Keywords: Gastroschisis, neighborhood, deprivation, socioeconomic, adverse pregnancy outcomes, disparity
Introduction
Pregnant women residing in low socioeconomic neighborhoods are at higher risk of experiencing adverse pregnancy outcomes.1–3 Neighborhood contextual factors encompass various physical, social, and service characteristics, such as ambient pollutants, levels of social cohesion, and access to goods and services, respectively. The combination of adverse conditions in these contextual factors is often correlated with the neighborhood’s socioeconomic position (nSEP) and is hypothesized to influence maternal health through psychosocial, behavioral, and biologic mechanisms.4,5 Exploratory etiologic studies have reported modest associations between measures of nSEP and birth outcomes, including preterm birth,1,3 low birth weight,6 neural tube defects,7 and, more recently, orofacial clefts8 and conotruncal heart defects,9,10 after accounting for individual-level SEP (iSEP). However, few studies have examined this association with gastroschisis.11
Gastroschisis is an abdominal birth defect characterized by the herniation of intestines and sometimes other abdominal organs outside the fetal body.12,13 In the United States, the gastroschisis prevalence increased from 3.6 (1995 – 2005)13 to 4.3 (2012–2016)12 cases per 10,000 livebirths, suggesting that environmental factors may have an etiologic role in the development of gastroschisis. Gastroschisis disproportionately affects infants of mothers who are young (<20 years),14–16 have low body mass index (BMI),17–20 smoke,16 and are of non-Hispanic White and Hispanic race–ethnicity.16,21,22 Despite these findings, the etiology remains unknown. Proposed underlying mechanisms include rupture of the amnion due to unidentified teratogens during normal physiologic hernia23,24 and disruption of inflammatory pathways.25,26 Identifying neighborhood-level factors that may influence teratogenic exposures or induce an inflammatory response, such as neighborhood deprivation-associated psychosocial stress, may provide further insight into the etiology of gastroschisis.
To our knowledge, only one study, using data from North Carolina (1998 – 2004), investigated the relationship between contextual factors and gastroschisis. The study reported a slight increase in the risk of gastroschisis associated with neighborhoods characterized by high poverty and unemployment, after adjustment for maternal age, race–ethnicity, smoking, and Medicaid status; however, estimates were imprecise.11 Our analysis expands this study by including a larger sample of gastroschisis cases spanning a wider geographical area, additional measures of iSEP, use of composite neighborhood indices, and neighborhood-based periconception addresses.
Neighborhoods may influence known risk factors of gastroschisis through shaping health behaviors, access to resources, and individual opportunities. The association between these risk factors and gastroschisis may, in part, be explained by neighborhood-level factors. Thus, the purpose of this study is to examine the overall association between nSEP during early pregnancy and the risk of gastroschisis.
Methods
Study population
We analyzed data from the National Birth Defects Prevention Study (NBDPS). Details about the NBDPS have been described previously.27 Briefly, the NBDPS is a large, multi-center case–control study sponsored by the Centers for Disease Control and Prevention (CDC) to examine potential risk factors of major structural birth defects, including gastroschisis. Eligible pregnancies between 1997 and 2011 were included from ten participating centers in the following states: Arkansas, California, Georgia, Iowa, Massachusetts, North Carolina, New York, New Jersey, Texas, and Utah. However, in this analysis, New Jersey participants were excluded since geocoded addresses were not available from this center. The NBDPS was approved by the Institutional Review Board (IRB) at the CDC and at each participating center.
We ascertained liveborn, stillborn, or terminated pregnancies with a diagnosis of gastroschisis (cases) (British Pediatric Association (BPA) modification of the International Classification of Diseases, 9th revision (ICD-9): 756.710) through surveillance registries from selected counties (in CA, GA, MA, NC, NY, TX) or the entire state (AR, IA, UT). Clinical geneticists verified cases and classified them as isolated (vs. non-isolated) if gastroschisis was the only major defect or if it occurred with another developmentally related defect.27,28 In this analysis, we included isolated (90.7%) and non-isolated (9.3%) singleton cases. We randomly sampled singleton liveborn infants without a birth defect (controls) using birth certificates or hospital records from the same geographic catchment area and time period as the cases. Mothers were invited to participate in a computer-assisted telephone interview (CATI) between 6 weeks and 24 months after their estimated date of delivery. Approximately 65% of case mothers and 65% of control mothers participated in the interview. Mothers were asked to report information on a variety of exposures and lifestyle factors, including their residential history.
Geocoding addresses and linkage to U.S. census-tract socioeconomic indicators
During the interview, mothers self-reported all residential addresses where they lived for at least 30 days between the three months before pregnancy to the end of pregnancy. The Agency for Toxic Substances and Disease Registry’s Geospatial Research, Analysis, and Services Program successfully geocoded addresses using the Centrus software version 6.00.00N for 97% of NBDPS participants and subsequently linked by the CDC to the 2000 and 2010 U.S. Census Tracts using ArcGIS. We linked census-tract level data from the 2000 US Census and 5-year American Community Survey (ACS) to maternal addresses using Federal Information Processing Standards (FIPS) codes and infant birth year (eTable 1).
Maternal periconceptional neighborhood
We defined “maternal neighborhood” as the census tract corresponding to each participant’s self-reported address during the periconceptional period of her pregnancy (i.e., one month before conception to the third month of pregnancy) to ensure nSEP is captured during the critical period of gastroschisis development (i.e., 8th – 11th gestational week23). If multiple addresses were reported for that period, we selected the address with the longest duration. Participants were excluded if they (1) reported multiple addresses during the periconceptional period with the same duration since it was unclear which address would have a larger influence on the risk of gastroschisis (n = 29) or (2) reported only one address with a duration of < 30 days (n = 3) (Figure 1).
Figure 1.
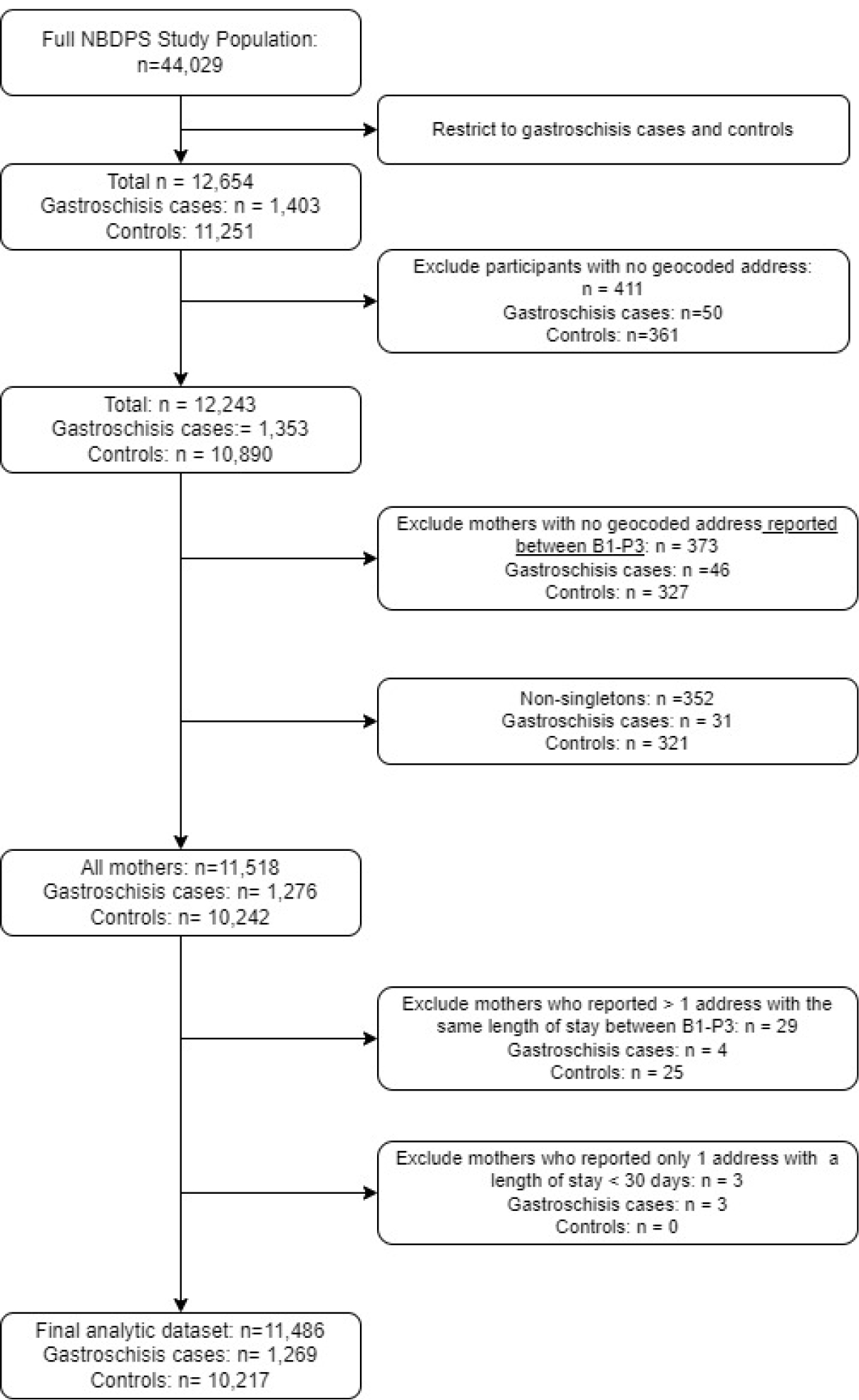
Study population
Among all 12,243 NBDPS gastroschisis cases and controls with at least one geocoded address, we assigned a periconceptional address as described above to 97% (n=11,838). Overall, we excluded ~6% of cases and controls due to missing geocoded addresses during the periconceptional period. Geocoding at the census tract level for maternal neighborhoods was successful for 93% of cases and 94% of controls. 6,315 census tracts were represented in our study sample.
Neighborhood Deprivation Index
To characterize nSEP, we used two indices: the neighborhood deprivation index (NDI) developed by Messer et al.29 and the neighborhood socioeconomic position index (nSEPI) we created using additional census indicators.
Detailed methods used to create the NDI are described elsewhere.29 In brief, eight census-tract level indicators representing five socioeconomic domains (income/poverty, occupation, housing, employment, and education) were selected to construct this index: percent of crowded housing, percent of males in management and professional occupations, percent of households in poverty, percent of households on public assistance, percent of female-headed households with dependents, percent of unemployed residents, percent of households earning < $30,000 per year, and percent of residents with less than a high school education (eTable 2).
We pooled census-tract level indicators across our study sample and performed principal component analysis (PCA). We retained the first principal component because it accounted for the largest proportion of the total variation among the component measures,29 and used component loadings to weight each census variable’s contribution to the index score. We standardized the index to have a mean of 0 and standard deviation of 1 (Figure 2, Range: −1.7, 5.6), with low scores indicating less deprivation and high scores indicating greater deprivation. We linked the NDI to NBDPS participants and categorized into tertiles (low [reference], moderate, and high neighborhood deprivation) to examine a potential gradient in the risk of gastroschisis. We categorized tertiles based on the distribution among controls since the control group best reflects the source population that gave rise to the cases.
Figure 2.
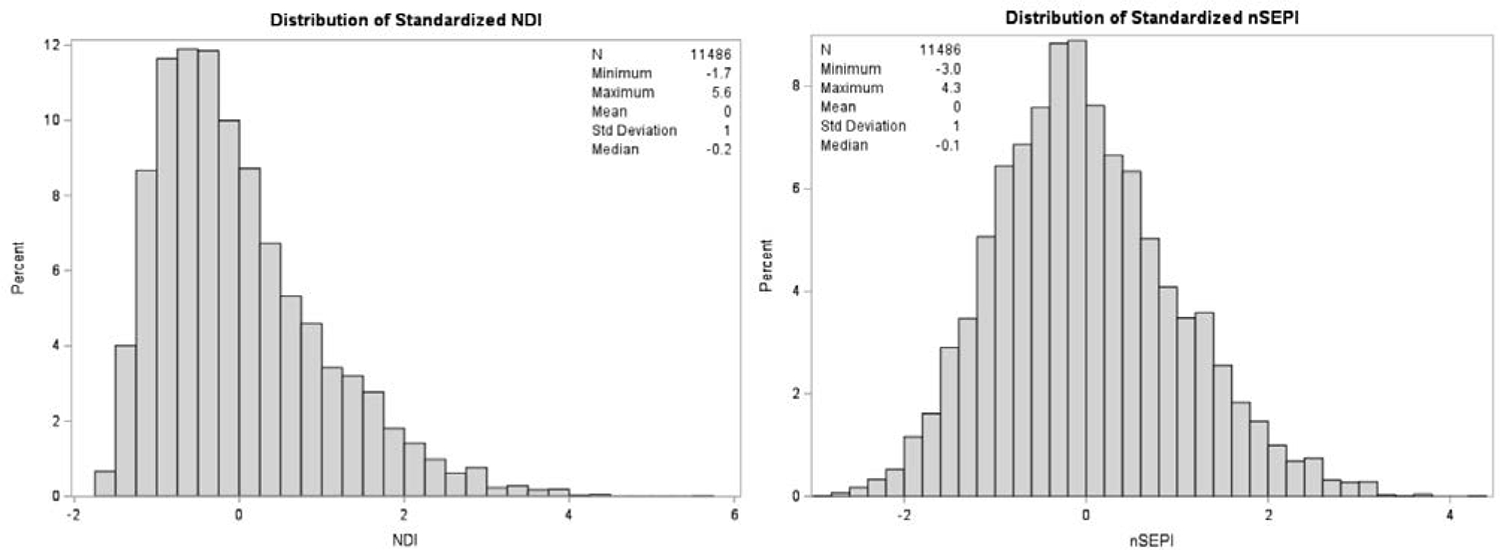
Distribution of Neighborhood Deprivation Index (NDI) and Neighborhood Socioeconomic Position Index (nSEPI), National Birth Defects Prevention Study, 1997 – 2011
Neighborhood Socioeconomic Position Index
Despite its being an established index of neighborhood deprivation, 1,30 the NDI was developed with data from a different time period and geographic area than our study which may reduce its validity for our study population.29 Furthermore, socioeconomic position (SEP), both at the individual and neighborhood level, is a multidimensional construct that should be measured with as many indicators as possible to reflect each socioeconomic domain.31 Although socioeconomic indicators are correlated, they are generally not strong enough to be proxies for one another.31 Thus, we constructed an independent data-driven index of nSEP within our study population, the nSEPI, which includes additional indicators, specifically measures of socioeconomic advantage, to provide a richer representation of each socioeconomic domain.
The nSEPI is composed of the eight NDI single-census indicators and nine additional indicators: percent of residents with a bachelor’s degree or higher; percent of employed residents who are nature, construction, or transportation workers; percent of employed residents who reported being an unpaid family worker or self-employed; median household income; percent of owner-occupied homes with values greater than $300,000; percent of owner-occupied homes with a mortgage and monthly owner costs of 30% or more of household income; percent of owner-occupied homes without a mortgage and monthly owner costs of 30% or more of household income; percent of renter-occupied units among total occupied housing units and percent of renter-occupied units with a monthly rent that costs 30% or more of household income (eTable 2). We constructed the nSEPI following the same methods as the NDI (Figure 2, Range: −3.0, 4.3), with low scores indicating high nSEP and high scores indicating low nSEP. Tertiles represent high (reference), moderate, and low nSEP.
Individual-level covariates
We obtained individual-level variables from the NBDPS interview. We selected potential confounders a priori using a directed acyclic graph (DAG)32 (Figure 3) and included infant birth year (1997–2004, 2005–2009, 2010–2011); iSEP [maternal years of education (0–11, 12, > 12 years) and household income (<$10,000, $10,000 - $50,000, >$50,000)]; self-identified maternal race–ethnicity (Non-Hispanic Black, Non-Hispanic White, Hispanic, and Other); and duration of residency (continuous). We conceptualized race–ethnicity as a socially constructed classification system, based on phenotypic characteristics, that is deeply entwined with residential and socioeconomic segregation. We included duration of residency because the time individuals spend living in a neighborhood likely impacts the amount of nSEP exposure and influences the risk of gastroschisis through unmeasured variables related to gastroschisis such as environmental exposures. Additional descriptive variables we included but not in the covariate adjustment set are in Table 1.
Figure 3.
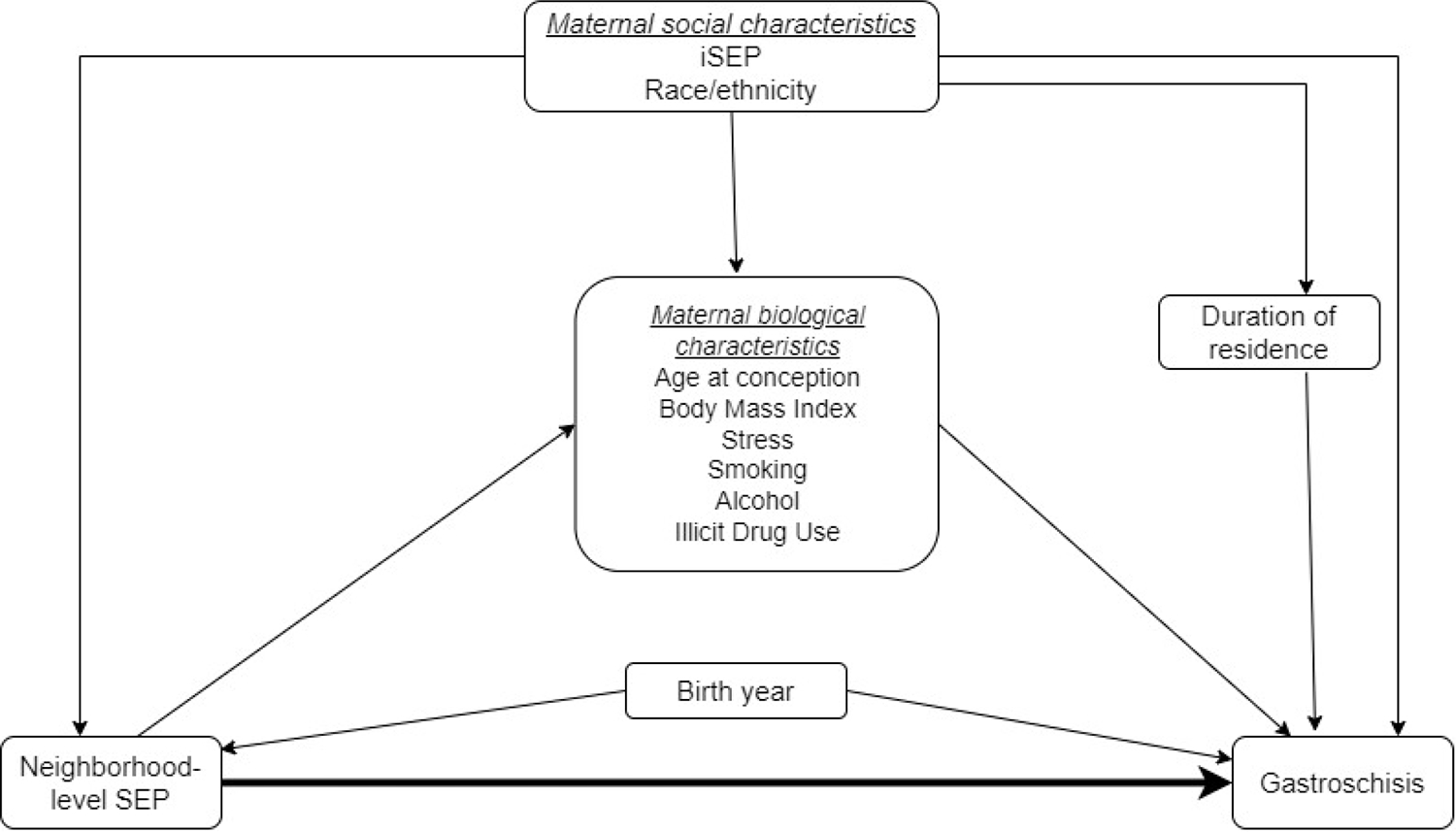
Simplified directed acyclic graph (DAG) for the association between neighborhood-level socioeconomic position during early pregnancy and risk of gastroschisis
Table 1.
Maternal and infant characteristics for mothers of participants with gastroschisis (cases) and infants without a birth defect (controls), National Birth Defects Prevention Study, 1997–2011
| Gastroschisis Cases n = 1,269 | Controls n = 10,217 | Total n = 11,486 | |
|---|---|---|---|
|
| |||
| Maternal age at conception (years), n(%) | |||
| <20 | 540 (43) | 1294 (13) | 1834 (16) |
| 20–25 | 541 (43) | 2969 (29) | 3510 (31) |
| 26–35 | 178 (14) | 5106 (50) | 5284 (46) |
| ≥36 | 10 (1) | 848 (8) | 858 (7) |
|
| |||
| Parity, n(%) | |||
| 0 | 830 (65) | 3986 (39) | 4816 (42) |
| 1 | 272 (21) | 3334 (33) | 3606 (31) |
| ≥2 | 166 (13) | 2891 (28) | 3057 (27) |
| Missing | 1 (0) | 6 (0) | 7 (0) |
|
| |||
| Maternal race/ethnicity, n(%) | |||
| Non-Hispanic White | 644 (51) | 5981 (59) | 6625 (58) |
| Non-Hispanic Black | 107 (8) | 1089 (11) | 1196 (10) |
| Hispanic | 410 (32) | 2486 (24) | 2896 (25) |
| Other | 108 (9) | 661 (6) | 769 (7) |
|
| |||
| Maternal pre-pregnancy BMI (kg/m2), n(%) | |||
| Underweight (<18.5) | 109 (9) | 508 (5.0) | 617 (5) |
| Normal weight (18.5≤BMI<25) | 830 (65) | 5198 (50.9) | 6028 (53) |
| Overweight (25≤BMI<30) | 223 (18) | 2251 (22.0) | 2474 (22) |
| Obese (≥30) | 71 (6) | 1858 (18.2) | 1929 (17) |
| Missing | 36 (3) | 402 (3.9) | 438 (4) |
|
| |||
| Maternal education (years), n(%) | |||
| <12 | 348 (27) | 1692 (17) | 2040 (18) |
| 12 | 488 (38) | 2437 (24) | 2925 (25.5) |
| >12 | 434 (34) | 6087 (60) | 6521 (57) |
|
| |||
| Annual household income ($USD), n(%) | |||
| <$10,000 | 438 (35) | 2018 (20) | 2456 (21.4) |
| $10,000 – $50,000 | 684 (54) | 4666 (46) | 5350 (46.6) |
| >$50,000 | 147 (12) | 3533 (35) | 3680 (32.0) |
|
| |||
| Maternal employment, n(%) | |||
| Employed | 852 (67) | 7213 (71) | 8065 (70) |
| Unemployed | 376 (30) | 2839 (28) | 3215 (28) |
| Unknown | 0 (0) | 5 (0) | 5 (0) |
| Missing | 41 (3) | 160 (2) | 201 (2) |
|
| |||
| Maternal recreational drug usea,b, n(%) | |||
| Yes | 171 (14) | 456 (5) | 627 (5) |
| No | 1057 (83) | 9600 (94) | 10657 (93) |
| Missing | 41 (3) | 161 (2) | 202 (2) |
|
| |||
| Maternal smokinga, n(%) | |||
| Yes | 438 (35) | 1812 (18) | 2250 (2) |
| No | 793 (63) | 8250 (81) | 9043 (79) |
| Missing | 38 (3) | 155 (2) | 193 (2) |
|
| |||
| Maternal alcohola, n(%) | |||
| Yes | 518 (41) | 3752 (37) | 4270 (37) |
| No | 708 (56) | 6284 (62) | 6992 (61) |
| Missing | 43 (3) | 181 (2) | 224 (2) |
|
| |||
| Gestational age at delivery (weeks), n(%) | |||
| Very preterm (<32 wks) | 80 (6) | 107 (1) | 187 (2) |
| Preterm (32–36 wks) | 710 (56) | 725 (7) | 1435 (12 |
| Term (37–45 wks) | 478 (38) | 9384 (92) | 9862 (86) |
| Missing | 1 (0) | 1 (0) | 2 (0) |
|
| |||
| Duration of residence (days), mean (range) | 952 (30 – 14,152) | 1,274 (30 – 14,456) | 1,239 (30 – 14,456) |
BMI: Body mass index
Self-reported use between one month prior to conception to third month of pregnancy
Recreational drug use includes: marijuana, hash, cocaine, crack, hallucinogens, heroin, hallucinogenic mushrooms
Statistical analysis
We used multiple imputation with fully conditional specification to account for missing data, including household income (9% missing), maternal education (2% missing), and census-tract SEP indicators (0.01% – 0.29% missing), assuming data were missing at random. We conducted ten imputation cycles. We conducted principal components analysis (PCA) and generalized estimating equations (GEEs) on each imputation dataset and pooled results using the proc mianalyze procedure in SAS.33
We used generalized estimating equations (GEEs) with logistic links and robust errors to estimate crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs). The use of GEEs accounted for potential correlation and non-independence of outcomes among mothers clustered within the same neighborhood. We modeled single-tract-level nSEP indicators on gastroschisis to explore associations between specific aspects of nSEP and gastroschisis, adjusting for the individual-level covariates identified above. We did not adjust for the larger set of census-tract-level indicators because they are likely affected by the single-tract-level indicator, and would thus, be mediators. Additionally, we examined the association between two neighborhood-level indices (NDI and nSEPI) and gastroschisis. We adjusted estimates for covariates identified in the directed acyclic graph (DAG) and examined the correlation between the two neighborhood-level indices using Spearman correlations. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Study population description
We analyzed data from 11,486 NBDPS participants, including 1,269 gastroschisis infants and 10,217 controls. Compared with control mothers, case mothers were more likely to be young (< 25 years), nulliparous, Hispanic, have a BMI < 25 kg/m2, smoke, use recreational drugs, have a household income of < $10,000, and have ≤ 12 years of education. The average total duration of residency was approximately 3 years, with case mothers having a shorter mean length of stay (~2.6 years) compared to control mothers (~3.5 years) (Table 1). Case mothers (45–46%) were more likely to reside in higher deprivation areas compared to control mothers (33%) (Table 2). Additionally, non-Hispanic Black and Hispanic mothers were more likely to reside in higher deprivation areas compared with non-Hispanic White mothers (Figure 4).
Table 2.
Distribution of gastroschisis cases and controls by neighborhood indices, NDI and nSEPI, n(%)
| Gastroschisis Cases n = 1,269 | Controls n = 10,217 | |
|---|---|---|
| Neighborhood Deprivation Index (NDI) | ||
| T1 (Low deprivation) | 246 (19) | 3406 (33) |
| T2 | 454 (36) | 3406 (33) |
| T3 (High deprivation) | 569 (45) | 3405 (33) |
| Neighborhood Socioeconomic Position Index (nSEPI) | ||
| T1 (High nSEP) | 240 (19) | 3406 (33) |
| T2 | 449 (35) | 3406 (33) |
| T3 (Low nSEP) | 580 (46) | 3405 (33) |
Neighborhood indices were created within each imputed dataset via principal component analysis. Counts and frequencies were averaged over the 10 imputed datasets.
NDI: Neighborhood Deprivation Index; nSEPI: Neighborhood Socioeconomic Position Index: nSEP: Neighborhood Socioeconomic Position
High tertile scores reflect high deprivation (NDI) or lower nSEP (nSEPI). Low tertile scores reflect low deprivation (NDI) or high nSEP (nSEPI).
Figure 4.
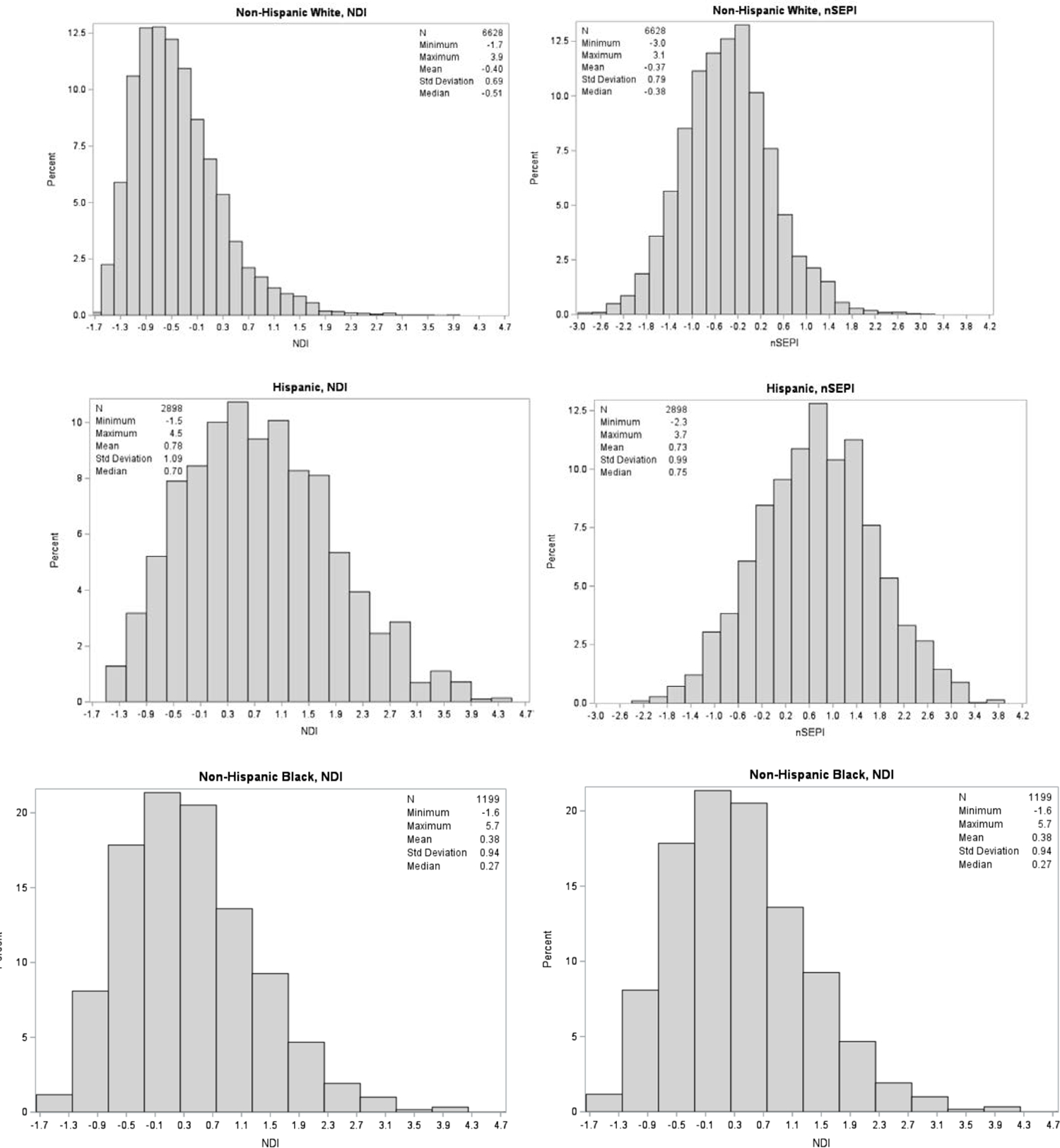
Distribution of Neighborhood Deprivation Index (NDI) and Neighborhood Socioeconomic Position Index (nSEPI) by maternal race/ethnicity, National Birth Defects Prevention Study, 1997 – 2011
Neighborhood-level indices were highly correlated (r = 0.99). The first principal component of the NDI and nSEPI explained about 57% and 41% of the total variability among the component measures, respectively. The top three indicators that were strongly correlated with the first principal component of both indices were low education, households earning < $30,000 per year, and poverty (eTable 3).
Single census-tract socioeconomic indicators
Mothers residing in areas characterized by adverse census indicators had a higher unadjusted risk of having an infant with gastroschisis, whereas mothers residing in areas characterized by favorable indicators had a reduced unadjusted risk. Adjustment for maternal-level characteristics attenuated the crude effect estimates such that the majority of single census-tract nSEP indicators had odds ratios around the null; however, few associations remained (Table 3).
Table 3.
Association between single census-tract level socioeconomic indicators and gastroschisis among women with at least one geocoded address during the periconceptional period, National Birth Defects Prevention Study, 1997 – 2011
| Gastroschisis Cases n = 1,269 | Crude ORs (95% CIs) | Adjusted ORs (95% CIs)a | |
|---|---|---|---|
|
| |||
| Crowdingb,c | |||
| T1 | 341 | Ref | Ref |
| T2 | 417 | 1.2 (1.1 – 1.4) | 1.1 (0.92 – 1.3) |
| T3 | 511 | 1.5 (1.3 – 1.7) | 1.1 (0.89 – 1.3) |
|
| |||
| Low educationb,c | |||
| T1 | 288 | Ref | Ref |
| T2 | 422 | 1.5 (1.3 – 1.7) | 1.1 (0.90 – 1.3) |
| T3 | 559 | 1.9 (1.7 – 2.3) | 1.1 (0.92 – 1.3) |
|
| |||
| Unemploymentb,c | |||
| T1 | 301 | Ref | Ref |
| T2 | 417 | 1.5 (1.3 – 1.7) | 1.2 (1.0 – 1.4) |
| T3 | 551 | 1.9 (1.6 – 2.2) | 1.3 (1.1 – 1.6) |
|
| |||
| Males in management and professional occupationb,c | |||
| T1 | 526 | Ref | Ref |
| T2 | 452 | 0.86 (0.75 – 0.98) | 1.1 (0.94 – 1.3) |
| T3 | 291 | 0.55 (0.47 – 0.65) | 0.87 (0.72 – 1.0) |
|
| |||
| Female-headed households w/ dependentsb,c | |||
| T1 | 288 | Ref | Ref |
| T2 | 458 | 1.6 (1.4 – 1.9) | 1.3 (1.1 – 1.5) |
| T3 | 523 | 1.8 (1.6 – 2.1) | 1.27 (1.06 – 1.52) |
|
| |||
| Povertyb,c | |||
| T1 | 264 | Ref | Ref |
| T2 | 455 | 1.7 (1.5 – 2.0) | 1.2 (1.0 – 1.4) |
| T3 | 550 | 2.1 (1.8 – 2.4) | 1.2 (0.98 – 1.4) |
|
| |||
| Households earning < $30,000b,c | |||
| T1 | 281 | Ref | Ref |
| T2 | 449 | 1.6 (1.4 – 1.9) | 1.1 (0.95 – 1.3) |
| T3 | 539 | 1.9 (1.7 – 2.2) | 1.1 (0.91 – 1.3) |
|
| |||
| Public Assistance Incomeb,c | |||
| T1 | 339 | Ref | Ref |
| T2 | 376 | 1.1 (0.95 – 1.3) | 0.91 (0.78 – 1.1) |
| T3 | 554 | 1.6 (1.4 – 1.9) | 1.1 (0.96 – 1.3) |
|
| |||
| High educationc | |||
| T1 | 575 | Ref | Ref |
| T2 | 444 | 0.77 (0.67 – 0.88) | 0.95 (0.83 – 1.1) |
| T3 | 250 | 0.44 (0.37 – 0.51) | 0.76 (0.64 – 0.92) |
|
| |||
| Affordable housing w/ mortgagec | |||
| T1 | 358 | Ref | Ref |
| T2 | 412 | 1.2 (0.99 – 1.3) | 1.1 (0.94 – 1.3) |
| T3 | 499 | 1.4 (1.2 – 1.6) | 1.2 (0.98 – 1.4) |
|
| |||
| Affordable housing w/out mortgagec | |||
| T1 | 415 | Ref | Ref |
| T2 | 445 | 1.1 (0.93 – 1.2) | 1.0 (0.88 – 1.2) |
| T3 | 409 | 0.99 (0.85 – 1.1) | 0.91 (0.78 – 1.1) |
|
| |||
| Manual occupationc | |||
| T1 | 276 | Ref | Ref |
| T2 | 443 | 1.6 (1.4 – 1.9) | 1.2 (0.98 – 1.4) |
| T3 | 550 | 2.0 (1.7 – 2.3) | 1.2 (1.0 – 1.4) |
|
| |||
| Workers classc | |||
| T1 | 446 | Ref | Ref |
| T2 | 404 | 0.91 (0.78 – 1.1) | 0.98 (0.85 – 1.1) |
| T3 | 419 | 0.94 (0.81 – 1.1) | 0.91 (0.79 – 1.1) |
|
| |||
| Renters affordable housingc | |||
| T1 | 363 | Ref | Ref |
| T2 | 427 | 1.2 (1.0 – 1.4) | 1.1 (0.94 – 1.3) |
| T3 | 479 | 1.3 (1.1 – 1.5) | 1.1 (0.91 – 1.3) |
|
| |||
| Renter occupancyc | |||
| T1 | 340 | Ref | Ref |
| T2 | 416 | 1.2 (1.1 – 1.4) | 1.0 (0.86 – 1.2) |
| T3 | 513 | 1.5 (1.3 – 1.8) | 1.1 (0.97 – 1.3) |
|
| |||
| Wealthc | |||
| T1 | 437 | Ref | Ref |
| T2 | 477 | 1.1 (0.95 – 1.3) | 1.2 (1.0 – 1.4) |
| T3 | 355 | 0.81 (0.70 – 0.94) | 1.1 (0.95 – 1.4) |
|
| |||
| Household median incomec | |||
| T1 | 503 | Ref | Ref |
| T2 | 492 | 0.98 (0.86 – 1.1) | 1.1 (0.97 – 1.3) |
| T3 | 274 | 0.55 (0.47 – 0.64) | 0.88 (0.72 – 1.1) |
NDI: Neighborhood Deprivation Index; nSEPI: Neighborhood Socioeconomic Position Index; T1: Tertile 1; T2: Tertile 2; T3: Tertile 3; OR: Odds Ratio; CI: Confidence Interval
High tertile scores reflect a high proportion of residents that meet census indicator definition. Low tertile scores reflect a low proportion of residents that meet census indicator definition.
Separate models were run for each census indicator.
Adjusted for maternal race/ethnicity (Non-Hispanic Black, Non-Hispanic White, Hispanic, Other), infant birth year (1997 – 2004, 2005 – 2009, 2010 – 2011), household income (<$10,000 $10,000 – $50,000, >$50,000), maternal education (0–11, 12 and > 12 years), and duration of residence
Census indicator used to create the NDI
Census indicator used to create the nSEPI
Neighborhood-level indices
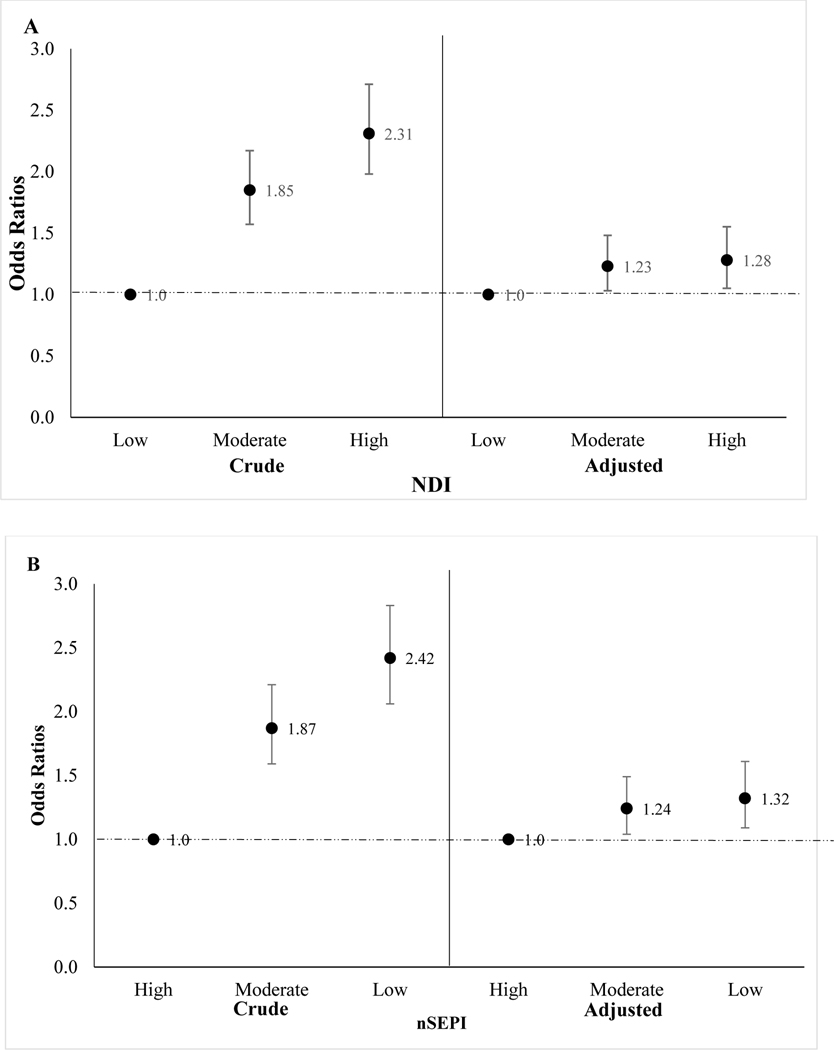
Results were similar for both neighborhood-level indices (Table 4). We observed a monotonic increase in the unadjusted odds of gastroschisis. Upon covariate adjustment, the patterns of association remained similar to the crude estimates, though estimates were attenuated towards the null. Mothers residing in moderate (NDI Tertile 2: aOR: 1.2; 95% CI: 1.0, 1.5) or high deprivation (NDI Tertile 3 aOR: 1.3; 95% CI: 1.1, 1.6) neighborhoods were more likely to deliver an infant with gastroschisis compared with mothers residing in low deprivation areas. Similarly, mothers residing in moderate (nSEPI Tertile 2 aOR: 1.2; 95% CI: 1.0, 1.5) or low nSEP areas (nSEPI Tertile 3 aOR: 1.3, 95% CI: 1.1, 1.6) had elevated risks of having an infant with gastroschisis (Figure 5) compared with mothers residing in high nSEP areas.
Table 4.
Association between neighborhood indices and gastroschisis among women with at least one geocoded address during the periconceptional period, National Birth Defects Prevention Study, 1997 – 2011
| Case n = 1,269 | Crude ORs (95% CIs) | Adjusted ORs (95% CIs)a | |
|---|---|---|---|
|
| |||
| NDI | |||
| T1 (Low deprivation) | 246 | Ref | Ref |
| T2 | 454 | 1.9 (1.6 – 2.2) | 1.2 (1.0 – 1.5) |
| T3 (High deprivation) | 569 | 2.3 (2.0 – 2.7) | 1.3 (1.1 – 1.6) |
|
| |||
| nSEPI | |||
| T1 (High nSEP) | 240 | Ref | Ref |
| T2 | 449 | 1.9 (1.6 – 2.2) | 1.2 (1.0 – 1.5) |
| T3 (Low nSEP) | 580 | 2.4 (2.1 – 2.8) | 1.3 (1.1 – 1.6) |
T1: Tertile 1; T2: Tertile 2; T3: Tertile 3; NDI: Neighborhood Deprivation Index; nSEPI: Neighborhood Socioeconomic Position Index
High tertile scores reflect high deprivation (NDI) or lower nSEP (nSEPI). Low tertile scores reflect low deprivation (NDI) or high nSEP (nSEPI).
Adjusted for maternal race/ethnicity (Non-Hispanic Black, Non-Hispanic White, Hispanic, Other), infant birth year (1997 – 2004, 2005 – 2009, 2010 – 2011), household income (<$10,000 $10,000 – $50,000, >$50,000), maternal education (0–11, 12 and > 12 years), and duration of residence
FIGURE 5.
Crude and adjusted odds ratios (95% confidence intervals) for the association between (A) Neighborhood Deprivation Index (NDI) (B) Neighborhood Socioeconomic Position Index (nSEPI), and gastroschisis. Adjusted for maternal race/ethnicity, education, household income, birth year, and duration of residence.
Discussion
Principal findings
This study examined the overall association between measures of nSEP during early pregnancy and risk of gastroschisis. We constructed two weighted neighborhood-level indices (NDI and nSEPI) and results were similar. Given the NDI is a standardized index that has been widely used to describe associations between neighborhood-level factors and birth outcomes, we believe the NDI may be a better measure of nSEP. Our findings suggest an overall elevation in risk of gastroschisis among mothers residing in neighborhoods characterized by moderate and high levels of socioeconomic deprivation during early pregnancy.
Literature review
To date, only one known study has explored this relationship. Root et al.11 conducted a case–control study in North Carolina (1998 – 2004) of 242 gastroschisis cases. Five SEP indicators were used to estimate nSEP including percent of residents living below 100% and 200% of the federal poverty level, percent of residents with less than a high school education, percent of residents unemployed, and percent of African American residents. Although estimates were imprecise, residing in census tracts characterized by high levels of residents living below 200% of the federal poverty level (aOR: 1.25; 95% CI: 0.81, 1.97) and unemployment (aOR: 1.21; 95% CI: 0.81,1.84) was modestly associated with gastroschisis, after adjustment for maternal age, race–ethnicity, parity, smoking, and Medicaid status.11
Similarly, we observed a modest increase in the risk of gastroschisis associated with neighborhoods characterized by moderate to high unemployment and poverty. However, direct comparison of results is difficult due to differences in nSEP measures, study size, covariate adjustment sets, and study population geographical areas. Our analysis expands meaningfully on Root et al.’s study11 in four ways. First, we evaluated this association in a larger sample of 1,269 gastroschisis cases spanning a national geographical range. Second, we examined single SEP indicators and composite neighborhood-level indices. Third, we used the address during the periconceptional period to define maternal neighborhood to reduce exposure misclassification. Last, we included additional measures of iSEP, specifically, maternal education and household income, to account for factors associated with residential selection.
Our findings add to a small body of literature examining the overall associations between nSEP and birth defects. In general, results are inconsistent. Two studies9,34 using similar nSEP factors as in our analysis reported a modest association with cleft lip with or without palate, whereas a study35 using the Carstairs index reported no associations. Inconsistent findings with neural tube defects7,36 and conotruncal heart defects have also been reported.9,10
Studies of neighborhood-level effects on pregnancy outcomes have typically adjusted for iSEP, as in our analysis. While adjustment theoretically mitigates bias due to confounding, there may be compelling reasons to consider the interpretation of unadjusted estimates when investigating nSEP. Epidemiologic evidence suggests that iSEP, measured by low household income, insurance status, and mothers whose fathers were absent, may be associated with gastroschisis, after adjustment for maternal age.15,22,37 If nSEP during the periconceptional period influences, at least in part, iSEP, unadjusted estimates may be more appropriate because adjusting for it may remove part of the neighborhood-level effect. For example, if residing in deprived neighborhoods with few career opportunities affects an individual’s income, adjusting for iSEP measures would lead to overadjustment bias,38 producing estimates that may be attenuated. If so, crude models may be more appropriate. However, if iSEP is more likely influenced by socioeconomic factors of maternal neighborhoods at birth or at another point along the life course, adjusting for it will account for residential selection during the periconceptional period and produce neighborhood-level effect estimates above and beyond individual-level factors. Furthermore, studies have shown that residents tend to remain in neighborhoods within a given socioeconomic stratum39,40 and that there is a low degree of social mobility in the United States.41 Thus, it is plausible that an individual’s current neighborhood has less of an impact on iSEP than originally thought and is the basis for including it as a confounder in this analysis.
Additionally, we did not identify maternal age at conception, one of the strongest risk factors for gastroschisis, as a confounder, but rather a mediator based on our DAG. Studies have shown there are many social, cultural, and economic factors that influence childbearing age.42,43 For instance, women with access to career opportunities are likely to pursue professional occupations, which may increase labor force participation, further postponing childbearing age.42,44 Moreover, given childrearing often follows childbearing, raising a child is intertwined with the social support and material resources available to mothers within their community, such as support among residents and quality childcare services.43–45 Thus, despite the strong association between maternal age and gastroschisis, we believe that nSEP in part influences when a woman conceives. This reasoning can similarly be applied to other risk factors of gastroschisis, such as BMI and risky health behaviors, given that nSEP likely influences other individual-level characteristics.2,4,46–49 Since our analysis is focused on examining the total effect of nSEP and gastroschisis, adjusting for maternal age at conception would introduce bias. However, it is important to acknowledge that the strong correlation between maternal age and iSEP is difficult to deconstruct. Adjustment for iSEP may inadvertently also adjust for maternal age. Thus, it may be more appropriate to consider the unadjusted and adjusted estimates as a range that contains a more accurate estimate of this overall association.50 Nevertheless, our results suggest that lower nSEP during early pregnancy may increase the risk of gastroschisis.
Potential mechanisms linking neighborhoods to gastroschisis
There are multiple mechanisms by which neighborhoods may influence individual health.4,48,49 It is hypothesized that the physical, social, and service environment of a neighborhood impacts individual health by mediating through biologic and/or social factors, such as psychosocial stress and health behaviors, that may directly or indirectly affect biologic processes.2,4,46–48 However, these mechanisms are unknown, partially because the etiology and pathogenesis of gastroschisis are unknown. To aid in hypothesizing these mechanisms, we considered theorized pathways proposed in other neighborhood-birth outcome studies.
The first hypothesized mechanism is through the neighborhood’s built environment. This includes exposure to air or noise pollution, environmental toxins, dilapidated housing, and overall physical space. Given some studies have shown increased odds of gastroschisis with pesticide exposure51 and overall environmental exposures, as assessed by the Environmental Quality Index,52 neighborhood physical characteristics may have direct biologic consequences on gastroschisis development.
Additionally, these characteristics may have indirect biological effects through its influence on the social environment of the neighborhood.49 This refers to the relationships among residents, level of cohesion or disorganization, and overall community integration.53 This mechanism may influence maternal health, and subsequently gastroschisis, through exposure to acute and chronic psychosocial stress. Although no studies to date have examined the association between stress-related biomarkers and gastroschisis, cumulative stress exposures defined to have biologic evidence of inducing an inflammatory stress response26 and stressful life events54 have been shown to increase the odds of gastroschisis, indicating that social factors may directly affect biologic processes and play a role in gastroschisis development. Furthermore, social factors may indirectly affect biological processes through downstream factors by influencing the adoption of stress-related health behaviors as coping mechanisms, such as smoking, which have been shown to be associated with gastroschisis.22
Depending on the extent to which residents cohesively work together to demand services for their community, the social environment may indirectly influence the service environment of the neighborhood. This reflects the availability of goods and services such as health care, transportation systems, police and fire safety, and healthy foods.48,55 Depending on the level of neighborhood deprivation, necessary and high-quality services, such as prenatal care and municipal services, may not exist or may be relatively inaccessible in certain communities. Areas with poor access to services may not only have a direct impact on the safety and health of residents but may also indirectly contribute to additional levels of psychosocial stress influencing the risk of gastroschisis, especially among women exposed to other negative aspects of their neighborhood.
Although our study was not aimed to assess a specific causal mechanism linking nSEP factors and gastroschisis, our findings confirm there is a contextual element that may directly or indirectly influence maternal factors associated with gastroschisis.
Strengths and limitations
The use of NBDPS data provided many strengths including geographic diversity, population-based ascertainment of cases and controls, a large sample of gastroschisis cases, clinically verified outcomes, and extensive covariate information. We extended the current literature on the relationship between nSEP and gastroschisis by including multiple SEP indicators and two neighborhood-level indices.29 Furthermore, this analysis was also strengthened by defining maternal neighborhood based on the address during the periconceptional period to ensure nSEP was present during the critical period of gastroschisis development.
This analysis, however, is not without limitations. First, although we adjusted for covariates identified in our DAG, residual confounding may occur due to unmeasured factors.31 Additionally, given that iSEP is strongly correlated with maternal age at conception, a mediator in this study, adjustment for iSEP may inadvertently adjust for maternal age. Second, neighborhoods were geographically defined using census tracts rather than maternal perception of her neighborhood. Nevertheless, census tracts are often used in neighborhood-level studies and have been shown to be meaningfully useful in relation to birth outcomes.56 Third, if factors associated with participation or having a geocoded address were systematically different from nonparticipants and women without a geocoded address, exclusion of both would lead to bias. However, a previous study reported that NBDPS control mothers were generally representative of their base populations.57 Also, given only 3% of NBDPS participants were excluded due to missing geocoded addresses, selection bias is likely minimal. Finally, assessment of neighborhood-level factors at one point in time may not only conceal how disparate neighborhoods truly are since neighborhoods may change over time, but may also inaccurately reflect a mother’s cumulative lifetime exposure to these contextual factors. Lack of data on the historical context of periconceptional neighborhoods and the neighborhoods mothers were born into and/or raised likely understates the true impact of neighborhoods on birth outcomes.39
Conclusions
Our study suggests that lower nSEP during early pregnancy is modestly associated with elevated odds of gastroschisis. These findings require replication in additional epidemiologic studies. Future studies could further explore the degree to which individual-level factors, such as maternal age at conception and risky health behaviors, account for the influence of nSEP on the risk of gastroschisis. Greater insight into mechanisms linking nSEP and gastroschisis will potentially help identify modifiable neighborhood characteristics that may be critical in shaping future public health interventions.
Supplementary Material
Sources of funding:
This work was supported by a cooperative agreement from the Centers for Disease Control and Prevention (CDC; U50CCU422096) to the North Carolina Center for Birth Defects Research and Prevention and through cooperative agreements under PA 96043, PA 02081, and FOA DD09–001 from the CDC to other Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study. This work was also supported in part by an institutional training grant from the National Institute of Child Health and Development (T32 HD 52468).
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Data availability:
The process for accessing the data used in this analysis is described here: https://www.cdc.gov/ncbddd/birthdefects/nbdps-public-access-procedures.html. Computing code may be requested from the corresponding author.
References
- 1.O’Campo P, Burke JG, Culhane J, et al. Neighborhood deprivation and preterm birth among non-Hispanic Black and white women in eight geographic areas in the United States. Am J Epidemiol. 2008;167(2):155–163. doi: 10.1093/aje/kwm277 [DOI] [PubMed] [Google Scholar]
- 2.Vinikoor-Imler LC, Messer LC, Evenson KR, Laraia BA. Neighborhood conditions are associated with maternal health behaviors and pregnancy outcomes. Soc Sci Med. 2011;73(9):1302–1311. doi: 10.1016/j.socscimed.2011.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickett KE, Ahern JE, Selvin S, Abrams B. Neighborhood socioeconomic status, maternal race and preterm delivery: A case–control study. Ann Epidemiol. 2002;12(6):410–418. doi: 10.1016/S1047-2797(01)00249-6 [DOI] [PubMed] [Google Scholar]
- 4.Culhane JF, Elo IT. Neighborhood context and reproductive health. doi: 10.1016/j.ajog.2005.01.071 [DOI] [PubMed] [Google Scholar]
- 5.Robert SA. Socioeconomic position and health: The independent contribution of community socioeconomic context. Annu Rev Sociol. 1999;25:489–516. doi: 10.1146/annurev.soc.25.1.489 [DOI] [Google Scholar]
- 6.O’Campo P, Xue X, Wang MC, Brien Caughy MO. Neighborhood risk factors for low birthweight in Baltimore: a multilevel analysis. Am J Public Health. 1997;87(7):1113. doi: 10.2105/AJPH.87.7.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasserman CR, Shaw GM, Selvin S, Gould JB, Syme SL. Socioeconomic status, neighborhood social conditions, and neural tube defects. Am J Public Health. 1998;88(11):1674–1680. doi: 10.2105/AJPH.88.11.1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lupo PJ, Danysh HE, Symanski E, Langlois PH, Cai Y, Swartz MD. Neighborhood-based socioeconomic position and risk of oral clefts among offspring. Am J Public Health. 2015;105(12):2518–2525. doi: 10.2105/AJPH.2015.302804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmichael SL, Ma C, Shaw GM. Socioeconomic Measures, Orofacial Clefts, and Conotruncal Heart Defects in California. Published online 2009. doi: 10.1002/bdra.20614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmichael SL, Nelson V, Shaw GM, Wasserman CR, Croen LA. Socio-economic status and risk of conotruncal heart defects and orofacial clefts. Paediatr Perinat Epidemiol. 2003;17(3):264–271. doi: 10.1046/j.1365-3016.2003.00498.x [DOI] [PubMed] [Google Scholar]
- 11.Root ED, Meyer RE, Emch M. Socioeconomic context and gastroschisis: Exploring associations at various geographic scales. Soc Sci Med. 2011;72(4):625–633. doi: 10.1016/j.socscimed.2010.11.025 [DOI] [PubMed] [Google Scholar]
- 12.Stallings EB, Isenburg JL, Short TD, et al. Population-based birth defects data in the United States, 2012–2016: A focus on abdominal wall defects. Birth Defects Res. 2019;111(18):1436–1447. doi: 10.1002/bdr2.1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones AM, Isenburg J, Salemi JL, et al. Increasing Prevalence of Gastroschisis — 14 States, 1995–2012. MMWR Morb Mortal Wkly Rep. 2016;65(2):23–26. doi: 10.15585/mmwr.mm6502a2 [DOI] [PubMed] [Google Scholar]
- 14.Kazaura MR, Lie RT, Irgens LM, et al. Increasing Risk of Gastroschisis in Norway: An Age-Period-Cohort Analysis. Am J Epidemiol Hopkins Bloom Sch Public Heal All rights Reserv. 2004;159(4):358–363. doi: 10.1093/aje/kwh051 [DOI] [PubMed] [Google Scholar]
- 15.Torfs CP, Velie EM, Oechsli FW, Bateson TF, Curry CJR. A Population-Based Study of Gastroschisis: Demographic, Pregnancy, and Lifestyle Risk Factors. Vol 50.; 1994. [DOI] [PubMed] [Google Scholar]
- 16.Mac Bird T, Robbins JM, Druschel C, Cleves MA, Yang S, Hobbs CA. Demographic and environmental risk factors for gastroschisis and omphalocele in the National Birth Defects Prevention Study ☆,☆☆. J Pediatr Surg. 2009;44:1546–1551. doi: 10.1016/j.jpedsurg.2008.10.109 [DOI] [PubMed] [Google Scholar]
- 17.Siega-Riz AM, Herring AH, Olshan AF, Smith J, Moore C. The joint effects of maternal prepregnancy body mass index and age on the risk of gastroschisis. Paediatr Perinat Epidemiol. 2009;23(1):51–57. doi: 10.1111/j.1365-3016.2008.00990.x [DOI] [PubMed] [Google Scholar]
- 18.Lam PK, Torfs CP, Brand RJ. A low prepregnancy body mass index is a risk factor for an offspring with gastroschisis. Epidemiology. 1999;10(6):717–721. doi: 10.1097/00001648-199911000-00012 [DOI] [PubMed] [Google Scholar]
- 19.Siega-Riz AM, Olshan AF, Werler MM, Moore C. Fat intake and the risk of gastroschisis. Birth Defects Res Part A Clin Mol Teratol. 2006;76(4):241–245. doi: 10.1002/bdra.20249 [DOI] [PubMed] [Google Scholar]
- 20.Waller DK, Shaw GM, Rasmussen SA, et al. Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med. 2007;161(8):745–750. doi: 10.1001/archpedi.161.8.745 [DOI] [PubMed] [Google Scholar]
- 21.Salihu HM, Pierre-Louis BJ, Druschel CM, Kirby RS. Omphalocele and gastroschisis in the State of New York, 1992–1999. Birth Defects Res Part A - Clin Mol Teratol. 2003;67(9):630–636. doi: 10.1002/bdra.10113 [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen SA, Frías JL. Non-genetic risk factors for gastroschisis. Am J Med Genet Part C Semin Med Genet. 2008;148(3):199–212. doi: 10.1002/ajmg.c.30175 [DOI] [PubMed] [Google Scholar]
- 23.Bargy F, Beaudoin S. Comprehensive developmental mechanisms in gastroschisis. Fetal Diagn Ther. 2014;36(3):223–230. doi: 10.1159/000360080 [DOI] [PubMed] [Google Scholar]
- 24.Beaudoin S Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg. 2018;27(5):283–288. doi: 10.1053/j.sempedsurg.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 25.Palmer SR, Evans A, Broughton H, et al. The role of maternal stress in early pregnancy in the aetiology of gastroschisis: An incident case control study. PLoS One. 2013;8(11):80103. doi: 10.1371/journal.pone.0080103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werler MM, Guéry E, Waller DK, Parker SE. Gastroschisis and cumulative stressor exposures. Epidemiology. 2018;29(5):721–728. doi: 10.1097/EDE.0000000000000860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reefhuis J, Gilboa SM, Anderka M, et al. The national birth defects prevention study: A review of the methods. Birth Defects Res Part A - Clin Mol Teratol. 2015;103(8):656–669. doi: 10.1002/bdra.23384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res Part A - Clin Mol Teratol 2003;67(3):193–201. doi: 10.1002/bdra.10012 [DOI] [PubMed] [Google Scholar]
- 29.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Heal. 2006;83(6):1041–1062. doi: 10.1007/s11524-006-9094-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janevic T, Stein CR, Savitz DA, Kaufman JS, Mason SM, Herring AH. Neighborhood deprivation and adverse birth outcomes among diverse ethnic groups. Ann Epidemiol. 2010;20(6):445–451. doi: 10.1016/j.annepidem.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic Status in Health Research. Jama. 2005;294(22):2879. doi: 10.1001/jama.294.22.2879 [DOI] [PubMed] [Google Scholar]
- 32.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. doi: 10.1097/00001648-199901000-00008 [DOI] [PubMed] [Google Scholar]
- 33.SAS/STAT® 13.1 User’s Guide The MIANALYZE Procedure. SAS/STAT ® 131 User’s Guid. Published online 2013:5172–5231. Accessed April 19, 2022. https://support.sas.com/documentation/onlinedoc/stat/131/mianalyze.pdf [Google Scholar]
- 34.Lupo PJ, Danysh HE, Symanski E, Langlois PH, Cai Y, Swartz MD. Neighborhood-based socioeconomic position and risk of oral clefts among offspring. Am J Public Health. 2015;105(12):2518–2525. doi: 10.2105/AJPH.2015.302804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vrijheid M, Dolk H, Stone D, Abramsky L, Alberman E, Scott JES. Socioeconomic Inequalities in Risk of Congenital Anomaly. Vol 82.; 2000. doi: 10.1136/adc.82.5.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grewal J, Carmichael SL, Song J, Shaw GM. Neural tube defects: An analysis of neighbourhood- and individual-level socio-economic characteristics. Paediatr Perinat Epidemiol. 2009;23(2):116–124. doi: 10.1111/j.1365-3016.2008.00992.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman AM, Ananth CV, Siddiq Z, D’Alton ME, Wright JD. Gastroschisis: epidemiology and mode of delivery, 2005–2013. Am J Obstet Gynecol. 2016;215(3):348.e1–348.e9. doi: 10.1016/j.ajog.2016.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schisterman EF, Cole SR, Platf RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glass TA, Bilal U. Are neighborhoods causal? Complications arising from the ‘stickiness’ of ZNA. Soc Sci Med. 2016;166:244–253. doi: 10.1016/j.socscimed.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 40.Wei F, Knox PL. Neighborhood Change in Metropolitan America, 1990 to 2010. Urban Aff Rev. 2014;50(4):459–489. doi: 10.1177/1078087413501640 [DOI] [Google Scholar]
- 41.Ferrie JP. History lessons: The end of American exceptionalism? Mobility in the United States since 1850. J Econ Perspect 2005;19(3):199–215. doi: 10.1257/089533005774357824 [DOI] [Google Scholar]
- 42.Mills M, Rindfuss RR, McDonald P, te Velde E. Why do people postpone parenthood? Reasons and social policy incentives. Hum Reprod Update. 2011;17(6):848–860. doi: 10.1093/humupd/dmr026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balbo N, Mills M. The effects of social capital and social pressure on the intention to have a second or third child in France, Germany, and Bulgaria, 2004–05. http://dx.doi.org.libproxy.lib.unc.edu/101080/003247282011579148.2011;65(3):335–351.doi: 10.1080/00324728.2011.579148 [DOI] [PubMed] [Google Scholar]
- 44.Brewster KL, Rindfuss RR. Fertility and women’s employment in industrialized nations. Annu Rev Sociol. 2000;26:271–296. doi: 10.1146/annurev.soc.26.1.271 [DOI] [Google Scholar]
- 45.Bernhardt EM. Fertility and employment. Eur Sociol Rev. 1993;9(1):25–42. doi: 10.1093/oxfordjournals.esr.a036659 [DOI] [Google Scholar]
- 46.Kawachi I, Berkman LF. Neighborhoods and Health. Oxford University Press; 2009. doi: 10.1093/acprof:oso/9780195138382.001.0001 [DOI] [Google Scholar]
- 47.Schempf A, Strobino D, O’Campo P. Neighborhood effects on birthweight: An exploration of psychosocial and behavioral pathways in Baltimore, 1995–1996. Soc Sci Med. 2009;68(1):100–110. doi: 10.1016/j.socscimed.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macintyre S, Ellaway A, Cummins S. Place effects on health: how can we conceptualise, operationalise and measure them? Soc Sci Med. 2002;55(1):125–139. doi: 10.1016/S0277-9536(01)00214-3 [DOI] [PubMed] [Google Scholar]
- 49.Diez Roux A V, Mair C Neighborhoods and health. Ann N Y Acad Sci. 2010;1186(1):125–145. doi: 10.1111/j.1749-6632.2009.05333.x [DOI] [PubMed] [Google Scholar]
- 50.Cubbin C, Marchi K, Lin M, et al. Is neighborhood deprivation independently associated with maternal and infant health? Evidence from Florida and Washington. Matern Child Health J. 2008;12(1):61–74. doi: 10.1007/s10995-007-0225-0 [DOI] [PubMed] [Google Scholar]
- 51.Waller SA, Paul K, Peterson SE, Hitti JE. Agricultural-related chemical exposures, season of conception, and risk of gastroschisis in Washington State. Am J Obstet Gynecol. 2010;202(3):241.e1–241.e6. doi: 10.1016/j.ajog.2010.01.023 [DOI] [PubMed] [Google Scholar]
- 52.Krajewski AK, Rappazzo KM, Langlois PH, Messer LC, Lobdell DT. Associations between cumulative environmental quality and ten selected birth defects in Texas. Birth Defects Res. 2021;113(2):161–172. doi: 10.1002/bdr2.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yen IH, Syme SL. The social environment and health: A discussion of the epidemiologic literature. Annu Rev Public Health. 1999;20:287–308. doi: 10.1146/annurev.publhealth.20.1.287 [DOI] [PubMed] [Google Scholar]
- 54.Palmer SR, Evans A, Broughton H, et al. The role of maternal stress in early pregnancy in the aetiology of gastroschisis: An incident case control study. PLoS One. 2013;8(11):80103. doi: 10.1371/journal.pone.0080103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawachi I, Berkman LF. Neighborhoods and Health. Oxford University Press; 2009. doi: 10.1093/acprof:oso/9780195138382.001.0001 [DOI] [Google Scholar]
- 56.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The public health disparities geocoding project (US). J Epidemiol Community Health. 2003;57(3):186–199. doi: 10.1136/jech.57.3.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cogswell ME, Bitsko RH, Anderka M, et al. Control selection and participation in an ongoing, population-based, case–control study of birth defects. Am J Epidemiol. 2009;170(8):975–985. doi: 10.1093/aje/kwp226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The process for accessing the data used in this analysis is described here: https://www.cdc.gov/ncbddd/birthdefects/nbdps-public-access-procedures.html. Computing code may be requested from the corresponding author.