Abstract
Background:
Individuals with kidney disease are at a high risk of bleeding and as such tools that identify those at highest risk may aid mitigation strategies.
Objective:
We set out to develop and validate a prediction equation (BLEED-HD) to identify patients on maintenance hemodialysis at high risk of bleeding.
Design:
International prospective cohort study (development); retrospective cohort study (validation).
Settings:
Development: 15 countries (Dialysis Outcomes and Practice Patterns Study [DOPPS] phase 2-6 from 2002 to 2018); Validation: Ontario, Canada.
Patients:
Development: 53 147 patients; Validation: 19 318 patients.
Measurements:
Hospitalization for a bleeding event.
Methods:
Cox proportional hazards models.
Results:
Among the DOPPS cohort (mean age, 63.7 years; female, 39.7%), a bleeding event occurred in 2773 patients (5.2%, event rate 32 per 1000 person-years), with a median follow-up of 1.6 (interquartile range [IQR], 0.9-2.1) years. BLEED-HD included 6 variables: age, sex, country, previous gastrointestinal bleeding, prosthetic heart valve, and vitamin K antagonist use. The observed 3-year probability of bleeding by deciles of risk ranged from 2.2% to 10.8%. Model discrimination was low to moderate (c-statistic = 0.65) with excellent calibration (Brier score range = 0.036-0.095). Discrimination and calibration of BLEED-HD were similar in an external validation of 19 318 patients from Ontario, Canada. Compared to existing bleeding scores, BLEED-HD demonstrated better discrimination and calibration (c-statistic: HEMORRHAGE = 0.59, HAS-BLED = 0.59, and ATRIA = 0.57, c-stat difference, net reclassification index [NRI], and integrated discrimination index [IDI] all P value <.0001).
Limitations:
Dialysis procedure anticoagulation was not available; validation cohort was considerably older than the development cohort.
Conclusion:
In patients on maintenance hemodialysis, BLEED-HD is a simple risk equation that may be more applicable than existing risk tools in predicting the risk of bleeding in this high-risk population.
Keywords: hemodialysis, bleeding, DOPPS, risk prediction models, international
Abrege
Contexte:
Les personnes atteintes d’insuffisance rénale présentent un risque élevé d’hémorragie. Des outils permettant de déceler les personnes les plus exposées au risque pourrait aider à mettre en œuvre des stratégies d’atténuation.
Objectifs:
Nous avons mis au point et validé une équation prédictive (BLEED-HD) afin d’identifier les patients sous hémodialyse d’entretien qui présentent un risque élevé d’hémorragie.
Type d’étude:
Étude de cohorte prospective internationale (développement); étude de cohorte rétrospective (validation)
Cadre:
Développement: dans 15 pays (étude DOPPS phases 2 à 6 entre 2002 et 2018); validation: en Ontario (Canada)
Sujets:
Développement: 53 147 patients; validation: 19 318 patients
Mesures:
Hospitalisation pour un événement hémorragique
Méthodologie:
Modèles à risques proportionnels de Cox
Résultats:
Dans la cohorte DOPPS (âge moyen: 63,7 ans; 39,7 % de femmes), 2 773 patients avaient subi un événement hémorragique (5,2 %; taux d’événements: 32 pour 1 000 années-personnes) avec un suivi médian de 1,6 an (ÉIQ: 0,9 à 2,1). BLEED-HD prend six variables en compte: âge, sexe, pays d’origine, saignement gastro-intestinal antérieur, présence d’une valve cardiaque prothétique et utilisation d’un antagoniste de la vitamine K. La probabilité observée de saignements dans les 3 ans par déciles de risque allait de 2,2 à 10,8 %. La discrimination du modèle variait de faible à modérée (statistique c: 0,65) avec un excellent étalonnage (plage de score de Brier: 0,036-0,095). La discrimination et l’étalonnage de se sont avérés semblables lors de la validation externe auprès de 19 318 patients de l’Ontario (Canada). Par rapport aux scores d’hémorragie existants, l’équation BLEED-HD a démontré une meilleure discrimination et un meilleur étalonnage (statistique c: HEMORRHAGE 0,59; HAS-BLED 0,59 et ATRIA 0,57; différence dans les c-stat, indices NRI et IDI toutes valeurs de p < 0,0001).
Limites:
L’information sur l’anticoagulant utilisé dans la procédure de dialyse n’était pas disponible; la cohorte de validation était beaucoup plus âgée que la cohorte de développement.
Conclusion:
Pour les patients sous hémodialyse d’entretien, BLEED-HD est une équation simple de calcul du risque qui peut être plus facilement applicable que les outils existants pour prédire le risque d’hémorragie dans cette population à haut risque.
What was known before: Individuals on long-term hemodialysis are at an exceedingly high bleeding risk. Prediction scores for bleeding derived from the general population often perform poorly when applied to those with dialysis.
What this adds: Using data from 53 147 hemodialysis patients from the Dialysis Outcomes and Practice Patterns Study (DOPPS) prospective study, we derived and externally validated a risk equation (BLEED-HD) incorporating 6 variables to predict bleeding risk. BLEED-HD demonstrated better discrimination and calibration than existing bleeding risk scores from the general population.
Introduction
Bleeding is a common diagnosis requiring hospitalization in patients with end-stage kidney disease often requiring invasive testing, interventions, and/or blood product transfusion.1-4 In older patients with end-stage kidney disease, 1 in 7 patients will experience a major bleeding event within 3 years of dialysis initiation, with an incidence rate of 52.6 per 1000 patient years from the international Dialysis Outcomes and Practice Patterns Study (DOPPS). 5 Multiple factors contribute to the high bleeding risk in dialysis patients, including (but not limited to) disorders of primary hemostasis (uremic platelet dysfunction), secondary hemostasis (reduced or abnormal coagulation factor activity), vessel wall disruption (vascular access needling or placement), and antithrombotic medication use (during the hemodialysis procedure as stroke prophylaxis with atrial fibrillation [AF]).6-8
Several bleeding scores, developed and validated in the non-dialysis population, are used to identify patients at high risk of bleeding with varying degrees of accuracy and applicability.9-12 However, a number of commonly used prediction models, such as the Hypertension, Abnormal kidney and liver function, Stroke, Bleeding, Labile INR, Elderly and Drugs or Alcohol (HASBLED), the Anticoagulation and Risk factors in Atrial Fibrillation (ATRIA), and the Hepatic or kidney disease, Ethanol abuse, Malignancy, Older age, Reduced platelet count or reduced platelet function, Hypertension, Anemia, Genetic factors, Excessive fall risk and Stroke (HEMORR2HAGES), did not include individuals receiving chronic dialysis and as such demonstrate questionable prediction in patients with advanced chronic kidney disease (CKD) and on dialysis. 4
As the risk of bleeding is high in long-term patients on dialysis with multiple factors unique to this population, it follows that a dialysis-specific risk equation may improve on existing risk scores. Furthermore, a risk algorithm may aid in clinical decision making such as initiation of anticoagulation in AF. As such, we set out to develop, validate, and compare a novel risk equation to predict bleeding events in patients on chronic hemodialysis (BLEED-HD) using the international DOPPS prospective cohort study.
Methods
Study Population
The DOPPS is an international prospective cohort study that collects information on adults (≥18 years) on maintenance, in-center hemodialysis in over 20 countries. 13 The current study included data from 53 147 patients participating in DOPPS phase 2-6 (2002-2018, Supplementary Table 1). The DOPPS uses a 2-stage stratified random cluster sample where facilities are randomly sampled within country-specific strata followed by a random selection of 20 to 40 patients within each facility. 13 The DOPPS collects detailed information on individual patients’ demographics, comorbid illnesses, medications, laboratory values, dialysis characteristics, hospitalizations, and mortality. Details on the DOPPS study design and methodology can be found elsewhere. 13 Regional ethics board approval was obtained for this de-identified retrospective study by the Ottawa Hospital Regional Ethic Board.
Study Design
Development of the risk equation required selection and identification of factors that were associated with bleeding events requiring hospitalization, creating a parsimonious model, and evaluating the model operating characteristics. Model validation was performed externally, in an independent dataset (the Canadian Organ Replacement Registry, CORR) linked and housed at ICES. 14 Finally, BLEED-HD was compared to existing bleeding risk models (HAS-BLED, ATRIA, HEMORR2GHAGE) in terms of discrimination and calibration.
Predictors
We created a large list of candidate list predictor variables a priori based on previous literature, clinical plausibility, and use in other bleeding risk scores.1-3,7,15,16 Variables included demographics (sex, age, country), era (DOPPS phase), comorbid illness (previous gastrointestinal bleeding, cardiovascular diseases, prosthetic heart valve, arrhythmias, diabetes, cancer, neurological disease, lung disease, HIV, psychiatric illness, recurrent cellulitis), dialysis characteristics (vintage, vascular access, pre-dialysis blood pressure, average ultrafiltration, average treatment time, adequacy), laboratory values (hemoglobin, albumin), and medications (anticoagulants, anti-platelet agents, gastric acid inhibitors). Characteristics are derived from patient charts and/or medical records by a trained study coordinator. Comorbid illnesses are based on prior medical history while vintage, vascular access, laboratory values, and medication use are based on information obtained at DOPPS enrollment. The average ultrafiltration (UF), treatment time, and pre-dialysis systolic blood pressure were based on 1 week of data at DOPPS enrollment (averaging of 3 values across 3 HD sessions in a week).
Study Outcome
The primary outcome definition was hospitalization for a bleeding event. A DOPPS-specific set of hospitalization diagnosis and procedure codes was used by study coordinators to classify hospitalizations based on chart abstraction; codes relevant to the outcome of interest were identified by the current study investigators. Bleeding events could include gastrointestinal bleeding, subdural hematoma, evacuation of a hematoma, abnormal bleeding, hemoptysis, hematuria, cerebral hemorrhage, epistaxis, or vascular access bleeding.
Statistical Analysis
Model development
We investigated the association between the predictor variables of interest and the study outcome of bleeding using time to event models. Follow-up time was censored upon the first of death, kidney transplantation, loss to follow-up, the study outcome, or end of the follow-up period. Predictor variables with >15% missing data were excluded. 17 Variables with <15% missingness were imputed using the predictive mean matching method for continuous variables and discriminant function method for categorical variables. 18 We prespecified all predictor variables in our initial model. Continuous variables were standardized and centered on their respective mean. 19 We used Cox proportional hazards models to examine the relationship between predictor variables and the outcome of interest. To develop a parsimonious model, we used the step-down procedure described by Ambler et al. 20 First, an initial model including all predictors was created followed by n-1 variable removal based on the beta coefficient. The final model contained the lowest number of predictors with a stable Akaike information criteria (AIC) value.20,21 The AIC is an estimate of the quality of a prediction model that optimizes the trade-off between model goodness of fit and simplicity. A plot of AICs versus n-1 predictive models is presented in Supplementary Figure 1 and Supplementary Table 2. Predictive performance was assessed and reported using measures of model discrimination (area under the receiver operator curve) and calibration (plots of the observed versus predicted risk of hemorrhage, Brier score) for the total cohort and stratified predictor variables included in the final model (ie, age [< or >= 65 years], sex, country, previous gastrointestinal bleeding, prosthetic heart valve, or vitamin K antagonist use). 22 Perfect calibration would yield a low Brier score with complete miscalibration yielding a Brier score of 1. 19 The time-averaged c-statistic at 3 years of follow-up was reported. We conducted all analyses with SAS software, SAS Enterprise guide version 7.1 (SAS Institute Inc., Cary, NC, USA).
External validation
We externally validated BLEED-HD in a cohort of adults (>66 years old) on chronic hemodialysis from Ontario, Canada, captured in the Canadian Organ Replacement Registry (CORR, a federally mandated, validated data registry for chronic dialysis patients) and housed at ICES.14,23-25 Individuals under 66 were excluded as medications are only captured in those 65 or older and an additional 1 year was added to allow for medication refills. We included patients from April 2008 to March 2019 with follow-up to March 2020 (Supplementary Table 3). Outcomes were defined using a validated algorithm for bleeding requiring an emergency department visit or hospitalization (94% sensitivity, positive predictive value 87%, Supplementary Table 4 for definitions used). 26 We calculated the event rate, area under the curve (AUC), and plots of deciles of risk for observed versus predicted events using the BLEED HD algorithm.
Comparison to HAS-BLED, ATRIA, and HEMORR2HAGE
We visually examined outcome events by raw score risk and AUC for our comparator bleeding scores.9,10,12 Definitions were modified to the dialysis population and/or based on data availability as required (Supplementary Table 5). To examine calibration, we plotted the observed and predicted probability of a bleeding event by risk score points for our comparator bleeding scores. Risk scores were compared to BLEED-HD by calculating the difference in Uno’s c-statistic with 95% confidence intervals, the net reclassification index, and integrated discrimination index.27,28
Results
A total of 53 147 hemodialysis patients with a median follow-up time of 1.6 (interquartile range [IQR], 0.9-2.1) years were examined (Table 1). Bleeding events occurred in 2770 (5.2%, event rate 32.0 per 1000 person-years) individuals. Bleeding events were more common with an older age, in those with cardiovascular disease (coronary artery disease, other cardiac disease, stroke, peripheral vascular disease, prosthetic heart valves), AF and other arrhythmias, neurological or lung disorders, previous gastrointestinal bleeding, and cancer. Bleeding events differed by geographic region (higher in Europe, North America, and Australia/NZ). In terms of dialysis characteristics, bleeding was higher in those with catheters, with longer dialysis vintage and lower mean ultrafiltration per session. No difference was noted by mean dialysis session time or pre-dialysis blood pressure. Anticoagulant or acetylsalicylic acid, proton pump inhibitors (PPIs), or H2-receptor blockers use was more commonly in those with bleeding events.
Table 1.
Baseline Characteristics of Hemodialysis Patients With and Without a Bleeding Event in the DOPPS.
| Variable | Total | Bleeding | |
|---|---|---|---|
| Event | No event | ||
| 53 147 | 2773 (5.2) | 50 374 (94.8) | |
| Age (years, mean, SD) | 63.7 (14.7) | 67.4 (13.1) | 63.5 (14.7) |
| Sex (%, female) | 39.7 | 39.5 | 39.7 |
| Era (%) | |||
| 2002-2004 | 22.0 | 17.4 | 22.2 |
| 2005-2008 | 19.8 | 25.1 | 19.5 |
| 2009-2011 | 21.9 | 23.3 | 21.9 |
| 2012-2015 | 30.6 | 31.2 | 30.6 |
| 2016-2018 | 5.7 | 3.1 | 5.8 |
| Country/region (%) | |||
| Australia-New Zealand | 4.8 | 5.6 | 4.8 |
| Europe a | 45.4 | 51.4 | 45.1 |
| North America | 21.9 | 22.7 | 21.8 |
| Other b | 27.9 | 20.3 | 28.4 |
| Comorbidities (%) | |||
| Coronary artery disease | 40.5 | 48.1 | 40.1 |
| Congestive heart failure | 27.5 | 32.5 | 27.2 |
| Other cardiovascular disease | 31.4 | 39.7 | 31.0 |
| Hypertension | 84.5 | 86.3 | 84.4 |
| Stroke | 16.0 | 21.1 | 15.7 |
| Diabetes | 40.6 | 39.7 | 40.7 |
| Peripheral vascular disease | 25.6 | 31.7 | 25.2 |
| Lung | 11.8 | 15.7 | 11.6 |
| Cancer | 13.6 | 17.5 | 13.4 |
| Previous gastrointestinal bleeding | 4.9 | 12.4 | 4.5 |
| Neurological disease | 10.7 | 13.9 | 10.5 |
| Atrial fibrillation | 12.3 | 17.2 | 12.1 |
| Other arrhythmias | 12.3 | 14.0 | 12.2 |
| Prosthetic heart valve | 2.2 | 3.9 | 2.2 |
| Dialysis-related | |||
| Vintage (median, IQR) | 2.0 (0.4, 5.4) | 2.5 (0.7, 6.0) | 2.0 (0.4, 5.3) |
| Catheter (%) | 24.3 | 26.3 | 24.1 |
| Treatment time (minutes) | 240 (210, 240) | 240 (210, 240) | 240 (210, 240) |
| Pre-dialysis SBP (mm Hg) | 143.7 (22.9) | 143.7 (23.1) | 143.7 (22.9) |
| Pre-dialysis DBP (mm Hg) | 75.1 (13.4) | 74.0 (13.5) | 75.2 (13.4) |
| Ultrafiltration (kg) c | −2.1 (1.8) | −2.0 (2.7) | −2.1 (1.8) |
| Laboratory values | |||
| Hemoglobin (g/L) | 11.0 (1.6) | 11.0 (1.6) | 11.0 (1.5) |
| Albumin (g/L) | 3.7 (0.5) | 3.6 (0.5) | 3.7 (0.5) |
| Medications (%) | |||
| Vitamin K antagonist | 8.9 | 14.1 | 8.6 |
| Other anticoagulant | 21.4 | 24.4 | 21.3 |
| Acetylsalicylic acid | 35.0 | 38.9 | 34.8 |
| Proton pump inhibitor | 41.7 | 48.2 | 41.3 |
| H2 blocker (antihistamine) | 20.6 | 21.1 | 20.6 |
Note. DOPPS = Dialysis Outcomes and Practice Patterns Study; SD = standard deviation; IQR = interquartile range; SBP = systolic blood pressure; DBP = diastolic blood pressure.
Belgium, France, Germany, Italy, Spain, and the United Kingdom.
Japan, Russia, Turkey, China, Gulf Cooperation Council (Saudi Arabia, Kuwait, the United Arab Emirates, Qatar, Bahrain, and Oman).
Ultrafiltration: average post weight minus pre-weight for first 3 dialysis sessions.
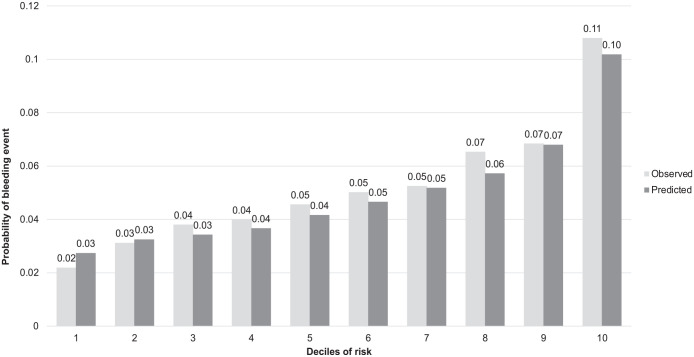
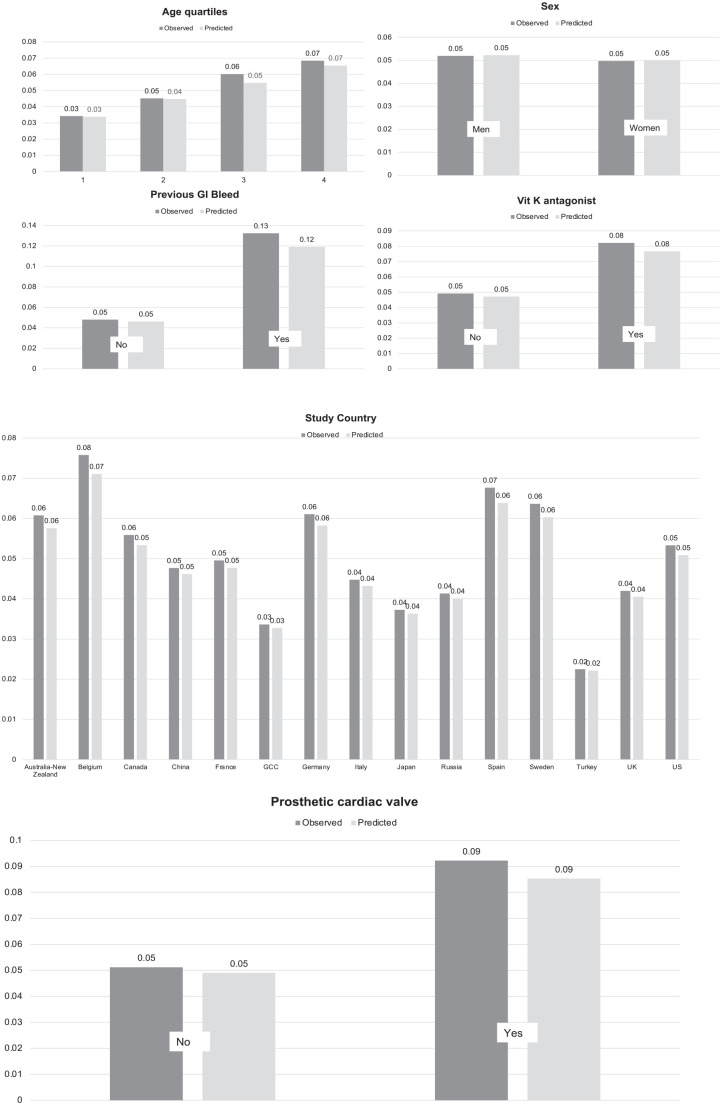
In time to event models, significant predictors of bleeding included in our final BLEED-HD model were age, sex, country, previous gastrointestinal bleed, prosthetic heart valve, and vitamin K antagonist use (Table 2, model equation is presented in Supplementary Box 1). No interactions were retained in the final model (based on AIC). The c-stat for BLEED-HD ranged from 0.66 to 0.69 over years 1 to 3, with a Brier score ranging from 0.035 to 0.095 (Table 3). The observed versus predicted risk difference did not exceed 2% across any decile risk category and the probability of predicted risk ranged from 3% to 10% (Figure 1). Calibration was consistent across individual predictors with no absolute difference between observed and predicted risk >2% (Figure 2).
Table 2.
Final Predictive Model for Bleeding in Individuals Who Receive Chronic Hemodialysis in the DOPPS Cohort (BLEED-HD).
| Parameter | Hazard ratio | 95% confidence interval |
|---|---|---|
| Age (increase by 1) | 1.02 | 1.02-1.02 |
| Sex (male) | 1.05 | 0.97-1.13 |
| Country | ||
| Australia/New Zealand | 1.17 | 0.97-1.41 |
| Belgium | 1.18 | 1.01-1.38 |
| Canada | 0.88 | 0.75-1.04 |
| China | 0.88 | 0.67-1.14 |
| France | 0.90 | 0.74-1.10 |
| Gulf States | 0.79 | 0.58-1.07 |
| Germany | 0.90 | 0.74-1.10 |
| Italy | 0.63 | 0.52-0.77 |
| Japan | 0.48 | 0.42-0.55 |
| Russia | 1.30 | 0.92-1.85 |
| Spain | 1.10 | 0.95-1.28 |
| Sweden | 1.08 | 0.92-1.27 |
| Turkey | 0.67 | 0.25-1.79 |
| UK | 0.82 | 0.68-1.00 |
| USA | referent | |
| Previous GI bleed | 2.92 | 2.61-3.27 |
| Prosthetic heart valve | 1.45 | 1.19-1.76 |
| Vitamin K antagonist | 1.50 | 1.34-1.67 |
Note. DOPPS = Dialysis Outcomes and Practice Patterns Study; GI = gastrointestinal; PPI = proton pump inhibitor.
Table 3.
Model Development c-Statistic and Brier Score by Year for BLEED-HD.
| Year | c-statistic (95% CI) | Brier score (95% CI) |
|---|---|---|
| 1 | 0.68 (0.66-0.69) | 0.04 (0.04-0.04) |
| 2 | 0.69 (0.67-0.70) | 0.06 (0.06-0.06) |
| 3 | 0.67 (0.65-0.68) | 0.10 (0.09-0.10) |
Note. CI = confidence interval.
Figure 1.
Plot of observed versus predicted probabilities across deciles of 3-year risk for bleeding for BLEED-HD among individuals on hemodialysis in the Dialysis Outcomes and Practice Patterns Study (DOPPS).
Figure 2.
Observed versus predicted probability of bleeding (y-axis) for BLEED-HD among individuals on hemodialysis in the Dialysis Outcomes and Practice Patterns Study (DOPPS) by model predictors: (1) age, (2) sex, (3) previous gastrointestinal (GI) bleeding, (4) vitamin K antagonist, (5) country, and (6) prosthetic cardiac valve.
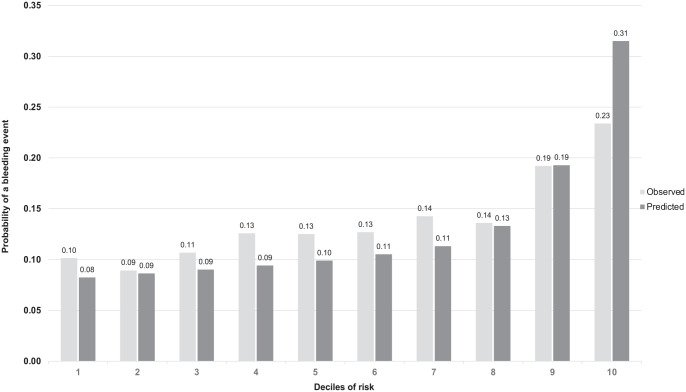
External validation was conducted on 19 318 hemodialysis patients from Ontario, Canada (Supplementary Table 6). In general, the external validation cohort was older (mean age 76.7), 40% female, 64% with diabetes mellitus, 58.7% coronary artery disease, 14% previous gastrointestinal bleed, and 3.6% prosthetic heart valve. Atrial fibrillation was present in 24.6% at baseline, 17.1% were on a vitamin K antagonist, and 46.8% were on a PPI. Overall, there were 2406 (12.5%, event rate 49.4 per 1000 person-years) bleeding events, with a median follow-up time of 1.95 years per patient. The BLEED-HD model discrimination yielded a time-averaged c-stat of 0.60. Examining observed versus predicted deciles of risk, BLEED HD underestimated most deciles of risk by 3% or less except in the highest risk decile (Figure 3).
Figure 3.
External validation: Plot of observed versus predicted probabilities across deciles of 3-year risk for bleeding for BLEED-HD among individuals on hemodialysis on external validation.
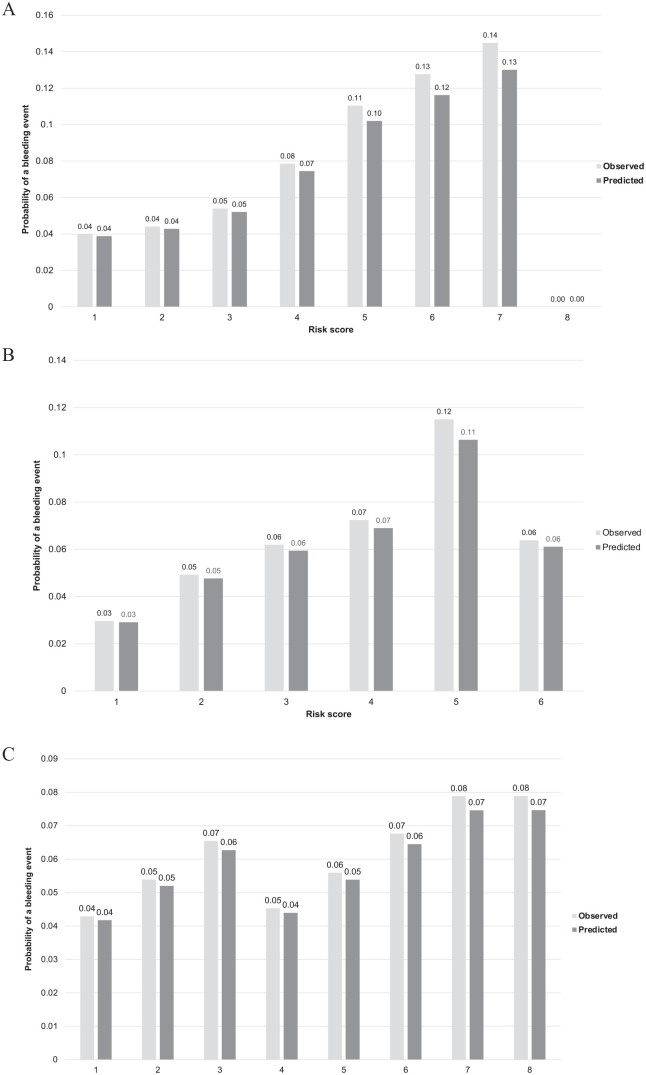
In comparison to existing bleeding risk scores, BLEED-HD demonstrated improved discrimination and calibration in the DOPPS-HD cohort (Supplementary Table 5). The c-statistic for the HAS-BLED, ATRIA, and HEMORR2HAGE scores were 0.55, 0.55, and 0.57, respectively, compared to 0.65 for BLEED-HD (P value for c-stat difference). BLEED-HD demonstrated statistically significant improvements in reclassification and integrated discrimination compared to existing bleeding score (Table 4). The predicted risk by HEMORR2HAGE ranged from 4% to 13% with no events among those with the highest risk score (Figure 4). The maximum score by HAS-BLED (6) predicted a lower risk than a score of 4 or 5. The ATRIA score predicted a very narrow risk ranging from 4% to 7% across a score range of 1 to 8.
Table 4.
Comparison of BLEED-HD Model Discrimination to Existing Bleeding Risk Prediction Models (1) HAS-BLED, (2) ATRIA, and (3) HEMORRHAGE in DOPPS Hemodialysis Patients.
| Prediction equation | c-statistic (95% CI) | c-stat. difference | P value | NRI | P value | IDI | P value |
|---|---|---|---|---|---|---|---|
| HAS-BLED score | 0.55 (0.56-0.59) | 0.06 | <.0001 | −0.20 | <.0001 | −0.6 × 10−2 | <.0001 |
| ATRIA score | 0.55 (0.52-0.55) | 0.10 | <.0001 | −0.29 | <.0001 | −0.8 × 10−2 | <.0001 |
| HEMORRHAGES | 0.57 (0.57-0.60) | 0.08 | <.0001 | −0.20 | <.0001 | −0.6 × 10−2 | <.0001 |
Note. DOPPS = Dialysis Outcomes and Practice Patterns Study; CI = confidence interval; NRI = net reclassification index; IDI = integrated discrimination index.
Figure 4.
Observed versus predicted probability of bleeding (y-axis) for (A) HEMORR2HAGES, (B) HAS-BLED, and (C) ATRIA among individuals on hemodialysis in the Dialysis Outcomes and Practice Patterns Study (DOPPS).
Discussion
Long-term hemodialysis patients represent one of the highest risk populations for major bleeding events and the ability to accurately identify those at high or low risk would aid in mitigation strategies and safe prescribing practices. Using data from an international, prospective cohort study of 53 147 individuals on long-term hemodialysis, we developed a clinical prediction equation (BLEED-HD) to help identify individuals at risk of clinically significant bleeding events requiring hospitalization. BLEED-HD included age, sex, country, previous diagnosis of a gastrointestinal (GI) bleeding, prosthetic heart valve, or vitamin K antagonist use in the final model. The overall model discrimination was low to moderate (c-stat = 0.65) with excellent calibration, was externally validated, and demonstrated some improvement to existing non-dialysis specific risk scores (HAS-BLED, ATRIA, HEMORR2GHAGE). Additional risk factors or novel biomarkers may further improve hemorrhage prediction in individuals on long-term dialysis.
Bleeding events are exceedingly common in the chronic hemodialysis population with estimates ranging from 40 to 80 per 1000 person-years.8,29-33 In our DOPPS cohort, we found a rate consistent and slightly lower than previous studies (32 per 1000 person-years).34,35 We previously reported international variation in bleeding rates ranging from 40 (Japan) to 160 (Sweden) per 1000 person-years in the DOPPS phase 1-4 cohort. 36 These events are not without consequence, as they are estimated to occur in 14.4% of patients within the first 3 years of initiating HD and associated with considerable procedure-related morbidity and health care costs. 37
The BLEED-HD risk equation included several established risk factors for bleeding. Comorbidity, age, and use of vitamin K antagonist are well-known risk factors for major bleeding. 38 Previous GI bleeding was the single strongest risk factor for predicting future bleeding risk consistent with a previous DOPPS study. 5 Surprisingly, anti-platelet agents and dialysis-related characteristics, such as treatment time or vascular access, were not retained in the final model, illustrating the importance of traditional bleeding-related risk factors in predicting risk. Whether additional risk factors not included in the present study such as anticoagulation use with the dialysis procedure itself or as a catheter locking solution are associated with bleeding events remains unknown.
Our findings add to the current body of evidence demonstrating the challenges of exiting bleeding risk scores developed in the general population when applied to those on hemodialysis.4,39-41 We demonstrated c-statistics <0.6 for HASBLED, ATRIA, and HEMORR2HAGES. A previous study of the NECOSAD dialysis cohort reported similar poor discrimination (c-statistics of 0.58 or lower) with currently available bleeding risk tools. 42 Calibration, a measure of more importance in prediction, was variable with existing risk scores with little ability to differentiate across risk groups and an inability to identify highest risk individuals. In contrast, BLEED-HD demonstrated improved calibration with a steady increase across risk deciles and an ability to predict from low (3%) to high (10%) risk. In some respects, these discrepancies are not surprising as many of the predictor definitions are difficult to apply directly to an HD population. For example, there is no clear definition of hypertension or anemia. Furthermore, existing scores were originally developed to identify bleeding risk in anticoagulated individuals with AF and not bleeding risk in general per se. Thus, BLEED-HD is targeted to estimate bleeding risk in the general hemodialysis population and demonstrates improvements in model operating characteristics (discrimination, calibration) to existing AF-based, general population tools.
Despite our large international cohort and data-driven model development approach, the BLEED-HD equation demonstrated low to moderate model discrimination and is of questionable clinical application. This suggests bleeding events may be relatively difficult to predict in the hemodialysis population and/or further studies or approaches may lead to model improvement. Key variables expanding on types, therapeutic effectiveness, routes of administration, and dosing of anticoagulation use may aid in that regard. Alternative approaches such as the accounting for the competing risk of death or model reduction techniques should be examined.
The clinical need for an accurate bleeding risk score in the hemodialysis population remains as it could aid in identifying and counseling individuals at risk in general or before/after the addition of an anticoagulant. Furthermore, a determination of high risk may prompt considerations of bleeding reduction strategies such as discontinuation of anti-platelet agents or prophylactic PPI use in individuals with AF. Routine PPI co-prescription has been estimated to have a number needed to treat of 25 to prevent one disabling or fatal upper gastrointestinal bleed in those aged 85 years or older with vascular events in the general population. 43 However, their use in the dialysis population can be more problematic due to the risk of hypomagnesemia, fracture, and/or Clostridium difficile infection. 44 Finally, scores may be used to identify bleeding risk for trial participation, particularly for evaluation of the newer anticoagulants.
The current study developed and validated a novel bleeding score using data from a large international and well-established hemodialysis cohort that substantively improves on existing prediction tools. Nevertheless, there are noteworthy limitations. Important factors that may be associated with bleeding were not readily available such as duration and dosage of anticoagulation, dialysis procedure-related anticoagulation use, INR lability, applicability in peritoneal dialysis, and there was a limited number of individuals using direct oral anticoagulants, an emerging anticoagulant in the dialysis population.45,46 Our external validation differed from the development cohort as they were in Canada only and older relative to the DOPPS. This may explain the mildly lower model discrimination observed in the validation cohort. Despite the use of a large international cohort, we were limited by a small number of events in examining subgroups of high clinical interest such as the bleeding risk in individuals with or without an anticoagulant. For the existing risk scores (HEMORR2HAGES, HAS-BLED, ATRIA), all risk factors were not available for inclusion and modifications were required.
In conclusion, BLEED-HD predicts the risk of bleeding in individuals on hemodialysis with potential clinical use in determining individual risk and trial enrollment. Further studies examining its utility in high-risk populations, such as those on peritoneal or home hemodialysis, are required.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581231169610 for Development and Validation of a Predictive Risk Algorithm for Bleeding in Individuals on Long-term Hemodialysis: An International Prospective Cohort Study (BLEED-HD) by Mohit Madken, Ranjeeta Mallick, Emily Rhodes, Roshanak Mahdavi, Anan Bader Eddeen, Gregory L. Hundemer, Dearbhla M. Kelly, Angelo Karaboyas, Bruce Robinson, Brian Bieber, Amber O. Molnar, Sunil V. Badve, Peter Tanuseputro, Gregory Knoll and Manish M. Sood in Canadian Journal of Kidney Health and Disease
Acknowledgments
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This study also received funding from: [list additional funders of this work, e.g., CIHR]. This document used data adapted from the Statistics Canada Postal Code OM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from ©Canada Post Corporation and Statistics Canada. Parts of this material are based on data and/or information compiled and provided by: [list relevant data sources, e.g., MOH, CIHI]. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Footnotes
Ethics Approval and Consent to Participate: Regional ethics board approval was obtained for this deidentified retrospective study by the Ottawa Hospital Regional Ethic Board.
Consent for Publication: Not applicable.
Availability of Data and Materials: The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g., healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification. DOPPS data is not freely available but access may be granted to investigators and collaborators via a data request.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: M.M.S. has received a CME speaker fee from AstraZeneca. A.K., B.R., and B.B. are employees of Arbor Research Collaborative for Health, which administers the DOPPS.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Global support for the ongoing DOPPS Programs is provided without restriction on publications. See https://www.dopps.org/AboutUs/Support.aspx for more information. All funding is provided to Arbor Research Collaborative for Health and not to the authors directly.
ORCID iDs: Mohit Madken  https://orcid.org/0000-0002-2975-0142
https://orcid.org/0000-0002-2975-0142
Gregory L. Hundemer  https://orcid.org/0000-0003-3559-3531
https://orcid.org/0000-0003-3559-3531
Amber O. Molnar  https://orcid.org/0000-0003-4549-0202
https://orcid.org/0000-0003-4549-0202
Manish M. Sood  https://orcid.org/0000-0002-9146-2344
https://orcid.org/0000-0002-9146-2344
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Huang KW, Leu HB, Luo JC, et al. Different peptic ulcer bleeding risk in chronic kidney disease and end-stage renal disease patients receiving different dialysis. Dig Dis Sci. 2014;59(4):807-813. doi: 10.1007/s10620-013-2973-6. [DOI] [PubMed] [Google Scholar]
- 2.Iseki K, Kinjo K, Kimura Y, Osawa A, Fukiyama K.Evidence for high risk of cerebral hemorrhage in chronic dialysis patients. Kidney Int. 1993;44(5):1086-1090. [DOI] [PubMed] [Google Scholar]
- 3.Kuo CC, Kuo HW, Lee IM, Lee CT, Yang CY.The risk of upper gastrointestinal bleeding in patients treated with hemodialysis: a population-based cohort study. BMC Nephrol. 2013;14:15. doi: 10.1186/1471-2369-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molnar AO, Sood MM.Predicting in a predicament: stroke and hemorrhage risk prediction in dialysis patients with atrial fibrillation. Semin Dial. 2018;31(1):37-47. doi: 10.1111/sdi.12637. [DOI] [PubMed] [Google Scholar]
- 5.Sood MM, Larkina M, Thumma JR, et al. Major bleeding events and risk stratification of antithrombotic agents in hemodialysis: results from the DOPPS. Kidney Int. 2013;84(3):600-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lutz J, Menke J, Sollinger D, Schinzel H, Thürmel K.Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):29-40. doi: 10.1093/ndt/gft209. [DOI] [PubMed] [Google Scholar]
- 7.Sohal AS, Gangji AS, Crowther MA, Treleaven D.Uremic bleeding: pathophysiology and clinical risk factors. Thromb Res. 2006;118(3):417-422. doi: 10.1016/j.thromres.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Holden RM, Harman GJ, Wang M, Holland D, Day AG.Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(1):105-110. doi: 10.2215/cjn.01810407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY.A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro heart survey. Chest. 2010;138(5):1093-1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 10.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151(3):713-719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Piccini JP, Stevens SR, Chang Y, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Circulation. 2013;127(2):224-232. doi: 10.1161/circulationaha.112.107128. [DOI] [PubMed] [Google Scholar]
- 12.Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Circulation. 2009;119(10):1363-1369. doi: 10.1161/circulationaha.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA.The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44(5suppl 2):7-15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Canadian Institute for Health Information (CIHI). Treatment of End-Stage Organ Failure in Canada, 1999 to 2008: CORR 2010 Annual Report. Toronto, ON, Canada: CIHI; 2010. [Google Scholar]
- 15.Fischer MJ, Ho PM, McDermott K, Lowy E, Parikh CR.Chronic kidney disease is associated with adverse outcomes among elderly patients taking clopidogrel after hospitalization for acute coronary syndrome. BMC Nephrol. 2013;14(1):107. doi: 10.1186/1471-2369-14-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedges SJ, Dehoney SB, Hooper JS, Amanzadeh J, Busti AJ.Evidence-based treatment recommendations for uremic bleeding. Nat Clin Pract Nephrol. 2007;3(3):138-153. doi: 10.1038/ncpneph0421. [DOI] [PubMed] [Google Scholar]
- 17.Little RJA, Rubin DB.Statistical Analysis with Missing Data. New York, NY: John Wiley Sons; 1986. [Google Scholar]
- 18.Yuan Y.Multiple imputation using SAS software. Journal of Statistical Software. 2011;45(6):1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steyerberg EW.Clinical Prediction Models: A Practical Approach to Development, Validation: Updating. Cham, Switzerland: Springer; 2010. [Google Scholar]
- 20.Ambler G, Brady AR, Royston P.Simplifying a prognostic model: a simulation study based on clinical data. Stat Med. 2002;21(24):3803-3822. doi: 10.1002/sim.1422. [DOI] [PubMed] [Google Scholar]
- 21.Rigatto C, Sood MM, Tangri N.Risk prediction in chronic kidney disease: pitfalls and caveats. Curr Opin Nephrol Hypertens. 2012;21(6):612-618. doi: 10.1097/MNH.0b013e328359072f. [DOI] [PubMed] [Google Scholar]
- 22.Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD). Circulation. 2015;131(2):211-219. doi: 10.1161/CIRCULATIONAHA.114.014508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canadian Institute for Health Information (CIHI). Data Quality Study on the Canadian Organ Replacement Register. Toronto, ON, Canada: CIHI; 2009. [Google Scholar]
- 24.Moist LM, Richards HA, Miskulin D, et al. A validation study of the Canadian Organ Replacement Register. Clin J Am Soc Nephrol. 2011;6(4):813-818. doi: 10.2215/cjn.06680810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sciences TIfCE. ICES Home Page. https://www.ices.on.ca/. Accessed September 5, 2018.
- 26.Arnason T, Wells PS, van Walraven C, Forster AJ.Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118(2):253-262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS.Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 28.Pencina MJ, Steyerberg EW, D’Agostino RB.Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11-21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo J-C, Leu H-B, Huang K-W, et al. Incidence of bleeding from gastroduodenal ulcers in patients with end-stage renal disease receiving hemodialysis. Can Med Assoc J. 2011;183(18):E1345-E1351. doi: 10.1503/cmaj.110299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott MJ, Zimmerman D, Holden RM.Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007;50(3):433-440. doi: 10.1053/j.ajkd.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Olesen JB, Lip GYH, Kamper A-L, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625-635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 32.Sakhuja A, Schold JD, Kumar G, Katzan I, Navaneethan SD.Nontraumatic subarachnoid hemorrhage in maintenance dialysis hospitalizations: trends and outcomes. Stroke. 2014;45(1):71-76. doi: 10.1161/strokeaha.113.003012. [DOI] [PubMed] [Google Scholar]
- 33.Wasse H, Gillen DL, Ball AM, et al. Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int. 2003;64(4):1455-1461. [DOI] [PubMed] [Google Scholar]
- 34.Sood MMBS, McArther E, Kapral MK, et al. The three-year incidence of major hemorrhage among older adults initiating chronic dialysis. Can J Kidney Health Dis. 2014;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Eck van der Sluijs A, Abrahams AC, Rookmaaker MB, et al. Bleeding risk of haemodialysis and peritoneal dialysis patients. Nephrol Dial Transp. 2020;36(1):170-175. doi: 10.1093/ndt/gfaa216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sood MM, Komenda P, Tangri N.Anticoagulation in patients with kidney disease: one size does not fit all. Thromb Res. 2013;131(6):469-471. doi: 10.1016/j.thromres.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 37.Sood MM, Bota SE, McArthur E, et al. The three-year incidence of major hemorrhage among older adults initiating chronic dialysis. Can J Kidney Health Dis. 2014;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan KE, Lazarus JM, Thadhani R, Hakim RM.Anticoagulant and antiplatelet usage associates with mortality among hemodialysis patients. J Am Soc Nephrol. 2009;20(4):872-881. doi: 10.1681/asn.2008080824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonde AN, Lip GYH, Kamper A-L, et al. Renal function and the risk of stroke and bleeding in patients with atrial fibrillation: an observational cohort study. Stroke. 2016;47(11):2707-2713. doi: 10.1161/strokeaha.116.014422. [DOI] [PubMed] [Google Scholar]
- 40.Keskar V, Sood MM.Use of oral anticoagulation in the management of atrial fibrillation in patients with ESRD: con. Clin J Am Soc Nephrol. 2016;11(11):2085-2092. doi: 10.2215/cjn.03200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McAlister FA, Wiebe N, Jun M, et al. Are existing risk scores for nonvalvular atrial fibrillation useful for prediction or risk adjustment in patients with chronic kidney disease? Can J Cardiol. 2017;33:243-252. doi: 10.1016/j.cjca.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Ocak G, Ramspek C, Rookmaaker MB, et al. Performance of bleeding risk scores in dialysis patients. Nephrol Dial Transp. 2019;34(7):1223-1231. doi: 10.1093/ndt/gfy387. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Geraghty OC, Mehta Z, Rothwell PM.Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390(10093):490-499. doi: 10.1016/s0140-6736(17)30770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Francisco ALM, Varas J, Ramos R, et al. Proton pump inhibitor usage and the risk of mortality in hemodialysis patients. Kidney Int Rep. 2018;3(2):374-384. doi: 10.1016/j.ekir.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK.Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6(11):2662-2668. doi: 10.2215/cjn.04550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldberg J, Patel P, Farrell A, et al. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol Dial Transp. 2018;34(2):265-277. doi: 10.1093/ndt/gfy031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581231169610 for Development and Validation of a Predictive Risk Algorithm for Bleeding in Individuals on Long-term Hemodialysis: An International Prospective Cohort Study (BLEED-HD) by Mohit Madken, Ranjeeta Mallick, Emily Rhodes, Roshanak Mahdavi, Anan Bader Eddeen, Gregory L. Hundemer, Dearbhla M. Kelly, Angelo Karaboyas, Bruce Robinson, Brian Bieber, Amber O. Molnar, Sunil V. Badve, Peter Tanuseputro, Gregory Knoll and Manish M. Sood in Canadian Journal of Kidney Health and Disease