Abstract
A strict localization into the neural retina and the capacity to survive many pathogenic conditions are unique characteristics of Müller glia. Retinal damage induces cellular and molecular changes that place Müller cells as focal points to either protect or further impair neuronal function. This work evaluated the involvement of extracellular vesicles (EVs) in the transduction of high N-methyl-D-aspartate (NMDA) concentration signal between Müller glia-to-Müller glia as part of several factors and signal transduction mechanisms dictating either a gliotic (damaging) or regenerative process. These EVs were obtained, characterized, and quantified from control and NMDA-treated Müller cells using murine Müller cell primary cultures to evaluate the functional outcome of these preparations. The obtained results support the existence of different subpopulations of EVs that may induce a differential response. These EVs did not affect the viability, migration, and wound healing capacity of cultured Müller cells. However, the EVs obtained from NMDA-treated Müller cultures, but not from untreated cells, increased actin polymerization and induced the expression of the multipotency genes nestin and lin28. We propose that, upon a lesion paradigm, Müller-derived EVs may induce the spread of molecular changes in neighboring cells resulting in the formation of the characteristic glial retinal scar in mammalians or else trigger a neurogenic process in regenerative species.
Summary Statement
Retinal Müller cells secrete extracellular vesicles that can be captured by other Müller cells. In response to a signal that may be deleterious for the retina, Müller glia-derived extracellular vesicles spread instructions to induce gene expression changes in other cells.
Keywords: extracellular vesicles, dedifferentiation, Müller glia, retina, stem cell
Introduction
Far from a passive structural role in the conformation of the retina, Müller glial cells contribute to retinal function by exerting multiple metabolic, trophic, and secretory tasks (Bringmann et al., 2006). In addition, Müller glia has the capacity to act as a resident “stem cell-like” population of the postnatal retina (Reichenbach & Bringmann, 2013). The capacity of differentiated Müller glia to dedifferentiate, proliferate, migrate, and acquire a neuronal phenotype, allows functional retinal regeneration in selected species such as the zebrafish (Danio rerio) (Raymond et al., 2006). In non-regenerative species, such as medaka fish (Oryzias latipes), chicken, and mammals, Müller glia are also able to dedifferentiate to acquire a “progenitor-like” phenotype (Fischer & Reh, 2001; Lust & Wittbrodt, 2018; Ooto et al., 2004). However, their capacity to repair the damaged tissue becomes increasingly restricted and a scar-forming gliotic reaction overcomes the regenerative response (Bringmann & Wiedemann, 2012).
The therapeutic exploitation of the silent neurogenic capacity of mammalian Müller cells demands a deep understanding of the molecular and cellular mechanisms that either sustain or impair their capacity and lead to a gliotic response. From a cellular perspective, Müller cells hypertrophy and retinal architecture modifications are a hallmark of the reactive response to damage (Graca et al., 2018; Lu et al., 2011). From a molecular point of view, two different types of experimental approaches aimed at evaluating the efficacy of Müller glia-derived progenitors on retinal function recovery reveal and reinforce the notion that secretory events from these cells play a crucial role in this regard. First, the analysis of the signaling pathways involved in Müller glia-directed regeneration in zebrafish, expose an autocrine/paracrine role to Müller-secreted cytokines and growth factors when promoting cell dedifferentiation after retinal injury (Nelson et al., 2013; Wan et al., 2014; Zhao et al., 2014). Second, cellular transplantation using either Müller-derived retinal neuronal cells (Becker et al., 2016), Müller glia isolated from induced pluripotent stem cell-derived retinal organoids (Eastlake et al., 2019), or NTPDase2+ Müller cells from young adult mouse retinas (Hoek et al., 2018), indicate that, while the integration of donor cells is a rare event, the partial restoration of visual function could be ascribed to donor-to-host cytoplasmic material transfer. However, any direct evidence supporting the occurrence of a bidirectional cell-to-cell material exchange has only been provided in photoreceptor transplantation assays (Pearson et al., 2016; Santos-Ferreira et al., 2016; Singh et al., 2016). The analysis of the underlying mechanisms of material transfer in transplantation assays suggests unique means of non-synaptic intercellular communication in the retina (Kalargyrou et al., 2021; Ortin-Martinez et al., 2021). Therefore, in this study, we decided to evaluate a novel intercellular pathway that involves the use of extracellular vesicles (EVs).
EVs are nanoscale particles formed by a lipid bilayer encapsulating and carrying a biologically active cargo of proteins and nucleic acids which are released into the extracellular space and then taken by recipient cells. The internalization of the vesicle-derived cargo may influence the recipient celĺs function and phenotype. It has been demonstrated that the exposure of cultured Müller cells to either human or mouse stem cell derived-EVs results in morphological and gene expression changes that promote the acquisition of a retinal progenitor phenotype, reactivating their neurogenic and regenerative capacities (Farber & Katsman, 2016; Gao et al., 2022; Peng et al., 2018). In this regard, a groundbreaking study demonstrated that Müller cells release distinct EV populations in the inner and outer retina of adult mice in vivo (Demais et al., 2022).
We speculated that the pathological context of the Müller response to retinal damage could be reflected by the number of Müller-derived EVs and their capacity to induce the expression of multipotency genes in cultured Müller cells. To test this hypothesis, we obtained, characterized, quantified, and functionally assessed EVs from both control and Müller cells treated with a high concentration (100 μM) of N-methyl-D-aspartate (NMDA), which is a selective agonist of NMDA glutamate receptors. NMDA is a well-known signaling molecule for experimentally mimicking retinal neurodegeneration, as NMDA receptors are abundant in the retina and glutamate excitotoxicity is known to lead further regeneration in degenerative retinal diseases (Boccuni & Fairless, 2022). However, it also exerts a paradoxical effect on Müller glia, since it promotes their proliferation in adult mice and in Müller-derived progenitor cell cultures (Karl et al., 2008; Ramírez & Lamas, 2009). Further, the activation of the glutamate receptor can induce the expression of multipotency genes, the acquisition of a “progenitor-like” phenotype and the dedifferentiation capacity of mammalian Müller glial cells (Reyes-Aguirre et al., 2013; Takeda et al., 2008; Xiao et al., 2017). In this study we found that treatment with NMDA did not increase the number of secreted EVs by Müller cells; regardless, it still affected the functional outcome of these EVs on recipient cells, where the exposure to Müller-derived EVs increased actin polymerization and altered the organization of the cytoskeleton, which was exacerbated due to NMDA treatment. Most importantly, we observed that only EVs obtained from NMDA treated cells were capable of inducing the expression of multipotency genes, i.e., nestin and lin28 on recipient cultured Müller cells.
Materials and Methods
Animal Subjects
Postnatal (8–12 days) C57BL/6J mice (RRID:IMSR_JAX:000664) were used for all experiments. In agreement with the prevalent notion that the regenerative potential of Müller cells becomes limited with increasing mouse age (Löffler et al., 2015), unpublished observations from our laboratory suggest that there is a greater opportunity to induce the expression of multipotency genes in primary Müller cell cultures derived from these mice. The laboratory animals were treated and handled in strict accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and the guidelines of the internal animal care committee (CICUAL-CINVESTAV; Project number: 0259-17).
Isolation and Culture of Müller Cells
C57BL/6J mice were euthanized and decapitated. Their heads were soaked in 70% ethanol, and the eyes were enucleated and placed in Dulbecco’s Minimal Essential Medium (DMEM; Gibco) containing 10% fetal bovine serum (FBS; Gibco #26140079) and 1% antibiotic antimycotic solution containing 10000 units penicillin, 10 mg streptomycin and 25 μg amphotericin B (Sigma-Aldrich #A5955). The samples were kept in the dark and in constant agitation at room temperature overnight. The following day, the eyes were incubated in DMEM containing 0.1% trypsin and 70 IU/mL collagenase (Sigma Chemical Co., St. Louis, MO) for 30 min at 37°C. The enzymatic digestion was stopped by adding DMEM containing 10% FBS and the samples were transferred to a Petri dish containing DMEM supplemented with 10% FBS (DMEM-FBS 10%). The eyes were hemisected with a circumferential cut posterior to the ora serrata. The anterior eyecup was discarded, and the retina was carefully excised from the posterior eyecup, clearing away vitreous residues and pigment epithelial attachments. Subsequently, the retinal tissue was transferred to an Eppendorf tube filled with 2 mL DMEM-FBS 10% and dissociated through vigorous pipetting. The dissociated cells from 8 to 10 retinas were seeded into a six-well plates with DMEM-FBS 10% and penicillin-streptomycin 1% and placed in an incubator at 37°C and 5% CO2. The cells were allowed to attach for 24 h and after, the cell cultures were washed with PBS 1X to eliminate non-adherent cells (neuronal cells), and the media was replaced. The cells were kept in culture and the medium was replaced after 4–5 days, after this, the medium was changed every 3 days. The cells were maintained for 2 weeks or until they were confluent (∼80% confluence). The purity of the culture was evaluated by immunofluorescence staining for Müller cells markers and lack of expression of neuronal markers as previously described (Das et al., 2006). EVs were isolated from passage 3. The cells were harvested using trypsin/EDTA 0.12% (Sigma-Aldrich #T4049) and re-seeded in three 25 cm2 flasks until confluent, then re-seeded in five 75 cm2 flasks and finally re-seeded in ten 175 cm2 flasks until they reached confluence (cell yield of ∼ 3.5–4 × 106 cells/flask). Five of these 175 cm2 flasks were used to isolate control EVs, while the remaining five flasks were treated with NMDA (100 µM) for 4 h before isolation the NMDA EVs.
Experimental Groups
Two experimental groups were established. In the first group, the cells were kept under control conditions (no stimulation), whereas in the second group, the cells were stimulated with NMDA 100 µM (Sigma-Aldrich #M3262).
Cell Viability
The Müller cells (1 × 105/mL) were plated in 6-well culture plates and cultured for 48 h at 37°C and 5% CO2. The media was then replaced with fresh DMEM-FBS 10% and supplemented with 100 µM NMDA, or with DMEM-FBS supplemented with 10% exosome-depleted FBS containing either control or NMDA EVs (10 and 30 µ/mL media, respectively). At the end of the incubation time with NMDA or EVs, the medium was removed, and the cells were detached using 0.5 mL trypsin/EDTA 0.12% (Sigma-Aldrich #T4049) and quenched by adding 0.5 mL medium supplemented with 10% FBS (using exosome-depleted FBS in EVs groups). The cells were centrifuged at 10,000 rpm for 5 min and gently resuspended in 1 mL of growth medium. Cell viability was measured using a Count & Viability kit (Millipore # MCH100102) in a MUSER cytometer following manufacturer’s instructions. Briefly, we stained the cell samples by mixing 50 µL of a uniform cell suspension with 450 µL of Muse™ Count & Viability Reagent in a 0.6 mL sample tube. We allowed the cells to stain for 5 min at room temperature, mixed the sample and loaded them on the instrument. Measurements were carried out in triplicate.
Isolation of EVs
After reaching 75%–80% confluency, the Müller cells were rinsed with 1× PBS and cultured with DMEM supplemented with 10% exosome-depleted FBS (Gibco #A2720801) for 48 h. After passage 3, the cells were either stimulated with 100 µM NMDA (Sigma-Aldrich #M3262) for 4 h or kept under control conditions. The conditioned media were collected into 50 mL polypropylene tubes (a total of 40 mL conditioned medium per tube).
To enrich EVs from conditioned media we applied the “ExtraPEG” method which includes a concentration step of vesicles from cell culture media using polyethylene glycol (PEG) and a wash and a re-pellet step of vesicles using ultracentrifugation (Rider et al., 2016). Briefly, the collected media was centrifugated at 400xg for 10 min followed by 2,000×g for 30 min at 4°C to remove the remaining cells and cellular debris respectively. A 50% stock solution of PEG (Sigma-Aldrich # P5413) in 0.5× PBS was added under sterile conditions to the conditioned media in a 2:10 ratio (2 mL of PEG per every 10 mL of conditioned medium) to achieve a final concentration of 8.3% PEG, which allow the precipitation of EVs by liquid exclusion (Martínez-Greene et al., 2021). The tubes were mixed by inversion and incubated overnight at 4°C. Then, the samples were centrifuged at 1,500×g for 30 min at 4°C in a Thermo Scientific SORVALL ST8 centrifuge. The supernatant was decanted, leaving behind approximately 5–10 mL of the medium in the tube and centrifuged again at 1,500×g for 5 min at 4°C. The supernatant was completely discarded using vacuum, taking care not to disrupt the EV pellet which was resuspended in 500 µL of sterile 1× PBS, avoiding the formation bubbles. The EV pellets from 80 mL of conditioned media were pooled according to their group and brought to a final volume of 3 mL. Subsequently, the samples were transferred to ultracentrifuge tubes (3.5 mL, Open-Top Thickwall Polycarbonate Tube, 13 × 51 mm) and ultra-centrifuged at 118,000×g (53,000 RPM, k-factor 54) for 1 h and 30 min at 4°C in a Beckman Coulter Optima MAX Ultracentrifuge using a TLA-100.3 fixed angle rotor to wash and re-pellet the EVs (Martínez-Greene et al., 2021). The pellets were finally resuspended in 30 µL of sterile PBS and stored at −80°C for further analysis. The amount of EV protein was measured by the BCA micro-assay (Thermo Scientific #23225) following the manufacturer’s instructions. Briefly, we prepared a BCA working reagent solution by mixing 50 parts of BCA Reagent A with 1 part of BCA Reagent B. The BSA standard curve was prepared by adding 20 μL of working reagent solution and 1 μL of the standard and mixing by gentle vortexing. After incubation at 37°C for 30 min, the samples were cooled to room temperature. The absorbance at 562 nm was measured in a NanoDrop Spectrophotometer and the protein concentration of each sample was extrapolated from the BSA standard curve.
Characterization of EVs
The morphology of the EVs was determined by transmission electron microscopy (JEOL JEM-1400 120 kV Transmission Electron Microscope). Briefly, the EV samples were thawed and kept at 4°C. 10 µL of each sample were deposited on a grid (G200-Cu) previously coated with Formvar and charcoal. The excess liquid was removed and 10 µL of 2% uranyl acetate was added and removed immediately, followed by a second addition lasting for 30 s, and a last incubation for 2 min. The surface of the grid was allowed to dry, mounted on the Jeol Electron Microscope Slide and viewed under 80 kV. An average of 4 to 6 images was taken of each sample at two different magnifications, 20 and 100 kV.
The size and concentration of EVs were determined through a nanoparticle tracking analysis (NTA) in a Nanosight NS300 (Malvern Instruments). Briefly, the primary cell cultures were expanded and maintained until 80% confluency in 1.5 mL in two 6-well culture plates. Then, the media was replaced with DMEM supplemented with 10% exosome depleted FBS for 48 h, as described above. One 6-well plate was incubated with 100 µM NMDA per well for 4 h, and the second was used as a control. The conditioned media from 2 wells was pooled, resulting in three samples per group, cleared of cells and debris as previously described, and injected (500 µL) into the Nanosight. A total of three 40-s-long videos were captured (camera setting:10 and detection threshold:8) for each sample. The concentration values and mean diameters were exported to Prism V8 (GraphPad Software) for statistical analysis and plotting.
Extracellular Vesicle Labeling and Uptake Analysis
The isolated EVs were labeled with the green fluorescent cell membrane linker dye PKH67 (Sigma-Aldrich #MKCH2226) following the manufacturer's instructions. Briefly, 20 µg of EVs were mixed with 6 μM cell-labeling solution and incubated for 5 min at room temperature. The unbound dye was removed by using Exosome Spin Columns MW3000 (Invitrogen #4484449). The labeled EVs were added into Müller cells cultured on coverslips, at a density of 20,000 cells, and incubated for 2 and 6 h. The cells were rinsed twice with 1× PBS, fixed in 4% paraformaldehyde, and stained with phalloidin and 4,6-diamidino-2-phenylindole (DAPI). Afterwards, the coverslips were mounted on slides, using ProLong Diamond Antifade Mountant (Invitrogen # P36970). The samples were allowed to dry at room temperature for 3 h and images were obtained using a ZEISS Axiovert 4° C/40 CLF inverted Fluorescent Microscope (Carl Zeiss, AXIO VISION Rel. 4.8 software) and an LSM 800 confocal system coupled to an inverted Axiovent AX10 microscope (Carl Zeiss, ZEN blue edition software) to determine the uptake of labeled EVs. The PKH67 dye with no EV contact was used as a negative control. The data shown are representative of at least three independent experiments, where at least eight non-overlapping fields were evaluated per group.
The detected immunoreactivity was assigned different colors. DAPI, (excitation wavelength 353 nm, emission wavelength 465 nm, detection wavelength 400–490 nm), AF488 (excitation wavelength 493 nm, emission wavelength 517 nm, detection wavelength 500–550 nm), and Rhodamine (excitation wavelength 558 nm, emission wavelength 575 nm, detection wavelength 565–700 nm). Images were captured in sequential acquisition to have separated images for each color. A pinhole of 1 Airy unit and a scan of four frame averaging was used.
Image analysis was performed using the open-source software ImageJ. The quantification of fluorescence was analyzed by the RGB method. Briefly, for each image, the channels were separated (Red-Green-Blue), the background was subtracted if necessary and a binary mask was created for the AF488 (green) and Rhodamine (red) channels and defined the regions of interest (ROIs). A Gaussian filter was applied to remove noise. Then, an automatic threshold was adjusted, the particles were detected (in the case of EVs) and the fluorescence intensity was measured. Quantitative fluorescence data were exported in tables from ImageJ to Microsoft Excel software for further analysis. The graphs were created using Prism V8 (GraphPad Software).
Immunocytochemistry
The harvested cells were seeded on glass coverslips in DMEM-FBS 10% and penicillin/streptomycin 1% at a density of 6,000 cells per coverslip and incubated for 48 h at 37°C and 5% CO2. After rinsing in 1× PBS, the cells were fixed in 4% paraformaldehyde (PFA) and cell auto-fluorescence was quenched with 50 mM NH4Cl2. The cells were permeabilized with 0.3% PBS-Triton and incubated in blocking solution for 2 h at room temperature (5% Normal Goat Serum (NGS), 3% Bovine Serum Albumin (BSA), and 0.3% Triton). The samples were incubated with Rabbit polyclonal anti-GS antibody (Abcam Cat# ab73593, RRID:AB_2247588) (1:500), mouse anti-CRALBP (Abcam Cat# ab15051, RRID:AB_2269474) (1:400); mouse monoclonal anti-GFAP (2E1) (Santa Cruz Biotechnology Cat# sc-33673, RRID:AB_627673), mouse monoclonal anti-CD63 antibody [NKI/C3] (Abcam Cat# ab213090, RRID:AB_2920802) (1:250), and mouse monoclonal anti-Alix antibody [3A9] (Abcam Cat# ab117600, RRID:AB_10899268) (1:250). All these antibodies were acquired from Abcam (Cambridge, UK) unless specified. The secondary goat anti-mouse IgG (Alexa 488) and goat anti-rabbit IgG (Alexa 546) antibodies were used at a 1:1000 dilution (Invitrogen), incubated for 2 h at room temperature, and the nuclei stained with DAPI (Sigma-Aldrich #D9542) at a dilution of 1:500. Finally, coverslips were mounted in microscope slides, using ProLong™ Diamond Antifade Mountant mounting medium (Invitrogen # P36970), dried at room temperature for 2 h and stored at 4°C. Fluorescent images were obtained using a ZEISS Axiovert 4°C/40 CLF inverted Fluorescent Microscope (Carl Zeiss, AXIO VISION Rel. 4.8 software) and an LSM 800 confocal system coupled to an inverted Axiovent AX10 microscope (Carl Zeiss, ZEN blue edition software) to determine the uptake of labeled EVs. Non-stained samples were used as negative control. The shown data is representative of at least three independent experiments, where at least five non-overlapping fields were evaluated per group to a total of at least 50 cells per condition.
Migration Assay
Cell migration was evaluated through a scratch-wound healing assay (Vera et al., 2021), with some modifications. In brief, Müller cells were seeded in 24-well plates until they reached a 90% confluence and serum starved for 12 h to prevent cell proliferation. A scratch “wound” was made on the cell monolayer with a 200 µL sterile-pipette tip. “Wound healing” capacity was evaluated at 0, 12, 24, and 48 h after the addition of culture media containing NMDA, control EVs, NMDA EVs, or EGF (100 ng/mL). EGF was used as a positive control since it can induce migration in this cell type (Pena et al., 2018). The images from these assays were acquired utilizing an inverted optical microscope (Leica, Wetzlar, Germany) with a 10× objective, using an EC3 camera coupled to the microscope and the Leica Application Suite (LAS) software version 3.2.0. Finally, acquired images were analyzed with the free ImageJ (RRID:SCR_003070) software (NIH).
Quantitative PCR
Total RNA was purified from Müller cells in two sets of experiments. In one set, the cells were left untreated or supplemented with NMDA at 50, 100, or 200 µM and incubated for 4 hours at 37°C and 5% CO2. In the second set, the cells were treated with either control or NMDA EVs. RNA was extracted using TRIzol reagent (Sigma-Aldrich) according to the manufacturer's instructions. The quality and quantity of isolated RNA were determined by spectrophotometric analysis (NanoDrop 2000, Thermo Scientific). The cDNA was synthesized using 200 ng of RNA and the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher), following the manufacturer's instructions. cDNA samples were stored at −70°C until further analysis. To assess the expression of some multipotency-associated genes, we employed SYBR green-based quantitative PCR (qPCR) that was performed on a CFX96 Real-Time PCR Detection System (BioRad). The qPCR reaction included 1 µL of cDNA and 0.5 µM of specific primers (Nestin, Ascl1, and Lin28) (Table 1). Thermal cycler conditions were 95°C for 10 min and 40 cycles of 15 s at 95°C followed by 60 s at 60°C in the case of Nestin, Ascl1, and Lin28 or followed by 60 s at 63°C in the case of Gapdh. All experiments were performed in triplicate, including the appropriate negative controls for each. The expression level of the analyzed genes was normalized against a housekeeper gene (Gapdh.) determining any fold-change through the 2–ΔΔCt method (Livak & Schmittgen, 2001). All data were expressed as the mean ± SEM. The significance of differences between means or ranks was assessed using one-way ANOVA or Kruskal–Wallis test, followed by a Tukey's or Dunn's test for post-hoc comparisons where necessary.
Table 1.
List of qPCR Primers.
| Gene | Sequence | |
|---|---|---|
| Gapdh. | Forward | ACTGGCATGGCCTTCCGTGTTCCTA |
| Reverse | TCAGTGTAGCCCAAGATGCCCTTC | |
| Nestin | Forward | AGGAGAAGCAGGGTCTACAGAG |
| Reverse | AGTTCTCAGCCTCCAGCAGAGT | |
| Ascl1 | Forward | GCAACCGGGTCAAGTTGGT |
| Reverse | GTCGTTGGAGTAGTTGGGGG | |
| Lin28 | Forward | GGTCTGGAATCCATCCGTGTCA |
| Reverse | TCCTTGGCATGATGGTCTAGCC | |
Statistical Analysis
All data were analyzed by using GraphPad Prism 8.0.1 software. Statistical analyses were performed by Shapiro–Wilk test for normality. Experimental groups were compared with non-treated controls using an unpaired Student´s t-test. The data are shown as the means and error bars corresponding to the standard error of the mean (SEM), or in violin plots as described in each figure caption. For each analysis, a value of p < 0.05 was considered significant.
Results
Expression of EVs Markers by Cultured Rodent Müller Cells
A first set of experiments included Müller-enriched mouse primary cultures to evaluate the secretion and functional outcome of Müller-derived EVs. We characterized these cultures and their response to NMDA exposure by evaluating the expression of Müller cell markers by immunofluorescence and induction of pluripotency gene expression by RT-qPCR. We found that more than 96% of the cells express the Müller cells markers GS and CRALBP (Figure 1(A)). Treatment with 100 μM NMDA for 6 h does not affect the number of cells that express GS and slightly reduces CRALBP immunofluorescence intensity as previously described to occur with other stimuli such as oxidative stress in cell culture (Abrahan et al., 2009) and in a retinal explant culture in a mouse regeneration assay (Schäfer & Karl, 2017). Also in agreement with previous reports, we observed that a small percentage of cultured Müller cells are immunoreactive to glial fibrillary acidic protein (GFAP) (Pereiro et al., 2020) (Figure 1(B)). NMDA treatment induces an increase in the number and complexity of immunoreactive cells. In addition, exposure of cultured Müller cells to 100 μM NMDA for 4 h induced the expression of the pluripotency genes Ascl1, Lin28, and Nestin (Figure 1(C)).
Figure 1.
(A) Representative images of the immunodetection of the Müller cells markers Glutamine Synthetase (GS) (purple) and CRALBP (green) in mouse primary Müller control cell cultures (A) or after treatment with 100 μM NMDA for 4 h. The actin cytoskeleton was labeled with rhodamine phalloidin (red) and cell nuclei counterstained with 4,6-diamino-2-phenylindole (DAPI) in blue. (B) Representative images of the immunodetection of GFAP CRALBP) in mouse primary Müller control cell cultures or after treatment with 100 μM NMDA for 4 h. The actin cytoskeleton was labeled with rhodamine phalloidin (red) and cell nuclei counterstained with 4,6-diamino-2-phenylindole (DAPI) in blue. (C) NMDA-treated Müller cells increase multipotency gene expression in culture. Cells were either left untreated or exposed to 50, 100 or 200 μM for four hours. The graphs show the relative expression values of Nestin, Ascl1 and lin28 calculated based on the 2−ΔΔCt method, from the Ct data obtained by real-time PCR. Means ± SEM of three different experiments performed in three independent biological replicates is shown (unpaired t-test, *p < 0.05).
As the expression of the canonical EV markers Alix and CD63 in photoreceptors and retinal-derived EVs has been recently described (Grillo et al., 2021; Mighty et al., 2020), we tested whether Alix and CD63 were also expressed in Müller-enriched primary cultures finding that, indeed, Alix and CD63 immunostaining was localized to the perinuclear region and the cytoplasm of these cells (Figure 2(A)-(F)). To determine possible changes, in either distribution or immunoreactivity after treatment with NMDA, the cells were exposed to 100 μM NMDA for 4 h. It was revealed that the cellular localization of these markers was unmodified by this treatment (Figure 2(G)-(L)) and its fluorescent signal intensity showed no difference between NMDA-treated and control cells.
Figure 2.
Representative immunostaining of control cultures stained with Alix (A-C) or CD63 (D-F), and NMDA treated Müller cells stained with Alix (G-I) or CD63 (J-L). The third panel of each row shows higher magnification images of individual cells where immunoreactivity can be observed in detail. Cell nuclei were labeled with 4,6-diamino-2-phenylindole (DAPI) in blue. Scale bar 20 µm. Data was acquired from at least three independent cultures and at least five non-overlapping fields were evaluated per condition.
Isolation and Characterization of Müller Glia-Secreted Extracellular Vesicles
Following the guidelines from the “Minimal Information for Studies of Extracellular Vesicles” (MISEV2018) (Théry et al., 2018), we isolated Müller glia-secreted extracellular vesicles from conditioned media by ultracentrifugation and processed them by scanning electron microscopy (EM) and NTA. The EM analysis showed that cultured Müller cells have an approximate extension of 50 μm and bear cytoplasmic protrusions (Figure 3(A)). Wide field and close up images of the EV fraction obtained from Müller cell culture supernatant after ultracentrifugation identify the presence of 100–150 nm particles, which is consistent with a small EV/exosomes (Figure 3(B)-(D)). Identical morphological characteristics were also observed in the EVs population from NMDA treated cell (Figure 3(E)). The Western blot analysis demonstrated the presence of EVs markers HSP70, TSG101, and CD63 in the evaluated samples (Figure 3(F)). Nanoparticle tracking analysis revealed that the concentration of particles isolated from control Müller cell cultures was 4.37 × 108 ± 2.47 × 107/mL, which was similar to that from NMDA treated cells (unpaired t-test, p = 0.1311) (Figure 3(G)). However, NMDA treatment induced a significant decrement in the modal size of the detected particles, which diminished from 194.1 nm in control cells to 106.8 nm in treated cultures (unpaired t-test, p < 0.0001) (Figure 3(H)–(J)).
Figure 3.
Em micrographs of (A) Müller cell in primary culture, (B-D) extracellular vesicles isolated from control Müller cell cultures supernatants and (E) extracellular vesicles isolated from supernatants from Müller cell cultures that had been treated for 4 h with 100 μm NMDA. (F) Western Blot analysis of extracellular vesicles isolated from control (C-EVs) and NMDA treated cells (N-EVs). 20 μg of protein were loaded per lane. Positive control were EVs obtained from MDA-MB-468 adenocarcinoma line. (G,H) Number of particles and modal size of extracellular vesicles isolated from control or NMDA-treated Müller cells determined by NTA. (-) Negative control: DMEM. (I,J) Graphical representation of the Nanosight analysis of particle size distribution of the EVs isolated from control (I) and NMDA-treated (J) Müller cells. The red shadow is the standard deviation and the black line is the mean of 3 separate analyses.
Müller Glia-Derived EV Uptake by Müller Primary Cultures
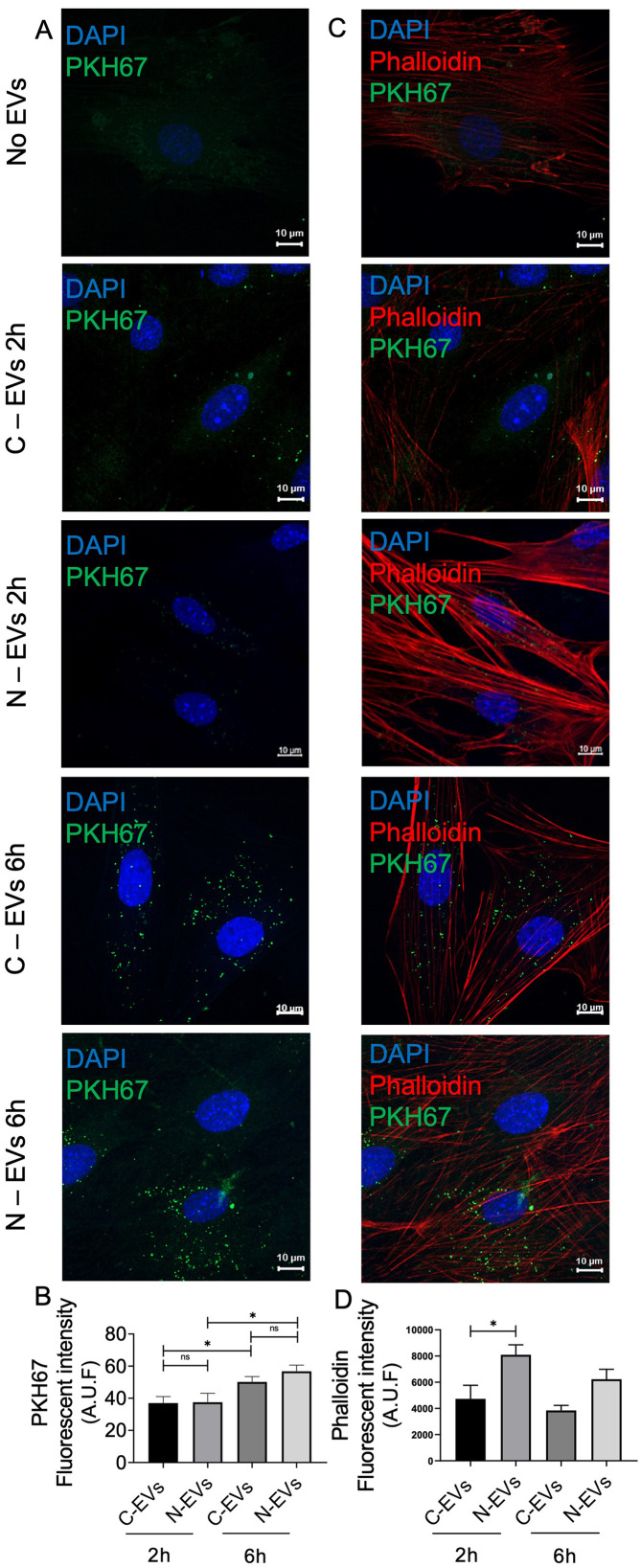
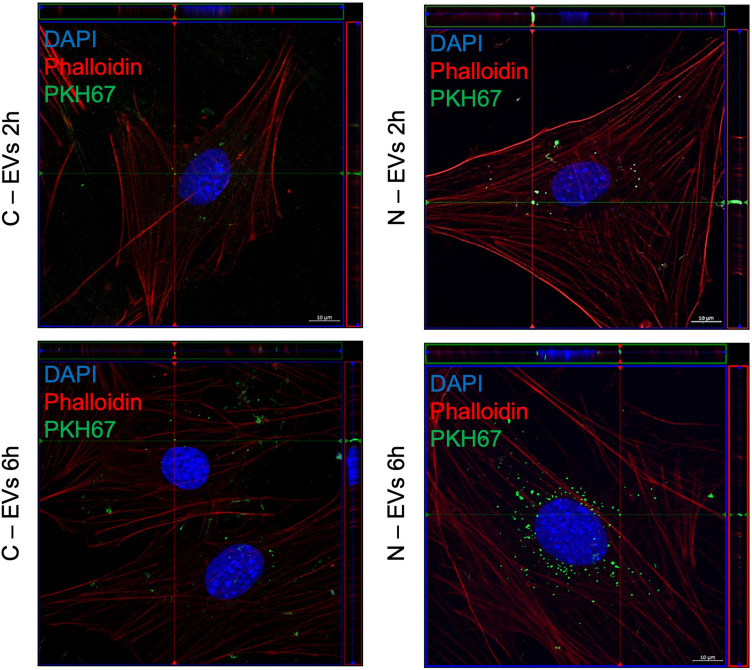
Cultured Müller cells were exposed to 30 μg of PKH67 labeled EVs obtained from control Müller cells (C-EVs) or from 100 μm NMDA (N-EVs), using unexposed cells as an untreated control. The cells were incubated with these EVs for 2 and 6 h and stained with phalloidin-Rhodamine to visualize the actin cytoskeleton, whereas that EVs uptake was evaluated by confocal microscopy (Figure 4(A)–(D)). Two main observations arose from these experiments. First, PKH67 fluorescence inside the cells gradually increased in a time-dependent manner (from 37.03 ± 2.673 arbitrary fluorescence units at 2 h to 56.76 ± 2.580 arbitrary fluorescence units at 6 h, unpaired t-test, p = 0.0001) (Figure 4(B)), regardless of EVs origin. Second, we observed that the exposure to NMDA EVs resulted in a transiently increased actin polymerization (71% in arbitrary fluorescence units) (Figure 4(C) and (D)) (p < 0.05; n = 3), which was pronounced 2 h after exposure and decreased to almost basal level after 6 h. This effect was specific to N-EVs. In contrast, C-EVs did not induce a significant increase in fluorescence intensity at this time point. The orthogonal projection analysis of confocal z-stacks confirmed that EVs were being efficiently internalized by the cells (Figure 5).
Figure 4.
Müller derived-EVs uptake in Müller cells culture. (A, B) Representative images of Müller cell cultures treated for 2 and 6 h with no EVs or with 30 μg/mL of control (C-EVs) or NMDA EVs (N-EVs), that were pre-stained with the fluorescent green dye PHK67. C-EVs: EVs derived from control Müller cells, N-EVs: EVs derived from NMDA-treated Müller cells. Cell nuclei were labeled with 4,6-diamino-2-phenylindole (DAPI) in blue. Objective 63X, scale bars, 10 µm. (B) The graph shows the fluorescence intensity of the green-labeled EVs inside muller cells that was quantified from eight images of four independent experiments using ImageJ software. The values on the graph represents the mean fluorescence of all the images per condition. Statistical significance was determined by an unpaired Student´s t-test. *p < 0.05. (C) Representative images of Müller cells in co-culture with control or NMDA EVs for 2 and 6 h. An increase in the intensity of rhodamine-phalloidin (red) in control Müller cells is evident after the exposure to NMDA EVs for 2 h. Objective 63X, scale bars, 10 µm. (D) The graph shows the fluorescence intensity of the red-labeled rhodamine-phalloidin in muller cells. It was quantified from five images of four independent experiments (at least 50 cells per condition) by ImageJ software. The values on the graph represents the mean fluorescence of all the images per condition. Statistical significance was determined by an unpaired Student´s t-test. *p < 0.05.
Figure 5.
Representative orthogonal projections of confocal z-stacks showing the localization of pre-stained green control (C-EVs) or NMDA EVs (N-EVs), inside the Müller cells after 2 h and 6 h exposure. Pre-stained EVs (green), rhodamine-phalloidin (red), and nuclei labeled with DAPI (blue). Objective 63X, scale bars, 10 µm. The data are representative of experiments performed in at least three independent biological replicates.
Müller to Müller EV-Mediated Signaling Enhances Cell Migration
To determine if these EVs could also impact the capacity of Müller cells to migrate, in addition to the induction of cytoskeleton remodeling, we performed an in vitro wound healing assay (Figure 6(A)). In brief, a scratch wound was created in a monolayer of Müller cells that were exposed to 100 ng/mL EGF as a positive control (Pena et al., 2018), 100 μM NMDA, 30 μg/mL C-EVs, 30 μg/mL N-EVs, or completely untreated (control), evaluating cell migration into the scratch wound at 12, 24 and 48 h. Our results show that EGF-treated Müller cells efficiently migrated into the scratch wound after 24 h. This effect was also observed when the cells were exposed to NMDA, C-EVs or N-EVs. It must be mentioned that the culture medium of the samples exposed to EVs tended to lose clarity, an effect that could be attributed to the presence of protein aggregates resulting from EV isolation. Regardless, a noticeably effect could still be observed concerning the cellular morphology since the cells exposed to these EVs acquired an elongated branched morphology that was not evident in either NMDA or EGF treated cells (Figure 6(B)). Therefore, the effect of EVs exposure on cell viability was tested through flow cytometry. However, the obtained results showed that the exposure of cultured Müller cells to C-EVs or N-EVs had no significant effect on this parameter, as all the samples presented over 93% of viability irrespective of treatment (Figure 7).
Figure 6.
(A) Müller cell-derived EVs enhance migration in cultured recipient Müller cells. Representative images of a wound healing assay performed on Müller cells that were cultured with 100 ng/mL of epidermal growth factor (EGF) as a positive control, without any treatment as a negative control, 100 µM NMDA, 30 μg/mL control EVs and 30 μg/mL NMDA EVs. Images were taken at 0, 12, 24 and 48 h after the scratch. Data was acquired from at least three independent cultures. The dotted lines indicate the border of the wound. (B) Müller cell derived EVs induce morphological changes on treated cultures. Magnification of photographs from Müller cells that were cultured for 48 h with no treatment (control), 100 µM NMDA, 30 μg/mL control EVs and 30 μg/mL NMDA EVs at 48 h.
Figure 7.
Effect of EV exposure on Müller cell viability. The graph represents the mean ± error standard of cell counts of recipient primary Müller cell cultures measured after no exposure (-) or exposure to 10 μg/mlL or 30 μg/mL EVs obtained from control cells (C-EVs) or cells that had been exposed to 100 μM NMDA (N-EVs), for 4 h. The experiment was performed in triplicates from three independent cultures. No significant statistical differences were found.
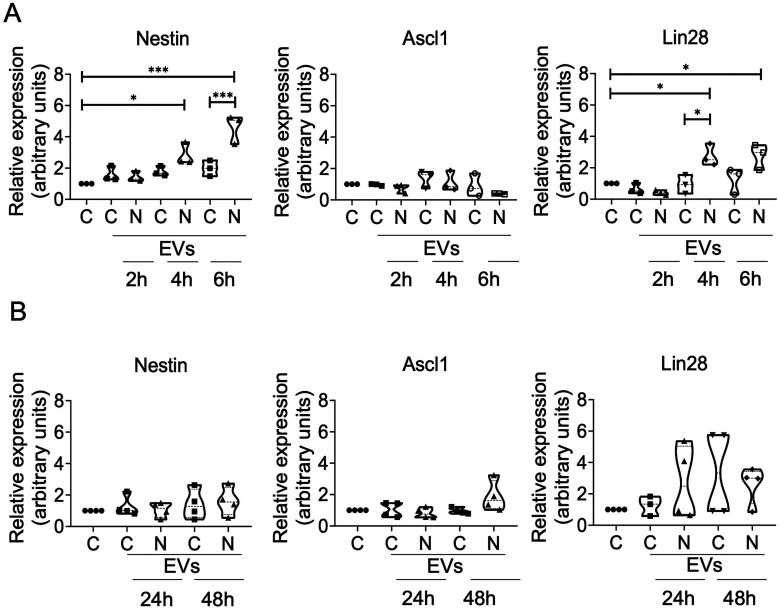
Müller to Müller EV-Mediated Signaling Induces Early Expression of Multipotency Genes Nestin and Lin28 but not Ascl-1 in Response to NMDA Exposure
The expression of the multipotency-associated genes Nestin, Lin28 and Ascl-1, was evaluated through RT-qPCR at different time points. The analysis showed that a single exposure event of 30 μg/mL EVs, obtained from non-treated cells or NMDA treated cells induced a significant increase of the nestin and lin28 genes, but only in samples that have been exposed for 4 and 6 h to N-EVs (Figure 8(A)). No effect was observed when the cells exposed to C-EVs. On the other hand, the expression of the Ascl1 gene was not induced in any of the analyzed samples. The analysis showed also that EV exposure had no significant effect on the expression of the selected genes after 24 and 48 h, time during which migration and morphology alterations of Müller cells were evident (Figure 8(B)).
Figure 8.
NMDA-treated Müller cell-derived EVs increase multipotency gene expression in cultured recipient Müller cells. Cells were exposed to EVs at a concentration of 30 µg/mL for 2, 4 and 6 h (A) or 24 and 48 h (B). Violin Graph showing the relative expression values of Nestin, Ascl1 and lin28 calculated based on the 2−ΔΔCt method, from the Ct data obtained by real-time PCR. Means ± SEM of three different experiments performed in three independent biological replicates is shown (one-way ANOVA multiple comparisons, *p < 0.05, ***p < 0.001).
Discussion
Extracellular vesicles (EVs) constitute a novel form of intercellular communication as they are secreted and captured by nearly all types of cells. Predictably, Müller glial cells are no exception and the ability to respond to embryonic stem cell-derived EVs (ESEVs) or C6 glioma cell-derived small EVs has been reported in human, mouse, and zebrafish Müller cells (Didiano et al., 2020; Katsman et al., 2012; Ke et al., 2021; Peng et al., 2018). On the whole, these previous studies coincide in that the exposure of Müller cells to EVs can induce the upregulation of genes and miRNAs involved in their dedifferentiation, proliferation and acquisition of neural-differentiation regenerative capacities. In addition, other reports have shown that cultured Müller glia cells secrete miRNA-loaded EVs into the supernatant (Eastlake et al., 2021) and, very recently, a groundbreaking study by Grosche’s group provided evidence for the release of EVs from Müller cells in adult mice (Demais et al., 2022). However, the physiological role of these Müller-derived vesicles remains elusive. In the present study, we evaluated the role of EVs in the transduction, Müller glia to Müller glia, of a high NMDA concentration that may be deleterious for retinal physiology. Our results suggest that the existence of different EVs subpopulations depends upon the presence or absence of a damage signal (NMDA), and thus promotes a differential response by the target cells.
As expected, the cultured Müller cells expressed the endosomal protein Alix and the tetraspanin CD63, both of which were immunolocalized to the perinuclear area. This is consistent with previous reports of Alix and CD63 subcellular localization in other cell types (Baba et al., 2020; Bongiovanni et al., 2012; Men et al., 2019). The treatment of Müller cells with NMDA did not affect CD63 or Alix distribution. It is known that an increased EVs biogenesis and secretion can be achieved through several physio/pathological stimuli that include, for example, hypoxia (Zhu et al., 2018), alcohol exposure (Bala et al., 2022) or aging (D'Anca et al., 2019). Specifically retinal hyperglycemia or stress can induce EV secretion from RPE cells, astrocytes, and photoreceptors, resulting in the production of functionally diverse EVs linked to the pathogenesis of diabetic retinopathy and AMD (age-related macular degeneration) (Maisto et al., 2020; Shah et al., 2018; Zhu et al., 2020). We speculated that exposure to a high concentration of NMDA could increase the number of secreted EVs by Müller cells in a similar manner. However, our results showed that NMDA does not affect the number of EVs; instead, it significantly decreased the size of the secreted particles. The physiological significance of this observation is currently not completely understood. Seminal works in this field have demonstrated how cells release subpopulations of EVs and exosomes that display different composition, size and/or functions (Kowal et al., 2016; Willms et al., 2016). One possible explanation to these observations may involve modifications to the cargo loaded into EVs, as it has been described that adaptive responses by other cells may drive cargo alterations and changes in biophysical characteristic such as size (Phillips et al., 2021).
This study was not without some limitations, one of which is that we did not attempt to further purify the preparation of Müller-derived EVs by successive centrifugations and parallel immunoprecipitations as previously suggested (Kowal et al., 2016). Therefore, we cannot exclude the possibility that we co-isolated mixed EV populations whose subcellular origin could be rather heterogeneous. Similarly, when we demonstrated that Müller cells are able to up-take Müller-derived EVs in vitro, we cannot discard the preferential capture of a specific EVs subpopulation by the receptor cells.
The prior statements notwithstanding, our functional analysis revealed a differential response by the exposed Müller cells depending on the provenance of the EVs. While no differential effects were observed in relation to cell viability or migration capacity, only EVs obtained from NMDA treated Müller cultures were able to increase actin polymerization rate at early time points and to trigger the induction of the expression of, otherwise silent, multipotency genes in cultured Müller cells. Together, both activities have been associated to the induction of the cellular dedifferentiation process. On the one hand, rearrangement of the actin cytoskeletal architecture and changes in cellular morphology have been associated with the dedifferentiation processes of different cell types (e.g., chondrocytes and smooth muscle cells) (Lauer et al., 2021; Qi et al., 2020) but also Müller cells in culture and in vivo (Lahne et al., 2015; Reyes-Aguirre et al., 2013). In fact, remodeling of the cytoskeleton has been even considered a key rate limiting step in reprogramming to pluripotency in IPSCs (Inducible Pluripotent Stem Cells) (Boraas et al., 2016).
More definite was the observation that only N-EVs could trigger the fast and transient induction of the expression of multipotency genes in cultured Müller cells. The observed induction kinetics of nestin and lin28 is consistent with previous descriptions made in rodents, both in vivo and in cultured Müller cells, i.e., early and transient gene upregulation after NMDA exposure (Reyes-Aguirre & Lamas, 2016). In addition, we do not observe induction of Ascl1 expression at early time points. This is notable because of the mounting evidence impelling the notion that Ascl1 is an essential factor in the reprogramming capacity of rodent Müller cells, suggesting its overexpression could substantially improve the neurogenic potential of Müller glia (Todd et al., 2021). Interestingly, Ascl1 tends to become upregulated after 48 h of exposure, although without reaching statistical significance. Further studies are thus necessary to determine the physiological meaning of this preliminary observation. Our results extend the notion that mammalian Müller cells have indeed the capacity to initiate a regenerative response, therefore introducing a novel mechanism that involves Müller glia to Müller glia EV-dependent signaling.
Past, paradigm-shifting works (Pearson et al., 2016; Santos-Ferreira et al., 2016) demonstrated that the transplantation of retinal photoreceptor results in the exchange of donor-to-host cytoplasmic biomaterial rather than in migration and integration of donor cells. However, this transfer does not seem to be mediated by photoreceptor-secreted EV but preferably by, recently described, nanotube-like structures (Kalargyrou et al., 2021; Ortin-Martinez et al., 2021). Regardless, it was demonstrated that cultured photoreceptors can also release EVs that are specifically taken up by Müller glial cells (Kalargyrou et al., 2021). This evidence, along with the confirmation that Müller glia are essential for efficient photoreceptor neuritogenesis (Tsai et al., 2019) has added a new perspective on the role that Müller glia may play in the injury process. In this regard, the observations made in this study drive us to expand the already complex role of Müller glia in response to damage.
In view of these revelations, we would like to propose that, in the context of a lesion paradigm, Müller-derived EVs may signal adjacent retinal cells to spread molecular changes that may result in the formation of the characteristic mammalian glial retinal scar or the triggering of a neurogenic process in regenerative species. The “required-to-be-tested” hypothesis behind this speculation includes the existence of an “injury-specific” differential EV cargo and the demonstration of a functional Muller-to-Müller effect of Müller-derived EVs in vivo. In this sense, a damage-specific cargo and functional effect of Müller-derived EVs on endothelial cells have been recently reported (Liu et al., 2021). Interestingly, specific effects on endothelial cell proliferation and migration were observed also in this system. Many other relevant questions arise: could Müller-derived EVs add up to the long list of EVs that are currently being considered as potential therapeutic tools (Mead & Tomarev, 2020)? Could it be possible to decipher the molecular mechanisms that impair the retinal regenerative capacity in mammals from the EV cargo alone? And, if Müller-derived EVs carry the instructions for glial scaring, could it be possible to counteract their effect? These and other questions could only benefit from further research seeking to shed light on this subject.
Acknowledgments
The authors would like to thank Cristina de los Santos, Marta I. Barragán-Bonilla, and Itzel G. Moreno for their involvement in this work; and Rene Garduño and Lourdes Rojas (Cinvestav) for assistance with confocal microscopy and TEM.
Footnotes
Author Contribution: A. K. C., E. J. M-C., D. S-V., R. Y. V-C. acquired, analyzed, and collaborated in the interpretation of data; I. L., provided critical technical assistance; E. M-M and M. L. designed the project, made substantial contributions to interpretation of data, drafted, and revised the article. All the authors approved the final version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Consejo Nacional de Ciencia y Tecnología (grant A1-S-25777) to M.L. and (grant 64382) to E. M; and PhD fellowships from Consejo Nacional de Ciencia y Tecnología to AK.C, EJ.M-C and D.S-V.
ORCID iDs: Eduardo Martinez-Martinez https://orcid.org/0000-0002-9020-559X
Monica Lamas https://orcid.org/0000-0002-0297-4218
References
- Abrahan C. E., Insua M. F., Politi L. E., German O. L., Rotstein N. P. (2009). Oxidative stress promotes proliferation and dedifferentiation of retina glial cells in vitro. Journal of Neuroscience Research, 87(4), 964–977. 10.1002/jnr.21903 [DOI] [PubMed] [Google Scholar]
- Baba K., Kuwada S., Nakao A., Li X., Okuda N., Nishida A., Mitsuda S., Fukuoka N., Kakeya H., Kataoka T. (2020). Different localization of lysosomal-associated membrane protein 1 (LAMP1) in mammalian cultured cell lines. Histochemistry and Cell Biology, 153(4), 199–213. 10.1007/s00418-019-01842-z [DOI] [PubMed] [Google Scholar]
- Bala S., Babuta M., Catalano D., Saiju A., Szabo G. (2022). Alcohol promotes exosome biogenesis and release via modulating rabs and miR-192 expression in human hepatocytes. Frontiers in Cell and Developmental Biology, 9, 787356. 10.3389/fcell.2021.787356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S., Eastlake K., Jayaram H., Jones M. F., Brown R. A., McLellan G. J., Charteris D. G., Khaw P. T., Limb G. A. (2016). Allogeneic transplantation of muller-derived retinal ganglion cells improves retinal function in a feline model of ganglion cell depletion. Stem Cells Translational Medicine, 5(2), 192–205. 10.5966/sctm.2015-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccuni I., Fairless R. (2022). Retinal glutamate neurotransmission: From physiology to pathophysiological mechanisms of retinal ganglion cell degeneration. Life (Chicago, Ill), 12(5), 638. 10.3390/life12050638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiovanni A., Romancino D. P., Campos Y., Paterniti G., Qiu X., Moshiach S., Di Felice V., Vergani N., Ustek D., d'Azzo A. (2012). Alix protein is substrate of Ozz-E3 ligase and modulates actin remodeling in skeletal muscle. Journal of Biological Chemistry, 287(15), 12159–12171. 10.1074/jbc.M111.297036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraas L. C., Guidry J. B., Pineda E. T., Ahsan T. (2016). Cytoskeletal expression and remodeling in pluripotent stem cells. PLoS One, 11(1), e0145084. 10.1371/journal.pone.0145084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann A., Pannicke T., Grosche J., Francke M., Wiedemann P., Skatchkov S. N., Osborne N. N., Reichenbach A. (2006). Müller cells in the healthy and diseased retina. Progress in Retinal and Eye Research, 25(4), 397–424. 10.1016/j.preteyeres.2006.05.003 [DOI] [PubMed] [Google Scholar]
- Bringmann A., Wiedemann P. (2012). Müller glial cells in retinal disease. Ophthalmologica, 227, 1–19. 10.1159/000328979 [DOI] [PubMed] [Google Scholar]
- D'Anca M., Fenoglio C., Serpente M., Arosio B., Cesari M., Scarpini E. A., Galimberti D. (2019). Exosome determinants of physiological aging and age-related neurodegenerative diseases. Frontiers in Aging Neuroscience, 11, 232. 10.3389/fnagi.2019.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. V., Mallya K. B., Zhao X., Ahmad F., Bhattacharya S., Thoreson W. B., Hegde G. V., Ahmad I. (2006). Neural stem cell properties of Müller glia in the mammalian retina: Regulation by Notch and Wnt signaling. Developmental Biology, 299(1), 283–302. 10.1016/j.ydbio.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Demais V., Pohl A., Wunderlich K. A., Pfaller A. M., Kaplan L., Barthélémy A., Dittrich R., Puig B., Giebel B., Hauck S. M., Pfrieger F. W., Grosche A. (2022). Release of VAMP5-positive extracellular vesicles by retinal Müller glia in vivo. Journal of Extracellular Vesicles, 11(9), e12254. 10.1002/jev2.12254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didiano D., Abner J. J., Hinger S. A., Flickinger Z., Kent M., Clement M. A., Balaiya S., Liu Q., Dai X., Levine E. M., Patton J. G. (2020). Induction of a proliferative response in the zebrafish retina by injection of extracellular vesicles. Experimental Eye Research, 200, 108254. 10.1016/j.exer.2020.108254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastlake K., Lamb W. D. B., Luis J., Khaw P. T., Jayaram H., Limb G. A. (2021). Prospects for the application of Müller glia and their derivatives in retinal regenerative therapies. Progress in Retinal and Eye Research, 85, 100970. 10.1016/j.preteyeres.2021.100970 [DOI] [PubMed] [Google Scholar]
- Eastlake K., Wang W., Jayaram H., Murray-Dunning C., Carr A. J. F., Ramsden C. M., Vugler A., Gore K., Clemo N., Stewart M., Coffey P., Khaw P. T., Limb G. A. (2019). Phenotypic and functional characterization of muller glia isolated from induced pluripotent stem cell-derived retinal organoids: improvement of retinal ganglion cell function upon transplantation. Stem Cells Translational Medicine, 8(8), 775–784. 10.1002/sctm.18-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber D. B., Katsman D. (2016). Embryonic stem cell-derived microvesicles: Could they be used for retinal regeneration? Advances in Medicine and Biology, 854, 563–569. 10.1007/978-3-319-17121-0_75 [DOI] [PubMed] [Google Scholar]
- Fischer A. J., Reh T. A. (2001). Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nature Neuroscience, 4, 247–252. 10.1038/85090 [DOI] [PubMed] [Google Scholar]
- Gao Y., Li H., Qin C., Yang B., Ke Y. (2022). Embryonic stem cells-derived exosomes enhance retrodifferentiation of retinal Müller cells by delivering BDNF protein to activate Wnt pathway. Immunobiology, 227(3), 152211. 10.1016/j.imbio.2022.152211 [DOI] [PubMed] [Google Scholar]
- Graca A. B., Hippert C., Pearson R. A. (2018). Müller Glia reactivity and development of gliosis in response to pathological conditions. Advances in Experimental Medicine and Biology, 1074, 303–308. 10.1007/978-3-319-75402-4_37 [DOI] [PubMed] [Google Scholar]
- Grillo S. L., Etzel J. D., Weber S. R., Ondeck C., Wang W., Zhao Y., Barber A. J., Sundstrom J. M. (2021). Descriptive analysis of Fibulin-3 and the extracellular vesicle marker, Alix, in drusen from a small cohort of postmortem human eyes. Experimental Eye Research, 203, 108422. 10.1016/j.exer.2020.108422 [DOI] [PubMed] [Google Scholar]
- Hoek R. M., Quinn P. M., Alves C. H., Hooibrink B., Wijnholds J. (2018). NTPDase2 as a surface marker to isolate flow cytometrically a Müller Glial cell enriched population from dissociated neural retinae. Journal of Neuroscience and Neurosurgery, 1(4), 117. 10.31021/jnn.20181117 [DOI] [Google Scholar]
- Kalargyrou A. A., Basche M., Hare A., West E. L., Smith A. J., Ali R. R., Pearson R. A. (2021). Nanotube-like processes facilitate material transfer between photoreceptors. EMBO reports, 22(11), e53732. 10.15252/embr.202153732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl M. O., Hayes S., Nelson B. R., Tan K., Buckingham B., Reh T. A. (2008). Stimulation of neural regeneration in the mouse retina. Proceedings of the National Academy of Sciences, 105, 19508–19513. 10.1073/pnas.0807453105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsman D., Stackpole E. J., Domin D. R., Farber D. B. (2012). Embryonic stem cell-derived microvesicles induce gene expression changes in Müller cells of the retina. PLoS One, 7(11), e50417. 10.1371/journal.pone.0050417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y., Fan X., Hao R., Dong L., Xue M., Tan L., Yang C., Li X., Ren X. (2021). Human embryonic stem cell-derived extracellular vesicles alleviate retinal degeneration by upregulating Oct4 to promote retinal Muller cell retrodifferentiation via HSP90. Stem Cell Research & Therapy, 12(1), 21. 10.1186/s13287-020-02034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J., Arras G., Colombo M., Jouve M., Morath J. P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., Théry C. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences of the United States of America, 113(8), E968–E977. 10.1073/pnas.1521230113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahne M., Li J., Marton R. M., Hyde D. R. (2015). Actin-Cytoskeleton- and rock-mediated INM are required for photoreceptor regeneration in the adult zebrafish retina. The Journal of Neuroscience, 35(47), 15612–15634. 10.1523/JNEUROSCI.5005-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer J. C., Selig M., Hart M. L., Kurz B., Rolauffs B. (2021). Articular chondrocyte phenotype regulation through the cytoskeleton and the signaling processes that originate from or converge on the cytoskeleton: Towards a novel understanding of the intersection between actin dynamics and chondrogenic function. International Journal of Molecular Sciences, 22(6), 3279. 10.3390/ijms22063279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Q., Fu H., Wang J., Yuan S., Li X., Xie P., Hu Z., Liu Q. (2021). Müller glia-derived exosomal miR-9-3p promotes angiogenesis by restricting sphingosine-1-phosphate receptor S1P1 in diabetic retinopathy. Molecular Therapy - Nucleic Acids, 27, 491–504. 10.1016/j.omtn.2021.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods, 25(4), 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Löffler K., Schäfer P., Völkner M., Holdt T., Karl M. O. (2015). Age-dependent Müller glia neurogenic competence in the mouse retina. Glia, 63(10), 1809–1824. 10.1002/glia.22846 [DOI] [PubMed] [Google Scholar]
- Lu Y. B., Iandiev I., Hollborn M., Körber N., Ulbricht E., Hirrlinger P. G., Pannicke T., Wei E. Q., Bringmann A., Wolburg H., Wilhelmsson U., Pekny M., Wiedemann P., Reichenbach A., Käs J. A. (2011). Reactive glial cells: Increased stiffness correlates with increased intermediate filament expression. The FASEB Journal, 25(2), 624–631. 10.1096/fj.10-163790 [DOI] [PubMed] [Google Scholar]
- Lust K., Wittbrodt J. (2018). Activating the regenerative potential of Müller glia cells in a regeneration deficient retina. Elife, 7, 1–23. 10.7554/eLife.32319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto R., Trotta M. C., Petrillo F., Izzo S., Cuomo G., Alfano R., Hermenean A., Barcia J. M., Galdiero M., Platania C. B. M., Bucolo C., D'Amico M. (2020). Resolvin D1 modulates the intracellular VEGF-related miRNAs of retinal photoreceptors challenged with high glucose. Frontiers in Pharmacology, 11, 235. 10.3389/fphar.2020.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Greene J. A., Hernández-Ortega K., Quiroz-Baez R., Resendis-Antonio O., Pichardo-Casas I., Sinclair D. A., Budnik B., Hidalgo-Miranda A., Uribe-Querol E., Ramos-Godínez M. D. P., Martínez-Martínez E. (2021). Quantitative proteomic analysis of extracellular vesicle subgroups isolated by an optimized method combining polymer-based precipitation and size exclusion chromatography. Journal of Extracellular Vesicles, 10(6), e12087. 10.1002/jev2.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead B., Tomarev S. (2020). Extracellular vesicle therapy for retinal diseases. Progress in Retinal and Eye Research, 79, 100849. 10.1016/j.preteyeres.2020.100849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men Y., Yelick J., Jin S., Tian Y., Chiang M. S. R., Higashimori H., Brown E., Jarvis R., Yang Y. (2019). Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nature Communications, 10(1), 4136. 10.1038/s41467-019-11534-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mighty J., Zhou J., Benito-Martin A., Sauma S., Hanna S., Onwumere O., Shi C., Muntzel M., Sauane M., Young M., Molina H., Cox D., Redenti S. (2020). Analysis of adult neural retina extracellular vesicle release, RNA transport and proteomic cargo. Investigative Opthalmology & Visual Science, 61(2), 30. 10.1167/iovs.61.2.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. M., Ackerman K. M., Hayer O., Bailey P., Gorsuch T. J., Hyde R. A., R D. (2013). Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Muller glia proliferation during zebrafish retina regeneration. The Journal of Neuroscience, 33, 6524–6539. 10.1523/JNEUROSCI.3838-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooto S., Akagi T., Kageyama R., Akita J., Mandai M., Honda Y., Takahashi M. (2004). Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proceedings of the National Academy of Sciences, 101, 13654–13659. 10.1073/pnas.0402129101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin-Martinez A., Yan N. E., Tsai E. L. S., Comanita L., Gurdita A., Tachibana N., Liu Z. C., Lu S., Dolati P., Pokrajac N. T., El-Sehemy A., Nickerson P. E. B., Schuurmans C., Bremner R., Wallace V. A. (2021). Photoreceptor nanotubes mediate the in vivo exchange of intracellular material. The EMBO Journal, 40(22), e107264. 10.15252/embj.2020107264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. A., Gonzalez-Cordero A., West E. L., Ribeiro J. R., Aghaizu N., Goh D., Sampson R. D., Georgiadis A., Waldron V., Duran Y., Naeem A., Kloc M., Cristante E., Kruczek K., Warre-Cornish K., Sowden J. C., Smith A. J., Ali R. R. (2016). Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nature Communications, 7, 13029. 10.1038/ncomms13029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena J., Dulger N., Singh T., Zhou J., Majeska R., Redenti S., Vazquez M. (2018). Controlled microenvironments to evaluate chemotactic properties of cultured Müller glia. Experimental Eye Research, 173, 129–137. 10.1016/j.exer.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Baulier E., Ke Y., Young A., Ahmedli N. B., Schwartz S. D., Farber D. B. (2018). Human embryonic stem cells extracellular vesicles and their effects on immortalized human retinal Müller cells. PLoS One, 13(3), e0194004. 10.1371/journal.pone.0194004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereiro X., Ruzafa N., Acera A., Urcola A., Vecino E. (2020). Optimization of a method to isolate and culture adult porcine, rats and mice Müller Glia in order to study retinal diseases. Frontiers in Cellular Neuroscience, 14, 7. 10.3389/fncel.2020.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W., Willms E., Hill A. F. (2021). Understanding extracellular vesicle and nanoparticle heterogeneity: Novel methods and considerations. Proteomics, 21(13-14), e2000118. 10.1002/pmic.202000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Liang X., Dai F., Guan H., Sun J., Yao W. (2020). Rhoa/ROCK pathway activation is regulated by AT1 receptor and participates in smooth muscle migration and dedifferentiation via promoting actin cytoskeleton polymerization. International Journal of Molecular Sciences, 21(15), 5398. 10.3390/ijms21155398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez M., Lamas M. (2009). NMDA Receptor mediates proliferation and CREB phosphorylation in postnatal Müller Glia-derived retinal progenitors. Molecular Vision, (15), 713–721. [PMC free article] [PubMed] [Google Scholar]
- Raymond P. A., Barthel L. K., Bernardos R. L., Perkowski J. J. (2006). Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Developmental Biology, 6, 36. 10.1186/1471-213X-6-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A., Bringmann A. (2013). New functions of Müller cells. Glia, 61(5), 651–678. 10.1002/glia.22477 [DOI] [PubMed] [Google Scholar]
- Reyes-Aguirre L. I., Ferraro S., Quintero H., Sánchez-Serrano S. L., Gómez-Montalvo A., Lamas M. (2013). Glutamate-induced epigenetic and morphological changes allow rat Müller cell dedifferentiation but not further acquisition of a photoreceptor phenotype. Neuroscience, 254, 347–360. 10.1016/j.neuroscience.2013.09.048 [DOI] [PubMed] [Google Scholar]
- Reyes-Aguirre L. I., Lamas M. (2016). Oct4 methylation-mediated silencing as an epigenetic barrier preventing Müller Glia dedifferentiation in a murine model of retinal injury. Frontiers in Neuroscience, 10, 523. 10.3389/fnins.2016.00523.:https://doi.org/PMC5108807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider M. A., Hurwitz S. N., Meckes D. G. J. (2016). ExtraPEG: A polyethylene glycol-based method for enrichment of extracellular vesicles. Scientific Reports, 6, 23978. 10.1038/srep23978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Ferreira T., Llonch S., Borsch O., Postel K., Haas J., Ader M. (2016). Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nature Communications, 7, 13028. 10.1038/ncomms13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer P., Karl M. O. (2017). Prospective purification and characterization of Müller glia in the mouse retina regeneration assay. Glia, 65(5), 828–847. 10.1002/glia.23130 [DOI] [PubMed] [Google Scholar]
- Shah N., Ishii M., Brandon C., Ablonczy Z., Cai J., Liu Y., Chou C. J., Rohrer B. (2018). Extracellular vesicle-mediated long-range communication in stressed retinal pigment epithelial cell monolayers. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1864(8), 2610–2622. 10.1016/j.bbadis.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. S., Balmer J., Barnard A. R., Aslam S. A., Moralli D., Green C. M., Barnea-Cramer A., Duncan I., MacLaren R. E. (2016). Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nature Communications, 7, 13537. 10.1038/ncomms13537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M., Takamiya A., Jiao J. W., Cho K. S., Trevino S. G., Matsuda T., Chen D. F. (2008). alpha-Aminoadipate induces progenitor cell properties of Müller glia in adult mice. Investigative Ophthalmology & Visual Science, 49(3), 1142–1150. 10.1167/iovs.07-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C., Witwer K. W., Aikawa E., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 7(1), 1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L., Hooper M. J., Haugan A. K., Finkbeiner C., Jorstad N., Radulovich N., Wong C. K., Donaldson P. C., Jenkins W., Chen Q., Rieke F., Reh T. A. (2021). Efficient stimulation of retinal regeneration from Müller glia in adult mice using combinations of proneural bHLH transcription factors. Cell Reports, 37(3), 109857. 10.1016/j.celrep.2021.109857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai E. L. S., Ortin-Martinez A., Gurdita A., Comanita L., Yan N., Smiley S., Delplace V., Shoichet M. S., Nickerson P. E. B., Wallace V. A. (2019). Modeling of photoreceptor donor-host interaction following transplantation reveals a role for Crx, Müller Glia, and Rho/ROCK signaling in neurite outgrowth. Stem Cells, 37(4), 529–541. 10.1002/stem.2985 [DOI] [PubMed] [Google Scholar]
- Vera M. S., Simón M. V., Prado Spalm F. H., Ayala-Peña V. B., German O. L., Politi L. E., Santiago-Valtierra F. X., Rotstein N. P. (2021). Ceramide-1-phosphate promotes the migration of retina Müller glial cells. Experimental Eye Research, 202, 108359. 10.1016/j.exer.2020.108359 [DOI] [PubMed] [Google Scholar]
- Wan J., Zhao X. F., Vojtek A., Goldman D. (2014). Retinal injury, growth factors, and cytokines converge on β-catenin and pStat3 signaling to stimulate retina regeneration. Cell Reports, 9(1), 285–297. 10.1016/j.celrep.2014.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms E., Johansson H., Mäger I Lee Y., Blomberg K. E., Sadik M., Alaarg A., Smith C. I., Lehtiö J., El Andaloussi S., Wood M. J., Vader P. (2016). Cells release subpopulations of exosomes with distinct molecular and biological properties. Scientific Reports, 6, 22519. 10.1038/srep22519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Li X., Chen L., Han X., Zhao W., Li L., Chen J. G. (2017). Apobec1 promotes neurotoxicity-induced dedifferentiation of Müller Glial cells. Neurochemical Research, 42(4), 1151–1164. 10.1007/s11064-016-2151-2 [DOI] [PubMed] [Google Scholar]
- Zhao X. F., Wan J., Powell C., Ramachandran R., Myers M. G., Jr., Goldman D. (2014). Leptin and IL-6 family cytokines synergize to stimulate Müller glia reprogramming and retina regeneration. Cell Reports, 9(1), 272–284. 10.1016/j.celrep.2014.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Lu K., Zhang N., Zhao Y., Ma Q., Shen J., Lin Y., Xiang P., Tang Y., Hu X. (2018). Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artificial Cells, Nanomedicine, and Biotechnology, 46(8), 1659–1670. 10.1080/21691401.2017.1388249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Zang J., Liu B., Yu G., Hao L., Liu L., Zhong J. (2020). Oxidative stress-induced RAC autophagy can improve the HUVEC functions by releasing exosomes. Journal of Cellular Physiology, 235(10), 7392–7409. 10.1002/jcp.29641 [DOI] [PMC free article] [PubMed] [Google Scholar]