Abstract
Background:
With an aging population and growing number of patients with chronic kidney disease (CKD), integrating the latest risk factors when deciding on a treatment plan can result in better patient care. Frailty remains a prevalent syndrome in CKD resulting in adverse health outcomes. However, measures of frailty and functional status remain excluded from clinical decision making.
Objective:
To examine the degree to which different measures of frailty and functional status are associated with mortality, hospitalization, and other clinical outcomes in patients with advanced CKD.
Design:
Systematic review.
Setting:
Observation studies including cohort study, case-control study, or cross-sectional study examining frailty and functional status on clinical outcomes. There were no restrictions on type of setting or country of origin.
Patients:
Adults with advanced CKD, including both types of dialysis patients.
Measurements:
Data including demographic information (e.g., sample size, follow-up time, age, country), assessments of frailty or functional status and their domains, and outcomes including mortality, hospitalization, cardiovascular events, kidney function, and composite outcomes were extracted.
Methods:
A search was conducted using databases Medline, Embase, and Cochrane Central Register for Controlled Trials. Studies were included from inception to March 17, 2021. The eligibility of studies was screened by 2 independent reviewers. Data were presented by instrument and clinical outcome. Point estimates and 95% confidence intervals from the fully adjusted statistical model were reported or calculated from the raw data.
Results:
A total of 117 unique instruments were found among 140 studies. The median sample size of studies was 319 (interquartile range, 161-893). Most studies focused on incident and chronic dialysis patient populations, with only 15% of studies examining non-dialysis CKD patients. Frailty and lower functional status were associated with an increased risk for adverse clinical outcomes such as mortality and hospitalization. The 5 individual domains of frailty were also found to be associated with poor health outcomes.
Limitations:
Meta-analysis could not be performed due to significant heterogeneity between studies and methods used to measure frailty and functional status. Many studies had issues with methodological rigor. Selection bias and the validity of data collection could not be ascertained for some studies.
Conclusion:
Frailty and functional status measures should be integrated to help guide clinical care decision making for a comprehensive assessment of risk for adverse outcomes among patients with advanced CKD.
Registration (PROSPERO):
CRD42016045251
Keywords: frailty, functional status, CKD, outcomes, dialysis patients
Abrégé
Contexte :
Compte tenu du vieillissement de la population et du nombre croissant de patients atteints d’insuffisance rénale chronique (IRC), l’intégration des plus récents facteurs de risque dans le processus de prise de décision d’un plan de traitement pourrait améliorer les soins aux patients. La fragilité demeure un syndrome prévalant en contexte d’IRC, qui entraîne des effets néfastes sur la santé. Pourtant, les mesures de la fragilité et de l’état fonctionnel demeurent exclues de la prise de décisions cliniques.
Objectif :
Déterminer à quel point les différentes mesures de la fragilité et de l’état fonctionnel sont associées à la mortalité, à l’hospitalisation et à d’autres résultats cliniques chez les patients atteints d’IRC avancée.
Type d’étude :
Examen systématique
Sources :
Des études d’observation, y compris des études de cohorte, des études cas-témoins ou des études transversales examinant le rôle de la fragilité et de l’état fonctionnel sur les résultats cliniques. Il n’y avait pas de restrictions quant au cadre ou au pays d’origine de l’étude.
Sujets :
Des adultes atteints d’IRC avancée, y compris les deux types de patients sous dialyse.
Mesures :
Les données suivantes ont été extraites : les données démographiques (taille de l’échantillon, temps de suivi, âge des patients, pays), les évaluations de la fragilité ou de l’état fonctionnel et de leurs domaines, et les résultats cliniques (mortalité, hospitalisation, événements cardiovasculaires, fonction rénale et résultats composites).
Méthodologie :
Une recherche a été effectuée dans les bases de données Medline, embase et Cochrane Central Register for Controlled Trials pour répertorier les études de la création jusqu’au 17 mars 2021. L’admissibilité des études a été déterminée par deux examinateurs indépendants. Les données ont été présentées par instrument et par résultat clinique. Des estimations ponctuelles et des intervalles de confiance à 95 % du modèle statistique ajusté ont été rapportés ou calculés à partir des données brutes.
Résultats :
Parmi les 140 études répertoriées, 117 instruments uniques ont été trouvés. La taille médiane des échantillons était de 319 patients (ÉIQ : 161 à 893). La plupart des études portaient sur des populations de patients incidents et sous dialyse chronique, seulement 15 % des études portaient sur des patients atteints d’IRC non dialysés. La fragilité et un faible état fonctionnel ont été associés à un risque accru de résultats cliniques défavorables comme une hospitalisation ou le décès. Les cinq domaines individuels de la fragilité ont également été associés à de mauvais résultats de santé.
Limites :
L’hétérogénéité significative entre les études et les méthodes utilisées pour mesurer la fragilité et l’état fonctionnel ne permettait pas de procéder à une méta-analyse. De nombreuses études n’étaient pas rigoureuses sur le plan méthodologique. Les biais de sélection et la validité de la collecte des données n’ont pas pu être vérifiés pour certaines études.
Conclusion :
Les mesures de la fragilité et de l’état fonctionnel devraient être intégrées au processus de prise de décision afin d’orienter les soins cliniques et de permettre une évaluation complète du risque d’effets indésirables chez les patients atteints d’IRC avancée.
Enregistrement (PROSPERO) :
CRD42016045251
Introduction
The prevalence of chronic kidney disease (CKD) and end-stage kidney disease has been growing, resulting in a greater need for renal replacement therapies including kidney transplantation. 1 Predicting outcomes in patients with CKD is an integral part of clinical care, decision making, and resource allocation. However, this remains a challenge, particularly in those eligible for kidney transplantation. 2 Prediction models have been developed to estimate survival of patients with CKD, assist clinicians with decisions on transplant eligibility, and identify risk factors for adverse outcomes.2-6 These models have variable predictive performances4-6 such that there is no standardized, accepted way of determining transplant eligibility.7,8
Frailty and functional status have emerged as novel risk factors associated with adverse outcomes among patients with CKD, subsequently impacting their quality of life and survival.9-13 Frailty has multiple causes and is defined as an increased state of vulnerability due to decreases in strength, endurance, and physiologic function.14,15 To accurately capture the syndrome of frailty, a comprehensive examination is required. This assessment should encompass the 5 domains that make up the Fried frailty phenotype. 16 Functional status reflects an individual’s ability to perform normal activities to meet their basic needs, maintain their health and well-being, as well as fulfill usual roles. 11 Frailty is highly prevalent among patients with CKD affecting up to 73% of patients on dialysis, and there is an increased risk of lower functional status among these patients.15,17 Despite the growing body of evidence, these risk factors remain excluded from most prediction models for adverse outcomes in CKD patients. Conventional comorbidity assessments do not accurately capture physiological decline associated with frailty and functional status. 18 The purpose of this systematic review was to examine the degree to which different measures of frailty and functional status are associated with mortality and adverse clinical outcomes in patients with advanced CKD.
Methods
The study methodology has been previously reported. 19 This systematic review was conducted in accordance with the guidelines outlined in the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement. 20 This review has been registered in the PROSPERO database (CRD42016045251).
Literature Search
A literature search was conducted using online databases Medline, Embase, and Cochrane Central Register for Controlled Trials. We searched for studies from inception to March 17, 2021, using search terms such as end-stage renal disease, frailty, sarcopenia, functional status, and activities of daily living (Item S1). Eligibility was restricted to articles published in the English language.
Peer reviewed published articles were included if they met our predefined inclusion criteria. Specifically, we included primary research studies that used the following designs: cohort study, case-control study, or cross-sectional study. Case series were included if they had more than 20 participants. Interventional studies were included if the intervention could not have influenced the outcomes of interest. There were no restrictions on length of follow-up, type of setting, or country of origin. Other inclusion criteria were as follows: (a) Population: Adults (≥18 years of age) with CKD stages 4 or 5 (including dialysis patients but excluding kidney transplant recipients and those waitlisted); (b) Instrument: An assessment of frailty or functional status using an instrument that specifically measures overall frailty or functional status or one of their individual domains. Frailty was defined as a syndrome resulting from various factors and contributors characterized by reduced strength, endurance, and physiological function, thus making an individual more susceptible to developing increased dependency and/or mortality. 21 Functional status was defined as an individual’s ability to carry out the normal activities of daily living required to meet basic needs, fulfill usual roles, and maintain health and well-being. 22 Performance-based measures and self-reported measures were accepted; (c) Outcome: Mortality was the primary outcome of interest. We also included other important clinical outcomes such as hospitalization, cardiovascular events, kidney function, composite outcomes (i.e., mortality or need for renal replacement therapy; mortality, hospitalization, or need for renal replacement therapy; mortality or hospitalization; mortality or functional status decline; mortality or cardiovascular disease; in-hospital mortality or discharge to assisted care facility), peritonitis, serious fall injuries, withdrawals from dialysis, discharge from assisted care facility, transplantation, dialysis-related complications, discharge home, and discharge to assisted care facility.
Article selection and data extraction
The eligibility of studies was examined by 2 independent reviewers. Titles and abstracts for all references were screened. Full texts were retrieved for articles passing this initial process, and subsequently screened in greater detail by 2 reviewers. Disagreements regarding the inclusion of studies were resolved by consensus or a third reviewer. The references of included studies were scanned for additional articles, and 2 further studies were included.
A standardized data abstraction form was created and used by reviewers to extract data from the included studies. To minimize any discrepancies, both reviewers compared their extractions to reach consensus. The following data were abstracted from each study: study design, subject characteristics, instrument used to assess frailty and/or functional status, outcomes, and results.
Quality assessment
The methodological quality of the included studies was evaluated using a modified version of the Quality in Prognosis Studies (QUIPS) tool.23-25 This tool assesses bias through several prompting questions across the following 6 domains: study participation, study attrition, instrument measurement, outcome measurement, study confounding, and statistical analysis and reporting. Each of the 6 domains was rated as having high, moderate, or low risk of bias by one reviewer and verified by a second.
Data analysis and presentation
Results were organized by subgroup of kidney disease: non-dialysis CKD, incident dialysis, and prevalent (chronic) dialysis. Frailty and functional status instruments were analyzed separately as the exposure for each of these subgroups and were grouped based on the domain the instrument was measuring (Box 1). Frailty instruments were classified according to the following domains of frailty: overall frailty, sarcopenia, slow gait, strength measurement, and physical activity and fatigue. 16 Although the World Health Organization’s International Classification of Functioning Disability and Health uses a biopsychosocial model incorporating the impact from environmental, social, and cognitive factors among others to overall functioning and disability, 26 the studies retrieved from our literature search used tools that mostly examined physical measures of functional status. These tools were classified into 3 categories, each of which have established measurement techniques: Activities of Daily Living (ADL),27,28 performance scale, 29 and physical performance. 30
Box 1.
Definition of frailty and functional status and their groupings.
| Frailty: “a medical syndrome with multiple causes and contributors that is characterized by diminished strength, endurance, and reduced physiological function that increases an individual’s vulnerability for developing increased dependency and/or death.”
21
• Sarcopenia/weight loss 16 • Slowness 16 • Weakness 16 • Poor endurance/exhaustion 16 • Low physical activity 16 |
| Functional status: an individual’s ability to carry out the normal activities of daily living required to meet basic needs, fulfill usual roles, and maintain health and well-being.
22
• ADL Impairments27,28 • Performance Scale 29 • Physical Performance 30 |
Outcome data were presented by instrument used and clinical outcome. We reported the point estimate and 95% confidence intervals from the fully adjusted statistical model, if available, otherwise the unadjusted estimate was reported. Hazard ratios, relative risks, and odds ratios were obtained directly from the study or calculated from the raw data provided. When studies reported the same measurements in different units, data were converted to the same units mathematically (e.g., studies reporting on the 6-minute walk test were all presented as 100m unit measures). Due to the large degree of heterogeneity between the study populations, instruments used, and study design, we did not statistically pool the results. Finally, main findings from studies were reported as assessments. Multiple assessments of instruments and/or outcomes were possible for 1 article. For example, if a study measured a particular frailty domain using 5 different instruments, this was reported as 5 separate assessments of that frailty domain.
Results
Overview
The literature search identified 7860 unique citations, and 478 articles were assessed for the eligibility criteria at the full-text level. At this stage, a further 338 articles were excluded, resulting in 140 articles included in the review (Figure 1).
Figure 1.
Search results and study selection.
aExcluded for the purpose of this study but will be the focus of another study.
The characteristics of the included studies are reported in Table S1 (references available in Item S2). In total, 68 studies used a prospective cohort design and 48 studies performed secondary analysis of established cohorts. Other data sources included hospital records (n = 17) and registry data (n = 7). Publication dates ranged from 1976 to 2021, with a median publication year of 2016. Most studies were from the United States (n = 45), followed by Japan (n = 16), Brazil (n = 10), and Canada (n = 9). Eighty-eight studies (62.8%) exclusively studied chronic dialysis patients with a total sample size of 1,574,214, n = 28 studies (20%) assessed incident dialysis patients accounting for 245,013 patients, n = 21 studies (15%) assessed non-dialysis CKD patients with a sample size of 9923, and 3 studies could not be grouped into any of these single patient populations and therefore categorized as “other” with a sample size of 2342. The overall median sample size of included studies was 319 (interquartile range [IQR], 161-893). Specifically, the median was 306 patients (IQR, 157-835) for chronic dialysis studies, 325 patients (IQR, 183-1516) for incident dialysis studies, 287 patients (IQR, 128-450) for non-dialysis CKD studies, and 907 patients (IQR, 679-946) among studies classified as other.
Instruments
Table S2 describes the frailty and functional status instruments used in the included studies. Overall, 117 unique instruments were reported in 140 studies. There were 91 different instruments that measured frailty across its 5 domains: 29 instruments for sarcopenia (e.g., Appendicular Skeletal Muscle Index) used across 28 studies; 27 for overall frailty (e.g., Fried Frailty Index) across 46 studies; 20 for measuring physical activity and fatigue (e.g., Exhaustion) across 34 studies; 10 for strength measurement (e.g., Handgrip Strength) across 32 studies; and 5 for gait (e.g., Gait Speed) across 19 studies.
There were 26 unique instruments that measured functional status among the included studies. Sixteen functional status instruments for ADL (e.g., Katz ADL) were used across 29 studies; 6 different performance scales (e.g., Karnofsky Performance Scale) were used across 14 studies; and 4 measuring physical performance (e.g., SF-36 Physical Component Summary) were used across 30 studies.
Mortality was the most frequent outcome examined (124 studies), followed by hospitalization (30 studies), cardiovascular events (14 studies), and kidney function (9 studies). Other reported clinical outcomes are listed in Table S3.
Critical appraisal of quality
The quality assessment of the studies is summarized in Table S4. Only 6 studies (4.3%) were assessed as having a low risk of bias across all 6 categories, and 23 studies (16.4%) had a low risk of bias across 5 of the categories. There were 82 studies (58.6%) assessed to have a high risk of bias in at least 1 of the categories. Overall, the studies performed the worst in the statistical analysis and reporting category, with 40 studies (28.6%) identified as high risk of bias in this category.
Mortality
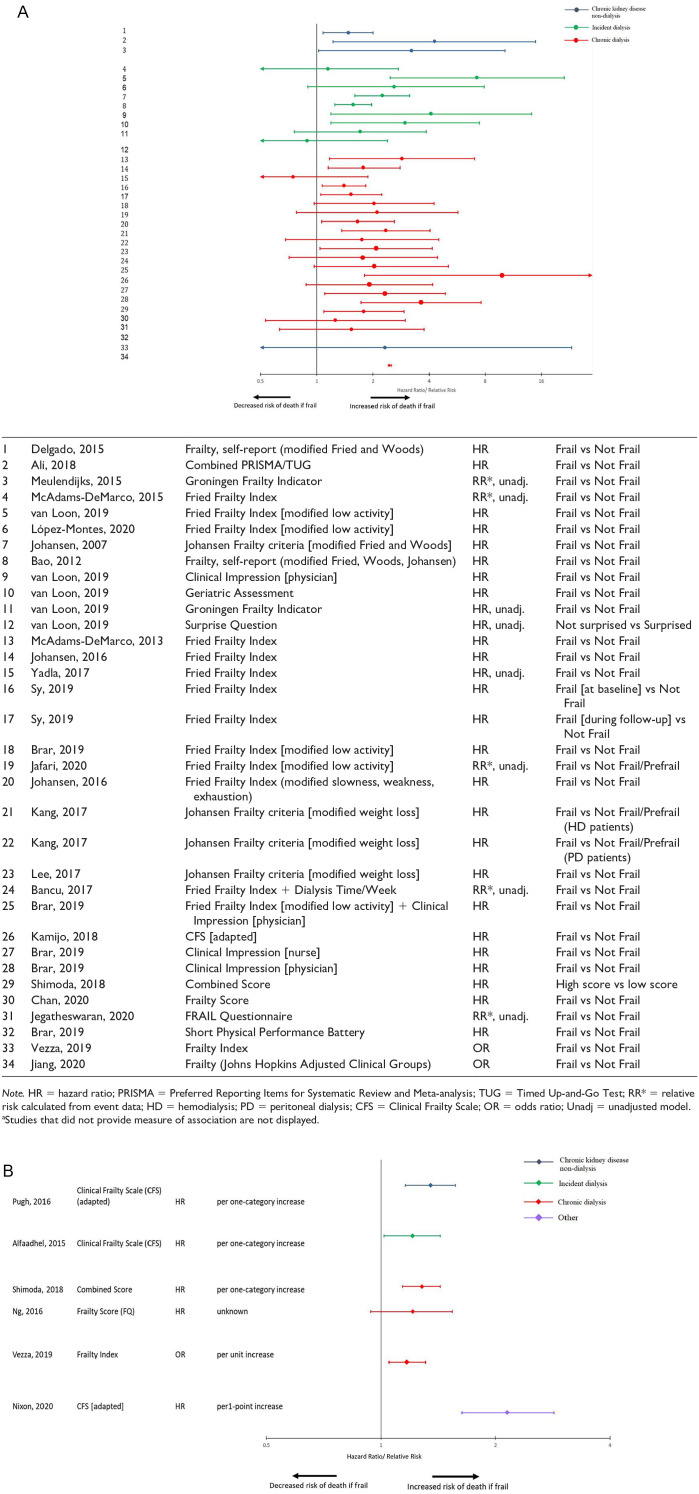
Table 1 provides an overview of the association between various instruments used to measure frailty and functional status and mortality, classified by patient population. The relationship between overall frailty and mortality was analyzed in non-dialysis CKD patients (5 assessments among 5 studies), incident dialysis patients (10 assessments among 6 studies), and chronic dialysis patients (24 assessments among 16 studies). One study examined patients listed in the “other” population category. When analyzed as a categorical variable, being frail was associated with a 2- to 4-fold increased risk of death in most included assessments. The findings were consistent across the different patient subgroups (Figure 2A). The findings were similar when frailty was assessed as a continuous variable (Figure 2B).
Table 1.
Overview of the Association Between Frailty and Functional Status Instruments and Mortality, Classified by Patient Population.
| Author, year | N | Tool | Follow-up | Analysis a | Main findings |
|---|---|---|---|---|---|
| CKD non-dialysis patients | |||||
| Frailty Tools, overall frailty or individual domains | |||||
| Delgado, 2015 | 812 | Frailty, self-report [modified Fried and Woods] | 17 years b | Not Frail [reference (ref)] vs: Intermediately Frail: aHR 1.43 (1.11-1.83) c Frail: aHR 1.48 (1.08-2.00) c |
Frailty was associated with ↑ risk of mortality. |
| Pugh, 2016 | 283 | Clinical Frailty Scale (CFS) [adapted] | 3 years | Per 1-category increase in CFS: aHR 1.35 (1.16-1.57) d | ↑ frailty was associated with ↑ risk of mortality. |
| Ali, 2018 | 104 | Combined PRISMA/Timed Up-and-Go (TUG) | 1.7 years e | Not Frail (ref) vs Frail: aHR 4.27 (1.22-14.9) f | Frailty was associated with ↑ risk of mortality. |
| Vezza, 2019 | 115 | Frailty Index | 1 year e | Not Frail (ref) vs Frail: aOR 2.32 (0.23-23.12)
c
Per unit increase: aOR 1.17 (1.05-1.31) c |
↑ frailty was associated with ↑ odds of mortality. |
| Meulendijks, 2015 | 63 | Groningen Frailty Indicator | 1 year | Not Frail (ref) vs Frail: RR 3.23 (1.02-10.2) g | Frailty was associated with ↑ risk of mortality. |
| Androga, 20176 | 1101 | Appendicular Skeletal Muscle Index (ASMI) | 9.4 yearsb,e | No Sarcopenia (ref) vs Sarcopenia: aHR 1.24 (0.98-1.58) c | Sarcopenia was not associated with mortality. |
| Kruse, 2020 | 351 | Skeletal Muscle Mass Index (SMI) | 7 years | Normal (ref) vs: Men Class I Sarcopenia: aHR 1.13 (0.82-1.57) c Class II Sarcopenia: aHR 1.20 (0.82-1.74) c Women Class I Sarcopenia: aHR 0.92 (0.74-1.15) c Class II Sarcopenia: aHR 0.98 (0.69-1.38) c |
Sarcopenia in men and women was not associated with mortality. |
| Pereira, 201595 | 287 | Sarcopenia Method A (Midarm Muscle Circumference [MAMC] + Handgrip Strength [HGS]) | 3.3 years e | No Sarcopenia (ref) vs Sarcopenia: aHR 1.62 (0.69-3.82) c | Sarcopenia Method A (MAMC + HGS) was not an independent predictor of mortality. |
| Pereira, 201595 | 287 | Sarcopenia Method B (Subjective Global Assessment [SGA]) + HGS) | 3.3 years e | No Sarcopenia (ref) vs Sarcopenia: aHR 1.80 (0.78-4.17) c | Sarcopenia Method B (SGA + HGS) was not an independent predictor of mortality. |
| Pereira, 201595 | 287 | Sarcopenia Method C (Skeletal Muscle Mass Index [SMI]) + HGS) | 3.3 years e | No Sarcopenia (ref) vs Sarcopenia: aHR 3.02 (1.30-7.05) c | Sarcopenia Method C (SMI + HGS) was associated with ↑ risk of mortality. |
| Roshanravan, 2013104 | 322 | Gait Speed | 3 years b | >0.8m/s (ref) vs ≤0.8m/s: aHR 2.45 (1.09-5.54)
c
Per 0.1 m/s slower: aHR 1.26 (1.09-1.47) c |
Slower gait speed was associated with ↑ risk of mortality. |
| Clarke, 201921 | 431 | Gait Speed [self-report] | 3.6 yearsb,e | ≥ 3 mph (ref) vs < 3 mph: aHR 2.70 (1.41-5.00)c,h | A faster walking pace was associated with ↑ risk of mortality. |
| Roshanravan, 2013104 | 309 | 6-Minute Walk Test (6MWT) | 3 years b | ≥350m (ref) vs <350m: aHR 2.82 (1.17-6.92)
c
Per 100m decrease: aHR 1.32 (0.96-1.85)c,i |
Shorter walk distance (<350m) was associated with ↑ risk of mortality. |
| Roshanravan, 2013104 | 362 | TUG | 3 years b | Fast (<12s) (ref) vs Slow (≥12s): aHR 1.81 (0.92-3.56)
c
Per 1s slower: aHR 1.08 (1.01-1.14) c |
Slower TUG (per 1s decrement) was associated with ↑ risk of mortality. |
| Roshanravan, 2013104 | 381 | HGS | 3 years b | Stronger (ref) vs Weak Grip: aHR 1.30 (0.71-2.37)
c
Per 1kg decrease: aHR 1.01 (0.98-1.04)c,i |
Lower HGS was not associated with mortality. |
| Watson, 2020 | 89 | Leg Extension Strength | 3.3 years j | Per 1kg decrease: aHR 1.04 (0.96-1.12) h | Muscle strength was not associated with mortality. |
| Navaneethan, 2014 | 2145 | Leisure Time Physical Activity (LTPA) | 4.5 person yearse,j | ≥450 metabolic equivalent (MET)/week (ref) vs <450 MET/week: aHR 1.36 (1.00-1.85)
c
Per log unit MET/week decrease: aHR 1.03 (1.00-1.05) h |
LTPA below the recommended level was associated with ↑ risk of mortality. |
| Androga, 20176 | 1101 | LTPA | 9.4 yearsb,e | <500 MET-min/week (ref) vs 0 MET-min/week: aHR 1.47 (1.11-1.96)
h
500-2000 MET-min/week (ref) vs 0 MET-min/week: aHR 1.43 (1.05-1.96) h >2000 MET-min/week (ref) vs 0 MET-min/week: aHR 1.59 (1.16-2.17) h |
Activity level was associated with ↑ risk of mortality. |
| Rampersad, 2021 | 569 | Physical Activity Scale for the Elderly (PASE) | 1194 days b | Light activity (ref) vs Low activity: aHR 1.11 (0.74-1.69)c,h Moderate to high activity (ref) vs Low activity: aHR 2.08 (1.18-3.70)c,h |
Low physical activity was associated with ↑ risk of mortality. |
| Clarke, 201921 | 437 | Walking | 3.6 yearsb,e | <1 walking hour/week (ref) vs 0 walking hours/week: aHR 2.08 (1.11-3.85)c,h 1-3 walking hours/week (ref) vs 0 walking hours/week: aHR 4.0 (1.75-9.09)c,h ≥3 walking hours/week (ref) vs 0 walking hours/week: aHR 2.08 (1.25-4.35)c,h |
No walking was associated with ↑ risk of mortality. |
| Functional status tools | |||||
| Clarke, 201921 | 450 | Duke Activity Status Index (DASI) | 3.6 yearsb,e | >19.2 summed METs (ref) vs ≤19.2 summed METs: aHR 1.96 (1.14-3.33)c,h Per 1-unit decrease: aHR 1.03 (1.01-1.05)c,h |
↓ physical function was associated with ↑ risk of mortality. |
| Ritchie, 2014 | 1515 | Karnofsky Performance Scale (KPS) | 2.9 years b | KPS = 100 (ref) vs: KPS = 90: aHR 1.20 (0.94-1.52) c KPS ≤ 80: aHR 1.80 (1.35-2.41) c |
Lower KPS is associated with ↑ risk of mortality. |
| Incident dialysis patients | |||||
| Frailty Tools, overall frailty or individual domains | |||||
| McAdams-DeMarco, 2015 | 324 | Fried Frailty Index | 1 year | Not Frail (ref) vs: Intermediately Frail: RR 1.23 (0.53-2.83) g Frail: RR 1.15 (0.48-2.74) g |
Frailty was not associated with mortality. |
| van Loon, 2019128 | 192 | Fried Frailty Index [modified low activity] | 1 year e | Not Frail (ref) vs Frail: aHR 7.22 (2.47-21.13) c | Frailty was associated with ↑ risk of mortality. |
| López-Montes, 2020 | 117 | Fried Frailty Index [modified low activity] | 1 year e | Not Frail (ref) vs Frail: aHR 2.6 (0.9-7.9) c | Frailty was not associated with mortality. |
| Johansen, 200748 | 2275 | Johansen Frailty Criteria [modified Fried and Woods] | 1 year | Not Frail (ref) vs Frail: aHR 2.24 (1.60-3.15) | Frailty was associated with ↑ risk of mortality. |
| Bao, 2012 | 1576 | Frailty, self-report [modified Fried, Woods, Johansen] | 2.9 years b | Not Frail (ref) vs Frail: aHR 1.57 (1.25-1.97) c | Frailty was associated with ↑ risk of mortality. |
| Alfaadhel, 2015 | 372 | CFS | 1.7 years b | Per 1-category increase: aHR 1.21 (1.02-1.43) c | ↑ frailty was associated with ↑ risk of mortality. |
| van Loon, 2019128 | 192 | Clinical Impression [physician] | 1 year e | Not Frail (ref) vs Frail: aHR 4.10 (1.19-14.14) c | Frailty was associated with ↑ risk of mortality. |
| van Loon, 2019128 | 192 | Geriatric Assessment | 1 year e | Not Frail (ref) vs Frail: aHR 2.97 (1.19-7.45) c | Frailty was associated with ↑ risk of mortality. |
| van Loon, 2019128 | 192 | Groningen Frailty Indicator | 1 year e | Not Frail (ref) vs Frail: HR 1.71 (0.76-3.86) k | Frailty was not associated with mortality. |
| van Loon, 2019128 | 192 | Surprise Question | 1 year e | Surprised (ref) vs Not Surprised: HR 0.89 (0.33-2.39) k | Frailty was not associated with mortality. |
| Isoyama, 201440 | 330 | Sarcopenia | 2.4 yearsb,e | Appropriate muscle mass and strength (ref) vs Sarcopenia (low muscle mass and strength): aHR 1.93 (1.01-3.71) c | Sarcopenia was associated with ↑ risk of mortality. |
| Xu, 2020136 | 229 | Sarcopenia (Lean Mass Index [LMI] + HGS) | 3 yearse,j | Normal HGS and LMI (ref) vs Sarcopenia (low HGS and LMI): aHR 2.49 (1.61-3.85) d | Sarcopenia was associated with ↑ risk of mortality. |
| van Loon, 2019128 | 192 | TUG | 1 year e | Not impaired (ref) vs Severely impaired: aHR 1.97 (0.80-4.85) c | Impairment was not associated with mortality. |
| Stenvinkel, 2002 | 169 | HGS | 3.1 yearse,j | Per 1kg decrease: Entire cohort: aHR 1.04 (0.99-1.08)d,h Men: aHR 1.08 (1.03-1.12)d,h Women: aHR 1.03 (0.96-1.11)d,h |
In men, decreasing HGS was associated with ↑ risk of mortality. |
| Hellberg, 201437 | Right: 132 Left: 130 |
HGS | 3.5 years b | Per unit decrease: Right hand: aHR 9.09 (0.99-100) h Left hand: aHR 9.09 (1.35-50.0) h |
Decreasing left HGS was associated with ↑ risk of mortality. |
| Isoyama, 201440 | 330 | HGS | 2.4 yearsb,e | Appropriate muscle strength (ref) vs Low muscle strength: aHR 1.79 (1.09-2.94)
c
Per 1 standard deviation (SD) decrease: aHR 3.13 (1.75-5.56)c,h |
Low muscle strength was associated with ↑ risk of mortality. |
| Xu, 2020136 | 327 | HGS | 3 yearse,j | High (ref) vs Low: aHR 1.96 (1.35-2.84) d | Low HGS was associated with ↑ risk of mortality. |
| Hellberg, 201437 | 100 | Isometric Quadriceps Strength | 3.5 years b | Per unit decrease: Right leg: HR 1.27 (0.17-9.09)c,h Left leg: HR 2.56 (0.28-25.0)c,h |
Decreasing isometric quadriceps strength was not associated with mortality. |
| Hellberg, 201437 | Right: 103 Left: 104 |
Standing Heel Rise | 3.5 years b | Per unit decrease: Right foot: aHR 1.32 (0.26-6.67) h Left foot: aHR 3.13 (0.61-16.7) h |
Decreasing heel raises was not associated with mortality. |
| Hellberg, 201437 | Right: 108 Left: 106 |
Toe Lift | 3.5 years b | Per unit decrease: Right foot: HR 4.55 (0.69-33.3)c,h Left foot: HR 5.26 (0.77-3.33)c,h |
Decreasing toe lifts was not associated with mortality. |
| Johansen, 200748 | 2275 | SF-36 Vitality Scale | 1 year | Score ≥55 (ref) vs <55: aHR 1.30 (0.97-1.76) c | Fatigue was not associated with mortality. |
| Johansen, 200748 | 2275 | Physical Activity | 1 year | Active (ref) vs Inactive: aHR 1.79 (1.42-2.25) c | Inactivity was associated with ↑ risk of mortality. |
| Functional status tools | |||||
| Inaguma, 2016 | 1496 | Barthel Index (BI) | 3.3 years e | High BI (score = 100) (ref) vs: Middle BI (75≤BI<100): aHR 1.61 (1.07-2.41) Low BI (<75): aHR 1.99 (1.46-2.70) |
Lower functional status was associated with ↑ risk of mortality. |
| Shum, 2014 | 157 | Basic Activities of Daily Living | 2.0 years b | Independent (ref) vs Impaired: HR 2.11 (1.28-3.46) c | Impaired activities of daily living was associated with ↑ risk of mortality. |
| Yazawa, 2016 | 7623 | Functional Status—Ability to perform Activities of Daily Living (ADL) | 1 year | Mild disability/none (ref) vs: Moderate: aRR 1.83 (1.54-2.16) c Severe: aRR 2.35 (1.97-2.81) c |
Lower functional status was associated with ↑ risk of mortality. |
| Shah, 2018 | 49645 | Functional Status—Form CMS-2728 | 1.8 yearse,j | Good functional status (ref) vs Poor functional status: aHR 1.28 (1.24-1.33) c | Poor functional status was associated with ↑ risk of mortality. |
| Wetmore, 2019 | 80284 | Functional Status Score | 0.5 years e | Score ≤ 0 (high functional status) (ref): Score 1-2: aOR 1.27 (1.20-1.34) Score 3-4: aOR 1.41 (1.33-1.49) Score 5-6: aOR 1.68 (1.54-1.84) Score ≥ 7 (low functional status): aOR 1.67 (1.45-1.92) |
Lower functional status was associated with ↑ odds of mortality. |
| van Loon, 2019128 | 192 | Katz’ ADL | 1 year e | Not impaired (ref) vs Impaired: aHR 3.20 (1.45-7.06) c | Impairment was associated with ↑ risk of mortality. |
| van Loon, 2019128 | 192 | Lawton and Brody’s Instrumental Activities of Daily Living (IADL) Scale | 1 year e | Not impaired vs Impaired [stratified by age, < or ≥ 80 years]: P value: <.01 c |
Impairment was associated with ↑ risk of mortality. |
| Hatakeyama, 2013 | 141 | Eastern Cooperative Oncology Group Performance Status (ECOG-PS) | 10 years e | ECOG-PS ≤1 (ref) vs >1: aHR 1.27 (1.08-1.49) | Lower functional status was associated with ↑ risk of mortality. |
| McClellan, 1991 | 294 | KPS | 479.6 days j | Per 10-unit decrease: aHR 1.35 (1.1-1.64)c,h | Lower functional status was associated with ↑ risk of mortality. |
| Chandna, 1999 | 292 | KPS | 5.3 years e | Per 10-point decrease: aHR 1.22 (1.10-1.34)c,d,h,i | Lower functional status was associated with ↑ risk of mortality. |
| Utas, 2001 | 334 | KPS | 2.0 yearse,j | aHR Not reported; P value: <.05 l | Lower functional status was associated with ↑ risk of mortality. |
| Joly, 2003 | 101 | KPS | 1 year | Normal Activity (KPS 80-100)/Requires Assistance (50-100) (ref) vs Dependent (10-40): aHR 2.34 (1.00-5.50)c,f | Lower functional status was associated with ↑ risk of mortality. |
| Revuelta, 200475 | 293 | KPS [modified] | 771 days b | Per 10-point decrease: aHR 1.13 (0.86-1.48)c,f | Decreasing functional status was not associated with mortality. |
| Arai, 2014 | 202 | Mobility—Criteria for Impaired Elderly | 0.5 yearse,j | Independent mobility before and after dialysis (ref) vs Independent before dialysis, but decline after dialysis: aHR 3.80 (1.02-14.1)
d
Independent mobility before and after dialysis (ref) vs Impaired mobility before dialysis: aHR 4.94 (1.42-17.1) d Independent mobility before dialysis (ref) vs Impaired mobility: aHR 2.76 (1.13-6.77) d No decline in mobility after starting dialysis (ref) vs Decline: aHR 4.82 (1.72-13.5) d |
Impaired mobility and declines in mobility were associated with ↑ risk of mortality. |
| Knight, 2003 | 14815 | SF-36 Physical Component Summary (PCS) | 1 year | Score ≥50 (ref) vs: ≥40 to <50: aHR 1.17 (0.98-1.41) c ≥30 to <40: aHR 1.32 (1.11-1.57) c ≥20 to <30: aHR 1.62 (1.36-1.92) c <20: aHR 1.97 (1.64-2.36) c Per 10-point decrease: aHR 1.25 (1.18-1.33) |
Impaired functional status was associated with ↑ risk of mortality. |
| Revuelta, 200475 | 293 | SF-36 PCS | 771 days b | Per 10-point decrease: aHR 1.16 (0.78-1.71)c,f | Decreasing functional status was not associated with mortality. |
| Johansen, 200748 | 2275 | SF-36 Physical Function (PF) Scale | 1 year | Score ≥75 (ref) vs <75: aHR 2.07 (1.33-3.24) c | Lower PF is associated with ↑ risk of mortality. |
| Argyropoulos, 2009 | 491 | SF-36 PF Scale | 3.5 years j | Per 10-point decrease: aHR 1.05 (1.01-1.11)c,h,i | Lower functional status was associated with ↑ risk of mortality. |
| Chronic dialysis patients | |||||
| Frailty Tools, overall frailty or individual domains | |||||
| McAdams-DeMarco, 2013 | 146 | Fried Frailty Index | 3.0 years b | Not Frail (ref) vs: Intermediately Frail: aHR 2.65 (1.05-6.67) c Frail: aHR 2.87 (1.17-7.03) c |
Frailty was associated with ↑ risk of mortality. |
| Johansen, 2016 49 | 728 | Fried Frailty Index | 1.7 years b | Not Frail (ref) vs Frail: aHR 1.78 (1.15-2.80) c | Frailty was associated with ↑ risk of mortality. |
| Yadla, 2017 | 205 | Fried Frailty Index | 1 year | Not Frail (ref) vs Frail: HR 0.75 (0.30-1.88) c | Frailty was not associated with mortality. |
| Sy, 2019 | 746 | Fried Frailty Index | 2 years | Not Frail (ref) vs Frail (at baseline): aHR 1.40 (1.07-1.83)
c
Not Frail (ref) vs Frail (at any point during follow-up): aHR 1.53 (1.05-2.23) c |
Frailty at baseline was associated with ↑ risk of mortality. Developing frailty was associated with ↑ risk of mortality. |
| Brar, 201915 | 109 | Fried Frailty Index [modified low activity] | 3.3 years b | Not Frail (ref) vs Frail: aHR 2.03 (0.97-4.24) | Frailty was not associated with mortality. |
| Jafari, 2020 | 97 | Fried Frailty Index [modified low activity] | 1 year | Not Frail/Pre-Frail (ref) vs Frail: RR 2.11 (0.78-5.72) g | Frailty was not associated with mortality. |
| Johansen, 201649 | 728 | Fried Frailty Index [modified slowness, weakness, exhaustion] | 1.7 years b | Not Frail (ref) vs Frail: aHR 1.66 (1.06-2.60) c | Frailty was associated with ↑ risk of mortality. |
| Kang, 201755 | 1250 (HD); 366 (PD) | Johansen Frailty Criteria [modified weight loss] | 489 days
j
(HD) 467 days j (PD) |
HD Not Frail/Pre-Frail (ref) vs Frail: aHR 2.35 (1.36-4.05) PD Not Frail/Pre-Frail (ref) vs Frail: aHR 1.75 (0.68-4.49) |
Frailty in hemodialysis patients was associated with ↑ risk of mortality. |
| Lee, 201770 | 1658 | Johansen Frailty Criteria [modified weight loss] | 1.4 yearsb,e | Not Frail (ref) vs: Pre-Frail: aHR 1.01 (0.48-2.12) Frail: aHR 2.08 (1.04-4.16) |
Frailty was associated with ↑ risk of mortality. |
| Bancu, 2017 | 320 | Fried Frailty Index + Dialysis Time/Week | 1 year | Not Frail (ref) vs Frail: RR 1.77 (0.71-4.42) g | Frailty was not associated with mortality. |
| Brar, 201915 | 109 | Fried Frailty Index [modified low activity] + Clinical Impression [physician] | 3.3 years b | Not Frail (ref) vs Frail: aHR 2.03 (0.97-5.08) | Frailty was not associated with mortality. |
| Kamijo, 201853 | 119 | CFS [adapted] | 589 days j | Not Frail (ref) vs Frail: aHR 9.83 (1.80-53.7) | Frailty was associated with ↑ risk of mortality. |
| Brar, 201915 | 109 | Clinical Impression [nurse] | 3.3 years b | Not Frail (ref) vs Frail: aHR 1.92 (0.88-4.18) | Frailty was not associated with mortality |
| Brar, 201915 | 109 | Clinical Impression [physician] | 3.3 years b | Not Frail (ref) vs Frail: aHR 2.32 (1.10-4.89) | Frailty was associated with ↑ risk of mortality. |
| Shimoda, 2018 | 314 | Combined Score | 6.5 years | Low score (<5) (ref) vs High score (≥5): aHR 3.63 (1.73-7.59)
c
Per 1-point increase: aHR 1.28 (1.14-1.43) c |
Higher Combined Score was associated with ↑ risk of mortality. |
| Jiang, 2020 | 1424026 | Frailty (Johns Hopkins Adjusted Clinical Groups) | Not reported | Not Frail (ref) vs Frail: aOR 2.46 (2.41-2.51) | Frailty was associated with ↑ odds of death while hospitalized for any reason. |
| Ng, 2016 | 193 | Frailty Score | 1.9 yearse,j | aHR: 1.21 (0.94-1.54)d,l | Frailty was not associated with mortality. |
| Chan, 2020 | 267 | Frailty Score | 2 years | Not Frail (ref) vs Frail: aHR 1.79 (1.09-2.94) d | Frailty was associated with ↑ risk of mortality. |
| Jegatheswaran, 2020 | 261 | FRAIL Questionnaire | 1.5 years e | Not Frail (ref) vs: Pre-Frail: RR 1.30 (0.68-2.48) g Frail: RR 1.26 (0.53-2.99) g |
Frailty was not associated with mortality. |
| Chao, 202020 | 33 | Laboratory Deficit-Based Frailty Index-1 | 2.7 yearse,j | Not Frail vs Frail: P value: .01 c |
Frailty was associated with mortality. |
| Chao, 202020 | 33 | Laboratory Deficit-Based Frailty Index-2 | 2.7 yearse,j | Not Frail vs Frail: P value: .07 c |
Frailty was not associated with mortality. |
| Brar, 201915 | 109 | Short Physical Performance Battery | 3.3 years b | Not Frail (ref) vs Frail: aHR 1.54 (0.63-3.77) | Frailty was not associated with mortality. |
| Kang, 201356 | 534 | ASMI | 3.7 yearse,j | Middle/High ASMI (ref) vs Low ASMI: Male: aHR 1.21 (0.74-1.98) d Female: aHR 1.52 (0.88-2.64) d |
Low ASMI was not associated with mortality. |
| Rymarz, 2018 | 48 | Lean Tissue Index | 2.5 yearse,j | No Sarcopenia vs Sarcopenia: P value: .055 c |
Sarcopenia was not associated with mortality. |
| Kang, 201356 | 534 | Limb/Trunk Lean Mass Ratio (LTLM) | 3.7 yearse,j | Middle/High LTLM (ref) vs Low LTLM: Male: aHR 1.88 (1.24-2.84)c,d Female: aHR 2.20 (1.36-3.54)c,d |
Low LTLM was associated with ↑ risk of mortality. |
| Noori, 2010 | 792 | MAMC | 730 days b | Highest quartile (Q4) (ref) vs Lowest quartile (Q1): aHR 1.59 (0.94-2.63)c,h Q3 (ref) vs Q1: aHR 1.45 (0.93-2.22)c,h Q2 (ref) vs Q1: aHR 1.16 (0.78-1.72)c,h |
Lower MAMC was not associated with mortality. |
| Jin, 2017 | 117 | Relative Appendicular Skeletal Muscle (RASM) | 5.0 yearse,j | No Sarcopenia (at 1 year) vs Sarcopenia (at 1 year): aHR 2.31 (1.11-4.81) d | Low RASM was associated with ↑ risk of mortality. |
| Lin, 2020 | 271 | SARC-F | 2 years | SARC-F <1 (ref) vs SARC-F ≥1: aHR 2.87 (1.11-7.38)
c
Per 1-point increase: aHR 1.12 (0.98-1.29) c |
High SARC-F score was associated with ↑ risk of mortality. |
| Mori, 2019 | 308 | Sarcopenia | 6.3 yearse,j | No Sarcopenia (ref) vs Sarcopenia: aHR 1.31 (0.81-2.10) | Sarcopenia was not associated with mortality. |
| Giglio, 201832 | 170 | Sarcopenia [modified] | 1.4 yearsb,e | No Sarcopenia (ref) vs Sarcopenia: aHR 2.09 (1.05-4.20) | Sarcopenia was associated with ↑ risk of mortality. |
| Yamamoto, 2021138 | 542 | Sarcopenia (Creatinine Index [CrI] + Gait Speed) | 3.0 years b | No Sarcopenia (ref) vs Sarcopenia: aHR 4.20 (2.38-7.41) | Sarcopenia was associated with ↑ risk of mortality. |
| Yamamoto, 2021138 | 542 | Sarcopenia (CrI + HGS) | 3.0 years b | No Sarcopenia (ref) vs Sarcopenia: aHR 3.79 (2.09-6.87) | Sarcopenia was associated with ↑ risk of mortality. |
| Souweine, 2020114 | 187 | Sarcopenia (CrI + Maximal Voluntary Force) | 2.0 yearse,j | No Sarcopenia (ref) vs Sarcopenia: aHR 1.60 (0.76-3.35) d | Sarcopenia was not associated with risk of mortality. |
| Kittiskulnam, 201758 | 643 | Sarcopenia (Muscle Mass/Height² + Weakness) | 1.9 years j | No Sarcopenia (ref) vs Sarcopenia: aHR 2.23 (0.99-5.00) c | Sarcopenia was not associated with mortality. |
| Kittiskulnam, 201758 | 643 | Sarcopenia (Muscle Mass/Body Weight (BW) + Weakness) | 1.9 years j | No Sarcopenia (ref) vs Sarcopenia: aHR 1.24 (0.63-2.43) c | Sarcopenia was not associated with mortality. |
| Kittiskulnam, 201758 | 643 | Sarcopenia (Muscle Mass/Body Surface Area (BSA) + Weakness) | 1.9 years j | No Sarcopenia (ref) vs Sarcopenia: aHR 1.53 (0.84-2.78) c | Sarcopenia was not associated with mortality. |
| Kittiskulnam, 201758 | 643 | Sarcopenia (Muscle Mass/body mass index (BMI) + Weakness) | 1.9 years j | No Sarcopenia (ref) vs Sarcopenia: aHR 1.65 (0.88-3.08) c | Sarcopenia was not associated with mortality. |
| Kittiskulnam, 201758 | 644 | Sarcopenia (Muscle Mass/Height² + Slowness) | 1.9 years j | No Sarcopenia (ref) vs Sarcopenia: aHR 2.92 (1.33-6.41) c | Sarcopenia was associated with ↑ risk of mortality. |
| Kittiskulnam, 201758 | 644 | Sarcopenia (Muscle Mass/BW + Slowness) | 1.9 years j | No Sarcopenia (ref) vs Sarcopenia: aHR 1.56 (0.85-2.83) c | Sarcopenia was not associated with mortality. |
| Kittiskulnam, 201758 | 644 | Sarcopenia (Muscle Mass/BSA + Slowness) | 1.9 years j | No Sarcopenia (ref) vs Sarcopenia: aHR 1.46 (0.83-2.58) c | Sarcopenia was not associated with mortality. |
| Kittiskulnam, 201758 | 644 | Sarcopenia (Muscle Mass/BMI + Slowness) | 1.9 years j | No Sarcopenia (ref) vs Sarcopenia: aHR 2.51 (1.41-4.66) c | Sarcopenia was associated with ↑ risk of mortality. |
| Kamijo, 201853 | 119 | Sarcopenia (RASM + HGS/Gait Speed) | 500 days | No Sarcopenia vs Sarcopenia: P value: <.001 c |
Sarcopenia was associated with mortality. |
| Lin, 202073 | 126 | Sarcopenia (SMI + HGS/Gait Speed) | 3 years | No Sarcopenia vs Sarcopenia: P value: .037 c |
Sarcopenia was associated with mortality. |
| Ren, 2016 | 131 | Sarcopenia Method C (SMI + HGS) | 1 year | No Sarcopenia (ref) vs Sarcopenia: RR 12.5 (1.20-131.4) g | Sarcopenia was associated with ↑ risk of mortality. |
| Kim, 201757 | 142 | Sarcopenia Status | 4.3 years j | No Sarcopenia (ref) vs Sarcopenia: aHR 6.99 (1.84-26.5) | Sarcopenia was associated with ↑ risk of mortality. |
| Song, 2020 | 88 | Sarcopenia Status | 5.2 years j | No Sarcopenia (ref) vs Sarcopenia: aHR 2.72 (1.11-6.63) | Sarcopenia was associated with ↑ risk of mortality. |
| Brar, 201915 | 109 | Weight Loss | 3.3 years b | No weight loss (ref) vs Weight loss: aHR 1.34 (0.57-3.14) | Weight loss was not associated with mortality. |
| Kutner, 2015 | 742 | Gait Speed | 703 days b | ≥0.6m/s (ref) vs: <0.6 m/s: aHR 2.17 (1.19-3.98) c Unable to perform walk: aHR 6.93 (4.01-11.9) c Per 0.1 m/s decrease: aHR 1.17 (1.05-1.31) |
Slower walk speed and being unable to walk was associated with ↑ risk of mortality. |
| Kittiskulnam, 201758 | 645 | Gait Speed | 1.9 years j | Normal (ref) vs Slow: aHR 2.25 (1.36-3.74)
c
Per 1 SD decrease in Gait Speed: aHR 1.35 (0.97-1.85)c,h |
Slow walking speed was associated with ↑ risk of mortality. |
| Kamijo, 201853 | 119 | Gait Speed | 589 days j | Normal (ref) vs Slow: aHR 19.3 (0.82-454.1) | Gait speed was not associated with mortality. |
| Brar, 201915 | 109 | Gait Speed | 3.3 years b | Normal (ref) vs Slow: aHR 1.28 (0.60-2.73) | Slowness was not associated with mortality. |
| Lin, 202073 | 126 | Gait Speed | 3 years | Normal vs Slow: P value: .020 c |
Slow gait speed was associated with mortality. |
| Yamamoto, 2021138 | 542 | Gait Speed | 3.0 years b | Per 1 SD (0.3 m/s) decrease: aHR 1.67 (1.56-1.79) h | Decreasing gait speed was associated with ↑ risk of mortality. |
| Kohl, 2012 | 52 | 6MWT | 12 years | Per 100m decrease: aHR 1.89 (1.35-2.7)d,h | Shorter walk distance was associated with ↑ risk of mortality. |
| Torino, 2014 | 296 | 6MWT | 3.3 years b | Per 100m decrease: aHR 1.76 (1.34-2.39)c,h,i | Shorter walk distance was associated with ↑ risk of mortality. |
| Shi, 2017 | 145 | 6MWT | 1.9 yearsb,e | Long (ref) vs Short 6MWT: RR 2.89 (1.1-7.64) g | Shorter walk distance was associated with ↑ risk of mortality. |
| Valenzuela, 2019125 | 30 | 6MWT | 1.5 years e | Long (ref) vs Short: RR 5.0 (1.31-19.07) c | Shorter walk distance was associated with ↑ risk of mortality. |
| Wang, 2005 | 180 | HGS | 2.5 yearse,j | Per 1kg decrease: aHR 1.05 (1.01-1.09)c,h | Decreasing HGS was associated with ↑ risk of mortality. |
| Matos, 2014 | 443 | HGS | 2.8 yearsb,e | High (ref) vs Low: Entire cohort: aHR 2.81 (1.62-4.88) c Men: aHR 3.57 (1.79-7.10) c Women: aHR 2.48 (0.87-7.03) c |
Low HGS in the entire cohort and in males only was associated with ↑ risk of mortality. |
| Vogt, 2016 | 265 | HGS | 1.1 yearse,j | High (ref) vs Low: aHR 2.04 (1.12-3.7)d,h | Low HGS was associated with ↑ risk of mortality. |
| Kim, 201757 | 142 | HGS | 4.3 years j | Appropriate Strength (ref) vs Low Strength: aHR 5.65 (1.99-16.0) | Low HGS was associated with ↑ risk of mortality. |
| Kittiskulnam, 201758 | 645 | HGS | 1.9 years j | Normal (ref) vs Weak: aHR 1.68 (1.01-2.79)
c
Per 1 SD decrease in HGS: aHR 1.49 (1.06-2.13)c,h |
Weak HGS was associated with ↑ risk of mortality. |
| Giglio, 201832 | 170 | HGS | 1.4 yearsb,e | Appropriate Strength (ref) vs Low Strength: aHR 1.84 (0.92-3.68) | Low HGS was not associated with mortality. |
| Kamijo, 201853 | 119 | HGS | 589 days j | Normal (ref) vs Low: aHR 0.95 (0.77-1.17) | HGS was not associated with mortality. |
| Brar, 201915 | 109 | HGS | 3.3 years b | Normal (ref) vs Weak: aHR 2.82 (1.36-5.83) | Weak HGS was associated with ↑ risk of mortality. |
| Valenzuela, 2019125 | 30 | HGS | 1.5 years e | High (ref) vs Low: RR 3.0 (1.01-8.95) c | Low HGS was associated with ↑ risk of mortality. |
| Lin, 202073 | 126 | HGS | 3 years | Normal vs Low: P value: .014 c |
Low HGS was associated with mortality. |
| Yamamoto, 2021138 | 542 | HGS | 3.0 years b | Per 1 SD (8.7 kg) decrease: aHR 1.96 (1.85-2.08) h | Decreasing HGS was associated with ↑ risk of mortality. |
| Zhang, 2020 | 174 | Biceps Muscle Strength | 1 year e | High (ref) vs Low: aHR 7.14 (1.28-50.0)c,h Per 1kg decrease: aHR 1.32 (1.10-1.59)c,h |
Low biceps muscle strength was associated with ↑ risk of mortality. |
| Souweine, 2020114 | 187 | Dynapenia | 2.0 yearse,j | No Dynapenia (ref) vs Dynapenia: aHR 2.99 (1.18-7.61) d | Low muscle strength was associated with ↑ risk of mortality. |
| Matsuzawa, 2014 | 190 | Lower extremity muscle strength | 3.0 yearsb,e | ≥40% (ref) vs <40%: aHR 2.73 (1.14-6.52) | Low lower extremity strength was associated with ↑ risk of mortality. |
| Valenzuela, 2019125 | 30 | 30-Second Chair Stand | 1.5 years e | More repetitions (ref) vs Less repetitions: RR 3.0 (1.01-8.95) c | Fewer sit-to-stand repetitions were associated with ↑ risk of mortality. |
| Brar, 201915 | 109 | Center for Epidemiologic Studies Depression Scale—Exhaustion | 3.3 years b | No exhaustion (ref) vs Exhaustion: aHR 1.16 (0.60-2.22) | Exhaustion was not associated with mortality. |
| Koyama, 2010 | 788 | Fukuda Fatigue Scale | 2.2 yearsb,e | Normal (ref) vs Highly fatigued: HR Not reported; P value >.05 c | Fatigue was not associated with mortality. |
| Ducharlet, 201928 | 102 | Palliative Care Outcome Scale Symptoms (POS-S) Renal—Weakness | 254 days j | No weakness/low energy (ref) vs Weakness/low energy: HR 2.0 (0.4-7.8) c | Weakness or low energy was not associated with mortality. |
| Mapes, 200379 | 10030 | SF-36 Vitality Scale | Not reported | Per 10-point decrease: aHR 1.09 (1.07-1.12) | ↑ fatigue was associated with ↑ risk of mortality. |
| Takaki, 2005117 | 490 | SF-36 Vitality Scale | 986 days j | Per 1 SD decrease: aHR Not reported; P value >.05 | ↑ fatigue was not associated with mortality |
| Jhamb, 2009 | 705 | SF-36 Vitality Scale | 1065 days b | Score >55 (ref) vs Score ≤55: aHR 1.33 (1.04-1.72)c,h | Fatigue was associated with ↑ risk of mortality. |
| Jhamb, 2011 | 1798 | SF-36 Vitality Scale | 2.8 years j | High vitality (Q4) (ref) vs: Q3: aHR 1.07 (0.84-1.35) c Q2: aHR 1.19 (0.98-1.45) c Low vitality (Q1): aHR 1.37 (1.12-1.67) c |
↑ fatigue was associated with ↑ risk of mortality. |
| Bossola, 2015 | 115 | SF-36 Vitality Scale | 3.6 yearse,j | Low fatigue (score ≥65) (ref) vs: ≥50 to <65: aHR 3.23 (1.23-8.46) c ≥35 to <50: aHR 5.11 (2.01-13.0) c High fatigue (score <35): aHR 5.29 (2.2-12.7) c |
↑ fatigue was associated with ↑ risk of mortality. |
| van Loon, 2017127 | 714 | SF-36 Vitality Scale | 2 years | Score >66 (ref) vs Score ≤66: aHR 1.37 (0.91-2.06)
c
Per 10-point decrease: aHR 1.12 (1.03-1.21) i |
↑ fatigue was associated with ↑ risk of mortality. |
| Kalantar, 201952 | 753 | SF-36 Vitality Scale | 5 years | High vitality (Q4) (ref) vs: Q3: aHR 1.03 (0.66-1.63) c Q2: aHR 1.00 (0.63-1.59) c Low vitality (Q1): aHR 1.88 (1.29-2.74) c Per 10-point decrease: aHR 1.11 (1.05-1.19)c,h |
↑ fatigue was associated with ↑ risk of mortality. |
| Torino, 2019121 | 245 | SF-36 Vitality Scale | 2.2 years b | Per unit decrease: aHR 1.09 (1.00-1.19)h,l | Fatigue was associated with ↑ risk of mortality. |
| Kurita, 2019 | 3667 | SF-12 Vitality Scale | 2.7 years b | Energy a little of the time (ref) vs None of the time: aHR 1.00 (0.75-1.33)c,h Energy some of the time (ref) vs None of the time: aHR 1.33 (1.04-1.69)c,h Energy most of the time (ref) vs None of the time: aHR 1.52 (1.08-2.13)c,h Energy all of the time (ref) vs None of the time: aHR 1.69 (0.84-3.45)c,h Per 1-level lower energy level: aHR 1.16 (1.04-1.28)c,h |
Lower energy was associated with ↑ risk of mortality. |
| Kutner, 199765 | 348 | Exercise Activity Score | 7 years | Per 3-unit shift toward less exercise: aOR 1.58 (CI, not reported); P value: .047 d | Decreasing exercise activity was associated with ↑ odds of mortality. |
| Tentori, 2010 | 20912 | Exercise Frequency | 1.7 years b | Regular (≥1/week) (ref) vs Non-regular (<1/week): aHR 1.37 (1.28-1.45)c,h Per decrease in each exercise frequency category: aHR 1.11 (1.09-1.14)c,h Exercise frequency: 1/week (ref) vs Never or <1/week: aHR 1.22 (1.1-1.37)c,h 2-3/week (ref) vs Never or <1/week: aHR 1.39 (1.27-1.52)c,h 4-5/week (ref) vs Never or <1/week: aHR 1.37 (1.16-1.61)c,h 6-7/week (ref) vs Never or <1/week: aHR 1.45 (1.32-1.59)c,h |
Low levels of physical activity were associated with ↑ risk of mortality. |
| Brar, 201915 | 109 | PASE | 3.3 years b | Normal physical activity (ref) vs Low physical activity: aHR 1.81 (0.88-3.71) | Low physical activity was not associated with mortality. |
| Kang, 201754 | 1611 | Physical Activity—World Health Organization Recommendations | 500 days | Active (ref) vs: Intermediate: RR 1.09 (0.59-2.01) g Inactive: RR 1.46 (0.84-2.54) g |
Low levels of physical activity were not associated with mortality. |
| Lopes, 2014 | 5763 | Rapid Assessment of Physical Activity | 1.6 years b | Infrequently active (ref) vs Never/rarely active: aHR 1.12 (0.91-1.39)f,h Sometimes active (ref) vs Never/rarely active: aHR 1.19 (0.95-1.49)f,h Often active (ref) vs Never/rarely active (ref): aHR 1.23 (1.04-1.47)f,h Very active (ref) vs Never/rarely active (ref): aHR 1.67 (1.3-2.13)f,h |
Low levels of physical activity were associated with ↑ risk of mortality. |
| Souweine, 2020114 | 187 | Voorrips Score | 2 yearse,j | Per unit decrease: aHR 3.57 (1.39-9.09)d,h | Decreased physical activity was associated with ↑ risk of mortality. |
| Functional status tools | |||||
| Anderson, 1990 | 44 | Activity of Daily Living Score | 0.41 patient yearse,j | Score ≥9.6 (ref) vs Score <9.6: aHR 2.6 (1.7-4.0) d | Lower ADL score was associated with ↑ risk of mortality. |
| Anderson, 1993 | 221 | Activity of Daily Living Score | 2.2 years e | Score >8 (ref) vs Score ≤8: aHR 2.0 (1.6-2.6) | Low functional status was associated with ↑ risk of mortality. |
| Anderson, 1997 | 109 | Activity of Daily Living Score | 1.1 yeare,j | Per 1-point lower: aHR 1.1 (1.04-1.15)d,h | Lower functional status was associated with ↑ risk of mortality. |
| Watanabe, 2021 | 300 | ADL Difficulty | 4.8 years b | Higher ADL (ref) vs Lower ADL: aHR 2.70 (1.57-4.64)
c
Per 1-point decrease in ADL: aHR 1.05 (1.02-1.08)c,h |
Lower ADL was associated with ↑ risk of mortality. |
| Kang, 201755 | 1250 (HD); 366 (PD) | Disability | 489 days
j
(HD) 467 days j (PD) |
HD No Disability (ref) vs Disability: aHR 2.13 (1.20-3.78) PD No Disability (ref) vs Disability: aHR 0.97 (0.40-2.36) |
Disability in HD patients was associated with ↑ risk of mortality. |
| Lee, 201770 | 1658 | Disability | 1.4 yearsb,e | No Disability (ref) vs Disability: HR 2.47 (1.59-3.82) c | Disability associated with ↑ risk of mortality. |
| Kutner, 1994 | 287 | Functional Limitations Score | 2.8 years e | Severe impairment vs Moderate to No impairment in functional status x time: aHR Not reported; P value: .01 | Severely low functional status was associated with ↑ risk of mortality. |
| Kutner, 199765 | 348 | Functional Limitations Score | 7 years | Functional status moderately or severely impaired vs no impairment: aOR Not reported; P value not reported d | Greater functional impairment at baseline was associated with ↑ odds of mortality. This effect varied based on patient age. An interaction between baseline functional impairment and age was reported. |
| Sood, 2011 | 1286 | Katz’ ADL | 7.5 days b | Per 1-point change toward more impaired: aOR 1.16 (1.11-1.22) | Increased impairment in functional status was associated with ↑ odds of in-hospital mortality. |
| Shavit, 2014 | 56 | Katz’ ADL | 2 years | Unimpaired (ref) vs Impaired: aOR Not reported; P value: .002 f | Functional impairment was associated with ↑ odds mortality. |
| Bossola, 201614 | 132 | Katz’ ADL | 7.5 years e | No functional impairment (ref) vs Impaired: aHR 2.47 (1.07-5.67) c | Functional impairment was associated with ↑ risk of mortality. |
| Farrokhi, 2013 | 167 | 4-Item Essential ADL Score | 5 years | Score 0 (no disability) (ref) vs: Score 1: aHR 2.18 (0.50-9.46) d Score 2: aHR 1.61 (0.35-7.26) d Score 3: aHR 2.50 (0.56-11.2) d Score 4 (severe disability): aHR 12.5 (2.44-65.0) d |
Severely low functional status was associated with ↑ risk of mortality. |
| Bossola, 201614 | 132 | Lawton and Brody’s Instrumental Activities of Daily Living (IADL) Scale | 7.5 years e | No functional impairment (ref) vs Impaired: aHR 0.80 (0.36-1.76) c | Functional impairment was not associated with mortality. |
| Jassal, 2016 | 7226 | Functional Status Score (ADL & IADL) | 1.4 yearsb,e | Functionally independent (score = 13) (ref) vs: Score 11 to <13: aHR 1.24 (1.03-1.48) c Score 8 to <11: aHR 1.65 (1.38-1.99) c Score <8: aHR 2.37 (1.92-2.94) c |
Lower functional status was associated with ↑ risk of mortality. |
| Tennankore, 2019 | 2593 | Functional Status Score (ADL & IADL) | 1.2 yearsb,e | Independent (score = 13) (ref) vs: Score 11 to <13: aHR 1.57 (1.13-2.20) Score 8 to <11: aHR 3.23 (2.27-4.60) Score <8: aHR 4.01 (2.44-6.61) |
Increased functional impairment was associated with ↑ risk of mortality. |
| Matsuzawa, 2019 | 817 | Functional Status Score (ADL & IADL) | 704 days b | No decline (ref) vs Decline: aHR 2.68 (1.31-5.50) No decline (ref) vs Decline in at least 1/13 functional status tasks: aHR 2.81 (1.25-6.33) |
A decline in Functional Status Score was associated with ↑ risk of mortality. A decline in at least 1 Functional Status Score task was associated with ↑ risk of mortality. |
| McClellan, 1992 | 2701 | KPS | 1 year | Score ≥ 70 (ref) vs Score <70: aHR 1.68 (1.32-2.13) | Lower functional status was associated with ↑ risk of mortality. |
| Ifudu, 1998 | 319 | KPS [modified] | 3 years | Score ≥70 (ref) vs Score <70: aHR Not reported; P value: .14 c | Decreasing functional status was not associated with mortality. |
| Freedman, 2001 | 3442 | KPS [modified] | 5 years | Highest functional status category (ref) vs: Second: aHR 0.9 (0.7-1.1) c Third: aHR 1.1 (0.9-1.4) c Lowest: aHR 1.6 (1.2-2.0) c |
Lower functional status was associated with ↑ risk of mortality. |
| Ducharlet, 201928 | 102 | POS-S Renal-Mobility | 254 days j | Normal mobility (ref) vs Low mobility: HR 4.6 (1.2-17.2) c | Low mobility was associated with ↑ risk of mortality. |
| Roberts, 1976 | 641 | State of Health | 5 years | Health Status 1 (ref) vs: Health Status 2: RR 1.21 (1.00-1.46) g Health Status 3: RR 1.57 (1.27-1.94) g Health Status 4: RR 1.58 (0.96-2.43) g Health Status 5: RR not compared due to small n |
Lower functional status was associated with ↑ risk of death. |
| DeOreo, 1997 | 1000 | SF-36 PCS | 531 days j | Per 10-unit decrease: aHR 1.25 (1.02-1.49)h,i | Decreasing PCS was associated with ↑ risk of mortality. |
| Lowrie, 2003 | 13952 | SF-36 PCS | 0.5 years e | Per 10-unit decrease: aOR 1.22 (1.20-1.25)h,i | Lower functional status was associated with ↑ odds of mortality. |
| Mapes, 200379 | 10030 | SF-36 PCS | Not reported | Score >46 (ref) vs: Score 39-46: aHR 1.03 (0.85-1.25) c Score 33-38: aHR 1.34 (1.10-1.63) c Score 26-32: aHR 1.50 (1.24-1.80) c Score <25: aHR 1.81 (1.49-2.20) c Per 10-point decrease: aHR 1.25 (1.20-1.30) c |
Decreasing PCS was associated with ↑ risk of mortality. |
| Takaki, 2005117 | 490 | SF-36 PCS | 986 days j | Per 1 SD decrease: aHR Not reported; P value >.05 | Decreased PCS was not associated with ↑ risk of mortality. |
| Lacson, 201068 | 44395 | SF-36 PCS | 1 year | Per 10-point decrease: aHR 1.28 (1.25-1.31)h,i | Lower PCS was associated with ↑ risk of mortality. |
| Peng, 2010 | 888 | SF-36 PCS | 7 years | Highest scores (Q4) (ref) vs: Q3: aHR 1.07 (0.70-1.65) Q2: aHR 1.69 (1.13-2.53) Lowest scores (Q1): aHR 1.85 (1.24-2.76) Per 10-point decrease: aHR 1.34 (1.10-1.63)h,i |
Decreased PCS was associated with ↑ risk of mortality. |
| Peng, 2013 | 816 | SF-36 PCS | 7 years | Per 10-point decrease: aHR 1.22 (1.10-1.48)h,i | Decreased PCS was associated with ↑ risk of mortality. |
| Turkmen, 2014 | 63 | SF-36 PCS | 7 years | aHR Not reported; P value >.05d,l | PCS was not associated with mortality. |
| Kang, 201755 | 1250 (HD); 366 (PD) | SF-36 PCS | 489 days
j
(HD) 467 days j (PD) |
HD High PCS tertile (ref) vs Middle/Low PCS tertile: aHR 1.01 (1.00-1.02) h PD High PCS tertile (ref) vs Middle/Low PCS tertile: aHR 1.03 (1.01-1.05) h |
Decreased PCS was associated with ↑ risk of mortality. |
| Kalantar, 201952 | 753 | SF-36 PCS | 5 years | Q4 (high score) (ref) vs: Q3: aHR 0.98 (0.61-1.59) c Q2: aHR 1.54 (0.99-2.39) c Q1 (low score): aHR 2.30 (1.53-3.47) c Per 10-point decrease: aHR 1.47 (1.27-1.72)c,h |
The lowest quartile of PCS was associated with ↑ risk of mortality. |
| Brito, 202016 | 670 | SF-36 PCS | 9 years | Per 1-point increase: aHR Not reported; P value >.05 | Physical function was not associated with risk of mortality. |
| Lacson, 201068 | 44395 | SF-12 PCS | 1 year | Per 10-point decrease: aHR 1.28 (1.24-1.31)h,i | Decreasing physical function was associated with ↑ risk of mortality. |
| Hall, 2019 | 1368 | SF-12 PCS | 151 days b | Per 10-point change m : aHR 0.82 (0.66-1.1)c,i | A change m in physical function was not associated with mortality. |
| Mapes, 200379 | 10030 | SF-36 PF Scale | Not reported | Per 10-point decrease: aHR 1.10 (1.08-1.11) | Decreasing physical function was associated with ↑ risk of mortality. |
| Takaki, 2005117 | 490 | SF-36 PF Scale | 986 days j | Per 1 SD decrease: aHR Not reported; P value >.05 | Decreasing physical function was not associated with mortality. |
| Santos, 2012 | 161 | SF-36 PF Scale | 1 year e | Per 10-unit decrease: HR 1.22 (1.04-1.44)c,h,i | Decreasing physical function was associated with ↑ risk of mortality. |
| de Oliveira, 2016 | 76 | SF-36 PF Scale | 2 years | Per 10-point decrease: aHR 1.20 (1.04-1.38)d,h,i | Decreased physical function was associated with ↑ risk of mortality. |
| van Loon, 2017126 | 679 | SF-36 PF Scale | 2 years | Good physical function vs: Intermediate: RR 1.41 (0.87-2.26) g Poor: RR 3.49 (2.31-5.27) g |
Decreased physical function was associated with ↑ risk of mortality. |
| van Loon, 2017127 | 714 | SF-36 PF Scale | 2 years | Score >66 (ref) vs Score ≤66: aHR 1.72 (1.02-2.73)
c
Per 10-point decrease: aHR 1.14 (1.06-1.21) i |
Decreased physical function was associated with ↑ risk of mortality. |
| Kalantar, 201952 | 753 | SF-36 PF Scale | 5 years | Q4 (high score) (ref) vs: Q3: aHR 0.98 (0.61-1.57) c Q2: aHR 1.04 (0.66-1.66) c Q1 (low score): aHR 1.87 (1.21-2.87) c Per 10-point decrease: aHR 1.11 (1.05-1.18)c,h |
Decreased physical function was associated with ↑ risk of mortality. |
| Torino, 2019121 | 245 | SF-36 PF Scale | 2.2 years b | Per unit decrease: aHR 1.14 (1.05-1.23)h,l | Decreasing physical function was associated with ↑ risk of mortality. |
| Brito, 202016 | 670 | SF-36 PF Scale | 9 years | Per 10-point decrease: aHR 1.1 (1.0-1.1)h,i | Physical function was associated with ↑ risk of mortality. |
| Fukuma, 2017 | 1376 | SF-12 PF Scale | 1 year | Score 100 (highest function) (ref) vs: Score 75: aOR 0.57 (0.23-1.42) Score 50: aOR 0.66 (0.31-1.40) Score 25: aOR 1.04 (0.47-2.29) Score 0 (lowest function): aOR 2.48 (1.26-4.91) |
Decreased physical function was associated with ↑ odds of mortality. |
| Other | |||||
| Frailty Tools, overall frailty or individual domains | |||||
| Nixon, 2020 | 450 | CFS [adapted] | 210 days b | Per 1-point increase: aHR 2.15 (1.63-2.85) | Each point increase in CFS score was associated with ↑ risk of mortality. |
| Dai, 2017 | 985 | HGS | 5 years e | % HGS > 74.07 (ref) vs % HGS < 74.07: aRR 1.19 (1.13-1.25) c | Lower HGS was associated with ↑ risk of mortality. |
| Beddhu, 2009 | Not reported | LTPA | 7 years j | Active (ref) vs Inactive: aHR 2.27 (1.72-3.03)c,h Insufficient (ref) vs Inactive: aHR 1.72 (1.27-2.38)c,h |
Activity level was associated with ↑ risk of mortality. |
Note. References are available in supplementary material; McClellan, 1991, KPS reported as 0-10, converted to 0-100. ADL = Activities of Daily Living; aHR = adjusted hazard ratio; aOR = adjusted odds ratio; aRR = adjusted relative risk; ASMI = Appendicular Skeletal Mass Index; BI = Barthel Index; BMI = body mass index; BSA = body surface area; BW = body weight; CFS = Clinical Frailty Scale; CI = 95% confidence interval; CKD = chronic kidney disease; CrI = Creatinine Index; DASI = Duke Activity Status Index; ECOG-PS = Eastern Cooperative Oncology Group Performance Status; HD = hemodialysis; HGS = handgrip strength; HR = unadjusted hazard ratio; IADL = Instrumental Activities of Daily Living; KPS = Karnofsky Performance Scale; LMI = Lean Mass Index; LTLM = Limb/Trunk Lean Mass Ratio; LTPA = Leisure Time Physical Activity; MAMC = midarm muscle circumference; MET = metabolic equivalent; OR = unadjusted odds ratio; PASE = Physical Activity Scale for the Elderly; PCS = Physical Component Summary; PD = peritoneal dialysis; PF = Physical Function; POS-S = Palliative Care Outcome Scale–Symptoms; PRISMA = Preferred Reporting Items for Systematic Review and Meta-analysis; RASM = Relative Appendicular Skeletal Muscle; Ref = reference value; SGA = Subjective Global Assessment; SMI = Skeletal Muscle Mass Index; TUG = Timed Up-and-Go Test; RR = unadjusted relative risk; 6MWT = 6-Minute Walk Test.
All models adjusted for a minimum of age and sex, unless otherwise noted. Where a choice of models exists, the most fully adjusted model is presented.
Median.
Multiple adjusted models available.
Model not adjusted for sex.
Converted to years.
Model not adjusted for age or sex.
RR calculated from event data, or cumulative survival event data.
Scale inverted.
Scale change.
Mean.
Unadjusted model.
Reference group and comparator not reported, unit of measure not clearly reported.
Change defined as a clinically relevant decline or improvement.
Figure 2.
(A) Forest plot of the association between frailty as a categorical variable and mortality.a (B) Forest plot of the association between frailty as a continuous variable and mortality.a
Twenty-five unique instruments were used to evaluate sarcopenia among 35 assessments. The point estimate for most of the categorical assessments (n = 32 of 34) were above 1.0 suggesting a positive association between the presence of sarcopenia and the risk of death (Figure 3). Effects were similar among both dialysis subgroups; however, a weaker association was noted among non-dialysis CKD patients. One study examined sarcopenia as a continuous measure and did not find a significant association (Figure S1).
Figure 3.
Forest plot of the association between sarcopenia as a categorical variable and mortality.a
Note. ASMI = Appendicular Skeletal Mass Index; HR = hazard ratio; HGS = handgrip strength; MAMC = midarm muscle circumference; SGA = Subjective Global Assessment; RASM = Relative Appendicular Skeletal Muscle; BW = body weight; BSA = body surface area; BMI = body mass index; RR* = relative risk calculated from event data; ¥ = comparison was inverted; Unadj = unadjusted model.
aStudies that did not provide measure of association are not displayed.
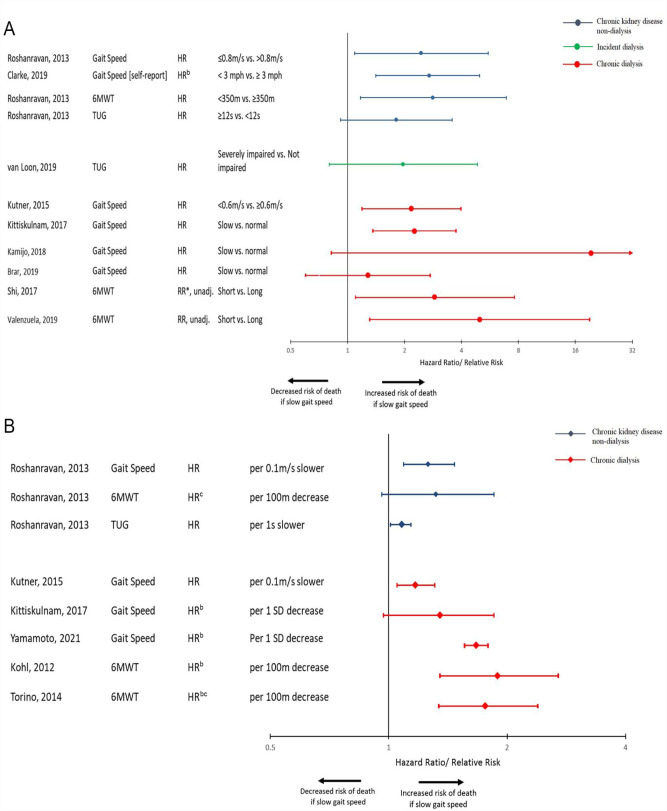
The association between frailty’s gait domain and mortality was examined in chronic dialysis patients (11 assessments among 9 studies), non-dialysis CKD patients (7 assessments among 2 studies), and incident dialysis patients (1 assessment among 1 study). Among categorical assessments of gait, most revealed a 2- to 3-fold risk of death consistent across all patient subgroups (Figure 4A). There was also a consistent increased risk of death when gait was examined as a continuous measure (Figure 4B).
Figure 4.
(A) Forest plot of the association between gait speed examined as a categorical variable and mortality.a (B) Forest plot of the association between gait speed examined as a continuous variable and mortality.a
Note. HR = hazard ratio; 6MWT = 6-Minute Walk Test; TUG = Timed Up-and-Go Test; RR* = relative risk calculated from event data; Unadj = unadjusted model.
aStudies that did not provide measure of association are not displayed.
bComparison was inverted.
cScale was transformed to be consistent with other values.
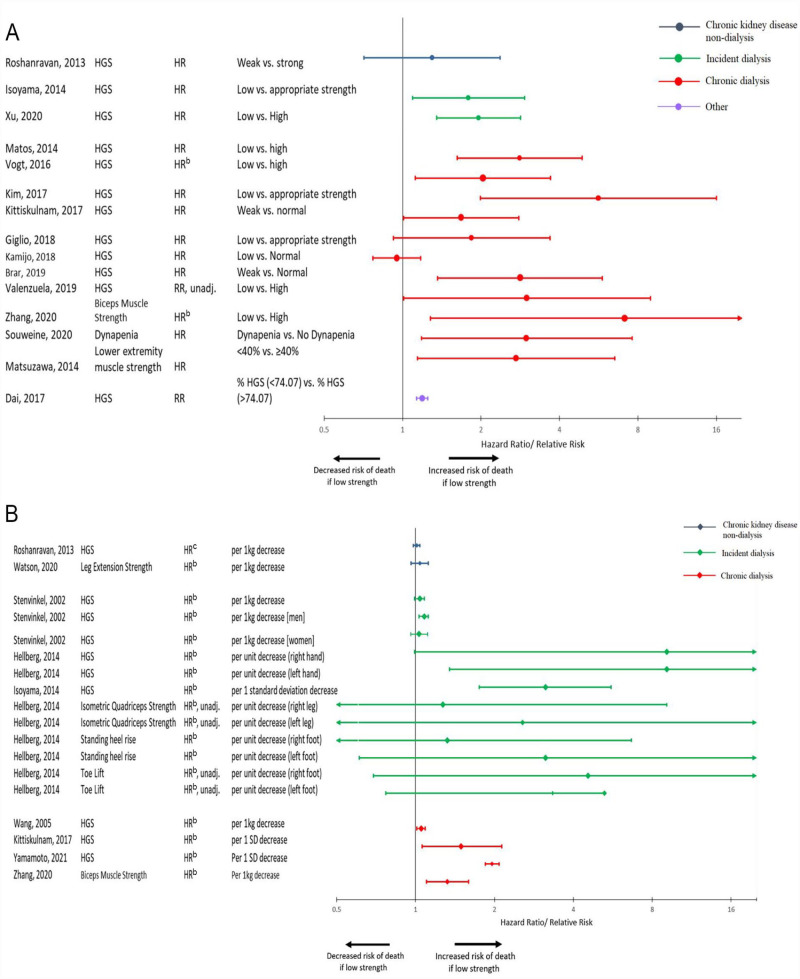
There were 33 assessments reported among 20 studies that examined the relationship between strength measurement and mortality in all patient subgroups. Categorical assessments of this frailty domain revealed an increased risk of death among patients with lower strength in nearly all assessments, with most estimates reporting around a 2- to 3-fold risk (Figure 5A). However, when strength was assessed as a continuous variable, risk estimates tended to be lower (Figure 5B). Effects were similar in the dialysis patient subgroups but less so among CKD non-dialysis patients where risk estimates were closer to 1.
Figure 5.
(A) Forest plot of the association between strength measurement as a categorical variable and mortality.a (B) Forest plot of the association between strength measurement as a continuous variable and mortality.a
Note. HGS = handgrip strength; HR = hazard ratio; RR = relative risk; Unadj = unadjusted model.
aStudies that did not provide measure of association are not displayed.
bComparison was inverted.
cScale was transformed to be consistent with other values.
Thirteen unique instruments were used to examine the relationship between physical activity and fatigue and mortality in all patient subgroups. Patients with lower physical activity and increased fatigue had a higher risk of death, with a point estimate between 1.5 and 2 among categorical assessments (Figure S2). All continuous assessments of physical activity and fatigue revealed a positive point estimate above 1, suggesting an increased risk of death (Figure S3).
The relationship between functional status and mortality was reported among 24 assessments in 19 studies. Most studies using categorical assessments of ADL found that patients with lower functional status had an increased risk of death, usually around 2- to 4-fold (Figure S4). Among continuous assessments of ADL impairment, all studies found a positive association between lower functional status and death (Figure S5).
There were 14 assessments among 11 studies that examined the relationship between performance scale and mortality in 3 patient subgroups. A positive association was reported between lower functional status and death. Specifically, a 1.5- to 4-fold increased risk of death was found among studies measuring performance scale as a categorical variable (Figure S6). Similarly, when assessed as a continuous variable, studies tended to show a positive association between lower performance and the risk of death (Figure S7).
Four instruments were used to assess physical performance in 20 studies among incident and chronic dialysis patients. All categorical assessments of physical performance were associated with a 1.5- to 4-fold increased risk of death (Figure S8). When examined as a continuous variable, decreased physical performance was associated with increased risk of death in the vast majority of reported assessments (Figure S9). Results were consistent in both dialysis populations.
Hospitalization
Table 2 provides an overview of the association between various instruments used to measure frailty and functional status and hospitalization, classified by patient subgroup.
Table 2.
Overview of the Association Between Frailty and Functional Status Instruments and Hospitalization, Classified by Patient Population.
| Author, year | N | Tool | Follow-up | Analysis a | Main findings |
|---|---|---|---|---|---|
| CKD non-dialysis patients | |||||
| Frailty, overall frailty or individual domains | |||||
| Vezza, 2019 | 115 | Frailty Index | 1 year b | Not Frail (reference [ref]) vs Frail: aOR 18.80 (2.36-150.0)
c
Per unit increase: aOR 1.07 (1.02-1.13) c |
Frailty was associated with ↑ odds of hospitalization. |
| Meulendijks, 2015 | 63 | Groningen Frailty Indicator | 1 year | Not Frail (ref) vs Frail: RR 1.68 (1.23-2.31) d | Frailty was associated with ↑ risk of hospitalization. |
| Tsai, 2017122 | 161 | 2-Minute Step | 2.4 yearsb,e | High 2-Minute Step (ref) vs Low 2-Minute Step: aHR 1.06 (0.04-25.0) f | Low 2-minute step was not associated with hospitalization. |
| Tsai, 2017122 | 161 | Handgrip Strength (HGS) | 2.4 yearsb,e | High HGS (ref) vs Low HGS: aHR 1.04 (0.98-1.11) f | Low HGS was not associated with hospitalization. |
| Watson, 2020 | 89 | Leg Extension Strength | 3.3 years e | Per 1kg decrease: aHR 1.01 (0.99-1.03) f | Muscle strength was not associated with unplanned hospitalization. |
| Tsai, 2017122 | 161 | 30-Second Chair Stand | 2.4 yearsb,e | Per unit decrease: aHR 1.19 (1.05-1.35) f | Chair stand performance was associated with ↑ risk of first hospitalization. |
| Incident dialysis patients | |||||
| Frailty, overall frailty or individual domains | |||||
| van Loon, 2019128 | 192 | Fried Frailty Index [modified low activity] | 0.5 years b | Not Frail (ref) vs Frail: aOR 2.31 (1.24-4.32) | Frailty was associated with ↑ odds of hospitalization. |
| Bao, 2012 | 1576 | Frailty, self-report [modified Fried, Woods, Johansen] | 1.2 years g | Not Frail (ref) vs Frail: aHR 1.26 (1.09-1.45) c | Frailty was associated with ↑ risk of first hospitalization. |
| Van Loon, 2019128 | 192 | Clinical Impression [physician] | 0.5 years b | Not Frail (ref) vs Frail: aOR 2.35 (1.14-4.86) | Frailty was associated with ↑ odds of hospitalization. |
| Van Loon, 2019128 | 192 | Geriatric Assessment | 0.5 years b | Not Frail (ref) vs Frail: OR 1.50 (0.84-2.65) h | Frailty was not associated with odds of hospitalization. |
| Van Loon, 2019128 | 192 | Groningen Frailty Indicator | 0.5 years b | Not Frail (ref) vs Frail: OR 1.27 (0.71-2.67) h | Frailty was not associated with odds of hospitalization. |
| Van Loon, 2019128 | 192 | Timed Up-and-Go | 0.5 years b | Not impaired (ref) vs Severely Impaired: aOR 1.97 (0.86-4.50) | Impaired mobility was not associated with odds of hospitalization. |
| Functional status | |||||
| Shum, 2014 | 157 | Basic Activities of Daily Living (BADL) | 1.96 years g |
Independent (ref) vs Impaired BADL: Emergency hospitalization rate: β = 0.20, P value <.01 i Number of emergency hospitalization days: β = 0.22, P value <.01 i |
BADL impairment was a predictor of emergency hospitalization and number of emergency hospitalization days. |
| Van Loon, 2019128 | 192 | Katz’ Activities of Daily Living (ADL) | 0.5 years b | Not Impaired (ref) vs Impaired: aOR 2.63 (1.31-5.34) | Impairment was associated with ↑ odds of hospitalization. |
| Van Loon, 2019128 | 192 | Lawton and Brody’s Instrumental Activities of Daily Living (IADL) Scale | 0.5 years b | Not Impaired (ref) vs Impaired: aOR 2.10 (0.99-4.45) | Impairment was associated with ↑ odds of hospitalization j |
| Utas, 2001 | 334 | Karnofsky Performance Scale (KPS) | 1.95 yearsb,e | Number of hospitalization days: Data not reported; P < .05k,l | Worse functional status was associated with more hospitalization days. |
| Revuelta, 200475 | 318 | KPS [modified] | 771 days g | Per 10-point decrease: aRR 1.12 (0.92-1.36)k,c | Karnofsky score was not associated with the number of days hospitalized. |
| Revuelta, 200475 | 318 | SF-36 Physical Component Summary (PCS) | 771 days g | Per 10-point decrease: aRR 1.13 (0.85-1.49)k,c | SF-36 PCS was not associated with the number of days hospitalized. |
| Chronic dialysis patients | |||||
| Frailty, overall frailty or individual domains | |||||
| McAdams-DeMarco, 2013 | 146 | Fried Frailty Index | 1 year | Not Frail (ref) vs: Intermediately Frail: aRR 0.74 (0.49-1.11) c Frail: aRR 1.47 (1.05-2.06) c |
Frailty was associated with ↑ risk of hospitalization. |
| Yadla, 2017 | 205 | Fried Frailty Index | 1 year | Not Frail (ref) vs Frail: HR 2.06 (1.18-3.58) h | Frailty was associated with ↑ risk of hospitalization. |
| Kang, 201755 | 1250 (HD) 366 (PD) |
Johansen Frailty Criteria [modified weight loss] | 489 days
e
(HD) 467 days e (PD) |
HD Not Frail/Pre-Frail (ref) vs Frail: aHR 1.56 (1.27-1.92) PD Not Frail/Pre-Frail (ref) vs Frail: aHR 1.41 (1.02-1.94) |
Frailty was associated with ↑ risk of first hospitalization in hemodialysis (HD) and peritoneal dialysis (PD) patients. |
| Lee, 201770 | 1658 | Johansen Frailty Criteria [modified weight loss] | 1.4 yearsb,g | Not Frail (ref) vs: Pre-Frail: aHR 1.29 (1.00-1.67) Frail: aHR 1.83 (1.41-2.37) |
Frailty was associated with ↑ risk of hospitalization. |
| Bancu, 2017 | 320 | Fried Frailty Index + Dialysis Time/Week | 1 year | Not Frail vs Frail: P = .005 h | The frailty group had significantly more hospital admissions per year compared to the not frail group. |
| Jiang, 2020 | 1424026 | Frailty (Johns Hopkins Adjusted Clinical Groups) | Not reported | Length of stay: Not Frail (ref) vs Frail: aβ = 4.82; P value <.05 |
Frailty was associated with longer hospital stays. |
| Ng, 2016 | 193 | Frailty Score | 1.9 yearsb,e | Number of hospitalizations for all causes: β = 0.29; P value <.0001i,l Total length of hospital stay: β = 0.34; P value <.0001i,l |
Frailty Score was associated with number of hospitalizations for all causes and total length of hospital stay. |
| Chan, 2020 | 267 | Frailty Score | 2 years | Number of all-cause hospital admissions: Not Frail (ref) vs Frail: aβ = 0.998; P value: .045 i Total length of hospital stay: Not Frail (ref) vs Frail: aβ = 14.295; P value: .049 i |
Frailty was associated with ↑ number of hospital admissions and ↑ duration of hospitalization. |
| Jegatheswaran, 2020 | 261 | FRAIL Questionnaire | 1.5 years b | Not Frail (ref) vs: Pre-Frail: RR 1.31 (0.98-1.75) d Frail: RR 1.57 (1.13-2.17) d |
Frailty was associated with ↑ risk of hospitalization. |
| Giglio, 201832 | 170 | Sarcopenia [modified] | 1.5 yearsb,g | No Sarcopenia (ref) vs Sarcopenia: aRR 2.07 (1.48-2.88) | Sarcopenia was associated with ↑ risk of hospitalization. |
| Lin, 202073 | 126 | Sarcopenia (Skeletal Muscle Mass Index + HGS/Gait Speed) | 3 years | No Sarcopenia vs Sarcopenia: P value: .294 h |
Sarcopenia was not associated with hospitalization. |
| Kutner, 2015 | 466 | Gait Speed | 1 year | Gait Speed ≥ 1.0m/s (ref) vs: 0.8 to < 1.0m/s: aOR 2.05 (1.30-3.25) 0.6 to < 0.8m/s: aOR 2.04 (1.19-3.49) |
Slower gait speed was associated with ↑ odds of hospitalization. |
| Lin, 202073 | 126 | Gait Speed | 3 years | Normal vs Slow: P value: .008 h |
Gait speed was associated with hospitalization. |
| Torino, 2014 | 296 | 6-Minute Walk Test | 3.3 years g | Per 100m decrease: aHR 1.22 (1.05-1.54)c,f,m | Shorter walk distance was associated with ↑ risk of all-cause hospitalization. |
| Giglio, 201832 | 170 | HGS | 1.5 yearsb,g | Appropriate Muscle Strength (ref) vs Low Muscle Strength: aRR 1.92 (1.38-2.57) | Low muscle strength was associated with ↑ risk of hospitalization. |
| Lin, 202073 | 126 | HGS | 3 years | Normal vs Low: P value: .01 h |
HGS was associated with hospitalization. |
| Mapes, 200379 | 10030 | SF-36 Vitality Scale | Not reported | Per 10-point decrease: aHR 1.05 (1.04-1.06) | Increasing fatigue was associated with ↑ risk of hospitalization. |
| Tentori, 2010 | 20920 | Exercise Frequency | 1.75 years g | Regular Exercise (≥ once/week) (ref) vs Non-Regular Exercise (< once/week or never): HR 1.00 (0.96-1.04) h | Exercise was not associated with all-cause hospitalization. |
| Functional status | |||||
| Kang, 201755 | 1250 (HD) 366 (PD) |
Disability | 489 days
e
(HD) 467 days e (PD) |
HD No Disability (ref) vs Disability: aHR 1.43 (1.12-1.84) PD No Disability (ref) vs Disability: aHR 1.16 (0.84-1.61) |
Disability was associated with ↑ risk of first hospitalization in HD patients only. |
| Lee, 201770 | 1658 | Disability | 1.4 yearsg¶ | No Disability (ref) vs Disability: HR 1.68 (1.40-2.02) h | Disability was associated with ↑ risk of hospitalization. |
| Jassal, 2016 | 3583 | Functional Status Score (ADL & IADL) | 1.4 yearsb,g | Functionally Independent (score = 13) (ref) vs Most Dependent (score <8): aHR 1.28 (1.14-1.44) | Functional dependence was associated with ↑ risk of first any-cause hospitalization. |
| Jones, 1991 | 527 | KPS | 0.5 years b | Per 10-unit decrease: aOR 1.22 (1.1-1.35)c,f,k,m | Lower KPS was associated with ↑ odds of hospitalization. |
| DeOreo, 1997 | 1000 | SF-36 PCS | 531 days e | Per 10-point decrease: aHR 1.12 (1.08-1.17)f,m | Decreasing functional status was associated with ↑ risk in the number of days in hospital. |
| Lowrie, 2003 | 13952 | SF-36 PCS | 0.5 years b | Per 10-point decrease: aOR 1.22 (1.19-1.26)f,m | Decreasing functional status was associated with ↑ odds of hospitalization. |
| Mapes, 200379 | 10030 | SF-36 PCS | Not reported | Score >46 (ref) vs: Score 39-46: aHR 1.16 (1.04-1.30) c Score 33-38: aHR 1.27 (1.14-1.42) c Score 26-32: aHR 1.40 (1.25-1.58) c Score <25: aHR 1.47 (1.30-1.67) c Per 10-point decrease: aHR 1.15 (1.11-1.18) c |
Decreasing functional status was associated with ↑ risk of hospitalization. |
| Lacson, 201068 | 44395 | SF-36 PCS | 1 year | Per 10-point decrease: aHR 1.04 (1.03-1.06)f,m | Decreasing PCS was associated with ↑ risk of hospitalization. |
| Kang, 201755 | 1250 (HD) 366 (PD) |
SF-36 PCS | 489 days
e
(HD) 467 days e (PD) |
HD High PCS tertile (ref) vs Middle/Low PCS tertile: aHR 1.00 (1.01-1.02) f PD High PCS tertile (ref) vs Middle/Low PCS tertile: aHR 1.01 (1.01-1.02) f |
Decreased PCS was associated with ↑ risk of first hospitalization in HD and PD patients. |
| Lacson, 201068 | 44395 | SF-12 PCS | 1 year | Per 10-point decrease: aHR 1.04 (1.03-1.06)f,m | Decreasing PCS was associated with ↑ risk of hospitalization. |
| Mapes, 200379 | 10030 | SF-36 Physical Function Scale | Not reported | Per 10-point decrease: aHR 1.05 (1.04-1.06) | Decreasing physical function was associated with ↑ risk of hospitalization. |
| Other | |||||
| Frailty, overall frailty or individual domains | |||||
| Nixon, 2020 | 450 | Clinical Frailty Scale [adapted] | 210 days g | Per 1-point increase: aHR 1.35 (1.20-1.53) c | Each point increase in Clinical Frailty Scale score was associated with ↑ risk of hospitalization. |
Note. References are available in supplementary material. ADL = Activities of Daily Living; aHR = adjusted hazard ratio; aOR = adjusted odds ratio; aRR = adjusted relative risk; aβ = adjusted beta; BADL = Basic Activities of Daily Living; CKD = chronic kidney disease; HD = hemodialysis; HGS = handgrip strength; HR = hazard ratio; IADL = Instrumental Activities of Daily Living; KPS = Karnofsky Performance Scale; PCS = Physical Component Summary; PD = Peritoneal dialysis; Ref = reference; RR = relative risk.
All models adjusted for a minimum of age and sex, unless otherwise noted. Where a choice of models exists, the most fully adjusted model is presented.
Converted to years.
Multiple adjusted models available.
RR calculated from event data, or cumulative survival event data.
Mean.
Scale inverted.
Median.
Unadjusted model.
Model not adjusted for sex.
Discrepancy reported between study data and conclusion.
Model not adjusted for age or sex.
Reference group and comparator not reported; unit of measure not clearly reported.
Scale change.
The relationship between frailty and hospitalization was assessed in 17 studies across all frailty domains in all patient subgroups. There was an approximately 2-fold increased risk of hospitalization among frail patients. This was consistent in the 3 patient subgroups. Frailty examined on a continuous scale also revealed a positive association with the risk for hospitalization (Figure S10). Few studies examined the association between measures of sarcopenia (n = 1, Figure S11), gait speed (n = 3, Figure S12), strength (n = 4, Figure S13), physical activity and fatigue (n = 2, Figure S14) and hospitalization; these studies tended to show a positive association among dialysis patients but revealed a weaker association among non-dialysis patients.
The relationship between functional status and hospitalization was reported among 18 assessments in 10 studies among incident and chronic dialysis patients. In both dialysis subgroups, there was a positive association between lower functional status, by categorical measurement of ADL impairment, and increased risk of hospitalization, around 1.5- to 2-fold (Figure S15). Only 2 studies examined the relationship of performance scale score and hospitalization (Figure S16). Finally, 10 studies assessed physical performance among dialysis patients (Figure S17). Decreased physical performance was associated with increased risk of hospitalization in most studies.
Finally, Table S3 provides additional details on the association of frailty and functional status tools with various other adverse effects.
Discussion
This systematic review identified 140 studies and 117 unique instruments used to examine the association of frailty and functional status with various clinical outcomes in patients with advanced CKD. Most studies focused on incident and chronic dialysis patient populations, with only 15% of studies examining non-dialysis CKD patients. Our study found that frailty was a predictor of mortality among all patient populations. When the specific domains of frailty were examined individually, they were also each found to be associated with mortality. Similarly, lower functional status was also associated with an increased risk of mortality among all patient populations. Parallel trends were noted when examining hospitalization as an outcome. These findings highlight that frailty and lower functional status are risk factors for adverse outcomes in patients with advanced CKD and on dialysis and emphasize the importance of considering them as prognostic metrics among these patients.
Previous systematic reviews have examined the association of frailty status and negative health outcomes in patients with CKD. The prevalence of frailty increases with kidney function decline, and these systematic reviews demonstrate a greater risk for adverse outcomes with frailty such as mortality and hospitalization.9,31-35 Our findings are consistent with these prior systematic reviews. Our study also assessed the relationship between functional status and adverse outcomes, something which has not been thoroughly considered in prior reviews. Therefore, our findings shed further light onto the significance of functional status in predicting adverse outcomes in CKD while further supporting the importance of frailty as a known prognostic factor.
Patients with advanced CKD often have various physiologic impairments resulting from chronic co-morbidities that are either caused by or associated with CKD. As a result of limited physiologic reserves, patients with CKD are much more susceptible to being frail 36 resulting in a high prevalence of frailty32,37 particularly among those undergoing dialysis, with rates ranging from 14% to 73%. 9 As a consistent predictor for adverse outcomes in CKD, it is not surprising that guidelines recommend evaluating frailty when assessing potential kidney transplant candidates, 38 similar to other factors such as the management of blood pressure and diabetes.39,40 However, in contrast to blood pressure and diabetes management which have clear indicators or adequate control, it remains unclear for both the degree to which frailty is potentially reversible and how interventions aimed at treating frailty may improve outcomes post-kidney transplant. Studies have explored the impact of an exercise intervention in patients prior to transplantation, finding significant improvements in frailty status and a reduction in adverse outcomes.41,42 Other interventions have explored the use of senolytic (removal of senescent cells) drugs and oral nutritional supplements to target frailty in the CKD population.43,44 Furthermore, by focusing on and improving functional status, relief from uremia and kidney failure, kidney transplantation may itself improve frailty. 45 The finding that patient frailty improves following kidney transplant complicates the decision-making process regarding the acceptable level of frailty for surgery. Excessive frailty puts the patient at clear risk for adverse outcomes, however, there is a potential for improvement with enhanced kidney function post-transplant. Additional research is needed to address how frailty should be considered when evaluating patients with advanced CKD for transplant candidacy, an area priority also highlighted by the 2020 KDIGO guidelines. 38
Given that frailty is a complex, multi-dimensional condition where deficits across multiple different domains (physical, cognitive, and social), are at play, the need for consistent and reliable measures for this concept/syndrome are extremely important. As underscored by our study and other systematic reviews,46,47 there is substantial heterogeneity in the tools used to measure frailty. Although the Fried frailty tool is one of the most used frailty measurement tools, 46 the optimal test to use in clinical practice to identify and grade the severity of frailty, particularly in the setting of CKD, has not been identified.48,49 This makes it difficult for clinicians to choose the optimal instrument when evaluating frailty. Most would be considered cumbersome, time-consuming, or require specific tools which would not make them practical for implementation into every day clinical practice, for example, in a dialysis unit or general nephrology clinics. If frailty is to become an important component of clinical care for primary care physicians and nephrologists, finding a practical and valid measurement tool will be crucial. Validation and standardization of frailty tools in patients with advanced CKD would enhance the clinician’s ability to properly counsel patients on their suitability for major medical procedures, but also improve the applicability of future interventions aimed at improving frailty.
Major strengths of our review are its size and broad scope, which increases the clinical applicability of our findings. We examined the effect of all domains of frailty on a variety of clinical outcomes, across all dialysis patients as well as non-dialysis CKD patients. Also, we examined the effect of functional status on adverse outcomes, something prior systematic reviews have not properly characterized. In addition, we did not restrict measurement methods; therefore, numerous instruments measuring functional status and the 5 domains of frailty were included in this review. Nonetheless, this study has limitations. There was considerable variation in methods used to measure frailty and functional status contributing to heterogeneity between studies. As such, conducting a meta-analysis and pooling statistics could not be performed. Second, most studies (n = 72) included in this review used data sources other than a primary cohort, including registry data, hospital charts, or performed secondary analysis of established cohort. This may impact the validity of data collection, assessment of exposure and outcomes, and the potential for selection bias in these studies, thus affecting the validity of the findings in our review. Furthermore, there were issues with the methodological rigor of some studies, as 28.6% of studies were rated as having a high risk of bias. Finally, we only included studies published in English.
Conclusion
Based on the findings summarized in this review, there is evidence to suggest that frailty and lower functional status are predictors of poor clinical outcomes such as mortality and hospitalization among patients with advanced CKD, including dialysis and non-dialysis patients. Our findings highlight the need to assess, monitor, and integrate frailty and functional status measures during clinical care decision making ensuring a comprehensive assessment of risk for adverse outcomes among these patients. Future research should focus on examining these findings among non-dialysis CKD patients, given the paucity of research among this population. Additional research is needed to identify the optimal method for measuring frailty in patients with CKD, and how best to incorporate frailty and functional status assessments in prognosis to guide decision-making surrounding eligibility for certain major medical interventions such as kidney transplant. Finally, studies are needed to identify targeted program initiatives to prevent frailty developing in CKD, treatments for reversal of frailty in CKD, and the role kidney transplantation plays in improving frailty in CKD.
Supplemental Material
Supplemental material, sj-pdf-1-cjk-10.1177_20543581231181026 for Physical Frailty and Functional Status in Patients With Advanced Chronic Kidney Disease: A Systematic Review by Priscilla Karnabi, David Massicotte-Azarniouch, Lindsay J. Ritchie, Shawn Marshall and Greg A. Knoll in Canadian Journal of Kidney Health and Disease
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a peer-reviewed grant from the Canadian Institutes of Health Research (Grant #FDN-143239).
ORCID iDs: Priscilla Karnabi  https://orcid.org/0000-0002-4142-6935
https://orcid.org/0000-0002-4142-6935
David Massicotte-Azarniouch  https://orcid.org/0000-0002-6954-2030
https://orcid.org/0000-0002-6954-2030
Lindsay J. Ritchie  https://orcid.org/0000-0003-4621-4197
https://orcid.org/0000-0003-4621-4197
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Canadian Institute for Health Information (CIHI). Annual Statistics on Organ Replacement in Canada Dialysis, Transplantation and Donation, 2009 to 2018. Ottawa, ON, Canada: CIHI; 2019. [Google Scholar]
- 2.Kabore R, Haller MC, Harambat J, et al. Risk prediction models for graft failure in kidney transplantation: a systematic review. Nephrol Dial Transplant. 2017;32:ii68-ii76. doi: 10.1093/ndt/gfw405. [DOI] [PubMed] [Google Scholar]
- 3.Bergler T, Hutchinson JA.Tools for predicting kidney transplant outcomes. Transplantation. 2017;101:1958-1959. doi: 10.1097/TP.0000000000001891. [DOI] [PubMed] [Google Scholar]
- 4.Patzer RE, Basu M, Larsen CP, et al. iChoose kidney: a clinical decision aid for kidney transplantation versus dialysis treatment. Transplantation. 2016;100:630-639. doi: 10.1097/TP.0000000000001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tangri N, Inker LA, Hiebert B, et al. A dynamic predictive model for progression of CKD. Am J Kidney Dis. 2017;69:514-520. doi: 10.1053/j.ajkd.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Loupy A, Aubert O, Orandi BJ, et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: international derivation and validation study. BMJ. 2019;366:l4923. doi: 10.1136/bmj.l4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong A, Hanson CS, Chapman JR, et al. The preferences and perspectives of nephrologists on patients’ access to kidney transplantation: a systematic review. Transplantation. 2014;98:682-691. doi: 10.1097/TP.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 8.Naylor KL, Dixon SN, Garg AX, et al. Variation in access to kidney transplantation across renal programs in Ontario, Canada. Am J Transplant. 2017;17:1585-1593. doi: 10.1111/ajt.14133. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury R, Peel NM, Krosch M, et al. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr. 2017;68:135-142. doi: 10.1016/j.archger.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Vermeiren S, Vella-Azzopardi R, Beckwee D, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17:1163.e1-1163.e17. doi: 10.1016/j.jamda.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Viswanath N, Harichandra Kumar KT, Haridasan S, et al. Functional status in hemodialysis—a comparative study with FIM, ADLQ and 7D5L instruments. Indian J Nephrol. 2019;29:172-178. doi: 10.4103/ijn.IJN_363_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallenberg MH, Kleinveld HA, Dekker FW, et al. Functional and cognitive impairment, frailty, and adverse health outcomes in older patients reaching ESRD —a systematic review. Clin J Am Soc Nephrol. 2016;11:1624-1639. doi: 10.2215/CJN.13611215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reese PP, Bloom RD, Shults J, et al. Functional status and survival after kidney transplantation. Transplantation. 2014;97:189-195. doi: 10.1097/TP.0b013e3182a89338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kojima G, Liljas AEM, Iliffe S.Frailty syndrome: implications and challenges for health care policy. Risk Manag Healthc Policy. 2019;12:23-30. doi: 10.2147/RMHP.S168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worthen G, Tennankore K.Frailty screening in chronic kidney disease: current perspectives. Int J Nephrol Renovasc Dis. 2019;12:229-239. doi: 10.2147/IJNRD.S228956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 17.Bowling CB, Sawyer P, Campbell RC, et al. Impact of chronic kidney disease on activities of daily living in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66:689-694. doi: 10.1093/gerona/glr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg 2012;147:190-193. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 19.Brett KE, Bennett A, Ritchie LJ, et al. Physical frailty and functional status in patients with advanced kidney disease: a protocol for a systematic review. Syst Rev. 2017;6:133. doi: 10.1186/s13643-017-0536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392-397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leidy NK.Functional status and the forward progress of merry-go-rounds: toward a coherent analytical framework. Nurs Res. 1994;43:196-202. [PubMed] [Google Scholar]
- 23.Drubbel I, Numans ME, Kranenburg G, et al. Screening for frailty in primary care: a systematic review of the psychometric properties of the frailty index in community-dwelling older people. BMC Geriatr. 2014;14:27. doi: 10.1186/1471-2318-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280-286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 25.Moulaert VR, Verbunt JA, van Heugten CM, et al. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;80:297-305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. International classification of functioning, disability and health (ICF). https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health (2023, accessed 27 April 2023).
- 27.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 28.Lawton MP, Brody EM.Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186. [PubMed] [Google Scholar]
- 29.Péus D, Newcomb N, Hofer S.Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak. 2013;13:72. doi: 10.1186/1472-6947-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30:473-483. [PubMed] [Google Scholar]
- 31.Lee HJ, Son YJ.Prevalence and associated factors of frailty and mortality in patients with end-stage renal disease undergoing hemodialysis: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18:3471. doi: 10.3390/ijerph18073471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Ma Y, Lin F, et al. Frailty and mortality among patients with chronic kidney disease and end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. 2020;52:363-370. doi: 10.1007/s11255-019-02369-x. [DOI] [PubMed] [Google Scholar]
- 33.Mei F, Gao Q, Chen F, et al. Frailty as a predictor of negative health outcomes in chronic kidney disease: a systematic review and meta-analysis. J Am Med Dir Assoc. 2021;22:535-543.e537. doi: 10.1016/j.jamda.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Walker SR, Gill K, Macdonald K, et al. Association of frailty and physical function in patients with non-dialysis CKD: a systematic review. BMC Nephrol. 2013;14:228. doi: 10.1186/1471-2369-14-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song YH, Cai GY, Xiao YF, et al. Risk factors for mortality in elderly haemodialysis patients: a systematic review and meta-analysis. BMC Nephrol. 2020;21:377. doi: 10.1186/s12882-020-02026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60:912-921. doi: 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roshanravan B, Patel KV.Assessment of physical functioning in the clinical care of the patient with advanced kidney disease. Semin Dial. 2019;32:351-360. doi: 10.1111/sdi.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chadban SJ, Ahn C, Axelrod DA, et al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. 2020;104:S11-S103. doi: 10.1097/TP.0000000000003136. [DOI] [PubMed] [Google Scholar]
- 39.Cheung AK, Chang TI, Cushman WC, et al. Executive summary of the KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:559-569. doi: 10.1016/j.kint.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 40.ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023;46:S97-S110. doi: 10.2337/dc23-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorenz EC, Hickson LJ, Weatherly RM, et al. Protocolized exercise improves frailty parameters and lower extremity impairment: a promising prehabilitation strategy for kidney transplant candidates. Clin Transplant. 2020;34:e14017. doi: 10.1111/ctr.14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, et al. Prehabilitation prior to kidney transplantation: results from a pilot study. Clin Transplant. 2019;33:e13450. doi: 10.1111/ctr.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. eBioMedicine. 2019;47:446-456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin-Alemany G, Espinosa-Cuevas MLA, Perez-Navarro M, et al. Effect of oral nutritional supplementation with and without exercise on nutritional status and physical function of adult hemodialysis patients: a parallel controlled clinical trial (AVANTE-HEMO Study). J Ren Nutr. 2020;30:126-136. doi: 10.1053/j.jrn.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 45.McAdams-DeMarco MA, Isaacs K, Darko L, et al. Changes in frailty after kidney transplantation. J Am Geriatr Soc. 2015;63:2152-2157. doi: 10.1111/jgs.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53-61. doi: 10.1016/j.arr.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Hoski S, Bean JF, Ma J, et al. Physical function and frailty for predicting adverse outcomes in older primary care patients. Arch Phys Med Rehabil. 2020;101:592-598. doi: 10.1016/j.apmr.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorenz EC, Kennedy CC, Rule AD, et al. Frailty in CKD and transplantation. Kidney Int Rep. 2021;6:2270-2280. doi: 10.1016/j.ekir.2021.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue QL, Tian J, Walston JD, et al. Discrepancy in frailty identification: move beyond predictive validity. J Gerontol A Biol Sci Med Sci. 2020;75:387-393. doi: 10.1093/gerona/glz052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cjk-10.1177_20543581231181026 for Physical Frailty and Functional Status in Patients With Advanced Chronic Kidney Disease: A Systematic Review by Priscilla Karnabi, David Massicotte-Azarniouch, Lindsay J. Ritchie, Shawn Marshall and Greg A. Knoll in Canadian Journal of Kidney Health and Disease