Abstract
Background:
Inflammatory bowel disease (IBD) in dogs is often characterized by a relapsing and remitting clinical course. Determination of inflammatory activity is important for assessing the disease extent, severity, and tailoring appropriate treatment.
Aims:
The study was conducted to record the macroscopic and microscopic changes associated with IBD to assess the usefulness of endoscopy in the diagnosis of the disease and to correlate the clinical activity index (CIBDAI) with endoscopic score.
Methods:
Thirty-three dogs with idiopathic IBD were selected after thorough examination and exclusion. Gastroduodenoscopy and colonoscopy were performed to document the gross macroscopic intestinal lesions. Histopathology of endoscopic aided biopsy samples was used to confirm the disease.
Results:
Mucosal erythema and increased friability were the most predominant endoscopic findings in the stomach, duodenum, and colon of IBD dogs. Lymphoplasmacytic infiltration was predominant in the mucosal samples on histopathology and diffuse form of IBD is more common in canines. Gastroduodenoscopy and colonoscopy in combination with endoscopically guided biopsy and histopathology are of value in the assessment and diagnosis of IBD. There was no correlation between the clinical inflammatory bowel disease activity index (CIBDAI) with the endoscopic score.
Conclusion:
A diffuse form of IBD and colitis is more common in dogs in comparison to human IBD where the disease manifests in two distinct forms. Colonoscopy with ileal biopsy could act as a gold standard in the confirmation of diffuse IBD in dogs. CIBDAI can be used as a reliable measure of clinical signs of inflammation and histopathology can be used as a definitive diagnosis of intestinal inflammation.
Key Words: Canine, CIBDAI, Endoscopy, Histopathology, IBD
Introduction
Inflammatory bowel disease (IBD) constitutes a group of emerging chronic inflammatory disorders of the gastrointestinal tract characterized by idiopathic mucosal inflammation that has been described in humans and several domestic animals including dogs. Although the naming is the same, canine and human IBD are not similar conditions. Crohn’s disease (CD) and ulcerative colitis (UC) are the main forms of IBD in humans. The differentiation between different types of IBD in dogs is made by naming the predominant cell type present viz lymphocytic-plasmacytic, eosinophilic, and granu-lomatous (Jergens et al., 1992). Diagnosis ultimately depends on the documentation of histopathologic changes in the endoscopic-aided biopsy specimens (Garcıa-Sancho et al., 2007; Cerquetella et al., 2010). Gastrointestinal endoscopy is, thus, an important diagnostic tool for distinguishing IBD from other causes of chronic enteropathy. Similarly, determining inflammatory activity is important for determining disease severity and, potentially, tailoring treatment. For this purpose, different indices for the assessment of disease activity have been proposed. These clinical indices utilize scoring systems derived from the gastrointestinal signs alone (CIBDAI) or in combination with laboratory testing (CCECAI) to quantify intestinal activity. Jergens et al. (2003) proposed the Clinical Inflammatory Bowel Disease Activity Index (CIBDAI), a simple numeric index based on the presence and frequency of the cardinal gastrointestinal signs, such as appetite, vomiting, faecal consistency, frequency of defecation, weight loss, and attitude/activity.
Currently, very little work has been carried out in India; hence, the present study was planned and conducted to record the macroscopic and microscopic changes associated with IBD to assess the usefulness of endoscopy in the diagnosis of disease and to correlate the clinical activity index with the endoscopic score.
Materials and Methods
Clinical cases
The study was conducted at the Small Animal Outpatient Unit of Madras Veterinary College Teaching Hospital, Tamil Nadu Veterinary and Animal Sciences University from 2014 to 2016. The dogs were included based on the WSAVA (Washabau et al., 2010) guidelines for the diagnosis of idiopathic IBD, viz (1) chronic (i.e. >3 weeks in duration) gastrointestinal signs (e.g., anorexia, vomiting, weight loss, diarrhoea, haematochezia, mucoid faeces), (2) histopathologic evidence of mucosal inflammation, (3) inability to document other causes of gastroenterocolitis by thorough diagnostic evaluation, (4) inadequate response to appropriately designed and implemented therapeutic trials (i.e. dietary, antibacterial, anthelmintic), and (5) clinical response to anti-inflammatory or immuno-suppressive agents.
Out of a total No. of 30,535 dogs presented to the out-patient unit, 67 dogs with a history and signs of chronic gastrointestinal disorders were selected for this study and subjected to detailed clinical examination, laboratory examination (haematology, serum bio-chemistry, and faecal analysis), radiography, ultra-sonographic examination, and pancreatic elastase 1 ELISA test. Six out of 67 dogs were diagnosed with exocrine pancreatic insufficiency based on the pancreatic elastase 1 ELISA test and were excluded from the study. In the remaining 61 cases, endoscopies were performed in 48 cases only based on the owners’ consent. Out of 48 cases with recurrent gastrointestinal signs and lymphoplasmacytic infiltration based on endoscopic histopathology, 15 cases were excluded from the study due to infestation of Trichuris vulpis (4), presence of rectal polyp (1), intestinal lymphoma (1), and Helicobacter spp. infection (8). Thirty-three confirmed cases of idiopathic IBD were selected for the study. The dogs ranged in age from 1 to 10 years (median is 5.5). There were 20 males and 13 females and the following breeds were represented: ten non-descript, eight German shepherds, five Labradors, four Dobermans, and one each of Rottweiler, Pug, Lhasapso, Dachshund, Rajapalayam, and Combai. The control group consisted of six apparently healthy dogs brought for routine health check-up and vaccination. The owners of the control and diseased dogs voluntarily consented to be part of the study.
Clinical Inflammatory Bowel Disease Activity Index (CIBDAI)
The clinical activity of affected dogs was recorded using CIBDAI by Jergens et al. (2003). Based on CIBDAI scoring, the IBD dogs were classified as dogs having mild IBD (4-5 score), moderate IBD (6-8 score), and severe IBD (>9 score).
Endoscopic assessment and biopsy
Gastroduodenoscopy and colonoscopy were performed using a Karl Storz type No. 60914 PKS (Germany) Veterinary Video endoscope with an outer diameter of 9.8 mm, a biopsy channel diameter of 2.8 mm, and a working length of 1400 mm. A fenestrated type, long oval cup biopsy forceps (Karl Storz) of 2.2 mm diameter was used for taking a biopsy. Endoscopic images were captured digitally.
Preparation and restraint of the animals were done as suggested by Zoran (2001). To improve the visualization of the mucosa and reduce the risk of aspiration, food was withheld for 24-48 h, and water was withheld for 4 h prior to the procedure. Oral laxative medication was given a day before and an electrolyte-based enema was given before the procedure. The endoscopic examination was performed with the dog in left lateral recumbency under general anaesthesia as per the method described by Guilford (2005) and Richter (2005).
Lesions observed during gastroscopy were recorded and scored by a standardized report form for upper and lower GI endoscopy published by WSAVA Gastrointestinal Standardization Group (Washabua et al., 2010) and four to five representative biopsy samples were taken by using pinch biopsy forceps (Jergens and Moore, 2010). Mucosal abnormalities such as hyperaemia, edema, discoloration, granularity, hemorrhage, erosions, and ulcers were examined in the gastrointestinal tract. Quantitatively, a score of 0-3 was given based on the presence/extent of abnormal mucosal appearances. Finally, composite score was calculated by adding the points obtained from each segment of the GI tract.
Histologic examination of biopsies
The characterization of the histologic changes in the endoscopic biopsy samples was performed according to the histopathologic standards established by the WSAVA Gastrointestinal Standardization Group (Washabau et al., 2010). The biopsy samples of affected dogs were looked at for inflammatory changes such as intraepithelial lymphocytes, lamina propria lymphocytes/plasma cells, lamina propria eosinophils, lamina propria neutrophils, gastric lymphofollicular hyperplasia, and lamina propria macrophages. Morphological changes to be looked for were surface epithelial injury, gastric pit epithelial injury, fibrosis/glandular, nesting/mucosal atrophy, villus stunting, epithelial injury, crypt distension, lacteal dilation, mucosal fibrosis, surface epithelial injury, crypt hyperplasia, and crypt dilation and distortion. The grading was given based on the severity as 0: Normal, 1: Mild, 2: Moderate, and 3: Marked.
Statistical analysis
All data for the scoring system is expressed as median. The Pearson correlation coefficient was used to determine the relationship between the two variables. The data obtained in the study were subjected to statistical analysis using SPSS 23.0 and discussed.
Results
In the present study, tenesmus combined with haematochezia and mucoid faeces (11/33), vomiting and diarrhea (8/33), watery diarrhoea alone (5/33), vomiting alone (4/33), haematochezia (2/33), and melena (3/33) were recorded as predominant signs. At the time of presentation, the median CIBDAI score was 7 (range 5-13). Based on CIBDAI scoring, mild IBD, moderate IBD, and severe IBD were observed in 9.09 (3/33), 54.54 (18/33), and 36.36 (12/33) percent of the cases, respectively. The highest numbers of cases were recorded in the moderate IBD group, followed by the severe disease group.
The endoscopic findings in the stomach, duodenum, ileum, and colon are given in Table 1 and Figs. 1a-d, Figs. 2a-d, Figs. 3a-d, and Figs. 4a-d. The appearance of the esophagus and lower esophageal sphincter was examined macroscopically during endoscopy and found to be normal in all dogs in the study. The most predominant endoscopic findings in the stomach, duodenum, and colon included mucosal erythema and increased friability.
Table 1.
Endoscopic findings in the gastrointestinal tract of IBD dogs (n=33)
| Parameters | Severity of lesion (%) | ||||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | Total | ||
| Stomach | |||||
| Hyperaemia (%) | 27.27 | 36.36 | 12.12 | 75.75 | |
| Edema (%) | 15.15 | 6.06 | - | 21.21 | |
| Discolouration (%) | 3.03 | 18.18 | - | 21.21 | |
| Friability (%) | 27.27 | 5.15 | 6.06 | 48.48 | |
| Haemorrhage (%) | 27.27 | 9.09 | 6.06 | 42.42 | |
| Erosion/Ulcer (%) | 12.12 | 15.15 | 9.09 | 36.36 | |
| Duodenum | |||||
| Hyperaemia (%) | 36.36 | 21.21 | 6.06 | 63.63 | |
| Edema (%) | 6.06 | 12.12 | - | 18.18 | |
| Discolouration (%) | 3.03 | 21.21 | - | 24.24 | |
| Friability (%) | 33.33 | 30.30 | 9.09 | 72.72 | |
| Haemorrhage (%) | 30.30 | 9.09 | 9.09 | 48.48 | |
| Erosion/Ulcer (%) | 12.12 | 9.09 | 12.12 | 33.33 | |
| Colon | |||||
| Hyperaemia (%) | 15.15 | 45.45 | 27.27 | 87.87 | |
| Discolouration (%) | 3.03 | 6.06 | - | 9.09 | |
| Friability/Haemorrhage (%) | 33.33 | 27.27 | 24.24 | 84.84 | |
| Erosion/Ulcer (%) | 24.24 | 6.06 | 27.27 | 57.57 | |
Fig. 1.

Endoscopic findings in the stomach of IBD dogs. These images represent the endoscopic appearance of hyperaemia of mucosa (a), friable mucosa (b), ulceration (c), and punctate ulcer (d)
Fig. 2.

Endoscopic findings in the duodenum of IBD dogs. These images represent the endoscopic appearance of friability (a), erosions (b), increased granularity (c), and irregular duodenal mucosa (d)
Fig. 3.

Endoscopic findings in the ileum of IBD dogs. These images represent the endoscopic appearance of closed ileocolic valve (a), relaxed ileocolic valve (b), mild hyperaemia (c), and irregular mucosa (d)
Fig. 4.

Endoscopic findings in the colon of IBD dogs. These images represent the endoscopic appearance of edema and loss of submucosal vessels (a), extensive erosion and haemorrhage (b), friability (c), and thickening and reduced lumen size (d)
In 6.06% of cases, bile was found mixed with scant contents in the stomach, and mucus was found in 3.03% of the cases. The pylorus was open in 27.27% of cases and the advancement of scope was easy, while in other cases the scope was introduced into the duodenum with difficulty.
Duodenal granularity was noticed in 27.27% of the cases and thick mucus was observed in 9.09% of the cases.
The ileum was explored in only five cases. In two of these, a relaxed ileal sphincter was observed, and the endoscope was easily passed. In the other two cases, a closed sphincter was noted and the scope was passed with difficulty. The ileum mucosa appeared hyperaemic in these cases. Among these, ileocolic valves were inflamed in two cases. The direct biopsy was done in four cases, while the blind biopsy was done in one.
A highly thickened wall of the distal colon was observed in six dogs, while proximal colon wall thickening was seen in one dog. In these cases, the scope could not be advanced further and there was severe bleeding. Due to the thickening of the colon, the absence of submucosal vessels was evident.
The median score of 6 with values ranging from 2 to 9 was observed as an endoscopic score in dogs with IBD.
On histopathology, the cellular infiltrate in all mucosal specimens was predominantly lymphocytes and plasma cells (Figs. 5a and b, Fig. 6, and Figs. 7a and b). The severity of the disease was classified as mild, moderate, and severe according to the presence of lymphoplasmacytic infiltration in the mucosa and lamina propria per field and is presented in Table 2.
Fig. 5.
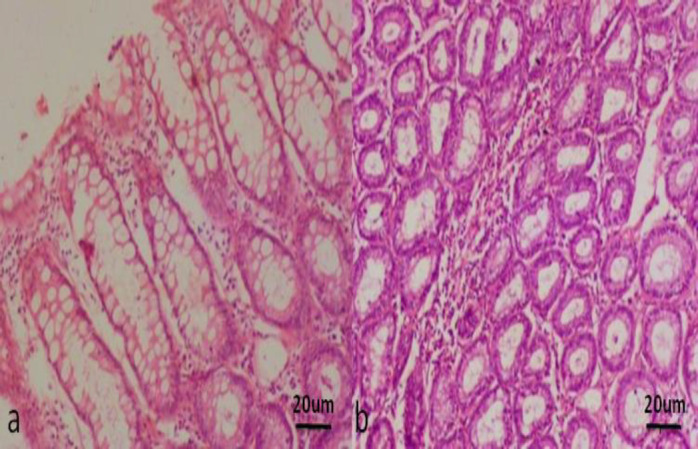
Histopathology of the stomach of IBD dogs (H&E staining, ×20). (a) Attenuation and degeneration of surface epithelium with mild lympho plasmacytic infiltration, and (b) Moderate lympho plasmacytic infiltration between the glands
Fig. 6.

Histopathology of the duodenum of IBD dogs. Mild to moderate lympho plasmacytic infiltration within the lamina propria (H&E staining, ×10)
Fig. 7.
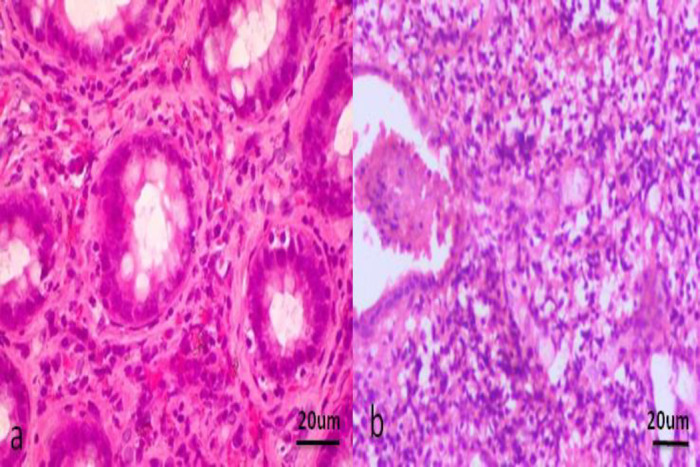
Histopathology of the colon of IBD dogs (H&E staining, ×20). (a) Distal colon - Moderate lymphoplasmacytic infiltration within the lamina propria, and (b) Distal colon - There is distortion/dilatation of the crypt with necrotic debris along with marked lymphoplasmacytic infiltration
Table 2.
Histopathological findings in the gastrointestinal tract of IBD dogs (n=33)
| Part of gastrointestinal tract | Mucosal lymphoplasmacytic infiltration percent (number of animals) | |||
|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | |
| Stomach | 30.30 (10/33) | 33.33 (11/33) | 30.30 (10/33) | 6.06 (2/33) |
| Duodenum | 33.33 (11/33) | 39.39 (13/33) | 21.21 (7/33) | 6.06 (2/33) |
| Ileum | - | 9.09 (3/33) | 6.06 (2/33) | - |
| Colon | - | 48.48 (16/33) | 39.39 (13/33) | 12.12 (4/33) |
Morphological changes such as mucosal surface epithelial degeneration and fibrosis or atrophy of the gastric glands were observed in 12.12% (4/33) of the cases. Epithelial injury (degeneration of surface epithelium) was observed in 6.06% (2/33) of the cases. Fibrosis/atrophy of mucosal glands of duodenum was recorded in 9.09% (3/33) of the cases. Lacteal dilatation was observed in 6.06% (2/33) of the cases. Degeneration and necrosis of the crypt were seen in 12.12% (4/33) of the cases. Degeneration of surface epithelium was evident in 15.15% (5/33) of the cases. Dilatation and crypt distortion was observed in 12.12% (4/33) of the cases.
According to the histopathology findings in the current study, diffuse IBD was found in 75.75% (25 out of 33) of the cases.
The Pearson correlation coefficient between the CIBDAI with the endoscopic score was 0.281. There was no correlation between the two statistical variables i.e. CIBDAI and the endoscopic score.
Discussion
Clinical signs in IBD dogs are highly variable. The clinical signs associated with canine inflammatory bowel disease are primarily gastrointestinal symptoms viz. vomiting, small and large bowel diarrhea, weight loss, and alterations in appetite. These signs are attributed to mucosal cellular infiltrates, inflammatory mediators, inflammation-associated enterocyte dysfunction, and intestinal dysmotility. Moreover, the clinical condition at a particular time may be influenced by the presence of active intestinal inflammation as well as secondary consequences of inflammation including anaemia and vitamin deficiencies that contribute to the diverse signs of gastrointestinal disease. Previous studies indicate that dogs with moderate-to-severe IBD accompanied by increased CRP concentrations are more likely to have significant histologic lesions than dogs with mild clinical signs. The severity of disease may differ considerably among patients depending on the localization and extent of affected regions of the gastrointestinal tract (Jergens et al., 2003). Thus, since IBD is a highly individualistic disease, its diagnosis involves both clinical signs and endoscopy. Macroscopic and microscopic changes are required to diagnose disease activity.
The clinical signs and CIBDAI score recorded in our study correlated with Suchodolski et al. (2010).
Jergens et al. (1992) indicated that on endoscopy in IBD dogs, increased granularity, increased friability, loss of submucosal vascularity, and erosions are the predominant mucosal lesions. Similarly, in our study, the most predominant endoscopic findings in the stomach, duodenum, and colon included mucosal erythema and increased friability. These were in agreement with Jergens et al. (2010b) and Slovak et al. (2014) who recorded similar findings of erythema, friability, enhanced granularity, and ulceration or erosion associated with predominantly lymphocytic plasmacytic mucosal inflammation.
In the current study, bile content in the stomach was found in a few cases, and the pylorus was passed with difficulty. A possible explanation was rendered by Tams (2010) that bile mixed contents in the stomach might be due to duodenogastral bile reflux. The author further stated that variable degrees of dilation of the pyloric canal may be observed on endoscopy. In some cases, the pylorus is persistently closed and this may represent normal pyloric closure.
Richter (2005) and Tams (2010) hypothesised that chronic colitis causes mucosal thickening and edema, which impairs the visualisation of submucosal blood vessels in the colon and it was also observed in our study.
In the present study, the ileal biopsy was taken by both direct and blind methods. Only in two cases was the ileocolic valve open and the scope was passed easily into ileum since the valve is closed in the majority of cases and only occasionally opened (Tams, 2010). Though ileoscopy may be technically difficult or limited by the instrumentation, the ileal biopsy is being recognized as potentially providing valuable information not always found in duodenal or colonic biopsies. The WSAVA International Gastrointestinal Standardization Group (Washabau et al., 2010) recommended obtaining ileal biopsies either by passing the endoscope into the ileum or blindly passing biopsy forceps through the ileocolic valve. The authors also recommend that ileal biopsies be obtained in animals whenever gastroduodenoscopy or colonoscopy appears to be indicated. In the present study, even though the majority of dogs presented with colitis signs (tenesmus and haematochezia), they were observed to have a diffuse form of IBD, which was evident in histopathology and which indicates the importance of ileal biopsy.
Histopathologically, mucosal inflammation com-prises the main feature of canine IBD that can be further decorated by epithelial damage, increased numbers of IELs, villus stunting, lacteal dilation, and varying degrees of mucosal architectural disruption (Hall and German, 2008; Washabau et al., 2010). In our present study, lymphocytes and plasmacytes were the common cell infiltration observed. Jergens et al. (2010a) and Suchodolski et al. (2012) observed similar mucosal infiltration in their studies on IBD dogs. In dogs, the lymphocytic-plasmacytic enteritis (LPE) is the most common type of IBD, in contrast to eosinophilic, neutrophilic, and granulomatous enteritis (Hall and German, 2010).
In our study, a mild and moderate form of ileal disease was identified histopathlogically. Casamian-Sorrosal et al. (2010) opined that ileal biopsy is important in the diagnosis of disease. The author compared the histology of duodenal and ileal biopsies and found that ileal biopsies often revealed lesions not found in duodenal samples. Colon lesions were seen invariably in all the IBD dogs in the present study which is in agreement with Jergens and Simpson (2012) who stated that the colon was found to be most commonly affected.
A diffuse form of IBD was recorded in the majority of the affected dogs in this study based on histopathology which was contrary to human IBD wherein the disease classically occurs in two major contrasting forms, Crohn’s disease and ulcerative colitis (UC) (Odze, 2003). The two forms of the disease differ in their clinical picture as Crohn’s disease is a marked transmural granulomatous process that can affect any part of the gastrointestinal tract from the mouth to the anus, whereas UC is a more superficial process, restricted to the colon (Odze, 2003). In our study, although the major clinical signs were pertaining to large bowel disease, the dogs had lesions both in the small and large intestine on histopathology which is in agreement with Guilford (1996) who stated that in dogs, the two major forms of IBD cannot be distinguished and that IBD is common in both the small and the large intestine.
Clinical trials utilizing endoscopic scoring for dogs with IBD are limited and have provided conflicting results on the utility of endoscopic scoring as a measure of disease activity. In the present study, an endoscope score of 6 (ranging from 2-9) was observed which was comparable to Heilmann et al. (2014). Moreover, there was no correlation between CIBDAI and the endoscopic score in the present study. Similarly, Allenpasch et al. (2007) observed that there was no correlation between CIBDAI and endoscopic score. Slovak et al. (2014) opined that the reason for this discrepancy could be interobserver variation in identifying endoscopic abnormalities based on the operator’s experience and lack of systematic endoscopic assessment.
In summary, mucosal erythema and increased friability were the most predominant endoscopic findings in the stomach, duodenum, and colon of IBD dogs. The diffuse form of IBD is more common in dogs in comparison to human IBD where the disease manifests in two distinct forms. Gastroduodenoscopy and colonoscopy in conjunction with endoscopically guided biopsy and histopathology are useful in assessing specific region involvement in IBD and distinguishing IBD from other chronic enteropathies. There was no correlation between clinical activity index and the endoscopic score.
Our present study concludes that a diffuse form of IBD is more common in dogs in comparison to human. Endoscopy enables direct accessibility of the lumen of the gastrointestinal tract and, when combined with the histopathology of mucosal biopsy, provides great diagnostic potential in the diagnosis of chronic inflammatory diseases of the gastrointestinal tract in dogs. Colonoscopy with ileal biopsy could act as the gold standard in the confirmation of diffuse IBD in dogs. It is concluded that CIBDAI can be used as a reliable measure of clinical signs of inflammation and histopathology can be used as a definitive diagnosis of intestinal inflammation in IBD dogs.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgment
The authors wish to thank the authorities of TANUVAS for the facilities provided.
References
- Allenspach K, Wieland B, Grone A, Gaschen F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J. Vet. Int. Med. 2007;21:700–708. doi: 10.1892/0891-6640(2007)21[700:ceideo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Casamian-Sorrosal D, Willard MD, Murray JK, Hall EJ, Taylor SS, Day MJ. Comparison of histopathologic findings in biopsies from the duodenum and ileum of dogs with enteropathy. J. Vet. Intern. Med. 2010;24:80–83. doi: 10.1111/j.1939-1676.2009.0427.x. [DOI] [PubMed] [Google Scholar]
- Cerquetella M, Spaterna A, Laus F, Tesei B, Rossi E, Antonelli G, Villanacci V, Bassotti G. Inflammatory bowel disease in the dog: Differences and similarities with human. World J. Gastroenterol. 2010;16:1050–1056. doi: 10.3748/wjg.v16.i9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day MJ, Blizer T, Mansell J, Wilcock B, Hall EJ, Jergens A, Minami T, Willard M, Washabau R. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardi-zation Group. J. Comp. Pathol. 2008;138:38–43. doi: 10.1016/j.jcpa.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Garcia-Sancho M, Rodriguez-Franco F, Sainz A, Mancho C, Rodriguez A. Evaluation of clinical, macroscopic, and histopathologic response to treatment in nonhypoproteinemic dogs with lymphocytic-plasmacytic enteritis. J. Vet. Intern. Med. 2007;21:11–17. doi: 10.1892/0891-6640(2007)21[11:eocmah]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Guilford WG. Idiopathic inflammatory bowel disease. In: Guilford WG;, Center SA, editors. Strombeck’s small animal gastroenterology. 3rd Edn. Philadelphia: W. B. Saunders; 1996. pp. 451–486. [Google Scholar]
- Guilford WG. Upper gastrointestinal endoscopy. In: McCarthy M, editor. Veterinary endoscopy for the small animal practitioner. USA: Elsevier Saunders; 2005. pp. 279–322. [Google Scholar]
- Heilmann RM, Grellet A, Allenspach K. Association between faecal S100A12 concentration and histologic, endoscopic, and clinical disease severity in dogs with idiopathic inflammatory bowel disease. Vet. Immunol. Immunopathol. 2014;158:156–166. doi: 10.1016/j.vetimm.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Jergens AE, Crandell J, Morrison JA, Deitz K, Pressel M, Ackermann M, Suchodolski JS, Steiner JM, Evans R. Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: A randomized-controlled trial. J. Vet. Intern. Med. 2010a;24:269–277. doi: 10.1111/j.1939-1676.2009.0447.x. [DOI] [PubMed] [Google Scholar]
- Jergens AE, Evans RB, Ackermann M, Hostetter J, Willard M, Mansell J, Bilzer T, Wilcock B, Washabau R, Hall EJ, Minami T, Wang C, Day MJ. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet. Pathol. 2010b;20:1–5. doi: 10.1177/0300985813511123. [DOI] [PubMed] [Google Scholar]
- Jergens AE, Moore FM, Haynes JS, Miles KG. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987-1990) J. Am. Vet. Med. Assoc. 1992;201:1603–1608. [PubMed] [Google Scholar]
- Jergens AE, Schreiner CA, Frank DE, Niyo Y, Ahrens FE, Eckersall PD, Benson TJ, Evans R. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 2003;17:291–297. doi: 10.1111/j.1939-1676.2003.tb02450.x. [DOI] [PubMed] [Google Scholar]
- Jergens AE, Simpson KW. Inflammatory bowel disease in veterinary medicine. Front. Biosci. 2012;E4:1404–1419. doi: 10.2741/e470. [DOI] [PubMed] [Google Scholar]
- Odze R. Diagnostic problems and advances in inflammatory bowel disease. Mod. Pathol. 2003;16:347–358. doi: 10.1097/01.MP.0000064746.82024.D1. [DOI] [PubMed] [Google Scholar]
- Richter KP. Endoscopic evaluation of the colon. In: McCarthy M, editor. Veterinary endoscopy for the small animal practitioner. USA: Elsevier Saunders; 2005. pp. 323–356. [Google Scholar]
- Slovak JE, Wang C, Sun Y, Otoni C, Morrison J. Development and validation of an endoscopic activity score for canine inflaymmatory bowel disease. Vet. J. 2014;203:290–295. doi: 10.1016/j.tvjl.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Suchodolski JS, Dowd SE, Wilke V, Steiner JM, Jergens AE. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS One. 2012;7:333–339. doi: 10.1371/journal.pone.0039333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchodolski JS, Steiner JM, Evans R. Comparison of oral prednisone and prednisone combined with metronidazole for induction therapy of canine inflammatory bowel disease: A randomized-controlled trial. J. Vet. Int. Med. 2010;24:269–277. doi: 10.1111/j.1939-1676.2009.0447.x. [DOI] [PubMed] [Google Scholar]
- Tams TR. Gastrointestinal endoscopy. In: Tams TR, editor. Small animal endoscopy. 3rd Edn. St. Luis, C. V: Mosby Company; 2010. pp. 275–376. [Google Scholar]
- Washabau R, Day M, Willard M, Hall EJ, Jergens AE, Mansell J, Minami T, Bilzer TW. ACVIM consensus statement: Endoscopic, biopsy and histo-pathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J. Vet. Intern. Med. 2010;24:10–26. doi: 10.1111/j.1939-1676.2009.0443.x. [DOI] [PubMed] [Google Scholar]
- Zoran DL. Gastroduodenoscopy in the dog and cat. Vet. Clin. Small Anim. 2001;31:631–656. doi: 10.1016/s0195-5616(01)50063-7. [DOI] [PubMed] [Google Scholar]


